Abstract
Background
Paramagnetic rim lesions (PRL) may be linked to relapse risk of people with relapsing-remitting multiple sclerosis (pwRRMS).
Methods
PRL load was compared between acutely relapsing pwRRMS and matched stable pwRRMS controls (each group n=21). Additionally, cognitive recovery was compared between acutely relapsing pwRRMS with at least one PRL (PRL+) and those without any PRL (PRL−).
Results
Acutely-relapsing pwRRMS had significantly greater prevalence and number of PRL (p=0.004 and p=0.003) compared to stable controls. These findings remained significant after adjusting for global neuroinflammatory burden (enhancing and non-enhancing lesions). Additionally, acutely-relapsing PRL+ pwRRMS (n=10) had worse recovery of verbal memory following relapse compared to acutely-relapsing PRL− pwRRMS (n=7; p=0.027).
Discussion
These findings may partially explain previously suggested associations between presence of PRL with more severe disease course.
Keywords: paramagnetic rim lesion, multiple sclerosis, relapse, cognitive recovery, SWI, QSM
Introduction
Relapsing-remitting multiple sclerosis (RRMS) is characterized by acute inflammatory demyelinating relapse that results in new or worsening symptoms or neurological signs lasting at least 24 hours and occur in absence of fever or infection.1 Acute inflammatory activity of contrast-enhancing lesions is a characteristic radiological sign of relapse and can be visualized through magnetic resonance imaging (MRI). Recovery of function varies widely and permanent deficits contribute to progressive disability.
Preliminary evidence suggests chronic inflammatory activity, in the form of paramagnetic rim lesions (PRL), may contribute to greater risk of MS relapse.2 However, it is unknown whether PRL allow for stratification of people with RRMS (pwRRMS) into high and low risk groups. Additionally, it is unknown if PRL influence functional recovery. We explored differences in PRL prevalence and number between acutely-relapsing pwRRMS and propensity-matched stable controls.
Methods
Study design:
Data were obtained from a prospective study of cognitive change around MS relapse.3 A group of relapsing (neurologist- and/ or MRI-defined) participants was identified from a baseline cohort of 592 pwRRMS followed for 5 years at three North American MS care centers. A second group of matched, clinically- and MRI-stable participants were subsequently recruited from the same cohort on a case-by-case basis as per a case-control design. For this retrospective analysis, all participants were studied at the Jacobs School of Medicine in Buffalo, New York. Alternate forms of the Symbol Digit Modalities Test (SDMT), Brief Visuospatial Memory Test – Revised (BVMT-R), and California Verbal Learning Test (CVLT) were assessed at clinically stable baseline, during an index timepoint (at relapse, or a stable point for controls), and at 3-month follow-up.
MRI Acquisition and Analysis:
As main outcome sequence, GRE was acquired from 21 acutely relapsing pwRRMS and 40 clinically stable pwRRMS controls on a 3T Signa Excite scanner (GE, Milwaukee, WI) during the index (relapse) timepoint. The MRI acquisition also included T2-FLAIR, pre- and post-contrast T1-weighted images, dual fast spin-echo proton density-weighted images, and high-resolution 3D T1-weighted sequences. The detailed MRI acquisition is described elsewhere.4
T2-FLAIR lesion volume (T2LV) and gadolinium-enhancing LV (GdLV) were quantified using a semi-automated contouring technique.5 Quantitative susceptibility mapping (QSM) was as previously described.6 PRL were identified on QSM using the proposed criteria determined during the 2022 NAIMS Consensus Statement on Imaging Chronic Active Lesions: 1) a paramagnetic rim continuous with at least 2/3 outer lesion edge that is discernable on at least two image slices; 2) a diamagnetic core relative to surrounding extra-lesional white matter; and 3) non-enhancement on post-contrast T1 sequence.7 PRL in pwRRMS without available T1-weighted post-contrast images were confirmed as chronic by identifying an existing T2 lesion at the same location in a previous FLAIR image acquired >6 months prior.
Statistical analyses and propensity matching:
Because GRE images were only obtained from one of three study sites, and some participants were lost to follow-up, the original case-control study design could not be maintained for this analysis. Therefore, stable pwRRMS were propensity matched to the 21 relapsing pwRRMS using the “MatchIt” R libraries, with optimal Mahalanobis distance matching. Matching used relapse cohort as the outcome variable and covariates of baseline age, sex, disease duration, baseline Expanded Disability Status Scale (EDSS), years of education, and time from baseline clinical exam to the index timepoint. Independent-samples T-tests and Mann-Whitney U-test were used to compare parametric and non-parametric measures between the relapsing and stable groups, whereas chi-squared tests were used to compare categorical variables. Additional T2-LV and Gd-LV-adjusted binary logistic regression analyses were used to assess the differences in presence and number of PRL between relapsing and stable pwRRMS groups. Kaplan-Meier survival curves determining the time to relapse in PRL+ and PRL− pwRRMS were then compared using a log-rank test, as well as with Cox regression accounting for baseline age, sex, disease duration, and treatment category (i.e. none, low/moderate efficacy, and high efficacy).8 Last, cognitive changes across the baseline-to-relapse and relapse-to-recovery intervals in relapsing pwRRMS were assessed for the PRL+ and PRL− subgroups using repeated-measures ANCOVA tests which controlled for the same variables as the Cox regression as well as time between the relapse and recovery timepoints. For all analyses, p-values lower than 0.05 were considered statistically significant.
Results
Baseline demographic, clinical and MRI-based information for both the relapsing and stable groups are shown in Table 1. Table 2 shows demographic, clinical, and MRI-based information for the relapsing PRL+ and PRL− groups. As per the propensity matching, the relapsing and stable groups were similar in baseline age, sex, disease duration, years of education, EDSS, SDMT, BVMTR, and CVLT (maximum standard mean difference < 0.69). At the index timepoint, relapsing pwRRMS had greater T2LV (10.0mL vs. 6.1mL, p=0.039), Gad-LV (0.35mL vs. 0.03mL p=0.006), PRL prevalence (57.1% vs. 14.3%, p=0.004), and median PRL number (1 vs. 0, p=0.003) when compared to the stable group. The positive association between the presence or number of PRL and MS relapse remained significant after adjusting for T2LV and Gad-LV (PRL presence exp(β)=6.88, p=0.017; PRL number exp(β)=1.64, p=0.049), while the positive association between PRL volume and MS relapse became marginally non-significant when accounting for these factors (exp(β)=1.003, p = 0.059).
Table 1.
Demographic and clinical characteristics of the study population.
| Demographic and clinical characteristics | Relapsing pwRRMS (n=21) | Stable pwRRMS (n=21) | p-value |
|---|---|---|---|
| At baseline timepoint | |||
| Age, mean ± SD | 39.4 ± 8.0 | 39.9 ± 6.5 | 0.833a |
|
| |||
| Sex | 15 female, 6 male | 17 female, 4 male | 0.469b |
|
| |||
| Disease Duration in years, mean ± SD | 9.0 ± 6.0 | 8.7 ± 4.9 | 0.868a |
|
| |||
| Years of Education, mean ± SD | 15.2 ± 1.9 | 14.6 ± 2.4 | 0.249a |
|
| |||
| EDSS, median (IQR) | 2.7 [1.0 – 6.5] | 2.5 [1.0 - 6.5] | 0.750c |
|
| |||
| SDMT score, mean ± SD | 53.8 ± 12.2 | 56.7 ± 15.4 | 0.501a |
|
| |||
| Short-delay BVMT score, mean ± SD | 24.5 ± 5.7 | 24.8 ± 7.8 | 0.490a |
|
| |||
| Immediate recall CVLT score, mean ± SD | 52.0 ± 12.0 | 54.6 ± 11.2 | 0.518a |
|
| |||
| Medication | 0.568 b | ||
|
| |||
| High efficacy | 19.0% (4/21) | 23.8% (5/21) | |
| Low/moderate efficacy | 52.4% (11/21) | 61.9% (13/21) | |
| None | 28.6% (6/21) | 14.3% (3/21) | |
|
| |||
| At the relapse timepoint | |||
|
| |||
| Time to relapse in days, mean ± SD | 671 ± 327 | 869 ± 315 | 0.053a |
|
| |||
| T2 LV, mean ± SD | 10.0 ± 7.4 | 6.1 ± 6.6 | 0.039 a |
|
| |||
| Gad LV, mean ± SD | 0.35 ± 0.54 | 0.03 ± 0.12 | 0.006 a |
|
| |||
| Presence of PRL % (n/n) | 57.1% (12/21) | 14.3% (3/21) | 0.004 b |
|
| |||
| PRL Number, median (IQR) | 1 [0 – 9] | 0 [0 – 5] | 0.003 c |
|
| |||
| PRL Volume, mean ± SD | 1.04 ± 1.89 | 0.03 ± 0.13 | 0.025 a |
pwRRMS – people with relapsing-remitting multiple sclerosis, EDSS – Expanded Disability Status Scale, SDMT – Symbol Digit Modalities Score, BVMT – Brief Visuospatial Memory Test, CVLT – California Verbal Learning Test, LV – lesion volume, Gad – gadolinium, PRL – paramagnetic rim lesion, SD – standard deviation, IQR – interquartile range.
P-values lower than 0.05 were considered statistically significant and shown in bold. T2 and Gad LV are shown in milliliters (mL). In all cognitive tests, higher scores indicate better cognitive performance. “Low/moderate” efficacy medications included dimethyl fumerate, glatiramer acetate, interferon beta-1a, peginterferon beta-1a, and teriflunomide. “High” efficacy medications included natalizumab and fingolimod.
Independent-samples t-test
Chi-square test, and
Mann-Whitney U test.
Table 2.
Demographic and clinical characteristics of the PRL+ and PRL− relapsing pwRRMS.
| Demographic and clinical characteristics | PRL+ (n=12) | PRL− (n=9) | p-value |
|---|---|---|---|
| At baseline timepoint | |||
|
| |||
| Age, mean ± SD | 39.2 ± 6.8 | 39.8 ± 9.8 | 0.874a |
|
| |||
| Sex | 7 female, 5 male | 8 female, 1 male | 0.178b |
|
| |||
| Disease Duration in years, mean ± SD | 8.3 ± 5.6 | 9.8 ± 6.9 | 0.613a |
|
| |||
| Years of Education, mean ± SD | 14.9 ± 2.1 | 15.6 ± 1.7 | 0.639a |
|
| |||
| EDSS, median (IQR) | 2.5 [1 – 6.5] | 1.5 [1.0 - 6.5] | 0.537c |
|
| |||
| Medication, % (n/n) | 0.126b | ||
|
| |||
| High efficacy | 25.0% (3/12) | 15.8% (1/9) | |
| Low/moderate efficacy | 33.3% (4/12) | 11.1% (7/9) | |
| None | 41.7% (5/12) | 11.1% (1/9) | |
|
| |||
| At the relapse timepoint | |||
|
| |||
| T2 LV, mean ± SD | 12.4 ± 7.4 | 6.8 ± 6.5 | 0.082a |
pwRRMS – people with relapsing-remitting multiple sclerosis, EDSS – Expanded Disability Status Scale, LV – lesion volume, PRL – paramagnetic rim lesion, SD – standard deviation, IQR – interquartile range.
P-values lower than 0.05 were considered statistically significant and shown in bold. T2 is shown in milliliters (mL). “Low/moderate” efficacy medications included dimethyl fumerate, glatiramer acetate, and interferon beta-1a. “High” efficacy medications included natalizumab and fingolimod.
Independent-samples t-test
Chi-square test, and
Mann-Whitney U test.
Figure 1A shows Kaplan-Meier survival curves for PRL+ and PRL− pwRRMS, and their time to relapse. PRL+ pwRRMS had greater and faster occurrence of relapse compared to the PRL− group (p=0.029). This difference became marginally non-significant when accounting for baseline age, sex, disease duration, and medication (p=0.055). Figure 1B shows the time-course of SDMT and CVLT scores within the relapsing pwRRMS, stratified into PRL+ and PRL− subgroups. The presence of at least one PRL significantly impacted CVLT recovery (p=0.027). Specifically, immediate recall CVLT scores in PRL+ cases continued to decline after relapse (mean 49.6 to 47.5; 7/10 (70%) decreased in score) whereas PRL− acutely relapsing pwRRMS evidenced numerical improvement (mean 58.7 to 66.4; 5/7 (71.4%) increased in score) over the same time period. This difference remained significant when accounting for baseline age, sex, disease duration, treatment, and time between relapse and recovery timepoints (p=0.012). No such changes were observed in the stable control group. Similarly, presence of PRL did not affect the SDMT recovery in either the relapsing and stable groups.
Figure 1.
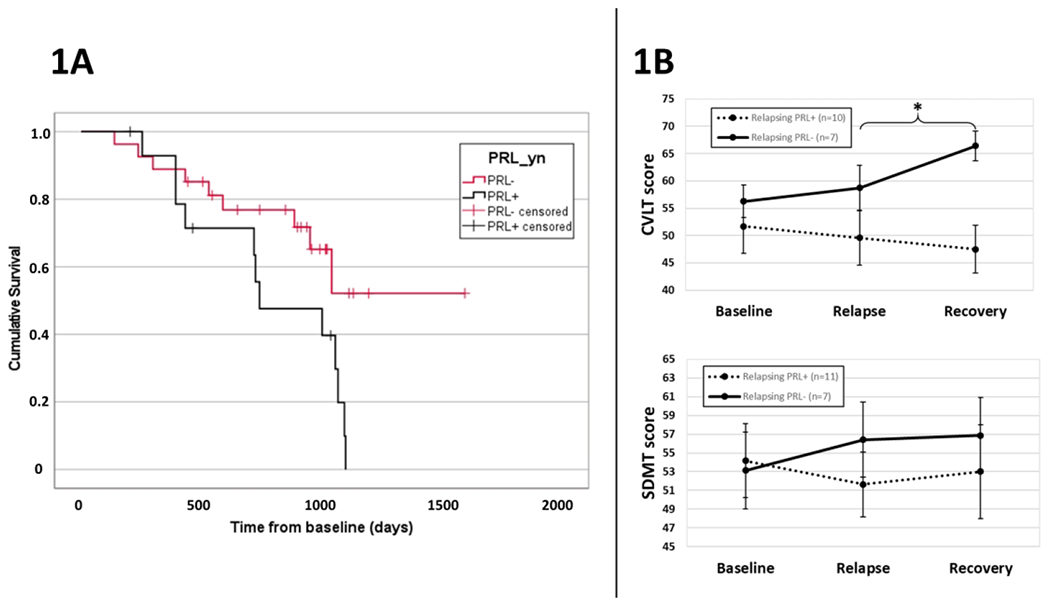
Survival curves for PRL+ and PRL− pwRRMS (1A) and cognitive trajectories in acutely-relapsing pwRRMS (1B).
CVLT – California Verbal Learning Test, PRL – paramagnetic rim lesion, PRL+/− – presence or absence of at least one PRL, pwRRMS – people with relapsing-remitting multiple sclerosis, SDMT – Symbol Digit Modalities Test. An asterisk (*) indicates an ANOVA time (relapse or recovery timepoint) by PRL presence (+/−) interaction effect with p-value lower than 0.05.
Discussion
The findings of this study expand on previous results by showing that PRL are positively associated with relapse occurrence independently of overall lesion burden and acute inflammatory activity. Additionally, we found that relapsing patients with at least one PRL had significantly worse recovery in verbal memory scores when compared to PRL− acutely relapsing pwRRMS. These findings may partially explain previously suggested associations between presence of PRL with more severe disease course, higher disability and higher rate of conversion from clinically isolated syndrome to MS.9, 10
Interestingly, the CVLT performance of the PRL− group, but not the PRL+ group nor the propensity-matched control group, recovered after the relapse and exceeded baseline levels. An increase relative to baseline can be attributed to a practice effect across the relatively brief follow-up periods.11 The continued worsening of their PRL+ peers further suggests that presence of PRL can interfere with the extent of cognitive recovery after acute relapse as well as limit the anticipated learning processes (lack of practice effect).
One limitation is the relatively small sample size, particularly when comparing cognitive changes between PRL+ and PRL− relapsing pwRRMS. Moreover, MRI scans were only available for the relapse timepoint, not for a baseline timepoint, and only at one center. Future prospective studies on a larger cohort comparing relapsing risk in PRL+ and PRL− patients are needed to confirm our results.
Acknowledgements
The authors would like to acknowledge Maryam Mohebbi for her assistance with PRL analysis and Daisy R. for her feedback on the manuscript.
Funding
Research reported in this publication was supported by grants from the National Institutes of Health (R01NS114227 from the National Institute of Neurological Disorders and Stroke and UL1TR001412 from the National Center for Advancing Translational Sciences) and the National MS Society (NMSS RG 5195A4/1). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the NMSS.
Declaration of Conflicting Interests
R.Z. has received personal compensation from Bristol Myers Squibb, EMD Serono, Sanofi, and Novartis for speaking and consultant fees. He received financial support for research activities from Sanofi, Novartis, Bristol Myers Squibb, Mapi Pharma, Keystone Heart, Protembis, and V-WAVE Medical. B.W.G. has participated in speaker’s bureaus and/or served as a consultant for Biogen, EMD Serono, Novartis, Genentech, Celgene/Bristol Meyers Squibb, Sanofi Genzyme, Bayer, Janssen and Horizon. She has also received grant/research support from the agencies listed in the previous sentence. She serves in the editorial board for BMJ Neurology, Children, CNS Drugs, MS International and Frontiers Epidemiology. M.D. received personal compensation from Bristol Myers Squibb, Novartis, EMD Serono and Keystone Heart, and financial support for research activities from Bristol Myers Squibb, Novartis, Mapi Pharma, Keystone Heart, Protembis, and V-WAVE Medical. R.H.B.B received honoraria, speaking, or consulting fees from Biogen, BMS, Celgene, EMD Serono, Genentech, Medday, Merck, Novartis, Roche, and Sanofi, and has received research support from Biogen, BMS, Genentech, Genzyme, and Novartis. He has received royalties from Psychological Assessment resources, Inc.
References
- 1.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018; 17: 162–173. 20171221. DOI: 10.1016/S1474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]
- 2.Guo Z, Long L, Qiu W, et al. The Distributional Characteristics of Multiple Sclerosis Lesions on Quantitative Susceptibility Mapping and Their Correlation With Clinical Severity. Front Neurol 2021; 12: 647519. 20210709. DOI: 10.3389/fneur.2021.647519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinstock Z, Morrow S, Conway D, et al. Interpreting change on the Symbol Digit Modalities Test in people with relapsing multiple sclerosis using the reliable change methodology. Mult Scler 2022; 28: 1101–1111. 20211006. DOI: 10.1177/13524585211049397. [DOI] [PubMed] [Google Scholar]
- 4.Morrow SA, Conway D, Fuchs T, et al. Quantifying cognition and fatigue to enhance the sensitivity of the EDSS during relapses. Mult Scler 2021; 27: 1077–1087. 20201201. DOI: 10.1177/1352458520973618. [DOI] [PubMed] [Google Scholar]
- 5.Zivadinov R, Heininen-Brown M, Schirda CV, et al. Abnormal subcortical deep-gray matter susceptibility-weighted imaging filtered phase measurements in patients with multiple sclerosis: a case-control study. Neuroimage 2012; 59: 331–339. 20110727. DOI: 10.1016/j.neuroimage.2011.07.045. [DOI] [PubMed] [Google Scholar]
- 6.Schweser F, Sommer K, Deistung A, et al. Quantitative susceptibility mapping for investigating subtle susceptibility variations in the human brain. Neuroimage 2012; 62: 2083–2100. 20120601. DOI: 10.1016/j.neuroimage.2012.05.067. [DOI] [PubMed] [Google Scholar]
- 7.Bagnato F NAIMS Symposium on Imaging Chronic Active White Matter Lesions. In: ACTRIMS Forum 2022 West Palm Beach, FL, United States, 2022. [Google Scholar]
- 8.Samjoo IA, Worthington E, Drudge C, et al. Efficacy classification of modern therapies in multiple sclerosis. J Comp Eff Res 2021; 10: 495–507. 20210223. DOI: 10.2217/cer-2020-0267. [DOI] [PubMed] [Google Scholar]
- 9.Clarke MA, Pareto D, Pessini-Ferreira L, et al. Value of 3T Susceptibility-Weighted Imaging in the Diagnosis of Multiple Sclerosis. AJNR Am J Neuroradiol 2020; 41: 1001–1008. 20200521. DOI: 10.3174/ajnr.A6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Absinta M, Sati P, Masuzzo F, et al. Association of Chronic Active Multiple Sclerosis Lesions With Disability In Vivo. JAMA Neurol 2019; 76: 1474–1483. DOI: 10.1001/jamaneurol.2019.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woods SP, Delis DC, Scott JC, et al. The California Verbal Learning Test--second edition: test-retest reliability, practice effects, and reliable change indices for the standard and alternate forms. Arch Clin Neuropsychol 2006; 21: 413–420. 20060714. DOI: 10.1016/j.acn.2006.06.002. [DOI] [PubMed] [Google Scholar]


