Abstract
Cancer initiation and progression drastically alter the microenvironment at the interface between healthy and malignant tissue. This site, termed the peritumor, bears unique physical and immune attributes that together further promote tumor progression through interconnected mechanical signaling and immune activity. In this review, we describe the distinct physical features of the peritumoral microenvironment and link their relationship to immune responses. The peritumor is a region rich in biomarkers and therapeutic targets, and thus is a key focus for future cancer research as well as clinical outlooks, particularly to understand and overcome novel mechanisms of immunotherapy resistance.
Keywords: Tumor microenvironment, peritumor, physical immunity, immunotherapy, physical oncology
The peritumor: a region rich in physical-immune cross-talk
The physical and biochemical interactions between cancer cells and tumor-associated stroma have long been studied and targeted as a source of tumor progression and immune evasion [1,2]. However, most of this research has focused on elements within the bounds of the tumor border, with significantly less attention given to the peritumor, which is the interface between the tumor and the neighboring normal tissue. The peritumor is phenotypically and functionally distinct from neoplastic and normal tissues, and represents a unique intermediary point between healthy and cancerous tissue [3]. Mechanical aberrations that arise in the peritumor during cancer growth contribute to shaping its unique intermediate profile, and investigations of this region are leading to advances in cancer diagnostic [4] and prognostic [5] capabilities. Therefore, the biophysical landscape of the peritumor provides a rich area of inquiry to better understand tumor progression, immune evasion, and treatment response.
Herein, we first summarize the four physical hallmarks of the peritumor, namely elevated solid mechanical stresses (see Glossary), heterogeneous fluid pressure and the consequent flow, altered tissue stiffness, and changes in tissue microarchitecture from the subcellular to tissue levels. We describe how these biophysical abnormalities affect immune responses, connecting the cell-level characterizations with their higher-order behavior, and highlight the links between the physical and immune tumor microenvironments. Moreover, we discuss how targeting the physical-immune cross-talk informs current treatment regimens and serves as a promising direction for future therapeutic strategies.
The physical microenvironment of the peritumor modulates pro- and anti-tumor immunity
Tumor mechanical aberrancies greatly influence the adaptive and innate tumor immune response in the peritumor. Immune cells such as T and B cells are often sparse within solid tumors and are instead sequestered to the peritumor region due to immunosuppressive biochemical mechanisms and physical barriers preventing their infiltration [6]. Abnormal mechanical cues such as increased stiffness and fluid flow also polarize tumor-associated macrophages to a tumor-supporting state. In the following sections categorized by the four physical hallmarks of cancer, we discuss the link between these abnormal mechanical cues and peritumor immunity (summarized in Table 1).
Table 1.
The significance of immune and immunomodulatory cell types in relation to the physical hallmarks of cancer
| Physical hallmark |
Cell type | Significance/interaction | Refs |
|---|---|---|---|
| Solid stress | Fibroblast | Increased expression of TGF-B and Collagen I | [18] |
| CD8+ T-cell | Impaired infiltration by means of HEV remodeling | [16] | |
| Epithelial cell | Wnt/β-catenin-mediated expression of pathways for immune-evasion | [22] | |
| Fluid flow | CAF | Invasive and immunosuppressive phenotype activated by shear stress | [36,63] |
| M2 macrophage | Polarized by Stat3/6 pathway | [45] | |
| Macrophage | Repressed activity by mechano-activated Notch expression | [49] | |
| T cell | Activated by mechanosensitive Piezo1 channel | [50] | |
| Stiffness | M2 macrophage | Enhanced polarization and recruitment | [82,83] |
| Macrophage | Enhanced activation via Piezo 1 | [72] | |
| CD8+ T cell | Inhibition of activity by means of elevated PD-L1 production | [81] | |
| Lysyl oxidase (LOX) mediated crosslinking guides metastatic remodeling | [79] | ||
| T cell | Stiffness-dependent proliferation, differentiation, and regulation of cytotoxic activity and regulatory markers | [67,69,70] | |
| Reduced microtubule organization indicative of reduced activation at high stiffness | [68] | ||
| Increased secretion of interferon-gamma (IFN-ɣ) at high stiffness | [68] | ||
| T helper type 1 (Th1) | Enhanced differentiation via Piezo1-mediated signaling in dendritic cells | [75] | |
| Regulatory T cell (Treg) | Enhanced differentiation via Piezo1-mediated signaling in dendritic cells | [75] | |
| Altered microarchitecture | T cells | Altered migration and infiltration | [6,99] |
| Neutrophil | Increases MMPs, thereby altering the microarchitecture | [43] | |
| Natural killer cell | Secrete IFN-γ, leading to increased deposition of fibronectin-1 by cancer cells | [93] |
Solid stress in the peritumor
Internal, growth-induced stresses arise as cells replicate, in addition to the stresses at the immediate tumor boundary as the tumor increases in volume and displaces surrounding tissues [7,8]. This solid stress transmission is scale-dependent, as tumor cells experience lower stress than measured at the tissue scale [9]. Tumor cells in metastatic tumors experience different levels of solid stresses compared to primary tumors from the same cancer cells, demonstrating the critical role of the host organ microenvironment in solid stress genesis [7,9]. The levels of solid stress critically affect tumor growth and progression by various mechanisms, including compressing surrounding vasculature [10], enhancing cell migration, and influencing cell proliferation rates [11,12] (Figures 1 and 2). Notably, the levels of radial and circumferential solid stress in the peritumor differentiate infiltrative tumors from nodular tumors in several cancers, including glioblastoma (GBM) and lung metastasis [8,13,14]. Compared to infiltrative tumors, nodular tumors exert higher stresses on the surrounding tissue, resulting in neuronal damage, reduced perfusion, compressed vasculature, and nuclear deformation in the peritumor [8,15]. This growth pattern effect is mirrored within lung metastases [13], where alveolar structure-function within the peritumor of nodular, but not infiltrative, tumors is compromised compared to healthy tissues.
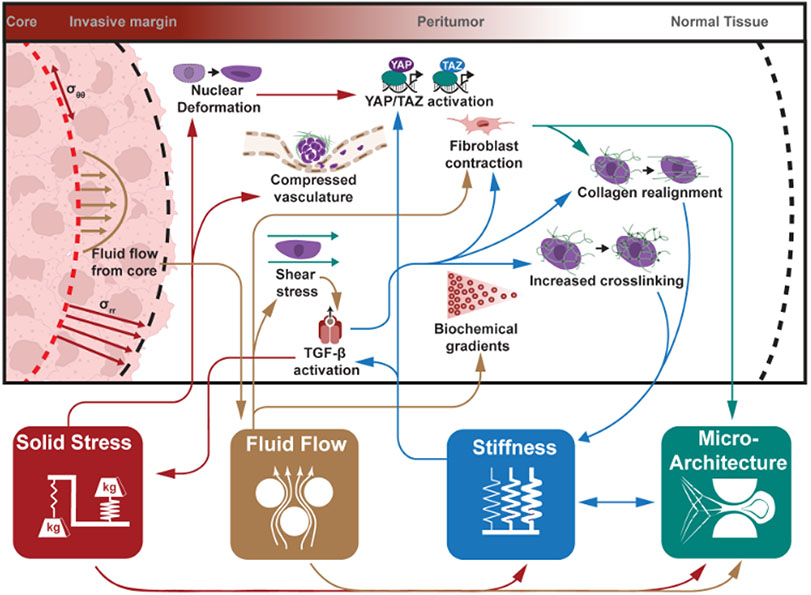
Figure 1. The highly interconnected physical landscape of the peritumor.
The four physical hallmarks of cancer – solid stress, fluid flow, stiffness, and microarchitecture – present as region-specific alterations in the peritumor. As changes occur within one hallmark, their downstream effects may create a positive feedback loop, potentially activating or exacerbating activity within other hallmarks. For example, increased solid stress (red arrows) from tumor growth results in radial and circumferential stress accumulating in the tissue. These stresses are capable of deforming cells and vasculature, while also contributing to the evolution of other physical hallmarks. A combination of leaky vasculature and faulty drainage contributes to a flow of fluid from the tumor through the peritumor. This flow (brown arrows) alters distributions of various molecular gradients (e.g., chemokines) and distributes them throughout the peritumor. Intriguingly, fluid flow and associated shear stresses activate cells (i.e., fibroblasts) as well as latent stores of growth factors, which have downstream implications in altering tissue stiffness and microarchitecture. Growth factors such as TGF-β activated by alterations in native stiffness (blue arrows) can exacerbate elevated matrix stiffness through such means as inducing fibroblast contraction, collagen realignment, or increases in matrix cross-linking. The latter two contribute to further increasing matrix stiffness. Finally, alterations in matrix and cellular microarchitecture (green arrows) arising from solid stress, fluid flow, matrix stiffness, and downstream activity of these pathways (i.e., fibroblast contraction, collagen realignment) contribute to the elevation of matrix stiffness. As shown by the mapping of physical interactions, these physical attributes are highly interconnected and foster the growth and development of each other.
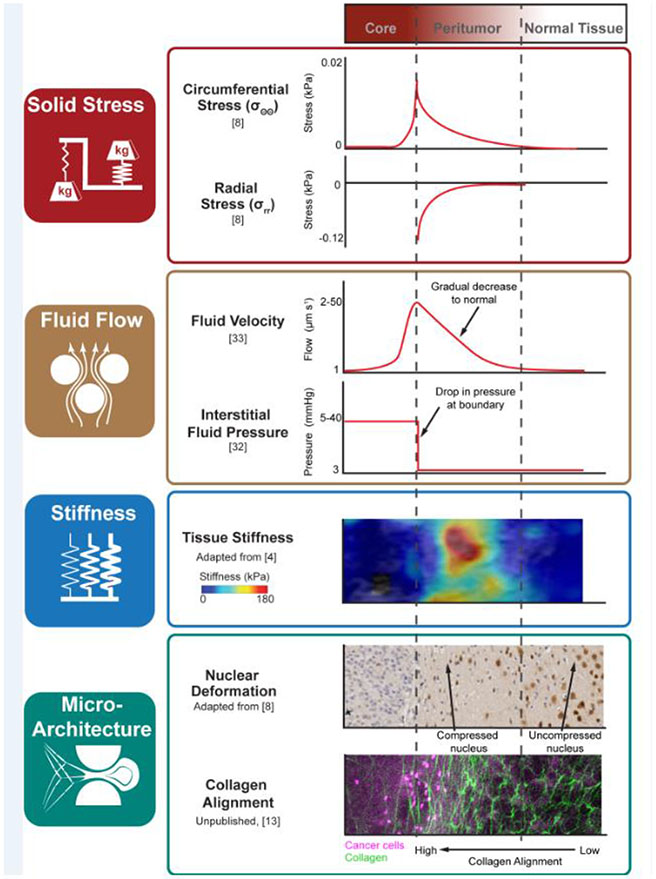
Figure 2. Profiling key physical hallmarks of the peritumor.
The peritumor exhibits alterations to four physical hallmarks: solid stress, fluid flow, stiffness, and microarchitecture. In regards to the tumor core, peritumor, and normal tissue, sample profiles of each hallmark demonstrate the distinct, region-specific characteristics of the peritumor. Solid stress arising from the growth of the tumor and resistance of the surrounding ECM acts in the circumferential (σθθ) and radial (σrr) directions. Consequently, single nuclei undergo varying degrees of nuclear compaction before reaching normal, spherical shapes within normal tissue. Fluid flow caused by leaky vasculature and faulty drainage mechanisms increase interstitial fluid pressure (IFP) at the tumor boundary, leading to positive fluid flow (Vf) from the tumor core that is capable of disseminating tumor components (e.g. cancer cells). Stiffness alterations within the peritumor may be detected using various elastography methods. Shown here are dramatically higher stiffnesses within the peritumor region of breast cancer tumors, represented by the color bar shown: highly stiff regions (red) are found in the peritumoral region compared to relatively less stiff (blue) regions towards healthy tissue. Finally, alterations in microarchitecture occur not only at the tumor surface but through the peritumoral stroma. Collagen images acquired using multiphoton microscopy and second harmonic generation show an alignment of collagen (green) at the boundary of a breast cancer tumor metastasized in the lung (pink). This gradient of alignment can be found near tumors undergoing growth and invasion as the peritumor region is modified to facilitate further cell movement.
Solid stress also modulates the immune response. Solid stress impedes the infiltration of lymphocytes/splenocytes by remodeling high endothelial venules (HEVs) in lymph node metastasis [16]. At the tumor periphery, high levels of circumferential tension and radial compression thwart immune cell trafficking to the tumor by stretching and collapsing blood and lymphatic vessels in the peritumor into elliptical shapes [17]. Additionally, compression of peritumoral stromal cells activates immunomodulatory pathways (Figure 3 and Table 1). Compression of fibroblasts in vitro increases their expression of transforming growth factor beta (TGF-β), collagen I, and alpha-smooth muscle actin (α-SMA) [18], suggesting an activated and immunosuppressive state [19]. TGF-β inhibits natural killer (NK) cell activity [20], cooperates with programmed cell death protein 1 (PD1) signaling to suppress T cell activity from tumordraining lymph nodes, and expands regulatory T cells (Tregs), which in turn inhibit T cell function via TGF-β signaling in a pathological feedback loop [21]. Moreover, compression of colon tissue activates Wnt/β-catenin signaling [22], which promotes immune evasion in colorectal cancer [23].
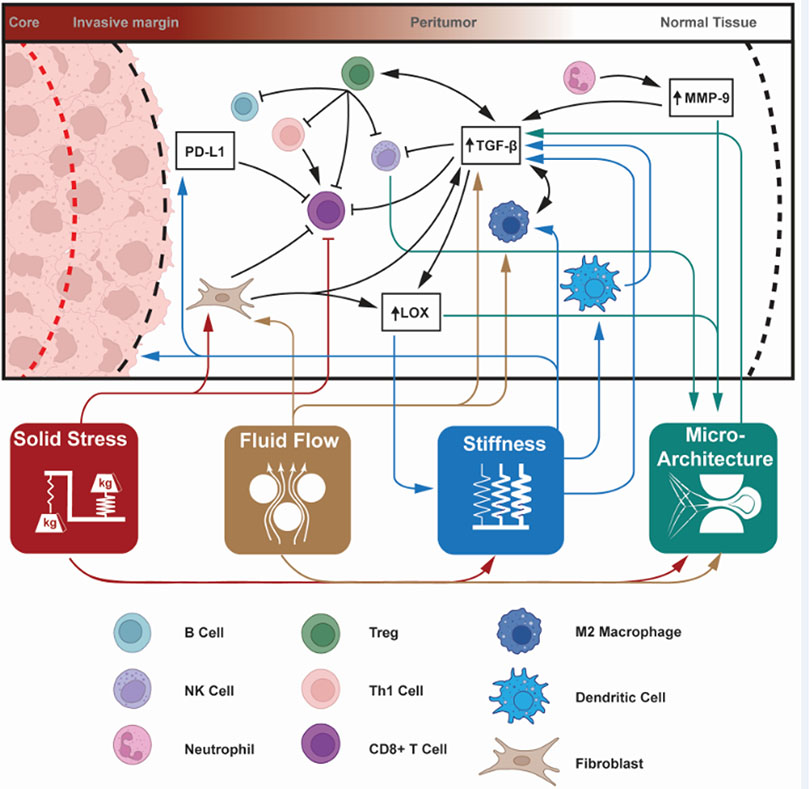
Figure 3. The physical landscape of the peritumor directly and indirectly affects the activity of immune cells to activate or suppress immunity.
Alterations to the four physical hallmarks of cancer – solid stress, fluid flow, stiffness, and microarchitecture – can have direct effects on immune cell activity (color-coded lines from attribute to specific cell type). These effects can be activating (sharp arrow) or inhibitory (blunt arrow) and are highly interconnected. Attributes may indirectly modulate immune cell behavior through intermediate signaling pathways. Prominent among these immunomodulatory molecules are: immune-inhibitory programmed death-ligand 1 (PD-L1), anti-inflammatory cytokine transforming growth factor β (TGF-β), matrix crosslinking enzyme lysyl oxidase (LOX), matrix remodeling matrix metalloproteinase-9 (MMP-9), and macrophage-specific transcription factors STAT3/6. The highly interrelated nature of the peritumor environment means that immune cells can be indirectly impacted by pathways which are up- or down-regulated by the physical microenvironment, which may then further activate (black sharp arrow) or inhibit (black blunt arrow) immune cells or immunomodulatory pathways. We illustrate the connections between the abundant elements that interact with mechano-immunity in the peritumor, and list the major components in Table 1 in the main text.
Solid stress that arises in the peritumor promotes mechanical cell competition [24]. Cells under mechanical competition experience cell fusion [25] and nuclear envelope rupture [26,27]. Nuclear rupture induces the cGAS-STING pathway, which stimulates expression of type I interferons and other pro-inflammatory genes in normal cells [28]. However, the cGAS-STING pathway is aberrant in cancer and compromises innate immune signaling; type I interferon, cGAS, and STING expression is disrupted, which promotes cell invasion, metastasis, and resistance to immunotherapy [29,30].
One of the challenges in probing the physical-immune crosstalk has been the lack of tools to quantify in vivo solid stress. Most key studies elucidating solid stress in mouse and human tumors used invasive ex vivo techniques [7,15,17]. However, non-invasive intravital imaging of in vivo solid stress in primary and metastatic tumors has been reported [9,13]. This technique allows for high resolution, spatiotemporal measurements of solid stress at a cellular-level in the native physical-immune microenvironment. Studies employing this technique reveal that peritumoral stresses are more anisotropic compared to intratumoral stresses. Non-invasive solid stress measurements preserve the existing in vivo physical and immune microenvironment and enable real-time dynamic evaluation of solid stress effects on cancer-immune cell interactions.
Studies over the past 20 years have highlighted the role of solid stress in the pathology and progression of cancer [1,31]. However, the relationship between solid stress and immunosuppression has only recently gained attention. As novel tools to study solid stress are developed, the mechanisms and consequences of the physical-immune crosstalk can be further investigated. This cross-talk is currently known to upregulate signaling axes implicated in facilitating cancer immune evasion, such as the Wnt/β-catenin, TGF-β, and PD-L1 signaling axes. Thus, combining therapeutic strategies that target these pathways to relieve solid stress may enhance efficacy of cancer therapies.
Fluid pressure and flow in the peritumor
Due to a combination of leaky vasculature and a lack of lymphatic drainage, the intratumoral environment exhibits a highly elevated interstitial fluid pressure (IFP) between 5 to 40 mmHg [1]. At the tumor margin, IFP reduces to normal ranges of −3 to 3 mmHg [32], causing fluid leakage into the peritumor at rates of 2-50 μm s−1 [33]. This is in contrast to normal interstitial flow rates of ~0.1-1 μm s−1 [34] (Figure 2). Increased fluid flow from the tumor core to peritumor alters immune cell distributions [35], modifies chemotactic gradients [36], and introduces immunomodulatory signaling factors [31] and cancer cells into the peritumor [37], thereby encouraging metastasis and facilitating cancer cell invasion (Figures 1 and 3) [37-39].
Fluid flow into a peritumoral matrix rich in glycosaminoglycans and proteoglycans promotes fluid retention, resulting in edema. Abnormal fluid flow is prevalent in brain cancers: flow into healthy tissues contributes to total peritumoral brain edema, intracranial pressure, and invasion of cancerous cells into the peritumor. A recent MRI study of GBM indicates that flow is not always directed radially outward, possibly due to the role of altered matrix microarchitecture [40]. Several of the potential pathways involving peritumor brain edema, including vascular endothelial growth factors (VEGF), matrix metalloproteinases (MMPs), interleukins [41-43], and the CXCR-4/CXCL12 chemokine axis [41], are known to be mechanically activated. As these pathways are activated, downstream products contribute to edema via enhanced pro-angiogenic behavior, additional fluid accumulation, and further activation of edematous pathways, resulting in a positive feedback loop. Alarmingly, recent evidence reports anti-PD1 immune checkpoint blockers can exacerbate MMP-mediated peritumoral edema in GBM patients [44], highlighting the complexities involving this positive feedback loop.
Fluid flow and flow-induced shear stresses induce pathways such as STAT3/6 [45], which contributes to polarizing macrophages towards an immunosuppressive M2-type [46] (Figure 3). However, the mechano-activated Notch pathway demonstrates context-specific modulation of pro- or anti-tumor effects [47]. While Notch in cancer cells can sustain the immunosuppressive STAT3 pathway [48], the expression of Notch in macrophages enhances antitumor effects by repressing tumor-associated macrophage activity [49]. Additionally, fluid shear stresses promote T cell activation via the mechanosensitive Ca2+ channel Piezo1 [50].
Shear stresses also indirectly influence the immune response. Stresses trigger the invasive and immunosuppressive phenotype of nearby fibroblasts either through mechanotransduction [36] or via the activation of latent stores of TGF-β [35,51]. Within the context of pancreatic tumors [52], the production of chemokine CXC ligand 12 (CXCL12) by stromal cells within the tumor prevents the infiltration of effector T cells, instead sequestering them to the boundary of the tumor. Shear stresses activate fibroblasts and increase the activity of MMPs [35], which alter the peritumoral microarchitecture. MMPs can induce further expression of TGF-β [53], suppress T cell proliferation [54], and affect leukocyte infiltration [55,56].
Fluid flow in the peritumor has wide-ranging implications in immunosuppression. Through the exudation of tumor cells and signaling factors, coupled with the application of shear stresses that trigger additional signaling cascades, fluid flow creates an environment that impacts immune cell activation and infiltration. The presence and consequences of peritumoral fluid flow are widely accepted and addressing the underlying sources and effects of this flow is an ongoing focus of treatment development.
Stiffness alteration in the peritumor
Stiffness, also referred to as rigidity or elasticity, promotes the initiation, progression, and invasion of tumors [57,58]. Changes in stiffness emerge as a result of extracellular matrix (ECM) production and remodeling at the tumor periphery as activated fibroblasts contract matrix elements, such as collagen, to reorganize the ECM in response to chemical or mechanical signaling [59,60] (Figure 1). Notably, collagen realignment stiffens the architecture via a phenomenon known as strain-stiffening [61] and disrupts the balance of intracellular tension at the single cell level [57]. Additionally, matrix crosslinking increases matrix stiffness, forming a continued positive-feedback cycle [62] that stimulates heightened fibroblast activity, TGF-β production, ECM alignment, and cancer cell invasion into the peritumor for further remodeling [35,36,63], while also contributing to tensile solid stresses exerted in the peritumor [7].
Local stiffness alterations disrupt mechanical feedback in cells and directly impact immune cell behavior (Figure 3). T cell behavior is highly dependent on local stiffness. One mechanism by which T cells sense their environmental stiffness is through yes-associated protein (YAP), a known effector of the mechanoresponsive Hippo pathway. In CD4+ and CD8+ cells, YAP is known to suppress T cell activation, differentiation, and proliferation [64]. YAP activation is hindered in T cells cultured on soft (4 kPa) alginate scaffolds, which reduces T cell proliferation and activation by restricting the translocation of NFAT1 into the nucleus [65]; conversely, stiff (40 kPa) alginate scaffolds promote T cell proliferation and activation. Similarly, stiff (100 kPa) polyacrylamide substrates promote CD4+ T cell activation, as determined by higher interferon-gamma (IFN-γ) and interleukin-2 (IL-2) production [66]. In contrast, increasing collagen concentrations from 1 to 4 mg/mL, which corresponds with increasing matrix stiffness from ~0.17 to 1.6 kPa, respectively [57], reduces T cell proliferation and infiltration, downregulates cytotoxic activity markers, and upregulates regulatory markers [67]. At high stiffness, the organization of CD4+ T cell microtubule structures decreases, implying reduced activation [68]. Studies suggest there is an optimal matrix stiffness for T cell activation and expansion; while one study reports this value as 25 kPa [69], another study cites an optimal value of ~1 kPa [70]. Evidently, there are variations in experimental findings on T cell activity in response to matrix stiffness; ultimately, matrix composition, relative substrate stiffness, and study design can influence observed T cell behavior.
In addition to T cells, macrophages and dendritic cells also respond to the stiffness of their surroundings. Macrophages sense and migrate towards fibroblast-mediated ECM deformation [71], with subsequent activation of mechanosensitive pathways such as Piezo1. In stiff environments, Piezo1 mediates actin polymerization in macrophages which further increases Piezo1 activity, resulting in a positive feedback loop that activates additional macrophages [72]. Inhibiting or deleting Piezo1, however, prevents the infiltration of immunosuppressive myeloid-derived suppressor cells (MDSCs) and subsequently enhances the activation of CD4+ and CD8+ T cells in pancreatic ductal adenocarcinoma (PDAC) tumor-bearing mice [73]. In the case of dendritic cells, which link the adaptive and innate immune systems, Piezo1 and YAP/TAZ pathways contribute to increased activation of cells [74]. Dendritic cell signaling via the Piezo1 pathway, as well as secretion of IL-12 and TGF-β, then directs the differentiation of T helper type 1 (Th1) and Treg cells [75].
Stiffening also activates latent TGF-β stores in peritumoral ECM [76]; as previously described, these stores are also activated by fluid flow. The presence of TGF-β induces invasiveness and upregulates the ECM cross-linking enzyme lysyl oxidase (LOX) in cancer-associated fibroblasts (CAFs) [77], thereby increasing ECM stiffness [78]. In the immune context, the relationship of LOX and immune cells is not well understood, but data suggest that following chemotherapy, CD8+ T cells expressing LOX contribute to the colonization of metastatic sites by remodeling premetastatic niches [79]. However, there are reports that LOX also acts as a tumor suppressor [80]. Conversely, matrix stiffness also promotes immune evasion via enhanced programmed death ligand 1 (PD-L1) expression [81] and M2 macrophage polarization and recruitment [82,83]. Matrix stiffness positively correlates with increased infiltration of activated macrophages as well as upregulated TGF-β signaling [84]. In turn, malignant cells manipulate the surrounding physical environment to further evade immune responses and promote metastasis [20,71].
Overall, stiffness in the peritumor plays an integral role in fibroblast and immune cell activation. This physical attribute has also recently emerged as its own powerful diagnostic (Figure 2) and prognostic tool in the clinic; stiffness in the peritumor, which can be higher than within the tumor itself, enables differentiation between malignant and benign breast tumors [4]. These prognostic indicators directly relate to the mechano-sensitivity and mechanically-induced activity of immune cells as observed within environments of altered stiffness.
Microarchitecture in the peritumor
Peritumoral cells and ECM undergo changes in morphology and architecture as the environment alters due to solid stress, fluid flow, and stiffening substrates (Figure 1). Certain tumor growth patterns (e.g., nodular) cause local tensile and compressive solid stresses that stretch the peritumoral ECM, causing collagen fibers to become highly organized and dense at the tumor periphery [7]. Additional ECM remodeling arises from the contractile behavior of CAFs and tumor cells: from this contraction, collagen fibers become highly aligned [85,86]. Altogether, such remodeling increases matrix stiffness, which, as previously discussed, impacts immune cell activity.
ECM microarchitecture remodeling also affects cancer and immune cell mobility. Highly aligned collagen fibers parallel to the tumor border hinder cell penetration, whereas orthogonally aligned collagen fibers facilitate cell migration across the peritumor [87,88] (Figure 2). In pancreatic cancer, dense collagen networks in the stroma and peritumor physically trap T cells, impeding their interaction with cancer cells [89]. Similarly, in vitro studies of T cell migration on lung tumor slices reveal that the dense matrix architecture at the peritumor severely limits T cell migration and infiltration, causing T cells to accumulate in the peritumor [6]. These observed cell migration patterns may have metabolic explanations; for example, highly motile cancer cells expend more energy when migrating through dense collagen matrices, and therefore migrate slower and shorter distances [90].
Although remodeled architecture reduces immune cell infiltration in some cases, restructured matrix around tumors can also be beneficial. A remodeled collagen rim around desmoplastic tumors (i.e., tumors with a dense fibrous stroma due to increased ECM deposition) likely buffers localized solid stresses to prevent compression of vessels in the peritumor [17,91]: patients with metastatic liver tumors surrounded by a collagen rim exhibit better prognosis compared to patients bearing tumors without a collagen rim [92]. However, structural changes also impact functional mechanics in the peritumor, as seen in lung tumors: collagen waviness, which plays a key role in the micromechanics of alveoli, is reduced at the peritumor [13]. The high degree of matrix restructuring compresses vasculature and leads to observed decreases in capillary function at the alveolar scale [13]; taken together, these observations highlight the role of the peritumor in understanding the dysfunction of alveoli surrounding tumors.
Like CAFs, immune cells also promote changes in ECM architecture (Figure 3). In the case of melanoma, NK cells secrete IFN-γ, which leads to increased deposition of fibronectin-1 by cancer cells and results in an altered tumor microarchitecture that impedes metastasis [93]. However, in breast cancers treated with chemotherapy, CD8+ T cells increase expression of LOX, resulting in ECM crosslinking and consequent pulmonary metastasis [79]. Additionally, neutrophils serve as a major source of MMP-9, a protease responsible for altering the surrounding matrix [43] and activating TGF-β [94]. Changes in matrix architecture arising from matrix degradation by MMPs and system remodeling hold prognostic significance: expression of certain MMPs (MMP7, MMP1, MMP14) at the invasive border of the peritumor are associated with larger tumor size, positive nodal status, and desmoplastic reaction respectively [95]. Taken together, the ability for immune cells to promote matrix modification highlights their role in altering the microarchitecture of the peritumoral stroma and modulating cancer progression.
Cells undergo alterations to their cellular morphology as a downstream effect of solid stress [11,96]. The application of compressive solid stresses at levels comparable to that of the in vivo peritumor region contributes to significant cell elongation in GBM cells in vitro [96], and in mouse and human models for GBM. Peritumoral cells which exhibit an elongated morphology also express death-associated protein kinase 1 (DAPK1) [97], which modulates T cell activity [98]. Morphological changes such as these can affect immune cell behavior directly. In melanoma, for example, elongated T cells in the peritumor migrate faster than rounded T cells in the tumor core [99].
Peritumoral matrix remodeling induces subcellular morphological changes, including the flattening and deformation of the nucleus and nuclear pores, and compromised integrity of the nuclear envelope. In vivo studies of solid stress in mice with glioma show that altered microarchitecture in healthy tissue surrounding nodular tumors with high solid stress results in local deformations of nuclei [8]. This result highlights the importance of solid stress transmission at the level of subcellular architecture. Altered nuclear shape coincides with swelling of the perinuclear space, indicating the high risk of developing neoplastic growth via lateral cancerization in the peritumor [100]. At the protein level, these subcellular changes trigger alterations in protein expression [101], protein unfolding energy requirements [101,102], pore resistance to chemical and molecular transport [102], and orientation and gene expression of chromosomes [103,104]. These effects, in turn, increase the stiffening of nuclei and the import of regulatory factors such as YAP [101], which promotes T cell exclusion from the tumor and inhibits CD4+ and CD8+ T cell activation and CD4+ T cell differentiation.
Thus, by analyzing changes to cell morphology and structural microarchitecture, the dynamic relationship between cells, microenvironment, and physical characteristics of the peritumor becomes clear. Characterizing changes to these microarchitectural features in the tumor and peritumor contributes to a better understanding of the underlying mechanisms and the development of potential interventions to combat tumor progression and cancer invasion.
Therapeutic significance of linking physical and immune attributes of the peritumor microenvironment
The tumor examples cited in the previous sections are mainly immune-excluded phenotypes, which are characterized by immune cells restricted to the peritumor due to their inability to penetrate the tumor. Understanding the mechanisms that lead to immune-exclusion and immunotherapy resistance remains a challenge and results in difficulties in specifically targeting the peritumor. However, several recent studies address this immune-excluded tumor phenotype by focusing on physical-immune cross-talk.
Therapeutics that target physical aspects of the peritumor can directly impact immune cell activity. For example, blocking CXCR4/CXCL12 signaling reduces CAF recruitment and solid stress in metastatic breast cancer models, which concurrently reduces immunosuppression and improves the outcome of immune checkpoint blockade therapies [105,106]. Additional reduction of solid stress and peritumoral edema in GBM models using losartan, a drug which lowers levels of hyaluronic acid and other ECM molecules, enhances the immune response by repolarizing immunosuppressive myeloid cells and increasing cytotoxic T cell infiltration, resulting in improved outcomes of immune checkpoint blockade [44].
Abnormal fluid flow and the resulting downstream effects represent additional targets to improve anti-tumor immunity. Lymphatics play a critical role in maintaining local fluid distributions; dysfunctional lymphatics increase peritumoral edema, which fosters accumulation of immunosuppressive cells and inflammatory cytokines, in addition to decreasing intratumoral accumulation of cytotoxic CD8+ T cells [107]. Thus, normalizing the lymphatics with antiangiogenic therapy could limit fluid convection into the peritumor to reduce edema and increase cytotoxic T cell accumulation intratumorally [108]. Lymphatic normalization is suggested to improve delivery of drugs into the tumor via decreasing IFP; indeed, several ongoing clinical trials are investigating the combination of antiangiogenic therapy and immune checkpoint blockers as a means of bolstering immune response [109]. However, reducing IFP to zero via removal of the tumor capsule did not improve drug penetration in a model of ovarian carcinoma; this study suggests that remaining physical barriers, including matrix and cellular components, should be targeted in conjunction with lowering IFP [110]. Indeed, design of therapeutic regimes should incorporate tumor-specific corrections to normalize vasculature and modify altered ECM to enhance delivery.
Intriguingly, impairing lymphatic flow in the lungs of non-tumor-bearing mice promotes the formation of tertiary lymphoid structures (TLS) [111]: lymph node-like structures rich in T-cell, B-cells, and dendritic cells that develop inside non-lymphoid organs [112]. TLS can be found at the tumor center and invasive margin [112] and are hypothesized to be a region for local activation and differentiation of tumor-infiltrating naïve lymphocytes. Thus, these are potential targets for immunotherapy. In breast cancer, a high density of peritumoral TLS is predictive of a worse disease-free survival (DFS) and overall survival (OS); patients lacking both adjacent TLS and distal TLS had the best clinical outcomes [113]. However, the link between mechanical forces and peritumoral TLS remains to be fully elucidated and is a promising opportunity for further research.
Altering the matrix architecture can increase T cell contact with cancer cells. When matrix content is reduced by using collagenase, T cells preferentially migrate to and localize in the peritumoral stroma of human lung tumors [6]. When combined with immune checkpoint blockade therapy, remodeling the collagen matrix also enhances T cell activation and infiltration into tumors and repolarizes suppressive macrophage populations [114]. Reducing collagen, hyaluronic acid, and other ECM molecules using losartan increases penetration of therapies into the tumor and improves immunotherapy in metastatic breast and GBM models [44,105]. Thus, targeting ECM or sources of ECM remodeling could effectively disrupt the physical barrier that prevents immune cell infiltration and drug delivery, while also slowing or halting stiffening to limit pathological progression [115,116]. This approach is particularly effective in PDAC, a cancer which often excludes immune cells to the peritumor and is therefore resistant to traditional immune checkpoint inhibitors (i.e., anti-PD1/PD-L1/CTLA-4) that augment CD8+ T cell infiltration and/or function. However, treatment with 6-diazo-5-oxo-l-norleucine (DON), a small molecule glutamine analog, results in ECM remodeling that facilitates immune cell infiltration from the peritumor while also targeting tumor cells by sensitizing them to anti-PD-1 therapy [117]. Indeed, in a model of PDAC that presents with T cells excluded to the peritumor, the depletion of CAFs expressing fibroblast activation protein by inhibiting CXC motif chemokine receptor 4 (CXCR4), which mediates desmoplasia in PDAC [118], allows for T cell accumulation among cancer cells and sensitizes them to α-PD-L1 therapy [52]. Concurrent improvement of T-cell-mediated cytotoxicity could be accomplished by increasing cell stiffness by depleting cholesterol within cancer cells via methyl-β-cyclodextrin [119].
Cell-based immunotherapy represents another clinical approach that may benefit from peritumoral targeting. Chimeric antigen receptor (CAR) T cells are highly efficacious in treating hematological cancers clinically. However, the translation of CAR T cell therapy to solid tumors remains a challenge in part due to the immunosuppressive peritumor microenvironment and physical barriers, such as the tumor stroma and dysfunctional vasculature, preventing effective and homogeneous CAR T cell infiltration. There have been efforts to engineer CAR T cells to overcome immunosuppression by providing immunostimulatory signals via inducible cytokines [120] and incurring resistance to inhibitory signals such as TGF-β [121]. Remodeling the tumor stroma by targeting fibroblast activation protein [122], administering antiangiogenic agents [123], or using ECM-degrading enzymes such as heparanase and hyaluronidase [124,125] may provide an avenue for enhanced CAR T cell penetration, although further investigation is needed to determine their efficacy due to mixed clinical results with the use of hyaluronidase [126]. However, little is known about how targeting the peritumor would affect CAR T cell therapy. Therefore, a better understanding of the physical-immune cross-talk in the peritumoral microenvironment would allow for the development of treatment strategies to simultaneously address immunosuppression and physical barriers of the tumor, which are closely interconnected.
Concluding remarks
Studying phenotypic, functional, and causal links between physics and immunity in the peritumor will enhance the current knowledge of cancer prognosis, diagnosis, and treatment strategies. By using state-of-the-art techniques and advancing technologies to couple immune response mechanisms to their related physical alterations of the peritumor microenvironment, we can further analyze clinical samples with a focus on the peritumor for increased prognostic and predictive power. It is important to note that although most mechanical and immunological characterizations are performed within the tumor, it is the peritumor that remains in situ after initial surgical resection and may contribute to local cancer recurrence. Consideration of the immune and mechanical components of the peritumor – and their cross-talk – must be included in treatment strategies, in addition to components of the resected tumor mass. By expanding the field of focus beyond just the tumor itself, novel therapeutic strategies will be proposed and evaluated that include tackling abnormal mechanics and bolstering immunological responses in the surrounding tissue to decrease recurrence/metastasis rates and improve successful treatment outcomes in cancer. While some links between the physical and immune interactions have been uncovered, there are still gaps in our knowledge of these mechanobiological relationships and how to design therapies that target the peritumor (see Outstanding questions). Thus we encourage further investigation into this exciting area at the intersection of cancer biology and mechanobiology.
Outstanding questions.
How do altered physical and immune microenvironments in the peritumor modulate tumor progression, invasion, metastasis, treatment response, and recurrence?
How does anti-cancer treatment affect the physical-immune cross-talk in the peritumor?
Do physical abnormalities in the peritumor linger post-resection and do they contribute to local cancer recurrence?
What signaling pathways that link peritumor physics and immunity provide diagnostic or prognostic information, and can they be targeted to improve treatment outcomes?
Would targeting physical hallmarks improve immunotherapy (e.g., immune checkpoint inhibitor and CAR-T therapy) outcomes?
How do physical abnormalities in the peritumor affect the role of the lymphatic system (e.g., lymph node and tertiary lymphoid structure (TLS)) in either pro- or anti-tumor immune responses?
Highlights.
Unique physical and immune signatures are present in the peritumor compared to the intratumor and healthy tissue.
Immune cell activity & signaling responds directly to physical cues from their microenvironment, resulting in altered differentiation and response.
Tumors respond to physical and immune cues that result in either a pro- or anti-tumorigenic response.
Exploiting weaknesses as well as targeting physical and immune components in the peritumor microenvironment is an emerging strategy for designing novel tumor-targeting therapeutics.
Acknowledgements
This work was supported in part by NIH R21EB031332 (H.T.N.) and DP2HL168562 (H.T.N.), Beckman Young Investigator Award (H.T.N.), K22CA258410 (M.D.), T32EB006359 (S.Z., M.W.G.), and Boston University Center for Multiscale and Translational Mechanobiology (S.Z., K.R., and H.T.N.)
Glossary
- Cancer-associated fibroblasts (CAFs)
a type of activated fibroblast that supports tumor progression and matrix remodeling through the secretion of chemokines, cytokines, collagen, ECM-remodeling enzymes, and growth factors
- Compressive solid stress
the application of inward (“pushing”) forces on a material
- Dendritic cell
a professional antigen-presenting cell important for initiating the adaptive immune response through the education and activation of T cells against a specific antigen
- Edema
the swelling of tissue resulting from fluid retention
- Fluid pressure
mechanical forces exerted by fluid components
- Growth-induced stress
solid stress generated from the proliferation of cells that causes strain on surrounding elements
- High endothelial venules (HEVs)
specialized blood vessels that permit the tissue extravasation of circulating lymphocytes
- Infiltrative tumors
tumor growth pattern where tumor boundaries are irregular, invading through tumor cell dissociation or along paths of least resistance. This growth pattern is also known as co-optive
- Interstitial fluid
fluid within the extracellular space
- Lateral cancerization
the progressive transformation of peritumoral cells that facilitates the lateral spread of cancer
- Macrophage
a phagocytic cell that ingests pathogens, cellular debris, and cancer cells, but which may also support cancer development and progression through the secretion of cytokines and pro-angiogenic growth factors
- Matrix crosslinking
reinforcement, reorganization, and stiffening of the ECM through the linkage of matrix fibers
- Mechanical cell competition
a process whereby mechanical compaction of slow-growing cells that are sensitive to mechanical stress results in elimination by fast-growing neighboring cells which are more resistant to mechanical stress
- Mechanical feedback
mechanosensing cells such as fibroblasts actively probe their surrounding environment to monitor the mechanical properties (tension, stress) and adjust their internal tensile homeostasis
- Myeloid-derived suppressor cells (MDSCs)
a heterogeneous group of immature myeloid cells which suppress effector T cell function
- Natural killer (NK) cell
an effector lymphocyte of the innate immune system responsible for controlling the anti-viral and anti-tumoral response via cytotoxic mechanisms
- Nodular tumors
tumor growth pattern where tumor boundaries are well-defined, resulting in cohesive invasive fronts and mechanical stress on surrounding tissue
- Perinuclear space
the space between the inner and outer nuclear membrane
- Shear stress
mechanical forces that are tangential to a surface
- Solid stress
mechanical forces (including compressive, tensile, and shear) that are contained in and transmitted by solid elements of cells and the ECM
- Strain-stiffening
positive feedback phenomenon where structures under tension activate further mechanosensing cell activity, resulting in increased stiffness and further cell activity
- Tensile solid stress
the application of outward (“pulling”) forces on a material
- Tissue microarchitecture
tissue-specific cell organization and matrix structures that are rearranged during tumor progression
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
None are declared by the authors.
References
- 1.Nia HT et al. (2020) Physical traits of cancer. Science 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Binnewies M et al. (2018) Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med 24, 541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aran D et al. (2017) Comprehensive analysis of normal adjacent to tumor transcriptomes. Nat. Commun 8, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tian J et al. (2017) Application of 3D and 2D quantitative shear wave elastography (SWE) to differentiate between benign and malignant breast masses. Sci. Rep 7, 41216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dai X et al. (2017) Positive Expression of Programmed Death Ligand 1 in Peritumoral Liver Tissue is Associated with Poor Survival after Curative Resection of Hepatocellular Carcinoma. Transl. Oncol 10, 511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salmon H et al. (2012) Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J. Clin. Invest 122, 899–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nia HT et al. (2016) Solid stress and elastic energy as measures of tumour mechanopathology. Nat. Biomed. Eng 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seano G et al. (2019) Solid stress in brain tumours causes neuronal loss and neurological dysfunction and can be reversed by lithium. Nat. Biomed. Eng 3, 230–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang S et al. (2023) In vivo multiscale measurements of solid stresses in tumors reveal scale-dependent stress transmission. Nat. Biomed. Eng, In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chauhan VP et al. (2013) Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat. Commun 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tse JM et al. (2012) Mechanical compression drives cancer cells toward invasive phenotype. Proc. Natl. Acad. Sci. U. S. A 109, 911–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helmlinger G et al. (1997) Solid stress inhibits the growth of multicellular tumor spheroids. Nat. Biotechnol 15, 778–783. [DOI] [PubMed] [Google Scholar]
- 13.Banerji R et al. (2023) Crystal ribcage: a platform for probing real-time lung function at cellular resolution in health and disease. bioRxiv, Preprint. Published online February 13th 2023. 10.1101/2022.10.28.514251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donnem T et al. (2018) Non-angiogenic tumours and their influence on cancer biology. Nat. Rev. Cancer 18, 323–336. [DOI] [PubMed] [Google Scholar]
- 15.Nia HT et al. (2018) Quantifying solid stress and elastic energy from excised or in situ tumors. Nat. Protoc 13, 1091–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones D et al. (2021) Solid stress impairs lymphocyte infiltration into lymph-node metastases. Nat. Biomed. Eng 5, 1426–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stylianopoulos T et al. (2013) Coevolution of solid stress and interstitial fluid pressure in tumors during progression: implications for vascular collapse. Cancer Res. 73, 3833–3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalli M et al. (2018) Solid Stress Facilitates Fibroblasts Activation to Promote Pancreatic Cancer Cell Migration. Ann. Biomed. Eng 46, 657–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang L et al. (2010) TGF-beta and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol. 31, 220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viel S et al. (2016) TGF-β inhibits the activation and functions of NK cells by repressing the mTOR pathway. Sci. Signal 9, ra19–ra19. [DOI] [PubMed] [Google Scholar]
- 21.Wei S et al. (2008) Tumor-induced Immune Suppression of In Vivo T Effector Cell Priming is Mediated by the B7-H1/PD-1 Axis and TGF-β. Cancer Res. 68, 5432–5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernández-Sánchez ME et al. (2015) Mechanical induction of the tumorigenic β-catenin pathway by tumour growth pressure. Nature 523, 92–95. [DOI] [PubMed] [Google Scholar]
- 23.Xiao Q et al. (2018) DKK2 imparts tumor immunity evasion through beta-catenin-independent suppression of cytotoxic immune-cell activation. Nat. Med 24, 262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levayer R (2019) Solid stress, competition for space and cancer: The opposing roles of mechanical cell competition in tumour initiation and growth. Semin. Cancer Biol 63, 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim JH et al. (2015) Mechanical tension drives cell membrane fusion. Dev. Cell 32, 561–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Denais CM et al. (2016) Nuclear envelope rupture and repair during cancer cell migration. Science 352, 353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Irianto J et al. (2017) DNA Damage Follows Repair Factor Depletion and Portends Genome Variation in Cancer Cells after Pore Migration. Curr. Biol 27, 210–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burleigh K et al. (2020) Human DNA-PK activates a STING-independent DNA sensing pathway. Sci. Immunol 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Falahat R et al. (2021) Epigenetic reprogramming of tumor cell-intrinsic STING function sculpts antigenicity and T cell recognition of melanoma. Proc. Natl. Acad. Sci. U. S. A 118. e2013598118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bakhoum SF et al. (2018) Chromosomal instability drives metastasis through a cytosolic DNA response. Nature 553, 467–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jain RK et al. (2014) The Role of Mechanical Forces in Tumor Growth and Therapy. Annu. Rev. Biomed. Eng 16, 321–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fukumura D and Jain RK (2007) Tumor microenvironment abnormalities: Causes, consequences, and strategies to normalize. J. Cell. Biochem 101, 937–949. [DOI] [PubMed] [Google Scholar]
- 33.Hompland T et al. (2012) Interstitial fluid pressure and associated lymph node metastasis revealed in tumors by dynamic contrast-enhanced MRI. Cancer Res. 72, 4899–4908. [DOI] [PubMed] [Google Scholar]
- 34.Chary SR and Jain RK (1989) Direct measurement of interstitial convection and diffusion of albumin in normal and neoplastic tissues by fluorescence photobleaching. Proc. Natl. Acad. Sci. U. S. A 86, 5385–5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swartz MA and Lund AW (2012) Lymphatic and interstitial flow in the tumour microenvironment: Linking mechanobiology with immunity. Nat. Rev. Cancer 12, 210–219. [DOI] [PubMed] [Google Scholar]
- 36.Munson JM and Shieh AC (2014) Interstitial fluid flow in cancer: implications for disease progression and treatment. Cancer Manag. Res, 6–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Polacheck WJ et al. (2011) Interstitial flow influences direction of tumor cell migration through competing mechanisms. Proc. Natl. Acad. Sci. U. S. A 108, 11115–11120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jain RK (2013) Normalizing tumor microenvironment to treat cancer: Bench to bedside to biomarkers. J. Clin. Oncol 31, 2205–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Munson JM et al. (2013) Interstitial flow in a 3d microenvironment increases glioma invasion by a cxcr4-dependent mechanism. Cancer Res. 73, 1536–1546. [DOI] [PubMed] [Google Scholar]
- 40.Kingsmore KM et al. (2018) MRI analysis to map interstitial flow in the brain tumor microenvironment. APL Bioeng. 2. 031905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang W et al. (2018) Expression of CXC-motif-chemokine 12 and the receptor C-X-C receptor 4 in glioma and the effect on peritumoral brain edema. Oncol. Lett 15, 2501–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berhouma M et al. (2017) Pathogenesis of peri-tumoral edema in inracranial meningiomas. Neurosurg. Rev 42, 59–71. [DOI] [PubMed] [Google Scholar]
- 43.Kuang DM et al. (2011) Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. J. Hepatol 54, 948–955. [DOI] [PubMed] [Google Scholar]
- 44.Datta M et al. (2023) Losartan controls immune checkpoint blocker-induced edema and improves survival in glioblastoma mouse models. Proc. Natl. Acad. Sci. U. S. A 120, e2219199120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li R et al. (2018) Interstitial flow promotes macrophage polarization toward an M2 phenotype. Mol. Biol. Cell 29, 1927–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu S et al. (2016) Fluid shear stress induces epithelial-mesenchymal transition (EMT) in Hep-2 cells. Oncotarget 7, 32876–32892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nowell CS and Radtke F (2017) Notch as a tumour suppressor. Nat. Rev. Cancer 17, 145–159. [DOI] [PubMed] [Google Scholar]
- 48.Peng D et al. (2016) Myeloid-Derived Suppressor Cells Endow Stem-like Qualities to Breast Cancer Cells through IL6/STAT3 and NO/NOTCH Cross-talk Signaling. Cancer Res. 76, 3156–3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao JL et al. (2016) Forced Activation of Notch in Macrophages Represses Tumor Growth by Upregulating miR-125a and Disabling Tumor-Associated Macrophages. Cancer Res. 76, 1403–1415. [DOI] [PubMed] [Google Scholar]
- 50.Hope JM et al. (2022) Fluid shear stress enhances T cell activation through Piezo1. BMC Biol. 20, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahamed J et al. (2008) In vitro and in vivo evidence for shear-induced activation of latent transforming growth factor-β1. Blood 112, 3650–3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feig C et al. (2013) Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc. Natl. Acad. Sci. U. S. A 110, 20212–20217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gorelik L and Flavell RA (2001) Immune-mediated eradication of tumors through the blockade of transforming growth factor-β signaling in T cells. Nat. Med 7, 1118–1122. [DOI] [PubMed] [Google Scholar]
- 54.Sheu B et al. (2001) A Novel Role of Metalloproteinase in Cancer-mediated Immunosuppression. Cancer Res.61, 237–242. [PubMed] [Google Scholar]
- 55.Germann M et al. (2020) Neutrophils suppress tumor-infiltrating T cells in colon cancer via matrix metalloproteinase-mediated activation of TGFbeta. EMBO Mol. Med 12, e10681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leifler KS et al. (2013) Inflammation induced by MMP-9 enhances tumor regression of experimental breast cancer. J. Immunol 190, 4420–4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paszek MJ et al. (2005) Tensional homeostasis and the malignant phenotype. Cancer Cell 8, 241–254. [DOI] [PubMed] [Google Scholar]
- 58.Provenzano PP et al. (2008) Collagen density promotes mammary tumor initiation and progression. BMC Med. 6, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dufort CCPMWV (2008) Balancing forces: architectural control of mechanotransduction. Nat. Rev. Mol. Cell Biol 23, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu R et al. (2009) Tissue architecture and function: Dynamic reciprocity via extra- and intracellular matrices. Cancer Metastasis Rev. 28, 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Storm C et al. (2005) Nonlinear elasticity in biological gels. Nature 435, 191–194. [DOI] [PubMed] [Google Scholar]
- 62.Levental KR et al. (2009) Matrix Crosslinking Forces Tumor Progression by Enhancing Integrin Signaling. Cell 139, 891–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ng CP et al. (2005) Interstitial fluid flow induces myofibroblast differentiation and collagen alignment in vitro. J. Cell. Sci 118, 4731–4739. [DOI] [PubMed] [Google Scholar]
- 64.Stampouloglou E et al. (2020) Yap suppresses T-cell function and infiltration in the tumor microenvironment. PLoS Biol. 18, e3000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meng KP et al. (2020) Mechanosensing through YAP controls T cell activation and metabolism. J. Exp. Med 217. e20200053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saitakis M et al. (2017) Different TCR-induced T lymphocyte responses are potentiated by stiffness with variable sensitivity. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kuczek DE et al. (2019) Collagen density regulates the activity of tumor-infiltrating T cells. J. Immunother. Cancer 7, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jin W et al. (2019) T cell activation and immune synapse organization respond to the microscale mechanics of structured surfaces. Proc. Natl. Acad. Sci. U. S. A 116, 19835–19840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yuan DJ et al. (2021) Biphasic response of T cell activation to substrate stiffness. Biomaterials 273, 120797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hickey JW et al. (2019) Engineering an Artificial T-Cell Stimulating Matrix for Immunotherapy. Adv. Mater 31, e1807359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hoffmann EJ and Ponik SM (2020) Biomechanical Contributions to Macrophage Activation in the Tumor Microenvironment. Front. Oncol 10, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Atcha H et al. (2021) Mechanically activated ion channel Piezo1 modulates macrophage polarization and stiffness sensing. Nat. Commun 12, 3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aykut B et al. (2020) Targeting Piezo1 unleashes innate immunity against cancer and infectious disease. Sci. Immunol 5. eabb5168. [DOI] [PubMed] [Google Scholar]
- 74.Chakraborty M et al. (2021) Mechanical Stiffness Controls Dendritic Cell Metabolism and Function. Cell. Rep 34, 108609. [DOI] [PubMed] [Google Scholar]
- 75.Wang Y et al. (2022) Dendritic cell Piezo1 directs the differentiation of T(H)1 and T(reg) cells in cancer. Elife 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wipff PJ et al. (2007) Myofibroblast contraction activates latent TGF-β1 from the extracellular matrix. J. Cell Biol 179, 1311–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pickup MW et al. (2013) Stromally derived lysyl oxidase promotes metastasis of transforming growth factor-beta-deficient mouse mammary carcinomas. Cancer Res. 73, 5336–5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang JY et al. (2021) Cancer-associated fibroblasts promote oral squamous cell carcinoma progression through LOX-mediated matrix stiffness. J. Transl. Med 19, 513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Haj-Shomaly J et al. (2022) T Cells Promote Metastasis by Regulating Extracellular Matrix Remodeling following Chemotherapy. Cancer Res. 82, 278–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang TH et al. (2016) Lysyl Oxidase and the Tumor Microenvironment. Int. J. Mol. Sci 18. 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miyazawa A et al. (2018) Regulation of PD-L1 expression by matrix stiffness in lung cancer cells. Biochem. Biophys. Res. Commun 495, 2344–2349. [DOI] [PubMed] [Google Scholar]
- 82.Xing X et al. (2021) Matrix stiffness - mediated effects on macrophages polarization and their LOXL2 expression. FEBS J. 288, 3465–3477. [DOI] [PubMed] [Google Scholar]
- 83.Taufalele PV et al. (2022) Matrix stiffness enhances cancer-macrophage interactions and M2-like macrophage accumulation in the breast tumor microenvironment. Acta Biomater. Apr 25:S1742-7061(22)00240-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Acerbi I et al. (2015) Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Int. J. Integr. Biol 7, 1120–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Goetz JG et al. (2011) Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell 146, 148–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Provenzano PP et al. (2006) Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 4, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Franchi M et al. (2019) Collagen Fiber Array of Peritumoral Stroma Influences Epithelial-to-Mesenchymal Transition and Invasive Potential of Mammary Cancer Cells. J. Clin. Med 8, 213–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ray A et al. (2022) Stromal architecture directs early dissemination in pancreatic ductal adenocarcinoma. J. Clin. Invest 8, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hartmann N et al. (2014) Prevailing role of contact guidance in intrastromal T-cell trapping in human pancreatic cancer. Clin. Cancer Res 20, 3422–3433. [DOI] [PubMed] [Google Scholar]
- 90.Zanotelli MR et al. (2022) Highly motile cells are metabolically responsive to collagen density. Proc. Natl. Acad. Sci. U. S. A 119, e2114672119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dafni H et al. (2002) Overexpression of Vascular Endothelial Growth Factor 165 Drives Peritumor Interstitial Convection and Induces Lymphatic Drain: Magnetic Resonance Imaging, Confocal Microscopy, and Histological Tracking of Triple-labeled Albumin 1. Cancer Res. 62. 6731–6739. [PubMed] [Google Scholar]
- 92.Eefsen RL et al. (2015) Growth pattern of colorectal liver metastasis as a marker of recurrence risk. Clin. Exp. Metastasis 32, 369–381. [DOI] [PubMed] [Google Scholar]
- 93.Glasner A et al. (2018) NKp46 Receptor-Mediated Interferon-gamma Production by Natural Killer Cells Increases Fibronectin 1 to Alter Tumor Architecture and Control Metastasis. Immunity 48, 107–119 e104. [DOI] [PubMed] [Google Scholar]
- 94.Kobayashi T et al. (2014) Matrix metalloproteinase-9 activates TGF-β and stimulates fibroblast contraction of collagen gels. Am. J. Physiol. Lung Cell Mol. Physiol 306, L1006–L1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Del Casar JM et al. (2009) Comparative analysis and clinical value of the expression of metalloproteases and their inhibitors by intratumor stromal fibroblasts and those at the invasive front of breast carcinomas. Breast Cancer Res. Treat 116, 39–52. [DOI] [PubMed] [Google Scholar]
- 96.Calhoun MA et al. (2020) MicroRNA-mRNA Interactions at Low Levels of Compressive Solid Stress Implicate mir-548 in Increased Glioblastoma Cell Motility. Sci. Rep 10, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gao X et al. (2015) Activation of death-associated protein kinase in human peritumoral tissue: A potential therapeutic target. J. Clin. Neurosci 22, 1655–1660. [DOI] [PubMed] [Google Scholar]
- 98.Wei Z et al. (2021) Death-associated protein kinase 1 (DAPK1) controls CD8(+) T cell activation, trafficking, and antitumor activity. FASEB J. 35, e21138. [DOI] [PubMed] [Google Scholar]
- 99.Lau D et al. (2020) Intravital Imaging of Adoptive T-Cell Morphology, Mobility and Trafficking Following Immune Checkpoint Inhibition in a Mouse Melanoma Model. Front. Immunol 11, 1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Osorio HL et al. (2018) Ultrastructure of colorectal adenocarcinoma and peritumoral tissue in untreated patients. Ultrastruct. Pathol 42, 81–90. [DOI] [PubMed] [Google Scholar]
- 101.Mohammadi H and Sahai E (2018) Mechanisms and impact of altered tumour mechanics. Nat. Cell Biol 20, 766–774. [DOI] [PubMed] [Google Scholar]
- 102.Elosegui-Artola A et al. (2017) Force Triggers YAP Nuclear Entry by Regulating Transport across Nuclear Pores. Cell 171, 1397–1410.el314. [DOI] [PubMed] [Google Scholar]
- 103.Ramdas NM and Shivashankar GV (2015) Cytoskeletal control of nuclear morphology and chromatin organization. J. Mol. Biol 427, 695–706. [DOI] [PubMed] [Google Scholar]
- 104.Wang Y et al. (2017) Orientation and repositioning of chromosomes correlate with cell geometry-dependent gene expression. Mol. Biol. Cell 28, 1997–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen IX et al. (2019) Blocking CXCR4 alleviates desmoplasia, increases T-lymphocyte infiltration, and improves immunotherapy in metastatic breast cancer. Proc. Natl. Acad. Sci. U. S. A 116, 4558–4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lakins MA et al. (2018) Cancer-associated fibroblasts induce antigen-specific deletion of CD8 + T Cells to protect tumour cells. Nat. Commun 9, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kataru RP et al. (2019) Tumor lymphatic function regulates tumor inflammatory and immunosuppressive microenvironments. Cancer Immunol. Res 7, 1345–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jain RK et al. (2007) Effect of vascular normalization by antiangiogenic therapy on interstitial hypertension, peritumor edema, and lymphatic metastasis: Insights from a mathematical model. Cancer Res. 67, 2729–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fukumura D et al. (2018) Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat. Rev. Clin. Oncol 15, 325–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Flessner M et al. (2005) Resistance of Tumor Interstitial Pressure to the Penetration of Intraperitoneally Delivered Antibodies into Metastatic Ovarian Tumors. Clin. Cancer Res 11, 3117–3125. [DOI] [PubMed] [Google Scholar]
- 111.Reed HA et al. (2019) Lymphatic impairment leads to pulmonary tertiary lymphoid organ formation and alveolar damage. J. Clin. Invest 1292, 2514–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dieu-Nosjean MC et al. (2014) Tertiary lymphoid structures in cancer and beyond. Trends Immunol. 35, 571–580. [DOI] [PubMed] [Google Scholar]
- 113.Sofopolous M et al. (2019) The prognostic significance of peritumoral tertiary lymphoid structures in breast cancer. Cancer Immunol. Immunother 68, 1733–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Horn LA et al. (2022) Remodeling the tumor microenvironment via blockade of LAIR-1 and TGF-beta signaling enables PD-L1-mediated tumor eradication. J. Clin. Invest 132. e155148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Elahi-Gedwillo KY et al. (2019) Antifibrotic Therapy Disrupts Stromal Barriers and Modulates the Immune Landscape in Pancreatic Ductal Adenocarcinoma. Cancer Res. 79, 372–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Provenzano PP et al. (2012) Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 21, 418–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sharma NS et al. (2020) Targeting tumor-intrinsic hexosamine biosynthesis sensitizes pancreatic cancer to anti-PD1 therapy. J. Clin. Invest 130, 451–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Morita T et al. (2020) CXCR4 in Tumor Epithelial Cells Mediates Desmoplastic Reaction in Pancreatic Ductal Adenocarcinoma. Cancer Res. 80, 4058–4070. [DOI] [PubMed] [Google Scholar]
- 119.Lei K et al. (2021) Cancer-cell stiffening via cholesterol depletion enhances adoptive T-cell immunotherapy. Nat. Biomed. Eng 5, 1411–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chmielewski M et al. (2014) Of CARs and TRUCKs: chimeric antigen receptor (CAR) T cells engineered with an inducible cytokine to modulate the tumor stroma. Immunol. Rev 257, 83–90. [DOI] [PubMed] [Google Scholar]
- 121.Kloss CC et al. (2018) Dominant-Negative TGF-beta Receptor Enhances PSMA-Targeted Human CAR T Cell Proliferation And Augments Prostate Cancer Eradication. Mol. Ther 26, 1855–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang LC et al. (2014) Targeting fibroblast activation protein in tumor stroma with chimeric antigen receptor T cells can inhibit tumor growth and augment host immunity without severe toxicity. Cancer Immunol. Res 2, 154–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dong X et al. (2023) Anti-VEGF therapy improves EGFR-vIII-CAR-T cell delivery and efficacy in syngeneic glioblastoma models in mice. J. Immunother. Cancer 11. e005583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Caruana I et al. (2015) Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes. Nat. Med 21, 524–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hingorani SR et al. (2018) HALO 202: Randomized Phase II Study of PEGPH20 Plus Nab-Paclitaxel/Gemcitabine Versus Nab-Paclitaxel/Gemcitabine in Patients With Untreated, Metastatic Pancreatic Ductal Adenocarcinoma. J. Clin. Oncol 36, 359–366. [DOI] [PubMed] [Google Scholar]
- 126.Ramanathan RK et al. (2019) Phase IB/II Randomized Study of FOLFIRINOX Plus Pegylated Recombinant Human Hyaluronidase Versus FOLFIRINOX Alone in Patients With Metastatic Pancreatic Adenocarcinoma: SWOG S1313. J. Clin. Oncol 37, 1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]