Abstract
In patients with hepatocellular carcinoma (HCC) awaiting liver transplantation (LT), there is a need to identify biomarkers that are superior to AFP in predicting prognosis. AFP-L3 and des-gamma-carboxyprothrombin (DCP) play a role in HCC detection, but their ability to predict waitlist dropout is unknown. In this prospective single-center study commenced in July 2017, 267 HCC patients had all three biomarkers obtained at LT listing. Among them, 96.2% received local-regional therapy and 18.8% had initial tumor stage beyond Milan criteria requiring tumor down-staging. At listing, median AFP was 7.0 ng/mL (IQR 3.4–21.5), median AFP-L3 was 7.1% (IQR 0.5–12.5), and median DCP was 1.0 ng/mL (IQR 0.2–3.8). After a median follow up of 19.3 months, 63 (23.6%) experienced waitlist dropout, while 145 (54.3%) received LT and 59 (22.1%) were still awaiting LT. Using Cox proportional hazards analysis, AFP-L3 ≥35% and DCP ≥7.5 ng/mL were associated with increased waitlist dropout whereas AFP at all tested cutoffs including ≥20, ≥100, and ≥250 ng/mL was not. In a multivariable model, AFP-L3 ≥35% (HR 2.25, p=0.04) and DCP ≥7.5 ng/mL (HR 2.20, p=0.02) remained associated with waitlist dropout as did time from HCC diagnosis to listing >1 year and increasing MELD-Na score. Kaplan-Meier probability of waitlist dropout within 2 years was 21.8% in those with AFP-L3 <35% and DCP <7.5 ng/mL, 59.9% with either AFP-L3 or DCP elevated, and 100% for those with both elevated (p<0.001). In this prospective study, listing AFP-L3% and DCP were superior to AFP in predicting waitlist dropout with the combination of AFP-L3 ≥35% and DCP ≥7.5 ng/mL associated with a 100% risk of waitlist dropout, thus clearly adding prognostic value to AFP alone.
Keywords: HCC, liver transplantation, des-gamma-carboxyprothrombin, lectin-reactive alpha-fetoprotein, BALAD
INTRODUCTION
In recent years, hepatocellular carcinoma (HCC) has become a leading indication for liver transplantation (LT) (1) though a combination of factors has led to increasing rates of waitlist dropout for HCC patients. Namely, HCC incidence has been increasing in the United States, including the proportion diagnosed with early stage HCC, leading to increased HCC waitlist registrations (2, 3) despite ongoing worldwide organ shortages. Additionally, a mandated waiting time of 6 months before awarding priority listing has been implemented in the US in an attempt to equalize access to LT for HCC and non-HCC patients (4). With increased demand and lengthening wait times resulting in a nearly 30% dropout rate for listed HCC patients (5), it is imperative to identify factors that can predict waitlist dropout.
Recent studies have focused on tumor biomarkers to identify aggressive HCC phenotypes and/or more advanced disease. Alpha-fetoprotein (AFP) has been increasingly recognized as an important prognostic marker among HCC patients being considered for LT. High AFP has been shown to predict waitlist dropout (5), the presence of microvascular invasion and worse tumor differentiation (6–8), and poor post-LT outcome (7–10). Based on these findings, in 2017 United Network for Organ Sharing (UNOS) adopted national policy where HCC LT candidates with an AFP >1,000 ng/mL are not eligible for priority listing until AFP falls to <500 with local-regional therapy (LRT) (4) though inferior post-LT outcome is seen with AFP levels as low as 20 ng/ml (10, 11).
While AFP is clearly an important marker of tumor biology, it is limited in its predictive power, especially in the LT setting. For example, median AFP in HCC patients is only ~10 ng/ml at the time of LT (11). Therefore, additional biomarkers including des-gamma-carboxyprothrombin (DCP) and lens culinaris agglutinin-reactive fraction of AFP (AFP-L3) have garnered increased attention recently. While these biomarkers have an important role in HCC detection (12, 13), tumors that express AFP-L3 and DCP are also thought to be more aggressive with the potential for rapid growth and early metastasis (14, 15). In patients undergoing resection, pre-operative DCP has been shown to correlate with micro-vascular invasion and post-surgical outcome (16, 17). Further, elevated DCP and AFP-L3% have been associated with high-risk explant pathology and worse survival after LT (18–21).
While elevations in AFP-L3 and DCP suggest aggressive tumor biology, their ability to predict waitlist dropout in HCC patients awaiting LT has not previously been investigated. Therefore, this prospective study was conducted to evaluate the prognostic power of all three biomarkers (AFP, AFP-L3, and DCP) measured at the time of LT listing to predict waitlist dropout and receipt of LT.
PATIENTS AND METHODS
Study Design and Patient Population
This is a prospective single-center study involving testing all 3 biomarkers – AFP, AFP-L3 and DCP in consecutive patients with the diagnosis of HCC and listed for LT at our institution since July 2017 with a minimum waitlist follow-up of 6 months required for inclusion. The diagnosis of HCC was based on characteristics on multi-phase computed tomography or magnetic resonance imaging meeting Liver Imaging Reporting and Data System (LI-RADS) 5 criteria (22) or needle biopsy confirming a histologic diagnosis of HCC if imaging diagnosis was equivocal. Patients initially exceeding Milan criteria underwent down-staging treatment(s) under a standardized protocol with all included patients having tumor(s) meeting Milan criteria at the time of listing. AFP ≥1000 ng/mL was an exclusion criterion unless the level decreased to <500 ng/mL with LRT (4) in accordance with UNOS policy for priority listing.
All patients had AFP, AFP-L3, and DCP measured at the time of LT listing. Blood samples were sent for testing at Quest Laboratory from July 2017 to October 2017 and to Associated Regional and University Pathologists, Inc. after October 2017. AFP-L3 was reported as a percentage of the total AFP. Per UNOS listing policy, patients underwent contrast-enhanced computed tomography or magnetic resonance imaging at a minimum of once every three months after listing for LT. The study was approved by the University of California, San Francisco Committee for Human Research and received expedited approval with minimal study risk assignment.
Outcomes and Statistical Analysis
The primary outcome was dropout from the transplant waiting list for any of the following reasons: death without LT, tumor progression, or being too sick to undergo LT. Patient characteristics were summarized using medians and inter-quartile ranges (IQR) for continuous variables and proportions for categorical variables. Patient follow-up time was measured from the date of first submitted MELD exception listing application to the waitlist outcome (dropout or LT) or last date on the waiting list. Patients remaining alive on the waitlist or removed for reasons other than death, tumor progression, or being too sick to undergo transplant were censored at their last date on the waitlist. Clinical characteristics were compared between groups experiencing dropout and receiving LT using Pearson’s chi-square and Wilcoxon tests, as appropriate.
Cox proportional hazards models estimated risk of waitlist dropout with hazard ratios (HR) and 95% confidence intervals (CI) for each explanatory variable as well as separately for the validated BALAD score which includes bilirubin, albumin, AFP-L3, AFP, and DCP (23). Multiple cut-offs for AFP (≥20, ≥100, ≥250 ng/mL), AFP-L3 (≥15% and ≥35%) and DCP (≥7.5 ng/mL) were tested (6, 20, 21). Factors with a univariate p-value less than 0.1 were evaluated in the multivariable analysis with the final model selected by backward elimination (p for removal greater than 0.05). The overall C-index assessed model discrimination. Net reclassification improvement was performed to quantify how well the multivariable model with AFP-L3 and DCP reclassified individuals in terms of estimated risk predictions (correctly or incorrectly), as compared to a multivariable model without AFP-L3 and DCP.
Kaplan-Meier methods were used to estimate cumulative probabilities of waitlist dropout with 95% confidence intervals and compared by presence of number of elevated biomarkers as well as by a biomarker based dropout risk score. Data were analyzed using Stata version 12 (Stata Corporation).
RESULTS
Baseline Characteristics
The study cohort included 267 patients with HCC who were listed for LT starting from July 2017 and had complete pre-listing biomarker data available. Baseline demographic and clinical characteristics of the entire cohort are summarized in Table 1. The median age of the cohort at listing was 63.5 years (IQR 58.1–66.78) with 74.5% men. White was the most common race (38.2%) with Hispanic ethnicity of 27.0%. Hepatitis C infection was the most common etiology of liver disease (52.1%). The median number of lesions at HCC diagnosis was 1 (IQR 1–2). At listing with MELD exception or before first LRT, the median size of largest tumor was 2.5 cm (IQR 2.1–3.5) and median initial total tumor diameter was 3.1 cm (IQR 2.2–4.7). Down-staging with LRT prior to listing was required in 50 patients (18.8%), the majority of whom initially met UNOS down-staging criteria. Overall, 257 (96.2%) received LRT with median of 2 (IQR 1–3) sessions of treatment. Trans-arterial chemoembolization was the most common LRT modality used. Median MELD-Na score at listing was 11 (IQR 8–14) and the median Child-Pugh score at listing was 6 (IQR 5–8).
Table 1.
Demographics and clinical characteristics (N=267)
| N (%) | |
|---|---|
| Age (median, years) | 63.5 (IQR 58.1–66.8) |
| Gender | |
| Men | 199 (74.5) |
| Women | 68 (25.5) |
| Race/Ethnicity | |
| White | 102 (38.2) |
| Asian/Pacific Islander | 49 (18.4) |
| Black | 11 (4.1) |
| Hispanic | 72 (27.0) |
| Other | 33 (12.4) |
| Etiology of liver disease | |
| HCV | 139 (52.1) |
| HBV | 43 (16.1) |
| Alcohol | 25 (9.4) |
| NASH | 43 (16.1) |
| Other | 17 (6.4) |
| Median number of HCC lesions at listing | 1 (IQR 1–2) |
| Median size of largest tumor at listing with MELD exception or before LRT (cm) | 2.5 (IQR 2.1–3.5) |
| Median total tumor diameter at listing with MELD exception or before LRT (cm) | 3.1 (IQR 2.2–4.7) |
| Tumor stage (worst classification) | |
| Milan criteria | 217 (81.3) |
| UNOS Downstaging | 41 (15.4) |
| All-comers | 9 (3.4) |
| Local regional therapy | |
| Ever received | 257 (96.2) |
| Median number of treatments | 2 (IQR 1–3) |
| Type of local regional therapy | |
| TACE | 202 (75.6) |
| RFA | 106 (39.7) |
| Y90 | 66 (24.7) |
| Median MELD-Na at listing | 11 (IQR 8–14) |
| Median Child-Pugh (CP) Score at listing (N=258) | 6 (IQR 5–8) |
| CP Class A (5–6) | 143 (55.4) |
| CP Class B (7–9) | 74 (28.7) |
| CP Class C (10–15) | 41 (15.9) |
| Bilirubin at listing (mg/dL) | 1.3 (IQR 0.8–2.3) |
| Albumin at listing (mg/dL) | 3.5 (3.0–4.0) |
| Platelets at listing (x 109/L) (N=256) | 87 (60–132) |
| Neutrophil to lymphocyte ratio (NLR) at listing (N=200) | 2.9 (1.9–4.7) |
Serum Biomarker Values Prior to Listing
At the time of HCC diagnosis, median AFP (n=244) was 9.0 ng/mL (IQR 4.1–31.8), median AFP-L3 (n=60) was 9.2% (5.5–19.3), and median DCP (n=55) was 1.3 ng/mL (IQR 0.2–5.6) (Table 2). At listing, median AFP was 7.0 ng/mL (IQR 3.4–21.5) with 4.5% having AFP ≥250 ng/mL and 8.2% having AFP ≥100 ng/mL. Median AFP-L3 at listing was 7.1% (IQR 0.5–12.5) with 9.0% having AFP-L3 ≥35%. Median DCP at listing was 1.0 ng/mL (IQR 0.2–3.8) with 17.7% having DCP ≥7.5 ng/mL.
Table 2.
Serum biomarkers at the time of HCC diagnosis and at LT listing
| Serum Biomarker | Median (IQR) or n (%) |
|---|---|
| Median AFP at diagnosis (ng/mL) (n=244) | 9.0 (IQR 4.1–31.8) |
| Median AFP at listing (n=267) | 7.0 (IQR 3.4–21.5) |
| AFP at listing ≥20 | 75 (28.1) |
| AFP at listing ≥100 | 22 (8.2) |
| AFP at listing ≥250 | 12 (4.5) |
| Median AFP-L3% at diagnosis (n=60) | 9.2 (IQR 5.5–19.3) |
| Median AFP-L3% at listing (n=267) | 7.1 (IQR 0.5–12.5) |
| AFP-L3 at listing ≥15% | 58 (21.7) |
| AFP at listing ≥35% | 24 (9.0) |
| Absolute AFP-L3 at listing ≥5 ng/mL | 48 (18.0) |
| Absolute AFP-L3 at listing ≥10 ng/mL | 36 (13.5) |
| Median DCP at diagnosis (ng/mL) (n=55) | 1.3 (IQR 0.2–5.6) |
| Median DCP at listing (n=267) | 1.0 (IQR 0.2–3.8) |
| DCP at listing ≥7.5 | 47 (17.7) |
Waitlist Outcomes and Association with Biomarkers
After a median waitlist follow up of 19 months, 145 (54.3%) underwent LT whereas 63 (23.6%) experienced waitlist dropout, including 30 (11.2%) related to tumor progression and 27 (10.1%) who died or experienced clinical deterioration. At the end of the study period, 59 (22.1%) were still active on the LT waitlist. Among patients who received LT (n=145 including n=18 who underwent live donor LT), median time from listing to LT was 10.8 months (IQR 7.5–14.6) months. There were no significant differences with regards to explant under-staging, microvascular invasion, or tumor differentiation between those who received deceased donor vs live donor LT.
In terms of clinical characteristics of those who received LT versus those who experienced waitlist dropout, the duration of time from diagnosis of HCC to LT listing was longer among those who experienced dropout (250 days) compared to those who received LT (179 days; p=0.01). Among those who received LT, 6.2% had pre-listing AFP ≥100 ng/mL compared with 14.3% among those who experienced waitlist dropout (p=0.06). Additionally, BALAD score was significantly higher in those who experienced waitlist dropout (p=0.01). For example, 74.6% of those who had waitlist dropout had a BALAD score ≥2 at listing compared with 56.6% of those who underwent LT.
Factors Associated with Waitlist Dropout
Predictors of waitlist dropout in univariate analysis are summarized in Table 3. Specifically, listing AFP-L3 ≥35% (HR 3.02, 95% CI 1.53–5.97, p=0.05), AFP-L3 ≥15% (HR 1.91, 95% CI 1.09–3.34, p=0.03), and DCP ≥7.5 ng/mL (HR 2.51, 95% CI 1.38–4.56, p=0.05) were associated with waitlist dropout along with initial tumor stage beyond Milan criteria, time from HCC diagnosis to listing, MELD-Na and Child-Pugh score at listing, and neutrophil to lymphocyte ratio (NLR). When waitlist follow-up was capped at 12 months, AFP-L3 ≥35% (HR 3.28, 95% CI 1.44–7.49, p=0.005), AFP-L3 ≥15% (HR 2.14, 95% CI 1.08–4.27, p=0.03), and DCP ≥7.5 ng/mL (HR 1.96, 95% CI 1.03–3.76, p=0.04) continued to be significantly associated with waitlist dropout. Interestingly, we failed to detect an association with AFP at all tested cutoffs including >20, >100, and >250 ng/mL and waitlist dropout either overall or within 12 months from listing.
Table 3.
Cox proportional hazards of features associated with waitlist dropout
| Univariate HR (95% CI) | p-value | Multivariate HR (95% CI) | p-value | |
|---|---|---|---|---|
| AFP pre-listing ≥250 ng/mL | 2.01 (0.86–4.73) | 0.14 | ||
| AFP pre-listing ≥100 ng/mL | 1.78 (0.87–3.64) | 0.14 | 1.84 (0.81–4.16) | 0.14 |
| AFP pre-listing ≥20 ng/mL | 1.02 (0.58–1.78) | 0.95 | ||
| AFP-L3 pre-listing ≥35% | 3.02 (1.53–5.97) | 0.005 | 2.25 (1.04–4.88) | 0.04 |
| AFP-L3 pre-listing ≥15% | 1.91 (1.09–3.34) | 0.03 | ||
| DCP pre-listing ≥7.5 ng/mL | 2.51 (1.38–4.56) | 0.005 | 2.20 (1.15–4.20) | 0.02 |
| Age | 0.99 (0.95–1.03) | 0.70 | ||
| Gender | 0.46 | |||
| Men | 1.23 (0.70–2.18) | |||
| Women | 1 | |||
| Race | 0.32 | |||
| White | 1 | |||
| Asian/Pacific Islander | 0.54 (0.23–1.24) | |||
| African American | 1.30 (0.45–3.75) | |||
| Hispanic | 1.26 (0.70–2.27) | |||
| Other | 0.91 (0.39–2.13) | |||
| Etiology | 0.64 | |||
| HCV | 1 | |||
| HBV | 0.71 (0.33–1.52) | |||
| Alcohol | 0.56 (0.17–1.82) | |||
| NASH | 0.92 (0.46–1.84) | |||
| Other | 0.50 (0.12–2.06) | |||
| Number of HCC lesions at listing | 0.92 (0.63–1.34) | 0.65 | ||
| Size of largest tumor at listing | 1.10 (0.92–1.32) | 0.32 | ||
| Total tumor diameter at listing | 1.03 (0.91–1.15) | 0.66 | ||
| Tumor stage beyond Milan criteria | 1.82 (1.03–3.22) | 0.05 | 1.62 (0.88–2.97) | 0.12 |
| Number of local regional therapy treatments | 1.07 (0.94–1.22) | 0.35 | ||
| Time to listing after diagnosis >1 year | 2.41 (1.42–4.08) | 0.002 | 2.70 (1.57–4.67) | <0.001 |
| MELD-Na at listing | 1.10 (1.05–1.15) | <0.001 | 1.08 (1.02–1.16) | 0.02 |
| MELD-Na ≥12 at listing | 2.39 (1.43–4.00) | 0.001 | ||
| Child-Pugh Score at listing | 1.28 (1.14–1.44) | 0.002 | 1.11 (0.94–1.31) | 0.23 |
| Neutrophil to lymphocyte ratio at listing | 1.08 (1.02–1.14) | 0.05 | - |
In a multivariable model, AFP-L3 ≥35% (HR 2.25, 95% CI 1.04–4.88, p=0.04) and DCP ≥7.5 ng/mL (HR 2.20, 95% CI 1.15–4.20, p=0.02) remained associated with waitlist dropout as did time from HCC diagnosis to listing >1 year (HR 2.70, 95% CI 1.57–4.67, p<0.001) and increasing listing MELD-Na score (HR 1.08 per point, 95% CI 1.02–1.16). The AUROC for this waitlist dropout model was 0.73. In the net reclassification analysis, this model including AFP-L3 and DCP predicted 32% of patients having more accurate dropout risk estimates at 2 years from listing than the model without AFP-L3 and DCP though the difference did not reach statistical significance (p>0.05).
The association of the BALAD score with waitlist dropout was also assessed. In multivariable Cox proportional hazards modelling (excluding individual biomarkers, MELD-Na score, and Child-Pugh score as these components are included in BALAD), increasing BALAD score predicted increased waitlist dropout risk (p=0.01) as did initial tumor stage beyond Milan criteria and time from HCC diagnosis to listing >1 year with an AUROC for this model of 0.72. Compared to a BALAD score of 0, multivariable dropout hazard ratios for BALAD scores of 1, 2, and 4 were 2.24 (95% CI 0.63–7.90), 4.55 (1.23–16.86), and 6.65 (1.21–36.61).
In exploratory subgroup analyses, additional multivariable Cox proportional hazards modelling was performed for waitlist dropout stratified by tumor burden, etiology of liver disease, and Child-Pugh class. In patients with single tumor at listing (n=185), DCP >7.5 ng/mL was associated with dropout (HR 3.30, 95% CI 1.60–6.79, p=0.001) whereas AFP >35% was not. For those with multiple tumors at listing (n=72), there was a trend towards higher dropout with AFP >35% (HR 4.05, 95% CI 0.80–20.45, p=0.09). AFP-L3 >35% (HR 3.35, 95% CI 1.42–7.90, p=0.006) and DCP >7.5 ng/mL (HR 2.16, 95% CI 1.03–4.51, p=0.04) were associated with waitlist dropout in those with viral etiology of liver disease (n=177). Finally, the association of biomarkers with waitlist dropout was strongest in the Child-Pugh A subgroup (n=143; AFP-L3 >35% HR 5.40, p=0.002; DCP >7.5 ng/mL HR 4.60, p<0.001).
Finally, we separately assessed the three biomarkers as continuous (rather than categorical variables) in multivariable Cox proportional hazards modelling for waitlist dropout. Only listing AFP-L3 (HR 1.02 per each 1% increase, 95% CI 1.00–1.03, p=0.03) remained significantly associated with waitlist dropout whereas continuous DCP (HR 1.01 per each 1-unit increase (ng/mL), 95% CI 0.99–1.03, p=0.18) and AFP (p=0.06) did not.
Probability of Waitlist Dropout Stratified by Biomarkers at Listing
Elevation in any biomarker, namely listing AFP ≥100 ng/mL or AFP-L3 ≥35% or DCP ≥7.5 ng/mL, led to a significant increase in the Kaplan-Meier cumulative probability of waitlist dropout (Figure 1a). The risk of dropout further increased when two or more biomarkers were elevated compared to elevation in a single biomarker (p<0.001). For example, the probability of waitlist dropout within 1 year of listing was 19.6% among those with one elevated biomarker compared with 51.6% among those with two or more elevated biomarkers at listing (p<0.001) (Figure 1a).
Figure 1.
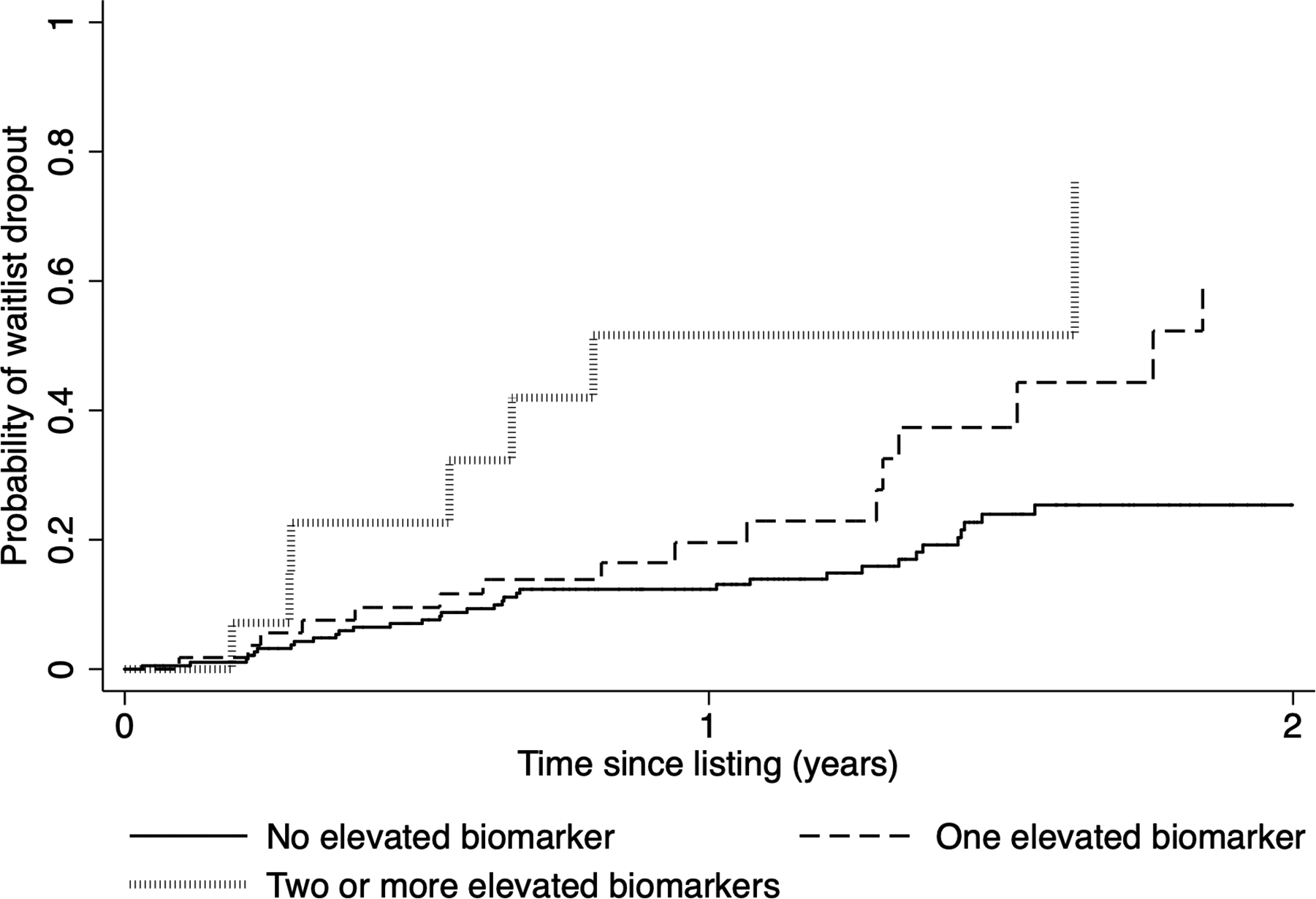
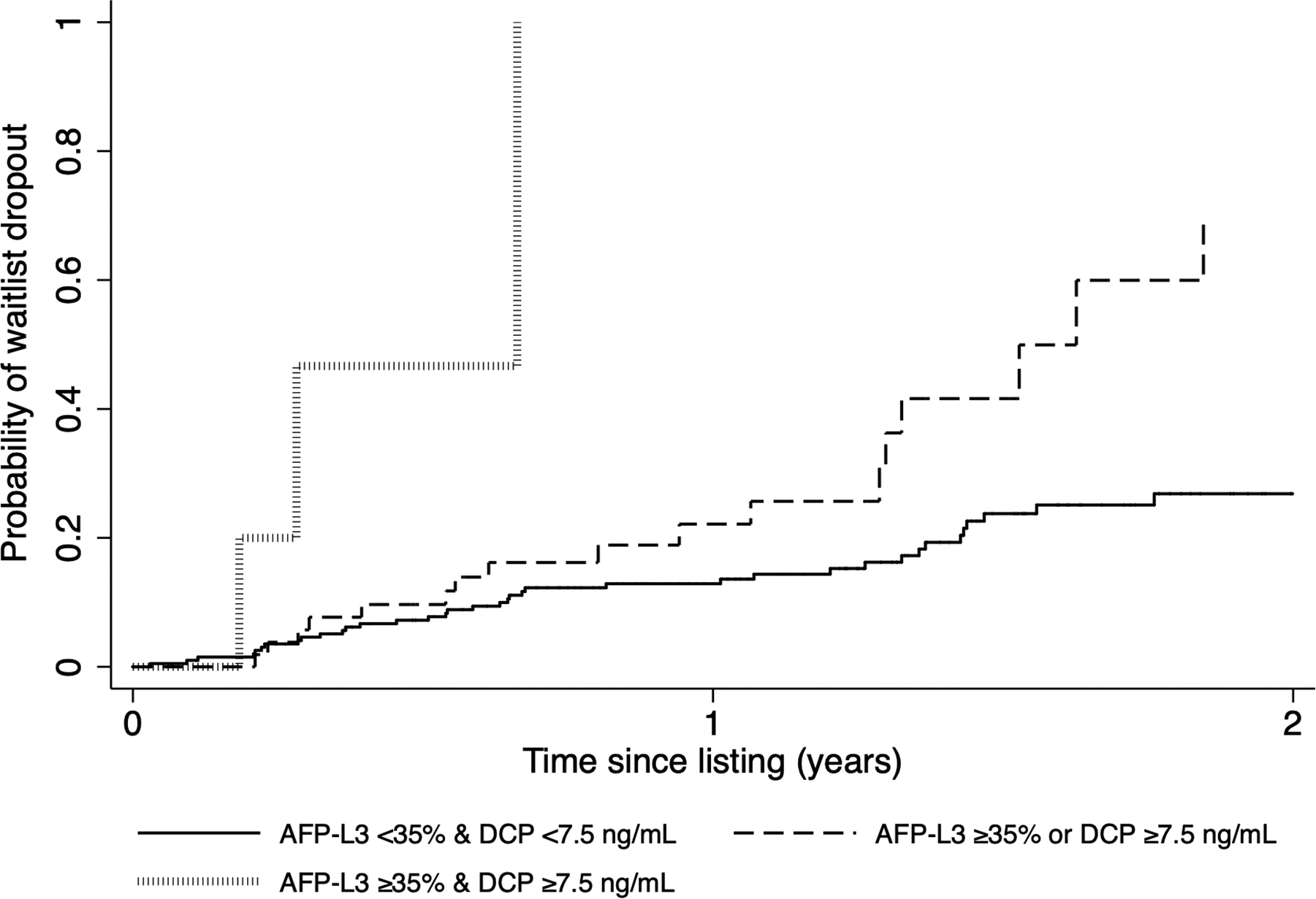
Kaplan-Meier cumulative probability of waitlist dropout based on number of elevated biomarkers at listing (AFP ≥100 ng/mL, AFP-L3 ≥35%, and DCP ≥7.5 ng/mL) when (a) all three biomarkers are considered and (b) only AFP-L3 and DCP are included
When only considering AFP-L3 and DCP, cumulative probability of waitlist dropout within 2 years of listing was 21.8% in those with AFP-L3 <35% and DCP <7.5 ng/mL (n=202) compared to 59.9% with either AFP-L3 or DCP elevated (n=59) (p<0.001). For those with both AFP-L3% and DCP elevated (n=6), waitlist dropout probability was 100% within 9 months of listing (Figure 1b) with median time to dropout of only 3.4 months. Further, among those with AFP <100 ng/mL at listing, having AFP-L3 ≥35% or DCP ≥7.5 ng/mL at listing led to a progressive increase in probability of waitlist dropout (p<0.001).
Increasing BALAD score at listing also led to a significant increase in the cumulative probability of waitlist dropout (Figure 2). For example, patients with a BALAD score of 0 had a 5.5% probability of dropout within 2 years of listing compared with 22.2% for those with a BALAD score of 1, and 52.9% for those with a BALAD score of ≥2 (p<0.001).
Figure 2.
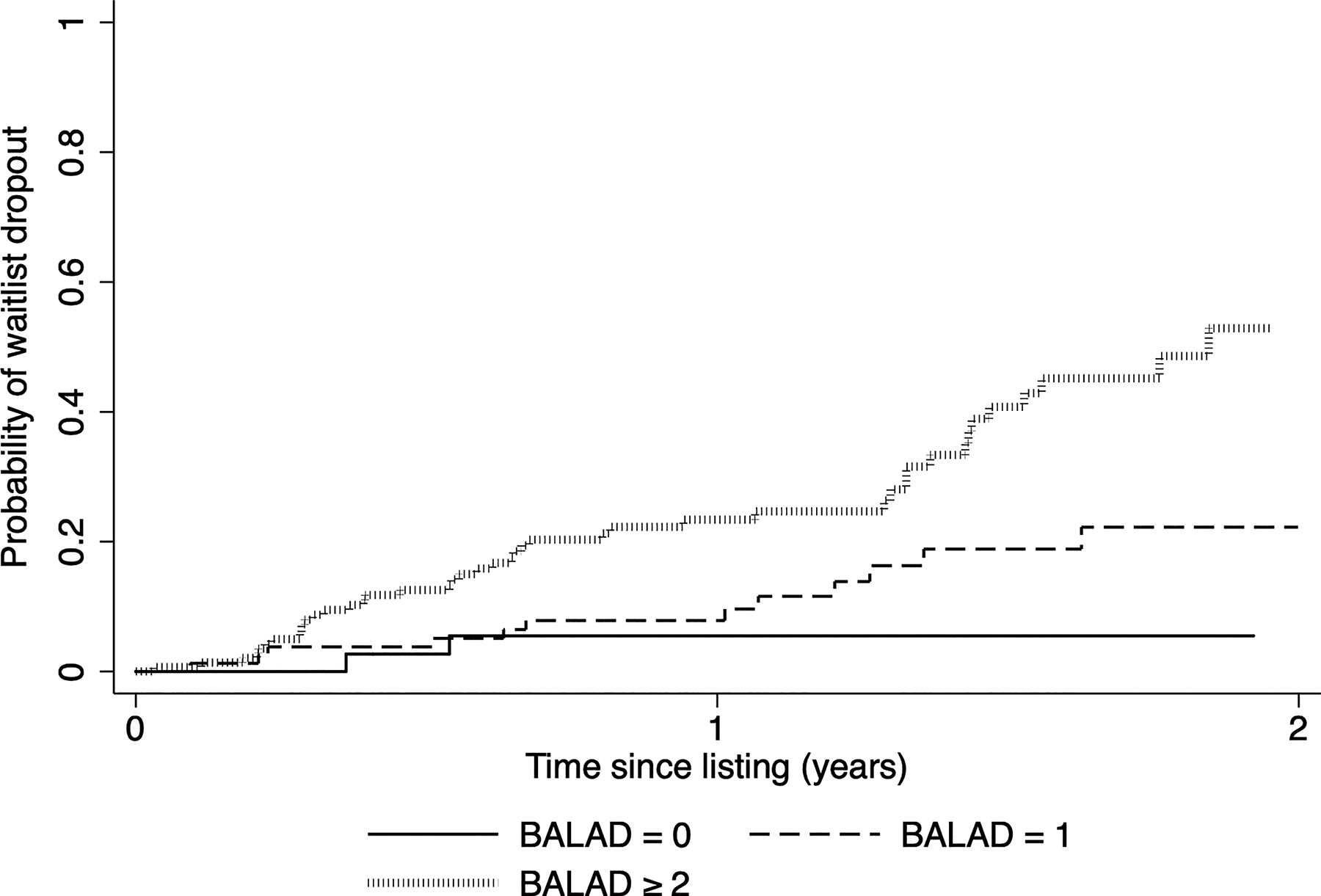
Kaplan-Meier cumulative probability of waitlist dropout based on BALAD score (bilirubin, age, AFP-L3, AFP, and DCP) at listing
Using the parameter estimates from the MV dropout model (Table 3), we created a biomarker-based rounded point risk score for manual calculation (6*(AFP >100 ng/mL) + 8*(AFP-L3 >35%) + 8*(DCP>7.5 ng/mL) + 5*(initial tumor stage beyond Milan criteria) +10*(Time to listing after HCC dx >1yr) + 1*(MELD-Na at listing) + 1*(Child-Pugh score at listing). Median risk score of the entire cohort was 23 (IQR 16–29). We then split the cohort (n=257) into quintiles with median values of 13, 17, 23, 27, and 40 for lowest to highest risk quintiles. Kaplan-Meier waitlist dropout probability within 1-year of listing for lowest to highest risk quintile was 1.9%, 8.0%, 13.4%, 22.2%, and 42.0%, respectively (p <0.001). Importantly, 2-year KM probability of dropout in the highest risk quintile (n=54) was 100%.
DISCUSSION
It is clear that a combination of liver-disease factors (e.g. MELD-Na score, Child Pugh class) and tumor characteristics (e.g. tumor size/number) predict waitlist dropout in HCC patients (5). However, there remains a need to incorporate biomarkers beyond AFP in prognosticating waitlist outcomes, especially since the majority of HCC patients being evaluated for LT have an AFP <20 ng/mL (5). While AFP-L3 and DCP appear to be promising HCC biomarkers with regards to early tumor detection (12, 13) published data supporting the utility of AFP-L3 and DCP in the LT setting have largely come from patients receiving LDLT in Asia (6, 18, 19). In a retrospective analysis of 127 LT recipients, investigators from the Mayo Clinic (20) observed the highest HCC recurrence risk in patients with a combination of AFP ≥250 ng/mL and DCP ≥7.5 ng/mL though AFP-L3 of ≥35% was also a significant predictor of HCC recurrence with a magnitude of risk comparable to that of AFP and DCP. More recently, in an ongoing prospective single-center analysis, AFP-L3 and DCP appeared to be superior to AFP in predicting high risk explant features (21). While these studies have shown the prognostic utility of AFP-L3 and DCP, the association of these biomarkers with waitlist dropout in HCC patients has not previously been investigated.
In this first prospective study evaluating the prognostic power of all three biomarkers (AFP, AFP-L3, and DCP) in patients listed for LT, we found that AFP-L3 >35% ng/mL and DCP ≥7.5 ng/mL measured at the time of listing were independent predictors of waitlist dropout whereas AFP at all tested cutoffs (e.g. >20, >100, and >250) was not. Probability of waitlist dropout within 2 years of listing was just over 20% in those with AFP-L3 <35% and DCP <7.5 ng/mL compared to nearly 60% with either AFP-L3 or DCP elevated. Further, all 6 patients with both elevated AFP-L3% and DCP experienced waitlist dropout within 9 months of listing. While AFP >1000 ng/ml is an exclusion criterion for LT, this excludes only a very small fraction (~2%) of early stage HCC patients and is therefore unlikely to account for the differential prognostic capability of these biomarkers. Additionally, in Kaplan-Meier survival analysis, among those with AFP <100 ng/mL at listing, the presence of either AFP-L3 ≥35% or DCP ≥7.5 ng/mL was associated with increased risk of waitlist dropout. This data thus clearly adds to previous literature that DCP and AFP-L3 are powerful markers of tumor biology and importantly, add prognostic value to AFP alone.
There are several ways to consider the clinical relevance of this data. First, measuring AFP-L3 and DCP in HCC patients at the time of listing is a simple, straightforward and relatively inexpensive way to prognosticate waitlist outcome. Additionally, given that elevated DCP and AFP-L3 predict high-risk explant features (21) and post-LT recurrence (20), it may be prudent to perform additional LRT for residual/recurrent HCC when these biomarkers are elevated to improve LT-related outcomes. This concept is similar to assessing AFP slope in response to LRT as a powerful predictor of post-LT recurrence and survival (24, 25). To this end, measuring all three biomarkers rather than only AFP every 3 months in HCC patients awaiting LT (especially in those with ≥1 biomarker elevated at listing) may provide a more comprehensive assessment of an individual’s response to LRT and waitlist dropout risk.
The usefulness of incorporating all three biomarkers with relevant clinical characteristics has been previously shown via the GALAD model (gender, age, AFP-L3, AFP, and DCP) to predict the presence of any HCC (12, 13) as well as early-stage HCC (26). Similarly, the validated BALAD score (bilirubin, albumin, AFP-L3, AFP, and DCP) has been shown to predict HCC-related survival (23). Therefore, in the present study, we assessed the association of BALAD score with waitlist dropout risk. Not surprisingly, we found that higher BALAD score predicts increasing dropout with more than twice the dropout risk for those with a BALAD score of 1 and more than five-fold increased risk for those with a score of ≥2 compared to BALAD score of 0. Additionally, the AUROC for the multivariable waitlist dropout model including BALAD was quite robust at 0.72, which aligns well with other prognostic models in the LT literature (27). In combining liver-disease and tumor-related characteristics, the BALAD model appears particularly useful in the LT setting for HCC patients given that elevated bilirubin may limit LRT options and as such identify a cohort of patients who are unlikely to ultimately receive LT.
Also of interest is the role of biomarkers in tumor down-staging and waiting time prior to LT. Previous studies on tumor down-staging have consistently demonstrated a strong association between AFP (or response of AFP to LRT) and post-LT outcomes (28–29) and increased wait time is a well-established risk factor for dropout (3). In the present cohort, 41 of the 267 patients (15%) had tumors successfully down-staged to within Milan criteria prior to LT listing. A recent multi-center prospective study from the MERITS-LT down-staging consortium showed that elevated pre-treatment AFP-L3% was associated with increased dropout risk (30) consistent with findings from the present study. Importantly, both initial tumor stage beyond Milan criteria and time from HCC diagnosis to listing >1 year were significant predictors of waitlist dropout in the present study. This data suggests that HCC patients with baseline elevated AFP-L3% and DCP who are likely to require multiple down-staging treatments prior to listing with MELD exception have a particularly high risk of waitlist dropout.
The strengths of the present study include the prospective study design and the homogeneous study population with well-defined LT selection criteria for HCC. Additionally, all three prognostic biomarkers are readily available and straightforward to obtain which increases the relevance and potential application of these findings. However, there are also several limitations. Due to the prospective, single-center design and ongoing nature of this study, the number of patients included (n=267) was relatively small as was the number of patients experiencing waitlist dropout (n=63). This limited our ability to create and test the performance of a biomarker dropout risk score. Additionally, AFP-L3 and DCP were not standardly measured every 3 months (as was AFP) and therefore we are unable to study the performance of these biomarkers in assessing dropout risk over time, most notably in relation to response to LRT. Finally, our center is located in a region of prolonged waiting time for LT, though recent incorporation of policy change to award median MELD at transplant minus 3 points (MMAT-3) (4) to equalize LT wait times for HCC patients nationally increases the generalizability of our findings.
In conclusion, in this first prospective study of HCC patients assessing all three biomarkers, AFP, AFP-L3 and DCP measured at the time of listing for LT, we found AFP-L3 and DCP to be superior to AFP in predicting waitlist dropout. Specifically, having AFP-L3 ≥35% or DCP ≥7.5 ng/mL at listing led to a progressive increase in probability of waitlist dropout, even when AFP was not significantly elevated and the combination of AFP-L3 ≥35% and DCP ≥7.5 ng/mL was associated with a 100% risk of waitlist dropout. Therefore, AFP-L3 and DCP clearly add prognostic value to AFP alone in predicting waitlist outcomes in HCC patients listed for LT, including those who have received tumor down-staging.
Financial Support:
This work was supported by the Clinical and Translational Core of the UCSF Liver Center (P30 DK026743).
Conflict of interest statement:
Neil Mehta has advised and received institutional grants from FujiFilm WAKO. He received instiutional grants from Glycotest and Target Pharmasolutions. Francis Yao received grants from FujiFilm Wako.
Abbreviations:
- HCC
Hepatocellular carcinoma
- LT
liver transplantation
- AFP
alpha-fetoprotein
- UNOS
United Network for Organ Sharing
- LRT
local regional therapy
- DCP
des-gamma-carboxyprothrombin
- AFP-L3
Lectin-reactive alpha-fetoprotein
- LI-RADS
Liver Imaging Reporting and Data System
- IQR
interquartile range
- MELD
Model for End Stage Liver Disease
- BALAD
bilirubin, age, AFP-L3, AFP, DCP
- HR
hazard ratio
- NLR
neutrophil-to-lymphocyte ratio
- AUROC
Area Under the Receiver Operating Characteristics
- GALAD
gender, age, AFP-L3, AFP, and DCP
REFERENCES
- 1.Kwong AJ, Kim WR, Lake JR, et al. OPTN/SRTR 2019 Annual Data Report: Liver. Am J Transplant. 2021;21 Suppl 2:208–315. doi: 10.1111/ajt.16494 [DOI] [PubMed] [Google Scholar]
- 2.Singal AG, Lampertico P, Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J Hepatol. 2020. Feb;72(2):250–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta N, Dodge JL, Hirose R, Roberts JP, Yao FY. Increasing Liver Transplantation Wait-List Dropout for Hepatocellular Carcinoma With Widening Geographical Disparities: Implications for Organ Allocation. Liver Transpl. 2018. Oct;24(10):1346–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.OPTN/UNOS Liver and Intestinal Organ Transplantation Committee. Available from: http://optn.transplant.hrsa.gov/ Accessed May 10, 2020
- 5.Mehta N, Dodge JL, Roberts JP, Yao FY. A novel waitlist dropout score for hepatocellular carcinoma - identifying a threshold that predicts worse post-transplant survival. J Hepatol. 2021. Apr;74(4):829–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujiki M, Takada Y, Ogura Y, Oike F, Kaido T, Teramukai S, Uemoto S. Significance of des-gamma-carboxy prothrombin in selection criteria for living donor liver transplantation for hepatocellular carcinoma. Am J Transplant. 2009. Oct;9(10):2362–71. [DOI] [PubMed] [Google Scholar]
- 7.Duvoux C, Roudot-Thoraval F, Decaens T, Pessione F, Badran H, Piardi T, Francoz C, et al. Liver transplantation for hepatocellular carcinoma: a model including alpha-fetoprotein improves the performance of Milan criteria. Gastroenterology 2012;143:986–994. [DOI] [PubMed] [Google Scholar]
- 8.Hameed B, Mehta N, Sapisochin G, Roberts JP, Yao FY. Alpha-fetoprotein level > 1000 ng/mL as an exclusion criterion for liver transplantation in patients with hepatocellular carcinoma meeting the Milan criteria. Liver Transpl 2014;20:945–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazzaferro V, Sposito C, Zhou J, et al. Metroticket 2.0 model for analysis of competing risks of death after liver transplantation for hepatocellular carcinoma. Gastroenterology 2018;154:128–139. [DOI] [PubMed] [Google Scholar]
- 10.Berry K, Ioannou GN. Serum alpha-fetoprotein level independently predicts posttransplant survival in patients with hepatocellular carcinoma. Liver Transpl 2013;19:634–645 [DOI] [PubMed] [Google Scholar]
- 11.Mehta N, Heimbach J, Harnois DM, et al. Validation of a risk estimation of tumor recurrence after transplant (RETREAT) score for hepatocellular carcinoma recurrence after liver transplant. JAMA Oncol 2017;3:493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson PJ, Pirrie SJ, Cox TF, et al. The detection of hepatocellular carcinoma using a prospectively developed and validated model based on serological biomarkers. Cancer Epidemiol Biomarkers Prev. 2014;23:144–153 [DOI] [PubMed] [Google Scholar]
- 13.Berhane S, Toyoda H, Tada T, et al. Role of the GALAD and BALAD-2 Serologic Models in Diagnosis of Hepatocellular Carcinoma and Prediction of Survival in Patients. Clin Gastroenterol Hepatol. 2016;14:875–886. [DOI] [PubMed] [Google Scholar]
- 14.Cheng J, Wang W, Zhang Y, et al. Prognostic role of pre-treatment serum AFP-L3% in hepatocellular carcinoma: systematic review and meta-analysis. PloS one. 2014;9:e87011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okuda H, Nakanishi T, Takatsu K, et al. Comparison of clinicopathological features of patients with hepatocellular carcinoma seropositive for α‐fetoprotein alone and those seropositive for des‐γ‐carboxy prothrombin alone. J Gastroenterol Hepatol. 2001;16:1290–1296. [DOI] [PubMed] [Google Scholar]
- 16.Suh SW, Lee KW, Lee JM, et al. Prediction of aggressiveness in early-stage hepatocellular carcinoma for selection of surgical resection. J Hepatol. 2014. Jun;60(6):1219–24.. [DOI] [PubMed] [Google Scholar]
- 17.Poté N, Cauchy F, Albuquerque M, et al. Performance of PIVKA-II for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion. J Hepatol. 2015. Apr;62(4):848–54. [DOI] [PubMed] [Google Scholar]
- 18.Kaido T, Ogawa K, Mori A, et al. Usefulness of the Kyoto criteria as expanded selection criteria for liver transplantation for hepatocellular carcinoma. Surgery. 2013;154(5):1053–1060. [DOI] [PubMed] [Google Scholar]
- 19.Lee JH, Cho Y, Kim HY, et al. Serum Tumor Markers Provide Refined Prognostication in Selecting Liver Transplantation Candidate for Hepatocellular Carcinoma Patients Beyond the Milan Criteria. Annals of Surgery. 2016;263(5):842–850. [DOI] [PubMed] [Google Scholar]
- 20.Chaiteerakij R, Zhang X, Addissie BD, et al. Combinations of biomarkers and Milan criteria for predicting hepatocellular carcinoma recurrence after liver transplantation. Liver Transpl. 2015;21(5):599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kotwani P, Chan W, Yao F, Mehta N. DCP and AFP-L3 Are Complementary to AFP in Predicting High-Risk Explant Features: Results of a Prospective Study. Clin Gastroenterol Hepatol. Published online January 29, 2021 [DOI] [PubMed] [Google Scholar]
- 22.Mitchell DG, Bruix J, Sherman M, Sirlin CB. LI-RADS (Liver Imaging Reporting and Data System): summary, discussion, and consensus of the LI-RADS Management Working Group and future directions. Hepatology 2015; 61:1056–1065. [DOI] [PubMed] [Google Scholar]
- 23.Wongjarupong N, Negron-Ocasio GM, Mara KC, et al. BALAD and BALAD-2 predict survival of hepatocellular carcinoma patients: a North American cohort study. HPB (Oxford). 2021;23(5):762–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halazun KJ, Rosenblatt RE, Mehta N, et al. Dynamic α-Fetoprotein Response and Outcomes After Liver Transplant for Hepatocellular Carcinoma. JAMA Surg. 2021. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giard JM, Mehta N, Dodge JL, et al. Alpha-Fetoprotein Slope >7.5 ng/mL per Month Predicts Microvascular Invasion and Tumor Recurrence After Liver Transplantation for Hepatocellular Carcinoma. Transplantation. 2018. May;102(5):816–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Best J, Bechmann LP, Sowa JP, et al. GALAD score detects early hepatocellular carcinoma in an international cohort of patients with nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2020;18:728–735. [DOI] [PubMed] [Google Scholar]
- 27.Godfrey EL, Malik TH, Lai JC, et al. The decreasing predictive power of MELD in an era of changing etiology of liver disease. Am J Transplant. 2019. Dec;19(12):3299–3307. [DOI] [PubMed] [Google Scholar]
- 28.Yao FY, Mehta N, Flemming JA, et al. Downstaging of hepatocellular cancer before liver transplant: Long-term outcome compared to tumors within Milan criteria. Hepatology 2015;61:1968–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehta N, Dodge JL, Grab JD, Yao FY. National experience on down-staging of hepatocellular carcinoma before liver transplant: Influence of initial tumor burden, alpha-fetoprotein, and wait Time. Hepatology 2020;71:943–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehta N, Frenette C, Tabrizian P, et al. Downstaging Outcomes for Hepatocellular Carcinoma: Results From the Multicenter Evaluation of Reduction in Tumor Size before Liver Transplantation (MERITS-LT) Consortium. Gastroenterology. 2021. Nov;161(5):1502–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]


