Abstract
Background and Aims
Endoscopist adenoma detection rates (ADR) vary widely and are associated with patients’ risk of post-colonoscopy colorectal cancers (PCCRC). However, few scalable physician-directed interventions demonstrably both improve ADR and reduce PCCRC risk.
Methods
Among patients undergoing colonoscopy, we evaluated a scalable online training’s influence on individual-level ADRs and PCCRC risk. The intervention was a 30-minute, interactive, online training, developed using behavior-change theory to address factors that potentially impede adenoma detection. Analyses included interrupted time series analyses for pre- vs. post-training individual-physician ADR changes (adjusted for temporal trends) and Cox regression for associations between ADR changes and patients’ PCCRC risk.
Results
Across 21 endoscopy centers and all 86 eligible endoscopists, ADRs increased immediately by an absolute 3.13% (95% confidence interval [CI]; 1.31–4.94) in the 3-month quarter following training compared with 0.58%/quarter (95%CI: 0.40–0.77) and 0.33%/quarter (95%CI: 0.16–0.49) in the 3-year pre- and post-training periods, respectively. Post-training ADR increases were higher among endoscopists with pre-training ADRs below the median. Among 146,786 post-training colonoscopies (all indications), each 1% absolute increase in screening ADR post-training was associated with a 4% decrease in their patients’ PCCRC risk (hazard ratio [HR]: 0.96, 95%CI: 0.93–0.99). An ADR increase of ≥10% vs. <1% was associated with a 55% reduced risk of PCCRC (HR: 0.45, 95%CI: 0.24–0.82).
Conclusions
A scalable online behavior-change training focused on modifiable factors was associated with significant and sustained improvements in ADR, particularly among endoscopists with lower ADRs. These ADR changes were associated with substantial reductions in their patients’ risk of PCCRC.
Keywords: Colonoscopy quality measure, behavioral intervention, screening, interval cancer, adenoma, colorectal cancer
INTRODUCTION
Colorectal cancer is a leading cause of cancer death in the United States.1 The use of colonoscopy for primary screening or for follow-up after other positive screening tests can reduce colorectal cancer incidence and deaths through the detection and removal of precancerous polyps (adenomas) and/or more treatable early-stage cancers.2 The quality of the colonoscopy examination influences the health benefits achieved, including the prevention of post-colonoscopy colorectal cancers (PCCRC) and related deaths.3–5
Physician adenoma detection rate (ADR), defined as the percentage of screening colonoscopies a physician performs that detect at least one adenoma, is an established colonoscopy quality metric.6 Its variation across settings is associated with a greater than two-fold variation in patient risk of PCCRC and related deaths.7–19 Numerous interventions have been tested to improve physician ADRs.7, 20 ADR feedback alone has not been associated with improvements in ADRs in individual randomized trials, although pooling trials suggested a benefit21 and it has been associated with increases in ADRs over time;7 other endoscopist-level interventions have had limited success or are not easily scalable to different settings.22–28 To our knowledge, no remotely available, generalizable, scalable endoscopist-level intervention has been demonstrated to both improve ADRs and evaluate if the ADR changes are associated with a reduced risk of post-colonoscopy cancers.
The current multi-center study sought to evaluate the impact of a behavior change theory-based 30-minute interactive online training on individual-level endoscopist ADRs and the influence of associated ADR changes on the endoscopists’ patients’ subsequent risk of PCCRC. This training was developed using research from a multidisciplinary group that included experts in behavioral change theory and evidence-based behavioral interventions, gastroenterologists, endoscopy nurses, and epidemiologists.29 This group’s findings were used to 1) create the training to address potential drivers of ADR variation; 2) implement the training; 3) evaluate the impact of this training on endoscopist ADRs, beyond temporal trends and periodic ADR feedback; and 4) then evaluate the associations between individual endoscopist-level changes in ADR post-training and their patients’ risk of PCCRC.
METHODS
Study Population and Oversight
The study setting was all 21 endoscopy centers across Kaiser Permanente Northern California (KPNC). KPNC is a large integrated healthcare delivery organization with approximately 4.5 million members, its membership’s demographic and socioeconomic characteristics closely approximate the region’s diverse census demographics and includes patients with Medicare, Medicaid, and commercial insurance.30
This study was conducted within the National Cancer Institute-funded Population-based Research Optimizing Screening through Personalized Regimens (PROSPR) consortium (U54 CA163262) and Population-based Research to Optimize the Screening Process II (PROSPR II) consortium (UM1 CA222035), which conducts multisite, coordinated, transdisciplinary research to evaluate and improve cancer-screening processes. The study was approved by the KPNC institutional review board, which waived the requirement for individual informed consent.
Study Design
This was a single-arm intervention study where endoscopists served as their own controls pre- vs. post-intervention, adjusted for temporal trends.
The study included a 3-year pre-training period, followed by a 3-month (one quarter) training period, followed by a 3-year post-training period. The first 3 months of the post-training period was the immediate post-training period (Figure 1); this immediate post-training period was used to evaluate the immediate impact of the training (see analysis section).
Figure 1.
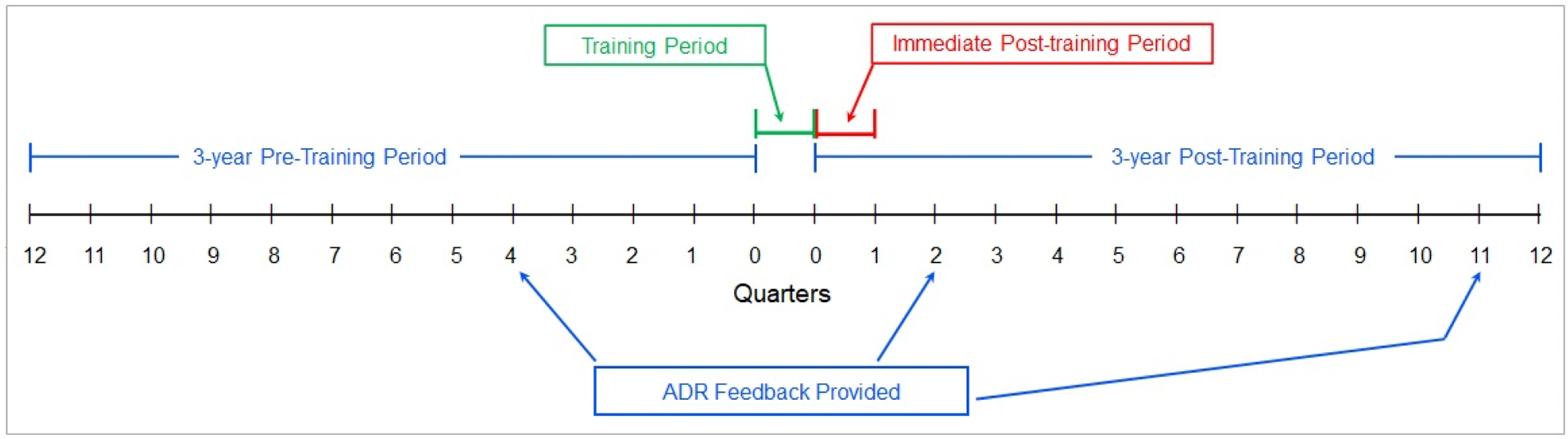
Four time periods of the study.
Eligibility Criteria
All KPNC gastroenterologists completed the mandated colonoscopy quality training between September and December, 2014; thus, study inclusion depended only on the endoscopist also performing ≥100 total colonoscopies annually, ≥25 of which were screening examinations, during the 3-year periods pre- and post-training. Colonoscopies were excluded if the patient 1) was <50 years old; 2) terminated health plan membership during the post-colonoscopy follow-up period; 3) was diagnosed with colorectal cancer within 6 months post-procedure (to allow for repeat procedures to make a cancer diagnosis); or 4) had a prior colorectal cancer.
Intervention
The colonoscopy quality training intervention used a theory-based performance improvement approach. The training addressed factors potentially associated with ADR variability identified from research among gastroenterologists and endoscopy unit staff in collaboration with a psychologist and researchers with expertise in behavior change theory and evidence-based interventions.29 Seven drivers of ADR variability were identified including four related to capability: 1) uncertainty about which types of polyps to remove; 2) style of endoscopy team leadership; 3) examination technique during withdrawal; and 4) difficulty detecting certain types of adenomas; two related to opportunity: 5) perceived pressure due to the number of examinations expected per shift and 6) social pressure to finish examinations before scheduled breaks or the end of a shift; and one related to motivation: 7) valuing a meticulous examination as the top priority.
To address these factors, a 30-minute, interactive, remotely accessible, online training was developed that included: 1) education on the evidence regarding associations between physician ADR and post-colonoscopy colorectal cancer, advanced-stage disease, and related deaths (addressing capability and motivation); 2) optimal colonoscopy examination techniques such as washing methods and second-looks in colon segments where polyps are frequently missed (addressing capability); 3) identification of difficult-to-see flat adenomas common to the proximal colon (addressing capability); and 4) social incentives for normalizing a quality-focused culture, peer testimonials about prioritizing quality, and the training program’s inclusion as a universal effort for all clinicians (addressing opportunity and motivation). The training utilized evidence-based learning theory methods for enhancing knowledge retention, including integrated questions and answers, group engagement, and interactive visual scenarios (e.g., for identifying difficult-to-see adenomas).31 The training is freely available at: https://deliveryscience-appliedresearch.kaiserpermanente.org/specialty-research-networks/gastroenterology-hepatology.
Separate from the training intervention, ADR feedback was provided approximately 24 months into the 3-year pre-training period and approximately 6 months and 32 months into the 3-year post-training period; no ADR feedback was provided during or in the months adjacent to the training period (Figure 1). For ADR feedback, endoscopist-level ADRs from screening colonoscopies were stratified by patient sex and distributed to medical center gastroenterology chiefs and to individual gastroenterologists. ADR reports included endoscopist ADRs and the ADR guideline-recommended benchmarks.6
Outcomes
The first outcome was the change in individual endoscopist-level ADRs based on screening colonoscopies in the post-training period compared to the pre-training period. The second outcome was the association between these changes in individual endoscopist-level ADRs and their patients’ risk of PCCRC following a colonoscopy in which cancer was not detected (a.k.a. a negative colonoscopy) performed in the post-training period.
ADRs were defined as the presence of at least one adenoma on a screening colonoscopy, using validated methods.32 PCCRCs were defined as a colorectal adenocarcinoma occurring ≥6 months and up to 3 years after a negative colonoscopy (done for any indication), using the World Endoscopy Organization’s definition.33 This metric provided comparable follow-up periods for statistical comparisons throughout the post-training period. Thus, as an example, for an endoscopist who completed colonoscopy training on December 15, 2014, the post-training period would have started on that date and extended for all negative colonoscopies performed in the next 3 years, to December 15, 2017. Three-year follow-up cancer data for 2017 examinations, i.e., through 2020, became available in the cancer registry in 2022, when the current analysis was completed. Consistent with prior studies, physician ADRs were calculated using screening colonoscopies and these ADRs were used to predict cancer outcomes after colonoscopies performed for any indication.3, 19
Colorectal adenocarcinoma definitions used Surveillance Epidemiology and End Results (SEER) cancer site group codes 21040 and 21050, and International Classification of Disease oncology codes: C18.0, C18.2-C18.9, C19.9, and C20.9.
Data sources
Patient characteristics were ascertained relative to the colonoscopy date. Endoscopist characteristics (i.e., age, sex, and years since medical school graduation) were determined relative to the training date. Colonoscopy procedures and indications, pathology findings and cancer diagnoses, and patient and endoscopist characteristics were obtained from previously validated electronic databases.3, 34 Colonoscopy procedures were identified using Current Procedural Terminology codes, International Classification of Disease procedure codes, Healthcare Common Procedure Coding System codes, and KPNC-specific internal codes for tracking the presence and year of colonoscopies performed prior to joining KPNC. Colonoscopy indication assignment used a validated algorithm that incorporates electronic consultations, International Classification of Diseases 10th revision codes, and laboratory, pathology, and radiologic tests to categorize colonoscopies as screening or non-screening (i.e., positive fecal test, surveillance, and diagnostic colonoscopies).34 ADRs were calculated by linking endoscopists with colonoscopies, ascertaining screening colonoscopy indication using a validated algorithm from pre-colonoscopy electronic data, and linking pathology results for ≥1 adenoma detected using Systematized Nomenclature of Medicine codes for colon location and histology.32 This approach was previously validated for identifying colonoscopies, assigning indication, and adenoma diagnosis, compared with chart review.3, 19, 34 Neither of the key factors measured (indication or pathology results) were modifiable by the performing endoscopist. Cancer data were obtained from a validated cancer registry. KPNC’s cancer registry completes validation and reports to SEER approximately two years after cancer diagnosis and has achieved >98% completeness in capture of cancer diagnosed detection and includes cancers diagnosed within California outside of KPNC facilities.
Statistical analyses
The analytic methods allowed evaluation of the training period’s effect on ADRs while controlling for pre-training temporal trends in ADR. For each endoscopist, we calculated ADRs for each three-month quarter as the unit of time during the four study periods (Figure 1). We then used interrupted time series analysis to assess ADR trends over the 3-year pre-training period, changes in the 3-month immediate post-training period, and the subsequent 3-year post-training period. The interrupted time series method allows for evaluation of a training intervention delivered within a discrete time period for associations between intervention and outcome that are independent of (adjusted for) temporal trends in ADR within the pre-training period.35 These analyses used a generalized linear mixed model with a random effect for endoscopist to account for physician clustering, and used a binomial response distribution and an identity link. A robust (sandwich) variance estimator was used for fixed effects. Following the interrupted time series methodology, the trends in ADR before and after training were modeled using segmented regression, allowing a change in slope associated with time (quarter) and an immediate change in the ADR level at an inflection point defined as the end of the immediate post-training period (i.e., first quarter after training).
Differences in ADR changes associated with training, by pre-training endoscopist ADR level, were assessed by including interaction terms in the regression model, allowing the pre-training ADR slope, immediate training effect, and post-training ADR slope to vary by endoscopist pre-training ADR level (< 29.2% vs. ≥ 29.2% [median ADR in the pre-training period]).
The associations between endoscopist-level changes in screening ADRs in the pre- vs. post-training periods and their patients’ subsequent PCCRC risk among all negative colonoscopies (regardless of indication) performed in the 3-year post-training period were evaluated using Cox proportional hazards regression. Each patient with a negative colonoscopy in the post-training period was followed to the earliest of 1) health plan disenrollment; 2) death; 3) colorectal cancer diagnosis; 4) a follow-up colonoscopy negative for colorectal cancer; or 5) 3-years after the colonoscopy date.
Each negative colonoscopy performed in the post-training period was assigned an ADR change value calculated as the difference between the performing endoscopist’s screening ADR for that post-training year and their screening ADR over the 3-year pre-training period. For example, a negative colonoscopy during the 2nd year post-training was assigned an absolute ADR change of 5% if the performing endoscopist’s screening ADR changed from 25% pre-training to 30% during the 2nd year of the post-training period.
Model covariates included patient age, sex, race, ethnicity, body mass index, Charlson comorbidity score and colonoscopy indication (screening, not screening), and endoscopist ADR status in the pre-training period (below, at or above the median). Marginal modeling accounted for within-physician clustering, with a robust sandwich estimate of the covariance matrix.36
Heterogeneity in associations by median pre-training endoscopist ADR level was evaluated using interaction terms. ADR change was evaluated as a continuous variable (i.e., for each 1% absolute change in ADR).
All statistical tests were two-sided; a p-value of <0.05 was considered statistically significant. SAS statistical software (version 9.3 Cary, NC) was used for analyses.
RESULTS
Endoscopist and patient characteristics
All KPNC gastroenterologists completed the training. Among these, 86 met the additional procedure volume and date eligibility criteria and are included in the analysis (Table 1). The demographic, body mass index, and comorbidity characteristics of patients who underwent colonoscopy in the pre-training vs. post-training periods were comparable (Table 1).
Table 1.
Endoscopist patient characteristics.
| Characteristics | At date of training | |
|---|---|---|
| Endoscopist characteristics | ||
| Physicians, n | 86 | |
| Age, years, median (interquartile range (IQR)) | 45 (40, 57) | |
| Male, n (%) | 63 (73.3) | |
| Time since medical school graduation, years, median (IQR) | 18 (8, 39) | |
| Endoscopist ADR in the pre-training period, %, median (IQR) | 29.2 (22.8, 35.1) | |
| Endoscopist ADR in the post-training period, %, median (IQR) | 35.5 (31.3, 44.5) | |
| Pre-training period | Post-training period | |
| Patient characteristics | ||
| Total colonoscopy procedures, n | 133,225 | 146,786 |
| Screening colonoscopy procedures, n | 31,643 (23.8) | 28,408 (19.4) |
| Age, years, median (IQR) | 63 (56, 69) | 63 (57,70) |
| Female, n (%) | 68,457 (51.4) | 74,244 (50.6) |
| Race and ethnicity, n (%) | ||
| Asian or Pacific Islander | 20,048 (15.1) | 23,038 (15.7) |
| Black | 8,924 (6.7) | 10,015 (6.8) |
| Hispanic | 16,940 (12.8) | 19,893(13.6) |
| White | 84,105 (63.1) | 89,665 (61.1) |
| Other | 1,595 (1.2) | 1,860 (1.3) |
| Missing | 1,613 (1.2) | 2,315 (1.6) |
| Body mass index, kg/m2, median (IQR) | 27.3 (24.1, 31.2) | 27.4 (24.1, 31.4) |
| Charlson comorbidity score, median (IQR) | 0 (0, 1) | 0 (0, 2) |
ADR, adenoma detection rate; IQR, interquartile range
133,225 colonoscopies were performed during the 3-year pre-training period, of which 31,643 (23.8%) were screening examinations, and 146,786 colonoscopies during the 3-year post-training period, of which 28,408 (19.4%) were screening examinations (Table 1). The median ADRs were 29.2% (interquartile range [IQR]: 22.8%, 35.1%) in the pre-training period and 35.5% (IQR: 31.3%, 44.5%) in the post-training period.
Endoscopist ADR changes
The immediate training effect on ADR for the quarter following colonoscopy quality training was significantly greater than temporal ADR trends, with an absolute mean increase of 3.13% (95% confidence interval [CI]: 1.31%, 4.94%). In contrast, during the 3-year pre-training period, mean ADRs increased by an absolute 0.58% per quarter (95% CI: 0.40%, 0.77%) and, during the 3-year post-training period, by 0.33% per quarter (95% CI: 0.16%, 0.49%) (Table 2 and Figure 2).
Table 2.
Endoscopist adenoma detection rate (ADR) changes by training period and median pre-training ADR.
| 3-year pre-training period | Immediately following training | 3-year post-training period | |
|---|---|---|---|
| All endoscopists, n=86 | 0.58 (0.40, 0.77) | 3.13 (1.31, 4.94) | 0.33 (0.16, 0.49) |
| Lower ADR endoscopists, n=43 | 0.43 (0.19, 0.67) | 4.89 (2.42, 7.36) | 0.27 (0.18, 0.51) |
| Higher ADR endoscopists, n=43 | 0.80 (0.54, 1.06) | 0.73 (−1.71, 3.17) | 0.40 (0.18, 0.63) |
Lower vs. higher ADR endoscopists were stratified using the median ADR of 29.2% in the pre-training period (see methods).
CI, confidence interval.
Figure 2.
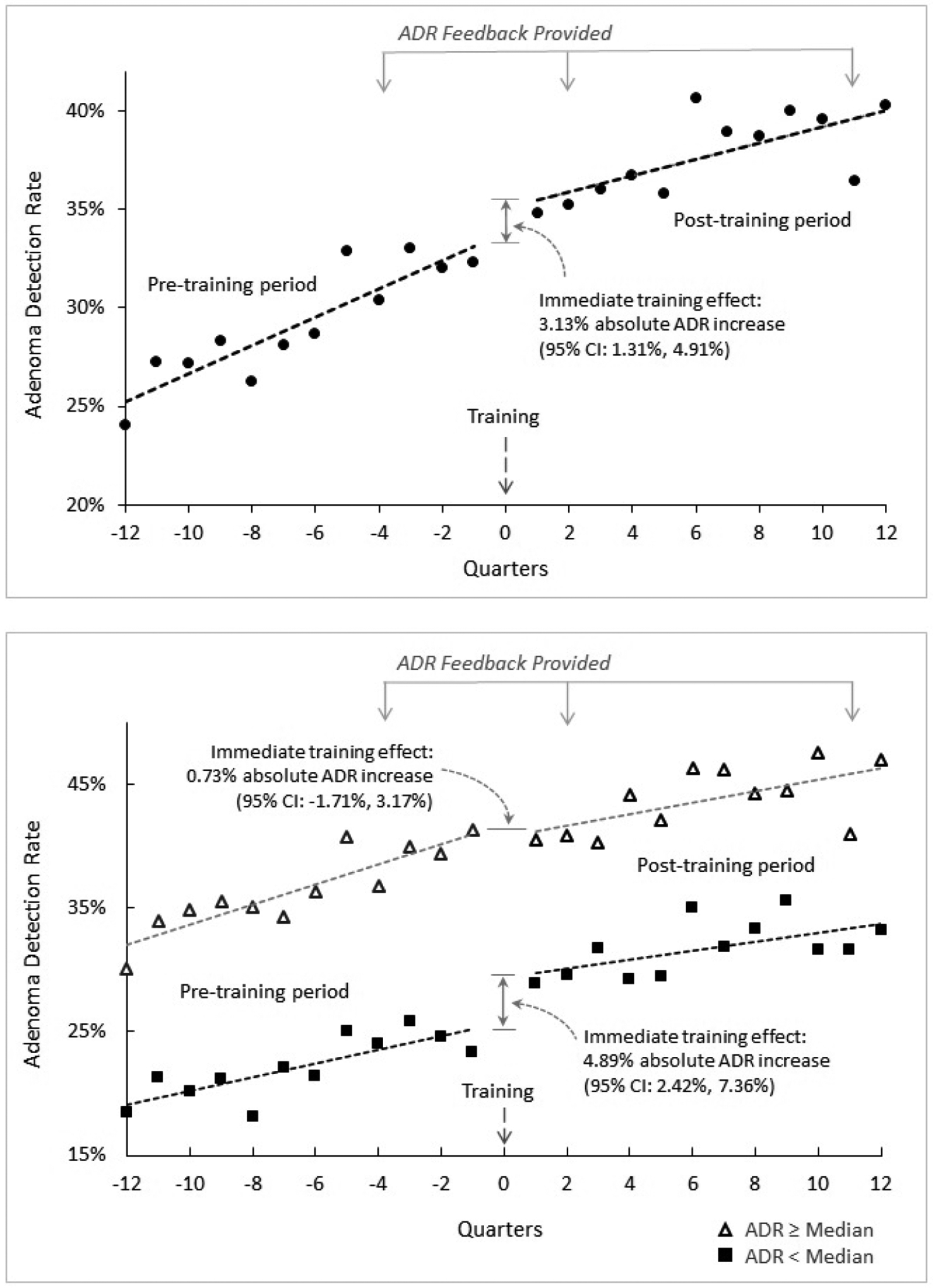
Endoscopist adenoma detection rates (ADR) pre-training, immediately after training, and post-training, for all endoscopists (panel A) and by pre-training median ADR (panel B).
ADR, adenoma detection rate; CI, confidence interval.
The immediate training effect was greater for endoscopists below the median pre-training ADR of 29.2% than for those at or above the median (absolute mean increase of 4.89%, 95% CI: 2.42%, 7.36% vs. 0.73%, 95% CI: −1.71%, 3.17%, respectively; p-interaction=0.02). In contrast, for the 3-year post-training period, mean quarterly absolute ADR increases were comparable for both groups of physicians (0.27% per quarter, 95% CI: 0.18%, 0.51% vs. 0.40% per quarter, 95% CI: 0.18%, 0.63%, respectively, p-interaction=0.37).
Post-training changes in endoscopist ADRs and their patients’ risk of PCCRC
Individual endoscopist-level increases in ADRs post-training were associated with substantial reductions in their patients’ risk of PCCRC (Figure 3, Table 3). Among patients who underwent 146,786 negative colonoscopy examinations performed post-training, 97 cancers were diagnosed during up to 3 years of follow-up (413,581 person-years of follow-up), including 53 cancers in the proximal colon, 39 in the distal colon, and 5 of unknown location. Each 1% absolute increase in endoscopist ADR was associated with a 4% decrease in their patients’ risk of PCCRC (adjusted hazard ratio [HR]: 0.96, 95% CI: 0.93, 0.99) (Table 3). While there was no heterogeneity in risk estimates according to physician pre-training ADR group (p=0.76 for interaction), the immediate absolute ADR increase of 3.13% associated with training for all endoscopists would estimate a 12.5% relative risk reduction in PCCRC within the next 3 years. For the lower ADR endoscopists, the 4.89% immediate absolute increase would estimate a 19.6% relative risk reduction, whereas in the higher ADR endoscopists, the 0.73% absolute immediate increase would estimate a 2.9% relative risk reduction in PCCRC within the next 3 years.
Figure 3.
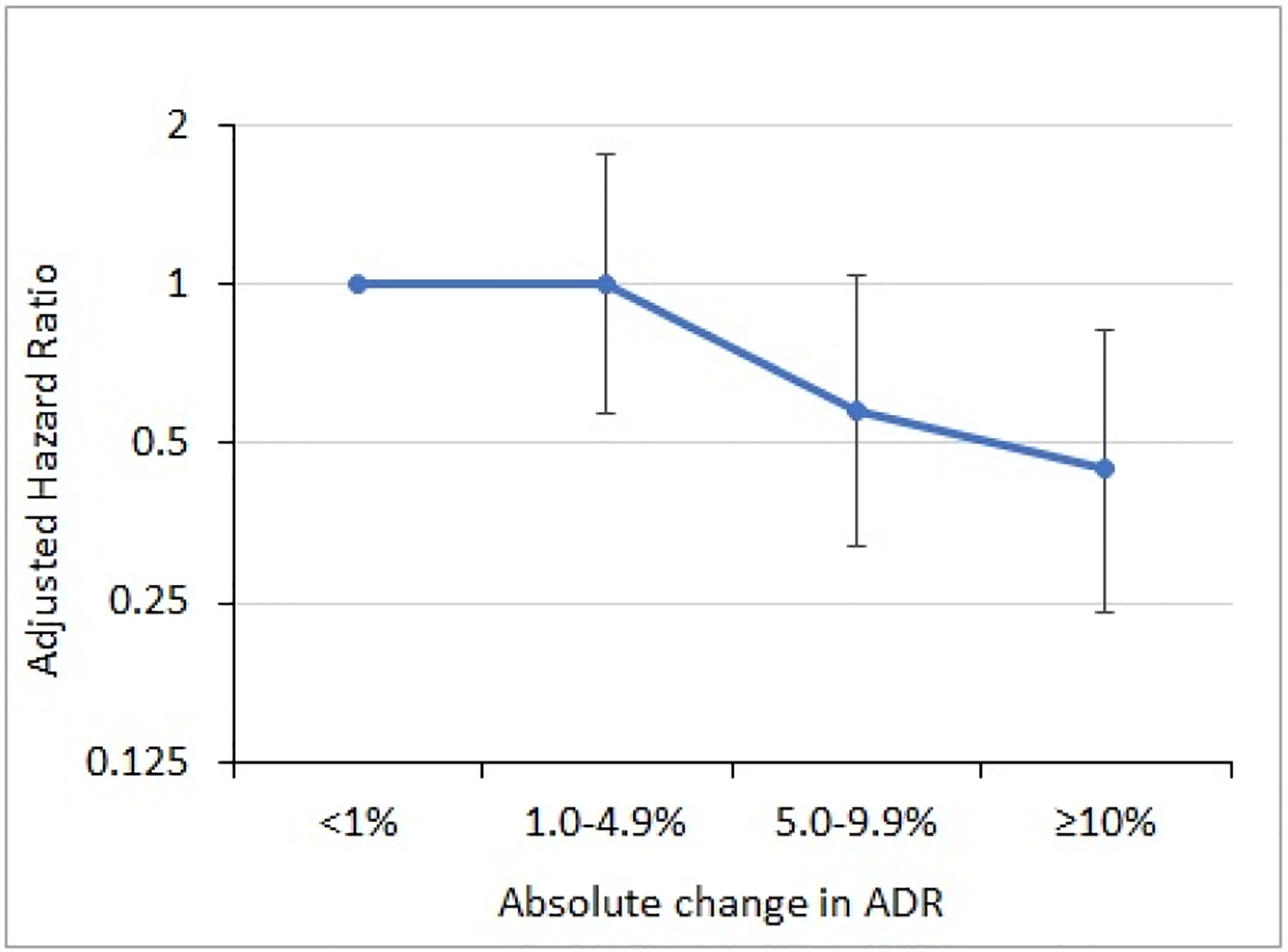
Adjusted hazard ratios for the association between categories of change in pre- vs. post-training endoscopist adenoma detection rate (ADR) and risk of post-colonoscopy colorectal cancer in the 3-year post-training period.
Changes in endoscopist ADR following training were calculated as the difference between the performing endoscopist’s ADR for the post-training year the negative colonoscopy was performed and their ADR over the 3-year pre-training period. Vertical bars represent 95% confidence intervals.
Table 3.
Adjusted hazard ratios for the associations between change in pre- vs. post-training endoscopist adenoma detection rate (ADR) and risk of post-colonoscopy colorectal cancer (PCCRC) in the 3-year post-training period, for all endoscopists and stratified by change in ADR.
| Absolute ADR change | Cancer-negative colonoscopies, n | PCCRC cases, n | Person-years | Crude cancer rate* | Adjusted hazard ratio (95% CI) |
|---|---|---|---|---|---|
| Per 1% (all endoscopists) | 146,786 | 97 | 413,581 | 23.5 | 0.96 (0.93, 0.99) |
| <1% | 24,750 | 22 | 69,677 | 31.6 | 1.00 (referent) |
| 1.0–4.9% | 30,648 | 30 | 86,457 | 34.7 | 1.00 (0.57, 1.77) |
| 5.0–9.9% | 44,032 | 25 | 124,185 | 20.1 | 0.58 (0.32, 1.04) |
| ≥10% | 47,356 | 20 | 133,261 | 15.0 | 0.45 (0.24, 0.82) |
Change in endoscopist ADR following training was calculated as the difference between the performing endoscopist’s ADR for the post-training year the negative colonoscopy was performed and their ADR over the 3-year pre-training period.
PCCRC cases/100,000 person-years.
CI, confidence interval
For analyses of risk estimates by categories of ADR change for the post-training period, an absolute increase in endoscopist ADR of ≥10% was associated with a 55% lower risk of PCCRC in their patients as compared to endoscopists with an absolute ADR change of <1% or a decrease in ADR (HR: 0.45, 95% CI: 0.24, 0.82) (Figure 3).
DISCUSSION
A 30-minute interactive online training based on behavior change theory that addressed factors potentially related to adenoma detection was associated with an immediate and substantial absolute increase in average endoscopist ADR, with a larger increase among those with lower ADRs (i.e., the ones most likely to benefit). These effects were independent from temporal ADR trends during the pre-training period, during which ADR feedback was provided, and were durable over a multi-year follow-up period. Increases in individual endoscopist-level ADRs following training, which included both the larger immediate impact of training and smaller quarterly increases in the post-training period, were associated with significant and substantial reductions in their patients’ risk of PCCRC; this association was independent of endoscopists’ pre-training ADR level.
The current study findings extend prior research in two important ways. First, our findings show that a scalable brief interactive online training may be a useful addition to ADR feedback for improving ADRs, especially among endoscopists with lower detection rates. A recent study that provided individualized feedback on colonoscopy inspection quality using instructional videos reported no impact on endoscopist ADRs overall, but an improvement in ADRs among the subgroup of endoscopists with lower ADRs; it did not evaluate post-colonoscopy cancer risk.37 More intensive training-based approaches have yielded mixed results. A pilot study of lecture-based training on inspection techniques combined with ADR feedback suggested an ability to improve ADRs;22, 23 however, a follow-up cluster randomized trial reported no significant improvement in ADRs. Also, neither the pilot study nor trial evaluated cancer outcomes and the strategy depended on an in-person training by the study investigator, which may limit adoption and scalability.24 Another study that evaluated a 2-day in-person colonoscopy quality training of endoscopy center leaders countrywide in Poland reported a subsequent increase in ADRs of the leaders and their centers;25 however, this type of in-person training may be difficult to replicate with fidelity and has not yet been widely adopted. Second, our findings demonstrate that individual endoscopist-level changes in ADR following training were associated with improvements in their patients’ outcomes. In the only other evaluation of whether intervention-related changes in ADRs influence patient outcomes, a Polish study reported that, for endoscopists with very low ADRs (mean 13.8%), ADR auditing and feedback was associated with ADR improvements and fewer PCCRCs.5
We observed an absolute mean increase of 3.13% immediately after training and a net absolute ADR increase of about 4% by the end of year 3 after training. This net change is comparable to but on the lower end of what has been reported for trials of other training approaches. For example, Kaminski et al, reported that in a trial comparing training of endoscopy center leaders to ADR feedback alone, training produced a net absolute ADR increase of 5.7% 2 years after training while feedback alone produced a net increase of 1.8%.25 In a cluster randomized trial, Wallace et al, reported that training with feedback yielded an absolute ADR increase of 11%, while in the no-intervention controls, ADRs increased by 3%, although the difference did not differ beyond chance.24
Study strengths include the diverse patient demographics and endoscopy centers. The comprehensive capture of colonoscopies and colorectal cancer outcomes among a large sample size of endoscopists also permitted stratified analyses according to pre-training ADR level. The study’s use of behavior change theory helped target potential areas for intervention with evidence-based methods for behavioral change.29 Such strategies can succeed even for topics that have largely failed leadership-initiated “best guess” top-down interventions, such as for handwashing, exercise, and weight loss.38, 39 The 100% endoscopist participation in the training minimized the potential for participation bias, where only those trained might be motivated to improve their performance. Importantly, the study evaluated cancer risk associated with individual endoscopist-level changes in ADR. In contrast, most prior studies have evaluated associations of different ADR levels vs. PCCRC risk across a population. Such studies are unable to directly evaluate if individual-level changes in ADR influence cancer outcomes and may even include different physicians over time.
The changes suggested by the current study are substantial. The immediate absolute increase of 4.89% associated with training for those below the median ADR, for example, would estimate a 19.6% relative risk reduction in PCCRC within the next 3 years. Endoscopists with larger post-training changes, such as absolute ADR changes of >10% vs. <1%, had PCCRC risk reductions of >50% within 3 years post-colonoscopy.
Study limitations include, first, that universal training precluded an untrained comparison group; however, the pre- and post-training design allowed endoscopists to serve as their own controls and the interrupted time series analysis controlled for temporal trends in ADRs.35 The significant increase in ADRs immediately following training combined with the sustained ADR increases over the 3-year post-training period argue against ascribing the improved performance only to a temporary heightened awareness of being measured (i.e., Hawthorne effect). In addition, there were no broad abrupt changes in colonoscopy technology or practices (i.e., bowel preparation) within the health system that would explain the rapid-onset post-treatment effects observed. Second, sample size calculations were not performed given all endoscopists received the training and all were included in the analyses if they performed an adequate number of procedures for calculating ADRs. The study sample size allowed for dichotomized analyses by pre-training median ADR, though not by finer ADR categories. Third, the training took place in a setting where periodic ADR feedback was also provided. However, any independent effects of ADR feedback alone are likely small given that the sharp increase in ADR observed immediately after training was not evident immediately after the delivery of ADR feedback at three time points in the study and feedback was not provided around the time of the intervention. Fourth, the training did not include the use of artificial intelligence-based technology for increasing adenoma detection or improving the characterization of polyps. Fifth, the 3-year follow-up period could not capture longer term effects of the intervention on cancer outcomes. A modeling study using a lifetime perspective estimated that increasing ADRs were even more strongly associated with lower lifetime risks of colorectal cancer and mortality than shorter duration studies suggest, given the preventive benefit of adenoma removal in some patients may not be evident for many years.40
In conclusion, a theory-based 30-minute interactive online training addressing factors that can impede adenoma detection was associated with immediate and sustained increases in endoscopist ADRs over 3 years of follow-up. The associations were greatest among the endoscopists most likely to benefit – those with lower ADRs. The training-associated increases were significantly beyond small quarterly temporal increases in ADR during ADR-feedback only periods. The endoscopist-level increases in ADR in the post-training period were strongly associated with substantial reductions in their patients’ risk of PCCRC. Inferences from this study must be tempered by the lack of control group which precludes elimination of potential confounders. Nonetheless, the 30-minute length of training, ready online access, testing of the intervention at multiple centers, and the sustained ADR increases observed post-training suggest these methods, coupled with ADR auditing and feedback, are generalizable to different settings and may be useful for increasing colonoscopy effectiveness and decreasing endoscopist-associated differences in patient colorectal cancer outcomes.
Grant support:
This study was conducted within the National Cancer Institute-funded Population-based Research Optimizing Screening through Personalized Regimens (PROSPR) consortium (U54 CA163262) and Population-based Research to Optimize the Screening Process II (PROSPR II) consortium (UM1 CA222035), which conducts multisite, coordinated, transdisciplinary research to evaluate and improve cancer-screening processes.
Acronyms and abbreviations:
- ADR
adenoma detection rate
- CI
confidence interval
- HR
adjusted hazard ratio
- IQR
interquartile range
- PCCRC
post-colonoscopy colorectal cancer
- PROSPR
Population-based Research Optimizing Screening through Personalized Regimens
- SEER
Surveillance Epidemiology and End Results
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: No conflicts of interest exist for any of the authors.
Author contributions:
Study concept and design: DAC, CDJ, WKZ, BHF, CPQ
Acquisition of data: WKZ
Analysis and interpretation of data: DAC, CDJ, WKZ, BHF, CPQ
Drafting of the manuscript: DAC, CDJ
Critical revision of the manuscript for important intellectual content: DAC, CDJ, JKL, TRL, WKZ, JES, NRG, CAD, EAH, CSS, NU, RC, BHF, CPQ
Data transparency statement:
Data, analytic methods, and study materials will be made available to other researchers upon request.
References
- 1.Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7–33. [DOI] [PubMed] [Google Scholar]
- 2.Force USPST, Davidson KW, Barry MJ, et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021;325:1965–1977. [DOI] [PubMed] [Google Scholar]
- 3.Corley DA, Jensen CD, Marks AR, et al. Adenoma detection rate and risk of colorectal cancer and death. The New England journal of medicine 2014;370:1298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. The New England journal of medicine 2010;362:1795–803. [DOI] [PubMed] [Google Scholar]
- 5.Kaminski MF, Wieszczy P, Rupinski M, et al. Increased Rate of Adenoma Detection Associates With Reduced Risk of Colorectal Cancer and Death. Gastroenterology 2017;153:98–105. [DOI] [PubMed] [Google Scholar]
- 6.Rex DK, Petrini JL, Baron TH, et al. Quality indicators for colonoscopy. The American journal of gastroenterology 2006;101:873–85. [DOI] [PubMed] [Google Scholar]
- 7.Corley DA, Jensen CD, Marks AR. Can we improve adenoma detection rates? A systematic review of intervention studies. Gastrointest Endosc 2011;74:656–65. [DOI] [PubMed] [Google Scholar]
- 8.Atkin W, Rogers P, Cardwell C, et al. Wide variation in adenoma detection rates at screening flexible sigmoidoscopy. Gastroenterology 2004;126:1247–56. [DOI] [PubMed] [Google Scholar]
- 9.Barclay RL, Vicari JJ, Doughty AS, et al. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med 2006;355:2533–41. [DOI] [PubMed] [Google Scholar]
- 10.Bressler B, Paszat LF, Vinden C, et al. Colonoscopic miss rates for right-sided colon cancer: a population-based analysis. Gastroenterology 2004;127:452–6. [DOI] [PubMed] [Google Scholar]
- 11.Bretthauer M, Skovlund E, Grotmol T, et al. Inter-endoscopist variation in polyp and neoplasia pick-up rates in flexible sigmoidoscopy screening for colorectal cancer. Scand J Gastroenterol 2003;38:1268–74. [DOI] [PubMed] [Google Scholar]
- 12.Chen SC, Rex DK. Endoscopist can be more powerful than age and male gender in predicting adenoma detection at colonoscopy. Am J Gastroenterol 2007;102:856–61. [DOI] [PubMed] [Google Scholar]
- 13.Hixson LJ, Fennerty MB, Sampliner RE, et al. Prospective study of the frequency and size distribution of polyps missed by colonoscopy. J Natl Cancer Inst 1990;82:1769–72. [DOI] [PubMed] [Google Scholar]
- 14.Hosokawa O, Shirasaki S, Kaizaki Y, et al. Invasive colorectal cancer detected up to 3 years after a colonoscopy negative for cancer. Endoscopy 2003;35:506–10. [DOI] [PubMed] [Google Scholar]
- 15.Leaper M, Johnston MJ, Barclay M, et al. Reasons for failure to diagnose colorectal carcinoma at colonoscopy. Endoscopy 2004;36:499–503. [DOI] [PubMed] [Google Scholar]
- 16.Pickhardt PJ, Nugent PA, Mysliwiec PA, et al. Location of adenomas missed by optical colonoscopy. Ann Intern Med 2004;141:352–9. [DOI] [PubMed] [Google Scholar]
- 17.Rex DK, Cutler CS, Lemmel GT, et al. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology 1997;112:24–8. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez W, Harewood GC, Petersen BT. Evaluation of polyp detection in relation to procedure time of screening or surveillance colonoscopy. Am J Gastroenterol 2004;99:1941–5. [DOI] [PubMed] [Google Scholar]
- 19.Schottinger JE, Jensen CD, Ghai NR, et al. Association of Physician Adenoma Detection Rates With Postcolonoscopy Colorectal Cancer. JAMA 2022;327:2114–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim S, Hammond S, Park J, et al. Training interventions to improve adenoma detection rates during colonoscopy: a systematic review and meta-analysis. Surg Endosc 2020;34:3870–3882. [DOI] [PubMed] [Google Scholar]
- 21.Boregowda U, Desai M, Nutalapati V, et al. Impact of feedback on adenoma detection rate: a systematic review and meta-analysis. Ann Gastroenterol 2021;34:214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coe SG, Crook JE, Diehl NN, et al. An endoscopic quality improvement program improves detection of colorectal adenomas. The American journal of gastroenterology 2013;108:219–26; quiz 227. [DOI] [PubMed] [Google Scholar]
- 23.Ussui V, Coe S, Rizk C, et al. Stability of Increased Adenoma Detection at Colonoscopy. Follow-Up of an Endoscopic Quality Improvement Program-EQUIP-II. The American journal of gastroenterology 2015;110:489–96. [DOI] [PubMed] [Google Scholar]
- 24.Wallace MB, Crook JE, Thomas CS, et al. Effect of an endoscopic quality improvement program on adenoma detection rates: a multicenter cluster-randomized controlled trial in a clinical practice setting (EQUIP-3). Gastrointest Endosc 2017;85:538–545 e4. [DOI] [PubMed] [Google Scholar]
- 25.Kaminski MF, Anderson J, Valori R, et al. Leadership training to improve adenoma detection rate in screening colonoscopy: a randomised trial. Gut 2016;65:616–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evans B, Pace D, Borgaonkar M, et al. Effect of an educational intervention on colonoscopy quality outcomes. Surg Endosc 2020;34:5142–5147. [DOI] [PubMed] [Google Scholar]
- 27.Seo JY, Jin EH, Bae JH, et al. Multidirectional Colonoscopy Quality Improvement Increases Adenoma Detection Rate: Results of the Seoul National University Hospital Healthcare System Gangnam Center Colonoscopy Quality Upgrade Project (Gangnam-CUP). Dig Dis Sci 2020;65:1806–1815. [DOI] [PubMed] [Google Scholar]
- 28.Rajasekhar PT, Rees CJ, Bramble MG, et al. A multicenter pragmatic study of an evidence-based intervention to improve adenoma detection: the Quality Improvement in Colonoscopy (QIC) study. Endoscopy 2015;47:217–24. [DOI] [PubMed] [Google Scholar]
- 29.Atkins L, Hunkeler EM, Jensen CD, et al. Factors influencing variation in physician adenoma detection rates: a theory-based approach for performance improvement. Gastrointest Endosc 2016;83:617–26 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gordon NP. How Does the Adult Kaiser Permanente Membership in Northern California Compare with the Larger Community? Oakland, CA: Kaiser Permanente Division of Research, 2006. [Google Scholar]
- 31.Improving Adenoma Detection Rates. https://deliveryscience-appliedresearch.kaiserpermanente.org/specialty-research-networks/gastroenterology-hepatology, 2021.
- 32.Corley DA, Jensen CD, Marks AR, et al. Variation of adenoma prevalence by age, sex, race, and colon location in a large population: implications for screening and quality programs. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association 2013;11:172–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rutter MD, Beintaris I, Valori R, et al. World Endoscopy Organization Consensus Statements on Post-Colonoscopy and Post-Imaging Colorectal Cancer. Gastroenterology 2018;155:909–925 e3. [DOI] [PubMed] [Google Scholar]
- 34.Lee JK, Jensen CD, Lee A, et al. Development and validation of an algorithm for classifying colonoscopy indication. Gastrointestinal Endoscopy 2015;81:575–582 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr 2013;13:S38–44. [DOI] [PubMed] [Google Scholar]
- 36.Wei LJ, Lin DY, Weissfeld L Regression analysis of multivariate incomplete failure time data by modeling marginal distributions. Journal of the American Statistical Association 1989;84:1065–1073. [Google Scholar]
- 37.Keswani RN, Wood M, Benson M, et al. Individualized feedback on colonoscopy skills improves group colonoscopy quality in providers with lower adenoma detection rates. Endosc Int Open 2022;10:E232–E237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fuller C, Besser S, Savage J, et al. Application of a theoretical framework for behavior change to hospital workers’ real-time explanations for noncompliance with hand hygiene guidelines. Am J Infect Control 2014;42:106–10. [DOI] [PubMed] [Google Scholar]
- 39.Silva MN, Vieira PN, Coutinho SR, et al. Using self-determination theory to promote physical activity and weight control: a randomized controlled trial in women. J Behav Med 2010;33:110–22. [DOI] [PubMed] [Google Scholar]
- 40.Meester RG, Doubeni CA, Lansdorp-Vogelaar I, et al. Variation in Adenoma Detection Rate and the Lifetime Benefits and Cost of Colorectal Cancer Screening: A Microsimulation Model. JAMA 2015;313:2349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data, analytic methods, and study materials will be made available to other researchers upon request.


