Abstract
Background:
Despite survival improvements, there is a paucity of data on neurocognitive outcomes in neuroblastoma survivors. This study addresses this literature gap.
Methods:
Neurocognitive impairments in survivors were compared to sibling controls from the Childhood Cancer Survivor Study (CCSS) using the CCSS Neurocognitive Questionnaire. Impaired emotional regulation, organization, task efficiency, and memory defined as scores ≥90th percentile of sibling norms. Modified Poisson regression models evaluated associations with treatment exposures, era of diagnosis, and chronic conditions. Analyses were stratified by age at diagnosis (≤1 and >1 year) as proxy for lower vs. higher risk disease.
Results:
Survivors (N=837; median[range] age 25[17–58] years, age diagnosed 1[0–21] years) were compared to sibling controls (N=728; age 32[16–43] years). Survivors had higher risk of impaired task efficiency (≤1 year relative risk [RR]=1.48, 95% confidence interval [95% CI]=1.08–2.03; >1 year RR=1.58, 95% CI=1.22–2.06) and emotional regulation (≤1 year RR=1.51, 95% CI=1.07–2.12; >1 year RR=1.44, 95% CI=1.06–1.95). Impaired task efficiency associated with platinum exposure (≤1 year RR=1.74, 95% CI=1.01–2.97), hearing loss (≤1 year RR=1.95, 95% CI=1.26–3.00; >1 year RR=1.56, 95% CI=1.09–2.24), cardiovascular (≤1 year RR=1.83, 95% CI=1.15–2.89; >1 year RR=1.74, 95% CI=1.12–2.69), neurologic (≤1 year RR=2.00, 95% CI=1.32–3.03; >1 year RR 2.29 95% CI=1.64–3.21), and respiratory (>1 year RR=2.35, 95% CI=1.60–3.45) conditions. Survivors ≤1 year; female sex (RR=1.54, 95% CI=1.02–2.33), cardiovascular (RR=1.71, 95% CI=1.08–2.70) and respiratory (RR=1.99, 95% CI=1.14–3.49) conditions associated impaired emotional regulation. Survivors were less likely to be employed full-time (p<0.0001), graduate college (p= 0.035), and live independently (p<0.0001).
Conclusions:
Neuroblastoma survivors report neurocognitive impairment impacting adult milestones. Identified health conditions and treatment exposures can be targeted to improve outcomes.
Keywords: cancer survivor, neuroblastoma, neurocognitive, Childhood Cancer Survivor Study
Lay summary:
Survival rates continue to improve in patients with neuroblastoma. There is a lack of information regarding neurocognitive outcomes in neuroblastoma survivors, most studies examined survivors of leukemia or brain tumors. This study 837 adult survivors of childhood neuroblastoma were compared to siblings from the Childhood Cancer Survivorship Study. Survivors had a 50% higher risk of impairment with attention/processing speed (task efficiency) and emotional reactivity/frustration tolerance (emotional regulation). Survivors were less likely to reach adult milestones such as living independently. Survivors with chronic health conditions are at higher risk of impairment. Early identification and aggressive management of chronic conditions may help mitigate level of impairment.
Condensed Abstract:
Survivors of neuroblastoma had 50% higher risk of impaired task efficiency and emotional regulation compared to siblings. Those with chronic health conditions were at higher risk of neurocognitive impairment.
Introduction
Neuroblastoma is the most common extra-cranial solid malignancy seen in children, with an average age at diagnosis of 19 months. Tumors can arise anywhere in the sympathetic nervous system, most commonly from the adrenal gland. Significant advances in treatment of neuroblastoma have resulted in overall survival rates of 80%.1, 2 Over the past 20 years treatment has been tailored to risk groups based on a combination of age at diagnosis, stage and biologic features of the tumor, resulting in improvements in survival for all groups. Risk-based approaches to treatment have resulted in a decrease in treatment exposures and intensity among low- and intermediate-risk groups without a decrease in survival, which is around 93%.3 Meanwhile, cure rates in high-risk patients have gone from near uniform fatality4 to 84 % 5-year survival, due to intensified treatment that includes chemotherapy, surgery, tandem stem cell transplantation, radiotherapy and immunotherapy.5, 6 We are at a critical juncture to better understand the detailed late effects of long-term survivors of neuroblastoma.
Neurocognitive outcomes are of particular importance to study in neuroblastoma, given the young age at which patients are treated corresponds to critical times in neural development and the long-term impact of cognitive deficits can have on attainment of milestones during adulthood and quality of life.7 Most prior work examining neurocognitive outcomes in childhood cancer survivors has focused on survivors of acute lymphoblastic leukemia (ALL), central nervous system (CNS) tumors and survivors exposed to traditional risk factors such as CNS radiation and intrathecal chemotherapy. Studies have found up to 50% of survivors being impaired on attention, memory, visuospatial abilities, executive functioning, and cognitive processing speed.8,9,10,11,12 Despite the intensive therapies patients with neuroblastoma receive at a very young age, little is known about their neurocognitive outcomes as adults13 or risk factors of impairment.
We sought to characterize neurocognitive outcomes in adult survivors of neuroblastoma diagnosed and treated for cancer over three decades (1970–1999), to examine potential differences in the proportion of impairment across treatment eras, to describe treatment-related risk factors for impairment, and to examine the impact of impairment on educational attainment, employment and ability to live independently.
Methods:
Childhood Cancer Survivor Study
The Childhood Cancer Survivor Study (CCSS) is a multi-institutional, retrospective cohort study with longitudinal follow-up of childhood cancer survivors diagnosed between January 1, 1970 and December 31, 1999 prior to the age of 21 years who survived at least 5 years. For this analysis, survivors of neuroblastoma were compared to a cohort of closest aged siblings of childhood cancer survivors recruited at the same time as survivors. The study design, procedure, and characteristics of CCSS have been described in detail previously.14,15 Briefly, baseline and follow-up questionnaires captured health and demographic information for all participants. The CCSS Neurocognitive Questionnaire (CCSS-NCQ) was collected through follow-up survey between five and seven years after entry into the cohort. To be eligible to complete the NCQ survivors had to be considered adults (age close to or greater than 18 years, living independently, or an emancipated minor) at the time of follow up. The baseline and follow-up surveys and the medical record abstraction form used for data collection at each institution can be found at http://ccss.stjude.org. The institutional review board at each of the participating institutions approved the CCSS protocol.
Cancer Treatment Information
Primary cancer characteristics and detailed treatment information were abstracted from medical records.15 Treatment exposures were examined as yes/no as well as by dose to determine if a dose dependent relationship existed. Chemotherapy exposures included alkylating agents, anthracyclines, platinum agents and retinoic acid. Cumulative doses of anthracyclines were expressed as doxorubicin equivalent dose.16 The majority of survivors who received neck radiation also received chest radiation. Similarly, the majority of patients treated with pelvic radiation also got abdominal radiation, therefore these exposure locations were grouped together. Exposure to cranial radiation, cranial spinal radiation and or total body irradiation (TBI) were combined into a single “brain radiation” category. Doses of radiation that were given as TBI were added to each body section.
Stage and risk classification were not collected in the cohort. Given age >12 months is a known prognostic factor we stratified our analysis by age. These strata serve as a proxy for risk group, low vs. intermediate/high risk disease respectively. Each of these strata were compared to siblings independently.
Chronic Health Conditions
Chronic health conditions were self-reported17 and graded using the National Cancer Institute’s Common Terminology Criteria for Adverse Events version 4.03.18 Events are graded from none (grade 0) to fatal (grade 5). Grade 1 conditions were described as asymptomatic or mild, grade 2 as moderate, grade 3 severe and disabling, while grade 4 were life-threatening. Conditions were grouped into neurologic, endocrine, cardiovascular, pulmonary, respiratory, and hearing loss.19, 20 Grade of chronic conditions reported at the time of neurocognitive questionnaire assessment were included in this analysis.
Outcome Measures
The Childhood Cancer Survivor Study Neurocognitive Questionnaire (CCSS-NCQ) was developed as a tool to identify neurocognitive problems in survivors of childhood cancer.9 It is a validated, self-reported 25-item questionnaire that evaluates four cognitive domains commonly affected in cancer survivors - emotional regulation (ER), organization, task efficiency (TE), and memory.21,22 The first two address executive functioning while the latter address processing speed/attention and working/long term memory, respectively. The instrument asks participants to report on a three-point scale the degree of impairment they experienced in the last 6 months. Scores are totaled by domain, with higher scores indicating more problems, and impairment is defined as a score ≥90th percentile of the sibling distribution.
Statistical Analysis
As neither disease stage nor risk categories were collected in the CCSS, survivors were stratified by age at diagnosis to serve as proxy for risk group, with those ≤1 year considered lower risk (Strata 1) and those >1 year, higher risk (Strata 2).23 Demographic characteristics for the survivors (overall and by strata) and siblings were provided. Treatment characteristics of the survivors were compared among treatment era within strata. Frequencies were provided for categorical variables and median and range were provided for continuous variable. Frequency of impairment on each neurocognitive measure were reported for two survivor strata and siblings and by treatment era within each strata, A modified Poisson approach24 with robust variance estimates was conducted to compare the relative risk of impairment on each neurocognitive measure, as well as chronic conditions to the sibling cohort for both strata, with adjustment on sex, age at evaluation and race.
The development of chronic conditions is expected to lie on the pathway from treatment exposure to neurocognitive measures and also the treatment exposures may be confounded with era, thus we evaluated their effects on neurocognitive measures in three separate models among survivors using modified Poisson approach; Model 1: Demographics and treatment, Model 2: Treatment era with adjustment on age at follow-up, sex, and race/ethnicity and Model 3: Chronic conditions with adjustment on age at follow-up, sex, and race/ethnicity. Frequency of chronic health condition by neurocognitive impairment among survivors were also reported. Most chronic conditions were examined comparing grade 2–4 vs < 2, except for hearing loss where grade1-4 was compared to < 1 due to evidence that grade 1 (Problems hearing, not requiring a hearing aid) can impact speech development and cognitive performance.25,26
Multivariable modified regression models were used to assess for the association between neurocognitive impairment and social attainment outcomes. Outcomes related to social attainment were restricted to survivors who were 25 years or older at the time of evaluations given that tthe outcomes are age depedant such as graduating from college.27 All models were appropriately adjusted, as indicated in the footnotes of the tables, and implemented in SAS (SAS9.4, SAS Institute, Cary NC) and GraphPad Prism (version 8.3.1 for Windows, GraphPad Software, San Diego, CA).
Results
Of the 1,039 potentially eligible 5-year neuroblastoma survivors 837 (80.5%) completed the CCSS-NCQ (Supplemental Figure 1: CONSORT diagram). Characteristics of the survivors and siblings are presented in Table 1 and treatment exposures for survivors in (Supplemental Table 1. The median [range] time since diagnosis of survivors was 23 (16–34) years and current age was 25 [16–43] years. 57% of survivors were female. The sibling group consisted of 728 siblings with median age 32 years [range: 17–58 years]. Survivors were younger, less likely to be white non-Hispanic (Table 1), graduate college (p=0.035), be married, have full-time employment (p<0.0001), and to be living independently (p<0.0001, Supplemental Table 2) compared to siblings.
Table 1:
Characteristics of Cancer Survivors and Siblings
| Characteristic | Survivors (N=837) | Siblings (N=728) | Age at diagnosis | |
|---|---|---|---|---|
| ≤1 year (N=428) | >1 year (N=409) | |||
| Sex, N (%) | ||||
| Female | 474 (56.6) | 410 (56.3) | 233 (54.4) | 241 (58.9) |
| Race, N (%) | ||||
| Other | 43 (5.26) | 14 (2.0) | 21 (5.0) | 22 (5.6) |
| Age at diagnosis (years) | ||||
| Median (range) | 0.97 (0.003–20.71) | 0.43 (0.002–0.99) | 2.42 (1–20.7) | |
| Age at evaluation (years) | ||||
| Median (range) | 25 (16–43) | 32 (17–58) | 24 (16–33) | 26 (18–43) |
| Time from diagnosis to evaluation (years) | ||||
| Mean (SD) | N/A | |||
| Median (range) | 23 (16–34) | 24 (16–33) | 23 (16–34) | |
| Treatment era, N (%) | ||||
| 1990–1999 | 262 (31.3) | 115 (43.9) | 147 (56.1) | |
Abbreviations: N= number; %= percent; SD= standard deviation; N/A; not applicable
Neurocognitive impairment
Frequency of impairment in survivors was 21.98% for task efficiency, 19.7% for emotional regulation, 25.3% for organization and 19.4% for memory. (Table 3) Survivors had 50% higher risk of impaired task efficiency (≤1 year relative risk [RR] 1.48, 95% confidence interval [CI] 1.08–2.03; >1 year RR 1.58, CI 1.22–2.06) and emotional regulation (≤1 year RR 1.51, CI 1.07–2.12; >1 year RR 1.44, CI 1.06–1.95) vs. siblings after adjusting for age at follow-up, sex, and race/ethnicity. (Figure 1). Few differences were identified between decade of treatment.
Table 3:
Frequency of chronic conditions and Survivors risk of chronic conditions compared to siblings
| Age≤ 1 at diagnosis | Age>1 at diagnosis | ||||
|---|---|---|---|---|---|
| Chronic health conditionb | Siblings, n (%) | Survivors, n (%) | RR, (95% CI) compared to siblings | Survivor, n (%) | RR, (95% CI) compared to siblings |
| Hearing loss | 24 (3.30) | 48 (11.21) | 3.58 (2.05–6.26) | 80 (19.56) | 5.89 (3.76–9.22) |
| Endocrine | 27 (3.71) | 44 (10.28) | 6.11 (3.51–10.62) | 85 (20.78) | 7.65 (5.10–11.47) |
| Respiratory | 24 (3.30) | 23 (5.37) | 1.71 (0.85–3.44) | 28 (6.85) | 1.97 (1.13–3.46) |
| Cardiovascular | 40 (5.49) | 49 (11.45) | 5.82 (3.32–10.21) | 42 (10.27) | 3.33 (2.14–5.18) |
| Neurologic | 11 (1.51) | 56 (13.08) | 8.11 (4.19–15.72) | 70 (17.11) | 10.16 (5.54–18.64) |
Abbreviations: N= Number; %= percent
Model adjusted for age at follow-up, sex, and race/ethnicity
Chronic health conditions examined comparing grade 2–4 vs < 2, except for hearing loss where grade 1–4 was compared to < 1
Figure 1:
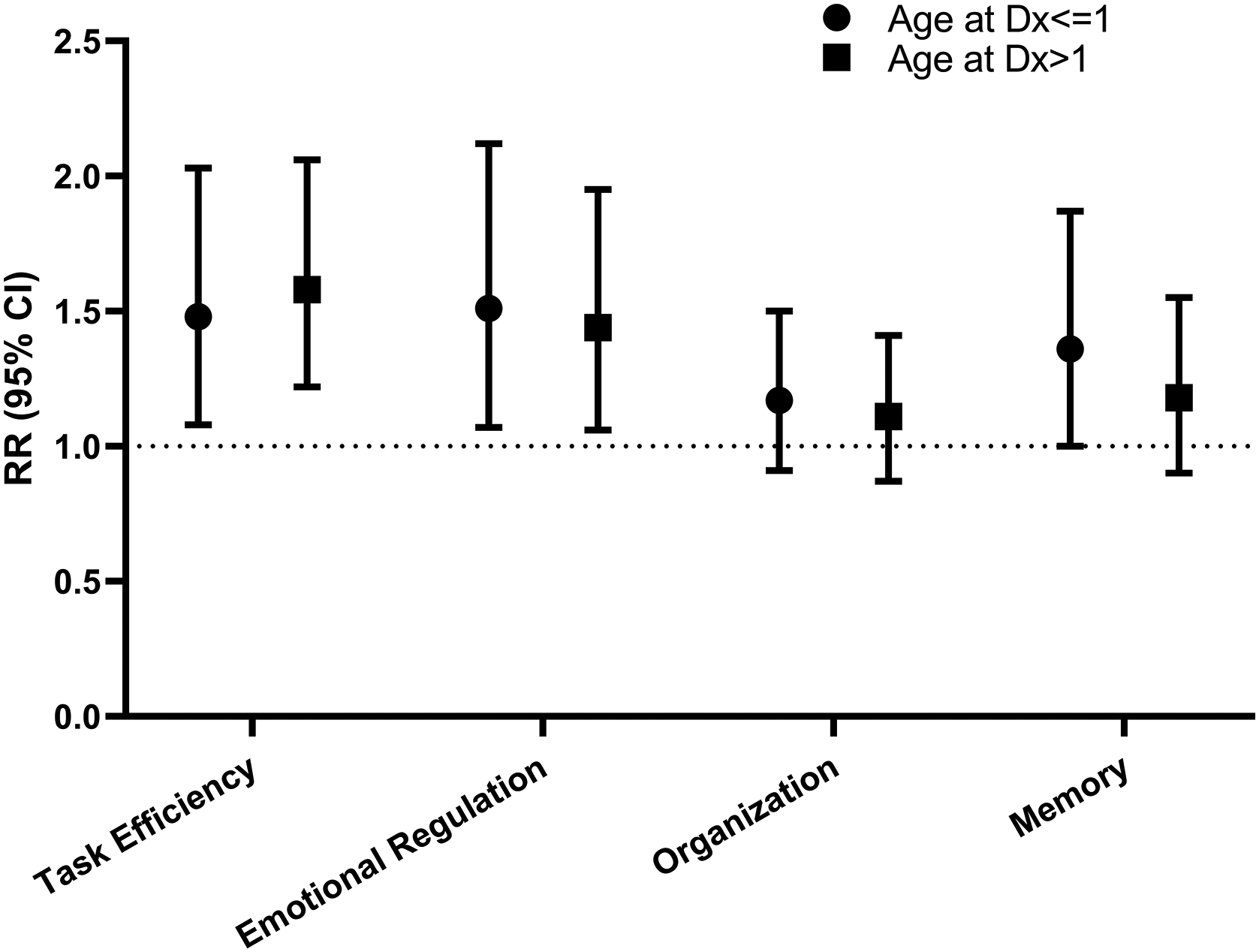
Relative Risk of Neurocognitive Impairment for Survivors compared to Siblings. Model adjusted for age at follow-up, sex, and race/ethnicity.
Association with demographic and treatment exposures
Among survivors diagnosed ≤1 year, there was an increased risk of impaired task efficiency observed in the multivariable model that was associated with platinum exposure (RR 1.74, CI 1.01–2.97). Females had a higher risk of impaired emotional regulation compared to males (RR=1.54, CI=1.02–2.33). For survivors >1 year at diagnosis, impairment in emotional regulation was associated with brain radiation (RR 1.96, CI 1.05–3.65) while non-Hispanic whites (RR 0.39, CI 0.26–0.58) and those treated with chest/neck/TBI radiation (RR 0.41, CI 0.23–0.76) were less likely to have impairment. Brain radiation was also associated with impairment in memory (RR 2.02, CI 1.14–3.58) (Table 2).
Table 2:
Risk of neurocognitive impairment associated with treatment exposures
| Domaina | ||||
|---|---|---|---|---|
| Exposure | Task efficiency | Emotional regulation | Organization | Memory |
| RR (95% CI) | ||||
| Age ≤ 1 at diagnosis | ||||
| Model 1: Demographics and treatmentb | ||||
| Male | 1.14 (0.77–1.71) | 0.65 (0.43–0.98) | 0.98 (0.69–1.38) | 1.05 (0.70– 1.58) |
| Non-Hispanic white | 1.35 (0.69–2.66) | 0.99 (0.54–1.81) | 1.28 (0.71–2.31) | 0.89 (0.50–1.58) |
| Abdominal/pelvic/TBI radiation | 1.11 (0.62–2.01) | 0.88 (0.45–1.70) | 1.24 (0.78–1.97) | 1.36 (0.78–2.38) |
| Thoracic/TBI radiationc | 0.49 (0.18–1.32) | 0.24 (0.06–1.06) | 0.84 (0.44–1.59) | 0.61 (0.24–1.56) |
| Brain radiationd | 0.80 (0.25–2.56) | 0.92 (0.26–3.26) | 0.67 (0.19–2.42) | 0.79 (0.22–2.85) |
| Platinum | 1.74 (1.01–2.97) | 1.36 (0.83–2.24) | 1.06 (0.67–1.68) | 1.27 (0.76–2.13) |
| Cyclophosphamide | 0.99 (0.97–1.02) | 1.01 (0.98–1.03) | 0.99 (0.97–1.01) | 0.99 (0.96–1.01) |
| Anthracycline | 0.99 (0.59–1.68) | 1.07 (0.66–1.72) | 1.18 (0.78–1.79) | 1.36 (0.82–2.26) |
| Model 2: Treatment Erae | ||||
| 1970–1979 | 1.31 (0.67–2.55) | 1.48 (0.74–2.95) | 1.89 (1.09–3.29) | 1.21 (0.61–2.40) |
| 1980–1989 | 0.92 (0.57–1.47) | 1.38 (0.82–2.33) | 1.48 (0.95–2.31) | 1.28 (0.76–2.16) |
| 1990–1999 | Reference | Reference | Reference | Reference |
| Age > 1 at diagnosis | ||||
| Model 1: Demographics and treatmentb | ||||
| Male | 0.66 (0.42–1.02) | 0.66 (0.43–1.03) | 0.98 (0.67–1.44) | 0.73 (0.47–1.15) |
| Non-Hispanic white | 0.86 (0.52–1.43) | 0.39 (0.26–0.58) | 1.13 (0.66–1.94) | 1.01 (0.57–1.80) |
| Abdominal/pelvic/TBI radiation | 1.27 (0.84–1.92) | 1.30 (0.82–2.05) | 1.10 (0.70–1.72) | 1.07 (0.65–1.76) |
| Thoracic/TBI radiationc | 0.95 (0.57–1.57) | 0.41 (0.23–0.76) | 0.88 (0.53–1.45) | 0.59 (0.33–1.05) |
| Brain radiationd | 1.22 (0.68–2.18) | 1.96 (1.05–3.65) | 0.67 (0.32–1.38) | 2.02 (1.14–3.58) |
| Platinum | 1.07 (0.60–1.92) | 0.79 (0.43–1.45) | 0.93 (0.49–1.74) | 1.25 (0.63–2.46) |
| Cyclophosphamide | 0.98 (0.95–1.01) | 1.00 (0.99–1.02) | 0.99 (0.96–1.02) | 0.99 (0.97–1.02) |
| Anthracycline | 1.06 (0.59–1.91) | 1.25 (0.69–2.26) | 1.22 (0.65–2.30) | 0.98 (0.48–1.98) |
| Model 2: Treatment Erae | ||||
| 1970–1979 | 1.16 (0.65–2.07) | 1.77 (1.02–3.06) | 1.2 (0.71–2.03) | 0.73 (0.39–1.39) |
| 1980–1989 | 1.12 (0.76–1.65) | 1.25 (0.79–1.98) | 1.1 (0.74–1.64) | 0.94 (0.61 −1.45) |
| 1990–1999 | Reference | Reference | Reference | Reference |
Abbreviations: RR= Relative Risk; 95% CI= confidence interval; TBI= Total body irritation
Impairment is defined as a score ≥90th percentile of the siblings’ distribution
Treatment is categorized as yes/no;
Thoracic/TBI includes patients who got chest radiation
Brain radiation includes exposure to cranial, cranial spinal and or TBI
Model adjusted for age at follow-up, sex, and race/ethnicity
Association with chronic health conditions
Compared to siblings, survivors were more likely to have chronic health conditions (Table 3), and survivors with chronic conditions were at higher risk of impairment than those without. (Supplemental Table 4) Among survivors ≤1 year at diagnosis, hearing loss (RR 1.95, CI 1.26–3.00), cardiovascular (RR 1.83, CI 1.15–2.89) and neurologic (RR 2.00, CI 1.32–3.03) conditions were associated with higher risk of impaired task efficiency while cardiovascular (RR 1.71, CI 1.08–2.70) and respiratory (RR 1.99, CI 1.14–3.49) conditions were associated with higher risk of impaired emotional regulation. Impairment in memory was also associated with hearing loss and neurologic conditions. For survivors >1 year at diagnosis, hearing loss (RR 1.56, CI 1.09–2.24) and respiratory (RR 2.35, CI 1.60–3.45) conditions were associated with higher risk of impaired task efficiency. Those with cardiovascular conditions were at 60–80% higher risk of impairment across all domains except memory. (Figure 2)
Figure 2:
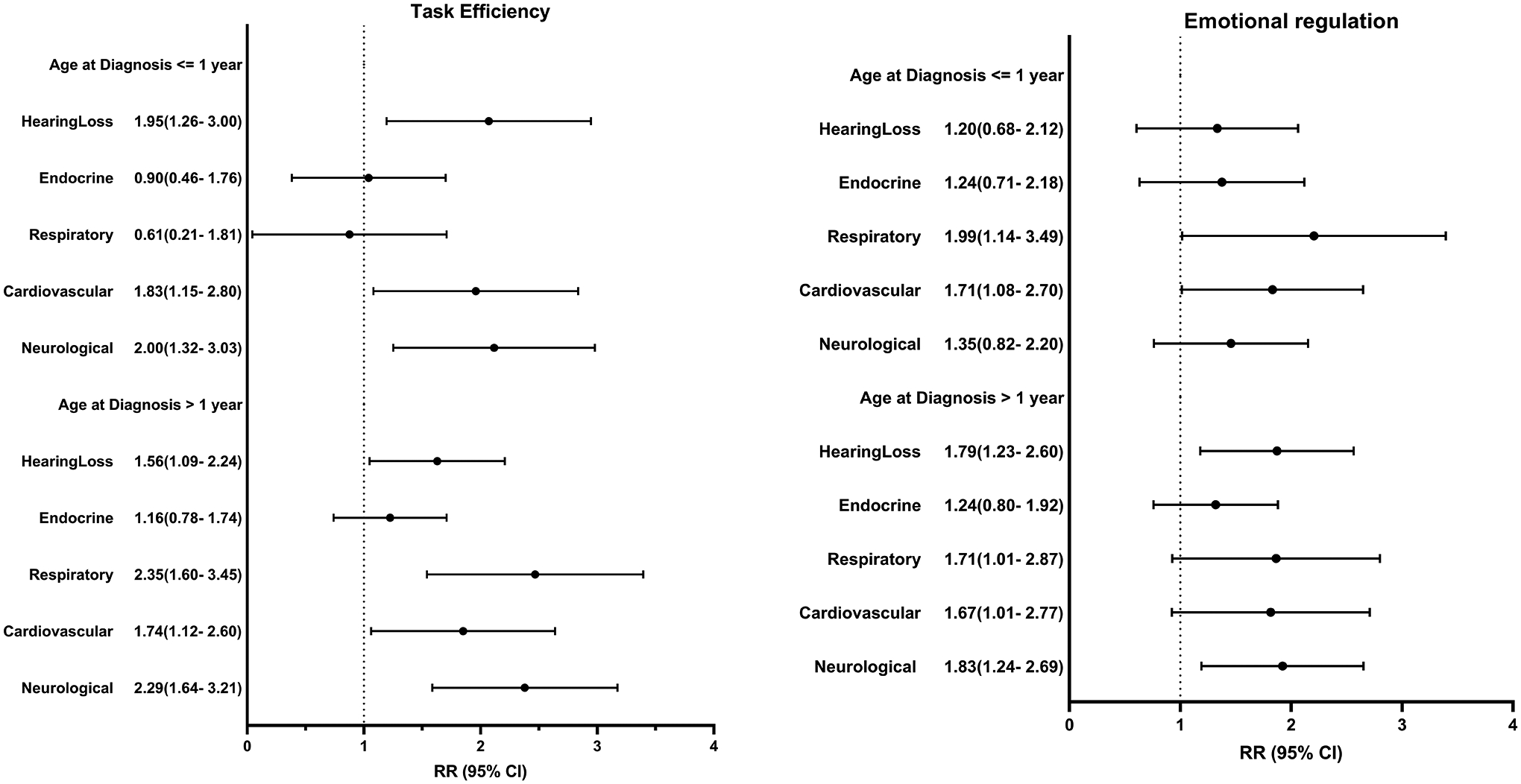
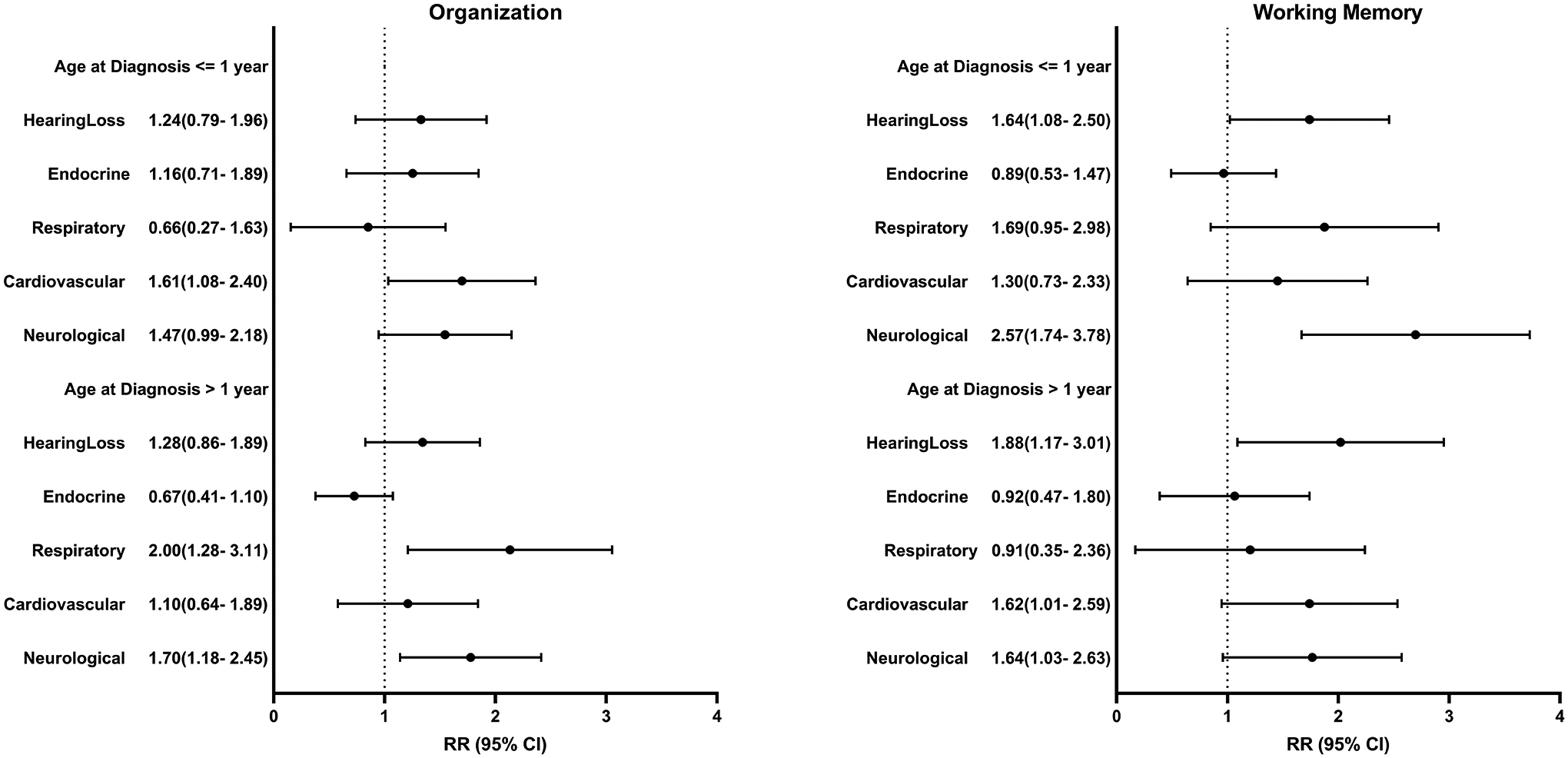
Associations between chronic health conditions and risk of neurocognitive impairment among survivors. Model adjusted for age at follow-up, sex, and race/ethnicity. Chronic health conditions examined comparing grade 2-4 versus <2, except for hearing loss where grade1-4 was compared to <1. Endocrine conditions include thyroid (hyper/hypo), diabetes, growth hormone deficiency, osteoporosis, and gonadal dysfunction. Respiratory conditions include emphysema and lung fibrosis. Cardiovascular conditions include hypertension, stroke, congestive heart failure, heart attack, arrhythmias, valvular dysfunction, and elevated cholesterol. Neurological conditions include epilepsy, paralysis, weakness, problems with balance, and memory dysfunction. Impairment is defined as a score ≥90th percentile of the siblings’ distribution.
Association of neurocognitive impairment with attainment of adult milestones
Among those ≥25 years of age, survivors were less likely than siblings to graduate from college (57.9 % vs. 64.6% p= 0.035), be employed full-time (58.3% vs. 73.3% p<0.0001), and more likely to be living dependently (24.3% vs. 10.1%, p<0.0001) (Supplemental Table 2). For survivors ≤1 year at diagnosis, those with impairment in task efficiency had a 65% higher risk of not graduating from college (RR 1.67, CI 1.18–2.25) or being fully employed (RR 1.64, CI 1.14–2.35) and were at twice the risk of living dependently (RR 2.05, CI 1.20–3.51) (Table 4).
Table 4:
Associations between neurocognitive impairment and educational obtainment, employment and independent living among survivors ≥25 year olds.
| Domain | Impairmenta | Non-college graduate N (%) | RR (CI) | Non-full-time employment N (%) | RR (CI) | Living dependently N (%) | RR (CI) |
|---|---|---|---|---|---|---|---|
| Age ≤ 1 | |||||||
| Task Efficiency | No | 56 (38.62) | 1 | 51 (35.92) | 1 | 29 (20.14) | 1 |
| Yes | 24 (63.16) | 1.67 (1.18– 2.35) | 22 (59.46) | 1.64 (1.14– 2.35) | 15 (39.47) | 2.05 (1.20– 3.51) | |
| Emotional Regulation | No | 53 (38.41) | 1 | 53 (38.69) | 1 | 34 (24.64) | 1 |
| Yes | 27 (60.00) | 1.52 (1.09– 2.10) | 20 (47.62) | 1.15 (0.78– 1.68) | 10 (22.73) | 0.94 (0.50– 1.79) | |
| Organization | No | 58 (42.65) | 1 | 54 (40.60) | 1 | 29 (21.48) | 1 |
| Yes | 22 (46.81) | 1.11 (0.77– 1.58) | 19 (41.30) | 1.01 (0.68– 1.50) | 15 (31.91) | 1.56 (0.93– 2.63) | |
| Memory | No | 51 (36.96) | 1 | 53 (38.97) | 1 | 32 (23.36) | 1 |
| Yes | 29 (64.44) | 1.77 (1.30– 2.39) | 20 (46.51) | 1.19 (0.81– 1.74) | 12 (26.67) | 1.13 (0.63– 2.05) | |
| Age > 1 | |||||||
| Task Efficiency | No | 67 (34.72) | 1 | 75 (39.27) | 1 | 41 (21.24) | 1 |
| Yes | 30 (68.18) | 2.1 (1.56– 2.84) | 23 (56.10) | 1.25 (0.89– 1.74) | 17 (37.78) | 1.48 (0.91– 2.41) | |
| Emotional Regulation | No | 66 (35.11) | 1 | 72 (38.71) | 1 | 44 (23.40) | 1 |
| Yes | 31 (63.27) | 1.87 (1.38– 2.54) | 26 (56.52) | 1.27 (0.93– 1.76) | 14 (28.00) | 0.83 (0.50– 1.38) | |
| Organization | No | 74 (40.00) | 1 | 79 (43.41) | 1 | 47 (25.41) | 1 |
| Yes | 23 (44.23) | 1.12 (0.78– 1.60) | 19 (38.00) | 0.91 (0.62– 1.33) | 11 (20.75) | 0.86 (0.50– 1.49) | |
| Memory | No | 75 (37.31) | 1 | 78 (39.20) | 1 | 45 (22.39) | 1 |
| Yes | 22 (61.11) | 1.67 (1.21– 2.30) | 20 (60.61) | 1.48 (1.07– 2.05) | 13 (35.14) | 1.39 (0.87– 2.23) | |
Abbreviations: RR, Relative Risk; CI, confidence interval; N, number; %; percent
Model adjusted for age at follow-up, sex, and race/ethnicity
Impairment is defined as a score ≥90th percentile of the siblings’ distribution
Discussion
To our knowledge, this is the first large, multi-institutional study of neurocognitive outcomes among a diverse sample of adult survivors of neuroblastoma treated over three decades. Importantly, we demonstrated that this population is at risk for neurocognitive impairment especially in the domains of task efficiency and emotional regulation. Risk was higher in the presence of chronic health conditions and specific treatment exposures. Survivors with impairment were less likely to attain important adult milestones such as graduating from college and living independently.
We hypothesized that survivors treated in more recent eras would have more impairment given the increase in intensity in therapy of high-risk disease, however we found that among survivors >1 year at diagnosis those treated in 1970s had an 80% higher risk of impaired emotional regulation when compared to those treated in the 1990s. This may reflect improvements in supportive care as well as increased awareness and understanding of late effects.28 Females ≤1 year at diagnosis were found to have a 35% higher risk for impairment in emotional regulation, which is a reflection of executive functioning.21 Similar results have been found in female survivors of ALL.29 It is unclear why females experience more problems with executive functioning, though differences in sequelae following traumatic brain injury are apparent between sexes.30 Hormonal regulation of brain development and response to injury may play a role. One perplexing result was the apparent protective effect of chest/neck radiation on ER in those older than 1 year at diagnosis. This may be due to use of more localized treatment and lower overall disease risk. Further study into this association is needed.
To date, understanding of neurocognitive late effects has primarily focused on survivors of ALL and brain tumors, who were exposed to CNS directed therapies like intrathecal methotrexate and cranial radiation.31,32 This study of neuroblastoma survivors found deficits despite the lack of traditional neurotoxic treatment risk factors. Animal studies have shown that neurotoxicity of different chemotherapy agents can occur by multiple mechanisms including direct neurotoxicity, hormonal changes, immunologic response, oxidative stress, DNA damage, and predisposing genetic polymorphisms33,34 that make the cells more susceptible to damage.35 For example, in vivo Cisplatin causes apoptosis of neurons in the cortex and thalamus, areas involved in processing speed and attention,36,37 which may explain impaired task efficiency in this cohort given platinum agents are integral to neuroblastoma treatment.
Studies of ALL survivors have shown higher risk of impairment with executive functioning and memory due to CNS directed therapy with methotrexate and brain radiotherapy.38 While overall, memory was not different in neuroblastoma survivors compared to siblings, those >1 year at diagnosis who were exposed to brain radiation did have impairment in memory and emotional regulation. This impairment occurred despite getting lower doses of radiation than is typical of leukemia protocols. This study helps build our understanding of neurocognitive dysfunction outside of the traditional risk factors of cranial radiation, high dose methotrexate and intrathecal chemotherapy.39,40
Neuroblastoma survivors in this cohort were more likely to have chronic health conditions compared to their siblings and survivors with chronic conditions were more likely to have neurocognitive impairment. We know from studies of patients with certain chronic diseases that they are more likely to have neurocognitive impairment.41 For example, children and adults with diabetes are more likely to have cognitive deficits and are at higher risk for dementia. The pathophysiologic mechanisms include systemic and cerebral vascular changes along with white matter changes due to hypo- and hyperglycemia.42 Similarly, hypertensive patients also show neurocognitive impairment.43 Interventions to improve glycemic control and intensive blood pressure management44 have been shown to improve cognition in these populations. It is possible that similar interventions could help ameliorate these effects in neuroblastoma survivors. Prevention of chronic conditions as a direct result of therapy may not be possible for patients with neuroblastoma, especially in high-risk disease as treatment continues to be intensified to improve survival. Therefore, identifying interventions such as lifestyle modifications (e.g., weight loss, smoking cessation, and exercise) to alter the impact of a certain conditions may be key to creating healthier survivors.
The impact of neurocognitive impairment on survivors’ ability to obtain adult milestones is striking. The inability of a survivor to live independently can have a multigenerational impact. This dependency can affect their parents and siblings’ ability to plan for the future. Survivors with neurocognitive impairment were less likely to be working full-time which has personal, familial, and societal consequences. Improving areas of impairment such as task efficiency with medication45 or cognitive interventions may result in improved independence and employment. These milestones are interconnected; lack of educational obtainment affects one’s ability to find employment which in turn can make living independently financially challenging, regardless of underlying medical conditions that may also be a hindrance. Aggressive early intervention while survivors are in school can have meaningful impacts. For example, we know that pediatric patients even with mild hearing loss (regardless of cause) can struggle academically. Simple interventions such as seating priority and teacher voice amplification systems are beneficial.46
The mean age at diagnosis for our study population was about 2 years, which is a critical time of development. Research on adverse childhood events (ACEs) shows that early childhood trauma/stress results in structural and functional changes in the brain, and that the effects of multiple ACEs compound each other.47 The diagnosis of cancer in a child is a traumatic event for themselves and their family, and the subsequent therapy has physical, emotional, and socioeconomical impacts. Using an early adversity framework, as suggested by Marusak48, the neurocognitive impairments found in neuroblastoma survivors may be a reflection of the collective family experience of their therapy as well as the medical treatments they received. Interventions to decrease compounding stressors (such as financial support, parental mental health counselling) may help mediate these effects.
Several limitations should be considered when interpreting our findings. Direct neuropsychological testing is the gold standard for assessing neurocognitive functioning,49 however it is not feasible in a large and geographically diverse cohort. The CCSS-NCQ is a self-reported tool that has been validated in subjects with known neurologic disorders and against direct neurocognitive testing. Given the relatively young age of survivors our data may underestimate the prevalence of chronic health conditions as these increase with age and the impact of these conditions on neurocognitive functioning may also change with age.12 Our data were stratified by age to serve as a proxy for risk group for those with non-high risk and high risk disease. It is likely an imperfect proxy. The retrospective nature of the cohort study design precludes studying the causal effect of mood on cognition. Indeed, the CCSS measures of anxiety/depression report on the past 7 days while the CCSS-NCQ questions are over the past 6 months, limiting direct comparisons. Previous studies of survivors have shown when anxiety and depression do occur, it is often driven by either chronic health conditions or academic/vocational problems that are secondary to neurocognitive problems.50 It should be noted that subtle differences were seen between survivors who did or did not completed Follow-up surveys (Supplemental Table 5). White female survivors were more likely to complete a Follow-up survey, which is consistent with many volunteer longitudinal clinical trials. Survivors who reported cardiovascular chronic health conditions were also slightly more likely to complete a Follow-up survey, which could result in bias towards slightly worse outcomes. Importantly, however, there was no differences found regarding sex or chronic conditions for survivors who completed Follow-up but did or did not complete the NCQ. Lastly, while 80.5% of eligible neuroblastoma survivors completed the CCSS-NCQ, we did find differences in participation based on race and treatment era. As expected, minority survivors and those closer to diagnosis were less likely to participate (Supplemental Table 6). Further, it is important to acknowledge that the overall CCSS cohort is predominately white, these need to be considered when generalizing our findings.
Advances in cancer genomics and personalized medicine have allowed for individualization of treatment based on personal likelihood of cure. A similar approach is needed in survivorship care. While we may still be decades away from changing upfront therapy based on an individual’s risk of late effects, knowing what groups are at greatest risk can help us create targeted interventions during and after treatment. For example, based on our findings, females and patients with hearing loss are more likely to have neurocognitive impairment. Interventions targeting female patients with mild hearing loss early in treatment may allow for improvement in neurocognitive outcomes. The high prevalence of neurocognitive impairment found in this study suggests all survivors of neuroblastoma should be carefully screened and there should be a low threshold for formal neuropsychological testing. Future work should continue to focus on understanding the outcomes of neuroblastoma survivors as treatments evolve, especially among the growing population of high-risk survivors treated with modern therapies (eg. tandem transplant, MIBG therapy, immunotherapy). Moreover, as research identifies survivors at risk for neurocognitive impairment, intervention studies aimed at preventing and mitigating these outcomes are needed.
Supplementary Material
Supplemental Figure 1: Cohort Diagram of Eligible and Enrolled Childhood Cancer Survivors
Funding:
This work was supported by grants from the National Cancer Institute (U24CA55727). Support to St. Jude Children’s Research Hospital was also provided by the American Lebanese-Syrian Associated Charities (ALSAC). The funding sources had no role in the design and conduct of the study; collection, management, analysis, or interpretation of data; or in preparation, review, approval, or decision to submit the manuscript for publication.
Footnotes
Author disclosures: The authors declare no conflicts of interest.
Data Availability:
The data underlying this article were provided by the Childhood Cancer Survivor Study by permission. Any investigator interested in potential use of this resource is encouraged to visit www.stjude.org/ccss.
References
- 1.DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64: 252–271. [DOI] [PubMed] [Google Scholar]
- 2.Park JR, Bagatell R, London WB, et al. Children’s Oncology Group’s 2013 blueprint for research: neuroblastoma. Pediatr Blood Cancer. 2013;60: 985–993. [DOI] [PubMed] [Google Scholar]
- 3.Baker DL, Schmidt ML, Cohn SL, et al. Outcome after reduced chemotherapy for intermediate-risk neuroblastoma. N Engl J Med. 2010;363: 1313–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans AE. Treatment of neuroblastoma. Cancer. 1972;30: 1595–1599. [DOI] [PubMed] [Google Scholar]
- 5.Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. N Engl J Med. 1999;341: 1165–1173. [DOI] [PubMed] [Google Scholar]
- 6.Park JR, Kreissman SG, London WB, et al. Effect of Tandem Autologous Stem Cell Transplant vs Single Transplant on Event-Free Survival in Patients With High-Risk Neuroblastoma: A Randomized Clinical Trial. Jama. 2019;322: 746–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson V, Spencer-Smith M, Leventer R, et al. Childhood brain insult: can age at insult help us predict outcome? Brain. 2009;132: 45–56. [DOI] [PubMed] [Google Scholar]
- 8.Nathan PC, Patel SK, Dilley K, et al. Guidelines for identification of, advocacy for, and intervention in neurocognitive problems in survivors of childhood cancer: a report from the Children’s Oncology Group. Arch Pediatr Adolesc Med. 2007;161: 798–806. [DOI] [PubMed] [Google Scholar]
- 9.Krull KR, Gioia G, Ness KK, et al. Reliability and validity of the Childhood Cancer Survivor Study Neurocognitive Questionnaire. Cancer. 2008;113: 2188–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butler RW, Sahler OJ, Askins MA, et al. Interventions to improve neuropsychological functioning in childhood cancer survivors. Dev Disabil Res Rev. 2008;14: 251–258. [DOI] [PubMed] [Google Scholar]
- 11.Duffner PK, Armstrong FD, Chen L, et al. Neurocognitive and Neuroradiologic Central Nervous System Late Effects in Children Treated on Pediatric Oncology Group (POG) P9605 (standard risk) and P9201 (lesser risk) Acute Lymphoblastic Leukemia Protocols (ACCL0131): A Methotrexate Consequence? A Report from the Children’s Oncology Group. Journal of pediatric hematology/oncology. 2014;36: 8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harila MJ, Winqvist S, Lanning M, Bloigu R, Harila-Saari AH. Progressive neurocognitive impairment in young adult survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2009;53: 156–161. [DOI] [PubMed] [Google Scholar]
- 13.Laverdiere C, Cheung NK, Kushner BH, et al. Long-term complications in survivors of advanced stage neuroblastoma. Pediatr Blood Cancer. 2005;45: 324–332. [DOI] [PubMed] [Google Scholar]
- 14.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27: 2308–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leisenring WM, Mertens AC, Armstrong GT, et al. Pediatric cancer survivorship research: experience of the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27: 2319–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feijen EAM, Leisenring WM, Stratton KL, et al. Equivalence Ratio for Daunorubicin to Doxorubicin in Relation to Late Heart Failure in Survivors of Childhood Cancer. Journal of Clinical Oncology. 2015;33: 3774–3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diller L, Chow EJ, Gurney JG, et al. Chronic disease in the Childhood Cancer Survivor Study cohort: a review of published findings. J Clin Oncol. 2009;27: 2339–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Common terminology criteria for adverse events : (CTCAE). [Bethesda, Md.] :: U.S. Department of Health and Human Services, 2010. [Google Scholar]
- 19.Gurney JG, Tersak JM, Ness KK, Landier W, Matthay KK, Schmidt ML. Hearing loss, quality of life, and academic problems in long-term neuroblastoma survivors: a report from the Children’s Oncology Group. Pediatrics. 2007;120: e1229–1236. [DOI] [PubMed] [Google Scholar]
- 20.Landier W, Knight K, Wong FL, et al. Ototoxicity in children with high-risk neuroblastoma: prevalence, risk factors, and concordance of grading scales--a report from the Children’s Oncology Group. J Clin Oncol. 2014;32: 527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kenzik KM, Huang IC, Brinkman TM, et al. The Childhood Cancer Survivor Study-Neurocognitive Questionnaire (CCSS-NCQ) Revised: Item Response Analysis and Concurrent Validity. Neuropsychology. 2015;29: 31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellenberg L, Liu Q, Gioia G, et al. Neurocognitive status in long-term survivors of childhood CNS malignancies: a report from the Childhood Cancer Survivor Study. Neuropsychology. 2009;23: 705–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Breslow N, McCann B. Statistical estimation of prognosis for children with neuroblastoma. Cancer Res. 1971;31: 2098–2103. [PubMed] [Google Scholar]
- 24.Zou G A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159: 702–706. [DOI] [PubMed] [Google Scholar]
- 25.Knight KR, Kraemer DF, Neuwelt EA. Ototoxicity in children receiving platinum chemotherapy: underestimating a commonly occurring toxicity that may influence academic and social development. J Clin Oncol. 2005;23: 8588–8596. [DOI] [PubMed] [Google Scholar]
- 26.Stelmachowicz PG, Pittman AL, Hoover BM, Lewis DE, Moeller MP. The Importance of High-Frequency Audibility in the Speech and Language Development of Children With Hearing Loss. JAMA Otolaryngology–Head & Neck Surgery. 2004;130: 556–562. [DOI] [PubMed] [Google Scholar]
- 27.OECD. Education at a Glance 2021. 2021.
- 28.Brouwers P Commentary: Study of the Neurobehavioral Consequences of Childhood Cancer: Entering the Genomic Era? Journal of Pediatric Psychology. 2005;30: 79–84. [DOI] [PubMed] [Google Scholar]
- 29.Moore BD III. Neurocognitive Outcomes in Survivors of Childhood Cancer. Journal of Pediatric Psychology. 2005;30: 51–63. [DOI] [PubMed] [Google Scholar]
- 30.Farace E, Alves WM. Do women fare worse: a metaanalysis of gender differences in traumatic brain injury outcome. J Neurosurg. 2000;93: 539–545. [DOI] [PubMed] [Google Scholar]
- 31.Robinson KE, Pearson MM, Cannistraci CJ, et al. Neuroimaging of executive function in survivors of pediatric brain tumors and healthy controls. Neuropsychology. 2014;28: 791–800. [DOI] [PubMed] [Google Scholar]
- 32.Schuitema I, Deprez S, Van Hecke W, et al. Accelerated aging, decreased white matter integrity, and associated neuropsychological dysfunction 25 years after pediatric lymphoid malignancies. J Clin Oncol. 2013;31: 3378–3388. [DOI] [PubMed] [Google Scholar]
- 33.Kamdar KY, Krull KR, El-Zein RA, et al. Folate pathway polymorphisms predict deficits in attention and processing speed after childhood leukemia therapy. Pediatr Blood Cancer. 2011;57: 454–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cole PD, Finkelstein Y, Stevenson KE, et al. Polymorphisms in Genes Related to Oxidative Stress Are Associated With Inferior Cognitive Function After Therapy for Childhood Acute Lymphoblastic Leukemia. J Clin Oncol. 2015;33: 2205–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sleurs C, Deprez S, Emsell L, Lemiere J, Uyttebroeck A. Chemotherapy-induced neurotoxicity in pediatric solid non-CNS tumor patients: An update on current state of research and recommended future directions. Crit Rev Oncol Hematol. 2016;103: 37–48. [DOI] [PubMed] [Google Scholar]
- 36.Rzeski W, Pruskil S, Macke A, et al. Anticancer agents are potent neurotoxins in vitro and in vivo. Ann Neurol. 2004;56: 351–360. [DOI] [PubMed] [Google Scholar]
- 37.Schmitt LI, Wimmer RD, Nakajima M, Happ M, Mofakham S, Halassa MM. Thalamic amplification of cortical connectivity sustains attentional control. Nature. 2017;545: 219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krull KR, Brinkman TM, Li C, et al. Neurocognitive outcomes decades after treatment for childhood acute lymphoblastic leukemia: a report from the St Jude lifetime cohort study. J Clin Oncol. 2013;31: 4407–4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krull KR, Sabin ND, Reddick WE, et al. Neurocognitive function and CNS integrity in adult survivors of childhood hodgkin lymphoma. J Clin Oncol. 2012;30: 3618–3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohrmann C, Henry J, Hauff M, Hayashi RJ. Neurocognitive outcomes and school performance in solid tumor cancer survivors lacking therapy to the central nervous system. J Pers Med. 2015;5: 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Compas BE, Jaser SS, Reeslund K, Patel N, Yarboi J. Neurocognitive deficits in children with chronic health conditions. Am Psychol. 2017;72: 326–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saedi E, Gheini MR, Faiz F, Arami MA. Diabetes mellitus and cognitive impairments. World J Diabetes. 2016;7: 412–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rovio SP, Pahkala K, Nevalainen J, et al. Cardiovascular Risk Factors From Childhood and Midlife Cognitive Performance: The Young Finns Study. J Am Coll Cardiol. 2017;69: 2279–2289. [DOI] [PubMed] [Google Scholar]
- 44.Williamson JD, Pajewski NM, Auchus AP, et al. Effect of Intensive vs Standard Blood Pressure Control on Probable Dementia: A Randomized Clinical Trial. Jama. 2019;321: 553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krull KR, Hardy KK, Kahalley LS, Schuitema I, Kesler SR. Neurocognitive Outcomes and Interventions in Long-Term Survivors of Childhood Cancer. J Clin Oncol. 2018;36: 2181–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tharpe AM, Gustafson S. Management of Children with Mild, Moderate, and Moderately Severe Sensorineural Hearing Loss. Otolaryngol Clin North Am. 2015;48: 983–994. [DOI] [PubMed] [Google Scholar]
- 47.Gilgoff R, Singh L, Koita K, Gentile B, Marques SS. Adverse Childhood Experiences, Outcomes, and Interventions. Pediatr Clin North Am. 2020;67: 259–273. [DOI] [PubMed] [Google Scholar]
- 48.Marusak HA, Iadipaolo AS, Harper FW, et al. Neurodevelopmental consequences of pediatric cancer and its treatment: applying an early adversity framework to understanding cognitive, behavioral, and emotional outcomes. Neuropsychology Review. 2018;28: 123–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jean-Pierre P, Johnson-Greene D, Burish TG. Neuropsychological care and rehabilitation of cancer patients with chemobrain: strategies for evaluation and intervention development. Support Care Cancer. 2014;22: 2251–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vuotto SC, Krull KR, Li C, et al. Impact of chronic disease on emotional distress in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer. 2017;123: 521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Cohort Diagram of Eligible and Enrolled Childhood Cancer Survivors
Data Availability Statement
The data underlying this article were provided by the Childhood Cancer Survivor Study by permission. Any investigator interested in potential use of this resource is encouraged to visit www.stjude.org/ccss.


