Abstract
Background:
Atrial fibrillation is a common cause of stroke among older adults and is often first detected during hospitalization, given frequent use of cardiac telemetry.
Methods:
In a 20% national sample of Medicare Fee-For-Service beneficiaries, we identified patients aged 65-or-older newly diagnosed with atrial fibrillation while hospitalized in 2016. Our primary outcome was oral anticoagulant claim within 7-days of discharge. Multivariable logistic regression analyses assessed relationships between anticoagulation initiation and thromboembolic and bleeding risk scores while controlling for demographics, frailty, comorbidities, and hospitalization characteristics.
Results:
Among 38,379 older adults newly diagnosed with atrial fibrillation while hospitalized (mean age 78.2 [SD 8.4]; 51.8% female; 83.3% white), 36,633 (95.4%) had an indication for anticoagulation and 24.6% (9,011) of those initiated an oral anticoagulant following discharge. Higher CHA2DS2-VASc score was associated with a small increase in oral anticoagulant initiation (predicted probability 20.5% [95% CI, 18.7% - 22.3%] for scores <2 and 24.9% [CI, 24.4% - 25.4%] for ≥4). Elevated HAS-BLED score was associated with a small decrease in probability of anticoagulant initiation (25.4% [CI, 24.4% - 26.4%] for score <2 and 23.1% [CI, 22.5% - 23.8%] for ≥3). Frailty was associated with decreased likelihood of oral anticoagulant initiation (24.7% [CI, 23.2% - 26.2%] for non-frail and 18.1% [CI, 16.6% - 19.6%] for moderately-severely frail). Anticoagulant initiation varied by primary reason for hospitalization, with predicted probability highest among patients with a primary diagnosis of atrial fibrillation (46.1% [CI, 45.0% - 47.3%]) and lowest among those with non-cardiovascular conditions (13.8% [CI, 13.3%−14.3%]) and bleeds (3.6% [CI, 2.4%−4.8%]).
Conclusions:
Oral anticoagulant initiation is uncommon among older adults newly diagnosed with atrial fibrillation during hospitalization, even among patients hospitalized primarily for atrial fibrillation and patients with high thromboembolic risk. Clinicians should discuss risks and benefits of oral anticoagulants with all inpatients found to have atrial fibrillation.
INTRODUCTION
Risk of atrial fibrillation-related stroke is high in older adults, with atrial fibrillation increasing stroke risk by five-fold and age 65 and above increasing risk by an additional three-fold.1 National guidelines recommend stroke prevention with oral anticoagulants for patients with atrial fibrillation and at least 2 non-sex-related risk factors for stroke, with therapy individualized based on shared decision-making focused on balancing the risks of thromboembolism and bleeding.2 Oral anticoagulants are underused among all older adults with atrial fibrillation, with prevalent use estimated at 50–60%, and even lower use for patients with frailty despite evidence of net benefit in this high-risk group.3–12
Hospitalization is a common setting for new atrial fibrillation diagnosis. Patients may be admitted for symptomatic atrial fibrillation or it may be discovered incidentally, driven in part by frequent inpatient use of cardiac monitoring and by acute illness triggering episodes of atrial fibrillation in susceptible and paroxysmal patients. Prior studies have investigated anticoagulant prescribing patterns in the outpatient setting, but little is known about care patterns for hospitalized patients newly diagnosed with atrial fibrillation.4,6,13,14 Moreover, hospitalized patients represent a high-risk group with greater medical comorbidity burden, and therefore increased stroke and bleeding risk, compared to healthy outpatients. Patients with frailty are also of particular interest, as the majority of older adults with atrial fibrillation have frailty or pre-frailty, and these states confer high risk of stroke but may have lower rates of anticoagulant prescribing.15
Thus, we examined a national sample of Medicare Part D claims to investigate rates and predictors of oral anticoagulant initiation for patients newly diagnosed with atrial fibrillation during hospitalization. We hypothesized that evidence-based risk scores of stroke and bleeding would be predictive of oral anticoagulant initiation, and that patients with frailty and those hospitalized for reasons other than atrial fibrillation would have reduced initiation rates.
METHODS
Study Population
We conducted a retrospective cohort study using a 20% national sample of Centers for Medicare and Medicaid Services (CMS) beneficiaries. We included adults age 65 years and older who received a new atrial fibrillation diagnosis during an acute hospitalization in 2016. We examined administrative and pharmacy claims from 2015 to 2017 and included patients enrolled in Medicare Parts A, B, and D for at least 1 year before hospitalization and 30 days after discharge.
We used the Medicare Chronic Conditions Warehouse (CCW) algorithm to identify hospital discharge diagnosis codes for atrial fibrillation (Table S1).16 We excluded patients with any prior diagnosis of atrial fibrillation while enrolled in Medicare and those who filled any oral anticoagulants in the prior year. We examined patients discharged home, and excluded patients discharged to nursing and long-term care facilities, as their medication use cannot be reliably ascertained using pharmacy claims. For all exclusion criteria, see the cohort diagram (Figure S1).
Outcomes
The primary outcome was any prescription drug claim for an oral anticoagulant (warfarin, apixaban, rivaroxaban, dabigatran, or edoxaban) within 7 days of discharge. This time-frame was selected to reflect prescribing patterns of the inpatient team at discharge while allowing a grace period for post-discharge prescriptions to be filled and for logistics such as prior authorization to be navigated. Anticoagulant claims were assessed using a validated generic drug name matching approach.17,18
Secondary outcomes included oral anticoagulant claims within 30 days of discharge and cardiac monitoring claims within 30 days of discharge. Cardiac event monitoring after discharge was included as a secondary outcome because outpatient monitoring is a potential alternative management strategy, particularly for patients with brief episodes of atrial fibrillation during hospitalization. Cardiac monitoring was identified based on outpatient Current Procedural Terminology codes billed within 30 days of discharge (Table S1).19 Additionally, to assess clinical follow-up, we examined the presence of subsequent outpatient diagnosis codes for atrial fibrillation within 1 year of discharge.
Primary Predictors
Primary predictors were thromboembolic and bleeding risks. Thromboembolic risk was assessed by calculating patients’ CHA2DS2-VASc score. History of heart failure, hypertension, diabetes, stroke, transient ischemic attack, ischemic heart disease, and peripheral vascular disease were identified using CCW algorithms (Table S1).20 We classified patients with CHA2DS2-VASc score less than 2 to have low thromboembolic risk, those with scores 2–3 to have moderate thromboembolic risk, and those with scores of 4 and above to have high thromboembolic risk.21 Patients were considered to have guideline-based indications for anticoagulation if they had at least 2 points on the CHA2DS2-VASc score not related to sex (score ≥2 for men and ≥3 for women).2
Bleeding risk was assessed using a modified HAS-BLED score.22 History of hypertension, chronic kidney disease, liver disease, stroke or transient ischemic attack, and alcohol use disorders were identified using CCW algorithms. Patients were assigned 1 point for history of major bleeding if they had a hospitalization for a bleeding event in the prior year (Table S1) and 1 point if they had filled a prescription for a non-steroidal anti-inflammatory or antiplatelet medication (Table S2).23 We were unable to assign a point for labile International Normalized Ratio given the lack of laboratory data.24,25,26 We classified patients with HAS-BLED scores of 0 or 1 to have low bleeding risk, scores of 2 to have moderate risk, and scores of 3 and above to have high risk.22
Covariates
Covariates included demographics, comorbidity, geriatric conditions, and reason for hospitalization (Table S1). Demographics included age, sex, and race. We did not adjust separately for age and sex, as they are both incorporated into the CHA2DS2-VASc score. Race and ethnicity were examined measured using the Research Triangle Institute Race Code, and categorized as Asian/Pacific Islander, Black, Hispanic, White, and other for the purposes of this study.27 Race was included because prior studies have demonstrated differences in anticoagulation use by race.14,25
Geriatric conditions assessed included those which may influence anticoagulation decision-making: frailty, history of falls, dementia, and delirium. Frailty was assessed using a claims-based frailty index validated for Medicare beneficiares.28,23 Consistent with prior studies, we grouped patients into non-frail, pre-frail, mildly frail, and moderately-severely frail groups.29 Dementia was identified using the CCW Algorithm for Alzheimer’s Disease, Related Disorders, or Senile Dementia.30 Delirium was identified based on discharge diagnosis codes while prior falls were identified based on ambulatory and inpatient codes in the year preceding hospitalization.31 Comorbidity was assessed using the Elixhauser Comorbidity Index.32
We also considered reason for hospitalization, as clinical decision-making may differ for patients hospitalized primarily for atrial fibrillation or for other conditions that impact the risks of anticoagulation.33 We classified patients into 5 clinical groups: hospitalized for atrial fibrillation, hospitalized for bleeding, hospitalized for cardiac surgery, hospitalized for other cardiovascular diagnoses, and all others (Table S1). We evaluated those hospitalized for cardiac surgery separately, as pathophysiology and guideline recommendations differ for atrial fibrillation post-cardiac surgery, and evaluated those hospitalized for cardiovascular conditions separately as those were more likely to have engaged with cardiovascular subspecialty clinicians during their admissions.33,34,35
Statistical Analysis
We first identified the proportions of patients initiated on oral anticoagulants by reason for hospitalization, frailty category, and clinical risk scores. We constructed multivariable logistic regression models to estimate the associations between the primary outcome of oral anticoagulation initiation within 7 days of discharge and the primary predictors of thromboembolic risk and bleeding risk, accounting for aforementioned covariates. To aid in interpretation of multivariable regression outcomes, we calculated post-estimation marginal predicted probabilities of anticoagulant initiation across subgroups.36
Secondary outcomes of oral anticoagulant and cardiac event monitoring within 30 days of discharge were assessed using the same multivariable logistic regression model structure. For subsequent atrial fibrillation billing within 1-year, unadjusted percentages are reported.
We determined statistical significance using 95% confidence intervals. All analyses were conducted using Stata v.16.1. This study was approved by the CMS Privacy Board and determined to be exempt from review by the Beth Israel Deaconess Medical Center Institutional Review Board.
RESULTS
The study cohort included 38,379 older adults (51.8% female, 61.6% age 75 and older, 83.0% white, 7.7% Black, 5.2% Hispanic) (Table 1). The majority of patients had high thromboembolic risk based on a CHA2DS2-VASc score greater than 4 (82.0%), 17.1% had moderate risk, and 0.91% had low thromboembolic risk. Bleeding risk was more evenly distributed, with 44.8% having high bleeding risk (HAS-BLED ≥3), 36.8% moderate risk, and 18.5% low risk. The majority of patients were pre-frail (55.5%) or frail (36.9%) and 15.1% had dementia. Categorized by reason for hospitalization, 20.4% of patients had a primary hospitalization diagnosis of atrial fibrillation, 28.7% were hospitalized for other cardiovascular diagnoses, 2.7% had cardiac surgery, 2.4% had primary bleeding diagnoses, and 45.7% were hospitalized for other non-cardiovascular conditions.
Table 1.
Cohort Characteristics
| Characteristic | N (%) |
|---|---|
| (n = 38,379) | |
| Age category, years | |
| 65–74 | 14,722 (38.4) |
| 75–84 | 14,149 (36.9) |
| ≥85 | 9508 (24.8) |
| Sex | |
| Male | 18,489 (48.2) |
| Female | 19,890 (51.8) |
| Race/Ethnicity | |
| Asian/Pacific Islander | 809 (2.1) |
| Black | 2,974 (7.7) |
| Hispanic | 2,011 (5.2) |
| White | 31,955 (83.3) |
| Other | 630 (1.6) |
| Thromboembolic Risk a | |
| Low - CHA2DS2-VASc <2 | 349 (0.9) |
| Moderate - CHA2DS2-VASc 2–3 | 6,567 (17.1) |
| High - CHA2DS2-VASc 4+ | 31,463 (82.0) |
| Bleeding Risk b | |
| Low – HAS-BLED <2 | 7,084 (18.5) |
| Moderate - HAS-BLED 2 | 14,106 (36.8) |
| High - HAS-BLED 3+ | 17,189 (44.8) |
| Geriatric Conditions | |
| Frailty | |
| Non-frail | 2,944 (7.7) |
| Prefrail | 21,294 (55.5) |
| Mildly Frail | 10,729 (28.0) |
| Moderately-severely frail | 3,412 (8.9) |
| Dementia | 5,779 (15.1) |
| Delirium | 2,002 (5.2) |
| Falls | 772 (2.0) |
| Primary Reason for Hospitalization | |
| Atrial fibrillation | 7,845 (20.4) |
| Bleeding | 935 (2.4) |
| Cardiac Surgery | 1,043 (2.7) |
| Cardiovascular | 11,001 (28.7) |
| Other | 17,555 (45.7) |
| Elixhauser Comorbidity Index, Median (IQR) | 9 (2–17) |
| Comorbidities | |
| Alcohol use disorder | 913 (2.4) |
| Antiplatelet or NSAID use | 9,583 (25.0) |
| Atherosclerotic cardiovascular diseasec | 25,438 (66.3) |
| Bleeding history | 2,136 (5.6) |
| Chronic kidney disease | 12,513 (32.6) |
| Cirrhosis or other liver conditions | 1,868 (4.9) |
| Congestive heart failure | 17,923 (46.7) |
| Diabetes | 17,209 (44.8) |
| Hypertension | 34,872 (90.9) |
| Stroke or transient ischemic attack history | 5,009 (13.1) |
Abbreviations: NSAID = non-steroidal anti-inflammatory drug.
CHA2DS2-VASc score includes congestive heart failure, hypertension, age, diabetes, stroke, vascular disease, and sex.
HAS-BLED score includes hypertension, abnormal renal or liver function, stroke, bleeding, labile international normalized ratio, elderly (age ≥65 years), and drugs or alcohol.
Atherosclerotic cardiovascular disease was considered a composite of coronary artery disease, peripheral vascular disease, or acute myocardial infarction, consistent with the CHA2DS2-VASc scoring system.
Initiation of oral anticoagulants
Among 36,633 patients (95.5% of study cohort) with guideline-based indications for anticoagulation, 9,011 (24.6%) filled an oral anticoagulant prescription within 7 days of discharge. Initiation was more frequent among patients with moderate to high CHA2DS2-VASc scores, with initiation rates of 23.0% for patients with scores less than 2, 26.8% for scores 2–3, and 24.2% for scores 4 and above. Initiation was more frequent among patients with primary hospitalization diagnosis of atrial fibrillation (48.1%) than those with secondary diagnosis of atrial fibrillation (18.5%). Initiation was less frequent among patients with frailty (17.6%) than among patients without frailty (28.9%) (Figure 1). Amongst the 1,746 patients who did not have guideline-based indications for anticoagulation, 22.9% initiated anticoagulants.
Figure 1.
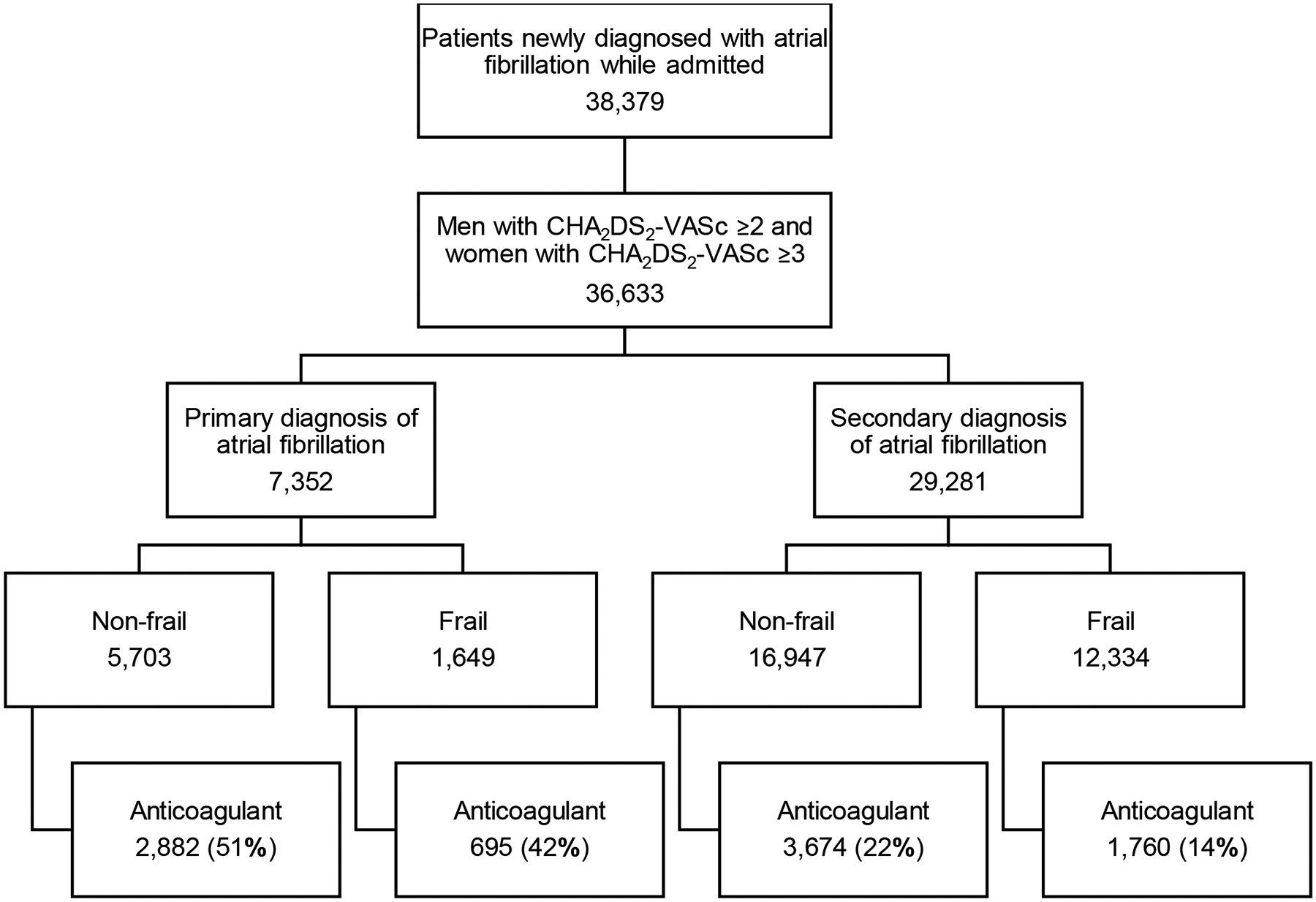
Unadjusted rates of anticoagulation initiation (bottom row) by primary discharge diagnosis (atrial fibrillation vs. other) and frailty status
Thromboembolic and bleeding risk
In multivariable models, both moderate and high thromboembolic risk were associated with greater odds of oral anticoagulant initiation (OR 1.24 [CI, 1.08–1.42] for CHA2DS2-VASc score 2–3, OR 1.33 [CI, 1.16–1.51] for score ≥4) (Table 2). However, the predicted probability of oral anticoagulant initiation was low for all thromboembolic-risk groups, increasing from 20.5% (CI, 18.7%−22.3%) for patients with low thromboembolic risk to 24.9% (CI, 24.4%−25.4%) for those with high thromboembolic risk (Figure 2A). Higher overall probabilities and similar initiation patterns were seen among patients with a primary hospitalization diagnosis of atrial fibrillation, for whom predicted probability of anticoagulant initiation was 39.9% (CI, 36.8% to 42.9%) among patients with low thromboembolic risk and 46.7% (CI, 45.5%−47.9%) among patients with high risk (Table S3).
Table 2.
Adjusted odds of oral anticoagulant initiation by thromboembolic risk, bleeding risk, frailty, and reason for hospitalization
| Initiated anticoagulation within 7 days | Initiated anticoagulation within 30 days | |||
|---|---|---|---|---|
| N (%) | Odds ratio (95% CI) | N (%) | Odds ratio (95% CI) | |
| Thromboembolic Risk a | ||||
| Low | 401/1,746 (22.0%) | Reference | 525/1,746 (30.1%) | Reference |
| Moderate | 1,386/5,170 (26.8%) | 1.24 (1.08,1.42) | 1,795/5,170 (34.7%) | 1.29 (1.13,1.47) |
| High | 7,625/31,463 (24.2%) | 1.33 (1.16,1.51) | 9,555/31,463 (30.4%) | 1.35 (1.19,1.52) |
| Bleeding Risk b | ||||
| Low | 1,959/7,084 (27.7%) | Reference | 2,491/7,084 (35.2%) | Reference |
| Moderate | 3,856/14,106 (27.3%) | 1.02 (0.95,1.09) | 4,854/14,106 (34.4%) | 1.02 (0.95,1.09) |
| High | 3,597/17,189 (20.9%) | 0.87 (0.81,0.94) | 4,530/17,189 (26.4%) | 0.87 (0.81,0.93) |
| Frailty Category | ||||
| Not frail | 903/2,944 (30.7%) | Reference | 1,190/2,9944 (40.4%) | Reference |
| Prefrail | 6,024/21,294 (28.3%) | 1.08 (0.98,1.18) | 7,590/21,294 (35.6%) | 0.999 (0.91,1.09) |
| Mildly frail | 2,076/10,729 (19.3%) | 0.89 (0.80,0.996) | 2,576/10,729 (24.0%) | 0.79 (0.72,0.88) |
| Moderately-severely frail | 409/3,412 (12.0%) | 0.64 (0.56,0.75) | 519/3,412 (15.2%) | 0.59 (0.51,0.67) |
| Primary Reason for Hospitalization | ||||
| Atrial fibrillation | 3,773/7,845 (48.1%) | Reference | 4,796/7,845 (61.1%) | Reference |
| Bleeding | 33/935 (3.5%) | 0.04 (0.03,0.06) | 50/935 (5.3%) | 0.04 (0.03,0.05) |
| Cardiac surgery | 474/1,043 (45.4%) | 0.85 (0.74,0.97) | 521/1,043 (50.0%) | 0.59 (0.52,0.67) |
| Other cardiovascular | 2,809/11,001 (25.5%) | 0.39 (0.37,0.42) | 3,506/11,001 (31.9%) | 0.32 (0.30,0.34) |
| Other non-cardiovascular | 2,323/17,555 (13.2%) | 0.18 (0.17,0.19) | 3,002/17,555 (17.1%) | 0.15 (0.14,0.16) |
For thromboembolic risk, CHA2DS2-VASc score <2 was considered low, 2–3 moderate, and ≥4 high.
For bleeding risk, HAS-BLED score <2 was considered low, 2 moderate and ≥3 high.
Figure 2.
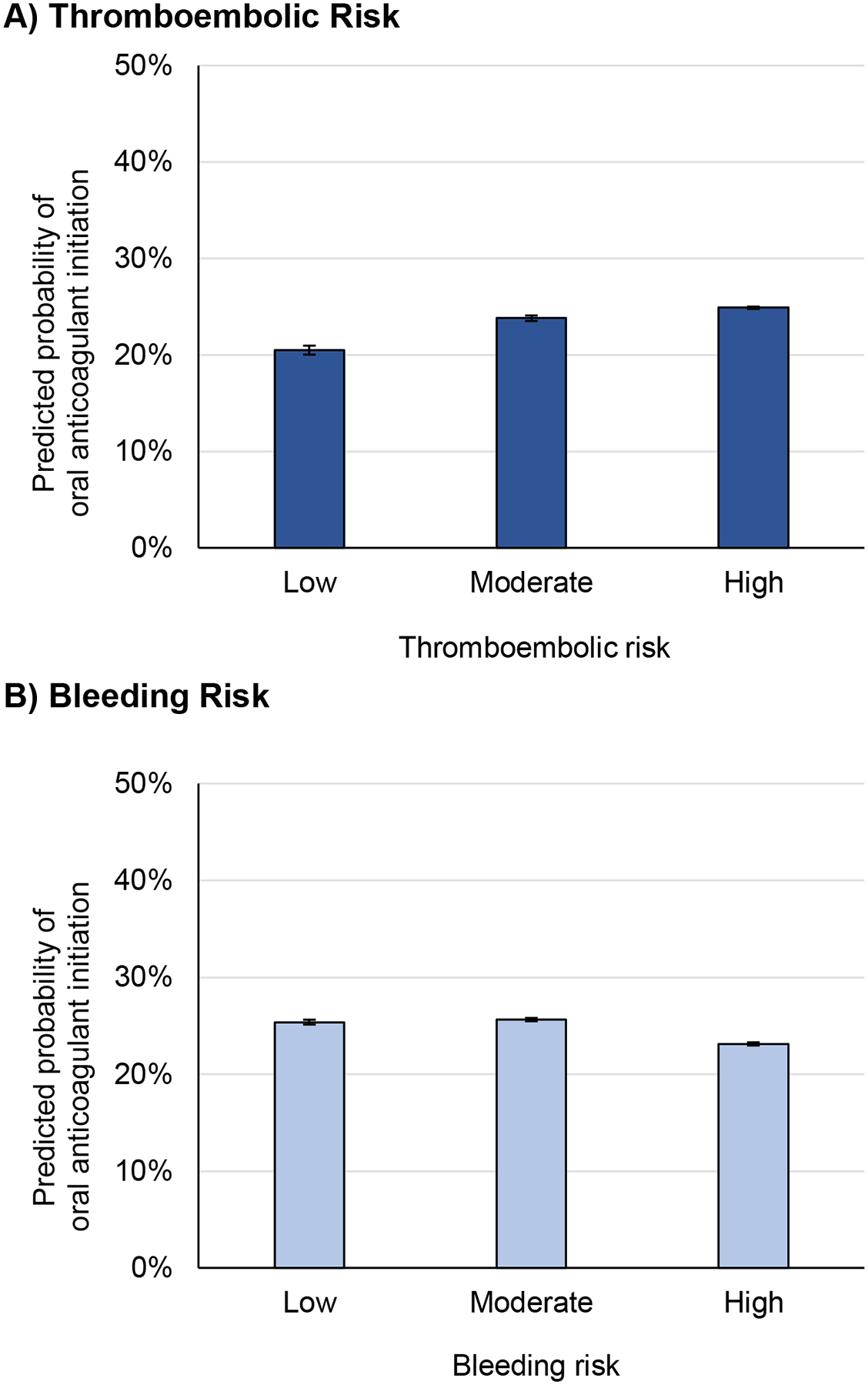
Predicted probability of anticoagulation initiation, by thromboembolic and bleeding risk category
a For thromboembolic risk, CHA2DS2-VASc score <2 was considered low, 2–3 moderate, and ≥4 high. Compared with patients at low thromboembolic risk, those with moderate and high thromboembolic risk had higher predicted probability of initiating anticoagulation (differences: +3.3% [CI +1.3% to +5.4%] for moderate thromboembolic risk, +4.4% [CI 2.5% to 6.3%] for high thromboembolic risk).
b For bleeding risk, HAS-BLED score <2 was considered low, 2 moderate and ≥3 high. Compared with patients at low bleeding risk, those with high bleeding risk had lower predicted probability of initiating anticoagulation, while those with moderate bleeding risk did not (differences: −0.26% [CI −0.92% to +1.4%] for moderate bleeding risk, −2.2% [CI −3.4% to −1.0%] for high bleeding risk).
Compared to low bleeding risk, high bleeding risk was associated with decreased odds of oral anticoagulant initiation, whereas moderate bleeding risk was not (OR 0.87 [CI, 0.81–0.94] for HAS-BLED score ≥3, OR 1.02 [CI, 0.95–1.09] for score 2–3) (Table 2). Predicted probability of oral anticoagulant initiation was 25.4% (CI, 24.4%−26.4%) for patients with low bleeding risk and 23.1% (CI, 22.5%−23.8%) for those with high bleeding risk (Figure 2B). Similar patterns and higher likelihood of initiation were seen among patients with a primary hospitalization diagnosis of atrial fibrillation (Table S3).
Geriatric syndromes
Mild and moderate-severe frailty were both associated with decreased likelihood of oral anticoagulant initiation (OR 0.89 [CI, 0.80–0.996] and OR 0.64 [CI, 0.56–0.75] respectively) (Table 2). Predicted probability of oral anticoagulant initiation was 24.7% (CI, 23.2%−26.2%) for non-frail patients, compared to 18.1% for moderately-severely frail patients (0.64 [CI, 0.56–0.75]) (Figure 3A). Dementia was associated with decreased oral anticoagulant initiation (OR 0.66 [CI, 0.61–0.72]) but delirium was not (OR 0.95 [CI, 0.83–1.09]).
Figure 3.
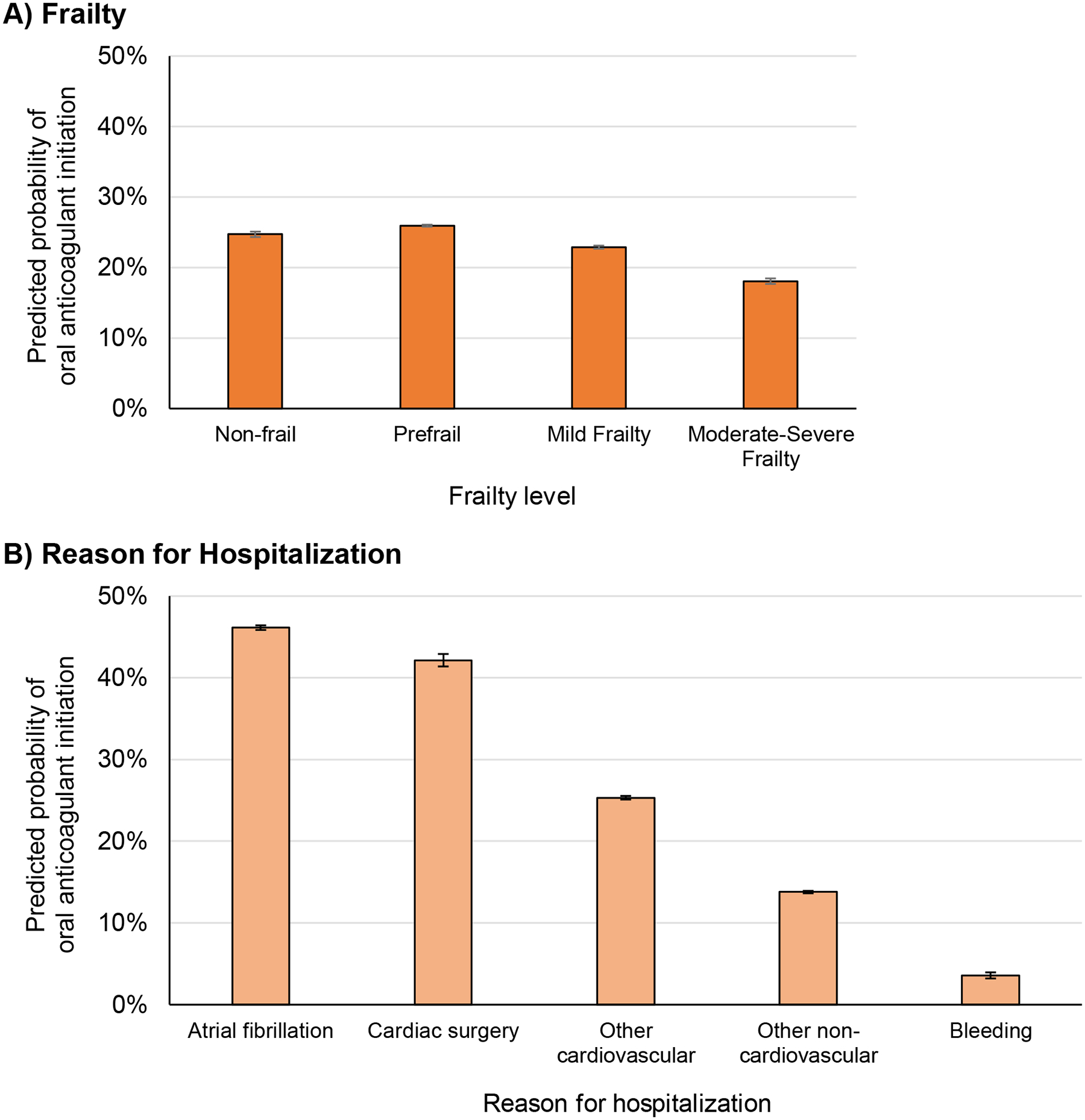
Predicted probability of oral anticoagulant initiation by frailty and reason for hospitalization among patients with guideline-based indications for anticoagulation
a Compared with non-frail patients, those with mild frailty had a 1.8% lower predicted probability of initiating anticoagulation (CI −3.6% to −0.04%) and those with moderate-severe frailty had 6.6% lower predicted probability of anticoagulation (CI −8.9% to −4.4%).
b Compared to patients hospitalized for atrial fibrillation, those hospitalized for all other reasons had lower predicted probability of initiating anticoagulation (differences: −4.0% [CI −7.1% to −0.83%] for cardiac surgery patients, −20.8% [CI −22.2% to −19.4%] for patients with other cardiovascular conditions, −32.4% [CI −33.6 to −31.1%] for patients with non-cardiovascular conditions, and −42.6% [CI −44.2% to −40.9%] for patients with bleeding).
Hospitalization category
Oral anticoagulant initiation rates differed by reason for hospitalization (Figure 3B, Table 2). The predicted probability of oral anticoagulant initiation was highest among patients hospitalized for atrial fibrillation (46.1% [CI, 45.0%−47.3%]) and those who had cardiac surgery during hospitalization (42.1% [CI, 39.2%−45.1%]). Predicted probability of initiation was lower among patients hospitalized for non-atrial fibrillation cardiovascular conditions (25.3% [CI, 24.5%−26.1%]), patients admitted for non-cardiovascular conditions (13.8% [CI, 13.3%−14.3%]), and lowest among those admitted for bleeding (3.6% [CI, 2.4%−4.8%]).
30-day anticoagulation initiation rates
By 30 days after discharge, 11,875 patients (30.9%) filled anticoagulant prescriptions compared with 9,412 (24.5%) within 7 days of discharge. Initiation patterns by thromboembolic risk, bleeding risk, frailty, dementia, and reason for hospitalization all mirrored 7-day fills (Table 2). The differences present at 30 days and not at 7 days were lower initiation rates among individuals with Black race, history of falls, or high comorbidity compared to those without these characteristics (Table S4).
Cardiac monitoring and follow-up
Post-discharge cardiac monitoring rates were low; 4.1% of patients had cardiac event monitoring within 30 days of discharge. Monitoring rates were higher among patients discharged on oral anticoagulants (5.6%) than among those who were not (3.6%). Similar to anticoagulant initiation, rates were lower in patients with mild frailty (2.8%) and moderate-severe frailty (1.5%) than in non-frail patients (6.0%), and higher among patients admitted for atrial fibrillation (9.1%) than other clinical groups (2.8%).
Most patients newly diagnosed with atrial fibrillation (59.3%) had at least 1 subsequent visit with a billing diagnosis of atrial fibrillation within a year of discharge. Subsequent atrial fibrillation billing was even more frequent in the subgroup admitted primarily for atrial fibrillation (79.5%).
DISCUSSION
In this national cohort of Medicare Part D beneficiaries, only one-quarter of older adults who were newly diagnosed with atrial fibrillation during hospitalization initiated oral anticoagulants within 7 days of discharge. Rates of anticoagulation were higher among patients whose primary reason for hospitalization was atrial fibrillation, but remained less than 50%. Frailty and reason for hospitalization were stronger predictors of oral anticoagulant initiation than thromboembolic and bleeding risk, with frail patients and those admitted for reasons other than atrial fibrillation or cardiac surgery having substantially lower likelihood of oral anticoagulant initiation. While some patients may have had uncaptured contraindications to anticoagulation, the lower rates observed in our population likely reflect a missed opportunity for stroke prevention for some patients, and an intentional choice based on contraindications and high near-term mortality risk for others.35,37,38
This study is the first to explore incident atrial fibrillation during hospital admission in the Medicare population. Prior estimates of anticoagulant initiation are generally higher than our own, though studied different populations. Prior registry studies have documented that anticoagulation was prescribed on discharge for 73% of patients with incident atrial fibrillation39 and 70–80% of patients with pre-existing atrial fibrillation, however over half of patients in these studies were on anticoagulation prior to hospitalization.14,38 Furthermore, registry studies provide information on prescribing behavior rather than filled prescriptions, thus, in conjunction with the current study, these data indicate that a proportion of patients prescribed anticoagulants may not be filling them following hospital discharge. Prior estimates using Medicare data have largely focused on the outpatient setting and demonstrate anticoagulant initiation rates for incident atrial fibrillation around 50%.3,4,12,13,40 One recent study of patients in an integrated health system with incident inpatient atrial fibrillation associated with sepsis reported anticoagulant initiation rates of 20%.41 Taken together with these studies, our results suggest that patients diagnosed with atrial fibrillation while admitted for other medical conditions—even those at high stroke risk—are infrequently initiated on anticoagulants or with ambulatory cardiac monitors.
Deferring oral anticoagulant initiation at hospital discharge may be reasonable in certain situations. First, deferring prescription would make sense if the risks of anticoagulation were deemed to outweigh the benefits after shared decision-making conversation. Although this is likely true for some patients, particularly those for whom bleeding risk was under-captured, clinicians’ fear of bleeding and falls are often inflated in this population.7,8,10,11 Second, atrial fibrillation might have been considered transient in some patients. Multiple findings in our study suggest that the atrial fibrillation events detected were often clinically significant enough to merit consideration of anticoagulation: anticoagulation rates were low even among patients whose atrial fibrillation was the primary hospitalization diagnosis and 60% of patients had subsequent visits for which atrial fibrillation within a year. Finally, some patients may prefer to discuss anticoagulation with their outpatient primary care physician or cardiologist rather than inpatient clinicians. However, the persistently low anticoagulant initiation rates observed at 30 days across all patient groups in our study (approximately 25% increase from 7 days) suggest this frequently did not occur, and are particularly concerning given emerging evidence around the acutely elevated stroke risk within 30 days following any episode of atrial fibrillation.42
Studies in outpatient populations have found that frailty and geriatric syndromes are associated with decreased rates of anticoagulant prescription among older adults with atrial fibrillation.5,12,15 Our study extends these findings to the inpatient setting. Although no prior US studies have examined inpatient anticoagulation by geriatric syndrome status, a meta-analysis of three small European studies that included a total of 1,204 hospitalized patients found that frail older adults were less likely to receive an anticoagulant.43 Rates of oral anticoagulant prescription were higher in these studies overall, ranging from 56–70%, which may reflect differential prescribing in European countries, though they also included patients with known atrial fibrillation and patients prescribed oral anticoagulants prior to admission.43
New diagnosis of atrial fibrillation in a hospitalized older adult should always trigger a shared decision-making conversation between patients and clinicians, regardless of reason for hospitalization. During these conversations, clinicians should emphasize objective risk factors for thromboembolism and bleeding, and acknowledge areas of uncertainty, and support the different risk-benefit ratios in patients at particularly high risk of mortality.35,44,45,46 To increase rates of appropriate prescribing nation-wide, systems-level interventions should be deployed, such as electronic medical record reminders to address oral anticoagulation for any patient found to have atrial fibrillation during their hospitalization, an approach which has had demonstrated success for heart failure guideline-directed medical therapy. Future prospective studies that examine the clinical decision-making of inpatient teams and hospitalized patients newly diagnosed with atrial fibrillation will be essential to inform interventions to reduce stroke risk.48 Additionally, drug pricing reform and reducing insurance barriers to anticoagulant initiation may help to increase the likelihood of filling prescriptions for prescribed anticoagulants, as DOACs’ high and rising prices may be adversely impacting initiation rates.49 Although DOACs have been guideline-recommended therapies for atrial fibrillation since 2014, their recommendation as first-line therapies in 2019 guidelines and increased DOAC penetration in Medicare Part D may have shifted practice patterns since our study year.2,12,49 However, improved safety profile and increased cost may have had opposing effects on prescription fills.49
This study has limitations. We used administrative claims and thus were unable to fully capture clinical decision-making, though we were able to measure HAS-BLED and CHA2DS2-VASc scores. Particularly, studies in smaller cohorts suggest that strict contraindications to anticoagulation were likely undercaptured, however contraindication prevalence reported in these studies would not be sufficient to explain the low initiation rates observed, especially compared with outpatient populations.37,38 Pharmacy claims do not reflect over the counter medications, including non-steroidal anti-inflammatory drugs, therefore HAS-BLED score was likely underestimated for some patients. We studied Medicare fee-for-service beneficiaries who were discharged home, thus our findings do not generalize to patients with Medicare Advantage or those discharged to skilled nursing facilities, for whom practice patterns may differ. Finally, as our primary endpoint was filled anticoagulant prescriptions, this study does not capture patients for whom anticoagulants were prescribed but not filled.
CONCLUSIONS
Oral anticoagulant initiation is uncommon among older adults newly diagnosed with atrial fibrillation during hospitalization, even among those hospitalized primarily for atrial fibrillation and those with high thromboembolic risk. Frailty and reason for hospitalization were more predictive of oral anticoagulant initiation than thromboembolic and bleeding risk. Shared decision-making conversations with all inpatients found to have atrial fibrillation will be necessary to improve oral anticoagulant prescribing for this high-risk patient population.
Supplementary Material
KEY POINTS.
Among older adults newly diagnosed with atrial fibrillation during hospitalization, only one in four with guideline-based indications for oral anticoagulation initiated an oral anticoagulant following discharge.
Higher frailty status and admission for reasons other than atrial fibrillation are associated with decreased likelihood of anticoagulant initiation.
Higher bleeding risk scores and lower thromboembolic risk scores are also associated with decreased likelihood of anticoagulant initiation, however to a lesser degree than frailty and reason for admission.
WHY DOES IT MATTER?
Oral anticoagulant initiation is uncommon among older adults diagnosed with atrial fibrillation during hospitalization, and initiation patterns do not appear to reflect clinical risk.
Acknowledgements:
Conflict of Interest Disclosures:
Dr. Anderson reports research grants from the American Heart Association, American College of Cardiology, Boston OAIC Pepper Center, and US Deprescribing Research Network outside of the submitted work and honoraria from Alosa Health. No other disclosures were reported.
Funding/Support:
This project was supported by grant K76AG074878 from the National Institute on Aging (Dr Anderson) and R01HS026215 from the Agency for Healthcare Research and Quality (Dr. Herzig).
Role of the Funder/Sponsor:
The National Institute on Aging and Agency for Healthcare Research and Quality had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Agency for Healthcare Research and Quality.
Supplementary Materials
Table S1. Variable Definitions
Table S2. NSAID and antiplatelet medications
Table S3. Predicted probability of anticoagulant initiation in patients with primary admission diagnosis of atrial fibrillation
Table S4. Adjusted odds of anticoagulant initiation among all patients by race and geriatric conditions
Figure S1. Cohort Diagram
REFERENCES
- 1.Marinigh R, Lip GYH, Fiotti N, Giansante C, Lane DA. Age as a Risk Factor for Stroke in Atrial Fibrillation Patients. Journal of the American College of Cardiology. 2010;56(11):827–837. doi: 10.1016/j.jacc.2010.05.028 [DOI] [PubMed] [Google Scholar]
- 2.January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation. 2019;140(2). doi: 10.1161/CIR.0000000000000665 [DOI] [PubMed] [Google Scholar]
- 3.Norby FL, Lutsey PL, Shippee ND, et al. Direct Oral Anticoagulants and Warfarin for Atrial Fibrillation Treatment: Rural and Urban Trends in Medicare Beneficiaries. Am J Cardiovasc Drugs. 2022;22(2):207–217. doi: 10.1007/s40256-021-00502-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Essien UR, Magnani JW, Chen N, Gellad WF, Fine MJ, Hernandez I. Race/Ethnicity and Sex-Related Differences in Direct Oral Anticoagulant Initiation in Newly Diagnosed Atrial Fibrillation: A Retrospective Study of Medicare Data. Journal of the National Medical Association. 2020;112(1):103–108. doi: 10.1016/j.jnma.2019.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah SJ, Fang MC, Jeon SY, Gregorich SE, Covinsky KE. Geriatric Syndromes and Atrial Fibrillation: Prevalence and Association with Anticoagulant Use in a National Cohort of Older Americans. J Am Geriatr Soc. 2021;69(2):349–356. doi: 10.1111/jgs.16822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lubitz SA, Khurshid S, Weng LC, et al. Predictors of oral anticoagulant non-prescription in patients with atrial fibrillation and elevated stroke risk. American Heart Journal. 2018;200:24–31. doi: 10.1016/j.ahj.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lip GYH, Clementy N, Pericart L, Banerjee A, Fauchier L. Stroke and Major Bleeding Risk in Elderly Patients Aged ≥75 Years With Atrial Fibrillation: The Loire Valley Atrial Fibrillation Project. Stroke. 2015;46(1):143–150. doi: 10.1161/STROKEAHA.114.007199 [DOI] [PubMed] [Google Scholar]
- 8.Gage BF, Birman-Deych E, Kerzner R, Radford MJ, Nilasena DS, Rich MW. Incidence of intracranial hemorrhage in patients with atrial fibrillation who are prone to fall. The American Journal of Medicine. 2005;118(6):612–617. doi: 10.1016/j.amjmed.2005.02.022 [DOI] [PubMed] [Google Scholar]
- 9.Kim D, Yang PS, Sung JH, et al. Effectiveness and Safety of Anticoagulation Therapy in Frail Patients With Atrial Fibrillation. Stroke. 2022;53(6):1873–1882. doi: 10.1161/STROKEAHA.121.036757 [DOI] [PubMed] [Google Scholar]
- 10.Donzé J, Clair C, Hug B, et al. Risk of Falls and Major Bleeds in Patients on Oral Anticoagulation Therapy. The American Journal of Medicine. 2012;125(8):773–778. doi: 10.1016/j.amjmed.2012.01.033 [DOI] [PubMed] [Google Scholar]
- 11.Wang W, Lessard D, Kiefe CI, et al. Differential effect of anticoagulation according to cognitive function and frailty in older patients with atrial fibrillation. Journal of the American Geriatrics Society. n/a(n/a). doi: 10.1111/jgs.18079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ko D, Lin KJ, Bessette LG, et al. Trends in Use of Oral Anticoagulants in Older Adults With Newly Diagnosed Atrial Fibrillation, 2010–2020. JAMA Network Open. 2022;5(11):e2242964. doi: 10.1001/jamanetworkopen.2022.42964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernandez I, Saba S, Zhang Y. Geographic Variation in the Use of Oral Anticoagulation Therapy in Stroke Prevention in Atrial Fibrillation. Stroke. 2017;48(8):2289–2291. doi: 10.1161/STROKEAHA.117.017683 [DOI] [PubMed] [Google Scholar]
- 14.Essien UR, Chiswell K, Kaltenbach LA, et al. Association of Race and Ethnicity With Oral Anticoagulation and Associated Outcomes in Patients With Atrial Fibrillation: Findings From the Get With The Guidelines–Atrial Fibrillation Registry. JAMA Cardiology. Published online October 26, 2022. doi: 10.1001/jamacardio.2022.3704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanghai SR, Liu W, Wang W, et al. Prevalence of Frailty and Associations with Oral Anticoagulant Prescribing in Atrial Fibrillation. J GEN INTERN MED. 2022;37(4):730–736. doi: 10.1007/s11606-021-06834-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Medicare and Medicaid Services. 27 CCW Chronic Conditions Algorithms: Atrial Fibrillation. Published online February 2022. https://www.ccwdata.org/documents/10280/19139608/ccw-cond-algo-atrialfib.pdf
- 17.Anderson TS, Xu E, Whitaker E, Steinman MA. A systematic review of methods for determining cross-sectional active medications using pharmacy databases. Pharmacoepidemiol Drug Saf. 2019;28(4):403–421. doi: 10.1002/pds.4706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson TS, Jing B, Wray CM, et al. Comparison of Pharmacy Database Methods for Determining Prevalent Chronic Medication Use. Med Care. 2019;57(10):836–842. doi: 10.1097/MLR.0000000000001188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Article - Billing and Coding: Electrocardiographic (EKG or ECG) Monitoring (Holter or Real-Time Monitoring) (A57476). Accessed August 26, 2022. https://www.cms.gov/medicare-coverage-database/view/article.aspx?articleid=57476&ver=11&
- 20.Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining Clinical Risk Stratification for Predicting Stroke and Thromboembolism in Atrial Fibrillation Using a Novel Risk Factor-Based Approach. Chest. 2010;137(2):263–272. doi: 10.1378/chest.09-1584 [DOI] [PubMed] [Google Scholar]
- 21.Friberg L, Rosenqvist M, Lip GYH. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. European Heart Journal. 2012;33(12):1500–1510. doi: 10.1093/eurheartj/ehr488 [DOI] [PubMed] [Google Scholar]
- 22.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJGM, Lip GYH. A Novel User-Friendly Score (HAS-BLED) To Assess 1-Year Risk of Major Bleeding in Patients With Atrial Fibrillation. Chest. 2010;138(5):1093–1100. doi: 10.1378/chest.10-0134 [DOI] [PubMed] [Google Scholar]
- 23.Brenner GM, Stevens CW. Pharmacology. Fifth edition. Elsevier; 2018. [Google Scholar]
- 24.Maura G, Blotière PO, Bouillon K, et al. Comparison of the Short-Term Risk of Bleeding and Arterial Thromboembolic Events in Nonvalvular Atrial Fibrillation Patients Newly Treated With Dabigatran or Rivaroxaban Versus Vitamin K Antagonists: A French Nationwide Propensity-Matched Cohort Study. Circulation. 2015;132(13):1252–1260. doi: 10.1161/CIRCULATIONAHA.115.015710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Essien UR, Kim N, Hausmann LRM, et al. Disparities in Anticoagulant Therapy Initiation for Incident Atrial Fibrillation by Race/Ethnicity Among Patients in the Veterans Health Administration System. JAMA Netw Open. 2021;4(7):e2114234. doi: 10.1001/jamanetworkopen.2021.14234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oger E, Botrel MA, Juchault C, Bouget J. Sensitivity and specificity of an algorithm based on medico-administrative data to identify hospitalized patients with major bleeding presenting to an emergency department. BMC Med Res Methodol. 2019;19(1):194. doi: 10.1186/s12874-019-0841-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jarrín OF, Nyandege AN, Grafova IB, Dong X, Lin H. Validity of Race and Ethnicity Codes in Medicare Administrative Data Compared With Gold-standard Self-reported Race Collected During Routine Home Health Care Visits. Medical Care. 2020;58(1):e1–e8. doi: 10.1097/MLR.0000000000001216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim DH, Schneeweiss S, Glynn RJ, Lipsitz LA, Rockwood K, Avorn J. Measuring Frailty in Medicare Data: Development and Validation of a Claims-Based Frailty Index. The Journals of Gerontology: Series A. 2018;73(7):980–987. doi: 10.1093/gerona/glx229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim DH, Glynn RJ, Avorn J, et al. Validation of a Claims-Based Frailty Index Against Physical Performance and Adverse Health Outcomes in the Health and Retirement Study. The Journals of Gerontology: Series A. 2019;74(8):1271–1276. doi: 10.1093/gerona/gly197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Centers for Medicare and Medicaid Services. 27 CCW Chronic Conditions Algorithms: Alzheimer’s Disease and Related Disorders or Senile Dementia. Published online February 2022. https://www2.ccwdata.org/documents/10280/19139608/ccw-cond-algo-alzdisorders.pdf
- 31.Kim DH, Lee J, Kim CA, et al. Evaluation of Algorithms to Identify Delirium in Administrative Claims and Drug Utilization Database. Pharmacoepidemiol Drug Saf. 2017;26(8):945–953. doi: 10.1002/pds.4226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity Measures for Use with Administrative Data: Medical Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004 [DOI] [PubMed] [Google Scholar]
- 33.Matos JD, Sellke FW, Zimetbaum P. Post–Cardiac Surgery Atrial Fibrillation. Cardiac Electrophysiology Clinics. 2021;13(1):133–140. doi: 10.1016/j.ccep.2020.11.011 [DOI] [PubMed] [Google Scholar]
- 34.Herzig SJ, Rudolph JL, Haime M, Ngo LH, Marcantonio ER. Atrial fibrillation at discharge in older cardiac surgery patients: A prospective study of prevalence and associated medication utilization. J Clin Trials. 2012;2(1):106. [PMC free article] [PubMed] [Google Scholar]
- 35.Siontis KC, Gersh BJ, Weston SA, et al. Associations of Atrial Fibrillation After Noncardiac Surgery With Stroke, Subsequent Arrhythmia, and Death : A Cohort Study. Ann Intern Med. 2022;175(8):1065–1072. doi: 10.7326/M22-0434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Norton EC, Dowd BE, Maciejewski ML. Marginal Effects—Quantifying the Effect of Changes in Risk Factors in Logistic Regression Models. JAMA. 2019;321(13):1304. doi: 10.1001/jama.2019.1954 [DOI] [PubMed] [Google Scholar]
- 37.McGrath ER, Go AS, Chang Y, et al. Use of Oral Anticoagulant Therapy in Older Adults with Atrial Fibrillation After Acute Ischemic Stroke. J Am Geriatr Soc. 2017;65(2):241–248. doi: 10.1111/jgs.14688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piccini JP, Xu H, Cox M, et al. Adherence to Guideline-Directed Stroke Prevention Therapy for Atrial Fibrillation Is Achievable. Circulation. 2019;139(12):1497–1506. doi: 10.1161/CIRCULATIONAHA.118.035909 [DOI] [PubMed] [Google Scholar]
- 39.Kir D, Zhang S, Kaltenbach LA, et al. Patterns of care for first-detected atrial fibrillation: Insights from the Get With The Guidelines® – Atrial Fibrillation registry. Heart Rhythm. 2022;19(7):1049–1057. doi: 10.1016/j.hrthm.2022.02.025 [DOI] [PubMed] [Google Scholar]
- 40.Guo J, He M, Magnani JW, Brooks MM, Gellad WF, Hernandez I. Comparison of Oral Anticoagulant Use and Stroke Risk Among Older Adults Newly-Diagnosed Atrial Fibrillation Living in Urban-Versus-Rural Areas. The American Journal of Cardiology. 2020;130:64–69. doi: 10.1016/j.amjcard.2020.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walkey AJ, Myers LC, Thai KK, et al. Practice Patterns and Outcomes Associated With Anticoagulation Use Following Sepsis Hospitalizations With New-Onset Atrial Fibrillation. Circulation: Cardiovascular Quality and Outcomes. 0(0):e009494. doi: 10.1161/CIRCOUTCOMES.122.009494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singer DE, Ziegler PD, Koehler JL, Sarkar S, Passman RS. Temporal Association Between Episodes of Atrial Fibrillation and Risk of Ischemic Stroke. JAMA Cardiol. 2021;6(12):1364. doi: 10.1001/jamacardio.2021.3702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oqab Z What is the Impact of Frailty on Prescription of Anticoagulation in Elderly Patients with Atrial Fibrillation? A Systematic Review and Meta-Analysis. Journal of Atrial Fibrillation. 2018;10(6):1870. doi: 10.4022/jafib.1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.John RM, Sharma D. Contraindication to Anticoagulation in Nonvalvular Atrial Fibrillation. JACC: Clinical Electrophysiology. 2019;5(12):1393–1395. doi: 10.1016/j.jacep.2019.07.008 [DOI] [PubMed] [Google Scholar]
- 45.Sen S, Dahlberg KW. Physician’s fear of anticoagulant therapy in nonvalvular atrial fibrillation. Am J Med Sci. 2014;348(6):513–521. doi: 10.1097/MAJ.0000000000000349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martinez KA, Hurwitz HM, Rothberg MB. Qualitative Analysis of Patient–Physician Discussions Regarding Anticoagulation for Atrial Fibrillation. JAMA Internal Medicine. Published online October 31, 2022. doi: 10.1001/jamainternmed.2022.4918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ghazi L, Yamamoto Y, Riello RJ, et al. Electronic Alerts to Improve Heart Failure Therapy in Outpatient Practice. Journal of the American College of Cardiology. 2022;79(22):2203–2213. doi: 10.1016/j.jacc.2022.03.338 [DOI] [PubMed] [Google Scholar]
- 48.Shah SJ, Covinsky KE. Do Anticoagulants Preserve Function and Quality of Life in Older Adults with Atrial Fibrillation? NEJM Evidence. 2022;1(3):EVIDtt2200010. doi: 10.1056/EVIDtt2200010 [DOI] [PubMed] [Google Scholar]
- 49.Troy A, Anderson TS. National Trends in Use of and Spending on Oral Anticoagulants Among US Medicare Beneficiaries From 2011 to 2019. JAMA Health Forum. 2021;2(7):e211693. doi: 10.1001/jamahealthforum.2021.1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


