Abstract
Background
Delayed access to care may contribute to disparities in prostate cancer (PCa). The Affordable Care Act (ACA) aimed at increasing access and reducing healthcare disparities, but its impact on timely treatment initiation for PCa men is unknown.
Methods
Men with intermediate‐ and high‐risk PCa diagnosed 2010–2016 and treated with curative surgery or radiotherapy were identified in the National Cancer Database. Multivariable logistic regression modeled the effect of race and insurance type on treatment delay >180 days after diagnosis. Cochran–Armitage test measured annual trends in delays, and joinpoint regression assessed if 2014, the year the ACA became fully operationalized, was significant for inflection in crude rates of major delays.
Results
Of 422,506 eligible men, 18,720 (4.4%) experienced >180‐day delay in treatment initiation. Compared to White patients, Black (OR 1.79, 95% CI 1.72–1.87, p < 0.001) and Hispanic (OR 1.37, 95% CI 1.28–1.48, p < 0.001) patients had higher odds of delay. Compared to uninsured, those with Medicaid had no difference in odds of delay (OR 0.94, 95% CI 0.84–1.06, p = 0.31), while those with private insurance (OR 0.57, 95% CI 0.52–0.63, p < 0.001) or Medicare (OR 0.64, 95% CI 0.58–0.70, p < 0.001) had lower odds of delay. Mean time to treatment significantly increased from 2010 to 2016 across all racial/ethnic groups (trend p < 0.001); 2014 was associated with a significant inflection for increase in rates of major delays.
Conclusions
Non‐White and Medicaid‐insured men with localized PCa are at risk of treatment delays in the United States. Treatment delays have been consistently rising, particularly after implementation of the ACA.
Keywords: ACA, disparities, Obamacare, prostate cancer, treatment delay
The Affordable Care Act (ACA) attempted to increase access to care and reduce healthcare disparities. However, we show that major delays (>6 months from diagnosis to starting treatment) for prostate cancer were more prevalent after the ACA was implemented, with Black patients having almost twice the odds of experiencing a major delay. Furthermore, patients with Medicaid were just as likely to experience major treatment delays as uninsured patients after the ACA, while patients with private insurance or Medicare had lower odds of treatment delays.
1. INTRODUCTION
In 2022, there will be over 260,000 new cases of prostate cancer in the United States. 1 Black men, compared with White men, are more likely to be diagnosed with and die of prostate cancer. 2 The root of these disparities is multifactorial, possibly due to intrinsic tumor biology, systemic racism, as well as medical mistrust. 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 Recent studies, however, suggest that racial disparities in prostate cancer outcomes in the United States may be driven predominantly by disparities in access to care and receipt of definitive therapy. 11 , 12 , 13 Specifically, Black men have historically experienced significantly longer delays from cancer diagnosis to treatment initiation compared with White men. 14
In the last decade, multiple structural changes have affected the management of prostate cancer as well as attempted to rectify disparities in access and care. In 2010, the Patient Protection and Affordable Care Act (ACA) was created to expand access to care and reduce healthcare disparities, 15 but was not fully operationalized with personal coverage mandates, development of insurance exchanges, elimination of pre‐existing condition plans, coverage of preventative care, and more until 2014. Evidence in breast, colon, and lung cancer suggest that while screening rates and early stage detection have improved after the ACA, disparities in timely cancer treatment initiation remained. 16 , 17 In the metastatic setting, Medicaid expansion under the ACA was associated with decreased racial disparities for timely initiation of systemic therapy. 18 One theory is that the ACA would disproportionately benefit non‐White patients who were uninsured, increasing their access to care through Medicaid. However, the impact of the ACA on timely access to prostate cancer care is unclear, particularly as patients with Medicaid more frequently encounter providers who refuse to accept their insurance. 19 Additionally, timely treatment initiation can be affected by patient preferences, shifting prostate cancer epidemiology, and lagging changes in the healthcare labor force to accommodate for potential increases in patient volume. In this analysis, we evaluated the impact of the ACA on timely access to care for men with intermediate or high risk localized prostate cancer in the United States, with a focus on its impact based on race.
2. METHODS
2.1. Data source and study population
The National Cancer Data Base (NCDB) is a nationwide hospital‐based registry sponsored by the American College of Surgeons and the American Cancer Society. It captures the first course of cancer treatment from more than 1500 Commission on Cancer‐accredited facilities, gathering data on approximately 70% of new cancer diagnoses in the United States. 20 , 21 Data accuracy are continually validated via quality review, site surveys, and internal monitoring. 20 , 21 , 22 Because the study used de‐identified data from the NCDB, the requirement for formal institutional review and the need for informed consent were waived.
We identified men ≥40 years old with a new diagnosis of clinical stage T1‐4, N0, M0 prostate adenocarcinoma between 2010 and 2016. Those pursuing definitive treatment with radical prostatectomy or radiation therapy (with or without concomitant androgen deprivation therapy [ADT]) were eligible. We excluded patients with low‐risk disease (i.e., T1‐2a and Gleason score 6 and PSA <10), patients pursuing active surveillance or palliative therapy, patients treated with chemotherapy or immunotherapy, patients with prior or synchronous diagnosis of other malignancy, and patients with missing date of surgery or start of radiation therapy or ADT (Figure S1).
The exposure of interest was non‐Hispanic White versus other races or ethnicities and insurance status. The primary outcome was timely initiation of any first‐course therapy (either radical prostatectomy, radiation therapy, or ADT) within 180 days from date of diagnosis. 23 , 24
2.2. Statistical methods
Descriptive statistics were used to present baseline characteristics as the NCDB registry captures data from the first course of treatment. Covariates included age (<60, 60–69, and ≥70 years old), clinical T stage (T1, T2, T3, and T4), prostate specific antigen (PSA) level (<10, 10–20, and >20), Gleason score (6, 7, and, 8–10), diagnosis year (2010–2013 vs. 2014–2016), race or ethnicity (White, Black, Hispanic, and Other or Unknown), hospital setting (academic vs. non‐academic), insurance type (not insured, Medicaid, Private, Medicare, or other Government), Charlson–Deyo comorbidity index (0 and ≥1), US region (Northeast, Central, South, and West), household income (<$38,000, $38,000–$47,999, $48,000–$62,999, and ≥$63,000), average education level of zip code where patient is from (≥21.0%, 13–20.9%, 7–12.9%, and <7% of residents with less than high school education), and distance from home to treatment facility (<25, 25–50, and >50 miles). Categorical variables were compared between groups via chi‐squared test and continuous variables via analysis of variance. We used a multivariable logistic regression model to assess the effect of race and insurance type on receipt of definitive therapy within 180 days from date of diagnosis. The final model was built by backward variable selection procedure with an alpha level of 0.10 for removal. The association of race with timely treatment initiation was compared across multiple subgroups, including patients diagnosed before and after 2014, as well as within men with private or Medicare insurance versus men with Medicaid or without insurance. Finally, Cochran–Armitage trend test was performed to assess year‐to‐year changes in proportion of patients with treatment delay >180 days stratified by race. Joinpoint regression was performed at the junction of 2010–2013 and 2014–2016, corresponding to intervals before and after full implementation of the ACA, to assess for significant changes in crude rates of patients with major delay amongst all patients, followed by White and Black patients, respectively. Statistical analysis was performed using SAS 9.4 (SAS Institute Inc.). Two‐sided p < 0.05 was considered statistically significant.
3. RESULTS
3.1. Association of clinicodemographic variables with time to treatment initiation
There were 422,506 men with intermediate or high risk localized prostate cancer diagnosed between 2010 and 2016 pursuing definitive treatment with primary surgery or radiotherapy. 311,398 patients started first‐course treatment (e.g., surgery, radiotherapy, or ADT) within 90 days of diagnosis (73.7%), 92,388 patients started treatment between 91 and 180 days of diagnosis (21.9%), and 18,720 patients started treatment >180 days after diagnosis (4.4%). By race or ethnicity, 6.9% of Black men, 6.1% of Hispanic men, 5.4% of men with other or unidentified race/ethnicity, and 3.8% of White men started treatment >180 days after diagnosis. Distribution of clinicodemographic variables and time to treatment initiation are summarized in Table 1.
TABLE 1.
Clinicodemographic variables and their association with time from prostate cancer to treatment initiation for patients with localized disease.
| Time from diagnosis to treatment initiation | ||||||
|---|---|---|---|---|---|---|
| Covariate | Level | Median (IQR) | <90 days n = 311,398 | 91–180 days n = 92,388 | >180 days n = 18,720 | Parametric p‐value |
| Age | <60 | 67 (45–98) | 78,546 (25.2%) | 27,337 (29.6%) | 5617 (30.0%) | <0.001 |
| 60–69 | 65 (42–96) | 13,7206 (44.1%) | 44,758 (48.5%) | 9054 (48.4%) | ||
| ≥70 | 50 (22–82) | 95,646 (30.7%) | 20,293 (22.0%) | 4049 (21.6%) | ||
| Prostate specific antigen level | <10 | 66 (44–96) | 184,514 (59.3%) | 61,306 (66.4%) | 11,595 (61.9%) | <0.001 |
| 10–20 | 63 (40–96) | 50,568 (16.2%) | 16,145 (17.5%) | 3808 (20.3%) | ||
| >20 | 45 (11–78) | 76,316 (24.5%) | 14,937 (16.2%) | 3317 (17.7%) | ||
| T‐Stage | T1 | 63 (40–95) | 187,452 (63.7%) | 59,209 (66.8%) | 12,327 (69.0%) | <0.001 |
| T2 | 62 (39–91) | 93,545 (31.8%) | 26,861 (30.3%) | 5058 (28.3%) | ||
| T3 | 50 (28–80) | 11,757 (4.0%) | 2453 (2.8%) | 445 (2.5%) | ||
| T4 | 10 (0–39) | 1411 (0.5%) | 99 (0.1%) | 24 (0.1%) | ||
| Gleason score | 6 | 65 (34–102) | 45,986 (15.8%) | 16,263 (18.2%) | 4508 (24.9%) | <0.001 |
| 7 | 68 (45–98) | 166,923 (57.2%) | 59,336 (66.5%) | 11,588 (64.0%) | ||
| 8–10 | 50 (28–76) | 78,773 (27.0%) | 13,656 (15.3%) | 2006 (11.1%) | ||
| Race or ethnicity | Others/Unknown | 63 (38–97) | 11,693 (3.8%) | 3734 (4.0%) | 887 (4.7%) | <0.001 |
| Hispanic | 65 (37–101) | 13,072 (4.2%) | 4651 (5.0%) | 1150 (6.1%) | ||
| Black | 69 (41–105) | 44,124 (14.2%) | 17,605 (19.1%) | 4568 (24.4%) | ||
| White | 61 (37–90) | 242,509 (77.9%) | 66,398 (71.9%) | 12,115 (64.7%) | ||
| Hospital setting | Non‐academic* | 57 (33–86) | 200,963 (64.5%) | 48,436 (52.4%) | 9623 (51.4%) | <0.001 |
| Academic | 70 (46–103) | 110,435 (35.5%) | 43,952 (47.6%) | 9097 (48.6%) | ||
| Charleson‐Deyo Comorbidity Score | ≥1 | 60 (34–91) | 60,852 (19.5%) | 170,70 (18.5%) | 3487 (18.6%) | <0.001 |
| 0 | 62 (38–93) | 250,546 (80.5%) | 75,318 (81.5%) | 15,233 (81.4%) | ||
| Insurance | Not insured | 68 (39–108) | 4657 (1.5%) | 1840 (2.0%) | 563 (3.1%) | <0.001 |
| Medicaid | 69 (40–109) | 8126 (2.7%) | 3220 (3.6%) | 987 (5.4%) | ||
| Private | 65 (43–95) | 14,0424 (46.0%) | 45,156 (50.2%) | 8368 (45.9%) | ||
| Medicare | 56 (30–88) | 145,808 (47.8%) | 36,802 (40.9%) | 7476 (41.0%) | ||
| Other government | 74 (44–114) | 6313 (2.1%) | 2977 (3.3%) | 858 (4.7%) | ||
| Geographical region | Northeast | 68 (42–99) | 59,663 (19.2%) | 21,780 (23.6%) | 4365 (23.3%) | <0.001 |
| Central | 57 (35–85) | 89,000 (28.6%) | 20,819 (22.5%) | 3817 (20.4%) | ||
| South | 62 (36–92) | 11,6253 (37.3%) | 34,747 (37.6%) | 6983 (37.3%) | ||
| West | 64 (40–97) | 46,482 (14.9%) | 15,042 (16.3%) | 3555 (19.0%) | ||
| Residence type | Urban/metropolitan | 62 (38–92) | 297,089 (97.8%) | 88,446 (98.3%) | 17,933 (98.3%) | <0.001 |
| Rural | 56 (31–84) | 6727 (2.2%) | 1527 (1.7%) | 303 (1.7%) | ||
| Income | <$38,000 | 61 (35–93) | 49,873 (16.1%) | 14,509 (15.8%) | 3395 (18.2%) | <0.001 |
| $38,000–$47,999 | 60 (35–91) | 68,777 (22.1%) | 19,105 (20.7%) | 3822 (20.5%) | ||
| $48,000–$62,999 | 62 (38–92) | 83,386 (26.8%) | 24,325 (26.4%) | 4776 (25.6%) | ||
| ≥$63,000 | 64 (41–94) | 108,657 (35.0%) | 34,206 (37.1%) | 6659 (35.7%) | ||
| Percent of zip code with less than high school education | ≥21.0% | 62 (35–95) | 46,484 (15.0%) | 14,295 (15.5%) | 3377 (18.1%) | <0.001 |
| 13%–20.9% | 62 (36–93) | 74,825 (24.1%) | 22,104 (24.0%) | 4750 (25.4%) | ||
| 7%–12.9% | 62 (38–92) | 102,673 (33.0%) | 30,170 (32.7%) | 5740 (30.7%) | ||
| <7% | 63 (40–92) | 86,886 (28.0%) | 25,635 (27.8%) | 4804 (25.7%) | ||
| Distance traveled to treatment | <25 miles | 61 (35–91) | 226,107 (72.7%) | 63,179 (68.5%) | 13,027 (69.8%) | <0.001 |
| 25–50 miles | 63 (39–92) | 42,911 (13.8%) | 12,812 (13.9%) | 2536 (13.6%) | ||
| >50 miles | 69 (46–101) | 41,980 (13.5%) | 16,259 (17.6%) | 3114 (16.7%) | ||
| Year of diagnosis | 2010–2013 | 61 (36–91) | 183,638 (59.0%) | 51,253 (55.5%) | 10,477 (56.0%) | <0.001 |
| 2014–2016 | 64 (40–96) | 127,760 (41.0%) | 41,135 (44.5%) | 8243 (44.0%) | ||
Non‐academic centers grouped as either community or comprehensive community centers.
On multivariable analysis, non‐White compared with White race was associated with higher odds of treatment delay >180 days. Compared with White men, Black men (OR 1.79, 95% CI 1.72–1.87, p < 0.001), Hispanic men (OR 1.37, 95% CI 1.28–1.48, p < 0.001), and men with other or unidentified race/ethnicity (OR 1.23, 95% CI 1.14–1.33, p < 0.001) had significantly greater odds of experiencing a treatment delay >180 days. Compared to uninsured patients, those with private insurance (OR 0.57, 95% CI 0.52–0.63, p < 0.001) or Medicare (OR 0.64, 95% CI 0.58–0.70, p < 0.001) had significantly lower odds of major treatment delay; however, patients with Medicaid had no significant difference in odds of treatment delay (OR 0.94, 95% CI 0.84–1.06, p = 0.307) compared to uninsured patients. Finally, diagnosis after 2014, was associated with significantly higher odds of major treatment delay (OR 1.11, 95% CI 1.07–1.15, p < 0.001). Complete univariable and multivariable associations of clinicodemographic factors with major treatment delays are shown in Table 2. On multiple associations testing, the odds of non‐White patients compared to White patients experiencing major delay was significantly lower after 2014 (OR 1.48; 95% CI 1.41–1.56, p < 0.001) compared to before 2014 (OR 1.70; 95% CI 1.62–1.78, p < 0.001) with Interaction p‐value <0.001.
TABLE 2.
Univariate and multivariable logistic regression analysis comparing associations of clinicodemographic covariates with proportion of patients experiencing major delay in time from diagnosis to treatment initiation (>180 days).
| Univariate analysis | Multivariable analysis | ||||||
|---|---|---|---|---|---|---|---|
| Covariate | Level | n | Odds ratio (95% CI) | p‐value | n | Odds ratio (95% CI) | p‐value |
| Age | <60 | 111,500 | ‐ | ‐ | 95,674 | ‐ | ‐ |
| 60–69 | 191,018 | 0.94 (0.91–0.97) | <0.001 | 167,241 | 1.02 (0.98–1.07) | 0.24 | |
| ≥70 | 119,988 | 0.66 (0.63–0.69) | <0.001 | 106,223 | 0.83 (0.79–0.88) | <0.001 | |
| Prostate specific antigen level | <10 | 257,415 | ‐ | ‐ | 233,512 | ‐ | ‐ |
| 10–20 | 70,521 | 1.21 (1.17–1.26) | <0.001 | 65,253 | 1.09 (1.04–1.13) | <0.001 | |
| >20 | 94,570 | 0.77 (0.74–0.80) | <0.001 | 70,373 | 0.74 (0.71–0.78) | <0.001 | |
| T‐Stage | T1 | 258,988 | ‐ | ‐ | 239,221 | ‐ | ‐ |
| T2 | 125,464 | 0.84 (0.81–0.87) | <0.001 | 115,399 | 0.82 (0.79–0.85) | <0.001 | |
| T3 | 14,655 | 0.63 (0.57–0.69) | <0.001 | 13,308 | 0.79 (0.71–0.87) | <0.001 | |
| T4 | 1534 | 0.32 (0.21–0.48) | <0.001 | 1210 | 0.60 (0.39–0.94) | 0.025 | |
| Gleason score | 6 | 66,757 | ‐ | ‐ | 58,930 | ‐ | ‐ |
| 7 | 237,847 | 0.71 (0.68–0.73) | <0.001 | 222,323 | 0.61 (0.58–0.63) | <0.001 | |
| 8–10 | 94,435 | 0.30 (0.28–0.32) | <0.001 | 87,885 | 0.28 (0.26–0.29) | <0.001 | |
| Race or ethnicity | Others/unknown | 16,314 | 1.47 (1.37–1.57) | <0.001 | 14,141 | 1.23 (1.14–1.33) | <0.001 |
| Hispanic | 18,873 | 1.65 (1.55–1.76) | <0.001 | 15,838 | 1.37 (1.28–1.48) | <0.001 | |
| Black | 66,297 | 1.89 (1.82–1.95) | <0.001 | 58,076 | 1.79 (1.72–1.87) | <0.001 | |
| White | 321,022 | ‐ | ‐ | 281,083 | ‐ | ‐ | |
| Hospital setting | Non‐academic* | 259,022 | ‐ | ‐ | 226,345 | ‐ | ‐ |
| Academic | 163,484 | 1.53 (1.48–1.57) | <0.001 | 142,793 | 1.41 (1.37–1.46) | <0.001 | |
| Charleson‐Deyo comorbidity score | ≥1 | 81,409 | 0.96 (0.92–0.99) | 0.023 | 72,281 | 0.99 (0.95–1.03) | 0.497 |
| 0 | 341,097 | ‐ | ‐ | 296,857 | ‐ | ‐ | |
| Insurance | Not insured | 7060 | ‐ | ‐ | 6014 | ‐ | ‐ |
| Medicaid | 12,333 | 1.00 (0.90–1.12) | 0.944 | 11,184 | 0.94 (0.84–1.06) | 0.307 | |
| Private | 193,948 | 0.52 (0.48–0.57) | <0.001 | 171,739 | 0.57 (0.52–0.63) | <0.001 | |
| Medicare | 190,086 | 0.47 (0.43–0.52) | <0.001 | 170,981 | 0.64 (0.58–0.70) | <0.001 | |
| Other government | 10,148 | 1.07 (0.95–1.19) | 0.259 | 9220 | 1.19 (1.06–1.35) | 0.004 | |
| Geographical region | Northeast | 85,808 | 0.93 (0.89–0.97) | 0.001 | 76,393 | 0.84 (0.80–0.88) | <0.001 |
| Central | 113,636 | 0.60 (0.57–0.63) | <0.001 | 100,904 | 0.59 (0.56–0.62) | <0.001 | |
| South | 157,983 | 0.80 (0.77–0.83) | <0.001 | 134,256 | 0.70 (0.67–0.73) | <0.001 | |
| West | 65,079 | ‐ | ‐ | 57,585 | ‐ | ‐ | |
| Residence Type | Urban/metropolitan | 403,468 | ‐ | ‐ | 361,288 | ‐ | ‐ |
| Rural | 8557 | 0.79 (0.70–0.89) | <0.001 | 7850 | 0.91 (0.80–1.03) | 0.118 | |
| Income | < $38,000 | 67,777 | 1.13 (1.08–1.18) | <0.001 | 59,892 | 0.89 (0.83–0.95) | <0.001 |
| $38,000–$47,999 | 91,704 | 0.93 (0.90–0.97) | <0.001 | 80,784 | 0.88 (0.83–0.93) | <0.001 | |
| $48,000–$62,999 | 112,487 | 0.95 (0.92–0.99) | 0.01 | 98,766 | 0.92 (0.88–0.96) | <0.001 | |
| ≥$63,000 | 149,522 | ‐ | ‐ | 129,696 | ‐ | ‐ | |
| Percent of zip code with less than high school education | ≥21.0% | 64,156 | 1.30 (1.24–1.36) | <0.001 | 55,867 | 1.12 (1.04–1.19) | 0.001 |
| 13%–20.9% | 101,679 | 1.15 (1.10–1.20) | <0.001 | 88,986 | 1.12 (1.06–1.19) | <0.001 | |
| 7%–12.9% | 138,583 | 1.01 (0.97–1.05) | 0.549 | 122,190 | 1.03 (0.99–1.08) | 0.184 | |
| <7% | 117,325 | ‐ | ‐ | 102,095 | ‐ | ‐ | |
| Distance traveled to treatment | <25 miles | 302,313 | ‐ | ‐ | 52,422 | 1.18 (1.13–1.24) | <0.001 |
| 25–50 miles | 58,259 | 1.01 (0.97–1.06) | 0.633 | 51,956 | 1.04 (0.99–1.09) | 0.083 | |
| >50 miles | 61,353 | 1.19 (1.14–1.24) | <0.001 | 264,760 | ‐ | ‐ | |
| Year of diagnosis | 2010–2013 | 245,368 | ‐ | ‐ | 211,655 | ‐ | ‐ |
| 2014–2016 | 177,138 | 1.09 (1.06–1.13) | <0.001 | 157,483 | 1.11 (1.07–1.15) | <0.001 | |
Non‐Academic centers grouped as either community or comprehensive community centers.
3.2. Trends in treatment initiation time after diagnosis over time
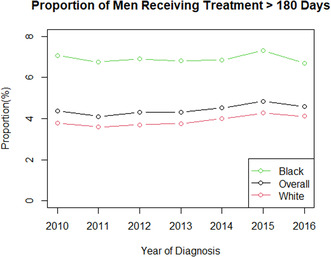
From 2010 to 2016, mean time from diagnosis to treatment initiation increased for all patients, regardless of race (Figure 1). Mean time from diagnosis to treatment initiation was consistently higher for Black men compared to White men. Median and interquartile range of number of days from diagnosis to treatment for the entire cohort is shown in Figure S2 with median time to treatment increasing from 60 days in 2010 to 66 days in 2016. The proportion of men with major treatment delay >180 days was higher for Black men compared to White men in every year from 2010 to 2016 (Figure 2). From 2010 to 2016, the proportion of Black patients experiencing major treatment delay decreased from 7.1% to 6.7%, while the proportion of White patients experiencing a major treatment delay increased from 3.8% to 4.1%. The number of patients included in this cohort treated per year is shown in Table S1.
FIGURE 1.
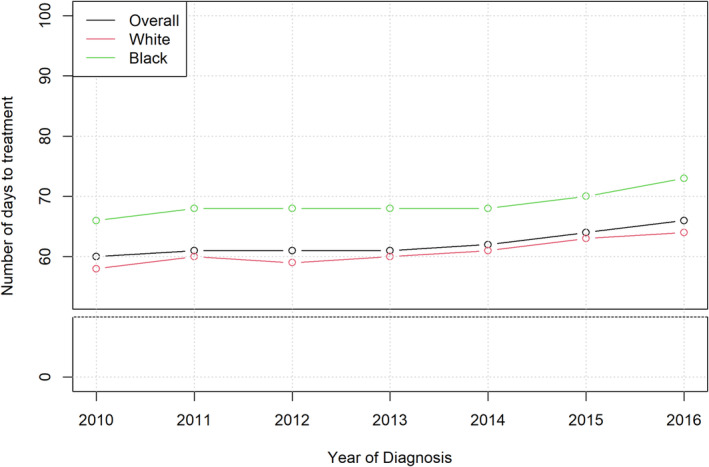
Mean number of days from prostate cancer diagnosis to treatment initiation by year (2010–2016) stratified by race.
FIGURE 2.
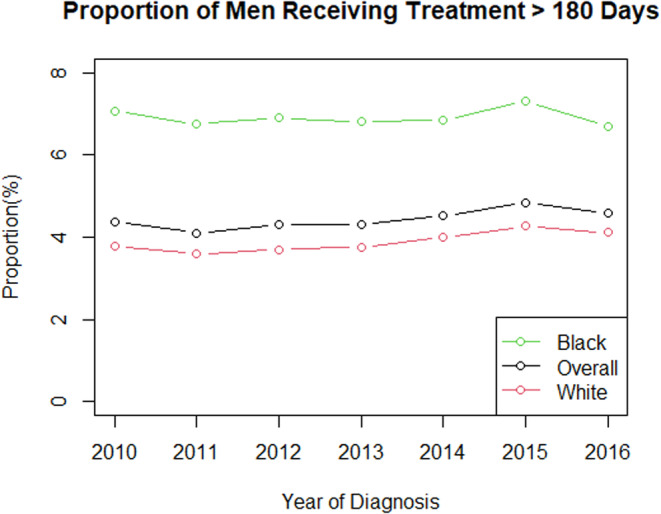
Proportion of patients with at least 180‐day delay from diagnosis to treatment initiation by year (2010–2016) stratified by race.
Within the entire cohort, the joinpoint between 2010–2013 and 2014–2016 was statistically significant for inflection between crude rates of patients experiencing >180‐day delay from diagnosis to treatment initiation (Figure S3). Additionally the joinpoint between 2010–2013 and 2014–2016 was significant for the subgroup of Black men (Figure S4) as well as for the subgroup of White men (Figure S5). Year‐over‐year, there was a significant trend for increasing time to treatment initiation for the entire cohort (trend p < 0.001). This trend remained significant among White men (p < 0.001), but not Black men (p = 0.98).
3.3. Interaction of insurance provider and ACA on associations with delays in treatment initiation
To further assess the impact of health insurance on timeliness of treatment during the study period before and after ACA was operationalized, we examined factors associated with major delay only in patients who had Medicare or private insurance. Within this subgroup, non‐White patients had significantly higher odds of major treatment delay on multivariable analysis, including those who were Black (OR 1.88, 95% CI 1.80–1.96, p < 0.001), Hispanic (OR 1.44, 95% CI 1.34–1.55, p < 0.001), or other/unidentified race/ethnicity (OR 1.27, 95% CI 1.17–1.37, p < 0.001). Furthermore, diagnosis after full implementation of the ACA in 2014 continued to be associated with higher odds of major treatment delay compared to diagnosis prior to 2014 (OR 1.12, 95% CI 1.09–1.16, p < 0.001). Complete univariable and multivariable associations of clinicodemographic factors with major treatment delays in subgroup with Medicare or private insurance shown in Table S2.
To further assess the impact of the implementation of the ACA, we analyzed factors associated with major delay for the subgroup of patients diagnosed in 2014–2016. On multivariable analysis, race and ethnicity continued to be significantly associated with odds of treatment delay. Compared to White patients, Black patients (OR 1.80, 95% CI 1.72–1.88, p < 0.001), Hispanic patients (OR 1.38, 95% CI 1.28–1.48, p < 0.001), and patients with other/unidentified race/ethnicity (OR 1.24, 95% CI 1.14–1.33, p < 0.001) had significantly greater odds of experiencing a treatment delay >180 days. There was no significant difference in odds of major delay between patients who were uninsured versus those with Medicaid coverage (OR 0.96, 95% CI 0.85–1.08, p = 0.473). Complete univariable and multivariable model of clinicodemographic factors associated with treatment delays in subgroup of men diagnosed after January 1, 2014 shown in Table S3.
4. DISCUSSION
In this large analysis of over 400,000 men with intermediate or high risk localized prostate cancer in the United States, we found that non‐White men have significantly greater odds of a >180‐day delay in time from diagnosis to definitive treatment initiation compared to White men. These results remained consistent even after accounting for insurance status or only assessing the years after the ACA was implemented. Furthermore, patients with Medicaid coverage experienced no significant difference in major treatment delays compared to patients without health insurance, while those with private insurance or Medicare coverage have significantly lower odds of major treatment delay. These disparities remained, but slightly decreased in the years after implementation of the ACA. Despite implementation of drastic health care reformative measures aimed at increasing access and reducing disparities, significant differences in disparate access to prostate cancer care remained for men eligible for curative management.
Interestingly, we found that delays in care increased significantly even after implementation of the ACA, perhaps most substantially for White men. It remains unclear why treatment initiation time may have increased over this period. It is plausible that the relatively rapid increase of 14 million newly insured patients through the ACA preceded expansion of provider or hospital capacity. Initial estimates projected 1.3 million additional Papanicolaou tests for cervical cancer screening and a need for more than 50,000 new primary care providers. 25 , 26 This mismatch, particularly in the first few years of the ACA, could at least in part explain why more men after 2014 experienced delay in accessing timely prostate cancer treatment in this analysis. Additionally, increase in time to treatment initiation could reflect need for patients to weigh the nuances of an increasingly varied selection of treatment options.
Nonetheless, the effect of the ACA on equitable healthcare access is unclear. While Medicaid expansion increased access to insurance coverage, the evidence for equitable access to care has been less promising. 27 For example, expansion of Medicaid in New York, prior to the ACA, did not impact racial disparities in utilization of surgical cancer services. 28 Furthermore, states with early expansion of Medicaid after the ACA saw a decrease in proportion of minority patients with private insurance. 29 Based on findings in this analysis, those patients who switch from private insurance to Medicaid may become at risk of significant delay in prostate cancer treatment initiation. Of note, many states had not expanded Medicaid access until after our study period, and 12 states have yet to expand Medicaid access as of 2022. Nonetheless, we found in this analysis no difference in timely access to care for patients with Medicaid even after the ACA became operationalized in 2014.
The public health impact of treatment initiation delays in prostate cancer is questionable. While modest differences in time to treatment initiation may not be impactful for favorable risk prostate cancer due to its insidious natural history, these delays can be significant for men with high‐risk or advanced prostate cancer at exceptionally high risk of metastatic dissemination. 30 Unfortunately, as the epidemiology of prostate cancer evolves in the United States, the impact of treatment delay could grow. Specifically, since the United States Preventive Services Task Force (USPSTF) recommended against routine PSA screening in 2012, the incidence of advanced prostate cancer has increased by 4% annually and incidence of de novo metastatic disease has increased by 6% annually. 31 Since then, the USPSTF revised recommendations in 2018 regarding PSA screening, specifically highlighting the importance of shared decision‐making with primary care providers, yet no significant difference in rates of screening have resulted. 32 If men continue to be diagnosed with more advanced prostate cancer, the risks of delays in treatment initiation may magnify disparate clinical outcomes in the United States. 33 , 34
Different healthcare systems have had divergent results in fulfilling pledges to reform and increase equity. Studies from the Veterans Health Administration (VHA) have shown no racial disparities in time from diagnosis to radical prostatectomy or mortality with definitive radiotherapy. 13 , 35 However, another study suggests potential over‐treatment of Black men with lower risk disease in parallel to undertreatment of higher risk disease at the VHA. 36 Concerningly, our results and others show that men managed at Commission on Cancer (CoC)‐accredited centers face significant variation in equitable care. Despite thorough use of quality metrics and data monitoring tools at participating facilities, 39% of facilities had higher rates of curative treatment for White men compared to Black men, but just 1% of facilities had the opposite. 37 Further, based on an analysis using Surveillance, Epidemiology, and End Results data, disparities in definitive treatment rates could be more profound amongst Black men with low compared to high income. 38 Beyond the standard of care, non‐White men are significantly underrepresented on clinical trials and have lower utilization of advanced radiotherapy techniques such as proton therapy. 39 , 40 , 41 , 42 , 43 , 44 , 45 The structural racism that Black patients face across healthcare settings perpetuates the disparate access and outcomes. 12 , 46 , 47 We show that timely access to definitive prostate cancer treatment remains a disproportionate albeit narrowing challenge for non‐White men even in the years after the ACA was implemented.
In addition to structural barriers, there are multiple individual‐level factors affecting men seeking prostate cancer care. Provider mistrust can limit timely access to definitive therapy. One study showed that Black men with newly diagnosed prostate cancer and men with fewer years of formal education had significantly higher levels of medical mistrust. 8 Additionally, when patient and provider race or ethnicity are concordant, patients report better experiences and are more likely to both visit and adhere to provider recommendations. 48 , 49 , 50 Unfortunately, Black and Hispanic providers are underrepresented relative to the general population with minimal increases in the last two decades. 51 , 52 Systematic efforts to increase trust in providers and the healthcare system will help men make complex, highly personalized decisions about management of their prostate cancer and may reduce population‐wide disparities in timely care. Apart from the ACA, structural changes in the health system such as hospital consolidation may have affected where patients seek care, which could in turn affect timely access to screening, diagnosis, and treatment. Additionally, increasing complexity of decision making with the development and use of MRI and genomic risk classifiers could prolong time from initial diagnosis to treatment for all men, while different treatment options could have different inherent delays (e.g., booking operating room time or planning radiotherapy treatment).
This study has multiple limitations to note. First, the NCDB is a hospital‐based cancer registry that captures only patients who are diagnosed or treated at CoC‐accredited facilities and may not generalize to the entire United States. However, given that the NCDB captures 70% of newly diagnosed cancer cases in the United States, we believe that this analysis is a representative reflection of general practice patterns. Second, given the retrospective design using a population‐based database, unmeasured confounders could be imbalanced and affect analyses. By adjusting our analyses for all relevant and available clinical and sociodemographic variables in the NCDB, we attempted to mitigate confounding. However, we cannot rule out that unmeasured patient‐level confounders, including performance status and level of social support, could explain treatment patterns. We were not able to account for performance status beyond what the Charlson–Deyo comorbidity score and social supports are not documented in the NCDB. Third, provider‐specific data, including age, gender, and race, are not available in the NCDB but would be impactful to further assess the effect of provider factors on racial and insurance disparities seen in this analysis. Additionally, changing insurance status or dual‐eligible Medicare‐Medicaid patients are not coded in the NCDB. As only a snapshot is provided, this study does not capture history of Medicaid enrollment, prior uninsured status, or if patients changed to a different insurance status after diagnosis. Fourth, granular prostate cancer characteristics aside from clinical T‐stage, documented PSA, and Gleason score, such as PSA kinetics or detailed histopathologic assessment (e.g., percent positive cores, perineural invasion, etc.), are not available in the NCDB. As such, we are unable to confirm if patients with substantial treatment delay may have been considering active surveillance. We attempted to control for this by excluding men with low‐risk disease and those documented as pursuing active surveillance. Fifth, while there is rigorous auditing of the database, it is possible that dates could be inaccurate. 20 , 21 Finally, with more routine use of advanced imaging and genetic testing, there could be increased delays in treatment initiation due to prolonged workup itself as well as increased complexity of decision making as patients are re‐categorized to different risk strata. These nuances are not captured in the NCDB.
5. CONCLUSION
In conclusion, this large registry analysis of men with localized, curable prostate cancer in the United States revealed striking racial and insurance disparities in timely access to treatment. Specifically, the impact of race and insurance status were independently associated with longer delays to treatment. Further, these disparities were unaffected by implementation of the ACA in 2014. In fact, in the years after implementation of ACA there were increased delays in treatment initiation for all men, regardless of race. Based on this data, the ACA and accompanying Medicaid expansion may not be modifying timely access to prostate cancer care, which is in line with studies in other cancer types. To increase equitable management of prostate cancer, additional work is needed, particularly exploring the differential effects for patients before and after Medicaid expansion in different states. Data on timely access to care in the years since 2016—particularly with more widespread implementation of Medicaid expansion—will be important to guide policy addressing new or continued barriers for patients. As the epidemiology of newly diagnosed prostate cancer in the United States continues to shift due to tempered screening and the COVID pandemic, further work will be needed to increase equity in prostate cancer care.
AUTHOR CONTRIBUTIONS
James R Janopaul‐Naylor: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); project administration (equal); visualization (equal); writing – original draft (lead); writing – review and editing (equal). Taylor J. Corriher: Writing – original draft (supporting); writing – review and editing (supporting). Jeffrey Switchenko: Data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); resources (equal); software (equal); validation (equal); visualization (equal); writing – review and editing (equal). Sheela Hanasoge: Writing – review and editing (supporting). Ashanda Esdaille: Investigation (supporting); methodology (supporting); writing – review and editing (supporting). Brandon A Mahal: Writing – review and editing (supporting). Christopher P Filson: Conceptualization (supporting); investigation (supporting); project administration (supporting); supervision (supporting); writing – original draft (supporting); writing – review and editing (supporting). Sagar A. Patel: Conceptualization (lead); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); project administration (equal); resources (equal); supervision (equal); validation (equal); writing – original draft (equal); writing – review and editing (equal).
FUNDING INFORMATION
Research reported in this publication was supported in part by the Biostatistics Shared Resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
CONFLICT OF INTEREST STATEMENT
None.
ETHICS STATEMENT
The study was conducted in accordance with local institutional review board policies, using de‐identified data from the NCDB.
PATIENT CONSENT STATEMENT
The requirement for patient consent was waived.
Supporting information
Figure S1.
Figure S2.
Figure S3.
Figure S4.
Figure S5.
Table S1.
Table S2.
Table S3.
Janopaul‐Naylor JR, Corriher TJ, Switchenko J, et al. Disparities in time to prostate cancer treatment initiation before and after the Affordable Care Act. Cancer Med. 2023;12:18258‐18268. doi: 10.1002/cam4.6419
DATA AVAILABILITY STATEMENT
Data are available through an application process to investigators associated with Commission on Cancer‐accredited cancer programs.
REFERENCES
- 1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7‐33. doi: 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- 2. DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69(3):211‐233. doi: 10.3322/CAAC.21555 [DOI] [PubMed] [Google Scholar]
- 3. Rebbeck TR. Prostate cancer genetics: variation by race, ethnicity, and geography. Semin Radiat Oncol. 2017;27(1):3‐10. doi: 10.1016/J.SEMRADONC.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kinlock BL, Parker LJ, Bowie JV, Howard DL, LaVeist TA, Thorpe RJ. High levels of medical mistrust are associated with low quality of life among black and white men with prostate cancer. Cancer Control. 2017;24(1):72‐77. doi: 10.1177/107327481702400112 [DOI] [PubMed] [Google Scholar]
- 5. Rogers CR, Rovito MJ, Hussein M, et al. Attitudes toward genomic testing and prostate cancer research among black men. Am J Prev Med. 2018;55(5):S103‐S111. doi: 10.1016/j.amepre.2018.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roberts LR, Wilson CM, Stiel L, Casiano CA, Montgomery SB. Prostate cancer screening among high‐risk black men. J Nurse Pract. 2018;14(9):677‐682. e2. doi: 10.1016/j.nurpra.2018.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pedersen VH, Armes J, Ream E. Perceptions of prostate cancer in black African and Black Caribbean men: a systematic review of the literature. Psychooncology. 2012;21(5):457‐468. doi: 10.1002/pon.2043 [DOI] [PubMed] [Google Scholar]
- 8. Halbert CH, Weathers B, Delmoor E, et al. Racial differences in medical mistrust among men diagnosed with prostate cancer. Cancer. 2009;115(11):2553‐2561. doi: 10.1002/CNCR.24249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Presley CJ, Raldow AC, Cramer LD, et al. A new approach to understanding racial disparities in prostate cancer treatment. J Geriatr Oncol. 2013;4(1):1‐8. doi: 10.1016/j.jgo.2012.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yamoah K, Johnson MH, Choeurng V, et al. Novel biomarker signature that may predict aggressive disease in African American men with prostate cancer. J Clin Oncol. 2015;33(25):2789‐2796. doi: 10.1200/JCO.2014.59.8912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dess RT, Hartman HE, Mahal BA, et al. Association of black race with prostate cancer‐specific and other‐cause mortality. JAMA Oncol. 2019;5(7):975‐983. doi: 10.1001/jamaoncol.2019.0826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deville C, Lee WR. Reconciling outcomes for black men with prostate cancer within and outside the veterans health administration. Cancer. 2021;127(3):342‐344. doi: 10.1002/CNCR.33225 [DOI] [PubMed] [Google Scholar]
- 13. McKay RR, Sarkar RR, Kumar A, et al. Outcomes of black men with prostate cancer treated with radiation therapy in the veterans health administration. Cancer. 2021;127(3):403‐411. doi: 10.1002/cncr.33224 [DOI] [PubMed] [Google Scholar]
- 14. Stokes WA, Hendrix LH, Royce TJ, et al. Racial differences in time from prostate cancer diagnosis to treatment initiation: a population‐based study. Cancer. 2013;119(13):2486‐2493. doi: 10.1002/CNCR.27975 [DOI] [PubMed] [Google Scholar]
- 15. Zhao J, Mao Z, Fedewa SA, et al. The Affordable Care Act and access to care across the cancer control continuum: a review at 10 years. CA Cancer J Clin. 2020;70(3):165‐181. doi: 10.3322/caac.21604 [DOI] [PubMed] [Google Scholar]
- 16. Jayakrishnan T, Bakalov V, Callander NS, Sadashiv S, Wagner R, Ailawadhi S. Impact of the affordable care act on timeliness to treatment for patients with multiple myeloma. Anticancer Res. 2020;40(10):5727‐5734. doi: 10.21873/ANTICANRES.14587 [DOI] [PubMed] [Google Scholar]
- 17. Takvorian SU, Oganisian A, Mamtani R, et al. Association of Medicaid Expansion under the Affordable Care act with insurance status, cancer stage, and timely treatment among patients with breast, colon, and lung cancer. JAMA Netw Open. 2020;3(2):e1921653. doi: 10.1001/JAMANETWORKOPEN.2019.21653 [DOI] [PubMed] [Google Scholar]
- 18. Adamson BJS, Cohen AB, Gross CP, et al. Aca medicaid expansion association with racial disparity reductions in timely cancer treatment. Am J Manag Care. 2021;27(7):274‐281. doi: 10.37765/AJMC.2021.88700 [DOI] [PubMed] [Google Scholar]
- 19. Bhandari N, Shi Y, Jung K. Patient experience of provider refusal of medicaid coverage and its implications. J Health Care Poor Underserved. 2016;27(2):479‐494. doi: 10.1353/hpu.2016.0096 [DOI] [PubMed] [Google Scholar]
- 20. Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15(3):683‐690. doi: 10.1245/s10434-007-9747-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boffa DJ, Rosen JE, Mallin K, et al. Using The National Cancer Database for outcomes research a review. JAMA Oncol. 2017;3(12):1722‐1728. doi: 10.1001/jamaoncol.2016.6905 [DOI] [PubMed] [Google Scholar]
- 22. Bilimoria KY, Bentrem DJ, Stewart AK, Winchester DP, Ko CY. Comparison of commission on cancer‐approved and ‐nonapproved hospitals in the United States: Implications For Studies That Use The National Cancer Data Base. J Clin Oncol. 2009;27(25):4177‐4181. doi: 10.1200/JCO.2008.21.7018 [DOI] [PubMed] [Google Scholar]
- 23. Cone EB, Marchese M, Paciotti M, et al. Assessment of time‐to‐treatment initiation and survival in a cohort of patients with common cancers. JAMA Netw Open. 2020;3(12):e2030072. doi: 10.1001/jamanetworkopen.2020.30072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Van Den Bergh RCN, Albertsen PC, Bangma CH, et al. Timing of curative treatment for prostate cancer: a systematic review. Eur Urol. 2013;64(2):204‐215. doi: 10.1016/j.eururo.2013.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Levy AR, Bruen BK, Ku L. Health care reform and women's insurance coverage for breast and cervical cancer screening. Prev Chronic Dis. 2012;9(10):E159. doi: 10.5888/pcd9.120069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Petterson SM, Liaw WR, Phillips RL, Rabin DL, Meyers DS, Bazemore AW. Projecting US primary care physician workforce needs: 2010‐2025. Ann Fam Med. 2012;10(6):503‐509. doi: 10.1370/afm.1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moss HA, Wu J, Kaplan SJ, Zafar SY. The Affordable Care Act's Medicaid expansion and impact along the cancer‐care continuum: a systematic review. J Natl Cancer Inst. 2020;112(8):779‐791. doi: 10.1093/JNCI/DJAA043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Al‐Refaie WB, Zheng C, Jindal M, et al. Did pre‐Affordable Care Act Medicaid expansion increase access to surgical cancer care? J Am Coll Surg. 2017;224(4):662‐669. doi: 10.1016/J.JAMCOLLSURG.2016.12.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Agarwal A, Katz AJ, Chen RC. The impact of the Affordable Care Act on disparities in private and Medicaid insurance coverage among patients under 65 with newly diagnosed cancer. Int J Radiat Oncol Biol Phys. 2019;105(1):25‐30. doi: 10.1016/J.IJROBP.2019.05.033 [DOI] [PubMed] [Google Scholar]
- 30. Hamdy FC, Donovan JL, Lane JA, et al. 10‐year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375(15):1415‐1424. doi: 10.1056/nejmoa1606220 [DOI] [PubMed] [Google Scholar]
- 31. Fleshner K, Carlsson SV, Roobol MJ. The effect of the USPSTF PSA screening recommendation on prostate cancer incidence patterns in the USA. Nat Rev Urol. 2017;14(1):26‐37. doi: 10.1038/NRUROL.2016.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jiang C, Fedewa SA, Wen Y, Jemal A, Han X. Shared decision making and prostate‐specific antigen based prostate cancer screening following the 2018 update of USPSTF screening guideline. Prostate Cancer Prostatic Dis. 2021;24(1):77‐80. doi: 10.1038/s41391-020-0227-1 [DOI] [PubMed] [Google Scholar]
- 33. Abern MR, Aronson WJ, Terris MK, et al. Delayed radical prostatectomy for intermediate‐risk prostate cancer is associated with biochemical recurrence: possible implications for active surveillance from the SEARCH database. Prostate. 2013;73(4):409‐417. doi: 10.1002/pros.22582 [DOI] [PubMed] [Google Scholar]
- 34. Siegel DA, O'Neil ME, Richards TB, Dowling NF, Weir HK. Prostate cancer incidence and survival, by stage and race/ethnicity—United States, 2001–2017. MMWR Morb Mortal Wkly Rep. 2020;69(41):1473‐1480. doi: 10.15585/mmwr.mm6941a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bañez LL, Terris MK, Aronson WJ, et al. Race and time from diagnosis to radical prostatectomy: does equal access mean equal timely access to the operating room?–results from the SEARCH database. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1208‐1212. doi: 10.1158/1055-9965.EPI-08-0502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rude T, Walter D, Ciprut S, et al. Interaction between race and prostate cancer treatment benefit in the Veterans Health Administration. Cancer. 2021;127(21):3985‐3990. doi: 10.1002/cncr.33643 [DOI] [PubMed] [Google Scholar]
- 37. Friedlander DF, Trinh QD, Krasnova A, et al. Racial disparity in delivering definitive therapy for intermediate/high‐risk localized prostate cancer: the impact of facility features and socioeconomic characteristics. Eur Urol. 2018;73(3):445‐451. doi: 10.1016/j.eururo.2017.07.023 [DOI] [PubMed] [Google Scholar]
- 38. Ziehr DR, Mahal BA, Aizer AA, et al. Income inequality and treatment of African American men with high‐risk prostate cancer. Urol Oncol. 2015;33(1):18.e7‐18.e13. doi: 10.1016/j.urolonc.2014.09.005 [DOI] [PubMed] [Google Scholar]
- 39. Ahaghotu C, Tyler R, Sartor O. African American participation in oncology clinical trials ‐ focus on prostate cancer: implications, barriers, and potential solutions. Clin Genitourin Cancer. 2016;14(2):105‐116. doi: 10.1016/j.clgc.2015.12.003 [DOI] [PubMed] [Google Scholar]
- 40. Wang W‐J, Ramsey SD, Bennette CS, Bansal A. Racial disparities in access to prostate cancer clinical trials: a county‐level analysis. JNCI Cancer Spectr. 2022;6(1):1‐7. doi: 10.1093/jncics/pkab093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gardner U, McClelland S, Deville C. Disparities in the utilization of radiation therapy for prostate cancer in the United States: a comprehensive review. Adv Radiat Oncol. 2022;7(4):100943. doi: 10.1016/j.adro.2022.100943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Woodhouse KD, Hwang WT, Vapiwala N, et al. Sociodemographic disparities in the utilization of proton therapy for prostate cancer at an urban academic center. Adv Radiat Oncol. 2017;2(2):132‐139. doi: 10.1016/J.ADRO.2017.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Janopaul‐Naylor JR, Liu T, Zhou B, et al. Longitudinal changes in U.S. parameters of neurovascular bundles suggest mechanism for radiation‐induced erectile dysfunction. Adv Radiat Oncol. 2022;7(5):100946. doi: 10.1016/j.adro.2022.100946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Von Eyben FE, Kiljunen T, Kangasmaki A, Kairemo K, Von Eyben R, Joensuu T. Radiotherapy boost for the dominant intraprostatic cancer lesion ‐ a systematic review and meta‐analysis. Clin Genitourin Cancer. 2016;14(3):189‐197. doi: 10.1016/j.clgc.2015.12.005 [DOI] [PubMed] [Google Scholar]
- 45. Zhou J, Yang X, Chang CW, et al. Dosimetric uncertainties in dominant intraprostatic lesion simultaneous boost using intensity modulated proton therapy. Adv Radiat Oncol. 2021;7(1):100826. doi: 10.1016/j.adro.2021.100826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dee EC, Pierce LJ, Winkfield KM, Lam MB. In pursuit of equity in cancer care: Moving Beyond The Affordable Care Act. Cancer. 2022;128(18):3278‐3283. doi: 10.1002/CNCR.34346 [DOI] [PubMed] [Google Scholar]
- 47. Cuevas AG, O'Brien K, Saha S. African American experiences in healthcare: “I always feel like I'm getting skipped over.”. Health Psychol. 2016;35(9):987‐995. doi: 10.1037/hea0000368 [DOI] [PubMed] [Google Scholar]
- 48. Ma A, Sanchez A, Ma M. The impact of patient‐provider race/ethnicity concordance on provider visits: updated evidence from the medical expenditure panel survey. J Racial Ethn Health Disparities. 2019;6(5):1011‐1020. doi: 10.1007/s40615-019-00602-y [DOI] [PubMed] [Google Scholar]
- 49. Takeshita J, Wang S, Loren AW, et al. Association of racial/ethnic and gender concordance between patients and physicians with patient experience ratings. JAMA Netw Open. 2020;3(11):e2024583. doi: 10.1001/jamanetworkopen.2020.24583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Traylor AH, Schmittdiel JA, Uratsu CS, Mangione CM, Subramanian U. Adherence to cardiovascular disease medications: does patient‐provider race/ethnicity and language concordance matter? J Gen Intern Med. 2010;25(11):1172‐1177. doi: 10.1007/s11606-010-1424-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Janopaul‐Naylor JR, Roberts SE, Shu HK, et al. Race, ethnicity, and sex among senior faculty in radiation oncology from 2000 to 2019. JAMA Netw Open. 2022;5(1):e2142720. doi: 10.1001/jamanetworkopen.2021.42720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kamran SC, Winkfield KM, Reede JY, Vapiwala N. Intersectional analysis of U.S. medical faculty diversity over four decades. N Engl J Med. 2022;386(14):1363‐1371. doi: 10.1056/nejmsr2114909 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1.
Figure S2.
Figure S3.
Figure S4.
Figure S5.
Table S1.
Table S2.
Table S3.
Data Availability Statement
Data are available through an application process to investigators associated with Commission on Cancer‐accredited cancer programs.


