Abstract
The development of immune checkpoint inhibitors (ICI) has transformed the treatment of advanced stage cutaneous melanoma; however, most trials did not include patients with conjunctival melanoma. Herein we describe a patient with recurrent conjunctival melanoma who developed locally advanced, BRAF-negative melanoma in her nasal cavity and extensive, metabolically active, bilateral lymphadenopathy in her thorax. Her nasal mass measured 4.3 × 1.7 cm and was determined to be unresectable. She was treated with four cycles of combination ipilimumab and nivolumab therapy followed by maintenance nivolumab. She experienced a dramatic treatment response with reduction in the size of her nasal mass to 3.0 × 1.1 cm and complete resolution of her adenopathy. She then underwent complete surgical resection of her residual mass (approximately 75% of her original tumor size) and remains melanoma-free at one year of follow-up. Given the underlying genetic similarities of conjunctival melanoma to cutaneous melanoma, providers should consider the use of neoadjuvant ICI for patients with locally advanced or limited metastatic disease.
Precis:
Here we describe a patient with metastatic conjunctival melanoma involving the nasal cavity and multiple lymph nodes who experienced a dramatic treatment response to immune checkpoint inhibitors, ultimately allowing complete surgical resection. She is now melanoma free one-year following her surgery.
Introduction
Conjunctival melanoma is a rare but aggressive ocular malignancy with a reported incidence of 0.2–0.6 per million people. Originating from melanocytes in the conjunctival basal epithelium, conjunctival melanoma is a distinct entity from intraocular tumors such as uveal melanoma and is more closely related to cutaneous and mucosal melanoma1. The mainstay treatment for a localized conjunctival disease is surgical excision of the tumor followed by cryotherapy to the surrounding tissue. A no-touch technique is employed during excision of conjunctival melanoma such that direct manipulation of the tumor is avoided to prevent tumor cells from seeding into a new area2. Despite adequate treatment of the primary tumor, 60% of patients with conjunctival melanoma experience local recurrence3. Patients with conjunctival melanoma often develop dissemination of disease to the head and neck lymph nodes and other body sites with a reported 10-year overall metastasis rate of 19%3. To date, there is no standardized treatment algorithm for metastatic conjunctival melanoma4.
Like cutaneous melanoma, conjunctival melanoma often harbors mutations in proto-oncogenes within the mitogen-activated protein kinase (MAPK) and phosphoinositide 3-kinase (PI3K) signaling cascades. The most common driver mutations occur within the small GTPase, NRAS, and its downstream effector, BRAF, as well as within the tumor suppressor protein NF15. These mutations independently result in constitutively active signaling leading to uncontrolled cell growth and are the focus in the development of targeted therapies5,6. One recent case-report showed successful neoadjuvant treatment with combined BRAF/MEK inhibition in a patient with BRAF/V600E mutated conjunctival melanoma7. However, BRAF mutations are only found in 35% of conjunctival melanoma6,8,9. Patients with unresectable or metastatic disease without BRAF mutations are typically treated with immune checkpoint inhibitors. Immune checkpoint inhibitors (ICIs) have revolutionized the prognosis of patients with metastatic cutaneous melanoma. These therapies work by blocking cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and/or programmed death protein-1 (PD-1) thereby promoting tumor recognition10,11. The successful use of both single-agent and combination ICI has been demonstrated in the treatment of recurrent, locally-advanced, and metastatic CM9,12–16.
ICIs have been shown to be successful in the management of unresectable locally advanced and metastatic conjunctival melanoma9,12. While these therapies have not yet been demonstrated in the neoadjuvant setting for conjunctival melanoma, they have be used for other tumor types, including cutaneous melanoma and non-small cell lung cancer (NSCLC)17,18 to reduce disease burden and increase the likelihood of complete surgical resection. Here, we present our experience employing ICI in a patient with unresectable conjunctival melanoma involving the nasal cavity and lymph nodes which resulted in dramatic disease reduction and enabled tumor resection. All collection and evaluation of protected patient health information were HIPPA compliant. All research adhered to the tenets of the Declaration of Helsinki.
Case
A 60-year-old female with no past ocular history presented with a lesion on her left cornea and adjacent conjunctiva inferiorly and nasally. It measured 5.5 × 4.5 mm (Figure 1A–C). Excisional biopsy by a no touch technique followed by cryotherapy revealed AJCC stage IIA, superficially invasive melanoma. However, three years later she developed recurrent disease. She underwent re-excision at that time but unfortunately experienced multiple episodes of recurrence. Exenteration of the left eye was offered, but the patient refused. Additional treatments performed included re-excision, cryotherapy, and external beam radiotherapy. Ten years after her original diagnosis she began having multiple weekly nosebleeds. She presented for evaluation and was found to have a soft-tissue mass in her left nasal cavity measuring 4.3 × 1.7 cm (Figure 2A). Biopsy of the nasal cavity mass demonstrated BRAF-negative metastatic melanoma. PD1/PDL1 status was unfortunately not evaluated in this patient. PET scan at that time also showed extensive, metabolically active, mediastinal and bilateral hilar lymphadenopathy (AJCC TNM stage: T3dN1M1). While the lymphadenopathy was concerning for metastatic disease, given the distribution, granulomatous inflammation was also in the differential. Biopsy of these lymph nodes was not pursued as the nasal mass was felt to be unresectable and the only option for treatment of the nasal mass was systemic therapy. She was then started on first-line ipilimumab 1mg/kg and nivolumab 3mg/kg (dose as per Checkmate 511 due to concern for side effects with ipilimumab 3mg/kg)19. She did not experience any treatment-related adverse events and her epistaxis steadily improved. After completion of 4 cycles, she had approximately 25% reduction in the size of the nasal cavity mass to 3.0 × 1.1 cm (Figure 2B). She continued maintenance nivolumab. A PET scan 16 months into treatment showed near complete resolution of the mediastinal and hilar hypermetabolic lymphadenopathy, however the nasal mass persisted. She continued to have no evidence of disease outside the nasal cavity on scans for the next 8 months. Given the persistence of the nasal mass and lack of systemic disease, she underwent an endoscopic left medial maxillectomy. Pathology findings at that time were consistent with metastatic melanoma. Repeat CT scan after 7 months showed post-surgical changes without evidence of recurrence (Figure 2C). She passed away due to an unrelated cause but did not have any evidence of melanoma recurrence at one year follow-up.
Figure 1.
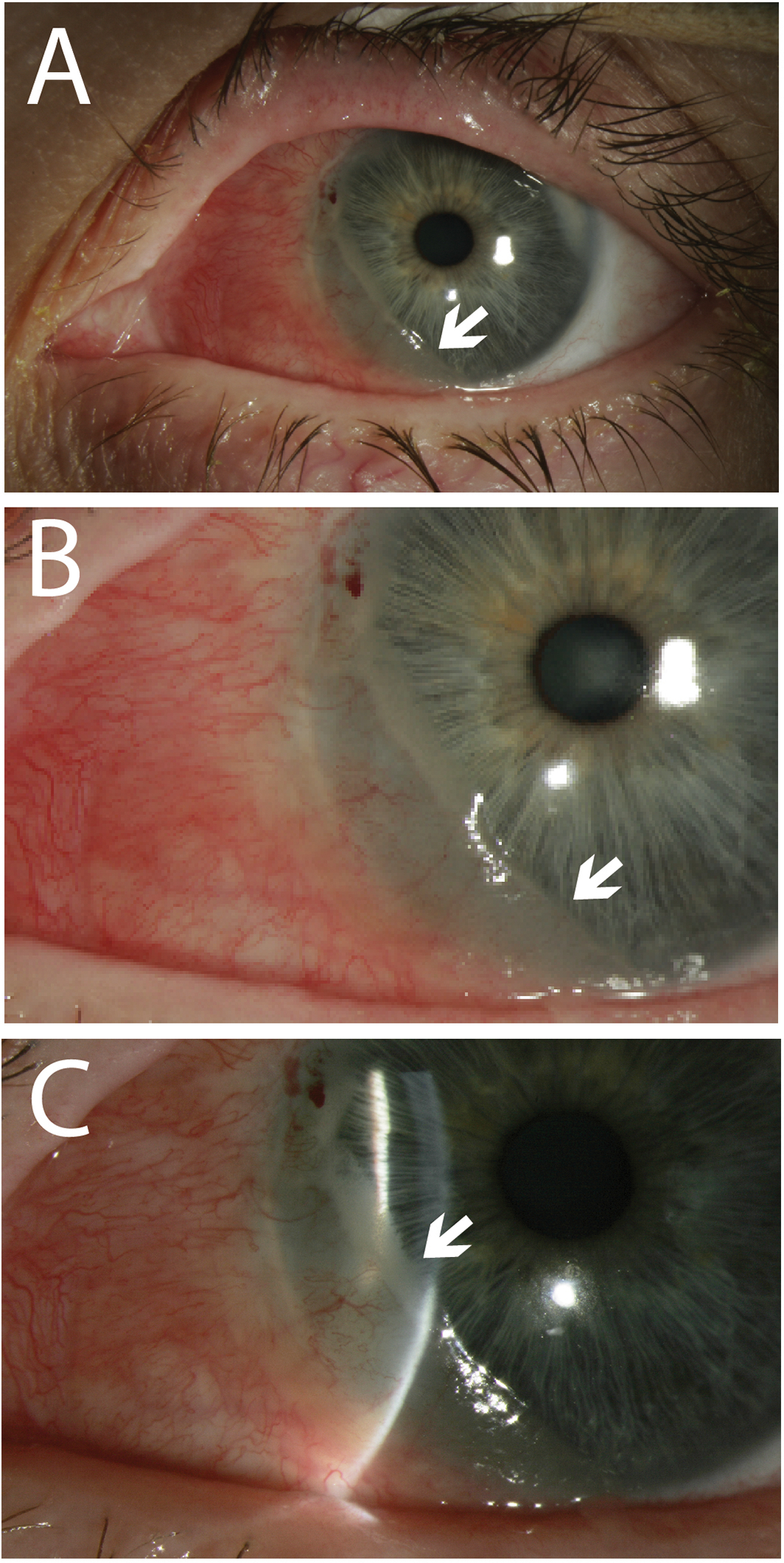
Slit lamp photos of the left eye showing recurrent amelanotic conjunctival melanoma extending from the 6 o’clock meridians with intrinsic vascularity (white arrows) (A, B). The conjunctival melanoma extends over the cornea nasally (white arrow) (C).
Figure 2.
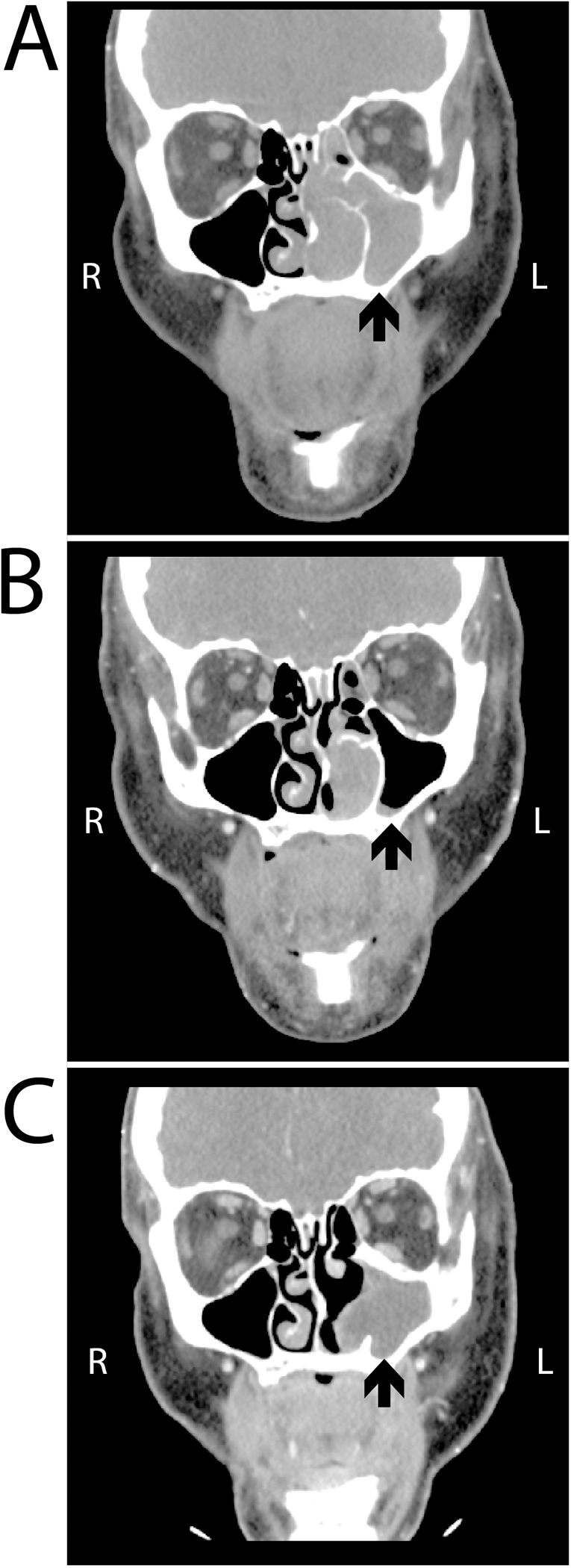
CT images upon identification of a mass in the left nasal, ethmoid and maxillary sinuses (A), after four cycles of combination ipilimumab/nivolumab, showing significant decrease in the size (B), and at most recent follow-up imaging approximately 7 months post-op, demonstrating no recurrence but evidence of a polyp in the maxillary sinus (C). Black arrows point out the mass in each image.
Discussion
Multiple randomized controlled trials, including Checkmate-238, EORTC 1325, and IMMUNED, have evaluated the utility of ICIs in the adjuvant setting for cutaneous melanoma20–22. In these studies, recurrence-free survival ranged from 70–75% at one year and 63–70% at two years demonstrating that these agents can have lasting benefit23. Though the above trials did not include patients with conjunctival melanoma, the efficacy of ICIs in locally advanced and metastatic disease has been demonstrated in a handful of cases in the literature.
Two independent retrospective case series described the successful use of anti-PD1 therapy in the treatment of both locally advanced and metastatic conjunctival melanoma10,12. Of the five patients included in the series by Sagiv et. al, four patients had a complete response and one patient had stable disease after 6 months of anti-PD1 therapy9. Similarly, two patients with metastatic conjunctival melanoma who were treated with either sequential or combination ICI had no evidence of disease two and three years after completion of therapy, respectively12.
In another report, Chang et al. described a patient with orbitally invasive conjunctival melanoma and liver metastasis who had disease regression following combination ICI16. Combination ICI for 5 months in a different patient with locally advanced and metastatic conjunctival melanoma also showed dramatic reduction in tumor size and preservation of visual function13. While there are reports of ICI efficacy as an adjuvant or salvage therapy, to our knowledge there is only one report of its use in the neoadjuvant setting for conjunctival melanoma. In attempts to avoid orbital exenteration and disfigurement, Hong et. al, treated a patient with locally advanced conjunctival melanoma with 12 months of pembrolizumab. This patient experienced near total clinical resolution of their conjunctival melanoma with evidence of complete pathologic response, defined as the absence of residual viable malignant cells13.
There are, however, studies that demonstrate efficacy of neoadjuvant ICI in advanced cutaneous melanoma17. For instance, an early study by Huang et. al, examined the usefulness of a single pre-operative dose of pembrolizumab in 27 patients with stage III and IV cutaneous melanoma. Major pathologic response was observed in 8/27 (29.6%) patients suggesting that even a short course of pre-operative ICI could provide clinical benefit24. These findings were further examined in randomized phase II study (SWOG 1801, NCT03698019)25. This trial demonstrated a significant improvement in event-free survival in patients with high-risk resectable melanoma who received neoadjuvant as opposed to adjuvant immunotherapy (HR: 0.59, 95% confidence interval (CI): 0.40–0.86)25. Other prospective studies examined the use of combination versus single-agent ICI in the neoadjuvant setting with high reported response rates for combination therapy (reported pathologic complete response (pCR), i.e. absence of residual viable malignant cells) ranging from 30–45%)26,27. The OpACIN trial (NCT02437279) also showed an increase in tumor infiltrating lymphocytes (TILs) following neoadjuvant treatment which is suggested to prolong relapse-free survival27. Unfortunately, TILs were not evaluated in the pathologic analysis of this patient’s tumor before or after ICI. While no patients with conjunctival melanoma were included in these trials, the underlying genetic similarities and mutation profile between conjunctival melanoma and cutaneous melanoma suggest that patients with metastatic conjunctival melanoma may also benefit from neoadjuvant ICI therapy.
In the present case we describe one such patient that had a remarkable response to systemic immunotherapy. Her response converted her disease from unresectable to resectable and she underwent successful surgical resection. She remained melanoma-free at her 1-year post-operative follow-up.
Financial support:
This study was funded by the Medical Scientist Training Program (MSTP) T32 (GM007863) and the University of Michigan Rackham Merit Fellowship to JJW and Richard N and Marilyn K Witham professorship to HD.
Footnotes
COI: None of the authors of this study have any conflicts of interest to report.
REFERENCES
- 1.Williams BK, Di Nicola M. Ocular Oncology-Primary and Metastatic Malignancies. Med Clin North Am. 2021;105(3):531–550. doi: 10.1016/j.mcna.2021.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Shields JA, Shields CL, De Potter P. Surgical management of circumscribed conjunctival melanomas. Ophthalmic Plast Reconstr Surg. 1998;14(3):208–215. doi: 10.1097/00002341-199805000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Shields CL, Markowitz JS, Belinsky I, et al. Conjunctival melanoma: outcomes based on tumor origin in 382 consecutive cases. Ophthalmology. 2011;118(2):389–95.e1–2. doi: 10.1016/j.ophtha.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 4.Nahon-Estève S, Bertolotto C, Picard-Gauci A, et al. Small but Challenging Conjunctival Melanoma: New Insights, Paradigms and Future Perspectives. Cancers. 2021;13(22):5691. doi: 10.3390/cancers13225691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lally SE, Milman T, Orloff M, et al. Mutational Landscape and Outcomes of Conjunctival Melanoma in 101 Patients. Ophthalmology. 2022;129(6):679–693. doi: 10.1016/j.ophtha.2022.01.016. [DOI] [PubMed] [Google Scholar]
- 6.Brouwer NJ, Verdijk RM, Heegaard S, Marinkovic M, Esmaeli B, Jager MJ. Conjunctival melanoma: New insights in tumour genetics and immunology, leading to new therapeutic options. Prog Retin Eye Res. 2022;86:100971. doi: 10.1016/j.preteyeres.2021.100971. [DOI] [PubMed] [Google Scholar]
- 7.Demirci H, Demirci FY, Ciftci S, et al. Integrative Exome and Transcriptome Analysis of Conjunctival Melanoma and Its Potential Application for Personalized Therapy. JAMA Ophthalmol. 2019;137(12):1444–1448. doi: 10.1001/jamaophthalmol.2019.4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larsen A-C, Dahl C, Dahmcke CM, et al. BRAF mutations in conjunctival melanoma: investigation of incidence, clinicopathological features, prognosis and paired premalignant lesions. Acta Ophthalmol. 2016;94(5):463–470. doi: 10.1111/aos.13007. [DOI] [PubMed] [Google Scholar]
- 9.Sagiv O, Thakar SD, Kandl TJ, et al. Immunotherapy With Programmed Cell Death 1 Inhibitors for 5 Patients With Conjunctival Melanoma. JAMA Ophthalmol. 2018;136(11):1236–1241. doi: 10.1001/jamaophthalmol.2018.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hodi FS, Chiarion-Sileni V, Gonzalez R, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19(11):1480–1492. doi: 10.1016/S1470-2045(18)30700-9. [DOI] [PubMed] [Google Scholar]
- 11.Lu JE, Chang JR, Berry JL, In GK, Zhang-Nunes S. Clinical Update on Checkpoint Inhibitor Therapy for Conjunctival and Eyelid Melanoma. Int Ophthalmol Clin. 2020;60(2):77–89. doi: 10.1097/IIO.0000000000000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finger PT, Pavlick AC. Checkpoint inhibition immunotherapy for advanced local and systemic conjunctival melanoma: a clinical case series. J Immunother Cancer. 2019;7(1):83. doi: 10.1186/s40425-019-0555-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong BY-B, Ford JR, Glitza IC, et al. Immune Checkpoint Inhibitor Therapy as an Eye-Preserving Treatment for Locally Advanced Conjunctival Melanoma. Ophthalmic Plast Reconstr Surg. 2021;37(1):e9–e13. doi: 10.1097/IOP.0000000000001700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kini A, Fu R, Compton C, Miller DM, Ramasubramanian A. Pembrolizumab for Recurrent Conjunctival Melanoma. JAMA Ophthalmol. 2017;135(8):891–892. doi: 10.1001/jamaophthalmol.2017.2279. [DOI] [PubMed] [Google Scholar]
- 15.Pinto Torres S, André T, Gouveia E, Costa L, Passos MJ. Systemic Treatment of Metastatic Conjunctival Melanoma. Case Rep Oncol Med. 2017;2017:4623964. doi: 10.1155/2017/4623964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang M, Lally SE, Dalvin LA, Orloff MM, Shields CL. Conjunctival melanoma with orbital invasion and liver metastasis managed with systemic immune checkpoint inhibitor therapy. Indian J Ophthalmol. 2019;67(12):2071–2073. doi: 10.4103/ijo.IJO_663_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Witt RG, Erstad DJ, Wargo JA. Neoadjuvant therapy for melanoma: rationale for neoadjuvant therapy and pivotal clinical trials. Ther Adv Med Oncol. 2022;14:17588359221083052. doi: 10.1177/17588359221083052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forde PM, Spicer J, Lu S, et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N Engl J Med. 2022;386(21):1973–1985. doi: 10.1056/NEJMoa2202170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lebbe C, Meyer N, Mortier L, et al. Evaluation of Two Dosing Regimens for Nivolumab in Combination With Ipilimumab in Patients With Advanced Melanoma: Results From the Phase IIIb/IV CheckMate 511 Trial. Journal of Clinical Oncology. 2019;37(11):867–875. doi: 10.1200/JCO.18.01998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ascierto PA, Del Vecchio M, Mandalá M, et al. Adjuvant nivolumab versus ipilimumab in resected stage IIIB-C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2020;21(11):1465–1477. doi: 10.1016/S1470-2045(20)30494-0. [DOI] [PubMed] [Google Scholar]
- 21.Eggermont AMM, Blank CU, Mandalá M, et al. Longer Follow-Up Confirms Recurrence-Free Survival Benefit of Adjuvant Pembrolizumab in High-Risk Stage III Melanoma: Updated Results From the EORTC 1325-MG/KEYNOTE-054 Trial. Journal of Clinical Oncology. 2020;38(33):3925–3936. doi: 10.1200/JCO.20.02110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zimmer L, Livingstone E, Hassel JC, et al. Adjuvant nivolumab plus ipilimumab or nivolumab monotherapy versus placebo in patients with resected stage IV melanoma with no evidence of disease (IMMUNED): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;395(10236):1558–1568. doi: 10.1016/S0140-6736(20)30417-7. [DOI] [PubMed] [Google Scholar]
- 23.Dimitriou F, Long GV, Menzies AM. Novel adjuvant options for cutaneous melanoma. Annals of Oncology. 2021;32(7):854–865. doi: 10.1016/j.annonc.2021.03.198. [DOI] [PubMed] [Google Scholar]
- 24.Huang AC, Orlowski RJ, Xu X, et al. A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nat Med. 2019;25(3):454–461. doi: 10.1038/s41591-019-0357-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel S, Othus M, Prieto V, et al. Neoadjuvant versus adjuvant pembrolizumab for resected Stage III-IV melanoma (SWOG 1801).
- 26.Amaria RN, Reddy SM, Tawbi HA, et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat Med. 2018;24(11):1649–1654. doi: 10.1038/s41591-018-0197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blank CU, Rozeman EA, Fanchi LF, et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat Med. 2018;24(11):1655–1661. doi: 10.1038/s41591-018-0198-0. [DOI] [PubMed] [Google Scholar]


