Abstract
Objective.
To examine fetal growth outcomes from agricultural worker households.
Methods.
Using Arizona 2006–2013 birth certificates with parental occupation, we identified N=623,185 live births by agricultural household status. Logistic regression models estimated adjusted Odds Ratios (aOR) for macrosomia (>4,000 grams), postterm birth (>41 weeks), low birth weight (LBW <2,500 grams), pre-term birth (PTB <37 weeks), large-for-gestational-age (LGA), small-for-gestational-age (SGA), and 5 min-APGAR (<7).
Results.
Newborns of agricultural households (n=6,371) had a higher risk of macrosomia (aOR 1.15, 95% CI: 1.05, 1.26), LGA (aOR 1.12, 95% CI: 1.03, 1.22), postterm birth (aOR 1.20, 95% CI: 1.09, 1.33), and low 5-min APGAR (aOR 1.39, 95% CI: 1.07, 1.81), whereas LBW (aOR: 0.85, 95% CI: 0.76, 0.96) and PTB (aOR: 0.82, 95% CI: 0.74, 0.92) were inversely related.
Conclusions.
Having an agriculture working parent increased the likelihood of fetal overgrowth and low APGAR.
Keywords: agricultural workers, farmworkers, fetal growth, pregnancy outcomes, vital statistics
Introduction
Women comprise approximately a quarter of the agricultural workforce.1 As the number of reproductive-aged women in farm labor continues to rise nationally, potential occupational exposures to reproductive toxins and teratogens may also increase. Female workers are particularly vulnerable to chronic, low-dose pesticide exposures that have the potential to cause hormone disruption.2 Further, evidence from animal models demonstrates that pesticides can induce epigenetic alterations in sperm.3 In addition to the increased risk from direct exposure, agricultural workers and their families are susceptible to chronic exposures via pesticide residues in dust, soil, or air, as well as track-in and cross-contamination from clothing.4 Agricultural laborers may also be subjected to ergonomic injuries5 and exposure to excessive heat6 from physically demanding work. Thus, due to these potential chronic exposures to pesticides, heat, and injuries, agricultural workers and/or their pregnant household members are particularly susceptible to adverse fetal outcomes.7 Few studies have assessed fetal growth outcomes among agricultural workers, perhaps due to the difficulty of studying this vulnerable population. (i.e., seasonal, migratory, temporary visas (H2A), other precarious legal status, and low wages).
Abnormal fetal growth, including both restriction and overgrowth, are major risk factors for mortality, morbidity, and lifelong metabolic diseases for mothers and children.8 Fetal growth is modulated by the maternal endocrine system and altered by nutrition, genetics, and environmental factors that can cause placental and metabolic dysfunction. While some variation in fetal development can be attributed to genetics and environmental interactions, most of the literature to date has focused on intrauterine growth restriction (IUGR), low birth weight (LBW), preterm birth (PTB), and small for gestational age (SGA).
Unlike fetal growth restriction, abnormalities of overgrowth, such as macrosomia (≥4,000 grams) and large for gestational age (LGA) (≥90th percentile for gestational age), are attributed to increased glucose in mothers that induce hyperinsulinemia, resulting in adipogenesis and increased oxygen demands upon the placenta.9 The effect of maternal overnutrition and excessive gestational weight gain increase the likelihood of women delivering LGA and macrosomic newborns.10 The short and long-term adverse effects of fetal overgrowth have been well-documented for high birthweight11, LGA12,13, macrosomia14–16, and postterm or prolonged births17–19, and include birth injury and mortality, neurodevelopmental deficits, and obesity-related health complications throughout the lifespan. The most established indicators of excessive fetal weight at delivery are macrosomia and LGA, accounting for approximately 9–10% of all births in the United States (U.S.).8 Although occupational exposures to chemical and physical agents are well-known to dysregulate fetal growth during pregnancy, few studies have examined these risk factors.20 For instance, two studies have evaluated variation in LBW by maternal occupation, and determined that the greatest risk was among manual laborers, such as farming and agricultural workers21,22, but there is a paucity of literature on gestational overgrowth and maternal employment.23
In this study, we assessed whether neonates born to agricultural worker households, corresponding to both mother and father farm workers, were at higher risk for macrosomia, postterm birth, LBW, PTB, LGA, SGA, and a poor 5 min-APGAR score derived from Arizona birth certificate data.
Methods
Study population
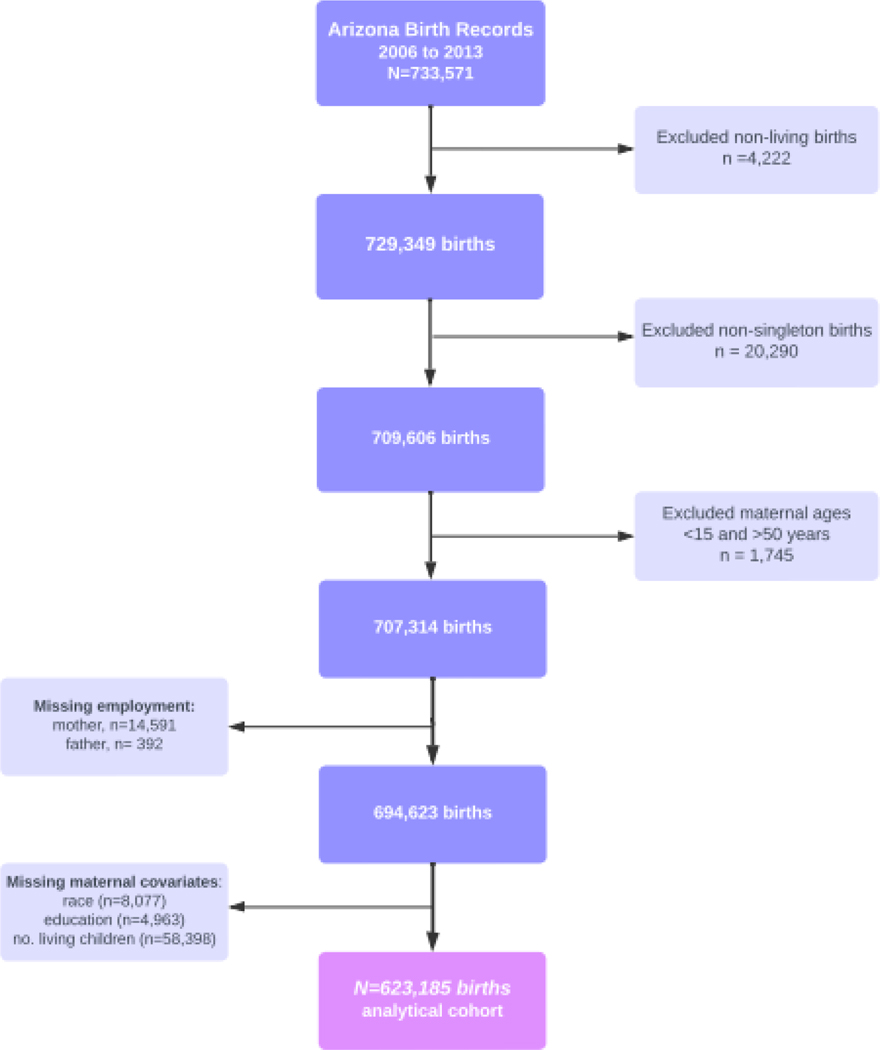
Birth certificate data were obtained from the Bureau of Vital Statistics, Arizona Department of Health Services and collected from January 1, 2006, to December 31, 2013. After restricting the population of 733,571 births to singleton, live-birth deliveries from individuals aged 15 to 50 years old, 707,314 birth records were retained. We excluded 14,591 births with missing occupational data, and 71,438 missing covariate data, resulting in data from 623,185 deliveries (Figure 1). This study was approved by the Human Subject Protection Program of the University of Arizona.
Figure 1:
Flowchart of live-births among agricultural worker households in Arizona (2006–2013).
Exposure
We utilized birth certificates from the period 2006 to 2013, since those years included occupational data for both mothers and fathers. Occupational data were open field, self-reported parental occupations in either English or Spanish, with agricultural workers classified using information on industry and/or occupation. Agricultural workers included: farmers, farmworkers (trabajador agrícola), field laborers (obrero del campo), field equipment operators, foremen/supervisors, growers, green house/nursery workers, harvesters, horticulturists, irrigation workers, cultivators (cultivador), packers (empacadora), sorters, graders, as well as pesticide handlers (mixers, loaders, and sprayers). As pesticide exposures could vary significantly within farm employment categories, we omitted from the definition occupations as florists, gardening/landscaping, office administration, pest control/termite/exterminators, pesticide manufacturers, produce inspectors, livestock, or dairy workers. Agricultural occupation exclusions were adopted from the U.S. Bureau of Labor Statistics, group 45–2000.24 Agricultural households were classified as having either the mother, father, or both reporting an occupation as agriculture or farming. Births missing both parental occupations were excluded from this analysis (n=14,983).
Birth outcomes
A newborn weighing less than 2,500 grams (g) was defined as low birth weight (LBW). For macrosomia, we used a graded scale to reflect increasing clinical morbidity at higher values. Grade 1 macrosomia was classified as more than 4,000g, grade 2 at 4,500g, and grade 3 at 5,000g. Large for gestational age (LGA) was defined as birth weight greater than the U.S. sex-specific 90th, 95th, and 97th percentile of weight for each week of gestation. Postterm birth (PTB) was defined as a delivery at 41 weeks and beyond, and a delivery occurring at ≤37 weeks was classified as a preterm birth (PTB). Gestational age estimate (weeks) and birth weight (grams) were numeric fields (and not checkboxes). APGAR is a standard assessment used to evaluate the physical fitness of neonates after 5-minutes of being born, and a score ≤7 was defined as low.
Statistical analysis
Covariates were selected a priori. The final models were adjusted for the following covariates: maternal age at delivery (years), maternal education (≤high school diploma, high school diploma/GED, college, and professional degree), race/ethnicity (White, Latina/Hispanic, Black, Other), previous number of living children, and having any diabetes (yes/no).
Baseline characteristics were presented as means (standard deviation [SD]) for continuous variables and numbers (percentage) for categorical variables. Multivariate logistic regression was used to calculate odds ratios (OR) and 95% confidence intervals (95% CI) to estimate associations of agricultural household exposure status with adverse neonatal outcomes. Exposure status was determined by the number and type of parents in farm work (mother, father, both). Adjusted ORs (aOR), which separately incorporated information on mother and father employment status, were also calculated.
We conducted several sensitivity analyses. First, since smoking and gestational weight gain were not included in the primary analyses due to the high levels of missingness in birth records (>10%), we evaluated smoking and gestational weight gain as potential confounders in the subset of births with these data. Second, due to concern about the potential impact of pre-existing chronic hypertension, we excluded these individuals from the model. Third, we re-defined agricultural workers to include primarily field workers and farmworkers, to exclude workers who were primarily administrative and/or with less physical tasks (e.g., horticulturists, supervisors/foremen, and growers). For these analyses, models were evaluated with reduced data subsets. A fourth sensitivity analysis examined the potential effect modification by fetal sex or maternal age (≤ 21 years old versus ≥ 35 years old). Interaction terms were added to the final models and the criterion of alpha of p < 0.05 for evidence of interaction between the variables using a likelihood ratio test was applied. Statistical analyses were conducted using RStudio version 1.1.453.
Results
Of the 623,185 births, a total of 6,371 were identified as an agricultural household. Most agricultural families had a father in farm work (N=6,143) rather than a mother in farm work (N=373), with 145 newborns having both a mother and a father employed in agriculture.
Table 1 describes these live births by parental employment in agricultural work and demographic characteristics of the mother and the household. Approximately 75% of the births from agricultural homes in Arizona came from mothers residing in Maricopa, Pinal, and Yuma counties, the most intensive regions of agriculture in Arizona. Agricultural households were more likely to have mothers who self-identified as Latina/Hispanic (76.3%) or foreign born (54.6%), and who had not completed a high school degree (41.3%). Compared to non-agricultural households, farm working women were more likely to be enrolled in Medicaid/Arizona Health Care Cost Containment System (AHCCCS) (72.2% vs. 51.6%) and to have low numbers of prenatal visits (22.7% vs. 8.9%).
Table 1:
Characteristics of households of live-births in Arizona from 2006 to 2013 by agricultural or farming household status using complete case analysis.
| Non-Agricultural Household n=616,814 | Agricultural Household n=6,371 | Total N=623,185 | |
|---|---|---|---|
| Maternal Age (years) | |||
| Mean (±SD) | 27.2 (±6.0) | 26.8 (±6.2) | 27.2 (±6.0) |
| Race/Ethnicity | |||
| White (Non-Hispanic) | 277,976 (45.1%) | 1,224 (19.2%) | 279,200 (44.8%) |
| Latina/Hispanic | 247,707 (40.2%) | 4,858 (76.3%) | 252,565 (40.5%) |
| Native American | 39,523 (6.4%) | 210 (3.3%) | 39,733 (6.4%) |
| Other | 51,608 (8.3%) | 79 (1.2%) | 91,420 (8.3%) |
| Maternal Education | |||
| < High School Degree | 135,184 (21.9%) | 2,629 (41.3%) | 137,813 (22.1%) |
| High School Degree | 190,182 (30.8%) | 2,144 (33.7%) | 192,326 (30.9%) |
| Some college | 147,119 (23.9%) | 934 (14.7%) | 148,053 (23.8%) |
| > College degree | 144,329 (23.4%) | 664 (10.4%) | 144,993 (23.3%) |
| No. living children | |||
| None | 254,312 (41.2%) | 2,013 (31.6%) | 256,325 (41.1%) |
| 1–2 | 321,989 (51.6%) | 3,762 (59.0%) | 325,751 (52.3%) |
| ≥3 | 40,513 (6.6%) | 596 (9.4%) | 41,109 (6.6%) |
| Insurance | |||
| Private | 268,132 (43.5%) | 1,269 (19.9%) | 269,401 (43.2%) |
| AHCCCS (Medicaid) | 318,087 (51.6%) | 4,601 (72.2%) | 322,688 (51.8%) |
| Other | 30,585 (4.9%) | 501 (7.9%) | 31,086 (5.0%) |
| Missing | 10 | 0 | 10 |
| No. of Prenatal Visits | |||
| High ≥ 11 | 349,703 (91.2%) | 2,543 (77.2%) | 352,246 (91.0%) |
| Medium 6–10 | 22,307 (5.8%) | 452 (13.7%) | 22,759 (5.9%) |
| Low ≤ 5 | 2,525 (0.7%) | 64 (1.9%) | 2,593 (0.7%) |
| None | 9,067 (2.4%) | 235 (7.1%) | 9,302 (2.4%) |
| Missing | 232,208 | 3,077 | 236,285 |
| Mother foreign born | 161,661 (26.2%) | 3,481 (54.6%) | 165,142 (26.5%) |
| Missing | 10 | 0 | 10 |
| Married | 336,620 (54.9%) | 3,544 (55.7%) | 340,164 (54.9%) |
| Missing | 3,189 | 3 | 3,192 |
| AZ County | |||
| Maricopa | 390,973 (63.4%) | 2,239 (35.1%) | 393,212 (63.2%) |
| Pinal | 31,859 (5.2%) | 764 (12.1%) | 32,623 (5.2%) |
| Yuma | 21,158 (3.4%) | 1,717 (27.0%) | 22,875 (3.7%) |
| Other | 172,769 (28.0%) | 1,650 (25.8%) | 174,419 (27.9%) |
| Missing | 55 | 1 | 56 |
Note: Analytic cohort using complete case analysis of covariate data: maternal age, education, ethnicity, and number of living children.
AHCCCS, Arizona Health Care Cost Containment System
Table 2 shows that among these farmworker households, 21.6% of neonates experienced an adverse outcome (macrosomia, postterm birth, PTB, SGA, LGA, or poor APGAR score). Neonates had a mean birth weight of 3,320.4 grams, and a mean gestational age of 39 weeks. The overall incidence of macrosomia (>4,000 g) was higher among agricultural households (8.4%) than non-agricultural households (7.5%). For pregnancies defined as grade 2 macrosomia (>4,500 g), the incidence was also higher for agricultural households (1.4% vs 1.0%) and similarly twice as high for grade 3 (>5,000 g) in these households as well. LGA incidence was also higher in agricultural households (above 90th percentile, 9.5% vs 8.5%) and this trend persisted for the 95th and 97th cutoffs. Of note, there was little difference between the households by whether the mother experienced a metabolic disorder during pregnancy (diabetes and/or gestational hypertensive disorder.
Table 2:
Characteristics of neonates in Arizona from 2006 to 2013 by agricultural or farming household status using complete case analysis.
| Non-Agricultural Household n=616,814 | Agricultural Household n=6,371 | Total N=623,185 | |
|---|---|---|---|
| Neonatal Sex | |||
| Male | 315,544 (51.2%) | 3,154 (49.5%) | 318,761 (51.2%) |
| Female | 301,264 (48.8%) | 3,217 (50.5%) | 304,418 (48.8%) |
| Missing | 6 | 0 | 6 |
| Gestational age (weeks) | |||
| Mean (±SD) | 38.7 (±1.6) | 38.8 (±1.6) | 38.7 (±1.6) |
| Missing | 17,346 | 168 | 17,514 |
| Child Birthweight (g) | |||
| Mean (±SD) | 3320.2 (±519.0) | 3342.1 (±519.4) | 3320.4 (±519.0) |
| Missing | 128 | 0 | 128 |
| Preterm Birth <37 weeks | 39,211 (6.5%) | 352 (5.7%) | 39,563 (6.5%) |
| Missing | 17,346 | 168 | 17,514 |
| Low Birth Weight <2500 g | 31,981 (5.2%) | 286 (4.5%) | 32,267 (5.2%) |
| Missing | 91 | 0 | 91 |
| Macrosomia >4,000 g | 46,543 (7.5%) | 537 (8.4%) | 47,080 (7.6%) |
| Missing | 91 | 0 | 91 |
| Postterm birth >41 weeks | 36,741 (6.1%) | 410 (6.6%) | 37,151 (6.1%) |
| Missing | 17,346 | 168 | 17,514 |
| APGAR score at 5 min <7 | 4,334 (0.7%) | 58 (0.9%) | 4,392 (0.7%) |
| Missing | 470 | 10 | 480 |
| Maternal Diabetes | 25,057 (4.1%) | 282 (4.4%) | 25,339 (4.1%) |
| Missing | 1 | 0 | 1 |
| Pre-existing hypertension | 4,774 (0.8%) | 47 (0.7%) | 4,821 (0.8%) |
| Missing | 1 | 0 | 1 |
| Eclampsia | 4,353 (0.7%) | 39 (0.6%) | 4,392 (0.7%) |
| Missing | 1 | 0 | 1 |
| Pregnancy Induced Hypertension | 23,844 (3.9%) | 165 (2.6%) | 24,009 (3.9%) |
| Missing | 1 | 0 | 1 |
Note: Analytic cohort using complete case analysis of covariate data: maternal age, education, ethnicity, and number of living children.
Neonatal outcomes
Table 3 shows estimates of the magnitude of associations between agriculture worker household status and various neonatal outcomes. Newborns born to a household where at least one parent worked in agriculture significantly increased the odds of grade 1 macrosomia (aOR 1.15, 95% CI: 1.05, 1.26), and grade 2 macrosomia (aOR 1.38, 95% CI: 1.11, 1.71). While the effect estimate continued to increase for grade 3 macrosomia (aOR 1.48, 95% CI: 0.79, 2.77), this association was no longer statistically significant, likely due to the small numbers of births from agricultural households (n=10). Similarly, parental employment in agriculture increased the likelihood of LGA at 90th, 95th, and 97th percentiles. An elevated risk of LGA was found for ≥90th percentile (OR 1.12, 95% CI: 1.03, 1.22), with steadily increased risk for ≥95th percentile (OR 1.26, 95% CI: 1.13, 1.41), and ≥97th percentile (OR 1.35, 95% CI: 1.17, 1.55). The estimated effect on LGA remained relatively unchanged even after adjustment. Agricultural household status was also associated with increased risk of postterm birth (aOR 1.20, 95% CI: 1.09, 1.33), and a low APGAR score at 5 minutes <7 (aOR 1.39, 95% CI: 1.07, 1.81). Conversely, LBW and PTB were inversely associated with agricultural household status (aOR: 0.85, 95% CI: 0.76, 0.96 and aOR: 0.82, 95% CI: 0.74, 0.92, respectively), and consistent with our findings that presence of a farmworker in the household is associated with larger birth size.
Table 3:
Odds ratios for associations between agricultural employment in household and adverse pregnancy outcomes in the analytical cohort (N=623,185)
| Non-Agricultural Household n = 616,814 | Agricultural Household n = 6,371 | Odds Ratio (95% CI) | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| N | events | % | events | % | Crude | Adjusteda | |
| PTB (<37 weeks) | 605,671 | 36,741 | 6.5 | 352 | 5.7 | 0.86 (0.77, 0.96) | 0.82 (0.74, 0.92) |
| LBW (≤2,500 g) | 623,094 | 31,981 | 5.2 | 286 | 4.5 | 0.86 (0.76, 0.97) | 0.85 (0.76, 0.96) |
| SGA (3rd percentile) | 623,184 | 11,480 | 1.9 | 106 | 1.7 | 0.89 (0.74, 1.08) | 0.93 (0.77, 1.13) |
| SGA (5th percentile) | 623,184 | 21,381 | 3.5 | 205 | 3.2 | 0.93 (0.81, 1.06) | 0.93 (0.81, 1.06) |
| SGA (10th percentile) | 623,184 | 48,415 | 7.8 | 444 | 7.0 | 0.88 (0.80, 0.97) | 0.91 (0.82, 1.00) |
| Postterm birth (≥41 weeks) | 605,671 | 36,741 | 6.1 | 410 | 6.6 | 1.08 (0.98, 1.20) | 1.20 (1.09, 1.33) |
| Grade 1 Macrosomia (≥4,000g) | 623,094 | 46,543 | 7.5 | 537 | 8.4 | 1.13 (1.03, 1.23) | 1.15 (1.05, 1.26) |
| Grade 2 Macrosomia (≥4,500g) | 623,094 | 5,983 | 1.0 | 87 | 1.4 | 1.41 (1.14, 1.75) | 1.38 (1.11, 1.71) |
| Grade 3 Macrosomia (≥5,000g) | 623,094 | 570 | 0.1 | 10 | 0.2 | 1.70 (0.91, 3.18) | 1.48 (0.79, 2.77) |
| LGA (90th percentile) | 623,184 | 52,698 | 8.5 | 605 | 9.5 | 1.12 (1.03, 1.22) | 1.14 (1.04, 1.24) |
| LGA (95th percentile) | 623,184 | 26,163 | 4.2 | 338 | 5.3 | 1.26 (1.13, 1.41) | 1.27 (1.13, 1.41) |
| LGA (97th percentile) | 623,184 | 15,146 | 2.5 | 209 | 3.3 | 1.35 (1.17, 1.55) | 1.33 (1.16, 1.53) |
| APGAR score at 5 min (≤7) | 622,705 | 4,334 | 0.7 | 58 | 0.9 | 1.30 (1.00, 1.69) | 1.39 (1.07, 1.81) |
CI, confidence interval; LBW, Low birth weight; PTB, Preterm birth; LGA, Large for gestational age; SGA, Small for gestational age
Adjusted for maternal age, education, ethnicity, number of living children, and diabetes.
Table 4 shows the effect when the agricultural worker was the mother or father. Firstly, the majority of the agricultural homes had a father as a farmworker. Overall, the findings remained statistically significant, except for low APGAR score. Associations with low APGAR score were much stronger for neonates born to mothers who were agricultural workers versus fathers (aOR 3.72 for mothers, aOR 1.34 for fathers), although these ORs were statistically significant for both fathers and mothers. Associations with postterm birth were similar by whether mothers or fathers were agricultural workers (aOR 1.39 and 1.20, respectively).
Table 4:
Odds ratios for associations between agricultural household and birth outcomes, stratified by maternal or paternal work status in the analytical cohort (N=623,185)
| Mother Farmworker n=373 | Odds Ratio (95% CI) | Father Farmworker n=6,143 | Odds Ratio (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| N | events | % | Crude | Adjusteda | events | % | Crude | Adjusteda | |
| PTB (<37 weeks) | 605,671 | 30 | 8.2 | 0.86 (0.77, 0.96) | 0.82 (0.74, 0.92) | 334 | 5.6 | 0.85 (0.76, 0.94) | 0.81 (0.72, 0.91) |
| LBW (≤2,500 g) | 623,094 | 16 | 4.3 | 0.82 (0.50, 1.35) | 0.79 (0.48, 1.31) | 281 | 4.6 | 0.88 (0.78, 0.99) | 0.87 (0.77, 0.98) |
| SGA (3rd percentile) | 623,184 | 6 | 1.6 | 0.86 (0.39, 1.93) | 0.91 (0.41, 2.04) | 100 | 1.6 | 0.87 (0.72, 1.06) | 0.91 (0.75, 1.11) |
| SGA (5th percentile) | 623,184 | 9 | 2.4 | 0.69 (0.36, 1.33) | 0.73 (0.38, 1.42) | 197 | 3.2 | 0.92 (0.80, 1.06) | 0.96 (0.83, 1.11) |
| SGA (10th percentile) | 623,184 | 16 | 4.3 | 0.53 (0.32, 0.87) | 0.56 (0.34, 0.92) | 434 | 7.1 | 0.89 (0.81, 0.98) | 0.92 (0.83, 1.01) |
| Postterm birth (≥41 weeks) | 605,671 | 27 | 7.4 | 1.23 (0.83, 1.82) | 1.39 (0.94, 2.07) | 391 | 6.5 | 1.07 (0.97, 1.19) | 1.20 (1.08, 1.33) |
| Grade 1 Macrosomia (≥4,000g) | 623,094 | 27 | 7.2 | 0.95 (0.65, 1.41) | 0.89 (0.60, 1.32) | 518 | 8.4 | 1.13 (1.03, 1.23) | 1.16 (1.06, 1.27) |
| Grade 2 Macrosomia (≥4,500g) | 623,094 | 4 | 1.1 | 1.10 (0.41, 2.95) | 0.93 (0.35, 2.50) | 85 | 1.4 | 1.43 (1.15, 1.78) | 1.40 (1.13, 1.74) |
| Grade 3 Macrosomia (≥5,000g) | 623,094 | 0 | — | — | — | 10 | 0.2 | 1.76 (0.94, 3.30) | 1.55 (0.82, 2.90) |
| LGA (90th percentile) | 623,184 | 30 | 8.0 | 0.94 (0.64, 1.36) | 0.86 (0.59, 1.25) | 583 | 9.5 | 1.12 (1.03, 1.22) | 1.14 (1.04, 1.24) |
| LGA (95th percentile) | 623,184 | 15 | 4.0 | 0.94 (0.56, 1.58) | 0.84 (0.50, 1.42) | 326 | 5.3 | 1.27 (1.13, 1.42) | 1.27 (1.14, 1.42) |
| LGA (97th percentile) | 623,184 | 11 | 2.9 | 1.20 (0.66, 2.19) | 1.05 (0.57, 1.92) | 201 | 3.3 | 1.34 (1.17, 1.55) | 1.34 (1.16, 1.54) |
| APGAR score at 5 min (≤7) | 622,705 | 9 | 2.4 | 3.52 (1.82, 6.83) | 3.72 (1.91, 7.22) | 54 | 0.9 | 1.25 (0.96, 1.64) | 1.34 (1.02, 1.76) |
CI, confidence interval; LBW, Low birth weight; PTB, Preterm birth; LGA, Large for gestational age; SGA, Small for gestational age
Sensitivity Analyses
Sensitivity analyses that included gestational weight gain and smoking during pregnancy as covariates did not change the magnitude of the effect estimates by more than 10% (see Table, Supplemental Digital Content 1). Similarly, when we excluded infants with a mother with chronic hypertension, the estimates of effects remained consistent (see Table, Supplemental Digital Content 2). Likewise, there was no change in effect measures after exclusion of births from parents who worked as farmers, growers, horticulturalists, and/or supervisors (n=372). Stratification by fetal sex showed a higher risk of LGA above the 90th percentile among boys (OR: 1.24, 95% CI: 1.10, 1.40) compared to girls (OR: 1.04, 95% CI: 0.92, 1.17) with agricultural household exposure (p-interaction=0.04), although we did not observe any interaction for the other outcomes.
Discussion
In this large, population-based study of Arizona birth certificate records with parental occupational data, newborns with parents working in agriculture had greater odds of macrosomia, LGA, postterm birth, and low APGAR score (≤7). We observed inverse associations with and/or did not observe positive associations with LBW, PTB, or SGA.
Fetal overgrowth risks
Abnormalities of fetal overgrowth are attributed to increased glucose in mothers that induce hyperinsulinemia, resulting in adipogenesis and increased oxygen demands upon the placenta.9 The effect of maternal overnutrition and excessive gestational weight gain increase the likelihood of women delivering LGA and macrosomic newborns. Moreover, fetal overnutrition leads to overgrowth, complicating labor and delivery for mothers, increasing the risk of neonatal mortality or birth trauma like respiratory distress and shoulder dystocia.8 Additionally, increased birth weight and conditions of overgrowth are associated with risk of obesity later on in childhood and adulthood.8 Women of reproductive age with co-morbidities like diabetes, hypertension, obesity and have had a prior macrosomic or postterm birth are at an increased risk of fetal overgrowth during gestation.
Agricultural occupation and birth outcomes
Results from environmental health studies utilizing maternal self-report on agricultural occupation and birth indicators, birth weight and gestational age (GA), have been inconsistent. We found positive associations with conditions of excessive fetal overgrowth and agricultural household status. Overall, the prevalence of macrosomia in the general US population is 7.8% of live births, similar to findings in our non-farmworker population.25 Given the adverse short-term and long-term outcomes for both the mother and child associated with macrosomia, some researchers have hypothesized the cost-effectiveness of labor induction with suspected macrosomia to prevent burdensome complications.26 In our study, agricultural workers were 15%, 38%, and 48% more at risk of delivering a macrosomic neonate at ≥4,000g, ≥4,500g, and ≥5,000g, respectively, than their non-farm work counterparts. In a similar manner, agricultural workers had higher odds ranging from 14 to 33% for delivering LGA neonates delivered at percentiles 90th, 95th, and 97th. Our observed associations are similar to findings from a study of Polish mothers who reported working in the fields during 1st and 2nd trimesters; these mothers delivered larger newborns compared to non-farming mothers. This positive association is also supported by findings among neonates born to agricultural workers in Denmark.27,28 Our findings that longer GA is also attributed to parental agricultural work aligns with a study of greenhouse workers.29 Similarly, in a study of paternal occupational exposure, higher birth weight of neonates are observed among father agricultural workers compared to non-agricultural fathers.30 In addition, studies of associations of fetal growth restriction indicators (i.e. LBW, PTB, SGA) among father agricultural workers show inverse associations.22,31 These findings suggest a plausible role for epigenetic influences of father’s sperm, although in our study it may also represent environmental exposures from track-in into the home.3,4
We also found lower APGAR scores for neonates born into agricultural households, which concurs with prior studies showing associations with exposure to manual labor.32 APGAR scores are an important marker for neurodevelopment in childhood and adulthood, and future research of workers exposed to agrochemicals will help characterize the risks posed to the general population of pregnant individuals. Preexisting literature suggests that the primary contributors of fetal overgrowth during gestation are related to exposures to heavy job strain and environmental endocrine disruptors, such as pesticides.
Agricultural Job Strain
While the effect of agricultural job tasks and working conditions (i.e., prolonged standing, repetitive lifting, long shifts) on birth outcomes have not been fully examined, one study found that mothers with heavy lifting/high job strain, increased their risk of delivering a LGA neonate compared to mothers with low physical/low stress jobs.33 Heavy manual labor is a known risk factor for pregnancy complications, and hypothesized to alter maternal plasma volume and blood flow to the placenta.7 Additionally, occupational stress has been linked to increased glucose levels that can give rise to diabetes, a risk factor for macrosomia and LGA.34 Working women, but in particular Latinas, are vulnerable to unsafe working conditions due to their low social status typically associated with their immigration status. Further, agricultural workers may experience language and cultural barriers that impede access to quality prenatal care. Metabolic effects of heavy job labor and stress may also be exacerbated by exposure to pesticides, which have been positively associated with type 2 diabetes among agricultural workers.35
Prenatal pesticide exposure
Pesticides have various toxicological mechanisms due to their different active ingredients, making it particularly challenging to examine their effects on fetal growth.36 However, many pesticides are endocrine disruptors that can impair hormonal function during pregnancy, as well as growth and development of the fetus.2 Chronic exposure to pesticides during pregnancy may cause deleterious harm; as pesticides are metabolized by the mother to cross the placental barrier.37 Although we do not have information specifically on which pesticides these farmworkers were exposed to during their work, we can presume that at least some of these exposures were organophosphate pesticides, carbamate pesticides, and/or pyrethroid pesticides, since OPs and pyrethroids were two of the most commonly used classes of insecticides during this decade.
OPs may regulate fetal growth through disruption of choline and acetylcholine receptors, which are critical components of fetal growth and development.38 In our study, positive findings of fetal overgrowth and farmworker household are consistent with studies measuring biomarkers of prenatal OPs exposures in longitudinal birth cohort studies. In an agricultural community in California, non-significant trends in birth weight with maternal urinary dialkyl-phosphate metabolites (DAPs, a general metabolite for most OPs) were observed, with the highest increase of 52 grams for every 10-fold increase in diethyl-phosphates metabolites (DEPs).39 However, a pooled analysis (N=1,169) from four U.S. birth cohorts found no significant association between OPs and birth weight.40 Conversely, the lesser studied carbamate pesticides, which have a similar mode of actions as OPs, have been inversely associated with birth weight.41,42
Pyrethroids may disrupt fetal growth and functioning through disruption of voltage gated sodium and ion channels, which are critical for neurodevelopment.43 They may also disrupt critical signaling processes in labor and delivery, as the voltage-gated ion channels targeted by pyrethroids play critical roles in initiating and maintain labor.44 In Asia, pyrethroids have been implicated45,46, but inverse associations have also been noted.27,47–49 Studies of other types of pesticides and pesticide mixtures are largely contradictory.20,27,45,50 More epidemiological research is needed to determine the co-exposures of chemicals that may impair fetal growth among pregnant populations.
Previous research from U.S. studies which examined GA have reported mostly inverse associations with pesticides, namely, OPs.39,51–53 Pyrethroids were implicated for longer GA and decreased risk of SGA and PTB in a Chinese population.45 Evidence to-date is largely speculative, which may be the result of GA acting as a mediator in the relationship between fetal size and prenatal environmental exposures. Studies of agricultural pesticides and adverse birth outcomes have likely yielded discordant results due to the disparate study designs, detection of parent compounds/metabolites, covariates, timing of exposures, PON1 enzymatic activity, dynamic employment statuses, background community-level exposures, and geographic variations in climate and pesticide levels around homes during pregnancy.
Strengths
Data from Arizona birth certificates over an 8-year period allowed investigation of potential risks of pregnancy complications from agricultural households. This allowed us to identify mothers and their partners who worked in agriculture and, therefore, to examine household exposures. Few studies have considered parental employment status and risk of adverse pregnancy outcomes.7,33 Next, this study could be considered a type of mixtures analysis. Traditionally, study design methods were limited to one environmental exposure to one pregnancy outcome. Misclassification could occur when a single chemical class of compounds is examined, because in the real world, joint exposures of industrial and consumer by-products could occur in-tandem. One study of non-persistent chemical exposure mixtures, including phthalates, bisphenols, and pesticides found that women with the highest levels of chemical mixtures exposures, compared to the lowest had greatest difference in fetal weight gain at delivery.50 By utilizing agriculture household status as a proxy for various exposures, we invariably account for multiple occupational exposures.
Limitations
Several potential limitations are due to the use of birth certificate data. Misclassification of exposure from self-reported classification of agricultural occupation may occur; however, research has shown good reliability compared to maternal interviews, suggesting that this bias is relatively minor.54 In this population, we assume minimal nondifferential misclassification due to high agreement, but also higher specificity and low sensitivity, so that fewer individuals are missing from the detected group of agricultural workers. Exposure misclassification may also occur as the result of workers shifting work status during the pregnancy from potentially higher exposure levels in early/mid-pregnancy to lower exposure status for the remainder of gestation and at the time of delivery. Birth records could also be subject to outcome misclassification, although other studies have shown that birth certificates performed well for GA and birth weight, respectively 98.9% and 98.6% in agreement.55 While there may be some discrepancy between birth certificate and medical records, records are also prone to missing data and recording errors and, unlike birth certificates, are not standardized across clinics/hospitals. Finally, another limitation could be the effect of unmeasured environmental confounders (i.e., pesticides). Additionally, we could not account for several other environmental factors, such as housing quality, which are known to be sources of pesticides exposures for women and children. Since environmental information is not included in birth certificates, we used parental farm work as a proxy for mixtures of environmental exposures. Other potential confounding variables like maternal diet, alcohol and/or drug use were not included in the final models, because of either high rates of missingness, or evidence that they were unreliable.
Conclusion
This study of births in Arizona is one of the few studies that considers parental exposures to agricultural work and adverse pregnancy outcomes. Agriculture and farm field laborers belong to one of the most dangerous and hazardous industries with limited occupational and legal protections to enforce workplace safety standards.56 In the U.S., the agricultural workforce comprises a vulnerable group of low-wage, immigrant workers and predominately foreign-born Latinos, making it challenging for research to address negative health outcomes. Future epidemiological studies should consider joint exposures of social and chemical stressors on fetal growth in under-resourced communities of color where environmental hazards are often most ubiquitous.
Supplementary Material
Supplemental Digital Content 1. Table that illustrates the associations between agricultural household and adverse birth outcomes in the analytical cohort that include maternal smoking and gestational weight gain. doc
Supplemental Digital Content 2. Table that illustrates the associations between agricultural household and adverse birth outcomes in the analytical cohort that excluded 4,823 births with maternal chronic hypertension. doc
Bulleted Learning Outcomes:
After reading this article, the reader will be able to:
Explain the potential cumulative effect of parental agricultural farm work on neonatal growth disorders likely attributed to occupational hazards and job strain.
Discuss the potential occupational exposure pathways that characterize risk of abnormal fetal growth among pregnant individuals, who report agricultural work at time of delivery.
Funding Sources:
This work was supported by NIH/NIEHS (R00ES028743, P30ES006694) and CDC-NIOSH WCAHS Pilot at UC Davis (2U54OH007550).
Footnotes
Ethical Considerations & Disclosures: This study was approved by the Human Subject Protection Program of the University of Arizona.
COI: None Declared.
References
- 1.US Department of Agriculture. Farm Labor. Accessed June 15, 2022. https://www.ers.usda.gov/topics/farm-economy/farm-labor/
- 2.Leemans M, Couderq S, Demeneix B, Fini J-B. Pesticides with potential thyroid hormone-disrupting effects: a review of recent data. Front Endocrinol. 2019;10:743. doi: 10.3389/fendo.2019.00743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehrpour O, Karrari P, Zamani N, Tsatsakis AM, Abdollahi M. Occupational exposure to pesticides and consequences on male semen and fertility: a review. Toxicol Lett. 2014;230(2):146–156. [DOI] [PubMed] [Google Scholar]
- 4.López-Gálvez N, Wagoner R, Quirós-Alcalá L, et al. Systematic Literature Review of the Take-Home Route of Pesticide Exposure via Biomonitoring and Environmental Monitoring. Int J Environ Res Public Health. 2019;16(12):2177. doi: 10.3390/ijerph16122177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Figa-Talamanca I. Occupational risk factors and reproductive health of women. Occup Med. 2006;56(8):521–31. doi: 10.1093/occmed/kql114 [DOI] [PubMed] [Google Scholar]
- 6.Flocks J, Vi Thien Mac V, Runkle J, Tovar-Aguilar JA, Economos J, McCauley LA. Female farmworkers’ perceptions of heat-related illness and pregnancy health. J Agromedicine. 2013;18(4):350–8. doi: 10.1080/1059924x.2013.826607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai C, Vandermeer B, Khurana R, et al. The impact of occupational activities during pregnancy on pregnancy outcomes: a systematic review and metaanalysis. Am J Obstet Gynecol. 2020;222(3):224–238. doi: 10.1016/j.ajog.2019.08.059 [DOI] [PubMed] [Google Scholar]
- 8.Damhuis SE, Ganzevoort W, Gordijn SJ. Abnormal Fetal Growth: Small for Gestational Age, Fetal Growth Restriction, Large for Gestational Age: Definitions and Epidemiology. Obstet Gynecol Clin North Am. 2021;48(2):267–279. doi: 10.1016/j.ogc.2021.02.002 [DOI] [PubMed] [Google Scholar]
- 9.Dumolt JH, Powell TL, Jansson T. Placental Function and the Development of Fetal Overgrowth and Fetal Growth Restriction. Obstet Gynecol Clin North Am. 2021;48(2):247–266. doi: 10.1016/j.ogc.2021.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstein RF, Abell SK, Ranasinha S, et al. Association of Gestational Weight Gain With Maternal and Infant Outcomes: A Systematic Review and Meta-analysis. JAMA. 2017;317(21):2207–2225. doi: 10.1001/jama.2017.3635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schellong K, Schulz S, Harder T, Plagemann A. Birth weight and long-term overweight risk: systematic review and a meta-analysis including 643,902 persons from 66 studies and 26 countries globally. PloS one. 2012;7(10):e47776. doi: 10.1371/journal.pone.0047776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scifres CM. Short- and Long-Term Outcomes Associated with Large for Gestational Age Birth Weight. Obstet Gynecol Clin North Am. 2021;48(2):325–337. doi: 10.1016/j.ogc.2021.02.005 [DOI] [PubMed] [Google Scholar]
- 13.Hong YH, Lee JE. Large for Gestational Age and Obesity-Related Comorbidities. J Obes Metab Syndr. 2021;30(2):124–131. doi: 10.7570/jomes20130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamai K, Yorifuji T, Takeuchi A, et al. Associations of birth weight for gestational age with child health and neurodevelopment among term infants: a nationwide Japanese population-based study. J Pediatr. 2020;226:135–141. e4. doi: 10.1016/j.jpeds.2020.06.075 [DOI] [PubMed] [Google Scholar]
- 15.Eide MG, Oyen N, Skjaerven R, Bjerkedal T. Associations of birth size, gestational age, and adult size with intellectual performance: evidence from a cohort of Norwegian men. Pediatr Res. 2007;62(5):636–42. doi: 10.1203/PDR.0b013e31815586e9 [DOI] [PubMed] [Google Scholar]
- 16.Wang D, Zhu L, Zhang S, et al. Predictive macrosomia birthweight thresholds for adverse maternal and neonatal outcomes. J Matern Fetal Med. 2016;29(23):3745–50. doi: 10.3109/14767058.2016.1147549 [DOI] [PubMed] [Google Scholar]
- 17.Andersson CB, Petersen JP, Johnsen SP, Jensen M, Kesmodel US. Risk of complications in the late vs early days of the 42nd week of pregnancy: A nationwide cohort study. Acta Obstet Gynecol Scand. 2022;101(2):200–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muglu J, Rather H, Arroyo-Manzano D, et al. Risks of stillbirth and neonatal death with advancing gestation at term: A systematic review and meta-analysis of cohort studies of 15 million pregnancies. PLoS Med. 2019;16(7):e1002838. doi: 10.1371/journal.pmed.1002838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenstein MG, Cheng YW, Snowden JM, Nicholson JM, Caughey AB. Risk of stillbirth and infant death stratified by gestational age. Obstet Gynecol. 2012;120(1):76–82. doi: 10.1097/AOG.0b013e31825bd286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birks L, Casas M, Garcia AM, et al. Occupational Exposure to Endocrine-Disrupting Chemicals and Birth Weight and Length of Gestation: A European Meta-Analysis. Environ Health Perspect. 2016;124(11):1785–1793. doi: 10.1289/ehp208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okui T, Ochiai M, Nakashima N. An Association between Maternal Occupations and Low Birth Weight Infants in Japan from 1995 to 2015. Int J Environ Res Public Health. 2021;18(15)doi: 10.3390/ijerph18158040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed P, Jaakkola JJ. Maternal occupation and adverse pregnancy outcomes: a Finnish population-based study. Occup Med. 2007;57(6):417–23. doi: 10.1093/occmed/kqm038 [DOI] [PubMed] [Google Scholar]
- 23.Rocheleau CM, Bertke SJ, Lawson CC, et al. Factors associated with employment status before and during pregnancy: Implications for studies of pregnancy outcomes. Am J Ind Med. 2017;60(4):329–341. doi: 10.1002/ajim.22700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.US Bureau of Labor Statistics. 2018 Standard Occupational Classification System. Accessed June 15 2022, https://www.bls.gov/soc/2018/major_groups.htm
- 25.ACOG. Macrosomia: ACOG Practice Bulletin, Number 216. Obstet Gynecol. 2020;135(1):e18–e35. doi: 10.1097/aog.0000000000003606 [DOI] [PubMed] [Google Scholar]
- 26.Herbst MA. Treatment of suspected fetal macrosomia: a cost-effectiveness analysis. Am J Obstet Gynecol. 2005;193(3 Pt 2):1035–9. doi: 10.1016/j.ajog.2005.06.030 [DOI] [PubMed] [Google Scholar]
- 27.Hanke W, Romitti P, Fuortes L, Sobala W, Mikulski M. The use of pesticides in a Polish rural population and its effect on birth weight. Int Arch Occup Environ Health. 2003;76(8):614–20. doi: 10.1007/s00420-003-0471-4 [DOI] [PubMed] [Google Scholar]
- 28.Zhu JL, Hjollund NH, Andersen AM, Olsen J. Occupational exposure to pesticides and pregnancy outcomes in gardeners and farmers: a study within the Danish National Birth Cohort. J Occup Environ Med. 2006;48(4):347–52. doi: 10.1097/01.jom.0000201566.42186.5f [DOI] [PubMed] [Google Scholar]
- 29.Bretveld RW, Hooiveld M, Zielhuis GA, Pellegrino A, van Rooij IA, Roeleveld N. Reproductive disorders among male and female greenhouse workers. Reprod Toxicol. 2008;25(1):107–14. doi: 10.1016/j.reprotox.2007.08.005 [DOI] [PubMed] [Google Scholar]
- 30.Ronda E, Regidor E. Higher birth weight and lower prevalence of low birth weight in children of agricultural workers than in those of workers in other occupations. J Occup Environ Med. 2003;45(1):34–40. doi: 10.1097/00043764-200301000-00010 [DOI] [PubMed] [Google Scholar]
- 31.Mayhoub F, Berton T, Bach V, et al. Self-reported parental exposure to pesticide during pregnancy and birth outcomes: the MecoExpo cohort study. PloS one. 2014;9(6):e99090. doi: 10.1371/journal.pone.0099090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Odd D, Lewis G, Gunnell D, Rasmussen F. Risk of low Apgar scores and socioeconomic status over a 30-year period. J Matern Fetal Med. 2014;27(6):603–607. [DOI] [PubMed] [Google Scholar]
- 33.Sejbaek CS, Bay H, Larsen AD, et al. Combined exposure to lifting and psychosocial strain at work and adverse pregnancy outcomes-A study in the Danish National Birth Cohort. PloS one. 2018;13(9):e0201842. doi: 10.1371/journal.pone.0201842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sancini A, Ricci S, Tomei F, et al. Work related stress and blood glucose levels. Ann Ig. 2017;29(2):123–133. doi: 10.7416/ai.2017.2139 [DOI] [PubMed] [Google Scholar]
- 35.Evangelou E, Ntritsos G, Chondrogiorgi M, et al. Exposure to Pesticides and Diabetes: A Systematic Review and Meta-Analysis. Environ Int. 2016;91doi: 10.1016/j.envint.2016.02.013 [DOI] [PubMed]
- 36.Kamai EM, McElrath TF, Ferguson KK. Fetal growth in environmental epidemiology: mechanisms, limitations, and a review of associations with biomarkers of non-persistent chemical exposures during pregnancy. Environ Health. 2019;18(1):43. doi: 10.1186/s12940-019-0480-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bradman A, Barr DB, Claus Henn BG, Drumheller T, Curry C, Eskenazi B. Measurement of pesticides and other toxicants in amniotic fluid as a potential biomarker of prenatal exposure: a validation study. Environ Health Perspect. 2003;111(14):1779–82. doi: 10.1289/ehp.6259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernhard W, Poets CF, Franz AR. Choline and choline-related nutrients in regular and preterm infant growth. Eur J Nutr. 2019;58(3):931–945. doi: 10.1007/s00394-018-1834-7 [DOI] [PubMed] [Google Scholar]
- 39.Eskenazi B, Harley K, Bradman A, et al. Association of in utero organophosphate pesticide exposure and fetal growth and length of gestation in an agricultural population. Environ Health Perspect. 2004;112(10):1116–24. doi: 10.1289/ehp.6789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harley KG, Engel SM, Vedar MG, et al. Prenatal Exposure to Organophosphorous Pesticides and Fetal Growth: Pooled Results from Four Longitudinal Birth Cohort Studies. Environ Health Perspect. 2016;124(7):1084–92. doi: 10.1289/ehp.1409362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wickerham EL, Lozoff B, Shao J, Kaciroti N, Xia Y, Meeker JD. Reduced birth weight in relation to pesticide mixtures detected in cord blood of full-term infants. Environ Int. 2012;47:80–5. doi: 10.1016/j.envint.2012.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Béranger R, Hardy EM, Binter AC, et al. Multiple pesticides in mothers’ hair samples and children’s measurements at birth: Results from the French national birth cohort (ELFE). Int J Hyg Environ Health. 2020;223(1):22–33. doi: 10.1016/j.ijheh.2019.10.010 [DOI] [PubMed] [Google Scholar]
- 43.D’Adamo MC, Liantonio A, Conte E, Pessia M, Imbrici P. Ion Channels Involvement in Neurodevelopmental Disorders. Neuroscience. 2020;440:337–359. doi: 10.1016/j.neuroscience.2020.05.032 [DOI] [PubMed] [Google Scholar]
- 44.Wray S, Arrowsmith S. Uterine Excitability and Ion Channels and Their Changes with Gestation and Hormonal Environment. Annu Rev Physiol. 2021;83:331–357. doi: 10.1146/annurev-physiol-032420-035509 [DOI] [PubMed] [Google Scholar]
- 45.Xu Q, Zhu B, Dong X, et al. Pyrethroid pesticide exposure during early pregnancy and birth outcomes in southwest China: a birth cohort study. J Toxicol Sci. 2020;45(5):281–291. doi: 10.2131/jts.45.281 [DOI] [PubMed] [Google Scholar]
- 46.Zhang J, Yoshinaga J, Hisada A, et al. Prenatal pyrethroid insecticide exposure and thyroid hormone levels and birth sizes of neonates. Sci Total Environ. 2014;488–489:275–9. doi: 10.1016/j.scitotenv.2014.04.104 [DOI] [PubMed]
- 47.Ding G, Cui C, Chen L, et al. Prenatal exposure to pyrethroid insecticides and birth outcomes in Rural Northern China. J Expo Sci Environ Epidemiol. 2015;25(3):264–70. doi: 10.1038/jes.2014.86 [DOI] [PubMed] [Google Scholar]
- 48.Ling C, Liew Z, von Ehrenstein OS, et al. Prenatal Exposure to Ambient Pesticides and Preterm Birth and Term Low Birthweight in Agricultural Regions of California. Toxics. 2018;6(3):41. doi: 10.3390/toxics6030041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsuki T, Ebara T, Tamada H, et al. Association between Prenatal Exposure to Household Pesticides and Neonatal Weight and Length Growth in the Japan Environment and Children’s Study. Int J Environ Res Public Health. 2020;17(12):4608. doi: 10.3390/ijerph17124608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van den Dries MA, Keil AP, Tiemeier H, et al. Prenatal Exposure to Nonpersistent Chemical Mixtures and Fetal Growth: A Population-Based Study. Environ Health Perspect. 2021;129(11):117008. doi: 10.1289/ehp9178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harley KG, Huen K, Aguilar Schall R, et al. Association of organophosphate pesticide exposure and paraoxonase with birth outcome in Mexican-American women. PloS one. 2011;6(8):e23923. doi: 10.1371/journal.pone.0023923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rauch SA, Braun JM, Barr DB, et al. Associations of Prenatal Exposure to Organophosphate Pesticide Metabolites with Gestational Age and Birth Weight. Environ Health Perspect. 2012;120(7):1055–60. doi: 10.1289/ehp.1104615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lesseur C, Pathak KV, Pirrotte P, et al. Urinary glyphosate concentration in pregnant women in relation to length of gestation. Environmental research. 2022;203:111811. doi: 10.1016/j.envres.2021.111811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brender JD, Suarez L, Langlois PH. Validity of parental work information on the birth certificate. BMC public health. 2008;8:95. doi: 10.1186/1471-2458-8-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ziogas C, Hillyer J, Saftlas AF, Spracklen CN. Validation of birth certificate and maternal recall of events in labor and delivery with medical records in the Iowa health in pregnancy study. BMC Pregnancy Childbirth. 2022;22(1):232. doi: 10.1186/s12884-022-04581-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liebman AK, Wiggins MF, Fraser C, Levin J, Sidebottom J, Arcury TA. Occupational health policy and immigrant workers in the agriculture, forestry, and fishing sector. Am J Ind Med. 2013;56(8):975–984. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1. Table that illustrates the associations between agricultural household and adverse birth outcomes in the analytical cohort that include maternal smoking and gestational weight gain. doc
Supplemental Digital Content 2. Table that illustrates the associations between agricultural household and adverse birth outcomes in the analytical cohort that excluded 4,823 births with maternal chronic hypertension. doc