Abstract
A recent article (C. G. Thornton et al., J. Clin. Microbiol. 36:1996–2003, 1998) reported a new specimen-processing method for improved recovery of mycobacteria. This method used C18-carboxypropylbetaine (CB-18) and increased both smear and culture sensitivity. The companion article (C. G. Thornton et al., J. Clin. Microbiol. 36:2004–2013, 1998) described initial improvements to this method. Additional significant parameters of the CB-18 processing method are identified herein. First, eliminating the incubation step was shown to further improve culture sensitivity. Subsequently, recovery of several mycobacterial isolates by the CB-18 method was compared to a contemporary processing method that combines NALC and NaOH (NALC-NaOH) and a Tween 80-based method. Recovery of the tuberculous isolates following NALC-NaOH processing averaged 20% and ranged from 1.6 to 45%, whereas recovery of the nontuberculous isolates averaged 11% and ranged from 0.1 to 55%. Recovery of the tuberculous and nontuberculous isolates by the Tween 80-based method ranged from 22 to 92% and 27 to 93%, respectively, with averages of 58 and 65%, respectively. Recovery of the tuberculous and nontuberculous mycobacteria following CB-18 processing averaged 86 and 73%, respectively, with ranges from 61 to over 100% and from 43 to over 100%, respectively. Other parameters of the CB-18 method were also examined, including recovery versus CB-18 concentration and the relationship between CB-18 concentration and the tuberculocidal effect. The tuberculocidal effect was time dependent but independent of concentration, whereas recovery was directly proportional to concentration. Increasing the CB-18 concentration to 4 mM provided quantitative recovery on solid medium; however, higher concentrations of CB-18 were not compatible with liquid culture. Examination of the relationship between increasing CB-18 and lecithin concentrations suggested that lecithin could not overcome the deleterious effects of CB-18 in liquid culture at these higher concentrations.
Several diagnostic techniques are currently used to evaluate the presence of mycobacteria in respiratory specimens. The principal methods include microscopic, cultural, and molecular techniques. As with most diagnostic methods, each technique has its advantages and disadvantages. For example, acid-fast staining is rapid, inexpensive, technically simple, and highly specific for acid-fast bacilli (AFB), but it lacks sensitivity. The culture method is extremely sensitive but is susceptible to contamination problems and is subject to inaccuracy unless some mycobacteria survive specimen processing. Current specimen-processing methods are very efficient at killing mycobacteria (9, 10, 24, 26, 28), yet processing is an essential prerequisite for culture due to the presence of other saprophytic and infectious organisms in specimens. Nucleic acid amplification is perhaps the most sensitive technique, as it requires a single target molecule (independent of viability), but it is expensive and technically complex. Amplification is also vulnerable to inhibition by components of the specimen and/or the solutions used to process the specimens.
The buoyant nature of the mycobacteria, and its effect on diagnostic sensitivity, is well known (3, 4, 7, 8, 11–14, 16, 17). The sensitivity of the aforementioned diagnostic techniques is compromised by buoyancy because all methods approved by the Centers for Disease Control and Prevention (CDC) for preparing clinical specimens for detection involve a centrifugation step (7). The degree to which the bacilli are buoyant will impact the ability to efficiently enrich the pellet with organisms (i.e., clear the supernatant). For example, if the specific gravity of the mycobacteria (ρmyco) is greater than (ρmyco > 1) or equal to (ρmyco = 1) that of the processing medium, then there is no buoyant force (FB) per se and centrifugation should permit quantitative recovery. However, if the specific gravity of the bacilli is less than that of the processing media (ρmyco < 1), quantitative recovery necessitates that the centrifugal force (FC) be greater than FB, and FC must act for such a time that the bacilli have the opportunity to completely sediment (i.e., enter the pellet).
None of the contemporary processing methods, save for sodium hypochlorite (NaOCl), a flocculation method (3, 7), counteracts buoyancy. The NaOCl procedure, however, cannot be used in conjunction with the culture method. The use of the zwitterionic detergent N,N-dimethyl-N-(n-octadecyl)-N-(3-carboxypropyl)ammonium inner salt (Chemical Abstract Service No. 78195-27-4), also known as C18-carboxypropylbetaine (CB-18), was recently introduced to improve detection of mycobacteria in respiratory specimens (18, 20). The improvements provided by this method were hypothesized to result from compensating for the innate buoyancy of the bacilli, having less of an impact on viability relative to NaOH, and to some degree dispersing cords of those mycobacteria that clump (19). In the present study quantitative culture was used to compare this new method in vitro with the processing method that combines N-acetyl-l-cysteine (NALC) and sodium hydroxide (NaOH) (7) and with a Tween 80-based method so as to assess the impacts of viability and buoyancy on mycobacterial recovery during specimen processing. Five different Mycobacterium tuberculosis complex isolates and four different mycobacteria other than tuberculosis (MOTT) isolates were evaluated. The CB-18 method provided the most consistent recovery regardless of species or strain, while the NALC-NaOH method generated the poorest results. Several other parameters of CB-18 processing were also investigated in an effort to determine optimal specimen-processing conditions.
MATERIALS AND METHODS
Mycobacterial stocks.
Several different mycobacterial isolates were used in these experiments. The M. tuberculosis complex isolates included the M. tuberculosis type strain, ATCC 27294 (American Type Culture Collection [ATCC], Rockville, Md.), the Mycobacterium bovis Calmette-Guérin (BCG) isolate ATCC 19274, and the Mycobacterium africanum type strain, ATCC 25420. The last two M. tuberculosis strains were the clinical isolates 571/573-S1 and 512-S3 isolates, which have been previously described (20). MOTT species included Mycobacterium avium (ATCC 15769), Mycobacterium chelonae (ATCC 35752), Mycobacterium fortuitum (ATCC 6841), and Mycobacterium kansasii (ATCC 12478). All isolates were maintained on 7H11-selective slants (Becton Dickinson, Cockeysville, Md.). Bacterial stocks were prepared for the in vitro experiments as previously described (21). Bacterial stocks were determined by microscopy to be >90% single-cell suspensions.
Smear and cultural analyses.
All acid-fast staining was performed with auramine-rhodamine according to the instructions of the manufacturer (DIFCO Laboratories, Detroit, Mich.). All solid media utilized for quantitative culture analyses were used with 7H11-selective plates (Becton-Dickinson). Plates were placed in sealed plastic bags, incubated in 5 to 10% CO2 at either 37°C (all but M. chelonae) or 30°C (M. chelonae only), and read at approximately 3 weeks for slow-growing mycobacteria or within the first week for rapid-growing mycobacteria. The BACTEC 12B/460TB system (Becton-Dickinson) was used with all liquid cultures. BACTEC 12B liquid cultures were supplemented with PANTA which had been fortified with ceftazidime (CAZ) to a final concentration of 8 μg/ml (12B-PANTA-CAZ) as previously described (20). In all experiments four replicate cultures were inoculated with the volumes indicated below and were monitored and analyzed as previously described (21). A growth index (GI) greater than or equal to 15 (GI ≥ 15) was considered positive. Growth curves were plotted as the average of the group and analyzed using a two-tailed, heteroscedastic Student’s t test. All positive cultures were subjected to acid-fast smear analysis to confirm the presence of AFB. In all experiments the identity of random samples was confirmed by AccuProbe (Gen-Probe, San Diego, Calif.) or biochemical analysis (7).
NALC-NaOH treatments.
The NALC-NaOH specimen-processing protocol used in these studies was that recommended by the CDC (7). Briefly, prepared samples were mixed with an equal volume of 2% NaOH (0.5 M) containing 0.5% NALC (Fluka) and 1.45% citric acid trisodium salt dihydrate (Sigma, St. Louis, Mo.) and then incubated for 15 min at room temperature. Neutralization buffer (67 mM NaKPO4 [pH 6.8]) was then added to each sample to a final volume of 40 ml, and samples were subjected to centrifugation (3,800 × g) for 20 min at 4°C. Decanted specimens were resuspended by addition of 1 ml of sterile filtered water (Life Technologies, Inc., Rockville, Md.) and analyzed as described below.
CB-18 treatments.
The CB-18 specimen-processing protocol involved treating prepared samples with a buffered solution of CB-18 in Tris-citrate buffer (50 mM Tris-HCl, 12.5 mM citrate [pH 7.6], 1.5 mM NaCl) containing 15 mM NALC. The Tris-citrate buffer was formulated as a 20-fold stock as previously described (21), and CB-18 (Ecochem Research, Inc., Chaska, Minn.) was made as a 100 mM concentrate in 1:1 isopropanol:water as previously described (20). Immediately prior to use the buffer was diluted 20:1 and the CB-18 was diluted into the buffer to achieve the desired concentrations described below. NALC was then added to this mixture to a final concentration of approximately 15 mM (0.25% [wt/vol]). In all experiments prepared samples were mixed with CB-18 and incubated as described for each experimental condition (below) prior to being subjected to centrifugation (3,800 × g) for 20 min at 35°C. Decanted specimens were resuspended by addition of 1 ml of 0.15% lecithin (wt/vol) and analyzed as previously described (21). A 100-fold concentrate of lecithin (7.5% [wt/vol]) was prepared for use as previously described (21).
Tween 80 treatments.
The Tween 80-based specimen-processing protocol involved treating the samples with a buffered solution of 1 mM (0.13% [wt/vol]) Tween 80 (Boehringer Mannheim, Indianapolis, Ind.) in the Tris-citrate buffer described above. The Tween 80 buffer was chilled to 4°C prior to use. In all experiments prepared samples were mixed with Tween 80 to a final volume of 40 ml and were immediately subjected to centrifugation (3,800 × g) for 20 min at 4°C. Decanted specimens were resuspended by addition of 1 ml of sterile water (Life Technologies) and analyzed as described above.
Processing assay.
The goal of this assay was to compare the efficiencies of different processing methods in collecting viable mycobacteria by centrifugation. Analysis of the impact of processing with NALC-NaOH, Tween 80, and CB-18 was accomplished by diluting a MacFarland standard 20,000:1. One-ml aliquots of this diluted stock were used to make three identical series of sample tubes, each series containing six identical 50-ml conical tubes. Sample tubes were initially prepared by addition of 2 ml of sterilized bronchial washing prior to addition of either the bacterial stock or any processing solutions. The bronchial washing was sterilized by autoclaving and used to simulate a clinical specimen in that cellular debris would form a fine sediment or “button” following centrifugation. The bronchial washing was sterilized to avoid culture contamination.
In the first series of six tubes, 3 ml of NALC-NaOH was added to the bacilli-bronchial washing mixture and processed as described above. In the second series, all tubes were brought to a final volume of 40 ml with Tween 80, and in the third series all tubes were brought to a final volume of 40 ml with 1 mM CB-18. The Tween 80 and CB-18 series tubes were immediately subjected to centrifugation as described above, whereas the NALC-NaOH tubes were incubated for 15 min at room temperature and then neutralized with buffer prior to centrifugation. Following centrifugation all tubes were decanted and resuspended as described for each method. Resuspended sediments of all replicates were analyzed by inoculating 7H11-selective plates in duplicate (200-μl aliquots each). When all aliquots were removed from processed tubes, the volume remaining in each was determined and used to adjust colony counts for quantitative culture. The volume of the completely resuspended sediments was typically 1.25 to 1.5 ml. Hence, each plate contained approximately 13 to 16% of a given sediment.
Input controls for these experiments involved analyzing quadruplicate 200-μl aliquots of the 20,000:1 stock on 7H11-selective plates. Recovery was calculated as the total CFU recovered in each sediment (adjusted for each volume) relative to the total number of CFU input prior to processing and centrifugation. The average recovery for the six sample tubes processed by a given method was determined and statistical comparisons within a given experiment were made using a two-tailed, heteroscedastic Student’s t test.
Tuberculocidal assay.
The tuberculocidal assay was performed and analyzed as previously described (21) with the exception that the MacFarland stock was diluted 200:1 in Tris-citrate buffer containing CB-18 at a final concentration of 0.1, 0.25, 1.0, or 4.0 mM.
Lecithin versus CB-18 titration.
By using a modified version of the assay previously described (21), different concentrations of lecithin were evaluated to determine how effective each was at overcoming the deleterious effects of increasing CB-18 concentrations. In these experiments the MacFarland stock of the 571/573-S1 isolate, the isolate most sensitive to CB-18 (21), was diluted 5,000:1 in Tris-citrate buffer and used directly (100 μl each) to inoculate all 12B-PANTA-CAZ sample bottles in a given experiment. A 4 by 4 matrix comparing the effects of different concentrations of lecithin on different concentrations of CB-18 was set up as follows: Sixteen mock sediments were formulated, each containing CB-18 at 0, 1.0, 2.0, or 4.0 mM. To these solutions were added appropriate volumes of the 100-fold concentrate of lecithin to achieve a 0, 0.075, 0.15, or 0.3% final concentration (400 μl of 95% ethanol was added to the 0% lecithin samples to appropriately control for the addition of ethanol to the system). The sixteen mock sediments comprised all possible combinations of these conditions. Each of these mock sediments was then used directly to supplement the 12B-PANTA-CAZ sample bottles (300 μl each), which were then inoculated with the 5,000:1 dilution of M. tuberculosis. Inoculations for all experimental conditions were done in quadruplicate, and cultures were monitored as previously described (21). Input CFU were estimated by further diluting the bacterial stock to a 20,000:1 final dilution and analyzing quadruplicate plates as described above.
RESULTS
CB-18 time course of recovery.
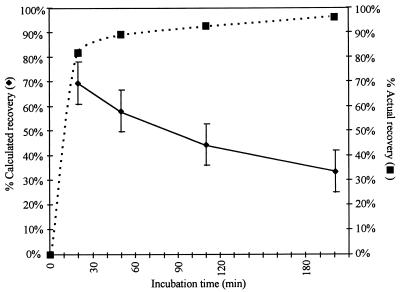
In the CB-18 pilot study (20) specimens were incubated for 90 min prior to centrifugation. In order to examine the effect of the incubation period on mycobacterial recovery, the M. tuberculosis type strain, ATCC 27294, was employed in experiments examining percent recovery versus incubation period. The zero time point was achieved by immediately subjecting the tubes to centrifugation for 20 min. This condition was compared to incubation periods of 30, 90, and 180 min prior to centrifugation. The average recovery of a typical time course experiment (Fig. 1) suggested that there was a consistent loss in viable CFU with increasing time. This curve was similar to the tuberculocidal effect caused by exposure to CB-18 (21). By combining the recovery data with the known tuberculocidal activity of CB-18 on this isolate (21), actual recovery could be estimated. Actual recovery is the observed CFU at a given time point divided by the difference between the calculated input and the number of CFU killed at this time point due to the tuberculocidal effect of CB-18 on this isolate (Fig. 1). For example, if the calculated recovery at 30 min was approximately 65%, and the results of the tuberculocidal assay with this isolate suggested that approximately 25% (see below) of the bacilli were killed in 30 min, then the actual recovery would approach 90%. The shape of this curve is time dependent and biphasic. CB-18 increased recovery rapidly: after 30 min only marginal increases in recovery were observed.
FIG. 1.
Time course of recovery versus incubation time in 1 mM CB-18 with M. tuberculosis 27294 (⧫). The input into this experiment was 185 ± 41 CFU. Actual recovery (■) is defined as the observed CFU at a given time point divided by the difference between the calculated input and the number of CFU killed at this time point due to the tuberculocidal effect of CB-18 on this isolate.
Comparison of processing methods.
The time course experiments suggested that recovery by culture was a trade-off: longer times provided increased recovery but at the expense of viability. The biphasic nature of the recovery curve suggested that eliminating the incubation step would further improve the efficacy of the CB-18 processing method relative to recovery by culture with only a modest reduction in overall recovery. Hence, for the comparison studies the CB-18 processing method was modified from that used in the CB-18 pilot study (20) in that the incubation step was eliminated to minimize the tuberculocidal effects of CB-18: samples were centrifuged immediately following addition of CB-18. The modified CB-18 format was compared to the contemporary NALC-NaOH method and a Tween 80-based method in an in vitro model by using quantitative culture. The NALC-NaOH method was used because it is recommended by the CDC, while the Tween 80-based method was used as an intermediate procedure because it utilized a surface-active agent that did not negatively impact mycobacterial viability. In these experiments the stocks of mycobacterial isolates were sonicated and clarified by centrifugation to minimize clumps and then divided so that the effects of the three different processing methods on recovery could be compared side-by-side. Five M. tuberculosis complex isolates (Table 1) and four MOTT isolates (Table 2) were examined. Three separate experiments were performed using each isolate. Each calculated recovery in a given experiment, for a given method, represented six identical samples, and each sample was analyzed in duplicate. Therefore, each reported recovery was the average of 12 plates (± standard deviation [SD]).
TABLE 1.
Recovery of M. tuberculosis complex isolates: comparison of specimen-processing methods
| Strain | Expt | Input (CFU) | % Recovery (± SD) by the following processing method:
|
Significance of differences (P)
|
||||
|---|---|---|---|---|---|---|---|---|
| 2% NALC-NaOH | 0.13% Tween 80 | 1 mM CB-18 | NaOH vs Tween 80 | NaOH vs CB-18 | Tween 80 vs CB-18 | |||
| M. tuberculosis ATCC 27294 | A | 988 ± 22 | 21.7 ± 3.0 | 43.0 ± 4.8 | 82.5 ± 8.8 | <0.001 | <0.001 | <0.001 |
| B | 501 ± 47 | 17.3 ± 4.0 | 58.7 ± 15.3 | 74.0 ± 18.9 | <0.001 | <0.001 | 0.046 | |
| C | 476 ± 49 | 37.9 ± 11.5 | 53.3 ± 13.7 | 74.5 ± 9.7 | 0.007 | <0.001 | <0.001 | |
| Avg CFU per platea | 23 ± 9 | 44 ± 13 | 81 ± 36 | <0.001 | <0.001 | <0.001 | ||
| M. bovis ATCC 19274 | A | 808 ± 75 | 2.3 ± 2.2 | 22.3 ± 10.4 | 84.4 ± 11.7 | <0.001 | <0.001 | <0.001 |
| B | 2,401 ± 103 | 1.9 ± 1.2 | 42.2 ± 5.9 | 86.1 ± 4.9 | <0.001 | <0.001 | <0.001 | |
| C | 383 ± 40 | 1.6 ± 1.6 | 21.7 ± 13.5 | 70.9 ± 14.4 | <0.001 | <0.001 | <0.001 | |
| Avg CFU per plate | 3 ± 3b | 61 ± 62 | 147 ± 117 | <0.001 | <0.001 | <0.001 | ||
| M. africanum ATCC 25420 | A | 301 ± 57 | 22.7 ± 7.0 | 64.8 ± 17.0 | 107.2 ± 15.5 | <0.001 | <0.001 | <0.001 |
| B | 118 ± 9 | 34.9 ± 17.6 | 83.9 ± 26.5 | 132.9 ± 35.6 | <0.001 | <0.001 | 0.002 | |
| C | 174 ± 28 | 44.8 ± 24.8 | 80.3 ± 21.6 | 143.0 ± 21.1 | <0.001 | <0.001 | 0.001 | |
| Avg CFU per plate | 9 ± 5 | 20 ± 8 | 36 ± 12 | <0.001 | <0.001 | <0.001 | ||
| M. tuberculosis 571/573-S1 | A | 199 ± 41 | 5.8 ± 3.5 | 56.2 ± 20.0 | 61.3 ± 11.0 | <0.001 | <0.001 | 0.45 |
| B | 134 ± 35 | 11.7 ± 8.2 | 34.5 ± 15.4 | 65.5 ± 28.6 | <0.001 | <0.001 | 0.004 | |
| C | 260 ± 39 | 15.0 ± 8.3 | 51.0 ± 9.8 | 73.1 ± 19.1 | <0.001 | <0.001 | 0.002 | |
| Avg CFU per plate | 3 ± 3c | 13 ± 7 | 21 ± 10 | <0.001 | <0.001 | <0.001 | ||
| M. tuberculosis 512-S3 | A | 288 ± 33 | 4.1 ± 3.1 | 80.8 ± 13.1 | 75.9 ± 13.8 | <0.001 | <0.001 | 0.38 |
| B | 228 ± 45 | 37.8 ± 10.7 | 85.6 ± 16.1 | 73.0 ± 12.0 | <0.001 | <0.001 | 0.042 | |
| C | 728 ± 86 | 42.9 ± 7.3 | 92.4 ± 9.9 | 92.9 ± 11.5 | <0.001 | <0.001 | 0.91 | |
| Avg CFU per plate | 19 ± 19d | 55 ± 35 | 55 ± 41 | <0.001 | <0.001 | 0.99 | ||
| Avg | 532 ± 566e | 20.1 ± 18.1 | 58.0 ± 26.6 | 86.0 ± 28.0 | <0.001 | <0.001 | <0.001 | |
| Avg CFU per platef | 11 ± 13 | 39 ± 37 | 68 ± 73 | <0.001 | <0.001 | <0.001 | ||
The average colony counts were calculated by taking the average of the 36 experimental results for a given isolate (i.e., six tubes analyzed in duplicate in the three experiments) for each processing method.
Of the 36 plates analyzed following processing with NALC-NaOH, 7 plates had 0 colonies, 7 plates had 1 colony, and 9 plates had 2 colonies.
Of the 36 plates analyzed following processing with NALC-NaOH, 3 plates had 0 colonies, 8 plates had 1 colony, and 11 plates had 2 colonies.
Of the 36 plates analyzed following processing with NALC-NaOH, 2 plates had 0 colonies, 3 plates had 1 colony, and 5 plates had 2 colonies.
When experiment B of the M. bovis series was eliminated, the average input in all experiments became 399 ± 266 CFU.
Colony counts for all M. tuberculosis complex isolates were calculated by taking the average of all experimental results for each processing method.
TABLE 2.
Recovery of MOTT isolates: comparison of specimen-processing methods
| Strain | Expt | Input (CFU) | % Recovery (± SD) by the following processing method:
|
Significance of differences (P)
|
||||
|---|---|---|---|---|---|---|---|---|
| 2% NALC-NaOH | 0.13% Tween 80 | 1 mM CB-18 | NaOH vs Tween 80 | NaOH vs CB-18 | Tween 80 vs CB-18 | |||
| M. avium ATCC 15769 (serovar 1) | A | 460 ± 52 | 23.6 ± 9.4 | 68.2 ± 12.5 | 53.1 ± 9.6 | <0.001 | <0.001 | 0.003 |
| B | 1,484 ± 117 | 55.2 ± 5.8 | 67.3 ± 4.5 | 68.8 ± 4.6 | <0.001 | <0.001 | 0.44 | |
| C | 2,161 ± 165 | 37.6 ± 4.7 | 54.6 ± 7.4 | 51.0 ± 5.8 | <0.001 | <0.001 | 0.20 | |
| Avg CFU per platea | 77 ± 46 | 117 ± 54 | 119 ± 61 | 0.001 | 0.001 | 0.85 | ||
| M. kansasii ATCC 12478 | A | 688 ± 73 | 3.4 ± 1.7 | 51.0 ± 5.5 | 94.6 ± 11.3 | <0.001 | <0.001 | <0.001 |
| B | 1,720 ± 90 | 5.4 ± 1.7 | 44.2 ± 5.1 | 94.0 ± 6.9 | <0.001 | <0.001 | <0.001 | |
| C | 1,329 ± 122 | 0.4 ± 0.5 | 60.7 ± 8.2 | 109.2 ± 9.9 | <0.001 | <0.001 | <0.001 | |
| Avg CFU per plate | 5 ± 6b | 92 ± 35 | 181 ± 64 | <0.001 | <0.001 | <0.001 | ||
| M. fortuitum ATCC 6841 | A | 1,529 ± 82 | 0.4 ± 0.5 | 88.9 ± 9.6 | 80.3 ± 9.8 | <0.001 | <0.001 | 0.041 |
| B | 511 ± 49 | 5.3 ± 6.7 | 77.8 ± 7.4 | 82.9 ± 18.0 | <0.001 | <0.001 | 0.38 | |
| C | 349 ± 47 | 0.7 ± 1.0 | 93.1 ± 12.9 | 82.0 ± 11.5 | <0.001 | <0.001 | 0.036 | |
| Avg CFU per plate | 2 ± 3c | 104 ± 74 | 101 ± 69 | <0.001 | <0.001 | 0.83 | ||
| M. chelonae ATCC 35752 | A | 151 ± 31 | 2.4 ± 3.2 | 27.3 ± 16.0 | 73.2 ± 13.9 | <0.001 | <0.001 | <0.001 |
| B | 575 ± 79 | 0.1 ± 0.3 | 93.3 ± 15.6 | 45.6 ± 6.6 | <0.001 | <0.001 | <0.001 | |
| C | 1,390 ± 63 | 0.5 ± 0.7 | 58.4 ± 16.1 | 43.0 ± 5.7 | <0.001 | <0.001 | 0.008 | |
| Avg CFU per plate | 1 ± 1d | 67 ± 52e | 48 ± 31 | <0.001 | <0.001 | 0.066 | ||
| Avg | 1,029 ± 629 | 11.2 ± 17.8 | 65.4 ± 22.2 | 73.1 ± 22.7 | <0.001 | <0.001 | 0.004 | |
| Avg CFU per platef | 21 ± 39 | 95 ± 58 | 112 ± 75 | <0.001 | <0.001 | 0.028 | ||
The average colony counts were calculated by taking the average of the 36 experimental results for a given isolate (i.e., six tubes analyzed in duplicate in the three experiments) for each processing method.
Of the 36 plates analyzed following processing with NALC-NaOH, 6 plates had 0 colonies, 7 plates had 1 colony, and 2 plates had 2 colonies.
Of the 36 plates analyzed following processing with NALC-NaOH, 17 plates had 0 colonies, 10 plates had 1 colony, and 5 plates had 2 colonies.
Of the 36 plates analyzed following processing with NALC-NaOH, 25 plates had 0 colonies, 7 plates had 1 colony, and 2 plates had 2 colonies.
Of the 36 plates analyzed following processing with Tween 80, one plate had 0 colonies and two plates had 2 colonies (these results were from experiment A).
Colony counts for all MOTT isolates were calculated by taking the average of all experimental results for each processing method.
All experiments were performed well below the limit of AFB smear detection (6, 27). Inputs for the M. tuberculosis isolates ranged from 118 ± 9 to 2,401 ± 103 CFU, with an average of 532 ± 566 CFU (Table 1). Inputs for the MOTT isolates ranged from 151 ± 31 to 2,161 ± 165 CFU (Table 2), with an average of 1,029 ± 629 CFU. When experiment B of the M. bovis BCG series was eliminated, the M. tuberculosis input average dropped to 399 ± 266 CFU.
The mycobacterial isolates tested each behaved differently. When processed with NALC-NaOH, recovery of the M. tuberculosis isolates ranged from under 2 to almost 45% and averaged approximately 20% (Table 1). Recovery of the nontuberculous isolates following NALC-NaOH processing ranged from 0.1% to approximately 55% and averaged approximately 11% (Table 2). Recovery of M. avium by the NALC-NaOH method was markedly higher than the recovery of the other MOTT isolates tested. When the M. avium data were omitted from the analysis, the average recovery of MOTT isolates following NALC-NaOH processing dropped to 2.1% ± 3.3%.
Recovery of the tuberculous isolates following processing with Tween 80 was similar to that of the nontuberculous isolates: recoveries ranged from 22 to 92% versus 27 to 93%, respectively, with averages of 58 and 65%, respectively. Omission of the Tween 80 result in experiment A of the M. chelonae series from the aggregate analysis increased the average recovery of the MOTT isolates following Tween 80 processing to 68.9% ± 19.3%, making the aggregate comparison between Tween 80 and CB-18 processing no longer statistically significant (P = 0.092).
Recovery of the tuberculous mycobacteria following CB-18 processing ranged from approximately 61 to 143% and averaged 86% (Table 1). Recovery of the nontuberculous mycobacteria following CB-18 processing ranged from 43 to over 100% and averaged approximately 73% (Table 2). After CB-18 processing recovery of the M. africanum isolate was consistently greater than 100%, possibly an artifact related to the alleviation of spontaneous cording. Omission of the M. africanum results from the analysis decreased the average recovery of M. tuberculosis complex isolates by the CB-18 method to 76.2% ± 16.7% (all aggregate comparisons remained statistically significant).
Upon examination, statistically significant differences were observed for all comparisons of the NALC-NaOH method with the CB-18 and the Tween 80 methods (Tables 1 and 2). Only six experiments comparing the CB-18 processing recoveries with the Tween 80 processing recoveries produced results that were not statistically significant. The M. avium, M. fortuitum, and 512-S3 experiments resulted in only minor differences among the methods in all the evaluations. In experiment A of the M. tuberculosis 571/573-S1 series, 2 of the 12 Tween 80 plates produced recoveries with nearly double the reported average, thereby introducing a large variation. When the results of these two plates were eliminated, the average Tween 80 recovery dropped to 49.3% ± 13.0% and the CB-18 to Tween 80 difference became significant (P = 0.033).
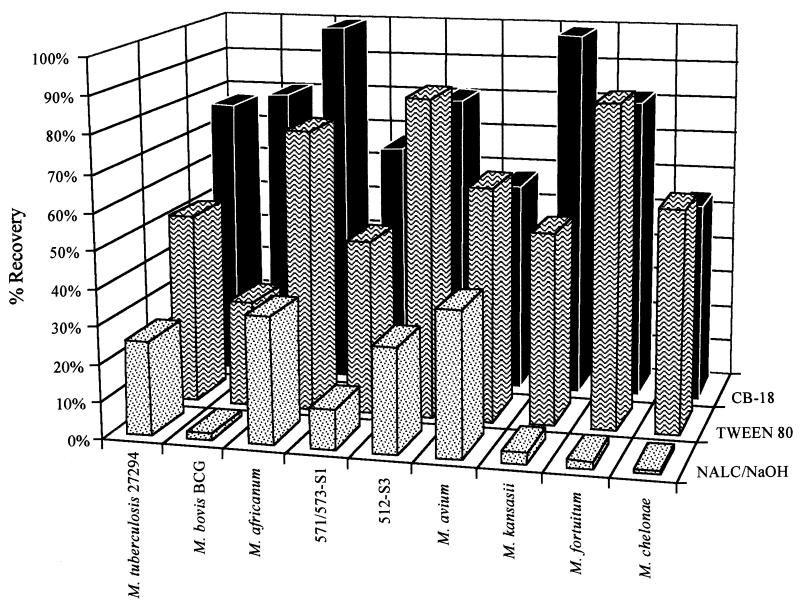
The average recovery of the three experiments for a given isolate and processing method was charted (Fig. 2), and the ratios of the average recoveries between the different processing methods were examined (Table 3). The Tween 80/NALC-NaOH ratios reflected the impact of NaOH on culture sensitivity, the CB-18/Tween 80 ratios reflected the impact of buoyancy on recovery, and the CB-18/NALC-NaOH ratios reflected the improvement in culture sensitivity expected when switching from NALC-NaOH to CB-18 processing in the clinical laboratory. Ratios ranged from 0.9 to 60 (Table 3).
FIG. 2.
Comparison of the average recoveries of the different specimen-processing methods for each of the isolates tested.
TABLE 3.
Analysis of average recovery ratiosa
| Isolate | Avg recovery ratio
|
||
|---|---|---|---|
| Tween 80/ NALC-NaOH | CB-18/ Tween 80 | CB-18/ NALC-NaOH | |
| M. tuberculosis ATCC 27294 | 2.0 | 1.5 | 3.0 |
| M. bovis BCG ATCC 19274 | 15 | 2.8 | 42 |
| M. africanum ATCC 25420 | 2.2 | 1.7 | 3.7 |
| M. tuberculosis 571/573-S1 | 4.4 | 1.4 | 6.2 |
| M. tuberculosis 512-S3 | 3.1 | 0.9 | 2.9 |
| Avg for M. tuberculosis complex isolates | 2.9 | 1.5 | 4.3 |
| M. avium ATCC 15769 | 1.6 | 0.9 | 1.5 |
| M. kansasii ATCC 12478 | 17 | 1.9 | 32 |
| M. fortuitum ATCC 6841 | 41 | 0.9 | 39 |
| M. chelonae ATCC 35752 | 60 | 0.9 | 54 |
| Avg for MOTT isolatesb | 5.8 | 1.1 | 6.5 |
The average recovery was determined by taking the average of the 36 experimental results of a given isolate (i.e., six tubes analyzed in duplicate in the three experiments) for a given processing method. The average recoveries for each processing method were then compared to obtain a given ratio.
Includes M. avium, M. kansasii, M. fortuitum, and M. chelonae.
Among tuberculous mycobacteria, switching to CB-18 processing improved culture sensitivity by more than fourfold. Buoyancy compensation alone improved sensitivity by 50%, while effects of viability produced a threefold increase in recovery. However, eliminating the M. bovis BCG data from the aggregate analysis decreased the Tween 80/NALC-NaOH ratio to 2.7, the CB-18/Tween 80 ratio to 1.3, and the CB-18/NALC-NaOH ratio to 3.6.
Improvements in the detection of MOTT infections by culture appear to be almost exclusively due to effects on viability; however, this might be an artifact caused by the use of isolates with minimal buoyant character. The M. avium isolate was markedly different from the other MOTT isolates in this model in that it was not buoyant, and it was the least affected by the action of NaOH. Buoyancy has been observed in other M. avium isolates (data not shown).
While recovery improvements among the tuberculous mycobacteria were generally less than improvements among the nontuberculous isolates, the sensitivity of the M. bovis BCG isolate to NaOH rivaled that of the M. kansasii isolate. Clearly, the effect of NaOH on viability was the single most significant factor affecting recovery by culture, whereas buoyancy as a factor was more significant among tuberculous isolates than among MOTT isolates. However, the M. kansasii isolate was more buoyant than several of the M. tuberculosis complex isolates.
Improvements were also consistent with the average CFU recovered for a given isolate (Tables 1 and 2). For example, comparison of the average colony counts of all plates in all three experiments (i.e., 36 plates) for the three different processing methods produced statistically significant results in all but four instances. These four instances were all comparisons between Tween 80 and CB-18 processing results, and three of these instances were among the MOTT isolates tested. The average CFU per plate following NALC-NaOH processing was generally higher among MOTT isolates than among M. tuberculosis complex isolates (21 versus 11 CFU, respectively); however, the M. avium results again skewed the MOTT recovery data. Omitting the M. avium colony counts decreased the MOTT average to 3 ± 4 CFU per plate.
When the colony counts of the 324 plates used in these experiments were examined further (i.e., the aggregate of all experiments; nine isolates with 36 plates each), it was noted that following NALC-NaOH processing, 214 (66.1%) of these plates had 0 to 10 CFU. Sixty plates (18.5%) had 0 CFU. Specifically, in 69.4, 47.2, 19.4, 16.7, 8.3, and 5.6% of the plates from M. chelonae, M. fortuitum, M. bovis BCG, M. kansasii, 571/573-S1, and 512-S3, respectively, no colonies were observed following NALC-NaOH processing. Conversely, after CB-18 processing of both tuberculous and nontuberculous isolates no plates with zero colonies were observed. Of the 324 plates analyzed in all experiments from all isolates following CB-18 processing, only 6 plates (1.8%) had 10 or fewer CFU. The Tween 80 method produced 35 plates (10.8%) with 10 or fewer CFU; only 1 of these plates had no colonies.
CB-18 concentration versus recovery.
In order to further improve mycobacterial recovery by the CB-18 method, the relationship between recovery and CB-18 concentration was examined. Effects of CB-18 concentrations of 0.1, 0.25, 1.0, and 4.0 mM on the recovery of the ATCC 27294 isolate were evaluated by using the modified processing format (i.e., no incubation). Recovery was found to be strongly dependent on concentration in three separate experiments (Table 4). In all experiments, differences in recovery between the different concentrations were statistically significant.
TABLE 4.
CB-18 processing: examination of recovery vs CB-18 concentration with M. tuberculosis ATCC 27294a
| Expt | Input (CFU) | Recovery (% ± SD) vs indicated CB-18 concentration (mM)
|
|||
|---|---|---|---|---|---|
| 0.1 | 0.25 | 1.0 | 4.0 | ||
| A | 185 ± 11 | 16.0 ± 14.4 | 29.1 ± 13.5 | 69.2 ± 23.8 | 93.0 ± 19.1 |
| B | 360 ± 34 | 18.2 ± 10.5 | 33.1 ± 13.9 | 83.7 ± 15.4 | 96.9 ± 12.4 |
| C | 374 ± 71 | 21.1 ± 7.3 | 34.8 ± 9.4 | 64.7 ± 14.5 | 91.0 ± 12.0 |
| Avg recoveryb | 18.4 ± 11.0 | 32.3 ± 12.3 | 72.5 ± 19.6 | 93.6 ± 14.6 | |
No incubation step was used in the CB-18 processing procedure for these experiments; in all cases sample tubes were immediately subjected to centrifugation.
The average recovery was calculated by taking the average of the 36 experimental results for a given concentration (i.e., six tubes analyzed in duplicate in the three experiments).
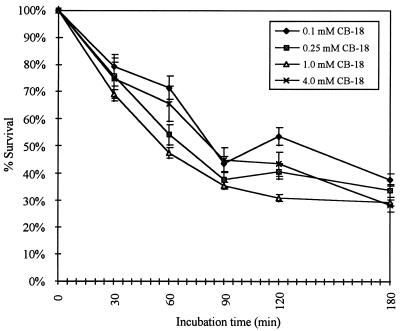
Interestingly, at 4.0 mM CB-18 recovery appeared to be quantitative, suggesting that the tuberculocidal activity of CB-18 was independent of concentration. The tuberculocidal effects of CB-18 concentrations of 0.1, 0.25, 1.0, and 4.0 mM were further examined. These experiments used the 571/573-S1 isolate because of its susceptibility to CB-18 (21) and confirmed that incubation time, not concentration, was the more significant factor (Fig. 3). For example, while there were a few statistically significant differences in survival between 0.1 and 1.0 mM CB-18 concentrations, there were very few other statistically significant comparisons among the various CB-18 concentrations. Repeated experiments at these concentrations confirmed no correlation between the tuberculocidal activity of CB-18 and the CB-18 concentration. In contrast, there were invariably statistically significant differences in CFU losses between those at 0 and 30 min; between those at 30 min and 90, 120, or 180 min; and between those at 60 min and 90, 120, or 180 min. While some statistically significant differences between 30 and 60 min, between 90 and 120 or 180 min, and between 120 and 180 min were observed, they were not consistently significant in all experiments performed.
FIG. 3.
Time-dependent killing of M. tuberculosis 571/573-S1 versus CB-18 concentration. The 1 mM CB-18 curve was taken from Thornton et al. (21) where the input during incubation was 154,800 ± 9,100 CFU. The inputs during incubation in the 0.1, 0.25, and 4.0 mM CB-18 experiments were 82,200 ± 6,400 CFU, 15,700 ± 3,900 CFU, and 20,000 ± 2,700 CFU, respectively.
Lecithin versus CB-18 titration.
The aforementioned experiments raised the question of whether increased CB-18 concentrations were compatible with the use of liquid culture. For example, while it was clear that higher CB-18 concentrations can be used with solid media and that minimizing the incubation time reduced the impact on viability caused by exposure to CB-18 during processing, exposure was previously shown to be less important to recovery in liquid culture than the amount of CB-18 that made it into the culture bottle as a result of inoculation (21). CB-18 concentrations above 5 μg/ml affected growth characteristics for the most-susceptible isolates, whereas CB-18 concentrations above 20 μg/ml, in the presence of PANTA-CAZ, substantively affected the viability of many more isolates. Increasing the concentration of CB-18 in the processing medium would increase the risk of exceeding 20 μg/ml in liquid cultures. For example, if 250 μl of supernatant at 4 mM (1.53 mg/ml) remained following decanting, and 1 ml of resuspension fluid was added to the sediment, inoculation of the BACTEC 12B bottle would result in a CB-18 concentration of approximately 35 μg/ml, a concentration that would inhibit the growth of most M. tuberculosis isolates, especially isolates such as the 571/573-S1 isolate (21).
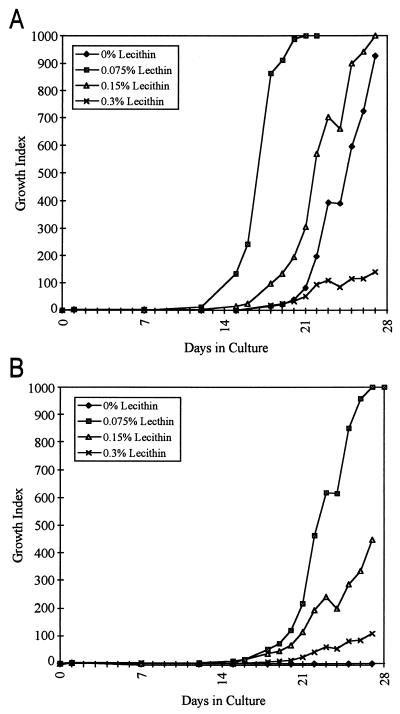
A matrix experiment examining combinations of different lecithin and CB-18 concentrations was performed with the 571/573-S1 isolate. Mock supernatants containing CB-18 concentrations of 0, 1, 2, or 4 mM were mixed with lecithin concentrations of 0, 0.075, 0.15, or 0.3% (all concentrations final) in all possible combinations. Analysis of the 0 mM CB-18 control series (Fig. 4A) indicated that there was a statistically significant reduction in the time to a positive result with 0.075% lecithin (13.0 ± 0.8 days) relative to that for the 0% lecithin control (18.5 ± 1.7 days [P = 0.004]). In contrast, the times to positive results for the 0.15 and 0.3% lecithin samples (16.0 ± 0.8 days and 17.2 ± 0.5 days, respectively) were not significantly different from that for the 0% lecithin controls (P = 0.056 and 0.25, respectively). The difference in time to maximum GI (GI = 999) was not statistically significant for the 0.15% lecithin series relative to that for the 0% lecithin controls (24.5 ± 1.9 days and 26.5 ± 2.5 days, respectively [P = 0.26]), but the times for the 0.075% and 0.3% lecithin series (18.2 ± 1.3 days and >28 days, respectively) were both significantly different from that for the 0% lecithin controls (P = 0.003 and < 0.001, respectively); however, at 0.075% lecithin, the time to maximum GI was decreased rather than increased, as was the case with 0.3% lecithin.
FIG. 4.
Lecithin matrix experiment with M. tuberculosis 571/573-S1 and either no CB-18 (A) or 1 mM CB-18 (B). Each bottle was inoculated with 406 ± 20 CFU.
When 1 mM CB-18 was introduced into the system (Fig. 4B), the controls with 0% lecithin did not grow, whereas all replicate samples containing lecithin became positive. The time to a positive result for 0.075% lecithin was not significantly different from that for 0.15% lecithin (16.5 ± 1.0 days and 17.0 ± 0.8 days, respectively [P = 0.47]), but both of these were significantly different from the time to positivity for the 0.3% lecithin series (20.8 ± 1.0 days [P < 0.001]). Differences in the time to maximum GI were statistically significant in all instances for the 1.0 mM concentration of CB-18.
Comparison of the time to a positive result in the experimental control (i.e., 0 mM CB-18 and 0% lecithin) to those for CB-18 concentrations of 1 mM combined with any amount of lecithin revealed that none of these comparisons were significantly different. In contrast, statistically significant differences were observed for the 0.075% lecithin with versus without CB-18 comparison and for the 0.3% lecithin with versus without CB-18 comparisons. Only the 0.15% lecithin with versus without CB-18 comparison was not significantly different (P = 0.13). Analysis by acid-fast staining of the 0.3% lecithin samples at maximum GI, with or without CB-18, revealed minor reductions in the number of observable bacilli relative to all other conditions that produced positive samples.
At 4.0 mM CB-18 none of the samples became positive, regardless of the amount of lecithin added (data not shown). Analysis of these samples by acid-fast staining at 4, 6, and 8 weeks revealed no bacilli. At 2.0 mM CB-18 all bottles eventually became positive but not until after 8 weeks. At these higher CB-18 concentrations, the solutions were clear, not cloudy, when mixed with lecithin, indicating that the lecithin was probably completely solubilized by CB-18.
DISCUSSION
The goal of this research was to define the important parameters of CB-18 processing in an effort to improve this method and then compare the CB-18 processing method with the CDC-recommended NALC-NaOH procedure (7) and a Tween 80-based method regarding their respective abilities to influence culture sensitivity in an in vitro processing model. Tween 80, a nonionic surface-active agent commonly used in mycobacteriology, was included in this study so that these two surface-active agents could be compared with the NALC-NaOH method to determine the impact of buoyancy on recovery and the effects of specimen processing on mycobacterial viability.
Initial experiments suggested that the CB-18 processing method could be improved by eliminating the 90-min incubation step used in the CB-18 pilot study (20). The hypothesis advanced in U.S. patent 5,658,749 (19) was that CB-18 is sequestered by the mycobacteria in such a manner as to form lipoidal bodies. These lipoidal bodies presumably increased the density of the bacilli thereby enhancing the efficiency of recovery during centrifugation. Therefore, exposure to CB-18 decreased both buoyancy and viability. For example, longer incubation times enhanced recovery but the tuberculocidal action of CB-18 reduced the number of viable CFU. By combining these data sets, we concluded that CB-18’s compensatory effect on buoyancy is more rapid than its deleterious effect. This conclusion is consistent with the observation of Weir et al. (25) in that M. smegmatis can sequester 25% of its dry weight in lipid in 30 min. Eliminating the incubation step from the CB-18 processing protocol further improved culture sensitivity without impacting increased recovery of the mycobacteria.
Comparison of the recovery rates following NALC-NaOH and Tween 80 processing suggests that the degree to which NaOH affects viability is species and strain dependent. In general, the viability of the MOTT isolates is more negatively affected by NaOH than that of the M. tuberculosis complex isolates; however, the M. bovis BCG isolate is significantly more sensitive to NaOH than the other M. tuberculosis complex isolates. The finding that the rapid growers (i.e., M. fortuitum and M. chelonae) are dramatically and negatively impacted by NaOH is consistent with the results of the CB-18 pilot study (20). While the susceptibility of the non-tuberculous isolates to NaOH was expected, the response of the M. bovis BCG isolate to NaOH was surprising and raises an issue as to the true number of M. tuberculosis complex isolates that are as sensitive or more sensitive to NaOH than the M. bovis BCG isolate and which generate false-negative culture results due to the contemporary processing procedures.
The toxicities of both CB-18 and NaOH are species and strain dependent. The apparent reduction in the recovery of the 571/573-S1 isolate following CB-18 processing, relative to the recovery of other M. tuberculosis complex isolates, is probably due to the extreme susceptibility of this isolate to CB-18 (21). In addition, in the CB-18 pilot study there was an apparent decrease in culture sensitivity among M. tuberculosis complex-positive specimens (20) due to the tuberculocidal activity of CB-18 and to the toxicity of CB-18 in liquid culture (21). These in vitro experiments eliminated the incubation step and included lecithin in the resuspension buffer to overcome these problems. Future studies incorporating these improvements should see an increase in culture sensitivity among M. tuberculosis complex-positive specimens.
Comparison of the recovery rates following Tween 80 and CB-18 processing suggests that buoyancy affects diagnostic sensitivity. While there was isolate-to-isolate variability, the CB-18/Tween 80 ratios did not vary to the same degree as was observed with the Tween 80/NALC-NaOH ratios. Buoyancy was most pronounced in the M. bovis BCG and M. kansasii isolates but was apparently absent in the M. avium isolate and the rapid growers. Smear sensitivity is independent of viability and solely dependent on buoyancy; therefore, the potential increase in smear sensitivity achieved by switching to the CB-18 processing method is reflected in the CB-18/Tween 80 ratio. This ratio suggests that overcoming buoyancy should increase smear sensitivity by approximately 50%. These results generally agree with the CB-18 pilot study (20), which achieved not only a 58% increase in aggregate smear sensitivity (P < 0.01) but also an increase in smear values when specimens were processed with CB-18. These increases were species-independent (20). The fact that the majority of the MOTT isolates chosen for this study were not buoyant was probably serendipitous. It is possible, however, that the increase in smear sensitivity among MOTT-positive specimens in the CB-18 pilot study (20) was also related to the caustic action of NaOH on the integrity of the cell wall of these bacilli. In addition, because the CB-18/Tween 80 ratios were determined by using culture, and since M. tuberculosis complex mycobacteria are more sensitive to the tuberculocidal action of CB-18 than the MOTT isolates (21), the actual CB-18/Tween 80 ratios among the tuberculous mycobacteria are probably greater than reported.
In general, buoyancy affects overall diagnostic sensitivity, independent of viability, a conclusion that is consistent with the results of Klein et al. (8). These authors showed that (i) 88.8 and 82.4% of all specimens spun for 15 min at 2,000 rpm or 3,000 rpm, respectively, contained cultivatable material in the supernatant following centrifugation; (ii) a small percentage of specimens (11.1 and 17.5%, respectively) contained cultivatable material in the sediment only; and (iii) a small percentage of specimens (2.7 and 2.2%, respectively) contained cultivatable material in the supernatant only. Other studies have examined the issue of buoyancy in vitro and have reported results which are different in some degree from those reported here. For example, the CDC manual (7) indicates that 95% of bacilli can be recovered in 20 min at 3,000 × g. Gebre et al. (3) used 51Cr-labeled M. bovis BCG and suggested that a 5 min spin at 2,400 × g was sufficient to sediment all cells. Ratnam and March (11) processed M. tuberculosis with NALC (without NaOH) and reported that 80.2% ± 8.2% of the bacilli could be recovered at 3,895 × g in 20 min. While the CDC manual does not provide extensive experimental details, Gebre et al. (3) used radioactivity to monitor recovery. Following 51Cr labeling, the cells were washed four to six times at 2,400 × g for 10 min (9a). Washing out the unincorporated 51Cr would leave a population of bacilli that would sediment efficiently at 2,400 × g, with recoveries approaching 100%, regardless of processing method.
The Ratnam and March (11) in vitro study closely resembles the design scheme employed in these CB-18 processing comparisons. The primary differences were the isolate(s) tested, the medium used to grow the isolates, and the solution used to prepare the bacterial stocks for processing. Ratnam and March (11) used L-J slants to grow the bacilli and prepared the bacterial stocks in Dubos-Tween 80 medium prior to sonication and clarifying centrifugation steps, while the CB-18 processing comparisons used isolates grown on 7H11-selective slants and utilized water to prepare the bacterial stocks for sonication and clarifying spins. Silverstolpe (16) examined the buoyant density of tubercle bacilli and reported the specific gravity as 0.79 to 1.07, with the average just below 1.0. More importantly, Silverstolpe (16) states that the specific gravity of the bacilli varies according to the cultivation medium, and bacilli grown on L-J slants have a higher specific gravity than bacilli grown on “…glycerin broth or Dubos’ broth.” Consequently, in the experiments of Ratnam and March (11), either the bacilli employed had a higher specific gravity as a result of the medium used to cultivate and/or prepare the cells or the selected isolates were not buoyant, as was observed with some of the isolates described herein.
It should be noted that the difference in recovery between the detergent-based Tween 80 and CB-18 methods demonstrates that surface tension is not a factor in losses during specimen processing because both detergents were used above their respective critical micellar concentrations (CMC): the CMC of CB-18 is 40 μM (23) and the CMC of Tween 80 is 12 μM (5). Therefore, surface tension was completely negated in both conditions and differences in recovery must result from the inherent buoyancy of the mycobacteria. This conclusion is also consistent with the report of Robinson and Stovall (14) wherein surface active agents were added to processing solutions in a failed attempt to improve recovery during centrifugation.
While it might seem remarkable that two-thirds of the plates analyzed following NALC-NaOH processing presented 10 or fewer CFU, these results are consistent with the previously described toxicity of NaOH (9, 10, 24, 26, 28). For example, if the average input in the M. tuberculosis complex experiments was approximately 400 CFU (see Table 1), approximately two-thirds of the bacilli were killed by NALC-NaOH, there was a twofold loss due to buoyancy, and approximately 15% of the resuspended sediment was analyzed per plate, then approximately 10 CFU per plate would be expected. By contrast, if approximately 10% of M. tuberculosis complex bacilli were killed during CB-18 processing, but none were lost due to buoyancy, and approximately 15% of the resuspended sediment was analyzed per plate, then approximately 54 CFU per plate would be expected. The higher than expected recovery may be related to CB-18’s ability to alleviate cording (19). While individual results would no doubt be species and strain dependent, these a priori calculations are consistent with the results of the study herein. Perhaps the most striking result of this study is the variability within this genus regarding behavior in the processing assay.
The ability of the CB-18 processing method to improve smear and culture sensitivity via increased recovery and dispersion should also translate to improved sensitivities of nucleic acid amplification assays. Two studies have combined the CB-18 processing method with PCR and significant improvements in the detection of mycobacterioses were reported (2, 22). Premarket approval submissions to the Food and Drug Administration for diagnostic kits based on nucleic acid amplification reported a false-negative rate of approximately 50% (1). The improvements provided by CB-18 processing should reduce this false-negative rate, thereby permitting amplification-based assays to be proficiently used as screening tests to detect tuberculosis in its earliest stages yet still allow cultural analysis of processed sediments for isolation and eventual susceptibility testing of the pathogen.
In this study, NALC-NaOH processing produced highly variable results while CB-18 and Tween 80 processing consistently recovered significantly more input CFU. Although Tween 80 processing provided greater viability than NALC-NaOH processing among those organisms recovered, Tween 80 has no decontamination capacity and cannot overcome buoyancy. In contrast, CB-18 processing also provides greater viability but does overcome buoyancy and does provide some decontamination capabilities (21). The CB-18 pilot study (20) confirmed that CB-18 processing provides increased diagnostic sensitivity by smear and culture. The companion study (21) showed that a resuspension buffer containing lytic enzymes and lecithin provides more consistent results in culture by reducing the contamination rate and alleviating the toxic effects of CB-18. The present in vitro study demonstrates that improvements in culture sensitivity can be achieved by eliminating the incubation step, and furthermore, that additional improvements in diagnostic sensitivity are possible by using greater concentrations of CB-18 when using solid medium. Unfortunately, higher concentrations of CB-18 do not produce similar improvements in liquid culture sensitivity.
In summary, the CB-18 procedure can be modified to include higher concentrations of CB-18 or longer incubation times when smear or amplification is the primary diagnostic technique for sediment analysis (i.e., when viability is not a prerequisite for analysis). However, shorter incubation times should be used if analysis by culture is to be included, and reduced CB-18 concentrations are essential if liquid culture is to be used. Incorporating the CB-18 processing method with the previously described improvements (21) as well as the additional refinements described in this article provides superior clinical detection of these important human pathogens.
REFERENCES
- 1.Catanzaro A, Davidson B L, Fujiwara P I, Goldberger M J, Gordin F, Salfinger M, Sbarbaro J, Schluger N W, Sierra M F, Woods G L. Rapid diagnostic tests for tuberculosis. What is the appropriate use? Am J Respir Crit Care Med. 1997;155:1804–1814. doi: 10.1164/ajrccm.155.5.9154896. [DOI] [PubMed] [Google Scholar]
- 2.Cornejo B J, Sahagún-Ruiz A, Suárez-Güemes F, Thornton C G, Ficht T A, Adams L G. Comparison between the use of C18-carboxypropylbetaine and glass bead DNA extraction methods for detection of Mycobacterium bovis in bovine milk samples and analysis of samples by PCR. Appl Environ Microbiol. 1998;64:3099–3101. doi: 10.1128/aem.64.8.3099-3101.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gebre N, Karlsson U, Jonsson G, Macaden R, Wolde A, Assefa A, Miorner H. Improved microscopical diagnosis of pulmonary tuberculosis in developing countries. Trans R Soc Trop Med Hyg. 1995;89:191–193. doi: 10.1016/0035-9203(95)90491-3. [DOI] [PubMed] [Google Scholar]
- 4.Hanks J H, Clark H F, Feldman H. Concentration of tubercle bacilli from sputum by chemical flocculation methods. J Lab Clin Med. 1938;23:736–746. [Google Scholar]
- 5.Helenius A, McCaslin D R, Fries E, Tanford C. Properties of detergents. Methods Enzymol. 1979;56:734–749. doi: 10.1016/0076-6879(79)56066-2. [DOI] [PubMed] [Google Scholar]
- 6.Hobby G L, Holman A P, Iseman M D, Jones J M. Enumeration of tubercle bacilli in sputum of patients with pulmonary tuberculosis. Antimicrob Agents Chemother. 1973;4:94–104. doi: 10.1128/aac.4.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kent P T, Kubica G P. Public health mycobacteriology. A guide for the level III laboratory. Atlanta, Ga: Centers for Disease Control and Prevention; 1985. [Google Scholar]
- 8.Klein G C, Maltz M, Cummings M M, Fish C H. Efficacy of centrifugation as a method of concentrating tubercle bacilli. Am J Clin Pathol. 1952;22:581–585. doi: 10.1093/ajcp/22.6_ts.581. [DOI] [PubMed] [Google Scholar]
- 9.Krasnow I, Wayne L G. Sputum digestion. I. The mortality rate of tubercle bacilli in various digestion systems. Am J Clin Pathol. 1966;45:352–355. [PubMed] [Google Scholar]
- 9a.Miörner, H. Personal communication.
- 10.Mitchison D A, Allen B W, Carrol L, Dickinson J M, Aber V R. A selective oleic acid albumin agar medium for tubercle bacilli. J Med Microbiol. 1972;5:165–175. doi: 10.1099/00222615-5-2-165. [DOI] [PubMed] [Google Scholar]
- 11.Ratnam S, March S B. Effect of relative centrifugal force and centrifugation time on sedimentation of mycobacteria in clinical specimens. J Clin Microbiol. 1986;23:582–585. doi: 10.1128/jcm.23.3.582-585.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rickman T W, Moyer N P. Increased sensitivity of acid-fast smears. J Clin Microbiol. 1980;11:618–620. doi: 10.1128/jcm.11.6.618-620.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts G D, Koneman E W, Kim Y K. Mycobacterium. In: Balows A, Hausler W J Jr, Herrmann K L, Isenberg H D, Shadomy H J, editors. Manual of clinical microbiology. 5th ed. Washington, D.C: American Society for Microbiology; 1991. pp. 304–339. [Google Scholar]
- 14.Robinson L, Stovall W D. Factors influencing the demonstration of tubercle bacilli by concentration methods. J Lab Clin Med. 1941;27:84–91. [Google Scholar]
- 15.Schaefer W B, Lewis W., Jr Effect of oleic acid on growth and cell structure of mycobacteria. J Bacteriol. 1965;90:1438–1447. doi: 10.1128/jb.90.5.1438-1447.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silverstolpe L. Förbättrad metod för påvisande av tuberkelbakterier. Nord Med. 1948;40/48:2220–2222. [Google Scholar]
- 17.Sommers H M, Good R C. Mycobacterium. In: Lennette E H, Barlows A, Hausler W J Jr, Shadomy H J, editors. Manual of clinical microbiology. 4th ed. Washington, D.C: American Society for Microbiology; 1985. pp. 216–248. [Google Scholar]
- 18.Thornton C G, Llorin O J, Wolfe D M, Romagnoli M, Hooper N, Turner J, Lim R G, Merz W G, Libonati J P, Joseph J M, Schwalbe R S, Moody M, Passen S. Abstracts of the 96th General Meeting of the American Society for Microbiology. Washington, D.C: American Society for Microbiology; 1996. A novel method for processing mycobacteria using C18-carboxypropylbetaine, abstr. U-50; p. 109. [Google Scholar]
- 19.Thornton, C. G. August 1997. Methods for processing mycobacteria. U.S. patent 5,658,749.
- 20.Thornton C G, MacLellan K M, Brink T L, Jr, Lockwood D E, Romagnoli M, Turner J, Merz W G, Schwalbe R S, Moody M, Lue Y, Passen S. Novel method for processing respiratory specimens for detection of mycobacteria by using C18-carboxypropylbetaine: blinded study. J Clin Microbiol. 1998;36:1996–2003. doi: 10.1128/jcm.36.7.1996-2003.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thornton C G, MacLellan K M, Brink T L, Jr, Wolfe D M, Llorin O J, Passen S. Processing respiratory specimens with C18-carboxypropylbetaine: development of a sediment resuspension buffer that contains lytic enzymes to reduce the contamination rate and lecithin to alleviate toxicity. J Clin Microbiol. 1998;36:2004–2013. doi: 10.1128/jcm.36.7.2004-2013.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thornton, C. G., M. R. Cranfield, K. M. MacLellan, T. L. Brink, J. D. Strandberg, E. A. Carlin, J. B. Torrelles, J. N. Maslow, J. L. B. Hasson, D. M. Heyl, S. J. Sarro, D. Chatterjee, and S. Passen. Processing postmortem specimens with C18-carboxypropylbetaine and analysis by PCR to develop an antemortem test for Mycobacterium avium infections in ducks. J. Zoo Wildlife Med., in press. [PubMed]
- 23.Tsujii K, Takkuchi T. Krafft point depression of some zwitter ionic surfactants by inorganic salts II. Yakagaku. 1981;30:495–499. [Google Scholar]
- 24.Wayne L G, Krasnow I, Kidd G. Finding the “hidden positive” in tuberculosis eradication programs. Am Rev Respir Dis. 1962;86:537–541. doi: 10.1164/arrd.1962.86.4.537. [DOI] [PubMed] [Google Scholar]
- 25.Weir M P, Langridge W H R, Walker R W. Relationships between oleic acid uptake and lipid metabolism. Am Rev Respir Dis. 1972;106:450–457. doi: 10.1164/arrd.1972.106.3.450. [DOI] [PubMed] [Google Scholar]
- 26.Yajko D M, Wagner C, Tevere V J, Kocagöz T, Hadley W K, Chambers H F. Quantitative culture of Mycobacterium tuberculosis from clinical sputum specimens and dilution endpoint of its detection by the Amplicor PCR assay. J Clin Microbiol. 1995;33:1944–1947. doi: 10.1128/jcm.33.7.1944-1947.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeager H, Jr, Lacy J, Smith L R, LeMaistre C A. Quantitative studies of mycobacterial populations in sputum and saliva. Am Rev Respir Dis. 1967;95:998–1004. doi: 10.1164/arrd.1967.95.6.998. [DOI] [PubMed] [Google Scholar]
- 28.Yegian D, Budd V. Toxic effect of sodium hydroxide on tubercle bacilli. Am J Clin Pathol. 1952;22:456–460. doi: 10.1093/ajcp/22.5.456. [DOI] [PubMed] [Google Scholar]