Abstract
Background
Despite the known efficacy of colorectal cancer (CRC) screening, the rates of individuals undergoing such testing have remained lower than target thresholds, even prior to the healthcare disruptions associated with the COVID‐19 pandemic. We evaluated the impact of the COVID‐19 pandemic on CRC screening within a nationally representative US population and assessed disparities in screening across racial/ethnic groups and socioeconomic (SES) strata.
Methods
We performed a retrospective cross‐sectional study using all eligible TRICARE beneficiaries aged 45–64 years between FY 2018 and 2021. High‐risk individuals, those with a previous or current CRC diagnosis, and/or a personal/family history of colonic polyps, were excluded. The pre‐COVID‐19 period (September 1, 2018–March 31, 2020) was compared to the COVID‐19 period (April 1, 2020–September 30, 2021). Secondary analyses were performed, evaluating the interaction between the COVID‐19 time period, race, and our proxy for socioeconomic status.
Results
During the study period, we identified 1,749,688 eligible individuals. Following the onset of the COVID‐19 pandemic, CRC screening overall decreased from 34% in the pre‐pandemic period to 30% following the onset of the pandemic (p < 0.001). This finding persisted even after adjusting for confounders in multivariable analysis (odds ratio [OR] for the pandemic timeframe: 0.79; 95% CI: 0.27, 0.31; p < 0.001). In the setting of SES, in the pandemic period, the odds of individuals from both Senior Enlisted (OR: 0.55; 95% CI: 0.54, 0.56) and Junior Enlisted sponsor ranks (OR: 0.27; 95% CI: 0.25, 0.30) were diminished as compared to Senior Officers.
Conclusions and Relevance
We found a 21% reduction in the odds of CRC screening in the context of the COVID‐19 pandemic. Reductions in colonoscopies and other types of screening tests were not offset by changes in the use of at‐home tests such as Cologuard.
Keywords: cancer prevention, colorectal cancer, epidemiology and prevention, screening
1. INTRODUCTION
Although the rates of colorectal cancer (CRC) diagnoses have diminished over the last four decades, this condition remains the second leading cause of cancer deaths in the United States, and the incidence is rising in individuals under age 50. 1 The lifetime risk of CRC overall is 4%, and in 2019, there were approximately 1.4 million people living with this condition in the US. 2 CRC is anticipated to cause over 50,000 deaths in 2023 alone and has the second highest treatment costs of any cancer at 23.7 billion USD per year. 3 Screening for CRC has been shown to assist in early detection, which not only improves treatment efficacy and survival but may also reduce healthcare expenditures. 1 , 3
Despite the known efficacy of CRC screening, the rates of individuals undergoing such testing have remained lower than target thresholds, even prior to the healthcare disruptions precipitated by the COVID‐19 pandemic. 1 , 2 , 4 , 5 Disparities were already identified in the pre‐COVID‐19 window among minorities and those from lower socioeconomic strata (SES). 6 , 7 , 8 These differences in access to screening as well as utilization may have been exacerbated further by disruptions resulting from COVID‐19, including the restricted performance of elective interventions such as colonoscopies. 4 , 5 , 9 , 10 , 11 , 12 At this time, reduced screening for CRC in the early stages of the pandemic is broadly acknowledged. 13 , 14 , 15 However, specific impacts on underserved communities and changes in the use of screening modalities such as colonoscopy, home fecal immunochemical, or DNA testing kits have not been adequately explored. Previous work in this area may have been limited by geographic and sociodemographic restrictions in the populations under study, surveillance windows that only considered the early phases of the COVID‐19 pandemic, and cohorts that may not have been representative of the broader population eligible for CRC screening on the whole.
In this context, we sought to evaluate the influence of the COVID‐19 pandemic on the utilization of CRC screening among a representative US population, specifically TRICARE beneficiaries receiving services from the Military Health System (MHS). The use of TRICARE claims data make it possible to examine a large, universally insured cohort of individuals aged 45–64 who come from different racial and ethnic backgrounds and possess diverse socioeconomic, educational, and occupational characteristics. 16 , 17 , 18 , 19 , 20 , 21 , 22 Use of TRICARE claims allowed us to determine individuals who received CRC screening regardless of their location, whether it was at home through mailed fecal testing kits or colonoscopy or other procedures performed in civilian healthcare settings (private sector care) or Department of Defense (DoD) hospitals (direct care). 18 , 20 , 21 We believe that these attributes enable a more accurate assessment of the impact of the pandemic on CRC screening over a longer time frame and in a representative US cohort. Informed by prior studies, 6 , 7 , 12 we hypothesized that CRC screening would decrease in the setting of the COVID‐19 pandemic and that disparities would be accentuated among racial minorities and individuals from lower SES.
2. METHODS
2.1. Data source and study cohort
This investigation employed TRICARE claims data that were derived from the Military Health System Data Repository (MDR). TRICARE, the health insurance plan of the DoD, provides healthcare coverage to active‐duty personnel, retirees, those medically retired with more than 30% disability, as well as dependents. 16 , 17 , 18 , 19 , 20 , 21 , 22 TRICARE beneficiaries are able to access care through direct care institutions maintained by the DoD, as well as via civilian facilities where TRICARE operates as an insurance product within a fee‐for‐service setting (private‐sector care). 17 , 18 , 20 , 21 , 22 As a result, TRICARE is among the largest healthcare insurance programs in the United States, with approximately 9.6 million participating beneficiaries. 16 , 17 The majority of these are civilian dependents or retirees, estimated at 80% of the covered population. 18
The processes used to collect TRICARE claims as well as the means by which data are prepared and made accessible to researchers have been outlined in prior publications. 18 , 19 , 20 , 22 The MDR captures claims associated with both inpatient and outpatient encounters, irrespective of healthcare resource utilization, and captures both inpatient and outpatient services regardless of the site of service (e.g., DoD or civilian facility). Care delivered through the Veterans Administration (VA) system is not administered through TRICARE or captured in the MDR. 18
We used the MDR records from September 1, 2018 to September 30, 2021, to identify TRICARE beneficiaries aged 45–64, at standard risk for CRC and eligible for screening. High‐risk individuals, as well as those with a previous or current diagnosis of CRC, related gastrointestinal disorders, and/or personal or family history of colonic polyps, were excluded (Figure 1). We abstracted data from the records of individuals eligible for inclusion to obtain age, race, biologic sex, sponsor rank, beneficiary category, branch of service affiliation, and healthcare service setting (direct or private sector). Race was recorded as documented in the MDR and based on individual self‐report. Our racial categories consisted of White, Black, Asian/Pacific Islander, American Indian/Alaskan Native, Other (e.g., mixed race/multiple race), and Missing. The MDR data does not specify ethnicity, so we were unable to identify those patients of Hispanic ethnicity. As such, White Hispanic patients are grouped in the White cohort, and Hispanic patients with Afro‐Caribbean ancestry would be categorized in the Black cohort. Sponsor rank was classified as Junior Enlisted, Senior Enlisted, Junior Officer, and Senior Officer. Aligned with previous research that supports the use of sponsor rank as a proxy for socioeconomic status, 18 , 19 , 20 , 23 , 24 we considered enlisted individuals as representative of lower SES and compared these classes to Senior Officers as the referent.
FIGURE 1.
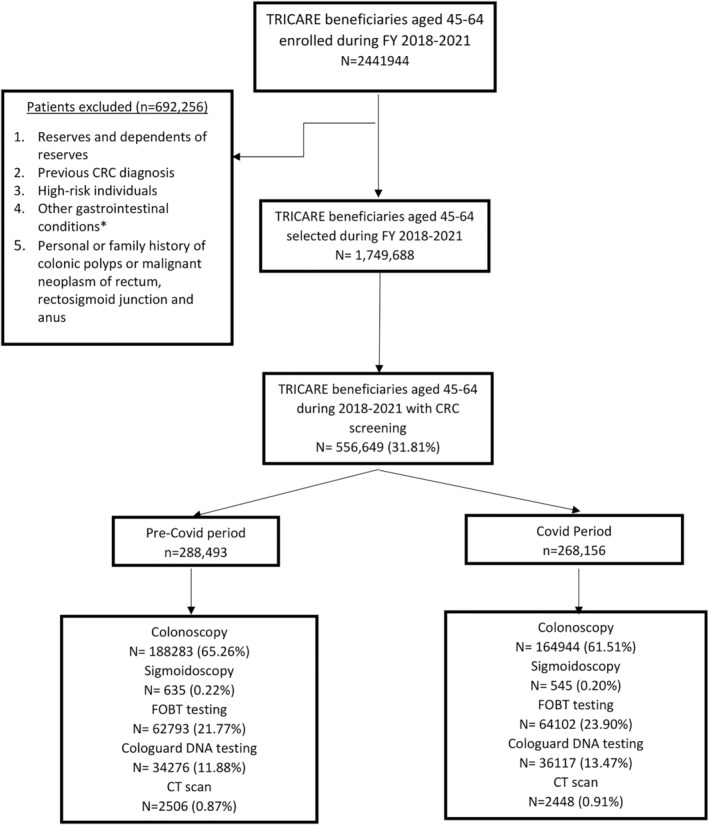
Study patients. CRC, colorectal cancer; FY, fiscal year. *prior diagnosis of diverticulitis, inflammatory bowel disease, Crohn's disease, ulcerative colitis or any benign/malignant lesions of colon.
CRC screening events were considered the first procedure performed within the study period based on Current Procedural Terminology and Healthcare Common Procedure Coding System codes (Table S1) for an eligible individual.
2.2. Statistical analysis
We compared the rates of CRC screening across two time‐windows 4 , 5 , 9 , 10 , 11 , 12 : the pre‐COVID‐19 period (September 1, 2018–March 31, 2020) and the COVID‐19 period (April 1, 2020–September 30, 2021). Bivariate and multivariable logistic regression were used to assess for changes in CRC screening based on the time period relative to the COVID‐19 pandemic. Multivariable testing was used to adjust for confounders in models that included age, biologic sex, race, sponsor rank, beneficiary category, and service branch. In line with prior research, 18 , 19 missing race was handled using imputation with reweighted estimating equations in all adjusted analyses. In this setting, enrollees with missing race were excluded, and reweighted estimating equations were applied to adjust the remaining data. This is a previously validated technique that has been suggested as the acceptable approach to address missing race data in military healthcare registries. 18 , 19
In secondary testing, we assessed the interaction between CRC screening and race and sponsor rank in the time periods relative to the COVID‐19 pandemic. Statistical significance was defined, a priori, as variables with an odds ratio (OR) and 95% confidence interval (CI) exclusive of 1.0 with a p < 0.05. This research was found exempt by the Institutional Review Board of the Uniformed Services University of the Health Sciences. All statistical analyses were performed using SAS v9.4 (SAS Institute).
3. RESULTS
During the study period, we identified 1,749,688 eligible individuals. Among these, 556,649 (32%) underwent CRC screening (Figure 1). There were 288,492 screenings performed in the pre‐COVID‐19 period and 268,156 in the COVID‐19 period. There were differences in the sociodemographic composition of the cohorts based on time periods that were statistically significant given the size of our sample (Table 1). In terms of the eligible population as a whole, all modes of testing were diminished between the two time frames, with essentially no meaningful change in the rate of Cologuard DNA testing (4% in both epochs). Following the onset of the COVID‐19 pandemic, CRC screening overall decreased from 34% in the pre‐pandemic period to 30% following the onset of the pandemic (p < 0.001). This finding persisted even after adjusting for confounders in multivariable analysis (OR for the pandemic timeframe: 0.79; 95% CI: 0.27, 0.31; p < 0.001; Table 2). Among those receiving testing, the use of colonoscopy significantly decreased from the pre‐COVID‐19 period (65%) to the COVID‐19 period (62%; p < 0.001), while the use of fecal occult blood testing (22% vs. 24%; p < 0.001) and Cologuard DNA testing (12% vs. 13%; p < 0.001) both significantly increased. When compared to colonoscopy as the referent, the odds of receiving fecal occult blood testing increased by 19% in the COVID‐19 period (OR: 1.19; 95% CI: 1.17, 1.21), while that of Cologuard DNA testing increased by 21% (OR: 1.21; 95% CI: 1.19, 1.24).
TABLE 1.
Baseline characteristics.
| All encounters | Pre‐COVID‐19 period | COVID‐19 period | p‐value | |
|---|---|---|---|---|
| N = 1,749,688 | N = 852,986 | N = 896,702 | ||
| N (%) | N (%) | N (%) | ||
| Sex | ||||
| Female | 873,081 (49.90) | 425,243 (49.85) | 447,838 (49.94) | Reference |
| Male | 876,590 (50.10) | 427,734 (50.15) | 448,856 (50.06) | 0.238 |
| Age group | ||||
| 45–49 | 613,708 (35.08) | 276,878 (32.46) | 336,830 (37.56) | <0.001 |
| 50–54 | 404,510 (23.12) | 206,799 (24.24) | 197,711 (22.05) | <0.001 |
| 55–59 | 391,864 (22.40) | 200,102 (23.46) | 191,762 (21.39) | <0.001 |
| 60–64 | 339,606 (19.41) | 169,207 (19.84) | 170,399 (19.00) | Reference |
| Race | ||||
| White | 727,645 (41.59) | 354,354 (41.54) | 373,291 (41.63) | Reference |
| Black | 260,559 (14.89) | 126,866 (14.87) | 133,703 (14.91) | 0.925 |
| Other | 55,648 (3.18) | 26,417 (3.10) | 29,231 (3.26) | <0.001 |
| Asian/Pacific Islander | 81,174 (4.64) | 38,794 (4.55) | 42,380 (4.73) | <0.001 |
| American Indian/Alaska Native | 9253 (0.53) | 4373 (0.51) | 4880 (0.54) | 0.006 |
| Missing | 615,399 (35.17) | 302,182 (35.43) | 313,217 (34.93) | <0.001 |
| Beneficiary category | ||||
| Dependent | 814,386 (46.55) | 393,394 (46.12) | 420,992 (46.95) | <0.001 |
| Retiree | 799,458 (45.69) | 398,524 (46.72) | 400,934 (44.71) | <0.001 |
| Active duty | 135,289 (7.73) | 60,815 (7.13) | 74,474 (8.31) | Reference |
| Other | 538 (0.03) | 245 (0.03) | 293 (0.03) | 0.787 |
| Service | ||||
| Army | 648,979 (37.09) | 313,546 (36.76) | 335,433 (37.41) | Reference |
| Air Force | 521,445 (29.80) | 257,813 (30.22) | 263,632 (29.40) | <0.001 |
| Navy | 425,977 (22.90) | 207,478 (23.12) | 218,499 (22.70) | <0.001 |
| Marines | 113,901 (6.51) | 54,601 (6.40) | 59,300 (6.61) | 0.019 |
| Other | 48,056 (2.75) | 22,875 (2.68) | 25,181 (2.81) | 0.003 |
| Rank | ||||
| Senior Enlisted | 1,262,607 (72.17) | 617,586 (72.41) | 645,021 (71.94) | 0.653 |
| Senior Officer | 257,393 (14.71) | 126,025 (14.78) | 131,368 (14.65) | Reference |
| Junior Officer | 207,964 (11.89) | 99,387 (11.65) | 108,577 (12.11) | <0.001 |
| Junior Enlisted | 21,567 (1.23) | 9903 (1.16) | 11,664 (1.30) | <0.001 |
| Sector (n = 556,649) | ||||
| Private | 389,292 (69.93) | 202,820 (70.30) | 186,472 (69.54) | <0.001 |
| Direct | 167,357 (30.07) | 85,673 (29.70) | 81,684 (30.46) | Reference |
| CRC Screening overall | 556,649 (31.81) | 288,493 (33.82) | 268,156 (29.90) | <0.001 |
| Colonoscopy | 353,227 (20.19) | 188,283 (22.07) | 164,944 (18.39) | Reference |
| Sigmoidoscopy | 1180 (0.07) | 635 (0.07) | 545 (0.06) | 0.727 |
| FOBT testing | 126,895 (7.25) | 62,793 (7.36) | 64,102 (7.15) | <0.001 |
| Cologuard DNA testing | 70,393 (4.02) | 34,276 (4.02) | 36,117 (4.03) | <0.001 |
| CT scan | 4954 (0.28) | 2506 (0.29) | 2448 (0.27) | <0.001 |
TABLE 2.
Factors associated with colorectal cancer screening for the cohort as a whole.
| CRC Screening No | CRC Screening Yes | Multivariable analysis Odds ratio (95% CI) | p‐value | |
|---|---|---|---|---|
| N = 1,193,039 | N = 556,649 | N = 1,134,289 | ||
| N (%) | N (%) | |||
| Sex | ||||
| Female | 584,537 (49.00) | 288,544 (51.84) | Reference | |
| Male | 608,486 (51.00) | 268,104 (48.16) | 0.97 (0.96–0.99) | <0.001 |
| Age group | ||||
| 45–49 | 430,166 (36.06) | 183,542 (32.97) | 1.09 (1.07–1.11) | <0.001 |
| 50–54 | 260,403 (21.83) | 144,107 (25.89) | 1.44 (1.42–1.46) | <0.001 |
| 55–59 | 254,074 (21.30) | 137,790 (24.75) | 1.48 (1.46–1.50) | <0.001 |
| 60–64 | 248,396 (20.82) | 91,210 (16.39) | Reference | – |
| Race | ||||
| White | 494,314 (41.43) | 233,331 (41.92) | – | Reference |
| Black | 178,836 (14.99) | 81,733 (14.68) | 1.07 (1.05–1.08) | <0.001 |
| Other | 36,933 (3.10) | 18,715 (3.36) | 1.08 (1.06–1.10) | <0.001 |
| Asian/Pacific Islander | 56,818 (4.76) | 24,356 (4.38) | 0.92 (0.90–0.93) | <0.001 |
| American Indian/Alaska Native | 6685 (0.56) | 2568 (0.46) | 0.81 (0.76–0.86) | <0.001 |
| Missing | 419,453 (35.16) | 195,946 (35.20) | – | – |
| Beneficiary category | ||||
| Active duty | 79,086 (6.63) | 56,203 (10.10) | – | Reference |
| Dependent | 547,425 (45.89) | 266,961 (47.96) | 0.70 (0.69–0.71) | <0.001 |
| Retiree | 566,071 (47.45) | 233,387 (41.93) | 0.63 (0.62–0.64) | <0.001 |
| Other | 448 (0.04) | 90 (0.02) | 0.21 (0.14–0.30) | <0.001 |
| Service | ||||
| Army | 445,558 (37.35) | 203,421 (36.54) | – | Reference |
| Air Force | 355,374 (29.79) | 166,071 (29.83) | 1.00 (0.99–1.02) | 0.202 |
| Navy | 283,340 (23.75) | 133,942 (24.06) | 0.99 (0.97–1.00) | <0.001 |
| Marines | 78,916 (6.61) | 34,985 (6.28) | 0.87 (0.85–0.89) | <0.001 |
| Other | 29,827 (2.50) | 18,229 (3.27) | 1.12 (1.08–1.15) | <0.001 |
| Rank | ||||
| Senior Officer | 883,853 (74.09) | 378,754 (68.05) | – | Reference |
| Senior Enlisted | 155,327 (13.02) | 102,066 (18.34) | 0.62 (0.61–0.63) | <0.001 |
| Junior Officer | 136,850 (11.47) | 71,114 (12.78) | 0.81 (0.79–0.83) | <0.001 |
| Junior Enlisted | 16,891 (1.42) | 4676 (0.84) | 0.28 (0.27–0.31) | <0.001 |
| COVID‐19 | 628,546 (52.68) | 268,156 (48.17) | 0.79 (0.78–0.80) | <0.001 |
In our secondary analyses, we found that prior to the COVID‐19 pandemic, Asian/Pacific Islanders (OR: 0.94; 95% CI: 0.92, 0.96) and American Indian/Alaskan natives (OR: 0.83; 95% CI: 0.78, 0.89) were less likely to receive CRC screening as compared to Whites (Table 3). Black beneficiaries had a slightly higher likelihood of undergoing screening (OR: 1.02; 95% CI: 1.00, 1.03) compared to Whites. In the COVID‐19 period, Black beneficiaries demonstrated lower odds of undergoing colorectal screening (OR: 0.87; 95% CI: 10.86, 0.89), while those of Asian/Pacific Islanders (OR: 0.76; 95% CI: 0.75, 0.78) and American Indians/Alaskan Natives were also lower (OR: 0.64; 95% CI: 0.60, 0.69).
TABLE 3.
Factors associated with colorectal cancer screening assessing the interaction between race and COVID‐19 period.
| Multivariable analysis Odds ratio (95% CI) | p‐value | |
|---|---|---|
| N = 1,134,289 | ||
| Sex | ||
| Female | Reference | |
| Male | 0.91 (0.90–0.92) | <0.001 |
| Age group | ||
| 45–49 | 1.04 (1.03–1.05) | <0.001 |
| 50–54 | 1.39 (1.37–1.41) | <0.001 |
| 55–59 | 1.43 (1.41–1.45) | <0.001 |
| 60–64 | Reference | – |
| Race | ||
| White pre‐COVID‐19 | – | Reference |
| Black pre‐COVID‐19 | 1.02 (1.00–1.03) | 0.014 |
| Asian/Pacific Islander pre‐COVID‐19 | 0.94 (0.92–0.96) | <0.001 |
| American Indian/Alaska Native pre‐COVID‐19 | 0.83 (0.78–0.89) | <0.001 |
| Other pre‐COVID‐19 | 1.11 (1.08–1.14) | <0.001 |
| White and COVID‐19 | 0.81 (0.80–0.82) | <0.001 |
| Black and COVID‐19 | 0.87 (0.86–0.89) | <0.001 |
| Asian/Pacific Islander and COVID‐19 | 0.76 (0.75–0.78) | <0.001 |
| American Indian/Alaska Native and COVID‐19 | 0.64 (0.60–0.69) | <0.001 |
| Other and COVID‐19 | 0.88 (0.86–0.90) | <0.001 |
| Beneficiary category | ||
| Active duty | – | Reference |
| Dependent | 0.75 (0.74–0.76) | <0.001 |
| Retiree | 0.61 (0.60–0.62) | <0.001 |
| Other | 1.20 (1.17–1.23) | <0.001 |
| Service | ||
| Army | – | Reference |
| Air Force | 1.06 (1.05–1.07) | <0.001 |
| Navy | 1.06 (1.05–1.07) | <0.001 |
| Marines | 0.97 (0.96–0.99) | 0.002 |
| Other | 1.20 (1.17–1.23) | <0.001 |
| Rank | ||
| Senior Officer | – | Reference |
| Senior Enlisted | 0.66 (0.65–0.67) | <0.001 |
| Junior Officer | 0.83 (0.82–0.84) | <0.001 |
| Junior Enlisted | 0.36 (0.34–0.38) | <0.001 |
In the setting of sponsor rank, our proxy for socioeconomic status, prior to the pandemic, both Senior Enlisted (OR: 0.66; 95% CI: 0.65, 0.67) and Junior Enlisted (OR: 0.40; 95% CI: 0.36, 0.43) were less likely to undergo CRC screening as compared to Senior Officers. These findings persisted in the pandemic period, with the odds of individuals from both Senior Enlisted (OR: 0.55; 95% CI: 0.54, 0.56) and Junior Enlisted sponsor ranks (OR: 0.27; 95% CI: 0.25, 0.30; Table 4) diminishing as compared to Senior Officers.
TABLE 4.
Factors associated with colorectal cancer screening assessing the interaction between sponsor rank (our proxy for socioeconomic status) and COVID‐19 period.
| Multivariable analysis × Odds ratio (95% CI) | p‐value | |
|---|---|---|
| N = 1,134,289 | ||
| Sex | ||
| Female | Reference | |
| Male | 0.91 (0.90–0.92) | <0.001 |
| Age group | ||
| 45–49 | 1.04 (1.03–1.05) | <0.001 |
| 50–54 | 1.39 (1.37–1.41) | <0.001 |
| 55–59 | 1.43 (1.41–1.45) | <0.001 |
| 60–64 | Reference | – |
| Race | ||
| White | – | Reference |
| Black | 1.07 (1.05–1.08) | <0.001 |
| Other | 1.10 (1.08–1.12) | <0.001 |
| Asian/Pacific Islander | 0.94 (0.92–0.95) | <0.001 |
| American Indian/Alaska Native | 0.81 (0.77–0.85) | <0.001 |
| Beneficiary category | ||
| Active duty | – | Reference |
| Dependent | 0.75 (0.74–0.76) | <0.001 |
| Retiree | 0.61 (0.60–0.62) | <0.001 |
| Other | 0.22 (0.15–0.31) | <0.001 |
| Service | ||
| Army | – | Reference |
| Air Force | 1.06 (1.05–1.07) | <0.001 |
| Navy | 1.06 (1.05–1.07) | <0.001 |
| Marines | 0.97 (0.96–0.99) | 0.002 |
| Other | 1.19 (1.17–1.23) | <0.001 |
| Rank | ||
| Senior Officer pre‐COVID‐19 | – | Reference |
| Senior Enlisted pre‐COVID‐19 | 0.66 (0.65–0.67) | <0.001 |
| Junior Officer pre‐COVID‐19 | 0.85 (0.83–0.87) | <0.001 |
| Junior Enlisted pre COVID‐19 | 0.40 (0.36–0.43) | <0.001 |
| Senior Officer and COVID‐19 | 0.83 (0.81–0.85) | <0.001 |
| Senior Enlisted and COVID‐19 | 0.55 (0.54–0.56) | <0.001 |
| Junior Officer and COVID‐19 | 0.68 (0.66–0.69) | <0.001 |
| Junior Enlisted and COVID‐19 | 0.27 (0.25–0.30) | <0.001 |
4. DISCUSSION
In this investigation, we sought to evaluate the impact of the COVID‐19 pandemic on rates of CRC screening among TRICARE beneficiaries in the MHS. While putative details on this topic have been published in the past, 13 , 14 , 15 , 25 we believed that our use of national healthcare data from a representative US population over a wider timeframe, beyond the initial phase of the pandemic, could provide more comprehensive information that would also enjoy greater generalizability. Further, the stability of the population insured through TRICARE 18 and lower concerns regarding loss of insurance associated with work restrictions during COVID‐19 may mean that our data provide more robust estimates regarding the impact of the pandemic itself on CRC screening. The results indicate a 21% reduction in the odds of CRC screening in 2020–2021 as compared to pre‐pandemic levels. When accounting for differences in the population of eligible individuals between the pre‐ and post‐pandemic periods, reductions across the board were seen in the use of colonoscopy, sigmoidoscopy, fecal occult testing, and CT screening. Moreover, disparities in receipt of CRC screening among Asians, Pacific Islanders, American Indians, and Alaskan Natives, as well as those of lower SES, either persisted or worsened during the COVID‐19 pandemic.
Our findings are consistent with several other investigations on the impact of COVID‐19 on cancer screening as well as the general utilization of such services among historically underserved populations. For example, researchers from the National Cancer Institute's Population‐based Research to Optimize the Screening Process (PROSPR) reported that CRC screening rates fell by 82% during the first 6 months of 2020 compared to 2019. 25 In an insured US population, Oakes and colleagues reported a 10%–18% reduction in the use of CRC screening during quarter 32,020 to quarter 42,021, as compared to pre‐pandemic numbers. 15 Similar figures were encountered for a Japanese cohort, 14 while a 23% reduction in CRC screening was reported in South Korea. 13 The 21% reduction in screening encountered in our own data appears consistent with the latter published reports and endorses the external validity of our findings. Similarly, the reduced use of screening among individuals of lower socioeconomic class, Asian, and the Native American population prior to the pandemic is aligned with other reports from this period. 6 , 7 , 8 , 26
Outside of our determinations regarding reduced colorectal screening overall, the most concerning findings are those that indicate persistent disparities in the use of these services among African Americans, Asians, Pacific Islanders, and Native Americans/Alaskans, as well as individuals of lower socioeconomic backgrounds. Worsening disparities among Native Americans/Alaskans, based on socioeconomic status, were also evident in the pandemic period. In a previous investigation, Perdue et al. had suggested that differences in the use of CRC screening among average‐risk American Indians and Alaskan Natives could be attributed to issues around access and were largely eliminated in the setting of equal access to care in the setting of health maintenance organizations. 8 As the MHS is a universally insured healthcare system, with several studies substantiating reduced racial healthcare disparities within the covered population, 16 , 20 , 22 the results of our work would appear to contest this claim.
The current findings also countermand reports that enhanced utilization of home‐based tests, such as Cologuard or fecal immunochemical assays, offset the downturn in colonoscopies that occurred over the course of the pandemic. For example, in their study using the NHIS Liu, and Murphy reported that the use of home tests resulted in an overall increase in screening in 2021, particularly among adults over 65, Blacks and Hispanics, and those with lower incomes and educational levels. 27 These results were not replicated in our investigation. Indeed, there was no demonstrable change in the use of Cologuard testing between the pre‐ and post‐pandemic periods, while all other forms of screening were reduced. The differences between our study and that of Liu and Murphy 27 may be explained by the fact that our population is under the age of 65 and possesses larger numbers of Asians, American Indians, and Alaskans, which enabled the detection of meaningful differences.
While additional validation in other studies is therefore warranted, given the characteristics of the population insured through TRICARE, 16 , 17 , 18 , 19 , 20 , 21 , 22 we believe our findings can be assumed to be translatable to the average‐risk US population between ages 50 and 64. At a minimum, the results herald the need for increased outreach to Asians, Native American, and Alaskans, as well as individuals of all races of lower socioeconomic status, to improve uptake of colorectal screening in these groups. There is also a need for increased investigation into the etiologies behind reductions in screening identified among Native Americans/Alaskans and those of lower socioeconomic status and whether these result from attitudes toward healthcare utilization in general, concerns around accessing healthcare in the pandemic environment, vaccine penetrance in these subpopulations, or other related factors. The lack of increased utilization of home tests for colorectal screening might indicate this is an opportunity to explore interest in and promote these more convenient alternatives to colonoscopy, in the overall covered population as well as in the subgroups that demonstrated significant disparities in screening. Experience in the VA has heralded the feasibility of institutional programs that use mailed fecal immunochemical testing to engage eligible patients. 28 The program is maintained to reach a greater number of eligible individuals, including those who did not seek in‐person primary care, and reduce demand for colonoscopies, which may be advantageous given the backlogs that have resulted from the COVID‐19 shutdowns.
There are several limitations that should be recognized. Foremost, this remains a retrospective investigation subject to the drawbacks associated with such study designs and the reliance on healthcare claims as the main substrate. Next, our research remains restricted in its scope to the pandemic period of April 2020–September 2021 and cannot assess behaviors or changes that occurred beyond this time window. In this regard, it is important to note that this is the most recent data available from the MHS at the time of this writing. As noted already, given the fact that the TRICARE population has only been found to be representative of the US population under age 64, 18 , 19 , 20 , 21 our results should not be extrapolated to those over 65 or individuals insured through Medicare. Patterns of utilization and approaches to colorectal screening among those 65 and older may be different from the cohort we studied here. We cannot rule out the possibility that small numbers of patients may have elected to receive screening covered by other insurance products or the VA. However, given prior work performed in this arena, 18 , 19 , 20 , 21 we do not anticipate that this small cohort of individuals would have an impact on our findings. Finally, some may consider the direct or indirect military affiliation of the covered population a limitation in terms of generalizing to the broader US demographic. We do not believe this is the case, as numerous prior investigations have substantiated the representative nature of the TRICARE population in terms of sociodemographic and clinical variation compared to US civilian cohorts aged 64 and younger. 16 , 17 , 18 , 19 , 20 , 21 , 22 In addition, we wish to emphasize that only 8% of the individuals under study in this analysis were on active duty. Nonetheless, further validation of our findings in other independent populations with similar clinical and demographic variation would be necessary before definitive investment in our determinations is possible.
In conclusion, in this large study examining changes in the use of colorectal screening between the pre‐ and COVID‐19 pandemic periods, we found a 21% reduction in the odds of screening overall. Reductions in colonoscopies and other types of screening tests were not offset by changes in the use of at‐home tests such as Cologuard. Pre‐pandemic disparities appreciated among Asians, Pacific Islanders, American Indians, Alaskan Natives, and those of lower socioeconomic backgrounds persisted in the pandemic timeframe and even worsened for American Indians/Alaskan Natives, and individuals from lower SES. Our findings suggest several avenues for further research into the drivers of these disparities as well as opportunities for improvement. These could include increased outreach and focused initiatives designed to increase utilization of colorectal screening in at‐risk subgroups as well as enhanced promotion of at‐home fecal immunochemical, or DNA testing.
AUTHOR CONTRIBUTIONS
Satish Munigala: Conceptualization (equal); data curation (equal); formal analysis (equal); methodology (equal); writing – original draft (equal); writing – review and editing (equal). Andrew J. Schoenfeld: Conceptualization (equal); methodology (equal); writing – original draft (equal); writing – review and editing (equal). Vivitha Mani: Conceptualization (equal); writing – review and editing (equal). Amanda Banaag: Conceptualization (equal); methodology (equal); writing – review and editing (equal). Ada Umoh: Writing – review and editing (equal). Christian L. Coles: Conceptualization (equal); methodology (equal); writing – original draft (equal); writing – review and editing (equal). Tracey Perez Koehlmoos: Conceptualization (equal); methodology (equal); resources (equal); supervision (equal); writing – original draft (equal); writing – review and editing (equal).
CONFLICT OF INTEREST STATEMENT
The contents, views, or opinions expressed in this manuscript are those of the author(s) and do not necessarily reflect the official policy or position of the Uniformed Services University of the Health Sciences, the Department of Defense, or the Departments of the Army, Navy, or Air Force, or the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. Mention of trade names, commercial products, or organizations does not imply endorsement by the US Government. The authors declare they have no conflicts of interest.
Supporting information
Table S1.
Munigala S, Schoenfeld AJ, Mani V, et al. Disparities in the use of colorectal cancer screening in a universally insured population during the COVID‐19 pandemic. Cancer Med. 2023;12:18201‐18210. doi: 10.1002/cam4.6400
DATA AVAILABILITY STATEMENT
The data contains personal identifiers and are not publicly available. The data that support the findings of this study are available on request from the corresponding author.
REFERENCES
- 1. American Cancer Society . Key statistics for colorectal cancer . 2023. Accessed January 20, 2023. https://www.cancer.org/cancer/colon‐rectal‐cancer/about/key‐statistics.html
- 2. National Cancer Institute . Cancer stat facts: colorectal cancer . 2023. Accessed January 20, 2023. https://seer.cancer.gov/statfacts/html/colorect.html
- 3. Centers for Disesae Control and Prevention . Health and economic benefits of colorectal cancer interventions . 2022. Accessed January 20, 2023. https://www.cdc.gov/chronicdisease/programs‐impact/pop/colorectal‐cancer.htm#:~:text=The%20High%20Cost%20of%20Colorectalof%20all%20cancer%20treatment%20costs.&text=The%20cost%20for%20medical%20services,%240.6%20billion%20for%20prescription%20drugs
- 4. Bakouny Z, Paciotti M, Schmidt AL, Lipsitz SR, Choueiri TK, Trinh QD. Cancer screening tests and cancer diagnoses during the COVID‐19 pandemic. JAMA Oncol. 2021;7:458‐460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fedewa SA, Star J, Bandi P, et al. Changes in cancer screening in the US during the COVID‐19 pandemic. JAMA Netw Open. 2022;5:e2215490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shah SK, Narcisse MR, Hallgren E, Felix HC, McElfish PA. Assessment of colorectal cancer screening disparties in US men and women using a demographically representative sample. Cancer Res Commun. 2022;2:561‐569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iyer HS, Gomez SL, Cheng I, Rebbeck TR. Relative impact of genetic ancestry and neigborhood socioeconomic status on all‐cause mortality in self‐identified African Americans. PLoS One. 2022;17:e0273735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Perdue DG, Chubak J, Bogart A, Dillard DA, Garroute EM, Buchwald D. A comparison of colorectal cancer screening uptake among average‐risk insured American Indian/Alaska Native and White women. J Health Care Poor Underserved. 2013;24:1125‐1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fuchs VR. Health care policy after the COVID‐19 pandemic. JAMA. 2020;324:233‐234. [DOI] [PubMed] [Google Scholar]
- 10. Mattingly AS, Rose L, Eddington HS, et al. Trends in US surgical procedures and health care system response to policies curtailing elective surgical operations during the COVID‐19 pandemic. JAMA Netw Open. 2021;4:e2138038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Webb Hooper M, Nápoles AM, Pérez‐Stable EJ. COVID‐19 and racial/ethnic disparities. JAMA. 2020;323:2466‐2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tsai TC, Bryan AF, Rosenthal N, et al. Variation in use of surgical care during the COVID‐19 pandemic by surgical urgency and race and ethnicity. JAMA Heal Forum. 2021;2:e214214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee K, Lee YY, Jun JK, Park B, Kim Y, Choi KS. IMpact of COVID‐19 on cancer screening in South Korea. Nat Sci Rep. 2022;12:11380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Machii R, Takahashi H. Japanese cancer screening programs durign the COVID‐19 pandemic: changes in participation between 2017–2020. Cancer Epidemiol. 2023;82:102313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Oakes AH, Boyce K, Patton C, Jain S. Rates of routine cancer screening and diagnosis before vs after the COVID‐19 pandemic. JAMA Oncol. 2023;9:145‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Crawford AM, Lightsey HM IV, Xiong GX, et al. Changes in elective and urgent surgery among TRICARE beneficiaries during the COVID‐19 pandemic. Mil Med. 2023;188:e2397‐e2404. doi: 10.1093/milmed/usac391 [DOI] [PubMed] [Google Scholar]
- 17. Tanielian T, Farmer C. The US military health system: promoting readiness and providing health care. Health Aff (Millwood). 2019;38:1259‐1267. [DOI] [PubMed] [Google Scholar]
- 18. Schoenfeld AJ, Kaji AH, Haider AH. Practical guide to surgical data sets: military health system tricare encounter data. JAMA Surg. 2018;153:679‐680. [DOI] [PubMed] [Google Scholar]
- 19. Chaudhary MA, Schoenfeld AJ, Harlow AF, et al. Incidence and predictors of opioid prescription at discharge after traumatic injury. JAMA Surg. 2017;152:930‐936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schoenfeld AJ, Jiang W, Harris MB, et al. Association between race and postoperative outcomes in a universally insured population versus patients in the state of California. Ann Surg. 2017;266:267‐273. [DOI] [PubMed] [Google Scholar]
- 21. Gimbel RW, Pangaro L, Barbour G. America's “undiscovered” laboratory for health services research. Med Care. 2010;48:751‐756. [DOI] [PubMed] [Google Scholar]
- 22. Chaudhary MA, de Jager E, Bhulani N, et al. No racial disparities in surgical care quality observed after coronary artery bypass grafting in TRICARE patients. Health Aff (Millwood). 2019;38:1307‐1312. [DOI] [PubMed] [Google Scholar]
- 23. Nurutdinova D, Chrusciel T, Zeringue A, et al. Mental health disorders and the risk of AIDS‐defining illness and death in HIV‐infected veterans. Aids. 2012;26:229‐234. [DOI] [PubMed] [Google Scholar]
- 24. Dalton MK, Manful A, Jarman MP, et al. Long‐term prescription opioid use among US military service members injured in combat. J Trauma Acute Care Surg. 2021;91(2S Suppl 2):S213‐S220. [DOI] [PubMed] [Google Scholar]
- 25. National Cancer Institute's PROSPR Consortium , Corley DA, Sedki M, et al. Cancer screening during the coronavirus disease‐2019 pandemic: a perspective from the National Cancer Institute's PROSPR consortium. Gastroenterology. 2021;160(4):999‐1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maxwell AE, Crespi CM, Antonio CM, Lu P. Explaining disparities in colorectal cancer screening among five Asian ethnic groups: a population‐based study in California. BMC Cancer. 2010;10:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu PH, Singal AG, Murphy CC. Stool‐based tests mitigate imapcts of COVID‐19 on colorectal cancer screening. Clin Gatroenterol Hepatol. 2023;21:1667‐1669.e2. doi: 10.1016/j.cgh.2022.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Deeds SA, Moore CB, Gunnink EJ, et al. Implementation of a mailed faecal immunochemical test programme for colorectal cancer screening among Veterans. BMJ Open Qual. 2022;11:e001927. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Data Availability Statement
The data contains personal identifiers and are not publicly available. The data that support the findings of this study are available on request from the corresponding author.


