INTRODUCTION
Chronic pain costs the U.S. $635 billion annually through medical expenditures and lost economic productivity, in large part because treatment remains a process of trial-and-error.[13] This is especially true of visceral pain conditions like Interstitial Cystitis/Bladder Pain Syndrome (IC/BPS) and Chronic Prostatitis/Chronic Pelvic Pain Syndrome (CP/CPPS), both which suffer from a lack of clear, evidence-based treatment algorithms.[16,42] Self-reported patient symptoms, while relatively easy to assess, are limited by issues of accuracy and reliability.[30] The question, then, remains: what tools can be effectively employed to improve subtyping efforts of patients with chronic pain?
Diagnostic categories are often based on anatomy, but some visceral pain patients consistently experience symptom exacerbation in association with particular activities while others do not.[26] One example of this symptom heterogeneity is the presence or absence of pain as the bladder fills in Interstitial Cystitis/Bladder Pain Syndrome and Chronic Prostatitis (collectively termed Urologic Chronic Pelvic Pain Syndrome [UCPPS]). This symptom is frequently assessed via self-report in these patient samples, but it is unclear if this information accurately identifies meaningful phenotypic information that can guide treatment decisions.[4,26,33] To address this limitation, the National Institute of Diabetes and Digestive and Kidney Diseases-sponsored Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network conducted a novel patient subtyping study that recreates pain during bladder filling and employs real-time patient reported measures with concomitant fMRI.[25] A previous MAPP analysis[23] found that roughly half of UCPPS participants experience painful bladder filling, and previous efforts to generate case definitions of UCPPS[2] have found that bladder filling pain occurs frequently in this population but did not include bladder filling pain as an essential component of the case definition. Therefore, we hypothesized that a bladder filling test would only evoke pain in a subgroup of patients with UCPPS, and that these patients would exhibit structural and functional changes in brain regions, such as anterior cingulate, insula, and somatosensory cortices that are key areas in the representation of pain in UCPPS.[7,21,22]
Here we demonstrate that this simple test meets these criteria as it is associated with clinical presentation of the disease, structural and functional changes in the brain, relatively stable patient subtypes, and an increased likelihood of future symptom flares as well as utilization of healthcare resources. This test appears to have high clinical and translational potential for patient subtyping in urologic chronic pain and may serve as a model for designing similar ecologically valid tests in other visceral pain conditions.
METHODS
Sample.
The MAPP Network Symptom Patterns Study (SPS) is a longitudinal, observational study of UCPPS symptom profiles with extensive, integrated, neurobiological phenotyping of participants.[5] In brief, primary eligibility criteria for UCPPS include 1) UCPPS symptoms present for a majority of the time during the most recent 3 months; 2) age ≥18 years; and 3) response ≥1 on the bladder/prostate or pelvic pain/pressure/discomfort scale during past 2 weeks. Healthy community participants primary eligibility criteria were 1) greater than or equal to 18 years of age, (2) zero on a pain, pressure or discomfort scale, (3) no chronic pain in the pelvic or bladder region, and no reports of chronic pain in any other body region, and (4) no urological symptoms. Full inclusion and exclusion criteria for UCPPS and control participants in the MAPP SPS study have been published.[5] In brief, The MAPP SPS project is registered at Clinicaltrials.gov: Trans-MAPP Symptom Patterns Study (MAPP II SPS [NCT02514265]): All procedures were approved by Institutional Review Boards at the participating institutions and all subjects provided informed consent.
Measures.
Demographic information and duration of symptoms in years was collected by patient self-report. Rand Interstitial Cystitis Epidemiology (RICE) criteria were used to identify self-reported pain on bladder filling, and painful urge.[33] The Genitourinary Pain Index (GUPI) and Interstitial Cystitis Symptoms/Problems Index were used to assess pelvic pain and urinary symptoms;[4,28] items were then scored into the Pelvic Pain Severity (PPS) and Urologic Symptom Severity (USS) instruments developed in the MAPP epidemiologic phenotyping study. PPS is a 0–28 scale of genitourinary pain severity in the past week.[14] Widespread pain was assessed by the Collaborative Health Outcomes Information Registry body map,[32] which was then scored into 13 painful regions (one pelvic, twelve extra-pelvic). Most bothersome symptom was captured via self-report across eight categories: 1) pubic/bladder pain 2) perineal pain, 3) pain with sex, 4) urinary urge, 5) daytime urinary frequency, 6) nighttime urinary frequency, 7) incomplete emptying, and 8) other.
Longitudinal healthcare utilization and symptom flares.
Health care utilization due to UCPPS symptoms was defined by a five-item questionnaire administered at months 3, 6, 9, 12, 15 and 18 of the SPS protocol. The question stem asked, “Have your urologic or pelvic pain symptoms been severe enough that they caused you to do any of the following in the past 2 weeks?” The items were a) contacted a healthcare provider by phone or email b) saw a healthcare provider in his/her office c) made a trip to the emergency room or urgent care d) had a medication change, and e) undergone a medical procedure, in the last two weeks. The total number of events over this timeframe was tallied for each patient. Flares were assessed at the same timepoints with a single question, “Are you currently experiencing a flare of your urologic or pelvic pain symptoms? By this we mean, are you currently experiencing symptoms that are much worse than usual?”. The number of affirmative responses were tallied for each patient.
Bladder filling test procedure.
The MAPP SPS used a novel natural bladder filling test to examine pain responses during the process of bladder filling and voiding. Once participants arrived at the magnetic resonance imaging (MRI) facilities, they were asked to fully void their bladder. The total void volume was recorded using graded specimen collection hats. After this void, participants drank 12 oz of water (~ 350 mL), with start/end times and volume consumed recorded. They provided an initial pain rating as soon as they finished drinking the water. Participants then waited for twenty minutes until providing a second pain rating. After an additional twenty minutes, participants rated their pain again and then entered the MRI scanner. Following acquisition of brain structural images and a roughly 10 minute resting-state scan, participants exited the scanner and again rated their pain. This portion of the test, representing the bladder filling period of the test, is the subject of the current analyses. The procedure was described previously. [25]
Neuroimaging data.
The Neuroimaging Core of the G. Oppenheimer Center for Neurobiology of Stress and Resilience (CNSR) at UCLA operated as the hub for neuroimaging operations in MAPP. The scanning parameters and harmonization procedures for rs-fMRI, T1, and DTI imaging have been described previously.[1,25] Throughout the study, scans from all sites were uploaded to the UCLA-CNSR repository where they were promptly reviewed for parameter compliance and image quality.
Resting-state functional magnetic resonance imaging (rs-fMRI) scans were acquired with echo planar imaging (EPI) pulse sequences. Basic pulse sequence parameters were as follows: repetition time (TR) of 2000 ms, echo time (TE) of 30 ms, flip angle of 77°, field-of-view (FoV) of 220 mm × 220 mm, resolution of 64 × 64, phase encode direction of anterior-to-posterior, slice thickness of 4.0 mm, slice gap of 0.5 mm, ascending slice acquisition, 30–40 slices per volume to cover the entire brain, and 300 volumes (10 min acquisition).
The MP-RAGE pulse sequence (or equivalent) was used for high resolution T1-weighted 3D volume imaging. Basic parameters were as follows: TR of 2300 ms, TE of 2.98 ms, inversion time (TI) of 900 ms, flip angle of 11° (GE scanners), 9° (Siemens/Philips scanners), FoV = 256 mm × 256 mm, Resolution of 256 × 256, Slices per volume = 240 (or maximum available), slice thickness of 1 mm.
Statistical Analyses:
Latent class mixed models for longitudinal outcomes-Overview.
Latent class models for longitudinal outcomes are a methodology used to relate a set of observed variables to a latent variable that changes over time. In practical terms, these models can be used to identify potential subgroups of observations that show similar temporal patterns.
Here we use the latent class model framework to identify potential subgroups of patients on the bladder filling test. We focus on a relatively simple framework that uses the first four observations during the test that represent pain immediately after drinking water and subsequent pain ratings through peak bladder filling (the last pain rating prior to voiding). Our purpose in using these timepoints is two-fold. One, pain and discomfort during bladder filling represents a clinical construct of interest in urologic pelvic pain syndromes, as indicated by the inclusion of bladder filling questions in the RICE criteria and genitourinary pain index questions for assessing IC/BPS symptoms. Two, while not always the case, simpler models are often more likely to generalize to new populations.
Latent classes
We use the “lcmm” package for the R programming language (v. 3.6.1) to perform these analyses.[31] Fixed effects included the intercept (post-ingestion pain rating), the linear slope of change in pain over the first four time points, patient sex, and void volume. Class-specific fixed effects and random effects included the intercept and linear slope. A non-structured matrix of variance-covariance was used to model class-specific random effects. We then iteratively increase the number of classes and compare Schwarz’s Bayesian Information Criteria (BIC) between models, where lower values indicate better model fit.[27] After identifying the initial model by BIC values, we consider the mean posterior probabilities for subjects in each class, where values closer to 1 indicate better classification.[31]
We repeat this exercise in the subsample of patients with data available at month 6 (n=361) to determine if any identified solution is similar with those found at baseline, and to determine if subjects belong to similar classes six months later, an important indication of temporal stability.
Clinical correlates of latent classes
Pelvic Pain Severity.
To determine the relationship between class membership and overall pelvic pain severity (PPS) we conducted a multivariable general linear model with overall PPS (0–28 scale) as the dependent variable and several previously identified factors associated with PPS as independent variables (IVs), plus class membership. Partial eta-square values were considered to determine the independent strength of the association between the IVs and overall PPS. RICE criteria for painful filling, painful urgency, degree of widespread pain (0–13 regions), symptom duration in years, and overall urinary symptom severity were included as IVs in addition to class membership.
Poisson regression models were used with healthcare utilization counts and flare counts as dependent variables. Covariates included RICE criteria for painful bladder filling, patient sex and age, and overall PPS. Class membership was the independent variable. For these analyses, we contrasted class one and class three only, as these groups represent two “extremes” of response to the test and were reasonably stable over time. Model fit compared to negative-binomial regression models was checked to affirm the appropriateness of Poisson models.
We compared the proportion of self-reported most bothersome symptom between classes using the Mantel-Haenszel test.
Brain neuroimaging preprocessing and outcome measures
Single-participant analyses.
Brain surfaces were reconstructed using recon-all T1-weighted images (FreeSurfer 6.0.1). Brain morphology outcome measures of cortical surface area and thickness on 5,124 vertices (fsaverage-4 space) were used as in multi-participant analyses.[24] Only structural scans that passed standardized quality assessment, performed independently of the authors, were used for further analysis: quality assessment of structural scans included visually inspection for subject motion artifacts, poor image contrast, and errors in image prescription.[1] rs-fMRI images from the full-bladder scan were preprocessed using fMRIPrep 20.2.0.[10] To account for the likelihood of increased head motion while participants remained in the scanner with a full bladder, we examined the sensitivity to motion criteria, starting with literature values and making the criteria more stringent (maximum framewise displacement < 3mm, varied threshold for mean framewise displacement: < 0.25mm, <0.2mm, <0.15mm).[25,29] Images were warped to Montreal Neurological Institute (MNI) standard space and resampled to 2 × 2 × 2 mm voxel dimension to allow for cross-participant comparison. The outcome measure for rs-fMRI was local activity quantified by the fractional amplitude of low frequency fluctuation (fALFF) in the slow-5 frequency band (0.01–0.027 Hz), as we have shown previously reduces with increased activation.[20,25,40] Slow-5 fALFF images were z-standardized by subtraction of the global image mean and division by the global standard deviation.
Multi-participant analyses.
Statistical analyses and data visualization were performed in MATLAB using custom scripts (release R2021a). Brain morphology maps were contrasted using un-paired t-tests without assumption of equal variance between BFP+ UCPPS patients and controls at baseline. False-discovery rate (FDR) correction (FDR < 0.05) was applied to the contrast maps. The set of all vertices showing FDR-corrected contrast between BFP+ patients and controls were defined to be a region-of-interest (ROI). To examine consistency of the morphologic changes, the morphology outcomes were averaged over the ROI from scans acquired at 6-months in individuals identified as BFP+ at baseline. We then performed a follow-up functional imaging analysis where the functional activity during full bladder was examined in ROI found to have morphology difference between BFP+ patients and controls. Brain surface locations within the structural ROI were mapped to MNI coordinates using previously-validated software.[39] A functional activity measure was derived for each participant by averaging fALFF during the full bladder scan over the structural ROI. Function activity data were pooled between baseline and 6-month scans, and a mixed effect linear model was used to assess the fixed effect of BFP status on the activity measure accounting for the random effect of participant, controlling for age, sex, imaging site, and mean framewise displacement.
RESULTS
Demographic Information
Data from 580 participants (72 healthy controls and 508 UCPPS patients) were examined. 79 UCPPS patients were excluded due to incomplete bladder filling data. Therefore, data from 501 participants (72 healthy controls and 429 UCPPS patients) were subjected to further analysis.
Sample demographic information is shown in Table 1. Briefly, UCPPS patients had an average age of 43.7 years (SD=15.6), with 155 males (36.1%) and 274 females (63.9%), and an average self-reported symptom duration 11.4 years (SD=11.1). Identical procedures were followed at the six month visit for the bladder filling test to those at baseline. The only significant difference was the smaller sample size due to normal study attrition. The total n at month six with bladder filling data was 361. Age (M=43.66, SD=15.56), gender distribution (men=36.8%, women=63.2%), and duration of symptoms in years (M=11.38, SD=11.07) were very similar to the baseline descriptive statistics.
Table 1:
Sample demographics. UCPPS = urologic chronic pelvic pain syndrome. BFP− = bladder filling pain negative, BFP+ = bladder filling pain positive, intermed. = intermediate between BFP− and BFP+
| Healthy Controls (N=72) | UCPPS all. (N=429) | UCPPS BFP− (N=109) | UCPPS Intermed. (N=175) | UCPPS BFP+ (N=145) | ||
|---|---|---|---|---|---|---|
| Age | Mean (SD) | 41.1 (14.8) | 43.7(15.6) | 46.4 (15.8) | 42.6 (15.9) | 42.8 (14.7) |
| Sex (F) | N (%) | 35 (48.6) | 274 (63.8) | 66 (60.5) | 103 (58.9) | 105 (72.4)* |
| Race | N (%) | |||||
| Caucasian | 47 (65.3) | 380 (88.6) | 98 (89.9) | 160 (91.4) | 122 (84.1) | |
| African American/Black | 9 (12.5) | 23 (5.4) | 5 (5.5) | 5 (2.9) | 12 (8.3) | |
| North American Indian/ Northern Native | 0 (0) | 2 (.5) | 0 (0) | 1 (.6) | 1 (.7) | |
| Native Hawaiian/Other Pacific Islander | 0 (0) | 1 (.2) | 0 (0) | 0 (0) | 1 (.7) | |
| Asian / Asian American | 7 (9.7) | 3 (.7) | 1 (.9) | 1 (.6) | 1 (.7) | |
| More than one race | 6 (8.3) | 15 (3.5) | 3 (2.8) | 6 (3.4) | 6 (4.1) | |
| other | 3 (4.2) | 5 (1.2) | 1 (.9) | 2 (1.1) | 2 (1.4) | |
| Ethnicity (Non-hispanic) | N (%) | 64 (88.9) | 400 (93.2) | 102 (93.6) | 162 (92.6) | 136 (93.8) |
| Duration of symptoms in years | Mean (SD) | N/A | 11.4 (11.1) | 11.4 (12.1) | 11.1 (11.0) | 11.7 (10.5) |
p=.030 by Pearson’s chi-square test
Latent class trajectories
A three-class model was identified according to BIC. Mean posterior probabilities were 0.89, 0.83, and 0.93 for the three classes. Additionally, each class represented a large proportion of the sample (25.4%, 40.8%, 33.8%)
The three classes are shown in Figure 1. Class one is characterized by very low levels of pain at the time of water ingestion (mean/SD = 0.17/.43), and little to no increase in pain as the bladder fills (Δ mean/SD =0.22/0.72). Class two shows mild pain at the time of water ingestion (mean/SD = 1.58/1.22), and a modest increase in pain as the bladder fills (Δ mean/SD =0.91/1.86). Class three, conversely, is characterized by moderate levels of pain at the time of water ingestion (mean/SD= 4.19/2.29) and a moderate increase in pain as the bladder fills (Δ mean/SD =1.71/3.01).
Figure 1.
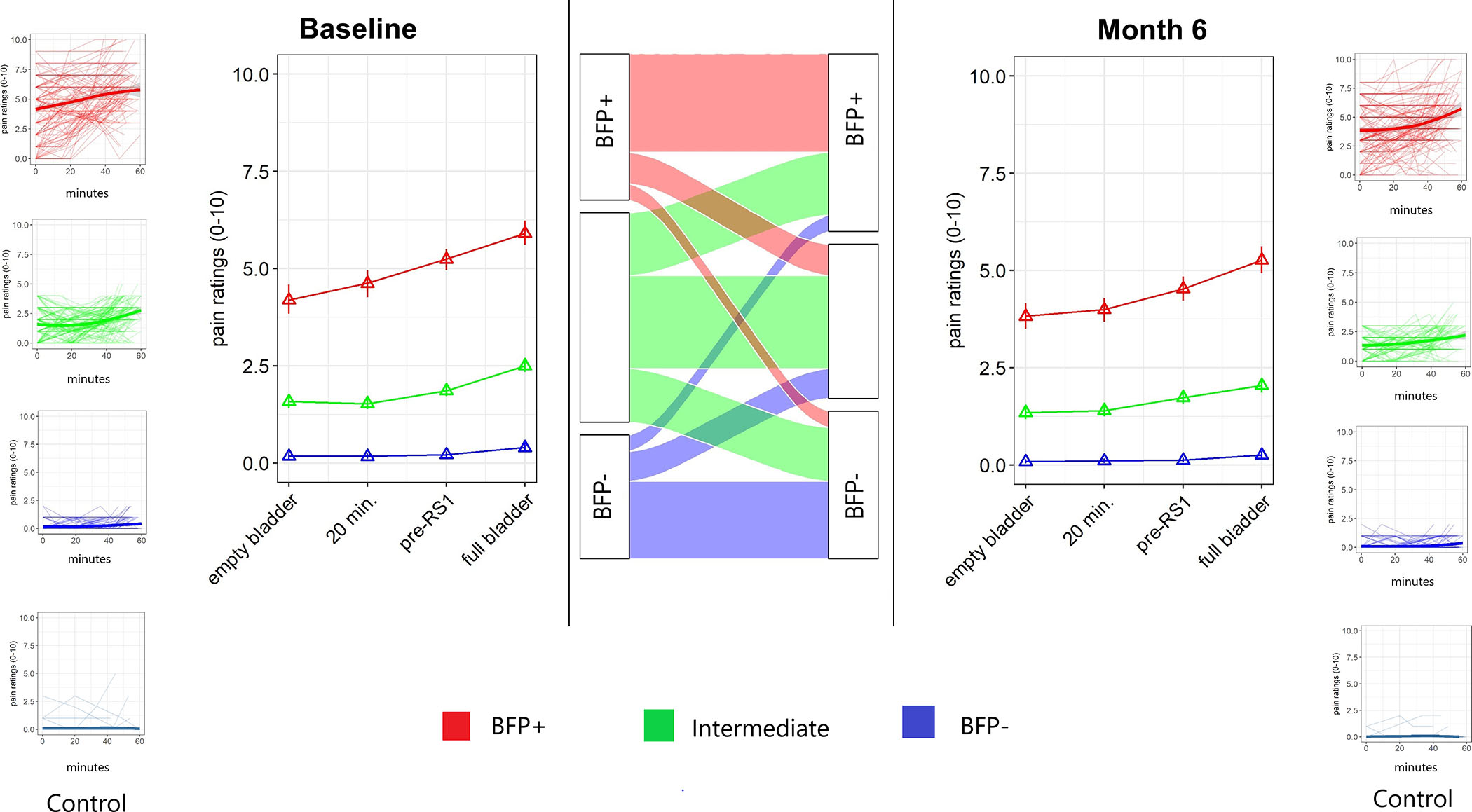
Latent class analysis reveals qualitatively different pain responses to bladder filling in UCPPS patients. Left panel is baseline data, right panel is the test repeated at a separate visit 6 months later, center panel shows alluvial plot consistency in class membership for individual participants. Pain was assessed at 4 time points: immediately after urine void, and then again at ~20 minutes, ~40 minutes, and ~50 minutes later. Between the 40 and 50 minute assessments, a 10-minute resting-state fMRI scan was performed. Three-class representation was optimal at both baseline and 6-months, with one class termed bladder filling pain positive (BFP+) expressing pain in response to the bladder filling test, one class termed bladder filling pain negative (BFP−) not expressing pain in response to bladder filling, and one intermediate class. The alluvial plot shows that transitions between BFP+ and BFP− response between the baseline and 6-month test were limited, with fewer than 12% of patients changing between BFP+ and BFP− classes.
At month 6, a three-class model was identified according to BIC. Mean posterior probabilities were 0.97, 0.77, and 0.92. Additionally, each class represented a large proportion of the sample (29.6%, 31.0%, 39.3%).
Month 6 classes were very similar to those identified at baseline. Class one is characterized by very low levels of pain at the time of water ingestion (mean/SD = 0.08/.31), and little to no increase in pain as the bladder fills (Δ mean/SD =0.17/0.49). Class two shows mild pain at the time of water ingestion (mean/SD = 1.35/0.93), and a modest increase in pain as the bladder fills (Δ mean/SD =0.70/1.46). Class three, conversely, is characterized by moderate levels of pain at the time of water ingestion (mean/SD= 3.82/2.11) and a moderate increase in pain as the bladder fills (Δ mean/SD =1.44/2.99).
Class stability
As seen in Figure 1, a majority of patients in class one and class three belonged to the analogous class six months later (Class one baseline to class one m6 = 62.7%; class two baseline to class two m6 = 44.3%; class three baseline to class three m6=67.3%). In class one baseline, 87% belonged to class one or two at m6, and in class three baseline, 89% belonged to class three or class two at m6. As class one and class three showed stability over time we refer to these as Bladder Filling Pain (BFP) positive (+) and negative (−) groups hereafter.
Pain Severity
Besides patient sex, all IVs were significantly associated with overall PPS (all p < .05). Of these, class membership showed the strongest independent relationship with overall PPS. See Supplemental Table 1.
Most bothersome symptom
The distribution of most bothersome symptom differed between the BFP+ and − participants such that a larger proportion of BFP+ participants reported painful symptoms vs urinary symptoms (pain=74.5%, urinary =22.8%) when compared to BFP− participants (pain= 59.3%, urinary = 35.2%; p = .012) (Figure 3).
Figure 3.
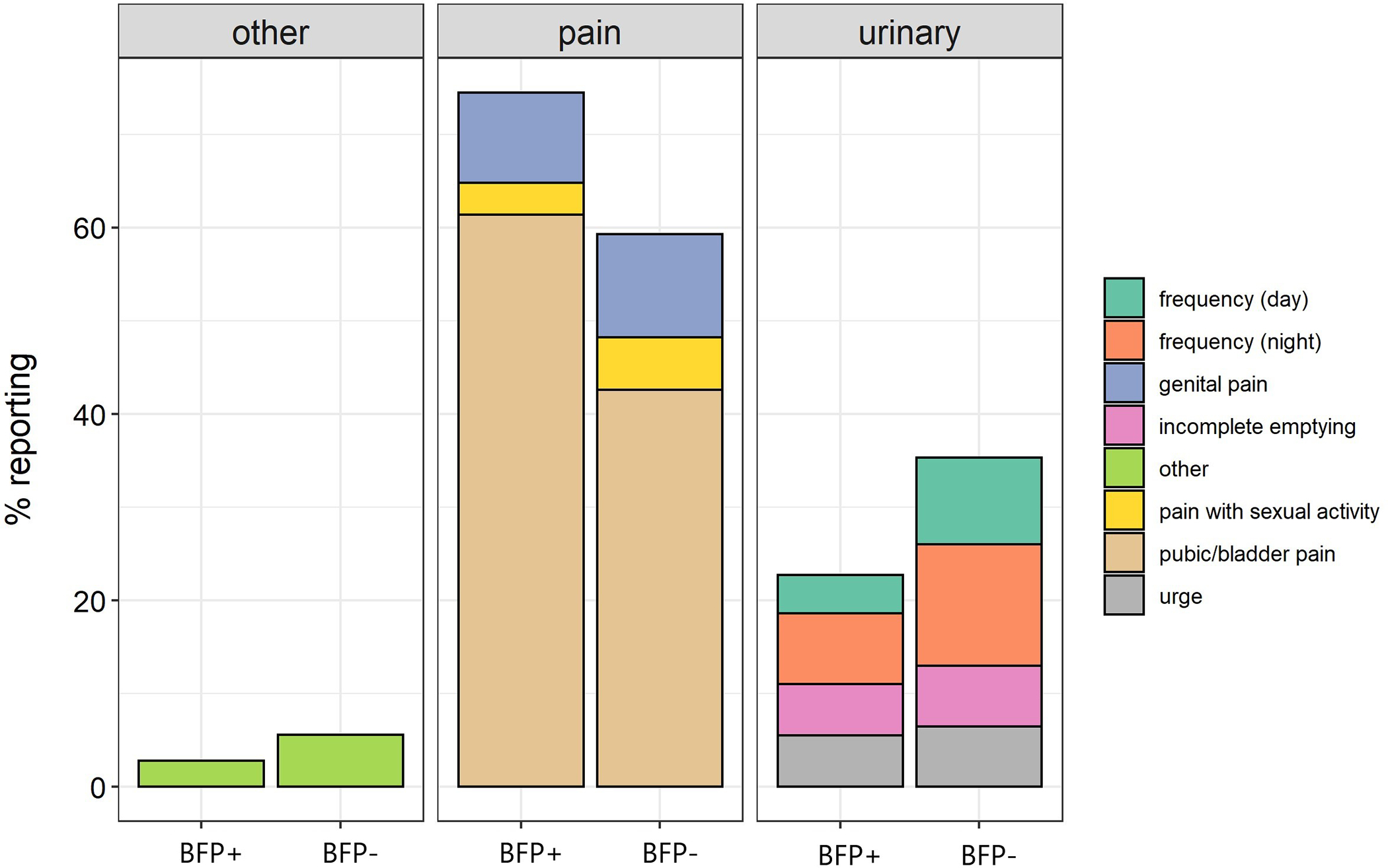
Most bothersome symptom by BFP+ and BFP− status. Panels show symptom domains and specific complaints are displayed with color coding in the legend.
Longitudinal outcomes
In Poisson regression models controlling for self-reported painful bladder filling (RICE criteria), patient sex / age, and overall PPS, BFP+ was significantly associated with more frequent healthcare utilization (p = .027). Because the lack of relationship with self-reported criteria was somewhat surprising, we conducted a contingency analysis to determine the overlap between self-reported bladder filling pain and the results of the test. While self-reported filling was slightly more common in BFP+ (71%) than in BFP− (56%; p = .021), the constructs appear to be largely independent.
BFP+ at baseline was associated with an estimated 2.11 (SE=0.15) health care utilization events compared to 1.62 (SE =.15) in BFP−. Similarly, when controlled for the same variables, symptom flares occurred more frequently in BFP+ participants versus BFP− (p = .020). BFP+ was associated with an estimated 0.97 flares (SE=0.10) versus 0.63 (SE=0.09) in BFP−. See Figure 2.
Figure 2.
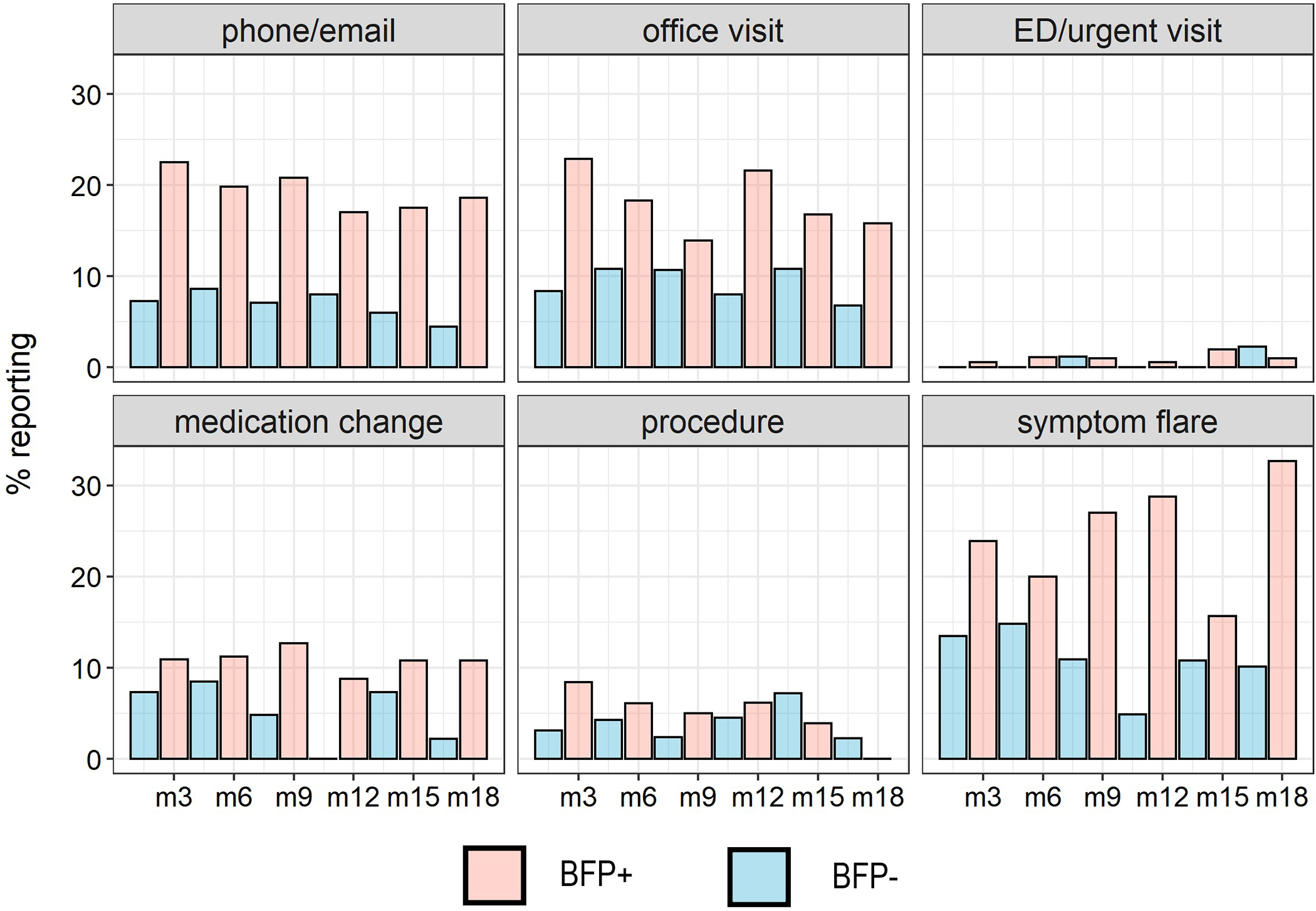
Proportion of BFP + and BFP− participants (classified from response at baseline visit) engaging in healthcare utilization or experiencing a symptom flare at each timepoint ranging from 3 months post-baseline (m3) to 18 months post-baseline (m18).
Neuroimaging correlates of BFP+ status
BFP+ UCPPS patients expressed changes in brain morphology that co-localized with increased activity during bladder filling. BFP+ patients had reduced cortical surface area widely distributed throughout the brain, most notably in prefrontal cortex, posterior cingulate cortex, anterior insular cortex, and primary somatosensory cortex (Figure 4A). These findings observed from baseline scans, were replicated in the same individuals that were scanned again 6-months later (Figure 4B). The reductions in cortical surface area remained significant when controlled for sex. No changes in cortical thickness were noted. Examining the functional activity in aggregate across these brain regions displaying morphological changes (Figure 4C) demonstrated that a clear signal of increased activity is present in these regions when the bladder is full in BFP+ patients compared to controls (Figure 4D). Activity increases between BFP+ and controls were not sensitive to motion criteria, and more stringent motion criteria showed the BFP− brain activity response was consistent with controls (Supplementary Figure 1). Depending on stringency of motion criteria, between 30% (N=125) and 11% (N=49) were included in the functional analyses, perhaps due to the exceptional difficulty of remaining very still when highly uncomfortable in the scanner with either urinary urge, pain, or both. While activity increases were observed in BFP+ patients, aggregated functional activity across the examined brain regions appeared not to correlate directly with the exact degree of pain experienced during the full bladder scan (p=0.27).
Figure 4.
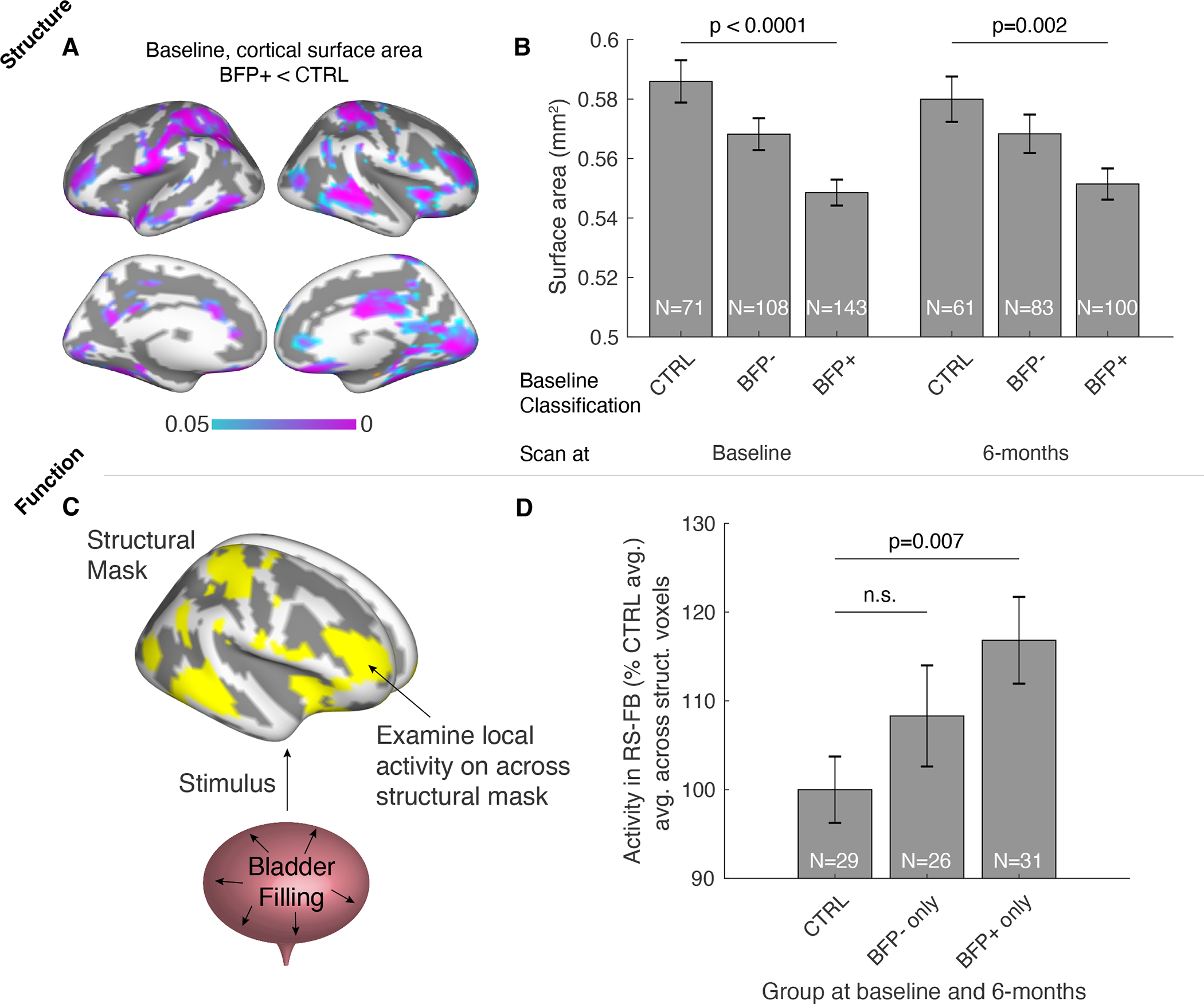
Changes in brain morphology and bladder filling function in BFP+ UCPPS patients. A. Cortical surface area decreases were observed at baseline in BFP+ patients compared to controls (p<0.05, FDR corrected for multiple comparisons across cortical mesh vertices). B. Decreases observed at baseline were observed again in the same individuals imaged 6-months later. C. Functional activity changes were observed across the brain masked to regions expressing structural changes shown in A. D. Activity was significantly higher during resting-state with full bladder (RS-FB) in BFP+ patients compared to controls (meanFD<0.20 shown here, see Supplemental Figure 1).
DISCUSSION
Improving pain management for millions worldwide affected by visceral chronic pain is an urgent priority for urologists and other clinicians who have been on the frontline of treating these challenging conditions for decades with little progress. For example, novel therapeutics appear to fail when tested in heterogeneous pain populations and existing treatments are poorly prioritized when based on clinical impressions alone. We have demonstrated here that a simple test designed to recapitulate a common feature of UCPPS provides rich clinical data with significant implications for the course of the disease. Specifically, we find that Bladder Filling + patients have more impactful genitourinary pain when controlling for a host of established risk factors, show specific and potentially pathophysiologic alterations in the structure and function of the brain, and are more likely to use healthcare resources and experience symptom flares over 18 months of observation. This feature is not simply a marker of disease severity: we find differences in the preponderance of most bothersome symptoms between Bladder Filling + and − patients, and worse longitudinal outcomes for BFP+ when controlling for overall pain severity. The simplicity of this test suggests high translational potential and the presented framework for subtyping chronic pain through a naturalistic paradigm may serve as a model for other conditions. It is worth noting that the lack of any specialized equipment means this framework could be implemented in under-resourced communities and developing countries.
While chronic pain is often described in terms of the overall prevalence in the population and with mean values for healthcare expenditures and loss productivity, it is well-understood that in most chronic pain conditions a smaller subset of patients with lower quality of life disproportionately drive healthcare utilization.[8] Independent analyses suggest that healthcare utilization and healthcare spending on interstitial cystitis is roughly twice that of community controls and the discrepancy appears heavily concentrated in outpatient services.[3,36] Estimates for healthcare utilization in chronic prostatitis are similar.[6] The current analyses strongly suggest that these differences are not evenly distributed amongst the population of patients with UCPPS, but rather are heavily concentrated in those patients who are bladder pain positive – who were responsible for fully two thirds of healthcare utilization events. Unfortunately, many studies attempting to establish risk factors for healthcare utilization have identified what are likely lagging indicators of poorly controlled disease such as low quality of life and use of opioids,[6,19] rather than pathophysiologic mechanisms that might serve as a starting point for improving clinical care. Naturalistic paradigms that evoke visceral hypersensitivity are advantageous because patients consider the symptoms central to their functional limitations.[34,35] In addition to identifying patients with bladder filling pain, the test also identified a group of participants meeting criteria for UCPPS that have almost no response to bladder filling – similar to healthy controls. This suggests intact neurobiological mechanisms for gauging bladder sensations, discussed further below.
Many patients with UCPPS suffer through a perplexing tradeoff between the consequences of dehydration and the exacerbation of symptoms that comes with consuming fluids -- patients are “punished” by their disease simply for attending to the basic necessities of life. In recognition of the importance of this symptom, various assessment tools for UCPPS have attempted to capture the experience of painful filling via self-report, including the RICE criteria and the GUPI.[4,6,19,33] In previous analyses conducted by the MAPP Network, self-reported painful filling was associated with more severe symptoms, lower quality of life, and the presence of comorbid pain.[23] However, in our current analyses where both the test response and self-report measures were included, only the bladder filling test response alone was significantly associated with more genitourinary pain and prospective disease burden.
There are compelling reasons that the bladder filling test may be a more salient predictor of clinical outcomes than self-reported painful filling. It may be that the fluid volume in the test is not sufficient to provoke the filling pain response in some patients. Additionally, because patients limit fluid consumption for symptom management, self-report reflects the patient’s individual efforts to balance physiologic needs and potential exacerbation of symptoms. Standardizing the timing of voids, fluid consumption, and symptom report reduces this variability. The test also avoids issues with the recall of pain where severity is often inflated compared to momentary assessment.[9]
The brain morphology differences we observed indicate either a trait risk factor for bladder filling pain development, or changes induced by powerful and on-going experience. Examining how the brain regions expressing morphology differences functionally respond to bladder filling allow us to interpret these regions as areas of excessive activity during bladder filling in individuals with bladder filling pain. We note important overlap between our findings and response to bladder filling in animal models of bladder pain induced by stress; excessive activity broadly in somatosensory cortex and posterior cingulate cortex.[38] Furthermore, we observe strong changes in lateral prefrontal cortex, a region implicated in the bladder filling control circuit that monitors the desired to void.[15] The preservation of this circuitry may therefore be a key underlying difference between BFP+ and − participants/healthy controls. We also observe changes in the anterior insula, a region associated with spontaneous pain in UCPPS patients.[11] Taken together, we interpret our finding of objective neurologic features of BFP+ UCPPS patients as a potential substrate of increased sensitivity to bladder filling. Since the increased activity profile did not correlate directly with the amount of pain experienced during the full bladder scan, it is likely that other brain regions and networks are involved in encoding pain intensity. However, since increased activity during bladder filling overlaps with morphological differences observed in BFP+ patients, we think that there is the possibility for consistent over-activity in these regions regardless of the variations in the exact pain intensity experienced across repeated bladder fillings. While the analyses described in this manuscript utilized stringent head motion criteria that may limit the practical clinical application of functional neuroimaging during this task, the results support the task by highlighting the plausible biological underpinnings of bladder filling pain.
Established efforts to phenotype patients with chronic pain have often involved diagnostic procedures or tests that are expensive but add little prognostic or clinical significance. For example, imaging for non-specific back pain and imaging for headache have both been identified as low-value procedures within the Medicare system.[41] Many invasive diagnostic procedures are predicated on the assumption that pain results from tissue damage or inflammation – nociceptive pain – while the extant literature suggests that many such findings, such as the extend and severity of endometriosis or the loss of intra-articular cartilage in osteoarthritis are at best weakly associated with the pain experience.[17,37] Pairing the pain response with naturalistic but standardized stimuli may therefore provide better information about the patient at substantially lower cost. Possible other naturalistic tests that could be further developed and exploited in chronic pain include standardized consumption of food and symptom ratings during the digestive process in IBS, chewing tests in temporomandibular disorder, provoked pain in vulvodynia (“tampon test”), or responses to light/odor/sound in migraine with aura.[12,17,18]
Limitations and Conclusions
This test requires further validation as it was performed in the context of an MRI. These results support the use of this test as a means of identifying UCPPS patient subgroups with a high degree of visceral hypersensitivity, or what has sometimes been called the “bladder-centric” patient. Importantly, our results suggest CNS changes associated with a symptom that is often presumed to be the result of bladder pathology. These findings support further clinical studies of the efficacy of centrally acting therapies, particularly those that might target the relevant neurologic substrates, for some phenotypic groups. The identification of a distinct BFP+ subgroup suggests examination of more aggressive treatment to diminish central vulnerabilities to chronic pain and more regular follow-up to manage emerging flares and coordinate care in future UCPPS trial design. The advantages provided by a simple, naturalistic test designed to evoke visceral pain are robust and should be considered a primary tool for phenotypic subtyping of chronic pain.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a cooperative agreement from the National Institute of Health, National Institute of Diabetes and Digestive and Kidney Diseases (Grant numbers DK082370, DK082342, DK082315, DK082344, DK082325, DK082345, and DK082316) as well as DK110669, DK121724, and DK123164. We thank all of the volunteers who participated in the study.
REFERENCES
- [1].Alger JR, Ellingson BM, Ashe-McNalley C, Woodworth DC, Labus JS, Farmer M, Huang L, Apkarian AV, Johnson KA, Mackey SC, Ness TJ, Deutsch G, Harris RE, Clauw DJ, Glover GH, Parrish TB, Hollander J den, Kusek JW, Mullins C, Mayer EA, MAPP Research Network Investigators. Multisite, multimodal neuroimaging of chronic urological pelvic pain: Methodology of the MAPP Research Network. Neuroimage Clin 2016;12:65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Berry SH, Elliott MN, Suttorp M, Bogart LM, Stoto MA, Eggers P, Nyberg L, Quentin Clemens J. Prevalence of Symptoms of Bladder Pain Syndrome/Interstitial Cystitis Among Adult Females in the United States. Journal of Urology 2011;186:540–544. doi: 10.1016/j.juro.2011.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chung S-D, Liu S-P, Li H-C, Lin H-C. Health Care Service Utilization among Patients with Bladder Pain Syndrome/Interstitial Cystitis in a Single Payer Healthcare System. PLoS ONE 2014;9:e87522. doi: 10.1371/journal.pone.0087522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Clemens JQ, Calhoun EA, Litwin MS, McNaughton-Collins M, Kusek JW, Crowley EM, Landis JR, Urologic Pelvic Pain Collaborative Research Network. Validation of a modified National Institutes of Health chronic prostatitis symptom index to assess genitourinary pain in both men and women. Urology 2009;74:983–7, quiz 987.e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Clemens JQ, Kutch JJ, Mayer EA, Naliboff BD, Rodriguez LV, Klumpp DJ, Schaeffer AJ, Kreder KJ, Clauw DJ, Harte SE, Schrepf AD, Williams DA, Andriole GL, Lai HH, Buchwald D, Lucia MS, van Bokhoven A, Mackey S, Moldwin RM, Pontari MA, Stephens-Shields AJ, Mullins C, Landis JR. The Multidisciplinary Approach to The Study of Chronic Pelvic Pain (MAPP) Research Network*: Design and implementation of the Symptom Patterns Study (SPS). Neurourol Urodyn 2020;39:1803–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Clemens JQ, Markossian T, Calhoun EA. Comparison of economic impact of chronic prostatitis/chronic pelvic pain syndrome and interstitial cystitis/painful bladder syndrome. Urology 2009;73:743–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Clemens JQ, Quentin Clemens J, on behalf of the MAPP Research Network Study Group, Mullins C, Lenore Ackerman A, Bavendam T, van Bokhoven A, Ellingson BM, Harte SE, Kutch JJ, Henry Lai H, Martucci KT, Moldwin R, Naliboff BD, Pontari MA, Sutcliffe S, Richard Landis J. Urologic chronic pelvic pain syndrome: insights from the MAPP Research Network. Nature Reviews Urology 2019;16:187–200. doi: 10.1038/s41585-018-0135-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Clemens JQ, Stephens-Shields A, Naliboff BD, Lai HH, Rodriguez L, Krieger JN, Williams DA, Kusek JW, Landis JR, MAPP Research Network. Correlates of Health Care Seeking Activities in Patients with Urological Chronic Pelvic Pain Syndromes: Findings from the MAPP Cohort. J Urol 2018;200:136–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Eich E, Reeves JL, Jaeger B, Graff-Radford SB. Memory for pain: relation between past and present pain intensity. Pain 1985;23:375–380. [DOI] [PubMed] [Google Scholar]
- [10].Esteban O, Markiewicz CJ, Blair RW, Moodie CA, Isik AI, Erramuzpe A, Kent JD, Goncalves M, DuPre E, Snyder M, Oya H, Ghosh SS, Wright J, Durnez J, Poldrack RA, Gorgolewski KJ. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods 2019;16:111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Farmer MA, Chanda ML, Parks EL, Baliki MN, Apkarian AV, Schaeffer AJ. Brain functional and anatomical changes in chronic prostatitis/chronic pelvic pain syndrome. J Urol 2011;186:117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Foster DC, Beth Kotok M, Huang L-S, Watts A, Oakes D, Howard FM, Stodgell CJ, Dworkin RH. The tampon test for vulvodynia treatment outcomes research: reliability, construct validity, and responsiveness. Obstet Gynecol 2009;113:825–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gaskin DJ, Richard P. The Economic Costs of Pain in the United States. The Journal of Pain 2012;13:715–724. doi: 10.1016/j.jpain.2012.03.009. [DOI] [PubMed] [Google Scholar]
- [14].Griffith JW, Stephens-Shields AJ, Hou X, Naliboff BD, Pontari M, Edwards TC, Williams DA, Quentin Clemens J, Afari N, Tu F, Brett Lloyd R, Patrick DL, Mullins C, Kusek JW, Sutcliffe S, Hong BA, Henry Lai H, Krieger JN, Bradley CS, Kim J, Richard Landis J. Pain and Urinary Symptoms Should Not be Combined into a Single Score: Psychometric Findings from the MAPP Research Network. Journal of Urology 2016;195:949–954. doi: 10.1016/j.juro.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Griffiths D. Neural control of micturition in humans: a working model. Nat Rev Urol 2015;12:695–705. [DOI] [PubMed] [Google Scholar]
- [16].Hanno PM, Erickson D, Moldwin R, Faraday MM, American Urological Association. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment. J Urol 2015;193:1545–1553. [DOI] [PubMed] [Google Scholar]
- [17].Hunter DJ, March L, Sambrook PN. The association of cartilage volume with knee pain. Osteoarthritis and Cartilage 2003;11:725–729. doi: 10.1016/s1063-4584(03)00160-2. [DOI] [PubMed] [Google Scholar]
- [18].Karibe H, Goddard G, Gear RW. Sex differences in masticatory muscle pain after chewing. J Dent Res 2003;82:112–116. [DOI] [PubMed] [Google Scholar]
- [19].Kidner CL, Mayer TG, Gatchel RJ. Higher opioid doses predict poorer functional outcome in patients with chronic disabling occupational musculoskeletal disorders. J Bone Joint Surg Am 2009;91:919–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kilpatrick LA, Kutch JJ, Tillisch K, Naliboff BD, Labus JS, Jiang Z, Farmer MA, Apkarian AV, Mackey S, Martucci KT, Clauw DJ, Harris RE, Deutsch G, Ness TJ, Yang CC, Maravilla K, Mullins C, Mayer EA. Alterations in resting state oscillations and connectivity in sensory and motor networks in women with interstitial cystitis/painful bladder syndrome. J Urol 2014;192:947–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kutch JJ, Ichesco E, Hampson JP, Labus JS, Farmer MA, Martucci KT, Ness TJ, Deutsch G, Apkarian AV, Mackey SC, Klumpp DJ, Schaeffer AJ, Rodriguez LV, Kreder KJ, Buchwald D, Andriole GL, Lai HH, Mullins C, Kusek JW, Landis JR, Mayer EA, Clemens JQ, Clauw DJ, Harris RE, MAPP Research Network. Brain signature and functional impact of centralized pain: a multidisciplinary approach to the study of chronic pelvic pain (MAPP) network study. Pain 2017;158:1979–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kutch JJ, Yani MS, Asavasopon S, Kirages DJ, Rana M, Cosand L, Labus JS, Kilpatrick LA, Ashe-McNalley C, Farmer MA, Johnson KA, Ness TJ, Deutsch G, Harris RE, Vania Apkarian A, Clauw DJ, Mackey SC, Mullins C, Mayer EA. Altered resting state neuromotor connectivity in men with chronic prostatitis/chronic pelvic pain syndrome: A MAPP. NeuroImage: Clinical 2015;8:493–502. doi: 10.1016/j.nicl.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lai HH, Krieger JN, Pontari MA, Buchwald D, Hou X, Landis JR, MAPP Research Network. Painful Bladder Filling and Painful Urgency are Distinct Characteristics in Men and Women with Urological Chronic Pelvic Pain Syndromes: A MAPP Research Network Study. J Urol 2015;194:1634–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Liem F, Varoquaux G, Kynast J, Beyer F, Kharabian Masouleh S, Huntenburg JM, Lampe L, Rahim M, Abraham A, Craddock RC, Riedel-Heller S, Luck T, Loeffler M, Schroeter ML, Witte AV, Villringer A, Margulies DS. Predicting brain-age from multimodal imaging data captures cognitive impairment. Neuroimage 2017;148:179–188. [DOI] [PubMed] [Google Scholar]
- [25].Mawla I, Schrepf A, Ichesco E, Harte SE, Klumpp DJ, Griffith JW, Strachan E, Yang CC, Lai H, Andriole G, Magnotta VA, Kreder K, Clauw DJ, Harris RE, Clemens JQ, Landis JR, Mullins C, Rodriguez LV, Mayer EA, Kutch JJ. Natural bladder filling alters resting brain function at multiple spatial scales: a proof-of-concept MAPP Network Neuroimaging Study. Sci Rep 2020;10:19901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mun CJ, Suk HW, Davis MC, Karoly P, Finan P, Tennen H, Jensen MP. Investigating intraindividual pain variability: methods, applications, issues, and directions. Pain 2019;160:2415–2429. [DOI] [PubMed] [Google Scholar]
- [27].Nylund KL, Asparouhov T, Muthén BO. Deciding on the Number of Classes in Latent Class Analysis and Growth Mixture Modeling: A Monte Carlo Simulation Study. Structural Equation Modeling: A Multidisciplinary Journal 2007;14:535–569. doi: 10.1080/10705510701575396. [DOI] [Google Scholar]
- [28].O’Leary MP, Sant GR, Fowler FJ, Whitmore KE, Spolarich-Kroll J. The interstitial cystitis symptom index and problem index. Urology 1997;49:58–63. doi: 10.1016/s0090-4295(99)80333-1. [DOI] [PubMed] [Google Scholar]
- [29].Parkes L, Fulcher B, Yücel M, Fornito A. An evaluation of the efficacy, reliability, and sensitivity of motion correction strategies for resting-state functional MRI. Neuroimage 2018;171:415–436. [DOI] [PubMed] [Google Scholar]
- [30].Pincus T, Morley S. Cognitive-processing bias in chronic pain: a review and integration. Psychol Bull 2001;127:599–617. [DOI] [PubMed] [Google Scholar]
- [31].Proust-Lima C, Philipps V, Liquet B. Estimation of Extended Mixed Models Using Latent Classes and Latent Processes: The R Package lcmm. Journal of Statistical Software 2017;78. doi: 10.18637/jss.v078.i02. [DOI] [Google Scholar]
- [32].Scherrer KH, Ziadni MS, Kong J-T, Sturgeon JA, Salmasi V, Hong J, Cramer E, Chen AL, Pacht T, Olson G, Darnall BD, Kao M-C, Mackey S. Development and validation of the Collaborative Health Outcomes Information Registry body map. Pain Rep 2021;6:e880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Suskind AM, Berry SH, Ewing BA, Elliott MN, Suttorp MJ, Clemens JQ. The prevalence and overlap of interstitial cystitis/bladder pain syndrome and chronic prostatitis/chronic pelvic pain syndrome in men: results of the RAND Interstitial Cystitis Epidemiology male study. J Urol 2013;189:141–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tu FF, Hellman KM, Roth GE, Dillane KE, Walker LS. Noninvasive bladder testing of adolescent females to assess visceral hypersensitivity. Pain 2022;163:100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Tu FF, Kane JN, Hellman KM. Noninvasive experimental bladder pain assessment in painful bladder syndrome. BJOG 2017;124:283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Tung A, Hepp Z, Bansal A, Devine EB. Characterizing Health Care Utilization, Direct Costs, and Comorbidities Associated with Interstitial Cystitis: A Retrospective Claims Analysis. J Manag Care Spec Pharm 2017;23:474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Vercellini P, Fedele L, Aimi G, Pietropaolo G, Consonni D, Crosignani PG. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum Reprod 2007;22:266–271. [DOI] [PubMed] [Google Scholar]
- [38].Wang Z, Chang HH, Gao Y, Zhang R, Guo Y, Holschneider DP, Rodriguez LV. Effects of water avoidance stress on peripheral and central responses during bladder filling in the rat: A multidisciplinary approach to the study of urologic chronic pelvic pain syndrome (MAPP) research network study. PLoS One 2017;12:e0182976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wu J, Ngo GH, Greve D, Li J, He T, Fischl B, Eickhoff SB, Yeo BTT. Accurate nonlinear mapping between MNI volumetric and FreeSurfer surface coordinate systems. Hum Brain Mapp 2018;39:3793–3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Yani MS, Fenske SJ, Rodriguez LV, Kutch JJ. Motor cortical neuromodulation of pelvic floor muscle tone: Potential implications for the treatment of urologic conditions. Neurourol Urodyn 2019;38:1517–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zajacova A, Grol-Prokopczyk H, Zimmer Z. Pain Trends Among American Adults, 2002–2018: Patterns, Disparities, and Correlates. Demography 2021;58:711–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhang J, Liang C, Shang X, Li H. Chronic Prostatitis/Chronic Pelvic Pain Syndrome: A Disease or Symptom? Current Perspectives on Diagnosis, Treatment, and Prognosis. American Journal of Men’s Health 2020;14:155798832090320. doi: 10.1177/1557988320903200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


