Abstract
Background:
Traditional approaches to guideline-directed medical therapy (GDMT) management often lead to delayed initiation and titration of therapies in heart failure. This study sought to characterize alternative models of care involving non-physician provider-led GDMT interventions and their associations with therapy utilization and clinical outcomes.
Methods:
We performed a systematic review and meta-analysis of randomized controlled trials (RCT) and observational studies comparing non-physician provider-led GDMT initiation and/or uptitration interventions versus usual physician care (PROSPERO ID: CRD42022334661). We queried PubMed, Embase, the Cochrane Library, and the World Health Organization International Clinical Trial Registry Platform for peer-reviewed studies from database inception to July 31, 2022. In the meta-analysis, we used RCT data only and leveraged random-effects models to estimate pooled outcomes. Primary outcomes were GDMT initiation and titration to target doses by therapeutic class. Secondary outcomes included all-cause mortality and HF hospitalizations.
Results:
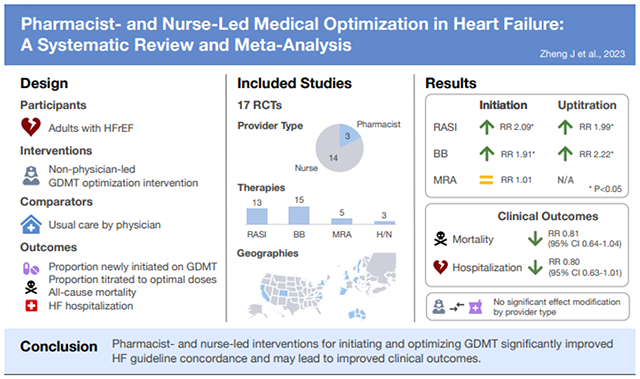
33 studies were reviewed, of which 17 (52%) were randomized controlled trials with median follow-up of 6 months. 14 (82%) trials evaluated nurse interventions, while the remainder assessed pharmacist interventions. The primary analysis pooled data from 16 RCTs, which enrolled 5,268 patients. Pooled risk ratios (RR) for RASI and BB initiation were 2.09 (95% CI 1.05-4.16; I2=68%) and 1.91 (95% CI 1.35-2.70; I2=37%), respectively. Outcomes were similar for uptitration of RASI (RR 1.99, 95% CI 1.24-3.20; I2=77%) and BB (RR 2.22, 95% CI 1.29-3.83; I2=66%). No association was found with MRA initiation (RR 1.01, 95% CI 0.47-2.19). There were lower rates of mortality (RR 0.82, 95% CI 0.67-1.04; I2=12%) and HF hospitalization (RR 0.80, 95% CI 0.63-1.01; I2=25%) across intervention arms, but these differences were small and not statistically significant. Prediction intervals were wide due to moderate-to-high heterogeneity across trial populations and interventions. Subgroup analyses by provider type did not show significant effect modification.
Conclusions and Relevance:
Pharmacist- and nurse-led interventions for GDMT initiation and/or uptitration improved guideline concordance. Further research evaluating newer therapies and titration strategies integrated with pharmacist- and/or nurse-based care may be valuable.
Keywords: Heart failure, guideline-directed medical therapy, titration, pharmacist, nurse
Graphical abstract
Lay Summary:
Several beneficial medicines are now available for patients with heart failure. However, many patients are currently not receiving these medications or taking lower-than-ideal doses. In this study, researchers reviewed published articles on initiatives led by nurses and pharmacists to start and adjust medical treatment for heart failure. Patients with heart failure who participated in these programs were more likely to receive recommended medications than patients who saw their usual physician. These patients also had fewer deaths and hospitalizations for heart failure. Health systems can use this information to create similar programs to help patients with heart failure receive optimal treatment in a timely manner.
Introduction
The management of heart failure with reduced ejection fraction (HFrEF) has progressed remarkably over several decades. The latest clinical practice guidelines support the timely initiation and optimization of guideline-directed medical therapy (GDMT) consisting of a renin-angiotensin system inhibitor (RASI), beta-blocker (BB), mineralocorticoid receptor antagonist (MRA), and sodium-glucose co-transporter 2 inhibitor (SGLT2I).1 Despite these advancements, epidemiological studies reveal steadily climbing risk of hospitalization and mortality for patients with heart failure.2,3 Inadequate uptake of GDMT represents an important driver of suboptimal cardiovascular outcomes. Moreover, longitudinal analyses of outpatient registries have demonstrated limited improvement in GDMT prescribing and dose achievement in recent years.4–7 This critical gap stresses the urgent need for alternative models of care.
Traditional paradigms of GDMT management usually involve sequential titration of each therapeutic class through intermittent outpatient visits with a primary care physician or cardiologist. At best, this process can take 6-12 months, twice the recommended timeline advocated by the American College of Cardiology Expert Consensus Pathways.8 Furthermore, optimal and timely uptitration rarely occurs in usual care.9,10 There has been emerging interest in leveraging non-physician healthcare professionals to facilitate HFrEF management. Prior reviews have examined a broad range of interventions, including nurse-directed patient education, pharmacy medication reconciliation, and multidisciplinary care.11–13 However, the evidence around interventions focusing on GDMT initiation and/or uptitration by non-physician providers remains unclear.
In this systematic review and meta-analysis, we aimed to characterize the impact of non-physician provider-led interventions on GDMT initiation and target dose optimization across randomized controlled trials (RCT) and non-randomized observational studies in heart failure. Our study also explored clinical outcomes of all-cause mortality and HF hospitalization and subgroup effects to assess possible heterogeneity among study interventions.
Methods
This study was conducted in accordance with the Cochrane methodology14 and reported according to the Preferred Reporting of Systematic Reviews and Meta-Analysis statement (supplementary Table S1).15 Our protocol was registered on the International Prospective Register of Systematic Reviews (PROSPERO; ID: CRD42022334661).16 The Stanford Institutional Review Board granted a waiver of patient informed consent based on minimal risk for collecting and synthesizing nonidentifiable data from published studies.
Search Strategy
We systematically searched PubMed, Embase, and the Cochrane Library database and identified key studies investigating GDMT management approaches in heart failure led by non-physician healthcare professionals published from database inception to July 31, 2022. The search queries used are outlined in supplementary Table S2. We additionally searched the World Health Organization International Clinical Trial Registry Platform and ClinicalTrials.gov (searched August 1, 2022). Reference lists from relevant studies and systematic reviews were extracted manually.
Eligibility Criteria
Randomized controlled trials and non-randomized observational studies were considered if they included adult patients with HFrEF, defined as EF≤40%, and evaluated the effectiveness of an intervention related to GDMT management compared with usual care. Usual care was defined as inpatient or outpatient follow-up by a clinician or medical team without active titration of GDMT by a non-physician healthcare professional (e.g., nurse, pharmacist). For inclusion, study interventions had to feature a non-physician provider either proposing GDMT recommendations to the primary clinician or prescribing GDMT independently or under physician supervision. Interventions solely focused on patient education, counseling, or medication reconciliation were excluded.
Full-length articles and brief reports presenting original data were considered eligible. Reviews, meta-analyses, and editorials were excluded from analysis, but their reference lists were extracted during publication screening. Other exclusion criteria included: 1) lack of a control group; 2) interventions only involving diuretic adjustment; 3) studies without a medication titration protocol; 4) absence of outcomes for patients with left ventricular systolic dysfunction specifically; and 5) secondary or post-hoc analyses.
Outcomes
Primary outcomes of interest included the proportion of patients newly initiated on GDMT and the proportion of patients uptitrated to target doses of GDMT, stratified by therapeutic class. Secondary outcomes included clinical endpoints: all-cause mortality and HF hospitalizations. GDMT was defined as angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker/angiotensin receptor-neprilysin inhibitor (ACEI/ARB/ARNI or RASI), BB, MRA, and SGLT2I. Outcomes related to GDMT management were only collected for therapeutic classes detailed in study-specific titration protocols.
Data Extraction and Risk of Bias Assessment
Two independent reviewers performed a manual screen of studies based on inclusion and exclusion criteria. Relevant information from eligible studies was obtained: author, date of publication, country, setting (e.g., hospital, clinic, home, telephone), target patient population, sample size, intervention and control arms, titration protocol, length of follow up, and outcomes. Studies were required to report at least one of the primary or secondary outcomes for meta-analysis. Outcomes at 6 and 12 months of follow-up were collected and aggregated. Where outcomes data were missing or incomplete from included studies, the reviewers planned to contact the respective corresponding authors via email. For all included studies, data required for meta-analysis were directly accessible from the published articles.
Risk of bias was assessed for meta-analyzed RCTs and non-randomized studies using the Cochrane risk of bias tool 2.017 and the Risk Of Bias In Non-randomized Studies–of Interventions tool18, respectively. Disagreements between reviewers were resolved by consensus or by arbitration by a third reviewer when necessary. Publication bias due to small-study effects was also evaluated using an inverted funnel plot as well as Egger’s test for outcomes with at least 10 studies.19
Primary and Secondary Analyses
Risk ratios (RR) were computed for the primary and secondary outcomes by pooling binary outcome data using pre-specified random-effects models to account for between-study heterogeneity. Test statistics and confidence intervals (CI) were adjusted using the Hartung-Knapp-Sidik-Jonkman approach.20 We estimated between-study variance (τ2) using the restricted maximum-likelihood estimator based on simulation studies comparing performance across multiple methods.21 We described statistical heterogeneity with the I2 statistic.22 We also reported prediction intervals, which present heterogeneity on the same scale as the outcomes. Prediction intervals are useful for estimating the variability of the intervention effect over different settings across similar studies. Cases of zero events in one arm were adjusted by adding the reciprocal of the size of the contrasting arm.14 Studies with zero events in both arms were given no weight in pooling estimates. To account for these studies, we also computed the risk difference between arms for each outcome as a secondary approach.
The primary meta-analysis only included RCTs to minimize potential confounding. Subgroup analyses to evaluate heterogeneity by provider type (pharmacist versus nurse) and care setting (inpatient versus outpatient) were pre-specified. We explored pooling RCTs and non-randomized studies at low risk of bias and the use of alternative meta-analytic approaches, including the DerSimonian-Laird and Sidik-Jonkman τ2 estimators and Mantel-Haenszel random-effects method, in pre-specified sensitivity analyses.23 A post-hoc exploratory analysis excluding trials with fewer than 50 subjects per arm was conducted. Meta-analyses were performed using R version 4.1.2 and the meta (v5.1-1)24 and robvis (v0.3.0)25 packages. Statistical tests were 2-sided and used a significance threshold of P<0.05.
Results
Study Characteristics
Our search identified 78 RCTs and non-randomized studies, 33 of which were deemed eligible for review (supplementary Figure S1 and Table S3). Study-level characteristics are summarized in Table 1. The median sample sizes were 93 (range: 12-1,090) and 97 (12-1,156) for the intervention and usual care arms, respectively. Median follow-up time was 6 months (range: 1-24 months). Included studies spanned most major continents except for South America, with the majority (21, 64%) conducted in North America.
Table 1.
Group-level Characteristics of Studies Included for Review
| Characteristic | All studies, n = 33 | RCT, n = 17 | Observational, n = 16 |
|---|---|---|---|
| Sample size, median (range) | |||
| Intervention arm | 93 (12-1,090) | 90 (14-1,090) | 101 (12-307) |
| Usual care arm | 97 (12-1,156) | 91 (12-1,074) | 97 (24-1,156) |
| Intervention arm: provider type | |||
| Pharmacist | 18 (55) | 3 (18) | 15 (94) |
| Nurse/nurse practitioner | 15 (45) | 14 (82) | 1 (6) |
| Intervention arm: practice scope | |||
| Independenta | 14 (42) | 7 (41) | 7 (44) |
| Non-independent | 19 (58) | 10 (59) | 9 (56) |
| Usual care arm: provider type b | |||
| General practicec | 20 (61) | 12 (71) | 8 (50) |
| Cardiologist | 16 (48) | 6 (35) | 10 (63) |
| Unspecified | 6 (18) | 3 (18) | 3 (19) |
| Therapies involved b | |||
| ACEI/ARB | 27 (82) | 13 (76) | 14 (88) |
| ARNI | 6 (18) | 1 (6) | 5 (31) |
| BB | 30 (91) | 15 (88) | 15 (94) |
| MRA | 14 (42) | 5 (29) | 9 (56) |
| Hydralazine/nitrates | 8 (24) | 3 (18) | 5 (31) |
| SGLT2I | 1 (3) | 0 (0) | 1 (6) |
| Geography | |||
| Africa | 1 (3) | 0 (0) | 1 (6) |
| Asia | 1 (3) | 0 (0) | 1 (6) |
| Australia | 2 (6) | 1 (6) | 1 (6) |
| Europe | 8 (24) | 8 (47) | 0 (0) |
| North America | 21 (64) | 8 (47) | 13 (81) |
| Care setting | |||
| Inpatient | 6 (18) | 0 (0) | 6 (38) |
| Outpatient | 23 (70) | 13 (76) | 10 (63) |
| Transitional | 4 (12) | 4 (24) | 0 (0) |
| Publication year, median (range) | 2014 (1999-2022) | 2006 (1999-2020) | 2019 (2010-2022) |
Values represent number of studies (percent) unless otherwise specified.
Expanded scope of practice under collaborative practice agreement that allows for independent patient assessment and therapy prescribing without need for physician approval
Percentages may not add to 100%, given overlap of multiple studies across categories
Includes physicians specialized or training in family medicine or internal medicine irrespective of care setting
Abbreviation: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor-neprilysin inhibitor; BB, β-blocker; IQR, interquartile range; MRA, mineralocorticoid receptor antagonist; RCT, randomized controlled trial; SGLT2I, sodium–glucose co-transporter 2 inhibitor
17 (52%) studies were randomized controlled trials (Table 2).26–42 Among these studies, 12 (71%) had inclusion criteria specifying EF restrictions, but thresholds varied between 40 and 50%.26,27,29–32,34–36,39,40,42 4 (24%) selected for more symptomatic patients based on New York Heart Association (NYHA) class.29,34,39,41 Most RCTs (14, 82%) evaluated interventions involving specialist heart failure nurses or nurse practitioners,26–30,32–36,39–42 while the remainder evaluated pharmacist-led interventions.31,37,38 7 (41%) featured non-physician healthcare professionals with an expanded, autonomous scope of practice for prescribing GDMT.27,29,32,35,37,39,42 Usual care varied across RCTs, with a majority defining the control arm as primary care (8, 47%)26,29,30,33,34,37,41,42 or a mix of primary and cardiologist care (4, 24%).27,32,35,38 Although none of the RCTs were conducted exclusively in the inpatient setting, 4 (24%) studied transitional interventions beginning during a HF admission followed by outpatient post-discharge care,32,35,36,42 whereas the remainder focused exclusively on outpatient interventions. ACEI/ARB (13, 76%) and BB (15, 88%) were well-represented across RCT titration protocols compared with MRA (5, 29%), hydralazine/nitrates (3, 18%), and ARNI (1, 6%). Prescribing of MRA was restricted in the following ways: NYHA class III-IV patients in 3 out of 5 studies,32,33,42 only if persistently symptomatic on RASI and BB therapy in 1 study,26 and only after full uptitration of ACEI/ARB therapy in another study.39 No RCT included digoxin initiation, but one study mentioned avoiding digoxin toxicity as part of its protocol.31 Of the 7 (41%) studies reporting safety outcomes, 5 found no significant differences in adverse events between arms,27–29,31,39 whereas 2 found higher rates of severe adverse events such as hospitalization for renal dysfunction in the control arm.35,37 Summaries of trial intervention protocols and cohort characteristics may be found in supplementary Table S4 and S5, respectively.
Table 2.
Characteristics of Included Randomized Controlled Trials
| Study Characteristics | Comparator Arms | Outcomes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Source | Setting | Country | Provider | Inclusion | Usual Care | n | Intervention | n | Endpoints | Follow-up (mo.) |
| Agvall et al,26 2013 | Outpatient | Sweden | Nurse | NYHAI-IV EF < 50% |
Annual or routine follow-up with GP | 81 | Initial visit with GP; follow-up by HF nurse at baseline, 1, 2, and 6 months for ACEI/ARB, BB, +/− MRA titration | 79 | All-cause mortality Change in EF, NT-proBNP, and QoL by SF-36 | 12 |
| Ansari et al,27 2003 | Outpatient | US | Nurse | Framingham criteria for CHF EF ≤ 45% |
Routine follow-up with primary, with GDMT education | 51 | Independent follow-up with NP upon approval by primary for BB titration until optimal doses reached | 54 | Proportion initiated on BB Proportion uptitrated on BB | 12 |
| Driscoll et al,28 2014 | Outpatient | Australia | Nurse | NYHA I-IV Confirmed LVSD Suboptimal BB |
Follow-up every 3 months with cardiologist | 14 | Follow-up with HF nurse weekly, biweekly, or monthly for BB titration, requiring cardiologist approval | 14 | Time to optimal BB dose All-cause or HF hospitalization Change in QoL by MLWHF | 6 |
| Ekman et al,29 2003 | Outpatient | Sweden | Nurse | NYHA III-IV EF < 40%b Age ≥ 65 |
Routine follow-up with primary | 75 | Initial visit with nurse; independent follow-up at least monthly for ACEI titration, education, and self-management training | 70 | Proportion receiving ACEI Proportion uptitrated on ACEI Mean ACEI dose at follow-up | 6 |
| Galbreath et al,30 2004 | Outpatient | US | Nurse | NYHA I-IV EF < 50%b |
Routine follow-up with primary | 244 | Scheduled telephone follow-up for ACEI/ARB, BB, MRA, H/N & diuretic titration, with education and counseling | 504 | All-cause mortality Proportion receiving GDMT Change in EF |
18 |
| Gattis et al,31 1999 | Outpatient | US | Pharmacist | NYHA I-IV EF < 45% |
Routine follow-up with primary | 91 | Telephone follow-up with pharmacist at baseline, 2, 12, and 24 weeks, with patient education and ACEI & H/N titration recommendations for physician | 90 | All-cause mortality HF hospitalization Proportion receiving ACEI |
6 |
| Güder et al,32 2015 | Inpatient/outpatient | Germany | Nurse | Admitted for HF NYHA I-IV EF ≤ 40% |
Post-discharge follow-up with primary | 363 | Inpatient education/self-management training; independent weekly telephone follow-up by HF nurse for ACEI/ARB, BB, and MRA titration | 343 | Proportion receiving GDMT Change in EF and QoL by SF-36 | 18 |
| Hancock et al,33 2012 | Outpatient | UK | Nurse | Confirmed LVSD Age ≥ 65 Long-term care facility resident |
Routine follow-up with GP, with consultant plan for HF management | 12 | Home visit by cardiologist; follow-up by HF nurse every 1-2 weeks for ACEI, BB, and MRA titration | 16 | Proportion uptitrated on GDMT All-cause mortality HF hospitalization |
12 |
| Kasper et al,34 2002 | Outpatient | US | Nurse | Admitted for HF NYHA III-IV EF < 45% |
Routine follow-up with primary, with cardiologist plan in patient chart | 98 | Telephone and in-person follow-up by HF nurse post-discharge for ACEI/ARB, BB, and diuretic titration under cardiologist direction | 102 | All-cause mortality HF hospitalization Change in QoL by MLWHF Proportion uptitrated on GDMT |
6 |
| Krantz et al,35 2008 | Inpatient/outpatient | US | Nurse | Admitted for HF EF ≤ 40% |
Routine follow-up with primary | 32 | Inpatient counseling with pre-discharge BB dose; independent biweekly telephone follow-up by nurse for BB titration and self-management training | 32 | HF hospitalization Change in EF Proportion receiving BB |
6 |
| Laramee et al,36 2003 | Inpatient/outpatient | US | Nurse | High-risk for readmission EF ≤ 40% |
Routine inpatient care and post-discharge follow-up with primary | 146 | Inpatient case management and education; weekly telephone follow-up with ACEI/ARB and BB titration recommendations for physician | 141 | All-cause hospitalization HF hospitalization Mean ACEI/ARB and BB dose |
3 |
| Lowrie et al,37 2012 | Outpatient | UK | Pharmacist | Confirmed LVSD | Routine follow-up with GP | 1074 | Initial visit with pharmacist; independent bi/weekly follow-up 3-4 times for ACEI/ARB and BB titration | 1090 | All-cause mortality HF hospitalization Proportion receiving or uptitrated on GDMT |
24 |
| McCarren et al,38 2013 | Outpatient | US | Pharmacist | Recent HF admissionb Suboptimal BB | Routine follow-up with primary | 98 | List of patients detailing guideline concordance provided to pharmacist | 122 | Proportion uptitrated on BB Mean BB dose | 6 |
| Oyanguren et al,39 2021 | Outpatient | Spain | Nurse | Prior HF admission NYHA II-III EF ≤ 40% |
Post-discharge follow-up with cardiologist and nurse support | 156 | Initial visit with cardiologist; independent bi/weekly follow-up with HF nurse for RASI, BB, and MRA titration | 164 | Mean GDMT dose All-cause mortality All-cause or HF hospitalization Change in QoL by MLWHF |
6 |
| Sisk et al,40 2006 | Outpatient | US | Nurse | NYHAI-IV EF ≤ 40% |
Routine follow-up with primary, with HF guidelines given to patient | 203 | Initial visit with nurse; telephone follow-up at 2, 4, 8, 12, and 24 weeks with ACEI/ARB, BB, and H/N titration recommendations for physician | 203 | All-cause mortality All-cause or HF hospitalization Change in QoL by SF-12 and MLWHF |
12 |
| Stromberg et al,41 2003 | Outpatient | Sweden | Nurse | NYHA II-IV HF confirmed by imaging |
Routine follow-up with primary | 54 | Initial visit with HF nurse with education and consultation with cardiologist for ACEI and BB titration | 52 | All-cause mortality All-cause hospitalization |
12 |
| Thompson et al,42 2005 | Inpatient/outpatient | UK | Nurse | Admitted for HF EF ≤ 45% |
Routine follow-up with GP | 48 | Inpatient intake with home visit within 10 days post-discharge; monthly in-clinic follow-up for ACEI/ARB, BB, and MRA titration | 58 | All-cause mortality Change in QoL by MLWHF Proportion receiving GDMT |
6 |
Includes primary and select secondary efficacy outcomes;
Included patients with preserved systolic function but reported outcomes separately or stated results were similar between groups.
Abbreviation: ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; BB, beta-blocker; EF, ejection fraction; GDMT, guideline-directed medical therapy; GP, general practitioner; HF, heart failure; H/N, hydralazine/nitrate; LVSD, left ventricular systolic dysfunction; MLWHF, Minnesota Living with Heart Failure; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; QoL, quality of life; RASI; renin-angiotensin system inhibitor
We also reviewed 16 (48%) non-randomized observational studies (supplementary Table S6).43–58 All but one analysis (15, 94%) focused on pharmacist-led interventions.49 More observational studies included cardiologist care in the control arm compared with RCTs (63% versus 35%).44,48,52,54,57 Other important differences from RCTs included median publication year (2019 [2010-2022] versus 2006 [1999-2020]); inclusion of ARNI (31% versus 6%), MRA (56% versus 29%), and hydralazine/nitrates (31% versus 18%); and inpatient interventions (38% versus 0%),7045,46,48,50,56,57 respectively.
Among all RCTs and non-randomized studies, the location and modality of interventions varied. Interventions were administered across integrated health care systems in 8 (24%) studies: 7 at Veterans Affairs (VA) medical centers27,38,46,51–53,55 and 1 at a safety-net hospital.35 In 10 (30%) studies, the intervention was delivered partly or completely through telephone follow-up.30–32,34–36,40,47,52,55 2 (6%) studies evaluated home visits as part of their interventions.42,49 One study predominantly included Black men at a VA medical center.53 Another study focused exclusively on older residents at long-term acute care facilities.33
Risk of Bias
Risk-of-bias assessments weighted by study sample size are shown in supplementary Figure S2. Most RCTs were judged to be at low risk of bias. We identified some concern for bias from the randomization process and from missing outcome data. One trial had a high overall risk of bias given significant deviation from usual care for unknown reasons.29 Several non-randomized studies carried higher risk of bias due to serious or critical concerns of confounding. Justifications for risk-of-bias assessments are shown in supplementary Figures S3 and S4. Funnel plots are displayed for each endpoint in supplementary Figure S5. Based on visual inspection, asymmetry is most notable for the RASI initiation and HF hospitalization endpoints. At least 10 studies were pooled for the BB initiation, all-cause mortality, and HF hospitalization endpoints. Eggers’ test found insufficient evidence of small-study effects for BB initiation (b=0.39, 95% CI −1.21-1.99; t=0.50, P=0.64) and all-cause mortality (b=−0.593, 95% CI −1.25-0.06; t=−1.774, P=0.10) but was significant for HF hospitalization (b=−1.128, 95% CI −1.98 to −0.28; t=−2.592, P=0.03).
Primary Analysis
For the main meta-analysis, we pooled 16 RCTs, which enrolled 5,268 patients and reported relevant data for primary and secondary outcome assessment. Among previously untreated patients, there was a median increase in RASI use of 45.8% (interquartile range [IQR] 29.8-66.7%) across intervention arms compared with 11.8% (IQR 7.1-18.1%) across usual care arms. As shown in Figure 1, pooling yielded a summary RR for RASI initiation of 2.09 (95% CI 1.05-4.16) with high heterogeneity across studies (I2=68%, P=0.002). Similarly, median BB initiation rates were 38.9% (IQR 16.6-69.5%) across intervention arms and 17.7% (IQR 9.3-28.6%) across usual care arms, corresponding to a summary RR of 1.91 (95% CI 1.35-2.70) with moderate heterogeneity (I2=37%, P=0.12). In contrast, median rates of MRA initiation were nearly equivalent between comparator arms (12.5% for intervention versus 12.4% for usual care), reflecting a summary RR of 1.01 (95% CI 0.47-2.19) with moderate heterogeneity (I2=31%, P=0.23). 95% prediction intervals for RASI and MRA initiation overlapped with the null. The estimated probabilities that the effect of the intervention on RASI, BB, and MRA initiation in a new study would be beneficial or greater than the null equaled 83.0%, 97.3%, and 50.9%, based on t-distributions with 7, 8, and 3 degrees of freedom, respectively.
Figure 1.
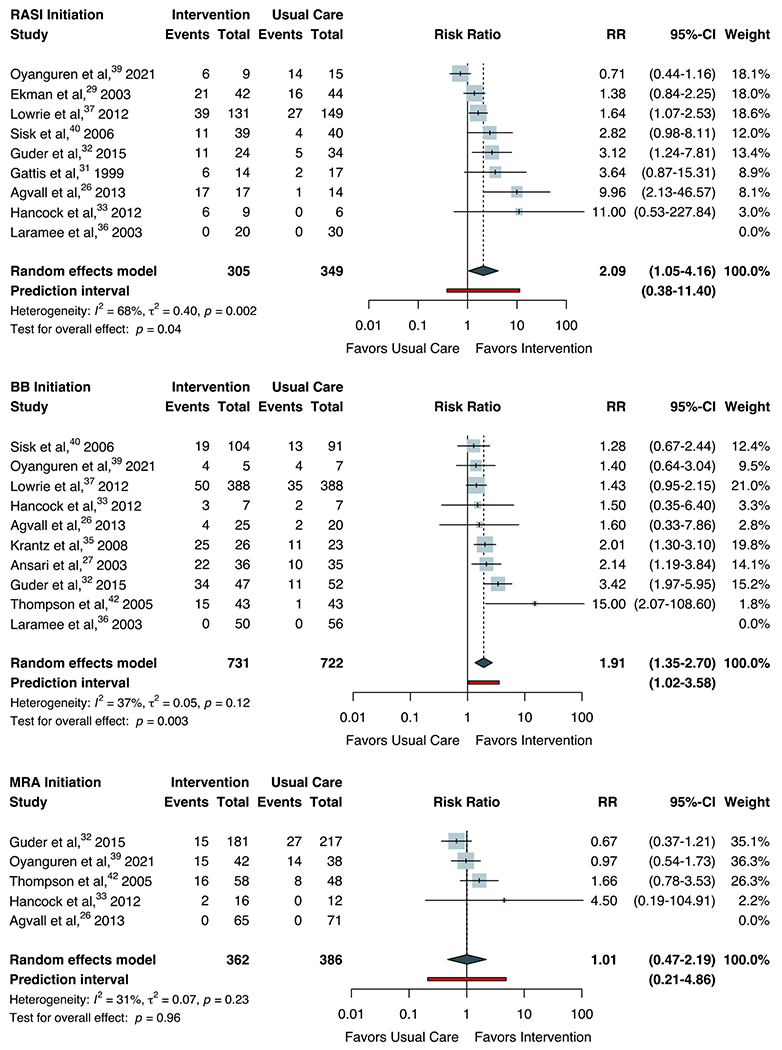
Random-effects Meta-analysis for Initiation of Guideline-directed Medical Therapy
Forest plots for random-effects meta-analysis are shown for the initiation of guideline-directed medical therapy by therapeutic class. The Hartung-Knapp-Sidik-Jonkman approach was used to adjust confidence intervals and test statistics. Heterogeneity (τ2) was estimated using the restricted maximum-likelihood variance estimator and described by I2 statistic. Statistical testing for overall effect and corresponding p-values are presented. Square size is proportional to the weight of each study. Horizontal lines indicate the 95% CI of each study. The diamond represents the pooled estimate with 95% CI. The red line represents the prediction interval, which accounts for heterogeneity in intervention effects across different settings. Abbreviation: BB, beta-blocker; RASI, renin-angiotensin system inhibitor; MRA, mineralocorticoid receptor antagonist; RR, risk ratio.
Outcomes were similar for the uptitration endpoints. Among treated patients, rates of RASI uptitration to target doses were 36.7% (IQR 30.1-52.8%) across intervention arms and 17.0% (IQR 10.2-42.2%) across usual care arms. As shown in Figure 2, this corresponded to a pooled RR of 1.99 (95% CI 1.24-3.20; I2=77%, P<0.001). Rates of BB uptitration to target doses were 27.5% (IQR 6.8%-50.8%) across intervention arms and 12.2% (4.3-26.3%) across usual care arms, corresponding to a summary RR of 2.22 (95% CI 1.29-3.83; I2=66%; P=0.007). Meta-analysis of MRA uptitration could not be performed as only 2 studies reported dose optimization outcomes.45,47 Based on the prediction intervals, the estimated probabilities that the effect of the intervention on RASI and BB uptitration in a new study would be beneficial were 90.7% and 90.9%, based on t-distributions with 5 and 8 degrees of freedom, respectively.
Figure 2.
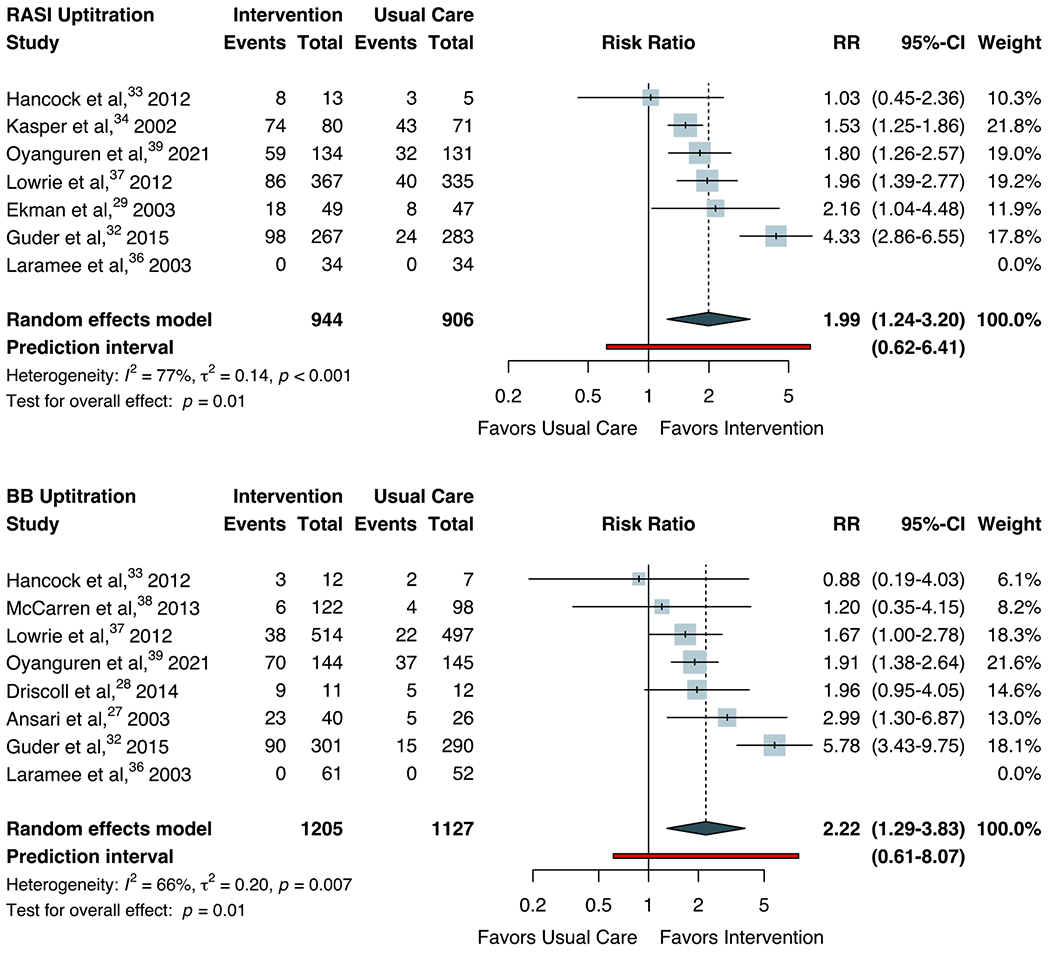
Random-effects Meta-analysis for Uptitration of Guideline-directed Medical Therapy
Forest plots for random-effects meta-analysis are shown for the titration of guideline-directed medical therapy to target dose by therapeutic class. Only endpoints with sufficient data for pooling study-level effects are shown. The Hartung-Knapp-Sidik-Jonkman approach was used to adjust confidence intervals and test statistics. Heterogeneity (τ2) was estimated using the restricted maximum-likelihood variance estimator and described by I2 statistic. Statistical testing for overall effect and corresponding p-values are presented. Square size is proportional to the weight of each study. Horizontal lines indicate the 95% CI of each study. The diamond represents the pooled estimate with 95% CI. The red line represents the prediction interval, which accounts for heterogeneity in intervention effects across different settings. Abbreviation: BB, beta-blocker; RASI, renin-angiotensin system inhibitor; RR, risk ratio.
We also assessed associations between study interventions and clinical endpoints (Figure 3). The summary RR for all-cause mortality was 0.81 (95% CI 0.64-1.04) with low heterogeneity (I2=12%, P=0.32). Similarly, the summary RR for HF hospitalization was 0.80 (95% CI 0.63-1.01) with moderate heterogeneity (I2=25%, P=0.22). The estimated probabilities that the effect of the intervention on mortality and HF hospitalizations in a new study would be beneficial were 80.0% and 94.7%, based on t-distributions with 14 and 8 degrees of freedom, respectively.
Figure 3.
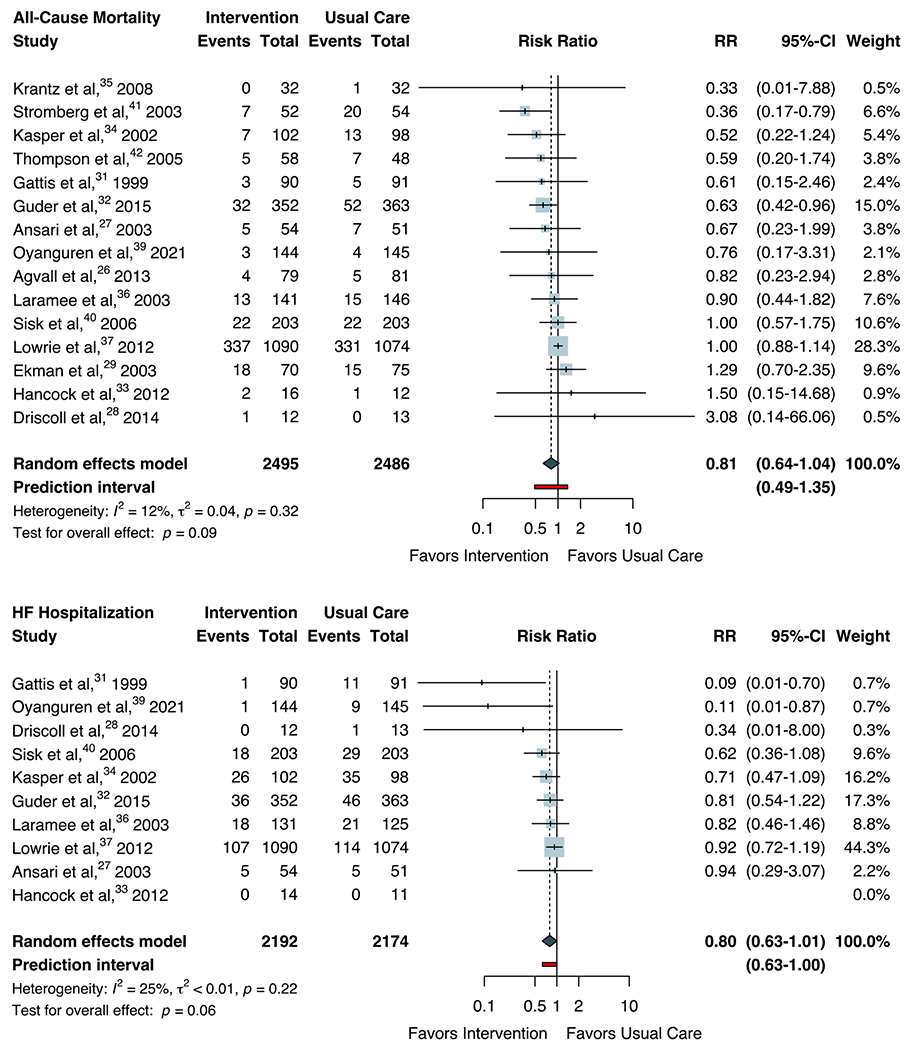
Random-effects Meta-analysis for Clinical Endpoints
Forest plots for random-effects meta-analysis are shown for all-cause mortality and heart failure hospitalization. The Hartung-Knapp-Sidik-Jonkman approach was used to adjust confidence intervals and test statistics. Heterogeneity (τ2) was estimated using the restricted maximum- likelihood variance estimator and described by I2 statistic. Statistical testing for overall effect and corresponding p-values are presented. Square size is proportional to the weight of each study. Horizontal lines indicate the 95% CI of each study. The red line represents the prediction interval, which accounts for heterogeneity in intervention effects across different settings. The diamond represents the pooled estimate with 95% CI. Abbreviation: HF, heart failure; RR, risk ratio.
Secondary Analyses
The pre-specified subgroup analyses did not show effect modification (supplementary Table S7). Pharmacist- and nurse-led interventions resulted in similar associations with both GDMT and clinical endpoints. Care setting (inpatient versus outpatient) could not be analyzed given none of the RCTs were conducted primarily in an inpatient setting (Table 1).
There were 3 studies with zero events in both arms for select outcomes.26,33,36 To incorporate these studies into pooled estimates, we computed risk differences (e.g., the differences in proportions) between arms for these endpoints. As shown in supplementary Figure S6, mean risk differences were 0.26 (95% CI −0.01-0.53) for RASI initiation, 0.17 (95% CI 0.05-0.29) for RASI uptitration, 0.21 (95% CI 0.05-0.36) for BB initiation, 0.14 (95% CI 0.01-0.27) for BB uptitration, and −0.00 (95% CI −0.05-0.04) for MRA initiation. We additionally observed small risk differences of −0.02 (95% CI −0.04 to 0.00) for all-cause mortality and −0.04 (95% CI −0.07 to −0.01) for HF hospitalization.
Pre-specified sensitivity analyses were conducted to evaluate only those studies at low risk of bias and the use of alternative meta-analytic approaches. Associations between study interventions and outcomes were largely consistent with the main analysis in both magnitude and direction. There were 10 RCTs and 3 non-randomized studies judged to be at low risk of bias. Pooling these studies resulted in RRs of 0.70 (95% CI 0.47-1.05) for all-cause mortality and 0.78 (95% CI 0.50-1.23) for HF hospitalization. There was minimal variability across meta-analytic methods and variance estimators (supplementary Table S8). We also conducted a post-hoc exploratory analysis restricted to RCTs with greater than 50 subjects in each arm. 4 RCTs were excluded.28,33,35,42 Pooled GDMT and clinical outcome RRs were similar to those of the main analysis.
Discussion
This systematic review found that non-physician based GDMT interventions were led primarily by specialist heart failure nurses or pharmacists. Practice arrangements ranged from completely autonomous prescribing of GDMT to supervised patient care under physicians. Reviewed RCTs featured pragmatic protocols that specified the initiation and/or uptitration of select GDMT classes, of which RASI and BB were most represented, but the exact steps of GDMT optimization were left to the discretion of the treating clinician. Given the narrow focus of our review, we conducted a meta-analysis of the RCTs reporting therapy prescription outcomes. Meta-analysis of 16 RCTs, which enrolled 5,268 patients, found significantly increased initiation and dose optimization of RASI and BB across pharmacist- and nurse-led GDMT interventions. We also observed small reductions in all-cause mortality and HF hospitalization after median follow-up of 6 months. Our results were robust to sensitivity analyses concerning risk of bias and meta-analytic methods. These findings support the potential impact of pharmacist- and nurse-led interventions on maximizing GDMT uptake among patients with HFrEF.
Traditional approaches to GDMT management have been unsuccessful in achieving widespread adoption of optimal therapy.4–6 Sequential titration to target doses across sporadic outpatient visits can take upwards of 12 months under ideal conditions, assuming therapeutic optimization remains a priority for both clinicians and patients at every visit.59 Pharmacist- and nurse-led GDMT interventions offer distinct advantages to overcome the inertia seen in conventional practice. Higher-frequency visits focused on a singular therapeutic goal facilitate incremental opportunities to optimize GDMT over usual care. The delivery of health services may also be customized to meet patient needs, such as home visits for nursing facility residents or telehealth for those living remotely.60 These benefits underscore the value of high-touch pharmacist- and nurse-based care in minimizing barriers to optimal HF management.
The lack of association between study interventions and MRA initiation deserves mention. Data for MRA endpoints were sparse, resulting in increased uncertainty around this endpoint. Of the studies included for meta-analysis, MRA was generally reserved for persistently symptomatic (e.g., NYHA Class III-IV) patients or those who had already completed titration of other GDMT classes.26,32,33,39,42 This likely restricted utilization of MRA compared with other therapeutic classes. Approaches involving sequential titration of GDMT often result in therapeutic inertia and undertreatment due to clinician hesitation to uptitrate medications when patients are “stable” and fear of increasing adverse effects. Medications such as MRA may be disproportionately affected in sequential strategies due to their side effect profiles. A mixed-methods study identified provider-level factors as key barriers to MRA adoption, including inexperience prescribing MRA and requisite safety monitoring to prevent hyperkalemia, particularly among non-cardiologist providers.61 The role of pharmacist- and nurse-led interventions in enhancing MRA utilization requires additional research, especially given consistently suboptimal MRA treatment rates across large outpatient registries.4,6
Our study addressed several gaps in the literature. First, prior meta-analyses included a wide range of study interventions without necessarily focusing on GDMT management.11,13 Koshman et al. (2008)11 and Parajuli et al. (2019)13 each focused on pharmacist care of patients with heart failure, but study interventions primarily addressed educational counseling, lifestyle modifications, and self-care behaviors; fewer than half of the included studies involved active modification of HF medications, and neither meta-analysis reported new prescription or dose achievement endpoints. For our systematic review, we specifically narrowed our search to studies focused on GDMT titration to facilitate interpretation of pooled outcomes. Second, our analysis includes interventions involving any type of non-physician healthcare professional. Previous studies have focused on either pharmacists11,13 or nurses,12 limiting their generalizability. A meta-analysis by Driscoll et al. (2016)12 only included RCTs investigating nurse-led GDMT interventions up to December 2014. Due to the limited number and size of RCTs included, the proportion of participants reaching maximal dose of GDMT could only be pooled for BB with a low quality of evidence. In our study, inclusion of more recent RCTs and pharmacist-led interventions enabled meta-analysis of other GDMT initiation and titration endpoints and more robust clinical outcomes assessment. Furthermore, we found minimal heterogeneity across GDMT outcomes between pharmacist- and nurse-led interventions, which suggests healthcare systems may have more flexibility when allocating resources for program implementation. Finally, we uniquely reviewed non-randomized comparative effectiveness studies in addition to RCTs. While randomization remains the gold standard of clinical investigation, high-quality observational research is frequently excluded a priori from meta-analyses.62 To mitigate concern for confounding, we pooled non-randomized study effects only as a secondary analysis and restricted to studies with low risk of bias in a sensitivity analysis.
Limitations
Our findings should be interpreted in the context of several limitations. First, high heterogeneity was observed across several therapy prescription endpoints. For select studies, the pattern of effect sizes across GDMT outcomes suggests contribution from patient-level factors. In the ETIFIC (Enfermera Titula Farmacos en Insuficiencia Cardiaca) study, 77-97% of patients were already on GDMT at baseline.39 While the intervention protocol included instructions for both therapy initiation and titration, the main outcomes of the trial were the mean doses achieved across therapies, indicating stronger emphasis on GDMT optimization. Accordingly, the intervention effects were substantially larger for GDMT uptitration than for initiation. Another trial studied nurse-based home care for long-term care facility residents, in which the average age was 84 years and no exclusions were made based on frailty.33 Although the intervention led to increased GDMT initiation, achievement of optimal doses was limited in the setting of comorbidities and adverse effects. In contrast, the Interdisciplinary Network for Heart Failure study found significant increases across most therapy prescription endpoints.32 This trial featured a relatively young cohort (average age of 66 years) with low comorbidity burden. Heterogeneity across interventions and populations deserves further investigation to identify the conditions necessary for successful implementation of nurse- and pharmacist-led GDMT interventions.
Second, visual inspection of funnel plots identified possible asymmetry in a couple endpoints, suggesting presence of small-study effects. One possible explanation is publication bias, since this study did not include unpublished or gray literature. Therefore, endpoints in which small-study effects are most prominent (i.e., RASI initiation, HF hospitalization) should be interpreted with caution, as the pooled outcomes may be overestimated. Third, few studies included management of ARNI and SGLT2I,39,45 and only 1 study protocol explicitly incorporated upfront simultaneous initiation of quadruple therapy in the intervention arm.45 Future implementation studies should integrate newer strategies for rapid sequence initiation and titration of GDMT incorporating all four therapeutic classes.63 Fourth, there is likely insufficient power to detect significant reductions in mortality or hospitalization endpoints given the relatively short follow-up across studies. However, the improved utilization of therapies recommended by guidelines for significant survival benefit and quality-of-life improvement should be sufficient to justify implementation efforts. Fifth, we defined a limited set of outcomes in our analysis, which excluded quality of life, change in ejection fraction, and medication adherence. These were noted during systematic review (Table 2 and supplementary Table S6) but deserve dedicated investigation. Finally, the small number of trials in select subgroup comparisons may limit the validity of statistical testing in those analyses.
Conclusion
Pharmacist- and nurse-led GDMT interventions were associated with significantly improved RASI and BB initiation and titration to target doses. Lower pooled rates of mortality and HF hospitalization were also observed for the intervention arm, though these effects were small and not statistically significant. Collaboration with experienced pharmacists and/or nurses to facilitate GDMT optimization may enhance guideline concordance and subsequent patient outcomes.
Supplementary Material
Sources of Funding:
ATS is supported by a grant from the NHLBI (1K23HL151672-01). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Biography

Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
None of the authors have relevant financial interests or relationships to disclose.
Data Statement:
Data extracted from included studies and used in all analyses along with analytic code may be obtained via request to the corresponding author.
References
- 1.Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure. J Am Coll Cardiol. Published online April 2022:S0735109721083959. doi: 10.1016/j.jacc.2021.12.012 [DOI] [Google Scholar]
- 2.Glynn P, Lloyd-Jones DM, Feinstein MJ, Carnethon M, Khan SS. Disparities in Cardiovascular Mortality Related to Heart Failure in the United States. J Am Coll Cardiol. 2019;73(18):2354–2355. doi: 10.1016/j.jacc.2019.02.042 [DOI] [PubMed] [Google Scholar]
- 3.Roger VL. Epidemiology of Heart Failure. Circ Res. 2021;128(10):1421–1434. doi: 10.1161/CIRCRESAHA.121.318172 [DOI] [PubMed] [Google Scholar]
- 4.Greene SJ, Butler J, Albert NM, et al. Medical Therapy for Heart Failure With Reduced Ejection Fraction. J Am Coll Cardiol. 2018;72(4):351–366. doi: 10.1016/j.jacc.2018.04.070 [DOI] [PubMed] [Google Scholar]
- 5.Maddox TM, Song Y, Allen J, et al. Trends in U.S. Ambulatory Cardiovascular Care 2013 to 2017. J Am Coll Cardiol. 2020;75(1):93–112. doi: 10.1016/j.jacc.2019.11.011 [DOI] [PubMed] [Google Scholar]
- 6.Savarese G, Bodegard J, Norhammar A, et al. Heart failure drug titration, discontinuation, mortality and heart failure hospitalization risk: a multinational observational study (US, UK and Sweden). Eur J Heart Fail. 2021;23(9):1499–1511. doi: 10.1002/ejhf.2271 [DOI] [PubMed] [Google Scholar]
- 7.Sandhu AT, Kohsaka S, Turakhia MP, Lewis EF, Heidenreich PA. Evaluation of Quality of Care for US Veterans With Recent-Onset Heart Failure With Reduced Ejection Fraction. JAMA Cardiol. 2022;7(2):130. doi: 10.1001/jamacardio.2021.4585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maddox TM, Januzzi JL, Allen LA, et al. 2021 Update to the 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 Pivotal Issues About Heart Failure With Reduced Ejection Fraction. J Am Coll Cardiol. 2021;77(6):772–810. doi: 10.1016/j.jacc.2020.11.022 [DOI] [PubMed] [Google Scholar]
- 9.Carnicelli AP, Lippmann SJ, Greene SJ, et al. Sacubitril/Valsartan Initiation and Postdischarge Adherence Among Patients Hospitalized for Heart Failure. J Card Fail. 2021;27(8):826–836. doi: 10.1016/j.cardfail.2021.03.012 [DOI] [PubMed] [Google Scholar]
- 10.Greene SJ, Fonarow GC, DeVore AD, et al. Titration of Medical Therapy for Heart Failure With Reduced Ejection Fraction. J Am Coll Cardiol. 2019;73(19):2365–2383. doi: 10.1016/j.jacc.2019.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koshman SL. Pharmacist Care of Patients With Heart Failure: A Systematic Review of Randomized Trials. Arch Intern Med. 2008;168(7):687. doi: 10.1001/archinte.168.7.687 [DOI] [PubMed] [Google Scholar]
- 12.Driscoll A, Currey J, Tonkin A, Krum H. Nurse-led titration of angiotensin converting enzyme inhibitors, beta-adrenergic blocking agents, and angiotensin receptor blockers for people with heart failure with reduced ejection fraction. Cochrane Heart Group, ed. Cochrane Database Syst Rev. 2015;2016(1). doi: 10.1002/14651858.CD009889.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parajuli DR, Kourbelis C, Franzon J, et al. Effectiveness of the Pharmacist-Involved Multidisciplinary Management of Heart Failure to Improve Hospitalizations and Mortality Rates in 4630 Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Card Fail. 2019;25(9):744–756. doi: 10.1016/j.cardfail.2019.07.455 [DOI] [PubMed] [Google Scholar]
- 14.Higgins J, Thomas J, Chander J, et al. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021). www.training.cochrane.org/handbook
- 15.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. Published online March 29, 2021:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng J, Mednick T, Sandhu AT. PROSPERO 2022 CRD42022334661. Published 2022. https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=334661
- 17.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. Published online August 28, 2019:14898. doi: 10.1136/bmj.14898 [DOI] [PubMed] [Google Scholar]
- 18.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. Published online October 12, 2016:i4919. doi: 10.1136/bmj.14919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sterne JAC, Sutton AJ, Ioannidis JPA, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343(jul22 1):d4002–d4002. doi: 10.1136/bmj.d4002 [DOI] [PubMed] [Google Scholar]
- 20.IntHout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014;14(1):25. doi: 10.1186/1471-2288-14-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langan D, Higgins JPT, Jackson D, et al. A comparison of heterogeneity variance estimators in simulated random-effects meta-analyses. Res Synth Methods. 2019;10(1):83–98. doi: 10.1002/jrsm.1316 [DOI] [PubMed] [Google Scholar]
- 22.Higgins JPT. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veroniki AA, Jackson D, Viechtbauer W, et al. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res Synth Methods. 2016;7(1):55–79. doi: 10.1002/jrsm.1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwarzer G, Carpenter JR, Rucker G. Meta-Analysis with R. Springer International Publishing; 2015. [Google Scholar]
- 25.McGuinness LA. robvis: An R package and web application for visualising risk-of-bias assessments. Published 2019. https://github.com/mcguinlu/robvis [DOI] [PubMed]
- 26.Agvall B, Alehagen U, Dahlström U. The benefits of using a heart failure management programme in Swedish primary healthcare. Eur J Heart Fail. 2013;15(2):228–236. doi: 10.1093/eurjhf/hfs159 [DOI] [PubMed] [Google Scholar]
- 27.Ansari M, Shlipak MG, Heidenreich PA, et al. Improving Guideline Adherence: A Randomized Trial Evaluating Strategies to Increase β-Blocker Use in Heart Failure. Circulation. 2003;107(22):2799–2804. doi: 10.1161/01.CIR.0000070952.08969.5B [DOI] [PubMed] [Google Scholar]
- 28.Driscoll A, Srivastava P, Toia D, Gibcus J, Hare DL. A nurse-led up-titration clinic improves chronic heart failure optimization of beta-adrenergic receptor blocking therapy - a randomized controlled trial. BMC Res Notes. 2014;7(1):668. doi: 10.1186/1756-0500-7-668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ekman I, Fagerberg B, Andersson B, Matejka G, Persson B. Can treatment with angiotensin-converting enzyme inhibitors in elderly patients with moderate to severe chronic heart failure be improved by a nurse-monitored structured care program? A randomized controlled trial. Heart Lung. 2003;32(1):3–9. doi: 10.1067/mhl.2003.5 [DOI] [PubMed] [Google Scholar]
- 30.Galbreath AD, Krasuski RA, Smith B, et al. Long-Term Healthcare and Cost Outcomes of Disease Management in a Large, Randomized, Community-Based Population With Heart Failure. Circulation. 2004;110(23):3518–3526. doi: 10.1161/01.CIR.0000148957.62328.89 [DOI] [PubMed] [Google Scholar]
- 31.Gattis WA, Hasselblad V, Whellan DJ, O’Connor CM. Reduction in Heart Failure Events by the Addition of a Clinical Pharmacist to the Heart Failure Management Team: Results of the Pharmacist in Heart Failure Assessment Recommendation and Monitoring (PHARM) Study. Arch Intern Med. 1999;159(16):1939. doi: 10.1001/archinte.159.16.1939 [DOI] [PubMed] [Google Scholar]
- 32.Güder G, Störk S, Gelbrich G, et al. Nurse-coordinated collaborative disease management improves the quality of guideline-recommended heart failure therapy, patient-reported outcomes, and left ventricular remodelling: Nurse-coordinated disease management improves therapy and outcomes in HF. Eur J Heart Fail. 2015;17(4):442–452. doi: 10.1002/ejhf.252 [DOI] [PubMed] [Google Scholar]
- 33.Hancock HC, Close H, Mason JM, et al. Feasibility of evidence-based diagnosis and management of heart failure in older people in care: a pilot randomised controlled trial. BMC Geriatr. 2012;12(1):70. doi: 10.1186/1471-2318-12-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kasper EK, Gerstenblith G, Hefter G, et al. A randomized trial of the efficacy of multidisciplinary care in heart failure outpatients at high risk of hospital readmission. J Am Coll Cardiol. 2002;39(3):471–480. doi: 10.1016/S0735-1097(01)01761-2 [DOI] [PubMed] [Google Scholar]
- 35.Krantz MJ, Havranek EP, Haynes DK, Smith I, Bucher-Bartelson B, Long CS. Inpatient Initiation of β-blockade Plus Nurse Management in Vulnerable Heart Failure Patients: A Randomized Study. J Card Fail. 2008;14(4):303–309. doi: 10.1016/j.cardfail.2007.12.008 [DOI] [PubMed] [Google Scholar]
- 36.Laramee AS, Levinsky SK, Sargent J, Ross R, Callas P. Case Management in a Heterogeneous Congestive Heart Failure Population: A Randomized Controlled Trial. Arch Intern Med. 2003; 163(7): 809. doi: 10.1001/archinte.163.7.809 [DOI] [PubMed] [Google Scholar]
- 37.Lowrie R, Mair FS, Greenlaw N, et al. Pharmacist intervention in primary care to improve outcomes in patients with left ventricular systolic dysfunction. Eur Heart J. 2012;33(3):314–324. doi: 10.1093/eurheartj/ehr433 [DOI] [PubMed] [Google Scholar]
- 38.McCarren M, Furmaga E, Jackevicius CA, et al. Improvement of Guideline Beta-Blocker Prescribing in Heart Failure: A Cluster-Randomized Pragmatic Trial of a Pharmacy Intervention. J Card Fail. 2013;19(8):525–532. doi: 10.1016/j.cardfail.2013.06.004 [DOI] [PubMed] [Google Scholar]
- 39.Oyanguren J, Garcia-Garrido L, Nebot-Margalef M, et al. Noninferiority of heart failure nurse titration versus heart failure cardiologist titration. ETIFIC multicenter randomized trial. Rev Esp Cardiol Engl Ed. 2021;74(6):533–543. doi: 10.1016/j.rec.2020.04.016 [DOI] [PubMed] [Google Scholar]
- 40.Sisk JE, Hebert PL, Horowitz CR, McLaughlin MA, Wang JJ, Chassin MR. Effects of Nurse Management on the Quality of Heart Failure Care in Minority Communities: A Randomized Trial. Ann Intern Med. 2006;145(4):273. doi: 10.7326/0003-4819-145-4-200608150-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stromberg A. Nurse-led heart failure clinics improve survival and self-care behaviour in patients with heart failureResults from a prospective, randomised trial. Eur Heart J. 2003;24(11):1014–1023. doi: 10.1016/S0195-668X(03)00112-X [DOI] [PubMed] [Google Scholar]
- 42.Thompson DR, Roebuck A, Stewart S. Effects of a nurse-led, clinic and home-based intervention on recurrent hospital use in chronic heart failure. Eur J Heart Fail. 2005;7(3):377–384. doi: 10.1016/j.ejheart.2004.10.008 [DOI] [PubMed] [Google Scholar]
- 43.Al-Bawardy R, Cheng-Lai A, Prlesi L, et al. Heart Failure Postdischarge Clinic: A Pharmacist-led Approach to Reduce Readmissions. Curr Probl Cardiol. 2019;44(10):100407. doi: 10.1016/j.cpcardiol.2018.12.004 [DOI] [PubMed] [Google Scholar]
- 44.Bhat S, Kansal M, Rondos GT, Groo V. Outcomes of a Pharmacist-Managed Heart Failure Medication Titration Assistance Clinic. Ann Pharmacother. 2018;52(8):724–732. doi: 10.1177/1060028018760568 [DOI] [PubMed] [Google Scholar]
- 45.Bhatt AS, Varshney AS, Nekoui M, et al. Virtual optimization of guideline-directed medical therapy in hospitalized patients with heart failure with reduced ejection fraction: the IMPLEMENT-HF pilot study. Eur J Heart Fail. 2021;23(7):1191–1201. doi: 10.1002/ejhf.2163 [DOI] [PubMed] [Google Scholar]
- 46.Blizzard S, Verbosky N, Stein B, et al. Evaluation of Pharmacist Impact Within an Interdisciplinary Inpatient Heart Failure Consult Service. Ann Pharmacother. 2019;53(9):905–915. doi: 10.1177/1060028019842656 [DOI] [PubMed] [Google Scholar]
- 47.Desai AS, Maclean T, Blood AJ, et al. Remote Optimization of Guideline-Directed Medical Therapy in Patients With Heart Failure With Reduced Ejection Fraction. JAMA Cardiol. 2020;5(12):1430. doi: 10.1001/jamacardio.2020.3757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Di Palo KE, Patel K, Assafin M, Piña IF. Implementation of a Patient Navigator Program to Reduce 30-day Heart Failure Readmission Rate. Prog Cardiovasc Dis. 2017;60(2):259–266. doi: 10.1016/j.pcad.2017.07.004 [DOI] [PubMed] [Google Scholar]
- 49.Driscoll A, Krum H, Wolfe R, Tonkin A. Nurse-Fed Titration of β-Adrenoreceptor Blocking Agents in Chronic Heart Failure Patients in the Community. J Card Fail. 2011; 17(3):224–230. doi: 10.1016/j.cardfail.2010.10.010 [DOI] [PubMed] [Google Scholar]
- 50.El Hadidi S, Samir Bazan N, Byrne S, Darweesh E, Bermingham M. Heart Failure Prescribing Quality at Discharge from a Critical Care Unit in Egypt: The Impact of Multidisciplinary Care. Pharmacy. 2020;8(3):159. doi: 10.3390/pharmacy8030159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu L, Jackevicius CA, de Feon NK, Warner AL, Chang DS, Mody FV. Impact of a Multidisciplinary Heart Failure Postdischarge Management Clinic on Medication Adherence. Clin Ther. 2017;39(6):1200–1209. doi: 10.1016/j.clinthera.2017.04.012 [DOI] [PubMed] [Google Scholar]
- 52.Martinez AS, Saef J, Paszczuk A, Bhatt-Chugani H. Implementation of a pharmacist-managed heart failure medication titration clinic. Am J Health Syst Pharm. 2013;70(12):1070–1076. doi: 10.2146/ajhpl20267 [DOI] [PubMed] [Google Scholar]
- 53.Noschese LA, Bergman CL, Brar CK, Kansal MM. The Pharmacist’s Role in Medication Optimization for Patients With Chronic Heart Failure. Fed Pract Health Care Prof VA DoD PHS. 2017;34(Suppl 10):S10–S15. [PMC free article] [PubMed] [Google Scholar]
- 54.Shah SP, Dixit NM, Mendoza K, et al. Integration of clinical pharmacists into a heart failure clinic within a safety-net hospital. J Am Pharm Assoc. 2022;62(2):575–579.e2. doi: 10.1016/j.japh.2021.11.012 [DOI] [PubMed] [Google Scholar]
- 55.Slade J, Lee M, Park J, Liu A, Heidenreich P, Allaudeen N. Harnessing the Potential of Primary Care Pharmacists to Improve Heart Failure Management. Jt Comm J Qual Patient Saf. 2022;48(1):25–32. doi: 10.1016/j.jcjq.2021.10.004 [DOI] [PubMed] [Google Scholar]
- 56.Sparks ER, Beavers JC. Evaluation of a Pharmacist-Driven Aldosterone Antagonist Stewardship Program in Patients With Heart Failure. J Pharm Pract. 2019;32(2): 158–162. doi: 10.1177/0897190017747083 [DOI] [PubMed] [Google Scholar]
- 57.Suzuki M, Matsue Y, Izumi S, et al. Pharmacist-led intervention in the multidisciplinary team approach optimizes heart failure medication. Heart Vessels. 2018;33(6):615–622. doi: 10.1007/s00380-017-1099-8 [DOI] [PubMed] [Google Scholar]
- 58.Upton AJ, Tilton R, Ogedengbe O, et al. Impact of a pharmacist-inclusive post-discharge clinic on outcomes in heart failure patients with reduced ejection fraction: Rates of hospital readmission, emergency department visits, or death. JACCP J Am Coll Clin Pharm. 2021;4(12):1516–1523. doi: 10.1002/jac5.1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McMurray JJV, Packer M. How Should We Sequence the Treatments for Heart Failure and a Reduced Ejection Fraction?: A Redefinition of Evidence-Based Medicine. Circulation. 2021;143(9):875–877. doi: 10.1161/CIRCULATIONAHA.120.052926 [DOI] [PubMed] [Google Scholar]
- 60.Thibodeau JT, Gorodeski EZ. Telehealth for Uptitration of Guideline-Directed Medical Therapy in Heart Failure. Circulation. 2020;142(16):1507–1509. doi: 10.1161/CIRCULATIONAHA.120.050582 [DOI] [PubMed] [Google Scholar]
- 61.Dev S, Hoffman TK, Kavalieratos D, et al. Barriers to Adoption of Mineralocorticoid Receptor Antagonists in Patients With Heart Failure: A Mixed-Methods Study. J Am Heart Assoc. 2016;5(3):e002493. doi: 10.1161/JAHA.115.002493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shrier I, Boivin JF, Steele RJ, et al. Should Meta-Analyses of Interventions Include Observational Studies in Addition to Randomized Controlled Trials? A Critical Examination of Underlying Principles. Am J Epidemiol. 2007;166(10):1203–1209. doi: 10.1093/aje/kwml89 [DOI] [PubMed] [Google Scholar]
- 63.Sharma A, Verma S, Bhatt DL, et al. Optimizing Foundational Therapies in Patients With HFrEF. .JACC Basic Transl Sci. Published online March 2022:S2452302X21003594. doi: 10.1016/j.jacbts.2021.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


