Abstract
Background:
Reoperative parathyroidectomy for recurrent/persistent primary hyperparathyroidism (PHPT) has high rates of failure. The goal of this study was to analyze our experience with imaging and parathyroid vein sampling (PAVS) for recurrent/persistent PHPT.
Methods:
We performed a retrospective cohort study (2002-2018) of patients with recurrent/persistent PHPT undergoing reoperative parathyroidectomy.
Results:
Among 181 patients, the most common imaging study was sestamibi (89.5%), followed by ultrasound (75.7%). CT had the highest rate of localization (70.8%) compared to sestamibi (58.0%) and ultrasound (47.4%). PAVS was performed in 25 patients, and localized in 96%. Ultrasound and sestamibi both demonstrated 62% PPV for operative pathology, compared to 41% in CT. PAVS was 95% sensitive with 95% PPV for predicting the correct side of abnormal parathyroid tissue.
Conclusions:
We recommend a sequential imaging evaluation for reoperative parathyroidectomy, with sestamibi and/or ultrasound followed by CT. PAVS should be considered if non-invasive imaging fails to localize.
Keywords: Reoperative parathyroidectomy, Parathyroidectomy, Jugular Venous Sampling, Primary hyperparathyroidism, Recurrent primary hyperparathyroidism, Parathyroid Venous Sampling
Introduction
Primary hyperparathyroidism (PHPT) is a common endocrine disorder characterized by the autonomous over-secretion of parathyroid hormone (PTH) from one or more parathyroid gland(s).1 The most common clinical manifestation of PHPT is asymptomatic hypercalcemia, with up to 80% of patients incidentally detected for high serum calcium levels through routine testing.1–3 Further biochemical testing, such as PTH levels, 24-hour urinary calcium, ionized calcium, is used to establish a diagnosis of PHPT.
The only curative option for PHPT is surgery.4,5 Although PHPT is a biochemical diagnosis, adjunctive imaging may help to identify surgical targets. Cure rates after initial parathyroidectomy are high, and in the hands of experienced surgeons, preoperative imaging may not significantly change outcomes.5–7 Rates of recurrent or persistent PHPT range from 1-10%.8,9 For patients with recurrent or persistent disease, reoperative parathyroidectomy represents a challenge, as failure rates are markedly higher.10–12 Common causes of recurrent or persistent primary hyperparathyroidism include missed multigland disease, failure to locate single adenomas, incomplete resection and/or the presence of ectopic glands.
In reoperative parathyroidectomy, imaging studies are of significant importance. An extensive literature exists comparing different imaging modalities. Ultrasound, Sestamibi, 4D CT and nuclear imaging studies are all utilized to identify recurrent or residual parathyroid tissue.13–16 A multi-modality approach may be required; for instance, 4D CT may have increased utility when Sestamibi is non-localizing.15–17 When noninvasive techniques used to localize abnormal parathyroid glands are exhausted, preoperative localization may be evaluated by minimally invasive procedures such as parathyroid venous sampling (PAVS).18,19
In this investigation, we sought to describe patterns of utilization of non-invasive and invasive localization techniques in patients with recurrent and/or persistent primary hyperparathyroidism undergoing reoperative parathyroidectomy.
Methods
Study cohort
This study was assessed by the Institutional Review Board of the Hospital of the University of Pennsylvania and deemed exempt (Protocol #831223). Subjects assessed for inclusion were identified from a prospectively maintained database of patients undergoing parathyroidectomy for hyperparathyroidism. Patients with a diagnosis of recurrent and or persistent sporadic primary hyperparathyroidism undergoing reoperative parathyroidectomy with intraoperative parathyroid hormone monitoring (2002-2018) were identified for inclusion. Patients who had more than one surgical procedure at the time of parathyroidectomy, such as a total thyroidectomy, thyroid lobectomy, or tracheostomy, were excluded from the study. The final cohort included 181 patients. Demographic, clinical, biochemical, imaging and histopathologic data were recorded.
Definitions and outcomes
The primary outcome was concordance, defined as agreement between imaging or PAVS and surgical findings assessed on 1) quadrant; and 2) side. Secondary outcomes included operative success and cure. Operative success was defined as >50% decrease in intraoperative parathyroid hormone (PTH) level. Cure was defined as eucalcemia >6 months after surgery. Localized was defined as a target identified on parathyroid venous sampling (PAVS).
Parathyroid venous sampling (PAVS) localization
Patients who had indeterminate findings from noninvasive imaging underwent parathyroid venous sampling and/or super-selective parathyroid venous sampling in the Department of Interventional Radiology as previously described.20 A subset of patients previously reported was included in this study. Intact PTH values were mapped anatomically. A ratio of target vein to superior vena cava which was greater than 2 was considered a positive gradient, consistent with prior studies.21
Clinical approach
Patients with biochemical recurrent or persistent primary hyperparathyroidism were assessed utilizing a multi-level approach. 1) Sestamibi and formal diagnostic radiology ultrasound were routinely the initial imaging studies; 2) If Sestamibi and/or ultrasound failed to identify a clear surgical target, 4D CT was performed; 3) If non-invasive imaging failed to identify a target, PAVS was performed. Subjects without a surgical target on non-invasive or invasive imaging were not considered candidates for reoperative parathyroidectomy.
Our institution does not have a 4D-CT protocol and therefore local practice is to utilize Sestamibi SPECT CT for preference. Our institutional Sestamibi SPECT protocol is as follows: Patients receive 20 mCi of Tc-99m Sestamibi injected intravenously. 10 minutes after injection, patients are positioned with neck hyperextended and head immobilized. SPECT is acquired in 128 x 128 matrix, 128 stops, 20 seconds/stop, including field of view extending from the parotid glands to the base of the heart, using dual head gamma camera with low energy high resolution parallel hole collimators. Delayed images are captured using the same parameters at 150 minutes post-injection.
Statistical analysis
Descriptive statistics were performed. Continuous variables were reported as mean with standard deviation (SD) for parametric and median with interquartile range (IQR) for non-parametric variables. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were assessed for imaging and PAVS. The relationship between covariates and concordance was assessed using univariate logistic regression. Data analysis was performed using Stata 17.1 (STATA Corp, College Station, TX).
Results
Patient demographics & biochemical data
In the final cohort consisting of 181 patients, the median age was 60 years old (IQR:50-68), as shown in Table 1. The majority were female (n=135, 74.6%) and Caucasian (n=116, 64.1%). On initial surgical evaluation, 131 patients (72.4%) reported neurocognitive symptoms and 33 patients (18.2%) had nephrolithiasis. Of the 101 patients with DXA scans, 39 (21.5%) had osteoporosis and 43 (23.8%) had osteopenia.
Table 1.
Cohort characteristics and biochemical data of patients (n=181) with recurrent/persistent primary hyperparathyroidism (PHPT) undergoing reoperative parathyroidectomy.
| CHARACTERISTICS/BIOCHEMICAL PARAMETERS | N, MEDIAN, OR MEAN |
|---|---|
| MEDIAN AGE, YEARS (IQR) | 60 (50-68) |
| GENDER (%) | |
| MALE | 46 (25.4) |
| FEMALE | 135 (74.6) |
| RACE (%) | |
| CAUCASIAN | 116 (64.1) |
| AFRICAN AMERICAN | 17 (9.4) |
| ASIAN | 3 (1.7) |
| MIXED RACE/OTHER | 9 (5.0) |
| NOT RECORDED | 36 (20.0) |
| NEUROCOGNITIVE SYMPTOMS (%) | 131 (72.4) |
| NEPHROLITHIASIS (%) | 33 (18.2) |
| DXA SCAN (%) | |
| NONE | 80 (44.2) |
| NORMAL | 19 (10.5) |
| OSTEOPENIA | 43 (23.8) |
| OSTEOPOROSIS | 39 (21.5) |
| MEDIAN PREOPERATIVE SERUM CALCIUM, MG/DL, (IQR) | 10.8 (10.4-11.3) |
| MEDIAN PREOPERATIVE URINARY CALCIUM, MG/DL, 24-HOUR (IQR) | 274 (181-368) |
| MEDIAN PREOPERATIVE INTACT PTH, PMOL/L (IQR) | 89 (52-129.7) |
| SITE OF INITIAL OPERATION (%) | |
| HOSPITAL OF THE UNIVERSITY OF PENNSYLVANIA | 51 (28.2) |
| OTHER SITE | 99 (54.7) |
| NOT RECORDED | 29 (16.0) |
| NUMBER OF GLANDS REMOVED AT INITIAL OPERATION (%) | |
| 1 | 86 (47.5) |
| 2 | 19 (10.5) |
| 3 | 9 (5.0) |
| 4 | 3 (1.7) |
The median preoperative serum calcium was slightly elevated at 10.8 mg/dl (IQR: 10.4-11.3). The median preoperative 24-hour urinary calcium was 274 mg/dl (IQR: 181-368). The median preoperative intact PTH was elevated at 89 pmol/l (IQR: 52-129.7).
In the initial operation, 8.3% of patients had no glands removed, 51.4% of patients had one gland removed, 12.2% of patients had two glands removed, and 7.7% of patients had three or more glands removed (subtotal parathyroidectomy). Prior to the initial operation, 51.9% of patients had a documented sestamibi scan performed. A minimally invasive approach was utilized in 26.5% of patients at the initial operation. The mean time interval between the initial parathyroidectomy and the reoperative parathyroidectomy was 7.6 ± 9.1 years.
Noninvasive Imaging studies
All patients underwent imaging prior to reoperative parathyroidectomy. The most commonly used study was sestamibi (n=162, 89.5%), followed by neck ultrasound (n=137, 75.7%), as shown in Table 2. CT was performed in only 24 patients. The highest rate of localization was observed in CT where 70.8% of studies performed identified localized parathyroid tissue (17/24), compared to 58.0% in sestamibi (94/162), and 47.4% on ultrasound (65/137). Frequencies of ectopic gland localization ranged from 6.1% on CT and ultrasound to 8.8% observed on sestamibi scan.
Table 2.
Imaging findings prior to reoperative parathyroidectomy for primary hyperparathyroidism.
| CHARACTERISTICS | N, MEDIAN, OR MEAN |
|---|---|
| CT SCAN (%) | |
| NON-LOCALIZING | 7 (3.9) |
| RIGHT SIDED | 5 (2.8) |
| LEFT SIDED | 1 (0.5) |
| BILATERAL | 0 (0.0) |
| ECTOPIC | 11 (6.1) |
| NOT PERFORMED | 157 (86.7) |
| SESTAMIBI SCAN (%) | |
| NON-LOCALIZING | 68 (37.6) |
| RIGHT SIDED | 41 (22.7) |
| LEFT SIDED | 33 (18.2) |
| BILATERAL | 4 (2.2) |
| ECTOPIC | 16 (8.8) |
| NOT PERFORMED | 19 (10.5) |
| ULTRASOUND (%) | |
| NON-LOCALIZING | 72 (39.8) |
| RIGHT SIDED | 28 (15.5) |
| LEFT SIDED | 23 (12.7) |
| BILATERAL | 3 (1.7) |
| ECTOPIC | 11 (6.1) |
| NOT PERFORMED | 44 (24.3) |
Operative findings
At the time of reoperative parathyroidectomy, the median intraoperative PTH decrease was 81.9% (IQR 66.4-89.6), shown in Table 3. 86.1% of patients met intraoperative PTH criteria for success. The final pathology was a single adenoma in 57.5% of patients, double adenoma in 7.2%, and hyperplasia in 22.7%. The median resected gland weight was 283.5 mg (IQR 126.0-736.0) and median size was 1.3 cm (IQR 0.9-1.9). For patients with biochemical follow up >6 months after surgery (n=62), 93.5% were cured.
Table 3.
Operative findings of patients with recurrent/persistent primary hyperparathyroidism undergoing reoperative parathyroidectomy (n=181).
| SURGICAL FINDINGS | N, MEDIAN, OR MEAN |
|---|---|
| MEDIAN INITIAL INTRAOPERATIVE PTH, PMOL/L (IQR) | 13.1 (10.1-21.4) |
| MEDIAN FINAL INTRAOPERATIVE PTH, PMOL/L (IQR) | 2.4 (1.6-4.6) |
| MEDIAN PERCENTAGE DECREASE IN IOPTH, % (IQR) | 81.9 (66.4-89.6) |
| ABNORMAL GLAND (%) | |
| RIGHT UPPER | 48 (26.5) |
| RIGHT LOWER | 35 (19.3) |
| LEFT UPPER | 38 (21.0) |
| LEFT LOWER | 37 (20.4) |
| SUPERNUMERARY | 8 (4.4) |
| NOT RECORDED | 9 (5.0) |
| MEDIAN WEIGHT, MG (IQR) | 283.5 (126.0-736.0) |
| MEDIAN SIZE, CM (SD) | 1.3 (0.9-1.9) |
| MEAN POST-OPERATIVE SERUM CALCIUM, MG/DL (SD) | 9.5 (0.7) |
| MEAN POST-OPERATIVE PTH, PMOL/L (IQR) | 6.5 (7.3) |
| OPERATIVE SUCCESS BY IOPTH (%) | |
| NO | 20 (11.6) |
| YES | 148 (86.1) |
| FINAL DIAGNOSIS (%) | |
| INCONCLUSIVE | 15 (8.3) |
| ADENOMA | 104 (57.5) |
| DOUBLE ADENOMA | 13 (7.2) |
| HYPERPLASIA | 41 (22.7) |
| PARATHYROID CANCER | 4 (2.2) |
Concordance of preoperative localization and operative findings
Sestamibi was concordant in with operative findings at the quadrant level in 31.3% and correctly predicted laterality in 40.6%, as shown in Table 5. Sestamibi scan demonstrated the best performance of the imaging studies, with 49% sensitivity and 17% specificity for predicting side of surgical pathology. Sestamibi PPV was 62% and 10% NPV for abnormal parathyroid tissue at the time of surgery. Ultrasound similarly had low concordance with both quadrant (30.9%) and side (34.6%) of operative pathology. Ultrasound had low sensitivity (38%) and specificity (22%) for laterality of abnormal parathyroid tissue, with PPV of 62% and NPV of 10%. CT was concordant with quadrant in 33.3% and side in 37.5%. CT demonstrated sensitivity 58%, specificity 17%, PPV 41% and NPV 29%. Conversely, PAVS was concordant with the quadrant of surgical findings in 60.0%, and with the side of the surgical findings in 72.0%. PAVS was 94% sensitive and had a 94% PPV for predicting the correct quadrant, and 95% sensitive and had a 95% PPV for predicting the correct side of the abnormal parathyroid tissue at the time of surgery. Additionally, PAVS demonstrated 0% sensitivity and 0% NPV for predicting the correct quadrant and similarly for the correct side.
Table 5.
Predictive value of imaging for abnormal parathyroid tissue by quadrant and side.
| CT | SESTAMIBI | ULTRASOUND | PAVS | |
|---|---|---|---|---|
| QUADRANT | ||||
| SENSITIVITY (%) | 55 | 42 | 35 | 94 |
| SPECIFICITY (%) | 15 | 12 | 19 | 0 |
| PPV (%) | 35 | 46 | 54 | 94 |
| NPV (%) | 29 | 10 | 10 | 0 |
| SIDE | ||||
| SENSITIVITY (%) | 58 | 49 | 38 | 95 |
| SPECIFICITY (%) | 17 | 17 | 22 | 0 |
| PPV (%) | 41 | 62 | 62 | 95 |
| NPV (%) | 29 | 10 | 10 | 0 |
Patients undergoing PAVS
A subset of patients (n=25) underwent PAVS for localization of abnormal parathyroid glands. PAVS was localizing in 24 patients (96%). PAVS identified a single quadrant in 20 subjects, a single side in 1 subject, and two quadrants on opposite sides in 3 subjects. Of note, PAVS localizing to a single quadrant was not a guarantee of single gland disease, however PAVS localizing to multiple quadrants was suggestive of multigland disease and therefore a more extensive operation was planned for patients in whom PAVS suggested multiple surgical targets. On surgical exploration, the median drop in PTH was 83.3% (IQR: 45.2-91.1), resulting in 72% of patients meeting the criteria for intraoperative success, as shown in Table 4. The final diagnosis was single adenoma in 14 patients, hyperplasia in 6, double adenoma in 1, parathyroid cancer in 1, and inconclusive in 3.
Table 4.
Operative findings of patients with recurrent/persistent primary hyperparathyroidism undergoing PAVS (n=25).
| SURGICAL FINDINGS | N, MEDIAN, OR MEAN |
|---|---|
| MEDIAN INITIAL INTRAOPERATIVE PTH, PMOL/L (IQR) | 12.4 (9.3-20.3) |
| MEDIAN FINAL INTRAOPERATIVE PTH, PMOL/L (IQR) | 2.2 (1.5-5.5) |
| MEDIAN PERCENTAGE DECREASE IN IOPTH, % (IQR) | 83.3 (45.2-91.1) |
| ABNORMAL GLAND (%) | |
| RIGHT UPPER | 3 (12.0) |
| RIGHT LOWER | 2 (8.0) |
| LEFT UPPER | 8 (32.0) |
| LEFT LOWER | 4 (16.0) |
| SUPERNUMERARY | 4 (16.0) |
| NOT RECORDED | 4 (16.0) |
| MEDIAN WEIGHT, MG (IQR) | 138.3 (30.8-301.0) |
| MEAN SIZE, CM (SD) | 1.1 (0.5) |
| OPERATIVE SUCCESS BY IOPTH (%) | |
| NO | 6 (24.0) |
| YES | 18 (72.0) |
| FINAL DIAGNOSIS (%) | |
| INCONCLUSIVE | 2 (8.0) |
| ADENOMA | 14 (56.0) |
| DOUBLE ADENOMA | 1 (4.0) |
| HYPERPLASIA | 7 (28.0) |
| PARATHYROID CANCER | 1 (4.0) |
| CONCORDANT WITH PAVS (%) | |
| QUADRANT CONCORDANT | 15 (60.0) |
| SIDE CONCORDANT | 18 (72.0) |
Discussion
Reoperative parathyroidectomy remains challenging even for the experienced surgeon. In this study, we assessed the performance of imaging studies and PAVS for predicting the location of parathyroid pathology in patients with recurrent/persistent PHPT undergoing reoperative parathyroidectomy. We found that non-invasive imaging had variable predictive value depending on the technique, but invasive imaging with PAVS consistently demonstrated the best positive predictive value. In our cohort, ultrasound and sestamibi both had low sensitivity (38% and 49% respectively) but moderate positive predictive value (62%) for the location of abnormal parathyroid tissue. The sensitivity of ultrasound and sestamibi observed in our study cohort are slightly lower than published sensitivities which range from 74-91% in ultrasound and 28-98% in sestamibi.22–24 However, our study was restricted to patients with recurrent/persistent primary hyperparathyroidism undergoing reoperative parathyroid surgery. Therefore, our study population would be anticipated to have both stigmata of prior surgery and a higher likelihood of multigland parathyroid disease, both of which can confound imaging findings. Sestamibi scan in particular has a particularly low sensitivity for multigland parathyroid disease.25,26
In contrast to our findings in US and Sestamibi, in our cohort CT demonstrated relatively high sensitivity but only 41% positive predictive value for the localization of abnormal parathyroid tissue. Multiple studies have shown that 4D CT has higher sensitivity than ultrasound and sestamibi.17,24,27 However, false positive findings may include lymph nodes, thyroid nodules, and other head and neck pathology. This may be of particular concern in the reoperative neck, where scar tissue, post-operative changes and retained foreign bodies such as clips or ties may distort the normal anatomy.
Although only performed in a small subset of our patient population, we found that PAVS demonstrated the best localization of all the imaging strategies we assessed. PAVS had a 94% PPV for quadrant, and 95% PPV for the affected side of abnormal parathyroid tissue. This is in agreement with other published series which show sensitivity ranging from 75-93%.33–35 Of note, as all patients who underwent PAVS did not undergo all three imaging studies, it is difficult to make a direct comparison between performance rates. However, given that patients were referred for PAVS when non-invasive imaging studies were negative, this selected population likely underestimates the performance of PAVS.
Based on these findings, we recommend a sequential approach for patients undergoing evaluation for reoperative parathyroidectomy, as shown in Figure 1. We suggest that the initial imaging consist of high specificity evaluation with neck ultrasound and parathyroid scan (preferably sestamibi SPECT CT). If these fail to localize, the next non-invasive imaging study would be a high sensitivity 4D CT. Similar strategies have been proposed for imaging of initial parathyroidectomy.28 Although ultrasound has significant user dependence and low sensitivity, ultrasound may have particular utility as a screening study, due to the low cost, absence of radiation exposure, and ability to be performed by the operative surgeon in a clinic-based setting.29–32 We uncommonly use MRI both due to the fact that there are multiple other imaging modalities which are well-established and due to the fact that it is difficult to obtain insurance approval.
Figure 1. Proposed algorithm for care of patients with recurrent or persistent primary hyperparathyroidism (PHPT) and indications for reoperative parathyroidectomy.
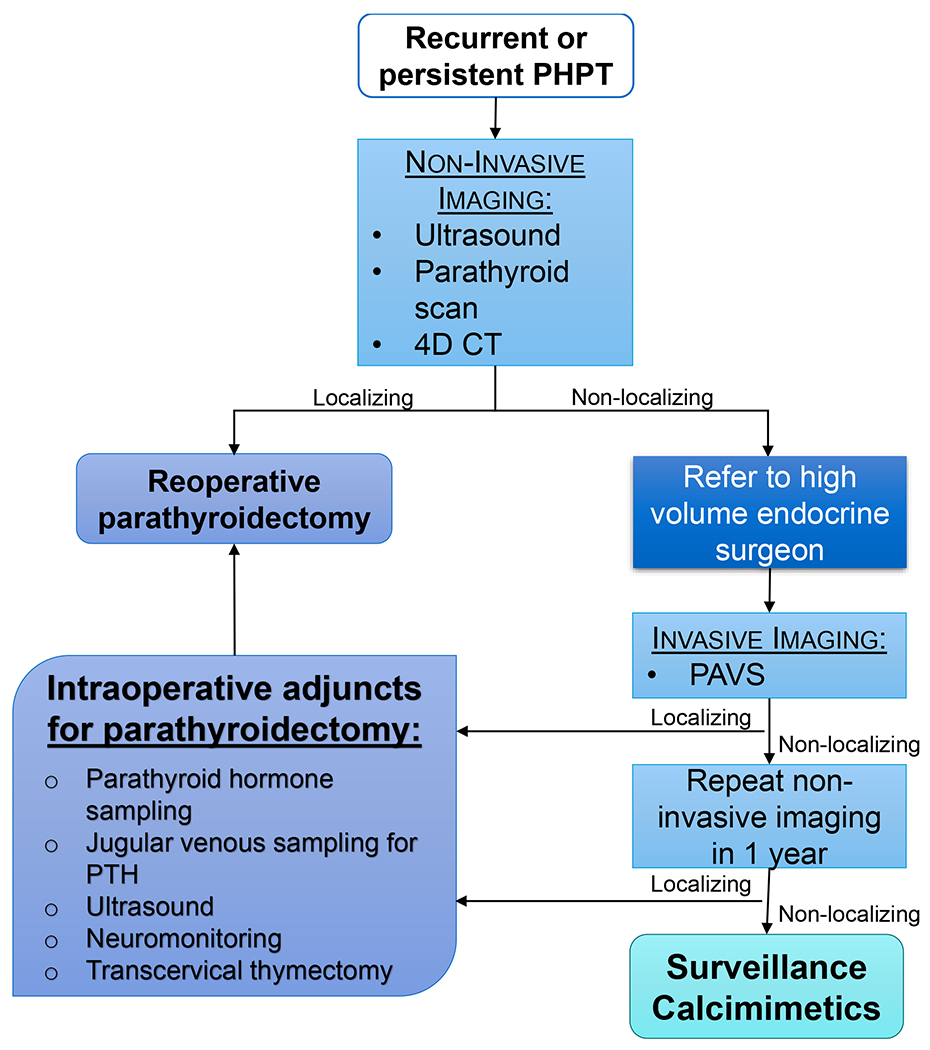
Evaluation should include non-invasive imaging; we suggest ultrasound and parathyroid scan with Sestamibi SPECT CT as the initial step, followed by 4D CT if ultrasound and parathyroid scan are non-localizing. For patients who fail to localize by 4D CT as well, we recommend referral to a tertiary care center with an experienced endocrine surgeon, ability to perform invasive imaging with PAVS, and availability of adjunctive technologies for reoperative parathyroidectomy. Patients in whom no potential operative target can be identified with any imaging modality are unlikely to have any benefit from reoperative parathyroidectomy. Therefore, for these patients we recommend repeat non-invasive imaging in 1 year as a subset may develop interval imaging findings which are amenable to surgery. For patients with no surgical targets, we recommend ongoing surveillance and consideration of calcimimetic therapy based on the clinical scenario.
For patients with recurrent/persistent primary hyperparathyroidism and clear indications for parathyroidectomy who fail to localize with sequential non-invasive imaging, we suggest referral to a tertiary care center for management by an expert parathyroid surgeon. In addition to surgical expertise, a tertiary care center can offer additional resources such as invasive imaging with PAVS and adjunctive intraoperative technologies. PAVS may identify potential operative targets where non-invasive imaging fails to localize.
For patients with an operative target identified on either invasive of non-invasive imaging, we recommend that reoperative parathyroidectomy be performed by an experienced surgeon and that neuromonitoring of the recurrent laryngeal nerve and intraoperative parathyroid hormone monitoring be used routinely. Other useful adjunctive technologies include intraoperative ultrasound, parathyroid venous sampling. If intraoperative parathyroid jugular venous sampling is utilized to determine laterality of abnormal parathyroid tissue, sampling should be performed at the most caudal point which is accessible and a gradient of more than 2- 3 to 1 is suggestive of lateralization. If parathyroid venous sampling is performed intraoperatively with the goal of identifying a specific quadrant rather than side, ipsilateral specimens should be drawn both proximal and distal to the middle thyroidal vein. If identified operative targets are in the mediastinum, formal transcervical thymectomy with thoracic surgeons may be an option at tertiary centers, potentially avoiding sternotomy.
Patients in whom no potential operative target can be identified with any imaging modality are unlikely to have any benefit from reoperative parathyroidectomy. Therefore, for these patients we recommend repeat non-invasive imaging in 1 year as a subset may develop interval imaging findings. For patients with no surgical targets on repeat imaging, we recommend ongoing biochemical surveillance and consideration of therapy with calcimimetics depending on the clinical scenario.
Utilizing a sequential approach to imaging for primary hyperparathyroidism is not novel. However, this investigation illustrates the application of the sequential imaging strategy to a large, real-world cohort of reoperative parathyroidectomies. Patients with recurrent and persistent primary hyperparathyroidism represent an ongoing challenge even to experienced surgeons, and therefore the proposed management algorithm presents an approach that has proved successful in a modern cohort of reoperative parathyroidectomies. This study is inherently limited by its retrospective nature. Evaluation of the performance characteristics is limited by the small sample size, particularly for CT. Additionally, due to the long study period, a heterogeneous group of CT scans was included encompassing a broad range of protocols. Therefore, these findings likely understate the performance of modern 4D parathyroid CT. There are two reasons that CT was infrequently utilized in this cohort: 1) this is an historic cohort and encompasses patients prior to widespread adoption of 4D CT; 2) our institutional 4D CT protocol is not well developed and therefore local practice is to utilize Sestamibi SPECT CT for preference. At our institution, MRI is uncommonly used for the evaluation of parathyroid disease, and therefore, was not included in our study. Furthermore, PAVS was performed by a small group of experienced interventional radiologists. While PAVS was a valuable adjunctive imaging in our study cohort, this is a highly specialized procedure which is limited to dedicated centers and may not be readily accessible. Therefore, these results may not be generalizable. However, given that reoperative parathyroidectomy is best managed by experienced surgeons at high volume centers, PAVS is applicable to this selected patient population and patients may benefit from referral to a tertiary care center with access to specialized care.
Conclusions
Ultrasound and sestamibi have low sensitivity but moderate predictive value for the location of parathyroid tissue in recurrent/persistent primary hyperparathyroidism. We recommend a sequential imaging evaluation for patients with persistent disease, using sestamibi and/or ultrasound followed by 4D parathyroid CT. PAVS demonstrates excellent predictive value and should be considered as an adjunctive study in patients who fail to localize with noninvasive imaging.
Table 6.
Cure rates and the number of patients undergoing an imaging modality for reoperative parathyroidectomy.
| NUMBER OF PATIENTS | CURE | % OF PATIENTS CURED | |
|---|---|---|---|
| SESTAMIBI ALONE | 27 | 20 | 74.1 |
| ULTRASOUND ALONE | 6 | 4 | 66.7 |
| SESTAMIBI + ULTRASOUND | 100 | 89 | 89.0 |
| SESTAMIBI + ULTRASOUND + 4D CT | 14 | 12 | 85.6 |
| SESTAMIBI + ULTRASOUND + PAVS | 11 | 9 | 81.8 |
Highlights.
Patients undergoing reoperative parathyroidectomy have higher rates of complications and failure
We compared the performance of localization techniques
CT had the highest sensitivity (58%) for abnormal parathyroid tissue
Sestamibi (62%) and ultrasound (62%) had higher positive predictive value than CT (41%)
We recommend a sequential imaging approach with a high specificity study such as ultrasound or sestamibi followed by high sensitivity study such as CT
Funding:
Support was provided to HW from the NIH NCATS grant #KL2 TR001879 and NIH NCI grant #K08 CA270385.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors declare no conflict of interest
References
- 1.Walker MD, Silverberg SJ. Primary hyperparathyroidism. Nat Rev Endocrinol. Feb 2018;14(2):115–125. doi: 10.1038/nrendo.2017.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bilezikian JP, Brandi ML, Eastell R, et al. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the Fourth International Workshop. J Clin Endocrinol Metab. Oct 2014;99(10):3561–9. doi: 10.1210/jc.2014-1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silverberg SJ, Bilezikian JP. Evaluation and management of primary hyperparathyroidism. J Clin Endocrinol Metab. Jun 1996;81(6):2036–40. doi: 10.1210/jcem.81.6.8964825 [DOI] [PubMed] [Google Scholar]
- 4.Noureldine SI, Gooi Z, Tufano RP. Minimally invasive parathyroid surgery. Gland Surg. Oct 2015;4(5):410–9. doi: 10.3978/j.issn.2227-684X.2015.03.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilhelm SM, Wang TS, Ruan DT, et al. The American Association of Endocrine Surgeons Guidelines for Definitive Management of Primary Hyperparathyroidism. JAMA Surg. Oct 1 2016;151(10):959–968. doi: 10.1001/jamasurg.2016.2310 [DOI] [PubMed] [Google Scholar]
- 6.Yip L, Pryma DA, Yim JH, Virji MA, Carty SE, Ogilvie JB. Can a lightbulb sestamibi SPECT accurately predict single-gland disease in sporadic primary hyperparathyroidism? World J Surg. May 2008;32(5):784–92; discussion 793-4. doi: 10.1007/s00268-008-9532-x [DOI] [PubMed] [Google Scholar]
- 7.Chander NR, Chidambaram S, Van Den Heede K, DiMarco AN, Tolley NS, Palazzo FF. Correlation of pre-operative imaging findings and parathyroidectomy outcomes support NICE 2019 guidance. J Clin Endocrinol Metab. Oct 13 2021;doi: 10.1210/clinem/dgab740 [DOI] [PubMed] [Google Scholar]
- 8.Shen W, Düren M, Morita E, et al. Reoperation for persistent or recurrent primary hyperparathyroidism. Arch Surg. Aug 1996;131(8):861–7; discussion 867-9. doi: 10.1001/archsurg.1996.01430200071013 [DOI] [PubMed] [Google Scholar]
- 9.Mundschenk J, Klose S, Lorenz K, Dralle H, Lehnert H. Diagnostic strategies and surgical procedures in persistent or recurrent primary hyperparathyroidism. Exp Clin Endocrinol Diabetes. 1999;107(6):331–6. doi: 10.1055/s-0029-1212122 [DOI] [PubMed] [Google Scholar]
- 10.Irvin GL 3rd, Molinari AS, Figueroa C, Carneiro DM. Improved success rate in reoperative parathyroidectomy with intraoperative PTH assay. Ann Surg. Jun 1999;229(6):874–8; discussion 878-9. doi: 10.1097/00000658-199906000-00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuo LE, Wachtel H, Fraker D, Kelz R. Reoperative parathyroidectomy: who is at risk and what is the risk? J Surg Res. Oct 2014;191(2):256–61. doi: 10.1016/j.jss.2014.05.073 [DOI] [PubMed] [Google Scholar]
- 12.Donatini G, Marciniak C, Lenne X, et al. Risk Factors of Redo Surgery After Unilateral Focused Parathyroidectomy: Conclusions From a Comprehensive Nationwide Database of 13,247 Interventions Over 6 Years. Ann Surg. Nov 2020;272(5):801–806. doi: 10.1097/sla.0000000000004269 [DOI] [PubMed] [Google Scholar]
- 13.Yen TW, Wang TS, Doffek KM, Krzywda EA, Wilson SD. Reoperative parathyroidectomy: an algorithm for imaging and monitoring of intraoperative parathyroid hormone levels that results in a successful focused approach. Surgery. Oct 2008;144(4):611–9; discussion 619-21. doi: 10.1016/j.surg.2008.06.017 [DOI] [PubMed] [Google Scholar]
- 14.Wells SA Jr., Debenedetti MK, Doherty GM. Recurrent or persistent hyperparathyroidism. J Bone Miner Res. Nov 2002;17 Suppl 2:N158–62. [PubMed] [Google Scholar]
- 15.Parikh AM, Grogan RH, Morón FE. Localization of Parathyroid Disease in Reoperative Patients with Primary Hyperparathyroidism. Int J Endocrinol. 2020;2020:9649564. doi: 10.1155/2020/9649564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cham S, Sepahdari AR, Hall KE, Yeh MW, Harari A. Dynamic Parathyroid Computed Tomography (4DCT) Facilitates Reoperative Parathyroidectomy and Enables Cure of Missed Hyperplasia. Ann Surg Oncol. Oct 2015;22(11):3537–42. doi: 10.1245/s10434-014-4331-0 [DOI] [PubMed] [Google Scholar]
- 17.Cheung K, Wang TS, Farrokhyar F, Roman SA, Sosa JA. A meta-analysis of preoperative localization techniques for patients with primary hyperparathyroidism. Ann Surg Oncol. Feb 2012;19(2):577–83. doi: 10.1245/s10434-011-1870-5 [DOI] [PubMed] [Google Scholar]
- 18.Taslakian B, Trerotola SO, Sacks B, Oklu R, Deipolyi A. The Essentials of Parathyroid Hormone Venous Sampling. Cardiovasc Intervent Radiol. Jan 2017;40(1):9–21. doi: 10.1007/s00270-016-1481-4 [DOI] [PubMed] [Google Scholar]
- 19.Powell AC, Alexander HR, Chang R, et al. Reoperation for parathyroid adenoma: a contemporary experience. Surgery. Dec 2009;146(6):1144–55. doi: 10.1016/j.surg.2009.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Habibollahi P, Shin B, Shamchi SP, Wachtel H, Fraker DL, Trerotola SO. Eleven-Year Retrospective Report of Super-Selective Venous Sampling for the Evaluation of Recurrent or Persistent Hyperparathyroidism in 32 Patients. Cardiovasc Intervent Radiol. Jan 2018;41(1):63–72. doi: 10.1007/s00270-017-1757-3 [DOI] [PubMed] [Google Scholar]
- 21.Chaffanjon PC, Voirin D, Vasdev A, Chabre O, Kenyon NM, Brichon PY. Selective venous sampling in recurrent and persistent hyperparathyroidism: indication, technique, and results. World J Surg. Oct 2004;28(10):958–61. doi: 10.1007/s00268-004-7449-6 [DOI] [PubMed] [Google Scholar]
- 22.Wong KK, Fig LM, Gross MD, Dwamena BA. Parathyroid adenoma localization with 99mTc-sestamibi SPECT/CT: a meta-analysis. Nucl Med Commun. Apr 2015;36(4):363–75. doi: 10.1097/mnm.0000000000000262 [DOI] [PubMed] [Google Scholar]
- 23.Steward DL, Danielson GP, Afman CE, Welge JA. Parathyroid adenoma localization: surgeon-performed ultrasound versus sestamibi. Laryngoscope. Aug 2006;116(8):1380–4. doi: 10.1097/01.mlg.0000227957.06529.22 [DOI] [PubMed] [Google Scholar]
- 24.Zafereo M, Yu J, Angelos P, et al. American Head and Neck Society Endocrine Surgery Section update on parathyroid imaging for surgical candidates with primary hyperparathyroidism. Head Neck. Jul 2019;41(7):2398–2409. doi: 10.1002/hed.25781 [DOI] [PubMed] [Google Scholar]
- 25.Chiu B, Sturgeon C, Angelos P. What is the link between nonlocalizing sestamibi scans, multigland disease, and persistent hypercalcemia? A study of 401 consecutive patients undergoing parathyroidectomy. Surgery. Sep 2006;140(3):418–22. doi: 10.1016/j.surg.2006.03.021 [DOI] [PubMed] [Google Scholar]
- 26.Nichols KJ, Tomas MB, Tronco GG, Palestro CJ. Sestamibi parathyroid scintigraphy in multigland disease. Nucl Med Commun. Jan 2012;33(1):43–50. doi: 10.1097/MNM.0b013e32834bfeb1 [DOI] [PubMed] [Google Scholar]
- 27.Hinson AM, Lee DR, Hobbs BA, Fitzgerald RT, Bodenner DL, Stack BC Jr. Preoperative 4D CT Localization of Nonlocalizing Parathyroid Adenomas by Ultrasound and SPECT-CT. Otolaryngol Head Neck Surg. Nov 2015;153(5):775–8. doi: 10.1177/0194599815599372 [DOI] [PubMed] [Google Scholar]
- 28.Hillyar CR, Rizki H, Begum R, et al. A Retrospective Cohort Study of the Utility of Ultrasound, 99mTc-Sestamibi Scintigraphy, and Four-Dimensional Computed Tomography for Pre-Operative Localization of Parathyroid Disease To Facilitate Minimally Invasive Parathyroidectomy. Cureus. Jan 2022;14(1):e21177. doi: 10.7759/cureus.21177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arora S, Balash PR, Yoo J, Smith GS, Prinz RA. Benefits of surgeon-performed ultrasound for primary hyperparathyroidism. Langenbecks Arch Surg. Sep 2009;394(5):861–7. doi: 10.1007/s00423-009-0522-8 [DOI] [PubMed] [Google Scholar]
- 30.Untch BR, Adam MA, Scheri RP, et al. Surgeon-performed ultrasound is superior to 99Tc-sestamibi scanning to localize parathyroid adenomas in patients with primary hyperparathyroidism: results in 516 patients over 10 years. J Am Coll Surg. Apr 2011;212(4):522–9; discussion 529-31. doi: 10.1016/j.jamcollsurg.2010.12.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang TS, Cheung K, Farrokhyar F, Roman SA, Sosa JA. Would scan, but which scan? A cost-utility analysis to optimize preoperative imaging for primary hyperparathyroidism. Surgery. Dec 2011;150(6):1286–94. doi: 10.1016/j.surg.2011.09.016 [DOI] [PubMed] [Google Scholar]
- 32.Solorzano CC, Carneiro-Pla DM, Irvin GL 3rd. Surgeon-performed ultrasonography as the initial and only localizing study in sporadic primary hyperparathyroidism. J Am Coll Surg. Jan 2006;202(1):18–24. doi: 10.1016/j.jamcollsurg.2005.08.014 [DOI] [PubMed] [Google Scholar]
- 33.Gücek Haciyanli S, Acar N, Balli Ö, Erdoğan N, Haciyanli M. Selective Venous Sampling in Primary Hyperparathyroidism: Is it worth doing? Turk J Med Sci. Oct 24 2021;doi: 10.3906/sag-2108-151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lebastchi AH, Aruny JE, Donovan PI, et al. Real-Time Super Selective Venous Sampling in Remedial Parathyroid Surgery. J Am Coll Surg. Jun 2015;220(6):994–1000. doi: 10.1016/j.jamcollsurg.2015.01.004 [DOI] [PubMed] [Google Scholar]
- 35.Sun PY, Thompson SM, Andrews JC, et al. Selective Parathyroid Hormone Venous Sampling in Patients with Persistent or Recurrent Primary Hyperparathyroidism and Negative, Equivocal or Discordant Noninvasive Imaging. World J Surg. Dec 2016;40(12):2956–2963. doi: 10.1007/s00268-016-3621-z [DOI] [PubMed] [Google Scholar]


