Abstract
Background:
After lung transplantation, both frailty and chronic lung allograft dysfunction (CLAD) commonly develop and, when they do, are associated with poorer outcomes. Given their potential shared mechanisms, we sought to explore the temporal relationship between frailty and CLAD onset.
Methods:
In a single center, we prospectively measured frailty by the Short Physical Performance Battery repeatedly after transplant. Since the nature of a relationship between frailty and CLAD is unknown, we tested the association between frailty, modeled as a time-dependent predictor, and CLAD development as well as CLAD development, modeled as a time-dependent predictor, and frailty development. To do so we used Cox proportional cause-specific hazards and conditional logistic regression models adjusted for age, sex, race, diagnosis, CMV serostatus, and post-transplant body mass index and acute cellular rejection episodes as time-dependent covariates. We tested SPPB frailty as a binary (≤9 points) and continuous predictor (12-point scale); as an outcome, we defined frailty as SPPB ≤9.
Results:
The 231 participants were mean age 55.7 years (SD 12.1). After adjusting for covariates, the development of frailty within 3-years after lung transplant was associated with cause-specific CLAD risk (cHR: 1.76, 95%CI: 1.05, 2.92 when defining frailty as SPPB ≤ 9 and cHR: 1.10, 95%CI: 1.03, 1.18 per 1 point worsening in SPPB). CLAD onset did not appear to be a risk factor for subsequent frailty (OR 4.0, 95%CI: 0.4, 197.0).
Conclusion:
Studying the mechanisms underlying frailty and CLAD could provide new insights into the pathobiology of both and potential targets for intervention.
INTRODUCTION
Chronic lung allograft dysfunction (CLAD) is a major barrier to long term well-being and survival after lung transplantation. Further, emerging work suggests that frailty, a syndrome of accelerated aging, commonly develops after transplant. When either CLAD or frailty develop, they are each independently associated with worse functioning, health-related quality of life, and life expectancy.1
Although the evidence base defining the pathobiology of frailty and CLAD is nascent, they share many putative mechanisms spanning multiple general pathways. Other frailty pathways have also been implicated in kidney and heart transplant rejection. Given these potential shared mechanisms, herein, we tested the hypothesis that frailty after lung transplantation may be associated with CLAD.
MATERIALS AND METHODS:
Study Design
We analyzed data from Breathe Again, a single-center prospective cohort study of adults who underwent first-time lung transplantation between 2010 and 2017. Participants completed study visits before and at 3-, 6-, 12-, 18-, 24-, 30-, and 36-months post-transplant. Study visits included measures of physical frailty and surveys. Clinicians were blinded to frailty assessment results and survey responses. Clinically, spirometry was performed after transplant at each of these study visit timepoints as well as multiple timepoints between. For this study, we focused only frailty measures performed after transplant and all clinically obtained spirometry values. Breathe Again was approved by our Institutional Review Board and participants provided written informed consent.
Variables of interest
Since the nature of the relationship between frailty and CLAD is undefined, we set up two overarching analyses made possible by the longitudinal, repeated measure nature of our study design. First, we hypothesized that the development of frailty might be a risk factor for subsequent CLAD. Alternatively, we hypothesized that the development of CLAD might be a risk factor for subsequent frailty. Thus, across these two analyses, frailty and CLAD were each primary predictor variables as well as outcome variables.
Frailty was quantified by the Short Physical Performance Battery (SPPB).2 The SPPB tests the ability for participants to perform repeated chair stands, and tests of balance and gait speed.2 Each test is scored 0 to 4 which are added together to make up a 0 to 12 scale. Higher scores reflect less frailty. For analyses testing whether frailty was a risk factor for CLAD onset, we used SPPB as both a binary (score ≤9) and a continuous predictor (range 0–12). When assessing whether CLAD was a risk factor for frailty, used SPPB as both a binary and continuous outcome. We selected the SPPB as our frailty measure of choice given our prior work demonstrating its superior face, construct, and predictive validity characteristics compared to the Fried Frailty Phenotype measure in lung transplantation and its acceptance in the broader field of frailty and geriatrics.1,3–7
CLAD:
Definite CLAD was first defined as 20% decline in forced expiratory volume in 1 second (FEV1) from post-transplant baseline that persisted for at least 3 months.8
Confounding Variables
Potential confounding variables were selected based on known or putative associations with frailty and CLAD, consistent with contemporary guidance on the selection of covariates.9 Pre-operative age at transplant, sex, race, pulmonary diagnosis, as well as candidate and donor CMV serostatus were abstracted from medical records. We also abstracted serial measures of BMI at the time of study visits as well as episodes of acute cellular rejection (ACR) graded per ISHLT guidelines. We did not include potentially inflammatory comorbidities such as obesity, sarcopenia, diabetes mellitus, and chronic kidney disease. Although these conditions are known risk factors for physical frailty, conceptually they lie “upstream” on the potential causal pathway between frailty and CLAD and would not act as confounders.
Analytic Approach
We tested whether frailty was associated with CLAD using Cox proportional cause-specific hazards models with frailty as a time-dependent binary or continuous predictor. Modeling frailty as a time-dependent predictor allowed us to test its association with the subsequent development of CLAD. Next, we tested whether CLAD was associated with subsequent frailty. Because participants transitioned between frailty states over time, testing this association required a matched cohort analysis. Participants who developed CLAD and were not frail at the time of CLAD diagnosis were matched by time post-transplant to participants who did not have CLAD and were not frail. Matching was performed in a blinded fashion and, within this matched cohort, we used conditional logistic regression to examine whether the onset of CLAD was associated with risk of subsequent frailty.
For the analysis testing whether frailty onset preceded CLAD development, the dataset was censored at 48 months. All models were adjusted for the covariates listed above. For the analyses testing whether CLAD preceded frailty onset, the dataset was censored at 36 months, reflecting the last frailty assessment study visit. We considered that ACR could confound the relationship between frailty and CLAD in two ways. First, treatment of ACR with high doses of corticosteroids could indirectly lead to frailty through generation of sarcopenia and adiposity.10,11 Second, the underlying immune mechanisms causing ACR could also cause frailty. Thus, we controlled for ACR in two separate models. First, we controlled for ACR grades A2 and above, reflecting the threshold that generally triggers treatment with high dose steroids at our center. Second, we controlled for ACR grade A1 or above.
Finally, we used the extended Kaplan-Meier estimator method to visualize the relationship between the onset of frailty during post-transplant follow-up as a time-varying predictor and development of CLAD. This extended estimator method is an extension of the standard Kaplan-Meier method which is limited to a time-independent predictor (i.e., a predictor that does not change over time). In this analysis, the development of frailty is a time-varying predictor which can assume different values over the duration of the follow-up period, whereas values for time-invariant variables such as sex and race/ethnicity are fixed. Because the frailty status for some patients changed over the course of follow-up, for example, changing not frail to frail the cohort size for frail and not frail groups were not fixed; participants could contribute to different cohorts at different times during follow-up. The extended Kaplan-Meier estimates of probabilities of freedom from CLAD stratified by the development of frailty were calculated and the extended Kaplan-Meier curves were plotted using the R ‘survival’ package.12
In two supplemental analyses, we compared baseline characteristics of our cohort. In the first analysis, we stratified our cohort by those who went on to develop CLAD compared to those who did not. In the second analysis, we stratified our cohort by those who ever developed frailty after transplant compared to those remained never frail.
Analyses were performed using SAS (version 9.4, SAS Institute), and R (version 4.2.2, R Foundation).
RESULTS
During the study period of the 259 participants enrolled, 231 underwent lung transplant and completed post-operative frailty assessments. These 231 participants were 55% male with a median age of 59 years (49 ,65) and formed our study cohort (Table 1 and Figure 1). Most participants underwent transplant for pulmonary fibrosis (71%) followed by non-suppurative obstructive lung diseases (16%). There were no differences in baseline characteristics between those who went on to develop CLAD after transplant compared to those who did not (all p > 0.04; Table 2). Baseline characteristics stratified by frailty status after transplant are presented in Table 3. Those who were ever frail after transplant were more likely have been female, older (median age 58 ± 11 versus 54 ± 13 years), have COPD, enter into transplant with lower 6MWD and pre-transplant SPPB scores, and undergo bilateral versus single lung transplant (all p < 0.05). The median duration of uncensored follow-up was 46.6 months post-transplant (24.0, 65.3), during which time 113 participants developed CLAD at a median of 2.9 years (1.6, 4.5). During the follow up period, 89 participants (39%) transitioned between frailty states (e.g., those who were not frail became so or vice versa) and some of them transitioned more than once.
Table 1.
Participant characteristics.
| No. of subjects | 231 |
| Male, No. (%) | 127 (55.0) |
| Age, mean ± SD | 55.7 ± 12.1 |
| Age, No. (%) | |
| < 35 years | 20 (8.7) |
| 35–49 years | 41 (17.8) |
| 50–64 years | 111 (48.1) |
| ≥65 years | 59 (25.5) |
| Race, No. (%) | |
| White | 165 (71.4) |
| Black | 19 (8.2) |
| Asian | 11 (4.8) |
| Hispanic | 32 (13.9) |
| Other | 4 (1.7) |
| Diagnosis, No. (%) | |
| A (e.g. Obstructive lung disease) | 37 (16.0) |
| B (e.g. Pulmonary Hypertension) | 10 (4.3) |
| C (e.g. Suppurative lung disease) | 20 (8.7) |
| D (e.g. Pulmonary Fibrosis) | 164 (71.0) |
| BMI (kg/m2) , mean ± SD | 25.5 ± 4.4 |
| FEV1 % predicted, mean ± SD | 45.4 ± 20.5 |
| FEV1 % predicted, median (Q1, Q3) | 46.0 (28.0, 60.0) |
| 6 MWD (m), mean ± SD | 254.4 ± 140.8 |
| 6 MWD (m), median (Q1, Q3) | 268.2 (142.7, 366.0) |
| LAS at transplant, mean ± SD | 58.5 ± 21.5 |
| LAS at transplant, median (Q1, Q3) | 52.0 (39.0, 79.0) |
| Transplant type, No. (%) | |
| Bilateral | 215 (93.1) |
| Single | 13 (5.6) |
| Heart/Lung | 3 (1.3) |
| Inpatient at transplant, No. (%) | 76 (32.9) |
| Ventilator at transplant, No. (%) | 21 (9.1) |
| ECMO at transplant, No. (%) | 18 (7.8) |
| Pre-transplant SPPB, mean ± SD | 9.0 ± 3.1 |
| Pre-transplant SPPB, median (Q1, Q3) | 10.0 (8.0, 11.0) |
| Time to CLAD, years, median (Q1, Q3) | 2.9 (1.6, 4.5) |
SPPB= Short Physical Performance Battery. COPD=Chronic Obstructive Pulmonary Disease, BMI=Body Mass Index, FEV1=Forced Expiratory Volume in the first second, 6 MWD=Six Minute Walk Distance, LAS=Lung Allocation Score, ECMO=Extracorporeal Membrane Oxygenation. Data are presented as number of patients (percentage) or mean ± standard deviation.
Figure 1.
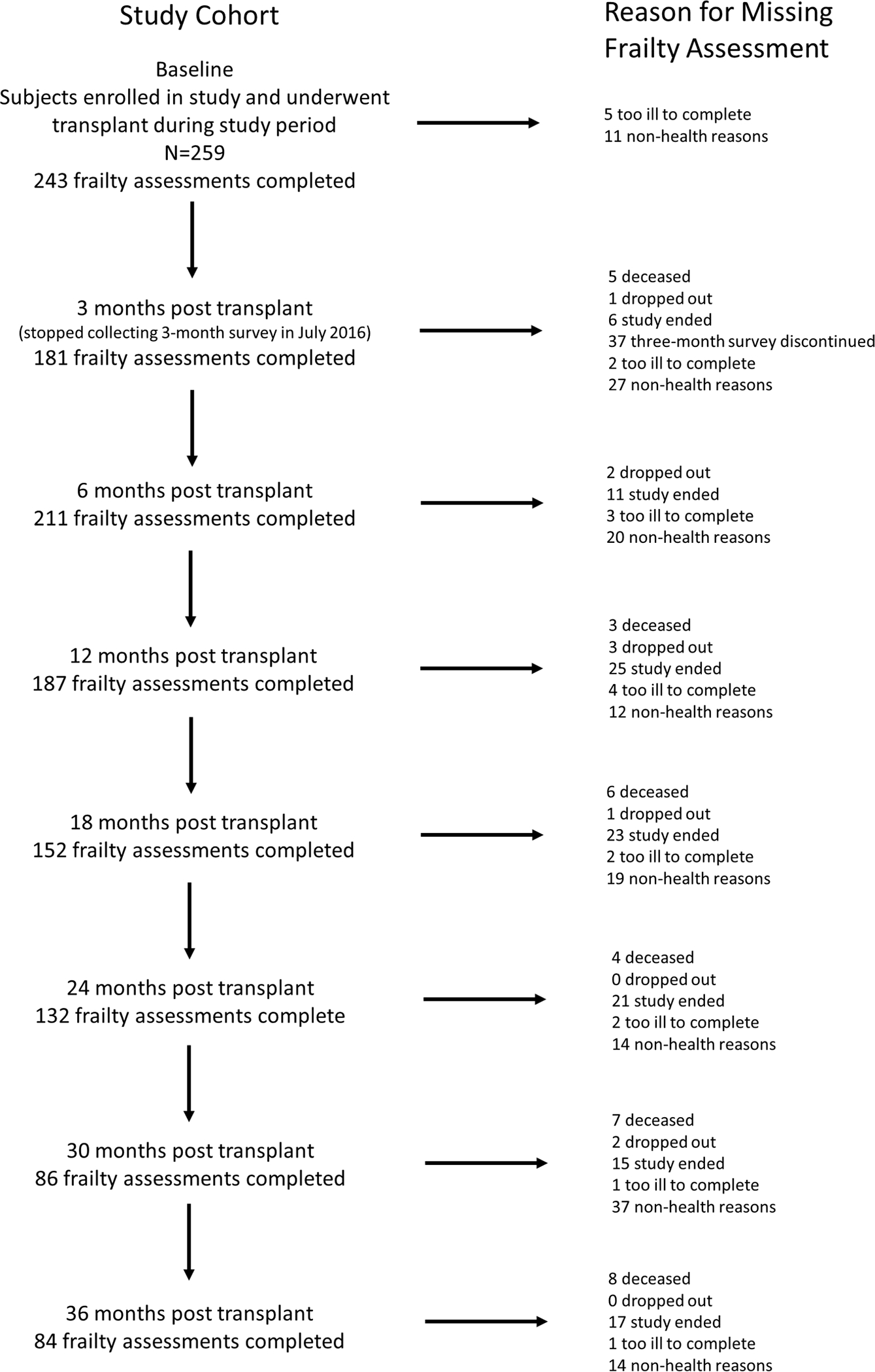
Study Flow
Table 2:
Participant characteristics stratified by CLAD
| Overall | Did not develop CLAD | Developed CLAD | P value | |
|---|---|---|---|---|
|
| ||||
| No. of subjects | 231 | 118 | 113 | |
| Male, No. (%) | 127 (55.0) | 67 (56.8) | 60 (53.1) | 0.574 |
| Age, mean ± SD | 55.7 ± 12.1 | 56.2 ± 12.2 | 55.1 ± 12.0 | 0.491 |
| Age, No. (%) | ||||
| < 35 years | 20 (8.7) | 10 (8.5) | 10 (8.9) | 0.965 |
| 35–49 years | 41 (17.8) | 22 (18.6) | 19 (16.8) | |
| 50–64 years | 111 (48.1) | 55 (46.6) | 56 (49.6) | |
| ≥65 years | 59 (25.5) | 31 (26.3) | 28 (24.8) | |
| Race, No. (%) | ||||
| White | 165 (71.4) | 83 (70.3) | 82 (72.6) | 0.884 |
| Black | 19 (8.2) | 10 (8.5) | 9 (8.0) | |
| Asian | 11 (4.8) | 5 (4.2) | 6 (5.3) | |
| Hispanic | 32 (13.9) | 17 (14.4) | 15 (13.3) | |
| Other | 4 (1.7) | 3 (2.5) | 1 (0.9) | |
| Diagnosis, No. (%) | ||||
| A (e.g. Obstructive lung disease) | 37 (16.0) | 18 (15.3) | 19 (16.8) | 0.770 |
| B (e.g. Pulmonary Hypertension) | 10 (4.3) | 4 (3.4) | 6 (5.3) | |
| C (e.g. Suppurative lung disease) | 20 (8.7) | 9 (7.6) | 11 (9.7) | |
| D (e.g. Pulmonary Fibrosis) | 164 (71.0) | 87 (73.7) | 77 (68.1) | |
| BMI (kg/m2) , mean ± SD | 25.5 ± 4.4 | 25.3 ± 4.3 | 25.8 ± 4.4 | 0.406 |
| FEV1 % predicted, mean ± SD | 45.4 ± 20.5 | 44.5 ± 19.8 | 46.3 ± 21.2 | 0.485 |
| FEV1 % predicted, median (Q1, Q3) | 46.0 (28.0, 60.0) | 43.0 (29.0, 59.0) | 47.0 (27.0, 62.0) | 0.544 |
| 6 MWD (m), mean ± SD | 254.4 ± 140.8 | 249.5 ± 144.8 | 259.6 ± 136.9 | 0.589 |
| 6 MWD (m), median (Q1, Q3) | 268.2 (142.7, 366.0) | 274.5 (122.0, 366.0) | 264.2 (155.0, 363.0) | 0.672 |
| LAS at transplant, mean ± SD | 58.5 ± 21.5 | 59.0 ± 21.1 | 58.0 ± 21.8 | 0.710 |
| LAS at transplant, median (Q1, Q3) | 52.0 (39.0, 79.0) | 52.5 (40.0, 80.0) | 50.0 (39.0, 73.0) | 0.645 |
| Transplant type, No. (%) | ||||
| Bilateral | 215 (93.1) | 111 (94.1) | 104 (92.0) | 0.550 |
| Single | 13 (5.6) | 5 (4.2) | 8 (7.1) | |
| Heart/Lung | 3 (1.3) | 2 (1.7) | 1 (0.9) | |
| Inpatient at transplant, No. (%) | 76 (32.9) | 41 (34.7) | 35 (31.0) | 0.542 |
| Ventilator at transplant, No. (%) | 21 (9.1) | 9 (7.6) | 12 (10.6) | 0.429 |
| ECMO at transplant, No. (%) | 18 (7.8) | 8 (6.8) | 10 (8.9) | 0.557 |
| Pre-transplant SPPB, mean ± SD | 9.0 ± 3.1 | 9.0 ± 3.1 | 9.0 ± 3.0 | 0.894 |
| Pre-transplant SPPB, median (Q1, Q3) | 10.0 (8.0, 11.0) | 9.5 (8.0, 11.0) | 10.0 (8.0, 11.0) | 0.952 |
Table 3.
Participant characteristics stratified by frailty status
| Overall | Never Frail | Ever Frail | P value | |
|---|---|---|---|---|
|
| ||||
| No. of subjects | 231 | 119 | 112 | |
| Male, No. (%) | 127 (55.0) | 78 (65.5) | 49 (43.8) | 0.001 |
| Age, mean ± SD | 55.7 ± 12.1 | 53.8 ± 12.9 | 57.7 ± 10.9 | 0.016 |
| Age, No. (%) | ||||
| < 35 years | 20 (8.7) | 13 (10.9) | 7 (6.3) | 0.006 |
| 35–49 years | 41 (17.8) | 30 (25.2) | 11 (9.8) | |
| 50–64 years | 111 (48.1) | 49 (41.2) | 62 (55.4) | |
| ≥65 years | 59 (25.5) | 27 (22.7) | 32 (28.6) | |
| Race, No. (%) | ||||
| White | 165 (71.4) | 85 (71.4) | 80 (71.4) | 0.611 |
| Black | 19 (8.2) | 8 (6.7) | 11 (9.8) | |
| Asian | 11 (4.8) | 8 (6.7) | 3 (2.7) | |
| Hispanic | 32 (13.9) | 16 (13.4) | 16 (14.3) | |
| Other | 4 (1.7) | 2 (1.7) | 2 (1.8) | |
| Diagnosis, No. (%) | ||||
| A (e.g. Obstructive lung disease) | 37 (16.0) | 11 (9.2) | 26 (23.2) | 0.008 |
| B (e.g. Pulmonary Hypertension) | 10 (4.3) | 6 (5.0) | 4 (3.6) | |
| C (e.g. Suppurative lung disease) | 20 (8.7) | 15 (12.6) | 5 (4.5) | |
| D (e.g. Pulmonary Fibrosis) | 164 (71.0) | 87 (73.1) | 77 (68.8) | |
| BMI (kg/m2), mean ± SD | 25.5 ± 4.4 | 25.0 ± 4.3 | 26.1 ± 4.3 | 0.063 |
| FEV1 % predicted, mean ± SD | 45.4 ± 20.5 | 45.9 ± 20.5 | 44.9 ± 20.5 | 0.715 |
| FEV1 % predicted, median (Q1, Q3) | 46.0 (28.0, 60.0) | 45.5 (28.0, 60.0) | 46.0 (29.0, 59.0) | 0.821 |
| 6 MWD (m), mean ± SD | 254.4 ± 140.8 | 275.8 ± 136.3 | 231.2 ± 142.5 | 0.017 |
| 6 MWD (m), median (Q1, Q3) | 268.2 (142.7, 366.0) | 303.9 (159.0, 376.0) | 228.6 (134.2, 332.5) | 0.007 |
| LAS at transplant, mean ± SD | 58.5 ± 21.5 | 57.4 ± 20.3 | 59.7 ± 22.6 | 0.413 |
| LAS at transplant, median (Q1, Q3) | 52.0 (39.0, 79.0) | 50.0 (41.0, 72.0) | 55.0 (38.5, 84.5) | 0.750 |
| Transplant type, No. (%) | ||||
| Bilateral | 215 (93.1) | 106 (89.1) | 109 (97.3) | 0.022 |
| Single | 13 (5.6) | 11 (9.2) | 2 (1.8) | |
| Heart/Lung | 3 (1.3) | 2 (1.7) | 1 (0.9) | |
| Inpatient at transplant, No. (%) | 76 (32.9) | 35 (29.4) | 41 (36.6) | 0.245 |
| Ventilator at transplant, No. (%) | 21 (9.1) | 13 (10.9) | 8 (7.1) | 0.317 |
| ECMO at transplant, No. (%) | 18 (7.8) | 11 (9.2) | 7 (6.3) | 0.396 |
| Pre-transplant SPPB, mean ± SD | 9.0 ± 3.1 | 9.7 ± 2.9 | 8.1 ± 3.0 | <0.001 |
| Pre-transplant SPPB, median (Q1, Q3) | 10.0 (8.0, 11.0) | 11.0 (9.0, 12.0) | 9.0 (7.0, 10.0) | <0.001 |
| Time to CLAD, years, median (Q1, Q3) | 2.9 (1.6, 4.5) | 3. 0 (1.8, 4.8) | 2.7 (1.2, 4.5) | 0.328 |
In the first three-years after transplant, frailty was associated with the risk of developing subsequent CLAD (Table 4). When frailty developed, it was associated with a 75% increased risk of subsequent CLAD development, controlling for covariates including ≥ A2 acute cellular rejection (adjusted cause-specific hazard ratio [cHR] 1.76, 95% Confidence Interval [CI]: 1.05, 2.92; p = 0.031; Figure 2). Further, a one-point worsening in SPPB frailty was associated with a 10% increased risk of developing CLAD (adjusted cHR 1.10, 95%CI: 1.03, 1.18; p = 0.007). Point estimates and confidence intervals were essentially unchanged when adjusting for ≥ A1 rather than ≥ A2 acute cellular rejection. In contrast, CLAD onset did not appear to be associated with increased risk of subsequent frailty (Odds Ratio: 4.0, 95%CI: 0.4, 197.0; p= 0.375), although the wide observed confidence interval underscored the uncertainty in this analysis.
Table 4:
Association of transplant-related frailty and CLAD
| Predictor | HR for CLAD (95% CI) | p-value | |
|---|---|---|---|
|
| |||
| Frailty (SPPB ≤9) | Unadjusted | 1.77 (1.11, 2.82) | 0.017 |
| Adjusted for CMV status, ACR grades ≥ A2 and other covariates* | 1.76 (1.05, 2.92) | 0.031 | |
| Adjusted for CMV status, ACR grades ≥ A1 and above, and other covariates* | 1.76 (1.06, 2.93) | 0.028 | |
|
| |||
| 1-point worsening in SPPB | Unadjusted | 1.10 (1.03, 1.17) | 0.005 |
| Adjusted for CMV status, ACR grades ≥ A2, and other covariates* | 1.10 (1.03, 1.18) | 0.007 | |
| Adjusted for CMV status, ACR grades ≥ A1 and other covariates* | 1.10 (1.03, 1.17) | 0.009 | |
Short Physical Performance Battery (SPPB), range from 0 to 12 (Minimal Important Difference [MID] = 1); The association between frailty and CLAD was quantified by Cox’s proportional hazards models with SPPB as a time-dependent predictor.
Other covariates included pre-operative age, sex, race, diagnosis, and BMI at each study visit.
Figure 2.
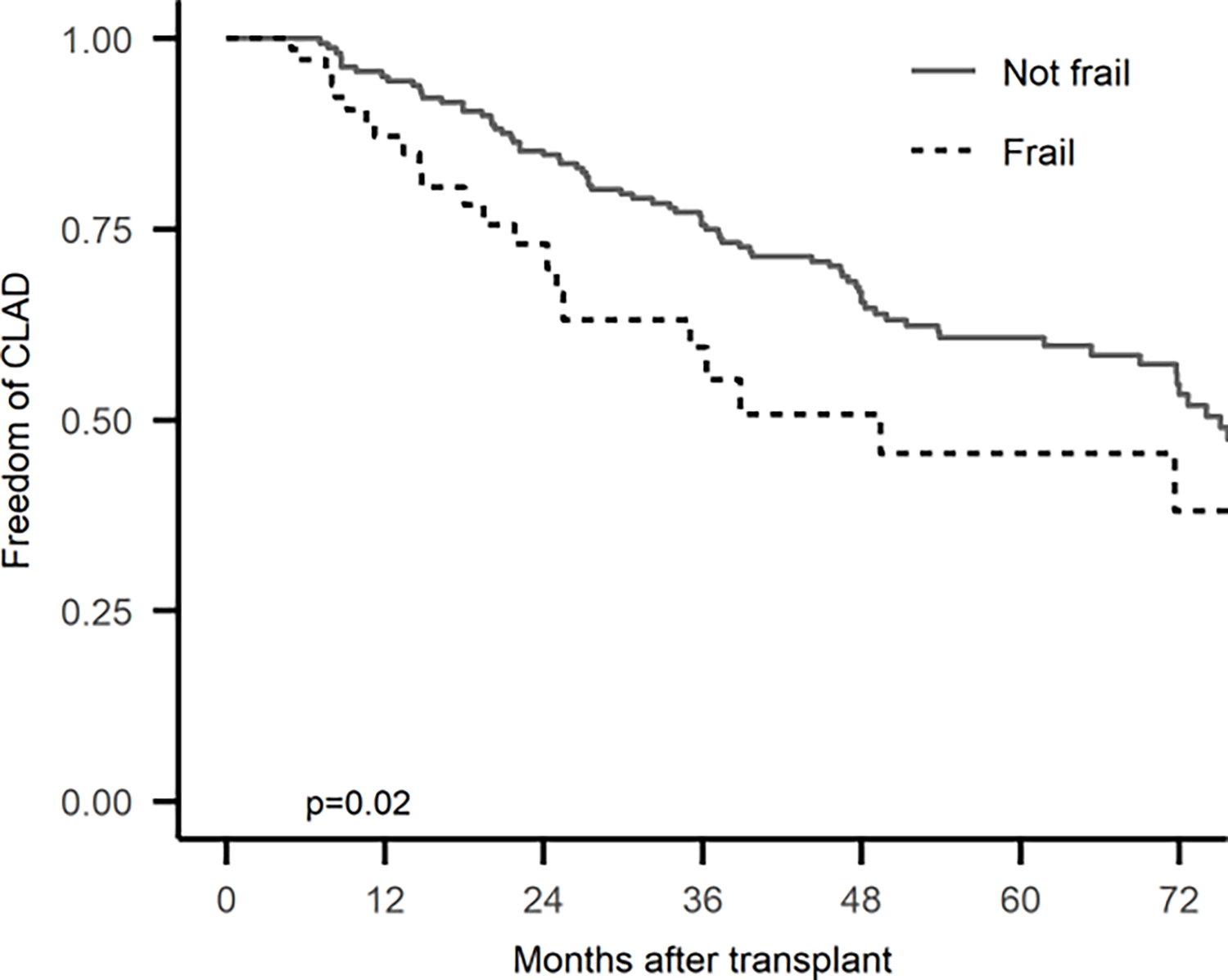
Extended Kaplan-Meier estimator of the association between transplant-related frailty and CLAD
DISCUSSION
In this single-center study, we found an association between frailty after lung transplant and risk of developing CLAD. These associations were consistent regardless of whether we defined frailty as a binary state or as a continuous gradient from resilient to frail. Further, we did not identify a statistically significant association between CLAD onset and the subsequent risk of developing frailty.
While our novel findings are consistent with the hypothesis that frailty and CLAD could share common underlying biology, disentangling the exact nature of the association between frailty and CLAD will require further study. We consider three broad potential relationships. First, the inflammatory state of frailty could drive allograft rejection. Second, subclinical allograft inflammation could also drive frailty, which may manifest earlier on objective frailty assessments than a 20% drop in FEV1 needed to define definite CLAD. Emerging research shows that lung transplant-related injury may lead to accelerated aging in the graft and that senescence can be transmitted from transplanted cells.13,14 Finally, it is possible that some latent driver of both frailty and CLAD could explain our findings. If it turns out that one of the latter two broad potential relationships are true, it would suggest that frailty may be an early “biomarker” of CLAD risk rather than lying on the causal pathway. Future studies with greater numbers of frailty cases and measurements of potential causal factors over time could help establish causal pathways and identify the most robust clinical determinants and biochemical markers of transplant-related frailty.
In addition to the limitation on causality noted above, our findings were derived from a single center with approaches to candidate selection and post-operative management that differ from other centers potentially limiting their generalizability. Although clinicians were blinded to frailty assessments, it is possible that some patients were referred for pulmonary rehabilitation and/or recommended to perform home-based exercises. Given the decentralized nature of post-transplant care, documentation of whether referrals to pulmonary rehabilitation resulted in program participation or whether patients reliably performed exercises at home is sparse. Thus, the potential impact of exercise on frailty (or CLAD) cannot be estimated. Our results might have differed had other measures of frailty such as the Fried Frailty Phenotype been employed. Additionally, our identified time to CLAD onset in this cohort differs from commonly cited CLAD onset times from registry-based analyses. It is unclear whether this reflects unique differences amongst our cohort of patients compared to the broader lung transplant population or differences in aggressive surveillance by our automated analysis of all spirometry data versus clinical staff reporting of CLAD onset to national registries. Although there is some uncertainty in the literature regarding time to CLAD onset15, until future studies with other cohorts resolve this issue, a degree of caution is recommended in interpreting our results. Because the evidence base for mechanisms causing frailty and CLAD after lung transplantation is nascent, it is possible and even likely that there are unknown confounders we did not consider or account for. Further, we lacked data on other potential confounders such as serious infections that could have confounded our observed relationship between frailty and CLAD. Future studies should consider how to design sampling timepoints and data collection methods to mitigate these limitations. Given the high incidence of frailty after transplant and its association with poorer HRQL, mortality risk, and possibly CLAD, research into risk factors for frailty development, including comorbidities such as CKD, is needed. Insights into risk factors may identify patients at risk for developing frailty or targets for preventive interventions.
Despite these limitations, our study has several strengths. We studied a relatively large and diverse cohort of lung recipients with repeated measures of frailty over several years. We had little missing data and no loss to follow-up. We leveraged the prospective collection of repeated measures to identify a novel relationship between physical frailty and the risk of CLAD after lung transplant. To our knowledge, this is the first study to assess the association between frailty after transplantation and graft dysfunction in solid-organ transplant. Although our findings generate as many, if not more, questions as they answer, it is possible that studies of the pathobiology of frailty after transplant may yield insights into CLAD and vice versa.
In sum, we found that persistent and emergent frailty after lung transplantation is associated with future CLAD risk.
Acknowledgements:
The authors are deeply appreciative of the time our patient participants provided to support this research and members of the UCSF clinical Advanced Lung Disease and Transplant program
Funding:
NHLBI R01 HL134851 (JPS) & HL151552 (JRG). VA CDA IK2CX002011 (JRG), U01HL145435 (JPS)
Footnotes
- JPS: Scientific Advisory Board: Altavant Therapeutics; Scientific Advisory Board: Mallinckrodt Pharmaceuticals
- SRH: Consulting fees from AI Therapeutics and CareDx; Scientific Advisory Board: CareDx
- JK: DSMB Lung Bioengineering
- JRG: Scientific Advisory Board and Research Funding: Theravance Biopharma
References
- 1.Venado A, Kolaitis NA, Huang CY, et al. Frailty after lung transplantation is associated with impaired health-related quality of life and mortality. Thorax. May 6 2020;75(8):669–678. Doi: 10.1136/thoraxjnl-2019-213988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. Mar 1994;49(2):M85–94. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin MR, Singer JP, Huang D, et al. Refining Low Physical Activity Measurement Improves Frailty Assessment in Advanced Lung Disease and Survivors of Critical Illness. Ann Am Thorac Soc. Apr 11 2017;14(8):1270–1279. Doi: 10.1513/AnnalsATS.201612-1008OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singer JP, Diamond JM, Gries C, et al. Frailty phenotypies, disability, and outcomes in adult candidates for lung transplantation. Am J Respir Crit Care Med. Dec 1 2015;192(11):1325–34. Doi: 10.1164/rccm.201506-1150OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singer JP, Diamond JM, Anderson MR, et al. Frailty phenotypes and mortality after lung transplantation: A prospective cohort study. Am J Transplant. Aug 2018;18(8):1995–2004. Doi: 10.1111/ajt.14873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landi F, Cesari M, Calvani R, et al. The “Sarcopenia and Physical fRailty IN older people: multi-componenT Treatment strategies” (SPRINTT) randomized controlled trial: design and methods. Aging clinical and experimental research. Feb 2017;29(1):89–100. Doi: 10.1007/s40520-016-0715-2 [DOI] [PubMed] [Google Scholar]
- 7.Cesari M, Landi F, Calvani R, et al. Rationale for a preliminary operational definition of physical frailty and sarcopenia in the SPRINTT trial. Aging clinical and experimental research. Feb 2017;29(1):81–88. Doi: 10.1007/s40520-016-0716-1 [DOI] [PubMed] [Google Scholar]
- 8.Dugger DT, Fung M, Hays SR, et al. Chronic lung allograft dysfunction small airways reveal a lymphocytic inflammation gene signature. Am J Transplant. Jan 2021;21(1):362–371. Doi: 10.1111/ajt.16293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lederer DJ, Bell SC, Branson RD, et al. Control of Confounding and Reporting of Results in Causal Inference Studies. Guidance for Authors from Editors of Respiratory, Sleep, and Critical Care Journals. Ann Am Thorac Soc. Jan 2019;16(1):22–28. Doi: 10.1513/AnnalsATS.201808-564PS [DOI] [PubMed] [Google Scholar]
- 10.Anderson MR, Kolaitis NA, Gao Y, et al. A nonlinear relationship between visceral adipose tissue and frailty in adult lung transplant candidates. Am J Transplant. Jul 6 2019;19(11):3155–3161. Doi: 10.1111/ajt.15525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maheshwari JA, Kolaitis NA, Anderson MR, et al. Construct and Predictive Validity of Sarcopenia in Lung Transplant Candidates. Ann Am Thorac Soc. Feb 10 2021;18(9):1464–1474. Doi: 10.1513/AnnalsATS.202007-796OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snapinn SM, Jiang Q, Iglewicz B. Illustrating the Impact of a Time-Varying Covariate With an Extended Kaplan-Meier Estimator. The American Statistician. 2005/11/01 2005;59(4):301–307. Doi: 10.1198/000313005X70371 [DOI] [Google Scholar]
- 13.Iske J, Matsunaga T, Zhou H, et al. Donor and Recipient Age-Mismatches: The Potential of Transferring Senescence. Mini Review. Frontiers in Immunology. 2021-April-28 2021;12Doi: 10.3389/fimmu.2021.671479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dugger DT, Calabrese DR, Gao Y, et al. Lung Allograft Epithelium DNA Methylation Age Is Associated With Graft Chronologic Age and Primary Graft Dysfunction. Front Immunol. 2021;12:704172. Doi: 10.3389/fimmu.2021.704172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kulkarni HS, Cherikh WS, Chambers DC, et al. Bronchiolitis obliterans syndrome-free survival after lung transplantation: An International Society for Heart and Lung Transplantation Thoracic Transplant Registry analysis. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. Jan 2019;38(1):5–16. Doi: 10.1016/j.healun.2018.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]


