Abstract
Socioeconomic status (SES) is associated with children’s brain and behavioral development Several theories propose that early experiences of adversity or low-SES can alter the pace of neurodevelopment during childhood and adolescence. These theories make contrasting predictions about whether adverse experiences and/or low-SES are associated with accelerated or delayed neurodevelopment We contextualize these predictions within the context of normative development of cortical and subcortical structure and review existing evidence on SES and structural brain development to adjudicate between competing hypotheses. Although none of these theories are fully consistent with observed SES-related differences in brain development, existing evidence suggests that low-SES is associated with brain structure trajectories more consistent with a delayed or simply different developmental pattern than an acceleration in neurodevelopment.
Keywords: socioeconomic status, poverty, adversity, structural brain development, acceleration, delay
SES, adversity, and the pace of neurodevelopment
Adverse childhood experiences and access to resources in childhood, as measured by socioeconomic status (SES), have been consistently linked to children’s neurodevelopment[1–5]. Recent theories have proposed that experiencing adversity or low-SES early in life may alter the pace of neurodevelopment[6–9]. While most of these models focus on adversity broadly[6–8], they have been expanded to include SES[9], given that SES likely impacts neurodevelopment via similar pathways[5,9,10] (although note that adversity and low-SES are related but not interchangeable constructs, see Box 1). Importantly, these theories make contrasting predictions about whether adverse environmental experiences are associated with an acceleration or delay in the pace of neurodevelopment While some models propose that adversity and/or low-SES may lead to an acceleration in the pace of brain maturation, another recent model argues that delayed development is also a possibility. Empirically testing these predictions has been challenging as a clear articulation of specific evidence that would align with either acceleration or delay has not been provided. Further, most studies have relied on cross-sectional designs that cannot be leveraged to investigate these questions.
Box 1: Defining adversity and socioeconomic status.
Many conceptual models on the associations between early experience and the pace of neurodevelopment focus on experiences of adversity broadly rather than SES specifically. It is important to acknowledge that while low-SES is a risk factor for adverse experiences, low-SES is not synonymous with adversity. Childhood adversity is defined as early-life stressors that are either chronic and/or severe and likely to require meaningful adaptation by an average child[129]. Adversity can be conceptualized in different ways, including cumulative risk and dimensions of adversity such as threat, deprivation, and unpredictability[15] (see Table I). Children from low-SES backgrounds are more likely to experience these forms of adversity than their peers from higher-SES backgrounds [10,130], although it is important to note that many children raised in low-SES environments do not encounter adversity. In addition, it is important to note that low-SES is associated with other exposures and experiences that do not neatly fit into any of these adversity definitions but may influence neurodevelopment such as crowding, pollution and toxicant exposure, high levels of noise, and lack of access to green spaces.
Table I.
Definitions of different conceptualizations of adversity.
| Cumulative risk | Dimensions |
||
|---|---|---|---|
| Threat | Deprivation | Unpredictability | |
| The cumulative risk approach focuses on the number of adverse experiences a child has encountered and assumes that these experiences have additive influences on developmental outcomes. Cumulative risk assumes that different types of adverse experiences influence behavioral and neural development through mechanisms that are largely universal or shared. | Threat refers to experiences that involve harm or the possibility of harm to one’s physical integrity. This includes experiences where the child is directly victimized, such as physical abuse, as well as situations where the child witnesses harm occurring to others, such as violence between caregivers. | Deprivation refers to reductions in social and cognitive inputs from the environment during development, leading to limited opportunities for learning. | Unpredictability can be described as a state of environmental instability, where there is a lack of routine and frequent, rapid, and/or unanticipated changes in the environment. It can also be defined as stochastic variation in extrinsic morbidity-mortality. |
SES is a broad and complex construct that represents access to or possession of both material resources, which is often indexed by income, and non-material resources such as educational attainment and neighborhood quality (see Table II). Subjective social status and parent occupational prestige have also been used as measures of SES, although these methods of measuring SES have rarely been studied in relation to neural outcomes in developmental studies outside of composite SES indices[1]. Generally, these different metrics of SES tend to be moderately correlated [118], which suggests that they capture unique aspects of the environment and may influence brain and behavioral development through pathways that are both shared and unique[68,94]. Importantly, each of these aspects of SES are associated with differences in exposure and experiences—like stress, adversity, stimulation, and support—to different extents.
Table II.
Definitions of commonly used indicators of SES.
| Educational attainment | Income | Neighborhood disadvantage | Composite SES |
|---|---|---|---|
| Educational attainment reflects human capital. Education is usually operationalized either as the highest or average level of completion (e.g., high school, college, professional degree) or as the total number of years of education completed. | Income reflects financial or economic resources. Income is typically measured as total monthly or annual household income, typically adjusted for household size by computing an income-to-needs ratio. | Neighborhood disadvantage reflects the socioeconomic characteristics of the neighborhood. It is typically computed by aggregating across multiple neighborhood-level measures of employment, education, and income. In addition, neighborhood measures can also capture opportunity levels in the neighborhood like access to early childcare centers and school quality. | Composite measures of SES are aggregate measures of the child’s socioeconomic environment. They can be operationalized by aggregating income, education, occupation (and other indicators) in measures such as the Hollingshead index, normalizing and averaging data across indicators, or constructing a composite measure using factor loadings of those indicators from a latent SES model. |
To adjudicate between these competing hypotheses, we first contextualize theoretical predictions within the context of normative structural neurodevelopment during infancy, childhood, and adolescence. We then review extant evidence from longitudinal studies to ascertain whether low-SES is associated with an accelerated, delayed, or a simply different trajectory of neurodevelopment We find that while none of these theories completely explain observed SES-related differences in structural neurodevelopment, current evidence indicates that low-SES is linked to brain structure trajectories that are more in line with a delayed or simply distinct developmental pattern rather than an acceleration in neurodevelopment. We suggest that low-SES may be associated with a distinct pattern of brain maturation that is less about the timing of the attainment of milestones (i.e., acceleration or delay) but the milestones themselves.
Evolutionary Development Theories
Theoretical models of how early experience might alter the pace of development are rooted in evolutionary developmental frameworks, which suggest that alterations in the pace of development may help children adapt to harsh and unpredictable environments[11–16]. These frameworks posit that evolution selected for enhanced plasticity during development such that early experiences could shape the pace of development to allow an individual to adapt to the demands of their current and future environment[15–17]. Resource-allocation trade-offs between growth, reproduction, and survival determine the pattern and timing of life history traits, including age of sexual maturation and reproduction, number of children, and investment in parenting. For example, in a harsh or threatening environment, faster development that results in earlier pubertal onset may be advantageous to maximize chances of reproduction prior to potential mortality[16,17]. In contrast, a slower and protracted developmental strategy may be adaptive in a safe and enriching environment with high parental investment[14]. The idea that early-life experiences may alter the pace of development has influenced developmental cognitive neuroscience, where it has been theorized that adversity is associated not only with the pace of pubertal development, but also with the pace of brain development
Neurodevelopmental frameworks
Numerous theoretical models make predictions about how early-life adversity and low-SES may influence the pace of neurodevelopment The Stress Acceleration Hypothesis (SAH) posits that adverse early-life experiences accelerate neurodevelopmental processes to reach ‘adult-like’ functioning earlier, specifically in brain circuits involved in emotion processing and regulation[6]. This model stipulates that early environments characterized by high levels of stress activate neural circuits underlying emotional learning and reactivity prematurely, accelerating the development of amygdala and medial prefrontal cortex (mPFC) functional connectivity. This acceleration of amygdala-mPFC circuit development is thought to be adaptive to allow for a faster transition from reliance on parents for emotion regulation to self-regulation[6]. The SAH focuses specifically on experiences of stress in caregiver-child relationships. Children from low-SES backgrounds are more likely to experience many forms of caregiving stress than their higher-SES peers, including parental separation, harsh parenting, family conflict, and low parental warmth and support[10,18–20]. Further, while the SAH refers specifically to caregiver-adversity and the acceleration of amygdala-mPFC circuit development, numerous studies have evaluated whether brain development is accelerated among children experiencing other types of adversity as well as low-SES[21–24].
A recent model extends the ideas of the SAH and applies them directly to SES, describing the types of experiences that may lead to accelerated neurodevelopment based on the valence and frequency of early experiences[9]. This model hypothesizes that negative and chronic childhood experiences, such as low-SES, are associated with faster brain development and reduced plasticity, while negative but uncommon experiences such as acute trauma are not[9]. This model posits that higher-SES is linked to a prolonged trajectory of neurodevelopment and enhanced plasticity that facilitates a longer trajectory of functional network segregation, ultimately resulting in more effective and refined neural circuits. Empirical studies testing these predictions are currently lacking.
Other theoretical models rooted in the Dimensional Model of Adversity[17,25–27], which distills adverse experiences into core underlying dimensions such as threat, deprivation, and unpredictability (see Box 1), make predictions about whether adversity is associated with accelerated or delayed development based on the dimension of adversity experienced. In the original dimensional model, reductions in social, cognitive, sensory, and linguistic stimulation associated with deprivation are argued to lead to excessive and exaggerated synaptic pruning, which leads to greater cortical thinning[7], a pattern typically interpreted to reflect accelerated cortical development More recent elaborations of these models, however, note that it is unclear whether a thinner cortex reflects acceleration or delay in neurodevelopment[15]. Rooted in the same conceptual framework, Colich et al.[24] hypothesized that threat, but not deprivation, would be associated with an acceleration in the pace of neurodevelopment specifically in cortical regions involved in social and emotional processing[24] that feature prominently in the SAH. It is therefore unclear whether SES should be associated with an acceleration or delay of neurodevelopment per these dimensional models.
Although most models predict acceleration of brain development as a function of adverse experiences, the recent “change of pace” model[8] considers both acceleration and delay in development This model suggests that the type of adversity encountered determines whether biological maturation is accelerated or delayed and that changes in the rate of development occur to eliminate gaps in parental caregiving. While the ‘change of pace’ model focuses on the parent-child dyad, we have extended it to apply to low-SES in our review given the strong links between SES and parenting behaviors[28]. The model purports that delaying maturation lowers children’s physiological requirements when there are unmet physiological needs in situations of deprivation, such as inadequate nutrition or parental care. In the event of threat or abuse, children may have unmet safety needs, and accelerated development may boost children’s ability to provide for their own safety. The model also predicts that the aforementioned acceleration is time-limited and may switch to slower or delayed development after puberty. Low-SES is characterized by higher levels of material deprivation, such as food insecurity and reduced access to other basic necessities[29,30]. Given this, the change of pace model is consistent with the idea that SES might be associated with slower neurodevelopment Indeed, some longitudinal studies report that low-SES is associated slower neurodevelopmental trajectories[e.g., 31]. However, low-SES is also associated with greater exposure to community violence and other forms of threat[10,20], which are argued to accelerate neurodevelopment in this model. It is therefore unclear whether SES should be associated with an acceleration or delay of neurodevelopment per this model. Empirical studies directly testing the predictions of the change of pace model are currently lacking.
Evaluating the validity of these frameworks has been challenging as concrete predictions about what evidence would be aligned with acceleration or delay have not been articulated clearly. More problematic, most studies use data from cross-sectional designs to make inferences about accelerated versus delayed patterns of brain development[9,23,24]. Longitudinal research is needed to test how adversity and SES are associated with deviations from typical developmental trajectories, yet such studies remain rare.
Theoretical predictions within the context of normative development
In the following sections, we ground the predictions of each theoretical model in the context of normative patterns of gray matter development, highlight the types of evidence needed to adjudicate among competing hypotheses, and review empirical studies on SES and brain structure to ascertain which framework is best aligned with the evidence. We focus on low-SES and gray matter structure as longitudinal studies of other forms of adversity and other metrics of brain structure are limited[2] and typical patterns of cortical and subcortical development have been relatively well characterized[32–38].
Normative development
To investigate whether empirical evidence is aligned with theoretical predictions, we must contextualize these predictions within normative developmental trajectories. The brain undergoes protracted gray matter development throughout childhood and adolescence characterized by changes in cortical thickness, surface area, volume, and subcortical volume[37]. Cortical thickness increases during the first two years of life, with more rapid increases in the first relative to the second year peaking somewhere between 12-24 months[37]. Thickness then decreases rapidly in early childhood and is followed by monotonic thinning from childhood to adolescence[32–36]. Cortical volume increases in the first two years of life[39] followed by a more gradual increase in volume during childhood peaking around 10 years of age[40], and non-linear decreases throughout adolescence, with varying rates of decreases across regions[36,41]. In contrast, cortical surface area greatly expands in the first years of life[42], continues to expand throughout childhood, peaking in late childhood or early adolescence, and then undergoes subtle decreases thereafter[32,34–36,43]. Finally, the volume of subcortical regions such as the amygdala and hippocampus increases throughout childhood and early adolescence, plateaus in middle to late adolescence, and decreases thereafter[40,44–47]. It is important to acknowledge however that these are average trajectories and that there is substantial individual variability in the magnitude and timing of the peak as well as in rates of change[48].
Theoretical predictions
Overall, models largely predict accelerated development as a function of adversity and low-SES, with the exception of one model that also considers the idea of delayed development. If the pace of brain development was accelerated[6,7,9] or delayed[8], we would expect to see a temporally shifted pattern of brain development That is, individuals with accelerated or delayed brain development would hit the same normative developmental milestones, but earlier or later, respectively. Below we briefly outline the expected patterns for different measures of cortical development as a function of low-SES. Since cortical thickness increases in the first two years of life, accelerated neurodevelopment in low-SES youth would be associated with more rapid growth trajectories resulting in an earlier peak and increased cortical thickness prior to age two years. Thereafter, accelerated development would manifest as more rapid cortical thinning, resulting in lower cortical thickness in low-SES relative to high-SES children beginning in early childhood and continuing through adolescence. If development were delayed, we would observe the opposite pattern—slower growth resulting in lower thickness during infancy, a later peak in cortical thickness, and slower thinning resulting a thicker cortex during childhood and adolescence in low relative to high-SES youth. Similarly, for volume and surface area, accelerated development would involve faster expansion and growth in early childhood, an earlier peak, and more rapid decreases during late childhood and adolescence. If development were delayed, the opposite pattern would be expected—slower growth in early childhood, a later peak, and slower decreases during adolescence. Finally, subcortical volume would exhibit more rapid growth resulting in higher volume if development were accelerated and slower growth resulting in lower volume if development were delayed. Figure 1 depicts these predictions using cortical thickness and subcortical volume as examples.
Figure 1: SES and the pace of neurodevelopment: theoretical predictions and empirical observations.
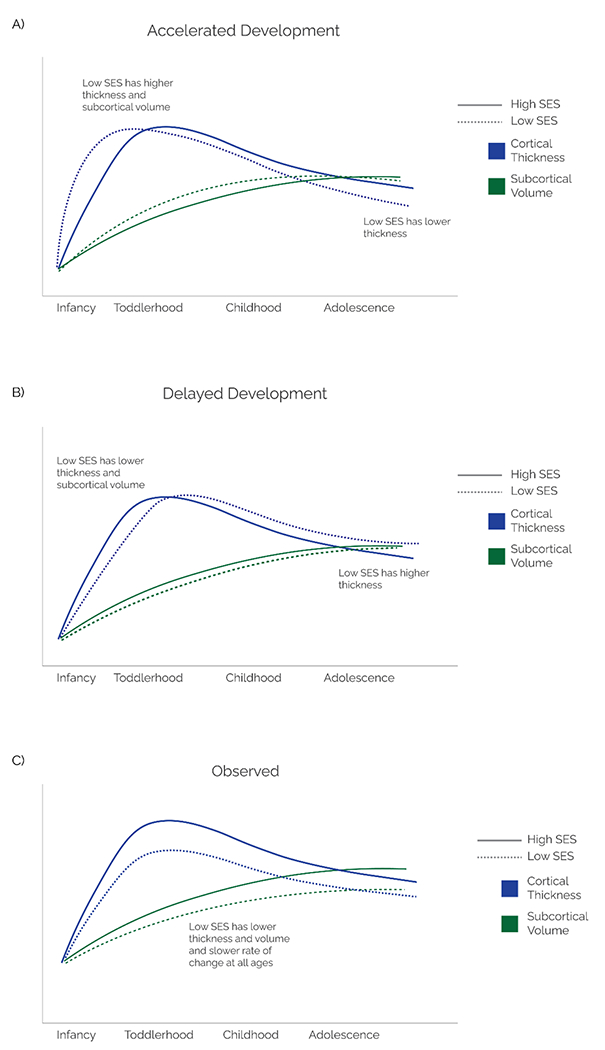
Expected trajectories of cortical thickness from infancy to late adolescence based on models of accelerated (A) and delayed (B) brain development Solid and dashed lines represent trajectories for high and low-SES youth, respectively. Panel C depicts the patterns observed in existing longitudinal studies. These patterns suggest that low-SES children have consistently lower cortical thickness, volume, surface area, and subcortical volume as well as slower rates of change during both growth and decline. Figures depict the starting point for low- and high-SES infants to coincide as evidence on SES-related differences in brain volume at birth is limited. Blue and green lines represent average trajectories for cortical thickness and subcortical volume—specifically amygdala and hippocampus, respectively.
Most studies have examined individual differences in the pace of neurodevelopment using cross-sectional data in adolescents, which has hindered our ability to truly test these theories. For example, cross-sectional data makes it impossible to disentangle whether lower thickness, surface area, or volume in low-SES adolescents[1] reflects a difference in the amount of cortical gray matter or in the rate of change over time, highlighting the need for longitudinal studies. Therefore, in order to assess which of these frameworks is best aligned with existing evidence, several pieces of information are needed in conjunction. First, information on SES-related differences in the rate of change in cortical grey matter and subcortical volume across development is required, as models differ in predictions about whether the rate of change in brain structure is faster versus slower during infancy, childhood, and adolescence. Second, models also differ in their predictions of whether cortical thickness and volume should be higher or lower in low-SES youth during infancy as well as childhood and adolescence (see Figure 1). Finally, knowledge about the timing of peak thickness and volume would help evaluate the predictions. Each of these pieces of information can be used to evaluate whether developmental trajectories are accelerated or delayed. We now review existing studies that provide the first and second pieces of information on SES and brain structure during infancy, childhood, and adolescence. Studies on differences in age at peak are currently lacking.
Empirical observations
Infancy
SES-related differences in cortical structure.
We identified six studies examining associations of SES with cortical structure in infants (Table 2). Four studies found that lower-SES was associated with lower cortical and subcortical volume[31,49–51] in neonates, infants, and toddlers. In contrast, one study reported both higher and lower cortical volume related to low-SES; infants aged 1-6 weeks from low-SES households had larger volumes in the occipital lobe, temporal pole, left inferior frontal regions, and anterior cingulate and lower volumes in the frontoparietal region and inferior temporal lobe relative to infants from high-SES households[52]. Partially in line with this, a study on a relatively large sample found low-SES to be associated with higher average cortical thickness and thickness of some frontal and temporal regions[53]. However, their findings could have been influenced by their adjustment for intracranial volume, which does not scale with thickness[54]. Although the literature is somewhat mixed, most findings, including those from well-powered samples of 756 infants aged 8-12 months and 280 neonates[51], suggest that low-SES is associated with lower cortical and subcortical volume early in life.
Table 2.
Infancy/childhood studies
| Study | Total N (Females) | Mean age (age range/SD) | SES measure | Imaging measure | Key Findings | Lower/higher in low-SES |
|---|---|---|---|---|---|---|
| Betancourt et al. (2016) [49] | 44 (25 low-SES, 19 high-SES), African American, 100%F | 5 ± 0.9 weeks | Composite SES (based on maternal education and income-to-needs) | Cortical gray, deep gray, and white matter volumes | Low SES infants had lower cortical gray matter and deep gray matter volume than high SES infants | Lower |
| Hanson et al. (2013) [31] | 77 (40%F) | 13.7 months (5 months to 4 years) | Household income | Total GMV and lobal volumes, white matter volume, total cerebral volume | Low SES toddlers had lower total, frontal, and parietal volume than high SES toddlers | Lower |
| Jha et al. (2019) [53] | 805 (47%F) | 30.6 days (6- to 144-days post birth) | Paternal and maternal education, income | Cortical thickness and surface area (average and regional) | Low paternal education was associated with higher average cortical thickness and thickness of some frontal and temporal regions (while adjusting for global brain size and ethnicity) | Higher |
| Knickmeyer et al. (2017) [50] | 756 (47%F) | 8-12 months | Maternal and paternal education and income | Total GM and WM | High paternal education was associated with higher gray matter and white matter volume (partially mediated through birth weight) | Lower |
| Spann et al. (2020) [52] | 37 (35%F) | 1-6 weeks | Hollingshead (education and occupation) | Volume | Infants born to mothers with lower SES had larger local volumes in the bilateral superior and middle occipital gyri, right middle frontal, and temporal pole, left inferior frontal and anterior cingulate regions. Low SES was associated with lower volumes in the frontoparietal region and the inferior temporal region. | Mixed |
| Triplett et al. (2022) [51] | 280 (47%F) | First weeks of life | Socioeconomic disadvantage: factor analysis of health insurance status, highest educational level, income-to-needs ratio, national Area Deprivation Index percentile at birth, and Healthy Eating Index. | Cortical and subcortical gray matter, white matter, and cerebellum volume, hippocampus and amygdala volume | Neonates born to mothers from disadvantaged backgrounds had lower cortical and subcortical gray matter volume and lower white matter volume | Lower |
| Qiu et al. (2017) [139] | 168 (44%F) | Mean post-conception age at scan 40 weeks | Household income | Subcortical volume and cortical thickness (whole brain) | Neonates with genetic profiles associated with heightened risk for developing depression showed a negative relationship between their family income and right amygdala and hippocampal volumes, whereas those with low genetic risk profiles showed no such association | Higher (only in those with high genetic risk for MDD) |
Abbreviations: GM = gray matter, GMV = gray matter volume, SES = socioeconomic status, WM = white matter
SES-related differences in rate of change.
To our knowledge, only one study has examined SES-related changes in cortical structure in infants or toddlers longitudinally. Low-SES infants had lower total, frontal, and parietal volume, and these differences became more pronounced with age[31], consistent with a slower pace of neurodevelopment.
Childhood and Adolescence
SES-related differences in cortical structure.
Numerous cross-sectional studies observe lower cortical thickness, surface area, volume, and subcortical volume among low-SES relative to high-SES children and adolescents[55–69]. For greater details see a recent systematic review[1]. Although studies vary in terms of specific regions where differences were observed, the evidence is remarkably consistent in the direction of the association between SES and brain structure.
SES-related differences in rate of change.
Longitudinal studies find low-SES to be associated with a lower rate of change (Table 3). For example, low-SES has been associated with reduced and slower growth in hippocampus[59,70,71] and overall subcortical[72] volume during childhood and adolescence. Three studies reported lower rate of change in cortical thickness and volume reported as a maturational lag in total gray matter, frontal, and temporal volume in low-compared to high-SES children[73], lower rate of cortical volume growth in parts of the insula and superior temporal gyrus[72], and less cortical thinning over time in low-SES adolescents[74], suggesting slower cortical development[75]. Finally, using a brain-predicted age framework based on both cortical and subcortical data, one study showed that low-SES children had higher brain age gap values at age 12 followed by a negative trajectory, reflecting slower brain development[22]. Finally, a recent paper shows higher SES to be associated with more rapid cortical thinning and area reduction[76]. Not all findings are aligned with lower rate of change in youth from lower SES backgrounds, however. Mixed sex-dependent findings of slower and faster change[77], more rapid amygdala growth in males[74], and greater decreases in surface area[78] in low-SES adolescents have also been reported.
Table 3.
Studies measuring rate of change
| Study | Total N (Females) | Mean age ± SD (range) | SES measure | Number of imaging time points | Imaging measure | Key Findings | Faster/Slower/Different |
|---|---|---|---|---|---|---|---|
| Barch et al. (2020) [70] | 167 (48.5%F) | 15.83 ± 1.11 (13-19) | Income-to-needs ratio (family income/federal poverty level based on family size) | 3 | Volume | Greater T1 poverty was associated with a shallower hippocampal slope | Slower |
| Barch et al. (2022) [72] | 210 (50%F) | 3-6 years at T1 and 15-21 at T5 | Income-to-needs | 5 | Volume | High INR was associated with faster subcortical volume growth and faster cortical volume growth (of a few regions) | Slower |
| Ellwood-Lowe et al. (2018) [71] | 116 (100% F) | 14.82 ± 3.09 (10-23). Time between first and last scans - 4.61 ± 2.15 | Family income and parental education | 4 | Volume | Hippocampal volume in girls from lower-income homes exhibited a modest decline during the teenage years, then an increase through to early adulthood. In contrast, hippocampal volume of high-income females peaked around the age of 19, and then started to decline. | Different (non-linear), high-income girls appear to reach a higher and earlier peak |
| Hair et al. (2015) [73] | 389 (52.5% F) | 11.1 (4-20) (first scan; follow-up period 24 months) | Household income | 3 | Volume | Low-income children exhibited maturational lag in total gray matter and the frontal lobe, temporal lobe, and hippocampus. | Slower |
| Hanson et al. (2013) [31] | 77 (40%F) | 13.5 ± 7.9 months at first scan, average time between scans = 6.5 ± 4.1 months | Average household income across time-points | 3 | Volume | Low SES was associated with a slower growth trajectory of development for total gray matter volume and volume of the frontal and parietal lobe | Slower |
| Judd et al. (2020) [78] | 551 (58%F) | T1: 14.44 ± 0.38 T2: 19.01 ± 0.7 |
Composite SES: sum of mother’s education score, father’s education score, family stress unemployment score, financial difficulties score, home inadequacy score, neighborhood score, financial crisis score, mother employed score, and father employed score | 2 | Surface area, thickness, subcortical volume | Greater decreases in surface area in low-SES individuals (NS for thickness and subcortical volume) | Faster |
| Kalantar-Hormozi et al. (2023) [76] | 183 (42%F) | Three repeated scans per subject, approximate interval 2.8 years; mean age: 11.2 ± 2.7, range 5-24.2 | Hollingshead two-factor index | 3 | Covariation across multiple cortical features (cortical thickness, surface area, local gyrification index, and mean curvature) | Higher SES was associated with accelerated cortical thinning and area reduction | Slower |
| King et al. (2020) [77] | 147 (57%F) | T1: 11 ± 1 (9-13) T2: 13 ± 1 (12-17) |
Income-to-needs | 2 | Volume | Higher INR was associated with less expansion, or greater contraction, in the right hippocampal cingulum in boys, but was weakly associated with greater volume expansion in girls Higher INR was associated with greater volume expansion in the left inferior temporal gyrus, bilateral lingual gyrus and in the right superior parietal lobule and less volume expansion (or more contraction) in an area of the bilateral superior frontal gyrus in boys |
Mixed and sex-dependent |
| McDermott et al. (2019) [59] | 623 (48%F) | 12 ±4 (5-25) | Hollingshead two-factor index | Long (344 individuals had > 1 scan) | Volume | High SES was associated with greater increases in hippocampal volume | Slower |
| Rakesh et al. (2021) [22] | 166 (52%F) | T1: 12.79 ± 0.43 T2: 16.7 ± 0.52 T3: 19.08 ± 0.46 |
Neighborhood disadvantage | 3 | Brain-predicted-age (based on surface area, thickness, and volume) | Low SES was associated with a negative brain-predicted-age trajectory (i.e., greater brain-predicted-age at earlier ages but not at later ages) | Slower trajectory |
| Whittle et al. (2017) [74] | 166 (52%F) | T1: 12.79 ± 0.43 T2: 16.7 ± 0.52 T3: 19.08 ± 0.46 |
Neighborhood disadvantage | 3 | Thickness and volume | Low SES was associated with less thinning (more thickening), greater increases in amygdala volume | Slower for cortical thickness, faster for amygdala in boys |
Abbreviations: INR = income-to-needs ratio, SES = socioeconomic status
Brain developmental trajectories associated with low-SES may be simply different
Collectively, the evidence suggests that low-SES is associated with lower thickness, surface area, and volume and slower rate of change throughout infancy, childhood, and adolescence (Table 1, Figure 1). In addition, the pattern of findings does not appear to vary based on the specific SES indicator used, although the number of studies of each specific SES indicator is small. There have been a limited number of studies examining SES and brain structure in infants, and even fewer longitudinal studies, which makes it challenging to make definitive conclusions about this time period. However, the available evidence is more consistent with delayed than accelerated brain development in low-SES infants. While lower thickness, area, and volume in childhood and adolescence is consistent with accelerated brain development, patterns of change over time are consistent with delay rather than acceleration during this period. Although most findings were consistent with delayed brain development, the lack of evidence for low-SES children exhibiting higher thickness or volume in childhood or adolescence than high-SES children is inconsistent with a delayed maturational trajectory.
Table 1.
Predicted versus observed data.
| Accelerated | Delayed | Observed | |
|---|---|---|---|
| Cortical Measures | |||
|
| |||
| Infancy, toddlerhood | Higher1,2,3 | Lower 1,2,3 | Lower3 |
| Earlier peak1 | Later peak1 | Slower change3 | |
| Faster change1,2,3 | Slower change 1,2,3 | ||
| Childhood, adolescence | Lower 1,2,3 | Higher1,2,3 | Lower1,2,3 |
| Earlier peak2,3 | Later peak2,3 | Slower change1,2,3 | |
| Faster change1,2,3 | Slower change 1,2,3 | ||
|
| |||
| Hippocampus and Amygdala Volume | |||
|
| |||
| Infancy, toddlerhood | Higher | Lower | Lower |
| Faster change | Slower change | Slower change | |
| Childhood, adolescence | Higher | Lower | Lower |
| Earlier peak | Later peak | Slower change | |
| Faster change | Slower change | ||
The table depicts the predictions of accelerated versus delayed development and compares them to the patterns observed in empirical studies, with the top panel referring to cortical measures and the bottom panel subcortical measures. Bolded text indicates when a prediction matches an empirical observation, listed in the observed column. Of note, for measures that peak during childhood and adolescence such as surface area, cortical volume, and subcortical volume, if development were accelerated, values would be higher before the peak and lower after the peak. On the other hand, if development was delayed, values during childhood and adolescence would be lower before the peak and higher after the peak.
= Cortical Thickness,
= Cortical Surface Area,
= Cortical Volume
Based on this review, we stipulate that none of the models fully captures the existing pattern of evidence of SES-related differences in structural brain maturation. Instead, it may be that low-SES is associated with a simply different developmental trajectory characterized by lower cortical thickness and volume at all ages from infancy through adolescence as well as slower growth and slower thinning over time (Figure 1). This trajectory is most consistent with the evidence, which shows lower thickness, volume, and surface area and slower rates of change in individuals from low-SES backgrounds at all ages. Of note, this proposed trajectory may be more applicable to cortical and subcortical volume given the limited number of longitudinal studies that have examined cortical thickness and surface area trajectories, as well as the presence of null and mixed findings. Clearly, more longitudinal studies examining changes in different brain structural metrics over time are needed, particularly in the first years of life.
Mechanisms contributing to SES-related differences in brain structure
Several factors that vary as a function of SES—including prenatal factors, exposure to stress, and reduced cognitive stimulation—likely influence changes in underlying neurobiological processes such as synaptic pruning and myelination and contribute to SES-related differences in large-scale brain morphology. These ideas have been discussed extensively[7,9,17,27,79]. We highlight some mechanisms that may explain SES-associated differences in the pace of brain development in each development period briefly.
Infancy
Local cellular events—such as rapid gains in dendritic complexity, myelination, synaptogenesis, glial proliferation, and axonal elongation—have been suggested to contribute to increases in cortical thickness and surface area in the first years of life[80–84]. Higher levels of enriching and stimulating experiences in high-SES households may alter cellular processes and contribute to SES-associated differences in brain structure. Evidence from animal models suggests that the expression of cellular signals involved in activity-dependent synaptic development is upregulated by enrichment including neurotrophins, brain-derived neurotrophic factor, synaptic proteins involved in synaptic proliferation and function, and factors implicated in glutamatergic signaling[79]. Low-SES is also associated with higher levels of family conflict and harsh parenting[85,86], meaningful sources of chronic stress in early life. Chronic stress also influences glial cell proliferation, which could contribute to differences in gray matter structure[87]. However, the mechanisms driving the associations between enrichment and stimulation, stress, and increases in cortical thickness and volume during the early years remain relatively unexplored.
Childhood and adolescence
Differences in brain structure are also evident in childhood and adolescence. It is possible that differences in proliferation during the first years of life simply carry forward into later developmental periods. Alternatively, differences in synaptic pruning could give rise to low-SES being associated with lower cortical thickness and volume in childhood and adolescence. For example, reduced dendritic spine density, branching and length of dendrites, and the number of synapses per neuron are all observed in animals raised in deprived environments[88–90]. In addition, greater chronic stress can cause spine loss[88], atrophy of apical dendrites[89], and suppress neurogenesis in the dentate gyrus[90], which could contribute to lower cortical thickness, volume, and subcortical volume. The slower rate of change reported in longitudinal studies suggests that greater pruning may not be a plausible explanation for SES-related differences in brain structure. Importantly, the biological mechanisms underlying reduced cortical thickness and surface area cannot solely be attributed to small-scale changes at the synapse level[37]. For example, changes in myelination and reduction in the number of glial cells can contribute to these developmental changes[37]. Understanding of how SES influences these processes remains limited.
Rate of change
To our knowledge, animal studies linking enrichment and stress with small-scale developmental changes at the level of synaptic pruning, myelination, and dendritic arborization have not been examined using longitudinal designs. The lack of such knowledge makes it challenging to comment on the mechanisms underlying slower rates of change. However, studies using the minimal bedding paradigm to mimic low-SES in rodents demonstrate impaired microglia-mediated synaptic pruning after this manipulation[91]. Less pruning could reflect slower circuit refinement Enrichment also contributes to newly produced neurons being integrated into functional circuits[79], and computational neuroscience models show that network abilities benefit from early synaptic overgrowth followed by pruning of weak synapses[92]. Accordingly, lower overall synaptic proliferation could partially explain the differences in brain functional integration and segregation observed as a function of low-SES both early in life[93], and during childhood and adolescence[1,94–101]. For example, measures of network efficiency, such as within-network connectivity and global efficiency, which typically increase with age during development[102–107] are lower in children from low-SES backgrounds[95,108,109]. However, given limited longitudinal research on functional and structural connectivity, caution is warranted in interpreting these patterns.
Importantly, we have focused on postnatal differences in this review. However, given differences in brain structure observed in the first weeks of life[51], it is possible and even likely that SES influences brain structure before birth, which may create a persistent offset that is observed as cross-sectional differences in brain morphology at all ages. Differences at birth could be due to a host of prenatal factors including maternal stress, nutrition, prenatal complications, drug and toxin exposure, and pre-term birth[79]. Higher levels of stress, higher infection rates, and poor nutrition can increase the levels of corticotropin-releasing factor and glucocorticoids in the mother and fetus[110–113]. These factors can lead to restricted fetal growth and premature birth[110,111,113]. More neuroimaging studies that examine associations between prenatal factors and fetal brain development are needed. Further, genetics may also play a confounding role. That is, genetics may in part determine both the parent’s SES as well as children’s brain structure. Past work has shown that both SES and genetic factors contribute to educational attainment and impact cognitive and brain development in adolescents[78]. It is also possible that the initial offset present at birth may influence rates of change in brain structure, however, this is speculative and longitudinal research is needed to test this hypothesis. Finally, even postnatally, low-SES is associated with numerous factors other than chronic stress and cognitive stimulation that can influence brain development, including nutrition, school environments, and exposure to toxins and pollutants[50,114–116]. Research examining how these factors might independently and jointly shape neurodevelopment is sorely needed.
Concluding remarks and future directions
We examined the predictions of influential conceptual models on adversity and the pace of brain development Across models, the predictions differ in how adversity and low-SES should be associated with brain structure during infancy as well as childhood and adolescence and whether changes in brain structure should occur at a slower or faster pace. The empirical data suggests that none of these models fully captures the observed differences in structural development between low and high-SES youth, and that low-SES may be associated with a simply different neurodevelopmental trajectory. However, in the absence of longitudinal data that spans infancy, childhood, and adolescence, it is challenging to make definitive conclusions about accelerated, delayed, or different trajectories. Despite the first years of life being marked by rapid and dynamic brain development, there has been very little research on SES- and adversity-related differences during this period of life. This is understandable given the challenges associated with infant neuroimaging. However, more longitudinal research that maps normative development as well as differences related to early experience from infancy to adolescence are needed to test these ideas thoroughly. Eventually, researchers will be able to combine data from studies such as Healthy Brain and Child Development and Adolescent Brain Cognitive Development to test associations between SES and changes in brain morphology from infancy to late adolescence.
Further, most of the conceptual models we evaluate focus on experiences of adversity broadly rather than SES specifically, with some exceptions[9]. We focus here on SES due to lack of longitudinal imaging studies and infant research on other forms of early-life adversity. However, it is important to acknowledge low-SES is not synonymous with adversity(Box 1) and that neurodevelopmental mechanisms beyond accelerated or delayed development may contribute to observed SES-related differences in brain structure. Many children raised in low-SES families receive enriching cognitive and social stimulation and are not exposed to harsh parenting or violence. Further, whether the patterns of structural brain maturation observed here apply to other forms of adversity is unknown and is a critical topic for future research, although similar patterns as those described here have been reported in relation to other forms of adversity in several studies. For example, numerous cross-sectional studies observe lower cortical thickness in children who have experienced maltreatment, exposure to violence, and severe deprivation related to institutional rearing[2], which is often interpreted to be consistent with accelerated development. Longitudinal work shows reduced growth in amygdala volume over time in adolescents exposed to maltreatment[117], which reflects a slower rate of development. More longitudinal research in this area is sorely needed. Further, SES is a broad and complex construct that can be operationalized in multiple ways—for example household income, parental education, and neighborhood SES as well as in the form of composite SES indices like Hollingshead Index. These indices tend to be moderately correlated[118] and may influence brain development through both distinct and similar pathways[see Box 1; 5]. More studies are needed to examine independent associations of different SES indicators with brain maturation.
Brain development is a profoundly complicated process. SES can influence brain development in numerous ways that vary meaningfully as a function of the presence of other risk and protective factors. Critically, the lack of longitudinal studies using other imaging modalities precluded us from examining associations between SES and the pace of maturation of white matter structure, structural and functional connectivity, and task-based activation(see Outstanding Questions), which also play an important role in behavioral outcomes. For example, although low-SES may be associated with lower cortical thickness and brain volumes on average, which has been shown to mediate links between SES and cognitive performance in young people[67,119,120], other neurodevelopmental changes associated with low-SES are likely to confer important advantages that help children adapt to the environment in which they are developing[121]. For example, the ability to switch between tasks or mental sets quickly and easily, and the capacity to track novel environmental information tend to be enhanced in children and adults who grew up in more unpredictable family environments[122,123]. Low-SES is likely associated with numerous brain adaptations that help children develop such skills and thrive in their environment[124]. Further, it is also important to consider the complex relationships of SES and adversity with systemic and interpersonal racism when examining associations with neurodevelopment[125,126]. In addition to variability in covariates included, some studies have covaried for race and ethnicity while others have not (see Supplementary Materials), which makes it somewhat challenging to compare findings. Further, results from studies that covary for race/ethnicity but do not have an even distribution of SES across racial and ethnic groups in the study need to be interpreted cautiously[127]. In addition, research in this area has relied heavily on data from Western, Educated, Industrialized, Rich, and Democratic(WEIRD) countries, limiting our ability to generalize findings to other countries and cultures. Finally, while parental SES does not change substantially during childhood for most individuals[128], given our limited knowledge about timing effects and when brain maturation may be most sensitive to SES or changes in SES, we are unable to comment on how these brain maturation curves(Figure 1) may change if SES were to increase or decrease. This is an important direction for future work, particularly in the context of interventions (see Box 2).
Outstanding Questions.
What are the normative brain developmental patterns in utero and during infancy, and how is SES associated with these trajectories?
How is SES associated with developmental changes in cortical thickness and surface area during childhood and adolescence?
How does SES relate to maturation of white matter structure, structural and functional connectivity, and task-based activation?
What are the neurobiological processes underlying change in cortical and subcortical brain structure across infancy, childhood, and adolescence, and what is the role of SES in shaping these mechanisms?
What are the proximal environmental factors that mediate the association between SES and changes in brain structure over time?
How do SES-associated differences in structural brain structure development impact functional network development and circuit refinement in the brain?
How can we disentangle the role of prenatal factors, genetics, and SES in shaping brain development?
Can this model be extended to other types of adversity including childhood abuse and traumatic experiences?
Box 2: Leveraging intervention studies to establish causal inferences.
It is crucial to acknowledge that the findings presented in this review are derived from observational studies, and as such, cannot establish a causal relationship between SES and brain development Intervention studies that involve changing income through cash transfers and quasi- experimental approaches can provide more definitive causal evidence for these associations. Numerous such studies support a causal relationship between increased income and improved academic outcomes for low-income students. For example, in the United States and Canada, quasi-experimental research has utilized income boosts to demonstrate that increases in income produce higher levels of school achievement in children[131–133]. Similar intervention studies that examine impacts on neurodevelopment have rarely been conducted. One key exception is the Baby’s First Years Study[134], which provides cash assistance to low-SES mothers during the first years of their child’s life and is collecting metrics of brain structure and function in the children across development. These types of studies can determine whether changes in income are causally associated with corresponding changes in brain structure as well as the pace of neurodevelopment Further, studies that intervene on specific environmental pathways that may mediate associations between SES and outcomes, such as by providing higher quality early education and child care, have also shown promise in improving a wide range of developmental outcomes[135–138]. Determining whether these interventions improve outcomes by contributing to changes in brain development is a critical question for future research. Such research can also help to identify periods when brain development is most responsive to intervention and inform optimal windows for intervention.
In sum, existing evidence is more, but not entirely consistent with low-SES predicting delayed rather than accelerated brain development. No existing model fully captures observed differences between low- and high-SES youth. Low-SES and other adverse environments are likely associated with brain developmental trajectories that differ in multiple ways considering the available evidence. Our understanding of how SES may influence the pace of neurodevelopment is limited and more longitudinal work, particularly during infancy and early childhood, is needed to establish normative developmental trajectories and to test the predictions of neurodevelopmental pace models more rigorously. Based on the available evidence, we suggest that low-SES may be associated with a distinct pattern of brain maturation that is less about the timing of the attainment of milestones (i.e., acceleration or delay) but the milestones themselves.
Supplementary Material
Highlights.
Theories make contrasting predictions about whether adverse experiences and low SES are associated with accelerated or delayed neurodevelopment.
Existing evidence is more consistent with low-SES predicting delayed rather than accelerated brain development. However, no existing model fully captures observed differences between low- and high-SES youth.
Low-SES and other adverse environments are likely associated with brain developmental trajectories that differ in multiple ways considering the available evidence.
We suggest that low SES is associated with brain maturation patterns characterized by lower volume and slower rates of change throughout development.
Longitudinal research, especially in the early years, is needed to rigorously test how adversity and SES are associated with deviations from typical developmental trajectories.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rakesh D and Whittle S (2021) Socioeconomic status and the developing brain - A systematic review of neuroimaging findings in youth. Neuroscience and Biobehavioral Reviews 130, 379–407 [DOI] [PubMed] [Google Scholar]
- 2.McLaughlin KA et al. (2019) Childhood Adversity and Neural Development: A Systematic Review. Annual Review of Developmental Psychology 1, 277–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCrory E et al. (2011) The impact of childhood maltreatment: A review of neurobiological and genetic factors. Frontiers in Psychiatry 2, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teicher MH et al. (2016) The effects of childhood maltreatment on brain structure, function and connectivity. Nature Reviews Neuroscience 17, 652–666 [DOI] [PubMed] [Google Scholar]
- 5.Farah MJ (2017) The Neuroscience of Socioeconomic Status: Correlates, Causes, and Consequences. Neuron 96, 56–71 [DOI] [PubMed] [Google Scholar]
- 6.Callaghan BL and Tottenham N (2016) The Stress Acceleration Hypothesis: Effects of early-life adversity on emotion circuits and behavior. Current Opinion in Behavioral Sciences 7, 76–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McLaughlin KA et al. (2017) Neglect as a Violation of Species-Expectant Experience: Neurodevelopmental Consequences. Biological Psychiatry 82, 462–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roubinov D et al. (2021) Change of pace: How developmental tempo varies to accommodate failed provision of early needs. Neuroscience and Biobehavioral Reviews 131, 120–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tooley UA et al. (2021) Environmental influences on the pace of brain development. Nature Reviews Neuroscience 22, 372–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans GW (2004) The Environment of Childhood Poverty. American Psychologist 59, 77–92 [DOI] [PubMed] [Google Scholar]
- 11.Belsky J (2012) The Development of Human Reproductive Strategies: Progress and Prospects. 10.1177/0963721412453588 21, 310–316 [DOI] [Google Scholar]
- 12.Belsky J et al. (1991) Childhood Experience, Interpersonal Development , and Reproductive Strategy: An Evolutionary Theory of Socialization. Child Development 62, 647–670 [DOI] [PubMed] [Google Scholar]
- 13.Giudice MD et al. (2011) The Adaptive Calibration Model of stress responsivity. Neuroscience and biobehavioral reviews 35, 1562–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belsky J et al. (2012) Beyond cumulative risk: Distinguishing harshness and unpredictability as determinants of parenting and early life history strategy. Developmental Psychology 48, 662–673 [DOI] [PubMed] [Google Scholar]
- 15.Ellis BJ et al. (2022) Why and how does early adversity influence development? Toward an integrated model of dimensions of environmental experience. Development and Psychopathology 34, 447–471 [DOI] [PubMed] [Google Scholar]
- 16.Belsky J (2019) Early-Life Adversity Accelerates Child and Adolescent Development. 10.1177/0963721419837670 28, 241–246 [DOI] [Google Scholar]
- 17.McLaughlin KA et al. (2014) Childhood adversity and neural development: Deprivation and threat as distinct dimensions of early experience. Neuroscience and Biobehavioral Reviews, 47, 578–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bradley RH and Corwyn RF (2002) Socioeconomic status and child development. Annual Review of Psychology 53, 371–399 [DOI] [PubMed] [Google Scholar]
- 19.Bradley RH et al. (2001) The Home Environments of Children in the United States Part I: Variations by Age, Ethnicity, and Poverty Status. Child Development 72, 1844–1867 [DOI] [PubMed] [Google Scholar]
- 20.Evans GW and Kim P (2010) Multiple risk exposure as a potential explanatory mechanism for the socioeconomic status-health gradient. Ann N Y Acad Sci 1186, 174–189 [DOI] [PubMed] [Google Scholar]
- 21.Keding TJ et al. (2021) Differential Patterns of Delayed Emotion Circuit Maturation in Abused Girls With and Without Internalizing Psychopathology. 10.1176/appi.ajp.2021.20081192 DOI: [DOI] [PMC free article] [PubMed]
- 22.Rakesh D et al. (2021) Neighborhood disadvantage and longitudinal brain-predicted-age trajectory during adolescence. Developmental Cognitive Neuroscience 51, 101002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gur RE et al. (2019) Burden of Environmental Adversity Associated with Psychopathology, Maturation, and Brain Behavior Parameters in Youths. JAMA Psychiatry 76, 966–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colich NL et al. (2020) Biological Aging in Childhood and Adolescence Following Experiences of Threat and Deprivation: A Systematic Review and Meta-Analysis. Psychological bulletin 146, 721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McLaughlin KA and Sheridan MA (2016) Beyond Cumulative Risk: A Dimensional Approach to Childhood Adversity. Current Directions in Psychological Science 25, 239–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLaughlin KA et al. (2021) The Value of Dimensional Models of Early Experience: Thinking Clearly About Concepts and Categories. Perspectives on Psychological Science 16, 1463–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheridan MA and McLaughlin KA (2014) Dimensions of early experience and neural development: deprivation and threat. Trends in Cognitive Sciences 18, 580–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roubinov DS and Boyce WT (2017) Parenting and SES: relative values or enduring principles? Current Opinion in Psychology 15, 162–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLaughlin KA et al. (2012) Food insecurity and mental disorders in a national sample of U.S. adolescents. Journal of the American Academy of Child and Adolescent Psychiatry 51, 1293–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Notten G and Kaplan J (2021) Material Deprivation: Measuring Poverty by Counting Necessities Households Cannot Afford. Canadian Public Policy, 47, 1–17 [Google Scholar]
- 31.Hanson JL et al. (2013) Family poverty affects the rate of human infant brain growth. PloS one 8, e80954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ducharme S et al. (2016) Trajectories of cortical thickness maturation in normal brain development — The importance of quality control procedures. NeuroImage 125, 267–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fjell AM et al. (2015) Development and aging of cortical thickness correspond to genetic organization patterns. Proceedings of the National Academy of Sciences 112, 15462–15467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mills KL et al. (2014) Developmental changes in the structure of the social brain in late childhood and adolescence. Social Cognitive and Affective Neuroscience 9, 123–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vidal-Pineiro D et al. (2020) Cellular correlates of cortical thinning throughout the lifespan. Scientific Reports 2020 10:1 10, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wierenga LM et al. (2014) Unique developmental trajectories of cortical thickness and surface area. NeuroImage 87, 120–126 [DOI] [PubMed] [Google Scholar]
- 37.Norbom LB et al. (2021) New insights into the dynamic development of the cerebral cortex in childhood and adolescence: Integrating macro- and microstructural MRI findings. Progress in Neurobiology 204, 102109. [DOI] [PubMed] [Google Scholar]
- 38.Tamnes CK et al. (2017) Development of the cerebral cortex across adolescence: A multisample study of inter-related longitudinal changes in cortical volume, surface area, and thickness. Journal of Neuroscience 37, 3402–3412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gilmore JH et al. (2012) Longitudinal development of cortical and subcortical gray matter from birth to 2 years. Cerebral Cortex 22, 2478–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gilmore JH et al. (2018) Imaging structural and functional brain development in early childhood. Nature Reviews Neuroscience 2018 19:3 19, 123–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mills KL et al. (2016) Structural brain development between childhood and adulthood: Convergence across four longitudinal samples. NeuroImage 141, 273–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li G et al. (2013) Mapping Region-Specific Longitudinal Cortical Surface Expansion from Birth to 2 Years of Age. Cerebral Cortex 23, 2724–2733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walhovd KB et al. (2016) Neurodevelopmental origins of lifespan changes in brain and cognition. Proceedings of the National Academy of Sciences 113, 9357–9362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wierenga LM et al. (2014) Typical development of basal ganglia, hippocampus, amygdala and cerebellum from age 7 to 24. NeuroImage 96, 67–72 [DOI] [PubMed] [Google Scholar]
- 45.Goddings A-LL et al. (2014) The influence of puberty on subcortical brain development. NeuroImage 88, 242–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herting ΜM et al. (2018) Development of subcortical volumes across adolescence in males and females: A multisample study of longitudinal changes. NeuroImage 172, 194–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.LeWinn KZ et al. (2017) Sample composition alters associations between age and brain structure. Nature communications 8, 874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McNeish D et al. (2021) Modeling individual differences in the timing of change onset and offset. Psychological Methods DOI: 10.1037/met0000407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Betancourt LM et al. (2016) Effect of socioeconomic status (SES) disparity on neural development in female African-American infants at age 1 month. Developmental science 19, 947–956 [DOI] [PubMed] [Google Scholar]
- 50.Knickmeyer RC et al. (2017) Impact of Demographic and Obstetric Factors on Infant Brain Volumes: A Population Neuroscience Study. Cerebral cortex (New York, N.Y. : 1991) 27, 5616–5625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Triplett RL et al. (2022) Association of Prenatal Exposure to Early-Life Adversity with Neonatal Brain Volumes at Birth. JAMA Network Open 5, E227045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spann MN et al. (2020) Prenatal socioeconomic status and social support are associated with neonatal brain morphology, toddler language and psychiatric symptoms. Child neuropsychology : a journal on normal and abnormal development in childhood and adolescence 26, 170–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jha SC et al. (2019) Environmental Influences on Infant Cortical Thickness and Surface Area. Cerebral Cortex 29, 1139–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vijayakumar N et al. (2018) Structural brain development: A review of methodological approaches and best practices. Developmental Cognitive Neuroscience 33, 129–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jednorog K et al. (2012) The Influence of Socioeconomic Status on Children’s Brain Structure. PLOS ONE 7, e42486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lawson GM et al. (2013) Associations between children’s socioeconomic status and prefrontal cortical thickness. Developmental Science 16, 641–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Machlin L et al. (2020) Brain structure mediates the association between socioeconomic status and attention-deficit/hyperactivity disorder. Developmental Science 23, el2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mackey AP et al. (2015) Neuroanatomical Correlates of the Income-Achievement Gap. Psychological Science 26, 925–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McDermott CL et al. (2019) Longitudinally mapping childhood socioeconomic status associations with cortical and subcortical morphology. The Journal of Neuroscience 39, 1365–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Romeo RR et al. (2018) Socioeconomic Status and Reading Disability: Neuroanatomy and Plasticity in Response to Intervention. Cerebral cortex (New York, N.Y. : 1991) 28, 2297–2312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brito NH and Noble KG (2018) The independent and interacting effects of socioeconomic status and dual-language use on brain structure and cognition. Developmental Science 21, el2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brito NH et al. (2017) Associations between cortical thickness and neurocognitive skills during childhood vary by family socioeconomic factors. Brain and Cognition 116, 54–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Assari S (2020) Race, Ethnicity, Family Socioeconomic Status, and Children’s Hippocampus Volume. Research In Health Science 5, p25. [DOI] [PubMed] [Google Scholar]
- 64.Dufford AJ et al. (2019) Socioeconomic disadvantage, brain morphometry, and attentional bias to threat in middle childhood. Cognitive, Affective & Behavioral Neuroscience 19, 309–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hanson JL et al. (2015) Behavioral problems after early life stress: Contributions of the hippocampus and amygdala. Biological Psychiatry 77, 314–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Taylor RL et al. (2020) Assessment of Neighborhood Poverty, Cognitive Function, and Prefrontal and Hippocampal Volumes in Children. JAMA network open 3, e2023774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Noble KG et al. (2015) Family income, parental education and brain structure in children and adolescents. Nature Neuroscience 18, 773–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rakesh D et al. (2022) Assessment of Parent Income and Education, Neighborhood Disadvantage, and Child Brain Structure. JAMA Network Open 5, e2226208–e2226208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Turesky TK et al. (2021) Brain morphometry and diminished physical growth in Bangladeshi children growing up in extreme poverty: A longitudinal study. Developmental Cognitive Neuroscience 52, 101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barch DM et al. (2020) Testosterone and hippocampal trajectories mediate relationship of poverty to emotion dysregulation and depression. Proceedings of the National Academy of Sciences of the United States of America 117, 22015–22023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ellwood-Lowe ME et al. (2018) Time-varying effects of income on hippocampal volume trajectories in adolescent girls. Developmental Cognitive Neuroscience 30, 41–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barch DM et al. (2022) Early Childhood Socioeconomic Status and Cognitive and Adaptive Outcomes at the Transition to Adulthood: The Mediating Role of Gray Matter Development Across Five Scan Waves. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 7, 34–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hair NL et al. (2015) Association of Child Poverty, Brain Development, and Academic Achievement. JAMA pediatrics 169, 822–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Whittle S et al. (2017) Role of positive parenting in the association between neighborhood social disadvantage and brain development across adolescence. JAMA Psychiatry ΊΑ, 824–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vijayakumar N et al. (2016) Brain development during adolescence: A mixed-longitudinal investigation of cortical thickness, surface area, and volume. Human Brain Mapping 37, 2027–2038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kalantar-Hormozi H et al. (2023) A cross-sectional and longitudinal study of human brain development: the integration of cortical thickness, surface area, gyrification index, and cortical curvature into a unified analytical framework. NeuroImage DOI: 10.1016/j.neuroimage.2023.119885 [DOI] [PubMed] [Google Scholar]
- 77.King LS et al. (2020) Cross-sectional and longitudinal associations of family income-to-needs ratio with cortical and subcortical brain volume in adolescent boys and girls. Developmental Cognitive Neuroscience 44, 100796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Judd N et al. (2020) Cognitive and brain development is independently influenced by socioeconomic status and polygenic scores for educational attainment. Proceedings of the National Academy of Sciences of the United States of America 117, 12411–12418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hackman DA et al. (2010) Socioeconomic status and the brain: Mechanistic insights from human and animal research. Nature Reviews Neuroscience 11, 651–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huttenlocher PR and Dabholkar AS (1997) Regional differences in synaptogenesis in human cerebral cortex. Journal of Comparative Neurology 387, 167–178 [DOI] [PubMed] [Google Scholar]
- 81.Koenderink MJT et al. (1994) Postnatal maturation of the layer III pyramidal neurons in the human prefrontal cortex: a quantitative Golgi analysis. Brain research 653, 173–182 [DOI] [PubMed] [Google Scholar]
- 82.Mrzljak L et al. (1991) Chapter 9 Neuronal development in human prefrontal cortex in prenatal and postnatal stages. Progress in Brain Research 85, 185–222 [DOI] [PubMed] [Google Scholar]
- 83.Petanjek Z et al. (2011) Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America 108, 13281–13286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rakic P et al. (1994) Synaptic development of the cerebral cortex: implications for learning, memory, and mental illness. Progress in Brain Research 102, 227–243 [DOI] [PubMed] [Google Scholar]
- 85.Mcewen CA and Mcewen BS (2017) Social structure, adversity, toxic stress, and intergenerational poverty: An early childhood model. Annual Review of Sociology 43, 445–472 [Google Scholar]
- 86.Boyce WT et al. (2012) Toward a new biology of social adversity. Proceedings of the National Academy of Sciences 109, 17143–17148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Popoli M et al. (2012) The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nature reviews. Neuroscience 13, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Woo E et al. (2021) Chronic Stress Weakens Connectivity in the Prefrontal Cortex: Architectural and Molecular Changes. Chronic Stress 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Magarifios AM et al. (1997) Chronic stress alters synaptic terminal structure in hippocampus. Proceedings of the National Academy of Sciences of the United States of America 94, 14002–14008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gould E and Tanapat P (1999) Stress and hippocampal neurogenesis. Biological Psychiatry 46, 1472–1479 [DOI] [PubMed] [Google Scholar]
- 91.Dayananda KK et al. (2023) Early life stress impairs synaptic pruning in the developing hippocampus. Brain, Behavior, and Immunity 107, 16–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chechik G et al. (1998) Synaptic Pruning in Development: A Novel Account in Neural Terms. Computational Neuroscience DOI: 10.1007/978-l-4615-4831-7_25 [DOI] [PubMed] [Google Scholar]
- 93.Gao W et al. (2015) Functional network development during the first year: Relative sequence and socioeconomic correlations. Cerebral Cortex 25, 2919–2928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rakesh D et al. (2021) Similar but distinct - Effects of different socioeconomic indicators on resting state functional connectivity: Findings from the Adolescent Brain Cognitive Development (ABCD) Study®. Developmental Cognitive Neuroscience 51, 101005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rakesh D et al. (2021) Associations Between Neighborhood Disadvantage, Resting-State Functional Connectivity, and Behavior in the Adolescent Brain Cognitive Development Study: The Moderating Role of Positive Family and School Environments. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 6, 877–886 [DOI] [PubMed] [Google Scholar]
- 96.Sripada C et al. (2022) Socioeconomic Resources are Associated with Distributed Alterations of the Brain’s Intrinsic Functional Architecture in Youth. Developmental Cognitive Neuroscience DOI: 10.1016/J.DCN.2022.101164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Marshall NA et al. (2018) Socioeconomic disadvantage and altered corticostriatal circuitry in urban youth. Human Brain Mapping 39, 1982–1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Barch DM et al. (2016) Effect of Hippocampal and Amygdala Connectivity on the Relationship Between Preschool Poverty and School-Age Depression. The American Journal of Psychiatry 173, 625–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hanson JL et al. (2019) A Family Focused Intervention Influences Hippocampal-Prefrontal Connectivity Through Gains in Self-Regulation. Child development 90, 1389–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Owens MM et al. (2020) Investigation of Psychiatric and Neuropsychological Correlates of Default Mode Network and Dorsal Attention Network Anticorrelation in Children. Cerebral Cortex 00, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sripada RK et al. (2014) Childhood poverty and stress reactivity are associated with aberrant functional connectivity in default mode network. Neuropsychopharmacology 39, 2244–2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rakesh D et al. (2021) Unraveling the Consequences of Childhood Maltreatment: Deviations From Typical Functional Neurodevelopment Mediate the Relationship Between Maltreatment History and Depressive Symptoms. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 6, 329–342 [DOI] [PubMed] [Google Scholar]
- 103.Truelove-hill M et al. (2020) A Multidimensional Neural Maturation Index Reveals Reproducible Developmental Patterns in Children and Adolescents. 40, 1265–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stevens MC (2016) The contributions of resting state and task-based functional connectivity studies to our understanding of adolescent brain network maturation. Neuroscience and Biobehavioral Reviews 70, 13–32 [DOI] [PubMed] [Google Scholar]
- 105.Dumontheil I (2016) Adolescent brain development. Current Opinion in Behavioral Sciences 10, 39–44 [Google Scholar]
- 106.Gozdas E et al. (2019) Developmental changes in functional brain networks from birth through adolescence. Human Brain Mapping 40, 1434–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rakesh D et al. (2021) Longitudinal changes in within-salience network functional connectivity mediate the relationship between childhood abuse and neglect, and mental health during adolescence. Psychological Medicine DOI: 10.1017/S0033291721003135 [DOI] [PubMed] [Google Scholar]
- 108.Kim D-JJ et al. (2019) Childhood poverty and the organization of structural brain connectome. NeuroImage 184, 409–416 [DOI] [PubMed] [Google Scholar]
- 109.Gellci K et al. (2019) Community and household-level socioeconomic disadvantage and functional organization of the salience and emotion network in children and adolescents. Neuroimage 184, 729–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Meaney MJ et al. (2007) Epigenetic mechanisms of perinatal programming of hypothalamic-pituitary-adrenal function and health. Trends In Molecular Medicine 13, 269–277 [DOI] [PubMed] [Google Scholar]
- 111.Olabi B and Seckl J (2011) Glucocorticoids, Developmental “Programming,” and the Risk of Affective Dysfunction. In Hormones In Neurodegeneration, Neuroprotection, and Neurogenesis, pp. 61–101, John Wiley & Sons, Ltd [Google Scholar]
- 112.Challis JRG et al. (2001) The fetal placental hypothalamic–pituitary–adrenal (HPA) axis, parturition and post natal health. Molecular and Cellular Endocrinology 185, 135–144 [DOI] [PubMed] [Google Scholar]
- 113.McGrath S and Smith R (2002) Prediction of preterm delivery using plasma corticotrophin-releasing hormone and other biochemical variables. Annals of Medicine 34, 28–36 [DOI] [PubMed] [Google Scholar]
- 114.Rakesh D et al. (2022) The role of school environment in brain structure, connectivity, and mental health in children – a multi-modal investigation. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging DOI: 10.1016/J.BPSC.2022.01.006 [DOI] [PubMed] [Google Scholar]
- 115.Dadvand P et al. (2018) The Association between Lifelong Greenspace Exposure and 3-Dimensional Brain Magnetic Resonance Imaging in Barcelona Schoolchildren. Environmental Health Perspectives 126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lanphear BP (2015) The Impact of Toxins on the Developing Brain. 10.1146/annurev-publhealth-031912-114413 36, 211–230 [DOI] [PubMed] [Google Scholar]
- 117.Whittle S et al. (2013) Childhood maltreatment and psychopathology affect brain development during adolescence. Journal of the American Academy of Child and Adolescent Psychiatry 52, 940–952.e1 [DOI] [PubMed] [Google Scholar]
- 118.Oakes JM and Rossi PH (2003) The measurement of SES in health research: Current practice and steps toward a new approach. Social Science and Medicine 56, 769–784 [DOI] [PubMed] [Google Scholar]
- 119.Merz EC et al. (2020) Socioeconomic Disparities in Language Input Are Associated With Children’s Language-Related Brain Structure and Reading Skills. Child Development 91, 846–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hackman DA et al. (2021) Association of Local Variation in Neighborhood Disadvantage in Metropolitan Areas with Youth Neurocognition and Brain Structure. JAMA Pediatrics DOI: 10.1001/jamapediatrics.2021.0426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ellwood-Lowe ME et al. (2016) The application of neuroimaging to social inequity and language disparity: A cautionary examination. Developmental Cognitive Neuroscience 22, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fields A et al. (2021) Adaptation in the face of adversity: Decrements and enhancements in children’s cognitive control behavior following early caregiving instability. Developmental science 24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mittal C et al. (2015) Cognitive adaptations to stressful environments: When childhood adversity enhances adult executive function. Journal of Personality and Social Psychology 109, 604–621 [DOI] [PubMed] [Google Scholar]
- 124.Ellis BJ et al. (2022) Hidden talents in harsh environments. Development and Psychopathology 34, 95–113 [DOI] [PubMed] [Google Scholar]
- 125.Dumornay NM et al. (2023) Racial Disparities in Adversity During Childhood and the False Appearance of Race-Related Differences in Brain Structure. AJP 180, 127–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Barch DM and Luby JL (2023) Understanding Social Determinants of Brain Health During Development. AJP 180, 108–110 [DOI] [PubMed] [Google Scholar]
- 127.Meghani SH and Chittams J (2015) Controlling for Socioeconomic Status in Pain Disparities Research: All-Else-Equal Analysis When “All Else” Is Not Equal. Pain Medicine 16, 2222–2225 [DOI] [PubMed] [Google Scholar]
- 128.Song X et al. (2022) Intergenerational income mobility table revisited: A trajectory group perspective. Research In Social Stratification and Mobility 80, 100713 [Google Scholar]
- 129.McLaughlin KA (2016) Future Directions in Childhood Adversity and Youth Psychopathology. Journal of Clinical Child and Adolescent Psychology 45, 361–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Evans GW and Kantrowitz E (2002) Socioeconomic status and health: the potential role of environmental risk exposure. Annu Rev Public Health 23, 303–331 [DOI] [PubMed] [Google Scholar]
- 131.Akee RKQ et al. (2010) Parents’ Incomes and Children’s Outcomes: A Quasi-Experiment. Am Econ J Appl Econ 2, 86–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Milligan K and Stabile M (2011) Do Child Tax Benefits Affect the Well-Being of Children? Evidence from Canadian Child Benefit Expansions. American Economic Journal: Economic Policy 3, 175–205 [Google Scholar]
- 133.Dahl GB and Lochner L (2012) The Impact of Family Income on Child Achievement: Evidence from the Earned Income Tax Credit. American Economic Review 102, 1927–1956 [Google Scholar]
- 134.Noble KG et al. (2021) Baby’s First Years: Design of a Randomized Controlled Trial of Poverty Reduction in the United States. Pediatrics DOI: 10.1542/PEDS.2020-049702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Campbell FA et al. (2001) The development of cognitive and academic abilities: Growth curves from an early childhood educational experiment. Developmental Psychology 37, 231–242 [DOI] [PubMed] [Google Scholar]
- 136.Diamond A et al. (2007) Preschool Program Improves Cognitive Control. Science 318, 1387–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bierman KL et al. (2008) Promoting academic and social-emotional school readiness: the head start REDI program. Child Dev 79, 1802–1817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Blair C and Raver CC (2014) Closing the Achievement Gap through Modification of Neurocognitive and Neuroendocrine Function: Results from a Cluster Randomized Controlled Trial of an Innovative Approach to the Education of Children in Kindergarten. PLoS ONE 9, e112393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Qiu A et al. (2017) Effects of Antenatal Maternal Depressive Symptoms and Socio-Economic Status on Neonatal Brain Development are Modulated by Genetic Risk. Cerebral Cortex 21, 3080–3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


