Abstract
Introduction:
The 2017 ACC/AHA blood pressure (BP) guideline redefined hypertension and lowered the BP treatment target. Empirical data on the guideline’s impact are needed.
Methods:
Data were analyzed from Atherosclerosis Risk in Communities (ARIC) Study participants who attended baseline pre-guideline (2016–2017) and post-guideline (2018–2019) visits with baseline systolic BP (SBP) between 120–159 mmHg. Participants were grouped according to baseline SBP by change in classification under the new guideline as follows: not reclassified (120–129 mmHg), reclassified to stage 1 hypertension (130–139 mmHg), and reclassified to stage 2 hypertension (140–159 mmHg). Means and 95% CIs for SBP changes between baseline and follow-up, changes in antihypertensive use, and percentages that achieved the post-guideline recommendation (SBP <130 mmHg) were calculated. Analyses were performed in 2021–2022.
Results:
Among 2,193 community-dwelling ARIC participants aged 71–95 years at baseline, SBP changes between baseline and follow-up visits differed among participants not reclassified (+4.1 [95% CI: 3.0, 5.3] mmHg), reclassified to stage 1 hypertension (−1.1 [−2.2, 0.1] mmHg), and reclassified to stage 2 hypertension (−5.7 [−6.8, −4.7] mmHg). Antihypertensive use changed from 77.3% to 78.4% (p=0.25) among participants reclassified to stage 1 hypertension and from 78.3% to 81.4% (p<0.01) among participants reclassified to stage 2 hypertension. At follow-up, 41.8% of the stage 1 and 22.4% of the stage 2 hypertension groups reached goal SBP <130 mmHg.
Conclusions:
There were small decreases in SBP and increases in antihypertensive therapy among older adults reclassified to stage 2 hypertension but not those reclassified to stage 1 hypertension by the 2017 ACC/AHA guideline.
Keywords: hypertension, blood pressure, guideline
Introduction
The 2017 American College of Cardiology/American Heart Association (ACC/AHA) Guideline for High Blood Pressure (BP) revised the cutoff for the diagnosis of hypertension to ≥130/80 from ≥140/90 mmHg. The target for treatment was also lowered to <130/80 from <140/90 mmHg once treatment is initiated.1 This downward shift in the hypertension diagnosis and treatment thresholds was based on the available observational and randomized clinical trial data that demonstrated lower rates of cardiovascular disease (CVD) at BP levels <130/80 mmHg.2–4 If full adherence to the new guideline recommendations for BP treatment would be achieved, simulation analyses suggest the potential to prevent hundreds of thousands of CVD events and deaths annually.5 However, nationally, rates of hypertension control declined between 2009 and March 2020 with 52.8% achieving the pre-2017 ACC/AHA Guideline level of <140/90mm Hg in 2009–2012 compared with 48.2% in 2017–2020.6,7
As older individuals derive the greatest mortality benefit of BP lowering,8 a goal systolic BP (SBP) <130 mmHg was recommended for all ambulatory, community-dwelling older adults (65 years or older).1 However, achieving this target in clinical practice may be challenging due to greater therapeutic inertia and concern for adverse events among older adults compared with younger adults.9 Empirical data on BP changes in community-based seetings before and after the release of the guidelines may inform quality improvement efforts to prevent cardiovascular morbidity and mortality in older adults. Therefore, this study sought to evaluate SBP changes among community-dwelling older adults in the Atherosclerosis Risk in Communities (ARIC) study who completed in-person visits in 2016–2017 (pre-guideline) and 2018–2019 (post-guideline).
Methods
Study Population
ARIC is a cohort study whose methods are described in detail elsewhere.10 In brief, the ARIC Study enrolled a cohort of 15,792 adults aged 45–65 in 1987 from four US communities (Washington County, MD; Forsyth County, NC; Jackson, MS; and Minneapolis, MN). Participants completed four study visits in the first decade of follow-up, which included detailed sociodemographic data collection and clinical examination, with interim telephone follow-ups. Additional in-person visits were conducted as the cohort reached older adulthood, including Visit 5 (2011–2013), Visit 6 (2016–2017), and Visit 7 (2018–2019); further annual visits are planned. All participants provided written informed consent at their local field centers, which were each locally approved to conduct the study. The Northwestern University IRB deemed this study not human research due to the secondary use of de-identified data.
ARIC participants who attended the 2016–2017 visit (pre-guideline) and 2018–2019 visit (post-guideline) were included in the primary analysis. Of 3,799 participants who attended the 2016–2017 visit, 839 did not attend the 2018–2019 visit. Those with missing SBP data (N= 21), with SBP <120 or ≥160 at the pre-guideline visit (N= 944), and with non-White and non-Black self-reported race (N= 2) were excluded (Appendix Figure 1). The SBP range was chosen to include participants who were recommended for more intensive BP control by the 2017 ACC/AHA Guideline between the pre-guideline and the post-guideline visit (SBP 130–159 mmHg), and a reference group which was not recommended for more intensive treatment by the guideline (SBP 120–129 mmHg). The final analytic sample included 2,193 participants.
Measures
The co-primary outcomes were change in mean SBP between the pre-guideline and the post-guideline visit and prevalence of controlled SBP (<130 mmHg) at the post-guideline visit. SBP at each examination was recorded as the average of the second and third of three measurements taken by the automated OMRON HEM-907XL sphygmomanometer,11 which was used in major BP trials such as SPRINT and ACCORD.12,13 BP measurement technique was consistent with AHA recommendations.14
Secondary outcomes included changes in the prevalence of antihypertensive medication use and the number of antihypertensive medications used. Participants were asked to bring in their medications at each study exam as well as self-report current medication use for common indications. Medication classes (defined by Medi-Span generic product identifier codes) were abstracted from the labels of medications that participants used within the past 4 weeks. Any antihypertensive medication use was defined according to the ARIC standard as either self-report of taking medication for BP (yes/no) or any current medication that lowers BP, including beta blockers, calcium channel blockers, renin-angiotensin-aldosterone system antagonists, diuretics, centrally acting sympatholytics, and vasodilators. Number of antihypertensive medications and use of first-line antihypertensives (angiotensin converting enzyme inhibitors, angiotensin receptor blockers, thiazide diuretics, and dihydropyridine calcium channel blockers) were also assessed, using only medication codes. Participants taking combination antihypertensives were considered as taking multiple antihypertensives (e.g., individuals taking lisinopril-hydrochlorothiazide had 2 antihypertensive medications).
Participants were categorized by baseline SBP at the pre-guideline visit into three SBP groups: (1) SBP 120–129 mmHg, which included those not reclassified by the 2017 ACC/AHA Guideline; (2) SBP 130–139 mmHg, which included those reclassified to stage 1 hypertension from prehypertension by the guideline; or (3) SBP 140–159 mmHg, which included those reclassified to stage 2 hypertension from stage 1 hypertension by the guideline. The groups with an SBP ≥130 mmHg at the pre-guideline visit represented those affected by the guideline change either with a new treatment target (stage 1 hypertension) or lower treatment target (stage 2 hypertension) of SBP <130 mmHg.
Statistical Analysis
Mean SBP was computed by baseline SBP group, and unadjusted changes in mean SBP from the pre-guideline visit (2016–2017) to the post-guideline visit (2018–2019) were estimated with paired t tests. As a complementary categorical measure, prevalence of controlled SBP (<130 mmHg) at the post-guideline visit was also computed (changes were not computed because at the pre-guideline visit, 100% of the not reclassified and 0% of the hypertension groups would be considered controlled). Changes in prevalence of antihypertensive medication use and number of antihypertensive medications used were computed using McNemar’s and Bowker’s tests, respectively. These analyses were restricted to participants with medication data available for both visits (99% for antihypertensive medication use and 97% for number of antihypertensive medications) due to the paired nature of the data.
In a secondary analysis, adjusted associations between baseline SBP group (SBP 120–129 mmHg or not reclassified, SBP 130–139 mmHg or reclassified to stage 1 hypertension, and SBP 140–159 mmHg or reclassified to stage 2 hypertension) and SBP change from the pre-guideline visit (2016–2017) to the post-guideline visit (2018–2019) were estimated using multivariable linear regression. These analyses were adjusted for traditional cardiovascular risk factors for participants with available data: age, sex, antihypertensive use, history of diabetes, total cholesterol, HDL cholesterol, smoking status, and history of CVD. In addition, a differences-in-differences approach was explored to account for a possible counterfactual trend that may have occurred in the absence of the 2017 guideline. However, using early pre-guideline data from 2011–2013, the parallel trends assumption was shown to be violated, and this analysis was not pursued further (see Appendix Methods).
Within the same study sample, analyses were repeated using two time periods both prior to the release of the guideline: an early pre-guideline visit (Visit 5: 2011–2013) as the baseline and the pre-guideline visit (Visit 6: 2016–2017) as the follow-up examination, in order to span the publication of the Eighth Joint National Committee (JNC 8) guideline15 in 2014 and the SPRINT trial12 in 2015. Because the natural histories of individuals with treated and untreated BP differ, subgroup analyses were conducted according to use of antihypertensive treatment at the baseline visit. Additional subgroups included stratification by sex (female and male), self-reported race (Black and White), diabetes at baseline, and history of CVD (coronary heart disease, stroke, or heart failure) at either baseline or follow-up. A further secondary analysis was conducted analyzing mean SBP changes among individuals with SBP ≥160 mmHg at baseline. In addition, a sensitivity analysis was conducted omitting participants who attended Visit 6 in November or December 2017, after the release of the guideline in November 2017 (N= 99).
All analyses were performed using Stata Version 15.1. A two-sided p-value <0.05 was considered statistically significant.
Results
Of 2,193 ARIC participants in the primary analytic sample, slightly more than half were women, and one in four self-identified as Black (Table 1). Mean (SD) age was 79.2 (4.6) years. Approximately three-quarters of participants were taking antihypertensives at baseline.
Table 1.
Baseline characteristics of included ARIC participants at the baseline pre-guideline visit (2016–2017)
| Characteristic | Overall | Not reclassified (SBP 120–129) | Reclassified to stage 1 hypertension (SBP 130–139) | Reclassified to stage 2 hypertension (SBP 140–159) |
|---|---|---|---|---|
| N | 2193 | 658 | 648 | 887 |
| Age (years), mean (SD) | 79.2 (4.6) | 78.8 (4.5) | 79.2 '4.5) | 79.6 (4.7) |
| Age category (years) | ||||
| 71–75 | 557 (25.4%) | 196 (29.8%) | 153 (23.6%) | 208 (23.4%) |
| 76–85 | 1383 (63.1%) | 394 (59.9%) | 426 (65.7%) | 563 (63.5%) |
| 86–95 | 253 (11.5%) | 68 (10.3%) | 69 (10.6%) | 116 (13.1%) |
| Sex | ||||
| Female | 1286 (58.6%) | 351 (53.3%) | 374 (57.7%) | 561 (63.2%) |
| Male | 907 (41.4%) | 307 (46.7%) | 274 (42.3%) | 326 (36.8%) |
| Race | ||||
| Black | 553 (25.2%) | 168 (25.5%) | 153 (23.6%) | 232 (26.2%) |
| White | 1640 (74.8%) | 490 (74.5%) | 495 (76.4%) | 655 (73.8%) |
| Education | ||||
| Less than high school | 267 (12.2%) | 79 (12.0%) | 66 (10.2%) | 122 (13.8%) |
| High school or some college | 875 (40.0%) | 252 (38.4%) | 268 (41.4%) | 355 (40.2%) |
| College graduate | 1046 (47.8%) | 326 (49.6%) | 313 (48.4%) | 407 (46.0%) |
| Household income | ||||
| <$25,000 | 499 (25.0%) | 160 (26.5%) | 144 (24.0%) | 195 (24.7%) |
| $25,000-$74,999 | 1036 (52.0%) | 303 (50.2%) | 305 (50.7%) | 428 (54.2%) |
| $75,000+ | 459 (23.0%) | 140 (23.2%) | 152 (25.3%) | 167 (21.1%) |
| History of CVD | 373 (17.0%) | 107 (16.3%) | 104 (16.0%) | 162 (18.3%) |
| History of Diabetes | 704 (32.8%) | 218 (33.8%) | 183 (28.7%) | 304 (35.1%) |
| Current smoking | 148 (6.9%) | 45 (7.0%) | 44 (6.9%) | 59 (6.8%) |
| Number of medications, | 9.0 (4.7) | 9.1 (4.8) | 9.0 (4.7) | 9.0 (4.5) |
| mean (SD) | ||||
| Hypertension treatment | 1677 (76.9%) | 486 (74.7%) | 500 (77.4%) | 691 (78.2%) |
| Total Cholesterol (mg/dL), mean (SD) | 174.8 (39.1) | 172.8 (40.1) | 172.9 (37.4) | 177.7 (39.4) |
| HDL-C Cholesterol (mg/dL), mean (SD) | 52.7 (14.1) | 52.2 (14.0) | 52.2 (13.8) | 53.4 (14.3) |
| eGFR (mL/min/1.73m2) | ||||
| <30 | 59 (2.8%) | 20 (3.1%) | 16 (2.6%) | 23 (2.7%) |
| 30–59 | 793 (37.5%) | 215 (33.8%) | 233 (37.2%) | 345 (40.5%) |
| 60–89 | 1131 (53.5%) | 358 (56.3%) | 345 (55.1%) | 428 (50.3%) |
| 90+ | 130 (6.2%) | 43 (6.8%) | 32 (5.1%) | 55 (6.5%) |
| Sport/exercise composite score,a mean (SD) | 2.6 (0.8) | 2.6 (0.8) | 2.6 (0.8) | 2.6 (0.8) |
| SPPB score,b mean (SD) | 9.2 (2.6) | 9.1 (2.6) | 9.3 (2.6) | 9.1 (2.7) |
| Chair stand,b mean (SD) | 2.2 (1.3) | 2.1 (1.3) | 2.3 (1.3) | 2.1 (1.3) |
| Summary balance score,b mean (SD) | 3.4 (1.1) | 3.4 (1.1) | 3.5 (1.1) | 3.4 (1.2) |
| 4 meter walk score,b mean (SD) | 3.6 (0.8) | 3.6 (0.8) | 3.6 (0.8) | 3.5 (0.8) |
| Grip strength (kg), mean (SD) | 25.9 (13.8) | 17.3 (13.3) | 35.6 (14.9) | 21.3 (4.2) |
| MMSE score,c mean (SD) | 27.8 (2.8) | 27.8 (3.0) | 27.9 (2.7) | 27.8 (2.6) |
| Dementia | 115 (5.3% ) | 41 (6.2%) | 34 (5.3%) | 40 (4.6%) |
| Moderate to severe hearing loss | 562 (27.2%) | 155 (25.4%) | 166 (26.8%) | 241 (28.9%) |
| CES-Depression scale,d mean (SD) | 2.5 (2.6) | 2.5 (2.7) | 2.5 (2.6) | 2.5 (2.6) |
Composite score ranging from 1 to 5 integrating intensity of and time spent exercising or playing sports during leisure time
Short physical performance battery score integrates three components scored 0 to 4 with a total score ranging from 0 to 12
Mini mental status examination score ranging from 0 to 30
Clinical Epidemiology Studies 11-item Depression scale ranging from 0 to 22
Data presented are N (%) unless otherwise specified. CVD: cardiovascular disease; SBP: systolic blood pressure; eGFR: estimated glomerular filtration rate
SBP changes from the pre-guideline (2016–2017) to post-guideline (2018–2019) visits were approximately normally distributed with a mean (SD) of −1.4 (16.1) mmHg (Appendix Figure 2). Changes in mean SBP (95% CI) were +4.1 (3.0, 5.3) mmHg among participants not reclassified (baseline SBP 120–129 mmHg), −1.1 (−2.3, 0.1) mmHg among participants reclassified to stage 1 hypertension (baseline SBP 130–139 mmHg), and −5.7 (−6.8, −4.7) mmHg among participants reclassified to stage 2 hypertension (baseline SBP 140–159 mmHg) (Table 2). Results were similar among the subset of participants not taking an antihypertensive at baseline, except there was no statistically significant change in mean SBP in the group not reclassified (baseline SBP 120–129 mmHg). Patterns were similar among subgroups defined by sex, self-reported race, diabetes at baseline, and absence of CVD at baseline and follow-up (Appendix Table 1), as well as in the sensitivity analysis excluding participants who attended Exam 6 in November or December 2017 (Appendix Table 2). By contrast, from the early pre-guideline to pre-guideline periods (Visit 5, 2011–2013 to Visit 6, 2016–2017), SBP increased in both the not reclassified and stage 1 hypertension groups (Appendix Table 3). Among participants with SBP ≥160 mmHg at baseline, mean SBP changed by −17.1 (−19.7, −14.5) mmHg (Appendix Table 4).
Table 2.
Mean systolic blood pressure change from the baseline pre-guideline (2016–2017) to the post-guideline (2018–2019) visit
| Group | Not reclassified (SBP 120–129) | Reclassified to stage 1 hypertension (SBP 130–139) | Reclassified to stage 2 hypertension (SBP 140–159) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-Guideline | Post-Guideline | Difference (95% CI) | Pre-Guideline | Post-Guideline | Difference (9 5% CI) | Pre-Guideline | Post-Guideline | Difference (95% CI) | |
| Overall | 124.7 | 128.9 | 4.1 (3.0, 5.3) | 134.7 | 133.6 | −1.1 (−2.3, 0.1) | 148.4 | 142.6 | −5.7 (−6.8, −4.7) |
| No antihypertensive | 124.7 | 126.4 | 1.7 (−0.2, 3.6) | 134.7 | 133.4 | −1.2 (−3.4, 0.9) | 147.5 | 143.7 | −3.8 (−6.0, −1.6) |
| Antihypertensive | 124.7 | 129.7 | 5.0 (3.6, 6.3) | 134.7 | 133.7 | −1.0 (−2.4, 0.3) | 148.6 | 142.3 | −6.3 (−7.6, −5.0) |
Data presented are in units of mmHg. SBP: systolic blood pressure
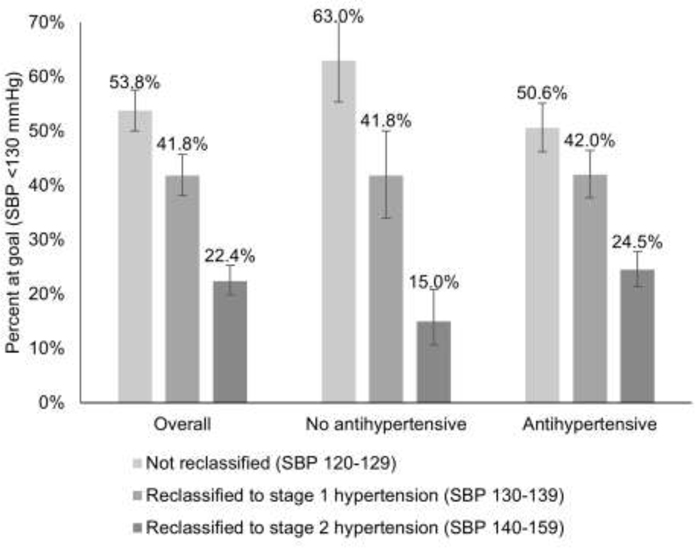
Attainment of the guideline goal of SBP <130 mmHg at follow-up (95% CI) was 53.8% (50.0, 57.6) in the not reclassified group, 41.8% (38.1, 45.7) in the stage 1 hypertension group, and 22.4% (19.8, 25.3) in the stage 2 hypertension group (Figure 1). Among participants not taking antihypertensives, the percentage at goal was higher in the not reclassified group and lower in the stage 2 hypertension group. Similar patterns were present by sex, self-reported race, diabetes, and absence of CVD (Appendix Figure 3). From the early pre-guideline (2011–2013) to pre-guideline (2016–2017) periods, a lower percentage of participants were at goal at follow-up in the not reclassified and stage 1 hypertension groups (Appendix Figure 4).
Figure 1. Percent of ARIC participants reaching goal systolic blood pressure at the post-guideline follow-up visit (2018–2019).
Participants were grouped by systolic blood pressure and use of antihypertensive therapy at the baseline pre-guideline visit (2016–2017). Percentages represent the percent of each subgroup that was at goal systolic blood pressure <130 mmHg at the post-guideline visit (2018–2019).
At baseline, use of any antihypertensive among the not reclassified, stage 1 hypertension, and stage 2 hypertension groups was 74.8%, 77.3%, and 78.3%, respectively, and use of a first-line antihypertensive was 64.5%, 64.9%, and 68.3% (Table 3). Approximately one-quarter of participants in all groups used each of 0, 1, 2, and 3 or more antihypertensives. At the post-guideline follow-up, there was a statistically significant increase in use of any antihypertensive medication among participants reclassified to stage 2 hypertension to 81.4%, and a statistically significant decrease in use of first-line antihypertensives among participants not reclassified to 60.4%, but no significant changes in antihypertensive medication use among participants reclassified to stage 1 hypertension. By contrast, there were statistically significant increases in antihypertensive use from the early pre-guideline to pre-guideline periods among all groups; for example, use of any antihypertensive increased among participants reclassified to stage 1 hypertension from 71.3% to 75.8% (Appendix Table 5).
Table 3.
Differences in use of antihypertensive medications, pre-guideline (2016–2017) to post-guideline (2018–2019) visits
| Characteristic | Not reclassified (SBP 120–129) | Reclassified to stage 1 hypertension (SBP 130–139) | Reclassified to stage 2 hypertension (SBP 140–159) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-Guideline | Post-Guideline | p-value for difference | Pre-Guideline | Post-Guideline | p-value for difference | Pre-Guideline | Post-Guideline | p-value for difference | |
| Any antihypertensivea | 74.8% | 74.9% | 0.86 | 77.3% | 78.4% | 0.25 | 78.3% | 81.4% | <0.001 |
| First-line antihypertensiveb,c | 64.5% | 60.4% | <0.001 | 64.9% | 66.7% | 0.10 | 68.3% | 70.1% | 0.08 |
| Number of antihypertensivesb | 0.003 | 0.09 | 0.002 | ||||||
| 0 | 25.6% | 25.9% | N/A | 23.1% | 21.9% | N/A | 22.5% | 19.8% | N/A |
| 1 | 22.0% | 24.0% | N/A | 23.8% | 24.6% | N/A | 21.7% | 23.4% | N/A |
| 2 | 23.9% | 25.0% | N/A | 30.1% | 28.1% | N/A | 27.9% | 26.5% | N/A |
| 3+ | 28.6% | 25.1% | N/A | 23.0% | 25.5% | N/A | 27.9% | 30.2% | N/A |
Based on self-report of taking an antihypertensive or objective antihypertensive medication use
Based on objective medication data only
Defined as angiotensin converting enzyme inhibitor, angiotensin receptor blocker, dihydropyridine calcium channel blocker, or thiazide diuretic
Note: Boldface indicates statistical significance. N/A: not applicable; SBP: systolic blood pressure
Among participants not taking antihypertensives at baseline, the proportion having initiated antihypertensive therapy at follow-up (95% CI) was 9.4% (5.7, 15.1) of those not reclassified and 20.3% (15.1, 26.7) of those reclassified to stage 2 hypertension (Appendix Table 6). However, among participants taking antihypertensives at baseline, the proportion having deintensified or stopped antihypertensive therapy at follow-up was 20.7% (17.3, 24.6) of those not reclassified and 16.6% (13.9, 19.6) of those reclassified to stage 2 hypertension. From the early pre-guideline to pre-guideline periods, a greater proportion of participants initiated antihypertensive therapy, while a similar proportion of participants deintensified or stopped antihypertensive therapy (Appendix Table 7).
Relative to the not reclassified group (baseline SBP 120–129), reclassification by the guideline in the stage 1 hypertension group (baseline SBP 130–139) was associated with a 5.2 (95% CI: −7.0, −3.5) mmHg lower SBP at follow-up compared to baseline, and in the stage 2 hypertension group (baseline SBP 140–159) was associated with a 10.0 (95% CI: −11.6, −8.3) mmHg lower SBP (Appendix Table 8). However, the same analysis conducted from the early pre-guideline to pre-guideline periods showed similar patterns.
DISCUSSION
Among older community-dwelling adults in the ARIC cohort aged 71–95 years, participants with SBP levels 130–139 mmHg at the pre-guideline visit, who were reclassified to Stage 1 hypertension based on the 2017 ACC/AHA guideline, had no statistically significant change in SBP at follow-up. Participants with SBP levels 140–159 mmHg at the baseline pre-guideline visit, who after the 2017 ACC/AHA guideline were reclassified to stage 2 hypertension, had a statistically significant decrease in SBP at follow-up by 6 mmHg. Results in the reclassified groups were similar whether or not participants were taking antihypertensives at baseline. Statistically significant but small increases in use of antihypertensive medications were observed only among those reclassified to Stage 2 hypertension and not among those reclassified to Stage 1 hypertension, despite the new recommendations released in between the visits by the 2017 ACC/AHA guideline to achieve a lower treatment target of SBP <130 mmHg. Less than half of the stage 1 hypertension (130–139 mmHg) and less than one-quarter of the stage 2 hypertension (140–159 mmHg) groups were at target SBP <130 mmHg at the follow-up post-guideline visit.
While participants with a pre-guideline visit SBP between 140–159 mmHg did have a statistically significant decrease in mean SBP and increase in antihypertensive medication use, the same pattern was observed in the pre-guideline period (2011–2013 to 2016–2017) suggesting this change was not a direct result of the guideline. Multivariable linear regression adjusted for traditional cardiovascular risk factors also showed similar associations of baseline group with changes in SBP between 2011–2013 to 2016–2017 and between 2016–2017 and 2018–2019. Taken together, these findings suggest the guideline release was not related to lower SBP levels or greater use of anti-hypertensive medications despite recommendations to intensify BP treatment with lower target SBP in this high-risk population. Further, in the pre-guideline period (2011–2013 to 2016–2017), during which the 2014 JNC 8 guideline was in effect recommending an SBP goal of 140–150 mmHg for older adults, SBP increased among participants with SBP 120–129 as well as those with SBP 130–139. This potentially indicates clinicians responded to a guideline that recommended de-intensification of antihypertensive therapy more readily than a later guideline that recommended intensification of therapy.
Another potential explanation for this null result is the 2017 American College of Physicians/American Academy of Family Practice hypertension guideline,16 which like JNC 8 recommends more liberal SBP targets of 140–150 mmHg for most older adults. Conflicting recommendations for hypertension treatment in older adults arise from data demonstrating higher risk of adverse events such as hypotension and syncope with intensification of treatment in older adults. While the absolute risk of syncope is low, and other symptomatic consequences of hypotension such as falls, fractures, kidney injury, and cognitive impairment are not increased due to BP lowering in most studies, concern by patients and clinicians may dissuade more intensive BP lowering.9,12,17 Concern for adverse effects may be higher among older adults with frailty, and further study is needed to establish the interaction between frailty and BP control.18
However, regardless of the guideline applied, appropriate intensification of antihypertensive therapy remains suboptimal.19 Improved implementation of guideline-directed BP control will require a multimodal approach as advised in the 2020 Surgeon General’s Call to Action to Control Hypertension.20 Clinical hypertension programs including the use of fixed-dose combination antihypertensives to decrease therapeutic inertia and pill burden resulted in large improvements in BP control in the Kaiser Permanente Northern California health system21,22 and could be potentially replicated in other settings. Significant racial and ethnic disparities in BP control reflect inequitable social and structural determinants of hypertension6,23–27 and will require investment in the built environment and health resources in communities most affected by hypertension. Innovative approaches integrating BP monitoring and hypertension treatment with patients’ homes and community spaces28–31 may help address disparities in access to care.
Limitations
This study has several limitations. First, given the lack of data on medication doses, participants may have changed the dosages of their existing BP medications at the follow-up exam and therefore intensified or de-intensified therapy to a greater extent than captured by our analysis. However, changes in doses would be expected to be reflected, in part, in the measured SBP. Second, by virtue of participation in a cardiovascular health study and receipt of BP interpretations after each study exam11 with encouragement to follow up with healthcare professionals for abnormal results, ARIC participants may be more engaged with their cardiovascular health than the general population; this may have led to earlier and more intensive antihypertensive treatment and would bias the analysis towards finding a significant difference in mean SBP and antihypertensive use. However, it is unknown whether participants had clinician visits between the exams included in our study because this question was not asked at the follow-up visit. Third, in this observational study design it is possible the results were influenced by external factors such as regression to the mean and publication of the SPRINT study; this was accounted for to the extent possible using stratification by groups plausibly affected by the new guideline, exclusion of outliers in SBP from the primary analysis, and secondary analysis of an earlier time period encompassing publication of SPRINT. Fourth, a second BP measurement several years after the 2017 guideline to better evaluate post-guideline trends was not available. Given the lead time that is required for uptake of new clinical guidelines, one potential explanation for our null results is insufficient time for participants to change BP management within the two-year period of the study. Future planned examinations in ARIC and other cohorts,32,33 as well as national data,34 may be useful to further evaluate BP trends in the period following the 2017 BP guideline, although further work will need to disentangle the negative effects of the COVID-19 pandemic on BP control.
Conclusions
Community-dwelling older adults in the ARIC study who were newly reclassified to stage 1 hypertension by the 2017 ACC/AHA guideline did not have significant improvement in SBP levels or intensification of antihypertensive therapy in the 2 years following release of the guideline compared with pre-guideline. Those reclassified to stage 2 hypertension, who were already recommended for antihypertensive therapy at baseline, had a significant decrease in SBP levels and small increase in antihypertensive therapy, which may not be related to the guideline.
Supplementary Material
Acknowledgements:
The authors thank the staff and participants of the ARIC study for their important contributions.
This study was supported by grants from the American Heart Association (#19TPA34890060) and NHLBI (HL161514, HL159250) to SSK.
The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (75N92022D00001, 75N92022D00002, 75N92022D00003, 75N92022D00004, 75N92022D00005).
The funding sponsors did not contribute to design and conduct of the study, collection, management, analysis, or interpretation of the data or preparation, review, or approval of the manuscript. The authors take responsibility for decision to submit the manuscript for publication. Dr. Khan had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
No financial disclosures have been reported by the authors of this paper.
Credit Statement
Michael C. Wang: Conceptualization, Methodology, Software, Writing – Original Draft. Lucia C. Petito: Methodology, Writing - Review & Editing. Lindsay R. Pool: Methodology, Writing - Review & Editing. Kathryn Foti: Methodology, Writing - Review & Editing. Stephen P. Juraschek: Methodology, Writing - Review & Editing. John W. McEvoy: Methodology, Writing - Review & Editing. Vijay Nambi: Methodology, Writing - Review & Editing. Mercedes R. Carnethon: Methodology, Writing - Review & Editing. Erin D. Michos: Conceptualization, Methodology, Writing - Review & Editing, Supervision; Sadiya S. Khan Conceptualization, Methodology, Writing - Review & Editing, Supervision, Funding acquisition.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. May 15 2018;71(19):e127–e248. doi: 10.1016/j.jacc.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 2.Karmali KN, Lloyd-Jones DM, van der Leeuw J, et al. Blood pressure-lowering treatment strategies based on cardiovascular risk versus blood pressure: A meta-analysis of individual participant data. PLoS Med. Mar 2018;15(3):e1002538. doi: 10.1371/journal.pmed.1002538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. Dec 14 2002;360(9349):1903–13. doi: 10.1016/s0140-6736(02)11911-8 [DOI] [PubMed] [Google Scholar]
- 4.Yano Y, Reis JP, Colangelo LA, et al. Association of Blood Pressure Classification in Young Adults Using the 2017 American College of Cardiology/American Heart Association Blood Pressure Guideline With Cardiovascular Events Later in Life. JAMA. Nov 6 2018;320(17):1774–1782. doi: 10.1001/jama.2018.13551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bundy JD, Mills KT, Chen J, Li C, Greenland P, He J. Estimating the Association of the 2017 and 2014 Hypertension Guidelines With Cardiovascular Events and Deaths in US Adults: An Analysis of National Data. JAMA Cardiol. Jul 1 2018;3(7):572–581. doi: 10.1001/jamacardio.2018.1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muntner P, Hardy ST, Fine LJ, et al. Trends in Blood Pressure Control Among US Adults With Hypertension, 1999–2000 to 2017–2018. JAMA. Sep 22 2020;324(12):1190–1200. doi: 10.1001/jama.2020.14545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muntner P, Miles MA, Jaeger BC, et al. Blood Pressure Control Among US Adults, 2009 to 2012 Through 2017 to 2020. Hypertension. May 26 2022:101161hypertensionaha12219222. doi: 10.1161/hypertensionaha.122.19222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs Standard Blood Pressure Control and Cardiovascular Disease Outcomes in Adults Aged ≥75 Years: A Randomized Clinical Trial. JAMA. Jun 28 2016;315(24):2673–82. doi: 10.1001/jama.2016.7050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss J, Freeman M, Low A, et al. Benefits and Harms of Intensive Blood Pressure Treatment in Adults Aged 60 Years or Older: A Systematic Review and Meta-analysis. Ann Intern Med. Mar 21 2017;166(6):419–429. doi: 10.7326/m16-1754 [DOI] [PubMed] [Google Scholar]
- 10.Wright JD, Folsom AR, Coresh J, et al. The ARIC (Atherosclerosis Risk In Communities) Study: JACC Focus Seminar 3/8. J Am Coll Cardiol. Jun 15 2021;77(23):2939–2959. doi: 10.1016/j.jacc.2021.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atherosclerosis Risk in Communities Study. Manual 2, Home and Field Center Procedures, ARIC Visit 7 Study Protocol. Accessed July 2, 2022, https://sites.cscc.unc.edu/aric/sites/default/files/public/manuals/MOP%202%20%20Home%20and%20Field%20Center%20Procedures_V7%20%20ver.%201.1%20%285.1.19%29.pdf
- 12.SPRINT Research Group. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373(22):2103–2116. doi: 10.1056/NEJMoa1511939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ACCORD Study Group. Effects of Intensive Blood-Pressure Control in Type 2 Diabetes Mellitus. N Engl J Med. 2010;362(17):1575–1585. doi: 10.1056/NEJMoa1001286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. Feb 8 2005;111(5):697–716. doi: 10.1161/01.CIR.0000154900.76284.F6 [DOI] [PubMed] [Google Scholar]
- 15.James PA, Oparil S, Carter BL, et al. 2014 Evidence-Based Guideline for the Management of High Blood Pressure in Adults: Report From the Panel Members Appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507–520. doi: 10.1001/jama.2013.284427 [DOI] [PubMed] [Google Scholar]
- 16.Clinical Guidelines Committee of the American College of Physicians and the Commission on Health of the Public and Science of the American Academy of Family Physicians. Pharmacologic Treatment of Hypertension in Adults Aged 60 Years or Older to Higher Versus Lower Blood Pressure Targets: A Clinical Practice Guideline From the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2017;166(6):430–437. doi: 10.7326/m16-1785%m28135725 [DOI] [PubMed] [Google Scholar]
- 17.Zhang W, Zhang S, Deng Y, et al. Trial of Intensive Blood-Pressure Control in Older Patients with Hypertension. N Engl J Med. 2021;385(14):1268–1279. doi: 10.1056/NEJMoa2111437 [DOI] [PubMed] [Google Scholar]
- 18.Benetos A, Petrovic M, Strandberg T. Hypertension Management in Older and Frail Older Patients. Circ Res. 2019;124(7):1045–1060. doi: 10.1161/CIRCRESAHA.118.313236 [DOI] [PubMed] [Google Scholar]
- 19.Chiu N, Chiu L, Aggarwal R, Raber I, Bhatt DL, Mukamal KJ. Trends in Blood Pressure Treatment Intensification in Older Adults With Hypertension in the United States, 2008 to 2018. Hypertension. 2022;0(0)doi:doi: 10.1161/HYPERTENSIONAHA.122.19882 [DOI] [PubMed] [Google Scholar]
- 20.U.S. Department of Health and Human Services. The Surgeon General’s Call to Action to Control Hypertension. U.S. Department of Health and Human Services, Office of the Surgeon General; 2020. [Google Scholar]
- 21.Jaffe MG, Lee GA, Young JD, Sidney S, Go AS. Improved Blood Pressure Control Associated With a Large-Scale Hypertension Program. JAMA. 2013;310(7):699–705. doi: 10.1001/jama.2013.108769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaffe MG, Young JD. The Kaiser Permanente Northern California Story: Improving Hypertension Control From 44% to 90% in 13 Years (2000 to 2013). J Clin Hypertens (Greenwich). Apr 2016;18(4):260–1. doi: 10.1111/jch.12803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hardy ST, Chen L, Cherrington AL, et al. Racial and Ethnic Differences in Blood Pressure Among US Adults, 1999–2018. Hypertension. Dec 2021;78(6):1730–1741. doi: 10.1161/hypertensionaha.121.18086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mujahid MS, Roux AVD, Cooper RC, Shea S, Williams DR. Neighborhood Stressors and Race/Ethnic Differences in Hypertension Prevalence (The Multi-Ethnic Study of Atherosclerosis). Am J Hypertens. 2011;24(2):187–193. doi: 10.1038/ajh.2010.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu EF, Rubinsky AD, Pacca L, et al. Examining Neighborhood Socioeconomic Status as a Mediator of Racial/Ethnic Disparities in Hypertension Control Across Two San Francisco Health Systems. Circ Cardiovasc Qual Outcomes. 2022;15(2):e008256. doi: 10.1161/CIRCOUTCOMES.121.008256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu Y, Liu Y, Dhingra LS, et al. National Trends in Racial and Ethnic Disparities in Antihypertensive Medication Use and Blood Pressure Control Among Adults With Hypertension, 2011–2018. Hypertension. Jan 2022;79(1):207–217. doi: 10.1161/hypertensionaha.121.18381 [DOI] [PubMed] [Google Scholar]
- 27.Fontil V, Pacca L, Bellows BK, et al. Association of Differences in Treatment Intensification, Missed Visits, and Scheduled Follow-up Interval With Racial or Ethnic Disparities in Blood Pressure Control. JAMA Cardiol. Feb 1 2022;7(2):204–212. doi: 10.1001/jamacardio.2021.4996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scirica BM, Cannon CP, Fisher NDL, et al. Digital Care Transformation: Interim Report From the First 5000 Patients Enrolled in a Remote Algorithm-Based Cardiovascular Risk Management Program to Improve Lipid and Hypertension Control. Circulation. Feb 2 2021;143(5):507–509. doi: 10.1161/circulationaha.120.051913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Victor RG, Lynch K, Li N, et al. A Cluster-Randomized Trial of Blood-Pressure Reduction in Black Barbershops. N Engl J Med. 2018;378(14):1291–1301. doi: 10.1056/NEJMoa1717250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheema E, Sutcliffe P, Singer DR. The impact of interventions by pharmacists in community pharmacies on control of hypertension: a systematic review and meta-analysis of randomized controlled trials. Br J Clin Pharmacol. Dec 2014;78(6):1238–47. doi: 10.1111/bcp.12452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pickering TG, Miller NH, Ogedegbe G, Krakoff LR, Artinian NT, Goff D. Call to action on use and reimbursement for home blood pressure monitoring: executive summary: a joint scientific statement from the American Heart Association, American Society Of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. Jul 2008;52(1):1–9. doi: 10.1161/hypertensionaha.107.189011 [DOI] [PubMed] [Google Scholar]
- 32.Bundy JD, Jaeger BC, Huffman MD, et al. Twenty-Five-Year Changes in Office and Ambulatory Blood Pressure: Results From the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Hypertens. May 22 2021;34(5):494–503. doi: 10.1093/ajh/hpaa189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osude N, Durazo-Arvizu R, Markossian T, et al. Age and sex disparities in hypertension control: The multi-ethnic study of atherosclerosis (MESA). Am J Prev Cardiol. Dec 2021;8:100230. doi: 10.1016/j.ajpc.2021.100230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paulose-Ram R, Graber JE, Woodwell D, Ahluwalia N. The National Health and Nutrition Examination Survey (NHANES), 2021–2022: Adapting Data Collection in a COVID-19 Environment. Am J Public Health. 2021;111(12):2149–2156. doi: 10.2105/ajph.2021.306517 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.