Abstract
Objective:
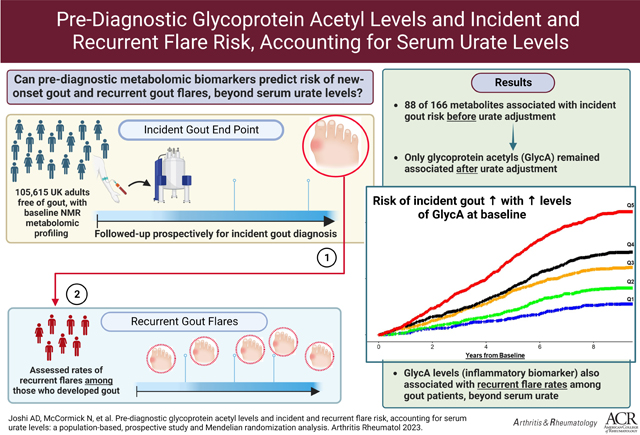
To prospectively investigate population-based metabolomics for incident gout and reproduce the findings for recurrent flares, accounting for serum urate.
Methods:
We conducted a pre-diagnostic metabolome-wide analysis among 105,615 UK Biobank participants with NMR metabolomic profiling (N=168 metabolites) from baseline blood samples (2006–2010), without history of gout. We calculated hazard ratios (HRs) for incident gout, adjusted for gout risk factors, excluding and including serum urate levels, overall and according to fasting duration of sampling. Potential causal effects were tested with two-sample Mendelian randomization. Poisson regression was used to calculate rate ratios (RR) for the association with recurrent flares among incident gout cases.
Results:
Correcting for multiple testing, 88 metabolites were associated with risk of incident gout (N=1303 cases) before urate adjustment, including glutamine and glycine (inversely), and lipids, branched-chain amino acids, and most prominently, glycoprotein acetyls (GlycA; P=9.17×10−32). Only GlycA remained associated following urate adjustment (HR=1.52 [95% CI: 1.22, 1.88] between extreme quintiles); the HR increased progressively with fasting duration, reaching 4.01 (1.36, 11.82) for ≥8 hours’ fasting. Corresponding HR per SD was 1.10 (1.04, 1.17) overall and 1.54 (1.21 to 1.96) for ≥8 hours’ fasting. GlycA levels were also associated with recurrent gout flares among incident gout cases (RR=1.90; 1.27, 2.85 between extreme quintiles) with larger associations with fasting. Mendelian randomization corroborated a potential causal role for GlycA on gout risk.
Conclusion:
This population-based prospective study implicates GlycA, a stable long-term biomarker reflecting neutrophil overactivity, in incident and recurrent gout flares (central manifestation from neutrophilic synovitis) beyond serum urate.
Graphical Abstract
INTRODUCTION
Gout, a metabolic condition causing the most common form of inflammatory arthritis, leads to excruciatingly painful flares and joint damage.(1, 2) With urate a causal metabolite,(3, 4) gout risk is affected by genetics as well as environmental factors,(5, 6) and its disease burden has risen globally,(7, 8) affecting 4% of US adults (>9 million)(1) with rising emergency room visits and hospitalizations.(9)
While substantial advances have been made over the past two decades in identifying these genetic and environmental risk factors for gout, fundamental questions remain in the progression to clinical gout,(3, 4) as prolonged hyperuricemia (HU) is necessary, but not sufficient (only 20% develop gout(10)). Similarly, the predictors for the risk of gout flares among gout patients beyond serum urate levels remain elusive. The metabolome, representing an intermediate trait between genome and phenome, is ideally suited for investigating disease mechanisms, predisposition, progression, and prediction,(11–13) especially for metabolic-inflammatory conditions like gout. A few prior, small-scale, cross-sectional studies of Asian men with preexisting gout implicated certain amino acids, including branched-chain amino acids (BCAAs).(14–19) While promising, it is unknown if these metabolites contribute to gout risk, or represent ‘markers’ of established disease (reverse causation from cross-sectional, prevalent case studies) or confounding (e.g., gout medications or lifestyle influence).(16–18) Importantly, none were population-based and their generalizability beyond Asian countries or males is unclear.
In this study, we first analyzed metabolomics data from a population-based cohort of >100,000 UK adults of European ancestry to prospectively identify novel metabolomic biomarkers associated with the future risk of incident gout (hypothesis-free, untargeted approach), and then reproduced the observed associations for recurrent flares (central target of clinical gout care) among the incident gout cases, accounting for serum urate levels. We also sought to prospectively evaluate metabolites that were associated with prevalent gout or hyperuricemia from those prior studies, in a hypothesis-driven, targeted approach.(14–16, 18–20) Finally, we corroborated our findings using Mendelian randomization analyses, leveraging genome-wide association study (GWAS) data from a European ancestry population, for a putative causal role of novel associations.
METHODS
Study Population
The UK Biobank resource (UKB) is a prospective cohort of more than 500,000 UK residents who were aged between 40 and 69 years upon enrollment (years 2006–2010).(21) Participants provided written informed consent and attended a baseline assessment where they provided information on sociodemographics, lifestyle exposures, medical history, and current medications through touch-screen questionnaires and an in-person interview; anthropometric measures were obtained, along with biospecimens for biomarker studies. These data were linked retrospectively and prospectively to primary care, hospitalization, and death records data. A subset of participants were invited to attend a follow-up visit over 2012–2013 at which additional data and biospecimens were obtained. The UK Biobank obtained ethical approval from the North West - Haydock Research Ethics Committee (16/NW/0274).
Assessment of metabolite levels
Details about the UKB blood collection,(21) and metabolomic biomarker assessment, quality control, and pre-processing procedures have been reported previously.(22, 23) Briefly, absolute concentrations of 168 nuclear magnetic resonance (NMR) metabolic biomarkers, including lipoprotein lipids, fatty acids, glycolysis metabolites, and amino acids were quantified from blood samples for a random sample of UKB participants, and have been made available for a total of 121,657 participants after quality control, including for 117,994 from the baseline measurement.
We performed a hypothesis-free, untargeted metabolome-wide study to identify novel biomarkers of incident gout among 105,615 participants of European ancestry with metabolomic biomarkers quantified at the baseline assessment, and no prior diagnosis of gout or urate lowering therapy use. For robustness of the untargeted metabolomic findings, we explored among a non-overlapping sample of European ancestry participants (n= 4,804) who had metabolomic biomarkers measured at the first follow-up assessment (average 4.2 years after the baseline assessment).(24) Furthermore, in a hypothesis-driven, targeted approach, we evaluated 8 metabolites implicated at least twice in prior studies (all from East Asia) based on prevalent cases (glycine, (14, 19) glutamine,(14, 18) BCAAs [leucine,(14, 16, 18) isoleucine,(14, 18, 19) valine(14–16)], alanine,(14, 15) tyrosine,(18, 20) and phenylalanine.(14, 16, 19) We required at least two prior reports for each target metabolite to minimize false positivity, given the small scale of these prior studies.(14–19) Nevertheless, stability of these metabolites is supported by their 10-year intraclass correlation coefficients (ICCs) (range, 0.39 ~ 0.64)(25) and these metabolites have been found associated with incident type 2 diabetes over a median 11.9 years of follow-up in the UKB.(26)
Ascertainment of incident and recurrent gout
In UKB, data on health-related conditions, including gout, were available by one or more of the following: self-report at the baseline or follow-up assessment, linkage to hospital inpatient records, linkage to primary care records, and linkage to death records. For the endpoint of incident gout, individuals were followed from the date of baseline assessment until the date of the first gout diagnosis recorded in any of the four modalities. The analysis for recurrent flares was performed among 835 incident gout cases from the first analysis whose individual primary care clinical diagnoses and prescription data were available to ascertain recurrent flares in UKB. Per a study recently conducted in the UK Clinical Practice Research Datalink,(27) recurrent flares were defined as (1) hospitalization with gout (ICD-10 M10) as the primary diagnosis; (2) primary care encounter with Read code for acute gout; or (3) primary care encounter with Read code for gout and prescription issued on the same day for corticosteroids, colchicine, or non-steroidal anti-inflammatory drugs (NSAIDs). Flare encounters occurring within 30 days of a previous flare encounter (or the date of gout diagnosis) were considered as part of the same episode.
Ascertainment of covariates
Covariates were ascertained from data collected at the UKB baseline assessment and linked medical records, and included age, sex, body mass index (BMI; kg/ m2, continuous),(28) smoking status, alcohol intake,(29) coffee intake,(30) red meat and poultry intake,(31) current use of diuretic medications,(28) prevalent diabetes and hypertension,(28) fasting time (categorical: < 4, 4 to 7, or ≥ 8 hours since last food or drink), and serum creatinine, urate, and high-sensitivity C-reactive protein (hs-CRP) levels (continuous). The latter three were measured on a Beckman Coulter AU5800 platform.(32) Creatinine levels were included as a measure of kidney function which could, in turn, impact serum urate levels.
Statistical Analysis
We conducted prospective analyses to examine associations between pre-diagnosis metabolome and risk of incident gout and to reproduce the findings for recurrent flares, accounting for serum urate. For the endpoint of incident gout, follow-up for each participant began 6 months after blood draw, to reduce chances of undiagnosed prevalent gout cases at blood draw, and ended at the occurrence of first recorded diagnosis of gout, death, or study period end (December 31, 2019), whichever came first. For the endpoint of recurrent gout, participants were followed from the date of first recorded gout diagnosis until death or end of study period. To test for associations between individual metabolites and gout risk, we used Cox Proportional hazards modeling to obtain hazard ratios (HRs) and 95% confidence intervals (CIs) comparing the highest and lowest quintiles of levels of each metabolite, as well as per standard deviation (SD) change in metabolite levels. Our multivariable model adjusted for age (continuous), sex, the first four genomic principal components, BMI, smoking status, frequency of alcohol intake, coffee intake, use of diuretics, serum creatinine levels (continuous), fasting time, history of diabetes, history of hypertension, and servings per week of red meat and poultry. We then additionally adjusted for serum urate levels; further adjustment for hs-CRP was also evaluated.
Of the 168 metabolites profiled in the UKB, two metabolites, creatinine (~4.7% missing) and 3-hydroxybutyrate (~1.5% missing), failed quality control based on >1% sample missingness. These two metabolites were dropped from subsequent analysis, though serum creatinine levels (assessed as baseline laboratory data) were retained as a covariate. Since missing values were present only for a very small proportion of UKB participants for the remaining 166 metabolites (ranging from 0% of values missing [for n=135 metabolites] to 0.28% of values missing for pyruvate), metabolite imputation was not undertaken, and each metabolite assessment was conducted only for participants with complete information.
For the metabolome-wide, hypothesis-free assessment of individual metabolites, we performed multiple comparisons correction using a principal components approach that accounts for the high correlation among metabolites, as done previously.(33, 34) We found 28 principal components explained 99.5% of the variation; as such, the number of effective tests was 28, and significance was met if P values were less than the Bonferroni-corrected α-threshold of 1.8×10−3 (0.05/28). For the hypothesis-driven, targeted analysis of the metabolites associated with prevalent cases, no multiple comparison correction was performed. As variability in metabolite measures may be affected by recent food intake,(35) we stratified by fasting time. We additionally conducted analyses stratified by sex. To test for associations between metabolites and recurrent gout rate, we used Poisson regression to obtain rate ratios (RR) and 95% CI comparing extreme quintiles and per-SD changes in levels of each metabolite identified in the above analysis, adjusting for the same covariates including serum urate.
Metabolites were grouped into clusters based on data-driven co-abundance patterns using weighted gene co‐expression network analysis (WGCNA).16 Co-abundance patterns were determined using Pearson correlation and modules were identified by unsupervised hierarchical clustering, with a soft thresholding power value of 6 (default values), followed by dynamic tree cutting specifying a minimum of 5 metabolites in a cluster. Functional enrichment for each module was determined by the ontology information/ class of majority of eigen-metabolites in each module.
Mendelian randomization
We used two-sample Mendelian randomization to assess the potential causal effects of the identified metabolites on gout risk. Summary statistics for the metabolites were sourced from a previously conducted GWAS of metabolic biomarkers in the UK Biobank (N=115,078 of European ancestry),(36) with estimates adjusted for sex, genotyping array and fasting time, and accounting for relatedness and population stratification. Summary statistics for gout were sourced from the largest-available GWAS of serum urate levels and gout (N=13,179 cases and 750,634 controls), adjusted for age, sex, and genetic principal components, and study-specific covariates.(37) The latter were generated from the Chronic Kidney Disease Genetics (CKDGen) consortium, which consisted of many European-ancestry cohorts, but not the UKB. Inverse-weighted variance (IVW) meta-analysis was used to assess the relationship between genetically predicted levels of the identified metabolites on gout risk. We assessed the presence of horizontal pleiotropy using the MR-Egger intercept test,(38) wherein the intercept represents the average pleiotropic effect. An intercept term that is significantly different from zero indicates the presence of unbalanced (directional) pleiotropy. The MR-PRESSO(39) test was then used to generate an outlier-corrected IVW estimate. Analyses were performed in R Studio using the TwoSampleMR package and the MR-Base portal.(40)
RESULTS
Our overall metabolome-wide analysis included 105,615 UKB participants (45% male, mean age 56.7 years at baseline) with no history of gout or current urate-lowering therapy use. Their baseline characteristics, as well as those for individuals who developed incident gout during the follow up, are summarized in Table 1. As expected, individuals who developed gout were characterized by older age at baseline, male predominance, higher urate and creatinine levels, cardiometabolic comorbidities, greater alcohol and red meat intake, and diuretic use. The mean and median time between participant’s last food or drink consumption and the blood draw were 3.75 and 3 hours, respectively, with 47,271 (45%) fasting for ≥ 4 hours, 11,216 (11%) for ≥ 6 hours, and 4,126 (4%) for ≥ 8 hours (Supplementary Table 1).
Table 1.
Baseline characteristics of study participants, by cohort
| Individuals without Gout at Baseline | Individuals with Incident Gout during Follow Up | |
|---|---|---|
|
| ||
| N | 105,615 | 835 |
| Male, n (%) | 47,216 (44.7) | 636 (76.2) |
| Age (years), Mean (SD) | 56.7 (8.1) | 58.6 (7.5) |
| BMI (kg/ m 2 ), Mean (SD) | 27.3 (4.7) | 29.9 (5.0) |
| Serum Urate (μmol/L), Mean (SD) | 308 (77.4) | 427 (87.8) |
| Creatinine (μmol/L), Mean (SD) | 72.0 (16.5) | 82.6 (17.4) |
| Hours of fasting, Mean (SD) | 3.75 (2.4) | 3.81 (2.4) |
| Smoking status, n (%) | ||
| Never | 56,849 (53.8) | 353 (42.3) |
| Former | 37,631 (35.6) | 409 (49.0) |
| Current | 11,135 (10.5) | 73 (8.7) |
| Alcohol consumption (frequency), n (%) | ||
| Never | 7087 (6.7) | 46 (5.5) |
| Special occasions only | 11,292 (10.7) | 59 (7.1) |
| One to three times a month | 12,059 (11.4) | 60 (7.2) |
| Once or twice a week | 28,027 (26.5) | 203 (24.3) |
| Three or four times a week | 25,292 (23.9) | 224 (26.8) |
| Daily or almost daily | 21,858 (20.7) | 243 (29.1) |
| Coffee consumption (cups/day) | ||
| 0 | 22,126 (20.9) | 187 (22.4) |
| 1–2 | 40,870 (38.7) | 316 (37.8) |
| 3–5 | 27,997 (26.5) | 225 (26.9) |
| >5 | 6943 (6.6) | 45 (5.4) |
| Missing/ Other | 7679 (7.3) | 62 (7.4) |
| Diabetes, n (%) | 4878 (4.6) | 69 (8.3) |
| Hypertension, n (%) | 10,278 (9.7) | 246 (29.5) |
| Red meat intake (servings/day) | ||
| ≤ 0.5 | 55,835 (52.9) | 361 (43.2) |
| > 0.5 | 49,780 (47.1) | 474 (56.8) |
| Poultry intake (servings/day) | ||
| ≤ 0.25 | 54,939 (52.0) | 427 (51.1) |
| > 0.25 | 50,676 (48.0) | 408 (48.9) |
| Diuretic use, n (%) | 1961 (1.9) | 54 (6.5) |
n, number; SD, standard deviation
Metabolites associated with risk of incident gout
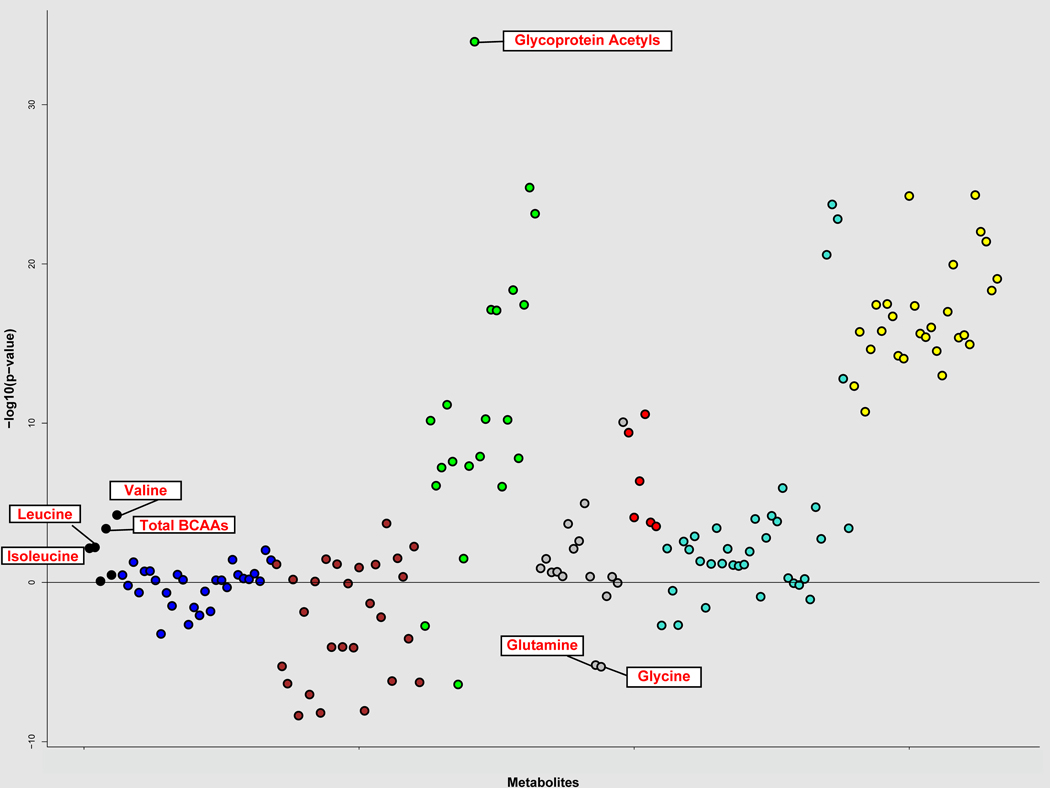
We documented 1303 cases of incident gout over a mean follow-up of 10.4 years. In our hypothesis-free, untargeted analysis, after correcting for multiple comparison, 88 individual metabolite markers were associated with incident gout from the multivariable models before adjusting for serum urate levels (Supplementary Table 2). There were seven distinct modules of interconnected metabolites with high topological overlap (Figure 1); the major functional classes consisted of triglycerides/very low-density lipoproteins (VLDL), high-density lipoprotein, cholines, and BCAAs. Among individual metabolites, glycine and glutamine were inversely associated with gout risk; glycoprotein acetyls showed the strongest association (P=1.66 × 10−21 for extreme quintile categorical comparison and 9.17 × 10−32 for per SD) (Figures 1 and 2 and Supplementary Table 2). Additionally, in our targeted approach for the metabolites previously identified by prior small cross-sectional studies, pre-diagnosis levels of isoleucine, leucine, valine, glycine, and glutamine were associated with the risk of incident gout (P <0.05), whereas alanine, phenylalanine, and tyrosine were not (Supplementary Table 2).
Figure 1. Association of individual metabolites with incident gout risk, by weighted correlation network analysis (WGCNA) modules.
These associations are adjusted for all covariates as in Table 1, except for serum urate levels. Glycoprotein acetyls showed the strongest association (P= 9.17 × 10−32 for per SD). The metabolites associated in our hypothesis-driven, targeted analysis are also marked with labels; glycine and glutamine were inversely associated with gout risk, whereas branched chain amino acids (BCAAs) were positively associated (see Results for details).
Black=branched chain amino acids; Blue=low density lipoproteins; Maroon=high-density lipoproteins; Green=very low-density lipoproteins and glycoprotein acetyls; Red=cholines; Teal=cholesterols, monounsaturated acids, polyunsaturated acids, and small very low-density lipoproteins; Yellow=triglycerides, chylomicrons, and large very low- density lipoproteins. Grey=unclassified. Total BCAAs, total concentration of branched chain amino acids (leucine + isoleucine + valine).
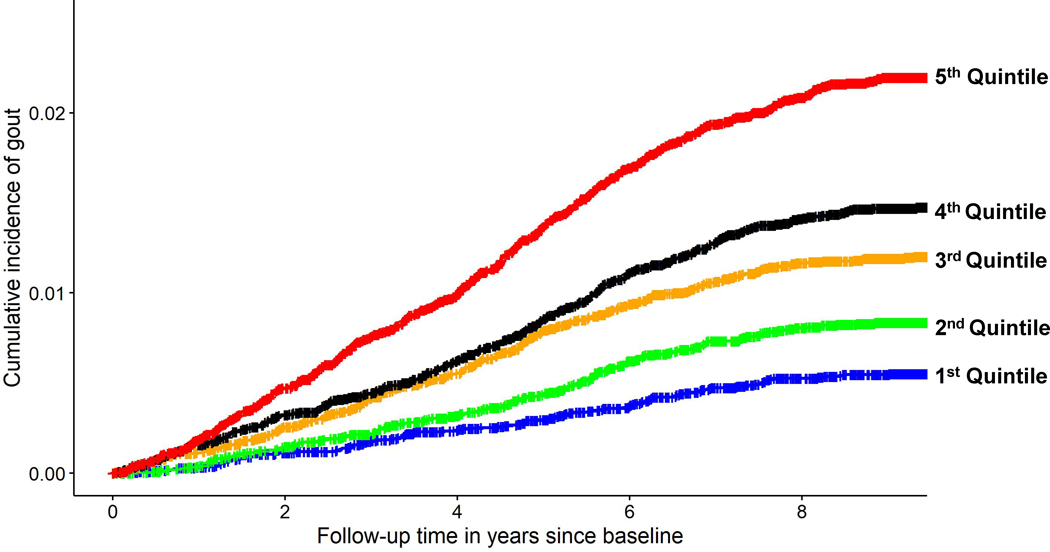
Figure 2. Cumulative incidence of gout according to quintiles of glycoprotein acetyl levels.
The range of values was as follows: first quintile, < 0.69 mmol/l; second quintile, ≥ 0.69 to 0.75 mmol/l; third quintile, ≥ 0.75 to 0.81 mmol/l; fourth quintile, ≥ 0.81 to 0.88 mmol/l; fifth quintile ≥ 0.88 mmol/l.
Upon further adjustment for serum urate levels, glycoprotein acetyls (GlycA) were the only metabolite significantly associated with the risk of incident gout, applying multiple testing criteria (Supplementary Table 2). The HR for incident gout comparing extreme quintiles of GlycA levels was 2.80 (95% CI: 2.27 to 3.47) before adjustment for urate levels and 1.52 (1.22 to 1.88) after adjustment; the corresponding HRs per SD were 1.38 (1.30 to 1.41) and 1.10 (1.04 to 1.17) (Table 2). These associations did not change materially with additional adjustment for hs-CRP level, which itself was not associated with the risk of gout in the same model (HR per mg/L = 1.00; 95% CI, 0.99 to 1.02). In the nonoverlapping follow-up cohort (N=4804), the HR of GlycA for the risk of incident gout was 1.56 (95% CI, 1.08 to 2.25) per SD before urate adjustment and 1.35 (0.89 to 2.02) after urate adjustment.
Table 2.
Associations of glycoprotein acetyls with risk of incident gout in the UK Biobank, overall and by fasting time
| Model | Cases, N | Cases Per 1000 PYs | Multivariable HR, (95% CI) | P | Multivariable HR, (95% CI), with serum urate adjustment | P |
|---|---|---|---|---|---|---|
| Overall (N=105,615) | 1303 | 1.22 | ||||
|
| ||||||
| Categorized as quintiles: | ||||||
| Q1 | 115 | 0.53 | 1.0 Ref | - | 1.0 Ref | - |
| Q2 | 175 | 0.81 | 1.34 (1.06 to 1.70) | 0.01515 | 1.20 (0.95 to 1.52) | 0.130 |
| Q3 | 252 | 1.17 | 1.74 (1.39 to 2.17) | 7.64 × 10−07 | 1.36 (1.09 to 1.70) | 6.50 × 10−3 |
| Q4 | 309 | 1.45 | 2.02 (1.62 to 2.51) | 2.68 × 10−10 | 1.43 (1.15 to 1.77) | 1.43 × 10−3 |
| Q5 | 452 | 2.14 | 2.80 (2.27 to 3.47) | 1.66 × 10−21 | 1.52 (1.22 to 1.88) | 1.48 × 10−4 |
|
| ||||||
| Per Standard deviation: | - | - | 1.38 (1.30 to 1.41) | 9.17 × 10−32 | 1.10 (1.04 to 1.17) | 6.54 × 10−4 |
|
| ||||||
| Fasting ≥ 4 hours (N=47,271) | 603 | 1.27 | ||||
|
| ||||||
| Categorized as quintiles: | ||||||
| Q1 | 45 | 0.50 | 1.0 Ref | - | 1.0 Ref | - |
| Q2 | 74 | 0.79 | 1.45 (1.00 to 2.11) | 0.049 | 1.29 (0.89 to 1.88) | 0.18 |
| Q3 | 115 | 1.19 | 1.94 (1.37 to 2.75) | 0.0002 | 1.48 (1.05 to 2.11) | 0.025 |
| Q4 | 151 | 1.56 | 2.39 (1.71 to 3.36) | 4.05E-07 | 1.71 (1.22 to 2.40) | 0.002 |
| Q5 | 218 | 2.25 | 3.27 (2.35 to 4.56) | 2.28E-12 | 1.72 (1.22 to 2.40) | 0.002 |
|
| ||||||
| Per Standard deviation: | - | - | 1.42 (1.31 to 1.53) | < 0.01 | 1.14 (1.05 to 1.23) | 0.002 |
|
| ||||||
| Fasting ≥ 6 hours (N= 11,216) | 158 | 1.40 | ||||
|
| ||||||
| Categorized as quintiles: | ||||||
| Q1 | 8 | 0.35 | 1.0 Ref | - | 1.0 Ref | - |
| Q2 | 17 | 0.76 | 1.93 (0.83 to 4.48) | 0.12 | 1.69 (0.73 to 3.94) | 0.22 |
| Q3 | 29 | 1.28 | 3.01 (1.37 to 6.61) | < 0.01 | 2.19 (0.99 to 4.84) | 0.05 |
| Q4 | 46 | 2.03 | 4.60 (2.15 to 9.84) | < 0.01 | 2.97 (1.39 to 6.38) | 0.005 |
| Q5 | 58 | 2.61 | 5.83 (2.73 to 12.45) | < 0.01 | 3.01 (1.39 to 6.49) | 0.005 |
|
| ||||||
| Per Standard deviation: | - | - | 1.62 (1.40 to 1.89) | 3.58 × 10−10 | 1.31 (1.11 to 1.54) | 0.0014 |
|
| ||||||
| Fasting ≥ 8 hours (N= 4,126) | 75 | 1.77 | ||||
|
| ||||||
| Categorized as quintiles: | ||||||
| Q1 | 4 | 0.49 | 1.0 Ref | - | 1.0 Ref | - |
| Q2 | 7 | 0.87 | 1.62 (0.74 to 3.53) | 0.60 | 1.36 (0.39 to 4.71) | 0.80 |
| Q3 | 12 | 1.38 | 2.41 (1.30 to 4.47) | 0.13 | 1.54 (0.49 to 4.89) | 0.44 |
| Q4 | 22 | 2.48 | 4.45 (2.71 to 7.32) | 0.006 | 2.86 (0.97 to 8.48) | 0.05 |
| Q5 | 30 | 3.49 | 6.90 (4.35 to 10.95) | 0.0004 | 4.01 (1.36 to 11.82) | 0.01 |
|
| ||||||
| Per Standard deviation: | - | - | 1.77 (1.42 to 2.22) | 6.28 × 10−7 | 1.54 (1.21 to 1.96) | 0.0003 |
N, number; HR, hazard ratio; PY, person-years
The associations between GlycA levels comparing extreme quintiles and risk of incident gout increased with fasting duration, from 1.72 (1.22 to 2.40) for ≥ 4 hours to 3.01 (1.39 to 6.49) for ≥ 6 hours, and 4.01 (1.36 to 11.82) for ≥ 8 hours of fasting, respectively. The corresponding HRs per SD were 1.14 (1.05 to 1.23), 1.31 (1.11 to 1.54), and 1.54 (1.21 to 1.96) for ≥ 4, 6, and 8 hours’ fasting, respectively.
There were 1014 cases of incident gout among males (2.14 per 1000 person-years) and 289 among females (0.48 per 1000 person-years). The fully adjusted HR for incident gout among males was 1.30 (1.03 to 1.65) comparing extreme quintiles of GlycA, and 1.06 (0.99 to 1.13) per SD. As with the overall result, the effect estimates were larger among males with greater fasting duration, with HRs of 1.53 (1.06 to 2.22), 2.55 (1.11 to 5.86), and 4.48 (1.31 to 15.39) with ≥ 4, ≥ 6, and ≥ 8 hours of fasting, respectively. The corresponding HRs among females were 2.70 (1.46 to 5.01) comparing extreme quintiles and 1.24 (1.11 to 1.40 per SD; P for interaction by sex=0.002.
Metabolites associated with recurrent gout flares
For the analysis of recurrent gout flares, 835 incident gout cases were eligible (76 % male, mean age 58.6 years at baseline) (Table 1), among whom 316 recurrent flares were documented over a mean 3.8 years of follow-up. Adjusting for covariates, including serum urate, GlycA levels were associated with recurrent gout flares, with RR of 1.90 (1.27 to 2.85) comparing extreme quintiles, and 1.20 (1.06 to 1.35) per SD (Table 3). These associations did not change materially with additional adjustment for hs-CRP level, which itself was not associated with the risk of recurrent flares in the same model (HR per mg/L = 1.01; 95% CI, 0.99 to 1.03). As with the incident gout endpoint, the effect estimates were larger among those with greater fasting duration, with corresponding RRs of 3.29 (1.66 to 6.54) and 1.37 (1.13 to 1.65) per SD among those fasting ≥ 4 hours (Table 3).
Table 3.
Associations of glycoprotein acetyls with rate of recurrent gout flare
| Model | N Flares | Flares Per 1000 PYs | Multivariable RR, (95% CI) | P | Multivariable RR, (95% CI), with serum urate adjustment | P |
|---|---|---|---|---|---|---|
| Overall (N= 835) | 316 | 100.3 | ||||
|
| ||||||
| Categorized as quintiles: | ||||||
| Q1 | 38 | 62.8 | 1.0 | - | 1.0 | - |
| Q2 | 61 | 93.6 | 1.33 (0.88 to 2.00) | 0.18 | 1.30 (0.86 to 1.96) | 0.21 |
| Q3 | 62 | 98.2 | 1.56 (1.03 to 2.37) | 0.03 | 1.51 (1.00 to 2.28) | 0.05 |
| Q4 | 69 | 111.0 | 1.75 (1.16 to 2.63) | 0.01 | 1.67 (1.11 to 2.51) | 0.01 |
| Q5 | 86 | 134.6 | 2.02 (1.35 to 3.02) | <0.001 | 1.90 (1.27 to 2.85) | 0.002 |
|
| ||||||
| Per Standard deviation: | - | - | 1.23 (1.09 to 1.38) | 0.001 | 1.20 (1.06 to 1.35) | 0.003 |
|
| ||||||
| Fasting ≥ 4 hours (N=364) | 127 | 93.5 | ||||
|
| ||||||
| Categorized as quintiles: | ||||||
| Q1 | 13 | 52.4 | 1.0 | - | 1.0 | - |
| Q2 | 18 | 73.9 | 1.56 (0.75 to 3.22) | 0.23 | 1.51 (0.73 to 3.13) | 0.27 |
| Q3 | 26 | 90.9 | 1.89 (0.94 to 3.81) | 0.07 | 1.83 (0.91 to 3.69) | 0.09 |
| Q4 | 22 | 78.5 | 1.88 (0.91 to 3.90) | 0.09 | 1.79 (0.85 to 3.74) | 0.12 |
| Q5 | 48 | 159.9 | 3.50 (1.78 to 6.88) | 0.00 | 3.29 (1.66 to 6.54) | <0.001 |
|
| ||||||
| Per Standard deviation: | - | - | 1.39 (1.16 to 1.68) | <0.001 | 1.37 (1.13 to 1.65) | 0.001 |
N, number; PY, person-years; RR, rate ratio
Mendelian randomization
The sixty-one SNPs associated with GlycA levels were pruned for linkage disequilibrium at a threshold of r2 < 0.001, leaving 58 independent SNPs; two palindromic SNPs were also excluded. As such, the genetic instrument for GlycA levels was comprised of 56 SNPs. Genetically predicted GlycA levels were associated with an increased risk of gout, with OR 1.52 (1.22 to 1.89) per SD change in GlycA. The MR-Egger intercept was 0.001 (p-value=0.91), consistent with the null hypothesis of no directional pleiotropy. Upon removal of the five SNPs identified by the MR-PRESSO test, the outlier-corrected OR was 1.19 (1.02 to 1.40) per SD change in GlycA.
DISCUSSION
In this first prospective population-based metabolomics study of over 100,000 UK individuals, with 1303 new gout cases documented over 10 years, we identified distinctive metabolites in pre-diagnostic plasma samples that were associated with incident gout, including glutamine and glycine (inversely), and lipids, BCAAs, and GlycA, which showed by far the most prominent association (Figure 1). In particular, GlycA (a stable inflammatory marker reflecting neutrophil overactivity(41–45)) predicted incident gout beyond serum urate levels. The association was progressively larger with increasing fasting time. Furthermore, GlycA was also associated with recurrent flares (central clinical target) among the same participants after they developed gout, and this association also was larger with fasting. Finally, Mendelian Randomization results corroborated a causal relationship between GlycA levels and gout risk. These findings may provide insight into its metabolic-neutrophilic synovitis pathways, with implications for biomarker-based flare prediction or therapeutic targets.
The GlycA is an NMR-derived novel biomarker of systemic inflammation, representing the integrated concentration and glycosylation of acute phase proteins (predominantly alpha-1- acid glycoprotein, haptoglobin, and alpha-1-antitrypsin) released in the inflammatory state.(41) Transcriptionally GlycA levels corresponded to a gene coexpression network for neutrophil activities, with most genes in the preserved module for peptides originating from neutrophil granules.(42) To that end, neutrophilic synovitis is the hallmark of a gout flare, which result from an innate immune response against monosodium urate crystal deposits, where macrophage crystal phagocytosis activates NLRP3 inflammasome, leading to the release of pro-inflammatory cytokines (e.g., IL-1ß, IL-6, and IL-8), attracting and stimulating blood neutrophils, including neutrophil extracellular traps (NETosis). GlycA has also been found to predict the risk of type 2 diabetes and cardiovascular diseases, independently of CRP,(43, 44) suggesting it may capture different inflammatory pathways.(45) Furthermore, GlycAs were better able to predict the early development of adverse cardiovascular disease risk profiles in young adults when compared with CRP.(45) To that end, GlycA and CRP’s representation of inflammatory burden appears to be analogous to HbA1c and fasting glucose for assessing blood sugar control, the former more representative of longer-term cumulative exposure, and the latter sensitive to acute changes.(45) We observed CRP was not independently associated with the risk of gout incidence or recurrent flares among gout patients, in contrast to GlycA, supporting the specific relevance of our findings. Furthermore, our Mendelian Randomization results supported a causal relationship between GlycA levels and gout risk.
The magnitude of association between GlycA levels and the risk of both incident and recurrent gout grew with longer fasting time. This suggests food intake impacts variability in glycoprotein levels, creating misclassification of its true usual level, and that the signal to noise ratio may be higher among fasting samples. Further research or potential application for prediction would be served better with fasting samples particularly for prospective sample collections. The stronger association among females reminded us of many differences in female gout, including sex hormone influence as well as higher prevalence of cardiometabolic and kidney comorbidities. Also, female gout predominantly affects postmenopausal and elderly women, which might contribute to the observed difference from males. Furthermore, it remains conceivable that differential neutrophile overactivity and associated systemic inflammatory propensity might explain the stronger associations among women.
While we primarily sought to identify metabolites beyond serum urate levels for disease mechanisms, progression, and recurrent flares, 88 metabolites were also associated with incident gout risk before urate adjustment, including glutamine and glycine (inversely), and lipids, and BCAAs. The nullification of the associations after urate adjustment suggests that these metabolites are upstream to serum urate in the causal pathway or predict gout risk fully by their association with serum urate levels. Prior epidemiologic studies employing conventional lipid profile data have reported associations with gout, although statin use has not been associated with lower serum urate or gout risk. More recently, small-scale, cross-sectional metabolomics studies of prevalent gout cases from China and Japan, with all but one employing mass spectrometry (as opposed to NMR), have also reported associations with various lipids, as well as certain amino acids including glutamine, glycine, and BCAAs.(14–19) In this prospective population-based analysis of pre-diagnosis metabolomics, we confirmed the positive association between BCAA levels and incident gout, and inverse associations for glutamine and glycine. BCAA levels have also been implicated in insulin resistance, obesity, type 2 diabetes, and cardiovascular sequalae(46–48) To that end, BCAA levels have been found amenable to lifestyle modifications in clinical trials.(49) Furthermore, several small-scale glycine supplementation trials found improvements in insulin sensitivity,(50) which in turn can lower serum urate levels.(51, 52) Finally, randomized controlled trials have found glutamine supplementation decreased the inflammatory response and abolished autophagy responses in cancer patients receiving radiotherapy,(53) and also reduced sickle-cell pain crises presumably by reducing oxidative stress.(54) Whether these presumed mechanisms and observed benefits are relevant to gout risk remains to be clarified.
Strength and limitations
Our study prospectively followed a large number of individuals without gout at baseline, tracing the occurrence of new diagnosis of gout in relation to pre-diagnosis metabolomics from the samples at baseline, with a six-month lag period. As such, the temporal relation between exposure and outcome was clear, unlike prior small-scale, cross-sectional studies of pre-existing gout patients, all from east Asian countries. (14–16, 18, 19) While we replicated some of the findings as discussed above, we found no associations with alanine, phenylalanine, and tyrosine, ones observed in those small-scale, cross-sectional Asian studies.(14, 15, 19) However, given the temporal ambiguity, as well as the potential for confounding associated with prevalent cases (e.g., impact of gout related medications), and selection bias, findings from those prior studies must be interpreted more cautiously. Furthermore, the association with recurrent gout among those with incident gout in our study tended to be stronger, which corroborated the prior observation that GlycA was stable within individuals up to 10 years.(42) Although we adjusted for known clinical and environmental risk factors, as with any observational study, our findings are subject to residual or unmeasured confounding. Conversely, the cardiometabolic sequelae adjusted in our model such as diabetes, hypertension, and kidney function, as well as others not included in our model, could also be causal intermediates, potentially underestimating the effect. While UKB participants tended to have a better socioeconomic position than the UK general population, with better health status and behaviours,(55) the biologic associations between metabolome and gout risk would likely be generalizable to other European ancestry populations.(55) While we sought to fill the gap in metabolomics evidence for gout among European ancestry population (beyond Asian(14–19)), by validly taking advantage of European metabolomics GWAS data(36) for Mendelian randomization analyses, further population-based prospective studies in non-European ancestry populations are warranted. Future studies could address the role of individual components of GlycA as well as mechanistic studies. Finally, as our assessment was limited to the 168 metabolites as profiled in the UKB sample, additional studies are needed to assess other metabolite classes.
Conclusions
This first population-based, prospective pre-diagnostic metabolomics study implicated GlycA (a stable inflammatory marker reflecting neutrophil overactivity), which was novelly associated with incident and recurrent gout beyond serum urate. These findings may provide insight into the metabolic-neutrophilic synovitis pathways in gout, and could lead to biomarkers to predict flares or become new therapeutic targets.
Supplementary Material
Funding:
This research was supported by grants P50-AR-060772 and R01 AR065944 from the National Institutes of Health. NM is supported by a Career Development Award from the National Institutes of Health [K99-AR080243]. RT also was supported by a grant from the VA Research Service (I01 BX005927).
Footnotes
Competing Interests: ADJ reports employment with Regeneron Pharmaceuticals. GC reports stock options for Allena Pharmaceuticals and is the Chief Medical Officer at OM1, Inc. HKC reports research support from Horizon, and consulting fees from Ani, LG, Horizon, Shanton, and Protalix. The remaining authors have no competing interests to declare.
A portion of these findings were selected for oral presentation at the 2022 EULAR European Congress of Rheumatology:
REFERENCES
- 1.Chen-Xu M, Yokose C, Rai SK, Pillinger MH, Choi HK. Contemporary Prevalence of Gout and Hyperuricemia in the United States and Decadal Trends: The National Health and Nutrition Examination Survey, 2007–2016. Arthritis & rheumatology. 2019;71(6):991–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khanna D, Fitzgerald JD, Khanna PP, Bae S, Singh MK, Neogi T, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: Systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis care & research. 2012;64(10):1431–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi HK, Mount DB, Reginato AM. Pathogenesis of gout. Ann Intern Med. 2005;143:499–516. [DOI] [PubMed] [Google Scholar]
- 4.Dalbeth N, Choi HK, Joosten LAB, Khanna PP, Matsuo H, Perez-Ruiz F, et al. Gout. Nat Rev Dis Primers. 2019;5(1):69. [DOI] [PubMed] [Google Scholar]
- 5.McCormick N, Rai SK, Lu N, Yokose C, Curhan GC, Choi HK. Estimation of primary prevention of gout in men through modification of obesity and other key lifestyle factors. JAMA Netw Open. 2020;3(11):e2027421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi HK, McCormick N, Lu N, Rai SK, Yokose C, Zhang Y. Population Impact Attributable to Modifiable Risk Factors for Hyperuricemia. Arthritis & rheumatology. 2020;72(1):157–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dehlin M, Jacobsson L, Roddy E. Global epidemiology of gout: prevalence, incidence, treatment patterns and risk factors. Nature reviews Rheumatology. 2020;16(7):380–90. [DOI] [PubMed] [Google Scholar]
- 8.Safiri S, Kolahi AA, Cross M, Carson-Chahhoud K, Hoy D, Almasi-Hashiani A, et al. Prevalence, Incidence, and Years Lived With Disability Due to Gout and Its Attributable Risk Factors for 195 Countries and Territories 1990–2017: A Systematic Analysis of the Global Burden of Disease Study 2017. Arthritis & rheumatology. 2020. [DOI] [PubMed] [Google Scholar]
- 9.Lim SY, Lu N, Oza A, Fisher M, Rai SK, Menendez ME, et al. Trends in Gout and Rheumatoid Arthritis Hospitalizations in the United States, 1993–2011. JAMA. 2016;315(21):2345–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalbeth N, Phipps-Green A, Frampton C, Neogi T, Taylor WJ, Merriman TR. Relationship between serum urate concentration and clinically evident incident gout: an individual participant data analysis. Ann Rheum Dis. 2018;77(7):1048–52. [DOI] [PubMed] [Google Scholar]
- 11.Chu SH, Huang M, Kelly RS, Benedetti E, Siddiqui JK, Zeleznik OA, et al. Integration of Metabolomic and Other Omics Data in Population-Based Study Designs: An Epidemiological Perspective. Metabolites. 2019;9(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicholson JK, Wilson ID. Opinion: understanding ‘global’ systems biology: metabonomics and the continuum of metabolism. Nat Rev Drug Discov. 2003;2(8):668–76. [DOI] [PubMed] [Google Scholar]
- 13.Menni C, Zierer J, Valdes AM, Spector TD. Mixing omics: combining genetics and metabolomics to study rheumatic diseases. Nature reviews Rheumatology. 2017;13(3):174–81. [DOI] [PubMed] [Google Scholar]
- 14.Mahbub MH, Yamaguchi N, Takahashi H, Hase R, Amano H, Kobayashi-Miura M, et al. Alteration in plasma free amino acid levels and its association with gout. Environ Health Prev Med. 2017;22(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang M, Chen T, Feng H, Zhang Y, Li L, Zhao A, et al. Serum metabolic signatures of four types of human arthritis. J Proteome Res. 2013;12(8):3769–79. [DOI] [PubMed] [Google Scholar]
- 16.Huang Y, Xiao M, Ou J, Lv Q, Wei Q, Chen Z, et al. Identification of the urine and serum metabolomics signature of gout. Rheumatology (Oxford). 2020;59(10):2960–9. [DOI] [PubMed] [Google Scholar]
- 17.Luo Y, Wang L, Liu XY, Chen X, Song YX, Li XH, et al. Plasma profiling of amino acids distinguishes acute gout from asymptomatic hyperuricemia. Amino Acids. 2018;50(11):1539–48. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Zhang H, Chang D, Guo F, Pan H, Yang Y. Metabolomics approach by (1)H NMR spectroscopy of serum reveals progression axes for asymptomatic hyperuricemia and gout. Arthritis Res Ther. 2018;20(1):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Q, Wei S, Wu D, Wen C, Zhou J. Urinary Metabolomics Study of Patients with Gout Using Gas Chromatography-Mass Spectrometry. Biomed Res Int. 2018;2018:3461572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahbub MH, Yamaguchi N, Takahashi H, Hase R, Ishimaru Y, Sunagawa H, et al. Association of plasma free amino acids with hyperuricemia in relation to diabetes mellitus, dyslipidemia, hypertension and metabolic syndrome. Scientific reports. 2017;7(1):17616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caleyachetty R, Littlejohns T, Lacey B, Besevic J, Conroy M, Collins R, et al. United Kingdom Biobank (UK Biobank): JACC Focus Seminar 6/8. J Am Coll Cardiol. 2021;78(1):56–65. [DOI] [PubMed] [Google Scholar]
- 22.Bell JA, Richardson TG, Wang Q, Sanderson E, Palmer T, Walker V, et al. Effects of general and central adiposity on circulating lipoprotein, lipid, and metabolite levels in UK Biobank: A multivariable Mendelian randomization study. Lancet Reg Health Eur. 2022;21:100457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mi J, Jiang L, Liu Z, Wu X, Zhao N, Wang Y, et al. Identification of blood metabolites linked to the risk of cholelithiasis: a comprehensive Mendelian randomization study. Hepatol Int. 2022;16(6):1484–93. [DOI] [PubMed] [Google Scholar]
- 24.Nightingale Health Metabolic Biomarkers: Phase 1 Release; 2021. [Google Scholar]
- 25.Zeleznik OA, Wittenbecher C, Deik A, Jeanfavre S, Avila-Pacheco J, Rosner B, et al. Intrapersonal Stability of Plasma Metabolomic Profiles over 10 Years among Women. Metabolites. 2022;12(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bragg F, Trichia E, Aguilar-Ramirez D, Besevic J, Lewington S, Emberson J. Predictive value of circulating NMR metabolic biomarkers for type 2 diabetes risk in the UK Biobank study . BMC medicine. 2022;20(1):159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cipolletta E, Tata LJ, Nakafero G, Avery AJ, Mamas MA, Abhishek A. Association Between Gout Flare and Subsequent Cardiovascular Events Among Patients With Gout. JAMA. 2022;328(5):440–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the health professionals follow-up study. Arch Intern Med. 2005;165(7):742–8. [DOI] [PubMed] [Google Scholar]
- 29.Choi HK, Atkinson K, Karlson EW, Willett WC, Curhan G. Alcohol Intake and Risk of Incident Gout in Men - A Prospective Study. Lancet. 2004;363:1277–81. [DOI] [PubMed] [Google Scholar]
- 30.Choi HK, Curhan G. Coffee consumption and risk of incident gout in women: the Nurses’ Health Study. Am J Clin Nutr. 2010;92(4):922–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi HK, Atkinson K, Karlson EW, Willett WC, Curhan G. Purine-Rich Foods, Dairy and Protein Intake, and the Risk of Gout in Men. New Eng J Med. 2004;350:1093–103. [DOI] [PubMed] [Google Scholar]
- 32.Biobank U. Companion document to accompany serum biomarker data. 2019. [cited; Available from: https://biobank.ctsu.ox.ac.uk/crystal/ukb/docs/serum_biochemistry.pdf
- 33.Gao X, Starmer J, Martin ER. A multiple testing correction method for genetic association studies using correlated single nucleotide polymorphisms. Genet Epidemiol. 2008;32(4):361–9. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Hu W, Wang Y, Wang W, Liao H, Zhang X, et al. Plasma metabolomic profiles of dementia: a prospective study of 110,655 participants in the UK Biobank. BMC medicine. 2022;20(1):252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Townsend MK, Clish CB, Kraft P, Wu C, Souza AL, Deik AA, et al. Reproducibility of metabolomic profiles among men and women in 2 large cohort studies. Clin Chem. 2013;59(11):1657–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Catalogue GWAS. [cited; Available from: https://www.ebi.ac.uk/gwas/ [Google Scholar]
- 37.Tin A, Marten J, Halperin Kuhns VL, Li Y, Wuttke M, Kirsten H, et al. Target genes, variants, tissues and transcriptional pathways influencing human serum urate levels. Nat Genet. 2019;51(10):1459–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32(5):377–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Otvos JD, Shalaurova I, Wolak-Dinsmore J, Connelly MA, Mackey RH, Stein JH, et al. GlycA: A Composite Nuclear Magnetic Resonance Biomarker of Systemic Inflammation. Clin Chem. 2015;61(5):714–23. [DOI] [PubMed] [Google Scholar]
- 42.Ritchie SC, Wurtz P, Nath AP, Abraham G, Havulinna AS, Fearnley LG, et al. The Biomarker GlycA Is Associated with Chronic Inflammation and Predicts Long-Term Risk of Severe Infection. Cell Syst. 2015;1(4):293–301. [DOI] [PubMed] [Google Scholar]
- 43.Lawler PR, Akinkuolie AO, Chandler PD, Moorthy MV, Vandenburgh MJ, Schaumberg DA, et al. Circulating N-Linked Glycoprotein Acetyls and Longitudinal Mortality Risk. Circ Res. 2016;118(7):1106–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akinkuolie AO, Pradhan AD, Buring JE, Ridker PM, Mora S. Novel protein glycan side-chain biomarker and risk of incident type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol. 2015;35(6):1544–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chiesa ST, Charakida M, Georgiopoulos G, Roberts JD, Stafford SJ, Park C, et al. Glycoprotein Acetyls: A Novel Inflammatory Biomarker of Early Cardiovascular Risk in the Young . J Am Heart Assoc. 2022;11(4):e024380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao H, Zhang F, Sun D, Wang X, Zhang X, Zhang J, et al. Branched-Chain Amino Acids Exacerbate Obesity-Related Hepatic Glucose and Lipid Metabolic Disorders via Attenuating Akt2 Signaling. Diabetes. 2020;69(6):1164–77. [DOI] [PubMed] [Google Scholar]
- 47.Vanweert F, Schrauwen P, Phielix E. Role of branched-chain amino acid metabolism in the pathogenesis of obesity and type 2 diabetes-related metabolic disturbances BCAA metabolism in type 2 diabetes. Nutr Diabetes. 2022;12(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tobias DK, Lawler PR, Harada PH, Demler OV, Ridker PM, Manson JE, et al. Circulating Branched-Chain Amino Acids and Incident Cardiovascular Disease in a Prospective Cohort of US Women . Circ Genom Precis Med. 2018;11(4):e002157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheng Y, Ceglarek U, Huang T, Li L, Rood J, Ryan DH, et al. Weight-loss diets and 2-y changes in circulating amino acids in 2 randomized intervention trials. Am J Clin Nutr. 2016;103(2):505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alves A, Bassot A, Bulteau AL, Pirola L, Morio B. Glycine Metabolism and Its Alterations in Obesity and Metabolic Diseases. Nutrients. 2019;11(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCormick N, O’Connor MJ, Yokose C, Merriman TR, Mount DB, Leong A, et al. Assessing the Causal Relationships Between Insulin Resistance and Hyperuricemia and Gout Using Bidirectional Mendelian Randomization. Arthritis & rheumatology. 2021;73(11):2096–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Facchini F, Chen YD, Hollenbeck CB, Reaven GM. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. JAMA. 1991;266(21):3008–11. [PubMed] [Google Scholar]
- 53.de Urbina JJO, San-Miguel B, Vidal-Casariego A, Crespo I, Sanchez DI, Mauriz JL, et al. Effects Of Oral Glutamine on Inflammatory and Autophagy Responses in Cancer Patients Treated With Abdominal Radiotherapy: A Pilot Randomized Trial. Int J Med Sci. 2017;14(11):1065–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Niihara Y, Miller ST, Kanter J, Lanzkron S, Smith WR, Hsu LL, et al. A Phase 3 Trial of l-Glutamine in Sickle Cell Disease. N Engl J Med. 2018;379(3):226–35. [DOI] [PubMed] [Google Scholar]
- 55.Fry A, Littlejohns TJ, Sudlow C, Doherty N, Adamska L, Sprosen T, et al. Comparison of Sociodemographic and Health-Related Characteristics of UK Biobank Participants With Those of the General Population. Am J Epidemiol. 2017;186(9):1026–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.