Abstract
CD8 T cells recognize cancers when they detect antigenic peptides presented on a tumor’s surface MHC I molecules. Since MHC I antigen presentation is not essential for cell growth or survival, many cancers inactivate this pathway, and thereby escape control by CD8 T cells. Such immune evasion allows cancers to progress and also become resistant to CD8 T cell-based immunotherapies, such as checkpoint blockade. Here we review recent findings about the various different mechanisms that cancers use to impair antigen presentation, the consequence of such changes, and, in some cases, the potential to reverse these defects.
Keywords: antigen presentation, MHC I, cancer immune evasion, immunotherapy
INTRODUCTION
Classical experiments by Robert Schreiber and coworkers demonstrated that in the absence of an adaptive immune system, mice developed much higher rates of carcinogen-induced or spontaneous tumors compared to their isogenic immune-sufficient counterparts [1, 2]. Moreover, the cancers that developed in these two settings differed from one another. Ones arising in immunodeficient mice were most often rejected when transplanted into immunocompetent mice, and hence were immunogenic, whereas in contrast, tumors from wild-type animals would generally grow upon similar transplantation [2]. Thus, the adaptive immune system nips many cancers in the bud, and those tumors that become clinically evident are ones that have evolved to evade these host defenses. Evidence suggests that the adaptive immune system in humans is similarly involved in immune surveillance and control of cancers.
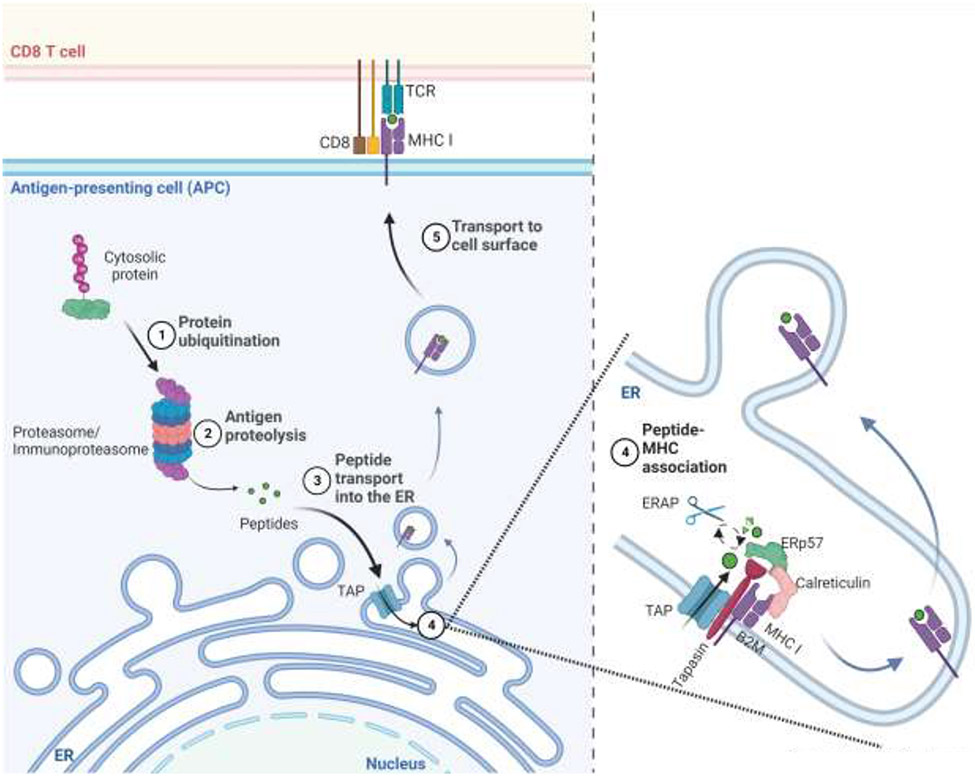
CD8 T lymphocytes are the most important adaptive immune defense against cancers. These immune cells recognize cancers when they detect antigenic peptides bound to MHC I molecules on the surface of tumor cells [3]. This MHC I antigen presentation pathway (Fig. 1) operates in all cells. In this pathway, proteasomes and immunoproteasomes hydrolyze endogenous proteins as part of normal cellular catabolism, and in this process create a library of oligopeptides derived from the polypeptides synthesized in cells [4, 5]. A fraction of these peptides is transferred into the ER by a dedicated peptide transporter, TAP, and once in this location may be further trimmed by the ER aminopeptidases, ERAP1 and/or ERAP2 [5-7]. With the aid of ER chaperones, such as Tapasin, MHC I molecules bind peptides of the correct length and sequence, and the resulting complexes are then transported to the cell surface for display to CD8 T cells [5, 6, 8]. The expression of all these antigen presentation components can be upregulated when cells are stimulated by type I and especially type 2 interferons [9]. This antigen presentation pathway allows CD8 T cells to detect and then eliminate cells that are synthesizing antigenic proteins, such as ones with mutated sequences in cancers [3, 5]. Additional molecules that participate in and regulate the MHC I antigen presentation pathway are being discovered and will be discussed in sections below.
Figure-1:
The MHC I antigen presentation pathway. Cytosolic and nuclear proteins are degraded by proteasomes and immunoproteasomes into oligopeptides. Some of these peptides are then translocated into the endoplasmic reticulum (ER) by the TAP transporter. In the ER, ERAPs may further trim these oligopeptides, and then ones of the right length and sequence bind to MHC I molecules within a peptide-loading complex, which contains Tapasin, TAP, calreticulin, and ERP57. Peptide-loaded MHCI molecules are then transported to the cell surface for display to CD8+ T cells. Created with BioRender.com.
The majority of the MHC I antigen presentation components, including immunoproteasome subunits, TAP, Tapasin, ERAP1/2, MHC I molecules (heavy chains + ß2M), and IFN receptors and their signaling components, are not required for cellular viability or proliferation. The consequence of losing any of these components is a reduction in the function of the pathway and the display of MHC I molecules on the cell surface. Therefore, cancers can escape control by CD8 T cells through genetic deletions, mutations, and/or epigenetic silencing of antigen presentation components. In fact, this is a very common occurrence in cancer as evidenced by the finding that a majority of human cancers have depressed levels of MHC I molecules on their cell surfaces [3]. Where examined, this is often clinically significant as it is associated with a worse prognosis [3]. Moreover, reduced MHC I expression can be associated with resistance of cancers to T cell-based cancer immunotherapies (check point blockade or adoptive transfer of anti-tumor CD8 T cells).
Here we review various mechanisms by which cancers inactivate the MHC I pathway, and in some cases, the potential to reverse such defects. We focus primarily on the recent literature and refer readers interested in a more comprehensive understanding of older publications to a recent review [3].
Loss or inactivation of MHC I Structural Genes
It has previously been recognized that cancers can inactivate MHC I structural genes and that the resulting MHC Ilow tumors are more resistant to immunotherapy [3]. Recent studies have confirmed and extended these observations. Maximal heterozygosity in MHC I alleles in melanoma and other cancers was correlated with improved outcomes to checkpoint blockade immunotherapy, whereas somatic loss of heterozygosity (LOH) had the opposite effect [10]. Presumably, the presence of more MHC I alleles allows greater presentation of tumor antigens, and LOH is a consequence of immunoselection against alleles that are presenting tumor antigens, which then allows immune evasion. Consistent with this, LOH can be frequent in cancers and affect immunotherapy. For example, recent analyses found that loss of heterozygosity (LOH) in MHC I genes occurred in 40% of NSCLCs [11], and that this was associated with poorer responses to checkpoint blockade [12]. Similarly, recent studies have documented that LOH of MHC I in melanoma [13], renal cell carcinoma [14], metastatic breast cancer [15], and myeloma [16] was associated with resistance to checkpoint blockade or adoptive T cell therapy. LOH, mutations and deletions in the MHC I light chain gene (ß2M) in melanomas, which reduce expression of all MHC I molecules, also impairs responses to checkpoint blockade [13]. Inactivating mutations in MHC I structural genes also occur [3]. Correcting the structural loss of MHC I genes to improve immunotherapy would require gene therapy or editing of all cancer cells in patients, which is currently not a practical therapeutic option.
Loss of MHC I Expression Through Transcriptional and Epigenetic Regulation
There are multiple transcription factors, including NLRC5, IRF1/IRF2, and NFkB, that drive expression of MHC I molecules, and/or their associated antigen presentation pathway genes. Loss of these factors was known to occur in some cancers and affect responses to immunotherapy [3]. Recent studies have extended such observations. NLRC5 expression in cancers was found to positively correlate with expression of MHC I, CD8+ T cell activation markers and better survival [17, 18]. In contrast, loss of NLRC5 expression was correlated with reduced expression of MHC I genes and impaired responses to immunotherapy [17, 18]. Reductions in NLRC5 expression in cancers can occur by epigenetic silencing through promoter methylation, coding region mutations, and loss of gene copy number. In multiple cancers, NLRC5 methylation negatively correlated with NLRC5 expression and MHC I pathway gene expression [17]. Similarly, NEDD4 Binding Protein 1(N4BP1)- and TNFAIP3-interacting protein 1 (TNIP1)-dependent suppression of NF-kB [19], or loss of NF-kB and interferon regulatory factor 1 (IRF1), resulted in loss of MHC I expression [19, 20]. Chemotherapy-induced activation of NF-κB upregulated MHC I antigen presentation, and improved immunotherapy responses in otherwise resistant tumors [21]. IRF2 expression was also discovered to be required for transcription of several antigen presentation pathway components [22]. A reduction of IRF2 transcripts was found in multiple human cancers. Loss of IRF2 reduced MHC I expression and antigen presentation [22], and such defects could be reversed by inducing IRF1 expression, which binds to the same gene regulatory elements as IRF2 [22].
On the other side of the coin, MHC I pathway genes can be a target for epigenetic regulation, e.g., silencing through promoter methylation [23]. Loss of the histone dimethyltransferase WHSC1 in cancers can reduce transcription of antigen presentation pathway components, resulting in decreased MHC I expression and immunotherapy resistance [24]. Cell linage specific transcriptional repression of MHC I, as occurs in tissue specific quiescent stem cells, is controlled by the polycomb repressive complex 2 (PRC2) and the cancers developing from these lineages are MHC llow [25]. There has been interest in restoring MHC I expression in cancers by reversing epigenetic silencing with drugs. Several recent studies have reduced resistance to immunotherapy by adding such agents, e.g. DNA methyltransferase inhibitors and histone deacetylase 6 inhibitors [26-28].
Post-Transcriptional MHC Loss via Non-coding RNA Silencing
MicroRNAs (miRNAs) can affect gene expression via RNA interference and silencing [29]. In melanomas, miR-200a-5p was found to bind to the TAP1 peptide-transporter’s 3′-UTR, reducing the transcripts levels, and thereby limiting peptide supply and downregulating MHC I expression [30]. Similarly, in esophageal adenocarcinoma cell lines, miRNAs miR-125a-5p and miR-148a-3p bind to the 3′-UTR regions of TAP2 peptide transporter and MHC I mRNAs respectively, resulting in downregulation of MHC I levels and impaired cytotoxic T cell killing. In esophageal cancer patient biopsies, TAP2 expression inversely correlates with miR-125a-5p expression [31]. Additionally, in in vivo colorectal cancer models, miR-27a was shown to target the 3′-UTR of the chaperone calreticulin, and levels of this miRNA inversely correlated with MHC I expression, infiltration of CD8+ T cells and cytotoxic activity in vivo [32]. For further information and earlier studies, we refer readers to the recent review articles [3, 29, 33].
Post-Translational Loss of MHC I function
Recent studies have revealed that MHC I antigen presentation can be affected through post-translational mechanisms. One novel and interesting mechanism arises from the loss of activity of the enzyme signal peptide peptidase-like 3 (SPPL3) [34]. In the absence of SPPL3 catalytic activity, glycosphingolipids (GSLs) shield MHC I molecules on the cell surface in ways to somehow impair the ability of MHC I molecules to interact with and stimulate CD8 T cells. Lower SPPL3 levels correlates with poor outcome in glioma patients, and anti-tumor responses were improved after pharmacological inhibition of GSL synthesis in glioma cell lines [34].
Optimal stimulation and activation CD8+ T cells also requires clustering of MHC I on the plasma membrane, which is promoted by tetraspanin-5 (Tspan5). Some cancers downregulated Tspan5 expression and the loss of Tspan5 reduces the size of the MHC I nanoclusters, resulting in impaired CD8 T cell stimulation [35]. This defect could be reversed by re-clustering MHC I molecules with anti-ß2M antibodies.
Loss of MHC I through degradation
Some tumors may reduce MHC I levels by degrading their MHC I molecules. One recent example involves staphylococcal nuclease and tudor domain containing 1 (SND1) oncoprotein, which interacts with MHC I molecules in the ER and targets them for degradation via the endoplasmic reticulum (ER)-associated degradation (ERAD) pathway [36]. Tumor cells that overexpress SND1 degrade their MHC I molecules, and thereby escape immune recognition through reduced MHC I levels [36]. This phenotype can be reversed via pharmacological inhibition of proteasomal degradation and the ERAD pathway [36].
Recently, pancreatic cancer cells were found to reduce their levels of MHC I molecules through autophagy [37]. In this example, the ubiquitin-binding autophagy receptor NBR1 targeted MHC I molecules for degradation in autophagosomes. Such loss of MHC I could be reversed by systemic administration of chloroquine, which inhibits intravesicular proteolysis, and such treatment enhanced the effect of checkpoint blockade in otherwise immunotherapy refractory pancreatic cancers [37]. Some tumors also use autophagy to degrade the NLRC5 transcription factor needed for MHC I expression (see above) [38].
In yet another mechanism, some breast cancers overexpress MAL2, a transmembrane protein involved in protein endocytosis, which then accelerates the endocytosis and degradation of surface MHC I molecules [39]. This results in an MHC Ilow phenotype and impaired killing by CD8 T cells [39].
MHC Loss as a Result of the Changes in Cellular Signaling and Stress
MHC I expression was downregulated as a consequence of elevated mitochondrial fission in a number of different cancer cells [40]. This reduction in MHC I molecules was secondary to mitochondrial fission in cancer cells causing oxidative stress and an unfolded protein response (UPR). It was suggested that the UPR reduced antigen presentation by upregulating the aminopeptidase tripeptidyl peptidase 2, which then destroyed antigenic peptides needed for antigen presentation [40]. Inhibition of dynamin-related protein-1-dependent mitochondrial fission in cancer cells, reversed the oxidative stress and UPR responses, restored MHC I antigen presentation, and immune control of transplanted tumors [40]. Hypoxia similarly downregulates MHC I expression in cancer cells in vivo and in vitro [41].
MHC I expression and antigen presentation are regulated by certain cytokines. Signaling through type I and type II interferon receptors strongly upregulates almost all of the components of the MHC I pathway and results in increased MHC I molecule expression and antigen presentation. Cancers can lose responsiveness to such stimulation by losing or inactivating their IFN receptors and their downstream Jak/STAT signaling components. When this occurs, cancers can drop their levels of MHC I and also fail to upregulate the pathway in response to IFNs that are made during immune responses. This has been seen, e.g. in melanomas and is associated with resistance to checkpoint blockade [42]. Conversely, IFN-signaling signatures in melanomas are correlated with responsiveness to checkpoint blockade [43]. However, surprisingly, and difficult to reconcile with these earlier results, a recent in vivo CRISPR screen across multiple cancers came to the opposite conclusion. IFN-signaling in cancers impaired responsiveness to checkpoint blockade and did so by inducing MHC class 1a and 1b molecules, which then inhibited immune responses by engaged NK inhibitory receptors both on CD8 T and NK cells [44].
There are other recent examples of signaling alterations in cancers that affect MHC I expression. In refractory melanomas, mammalian target of rapamycin (mTOR), Rho family GTPase B (RhoB GTPase), Wnt and Mitogen-activated protein kinase (MAPK) pathways negatively correlate with MHC I expression [45]. In immune evasive lung adenocarcinomas, reduced levels of 5' AMP-activated protein kinase or AMPK pathway results in lower expression B2M and MHC I [46].
CONCLUSIONS
The MHC I antigen presentation pathway allows CD8 T cells to find and eliminate cancer cells. Since this pathway is not required for cell viability or growth, tumors frequently evade immune control by losing or inactivating components of this pathway. In different studies, and depending on the cancer type, MHC I loss has been seen on average in about 40-70% of cases [3, 47]. Such immune evasion allows tumors to progress and also to become resistant to CD8 T cell-based immunotherapies. The molecular changes that cripple the MHC I pathway might be useful as biomarkers to identify patients that are likely to have poor responses to immunotherapy, and future studies are needed to examine this further. Although cancers can impair the MHC I pathway in a large number of different ways, there are subsets of cancer patients that will share the same lesions and some of these lesions are potentially reversible or could be bypassed. Investigating how to do this therapeutically is a high priority for future research. Where successful, one could imagine a future of personalized medicine, where a patient’s MHC I pathway lesions are defined and then agents that reverse the defect, if available, would be co-administered with immunotherapy.
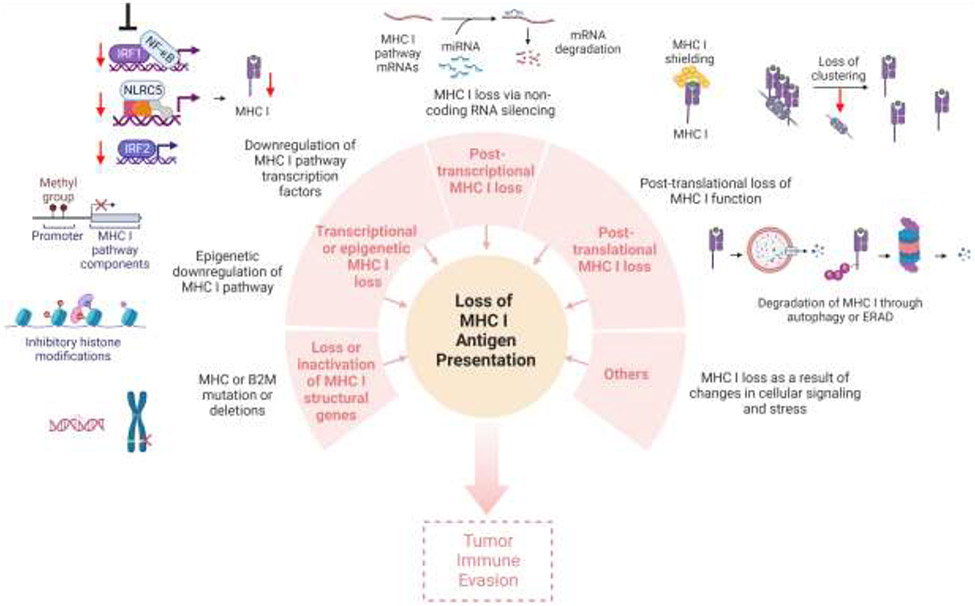
Figure-2:
Cancer immune evasion through loss of MHC I antigen presentation. MHC I antigen presentation may be lost or downregulated as a result of loss of, or inactivating mutations in MHC I and ß2M structural genes; loss or downregulation of transcriptional factors; inhibitory epigenetic modifications; non-coding RNA silencing; post translational loss of MHC I molecules through degradation; MHC I shielding or loss of MHC nanoclustering; and other reasons such as changes in the cellular signaling or stress. Loss of MHC I antigen presentation often leads to resistance to CD8 T cell-based immunotherapy and tumor immune evasion. Created with BioRender.com.
Highlights.
Cancers often escape immune control by inactivating their presentation of antigens on MHC I molecules.
Such immune evasion can arise from lesions in multiple antigen presentation components, and at multiple levels (in structural genes, transcription, post-transcriptional, post translational mechanisms).
Loss of MHC I antigen presentation often leads to resistance to CD8 T cell-based immunotherapy.
Some antigen presentation defects in cancers may be reversible.
Acknowledgements
This work was supported by NIH grants R01AI114495, R01AI145932 and R01CA247624 to KLR. Authors thank Larry Stern for his critical reading and editing of the manuscript.
Footnotes
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest.
- 1.Koebel CM, et al. , Adaptive immunity maintains occult cancer in an equilibrium state. Nature, 2007. 450(7171): p. 903–7. [DOI] [PubMed] [Google Scholar]
- 2.Shankaran V, et al. , IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature, 2001. 410(6832): p. 1107–11. [DOI] [PubMed] [Google Scholar]
- 3.Dhatchinamoorthy K, Colbert JD, and Rock KL, Cancer Immune Evasion Through Loss of MHC Class I Antigen Presentation. Front Immunol, 2021. 12: p. 636568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rock KL, et al. , Inhibitors of the Proteasome Block the Degradation of Most Cell-Proteins and the Generation of Peptides Presented on Mhc Class-1 Molecules. Cell, 1994. 78(5): p. 761–771. [DOI] [PubMed] [Google Scholar]
- 5.Rock KL, Reits E, and Neefjes J, Present Yourself! By MHC Class I and MHC Class II Molecules. Trends Immunol, 2016. 37(11): p. 724–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eggensperger S and Tampe R, The transporter associated with antigen processing: a key player in adaptive immunity. Biol Chem, 2015. 396(9-10): p. 1059–72. [DOI] [PubMed] [Google Scholar]
- 7.Evnouchidou I and van Endert P, Peptide trimming by endoplasmic reticulum aminopeptidases: Role of MHC class I binding and ERAP dimerization. Hum Immunol, 2019. 80(5): p. 290–295. [DOI] [PubMed] [Google Scholar]
- 8.Evnouchidou I, et al. , ERAP1-ERAP2 dimerization increases peptide-trimming efficiency. J Immunol, 2014. 193(2): p. 901–8. [DOI] [PubMed] [Google Scholar]
- 9.Zhou F, Molecular mechanisms of IFN-gamma to up-regulate MHC class I antigen processing and presentation. Int Rev Immunol, 2009. 28(3-4): p. 239–60. [DOI] [PubMed] [Google Scholar]
- 10.Chowell D, et al. , Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science, 2018. 359(6375): p. 582–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGranahan N, et al. , Allele-Specific HLA Loss and Immune Escape in Lung Cancer Evolution. Cell, 2017. 171(6): p. 1259–1271 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu S, et al. , HLA loss of heterozygosity-mediated discordant responses to immune checkpoint blockade in squamous cell lung cancer with renal metastasis. Immunotherapy, 2021. 13(3): p. 195–200. [DOI] [PubMed] [Google Scholar]
- 13.Sade-Feldman M, et al. , Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat Commun, 2017. 8(1): p. 1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Labriola MK, et al. , Characterization of tumor mutation burden, PD-L1 and DNA repair genes to assess relationship to immune checkpoint inhibitors response in metastatic renal cell carcinoma. J Immunother Cancer, 2020.8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Messaoudene M, et al. , T-cell bispecific antibodies in node-positive breast cancer: novel therapeutic avenue for MHC class I loss variants. Ann Oncol, 2019. 30(6): p. 934–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klippel ZK, et al. , Immune escape from NY-ESO-1-specific T-cell therapy via loss of heterozygosity in the MHC. Gene Ther, 2014. 21(3): p. 337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yoshihama S, et al. , NLRC5/CITA expression correlates with efficient response to checkpoint blockade immunotherapy. Sci Rep, 2021. 11(1): p. 3258. •This study shows that the transcription factor NLRC5 is critical for MHC I dependent T cell cytotoxicity and is a novel predictive biomarker for responses to immunotherapy in melanomas.
- 18.Yoshihama S, et al. , NLRC5/MHC class I transactivator is a target for immune evasion in cancer. Proc Natl Acad Sci U S A, 2016. 113(21): p. 5999–6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spel L, et al. , Nedd4-Binding Protein 1 and TNFAIP3-Interacting Protein 1 Control MHC-1 Display in Neuroblastoma. Cancer Res, 2018. 78(23): p. 6621–6631. [DOI] [PubMed] [Google Scholar]
- 20.Lorenzi S, et al. , IRF1 and NF-kB restore MHC class I-restricted tumor antigen processing and presentation to cytotoxic T cells in aggressive neuroblastoma. PLoS One, 2012. 7(10): p. e46928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou Y, et al. , Activation of NF-kappaB and p300/CBP potentiates cancer chemoimmunotherapy through induction of MHC-I antigen presentation. Proc Natl Acad Sci U S A, 2021. 118(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kriegsman BA, et al. , Frequent Loss of IRF2 in Cancers Leads to Immune Evasion through Decreased MHC Class I Antigen Presentation and Increased PD-L1 Expression. J Immunol, 2019. 203(7): p. 1999–2010. • This study shows that loss of the transcription factor IRF2 causes cancer immune evasion through a low MHC I and high PD-L1 phenotype and, that this phenotype can be reversed by interferon stimulation.
- 23.Ye Q, et al. , Hypermethylation of HLA class I gene is associated with HLA class I downregulation in human gastric cancer. Tissue Antigens, 2010. 75(1): p. 30–9. [DOI] [PubMed] [Google Scholar]
- 24. Ren J, et al. , Histone methyltransferase WHSC1 loss dampens MHC-I antigen presentation pathway to impair IFN-gamma-stimulated antitumor immunity. J Clin Invest, 2022. 132(8). • This study shows that downregulation of the histone dimethyltransferase WHSC1 results in a low MHC I phenotype and impaired IFN-γ signaling in colorectal cancers.
- 25. Burr ML, et al. , An Evolutionarily Conserved Function of Polycomb Silences the MHC Class I Antigen Presentation Pathway and Enables Immune Evasion in Cancer. Cancer Cell, 2019. 36(4): p. 385–401 e8. • •This study identifies that the polycomb repressive complex 2 transcriptionally silences the MHC I pathway, which results in cancer immune evasion.
- 26.Luo N, et al. , DNA methyltransferase inhibition upregulates MHC-I to potentiate cytotoxic T lymphocyte responses in breast cancer. Nat Commun, 2018. 9(1): p. 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moufarrij S, et al. , Combining DNMT and HDAC6 inhibitors increases anti-tumor immune signaling and decreases tumor burden in ovarian cancer. Sci Rep, 2020. 10(1): p. 3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun T, et al. , Histone deacetylase inhibition up-regulates MHC class I to facilitate cytotoxic T lymphocyte-mediated tumor cell killing in glioma cells. J Cancer, 2019. 10(23): p. 5638–5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu L, et al. , Noncoding RNAs: the shot callers in tumor immune escape. Signal Transduct Target Ther, 2020. 5(1): p. 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lazaridou MF, et al. , Identification of miR-200a-5p targeting the peptide transporter TAP1 and its association with the clinical outcome of melanoma patients. Oncoimmunology, 2020. 9(1): p. 1774323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mari L, et al. , microRNA 125a Regulates MHC-I Expression on Esophageal Adenocarcinoma Cells, Associated With Suppression of Antitumor Immune Response and Poor Outcomes of Patients. Gastroenterology, 2018. 155(3): p. 784–798. [DOI] [PubMed] [Google Scholar]
- 32.Colangelo T, et al. , Proteomic screening identifies calreticulin as a miR-27a direct target repressing MHC class I cell surface exposure in colorectal cancer. Cell Death Dis, 2016. 7: p. e2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yi M, et al. , The role of cancer-derived microRNAs in cancer immune escape. J Hematol Oncol, 2020. 13(1): p. 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jongsma MLM, et al. , The SPPL3-defined glycosphingolipid repertoire orchestrates HLA class I-mediated immune responses. Immunity, 2021. 54(2): p. 387. • •This study defines a novel mechanisim where MHC I antigen presentation is silenced through shielding of MHC I molecules with cell surface glycosphingolipids.
- 35. Colbert JD, et al. , Tetraspanin-5-mediated MHC class I clustering is required for optimal CD8 T cell activation. Proc Natl Acad Sci U S A, 2022. 119(42): p. e2122188119. •This study defines how MHC I molecules are clustered by tetraspanin-5 for more efficient and optimal antigen presentation by MHC I to simulate CD8 T cells.
- 36. Wang Y, et al. , Oncoprotein SND1 hijacks nascent MHC-I heavy chain to ER-associated degradation, leading to impaired CD8(+) T cell response in tumor. Sci Adv, 2020. 6(22). • •Authors here decsribe that the oncopotein SND1 interacts with heavy chain of MHC I molecules in the ER and promotes their degradation via ERAD pathway, which in return facilitates cancer immune evasion.
- 37. Yamamoto K, et al. , Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Nature, 2020. 581(7806): p. 100–105. •This study shows that pancreatic cancer cells degrade MHC I molecules though an NBR1-dependent autophagy pathway, allowing escape from CD8 T cell killing and resistance to immunotherapy.
- 38.Zhan L, et al. , LC3 and NLRC5 interaction inhibits NLRC5-mediated MHC class I antigen presentation pathway in endometrial cancer. Cancer Lett, 2022. 529: p. 37–52. [DOI] [PubMed] [Google Scholar]
- 39.Fang Y, et al. , MAL2 drives immune evasion in breast cancer by suppressing tumor antigen presentation. J Clin Invest, 2021. 131(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lei X, et al. , Mitochondrial fission induces immunoescape in solid tumors through decreasing MHC-I surface expression. Nat Commun, 2022. 13(1): p. 3882. • •This study shows that inhibiting mitochondrial fission with Mdivi-1 increases cell surface MHC I expression and promotes the efficacy of immunotherapies in patient-derived tumor models.
- 41.Sethumadhavan S, et al. , Hypoxia and hypoxia-inducible factor (HIF) downregulate antigen-presenting MHC class I molecules limiting tumor cell recognition by T cells. PLoS One, 2017. 12(11): p. e0187314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sucker A, et al. , Acquired IFNgamma resistance impairs anti-tumor immunity and gives rise to T-cell-resistant melanoma lesions. Nat Commun, 2017. 8: p. 15440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grasso CS, et al. , Conserved Interferon-gamma Signaling Drives Clinical Response to Immune Checkpoint Blockade Therapy in Melanoma. Cancer Cell, 2021. 39(1): p. 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dubrot J, et al. , In vivo CRISPR screens reveal the landscape of immune evasion pathways across cancer. Nat Immunol, 2022. 23(10): p. 1495–1506. [DOI] [PubMed] [Google Scholar]
- 45.Yu J, et al. , Loss of MHC-I antigen presentation correlated with immune checkpoint blockade tolerance in MAPK inhibitor-resistant melanoma. Front Pharmacol, 2022. 13: p. 928226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao Y, et al. , Inactivation of AMPK Leads to Attenuation of Antigen Presentation and Immune Evasion in Lung Adenocarcinoma. Clin Cancer Res, 2022. 28(1): p. 227–237. [DOI] [PubMed] [Google Scholar]
- 47.Cornel AM, Mimpen IL, and Nierkens S, MHC Class I Downregulation in Cancer: Underlying Mechanisms and Potential Targets for Cancer Immunotherapy. Cancers (Basel), 2020. 12(7). [DOI] [PMC free article] [PubMed] [Google Scholar]