Abstract
The advent of highly-effective disease modifying therapy has transformed the landscape of multiple sclerosis (MS) care over the last two decades. However, there remains a critical, unmet need for sensitive and specific biomarkers to aid in diagnosis, prognosis, treatment monitoring, and the development of new interventions, particularly for people with progressive disease. This review evaluates the current data for several emerging imaging and liquid biomarkers in people with MS. MRI findings such as the central vein sign and paramagnetic rim lesions may improve MS diagnostic accuracy and evaluation of therapy efficacy in progressive disease. Serum and cerebrospinal fluid levels of several neuroglial proteins such as neurofilament light chain (NfL) and glial fibrillary acidic protein (GFAP) show potential to be sensitive biomarkers of pathologic processes such as neuro-axonal injury or glial-inflammation. Additional promising biomarkers including optical coherence tomography, cytokines and chemokines, microRNAs, and extracellular vesicles/exosomes are also reviewed, among others. Beyond their potential integration into MS clinical care and interventional trials, several of these biomarkers may be informative of MS pathogenesis and help elucidate novel targets for treatment strategies.
Keywords: central vein sign, paramagnetic rim lesion, neurofilament light chain, glial fibrillary acidic protein, optical coherence tomography
Graphical Abstract
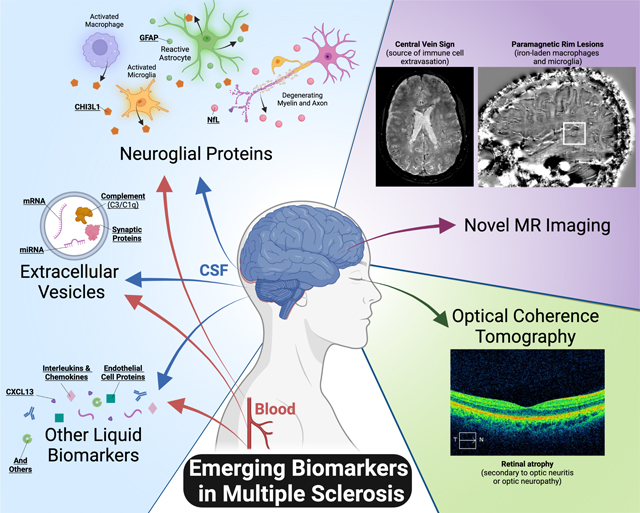
Several emerging imaging and liquid biomarkers reflecting underlying immunopathology have potential to aid in the diagnosis, prognosis, and treatment monitoring in people living with multiple sclerosis. Of particular note are novel MRI techniques and quantification of relevant neuroglial proteins in the blood.
Introduction
Multiple sclerosis (MS) is a complex neurologic disease with neuroinflammatory and neurodegenerative components that affects over 2 million people worldwide1. Current MS diagnostic criteria rely largely on clinical presentation and non-specific imaging and laboratory findings and thus misdiagnosis remains a significant issue2–4. Even after an accurate diagnosis of MS is made, the disease course and response to disease modifying therapy (DMT) are highly variable and are poorly predicted by currently clinically available biomarkers. With a wide array of medications of varying efficacy and safety available, treatments for prevention of the inflammatory, relapsing component of the disease have expanded significantly in recent years, but there has been far less forward movement in develop of therapy for insidious progressive decline or remyelination. This lack of reliable, accurate, non-invasive, and easily applied biomarkers significantly hinders MS research, prognostication, and DMT management decisions, particularly in progressive disease. This review highlights some of the emerging imaging and liquid biomarkers in people living with MS (PwMS) that have potential for improving MS diagnosis, quantifying current disease activity, assessing response to therapy, and prognosticating future disease activity and disability.
MS Pathogenesis, Diagnosis, and Clinically Established Biomarkers
MS pathogenesis
In the setting of genetic predisposition and a range of possible environmental triggers such as viral5 or toxin exposure6, the CNS undergoes autoimmune inflammatory injury resulting in demyelination and axonal transection followed by varying degrees of remyelination, neurodegeneration, and gliosis. In PwMS, these processes occur in focal, characteristic lesions as well as more diffusely throughout the CNS, the extent of which varies by the individual and by phase of the disease. While classically MS was thought of as predominantly affecting the white matter of the CNS, MS is known to significantly involve the gray matter as well7–10. Potential antigenic triggers remain uncertain, but are likely CNS-derived; examples of candidates include myelin basic protein (MBP), myelin oligodendrocyte glycoprotein (MOG), and proteolipoprotein (PLP)11. New inflammatory demyelinating lesions in PwMS are largely driven by the adaptive immune system, with bouts of peripheral lymphocyte activation and infiltration into the CNS (initially, predominantly CD8+ T cells, but also B-cells and macrophages) and subsequent activation of local glia12,13. MS-related neurodegeneration and progressive decline in PwMS independent of new inflammatory lesions is not well understood, but is thought to be driven more by compartmentalized processes within the CNS and leptomeninges, such as reactive glia, ectopic meningeal lymphoid follicles, oxidative stress, and age-related iron deposition14–16. This smoldering, CNS-compartmentalized pathological activity is more difficult to monitor and current DMTs largely target the peripheral immune system and thus may be of limited benefit.
Diagnostic and monitoring challenges
Diagnosis of MS is frequently challenging due to complex, variable clinical presentations, relative non-specificity of biomarkers and imaging findings, and many potential MS mimics. Two important mimics, among many, are neuromyelitis optica (NMO) and myelin oligodendrocyte antibody disease (MOGAD). Both of these diseases cause inflammatory, demyelinating lesions in the CNS with significant clinical overlap with MS. MOG has more cortical features and better recovery, and is more likely to be monophasic than MS17. NMO is an astrocytopathy with severe attacks (such as simultaneous bilateral optic neuritis, or longitudinally-extensive cord lesions) with poor recovery18. Unlike MS, NMO does not seem to have a prominent neurodegenerative component. Over the last two decades disease-specific autoantibodies (anti-aquaporin-4 for NMO and anti-MOG for MOGAD) have been identified. Clinically available testing for these antibodies has made assessing for these conditions in an MS workup more routine, but seronegative MOGAD or NMOSD remains a challenge. Additionally, many other MS mimics remain resulting in both over and under diagnosis. Studies show that about 25% of those referred to an academic center with a diagnosis of MS are misdiagnosed and many people with an erroneous MS diagnosis are started on disease modifying therapy (DMT)2–4. There is urgent need for more sensitive and specific biomarkers in MS, as misdiagnosis, often lasting years, may result in preventable morbidity, unnecessary expenditures, and psychologic burden2.
The current consensus diagnostic criteria for MS are the 2017 McDonald criteria19. The core of MS diagnosis requires clinical symptoms and radiologic CNS lesions disseminated in both space and time. Clinically definite MS is defined as two typical symptomatic clinical attacks along with certain paraclinical (imaging/cerebrospinal fluid (CSF)) findings; the 2017 McDonald criteria19 also allows for MS diagnosis with only one clinical event with certain imaging/CSF findings that satisfy dissemination in time and space, and this has been shown to offer at least moderate specifity20.
MS is currently clinically categorized as relapsing-remitting (RRMS), relapsing with secondary progression (SPMS), and primary progressive MS (PPMS). While lines between these categories are often blurred, RRMS consists of discrete clinical and radiologic attacks or relapses without disability progression between; SPMS begins as RRMS but in a later phase disability progression occurs between and distinct from acute relapses; and PPMS consists of insidious disease progression without any acute clinical attacks initially. SPMS and PPMS are often referred to collectively as progressive forms of MS (PMS). The vast majority of PwMS present as RRMS. Other entities on the demyelinating disease spectrum include clinically isolated syndrome (CIS) which defines one suspicious clinical event, but without adequately specific clinical or paraclinical data to satisfy current MS diagnostic criteria21. Radiologically isolated syndrome (RIS) involves imaging findings suspicious for MS but without any clinical symptoms attributable to MS22. Some people with CIS and RIS will go on to develop MS, but sensitive and specific biomarkers to predict who will convert to MS are needed and would allow for earlier initiation of DMT.
Current clinically established biomarkers
Key MRI features including location, morphology, number of lesions, and enhancement of lesions aid in MS diagnosis and prognosis. Gadolinium enhancement on MRI demonstrates an actively disturbed blood-brain barrier associated with peripheral-immune cell infiltration in MS. MS lesions tend to be ovoid and tend to occur in specific regions: periventricular, juxtacortical or cortical, infratentorial, or spinal cord. Despite some typical characteristics, MS lesions can sometimes be difficult to distinguish from other causes of white matter lesions such as microvascular disease, vasculopathies, systemic inflammatory conditions (e.g. Sjogren’s), and leukodystrophies. Thus, routine MRI imaging techniques may have low specificity for a diagnosis of MS, particularly in people with CIS and RIS, though newer imaging techniques discussed below significantly improve imaging-based diagnosis of MS. In PwMS, neurologists monitor for disease activity and response to current therapies by assessing for new, demyelinating-appearing lesions, and by enhancement which suggests recent/current inflammatory activity. Newer imaging markers of disease activity, particularly progressive disease, under investigation are discussed below.
In addition to MRI imaging, CSF can help support or dissuade an MS diagnosis, such as in CIS or in suspected MS with atypical features. Oligoclonal bands (OCBs) unique to the CSF, in the setting of a clinically suspicious event or suspicious imaging finds, are an established risk factor for future MS disease activity, and greater than 90% of those with MS have CSF-restricted OCBs23–25. OCBs unique to the CSF are nearly always abnormal, and reflect intrathecal IgG production from B cell clones that reside within the CNS compartment. Importantly, OCBs are not specific for MS. The long list of other CNS inflammatory conditions that may have OCBs unique to the CSF include neurosarcoidosis, paraneoplastic disorders, many neuro-infections, and Sjogren’s. One systematic review found that, when considering only neuroinflammatory differentials, CSF-unique OCBs were only 61% specific for MS26. CSF IgG, in normal conditions, is present via diffusion from serum, and therefore, polyclonal OCBs that mirror those in the serum (which must be simultaneously assessed) do not suggest CNS-specific inflammation. A potential alternative to OCBs that is gaining interest is CSF kappa free light chain index27, with some studies suggest it may be a more sensitive indicator for intrathecal IgG production28.
The current gold standard to quantify disability is the Expanded Disability Status Scale (EDSS), which is vulnerable to intra- and inter-rater variability16 and in many cases over-emphasizes ambulation and may not capture meaningful but subtler symptoms. Other clinical markers include patient-reported outcomes like the Patient Determined Disease Steps (PDDS). Potentially more nuanced clinical measures, such as actigraphy measuring daily steps and activity patterns, are undergoing investigation as a clinical tool as well.
Despite the current imaging and CSF biomarkers used in the clinic, there is a critical, unmet need for accurate, reliable, objective, and trackable biomarkers. This review highlights some of the emerging imaging and liquid biomarkers in field of MS that have potential for improving MS diagnosis, quantifying current disease activity, assessing response to therapy, and prognosticating future disease activity and disability.
Emerging Imaging Biomarkers
Central vein sign
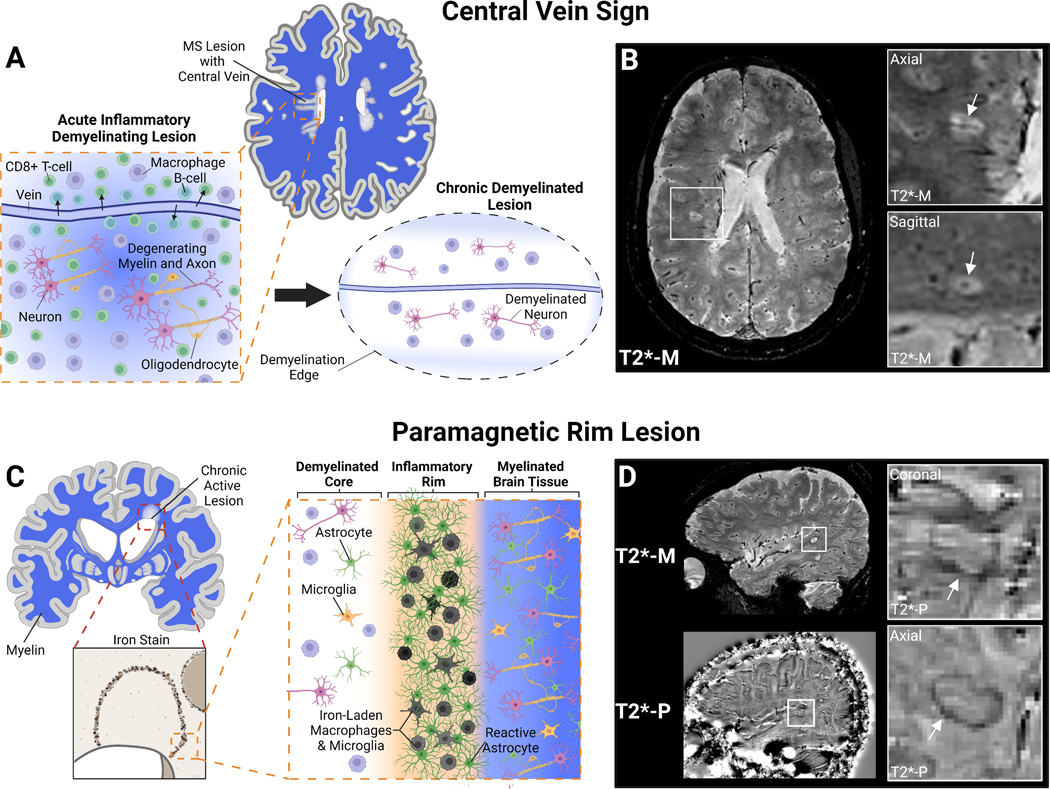
For decades pathology studies have demonstrated that acute white matter lesions in MS are characterized by infiltration of monocytes and lymphocytes from a small central vein29. Recent studies have demonstrated that the presence of a central vein, or “central vein sign” (CVS), within white matter lesions can be reliably imaged by MRI (Figure 1, A and B). The CVS is relatively specific for MS pathologic processes and has the potential differentiate MS from mimicking diseases including migraine30, cerebral small vessel ischemic disease31,32, NMO spectrum disorder33, and inflammatory vasculopathies34, among others35,36. Some rare diseases like Behcet’s disease may have a perivenular lesion burden similar to MS34. While different optimized MRI sequences have been developed to detect the CVS and several different CVS-based criteria (e.g. percentage of CVS lesions vs. CVS lesion count) have been proposed to date to distinguish MS from other mimicking neurological conditions, retrospective studies have shown excellent diagnostic discrimination in a meta-analysis (sensitivity 91%, specificity 96%)37. CVS is also detectable in patients diagnosed with RIS and CIS and may be able to help prognosticate those who will convert to MS36,38,39. While CVS seems poised to be a clinically useful biomarker for MS diagnosis, it does not appear to differentiate between MS subtypes (ie. RRMS vs. PMS) in the above studies. To date, the utility of CVS in MS diagnosis has mainly been studied in cross-sectional and retrospective studies. The “Central Vein Sign” A Diagnostic Biomarker in Multiple Sclerosis” (CAVS-MS) is a prospective, international, multicenter study that recently completed its recruitment of >400 subjects with and without typical MS presentations to evaluate CVS as an MS diagnostic biomarker, with the goal to rapidly translate CVS into clinical care40.
Figure 1: Emerging MRI Biomarkers in MS: Central Vein Sign and Paramagnetic Rim Lesions.
A) White matter lesions in MS are acutely result of immune cell infiltration, particularly CD8+ T-cells, from the periphery into the CNS via small penetrating veins. These inflammatory lesions result in oligodendrocyte and myelin damage as well as neuro-axonal degeneration. After peripheral lymphocyte infiltration resolves a chronic demyelinated lesion centered around a vein remains B) Certain MRI sequences can depict white matter pathology and small CNS vessels simultaneously (e.g., T2*-weighted magnitude reconstruction; T2*-M). These small veins within classic ovoid MS lesions can be visualized and quantified to aid in MS diagnosis. Inserts show confirmation of central vein in two planes. C) Chronic active lesions in MS can be identified pathologically by an iron-rim at the lesion edge that contains iron-laden macrophages and microglia as well as activated astrocytes. D) These iron-rimed chronic active lesions can be visualized on MRI as paramagnetic rim lesions (PRLs) by “unwrapping” the phase reconstruction of the same T2*-weighted imaging (T2*-P)175. Paramagnetic rim lesions may represent a biomarker of at least one cause of progressive disease in MS. Created with BioRender.com
Paramagnetic rim lesions
Many MS lesions after the resolution of the acute inflammatory phase remain demyelinated and a small subset can become chronic active lesions (CALs). CAL have a hypocellular, demyelinated center with peripheral iron-laded activated CD68+ microglia/macrophages and reactive astrocytes41–43. These lesions can slowly expand over time likely reflecting at least one potential mechanism of progressive disease44,45. Pathology studies have shown CALs are present in the majority of PwMS with a higher prevalence in more severe disease and progressive disease courses8,46. Susceptibility-based MRI sequences (ie. Gd-T2*-EPI) identify paramagnetic rims around some non-enhancing chronic lesions (paramagnetic rim lesions, PRLs) that MRI-pathological correlation studies have been shown to identify with high-accuracy iron-enriched microglia at the edge of CALs45,47,48. PRLs are thus a reliable imaging surrogate of a at least a subset of CALs (Figure 1, C and D).
The presence of at least one PRL in the supratentorial brain is common in all MS phenotypes (~50% in RRMS and CIS) and a higher prevalence of PRLs are found in patients with progressive disease, highlighting potential prognostic implications44. PRLs are relatively specific for MS compared to other neurologic inflammatory disorders such as NMOSD, systemic lupus erythematosus, Behcet’s disease, Sjogren’s syndrome, CNS vasculitis, and neurosarcoidosis, with the exception of Susac Syndrome which features lesions resembling PRL in the corpus collosum (notably without CVS)49. This specificity is affirmed in a study that demonstrated that all CIS patients with at least one PRL developed RRMS over mean follow-up period of 4.6 years, outperforming oligoclonal bands39. Despite the high specificity, as only about half of MS cases have at least one PRL, the diagnostic sensitivity is poor. In the largest published study to date, the presence of at least one PRL had sensitivity of 52% and specificity of 93% for MS diagnosis49. In this same cohort CVS (present in >40% of an individual’s visualized lesions) could significantly better discriminate MS from non-MS cases with high specificity (96%) and sensitivity (99%), though combining PRL and CVS criteria did improve specificity to 99%. This and other studies suggest that PRL might improve diagnostic specificity when combined with CVS, particularly in setting of high-suspicion of MS despite low frequency of CVS such as in the context of small vessel disease comorbidity. While the diagnostic utility of PRLs may be limited, a higher PRL burden associates with higher disability and MS severity49, suggesting a role in prognostication particularly in progressive disease. This is underscored by the finding that more than half of MS cases on DMT continue to have PRLs, a marker of chronically inflamed lesions, even in PwMS receiving highly-effective antibody-based therapy. PRLs may therefore serve as a marker of persistent, low-level inflammation that may require adjunctive therapies to target and prevent lesion expansion and insidious clinical decline.
Other MRI biomarkers
Several other MRI imaging findings have evidence as biomarkers in MS prognosis, including brain atrophy7,50,51, spinal cord atrophy52–54, cortical lesions9,55,56, enlarged perivascular spaces57, and leptomeningeal enhancement58. CNS atrophy, both global and regional, serves as a surrogate of neurodegeneration. Cortical lesions also associate with progressive disease as well as cognitive symptoms59–61. Cortical lesions, particularly intracortical and subpial lesions, are difficult to detect on clinically available MRI, currently limiting their potential utility62,63. A number of other challenges remain to be overcome before reliable detection of cortical lesions or quantitative MRI can be translated from the research setting to the clinic64,65.
Optical coherence tomography
Retinal optical coherence tomography (OCT) is an emerging imaging biomarker that is rapid, practical, and non-invasive. OCT is increasingly utilized in trials and clinical centers in MS and in optic neuropathy patients, as adjunct information that in some situations can aid in differential diagnosis, prognosis, and monitoring response to therapy in MS and related conditions66,67. OCT allows for measurement of retinal layers on the scale of microns via an interference pattern generated by infrared light beam reflection. The retina is the most easily accessible part of the CNS and is a common site of blood-retina barrier breakdown, local inflammation, and degeneration. Specific measures of interest include the retinal nerve fiber layer (RNFL) and the ganglion cell-inner plexiform layer (GCIPL). Both these layers thin with loss or damage to the retinal ganglion cells whose axons make up the optic nerve. These measures correlate with and predict cerebral atrophy, MS course and disability, response to DMT, as well as low-contrast visual acuity68–70. Symptomatic optic neuritis (ON) occurs in at least half of MS cases, but regardless of known ON history, some immune mediated demyelination of the optic nerve and subsequent retinal ganglion cell pathology occurs in nearly all people with MS. RNFL data may be more limited by expected transient thickening during acute ON, gliosis-obscured atrophy, and poorer RNFL inter-scan reliability, versus the GCIPL71. Growing data is emerging about other retinal measures of interest. For example, about 5% of those with MS have microcystic macular pathology, which may be associated with a more severe MS course72. How differing pathological patterns in OCT may serve as a proxy for disease processes in the cerebrum remains an area of active investigation.
Emerging Liquid Biomarkers
Clinical history, physical exam, and MRI are currently the gold standard to diagnose and monitor clinical activity over time in MS. While current imaging biomarkers are excellent at identifying new active inflammatory lesions and novel biomarkers such as CVS seem most likely to contribute to increasing MS diagnostic accuracy, current clinically available imaging is limited in its ability to differentiate MS subtypes or identify and quantify subclinical disease and progressive disease where acute inflammation and new lesions may be absent. Additionally, imaging is expensive, time consuming (particularly for more advanced analyses), and difficult to standardize. Liquid biomarkers, particularly in the blood or other non-invasive body fluid, have great potential to meet the unmet need for pragmatic, cost-efficient, and repeatable markers.
Neuroglial proteins
Neurofilament Light Chain
Neurofilament light chain (NfL) is an emerging biomarker of neuro-axonal injury in several neurological conditions including MS. Neurofilaments are neuron-specific intermediate filaments that are components of the cytoskeleton. With loss of neuronal membrane integrity in the CNS, neurofilaments are released into the extracellular space and ultimately into the cerebrospinal fluid and the blood. NfL and other neurofilaments are not specific to MS and can be elevated from any cause of neuronal injury, including other CNS neuroinflammatory disorders such as NMO or MOGAD73,74. While initial studies focused on detection of NfL in the CSF, newer more sensitive assays have allowed for detection of plasma and serum NfL (sNfL). Several studies have demonstrated that sNfL correlates tightly with CSF NfL in CNS disease, including in PwMS75–77, making sNfL a potential non-invasive biomarker of neuronal damage within the CNS.
CSF and sNfL are higher in MS compared to healthy controls or individuals with non-inflammatory neurologic disorders75–78, suggesting sNfL may improve MS diagnosis. Additionally, sNfL may be increased up to six years prior to first clinical symptoms suggesting neuroaxonal damage occurs during a prolonged MS prodromal phase79. Consistent with the above, several studies demonstrate that sNfL increases the sensitivity and specificity of differentiating patients with MS from both CIS and RIS80–83, which could enable early DMT initiation in CIS and RIS patients with high-risk for conversion to MS.
In addition to aiding in diagnosis, sNfL may also be a biomarker for both concomitant and future disease activity. Several studies have shown that sNfL correlates with disease activity and baseline MRI lesion burden and can predict future acute clinical activity and new gadolinium-enhancing and T2 MRI lesions75,76,80,84–87, as well as future brain and spinal cord atrophy and disability worsening75,84–88. Consistent with sNfL’s role as a disease activity indicator, DMT use correlates with lower sNfL levels. More specifically, higher-efficacy monoclonal antibody therapies (i.e. ocrelizumab, natalizumab, alemtuzumab, rituximab) seem to lower sNfL levels with greater efficacy than oral therapies (i.e. dimethyl fumarate, fingolimod, teriflunomide), all of which were more efficacious in lowering sNfL than platform therapies (interferons and glatiramer acetate)80,84. This data suggests that sNfL may also be able to assess DMT-efficacy, with stable or low sNfL levels able to help exclude clinical or subclinical inflammatory disease activity.
While the growing evidence for sNfL’s utility in inflammatory MS activity is strong, its role in PMS, where biomarkers are particularly needed, is less clear. Current data highlights that active inflammation is a major contributor to sNfL even in patients with progressive disease. Separating this acute inflammatory disease activity from insidious disease and gradual disability worsening that defines progressive MS presents a difficult challenge. There is significant disagreement in reported data comparing NfL (CSF and serum) between patients with RRMS and PMS, with several studies reporting higher NfL in PMS compared to RRMS, several lower in PMS compared to RRMS, and the majority finding no difference (reviewed in89). These discrepancies are likely explained by associations with other factors that differ between these groups and associate with NfL levels, especially age. Supporting this, analyses including age, disability status, recent relapses, and DMT-treatment status as covariates often results in loss of significance between these groups75,85,90. Similarly, some studies found a significant, albeit marginal, relationship between baseline sNfL and conversion from RRMS to SPMS, whereas other studies did not91–93.
Overall, current data support that sNfL levels provide a good reflection of ongoing and future neuroaxonal damage in the setting of inflammatory disease activity in MS. While sNfL may be a useful biomarker across several aspects of MS care, to date sNfL has largely been investigated on a group level and only a handful of studies have looked at predictions on an individual level. Interpretation of individual sNfL levels are also complicated by several common factors that associate with higher sNfL levels including age, body mass index, impaired renal function, diabetes mellitus, and active smoking, underscoring the need for normative sNfL data to correct for these factors84,87,94. Prospective clinical use of sNfL on an individual MS patient level has not yet been established. Its recent commercial approval will likely shed more light on its role in this context.
Chitinase-3-like 1
Chitinase-3-like 1 (CHI3L1/YKL-40) is a pro-inflammatory secreted glycoprotein of unclear function that has been purported as a potential marker of reactive astrocytes and microglia/macrophages, though it is also expressed on peripheral cells including monocytes, chondrocytes, neutrophils, and vascular smooth muscle cells, among other cell types95,96. Initial studies suggested that CSF CHI3L1 is primarily intrathecally produced and that CSF levels do not correlate with serum levels 95,97,98. Elevated CSF CHI3L1 has also been shown to associate with higher-risk and faster time for conversion from CIS to MS, faster development of disability, brain MRI lesions, and brain atrophy and may decrease with DMT initiation83,95–100. A recent meta-analysis found that CSF CHI3L1 levels were higher in patients with MS compared to healthy controls, higher in people with PPMS compared to both RRMS and SPMS, and higher in those with CIS who converted to MS compared to those that did not convert101. Interestingly, pathologic studies have shown that in CALs with high inflammatory activity, CHI3L1 is expressed highly at the lesion edge in reactive astrocytes and CD68+ macrophages/microglia95, emphasizing the possibility that CHI3L1 may in part associate with CALs with iron rims. Supporting this potential relationship, a recent study found that CSF CHI3L1 associates with PRLs in PwMS after their first demyelinating event 102. In summary, CSF CHI3L1 is a potentially useful CSF biomarker in MS, perhaps particularly in CIS, but less invasive biomarkers (serum and imaging) may be more beneficial for tracking disease activity and response to therapy over time.
Glial Fibrillary Acidic Protein
Glial fibrillary acidic protein (GFAP) is the primary cytoskeletal protein in astrocytes and an established marker of astroglial reactivity (astrogliosis) and astrocyte damage. GFAP is also found in non-myelinating Schwann cells in the peripheral nervous system, Mueller cells in the retina, and in glia of the enteric nervous system, and to a lesser extent among other neurological and non-neurological cells. GFAP plays a role in the extension of astrocyte processes formed in response to injury, as part of the dynamic intermediate filament network, and supports interactions with neighboring neurons and the blood-brain barrier.
Similar to NfL, cell membrane permeability changes may facilitate leakage of GFAP. There may also be an increase in intracellular GFAP levels from increased expression as part of physiological or pathological injury response. GFAP may be released into the CSF and then through the CSF-blood barrier, and likely also released into the glymphatic system/direct venous drainage103. Correlation is high between CSF and blood GFAP in MS patients and controls104, suggesting serum levels reflect CNS pathology. Though some degree of astrocyte response is likely beneficial, pathologically reactive astrocytes are proposed to be key drivers of neurologic damage in MS105. Much of the clinical data regarding GFAP is from post-traumatic brain injury outcomes106, though recent studies highlight its promise for use in MS. As with many liquid biomarkers in MS, differences between relapsing and progressive disease have been variable, largely complicated by variability of RRMS data gathered around time of active lesions versus in stable periods, expected and typical age differences between PMS vs RRMS, with PMS patients tending to be older and GFAP, as with NfL, higher with advancing age104,107–109.
Some studies reported GFAP is mildly elevated in RRMS versus healthy controls and patients with non-inflammatory neurological disorders, though these findings may be driven by RRMS patients with recent relapses74,104,110; those with stable, inactive RRMS may have similar blood GFAP levels to healthy controls74. Interestingly, GFAP elevation or lack thereof around time of acute relapse has been inconsistent across studies74. Promisingly, some studies suggest that serum GFAP is elevated in progressive MS versus RRMS78,108,111, though some of these studies found no difference between progressive versus RRMS after adjusting for age104. Blood GFAP has been shown to correlate with clinical disability in MS as measured by EDSS107,109,111 and with lesion burden104,107,111. Though GFAP and NfL often correlate104,107,109,111, instances where they diverge may provide particularly useful information. One large recent study found that elevated blood GFAP predicted poorer disability status in six months in PwMS, particularly in those with low sNfL and who did not have recent relapses108. Another group suggested a “glia score” that integrates multiple biomarkers (GFAP x CHI3L1 / sNfL), which they found was higher in SPMS than RRMS patients, and correlated with EDSS in SPMS patients112. Blood GFAP has also proven to be increased in NMO versus healthy controls or MS, with early data supporting exploration of use of GFAP:NfL ratio during acute relapse74. Assessing both NfL, GFAP, and perhaps other biomarkers simultaneously may be useful for differentiating MS across different stages of the disease and may assist with prognosis and response to therapy. Overall, data suggests it is possible that GFAP may better reflect progressive disease in PMS without relapse whereas sNfL may better reflect acute relapsing disease activity.
Parvalbumin
The budding success of NFL, GFAP, CHI3L1 and other neuronal and glial proteins as biomarkers of neurologic disease has led to the search of even more nuanced, cell-specific based markers of distinct CNS pathology. For example, parvalbumin is a protein specifically expressed in GABAergic interneurons and CSF levels could be a specific marker of grey-matter neurodegeneration in MS. Cortical neurodegeneration is associated with meningeal B-cell follicles and progressive disease. A recent study by Magliozzi et al. showed parvalbumin gene expression and parvalbumin-positive cell density in the motor cortex are decreased in PwMS versus controls. CSF parvalbumin levels negatively correlated with parvalbumin-positive cell density and were increased in MS compared to controls. CSF parvalbumin levels also associated positively with cortical lesion number and global cortical thickness on MRI, microglia density in the motor cortex, earlier age of MS onset, faster disability progression, and severity of cognitive impairment113. These initial results suggest CSF parvalbumin may reflect loss of cortical interneurons in MS and associated cortical neurodegeneration, atrophy, and cognitive decline. However, parvalbumin is also highly expressed in fast-contracting muscle fibers and thus serum parvalbumin is thought to be an indicator of muscle pathology, limiting the ability of serum parvalbumin to be a useful biomarker for CNS disease.
Extracellular vesicles
Extracellular vesicles (EVs) are small lipid-bound particles that facilitate cellular communication through their contents of bioactive proteins, nucleic acids, and lipids. EVs released from CNS cells such as neurons, astrocytes, microglia, and oligodendrocytes can cross into the blood, urine, and tears. Cell surface receptors on EVs as well as their contents can be used to identify their parental cell of origin. This makes CNS-derived EVs in blood a non-invasive source of potential cell-type specific biomarkers. CNS-derived EVs from several cell types have been shown to play important roles in MS and animal models of demyelination including myelin damage, inflammatory signaling, blood-brain-barrier breakdown, and neuroplasticity. CNS-derived EVs, including myeloid and endothelial-derived EVs have been shown to be elevated in the CSF of PwMS particularly in association with acute active disease114,115, suggesting they may serve as measures of neuroinflammation. Myeloid EVs are elevated in CIS and higher levels associate with a shorter time to further disease activity81. Recently several studies have started examining the EVs cargo including microRNAs, proteins, and lipids as potential biomarkers in MS (reviewed in116). One study by Galazka et al., showed that MOG was elevated in serum-derived EVs in RRMS during relapse and also in SPMS, potentially reflecting MOG within CSF-derived EV117. MOG, an immunogenic myelin protein expressed only on the surface of myelin sheaths and oligodendrocyte membrane, likely directly reflects oligodendrocyte pathology in this context.
The research of EVs as biomarkers in MS is in its infancy and much remains unexplored particularly regarding cell-type specific EVs and their relation to disease activity and prognosis. A recent study found that neuronal-enriched EVs had lower levels of synaptopodin and synaptophysin in MS compared to controls potentially reflecting synaptic loss in MS118. Versus controls, PwMS were found to have higher levels of multiple early classical complement cascade components in astrocyte-derived EVs. This suggests a potential link to astrocyte complement production, which is thought to opsonize synapses and has been implicated in several neurodegenerative disorders including MS. Importantly, these differences in synaptic and complement proteins were not found in total EVs or neat plasma, demonstrating that CNS-enriched EVs may prove to be unique reservoirs of biomarkers in neuroinflammatory diseases. In a follow-up study119, altered mitochondrial complex activity in neuronally-enriched EVs was significantly associated with faster brain and retinal atrophy in MS, exemplifying that neuronal-derived EVs also have potential to provide a unique assessment of neuronal health and pathology in MS. Expanding the repertoire of cell-specific EVs, a recent study by Mazzucco et al., presented a method to isolate CNS-endothelial derived EVs from plasma with results from their pilot study suggesting increased levels of these EVs in PwMS with active disease compared to healthy controls and PwMS with stable disease or on high-efficacy therapy120. Thus CNS-derived EVs may be a biomarker of blood-brain barrier permeability and active disease in MS. Overall, preliminary findings support CNS-derived EVs and their contents as promising candidates to serve as novel biomarkers of disease activity and progression in MS and other neurological conditions.
CSF and serum inflammatory mediators and other potential liquid biomarkers
CSF and Serum Inflammatory Mediators
Several cytokines, chemokines, and other inflammatory mediators are altered in the CSF and sometimes blood in MS and some have correlated with disease activity and future disability (Table 1). One example is CXCL13, a major chemoattractant involved in recruiting B-cell and some T-cell subsets, including follicular T-helper cells, into the CNS. CXCL13 has been implicated in the formation of ectopic lymphoid follicles in the CNS in MS, particularly progressive MS121. CXCL13 has repeatedly been shown to be elevated in the CSF in MS, especially RRMS122–127. Some studies report a correlation between CSF and serum CXCL13 levels, including in PwMS128. CSF CXCL13 can be used to predict conversion to MS from CIS129–131. CXCL13 CSF levels also correlated with current and future disease activity, particularly in RRMS, including relapse rate, disability, and MRI lesions124,127,128. CSF and serum CXCL3 levels decreased with steroids, with DMT including B-cell depleting therapy, and in some cases may predict response to therapy127,132–134.
Table 1:
Summary of diagnostic and prognostic features of select emerging biomarkers in MS.
| Biomarker | Compartment | Diagnosis/Classification | Disease Activity/Prognosis |
|---|---|---|---|
| Neuroglial Proteins | |||
| NfL | CSF, serum, plasma. Good correlation. | CSF & Serum: ↑ in RMS & PMS vs. HC75–78; ↑↓ in PMS vs. RMS (conflicting results) 89,176 | Serum: ↑ CIS or RIS that converts to MS80–83; ↓ with DMT in RMS or PMS80,84,177; ↑ disease activity in RMS75,76,80,84–86; ↑ risk of new MRI lesions 76,80,84,86; ↑ risk of brain / spinal cord atrophy85 |
| GFAP | CSF, serum, plasma. Good correlation104 | Serum: ↑ in active RMS vs. NIND & HC 74,104,110; ↑↓ in PMS vs. RMS (conflicting results) 78,104,108,111; ↑ in NMOSD vs. MS or HC 74 | Serum: ↑ risk of worsening EDSS107,109,111; ↑ lesion progression on MRI104,107,111 |
| CHI3L1/YKL-40 | CSF. CSF levels do not correlate with serum | CSF: ↑ in RMS vs. HC; ↑ in PMS vs. RMS & SPMS 101 | CSF: ↑CIS that converts to MS; ↑ likelihood of disability progression; ↓ with DMT; ↑ risk of new MRI lesions 83,95–100 |
| Parvalbumin | CSF | CSF: ↑ PMS vs. HC113 | CSF: ↑ increased cortical lesion number; ↑ cortical thinning; ↑ cognitive impairment113 |
| BDNF | Serum, plasma | Serum and plasma: ↓ in MS compared to HC178,179 | Serum: ↓ increased MRI lesions180 |
| Osteopontin | CSF, serum. Some correlation between CSF and serum | CSF and serum: ↑ in PMS & RMS vs. NIND & HC181 Serum: ↑ in RMS vs. CIS & SPMS181 |
CSF: ↑ in active MS vs. stable MS182; ↑ risk of brain atrophy181,183 |
| Neurogranin | CSF | CSF: conflicting results, no difference or ↓ in MS vs. HC184,185 | CSF: ↑ in MS with enhancing lesions vs. MS without enhancing lesions185 |
| NSE | Serum, plasma, CSF. Good correlation between sources | CSF: ↓ in CIS vs. HC186; CSF and plasma: no change between RMS & PMS vs. HC187,188 |
Serum and plasma: conflicting results, no change or negative correlation with EDSS and MSSS187–189 |
| KIF5A | CSF | CSF: ↑ in PMS vs. RMS, NIND, HC190 | CSF: ↑ in RMS correlates with worsened EDSS, MSSS and ARMSSS at 2 year follow up190 |
| Cytokines and Chemokines | |||
| TNFα | Serum, CSF | CSF & serum: ↑ MS vs. healthy control 191,192; CSF: ↑ RMC > PMS > HC193 | |
| IL-4 | Serum, CSF | Serum and CSF: Conflicting results. Large meta-analysis shows no difference in MS vs. HC191 | |
| IL-6 | Serum, CSF | Serum and CSF: Conflicting results. Large meta-analysis shows no difference in MS vs. HC191 | Serum: ↓ with DMT191 |
| IL-8 (CXCL8) | Serum, CSF. Serum does not correlate with CSF194 | Serum: ↑↓ MS vs. healthy control191,194 (conflicting results) CSF: ↑ RMS & PMS vs. healthy control193,194 |
|
| IL-10 | Serum, CSF | CSF: ↑ in RMS & PMS vs. HC193 | Serum: ↑ CIS that converts to MS or NMOSD195; ↑ with DMT (IFN-b1a)196; ↑ in MS remission compared to relapse197; ↓ increased risk of relapse in pediatric MS198 |
| CCL11 (Eotxain-1) | Plasma, CSF | CSF & plasma: ↑ SPMS vs. RMS199 | CSF & plasma: ↑ longer duration of disease199 |
| IL-12p40 | Serum, plasma, CSF | CSF, plasma, serum: ↑ MS vs. healthy control191,199 & NIND CSF: ↑ in CIS vs. HC166 |
|
| CXCL13 | CSF. Serum does not correlate with CSF 128,137 | CSF: ↑RMS and PMS vs. NIND & OIND122–127 | CSF: ↑CIS that convert to MS129–131; ↑ disease activity in RMS124,127–129
CSF>Serum: ↓ with DMT 127,132,133 Serum: Predict response to fingolimod134 |
| IL-17A | Serum, CSF | Serum: ↑ MS vs. healthy control191,200; CSF: Large meta-analysis with nearly-significant ↑ in MS vs. HC191. ↑ RMS > PMS > HC196 | Serum: ↓ with DMT (IFN-b1a)196 |
| IL-23 | Serum | Serum: ↑ MS vs. healthy control191 | |
| IL-27 | Serum, CSF | Serum: Large meta-analysis shows no difference in MS vs. HC191. CSF: ↑ in RMS vs. HC193,201 | |
| IL-33 | Plasma | Plasma: non-significant ↑ RMS vs. HC202,203 | Plasma: ↑ decreased lesions number; ↓ with DMT (IFN-b1a)203 |
| IL-36 | Serum | Serum: ↑ RMS vs. HC204 | |
| IL-37 | Serum | Serum: ↑ RMS vs. HC205 | Serum: ↑ with DMT (fingolimod)206; ↑ during MS relapse; ↑ increased EDSS206 |
| IL-38 | Serum | Serum: ↑ in newly diagnosed MS compared to treated207 | |
| Endothelial Cell-Related Proteins | |||
| Endothelian-1 | Plasma | Plasma: RMS or PMS vs. HC208 | CSF: ↑ increased risk of poor visual recovery from optic neuritis209; increased in active vs. stable MS210 |
| Endothelian-3 | Plasma, CSF | Plasma: ↑ MS vs. HC211 | Plasma: ↑ MS disease duration |
| VEGF (total) | Serum | Serum: ↑ RMS vs. HC212 | Serum: ↑ shortened duration between first and second relapses212 |
| VEGF-A | mRNA, CSF | CSF: ↑ in RMS & PMS vs. HC193 | mRNA (from serum monocytes): ↓ in SPMS vs. RMS213 |
| TGF-α / VEGF-β (ratio) | Serum | Serum: ↑ in CIS vs. RMS214 | Serum: ↓ stable RMS vs. HC; ↑ active RMS vs. HC; ↓ increased EDSS214 |
| sNCAM | CSF | CSF: ↓ in MS vs. HC; ↓ in SPMS > RMS > CIS215–218 | CSF: ↑ after natalizumab or mitoxantrone; ↓ after fingolimod treatment216; ↓ increased EDSS215 |
| VCAM1 | Serum, CSF | Serum and CSF: ↑ in active RMS & PMS vs. HC219 | Serum and CSF: ↑ during MS relapse vs. remission219 Serum: ↑ decreased number of MRI lesions. ↑ with DMT (IFNB-1b)220 |
| Mitochondria and Autophagy-Related Proteins | |||
| Parkin | Serum, CSF | Serum and CSF: ↑ in RMS & PMS vs. HC221–223 | Serum and CSF: ↑ in MS with enhancing lesions vs. MS without enhancing lesions224 |
| ATG5 | Serum, CSF | Serum and CSF: ↑ in RMS vs. HC222,223 | Serum and CSF: ↑ in MS with enhancing lesions vs. MS without enhancing lesions224 |
| ANT1 | Serum, CSF | Serum and CSF: ↓ in RMS vs. HC222,223 | |
| Other | |||
| Kynurenine | Serum, CSF | CSF: ↓ in remission RMS vs. HC225; ↑ in relapsing RMS vs. HC226
Serum: ↑ in active RMS vs. HC and PMS226–228 |
|
| CCN3 | Plasma, CSF. Good correlation between sources229 | Plasma: ↑ in PMS vs. RMS229 | |
| Vitamin D | Serum | Serum: ↑ lower risk of developing MS152,230; ↓ neonatal vitamin D increased risk of developing MS231; ↑ lower degree of brain atrophy; ↑ less clinical progression at 5 year232,233; ↓ increased relapse risk232 | |
Up (↑) and down (↓) arrows designate increase or decrease of the biomarker collected from the designated compartment(s). Correlations are statistically significant except where otherwise noted; topics with conflicting results are reported with up/down (↑↓) arrow.
Abbreviations: CSF = cerebrospinal fluid; MS = multiple sclerosis; RMS = relapsing multiple sclerosis; PMS = progressive multiple sclerosis; SPMS = secondarily progressive multiple sclerosis; CIS = clinically isolated syndrome; RIS = radiologically isolated syndrome; HC = healthy control; NIND = non-inflammatory neurologic disorder; EDSS = expanded disability status scale; NMOSD = neuromyelitis optica spectrum disorder; DMT = disease modifying therapy; NfL = neurofilament light chain; GFAP = glial fibrillary acidic protein; CHI3L1 = chitinase-3-like protein 1; BDNF = brain derived neurotrophic factor; NSE = neuron specific enolase; KIF5A = Kinesin family member 5A protein; VEGF = vascular endothelial growth factor; sNCAM = soluble neural cell adhesion molecule; VCAM = vascular cell adhesion molecule; ATG5 = autophagy related 5 protein; ANT1 = adenine nucleoside translocator 1; CCN3 = cellular communication network factor 3
However, several common issues exist with many of these inflammatory mediators as biomarkers in MS. Many of these molecules are elevated in other inflammatory neurologic conditions, limiting their diagnostic specificity. For example, CXCL13 has been shown to be elevated in NMO, neurosarcoidosis, primary CNS lymphoma, idiopathic transverse myelitis, Lyme neuroborreliosis, and viral and bacterial meningitis123,124,126,135,136. Moreover, many are relatively small molecules that can cross the blood-CSF and/or blood-brain barrier, so serum levels may contribute greatly to CSF concentrations, requiring correction137. While CXCL13 levels correlate between CSF and serum in PwMS in some studies, this correlation is markedly stronger in people with non-inflammatory neurologic conditions128 Thus, serum levels are not a good predictor of CSF levels, particularly when CSF levels are high. Many of these potential biomarkers that are produced in the periphery also do not correlate, or do not correlate well, with their CSF levels, which may more accurately reflect intrathecal pathology. Additionally, some of these potential biomarkers that are only produced significantly in the CNS are not detectable reliably in the blood using standard commercially available assays, with more sensitive assays not readily available in clinical laboratories. The inability to assay these biomarkers in the blood limits their promise as applicable biomarkers.
Micro RNAs
MicroRNAs (miRNA/miR) play a vital role in gene-regulation, through targeting messenger RNAs for cleavage or translational repression. Several miRNAs have been shown to regulate processes critical to MS including oligodendrocyte development, myelination, and inflammatory responses138,139. Several studies have profiled miRNAs in blood, other biological fluids, or cells in MS, with some miRNAs differing between MS and healthy controls, MS subtypes, or with outcomes such as MRI lesions, disability, or response to therapy138,140–143. Though there are conflicting results and lack of replication in the miRNA biomarker literature, several miRNAs with known roles in inflammatory signaling including regulation of lymphocyte subsets have been identified in multiple MS studies including miR-145, miR-155, and miR-92a 140,143–148. The role of miRNAs as biomarkers and as potential therapies in MS are reviewed in detail elsewhere149,150.
Vitamin D
Decades ago epidemiological studies first noted that MS prevalence is lowest along the equator and increases with increasing latitude 151. Scientists hypothesized that perhaps sunlight dependent biology, such as Vitamin D synthesis, was involved in this phenomenon. Observational studies supported this notion in finding that individuals with low serum 25-hydroxyvitamin D levels or lower vitamin D intake have higher risk of developing MS and having more severe MS disease152–155. Vitamin D has known immunologic effects on both the innate and adaptive immune system156. Further supporting a role for Vitamin D in CNS autoimmunity, Vitamin D supplementation suppressed experimental autoimmune encephalomyelitis157,158, the CD4+ T-cell dependent demyelinating mouse model of MS, and moreover the therapeutic effects required Vitamin D receptor function in T cells159. However, numerous trials indicate that Vitamin D supplementation provides little, if any, benefit in PwMS160. Furthermore, risk for lower serum 25-hydroxyvitamin D as determined by polygenic risk scores was not associated with worse disease outcomes in PwMS161. Several ongoing Vitamin D supplementation studies in MS may provide additional insight into the potential role of Vitamin D in PwMS. At the current time, Vitamin D levels are not an adequately sensitive or specific diagnostic or prognostic tool.
Epstein Barr virus related-biomarkers
Epidemiologic research to identify a viral trigger of MS over the last few decades has suggested that Epstein Barr Virus (EBV) infection may be necessary but not sufficient for development of MS in most, if not all, cases162–164. A recent study by Bjornevik et al. demonstrated a 32-fold increased risk of MS after EBV infection, but not other viruses, in a large U.S. military cohort165, reigniting the field’s interest in EBV as a putative causal agent of MS. Several studies suggest that symptomatic EBV infection (i.e. infectious mononucleosis) confers a higher risk of MS than asymptomatic EBV infection163,166–168. Additionally, higher titers of anti-EBV-nuclear antigen (EBNA) antibodies associate with increased MS risk162,166. These findings may suggest that individuals with decreased ability to control EBV infection may be at most increased risk due to higher chance for EBV to activate the pathologic processes that lead to MS, although this could also be a reflection of a heightened humoral response in PwMS. Consistent with the hypothesis that EBV is an immunological driver of disease, there are possible interactions between HLA and other genes involved with B and T-cell activation, anti-EBNA-1 antibody levels, and MS risk169–171. There is high sequence homology between an EBV peptide and the encephalitogenic epitope of myelin basic protein that is presented by a major MS risk allele, HLADRB1*1501172. In addition to postulating cell-mediated molecular mimicry, EBV may evoke cross-reactive antibodies produced by clonally expand CSF B-cells in MS bind to EBV EBNA1 and cross-react to the CNS protein GlialCAM173. Several additional potential mechanisms by which EBV might alter the host immune system to promote CNS autoimmunity have been postulated and described (reviewed in174). Notably, while large and well-designed epidemiologic studies support the causal role of EBV in MS, experimental evidence of causation is lacking.
While the potential causality of EBV in MS engenders thoughts of the potential therapeutic implications, namely would vaccinations or treatments against EBV decrease MS risk or severity, it also suggests that EBV infection and its downstream pathogenic mechanisms could be useful biomarkers in MS as well as in other diseases that implicate EBV. The high seroprevalence of EBV (~95% in health adults) makes EBV serology or other measures of past-infection of limited utility to rule-in MS, but the absence of evidence of prior EBV infection, particularly if confirmed by multiple methods, could be a red flag against a diagnosis of MS162–165. Studies have not found an association between anti-EBNA1 IgG levels and risk of conversion from CIS to MS, but the potential remains for markers of EBV-specific biology to serve as diagnostic, disease activity, and prognostic biomarkers. As more is understood about the immunologic pathomechanisms that EBV exerts on the immune system to potentially cause MS, the field may identify a molecular signature unique to MS pathogenesis that can aid in diagnosis and prognosis.
Conclusion
An ideal biomarker is highly sensitive and specific, reproducible, non-invasive, and easy to interpret. Such biomarkers are currently lacking in MS care, limiting ability to diagnose, prognosticate, and monitor treatment response in PwMS, and also hindering development of new interventions particularly for progressive disease. However, several biomarkers seem poised for integration into routine MS clinical care and interventional trials in the next decade. If ongoing clinical trials affirm the ability of CVS to aid in MS diagnosis this imaging biomarker has the potential to be incorporated rapidly into MS diagnostic work-up. While additional studies are needed to validate appropriate interpretation in various clinical scenarios, tests for NfL levels both in the serum and CSF are clinically available and there is considerable evidence suggesting they may be a useful biomarker of ongoing neuro-axonal injury in MS and may aid in prognosis and treatment decisions. Additional biomarkers that represent cell-specific and pathology-specific processes occurring in various stages of MS, especially progressive disease, are greatly needed, and several candidates have been presented in this review. These biomarkers have the potential both for MS clinical care and in research studies and clinical trials to define and develop novel therapeutic approaches. There are several potential candidate biomarkers and biomarker reservoirs that are promising, and in the future MS clinicians will hopefully have a panel of biomarkers capable of aiding in predicting and monitoring disease activity and treatment response in PwMS.
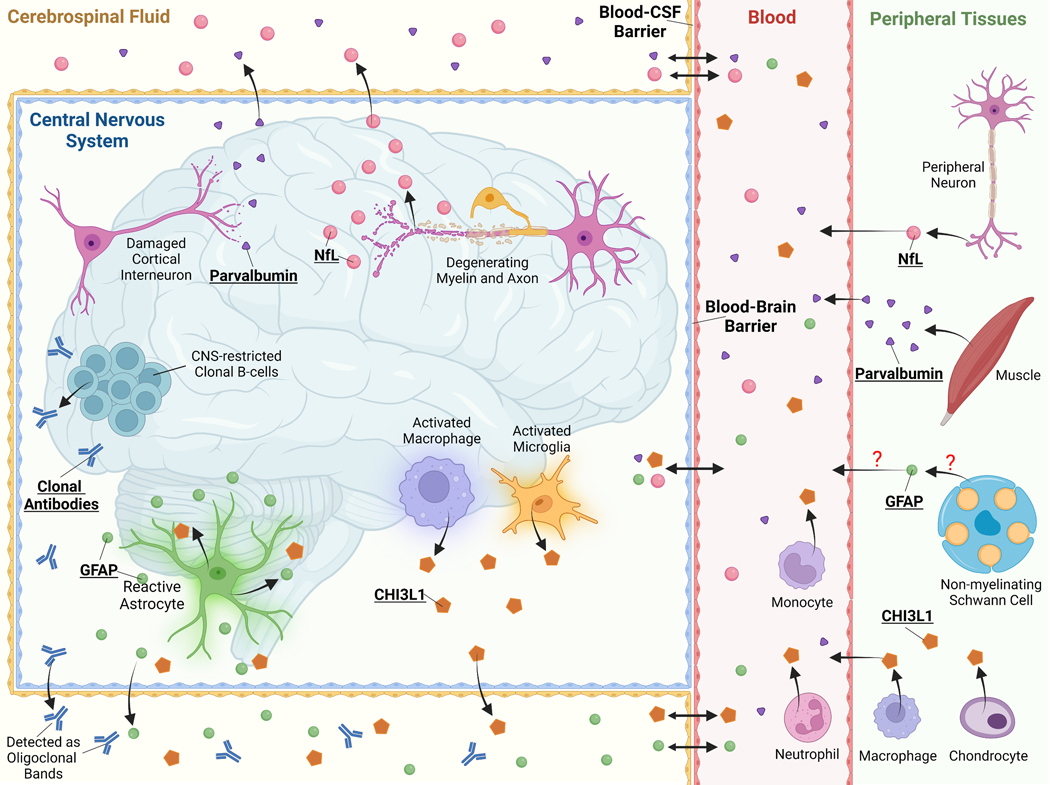
Figure 2: Emerging Neuroglial Biomarkers in MS.
Schematic of the CNS, periphery, and blood-brain barrier, and blood-CSF barrier cell types relevant to emerging neuroglial biomarkers and CSF-specific oligoclonal bands. Released neuroglial protein biomarkers are released from one or a select few CNS resident cell types where they can traffic to the CSF and blood. These cell-specific biomarkers may thus reflect cell-type specific pathology, such as axonal damage in the case of Nfl. Many neuroglial biomarkers also have identified or potential peripheral sources that may, if a significant source, limit or prevent the use of blood levels to be a useful as a biomarker, such as the case with parvalbumin and CHI3L1. Many neuroglial biomarkers cross the CSF-blood barrier and even the blood-brain barrier, particularly in the setting of blood-brain barrier injury such as occurs in an active MS lesion. This equilibrium between CSF and serum or plasma levels is important to determine for each biomarker as high peripheral levels from a non-CNS source may impact CSF levels requiring correction of obtained CSF values. Abbreviations: CHI3L1, chitinase-3 like protein 1; GFAP, glial fibrillary acidic protein; Nfl, neurofilament. Created with BioRender.com
Acknowledgements
A.J.G. is supported by NMSS and ABF FAN-2007-36944. S.P.G. is supported by NMSS and ABF FAN-2106-37832. P.A.C. is supported by NIH R01NS041435 and NMSS RG-1907-34756.
EMS has received fellowship funding from Biogen. PAC has received consulting fees from Biogen, Eli Lilly, Idorsia, and Vaccitech and is PI on a grant to Johns Hopkins University from Genentech.
Footnotes
Conflicts of Interest Disclosure:
AJG and SPG have no conflicts of interest.
Data Availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- 1.Reich DS, Lucchinetti CF, Calabresi PA. Multiple Sclerosis. N Engl J Med 2018;378(2):169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Solomon AJ, Bourdette DN, Cross AH, et al. The contemporary spectrum of multiple sclerosis misdiagnosis: A multicenter study. Neurology 2016;87(13):1393–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamout BI, Khoury SJ, Ayyoubi N, et al. Alternative diagnoses in patients referred to specialized centers for suspected MS. Mult Scler Relat Disord 2017;18:85–89. [DOI] [PubMed] [Google Scholar]
- 4.Kaisey M, Solomon AJ, Luu M, Giesser BS, Sicotte NL. Incidence of multiple sclerosis misdiagnosis in referrals to two academic centers. Mult Scler Relat Disord 2019;30:51–56. [DOI] [PubMed] [Google Scholar]
- 5.Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part I: the role of infection. Ann Neurol 2007;61(4):288–99. [DOI] [PubMed] [Google Scholar]
- 6.Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part II: Noninfectious factors. Ann Neurol 2007;61(6):504–13. [DOI] [PubMed] [Google Scholar]
- 7.Eshaghi A, Marinescu RV, Young AL, et al. Progression of regional grey matter atrophy in multiple sclerosis. Brain 2018;141(6):1665–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luchetti S, Fransen NL, van Eden CG, Ramaglia V, Mason M, Huitinga I. Progressive multiple sclerosis patients show substantial lesion activity that correlates with clinical disease severity and sex: a retrospective autopsy cohort analysis. Acta Neuropathol 2018;135(4):511–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calabrese M, Poretto V, Favaretto A, et al. Cortical lesion load associates with progression of disability in multiple sclerosis. Brain 2012;135(Pt 10):2952–61. [DOI] [PubMed] [Google Scholar]
- 10.Ontaneda D, Raza PC, Mahajan KR, et al. Deep grey matter injury in multiple sclerosis: a NAIMS consensus statement. Brain 2021;144(7):1974–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hohlfeld R, Dornmair K, Meinl E, Wekerle H. The search for the target antigens of multiple sclerosis, part 1: autoreactive CD4+ T lymphocytes as pathogenic effectors and therapeutic targets. Lancet Neurol 2016;15(2):198–209. [DOI] [PubMed] [Google Scholar]
- 12.Healy LM, Stratton JA, Kuhlmann T, Antel J. The role of glial cells in multiple sclerosis disease progression. Nat Rev Neurol 2022;18(4):237–248. [DOI] [PubMed] [Google Scholar]
- 13.Lassmann H. Pathogenic Mechanisms Associated With Different Clinical Courses of Multiple Sclerosis. Front Immunol 2018;9:3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wicken C, Nguyen J, Karna R, Bhargava P. Leptomeningeal inflammation in multiple sclerosis: Insights from animal and human studies. Mult Scler Relat Disord 2018;26:173–182. [DOI] [PubMed] [Google Scholar]
- 15.Hametner S, Wimmer I, Haider L, Pfeifenbring S, Brück W, Lassmann H. Iron and neurodegeneration in the multiple sclerosis brain. Ann Neurol 2013;74(6):848–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barcelos IP, Troxell RM, Graves JS. Mitochondrial Dysfunction and Multiple Sclerosis. Biology (Basel) 2019;8(2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marignier R, Hacohen Y, Cobo-Calvo A, et al. Myelin-oligodendrocyte glycoprotein antibody-associated disease. Lancet Neurol 2021;20(9):762–772. [DOI] [PubMed] [Google Scholar]
- 18.Huda S, Whittam D, Bhojak M, Chamberlain J, Noonan C, Jacob A. Neuromyelitis optica spectrum disorders. Clin Med (Lond) 2019;19(2):169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018;17(2):162–173. [DOI] [PubMed] [Google Scholar]
- 20.van der Vuurst de Vries RM, Mescheriakova JY, Wong YYM, et al. Application of the 2017 Revised McDonald Criteria for Multiple Sclerosis to Patients With a Typical Clinically Isolated Syndrome. JAMA Neurol 2018;75(11):1392–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller DH, Chard DT, Ciccarelli O. Clinically isolated syndromes. Lancet Neurol 2012;11(2):157–69. [DOI] [PubMed] [Google Scholar]
- 22.Hosseiny M, Newsome SD, Yousem DM. Radiologically Isolated Syndrome: A Review for Neuroradiologists. AJNR Am J Neuroradiol 2020;41(9):1542–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez-Gomez J, Sacristan-Enciso B, Caro-Miro MA, Querol Pascual MR. Clinically isolated syndrome: diagnosis and risk of developing clinically definite multiple sclerosis. Neurologia (Engl Ed) 2021 [DOI] [PubMed] [Google Scholar]
- 24.Skov AG, Skov T, Frederiksen JL. Oligoclonal bands predict multiple sclerosis after optic neuritis: a literature survey. Mult Scler 2011;17(4):404–10. [DOI] [PubMed] [Google Scholar]
- 25.Lebrun-Frenay C, Rollot F, Mondot L, et al. Risk Factors and Time to Clinical Symptoms of Multiple Sclerosis Among Patients With Radiologically Isolated Syndrome. JAMA Netw Open 2021;4(10):e2128271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petzold A. Intrathecal oligoclonal IgG synthesis in multiple sclerosis. J Neuroimmunol 2013;262(1–2):1–10. [DOI] [PubMed] [Google Scholar]
- 27.Gaetani L, Di Carlo M, Brachelente G, et al. Cerebrospinal fluid free light chains compared to oligoclonal bands as biomarkers in multiple sclerosis. J Neuroimmunol 2020;339:577108. [DOI] [PubMed] [Google Scholar]
- 28.Vecchio D, Bellomo G, Serino R, et al. Intrathecal kappa free light chains as markers for multiple sclerosis. Sci Rep 2020;10(1):20329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adams CW. The onset and progression of the lesion in multiple sclerosis. J Neurol Sci 1975;25(2):165–82. [DOI] [PubMed] [Google Scholar]
- 30.Solomon AJ, Schindler MK, Howard DB, et al. “Central vessel sign” on 3T FLAIR* MRI for the differentiation of multiple sclerosis from migraine. Ann Clin Transl Neurol 2016;3(2):82–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campion T, Smith RJP, Altmann DR, et al. FLAIR* to visualize veins in white matter lesions: A new tool for the diagnosis of multiple sclerosis? Eur Radiol 2017;27(10):4257–4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mistry N, Abdel-Fahim R, Samaraweera A, et al. Imaging central veins in brain lesions with 3-T T2*-weighted magnetic resonance imaging differentiates multiple sclerosis from microangiopathic brain lesions. Mult Scler 2016;22(10):1289–96. [DOI] [PubMed] [Google Scholar]
- 33.Cortese R, Magnollay L, Tur C, et al. Value of the central vein sign at 3T to differentiate MS from seropositive NMOSD. Neurology 2018;90(14):e1183–e1190. [DOI] [PubMed] [Google Scholar]
- 34.Maggi P, Absinta M, Grammatico M, et al. Central vein sign differentiates Multiple Sclerosis from central nervous system inflammatory vasculopathies. Ann Neurol 2018;83(2):283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teunissen CE, Iacobaeus E, Khademi M, et al. Combination of CSF N-acetylaspartate and neurofilaments in multiple sclerosis. Neurology 2009;72(15):1322–9. [DOI] [PubMed] [Google Scholar]
- 36.Sinnecker T, Clarke MA, Meier D, et al. Evaluation of the Central Vein Sign as a Diagnostic Imaging Biomarker in Multiple Sclerosis. JAMA Neurol 2019;76(12):1446–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castellaro M, Tamanti A, Pisani AI, Pizzini FB, Crescenzo F, Calabrese M. The Use of the Central Vein Sign in the Diagnosis of Multiple Sclerosis: A Systematic Review and Meta-analysis. Diagnostics (Basel) 2020;10(12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suthiphosuwan S, Sati P, Guenette M, et al. The Central Vein Sign in Radiologically Isolated Syndrome. AJNR Am J Neuroradiol 2019;40(5):776–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clarke MA, Pareto D, Pessini-Ferreira L, et al. Value of 3T Susceptibility-Weighted Imaging in the Diagnosis of Multiple Sclerosis. AJNR Am J Neuroradiol 2020;41(6):1001–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ontaneda D, Sati P, Raza P, et al. Central vein sign: A diagnostic biomarker in multiple sclerosis (CAVS-MS) study protocol for a prospective multicenter trial. Neuroimage Clin 2021;32:102834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Absinta M, Maric D, Gharagozloo M, et al. A lymphocyte-microglia-astrocyte axis in chronic active multiple sclerosis. Nature 2021;597(7878):709–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frischer JM, Weigand SD, Guo Y, et al. Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque. Ann Neurol 2015;78(5):710–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Absinta M, Sati P, Masuzzo F, et al. Association of Chronic Active Multiple Sclerosis Lesions With Disability In Vivo. JAMA Neurol 2019;76(12):1474–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martire MS, Moiola L, Rocca MA, Filippi M, Absinta M. What is the potential of paramagnetic rim lesions as diagnostic indicators in multiple sclerosis? Expert Rev Neurother 2022;22(10):829–837. [DOI] [PubMed] [Google Scholar]
- 45.Dal-Bianco A, Grabner G, Kronnerwetter C, et al. Slow expansion of multiple sclerosis iron rim lesions: pathology and 7 T magnetic resonance imaging. Acta Neuropathol 2017;133(1):25–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fischer MT, Wimmer I, Hoftberger R, et al. Disease-specific molecular events in cortical multiple sclerosis lesions. Brain 2013;136(Pt 6):1799–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bagnato F, Hametner S, Yao B, et al. Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 Tesla. Brain 2011;134(Pt 12):3602–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Absinta M, Sati P, Schindler M, et al. Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J Clin Invest 2016;126(7):2597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maggi P, Sati P, Nair G, et al. Paramagnetic Rim Lesions are Specific to Multiple Sclerosis: An International Multicenter 3T MRI Study. Ann Neurol 2020;88(5):1034–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bermel RA, Bakshi R. The measurement and clinical relevance of brain atrophy in multiple sclerosis. Lancet Neurol 2006;5(2):158–70. [DOI] [PubMed] [Google Scholar]
- 51.Radue EW, Barkhof F, Kappos L, et al. Correlation between brain volume loss and clinical and MRI outcomes in multiple sclerosis. Neurology 2015;84(8):784–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kearney H, Miller DH, Ciccarelli O. Spinal cord MRI in multiple sclerosis--diagnostic, prognostic and clinical value. Nat Rev Neurol 2015;11(6):327–38. [DOI] [PubMed] [Google Scholar]
- 53.Losseff NA, Webb SL, O’Riordan JI, et al. Spinal cord atrophy and disability in multiple sclerosis. A new reproducible and sensitive MRI method with potential to monitor disease progression. Brain 1996;119 ( Pt 3):701–8. [DOI] [PubMed] [Google Scholar]
- 54.Bischof A, Papinutto N, Keshavan A, et al. Spinal Cord Atrophy Predicts Progressive Disease in Relapsing Multiple Sclerosis. Ann Neurol 2022;91(2):268–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jonkman LE, Fleysher L, Steenwijk MD, et al. Ultra-high field MTR and qR2* differentiates subpial cortical lesions from normal-appearing gray matter in multiple sclerosis. Mult Scler 2016;22(10):1306–14. [DOI] [PubMed] [Google Scholar]
- 56.Calabrese M, Filippi M, Rovaris M, et al. Evidence for relative cortical sparing in benign multiple sclerosis: a longitudinal magnetic resonance imaging study. Mult Scler 2009;15(1):36–41. [DOI] [PubMed] [Google Scholar]
- 57.Granberg T, Moridi T, Brand JS, et al. Enlarged perivascular spaces in multiple sclerosis on magnetic resonance imaging: a systematic review and meta-analysis. J Neurol 2020;267(11):3199–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ineichen BV, Tsagkas C, Absinta M, Reich DS. Leptomeningeal enhancement in multiple sclerosis and other neurological diseases: A systematic review and Meta-Analysis. Neuroimage Clin 2022;33:102939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Curti E, Graziuso S, Tsantes E, Crisi G, Granella F. Correlation between cortical lesions and cognitive impairment in multiple sclerosis. Brain Behav 2018;8(6):e00955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Calabrese M, Rinaldi F, Grossi P, Gallo P. Cortical pathology and cognitive impairment in multiple sclerosis. Expert Rev Neurother 2011;11(3):425–32. [DOI] [PubMed] [Google Scholar]
- 61.Morgen K, Sammer G, Courtney SM, et al. Evidence for a direct association between cortical atrophy and cognitive impairment in relapsing-remitting MS. Neuroimage 2006;30(3):891–8. [DOI] [PubMed] [Google Scholar]
- 62.Bouman PM, Steenwijk MD, Pouwels PJW, et al. Histopathology-validated recommendations for cortical lesion imaging in multiple sclerosis. Brain 2020;143(10):2988–2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maranzano J, Dadar M, Rudko DA, et al. Comparison of Multiple Sclerosis Cortical Lesion Types Detected by Multicontrast 3T and 7T MRI. AJNR Am J Neuroradiol 2019;40(7):1162–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martin AR, Aleksanderek I, Cohen-Adad J, et al. Translating state-of-the-art spinal cord MRI techniques to clinical use: A systematic review of clinical studies utilizing DTI, MT, MWF, MRS, and fMRI. Neuroimage Clin 2016;10:192–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.La Rosa F, Wynen M, Al-Louzi O, et al. Cortical lesions, central vein sign, and paramagnetic rim lesions in multiple sclerosis: Emerging machine learning techniques and future avenues. Neuroimage Clin 2022;36:103205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brandt AU, Zimmermann H, Kaufhold F, et al. Patterns of retinal damage facilitate differential diagnosis between Susac syndrome and MS. PLoS One 2012;7(6):e38741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schorr EM, Gill AJ, Saidha S, Calabresi PA. Retinal optical coherence tomography in MS. . Practical Neurology 2022;21(7):30–34. [Google Scholar]
- 68.Rothman A, Murphy OC, Fitzgerald KC, et al. Retinal measurements predict 10-year disability in multiple sclerosis. Ann Clin Transl Neurol 2019;6(2):222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Button J, Al-Louzi O, Lang A, et al. Disease-modifying therapies modulate retinal atrophy in multiple sclerosis: A retrospective study. Neurology 2017;88(6):525–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bsteh G, Hegen H, Altmann P, et al. Retinal layer thinning predicts treatment failure in relapsing multiple sclerosis. Eur J Neurol 2021;28(6):2037–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nguyen J, Rothman A, Gonzalez N, et al. Macular Ganglion Cell and Inner Plexiform Layer Thickness Is More Strongly Associated With Visual Function in Multiple Sclerosis Than Bruch Membrane Opening-Minimum Rim Width or Peripapillary Retinal Nerve Fiber Layer Thicknesses. J Neuroophthalmol 2019;39(4):444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Al-Louzi O, Sotirchos ES, Vidal-Jordana A, et al. Characteristics of morphologic macular abnormalities in neuroimmunology practice. Mult Scler 2019;25(3):361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim H, Lee EJ, Kim S, et al. Serum biomarkers in myelin oligodendrocyte glycoprotein antibody-associated disease. Neurol Neuroimmunol Neuroinflamm 2020;7(3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Watanabe M, Nakamura Y, Michalak Z, et al. Serum GFAP and neurofilament light as biomarkers of disease activity and disability in NMOSD. Neurology 2019;93(13):e1299–e1311. [DOI] [PubMed] [Google Scholar]
- 75.Disanto G, Barro C, Benkert P, et al. Serum Neurofilament light: A biomarker of neuronal damage in multiple sclerosis. Ann Neurol 2017;81(6):857–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kuhle J, Barro C, Disanto G, et al. Serum neurofilament light chain in early relapsing remitting MS is increased and correlates with CSF levels and with MRI measures of disease severity. Mult Scler 2016;22(12):1550–1559. [DOI] [PubMed] [Google Scholar]
- 77.Novakova L, Zetterberg H, Sundstrom P, et al. Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology 2017;89(22):2230–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Loonstra FC, de Ruiter LRJ, Koel-Simmelink MJA, et al. Neuroaxonal and Glial Markers in Patients of the Same Age With Multiple Sclerosis. Neurol Neuroimmunol Neuroinflamm 2023;10(2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bjornevik K, Munger KL, Cortese M, et al. Serum Neurofilament Light Chain Levels in Patients With Presymptomatic Multiple Sclerosis. JAMA Neurol 2020;77(1):58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bittner S, Steffen F, Uphaus T, et al. Clinical implications of serum neurofilament in newly diagnosed MS patients: A longitudinal multicentre cohort study. EBioMedicine 2020;56:102807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dalla Costa G, Martinelli V, Sangalli F, et al. Prognostic value of serum neurofilaments in patients with clinically isolated syndromes. Neurology 2019;92(7):e733–e741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Matute-Blanch C, Villar LM, Alvarez-Cermeno JC, et al. Neurofilament light chain and oligoclonal bands are prognostic biomarkers in radiologically isolated syndrome. Brain 2018;141(4):1085–1093. [DOI] [PubMed] [Google Scholar]
- 83.Martinez MA, Olsson B, Bau L, et al. Glial and neuronal markers in cerebrospinal fluid predict progression in multiple sclerosis. Mult Scler 2015;21(5):550–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Benkert P, Meier S, Schaedelin S, et al. Serum neurofilament light chain for individual prognostication of disease activity in people with multiple sclerosis: a retrospective modelling and validation study. Lancet Neurol 2022;21(3):246–257. [DOI] [PubMed] [Google Scholar]
- 85.Barro C, Benkert P, Disanto G, et al. Serum neurofilament as a predictor of disease worsening and brain and spinal cord atrophy in multiple sclerosis. Brain 2018;141(8):2382–2391. [DOI] [PubMed] [Google Scholar]
- 86.Chitnis T, Gonzalez C, Healy BC, et al. Neurofilament light chain serum levels correlate with 10-year MRI outcomes in multiple sclerosis. Ann Clin Transl Neurol 2018;5(12):1478–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sotirchos ES, Fitzgerald KC, Singh CM, et al. Associations of sNfL with clinico-radiological measures in a large MS population. Ann Clin Transl Neurol 2023;10(1):84–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kuhle J, Nourbakhsh B, Grant D, et al. Serum neurofilament is associated with progression of brain atrophy and disability in early MS. Neurology 2017;88(9):826–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Williams T, Zetterberg H, Chataway J. Neurofilaments in progressive multiple sclerosis: a systematic review. J Neurol 2021;268(9):3212–3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Martin SJ, McGlasson S, Hunt D, Overell J. Cerebrospinal fluid neurofilament light chain in multiple sclerosis and its subtypes: a meta-analysis of case-control studies. J Neurol Neurosurg Psychiatry 2019;90(9):1059–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Manouchehrinia A, Stridh P, Khademi M, et al. Plasma neurofilament light levels are associated with risk of disability in multiple sclerosis. Neurology 2020;94(23):e2457–e2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bhan A, Jacobsen C, Myhr KM, Dalen I, Lode K, Farbu E. Neurofilaments and 10-year follow-up in multiple sclerosis. Mult Scler 2018;24(10):1301–1307. [DOI] [PubMed] [Google Scholar]
- 93.Sellebjerg F, Royen L, Soelberg Sorensen P, Oturai AB, Jensen PEH. Prognostic value of cerebrospinal fluid neurofilament light chain and chitinase-3-like-1 in newly diagnosed patients with multiple sclerosis. Mult Scler 2019;25(11):1444–1451. [DOI] [PubMed] [Google Scholar]
- 94.Vermunt L, Otte M, Verberk IMW, et al. Age- and disease-specific reference values for neurofilament light presented in an online interactive support interface. Ann Clin Transl Neurol 2022;9(11):1832–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Canto E, Tintore M, Villar LM, et al. Chitinase 3-like 1: prognostic biomarker in clinically isolated syndromes. Brain 2015;138(Pt 4):918–31. [DOI] [PubMed] [Google Scholar]
- 96.Bonneh-Barkay D, Wang G, Starkey A, Hamilton RL, Wiley CA. In vivo CHI3L1 (YKL-40) expression in astrocytes in acute and chronic neurological diseases. J Neuroinflammation 2010;7:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tamam Y, Gunes B, Akbayir E, et al. CSF levels of HoxB3 and YKL-40 may predict conversion from clinically isolated syndrome to relapsing remitting multiple sclerosis. Mult Scler Relat Disord 2021;48:102697. [DOI] [PubMed] [Google Scholar]
- 98.Comabella M, Fernandez M, Martin R, et al. Cerebrospinal fluid chitinase 3-like 1 levels are associated with conversion to multiple sclerosis. Brain 2010;133(Pt 4):1082–93. [DOI] [PubMed] [Google Scholar]
- 99.Malmestrom C, Axelsson M, Lycke J, Zetterberg H, Blennow K, Olsson B. CSF levels of YKL-40 are increased in MS and replaces with immunosuppressive treatment. J Neuroimmunol 2014;269(1–2):87–9. [DOI] [PubMed] [Google Scholar]
- 100.Burman J, Raininko R, Blennow K, Zetterberg H, Axelsson M, Malmestrom C. YKL-40 is a CSF biomarker of intrathecal inflammation in secondary progressive multiple sclerosis. J Neuroimmunol 2016;292:52–7. [DOI] [PubMed] [Google Scholar]
- 101.Floro S, Carandini T, Pietroboni AM, De Riz MA, Scarpini E, Galimberti D. Role of Chitinase 3-like 1 as a Biomarker in Multiple Sclerosis: A Systematic Review and Meta-analysis. Neurol Neuroimmunol Neuroinflamm 2022;9(4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Comabella M, Clarke MA, Schaedelin S, et al. CSF chitinase 3-like 1 is associated with iron rims in patients with a first demyelinating event. Mult Scler 2022;28(1):71–81. [DOI] [PubMed] [Google Scholar]
- 103.Plog BA, Dashnaw ML, Hitomi E, et al. Biomarkers of traumatic injury are transported from brain to blood via the glymphatic system. J Neurosci 2015;35(2):518–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Abdelhak A, Huss A, Kassubek J, Tumani H, Otto M. Serum GFAP as a biomarker for disease severity in multiple sclerosis. Sci Rep 2018;8(1):14798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ponath G, Park C, Pitt D. The Role of Astrocytes in Multiple Sclerosis. Front Immunol 2018;9:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yue JK, Yuh EL, Korley FK, et al. Association between plasma GFAP concentrations and MRI abnormalities in patients with CT-negative traumatic brain injury in the TRACK-TBI cohort: a prospective multicentre study. Lancet Neurol 2019;18(10):953–961. [DOI] [PubMed] [Google Scholar]
- 107.Ayrignac X, Le Bars E, Duflos C, et al. Serum GFAP in multiple sclerosis: correlation with disease type and MRI markers of disease severity. Sci Rep 2020;10(1):10923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Barro C, Healy BC, Liu Y, et al. Serum GFAP and NfL Levels Differentiate Subsequent Progression and Disease Activity in Patients With Progressive Multiple Sclerosis. Neurol Neuroimmunol Neuroinflamm 2023;10(1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee EJ, Lim YM, Kim S, et al. Clinical implication of serum biomarkers and patient age in inflammatory demyelinating diseases. Ann Clin Transl Neurol 2020;7(6):992–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Niiranen M, Kontkanen A, Jaaskelainen O, et al. Serum GFAP and NfL levels in benign relapsing-remitting multiple sclerosis. Mult Scler Relat Disord 2021;56:103280. [DOI] [PubMed] [Google Scholar]
- 111.Högel H, Rissanen E, Barro C, et al. Serum glial fibrillary acidic protein correlates with multiple sclerosis disease severity. Mult Scler 2020;26(2):210–219. [DOI] [PubMed] [Google Scholar]
- 112.Huss A, Otto M, Senel M, Ludolph AC, Abdelhak A, Tumani H. A Score Based on NfL and Glial Markers May Differentiate Between Relapsing-Remitting and Progressive MS Course. Front Neurol 2020;11:608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Magliozzi R, Pitteri M, Ziccardi S, et al. CSF parvalbumin levels reflect interneuron loss linked with cortical pathology in multiple sclerosis. Ann Clin Transl Neurol 2021;8(3):534–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Minagar A, Jy W, Jimenez JJ, et al. Elevated plasma endothelial microparticles in multiple sclerosis. Neurology 2001;56(10):1319–24. [DOI] [PubMed] [Google Scholar]
- 115.Verderio C, Muzio L, Turola E, et al. Myeloid microvesicles are a marker and therapeutic target for neuroinflammation. Ann Neurol 2012;72(4):610–24. [DOI] [PubMed] [Google Scholar]
- 116.D’Anca M, Fenoglio C, Buccellato FR, Visconte C, Galimberti D, Scarpini E. Extracellular Vesicles in Multiple Sclerosis: Role in the Pathogenesis and Potential Usefulness as Biomarkers and Therapeutic Tools. Cells 2021;10(7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Galazka G, Mycko MP, Selmaj I, Raine CS, Selmaj KW. Multiple sclerosis: Serum-derived exosomes express myelin proteins. Mult Scler 2018;24(4):449–458. [DOI] [PubMed] [Google Scholar]
- 118.Bhargava P, Nogueras-Ortiz C, Kim S, Delgado-Peraza F, Calabresi PA, Kapogiannis D. Synaptic and complement markers in extracellular vesicles in multiple sclerosis. Mult Scler 2021;27(4):509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ladakis DC, Yao PJ, Vreones M, et al. Mitochondrial measures in neuronally enriched extracellular vesicles predict brain and retinal atrophy in multiple sclerosis. Mult Scler 2022;28(13):2020–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mazzucco M, Mannheim W, Shetty SV, Linden JR. CNS endothelial derived extracellular vesicles are biomarkers of active disease in multiple sclerosis. Fluids Barriers CNS 2022;19(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhan J, Kipp M, Han W, Kaddatz H. Ectopic lymphoid follicles in progressive multiple sclerosis: From patients to animal models. Immunology 2021;164(3):450–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Krumbholz M, Theil D, Cepok S, et al. Chemokines in multiple sclerosis: CXCL12 and CXCL13 up-regulation is differentially linked to CNS immune cell recruitment. Brain 2006;129(Pt 1):200–11. [DOI] [PubMed] [Google Scholar]
- 123.Alvarez E, Piccio L, Mikesell RJ, et al. CXCL13 is a biomarker of inflammation in multiple sclerosis, neuromyelitis optica, and other neurological conditions. Mult Scler 2013;19(9):1204–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Khademi M, Kockum I, Andersson ML, et al. Cerebrospinal fluid CXCL13 in multiple sclerosis: a suggestive prognostic marker for the disease course. Mult Scler 2011;17(3):335–43. [DOI] [PubMed] [Google Scholar]
- 125.Ragheb S, Li Y, Simon K, et al. Multiple sclerosis: BAFF and CXCL13 in cerebrospinal fluid. Mult Scler 2011;17(7):819–29. [DOI] [PubMed] [Google Scholar]
- 126.Bielekova B, Komori M, Xu Q, Reich DS, Wu T. Cerebrospinal fluid IL-12p40, CXCL13 and IL-8 as a combinatorial biomarker of active intrathecal inflammation. PLoS One 2012;7(11):e48370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sellebjerg F, Bornsen L, Khademi M, et al. Increased cerebrospinal fluid concentrations of the chemokine CXCL13 in active MS. Neurology 2009;73(23):2003–10. [DOI] [PubMed] [Google Scholar]
- 128.DiSano KD, Gilli F, Pachner AR. Intrathecally produced CXCL13: A predictive biomarker in multiple sclerosis. Mult Scler J Exp Transl Clin 2020;6(4):2055217320981396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lucchini M, De Arcangelis V, Piro G, et al. CSF CXCL13 and Chitinase 3-like-1 Levels Predict Disease Course in Relapsing Multiple Sclerosis. Mol Neurobiol 2023;60(1):36–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Brettschneider J, Czerwoniak A, Senel M, et al. The chemokine CXCL13 is a prognostic marker in clinically isolated syndrome (CIS). PLoS One 2010;5(8):e11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Olesen MN, Soelberg K, Debrabant B, et al. Cerebrospinal fluid biomarkers for predicting development of multiple sclerosis in acute optic neuritis: a population-based prospective cohort study. J Neuroinflammation 2019;16(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Alvarez E, Piccio L, Mikesell RJ, et al. Predicting optimal response to B-cell depletion with rituximab in multiple sclerosis using CXCL13 index, magnetic resonance imaging and clinical measures. Mult Scler J Exp Transl Clin 2015;1:2055217315623800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fissolo N, Pappolla A, Rio J, et al. Serum Levels of CXCL13 Are Associated With Teriflunomide Response in Patients With Multiple Sclerosis. Neurol Neuroimmunol Neuroinflamm 2023;10(1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Karaaslan Z, Kurtuncu M, Akcay HI, et al. CXCL13 Levels Indicate Treatment Responsiveness to Fingolimod in MS Patients. Eur Neurol 2022;85(1):69–71. [DOI] [PubMed] [Google Scholar]
- 135.Irani DN. Regulated Production of CXCL13 within the Central Nervous System. J Clin Cell Immunol 2016;7(5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yang X, Peng J, Huang X, et al. Association of Circulating Follicular Helper T Cells and Serum CXCL13 With Neuromyelitis Optica Spectrum Disorders. Front Immunol 2021;12:677190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.DiSano KD, Gilli F, Pachner AR. Are CSF CXCL13 concentrations solely dependent on intrathecal production? A commentary on “Chemokine CXCL13 in serum, CSF, and blood-CSF barrier function”. Fluids Barriers CNS 2021;18(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ngo C, Kothary R. MicroRNAs in oligodendrocyte development and remyelination. J Neurochem 2022;162(4):310–321. [DOI] [PubMed] [Google Scholar]
- 139.Singh RP, Massachi I, Manickavel S, et al. The role of miRNA in inflammation and autoimmunity. Autoimmun Rev 2013;12(12):1160–5. [DOI] [PubMed] [Google Scholar]
- 140.Gandhi R, Healy B, Gholipour T, et al. Circulating microRNAs as biomarkers for disease staging in multiple sclerosis. Ann Neurol 2013;73(6):729–40. [DOI] [PubMed] [Google Scholar]
- 141.Hemond CC, Healy BC, Tauhid S, et al. MRI phenotypes in MS: Longitudinal changes and miRNA signatures. Neurol Neuroimmunol Neuroinflamm 2019;6(2):e530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Haghikia A, Haghikia A, Hellwig K, et al. Regulated microRNAs in the CSF of patients with multiple sclerosis: a case-control study. Neurology 2012;79(22):2166–70. [DOI] [PubMed] [Google Scholar]
- 143.Regev K, Healy BC, Khalid F, et al. Association Between Serum MicroRNAs and Magnetic Resonance Imaging Measures of Multiple Sclerosis Severity. JAMA Neurol 2017;74(3):275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fujiwara M, Raheja R, Garo LP, et al. microRNA-92a promotes CNS autoimmunity by modulating the regulatory and inflammatory T cell balance. J Clin Invest 2022;132(10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ehtesham N, Khorvash F, Kheirollahi M. miR-145 and miR20a-5p Potentially Mediate Pleiotropic Effects of Interferon-Beta Through Mitogen-Activated Protein Kinase Signaling Pathway in Multiple Sclerosis Patients. J Mol Neurosci 2017;61(1):16–24. [DOI] [PubMed] [Google Scholar]
- 146.Sharaf-Eldin WE, Kishk NA, Gad YZ, et al. Extracellular miR-145, miR-223 and miR-326 expression signature allow for differential diagnosis of immune-mediated neuroinflammatory diseases. J Neurol Sci 2017;383:188–198. [DOI] [PubMed] [Google Scholar]
- 147.Ali Ashrafi S, Asadi M, Shanehbandi D, et al. Association between miRNA-145 and miRNA-155 expression in peripheral blood mononuclear cells of patients with multiple sclerosis: a case-control study. BMC Neurol 2022;22(1):405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Maciak K, Dziedzic A, Miller E, Saluk-Bijak J. miR-155 as an Important Regulator of Multiple Sclerosis Pathogenesis. A Review. Int J Mol Sci 2021;22(9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang H. MicroRNAs, Multiple Sclerosis, and Depression. Int J Mol Sci 2021;22(15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gao Y, Han D, Feng J. MicroRNA in multiple sclerosis. Clin Chim Acta 2021;516:92–99. [DOI] [PubMed] [Google Scholar]
- 151.Acheson ED, Bachrach CA. The distribution of multiple sclerosis in U. S. veterans by birthplace. Am J Hyg 1960;72:88–99. [DOI] [PubMed] [Google Scholar]
- 152.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 2006;296(23):2832–8. [DOI] [PubMed] [Google Scholar]
- 153.Munger KL, Zhang SM, O’Reilly E, et al. Vitamin D intake and incidence of multiple sclerosis. Neurology 2004;62(1):60–5. [DOI] [PubMed] [Google Scholar]
- 154.Runia TF, Hop WC, de Rijke YB, Buljevac D, Hintzen RQ. Lower serum vitamin D levels are associated with a higher relapse risk in multiple sclerosis. Neurology 2012;79(3):261–6. [DOI] [PubMed] [Google Scholar]
- 155.Simpson S Jr., Taylor B, Blizzard L, et al. Higher 25-hydroxyvitamin D is associated with lower relapse risk in multiple sclerosis. Ann Neurol 2010;68(2):193–203. [DOI] [PubMed] [Google Scholar]
- 156.Charoenngam N, Holick MF. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020;12(7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lemire JM, Archer DC. 1,25-dihydroxyvitamin D3 prevents the in vivo induction of murine experimental autoimmune encephalomyelitis. J Clin Invest 1991;87(3):1103–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nashold FE, Miller DJ, Hayes CE. 1,25-dihydroxyvitamin D3 treatment decreases macrophage accumulation in the CNS of mice with experimental autoimmune encephalomyelitis. J Neuroimmunol 2000;103(2):171–9. [DOI] [PubMed] [Google Scholar]
- 159.Mayne CG, Spanier JA, Relland LM, Williams CB, Hayes CE. 1,25-Dihydroxyvitamin D3 acts directly on the T lymphocyte vitamin D receptor to inhibit experimental autoimmune encephalomyelitis. Eur J Immunol 2011;41(3):822–32. [DOI] [PubMed] [Google Scholar]
- 160.Jagannath VA, Filippini G, Di Pietrantonj C, et al. Vitamin D for the management of multiple sclerosis. Cochrane Database Syst Rev 2018;9(9):CD008422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Vasileiou ES, Hu C, Bernstein CN, et al. Association of Vitamin D Polygenic Risk Scores and Disease Outcome in People With Multiple Sclerosis. Neurol Neuroimmunol Neuroinflamm 2023;10(1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Munger KL, Levin LI, O’Reilly EJ, Falk KI, Ascherio A. Anti-Epstein-Barr virus antibodies as serological markers of multiple sclerosis: a prospective study among United States military personnel. Mult Scler 2011;17(10):1185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Levin LI, Munger KL, O’Reilly EJ, Falk KI, Ascherio A. Primary infection with the Epstein-Barr virus and risk of multiple sclerosis. Ann Neurol 2010;67(6):824–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dobson R, Kuhle J, Middeldorp J, Giovannoni G. Epstein-Barr-negative MS: a true phenomenon? Neurol Neuroimmunol Neuroinflamm 2017;4(2):e318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bjornevik K, Cortese M, Healy BC, et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 2022;375(6578):296–301. [DOI] [PubMed] [Google Scholar]
- 166.Hedstrom AK, Huang J, Michel A, et al. High Levels of Epstein-Barr Virus Nuclear Antigen-1-Specific Antibodies and Infectious Mononucleosis Act Both Independently and Synergistically to Increase Multiple Sclerosis Risk. Front Neurol 2019;10:1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Handel AE, Williamson AJ, Disanto G, Handunnetthi L, Giovannoni G, Ramagopalan SV. An updated meta-analysis of risk of multiple sclerosis following infectious mononucleosis. PLoS One 2010;5(9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Thacker EL, Mirzaei F, Ascherio A. Infectious mononucleosis and risk for multiple sclerosis: a meta-analysis. Ann Neurol 2006;59(3):499–503. [DOI] [PubMed] [Google Scholar]
- 169.De Jager PL, Simon KC, Munger KL, Rioux JD, Hafler DA, Ascherio A. Integrating risk factors: HLADRB1*1501 and Epstein-Barr virus in multiple sclerosis. Neurology 2008;70(13 Pt 2):1113–8. [DOI] [PubMed] [Google Scholar]
- 170.Kreft KL, Van Nierop GP, Scherbeijn SMJ, Janssen M, Verjans G, Hintzen RQ. Elevated EBNA-1 IgG in MS is associated with genetic MS risk variants. Neurol Neuroimmunol Neuroinflamm 2017;4(6):e406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sundqvist E, Sundstrom P, Linden M, et al. Epstein-Barr virus and multiple sclerosis: interaction with HLA. Genes Immun 2012;13(1):14–20. [DOI] [PubMed] [Google Scholar]
- 172.Laderach F, Munz C. Epstein Barr Virus Exploits Genetic Susceptibility to Increase Multiple Sclerosis Risk. Microorganisms 2021;9(11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lanz TV, Brewer RC, Ho PP, et al. Clonally expanded B cells in multiple sclerosis bind EBV EBNA1 and GlialCAM. Nature 2022;603(7900):321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Veroni C, Aloisi F. The CD8 T Cell-Epstein-Barr Virus-B Cell Trialogue: A Central Issue in Multiple Sclerosis Pathogenesis. Front Immunol 2021;12:665718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Dewey B. Laplacian-based Phase Unwrapping in Python (v1.0), Zenodo; 2022. 10.5281/zenodo.7198991. [DOI] [Google Scholar]
- 176.Reyes S, Smets I, Holden D, et al. CSF neurofilament light chain testing as an aid to determine treatment strategies in MS. Neurol Neuroimmunol Neuroinflamm 2020;7(6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Axelsson M, Malmeström C, Gunnarsson M, et al. Immunosuppressive therapy reduces axonal damage in progressive multiple sclerosis. Mult Scler 2014;20(1):43–50. [DOI] [PubMed] [Google Scholar]
- 178.Islas-Hernandez A, Aguilar-Talamantes HS, Bertado-Cortes B, et al. BDNF and Tau as biomarkers of severity in multiple sclerosis. Biomark Med 2018;12(7):717–726. [DOI] [PubMed] [Google Scholar]
- 179.Karimi N, Ashourizadeh H, Akbarzadeh Pasha B, et al. Blood levels of brain-derived neurotrophic factor (BDNF) in people with multiple sclerosis (MS): A systematic review and meta-analysis. Mult Scler Relat Disord 2022;65:103984. [DOI] [PubMed] [Google Scholar]
- 180.Comini-Frota ER, Rodrigues DH, Miranda EC, et al. Serum levels of brain-derived neurotrophic factor correlate with the number of T2 MRI lesions in multiple sclerosis. Braz J Med Biol Res 2012;45(1):68–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Agah E, Zardoui A, Saghazadeh A, Ahmadi M, Tafakhori A, Rezaei N. Osteopontin (OPN) as a CSF and blood biomarker for multiple sclerosis: A systematic review and meta-analysis. PLoS One 2018;13(1):e0190252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chowdhury SA, Lin J, Sadiq SA. Specificity and correlation with disease activity of cerebrospinal fluid osteopontin levels in patients with multiple sclerosis. Arch Neurol 2008;65(2):232–5. [DOI] [PubMed] [Google Scholar]
- 183.Orsi G, Hayden Z, Cseh T, Berki T, Illes Z. Osteopontin levels are associated with late-time lower regional brain volumes in multiple sclerosis. Sci Rep 2021;11(1):23604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Novakova L, Axelsson M, Khademi M, et al. Cerebrospinal fluid biomarkers of inflammation and degeneration as measures of fingolimod efficacy in multiple sclerosis. Mult Scler 2017;23(1):62–71. [DOI] [PubMed] [Google Scholar]
- 185.Novakova L, Axelsson M, Khademi M, et al. Cerebrospinal fluid biomarkers as a measure of disease activity and treatment efficacy in relapsing-remitting multiple sclerosis. J Neurochem 2017;141(2):296–304. [DOI] [PubMed] [Google Scholar]
- 186.Hein Née Maier K, Köhler A, Diem R, et al. Biological markers for axonal degeneration in CSF and blood of patients with the first event indicative for multiple sclerosis. Neurosci Lett 2008;436(1):72–6. [DOI] [PubMed] [Google Scholar]
- 187.Malmestrom C, Haghighi S, Rosengren L, Andersen O, Lycke J. Neurofilament light protein and glial fibrillary acidic protein as biological markers in MS. Neurology 2003;61(12):1720–5. [DOI] [PubMed] [Google Scholar]
- 188.Koch M, Mostert J, Heersema D, Teelken A, De Keyser J. Plasma S100beta and NSE levels and progression in multiple sclerosis. J Neurol Sci 2007;252(2):154–8. [DOI] [PubMed] [Google Scholar]
- 189.Cunningham RT, Morrow JI, Johnston CF, Buchanan KD. Serum neurone-specific enolase concentrations in patients with neurological disorders. Clin Chim Acta 1994;230(2):117–24. [DOI] [PubMed] [Google Scholar]
- 190.Hares K, Kemp K, Loveless S, et al. KIF5A and the contribution of susceptibility genotypes as a predictive biomarker for multiple sclerosis. J Neurol 2021;268(6):2175–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bai Z, Chen D, Wang L, et al. Cerebrospinal Fluid and Blood Cytokines as Biomarkers for Multiple Sclerosis: A Systematic Review and Meta-Analysis of 226 Studies With 13,526 Multiple Sclerosis Patients. Front Neurosci 2019;13:1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Grzegorski T, Iwanowski P, Kozubski W, Losy J. The alterations of cerebrospinal fluid TNF-alpha and TGF-beta2 levels in early relapsing-remitting multiple sclerosis. Immunol Res 2022;70(5):708–713. [DOI] [PubMed] [Google Scholar]
- 193.Talbot J, Højsgaard Chow H, Mahler M, et al. Relationship between cerebrospinal fluid biomarkers of inflammation and tissue damage in primary progressive multiple sclerosis. Mult Scler Relat Disord 2022;68:104209. [DOI] [PubMed] [Google Scholar]
- 194.Matejčíková Z, Mareš J, Sládková V, et al. Cerebrospinal fluid and serum levels of interleukin-8 in patients with multiple sclerosis and its correlation with Q-albumin. Mult Scler Relat Disord 2017;14:12–15. [DOI] [PubMed] [Google Scholar]
- 195.Wei Y, Chang H, Feng H, Li X, Zhang X, Yin L. Low Serum Interleukin-10 Is an Independent Predictive Factor for the Risk of Second Event in Clinically Isolated Syndromes. Front Neurol 2019;10:604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Balasa R, Maier S, Voidazan S, et al. Assessment of Interleukin-17A, Interleukin-10 and Transforming Growth Factor-Beta1 Serum Titers in Relapsing Remitting Multiple Sclerosis Patients Treated with Avonex, Possible Biomarkers for Treatment Response. CNS Neurol Disord Drug Targets 2017;16(1):93–101. [DOI] [PubMed] [Google Scholar]
- 197.Sedeeq MS, El-Nahrery EMA, Shalaby N, et al. Micro-RNA-96 and interleukin-10 are independent biomarkers for multiple sclerosis activity. J Neurol Sci 2019;403:92–96. [DOI] [PubMed] [Google Scholar]
- 198.Cala CM, Moseley CE, Steele C, et al. T cell cytokine signatures: Biomarkers in pediatric multiple sclerosis. J Neuroimmunol 2016;297:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Huang J, Khademi M, Fugger L, et al. Inflammation-related plasma and CSF biomarkers for multiple sclerosis. Proc Natl Acad Sci U S A 2020;117(23):12952–12960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ashtari F, Madanian R, Shaygannejad V, Zarkesh SH, Ghadimi K. Serum levels of IL-6 and IL-17 in multiple sclerosis, neuromyelitis optica patients and healthy subjects. Int J Physiol Pathophysiol Pharmacol 2019;11(6):267–273. [PMC free article] [PubMed] [Google Scholar]
- 201.Lalive PH, Kreutzfeldt M, Devergne O, et al. Increased interleukin-27 cytokine expression in the central nervous system of multiple sclerosis patients. J Neuroinflammation 2017;14(1):144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Mado H, Adamczyk-Sowa M, Bartman W, Wierzbicki K, Tadeusiak B, Sowa P. Plasma Interleukin-33 level in relapsing-remitting multiple sclerosis. Is it negatively correlated with central nervous system lesions in patients with mild disability? Clin Neurol Neurosurg 2021;206:106700. [DOI] [PubMed] [Google Scholar]
- 203.Alsahebfosoul F, Rahimmanesh I, Shajarian M, et al. Interleukin-33 plasma levels in patients with relapsing-remitting multiple sclerosis. Biomol Concepts 2017;8(1):55–60. [DOI] [PubMed] [Google Scholar]
- 204.Alsahebfosoul F, Jahanbani-Ardakani H, Ghavimi R, Sedaghat N, Etemadifar M. Serum level of interleukin 36 in patients with multiple sclerosis. J Immunoassay Immunochem 2018;39(5):558–564. [DOI] [PubMed] [Google Scholar]
- 205.Farrokhi M, Rezaei A, Amani-Beni A, Etemadifar M, Kouchaki E, Zahedi A. Increased serum level of IL-37 in patients with multiple sclerosis and neuromyelitis optica. Acta Neurol Belg 2015;115(4):609–14. [DOI] [PubMed] [Google Scholar]
- 206.Cavalli E, Mazzon E, Basile MS, et al. In Silico and In Vivo Analysis of IL37 in Multiple Sclerosis Reveals Its Probable Homeostatic Role on the Clinical Activity, Disability, and Treatment with Fingolimod. Molecules 2019;25(1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zarrabi M, Nazarinia M, Rahimi Jaberi A, Gholijani N, Amirghofran Z. Elevated IL-38 Serum Levels in Newly Diagnosed Multiple Sclerosis and Systemic Sclerosis Patients. Med Princ Pract 2021;30(2):146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Haufschild T, Shaw SG, Kesselring J, Flammer J. Increased endothelin-1 plasma levels in patients with multiple sclerosis. J Neuroophthalmol 2001;21(1):37–8. [DOI] [PubMed] [Google Scholar]
- 209.Castellazzi M, Lamberti G, Resi MV, et al. Increased Levels of Endothelin-1 in Cerebrospinal Fluid Are a Marker of Poor Visual Recovery after Optic Neuritis in Multiple Sclerosis Patients. Dis Markers 2019;2019:9320791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Speciale L, Sarasella M, Ruzzante S, et al. Endothelin and nitric oxide levels in cerebrospinal fluid of patients with multiple sclerosis. J Neurovirol 2000;6 Suppl 2:S62–6. [PubMed] [Google Scholar]
- 211.Monti L, Arrigucci U, Rossi A. Insights into Endothelin-3 and Multiple Sclerosis. Biomol Concepts 2020;11(1):137–141. [DOI] [PubMed] [Google Scholar]
- 212.Amini Harandi A, Siavoshi F, Shirzadeh Barough S, et al. Vascular Endothelial Growth Factor as a Predictive and Prognostic Biomarker for Multiple Sclerosis. Neuroimmunomodulation 2022;29(4):476–485. [DOI] [PubMed] [Google Scholar]
- 213.Iacobaeus E, Amoudruz P, Ström M, et al. The expression of VEGF-A is down regulated in peripheral blood mononuclear cells of patients with secondary progressive multiple sclerosis. PLoS One 2011;6(5):e19138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Cirac A, Tsaktanis T, Beyer T, et al. The Aryl Hydrocarbon Receptor-Dependent TGF-α/VEGF-B Ratio Correlates With Disease Subtype and Prognosis in Multiple Sclerosis. Neurol Neuroimmunol Neuroinflamm 2021;8(5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Gnanapavan S, Ho P, Heywood W, et al. Progression in multiple sclerosis is associated with low endogenous NCAM. J Neurochem 2013;125(5):766–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Axelsson M, Dubuisson N, Novakova L, et al. Cerebrospinal fluid NCAM levels are modulated by disease-modifying therapies. Acta Neurol Scand 2019;139(5):422–427. [DOI] [PubMed] [Google Scholar]
- 217.Massaro AR, Albrechtsen M, Bock E. N-CAM in cerebrospinal fluid: a marker of synaptic remodelling after acute phases of multiple sclerosis? Ital J Neurol Sci 1987;Suppl 6:85–8. [PubMed] [Google Scholar]
- 218.Massaro AR. The role of NCAM in remyelination. Neurol Sci 2002;22(6):429–35. [DOI] [PubMed] [Google Scholar]
- 219.Matsuda M, Tsukada N, Miyagi K, Yanagisawa N. Increased levels of soluble vascular cell adhesion molecule-1 (VCAM-1) in the cerebrospinal fluid and sera of patients with multiple sclerosis and human T lymphotropic virus type-1-associated myelopathy. J Neuroimmunol 1995;59(1–2):35–40. [DOI] [PubMed] [Google Scholar]
- 220.Calabresi PA, Tranquill LR, Dambrosia JM, et al. Increases in soluble VCAM-1 correlate with a decrease in MRI lesions in multiple sclerosis treated with interferon beta-1b. Ann Neurol 1997;41(5):669–74. [DOI] [PubMed] [Google Scholar]
- 221.Cossu D, Yokoyama K, Sechi LA, Hattori N. Potential of PINK1 and PARKIN Proteins as Biomarkers for Active Multiple Sclerosis: A Japanese Cohort Study. Front Immunol 2021;12:681386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Hassanpour M, Cheraghi O, Laghusi D, Nouri M, Panahi Y . The relationship between ANT1 and NFL with autophagy and mitophagy markers in patients with multiple sclerosis. J Clin Neurosci 2020;78:307–312. [DOI] [PubMed] [Google Scholar]
- 223.Joodi Khanghah O, Nourazarian A, Khaki-Khatibi F, et al. Evaluation of the Diagnostic and Predictive Value of Serum Levels of ANT1, ATG5, and Parkin in Multiple Sclerosis. Clin Neurol Neurosurg 2020;197:106197. [DOI] [PubMed] [Google Scholar]
- 224.Castellazzi M, Patergnani S, Donadio M, et al. Correlation between auto/mitophagic processes and magnetic resonance imaging activity in multiple sclerosis patients. J Neuroinflammation 2019;16(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Rejdak K, Bartosik-Psujek H, Dobosz B, et al. Decreased level of kynurenic acid in cerebrospinal fluid of relapsing-onset multiple sclerosis patients. Neurosci Lett 2002;331(1):63–5. [DOI] [PubMed] [Google Scholar]
- 226.Hartai Z, Klivenyi P, Janaky T, Penke B, Dux L, Vecsei L. Kynurenine metabolism in multiple sclerosis. Acta Neurol Scand 2005;112(2):93–6. [DOI] [PubMed] [Google Scholar]
- 227.Rejdak K, Petzold A, Kocki T, et al. Astrocytic activation in relation to inflammatory markers during clinical exacerbation of relapsing-remitting multiple sclerosis. J Neural Transm (Vienna) 2007;114(8):1011–5. [DOI] [PubMed] [Google Scholar]
- 228.Lim CK, Bilgin A, Lovejoy DB, et al. Kynurenine pathway metabolomics predicts and provides mechanistic insight into multiple sclerosis progression. Sci Rep 2017;7:41473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Naughton M, Moffat J, Eleftheriadis G, et al. CCN3 is dynamically regulated by treatment and disease state in multiple sclerosis. J Neuroinflammation 2020;17(1):349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Munger KL, Hongell K, Åivo J, Soilu-Hänninen M, Surcel HM, Ascherio A. 25-Hydroxyvitamin D deficiency and risk of MS among women in the Finnish Maternity Cohort. Neurology 2017;89(15):1578–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Nielsen NM, Munger KL, Koch-Henriksen N, et al. Neonatal vitamin D status and risk of multiple sclerosis: A population-based case-control study. Neurology 2017;88(1):44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Salzer J, Hallmans G, Nyström M, Stenlund H, Wadell G, Sundström P. Vitamin D as a protective factor in multiple sclerosis. Neurology 2012;79(21):2140–5. [DOI] [PubMed] [Google Scholar]
- 233.Ascherio A, Munger KL, White R, et al. Vitamin D as an early predictor of multiple sclerosis activity and progression. JAMA Neurol 2014;71(3):306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.