Abstract
Introduction:
We investigated the effect of daily oral Lactobacillus rhamnosus GG (LGG) in reducing liver injury/severity and drinking in patients with alcohol use disorder (AUD) and moderately severe alcohol-associated hepatitis (mAH).
Patients/Methods:
Forty-six males and females with AUD and mAH (12≤MELD<20, aged 21–67) received either LGG (n=24) or placebo, (n=22). Data were collected/assessed at baseline, 1-month, 3-months, and 6-months.
Results:
LGG treatment was associated with a significant reduction in liver injury after one month. Six months of LGG reduced heavy drinking levels to social or abstinence levels
Conclusion:
LGG treatment was associated with an improvement in both liver injury and drinking.
Introduction
Alcohol Use Disorder (AUD) affects almost 15 million people in the US over the age of 12. Alcohol misuse can cause alterations in the gut microbiome resulting in a pro-inflammatory response, with endotoxemia and alterations in the gut-brain axis[1, 2]. Recent studies have reported that treatment with probiotics can result in positive behavioral changes such as attenuating anxiety and depression; however, hypothesis-driven clinical studies of probiotic efficacy for treating AUD are lacking[3].
Heavy drinkers frequently suffer from alcohol-associated liver disease (ALD)[4]. Unfortunately, there is no FDA-approved therapy for any stage of ALD [5]. Finding an intervention with the potential to treat AUD along with ALD is a major unmet need. Increasing evidence indicates a critical role for dysbiosis in ALD pathogenesis and the potential for gut-microbiome modulating probiotics in the treatment of ALD [6–8]. Indeed, several research groups have shown that the probiotic, Lactobacillus rhamnosus GG (LGG, used in this study) was effective at both preventing and treating experimental ALD through multiple mechanisms [6–8].
Moderate Alcohol-associated Hepatitis (mAH) is increasingly recognized as an important subset of ALD. Appropriate treatment could potentially prevent progression to poor quality of life, severe AH, or decompensated cirrhosis. The AASLD and EASL declared that mAH is an area for “consensus, unmet needs, and opportunities for future study”[9].
This randomized, double-blind, placebo-controlled study evaluated the safety and efficacy of 6-month LGG supplementation versus placebo in treating patients with both AUD and mAH. The primary efficacy endpoint was reduction of Model for End-stage Liver Disease (MELD) score at one month and secondary endpoints included improvement in liver biochemistries and drinking behavior.
Patients and Methods
Patients
This study was approved by the Institutional Review Boards at University of Louisville, University of Texas Southwestern and Cleveland Clinic. This study was part of a large NIAAA-funded clinical trial indexed at clinicaltrials.gov: NCT01922895. Inclusion criteria included: age 21 years or older, reported heavy drinking for at least six months, and diagnosis of mAH (12≤MELD<20), and were similar to those published by us for severe AH, except for the lower MELD score indicating mAH[5] (S-Table1).
Study Paradigm
Forty-six male and female patients with AUD and mAH aged 21–67 were randomized to receive LGG or placebo orally once/day for 6 months: LGG, n=24; placebo, n=22 (S-Fig.1). All patients received standard-of-care for their liver disease and counseling for AUD. Data on demographics, drinking, and liver disease were collected and assessed at baseline, 1-, 3-, and 6-months. Other liver assessments included ALT (Alanine Aminotransferase), AST (Aspartate Aminotransferase), AST:ALT ratio, and Maddrey Discriminant Function (MDF). Drinking history (drinks/week) was collected at baseline (using TLFB [Timeline follow-back])[10] and at follow-up times. Alcohol Use Disorder Identification Test (AUDIT)[11] and Lifetime Drinking History (LTDH)[12] were also collected at baseline to assess drinking.
Statistical Design and Analysis
Demographics, liver injury biomarkers, and drinking history markers were analyzed using one-way ANOVA with Bonferroni’s post-hoc correction and t-tests. Analysis of data over time was performed using repeated analyses of variance (RANOVA). Distribution analyses used Chi-square test. We used Microsoft Office 365 (MS Corp., Redmond WA), SPSS 28.0 Version (IBM, Armonk NY), and GraphPad Prism version 9.4.1. (GraphPad Software, San Diego CA). Significance level was set at p<0.05. Data are presented as mean±standard deviation (M±SD) in tables and figures, unless otherwise noted.
Results
Baseline Demographics, Liver Assessments and Alcohol Consumption
There were no significant differences among baseline characteristics between the two groups; however, BMI was higher in the LGG arm and there were fewer females in the LGG arm compared to the placebo arm (Table 1).
Table 1:
Key Baseline Characteristics by Treatment Group
| LGG Treated Group | Placebo Treated Group | Total | |
|---|---|---|---|
| Demographics and Drinking [Mean ± SD) | |||
| Age (yrs.) | 44.2 ± 12.0 | 45.0 ± 9.4 | 44.6 ± 10.7 |
| Sex (F=Female, n=numbers) | F=9 (37.5%) | F=11 (50%) | F=20, M=25 |
| BMI (Unit Count) |
30±7.6 |
26±5.5 | 27.7 ± 6.9 |
| AUDIT (Unit Count) | 23.18 ± 8.4 | 25.05 ± 9.7 | 24.1 ± 9.0 |
| LTDH (yrs.) | 16.7 ± 11.7 | 23 ± 13.6 | 19.6 ± 13 |
| Clinical Characteristics | |||
| Albumin (g/dL) | 2.7 ± 0.6 | 2.8 ± 0.5 | 2.7 ± 0.6 |
| Alkaline phosphatase (IU) | 196.8 ± 94.1 | 197.6 ± 100.6 | 197.2 ± 96.1 |
| ALT (IU) | 73.7 ± 47.2 | 55.4 ± 33.9 | 65.1 ± 42.1 |
| AST (IU) | 169.2 ± 89.4 | 170.5 ± 80.2 | 169.8 ± 84.3 |
| AST:ALT (Unit score) | 2.7 ± 1.5 | 3.5 ± 1.3 | 3.1 ± 1.5 |
| Bilirubin (mg/dL) | 7.2 ± 4.5 | 9.5 ± 7.7 | 8.3 ± 6.2 |
| BMI (kg/m2) | 29.6 ± 7.7 | 25.7 ± 5.5 | 27.7 ± 7.0 |
| Creatinine (mg/dL) | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.6 ± 0.2 |
| CONUT Score* | 6.8 ± 3.2 | 4.9 ± 2.0 | 6.2 ± 2.9 |
| Globulin (g/dL) | 3.4 ± 1.1 | 3.7 ± 1.0 | 3.5 ± 1.1 |
| Maximum Hand Grip Score (psi) | 39.1 ± 22.9 | 35.3 ± 20.6 | 37.4 ± 21.7 |
| MELD (Unit Count) | 17.4 ± 1.8 | 16.7 ± 2.4 | 17.1 ± 2.1 |
| Total Protein (g/dL) | 6.2 ± 1.2 | 6.6 ± 1.1 | 6.4 ± 1.2 |
| Maddrey DF (Unit Count) | 21.8 ± 13.1 | 18.18 ± 10.3 | 20.1 ± 11.9 |
| Platelets (10^9) (Unit Count) | 104.9 ± 13.8 | 109.1 ± 12.5 | 106.8 ± 13.3 |
LGG - Lactobacillus GG; F – females; AUDIT - alcohol use disorder identification test (Unit – unit score); BMI - body mass index (Unit: unit score); LTDH - lifetime drinking history (Unit: years); Platelets - Unit: 10^9/L; AST:ALT ratio - aspartate aminotransferase to alanine aminotransferase ratio (Unit: unit score;, MELD - Model for end–stage liver disease score (Unit: unit score); MDF – Maddrey’s Discriminant Function (Unit: unit score).
CONUT Score is a measure of undernutrition degree where 0-1=normal, 2-4=light, 5-8=moderate, 9-12=severe. Data presented as Mean ± SD.
Effects of LGG on Liver and Drinking Assessments
MELD score and AST/ALT ratio were significantly decreased at one-month (Fig. 1) in the LGG group. There were positive but statistically non-significant changes in other liver biomarkers (ALT, AST, Bilirubin, etc.-Suppl. Table 2).
Figure 1:
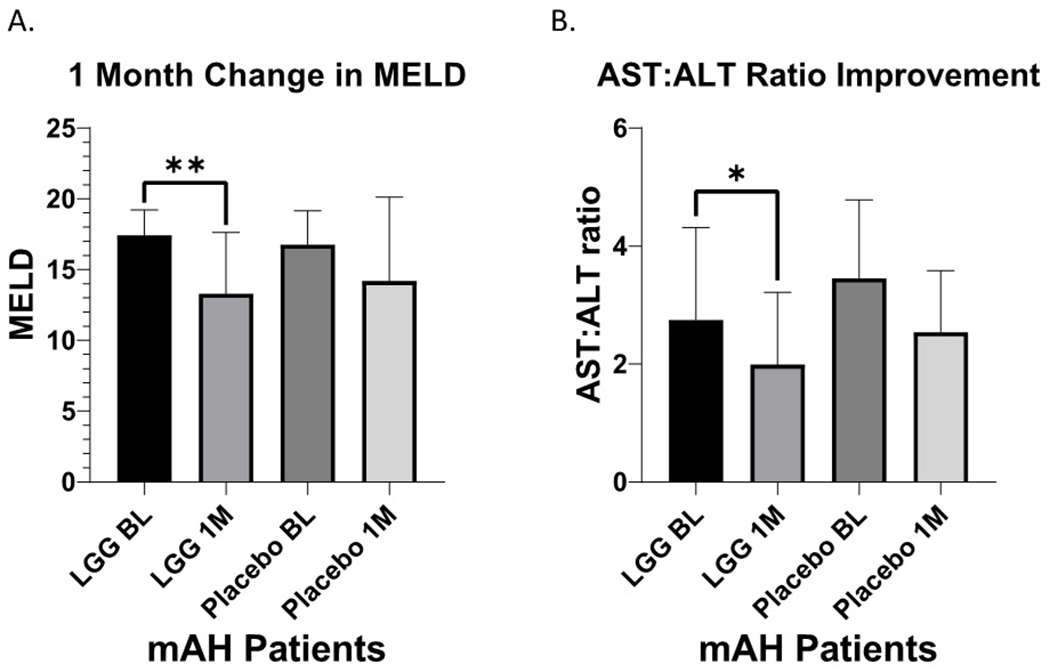
Liver Injury. A. Liver disease severity as determined by MELD in patients with moderate alcohol-associated hepatitis (mAH) receiving LGG or placebo at baseline and at 1-month time-period. BL: Baseline, LGG: Lactobacillus GG, 1M: 1-month timeline, PL: Placebo, MELD: Model for End-Stage Liver Disease. B. Liver injury as determined by the AST:ALT ratio in patients with moderate alcohol-associated hepatitis (mAH) receiving LGG or placebo at baseline and at 1-month. BL: Baseline, LGG: Lactobacillus GG, 1M: 1-month, PL: Placebo. AST: Aspartate aminotransferase. ALT: Alanine aminotransferase. Data presented as Mean ± Standard Deviation. Statistical significance was set at p<0.05. *p<0.05; **p<0.01.
Sixteen of 24 LGG treated patients showed a significant reduction in drinking (p<0.001), to the social or abstinence level of <4 drinks/week, at 6-months compared to four of 22 placebo treated patients (Fig. 2). There was a significant drop (p=0.0128) at the 6-month assessment for average drinks/week in LGG arm compared to the placebo. It is likely that counseling/standard-of-care was a contributing factor to the reduction in alcohol intake in the placebo arm.
Figure 2:
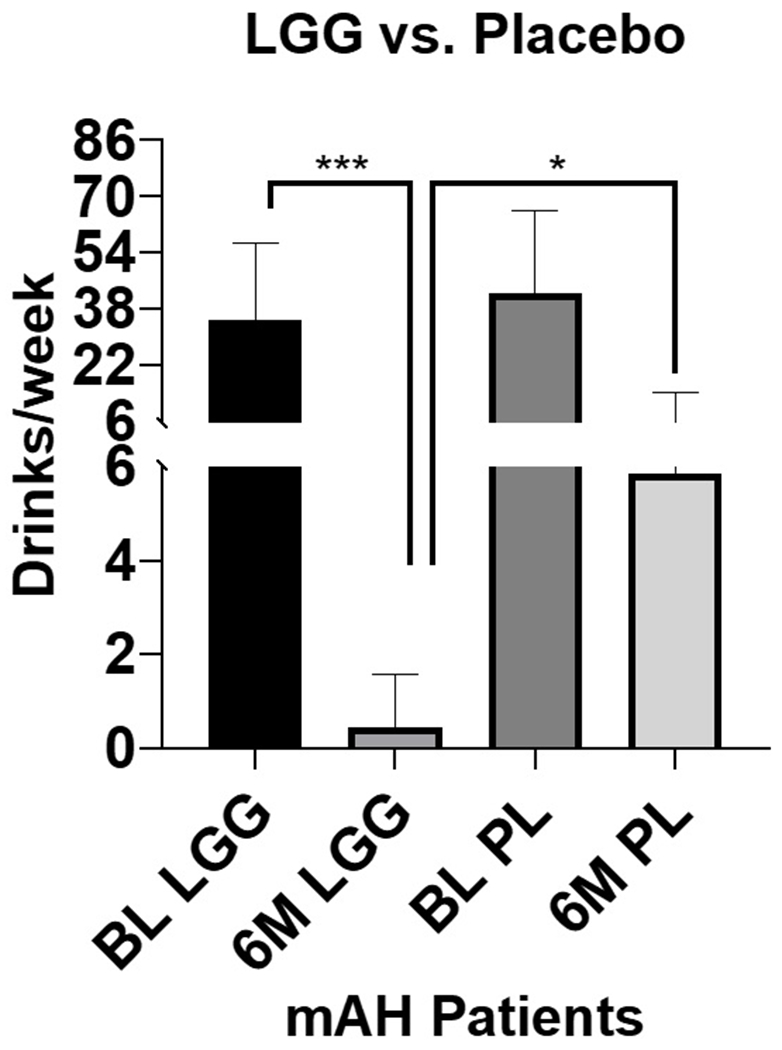
Drinks per week in patients with moderate alcohol-associated hepatitis (mAH) receiving LGG or placebo at baseline, and at 6-month time-period. BL: Baseline, LGG: Lactobacillus GG, 6M: 6-month timeline, PL: Placebo. Data presented as Mean ± Standard Deviation. Statistical significance was set at p<0.05. ***p<0.001, *p<0.05.
Adherence, Retention, and Safety
Nine of 22 Placebo treated patients and eight of the 24 LGG-treated patients did not continue with the medical management through the full six months. Adverse and serious adverse events were similar between groups, and one patient died in the placebo arm (Supplement Fig. 2).
Discussion
We showed that treatment with LGG compared to placebo caused a moderate, but significant, reduction in MELD at 1 month (primary endpoint) as well as a significant reduction in AST:ALT ratio, which is a biomarker of severity of AH. LGG also significantly reduced heavy drinking at 6 months. There were some limitations to this clinical trial. This was a pilot study and no dose response was evaluated. This patient population had poor medical compliance, which limited our analyses of liver injury beyond one month and prevented evaluating mechanisms of action. Alcohol consumption was based solely on history. In spite of these limitations, multiple endpoints were met.
Our findings support and extend other recent translational studies. Kirpich and co-workers reported short-term probiotic treatment caused a more rapid reduction in liver enzymes compared to standard-of-care in patients hospitalized for AUD ([13]). Recent preclinical/clinical studies have demonstrated that fecal microbial transplant (FMT) from patients with AUD can modulate the intestinal barrier and brain function. Bajaj and associates performed a placebo-controlled randomized clinical trial in patients with cirrhosis and AUD in which FMT from a donor led to short-term lowered alcohol craving/consumption in the FMT group, but not placebo[14]. They subsequently transplanted stool from these cirrhotic patients into germ-free mice and showed that alcohol preference/intake was conferred through FMT ([15]). Cumulatively, these data suggest a role for dysbiosis and fecal metabolites in both ALD and AUD.
In summary, this 6-month pilot study showed that Lactobacillus rhamnosus GG was safe, attenuated liver injury, and reduced heavy alcohol intake in AUD patients with mAH, lending support for a role for the gut-liver-brain axis in both AUD and ALD.
Supplementary Material
Acknowledgments:
Authors acknowledge clinical staff from all participating institutions (DASH U01 Consortium) and the NIAAA support for all U01 DASH sites. We thank Ms. Marion McClain for careful editing of this manuscript.
Financial/Grant Support:
Study was supported by 1U01AA026934-01, 1U01AA026936-01, 1U01AA026980-01, (CJM), 1I01BX002996-01A2 (CJM), U01AA026264 (LEN), and K23AA029198 (VV). Research reported in this publication was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM113226 (CJM), and the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under Award Number P50AA024337 (CJM). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- AUD
Alcohol use disorder
- ALD
Alcohol-associated liver disease
- ALT
Alanine transaminase
- AST
Aspartate aminotransferase
- mAH
moderate Alcohol-associated Hepatitis
- AvgDW
Average Drinks per Week
- MELD
Model for end-stage liver disease
Footnotes
Trial registration: ClinicalTrials.gov identifier # NCT01922895.
Disclosures/Conflicts of Interest: All authors declare no conflicts of interest.
References
- 1.Kirpich IA, et al. , Liver injury and endotoxemia in male and female alcohol-dependent individuals admitted to an alcohol treatment program. Alcoholism: Clinical and Experimental Research, 2017. 41(4): p. 747–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Szabo G and Lippai D, Converging actions of alcohol on liver and brain immune signaling. International review of neurobiology, 2014. 118: p. 359–380. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt C, Thinking from the Gut. Nature, 2015. 518(7540): p. S12–S14. [DOI] [PubMed] [Google Scholar]
- 4.Crabb DW, et al. , Diagnosis and Treatment of Alcohol-Associated Liver Diseases: 2019 Practice Guidance From the American Association for the Study of Liver Diseases. Hepatology, 2020. 71(1): p. 306–333. [DOI] [PubMed] [Google Scholar]
- 5.Szabo G, et al. , IL-1 receptor antagonist plus pentoxifylline and zinc for severe alcohol-associated hepatitis. Hepatology, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, et al. , Lactobacillus rhamnosus GG culture supernatant ameliorates acute alcohol-induced intestinal permeability and liver injury. American Journal of Physiology-Gastrointestinal and Liver Physiology, 2012. 303(1): p. G32–G41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forsyth CB, et al. , Lactobacillus GG treatment ameliorates alcohol-induced intestinal oxidative stress, gut leakiness, and liver injury in a rat model of alcoholic steatohepatitis. Alcohol, 2009. 43(2): p. 163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, et al. , Probiotic LGG prevents liver fibrosis through inhibiting hepatic bile acid synthesis and enhancing bile acid excretion in mice. Hepatology (Baltimore, Md.), 2020. 71(6): p. 2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thursz M, et al. , Alcohol-related liver disease: Areas of consensus, unmet needs and opportunities for further study. J Hepatol, 2019. 70(3): p. 521–530. [DOI] [PubMed] [Google Scholar]
- 10.Sobell LC and Sobell MB, Timeline follow-back, in Measuring alcohol consumption. 1992, Springer. p. 41–72. [Google Scholar]
- 11.Saunders JB, et al. , Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction, 1993. 88(6): p. 791–804. [DOI] [PubMed] [Google Scholar]
- 12.Skinner HA and Sheu W-J, Reliability of alcohol use indices. The Lifetime Drinking History and the MAST. Journal of studies on alcohol, 1982. 43(11): p. 1157–1170. [DOI] [PubMed] [Google Scholar]
- 13.Kirpich IA, et al. , Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol, 2008. 42(8): p. 675–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bajaj JS, et al. , A randomized clinical trial of fecal microbiota transplant for alcohol use disorder. Hepatology, 2021. 73(5): p. 1688–1700. [DOI] [PubMed] [Google Scholar]
- 15.Wolstenholme JT, et al. , Reduced alcohol preference and intake after fecal transplant in patients with alcohol use disorder is transmissible to germ-free mice. Nat Commun, 2022. 13(1): p. 6198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


