Abstract
Objective.
Yes-associated protein (YAP) has been widely studied as a mechanotransducer in many cell types, but its function in cartilage is controversial. The aim of this study was to identify the effect of YAP phosphorylation and nuclear translocation on the chondrocyte response to stimuli relevant to osteoarthritis (OA).
Design.
Cultured normal human articular chondrocytes from 81 donors were treated with increased osmolarity media as an in vitro model of mechanical stimulation, fibronectin fragments (FN-f) or IL-1β as catabolic stimuli, and IGF-1 as an anabolic stimulus. YAP function was assessed with gene knockdown and inhibition by verteporfin. Nuclear translocation of YAP and its transcriptional co-activator TAZ and site-specific YAP phosphorylation were determined by immunoblotting. Immunohistochemistry and immunofluorescence to detect YAP were performed on normal and OA human cartilage with different degrees of damage.
Results.
Chondrocyte YAP/TAZ nuclear translocation increased under physiological osmolarity (400 mOsm) and IGF-1 stimulation, which was associated with YAP phosphorylation at Ser128. In contrast, catabolic stimulation decreased the levels of nuclear YAP/TAZ through YAP phosphorylation at Ser127. Following YAP inhibition, anabolic gene expression and transcriptional activity decreased. Additionally, YAP knockdown reduced proteoglycan staining and levels of type II collagen. Total YAP immunostaining was greater in OA cartilage, but YAP was sequestered in the cytosol in cartilage areas with more severe damage.
Conclusions.
YAP chondrocyte nuclear translocation is regulated by differential phosphorylation in response to anabolic and catabolic stimuli. Decreased nuclear YAP in OA chondrocytes may contribute to reduced anabolic activity and promotion of further cartilage loss.
Keywords: Yes-associated protein, nuclear translocation, differential phosphorylation, mechanotransduction, chondrocytes
Introduction
Normal physiologic loading promotes joint tissue homeostasis while abnormal or excessive mechanical loading leads to increased catabolic activity and promotes the onset and progression of osteoarthritis (OA) 1–3. Articular chondrocytes translate external mechanical cues into signaling conduits that impact joint tissue homeostasis through a wide range of molecular pathways 4. Mechanical signaling often operates in conjunction with cues provided by soluble factors present in the pericellular matrix. To identify potential targets for the treatment of OA, it is important to characterize the interaction of mechanical and catabolic mediators at the molecular level. Among the molecules that are known to sense and mediate mechanical signals, Yes-associated protein (YAP) and its transcriptional coactivator with PDZ-binding motif (TAZ) are direct mechanotransducers 5. In addition to mechanical cues, YAP/TAZ pathway activation promotes YAP/TAZ nuclear translocation which can be modified by different stimuli including cell density, growth factors and cytokines 6,7. Translocation of YAP into the nucleus regulates gene expression by interacting with transcription factors to control proliferation, apoptosis, differentiation, and maturation in various tissues8,9. However, the mechanism by which nuclear translocation of YAP is regulated in articular cartilage remains unclear.
Studies of YAP in chondrocytes thus far have produced conflicting results. Pro-inflammatory cytokines induced YAP degradation in chondrocytes and promoted catabolic effects10. In contrast, increased extracellular matrix (ECM) stiffness, which is seen in OA cartilage, triggered YAP activation to worsen cartilage damage11,12. In OA cartilage samples, YAP levels were reduced in association with increased OA severity in one study10, whereas two other studies reported higher levels of YAP in human OA cartilage compared to normal11,13. In terms of YAP activation, YAP nuclear localization was noted to be higher in OA than normal cartilage12. Furthermore, targeting YAP in preclinical OA models has also shown inconsistent outcomes. Conditional YAP knockout in cartilage using a Col2a1 Cre driver either aggravated or ameliorated cartilage damage in the destabilized medial meniscus (DMM) model of OA in mice in two conflicting studies10,12. YAP overexpression using intra-articular (IA) injection of YAP-expressing lentivirus attenuated experimental OA induced by anterior cruciate ligament transection14 while a study that used IA lentivirus to express YAP siRNA for YAP knockdown reported alleviation of OA in the same mouse OA model11. Therefore, the effect of YAP on cartilage homeostasis under different cellular and environmental conditions and whether it promotes anabolic or catabolic activity is not clear and varies widely among studies.
The present study aimed to investigate the role of YAP in chondrocyte homeostasis in response to different physiologic and OA-related stimuli to gain a better understanding of the expression, regulation, and function of YAP in human cartilage. Joint loading during normal daily activities increases the osmolarity of the cartilage extracellular matrix by promoting water to be exuded from the tissue, retaining negatively charged proteoglycans and attracting positive counter-ions15,16. Hence, increased osmolarity was used as an in vitro model of joint loading in our study. Human articular chondrocytes were cultured in 400 mOsm media which is within physiological osmolarity in cartilage, in conditions of pathological osmolarity (600 mOsm media) or in standard DMEM/F12 media (300 mOsm) as control15,17. We evaluated whether osmotic stimulation induced YAP and TAZ nuclear translocation and determined if this was accompanied by changes in anabolic effects. YAP/TAZ nuclear translocation in response to anabolic and catabolic stimuli and regulation by site-specific YAP phosphorylation were also studied as was the location and regulation of YAP in normal human and OA cartilage. Our overall findings support a positive role for YAP/TAZ in maintaining cartilage homeostasis.
Materials and Methods
Antibodies and Reagents
Antibodies against phospho-YAP (Ser127) (#13008), total-YAP (#14074), phospho-p65 (Ser536) (#3033), total-P65 (#8242), phospho-IκBα (Ser32) (#2859), total-IκBα (#4812), TBP (#8515), and GAPDH (#2118) were from Cell Signaling Technology. Antibodies against phospho-YAP (Ser128) (#PA5–117264) and 14–3-3 (#51–0700) were from Thermo Fisher. The antibody against total TAZ (#HPA007415) was from EMD Millipore. The antibody against LDH (#20-LR22) was from Fitzgerald. Purified endotoxin-free recombinant 42 kDa FN-f was produced as previously described 18. IGF-1 was from AUSTRAL Biologicals (# GF-050–4), and IL-1β was purchased from R&D Systems (#201-LB-005). The YAP inhibitor verteporfin was from Sigma (#SML0534), and an activator, lysophosphatidic acid, was from Santa Cruz (#sc-201053).
Primary chondrocyte isolation, culture, and stimulation
Normal human cartilage was obtained from talocrural joints of tissue donors (n=81, mean age ±SD= 58.46±11.34, 17 females and 64 males) through the Gift of Hope Organ and Tissue Donor Network (Itasca, IL). The donors had no known history of joint disease. The tissue was graded as described 19 using the modified Collins grading system (details in Supplementary Methods). Only cartilage with Collin’s grade 0–1 or normal appearing cartilage from joints with Collins’s grade 2 was used. Osteoarthritic cartilage samples (n = 32 donors, mean age 62.53±8.47, 20 females and 12 males) were obtained from patients undergoing total knee arthroplasty at the University of North Carolina Hospital Hillsborough Campus (Hillsborough, NC, USA). Chondrocytes were isolated and cultured in DMEM/F12 media supplemented with 10% fetal bovine serum (FBS) (#97068–085; VWR Seradigm) as described20. Experiments were performed on unpassaged primary chondrocyte monolayers upon 80–90% confluency. For each experiment, chondrocytes isolated from the joints of one donor were treated with stimuli for different time points and regarded as one independent biological experiment. The experiments were repeated at least three times using chondrocytes from 3 different donors as biological repeats. For the experiments using chondrocytes from homozygous mice carrying the YAP1flox allele, femoral cap cartilage was pooled from one batch of mice (10 to 22 mice per batch), enzymatically digested, and cultured as described above. Experiments with cells from one batch of mice were regarded as one independent biological experiment and further biological repeats were done using chondrocytes from another two batches of different mice.
Osmotic stress
Media osmolarity was determined with a vapor pressure osmometer (VAPOR™ Model 5520; WESCOR) using 300 mOsm as the iso-osmotic point. DMEM/F12 media without FBS (300 mOsm) was used as iso-osmotic media. Osmotic media (400 mOsm) and hyperosmotic media (600 mOsm) were prepared by adding sucrose or NaCl to iso-osmotic media to achieve the desired osmolarity.
Cell lysate preparation and immunoblotting
Chondrocytes were lysed, nuclear and cytosolic preparations prepared, and immunoblotting performed and analyzed as described in the Supplementary Methods.
Quantitative real-time PCR (RT–qPCR)
Total RNA was isolated from chondrocytes using an RNeasy Mini Kit (#74004; Qiagen) according to the manufacturer’s instructions. Details of RT-PCR and primers used are provided in the Supplementary Methods.
Nucleofection and adenoviral transduction
A luciferase reporter plasmid with SOX9-dependent COL2A1 enhancer elements (a kind gift from Veronique Lefebvre, University of Pennsylvania) was used to quantify the transcriptional activity of SOX9 and adenovirus expressing the NFκB luciferase reporter (Ad5 HSV-NFκB-luc) (#Ad4059; Gene Transfer Vector Core, U of Iowa) was used for NFκB. A TEA/ATTS domain (TEAD) promoter-luciferase reporter plasmid was from Addgene (#83467). Details of nucleofection and adenoviral transduction are provided in the Supplementary Methods. Luciferase activity was analyzed using a luciferase assay system (#E1500; Promega) according to the manufacturer’s instructions.
Live cell imaging
Human chondrocytes were transfected with YAP-EGFP plasmid (#17843; Addgene) and seeded into glass-bottom dishes (Cellvis, Cat. No. D35–20-1.5H). 48 hrs later, the cells were serum starved overnight before experiments. Imaging was done at 37°C using a confocal microscope system (Zeiss LSM 710) equipped with a temperature- and CO2 (5%)-controlled incubator (Pecon, Incubator PM 2000 RBT). Images were acquired each 2 min with sucrose or NaCl (400 mOsm) being added after the third scan and recorded for 1 hr. Nucleus was stained with Hoechst 33342 (#62249; Thermo Fisher).
Pellet culture and Safranin O staining
Mouse chondrocytes were washed with serum free DMEM-high glucose (DMEM-HG, #11995–065; Sigma) and seeded at 2×105 cells per well into polypropylene, v-bottom 96-well plates (#249946; Thermo Fisher). Pellets were collected for histological analysis on day 14. Details for pellet cultures and Safranin O staining are provided in the Supplementary Methods.
Immunohistochemistry
Human cartilage and mouse chondrocyte pellets were embedded in paraffin, sectioned at 6 μm thickness and processed for immunohistochemistry with primary antibodies against total (t)-YAP, phosphorylated (p)-YAP (Ser127) and 14–3-3 as detailed in the Supplementary Methods. Quantification was performed by counting the number of chondrocytes that stained positive and the total number of chondrocytes. For each donor, 9 fields (10X magnification) from at least 4 stained cartilage sections were examined by two independent readers. Final values were the average of the percentage of positive cells based on the nine fields.
Immunofluorescence staining and image analysis
Frozen sections of human cartilage (20 μm thick) were processed for immunofluorescence and image analysis using primary antibody for t-YAP as detailed in the Supplementary Methods. Images of human cartilage tissues were captured using a Zeiss LSM 700 confocal microscope system (Carl Zeiss).
Statistical analysis
The data were analyzed and graphed using GraphPad Prism version 8 (GraphPad Software, Inc.) with further analysis using R version 4.2.1. The results are presented graphically as mean values with standard deviations (SD). The means and 95% confidence intervals (CIs) are provided in the text for selected results. Exact biological replicates for each experiment are provided in the figure legends where each number (n) indicates the number of biologically independent donors. Details of the statistical analysis are provided in the Supplementary Methods.
Results
Osmotic but not hyperosmotic stimulation induces YAP/TAZ nuclear translocation and promotes anabolic gene expression in human chondrocytes
We tested whether increased osmolarity, as a model for mechanotransduction, induces YAP nuclear translocation together with its transcriptional coactivator TAZ. Immunoblot analysis revealed that compared to standard media of 300 mOsm, 400 mOsm media produced using sucrose or NaCl increased YAP nuclear translocation (mean difference (95% CIs) at 1 hr, Sucrose: 0.89 (0.43, 1.35), NaCl: 0.89 (0.14, 1.63)). However, increasing osmolarity to 600 mOsm using sucrose lowered nuclear YAP abundance at all time points. Similar trends were observed for nuclear TAZ (Fig. 1 A–D). In line with these observations, live-cell imaging by confocal microscopy of chondrocytes expressing YAP-EGFP also indicated that 400 mOsm sucrose or NaCl treatment induced YAP nuclear translocation (Fig. 1E, F).
Fig. 1. YAP/TAZ nuclear translocation in response to osmotic stimulation.
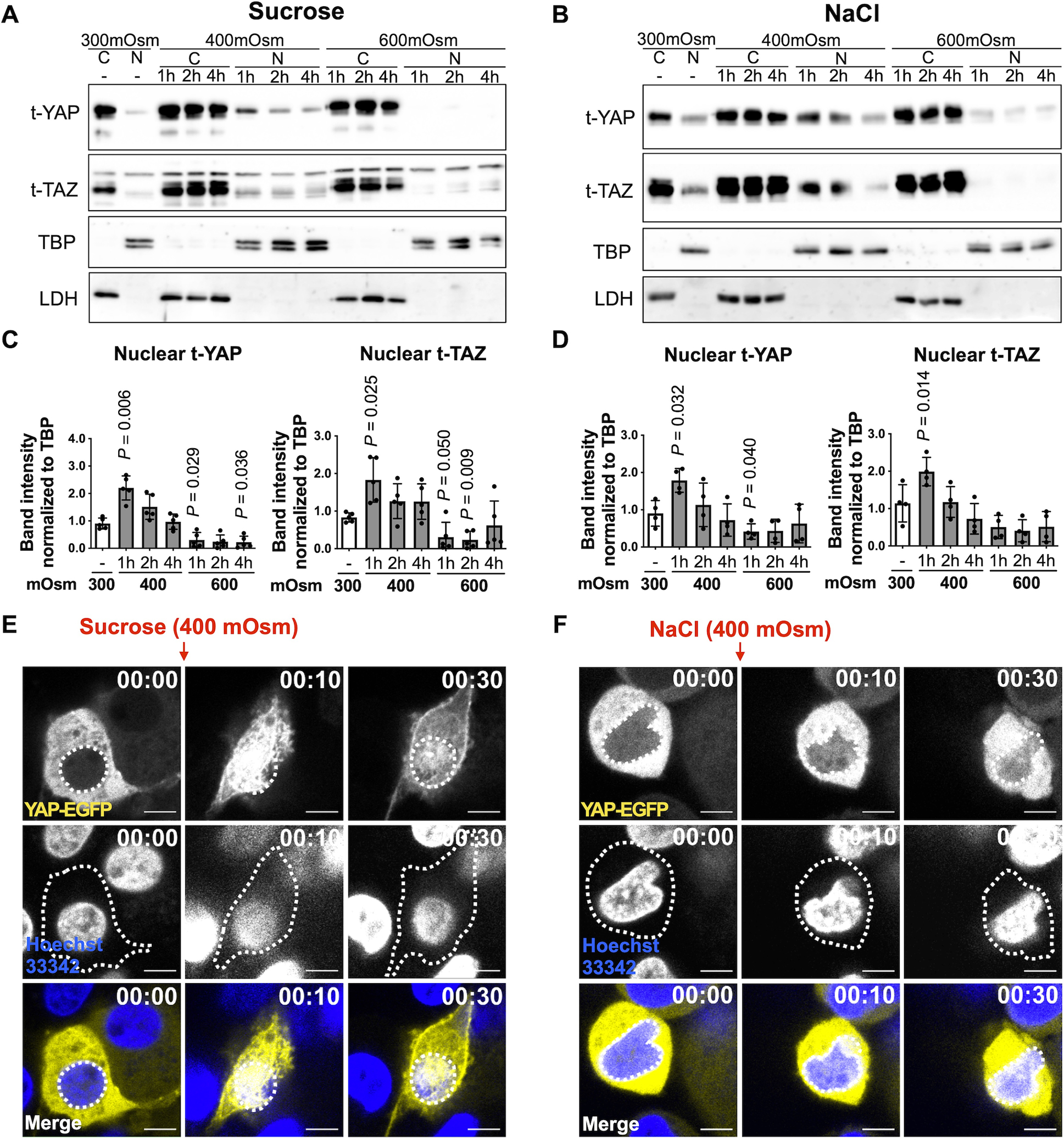
(A, B) Human chondrocytes were incubated for 1–4 hrs in standard iso-osmotic 300 milliosmole (mOsm) media, 400 mOsm media, or 600 mOsm media produced using sucrose or NaCl. Cytosolic and nuclear fractions were prepared from cell lysates and immunoblotted for total YAP and TAZ. LDH and TBP served as loading controls for cytosolic and nuclear fractions, respectively. (A) Representative immunoblots from n = 5 independent donors under sucrose-induced osmotic stimulation and (B) representative immunoblots from n = 4 independent donors under NaCl-induced osmotic stimulation (t, total; SE, short exposure; LE, long exposure). (C, D) Densitometric analysis of immunoblots. The relative band intensities of nuclear YAP (n =5 independent donors) and TAZ (n = 4 independent donors) were normalized to the loading control TBP and are presented as the mean ± standard deviation; indicated p-values were determined by paired t-tests used to compare different conditions to 300 mOsm controls. The values for Figure 1C (YAP) were logarithmically transformed to achieve normality. (E, F) Human chondrocytes expressing YAP-EGFP were incubated in 400 mOsm media produced using sucrose or NaCl and subjected to live-cell imaging using confocal microscopy over 1hr. Nuclei were detected with Hoechst 33342 (blue staining). The top row shows representative images of YAP-EGFP, and white dotted lines indicate the contour of the nucleus. The middle row shows representative images of the nucleus, and white dotted lines indicate the contour of corresponding chondrocytes. Representative live-cell imaging for merged images (Hoechst 33342 and YAP-EGFP) are presented in the bottom row. White dotted lines indicate the contour of the nucleus. Scale bar, 5 μm.
To investigate if the 400 mOsm media that increased YAP/TAZ nuclear levels was associated with changes in anabolic gene expression, we used a SOX9-dependent COL2A1 enhancer-luciferase reporter (Fig. 2 A). Compared to standard 300 mOsm media, 400 mOsm induced by either sucrose or NaCl increased the activity of this enhancer after 12 hr by 2.8-fold (95% CIs: 2.5–3.1) and 3.5-fold (95% CIs: 2.5–5.1), respectively (Fig. 2 B, C). We examined the expression of two YAP downstream target genes, CTGF and CYR61, and three anabolic genes, SOX9, ACAN and COL2A1, starting at 3 hrs after incubation in 300 mOsm and 400 mOsm media. (Fig. 2 D, E). The 400 mOsm media produced using either sucrose or NaCl increased the expression of CTFG at 3, 6 or 18 hrs and increased CYR61 at 6 or 18 hrs. SOX9 was increased at 3, 6 or 18 hrs; ACAN was increased at 3 and 6 hrs in 400 mOsm media created using sucrose but not NaCl.
Fig. 2. Anabolic gene expression in response to osmotic stimulation.
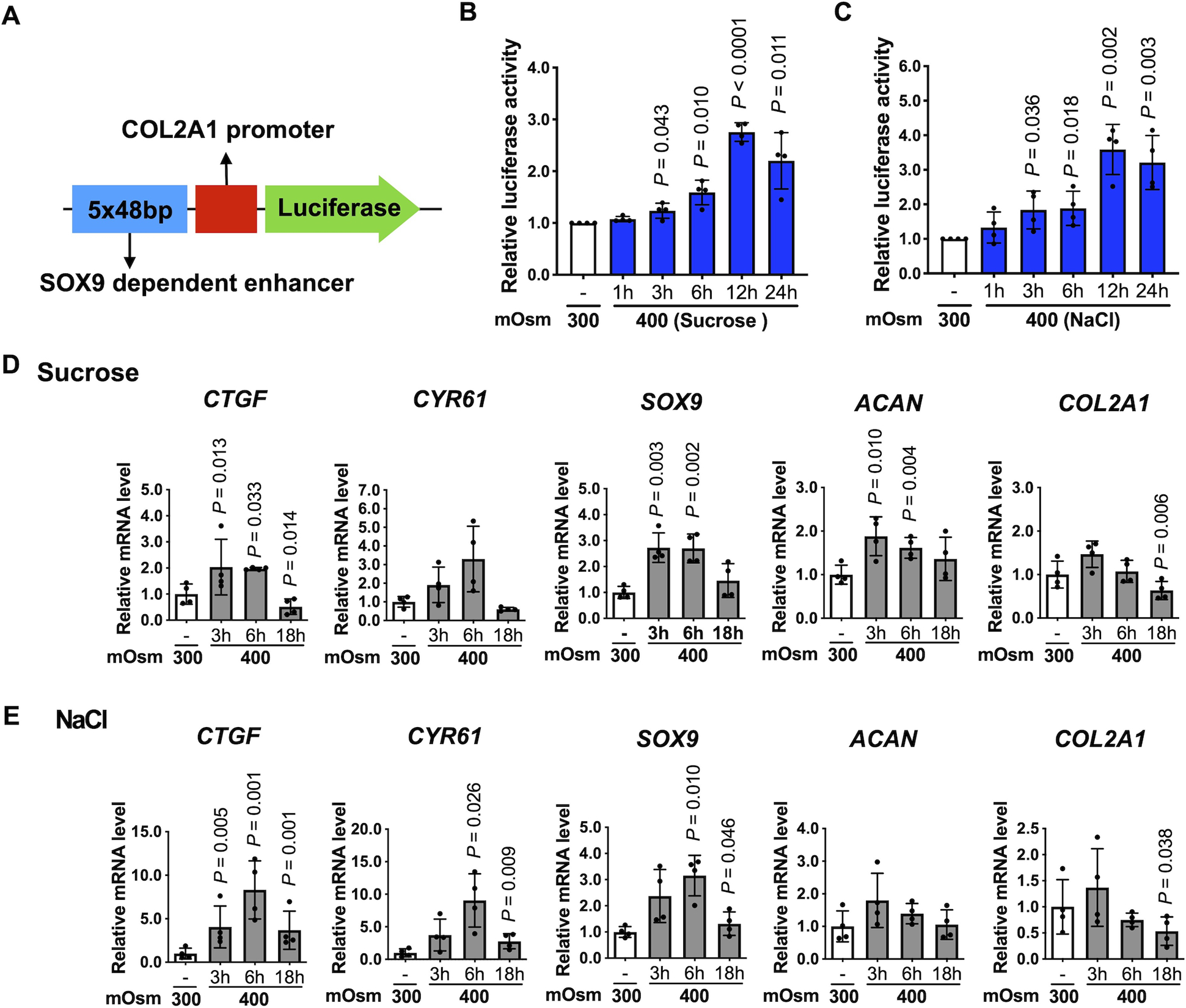
(A) Schematic of the SOX9-dependent COL2A1 enhancer-luciferase reporter construct. The 5 tandem copies of 48 bp enhancer elements (blue box) and the COL2A1 promoter (red box) are indicated. (B, C) Chondrocytes were transiently transfected with the SOX9-dependent COL2A1 reporter construct for 48 hr, followed by incubation in standard 300 mOsm media or 400 mOsm media produced using sucrose or NaCl for the indicated times, and then the luciferase activity was measured. The data are expressed as fold changes relative to 300 mOsm controls. (n = 4 independent donors). For each condition, one-sample t-test was applied to compare logged 2 transformed fold change to zero, which corresponds to the fold change of 1 indicating no difference. (D, E) Chondrocytes were incubated in 300 mOsm or 400 mOsm media for 3 hr, 6 hr and 18 hr. Expression of CTGF, CYR61, SOX9, ACAN and COL2A1 mRNA was normalized to TBP. Data are presented as the mean ± standard deviation. (n = 4 independent donors); indicated p-values were determined by paired t-tests used to compare different conditions to 300 mOsm controls. The values for Figure 2D (CTGF, ACAN), 2E (CTFF, SOX9, COL2A1) were logarithmically transformed to achieve normality.
To examine the effect of YAP/TAZ nuclear translocation on catabolic signaling under 400 mOsm conditions, we incubated chondrocytes in 300 mOsm control media or 400 mOsm media for 30 min and then added 10 ng/ml IL-1β. Medium (400 mOsm) produced using either sucrose or NaCl promoted YAP nuclear translocation and inhibited IL-1β-induced NFκB signaling, as indicated by decreased cytosolic p-P65, p-IκBα and nuclear t-P65 levels as well as reduced NFκB luciferase activity (Supplementary Fig. S1). These findings, combined with the effects on anabolic gene expression, suggest that increased nuclear YAP/TAZ seen in cells incubated in 400 mOsm media is associated with increased anabolic and reduced catabolic activity.
Pharmacological inhibition and gene knockdown of YAP reduces chondrocyte anabolic activity
To determine if YAP nuclear translocation promotes chondrocyte anabolic activities, the YAP-specific inhibitor verteporfin (VP) was used. Treatment of chondrocytes with VP inhibited YAP nuclear translocation (Fig. 3 A, B) and the expression of the YAP target genes CTGF and CYR61 (Fig. 3 C), verifying the YAP inhibitory effect of VP. Inhibition of YAP with 1 μM VP also reduced the basal expression of SOX9, COL2A1 and ACAN (Fig. 3 D) and blocked the elevated activity of the SOX9-dependent COL2A1 enhancer induced by 400 mOsm media (Fig. 3 E). To further test the pro-anabolic effect of YAP, we isolated chondrocytes from homozygous mice carrying the YAP1flox allele (YAP1flox/flox) and knocked down YAP expression by transduction with an adenovirus that expresses Cre recombinase with a GFP tag (Ad-Cre-GFP) or an EGFP-expressing adenovirus (Ad-EGFP) as a control (Fig. 4 A). Adenoviral transduction was verified by fluorescence microscopy (Fig. 4 B), and YAP knockdown was verified by immunoblotting (Fig. 4 C, D). Pellet cultures generated from YAP knockdown and wild-type control chondrocytes were generated. YAP knockdown was confirmed by IHC and was associated with reduced proteoglycan staining using safranin-O as well as decreased levels of type II collagen via IHC (Fig. 4 E). We also tested a proposed YAP activator, lysophosphatidic acid (LPA) 21, which can increase YAP nuclear translocation (Supplementary Fig. S2 A, B). LPA increased the activity of the SOX9-dependent COL2A1 enhancer-luciferase reporter by 2.2-fold (95% CIs: 1.2–1.9) (Fig. S2 C) and the expression of the YAP target genes CTFG (mean difference (95% CIs): 1.56 (0.38, 2.75)) and CYR61 (mean difference (95% CIs): 0.70 (0.29, 1.11)). LPA also increased the expression of COL2A1 (mean difference (95% CIs): 1.30 (0.08, 2.52)) but not SOX9 or ACAN (Fig. S2 D). Taken together these findings support an anabolic effect of YAP nuclear translocation.
Fig. 3. Effects of YAP inhibition on chondrocyte anabolic activity.
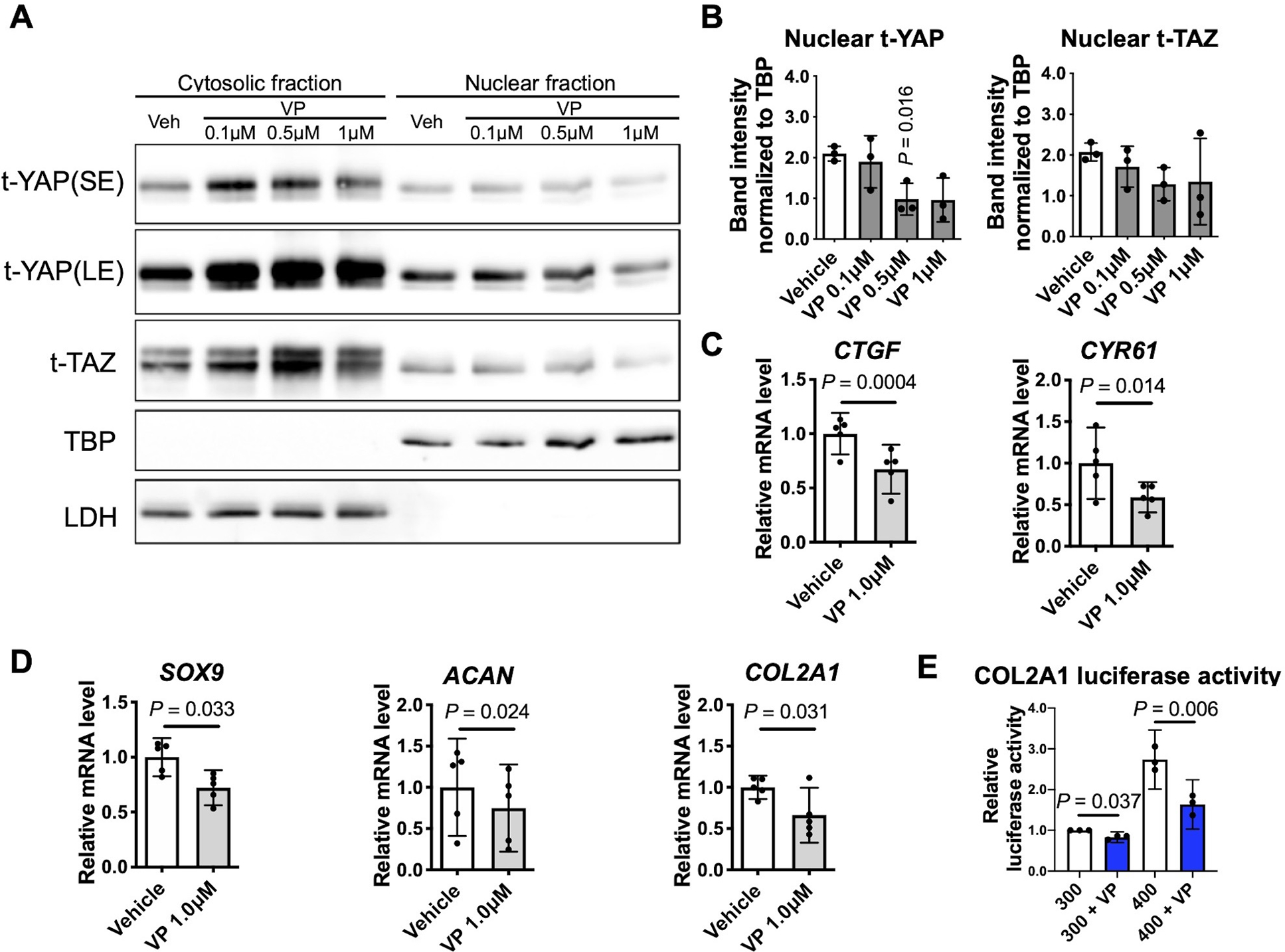
(A) Chondrocytes incubated in standard 300 mOsm media were treated for 6 hr with the indicated concentration of the YAP inhibitor verteporfin (VP). Protein levels of YAP and TAZ in cytosolic and nuclear fractions were determined by immunoblotting. The immunoblots shown are representative of three independent donors (t, total; SE, short exposure; LE, long exposure). (B) Densitometry analysis of YAP/TAZ nuclear abundance. Band intensities of nuclear YAP (n=3 independent donors) and TAZ (n=3 independent donors) were normalized to the loading control TBP and are presented as the mean ± standard deviation; indicated p-values were determined by paired t-tests used to compare different conditions to vehicle controls. (C, D) Quantification of the expression of the indicated genes in response to 6 hr of 1 μM VP treatment. Data are presented as the mean with 95% confidence interval (CI). Differences from vehicle controls are indicated (n = 5 independent donors). (E) Chondrocytes were transiently transfected with the SOX9-dependent COL2A1 luciferase reporter construct for 48 hr, followed by incubation in 300 mOsm media or 400 mOsm media produced using sucrose for 12 h after pretreatment with VP at 1 μM for 6 h and measurement of luciferase activity. The data are expressed as fold changes relative to 300 mOsm controls (n = 3 independent donors). For each condition, one-sample t-test was applied to compare logged 2 transformed fold change to zero, which corresponds to the fold change of 1 indicating no difference.
Fig. 4. Effects of YAP knockdown on chondrocyte anabolic activity.
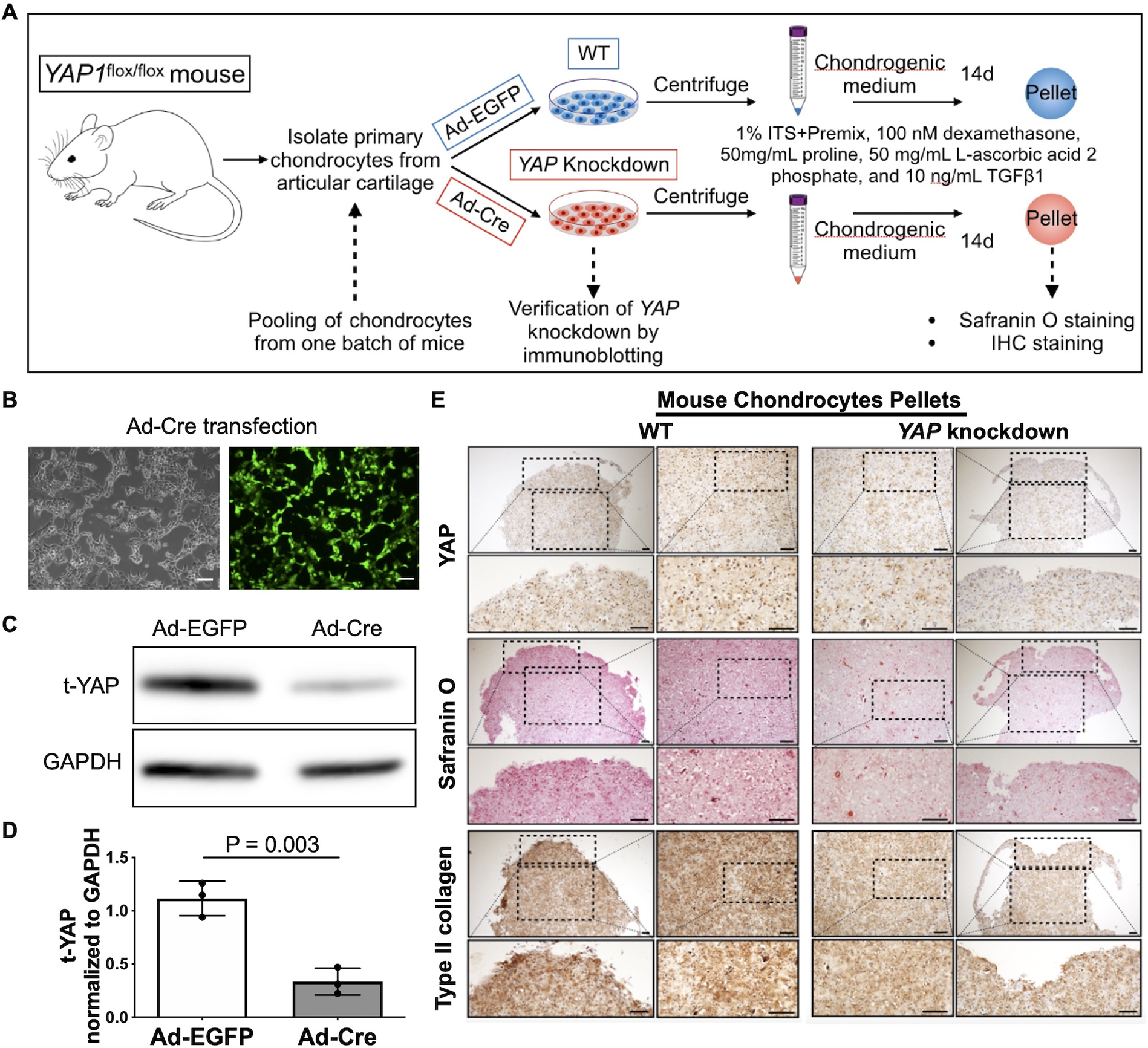
(A) Experimental design. Articular chondrocytes isolated from 22 homozygous mice carrying the YAP1flox allele (YAP1flox/flox) were pooled and transduced with an adenovirus that expresses Cre recombinase with a GFP tag (Ad-Cre) to knockdown YAP expression or Ad-EGFP as a control. After 48 hr, the chondrocytes were centrifuged to form pellets and cultured in chondrogenic media for 14 days. (B) Light and fluorescence microscopy demonstrating Ad-Cre-GFP transduction in YAP1flox/flox mouse chondrocytes. Scale bar, 100 μm. (C) YAP1flox/flox mouse chondrocytes were transduced with the indicated adenovirus and immunoblotted for YAP. (D) Densitometric analysis showing the levels of YAP in total lysates. The relative band intensity of YAP was calculated by normalizing to the loading control GAPDH and is presented as the mean ± standard deviation (n = 3 independent experiments from 3 batches of mice each); indicated p-values were determined by paired t-tests. (E) Sections comparing WT and YAP knockdown mouse chondrocyte pellets with safranin-O staining and IHC staining of YAP and type II collagen.
YAP nuclear translocation mediated by differential phosphorylation is induced by an anabolic stimulus and inhibited by catabolic stimuli
We further investigated YAP/TAZ nuclear translocation in response to pro-anabolic and catabolic stimuli in human chondrocytes. Following treatment with IGF-1, a pro-anabolic factor, YAP nuclear translocation was induced starting at 1 hr (mean difference (95% CIs): 0.65 (0.25, 1.05)) and decreased back to basal levels at 6 hrs (Fig. 5 A, D). A similar trend was observed for nuclear TAZ. On the other hand, fibronectin fragments (FN-F) that are found in OA cartilage and synovial fluid and activate chondrocyte catabolic signaling 22 gradually decreased the levels of nuclear YAP and TAZ, as did IL-1β (Fig. 5 B, C, E, F). These results demonstrate differential YAP translocation in response to anabolic vs catabolic mediators.
Fig. 5. Regulation of YAP/TAZ nuclear translocation by anabolic and catabolic stimuli.
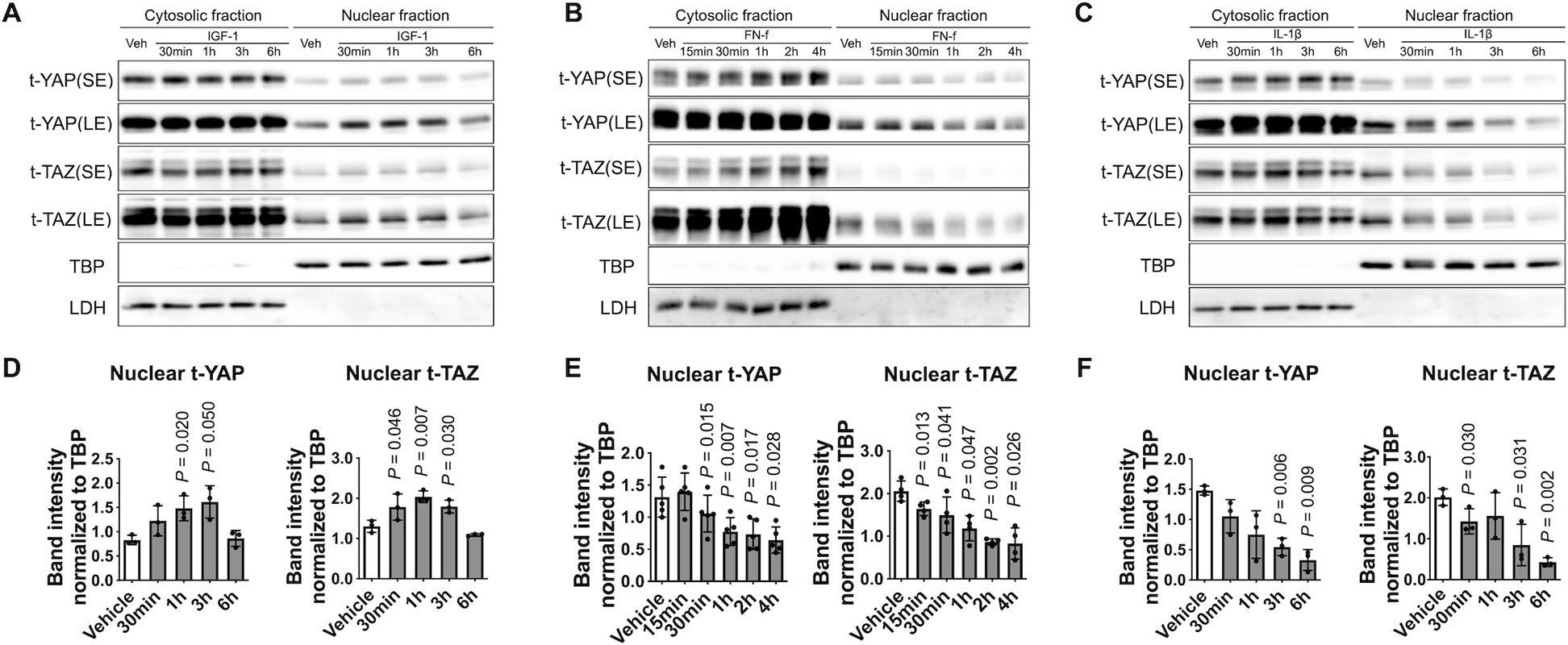
(A - C) Chondrocytes were treated with IGF-1 (100 ng/ml), FN-f (1 μM), or IL-1β (10 ng/ml) for the indicated times, and then cell lysates were prepared. Protein levels of YAP and TAZ were measured in cytosolic and nuclear fractions by immunoblotting. The immunoblots shown are representative of three independent donors of IGF-1 and IL-1β treatment and of five independent donors of FN-f treatment (t, total; SE, short exposure; LE, long exposure). (D - F) Densitometric analysis of YAP/TAZ nuclear abundance. Band intensities of nuclear YAP and TAZ were normalized to the loading control TBP and are presented as the mean ± standard deviation; indicated p-values were determined by paired t-tests used to compare different conditions to vehicle controls.
YAP phosphorylation at Ser127 leads to its cytosolic retention, while phosphorylation at Ser128 blocks its sequestration in the cytosol and enhances nuclear translocation 23,24. To determine if differential chondrocyte YAP nuclear translocation in response to anabolic and catabolic stimuli was regulated by differential YAP phosphorylation, we treated human chondrocytes with two different anabolic stimuli (incubation in 400 mOsm media or stimulation with IGF-1) and two different catabolic stimuli (FN-f or IL-1β). Culture with both anabolic stimuli increased YAP phosphorylation at Ser128, while the level of YAP phosphorylated at Ser127 did not change, resulting in an increased ratio of p-YAP (Ser128) to p-YAP (Ser127) (Fig. 6 A–D). In contrast, FN-f and IL-1β induced YAP phosphorylation at Ser127. The Ser128 phosphorylation remained the same with FN-f and was slightly decreased with IL-1β (Fig. 6 E–H). As a result, the ratio of p-YAP (Ser128) to p-YAP (Ser127) decreased with the two catabolic stimuli.
Fig. 6. YAP phosphorylation in response to changes in osmolarity, IGF-1, FN-f, and IL-1β.
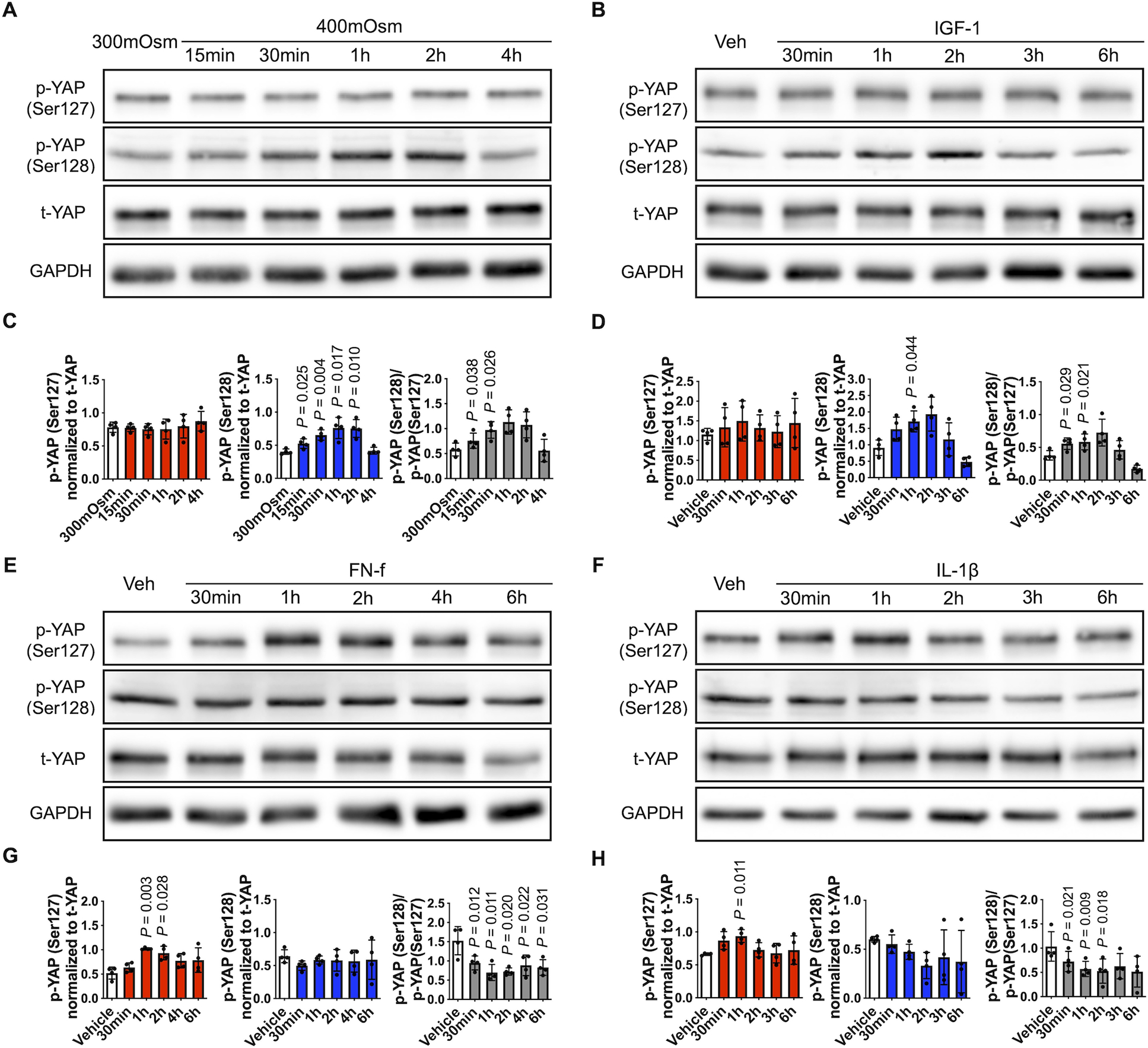
(A) Chondrocytes were incubated in 300 mOsm media or 400 mOsm media generated using sucrose for the indicated times. (B, E, F) Chondrocytes incubated in 300 mOsm media were treated with IGF-1 (100 ng/ml), FN-f (1 μM) or IL-1β (10 ng/ml) for the indicated times. Cell lysates were prepared and subjected to immunoblot analysis for p-YAP (Ser127), p-YAP (Ser128) and t-YAP. The immunoblots shown are representative of four independent donors (p, phospho-; t, total). (C, D, G, H) Densitometric analysis showing YAP phosphorylation at Ser127 (red column) and Ser128 (blue column). Phosphorylated bands were normalized to total protein as loading controls. The ratio of p-YAP (Ser128) to p-YAP (Ser127) was also analyzed (gray column). Data are presented as the mean ± standard deviation; indicated p-values were determined by paired t-tests used to compare different conditions either to 300 mOsm controls or vehicle controls.
To further investigate the effects of IGF-1 on YAP activity, we transfected chondrocytes with a TEA/ATTS domain (TEAD) promoter-luciferase reporter construct. YAP/TAZ are the primary co-activators of TEAD-mediated transcription and we noted increased TEAD activity 3 hrs after IGF-1 treatment (Fig. S3) which matches the time when YAP and TAZ have translocated to the nucleus (Fig. 5). We investigated upstream kinases responsible for IGF-1 mediated YAP nuclear translocation by treating chondrocytes with inhibitors to nemo-like kinase (NLK), GSK-3β and PI-3 kinase, as detailed in the Supplementary Methods. We found that inhibition of NLK or PI-3 kinase but not GSK-3β reduced YAP nuclear translocation (Fig. S3) suggesting both kinases are involved.
YAP cytosolic sequestration is associated with YAP Ser127 phosphorylation in human cartilage
To investigate the regulation of YAP in human OA cartilage, we isolated chondrocytes from less damaged and damaged regions of cartilage from knee arthroplasties from the same patients with OA. Immunoblotting of fractionated cell lysates was used to evaluate the levels of nuclear YAP and cytosolic YAP. Chondrocytes isolated from the damaged region of OA cartilage exhibited a lower level of nuclear YAP than cells from the less damaged regions (Fig. 7 A, D, E).
Fig. 7. YAP cytosolic sequestration is associated with YAP Ser127 phosphorylation in human cartilage.
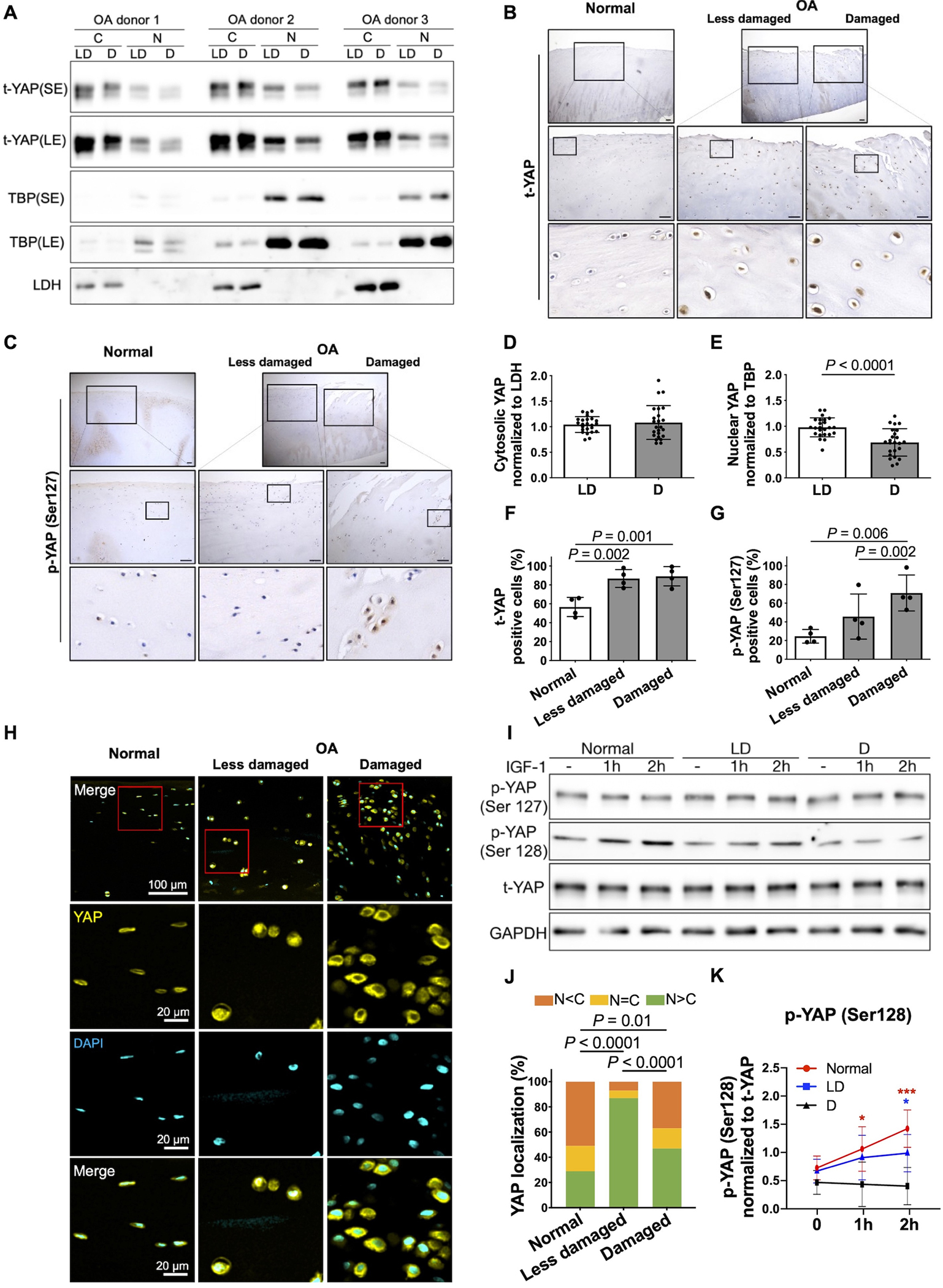
(A) Representative immunoblots of nuclear and cytosolic t-YAP in cultured chondrocytes from less damaged (LD) and damaged (D) areas of the same knee OA donor (t, total; SE, short exposure; LE, long exposure). (B, C) IHC staining for t-YAP and p-YAP (Ser127) in normal femoral cartilage and less damaged and damaged areas from the same OA donor’s femoral cartilage. Representative images from four different donors for each group are shown. Magnified images of regions are marked by black boxes. Scale bar, 100 μm. (D, E) Densitometric analysis of immunoblots of nuclear and cytosolic YAP. The relative band intensity of nuclear and cytosolic YAP was calculated by normalizing to the loading control TBP and LDH, respectively. Data are presented as the mean values ± standard deviation; indicated p-values were determined by paired t-tests used to compare LD to D areas. (n = 24 independent samples from 24 donors). (F, G) The percentages of t-YAP- and p-YAP (Ser127)-positive cells in IHC staining are shown. Data are presented as the mean values ± standard deviation; indicated p-values were determined by paired t-tests used to compare LD to D areas and Welch’s t-tests used to compare normal cartilage to less damaged or more damaged areas in OA donor’s cartilage. (H) Immunofluorescence staining for YAP cellular localization in normal femoral cartilage and less damaged and damaged areas from the same OA donor’s femoral cartilage. Representative images from four normal and four OA donors are shown. Scale bars, 100 μm for low and 20 μm for high magnification (red box). (J) The statistical analysis for YAP cellular distribution (% percentage) (n = 4 for both normal and OA donors). Data are presented as proportion of distribution in the nucleus (N) and cytosol (C) in normal and less damaged and damaged OA cartilage; indicated p-values were determined by Fisher’s exact t-tests used to compare normal cartilage to less damaged or more damaged areas in OA donor’s cartilage. McNemar’s tests were used to compare less damaged to more damaged areas in OA donor’s cartilage. (I) Chondrocytes from normal donors and less damaged (DL) and damaged (D) areas of the same OA donors were treated with IGF-1 (100 ng/ml) for 1 h and 2 h. Representative immunoblots of p-YAP (Ser127), p-YAP (Ser128) and t-YAP are shown (p, phospho-; t, total). (K) Densitometric analysis of p-YAP (Ser128). Data are presented as the mean values with 95% confidence intervals (CIs) (n = 4 independent donors). P-values and confidence intervals were determined by linear mixed effect model. Linear mixed effects model was fit with unstructured covariance structure to account for repeated measures over time. Fixed-effect terms included indicators for group (normal, less damaged and damaged), time in hours and their interaction. To account for more and less damaged regions being collected from a same donor, donor random effect was included in the model.
We analyzed the levels of YAP and YAP phosphorylated at Ser127 in normal human cartilage and OA cartilage by IHC. Levels of YAP were higher in both damaged and less damaged OA cartilage compared to normal (Fig. 7 B, F) while levels of p-YAP (Ser127) were higher in OA cartilage compared to normal and higher in the more damaged region of OA cartilage compared to the less damaged region (Fig. 7 C, G). These results were confirmed using immunofluorescence, which demonstrated higher nuclear YAP in less damaged regions compared with both damaged OA regions and normal cartilage. (Fig. 7 H, J). Finally, we treated chondrocytes isolated from normal cartilage, less damaged, and damaged OA cartilage with IGF-1 for 2 hrs. There was no change of p-YAP (Ser128) in response to IGF-1 in chondrocytes from damaged regions, indicating a reduced responsiveness to IGF-1 in terms of YAP activation in OA chondrocytes (Fig. 7 I, K, Supplementary Table S1).
Discussion
The activation of YAP relies on translocation from the cytoplasm to the nucleus in response to diverse upstream stimuli 7,25. We found that differential YAP phosphorylation and nuclear translocation regulates anabolic activity in response to OA-relevant stimuli in primary human chondrocytes (summarized in Fig. 8). Catabolic stimuli (IL-1β and fibronectin fragments) resulted in phosphorylation of YAP at Ser127, leading to YAP cytosolic retention, while anabolic stimuli (physiologic osmotic stimulation and IGF-1) induced YAP phosphorylation at Ser128 with enhanced YAP nuclear translocation. The findings that YAP nuclear translocation increased chondrocyte anabolic activity and that YAP was retained in the cytosol in severely damaged regions of human OA cartilage are consistent with a role for YAP in maintaining cartilage homeostasis that is lost in advanced OA. Our results support previous studies suggesting a positive role for YAP in the joint 10,14 and indicate the importance of considering YAP phosphorylation and evaluating both cytosolic and nuclear levels of YAP and its co-regulator TAZ to determine YAP/TAZ activation status.
Fig. 8. Schematic model of the regulation of YAP nuclear translocation in response to different stimuli and the downstream effects on chondrocyte gene expression.
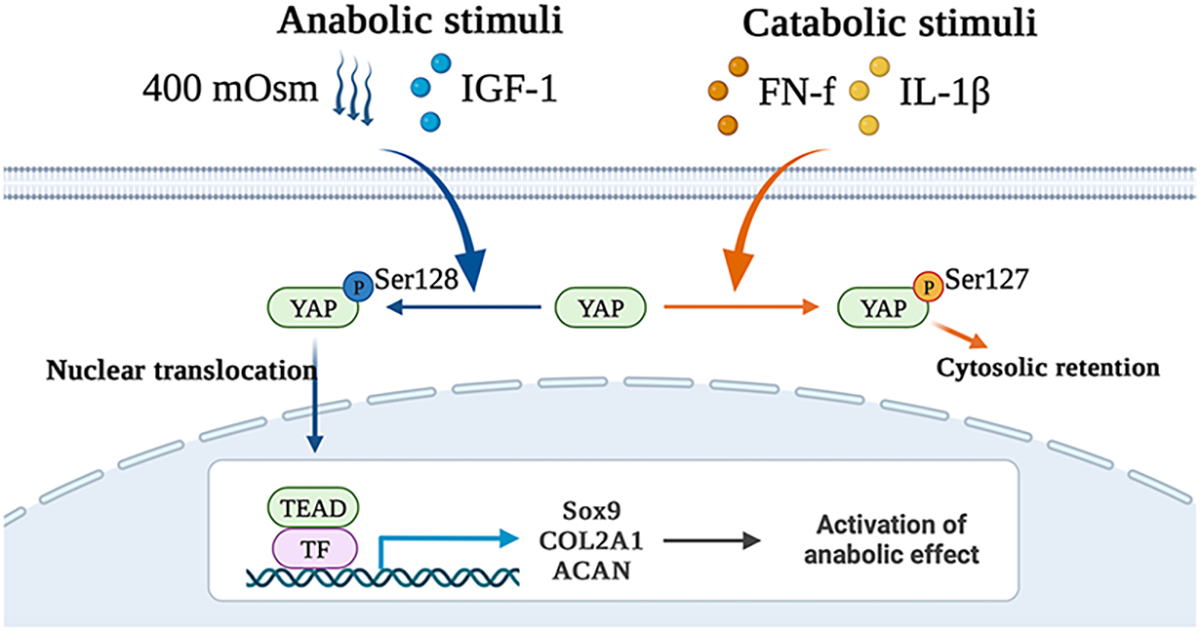
The catabolic stimuli FN-f and IL-1β result in phosphorylation of YAP at Ser127, leading to YAP cytosolic retention. In contrast, anabolic stimuli, including an osmotic stimulus using 400 mOsm media and IGF-1, result in YAP phosphorylation at Ser128, which enhances YAP nuclear translocation and subsequent binding to transcription factors (TFs), e.g., TEAD, to promote downstream anabolic gene expression.
Using increased osmolarity of the chondrocyte media as an in vitro model of joint loading, we found that YAP/TAZ nuclear translocation was induced by 400 mOsm media but blocked by higher osmolarity at 600 mOsm, compared with iso-osmotic media of 300 mOsm. Importantly, 400 mOsm is within the physiological osmotic range of normal healthy cartilage which ranges from 350 mOsm to 480 mOsm 26,27. Previous studies have shown promotion of anabolic activity at physiological osmotic pressures 17,28,29 consistent with our finding that increased YAP/TAZ nuclear translocation at 400 mOsm was associated with enhanced SOX9-dependent COL2A1 transcriptional activity and promotion of anabolic genes (SOX9, ACAN and COL2A1)..
In chondrocytes, catabolic cytokines (such as IL-1β and TNFα) were previously found to promote the degradation of YAP through TAK1-mediated phosphorylation at multiple sites 10. We noted that catabolic stimuli, including IL-1β and fibronectin fragments, induced YAP/TAZ cytosolic retention associated with YAP phosphorylation at Ser127 while anabolic stimuli, including IGF-1 and 400 mOsm media, promoted YAP/TAZ nuclear translocation associated with YAP phosphorylation at Ser128. In contrast, in a 2D chondrocyte culture model with increased ECM stiffness that exerted a catabolic effect, YAP nuclear translocation was induced rather than inhibited 12. Therefore, YAP nuclear translocation is a complex context-dependent process 30,31. Similarly, in the HEK293 cell line, hyperosmotic stress (800 mOsm) was found to phosphorylate YAP at Ser127 and lead to YAP cytosolic retention 32, while an osmotic stimulus, 500 mOsm, induced YAP nuclear translocation via Ser128 phosphorylation 24. A study in endothelial cells found that oscillatory shear stress enhanced the level of nuclear YAP via Tyr357 phosphorylation 33. Taken together, the nuclear translocation of YAP depends on upstream stimulation and regulation by post-translational modifications that include specific sites of Ser and Tyr phosphorylation. Understanding the context-specific regulation of YAP activity is important for understanding its role in osteoarthritis.
Our study showed that YAP promotes anabolic activity in normal human primary chondrocytes, as indicated by the observations that a YAP specific inhibitor reduced, while a YAP activator enhanced anabolic gene expression. The promotion of anabolic activity was also verified in pellet cultures generated from YAP knockdown chondrocytes. In addition to anabolic activity, YAP has been found to impact other OA-associated phenotypes. Chondrocyte hypertrophy is a feature of OA, and chondrocyte hypertrophy-like changes play a role in the development of OA 34,35. Runx2 and its target gene collagen X are essential for chondrocyte hypertrophy 36. YAP has been shown to inhibit chondrocyte hypertrophy by directly interacting with Runx2 37,38. Cellular senescence in joint tissues is associated with OA 39,40 and declining YAP activity was proposed to drive senescence in stromal cells 41. YAP was shown to safeguard mesenchymal stem cells (MSCs) from senescence and thus, play a protective role in cartilage degradation14. As to cartilage repair, Patel et al. 42 designed a hyaluronic acid (HA) hydrogel system and found HA therapy of degenerated cartilage improved MSC mechanosensation, indicated by YAP nuclear translocation. As a result, it enhanced fortification and reestablished a superficial zone of the degenerate cartilage 42, which also supports a protective role of YAP in cartilage.
We noted that total YAP levels were increased in OA cartilage, which was similar to previous studies 11,12,43. We also found increased YAP nuclear localization in less damaged OA cartilage, compared with normal. However, in the same OA cartilage, the level of nuclear YAP was lower in severely damaged regions than in less damaged areas, accompanied by a higher level of YAP phosphorylation at Ser127. Furthermore, unlike normal chondrocytes, chondrocytes isolated from severely damaged areas did not show an increase of YAP phosphorylation at Ser128 in response to IGF-1 stimulation. Since we found that YAP Ser128 phosphorylation and nuclear translocation promotes anabolic activity in chondrocytes, taken together, our findings implicated that in less damaged OA cartilage, the nuclear translocation of YAP could be needed to help maintain cartilage homeostasis. In areas with more severe damage, YAP is sequestered in the cytosol due to increased YAP phosphorylation at Ser127 and chondrocytes from damaged areas have lost the ability to respond to stimuli such as IGF-1 that promote YAP Ser128 phosphorylation.
There are some potential limitations to this study. We only used the method of modifying osmolarity to simulate the effects of joint loading. Further studies should investigate other methods of stimulating mechanotransduction. Although LPA is a proposed YAP activator 44, it is not specific to YAP activation and may have other effects on cells. Hence, we used YAP knockdown in mouse chondrocytes to verify our results. Further studies with YAP knockout or overexpression in chondrocytes are warranted. Additionally, we mainly used human primary articular chondrocytes in this study. The variability among primary cells isolated from different human donors can reduce the chances of detecting significant results when differences are small, but the advantage of using human cells over cell lines or cells from other species counters this limitation. In this study, phosphorylation of YAP at Ser127 and Ser128 were identified to regulate subcellular localization of YAP. Future studies could also evaluate the contribution of Tyr357 phosphorylation to YAP nuclear translocation in response to 400 mOsm media and IGF-1. Further studies are also needed to determine if YAP binding to the 14–3-3 protein sequesters YAP in the cytoplasm when it is phosphorylated at Ser12745.
In conclusion, YAP nuclear translocation is mediated by site-specific YAP phosphorylation in response to OA-related stimuli in human chondrocytes. Increased YAP activity was found to be associated with anabolic effects consistent with studies showing a positive role of YAP/TAZ in maintaining cartilage homeostasis. Decreased nuclear YAP in OA chondrocytes may contribute to reduced anabolic activity and promotion of further cartilage loss. Further studies are needed to determine how to best maintain the proper level of YAP activity in the joint by promoting YAP Ser128 phosphorylation or inhibiting phosphorylation at Ser127.
Supplementary Material
Acknowledgments
We would like to acknowledge the Gift of Hope Organ and Tissue Donor Network (Itasca, IL) and the donor families for providing normal human joints from tissue donors, and we would like to acknowledge the Department of Orthopedics, University of North Carolina at Chapel Hill, for providing osteoarthritic human cartilage tissues. We thank Dr. Veronique Lefebvre (University of Pennsylvania) for providing constructs. We thank Dr. Brian Button (University of North Carolina at Chapel Hill) for sharing the vapor pressure osmometer. We thank Dr. Pablo Ariel and the Microscopy Services Laboratory (University of North Carolina at Chapel Hill) for providing training and technical support on microscopy. We thank Matthew Rich (University of North Carolina at Chapel Hill) for critically discussing the function of YAP. We also appreciate Kathryn Kelly and Helen Willcockson (University of North Carolina at Chapel Hill) for all their generous technical support.
Role of the funding source
This project was supported by grants from the National Institute of Arthritis, Musculoskeletal, and Skin Disease (R37-AR049003), Australia National Health and Medical Council (APP1177374; to QPS), Australia National Heart Foundation (102592; to QPS) and Australia Research Council (DP200101970; to QPS). The study sponsors had no role in the study design, collection, analysis and interpretation of data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Footnotes
Competing Interest Statement
The authors declare that they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fu S, Thompson CL, Ali A, Wang W, Chapple JP, Mitchison HM, et al. Mechanical loading inhibits cartilage inflammatory signalling via an HDAC6 and IFT-dependent mechanism regulating primary cilia elongation. Osteoarthritis Cartilage 2019;27(7):1064–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang SH, Mori D, Kobayashi H, Mori Y, Nakamoto H, Okada K, et al. Excessive mechanical loading promotes osteoarthritis through the gremlin-1-NF-κB pathway. Nat Commun 2019;10(1):1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayala S, Delco ML, Fortier LA, Cohen I, Bonassar LJ. Cartilage articulation exacerbates chondrocyte damage and death after impact injury. J Orthop Res 2021;39(10):2130–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vincent TL. Targeting mechanotransduction pathways in osteoarthritis: a focus on the pericellular matrix. Curr Opin Pharmacol 2013;13(3):449–54. [DOI] [PubMed] [Google Scholar]
- 5.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, et al. Role of YAP/TAZ in mechanotransduction. Nature 2011;474(7350):179–83. [DOI] [PubMed] [Google Scholar]
- 6.Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev 2014;94(4):1287–312. [DOI] [PubMed] [Google Scholar]
- 7.Totaro A, Panciera T, Piccolo S. YAP/TAZ upstream signals and downstream responses. Nat Cell Biol 2018;20(8):888–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu F-X, Zhao B, Guan K-L. Hippo Pathway in Organ Size Control, Tissue Homeostasis, and Cancer. Cell 2015;163(4):811–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cosgrove BD, Mui KL, Driscoll TP, Caliari SR, Mehta KD, Assoian RK, et al. N-cadherin adhesive interactions modulate matrix mechanosensing and fate commitment of mesenchymal stem cells. Nat Mater 2016;15(12):1297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng Y, Lu J, Li W, Wu A, Zhang X, Tong W, et al. Reciprocal inhibition of YAP/TAZ and NF-κB regulates osteoarthritic cartilage degradation. Nat Commun 2018;9(1):4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong Y, Li S-J, Liu R, Zhan J-F, Tan C, Fang Y-F, et al. Inhibition of YAP with siRNA prevents cartilage degradation and ameliorates osteoarthritis development. J Mol Med (Berl) 2019;97(1):103–14. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X, Cai D, Zhou F, Yu J, Wu X, Yu D, et al. Targeting downstream subcellular YAP activity as a function of matrix stiffness with Verteporfin-encapsulated chitosan microsphere attenuates osteoarthritis. Biomaterials 2020;232(119724. [DOI] [PubMed] [Google Scholar]
- 13.Zhong W, Li Y, Li L, Zhang W, Wang S, Zheng X. YAP-mediated regulation of the chondrogenic phenotype in response to matrix elasticity. J Mol Histol 2013;44(5):587–95. [DOI] [PubMed] [Google Scholar]
- 14.Fu L, Hu Y, Song M, Liu Z, Zhang W, Yu F-X, et al. Up-regulation of FOXD1 by YAP alleviates senescence and osteoarthritis. PLoS Biol 2019;17(4):e3000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phan MN, Leddy HA, Votta BJ, Kumar S, Levy DS, Lipshutz DB, et al. Functional characterization of TRPV4 as an osmotically sensitive ion channel in porcine articular chondrocytes. Arthritis Rheum 2009;60(10):3028–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark AL, Votta BJ, Kumar S, Liedtke W, Guilak F. Chondroprotective role of the osmotically sensitive ion channel transient receptor potential vanilloid 4: age- and sex-dependent progression of osteoarthritis in Trpv4-deficient mice. Arthritis Rheum 2010;62(10):2973–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Windt AE, Haak E, Kops N, Verhaar JAN, Weinans H, Jahr H. Inhibiting calcineurin activity under physiologic tonicity elevates anabolic but suppresses catabolic chondrocyte markers. Arthritis Rheum 2012;64(6):1929–39. [DOI] [PubMed] [Google Scholar]
- 18.Yin W, Park J-I, Loeser RF. Oxidative stress inhibits insulin-like growth factor-I induction of chondrocyte proteoglycan synthesis through differential regulation of phosphatidylinositol 3-Kinase-Akt and MEK-ERK MAPK signaling pathways. J Biol Chem 2009;284(46):31972–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muehleman C, Bareither D, Huch K, Cole AA, Kuettner KE. Prevalence of degenerative morphological changes in the joints of the lower extremity. Osteoarthritis Cartilage 1997;5(1):23–37. [DOI] [PubMed] [Google Scholar]
- 20.Loeser RF, Pacione CA, Chubinskaya S. The combination of insulin-like growth factor 1 and osteogenic protein 1 promotes increased survival of and matrix synthesis by normal and osteoarthritic human articular chondrocytes. Arthritis Rheum 2003;48(8):2188–96. [DOI] [PubMed] [Google Scholar]
- 21.Yu F-X, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 2012;150(4):780–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loeser RF. Integrins and chondrocyte-matrix interactions in articular cartilage. Matrix Biol 2014;39(11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moon S, Kim W, Kim S, Kim Y, Song Y, Bilousov O, et al. Phosphorylation by NLK inhibits YAP-14–3-3-interactions and induces its nuclear localization. EMBO Rep 2017;18(1):61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong AW, Meng Z, Yuan H-X, Plouffe SW, Moon S, Kim W, et al. Osmotic stress-induced phosphorylation by NLK at Ser128 activates YAP. EMBO Rep 2017;18(1):72–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heng BC, Zhang X, Aubel D, Bai Y, Li X, Wei Y, et al. An overview of signaling pathways regulating YAP/TAZ activity. Cell Mol Life Sci 2021;78(2):497–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maroudas AI. Balance between swelling pressure and collagen tension in normal and degenerate cartilage. Nature 1976;260(5554):808–09. [DOI] [PubMed] [Google Scholar]
- 27.Urban JP, Hall AC, Gehl KA. Regulation of matrix synthesis rates by the ionic and osmotic environment of articular chondrocytes. J Cell Physiol 1993;154(2):262–70. [DOI] [PubMed] [Google Scholar]
- 28.Xu X, Urban JPG, Tirlapur UK, Cui Z. Osmolarity effects on bovine articular chondrocytes during three-dimensional culture in alginate beads. Osteoarthritis Cartilage 2010;18(3):433–39. [DOI] [PubMed] [Google Scholar]
- 29.van der Windt AE, Haak E, Das RHJ, Kops N, Welting TJM, Caron MMJ, et al. Physiological tonicity improves human chondrogenic marker expression through nuclear factor of activated T-cells 5 in vitro. Arthritis Res Ther 2010;12(3):R100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scott KE, Fraley SI, Rangamani P. A spatial model of YAP/TAZ signaling reveals how stiffness, dimensionality, and shape contribute to emergent outcomes. Proc Natl Acad Sci U S A 2021;118(20):e2021571118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo J, Li P. Context-dependent transcriptional regulations of YAP/TAZ in stem cell and differentiation. Stem Cell Res Ther 2022;13(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin KC, Moroishi T, Meng Z, Jeong H-S, Plouffe SW, Sekido Y, et al. Regulation of Hippo pathway transcription factor TEAD by p38 MAPK-induced cytoplasmic translocation. Nat Cell Biol 2017;19(8):996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li B, He J, Lv H, Liu Y, Lv X, Zhang C, et al. c-Abl regulates YAPY357 phosphorylation to activate endothelial atherogenic responses to disturbed flow. J Clin Invest 2019;129(3):1167–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pitsillides AA, Beier F. Cartilage biology in osteoarthritis--lessons from developmental biology. Nat Rev Rheumatol 2011;7(11):654–63. [DOI] [PubMed] [Google Scholar]
- 35.van der Kraan PM, van den Berg WB. Chondrocyte hypertrophy and osteoarthritis: role in initiation and progression of cartilage degeneration? Osteoarthritis Cartilage 2012;20(3):223–32. [DOI] [PubMed] [Google Scholar]
- 36.Zheng Q, Zhou G, Morello R, Chen Y, Garcia-Rojas X, Lee B. Type X collagen gene regulation by Runx2 contributes directly to its hypertrophic chondrocyte-specific expression in vivo. J Cell Biol 2003;162(5):833–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deng Y, Wu A, Li P, Li G, Qin L, Song H, et al. Yap1 Regulates Multiple Steps of Chondrocyte Differentiation during Skeletal Development and Bone Repair. Cell Rep 2016;14(9):2224–37. [DOI] [PubMed] [Google Scholar]
- 38.Abou-Jaoude A, Courtes M, Badique L, Elhaj Mahmoud D, Abboud C, Mlih M, et al. ShcA promotes chondrocyte hypertrophic commitment and osteoarthritis in mice through RunX2 nuclear translocation and YAP1 inactivation. Osteoarthritis Cartilage 2022. [DOI] [PubMed] [Google Scholar]
- 39.Coryell PR, Diekman BO, Loeser RF. Mechanisms and therapeutic implications of cellular senescence in osteoarthritis. Nat Rev Rheumatol 2021;17(1):47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y, Zhang Z, Li T, Xu H, Zhang H. Senescence in osteoarthritis: from mechanism to potential treatment. Arthritis Res Ther 2022;24(1):174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sladitschek-Martens HL, Guarnieri A, Brumana G, Zanconato F, Battilana G, Xiccato RL, et al. YAP/TAZ activity in stromal cells prevents ageing by controlling cGAS-STING. Nature 2022;607(7920):790–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel JM, Loebel C, Saleh KS, Wise BC, Bonnevie ED, Miller LM, et al. Stabilization of Damaged Articular Cartilage with Hydrogel-Mediated Reinforcement and Sealing. Adv Healthc Mater 2021;10(10):e2100315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thorup A-S, Strachan D, Caxaria S, Poulet B, Thomas BL, Eldridge SE, et al. ROR2 blockade as a therapy for osteoarthritis. Sci Transl Med 2020;12(561):eaax3063. [DOI] [PubMed] [Google Scholar]
- 44.Kim J, Lim H, Moon S, Cho SY, Kim M, Park JH, et al. Hot Spot Analysis of YAP-TEAD Protein-Protein Interaction Using the Fragment Molecular Orbital Method and Its Application for Inhibitor Discovery. Cancers (Basel) 2021;13(16):4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev 2007;21(21):2747–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


