Abstract
Seasonal changes in food intake and adiposity in many animal species are triggered by changes in the photoperiod. These latter changes are faithfully transduced into a biochemical signal by melatonin secreted by the pineal gland. Seasonal variations, encoded by melatonin, are integrated by third ventricular tanycytes of the mediobasal hypothalamus through the detection of the thyroid-stimulating hormone (TSH) released from the pars tuberalis. The mediobasal hypothalamus is a critical brain region that maintains energy homeostasis by acting as an interface between the neural networks of the central nervous system and the periphery to control metabolic functions, including ingestive behavior, energy homeostasis, and reproduction. Among the cells involved in the regulation of energy balance and the blood–hypothalamus barrier (BHB) plasticity are tanycytes. Increasing evidence suggests that anterior pituitary hormones, specifically TSH, traditionally considered to have unitary functions in targeting single endocrine sites, display actions on multiple somatic tissues and central neurons. Notably, modulation of tanycytic TSH receptors seems critical for BHB plasticity in relation to energy homeostasis, but this needs to be proven.
Keywords: tanycyte, pituitary glycoprotein hormone receptor, blood–hypothalamus barrier
Graphical Abstract
The mediobasal hypothalamus is a critical brain region that maintains energy homeostasis. Among the cells involved in the regulation of energy balance and the blood–hypothalamus barrier plasticity are tanycytes. We discuss how third ventricular tanycytes of the mediobasal hypothalamus circulate metabolic signals to hypothalamic neurons as critical metabolic processors of communication between the central nervous system and the periphery.
INTRODUCTION
Changes in the photoperiod are faithfully transduced into neuroendocrine signals by melatonin secreted by the pineal gland, which, in turn, triggers seasonal adaptive responses in many animal species. Most of these species decrease their food intake and adiposity as they are reproductively quiescent during a short photoperiod, when ambient temperatures decline and the availability of food is low. They increase their food intake and adiposity upon resuming reproductive activities during a long photoperiod when ambient temperature and the food supply are more favorable. The pars tuberalis (PT) of the pituitary gland, comprising the anterior lobe of the pituitary stalk that links the mediobasal hypothalamus with the posterior pituitary, is a key site involved in the regulation of seasonality across birds and mammals. That is, short/long photoperiod-dependent seasonal variations, encoded by melatonin, are integrated by tanycytes via the detection of the thyroid-stimulating hormone (TSH) released from the PT (1–4). The switch to a long photoperiod triggers melatonin-responsive cells in the PT of the anterior pituitary to increase TSH production, which, in turn, acts locally on TSH receptor (TSHR)–expressing cells of the mediobasal hypothalamus, resulting in increased type 2 deiodinase (DiO2) expression (2). Notably, tanycytes of the ependymal layer express very high levels of DiO2, an enzyme that converts l-thyroxine (T4) to triiodothyronine (T3) (3, 5). This calls into question whether central TSH actions regulate thyroid hormone metabolism within tanycytes, and in doing so, directly modulate thyrotropin-releasing hormone (TRH) neuronal signaling.
In addition to the PT-synthesized TSH, several groups have reported that TSH is also present in the rat hypothalamus (6, 7). Using double antibody immunoassay, TSH was recovered preferentially from the synaptosome-rich hypothalamic layers (6). The physiological in vivo significance of these findings is not clear, however. Although the concentration of TSH is markedly low relative to the PT, it may function locally as a neuromodulator, as proposed for other peptide hormones (8).
To our knowledge, the first mention of TSHRs broadly in the hypothalamus of the laboratory rat was made by Lawrence and colleagues (9). Subsequently, others reported TSHR expression in the hippocampus of the mouse (10); hypothalamus, hippocampus, pyriform and postcingulate cortex of the rat (11, 12); hypothalamus of the quail (13); and amygdala, cingulate gyrus, frontal cortex, hippocampus, hypothalamus, and thalamus of the adult human (14). However, the first mention of tanycytic TSHR expression, to our knowledge, was 15 years earlier by the Hazlerigg laboratory, describing strong expression of TSHRs both within the PT itself and in adjacent cells in the median eminence, extending into the ependymal paraventricular region surrounding the base of the third ventricle in sheep (2).
Given our recent discovery of a broad array of anterior pituitary hormone receptors, notably TSHRs, follicle-stimulating hormone receptors (FSHRs), and luteinizing hormone receptors (LHCGRs), in multiple brain sites (15), our perspective will focus on the question—what is the role of ependymal TSHRs in mediating blood–hypothalamus barrier (BHB) plasticity in relation to ingestive behavior and energy homeostasis? While little is known about this topic, the main functions and neuroanatomy of the BHB are well known. A specific question we address is how tanycytes, which border the periphery of the third ventricle (3V), circulate metabolic signals to hypothalamic neurons as critical metabolic processors of communication between the central nervous system (CNS) and the periphery. Evidence supports the premise that tanycytes influence energy metabolism through the organization of hormonal and nervous signals (16). Research regarding tanycytic regulation of food intake, glucagon and insulin release, and fatty acid metabolism in adipose tissue and skeletal muscle may provide answers to such questions (16).
TANYCYTES AND THE BLOOD–BRAIN BARRIER
Lining the border of the 3V, tanycytes are specialized ependymoglial cells that reside along the regions of the dorsomedial nucleus (DMH), ventromedial nucleus (VMH), arcuate nucleus (Arc), and median eminence (ME) of the hypothalamus (16–18). Although sharing close resemblance with astrocytes, tanycytes possess a unique morphology and distinct functional characteristics (19). They display a cell soma intact along the mediobasal wall of the hypothalamus, which is in direct contact with CSF through extended microvilli. Long processes extend from their soma into the parenchyma of the hypothalamic Arc, VMH, DMH, ME, and the brain pial surface, thus enabling a specific range of functions that maintain energy balance. Because of this peculiar morphology and their stem-cell–like features, tanycytes are also considered to be radial glia cells of the mature brain (20). ME tanycytes also contribute to the regulation of metabolic functions and reproduction. They dynamically control the secretion of neuropeptides into the hypothalamus–pituitary portal vasculature, sense glucose levels, produce and secrete the active form of thyroid hormones, and regulate local homeostasis via their ability to control the exchange of molecules, such as leptin, between the blood and the hypothalamic extracellular fluid (20).
Tanycytes are composed of four different subtypes: α1, α2, β1, and β2 (16–18). α1 Tanycyte processes extend directly into the VMH, while α2 processes extend directly into the arcuate nucleus (18). α Tanycytes thus create a CSF–hypothalamic barrier in this region (16, 18, 21). β1 Tanycytic processes project to the lateral ME and Arc, while β2 tanycytic processes project to fenestrated capillaries of the ME, allowing contact with nutritional signals (18). β2 Tanycytes are joined together by tight junctions, zonula occludens, and claudins, which help form the BHB. Such organized tight-junction complexes at the level of tanycytic soma seal the intercellular space to prevent free diffusion of blood-borne molecules extravasating from fenestrated capillaries of the ME into the CSF.
For energy metabolism and ingestive behavior studies, tanycytes can be readily identified through specific markers including G protein–coupled receptor 50 (GPR50), which is related to the melatonin family receptors found in tanycytes, as well as vimentin (VIM), nestin (NES), solute carrier family 1 member 3 (SLC1A3), and retina and anterior neural fold homeobox (RAX) (17, 22). Of note, vimentin and nestin were also found to label hypothalamic pericytes (23, 24), as well as pituitary cells that are neither hormonal nor typical folliculo-stellate (25).
TANYCYTES CONTROL FOOD INTAKE AND ENERGY METABOLISM
Food intake and energy homeostasis are processes controlled through central neural networks that interact with the periphery (16, 20, 21). This interaction is achieved through the coordination of thermoregulation, insulin and glucagon release, and fatty acid metabolism in adipose tissues and skeletal muscles (16). Among the cells regulating energy balance and glucose homeostasis, tanycytes have begun to attract considerable attention (20, 26, 27). The pervasive assumption that tanycytes are involved in regulation of food intake has been tested through tanycyte ablation studies, where alloxan-induced suppression of the tanycytic layer triggered hyperphagia following overnight fasting (28). Another elegant study also showed that 8-week tamoxifen treatment of Rax-CreER;Eno2-lsl-DTA mice, which selectively ablated tanycyte-derived neurons without killing the tanycytes themselves, induced hyperphagia in response to fasting and, as a result, an increased epidydymal white adipose tissue mass (29). Nevertheless, several studies, employing genetic approaches, reveal less profound anorexigenic effects of tanycyte manipulation (30–32), while optogenetically activated tanycytes trigger food consumption. Support for tanycytic sufficiency to modulate food intake comes from several studies showing that food intake is modulated by Arc neuropeptide Y, agouti-related peptide (AGRP), proopiomelanocortin, cocaine- and amphetamine-regulated transcript (CART), [(28, 30, 31); for review, see Ref. 16].
There are indirect and direct mechanisms through which tanycytes elicit neuronal modulation. The first involves BHB remodeling. For example, during fasting, tanycytes undergo structural alterations favoring increased accessibility of the hypothalamic Arc to peripheral metabolic cues, which, in turn, activate Arc orexigenic neurons to initiate a feeding response (28, 30, 33–36). The latter indicates a direct role of so-called “tanykines”, that is, tanycytic molecules with orexigenic actions such as T3 (37), anorexigenic actions such as diazepam-binding inhibitor (38, 39) and prostaglandin E2 (40), and tanykines with dual orexigenic and anorexigenic properties such as chemerin (41) and purines (16, 42–44).
As the key integrative hypothalamic area for energy balance, the Arc also plays an important role in the seasonal cycles of ingestive behavior and body weight regulation. Species exhibiting seasonal adiposity can be divided into two major categories. The first includes species that respond to changes in the photoperiod (e.g., hamsters and voles), whereas the second category includes species responding to the timing of an endogenous clock of unknown location (e.g., ground squirrels, wood chucks, and marmots) (45). The orexigenic action of the Arc-NPY/AGRP neuronal population is countered by the Arc-POMC/CART neuronal population (46). Both neuronal populations are regulated by leptin, which is transported to the Arc through the tanycytes (21, 47). Surprisingly, the finding of POMC mRNA and protein not only in the hypothalamic neurons that were thought to be its exclusive province, but also in tanycytes of seasonal and nonseasonal rats, supports an anorexigenic role of tanycytes in ingestive behavior (48–50). In the seasonal context, the photoperiod regulates pituitary TSH production via melatonin, which, in turn, controls seasonal cycles of thyroid hormone synthesis and metabolism. Decreases in tanycytic T3 levels are hypothesized to trigger apoptosis in a small fraction of key hypothalamic neurons involved in energy balance and reproduction, thus affecting physiological status, namely body weight and testes size, respectively (51).
The mediobasal hypothalamus, comprising 3V tanycytes, is a critical brain region that maintains energy homeostasis by acting as an interface between the neural networks of the CNS and the periphery to control metabolic functions, including ingestive behavior, energy homeostasis, and reproduction. The link between ependymal TSHR regulation of the BHB vascular permeability and ingestive behavior has not been established, but we believe it is more than coincidental that TSHRs cluster in the tanycytic region bordering brain centers that control food intake. We have recently discovered that the BHB undergoes dynamic and reversible structural alterations that modulate its permeability in response to TSHR modulation during fasting, thereby acting as a checkpoint for the access of peripheral metabolic signals to surrounding feeding centers, such as the Arc, paraventricular nucleus of the hypothalamus, VMH, and DMH (Figure 1).
Figure 1:
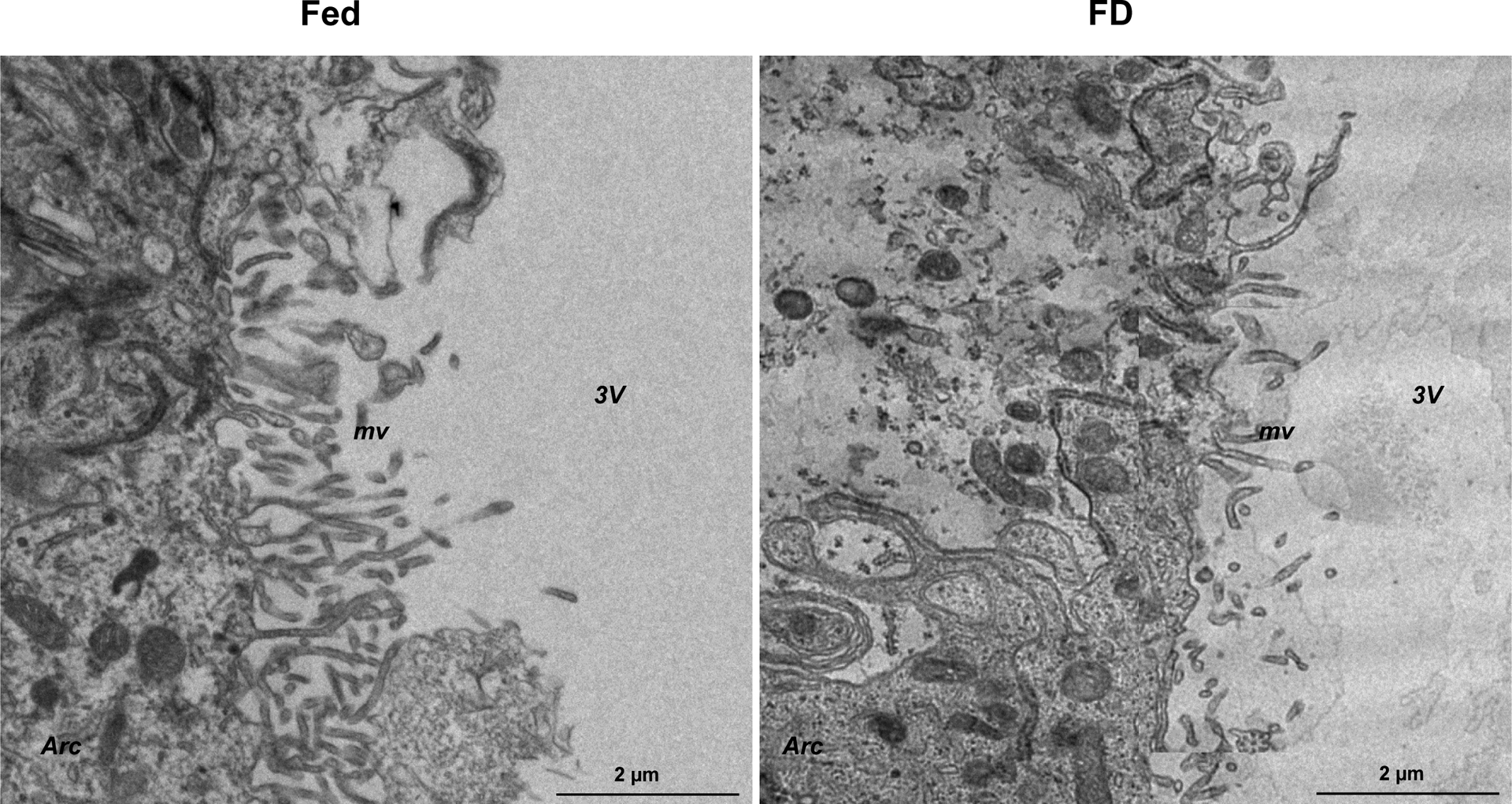
Food deprivation reduces microvilli surface area of α2 tanycytes, suggesting decreased CSF–hypothalamus permeability. Transmission electron microscopy of α2 tanycytes in the mouse mediobasal hypothalamus bordering the 3V, ventromedial nucleus, and arcuate nucleus of the hypothalamus showed that, compared to control mice (Fed), mice that have been food deprived (FD) have shorter microvilli with markedly lower surface area facing the 3V. These data suggest that in the FD mice the diffusion of circulating factors is more restricted through the tanycytes that ultimately project to the Arc and 3V CSF. 3V, third ventricle; Arc, arcuate nucleus; FD, food deprivation; mv, microvilli; VMH, ventromedial nucleus. Scale bar = 2 μm.
TANYCYTES ARE A CONDUIT FOR LEPTIN TRANSPORT
Leptin, a primary white adipose tissue adipokine, is traditionally thought to convey lipid reserve information to the brain through the circulation. Leptin is released into the bloodstream from adipocytes and circulates to the brain where it crosses the blood–brain barrier and BHB to act on leptin receptors (LEPRs) in multiple brain sites to decrease food intake and increase energy expenditure. Its deficiency results in hyperphagia and obesity (52). While leptin has been traditionally considered as a circulating factor that may inform the brain of adiposity levels (53), its accuracy to do so is questionable. For example, in cold-exposed rats (54) and humans (55), circulating leptin levels decrease rapidly, but there are no measurable alterations in adiposity. Furthermore, although plasma leptin levels in humans and rodents correlate with the degree of adiposity more generally, a considerable part of the between-individual variance in plasma leptin is unrelated to the degree of adiposity in humans [for review, see Ref 56]. Thus, understanding leptin transport to convey adiposity-level information of the specific fat pad becomes fundamental.
To this end, Balland et al. (21) showed that tanycytes are the key conduit for leptin transport into the hypothalamus. Notably, this process requires tanycytic ERK signaling to enable the passage of peripheral leptin via the CSF (21). Furthermore, LEPR-deficient db/db mice or mice treated with a leptin antagonist display disrupted leptin transport into the hypothalamic sites, as evidenced by stagnant leptin accumulation in the ME (21). This study suggests that ERK-dependent leptin transport via tanycytes may be an actionable target for overcoming leptin resistance. Another study by Duquenne et al. showed that tanycytic LEPRs respond to leptin by triggering Ca2+ waves and target protein phosphorylation (47). Transcytotic transport of leptin into the hypothalamus occurs through the sequential activation of a LEPR:EGFR complex triggered by leptin and EGF (47). Importantly, the authors found a link between deficient tanycytic leptin transport and the pathophysiology of pancreatic β-cell failure and lipid dysmetabolism in relation to moderate weight gain, thus providing vital pointers toward improving pharmacological interventions for controlling metabolic disorders (47).
NUTRIENT AND HORMONE SENSING BY TANYCYTES
Mounting evidence suggests that tanycytes regulate sensing of CSF glucose concentrations (termed glucosensing) (57). In situ studies reveal that tanycytes not only sense glucose, evoking robust ATP-mediated calcium responses, but also respond to nonmetabolizable glucose analogs, such as 2-deoxy-d-glucose (2DG) (58). It has been reported that tanycytes express the glucose transporter GLUT2 and glucolytic enzymes, such as glucokinase (GCK) (59). The latter is a critical enzyme that senses glucose and modulates neuronal responses to glucose levels (60). Direct evidence for the tanycytic role in glucosensing was shown in the rat, wherein the central injection of a glucokinase inhibitor, alloxan, destroyed specific glucose-sensitive tanycytes and induced higher fasting glycemia (28). Interestingly, a similar phenotype was recently reported in a murine model, where tanycyte lipid sensing and lipid metabolism in the subcutaneous white adipose tissue were both altered by Fgf21 ablation (61). These mice had higher post-fasting glycemia and elevated glucose levels in glucose tolerance tests (61). In another study, the ablation of GCK-expressing tanycytes led not only to increased adiposity, specifically in the epididymal white adipose tissue, but also to leaner brown adipose tissue, suggesting decreased thermogenesis (60).
There is also direct evidence of glucose sensing via the sweet taste receptors TAS1R2 and TAS1R3 expressed in tanycytes (62). In taste receptor tanycytes, the calcium-sensitive monovalent cation channel TRPM5 is required for cell depolarization (63), followed by ATP release from sweet taste receptor tanycytes to activate gustatory nerves (64). Furthermore, in addition to glucose and nonmetabolizable analogs of glucose, tanycytes also respond to three different ligands of the sweet taste receptor, namely sucralose, AceK, and RebA (62). Therefore, the nodes of glucosensitive tanycytes and neurons in the Arc and VMH appear to be perfectly positioned to control ingestive behavior and energy balance. Heterodimerization of TAS1R1/R3 with mGluR4 allows CSF amino acid sensing (65). It was hypothesized that in rodents that lack the Tas1r1 gene, this sweet taste receptor underwent adaptive changes to sense umami (monosodium glutamate) taste (65).
Tanycytes also appear to modulate insulin and glucagon sensitivity; that is, ablation of the ME and Arc tanycytes alters insulin sensitivity (29), whereas 2DG-induced glucoprivation lowers glucagon secretion (60). 2DG and fasting have been shown to regulate remodeling of vascular permeability as shown by altered tanycytic tight junction complexes due to the upregulation of tanycyte vascular endothelial growth factor A (VEGFA) expression (34). Interruption of VEGFA signaling blocks fasting-induced hypothalamic barrier remodeling and markedly impairs the physiological response to refeeding (34). Finally, a very recent study revealed the surprising ability of Arc tanycytes to convert glucose into lactate that is then transmitted via monocarboxylate transporters to the Arc POMC neurons to fuel their activity in regulating ingestive behavior and energy homeostasis (66).
It has recently been reported that insulin receptors are expressed in tanycytes but not in brain endothelial cells. These cells are required to shuttle insulin to the hypothalamic arcuate nucleus (67). Deletion of insulin receptors specifically in tanycytes led to systemic insulin resistance without affecting normal food intake and energy expenditure. In addition, ablation of tanycytic insulin receptors abrogated the feeding-regulatory effect of peripherally injected ghrelin. This explains the necessity of tanycytic insulin receptor signaling for the orexigenic effects of ghrelin (67).
In attempting to understand how liraglutide, an anti-diabetic agent and a glucagon-like peptide one receptor (GLP1R) agonist, enters the mediobasal hypothalamus to regulate energy homeostasis, Imbernon et al. injected fluorescently labeled liraglutide peripherally (68). Surprisingly, blood-borne liraglutide did not cross the blood–brain barrier via the GLP1R-negative endothelial cells, but did so by transcytosis via hypothalamic tanycytes using routes of entry previously described for leptin, ghrelin, and insulin (21, 33, 67, 68). Selective silencing of tanycytic GLP1R or inhibition of tanycytic transcytosis not only prevented liraglutide shuttling into the hypothalamus but also blocked its anti-obesity effects (68).
An additional therapeutic, FGF21, which affects energy homeostasis, not only acts directly on peripheral targets but also by way of its action in the brain. A recent study has detected the FGF21 protein in the tanycytes of the median eminence, suggesting that the tanycytic shuttle is used to transport liver-derived FGF21 into the hypothalamus (69). Entering into a controversy, Zhou et al. showed that FGF21 is not expressed in hypothalamic tanycytes, but is instead found in select brain sites, including the retrosplenial cortex and thalamic nuclei (70). Modulation of FGF receptor 1 (FGFR1) signaling is another potential mechanism underlying adaptation of energy expenditure to negative energy balance. FGFR1 signaling is crucially involved in FGF21-mediated regulation of energy metabolism by regulating genes implicated in energy consumption, adiposity reduction, and weight loss (71, 72). In this regard, the selective inhibition of tanycytic FGFR1 by the monoclonal antibody IMC-H7 suppressed appetite and increased energy expenditure in the Siberian hamster (73). It is especially noteworthy that reductions in food intake and body weight are always paralleled by decreased tanycytic DIO2 expression (73).
TANYCYTES EXPRESS PITUITARY GLYCOPROTEIN HORMONE RECEPTORS
Increasing evidence suggest that anterior pituitary glycoprotein hormones, traditionally considered to have unitary functions in targeting single endocrine sites, act on multiple somatic tissues (74–77). Furthermore, in light of emerging evidence for anterior pituitary hormone action on brain receptors in regulating central neural and peripheral somatic functions (77), we have recently discovered additional multiple brain sites expressing TSHRs (15). Tanycytes of the 3V not only possess the highest number of Tshr transcripts, but also display the highest transcript density [(15), Figures 2 and 3]. Furthermore, and as noted above, physiological adaption to seasonal changes in the day length, encoded by melatonin, are integrated by hypothalamic tanycytes through the detection of TSH released from the PT (1, 2, 4). TSH, in turn, acts locally on tanycytic TSHRs, resulting in increased DIO2 expression and thyroid hormone synthesis (2). In line with a unique physiological role for locally produced TSH, PT-derived TSH is distinctly glycosylated compared with pars distalis–derived TSH. Furthermore, PT-TSH, detectable in the circulation, does not stimulate the thyroid gland (78). Collectively, hypothalamic tanycytes serve as a gateway to relay photoperiodic information to the brain, underpinning mechanisms responsible for seasonal changes in ingestive behavior and adiposity in many animal species. Therefore, it is plausible, although not proven, that an uncharacterized central TSH–TSHR feedback loop may directly regulate the hypothalamic–pituitary–thyroid axis, previously thought to be exclusively controlled by thyroid hormones (79).
Figure 2:
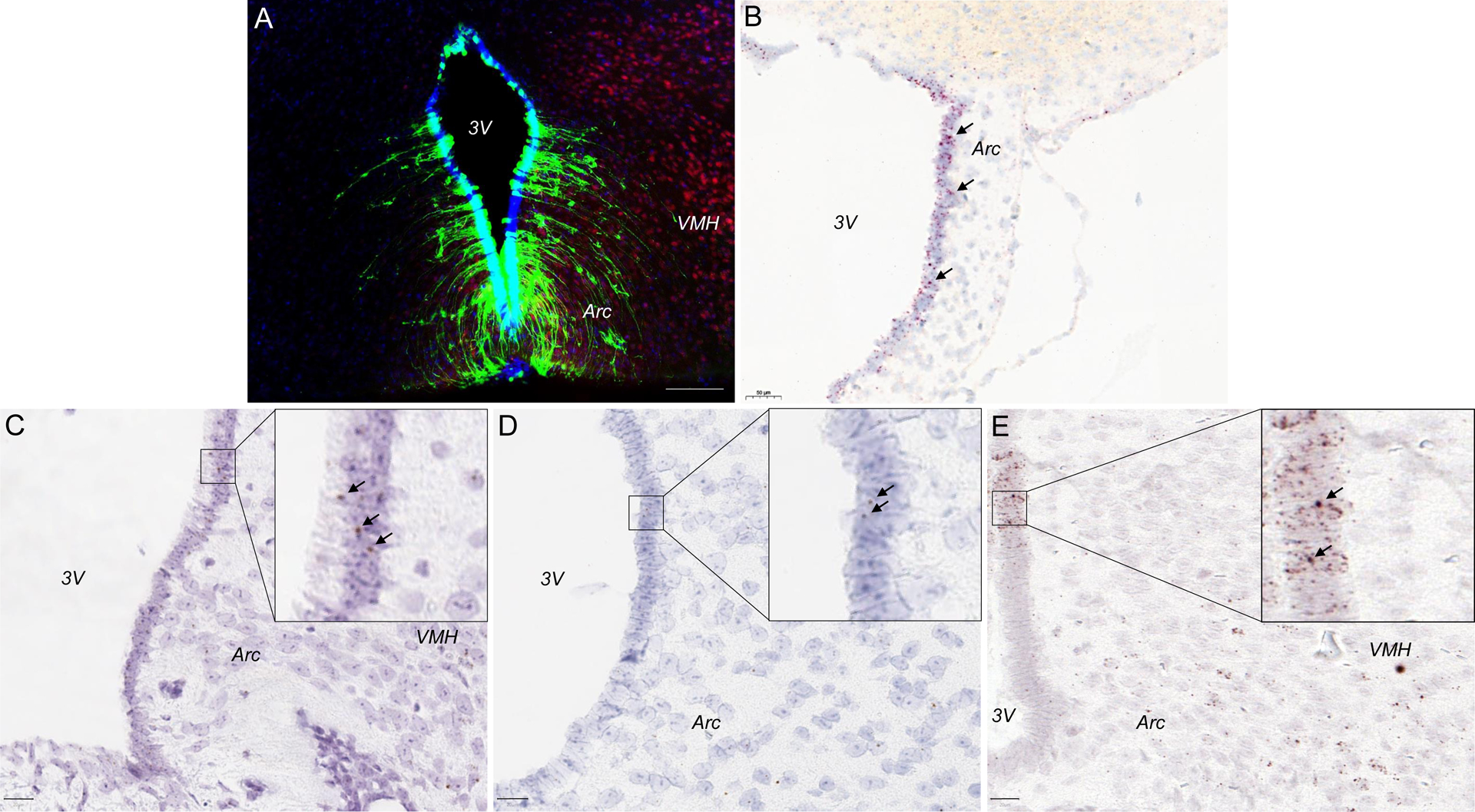
TSHR is highly expressed in the tanycytes. (A). A representative photomicrograph showing immunofluorescence of TSHR in the 3V tanycytes. We utilize a complementary approach to examine tanycytic Tshr expression, that is, the Tshr-deficient mouse in which exon 1 of the Tshr gene is replaced by a Gfp cassette. This allows for the in vivo display of TSHR using GFP immunoreactivity (GFP-ir) as a surrogate for TSHR expression. Abundant GFP-ir (green) was detected in the ependymal layer of the 3V in Tshr+/− mice whereas GFP-ir was absent in Tshr+/+ mice. Sections were co-stained with DAPI (blue) and the neuronal marker NeuN (red). (B). Tshr transcripts in the 3V tanycytes in a sagittal brain section (RNAscope, red dots). Fshr (C), Lhcgr (D), and Oxtr (E) transcripts in the 3V tanycytes in a coronal brain section (RNAscope, brown dots). 3V, third ventricle; Arc, arcuate nucleus; VMH, ventromedial nucleus. Scale bar = 100 μm (A), 50 μm (B), and 20 μm (C–E).
Figure 3:
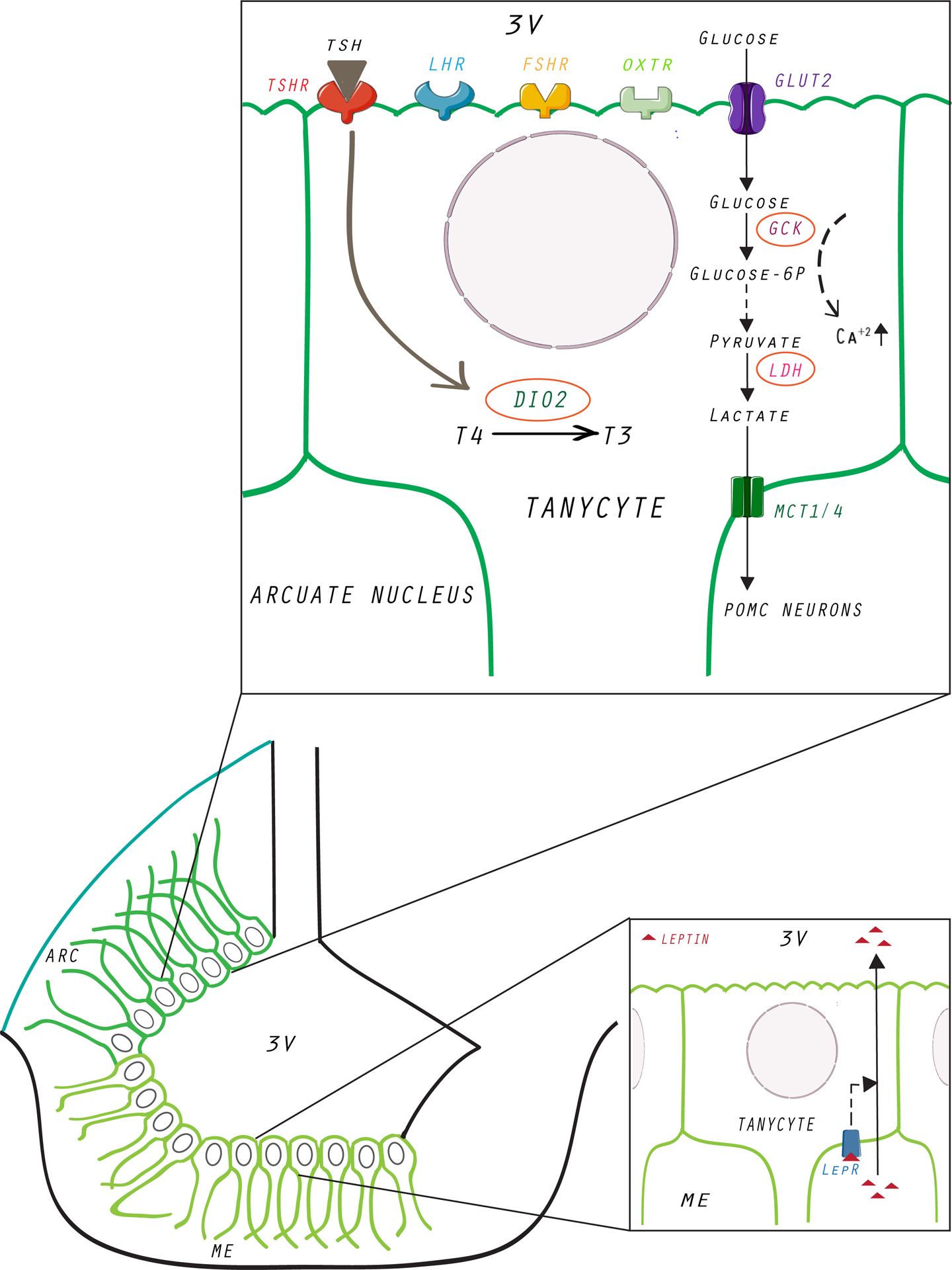
Pituitary glycoprotein hormone receptors, GLUT2, and leptin receptors are found in 3V tanycytes. Thyroid-stimulating hormone (TSH) is stimulated and secreted in response to the hypothalamic thyrotropin-releasing hormone (TRH). The conversion of thyroxine to triiodothyronine (T3) occurs in the tanycytes, and this process is thought to be the critical regulator of the TRH neurons. In turn, T3 itself has been reported to have orexigenic properties. Tanycytes express the glucose transporter GLUT2 and glycolytic enzyme GCK, which is a critical enzymatic component of glucosensing. Additionally, the adipokine leptin acts via LEPR found on the ME tanycytes to regulate appetite and energy expenditure. We also found expression of FSHRs, LHCGRs, and OXTRs in 3V tanycytes, but their functions are currently unknown. 3V, third ventricle; ARC, arcuate nucleus; DIO2, iodothyronine deiodinase 2; FSHR, follicle-stimulating hormone receptor; GCK, glucokinase; GLUT2, glucose transporter 2; LDH, lactate dehydrogenase; LEPR, leptin receptor; LHCGR, luteinizing hormone/choriogonadotropin receptor; MCT1/4, lactate transporter 1 and 4; ME, median eminence; OXTR, oxytocin receptor; POMC, proopiomelanocortin; T3, triiodothyronine; T4, l-thyroxine; TSH, thyroid-stimulating hormone; TSHR, thyroid-stimulating hormone receptor.
Utilizing RNAscope, we recently reported the expression of another glycoprotein hormone receptor, FSHR, in multiple brain sites (15, 77). Notably, the highest Fshr density was also found in the 3V tanycytes, which was not surprising, given its anatomical proximity to the anterior pituitary gland where FSH is synthesized in response to the hypothalamic gonadotropin-releasing hormone GnRH. Likewise, tanycytes also expressed the LHCGR (15), although at much lower transcript number or density (Figure 2). Finally, we also recently documented the expression of oxytocin receptors in 3V tanycytes. However, functional evidence for the exact roles of the pituitary hormone receptors is currently unknown.
CONCLUSIONS AND PERSPECTIVES
We have summarized the role of this unique cell population of tanycytes in the brain in regulating BHB plasticity and energy homeostasis. Clearly, nutrient and hormone sensing by tancytyes and modulation of BHB plasticity appear critical in relation to ingestive behavior. With increases in BHB permeability, increased food ingestion is triggered, perhaps due to greater accessibility of hunger factors to food-related hypothalamic and hindbrain centers resulting in sensitization to orexigenic cues, whereas decreases in BHB permeability have the opposite effects. We and others have found ubiquitous expression of pituitary hormone receptors in the tanycytes, with TSHRs having both the highest transcript number and density. It is apparent that our understanding of the functional roles of these glycoprotein hormone receptors in various brain sites, and specifically in the 3V tanycytes in relation to appetite is lacking. We therefore hope that our review will stimulate further investigations by others.
ACKNOWLEDGEMENTS
Work at Icahn School of Medicine at Mount Sinai carried out at the Center for Translational Medicine and Pharmacology was supported by R01 AG071870 to M.Z., T.Y., and S.-M.K.; R01 AG074092 and U01AG073148 to T.Y. and M.Z.; U19 AG060917 to M.Z. and C.J.R.; and R01 DK113627 to M.Z.
REFERENCES
- 1.Saenz de Miera C, Sage-Ciocca D, Simonneaux V, Pevet P, Monecke S, Melatonin-independent Photoperiodic Entrainment of the Circannual TSH Rhythm in the Pars Tuberalis of the European Hamster. J Biol Rhythms 33, 302–317 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Hanon EA et al. , Ancestral TSH mechanism signals summer in a photoperiodic mammal. Curr Biol 18, 1147–1152 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Sanchez E et al. , Contribution of TNF-alpha and nuclear factor-kappaB signaling to type 2 iodothyronine deiodinase activation in the mediobasal hypothalamus after lipopolysaccharide administration. Endocrinology 151, 3827–3835 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrett P, Bolborea M, Molecular pathways involved in seasonal body weight and reproductive responses governed by melatonin. J Pineal Res 52, 376–388 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Fonseca TL et al. , Coordination of hypothalamic and pituitary T3 production regulates TSH expression. J Clin Invest 123, 1492–1500 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hojvat S et al. , Immunoreactive thyroid stimulating hormone (TSH)(: association with synaptosomally-rich fractions in the rat hypothalamus. Brain Res 265, 259–263 (1983). [DOI] [PubMed] [Google Scholar]
- 7.DeVito WJ, Spearman TN, Connors JM, Hedge GA, Subcellular localization of immunoreactive thyroid-stimulating hormone in the rat hypothalamus. Neuroendocrinology 42, 459–466 (1986). [DOI] [PubMed] [Google Scholar]
- 8.Barchas JD, Akil H, Elliott GR, Holman RB, Watson SJ, Behavioral neurochemistry: neuroregulators and behavioral states. Science 200, 964–973 (1978). [DOI] [PubMed] [Google Scholar]
- 9.Emanuele NV, Baker G, McDonald D, Kirsteins L, Lawrence AM, The impact of aging on luteinizing hormone (LH) and thyroid-stimulating hormone (TSH) in the rat brain. Brain Res 352, 179–183 (1985). [DOI] [PubMed] [Google Scholar]
- 10.Luan S et al. , Thyrotropin receptor signaling deficiency impairs spatial learning and memory in mice. J Endocrinol 246, 41–55 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Crisanti P et al. , The expression of thyrotropin receptor in the brain. Endocrinology 142, 812–822 (2001). [DOI] [PubMed] [Google Scholar]
- 12.Burgos JR, Iresjo BM, Warnaker S, Smedh U, Presence of TSH receptors in discrete areas of the hypothalamus and caudal brainstem with relevance for feeding controls-Support for functional significance. Brain Res 1642, 278–286 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Williams GR, Extrathyroidal expression of TSH receptor. Ann Endocrinol (Paris) 72, 68–73 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Naicker M, Naidoo S, Expression of thyroid-stimulating hormone receptors and thyroglobulin in limbic regions in the adult human brain. Metab Brain Dis 33, 481–489 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Ryu V et al. , Brain atlas for glycoprotein hormone receptors at single-transcript level. Elife 11 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bolborea M, Langlet F, What is the physiological role of hypothalamic tanycytes in metabolism? Am J Physiol Regul Integr Comp Physiol 320, R994–R1003 (2021). [DOI] [PubMed] [Google Scholar]
- 17.Ebling FJP, Lewis JE, Tanycytes and hypothalamic control of energy metabolism. Glia 66, 1176–1184 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Elizondo-Vega RJ, Recabal A, Oyarce K, Nutrient Sensing by Hypothalamic Tanycytes. Front Endocrinol (Lausanne) 10, 244 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clasadonte J, Prevot V, The special relationship: glia-neuron interactions in the neuroendocrine hypothalamus. Nat Rev Endocrinol 14, 25–44 (2018). [DOI] [PubMed] [Google Scholar]
- 20.Prevot V et al. , The Versatile Tanycyte: A Hypothalamic Integrator of Reproduction and Energy Metabolism. Endocr Rev 39, 333–368 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Balland E et al. , Hypothalamic tanycytes are an ERK-gated conduit for leptin into the brain. Cell Metab 19, 293–301 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langlet F, Targeting Tanycytes: Balance between Efficiency and Specificity. Neuroendocrinology 110, 574–581 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Alliot F, Rutin J, Leenen PJ, Pessac B, Pericytes and periendothelial cells of brain parenchyma vessels co-express aminopeptidase N, aminopeptidase A, and nestin. J Neurosci Res 58, 367–378 (1999). [PubMed] [Google Scholar]
- 24.Geranmayeh MH, Rahbarghazi R, Farhoudi M, Targeting pericytes for neurovascular regeneration. Cell Commun Signal 17, 26 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krylyshkina O, Chen J, Mebis L, Denef C, Vankelecom H, Nestin-immunoreactive cells in rat pituitary are neither hormonal nor typical folliculo-stellate cells. Endocrinology 146, 2376–2387 (2005). [DOI] [PubMed] [Google Scholar]
- 26.Bolborea M, Dale N, Hypothalamic tanycytes: potential roles in the control of feeding and energy balance. Trends Neurosci 36, 91–100 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia-Caceres C et al. , Role of astrocytes, microglia, and tanycytes in brain control of systemic metabolism. Nat Neurosci 22, 7–14 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Sanders NM, Dunn-Meynell AA, Levin BE, Third ventricular alloxan reversibly impairs glucose counterregulatory responses. Diabetes 53, 1230–1236 (2004). [DOI] [PubMed] [Google Scholar]
- 29.Yoo S et al. , Tanycyte ablation in the arcuate nucleus and median eminence increases obesity susceptibility by increasing body fat content in male mice. Glia 68, 1987–2000 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barahona MJ et al. , Glial hypothalamic inhibition of GLUT2 expression alters satiety, impacting eating behavior. Glia 66, 592–605 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elizondo-Vega R et al. , Inhibition of hypothalamic MCT1 expression increases food intake and alters orexigenic and anorexigenic neuropeptide expression. Sci Rep 6, 33606 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uranga RM et al. , Adenovirus-mediated suppression of hypothalamic glucokinase affects feeding behavior. Sci Rep 7, 3697 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collden G et al. , Neonatal overnutrition causes early alterations in the central response to peripheral ghrelin. Mol Metab 4, 15–24 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langlet F et al. , Tanycytic VEGF-A boosts blood-hypothalamus barrier plasticity and access of metabolic signals to the arcuate nucleus in response to fasting. Cell Metab 17, 607–617 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Langlet F, Mullier A, Bouret SG, Prevot V, Dehouck B, Tanycyte-like cells form a blood-cerebrospinal fluid barrier in the circumventricular organs of the mouse brain. J Comp Neurol 521, 3389–3405 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elizondo-Vega R et al. , Inhibition of Hypothalamic MCT4 and MCT1-MCT4 Expressions Affects Food Intake and Alters Orexigenic and Anorexigenic Neuropeptide Expressions. Mol Neurobiol 57, 896–909 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coppola A et al. , A central thermogenic-like mechanism in feeding regulation: an interplay between arcuate nucleus T3 and UCP2. Cell Metab 5, 21–33 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lanfray D et al. , Gliotransmission and brain glucose sensing: critical role of endozepines. Diabetes 62, 801–810 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guillebaud F et al. , Glial Endozepines Inhibit Feeding-Related Autonomic Functions by Acting at the Brainstem Level. Front Neurosci 11, 308 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bottcher M et al. , NF-kappaB signaling in tanycytes mediates inflammation-induced anorexia. Mol Metab 39, 101022 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Helfer G et al. , A neuroendocrine role for chemerin in hypothalamic remodelling and photoperiodic control of energy balance. Sci Rep 6, 26830 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bolborea M, Pollatzek E, Benford H, Sotelo-Hitschfeld T, Dale N, Hypothalamic tanycytes generate acute hyperphagia through activation of the arcuate neuronal network. Proc Natl Acad Sci U S A 117, 14473–14481 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bolborea M, Helfer G, Ebling FJ, Barrett P, Dual signal transduction pathways activated by TSH receptors in rat primary tanycyte cultures. J Mol Endocrinol 54, 241–250 (2015). [DOI] [PubMed] [Google Scholar]
- 44.Recabal A et al. , The FGF2-induced tanycyte proliferation involves a connexin 43 hemichannel/purinergic-dependent pathway. J Neurochem 156, 182–199 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mrosovsky N, Faust IM, Cycles of body fat in hibernators. Int J Obes 9 Suppl 1, 93–98 (1985). [PubMed] [Google Scholar]
- 46.Webber ES, Bonci A, Krashes MJ, The elegance of energy balance: Insight from circuit-level manipulations. Synapse 69, 461–474 (2015). [DOI] [PubMed] [Google Scholar]
- 47.Duquenne M et al. , Leptin brain entry via a tanycytic LepR-EGFR shuttle controls lipid metabolism and pancreas function. Nat Metab 3, 1071–1090 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ross AW et al. , Photoperiod regulates lean mass accretion, but not adiposity, in growing F344 rats fed a high fat diet. PLoS One 10, e0119763 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ross AW et al. , Divergent regulation of hypothalamic neuropeptide Y and agouti-related protein by photoperiod in F344 rats with differential food intake and growth. J Neuroendocrinol 21, 610–619 (2009). [DOI] [PubMed] [Google Scholar]
- 50.Wittmann G et al. , Variable proopiomelanocortin expression in tanycytes of the adult rat hypothalamus and pituitary stalk. J Comp Neurol 525, 411–441 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Helfer G, Barrett P, Morgan PJ, A unifying hypothesis for control of body weight and reproduction in seasonally breeding mammals. J Neuroendocrinol 31, e12680 (2019). [DOI] [PubMed] [Google Scholar]
- 52.Friedman J, The long road to leptin. J Clin Invest 126, 4727–4734 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woods SC, Seeley RJ, Adiposity signals and the control of energy homeostasis. Nutrition 16, 894–902 (2000). [DOI] [PubMed] [Google Scholar]
- 54.Hardie LJ, Rayner DV, Holmes S, Trayhurn P, Circulating leptin levels are modulated by fasting, cold exposure and insulin administration in lean but not Zucker (fa/fa) rats as measured by ELISA. Biochem Biophys Res Commun 223, 660–665 (1996). [DOI] [PubMed] [Google Scholar]
- 55.Ricci MR, Fried SK, Mittleman KD, Acute cold exposure decreases plasma leptin in women. Metabolism 49, 421–423 (2000). [DOI] [PubMed] [Google Scholar]
- 56.Jequier E, Leptin signaling, adiposity, and energy balance. Ann N Y Acad Sci 967, 379–388 (2002). [DOI] [PubMed] [Google Scholar]
- 57.Elizondo-Vega R et al. , The role of tanycytes in hypothalamic glucosensing. J Cell Mol Med 19, 1471–1482 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frayling C, Britton R, Dale N, ATP-mediated glucosensing by hypothalamic tanycytes. J Physiol 589, 2275–2286 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garcia M et al. , Hypothalamic ependymal-glial cells express the glucose transporter GLUT2, a protein involved in glucose sensing. J Neurochem 86, 709–724 (2003). [DOI] [PubMed] [Google Scholar]
- 60.Rohrbach A et al. , Ablation of glucokinase-expressing tanycytes impacts energy balance and increases adiposity in mice. Mol Metab 53, 101311 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Geller S et al. , Tanycytes Regulate Lipid Homeostasis by Sensing Free Fatty Acids and Signaling to Key Hypothalamic Neuronal Populations via FGF21 Secretion. Cell Metab 30, 833–844 e837 (2019). [DOI] [PubMed] [Google Scholar]
- 62.Benford H et al. , A sweet taste receptor-dependent mechanism of glucosensing in hypothalamic tanycytes. Glia 65, 773–789 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Damak S et al. , Trpm5 null mice respond to bitter, sweet, and umami compounds. Chem Senses 31, 253–264 (2006). [DOI] [PubMed] [Google Scholar]
- 64.Chaudhari N, Roper SD, The cell biology of taste. J Cell Biol 190, 285–296 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lazutkaite G, Solda A, Lossow K, Meyerhof W, Dale N, Amino acid sensing in hypothalamic tanycytes via umami taste receptors. Mol Metab 6, 1480–1492 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lhomme T et al. , Tanycytic networks mediate energy balance by feeding lactate to glucose-insensitive POMC neurons. J Clin Invest 131 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Porniece Kumar M et al. , Insulin signalling in tanycytes gates hypothalamic insulin uptake and regulation of AgRP neuron activity. Nat Metab 3, 1662–1679 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Imbernon M et al. , Tanycytes control hypothalamic liraglutide uptake and its anti-obesity actions. Cell Metab 34, 1054–1063 e1057 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pena-Leon V et al. , Prolonged breastfeeding protects from obesity by hypothalamic action of hepatic FGF21. Nat Metab 4, 901–917 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou B et al. , Central FGF21 production regulates memory but not peripheral metabolism. Cell Rep 40, 111239 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ye M et al. , FGF21-FGFR1 Coordinates Phospholipid Homeostasis, Lipid Droplet Function, and ER Stress in Obesity. Endocrinology 157, 4754–4769 (2016). [DOI] [PubMed] [Google Scholar]
- 72.Baruch A et al. , Antibody-mediated activation of the FGFR1/Klothobeta complex corrects metabolic dysfunction and alters food preference in obese humans. Proc Natl Acad Sci U S A 117, 28992–29000 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Samms RJ et al. , Antibody-Mediated Inhibition of the FGFR1c Isoform Induces a Catabolic Lean State in Siberian Hamsters. Curr Biol 25, 2997–3003 (2015). [DOI] [PubMed] [Google Scholar]
- 74.Abe E et al. , TSH is a negative regulator of skeletal remodeling. Cell 115, 151–162 (2003). [DOI] [PubMed] [Google Scholar]
- 75.Sun L et al. , FSH directly regulates bone mass. Cell 125, 247–260 (2006). [DOI] [PubMed] [Google Scholar]
- 76.Liu P et al. , Blocking FSH induces thermogenic adipose tissue and reduces body fat. Nature 546, 107–112 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xiong J et al. , FSH blockade improves cognition in mice with Alzheimer’s disease. Nature 603, 470–476 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ikegami K et al. , Tissue-specific posttranslational modification allows functional targeting of thyrotropin. Cell Rep 9, 801–810 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abel ED et al. , Novel insight from transgenic mice into thyroid hormone resistance and the regulation of thyrotropin. J Clin Invest 103, 271–279 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]


