Abstract
The purpose of the present study was to investigate the transmission of a human isolate of the agent of human granulocytic ehrlichiosis (HGE agent) from infected mice to larval ticks and to examine the population kinetics of the HGE agent in different stages of the tick life cycle. The HGE agent was quantitated by competitive PCR with blood from infected mice and with Ixodes scapularis ticks. The median infectious dose for C3H mice was 104 to 105 organisms when blood from an infected severe combined immunodeficient mouse was used as an inoculum. Uninfected larval ticks began to acquire infection from infected mice within 24 h of attachment, and the number of HGE agent organisms increased in larval ticks during feeding and after detachment of replete ticks. Molted nymphal ticks, infected as larvae, transmitted infection to mice between 40 and 48 h of attachment. Onset of feeding stimulated replication of the HGE agent within nymphal ticks. These studies suggest that replication of the HGE agent during and after feeding in larvae and during feeding in nymphs is a means by which the HGE agent overcomes inefficiencies in acquisition of infection by ticks and in tick-borne transmission to mammalian hosts.
Human granulocytic ehrlichiosis (HGE) is a newly recognized zoonotic disease caused by an obligate intracellular member of the class Proteobacteria. The agent of HGE has not been officially named. The HGE agent has 99.8 to 99.9% 16S rRNA gene homology with Ehrlichia equi and Ehrlichia phagocytophila, respectively, suggesting that the HGE agent belongs to a group of very closely related granulocytic ehrlichiae (4). The HGE agent infects a broad range of mammalian species, including dogs, rodents, and humans, as incidental hosts (2, 12, 26, 29).
Since 1994, more than 200 cases of HGE have been diagnosed in humans, most commonly in northeastern and upper midwestern regions of the United States in which the principal tick vector, Ixodes scapularis, is most abundant. In these regions, I. scapularis also serves as the vector for Borrelia burgdorferi, the agent of Lyme disease, and Babesia microti (1, 3, 7, 13, 18, 23, 27). Indeed, mixed infections with these agents have been documented in human patients (16, 17). A recently discovered encephalitis virus that is related to the tick-borne encephalitis virus group has been added to this guild of I. scapularis-transmitted agents (25).
Larval ticks acquire the HGE agent by feeding on reservoir-competent hosts, such as the white-footed mouse (Peromyscus leucopus) (26, 29). The principal host for adult I. scapularis ticks is the white-tailed deer (2, 26). Tick-borne infection can be transmitted to mammalian hosts transstadially when either larval or nymphal ticks become infected and then transmit the agent during successive life stages (as nymphs or adults, respectively) or can be transmitted intrastadially (as adults) when a tick becomes infected and transmits the pathogen within the same life stage (8, 26). Unlike other rickettsial agents, ehrlichiae are not known to be maintained through transovarial transmission in ticks. In the absence of such transmission, E. phagocytophila, for example, is horizontally maintained within an I. ricinus-domestic animal (primarily sheep and goat) cycle (15, 30).
Transmission of the HGE agent by ticks relies on successful acquisition of the pathogen from reservoir hosts, but this is likely to be impeded if such hosts have only low-level bacteremia or transient infections. In a recent study, the rate of transmission of the HGE agent from infected mice to nymphal I. scapularis ticks correlated with the level of bacteremia in the host mouse blood, as assessed by the percentage of peripheral blood granulocytes with morulae (membrane-bound intracytoplasmic clusters of ehrlichiae). During early stages of infection, in which there was a high percentage of peripheral blood granulocytes with morulae, ticks readily acquired infection, but during late stages of infection of mice, in which there was no or a low percentage of granulocytes with morulae, ticks only occasionally became infected (14). This suggested that bacteremia levels in mice influenced the rate of transmission of the HGE agent to ticks during feeding.
Furthermore, efficient transmission of the HGE agent from ticks to mammals is likely to be dose dependent. However, once ticks are infected, even with a low number of bacteria, subsequent replication of the agent in the tick may compensate and enhance transmission, as shown with Anaplasma marginale, another tick-borne rickettsial agent (8). In addition, B. burgdorferi, which is cotransmitted by I. scapularis ticks, becomes activated in feeding ticks, with replication and enhanced infectivity (5, 6, 20, 22). These factors may explain why infection of ticks with the HGE agent in areas of endemicity is less prevalent than infection with B. burgdorferi, despite their common reservoir hosts and tick vector (26).
The goal of this study was to investigate transmission of the HGE agent from infected mice to larval ticks and to examine the population kinetics of the HGE agent in different stages of the tick life cycle in order to better understand the factors involved in the transmission of the HGE agent by vector ticks.
MATERIALS AND METHODS
Mice.
Three- to 5-week-old pathogen-free C3H/HeJ (C3H) and C3H/Smn.CIcrHSD/scid (severe combined immunodeficient [SCID]) mice were purchased from The Jackson Laboratory, Bar Harbor, Maine, and Harlan Sprague Dawley, Inc., Indianapolis, Ind., respectively. These mice were pathogen-free and were maintained in isolator cages within an infectious disease containment room following arrival.
HGE agent.
The NCH-1 isolate of the HGE agent (26) was maintained by serial passage from infected SCID mice to naive SCID mice by intraperitoneal (i.p.) inoculation of 0.1 ml of EDTA-anticoagulated blood at 3-week intervals. Blood from infected SCID mice was used to inoculate C3H mice. The percentage of blood granulocytes with morulae in peripheral blood smears was determined prior to inoculation. Peripheral blood smears were air dried, fixed in methanol, stained with Giemsa, and then examined for morulae. The percentage of granulocytes in peripheral blood smears among 200 granulocytes examined in each smear was recorded.
The HGE agent was also maintained in HL-60 cells (ATCC 240-CCL) as described previously (12). Purified HGE agent was prepared with a discontinuous Renografin (Bracco Diagnostics, New Brunswick, N.J.) density gradient as described previously (5), with some modifications. Infected HL-60 cultures were pelleted and resuspended in phosphate-buffered saline–glucose. HGE agents were liberated by lysing the HL-60 cells by repeated aspiration with a 22-gauge needle. Cell debris was pelleted by low-speed centrifugation. The supernatant was incubated with DNase and RNase (50 μg/ml), layered on top of a discontinuous (42 and 30%) Renografin gradient, and ultracentrifuged at 58,000 × g for 90 min at 4°C. The interface band was collected, washed with SPGN (7.5% sucrose, 3.7 mM K2HPO4, 5 mM l-glutamine), pelleted at 15,000 × g, resuspended in SPGN at 2 g/ml, and stored at −70°C.
Ticks.
The I. scapularis ticks used in this study were from a tick colony established from field-collected adults derived from southern Connecticut. In this region, both B. burgdorferi and the HGE agent are endemic, but it has been our experience that neither agent is transmitted transovarially.
To ensure that transovarial transmission was not a factor, we tested sera from 50 mice used to rear ticks in our colony. We used an indirect immunofluorescence assay, as described previously (14), with USG3, a Westchester County, N.Y., isolate cultured in HL-60 cells (provided by Richard T. Coughlin, Aquilla Biopharmaceuticals, Worcester, Mass.). Serum was diluted 1:40 for screening. Field-collected adults were fed upon rabbits or sheep and then allowed to oviposit in vials kept at 21°C and 95% relative humidity in an environmental chamber. A total of 4,251 larvae and 1,649 nymphs from this tick colony were fed on these 50 mice, which were bled at least 14 days since the last infestation. Larval infestations ranged from 100 to 400 per mouse, and nymphal infestations ranged from 4 to 50 per mouse. Twenty-nine mice were exposed to multiple infestations of either the same or mixed stages of ticks. All 50 of these mice were seronegative for the HGE agent. In contrast, serum from a mouse infected with HGE by nymphal ticks was seropositive. In addition, larval ticks from this colony are periodically monitored for both the HGE agent and B. burgdorferi by PCR (data not shown). So far, no evidence of transovarial infection of larvae by either agent has been observed among the progeny of more than 200 females collected from this location of endemicity.
In the series of experiments featured in this study, all ticks were derived from a single cohort of larvae. Some of the larvae were used to test acquisition of infection by larvae, and other larvae were fed on infected (or uninfected) mice and then allowed to molt into nymphs for testing of the transmission of infection by nymphs. To verify that this cohort of ticks was not infected, we tested 43 nymphal ticks from this cohort that fed upon uninfected (control) mice as larvae. None was PCR reactive, confirming that no unintentional infection was present in this cohort of ticks. Only ticks infected by feeding upon experimentally inoculated mice were PCR positive, thereby confirming the specificity of the PCR primers for the detection of the single human isolate used in this study.
PCR.
HGE agent DNA from culture, ticks, and blood was extracted with the QIAmp tissue kit, according to the manufacturer’s instructions for body fluids (Qiagen, Santa Clara, Calif.). For amplification of HGE agent DNA from ticks, ticks were individually crushed with a plastic grinder immediately after detachment from mice. For DNA extraction from blood, 50 μl of blood was lysed in erythrocyte lysis buffer (155 mM NH4Cl, 10 mM KHCO3, 1 mM EDTA), treated with 10 mg of proteinase K per ml at 56°C for 1 h, and then boiled for 15 min. After purification, DNA from each dilution was used in a competitive PCR. Primers ehr 521 (5′-TGTAGGCGGTTCGGTAAGTTAAAG-3′) and ehr 747 (5′-GCACTCATCGTTTACAGCGTG-3′) were synthesized to amplify a variable region of the 16S rRNA gene sequence specific for E. equi, E. phagocytophila, and the HGE agent (18). Each 50 μl of PCR mixture contained 10 μl of tick-extracted genomic DNA or 5 μl of infected mouse blood-extracted DNA, 5 μl of 1× PCR buffer, and 1.5 mM MgCl2. The final concentrations of the other reagents were 0.2 mM for each deoxynucleoside triphosphate dNTP, 2 U for Taq polymerase, and 100 pmol for each primer. Water was added to make up a final volume of 50 μl. Amplification was performed in a thermal cycler (Perkin-Elmer Corp., Norwalk, Conn.) with a three-step cycling program. DNA was denatured for 5 min at 94°C, followed by 40 cycles of three steps: 45 s of denaturation at 94°C, 45 s of annealing at 60°C, and 45 s of extension at 72°C. The final step was 5 min of extension at 72°C.
Quantitative PCR was performed with a heterologous 549-bp competitive DNA target that was amplified by PCR with external HGE agent-specific primers joined to an irrelevant internal 504-bp B. burgdorferi ospA fragment. Primers consisted of a 45-mer ehr521-ospA5 (5′-TGTAGGCGGTTCGGTAAGTTAAAG-GGAATAGGTCTAATATTAGCC-3′) and a 39-mer ehr747-ospA3 (5′-GCACTCATCGTTTACAGCGTG-TTCAGCAGTTAGAGTTCC-3′) (the underlined sequences represent the sequences of the underlined primers). Because primer specificity is determined by the internal sequences, the competitive target was amplified from B. burgdorferi genomic DNA. The DNA concentration was determined by measuring the optical density. A second round of quantitative PCR was performed with HGE agent-specific primers. A decreasing known amount of competitor in 10 μl and constant amounts of target DNA (HGE agent DNA from infected ticks or blood) were added to a series of tubes containing all PCR reagents. The amplification products (generated as described above) were distinguished by size on an agarose gel stained with ethidium bromide. Because the competitor contained the same primer templates as the target HGE agent DNA, both were amplified with HGE agent DNA-specific primers. Thus, the amount of target (HGE agent DNA) in the test sample was the amount at which the competitor and target densities were equivalent (24). To estimate the maximal number of ehrlichia bacterial cells that could possibly be present in each sample, we made the conservative assumption (for calculation purposes only) that the HGE agent, like Rickettsia prowazekii, to which the HGE agent is phylogenetically distantly related (19), possesses a single copy of the 16S rRNA gene per cell. We therefore estimated that 50 fg of HGE agent DNA had 1.9 × 105 bacterial cells (0.25 kb of DNA = 1.9 × 50 × 105/50 molecules/ml).
RESULTS
Validation of competitive PCR.
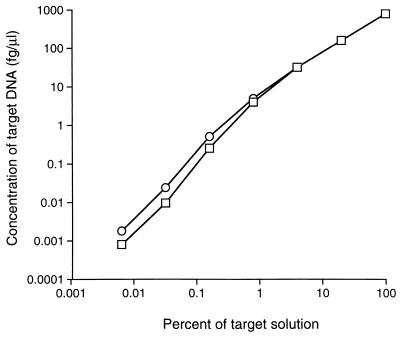
The competitive PCR was validated and had equal sensitivities with density gradient-purified HGE agent DNA, serial dilutions of HGE agent-infected HL-60 cells, DNA extracted from several HGE agent-infected ticks, and DNA extracted from the blood of HGE agent-infected mice (Fig. 1). The accuracy of the competitive PCR was evaluated with HGE agent-infected HL-60 cells in two series of assays: (i) a known, decreasing amount of competitor in 10 μl was mixed with a constant volume (5 μl) of target from each aliquot of a fivefold serial dilution of the target, and (ii) a known, constant amount of competitor was mixed with a decreasing, unknown concentration of the target. No significant differences were found when constant amounts of competitor and decreasing amounts of target and when decreasing amounts of competitor and constant amounts of target were used (Fig. 2). There was a strong, positive correlation between the concentration of DNA in target solution and the measured amount of target DNA (ρ = >0.99), regardless of whether the amount of competitor or target was held constant.
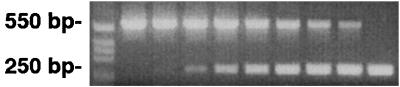
FIG. 1.
Quantitation of HGE agent DNA by competitive PCR with primers specific for a 250-bp 16S rRNA target of the HGE agent and a 549-bp competitive target containing an irrelevant 504-bp internal B. burgdorferi ospA segment. Fivefold decreasing amounts (from 1 pg/μl to 0.04 fg/μl) of competitor (top row, 549 bp) were added to a constant amount of HGE agent target DNA (bottom row, 250 bp). The PCR mixtures were amplified for 40 cycles, and the products were resolved on a 1.6% agarose gel stained with ethidium bromide. When competitor and target band intensities were equivalent, the amount of target DNA was presumed to equal the known amount of competitor DNA.
FIG. 2.
The accuracy of the competitive PCR was evaluated by performing a series of competitive PCR assays with a decreasing amount of competitor mixed with a constant volume from each of seven different dilutions of target solution ( ). In another series of seven assays, for each assay a constant amount of competitor was mixed with a decreasing amount of target (○). The x axis is a log10 scale of the percentage of HGE agent-infected HL-60 cell solution (target solution) in a fivefold serial dilution. The y axis is a log10 scale of the DNA concentration at equivalent competitor and target intensities. For each curve, linear analysis revealed P values of <0.001 and correlation coefficients (ρ) of >0.99.
We have shown that the level of morulae in peripheral blood granulocytes in mice correlated with the inoculum dose, but morulae decreased to undetectable levels with low doses of inocula (14). To estimate the number of ehrlichiae in blood inocula and to determine the infectious dose, blood from an infected SCID mouse (26% granulocytes with morulae) was serially diluted in phosphate-buffered saline. Each 10-fold serial dilution was divided: one part of each dilution was inoculated i.p. into four C3H mice (0.1 ml each), and DNA was extracted from the other part of each dilution for quantitative PCR. Each of four control mice received 0.1 ml of uninfected SCID mouse blood. All C3H mice were necropsied at 10 days after inoculation, and peripheral blood smears were examined for the presence of morulae in granulocytes, mice were examined for splenomegaly, and blood was tested by PCR for the HGE agent-specific 16S rRNA gene (Table 1). Undiluted blood from the infected SCID mouse contained 1.2 × 108 HGE agent bacterial cells/ml of blood, and the number of bacteria decreased exponentially with serial dilutions of the blood. The terminal dilution (1:1,000; 1.2 × 104 bacterial cells in 0.1 ml of inoculum) did not induce detectable morulae or splenomegaly in inoculated mice, but blood samples from two of four of the mice were PCR positive. On the basis of these results, we concluded that the median infectious dose was approximately 104 bacteria, but the disease-inducing dose (resulting in morulae and splenomegaly) required a higher dose (105 bacteria) of inoculum.
TABLE 1.
Quantification of HGE agent organisms by competitive PCR, percentage of morulae in granulocytes, and infectivity of blood from an SCID mouse infected with the HGE agent
| Blood | Dilution | No. of bacteria/mla | % Morulae (mean ± SD)b | Infectivityc |
|---|---|---|---|---|
| Infected | Neat | 1.2 × 108 | 9.0 ± 0.0 | 4/4 |
| 1:10 | 1.2 × 107 | 11.4 ± 0.6 | 4/4 | |
| 1:100 | 9.6 × 105 | 5.6 ± 0.4 | 4/4 | |
| 1:1,000 | 1.2 × 105 | 0 | 2/4 | |
| Control | Neat | 0 | 0 | 0/4 |
HGE agent bacterial numbers were estimated on the basis of 1.9 × 105 bacteria/50 fg of target DNA.
Percentage of granulocytes with morulae among 200 granulocytes examined per mouse.
Number of C3H mice with PCR-positive blood/number of mice inoculated with 0.1 ml of each dilution of blood.
Acquisition of HGE agent by larval ticks.
In our experience we have found no evidence of transovarial transmission of the HGE agent. We assume that larval ticks must acquire infection by the HGE agent through feeding upon an infected rodent and then subsequently transmit the HGE agent transstadially following molting into nymphs. We therefore sought to determine the interval at which the HGE agent was transmitted from infected mice to larval ticks during the attachment and feeding process. To infect mice that served as infected hosts for larval ticks, C3H mice were inoculated i.p. with 0.1 ml of infected SCID mouse blood (18.5% granulocytes with morulae). Two hundred uninfected larval ticks were placed on each of four HGE agent-infected mice at 8 days of infection. Two hundred larvae were placed on each mouse because our experience indicated that this number was well tolerated by the mice. The expected yield was 50 to 60% successfully fed ticks. At 24, 48, and 72 h after tick attachment, 5 ticks were removed with forceps from each of the four mice at each time point (20 ticks/interval). An additional group of 20 ticks (5 ticks from each mouse) was allowed to feed to repletion and detach, and the ticks were then kept in a humidified chamber at 21°C for 10 days. The remaining replete ticks were placed in a humified chamber for 8 to 9 weeks and then allowed to molt and harden into nymphs for subsequent experiments.
In addition, larval ticks from the same cohort were fed to repletion on uninfected (control) mice and were then allowed to molt and harden as described above. As a negative control to prove a lack of infection of this entire cohort of ticks, 43 of these uninfected nymphs were tested by PCR (see the next experiment described below).
The number of ehrlichiae in each individual tick (20 ticks/interval, 5 ticks/mouse) was determined by competitive PCR (Table 2). The HGE agent was detected in approximately one-third of the feeding ticks within 24 h of attachment. The prevalence of tick infection and the number of organisms within infected ticks increased with time, with a marked increase after detachment, suggesting replication within replete ticks. However, the prevalence of HGE agent infection among larval ticks examined 10 days after initial attachment was lower (13 of 20) compared to that among the larvae removed from mice at 72 h (20 of 20) (P < 0.001 by chi-square analysis).
TABLE 2.
Quantification by competitive PCR of the HGE agent within feeding larval ticks at intervals relative to time of attachment to HGE agent-infected C3H mice
| Interval after attachment | Prevalence of tick infectiona | No. of bacteria/tick (mean ± SD) |
|---|---|---|
| 24 h | 6/20 | 3.3 ± 3.9 (× 102) |
| 48 h | 14/20 | 8.0 ± 2.3 (× 102) |
| 72 h | 20/20 | 1.0 ± 0.4 (× 103) |
| 10 days | 13/20 | 1.3 ± 0.3 (× 105) |
Number of PCR-positive ticks/number of ticks tested.
Growth of HGE agent in infected nymphal ticks.
Because infected nymphal ticks are a means of transmission of the HGE agent to humans, we next sought to examine population kinetics in transstadially infected nymphal ticks before, during, and after feeding on uninfected mice. Molted nymphs, infected as larvae in the previous study, were used. A random sample of 20 flat (unfed) nymphs was initially tested by PCR, and 6 of 20 were positive. Since these nymphal ticks represented individuals from the same pool of replete larval ticks tested at 10 days after attachment as larvae, data suggested a further decline in prevalence (13 of 20 versus 6 of 20) but a continued increase in the number (1.3 × 105 versus 2.6 × 106) of HGE agent bacteria within positive ticks during the transstadial period.
To determine the effect of feeding on the number of HGE agent organisms within infected nymphal ticks during feeding, six uninfected C3H mice were each infested with 12 flat nymphal ticks that had fed upon infected mice. Four ticks were removed from each mouse (24 total) at 24, 48, and 96 h after attachment. Four control uninfected C3H mice were each infested with 12 uninfected nymphal ticks, and the ticks were allowed to feed to repletion.
Feeding stimulated a significant (nearly 20-fold) rise in organism numbers within nymphal ticks (Table 3). Ten days after the ticks had detached, mice were necropsied and the infection status of the mice was determined by the presence of morulae in blood smears, the presence of splenomegaly, and PCR of blood. Infection was verified in all mice by all indices. Morulae were visible in peripheral blood granulocytes of all mice (mean ± standard deviation [SD], 5.6% ± 1.9%). None of the control mice infested with naive nymphs were infected. An additional group of nymphs was allowed to feed to repletion and was then examined after the nymphs molted into adults. Of 20 tested adults (10 females and 10 males), 7 females and 3 males were infected. These data suggest that the HGE agent replicates within feeding ticks as a possible mechanism that enhances transmission.
TABLE 3.
Quantification by competitive PCR of the HGE agent within flat and feeding nymphal ticks and subsequently molted adult ticks at intervals relative to time of attachment to uninfected mice
| Interval after attachment or stage | Prevalence of tick infection | No. of bacteria/tick (mean ± SD) |
|---|---|---|
| Prefeeding | 6/20 | 2.6 ± 4.1 (× 106) |
| 24 h | 8/24 | 7.0 ± 0.3 (× 105) |
| 48 h | 7/24 | 4.5 ± 3.8 (× 107) |
| 96 h | 7/24 | 8.0 ± 2.3 (× 107) |
| Uninfected nymphs | 0/43 | 0 |
| Molted nymphs | ||
| Female | 7/10 | 2.3 ± 3.9 (× 106) |
| Male | 3/10 | 4.7 ± 4.0 (× 106) |
Duration of feeding and efficiency of HGE agent transmission.
We next performed a pilot experiment to evaluate the effect of duration of nymphal tick feeding on the efficiency of transmission of the HGE agent. Six uninfected C3H mice were each infested with 12 flat nymphs from a pool of ticks that had fed upon HGE agent-infected mice as larvae (12 PCR-positive ticks within a random sample of 40 ticks). At 16, 24, 40, 48, 72, or 96 h after tick attachment, single mice were anesthetized and all attached ticks were removed. Ticks were tested for infection by PCR, and mice were necropsied at 10 days after initial tick attachment. Infection of mice was determined by examination of blood smears for the presence of morulae and splenomegaly and by PCR of blood.
None of the mice became infected when ticks fed for 16, 24, or 40 h, whereas mice became infected when ticks fed for 48, 72, or 96 h (Table 4). On the basis of the results of this pilot experiment with single mice at each interval, the time required for transmission of the HGE agent to mice appeared to be between 40 and 48 h. Results were verified by infesting each of eight mice with 12 nymphal ticks from the same pool of ticks that had fed upon infected mice. All attached ticks were removed from one group of four mice at 40 h and from another group of four mice at 48 h. Mice were necropsied and evaluated for infection as described above. None of four mice became infected when ticks fed for 40 h, but all four mice were infected when ticks were allowed to feed for 48 h (P < 0.001 by Fisher’s exact test). These data suggest a delay in transmission that may be explained by the need for the HGE agent to replicate within the vector (supported by our other experiments described in this article) prior to reaching optimal doses for transmission. They do not discount the possibility that transmission might take place at earlier intervals.
TABLE 4.
Duration of HGE agent-infected nymphal tick attachment required for transmission of the HGE agent to uninfected mice
| Duration of attachment (h) | Prevalence of tick infectiona | Indices of mouse
infection
|
||
|---|---|---|---|---|
| % Morulae | Presence of splenomegaly | PCR result | ||
| 16 | 2/10 | 0 | − | − |
| 24 | 6/12 | 0 | − | − |
| 40 | 3/11 | 0 | − | − |
| 48 | 6/12 | 2.5 | + | + |
| 72 | 6/10 | 5.0 | + | + |
| 96 | 6/10 | 7.5 | + | + |
Number of PCR-positive ticks/number of ticks tested.
DISCUSSION
Competitive PCR for quantification of the HGE agent provided a sensitive and reproducible means of assessment of the number of ehrlichia organisms in ticks during and after feeding and molting. Our results indicated that the majority of I. scapularis larval ticks acquired infection within 24 to 48 h of attachment on infected mice and that the number of HGE agent organisms increased during the larval tick molting period. Furthermore, replication of the HGE agent also occurred during feeding within nymphal ticks. Both of these factors are likely to influence the effectiveness of transmission from the tick to the host, ensuring sufficient numbers of bacteria for attainment of an infectious dose for the mammalian host.
Dosage studies with Ehrlichia risticii have indicated that innate defense mechanisms can protect against or eliminate low-dose inoculation (21). Only at higher doses could E. risticii cause infection and disease. A similar effect has been shown with Ehrlichia canis (10). The lethal dose of Rickettsia australis organisms for mice was 2 × 106 (9), and that of Rickettsia conorii was 2.25 × 105 (28). Evaluation of the HGE agent infectious dose for mice in the current study revealed that relatively large numbers of organisms are needed (104 to 105) to infect mice. This must be interpreted with caution, because the quantitation is based upon the amount of DNA, which does not necessarily directly reflect the infectivity of the test material. Mouse blood, for example, may contain more or fewer infectious units/DNA unit than tick-derived material. Assuming that the HGE agents in infected SCID mouse blood and in ticks are equivalent in infectivity (which may not necessarily be true), tick-transmitted infection induced detectable morulae in peripheral blood granulocytes equivalent to the effect of 105 organisms within an inoculum of infected SCID mouse blood. Furthermore, previous studies suggested that dermal inoculation required higher infectious doses compared to the amount required for i.p. inoculation (14). Thus, although we cannot accurately determine the tick-borne infectious dose, data suggest that infection with the HGE agent, like infections with related organisms, is dose dependent and that relatively high doses of organisms appear to be needed to infect a mouse.
The HGE agent grows slowly in HL-60 cells (11), and if it is anything like its distantly related species, R. prowazekii, its generation time is probably about 10 h (31). Nevertheless, the number of HGE agent organisms that a larval tick obtains during its blood meal is likely to be quite small, because it is dependent upon the concentration of bacteria in the blood. Therefore, to achieve the number of ehrlichiae needed to be efficiently transmitted, the HGE agent must rely on replication within the vector rather than the host, either by replication within ticks after feeding as larvae or by replication during the process of feeding as nymphs, or both. Our data support both possibilities. We have shown that transmission occurs within a narrow window of 40 to 48 h of feeding. This does not discount the possibility that transmission might take place at earlier intervals with some ticks and some HGE agent isolates, but our data support the contention that the HGE agent requires the process of replication within the tick to be efficiently transmitted. Our data also confirm findings by others, who examined transmission of the HGE agent by nymphal ticks at 12, 24, 30, 36, and 50 h of tick attachment and who found that few mice became infected when ticks were removed prior to 36 h of tick attachment (14a).
Furthermore, our previous studies (14) indicate that the number of organisms and the efficiency of transmission to ticks declines significantly over the course of infection in mice. It remains to be determined if this is the case in Peromyscus mice, the natural reservoir host (26). However, if these factors are true in Peromyscus mice as well, they suggest that the HGE agent may be inefficiently transmitted and inefficiently acquired by the vector, requiring some compensatory mechanism on the part of the organism to optimize its force of transmission. Our current studies suggest that replication within ticks during and after feeding in larvae and during feeding in nymphs appears to be a mechanism that enhances the effectiveness of transmission of the HGE agent by ticks.
ACKNOWLEDGMENTS
This study was supported in part by the G. Harold and Leila Y. Mathers Charitable Foundation and NIH grants AI 41440, AI 28956, and RR 07038.
REFERENCES
- 1.Bakken J S, Dumler J S, Chen S-M, Eckman M R, VanEtta L L, Walker D H. Human granulocytic ehrlichiosis in the upper Midwest United States: a new species emerging? JAMA. 1994;272:212–218. [PubMed] [Google Scholar]
- 2.Belognia E A, Reed K D, Mitchell P D, Kolbert C P, Persing D H, Gill J S, Kazmierczak J J. Prevalence of granulocytic Ehrlichiainfection among white-tailed deer in Wisconsin. J Clin Microbiol. 1997;35:1465–1468. doi: 10.1128/jcm.35.6.1465-1468.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Human granulocytic ehrlichiosis—New York. Morbid Mortal Weekly Rep. 1995;4:593–595. [Google Scholar]
- 4.Chen S-M, Dumler J S, Bakken J S, Walker D H. Identification of a granulocytotropic Ehrlichiaspecies as the etiologic agent of human disease. J Clin Microbiol. 1994;32:589–595. doi: 10.1128/jcm.32.3.589-595.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.deSilva A, Fikrig E. Growth and migration of Borrelia burgdorferi in Ixodesticks during blood feeding. Am J Trop Med Hyg. 1995;53:397–404. doi: 10.4269/ajtmh.1995.53.397. [DOI] [PubMed] [Google Scholar]
- 6.deSilva A, Telford S R, Brunet L R, Barthold S W, Fikrig E. Borrelia burgdorferiOspA arthropod-specific transmission-blocking Lyme disease vaccine. J Exp Med. 1996;183:271–275. doi: 10.1084/jem.183.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.desVignes F, Fish D. Transmission of the agent of human granulocytic ehrlichiosis by host-seeking blacklegged tick (Acari: Ixodidae) in southern New York state. J Med Entomol. 1997;34:379–382. doi: 10.1093/jmedent/34.4.379. [DOI] [PubMed] [Google Scholar]
- 8.Eriks I S, Stiller D, Palmer G H. Impact of persistent Anaplasma marginalerickettsemia on tick infection and transmission. J Clin Microbiol. 1993;31:2091–2096. doi: 10.1128/jcm.31.8.2091-2096.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng H M, Wen J, Walker D H. Rickettsia australisinfection: a murine model of a highly invasive vasculopathic rickettsiosis. Am J Pathol. 1993;142:1471–1482. [PMC free article] [PubMed] [Google Scholar]
- 10.Gaunt S D, Corstvet R E, Berry C M, Brennan B. Isolation of Ehrlichia canisfrom dogs following subcutaneous inoculation. J Clin Microbiol. 1996;34:1429–1432. doi: 10.1128/jcm.34.6.1429-1432.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodman J L, Nelson C, Vitale B, Madigan J E, Dumler J S, Kurtti T J, Munderloh U G. Direct cultivation of the causative agent of human granulocytic ehrlichiosis. N Engl J Med. 1996;334:209–215. doi: 10.1056/NEJM199601253340401. [DOI] [PubMed] [Google Scholar]
- 12.Greig B, Asanovich K M, Armstrong P J, Dumler J S. Geographic, clinical, serologic, and molecular evidence of granulocytic ehrlichiosis, a likely zoonotic disease, in Minnesota and Wisconsin dogs. J Clin Microbiol. 1996;34:44–48. doi: 10.1128/jcm.34.1.44-48.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardalo C J, Quagliarello V, Dumler J S. Human granulocytic ehrlichiosis in Connecticut: report of a fatal case. Clin Infect Dis. 1995;21:910–914. doi: 10.1093/clinids/21.4.910. [DOI] [PubMed] [Google Scholar]
- 14.Hodzic E, IJdo J W I, Feng S, Katavolos P, Sun W, Maretzki C H, Fish D, Fikrig E, Telford III S R, Barthold S W. Granulocytic ehrlichiosis in the laboratory mouse. J Infect Dis. 1998;177:737–745. doi: 10.1086/514236. [DOI] [PubMed] [Google Scholar]
- 14a.Katavolos P, Armstrong P M, Dawson J E, Telford S R., III Duration of tick attachment required for transmission of granulocytic ehrlichiosis. J Infect Dis. 1998;177:1422–1425. doi: 10.1086/517829. [DOI] [PubMed] [Google Scholar]
- 15.MacLeod J, Gordon W S. Studies on tick-borne fever of sheep. I. Transmission by the tick Ixodes ricinuswith a description of the disease produced. Parasitology. 1933;25:273–283. [Google Scholar]
- 16.Mitchell P D, Reed K D, Hofkes J M. Immunoserologic evidence of coinfection with Borrelia burgdorferi, Babesia microti, and human granulocytic Ehrlichiaspecies in residents of Wisconsin and Minnesota. J Clin Microbiol. 1996;34:724–727. doi: 10.1128/jcm.34.3.724-727.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nadelman B R, Horwitz H W, Hsieh T C, Wu J M, Aguero-Rosenfeld M E, Schwartz I, Nowakowski J, Varde S, Wormser G P. Simultaneous human granulocytic ehrlichiosis and Lyme borreliosis. N Engl J Med. 1997;337:27–30. doi: 10.1056/NEJM199707033370105. [DOI] [PubMed] [Google Scholar]
- 18.Pancholi P, Kolbert C P, Mitchell P D, Reed K D, Dumler J S, Bakken J S, Telford III S R, Persing D H. Ixodes damminias a potential vector of human granulocytic ehrlichiosis. J Infect Dis. 1995;172:1007–1012. doi: 10.1093/infdis/172.4.1007. [DOI] [PubMed] [Google Scholar]
- 19.Pang H, Winkler H H. Copy number of the 16S rRNA gene in Rickettsia prowazekii. J Bacteriol. 1993;175:3893–3896. doi: 10.1128/jb.175.12.3893-3896.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piesman J. Dynamics of Borrelia burgdorferi transmission by nymphal Ixodes damminiticks. J Infect Dis. 1993;167:1082–1085. doi: 10.1093/infdis/167.5.1082. [DOI] [PubMed] [Google Scholar]
- 21.Rikihisa Y. The tribe Ehrlichieaeand ehrlichial diseases. Clin Microbiol Rev. 1991;4:286–308. doi: 10.1128/cmr.4.3.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwan T G, Piesman J, Golde W T, Dolan M C, Rosa P A. Induction of outer surface protein on Borrelia burgdorferiduring tick-feeding. Proc Natl Acad Sci USA. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz I, Fish D, Varde S, desVignes F, Daniels T J. Prevalence of the rickettsial agent of human granulocytic ehrlichiosis in ticks from a hyperendemic focus of Lyme disease. N Engl J Med. 1997;337:49–50. doi: 10.1056/NEJM199707033370111. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 24.Siebert D P, Larrick J W. Competitive PCR. Nature. 1992;359:557–558. doi: 10.1038/359557a0. [DOI] [PubMed] [Google Scholar]
- 25.Telford S R, III, Armstrong P M, Katavolos P, Foppa I, Garcia A S O, Wilson M L, Spielman A. A new tick-borne encephalitis-like virus infecting New England deer ticks, Ixodes dammini. Emerg Infect Dis. 1997;3:165–170. doi: 10.3201/eid0302.970209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Telford S R, III, Dawson J E, Katavolos P, Warner C K, Kolbert C P, Persing D H. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc Natl Acad Sci USA. 1996;93:6209–6214. doi: 10.1073/pnas.93.12.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Telford S R, III, Lepore T J, Snow P, Warner C K, Dawson J E. Human granulocytic ehrlichiosis in Massachusetts. Ann Intern Med. 1995;123:277–279. doi: 10.7326/0003-4819-123-4-199508150-00006. [DOI] [PubMed] [Google Scholar]
- 28.Walker D H, Popov V L, Wen J, Feng H M. Rickettsia conoriiinfection of C3H/HeN mice. A model of endothelial-target rickettsiosis. Lab Invest. 1994;70:358–368. [PubMed] [Google Scholar]
- 29.Walls J J, Greig B, Neitzel D F, Dumler J S. Natural infection of small mammal species in Minnesota with the agent of human granulocytic ehrlichiosis. J Clin Microbiol. 1997;35:853–855. doi: 10.1128/jcm.35.4.853-855.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Webster K A, Mitchell G B B. An electron microscopic study of Cytoecetes phagocytophila infection in Ixodes ricinus. Res Vet Sci. 1989;47:30–33. [PubMed] [Google Scholar]
- 31.Winkler H H. Rickettsia prowazekii, ribosomes and slow growth. Trends Microbiol. 1995;3:196–198. doi: 10.1016/s0966-842x(00)88920-9. [DOI] [PubMed] [Google Scholar]