Abstract
The field of organic chemistry began with 19th century scientists identifying and then expanding upon synthetic dye molecules for textiles. In the 20th century, dye chemistry continued with the aim of developing photographic sensitizers and laser dyes. Now, in the 21st century, the rapid evolution of biological imaging techniques provides a new driving force for dye chemistry. Of the extant collection of synthetic fluorescent dyes for biological imaging, two classes reign supreme: rhodamines and cyanines. Here, we provide an overview of recent examples where modern chemistry is used to build these old-but-venerable classes of optically responsive molecules. These new synthetic methods access new fluorophores, which then enable sophisticated imaging experiments leading to new biological insights.
Keywords: fluorescence, organic chemistry, rhodamine, cyanine, microscopy, imaging
Introduction
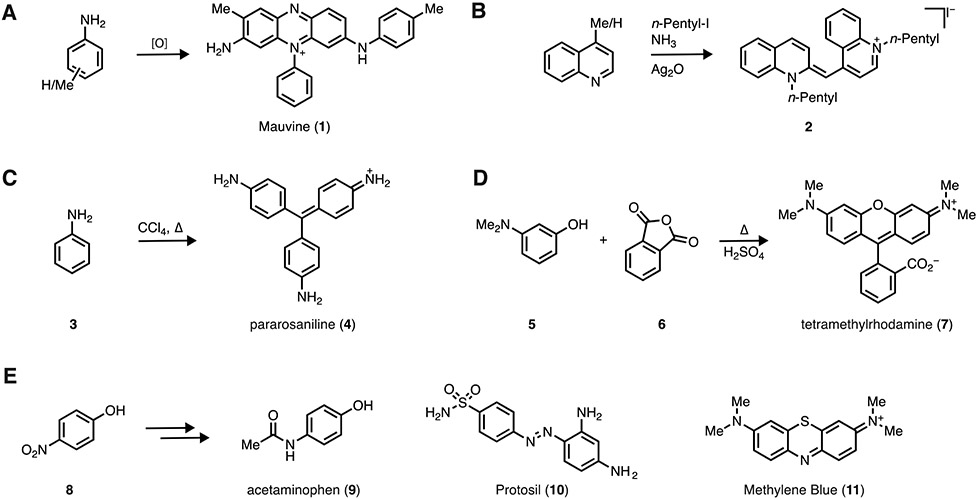
In 1856, 18-year-old William Perkin accidentally synthesized the first synthetic dye, Mauveine (1) by condensing a mixture of toluidines (Figure 1A). This preparation of a synthetic colorant was the spark that transformed the field of organic chemistry from a curiosity to a viable industry [1]. That same year the first cyanine dye was synthesized through base-promoted condensation of a mixture of N-alkylated quinoline and 4-methyl quinoline to yield 2 (Figure 1B) [2]. In addition to early cyanines, triarylmethane dyes were discovered during this decade via condensation of aniline and its derivatives. Reaction of aniline (3) with CCl4 at elevated temperature produced pararosaniline (4), a component of the magenta dye Fuchsine, in 1859 (Figure 1C) [3]. Dye discovery continued, with the synthesis of fluorescein in 1871 by Bayer [4] and rhodamine dyes at Badische Anilin- und Sodafabrik (BASF) in 1887 [5]. An key example of rhodamine dye synthesis is the acid-catalyzed condensation of 3-N,N-dimethylaminophenol (5) and phthalic anhydride (6) to yield tetramethylrhodamine (TMR; 7; Figure 1D). These 19th-century advances provided the chemical foundation that enabled further development of these highly absorbing and sometimes fluorescent molecular scaffolds.
Figure 1. Early developments in dye chemistry.
(A) Synthesis of mauveine (1). (B) Synthesis of cyanine dye 2. (C) Synthesis of pararosaniline (4). (D) Synthesis of tetramethylrhodamine (7). (E) Examples of dye chemistry inspiring medicinal chemistry.
Dyes dominated the field of organic chemistry for decades and played an important role in the development of pharmacological agents. The synthesis of Benzoazurine G, an important blue textile dye that competed with natural indigo, resulted in the production of large quantities of p-nitrophenol as a byproduct (Figure 1E. 8); this material was eventually repurposed by Bayer to create the analgesic acetaminophen (i.e., paracetamol; 9) [6]. The first antibiotic, Prontosil (10) was discovered by screening a collection of synthetic dyes for antibacterial activity [7, 8]. Methylene Blue (11), another BASF product, found use as a treatment for several maladies including malaria [9]. In the mid 20th century, synthetic dyes gained prominence due to the development of color photography and laser dyes [10]. This was further bolstered by the rise of modern fluorescence imaging techniques, giving rise to the excellent collections of fluorescent labels (e.g., CyDye, Alexa Fluor, ATTO) that consist primarily of rhodamine and cyanine dyes [11].
Despite the 150-year history of synthetic dyes, the chemistry underpinning synthetic colorants remained stubbornly rooted in 19th-centrury chemical methods [12]. These typically involved harsh reaction conditions such as acid catalyzed condensations, which severely limited the scope of dye synthesis. Now, in the 21st century, fluorescent dyes are enjoying a renaissance that is driven by the application of modern synthetic techniques to these classic dye scaffolds. As synthetic organic chemists trained in total synthesis, we advocate that probe discovery be given the same treatment as natural products, replete with retrosynthetic analysis along with adaptation and refinement of modern synthetic methodology for these important targets. Here, we showcase examples from our laboratories and others where new chemistry yields new dyes that enable new, sophisticated biological imaging experiments.
Rhodamines
The first rhodamine dyes absorbed relatively short wavelength light giving rise to a red color—their name stems from the Greek rhodon (rose). The high brightness and photostability of rhodamines made them prominent laser dyes and many of these dye scaffolds were repurposed in the 1990s for use as antibody or oligonucleotide labels—most Alexa Fluor and ATTO dyes are simply sulfonated or amidated laser dyes, respectively [11, 13]. Still, as mentioned above, the use of harsh 19th century chemistry—acid-catalyzed condensation at elevated temperature—is incompatible with all but the simplest functional groups, which severely limited the scope of rhodamine synthesis. This is reflected in the structures of many rhodamines, which incorporate N-methyl or N-ethyl anilines along with quinoline and julolidine moieties. This limited chemistry produced suboptimal dyes such as TMR that exhibits a relatively low quantum yield (ΦF = 0.41) due to nonradiative decay involving twisted internal charge transfer (TICT) [14].
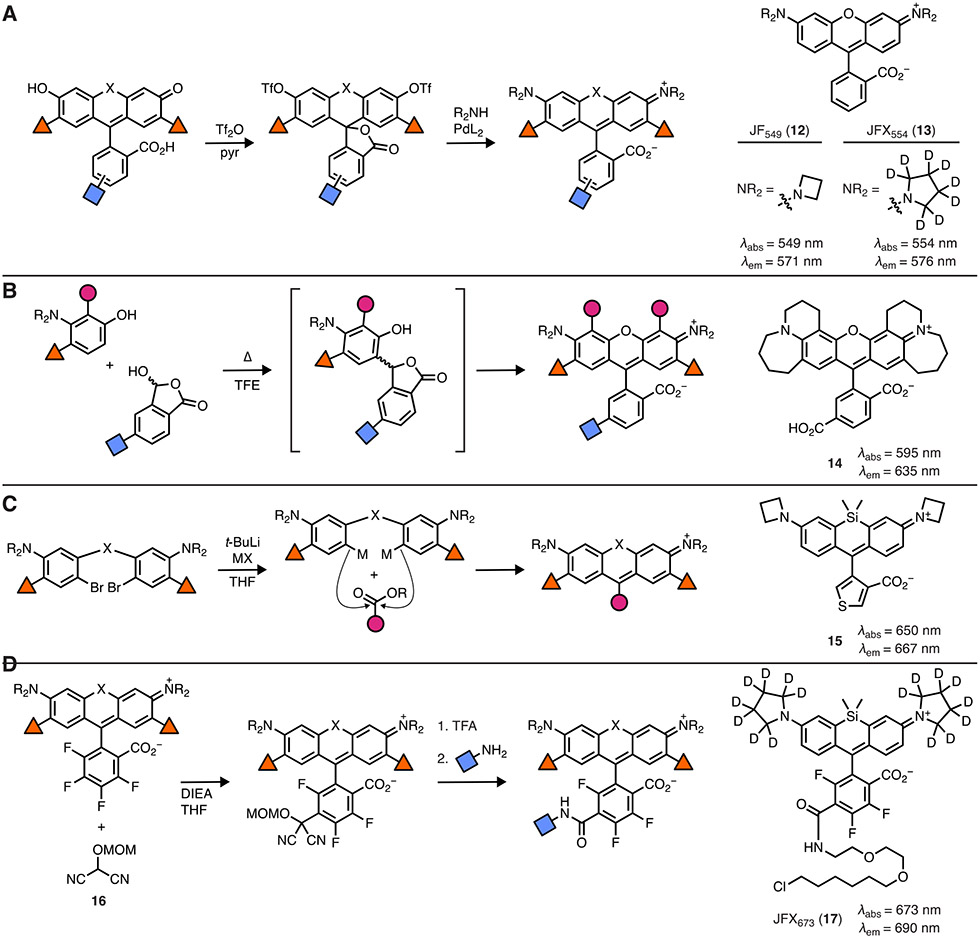
The first step in solving this problem is the development of new chemistry that would allow the exploration of a more diverse set of auxochromes beyond N,N-dimethylamine. The Lavis lab developed a simple strategy starting from dibromofluoran [15] or fluorescein ditriflate [16, 17], using Pd-catalyzed cross-coupling reactions to generate rhodamines (Figure 2A). This idea was supported by the tremendous effort by the Buchwald lab and others developing ligands to facilitate general Pd-mediated reactions [18]. It is important to note that ditriflates of electron-deficient fluoresceins bearing fluorines or chlorines are prone to aminolysis when using primary or secondary amine reactants. This can be remedied by using t-Boc-protected amines or switching from ditriflates to dibromides or diiodides, which can be accessed through direct synthesis [15, 19] or via metal-catalyzed reactions [20].
Figure 2. Modern chemical approaches for rhodamine dye synthesis.
(A) Synthesis of rhodamines using Pd-catalyzed cross-coupling and structures of exemplar dyes: JF549 (12) and JFX554 (13). (B) Condensation–oxidation of 3-aminophenols and lactols yields dyes such as 14. (C) Synthesis of rhodamines using metalation of dibromides and structure of thiophene-containing Si-rhodamine 15. (D) Use of masked acyl cyanide (16) to derivatize fluorinated rhodamines via SNAr and structure of bioavailable JFX673-HaloTag ligand (17).
This cross-coupling chemistry allows almost any amino auxochrome to be installed into a rhodamine, making many new derivatives possible. Inspired by photochemistry principles and computational chemistry, the Lavis lab hypothesized that switching the N,N-dimethylamino group to the diminutive four-membered azetidine could improve fluorophore properties [14]. Azetidine exhibits a higher oxidation potential than dimethylamine and ground-state calculations of azetidinylrhodamines suggested a planar structure necessary for a fluorescent species; together these data suggested a fluorophore with improved properties. This hypothesis was confirmed with the azetidine-containing dye 12 (i.e., Janelia Fluor 549, JF549; Figure 2A) showing substantially higher fluorescence quantum yield (ΦF = 0.88) and better photostability. This general strategy was applied to many different rhodamines, resulting in a palette of dyes spanning a substantial section of the visible spectrum [21, 22]. To further improve brightness and photostability, deuteration of the N-alkyl groups was proposed since the oxidation of amines shows a surprisingly high secondary isotope effect [23]. Deuterated azetidine and deuterated pyrrolidine could be incorporated into rhodamines via Pd-catalyzed cross-coupling, yielding bright and photostable dyes such as JFX554 (13; Figure 2A). Combined with the self-labeling HaloTag system [24], the JF and JFX dyes have enabled sophisticated single-molecule experiments in live cells [25-27]. This cross-coupling approach has been adopted to prepare other useful derivatives such as photoactivatable (“caged”) fluorophores [16, 28].
The Pd-catalyzed cross-coupling approach is generalizable but has two limitations. First, it does not allow the construction of rhodamines with fused rings. This problem was addressed by scientists at Promega Biosciences, who showed that condensation of 3-aminophenols and lactols could produce rhodamine dyes in the presence of O2 (Figure 2B) [29]. This allowed the synthesis of rhodamine 14, which is an excellent acceptor dye for bioluminescence resonance transfer (BRET). Second, the cross-coupling approach requires synthesis of fluorescein starting materials. This is straightforward for classic, oxygen-containing dyes but not for other variants where the xanthene oxygen is replaced with another moiety such as carbon or silicon—such fluorescein starting materials can be difficult to synthesize [17]. To complement the cross-coupling approach, the Lavis lab developed an alternative approach where diarylbromides can be metalated and added to either esters or anhydrides (Figure 2C) [30]. Suitable dibromide starting materials can be synthesized in a few steps and this method allows direct access to many rhodamines and their analogs; this strategy can also be used to prepare fluorescein analogs. This so-called “dibromide” synthetic approach has been expanded upon by the Lavis lab and several other groups to prepare numerous rhodamine dyes [22, 31, 32]. In addition to allowing direct access to standard rhodamine derivatives, the approach allows introduction of alternative substituents at the 9-position of the xanthene system, replacing the classic o-carboxy moiety. An example is dye 15, which contains a carboxy-thiophene moiety. This dye shows high visible absorption in aqueous solution and can be used as a photocatalyst to oxidize dihydrotetrazines, allowing light directed polymerization in vivo [33].
Another useful structural modification is fluorination of the pendant phenyl ring. Dyes with this motif exhibit a bathochromic shift in absorption and emission maxima and fluorination also adjusts chemical properties. The electron-deficient phenyl ring is also a good substrate for nucleophilic aromatic substitution reactions (SNAr), which allow facile installation of a chemical handle for bioconjugation. This has typically been realized by reaction with thiols [34, 35], but the Lavis lab discovered that masked acyl cyanide (MAC) reagents [36] such as compound 16 add in a regioselective manner to the 6-postion of the pendant ring, deprotection of the MOM group yields a putative acyl cyanide that can be transformed to an acid, ester, or anhydride (Figure 2D) [22]. This approach allows the facile synthesis of functional derivatives of dyes such as JFX673-HaloTag ligand (17) which combines deuteration, fluorination, and this umpoloung chemistry to yield a bright, photostable, and bioavailable dye ligand suitable for pulse–chase experiments in vivo [37].
Cyanines
Since the introduction of the “CyDye’ series in the early 1990s, polymethine indocyanines have become the molecules of choice as covalent labels to visualize proteins and nucleic acids. More recently, polymethine cyanine dyes are emerging as the molecular core of responsive fluorescent sensors and photocages [38-46]. Despite finding use across the spectrum of preclinical to clinical biomedical applications, the chemistries used to assemble and diversify these probes had changed relatively little until recent years. Here we highlight recent synthetic advances, which are enabling the creation of promising probes for challenging imaging applications.
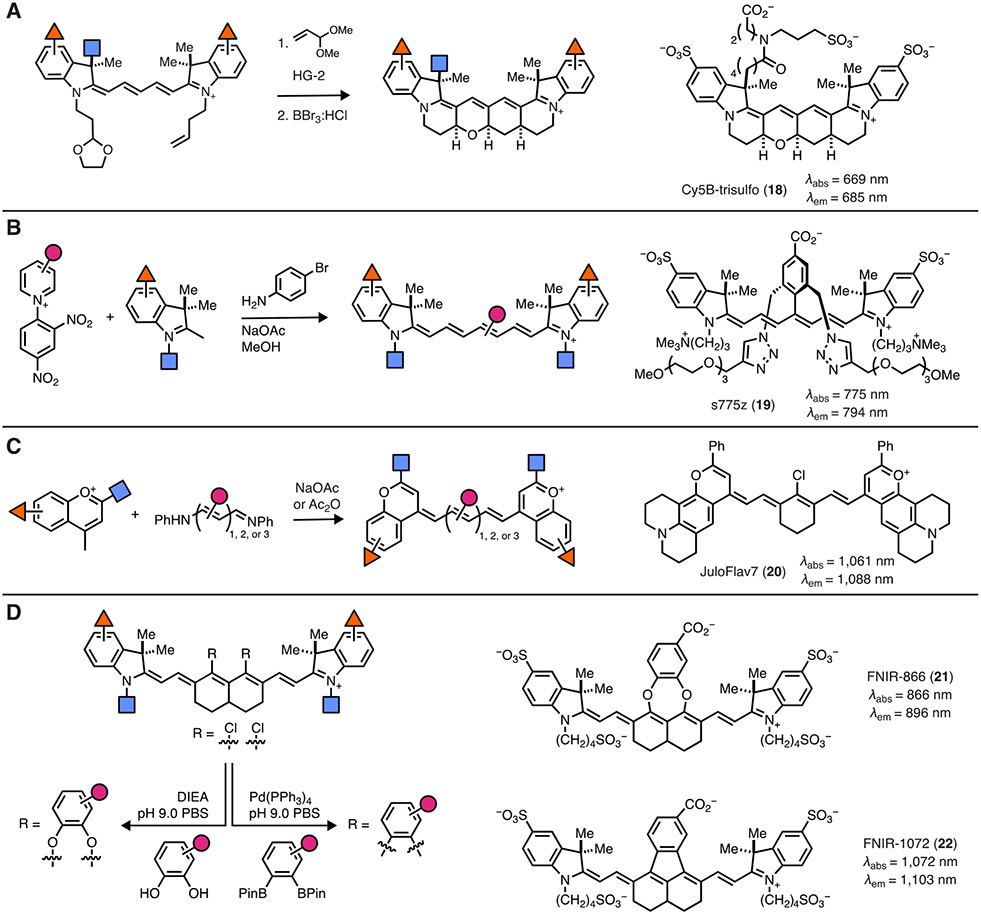
The quantum yield of a typical pentamethine cyanine (ΦF ≈ 0.1 to 0.2 in aqueous solvent) can present a limitation, particularly for demanding single molecule applications. A major non-emissive pathway for excited-state deactivation involves polymethine isomerization [43, 47]. Using the trimethine scaffold, this pathway was blocked by installing 6-membered rings along the polyene chain to form Cy3B, leading to a dramatic improvement in quantum yield ΦF = 0.09 for Cy3 to ΦF = 0.85 for Cy3B [48]. Applying this approach to penta- and hepta-methine congeners required the development of new synthetic strategies to assemble the more complex polycyclic ring systems. This challenge was recently addressed by the Schnermann lab using the synthetic strategy shown in Figure 3A. In the key sequence, a protected aldehyde precursor undergoes a ruthenium-mediated cross-metathesis reaction followed by an intramolecular Michael addition and dihydropyran ring-forming cascade to form the desired tetracyclic ring system. These compounds exhibit dramatically improved ΦF relative to a typical Cy5-type fluorophore (ΦF = 0.69 vs. ΦF = 0.15) and extended fluorescence lifetimes [49]. While mono-sulfonated variants were only suitable for small molecule labeling, second-generation di- and tri-sulfonated Cy5B probes such as 18 enabled the preparation of labeled mAb and nucleic acid conjugates. These probes have been applied for single molecule FRET and single molecule localization microscopy where they improve photon output relative to non-constrained cyanine variants [50, 51]. The Schnermann lab also applied the cross-metathesis/cyclization chemistry on heptamethine cyanine scaffold—an approach that entailed a pentacyclization reaction [52]. Surprisingly, the resulting conformationally restrained heptamethine cyanine did not exhibit significantly improved quantum yields or lifetimes at room temperature in protic solvents. This finding reveals an important insight about the excited state-quenching of heptamethine cyanines—the role of polymethine isomerization is modest relative to the dominant role of water/solvent-mediated excited-state deactivation [53]. Critically, this observation suggests future studies should not focus on the issue of photoisomerization in the development of cyanine probes that absorb at longer wavelengths (>700 nm) [54].
Figure 3. Modern chemical approaches for cyanine dye synthesis.
(A) Synthesis of conformationally restricted cyanine dyes and representative compound 18. (B) Use of Zincke salts in the synthesis of heptamethine dyes such as 19. (C) Replacing the indoline units in cyanine dyes with flavylium moieties yields SWIR-absorbing dyes including 20. (D) Synthesis of nonamethine dyes and representative compounds 21 and 22.
The most broadly used approach to modify the heptamethine cyanine chromophore entails sequential derivatization of cyclohexanone derived precursors [55-58]. Stacko, Klan and coworkers recently reported a new approach to rapidly prepare a range of substituted polymethine probes [59]. This chemistry converts 2,4-dinitroaryl pyridine (Zincke) salts to the corresponding ring-opened dianiline intermediate, which can be carried on to various heptamethine cyanines (Figure 3B). This new assembly strategy has been adopted for a variety of applications, including the development of novel cyanine-based photocages as well as aggregation-resistant fluorophores [40, 45]. Representative of these efforts, s775z (19), developed by Smith and colleagues, is a promising compound for in vivo imaging applications [60]. This new chemistry has the strategic benefit of starting from broadly available pyridine precursors to enable chemical diversification of the polymethine chromophore unit.
Over the last decade, a variety of studies have shown that wavelengths between 1000 and 2000 nm can enable high-resolution in vivo multicolor imaging at depths not possible with conventional optical wavelengths [61]. While significant early efforts in this area were enabled by a range of nanoparticles quantum dots, and carbon nanotubes, there are significant benefits to using small molecule organic probes [62-64]. Recently it has been found that heptamethine cyanines emit readily observed short wavelength infrared (SWIR) signal, which has been broadly applied including in clinical efforts with ICG [65, 66]. To enable multicolor imaging in this wavelength regime, bright, readily accessible, and easily modified probes with absorbance and emission maxima beyond the Cy7 range are needed. However, the design and synthesis of such molecules represents a significant, but now approachable, chemical challenge.
Towards the goal of shifting cyanine absorption wavelengths into the SWIR, recent studies by Sletten and colleagues replaced the conventional indoline ring with modified flavylium heterocycles to provide tunable agents with emission beyond 1000 nm [67, 68]. The critical synthetic advance used cyanine precursors to assemble the array of tri-, penta-, and hepta-methine probes, which could be applied for a range of mutlicolor imaging efforts (Figure 3C) [68]. Further tuning of the heterocycle has led to compounds such as JuloFlav7 (20), which is ideally matched for excitation with the broadly available 1064 nm laser [69]. There remains a significant need for the type of bioconjugatable probes that have proven invaluable for multiplexed imaging in the visible and NIR range. The Schnermann lab hypothesized that nonamethine cyanines, which had been only sparingly reported, might be suitably modified to enable targeted imaging efforts. A rational design process led to the insight that direct aryl fusion onto the nonamethine scaffold might lead to a dramatic bathochromic shift. This supposition was validated through the preparation of the persulfonated indocyanine dyes, FNIR-866 (21) and FNIR-1072 (22) through catechol- and aryl-ring fusion, respectively, onto the nonamethine scaffold (Figure 3D). These probes were applied in a range of imaging applications, including three-color imaging in a model surgical setting [69].
Conclusion and Future Outlook
The century old rhodamine and cyanine dyes continue to dominate biological imaging. First-generation rhodamines held the blue-orange excitation window and were primary used as antibody labels and live-cell stains. Cyanine dyes filled the far-red and NIR region of the spectrum making them useful for in vivo imaging, while also finding broad use as oligonucleotide and protein labels. Although the inherent advantages of the rhodamine and cyanine scaffolds will ensure different utility, the traditional divisions of these two classes are beginning to blur. This is due largely to new chemistry. The improved methods to make rhodamine dyes are pushing excitation wavelengths longer by incorporating silane, phosphine oxide, and ketone functionalities; these also allow fine-tuning of chemical properties [22]. Likewise, chemistry is providing strategies to broadly tune the cyanine chromophore, enabling a broader set of applications. Looking forward, we hypothesize that the union of advances in the synthetic chemistry and imaging probe design will provide versatile molecules to interrogate complex biological systems with increasing precision.
Acknowledgements
Related work in our laboratories is funded by the Howard Hughes Medical Institute (HHMI) and the National Institutes of Health (NIH) Intramural Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Competing Interests Statement
Patents and patent applications covering rhodamine dyes (with inventor L.D. Lavis) are assigned to HHMI. Patents and patent applications covering cyanine dyes (with inventor M. Schnermann) are assigned to the NIH.
References
- [1].Brightman R. Perkin and the dyestuffs industry in Britain. Nature 1956; 177:815. [Google Scholar]
- [2].Ilina K, Henary M. Cyanine dyes containing quinoline moieties: History, synthesis, optical properties, and applications. Chemistry 2021; 27:4230–4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Einwirkung des Chlorkohlenstoffs auf Anilin. Cyantriphenyldiamin. J. Prakt. Chem 1859; 77:190–191. [Google Scholar]
- [4].Baeyer A. Ueber eine neue Klasse von Farbstoffen. Ber. Dtsch. Chem. Ges 1871; 4:555–558. [Google Scholar]
- [5].Ceresole M. Verfahren zur Darstellung von Farbstoffen aus der Gruppe des Meta-amidophenolphtaleïns. In: Germany: 1887. p. D.R. Patent No. 44002.
- [6].Dronsfield A, Ellis P. The rise and fall of phenacetin. RSC Historical Group Newsletter; 2021; 80:18–26. [Google Scholar]
- [7].Domagk G. Ein Beitrag zur Chemotherapie der bakteriellen Infektionen. Dtsch. Med. Wochenschr 1935; 61:250–253. [Google Scholar]
- [8].Lesch JE. Chapter 3: Prontosil. In: The first miracle drugs: How the sulfa drugs transformed medicine. Oxford, England: Oxford University Press; 2007. [Google Scholar]
- [9].Lu G, Nagbanshi M, Goldau N et al. Efficacy and safety of methylene blue in the treatment of malaria: A systematic review. BMC Med 2018; 16:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Schäfer FP, Ed. Dye Lasers. Berlin: Springer-Verlag; 1977. [Google Scholar]
- [11].Lavis LD, Raines RT. Bright ideas for chemical biology. ACS Chem. Biol 2008; 3:142–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lavis LD, Raines RT. Bright building blocks for chemical biology. ACS Chem. Biol 2014;9:855–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Panchuk-Voloshina N, Haugland RP, Bishop-Stewart J et al. Alexa Dyes, a series of new fluorescent dyes that yield exceptionally bright, photostable conjugates. J. Histochem. Cytochem 1999; 47:1179–1188. [DOI] [PubMed] [Google Scholar]
- [14].Grimm JB, English BP, Chen J et al. A general method to improve fluorophores for live-cell and single-molecule microscopy. Nat. Methods 2015; 12:244–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wysocki LM, Grimm JB, Tkachuk AN et al. Facile and general synthesis of photoactivatable xanthene dyes. Angew. Chem. Int. Ed. Engl 2011; 50:11206–11209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Grimm JB, Lavis LD. Synthesis of rhodamines from fluoresceins using Pd-catalyzed C-N cross-coupling. Org. Lett 2011; 13:6354–6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Grimm JB, Sung AJ, Legant WR et al. Carbofluoresceins and carborhodamines as scaffolds for high-contrast fluorogenic probes. ACS Chem. Biol 2013; 8:1303–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ruiz-Castillo P, Buchwald SL. Applications of palladium-catalyzed C-N cross-coupling reactions. Chem. Rev 2016; 116:12564–12649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zheng Q, Ayala AX, Chung I et al. Rational design of fluorogenic and spontaneously blinking labels for super-resolution imaging. ACS Cent. Sci 2019; 5:1602–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Butkevich AN, Bossi ML, Lukinavicius G, Hell SW. Triarylmethane fluorophores resistant to oxidative photobluing. J. Am. Chem. Soc 2019; 141:981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Grimm JB, Muthusamy AK, Liang Y et al. A general method to fine-tune fluorophores for live-cell and in vivo imaging. Nat. Methods 2017; 14:987–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[22]. Grimm JB, Tkachuk AN, Xie L et al. A general method to optimize and functionalize red-shifted rhodamine dyes. Nat. Methods 2020; 17:815–821. Using chemisty to fine-tune dyes across the visible spectrum with an emphasis on long wavelength-absorbing dyes.
- [23].Grimm J, Xie L, Casler J et al. A general method to improve fluorophores using deuterated auxochromes. JACS Au 2021; 5:690–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Los GV, Encell LP, McDougall MG et al. HaloTag: A novel protein labeling technology for cell imaging and protein analysis. ACS Chem. Biol 2008; 3:373–382. [DOI] [PubMed] [Google Scholar]
- [25].Liu Z, Legant WR, Chen BC et al. 3D imaging of Sox2 enhancer clusters in embryonic stem cells. eLife 2014; 3:e04236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Li L, Liu H, Dong P et al. Real-time imaging of Huntingtin aggregates diverting target search and gene transcription. eLife 2016; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chong S, Dugast-Darzacq C, Liu Z et al. Imaging dynamic and selective low-complexity domain interactions that control gene transcription. Science 2018; 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Butkevich AN, Weber M, Cereceda Delgado AR et al. Photoactivatable fluorescent dyes with hydrophilic caging groups and their use in multicolor nanoscopy. J. Am. Chem. Soc 2021; 143:18388–18393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[29]. Dwight SJ, Levin S. Scalable regioselective synthesis of rhodamine dyes. Org. Lett 2016; 18:5316–5319. A simple and straightforward method for synthesizing single-isomer rhodamine dyes from accessable starting materials.
- [30].Grimm JB, Brown TA, Tkachuk AN, Lavis LD. General synthetic method for Si-fluoresceins and Si-rhodamines. ACS Cent. Sci 2017; 3:975–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Fischer C, Sparr C. Direct transformation of esters into heterocyclic fluorophores. Angew. Chem. Int. Ed. Engl 2018; 57:2436–2440. [DOI] [PubMed] [Google Scholar]
- *[32]. Butkevich AN. Modular synthetic approach to silicon-rhodamine homologues and analogues via ais-aryllanthanum reagents. Org. Lett 2021; 23:2604–2609. A large collection of dyes accessed using dimetalation of bis(arylbromides) and addition to esters or anhydrides.
- [33].Wang C, Zhang H, Zhang T et al. Enabling in vivo photocatalytic activation of rapid bioorthogonal chemistry by repurposing silicon-rhodamine fluorophores as cytocompatible far-red photocatalysts. J. Am. Chem. Soc 2021; 143:10793–10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gee KR, Sun W-C, Klaubert DH et al. Novel derivatization of protein thiols with fluorinated fluoresceins. Tetrahedron Lett. 1996; 37:7905–7908. [Google Scholar]
- [35].Mitronova GY, Polyakova S, Wurm CA et al. Functionalization of the meso-phenyl ring of rhodamine dyes through SNAr with sulfur nucleophiles: Synthesis, biophysical characterizations, and comprehensive NMR analysis. Eur. J. Org. Chem 2015; 2015:337–349. [Google Scholar]
- [36].Nemoto H, Kubota Y, Yamamoto Y. Development of a new acyl anion equivalent for the preparation of masked activated esters, and their use to prepare a dipeptide. J. Org. Chem 1990; 55:4515–4516. [Google Scholar]
- [37].Mohar B, Grimm JB, Patel R et al. Brain-wide measurement of protein turnover with high spatial and temporal resolution. bioRxiv 2022:2022.2011.2012.516226. [Google Scholar]
- [38].Gorka AP, Nani RR, Schnermann MJ. Cyanine polyene reactivity: Scope and biomedical applications. Org. Biomol. Chem 2015; 13:7584–7598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gorka AP, Nani RR, Schnermann MJ. Harnessing cyanine reactivity for optical imaging and drug delivery. Acc. Chem. Res 2018; 51:3226–3235. [DOI] [PubMed] [Google Scholar]
- [40].Janekova H, Russo M, Ziegler U, Stacko P. Photouncaging of carboxylic acids from cyanine dyes with near-Infrared light. Angew. Chem. Int. Ed. Engl 2022; 61:e202204391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Usama SM, Inagaki F, Kobayashi H, Schnermann MJ. Norcyanine-carbamates are versatile near-infrared fluorogenic probes. J. Am. Chem. Soc 2021; 143:5674–5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lin VS, Chen W, Xian M, Chang CJ. Chemical probes for molecular imaging and detection of hydrogen sulfide and reactive sulfur species in biological systems. Chemical Society reviews 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Levitus M, Ranjit S. Cyanine dyes in biophysical research: the photophysics of polymethine fluorescent dyes in biomolecular environments. Q Rev Biophys 2011; 44:123–151. [DOI] [PubMed] [Google Scholar]
- [44].Waggoner A. Fluorescent labels for proteomics and genomics. Current opinion in chemical biology 2006; 10:62–66. [DOI] [PubMed] [Google Scholar]
- [45].Alachouzos G, Schulte AM, Mondal A et al. Computational design, synthesis, and photochemistry of Cy7-PPG, an efficient NIR-activated photolabile protecting group for therapeutic applications. Angew. Chem. Int. Ed. Engl 2022; 61:e202201308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Usama SM, Marker SC, Caldwell DR et al. Targeted fluorogenic cyanine carbamates enable in vivo analysis of antibody-drug conjugate linker chemistry. J. Am. Chem. Soc 2021; 143:21667–21675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Gratz H, Penzkofer A, Abels C et al. Photo-isomerisation, triplet formation, and photo-degradation dynamics of indocyanine green solutions. J. Photoch. Photobio. A 1999; 128:101–109. [Google Scholar]
- [48].See: https://www.gelifesciences.com/gehcls_images/GELS/Related%20Content/Files/1314735988470/litdocflprobcy_20161013183116.pdf
- [49].Michie MS, Gotz R, Franke C et al. Cyanine conformational restraint in the far-red range. J. Am. Chem. Soc 2017; 139:12406–12409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Beliu G, Kurz AJ, Kuhlemann AC et al. Bioorthogonal labeling with tetrazine-dyes for super-resolution microscopy. Commun. Biol 2019; 2:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Waldchen F, Schlegel J, Gotz R et al. Whole-cell imaging of plasma membrane receptors by 3D lattice light-sheet dSTORM. Nat. Commun 2020; 11:887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Matikonda SS, Hammersley G, Kumari N et al. Impact of cyanine conformational restraint in the near-infrared range. J. Org. Chem 2020; 85:5907–5915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Maillard J, Klehs K, Rumble C et al. Universal quenching of common fluorescent probes by water and alcohols. Chem. Sci 2020; 12:1352–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Friedman HC, Cosco ED, Atallah TL et al. Establishing design principles for emissive organic SWIR chromophores from energy gap laws. Chem 2021; 7:3359–3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Strekowski L, Lipowska M, Patonay G. Substitution-reactions of a nucleofugal group in heptamethine cyanine dyes—Synthesis of an isothiocyanato derivative for labeling of proteins with a near-infrared chromophore. J. Org. Chem 1992; 57:4578–4580. [Google Scholar]
- [56].Lee H, Mason JC, Achilefu S. Heptamethine cyanine dyes with a robust C-C bond at the central position of the chromophore. J. Org. Chem 2006; 71:7862–7865. [DOI] [PubMed] [Google Scholar]
- [57].Sato K, Gorka AP, Nagaya T et al. Effect of charge localization on the in vivo optical imaging properties of near-infrared cyanine dye/monoclonal antibody conjugates. Mol. Biosyst 2016; 12:3046–3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Luciano MP, Crooke SN, Nourian S et al. A nonaggregating heptamethine cyanine for building brighter labeled biomolecules. ACS Chem. Biol 2019; 14:934–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[59]. Stackova L, Stacko P, Klan P. Approach to a substituted heptamethine cyanine chain by the ring opening of zincke salts. J. Am. Chem. Soc 2019; 141:7155–7162. An elegant synthetic method for synthesizing cyanine dyes in a single step.
- [60].Li DH, Schreiber CL, Smith BD. Sterically shielded heptamethine cyanine dyes for bioconjugation and high performance near-infrared fluorescence imaging. Angew. Chem. Int. Ed. Engl 2020; 59:12154–12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Wong KCY, Sletten EM. Extending optical chemical tools and technologies to mice by shifting to the shortwave infrared region. Curr. Opinion Chem. Biol 2022; 68:102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Cao J, Zhu B, Zheng K et al. Recent progress in NIR-II contrast agent for biological imaging. Front. Bioeng. Biotechnol 2019; 7:487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Wilson RH, Nadeau KP, Jaworski FB et al. Review of short-wave infrared spectroscopy and imaging methods for biological tissue characterization. J. Biomed. Opt 2015; 20:030901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Bruns OT, Bischof TS, Harris DK et al. Next-generation in vivo optical imaging with short-wave infrared quantum dots. Nat. Biomed. Eng 2017; 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Carr JA, Franke D, Caram JR et al. Shortwave infrared fluorescence imaging with the clinically approved near-infrared dye indocyanine green. Proc. Natl. Acad. Sci. U. S. A 2018; 115:4465–4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Hu Z, Fang C, Li B et al. First-in-human liver-tumour surgery guided by multispectral fluorescence imaging in the visible and near-infrared-I/II windows. Nat. Biomed. Eng 2020; 4:259–271. [DOI] [PubMed] [Google Scholar]
- [67].Cosco ED, Caram JR, Bruns OT et al. Flavylium polymethine fluorophores for near- and shortwave infrared imaging. Angew. Chem. Int. Ed. Engl 2017; 56:13126–13129. [DOI] [PubMed] [Google Scholar]
- *[68]. Cosco ED, Spearman AL, Ramakrishnan S et al. Shortwave infrared polymethine fluorophores matched to excitation lasers enable non-invasive, multicolour in vivo imaging in real time. Nat. Chem 2020; 12:1123–1130. A tour-de-force synthesis of a portfolio of tuned SWIR dyes useful for in vivo imaging.
- *[69]. Bandi VG, Luciano MP, Saccomano M et al. Targeted multicolor in vivo imaging over 1,000 nm enabled by nonamethine cyanines. Nat. Methods 2022; 19:353–358. Pushing cyanine dyes to longer wavelengths using a nona-methine bridge.