Abstract
Purpose:
Limited evidence exists regarding outcomes associated with different correction rates of severe hyponatremia.
Materials and Methods:
This retrospective cohort analysis employed a multi-center ICU database to identify patients with sodium ≤ 120 mEq/L during ICU admission. We determined correction rates over the first 24 hours and categorized them as rapid (> 8 mEq/L/day) or slow (≤ 8 mEq/L/day). The primary outcome was in-hospital mortality. Secondary outcomes included hospital-free days, ICU-free days, and neurological complications. We used inverse probability weighting for confounder adjustment.
Results:
Our cohort included 1,024 patients; 451 rapid and 573 slow correctors. Rapid correction was associated with lower in-hospital mortality (absolute difference: −4.37%; 95% CI, −8.47 to −0.26%), longer hospital-free days (1.80 days; 95% CI, 0.82 to 2.79 days), and longer ICU-free days (1.16 days; 95% CI, 0.15 to 2.17 days). There was no significant difference in neurological complications (2.31%; 95% CI, −0.77 to 5.40%).
Conclusion:
Rapid correction (>8 mEq/L/day) of severe hyponatremia within the first 24 hours was associated with lower in-hospital mortality and longer ICU and hospital-free days without an increase in neurological complication. Despite major limitations, including the inability to identify the chronicity of hyponatremia, the results have important implications and warrant prospective studies.
Keywords: Hyponatremia, Sodium, Electrolytes, Intensive Care Units, Nephrology, Mortality
Introduction
Hyponatremia is a potentially life-threatening condition that may lead to permanent neurological impairment [1]. It has been shown to be an independent risk factor for mortality in hospitalized patients, including those in the intensive care unit (ICU) [2–4], and any improvement of hyponatremia appears to be associated with reduced mortality, with mortality benefit persisting for up to 12 months after discharge [5]. However, too rapid correction once hyponatremia has been present long enough for the body to compensate for the osmotic imbalance has been associated with the development of osmotic demyelination syndrome (ODS). ODS is rare but can lead to permanent neurological complications and death [6–8].
The relationship between the correction rates of serum sodium concentration and the incidence of ODS remains unclear. While some studies have suggested that ODS is more common with higher rates of correction, with most cases having a 24-hour correction rate >12 mEq/L [6, 9–12], other studies have failed to demonstrate any association between the rate of correction and patient outcomes [13–15]. In a 2013 review, an expert panel on hyponatremia provided their recommendations on correction rates in chronic hyponatremia, which included a minimum correction rate of 4 to 8 mmol/L/day (with a lower threshold of 4 to 6 mmol/L/day for those at high risk of ODS) and an upper limit of 8 mmol/L/day for high risk of ODS and 10 to 12 mmol/L/day for normal risk of ODS [8]. Small observational studies have suggested that more rapid correction of chronic hyponatremia of up to 12 mmol/L/day may improve mortality and length of stay; however, the evidence supporting the benefits of rapid correction on patient outcomes is still limited [16, 17].
The aim of this study was to investigate the association between “slow correction” vs. “rapid correction” and in-hospital mortality, hospital and ICU-free days, and development of neurological complications in ICU patients with serum [Na+] ≤120 mEq/L. We set the cut-off for slow vs rapid correction at 8 mmol/L/day. This cut-off was chosen because it falls within the recommended correction range for individuals at high risk for ODS in several publications [8, 18] and could serve as the maximum correction rate for many patients with severe hyponatremia in the absence of sufficient information regarding whether they have acute or chronic hyponatremia or risk factors of ODS on ICU admission. We hypothesized based on the aforementioned studies that patients in the rapid correction group would have lower mortality and greater hospital and ICU-free days, but would have more frequent neurological complications, compared to patients in the slow correction group.
Materials and Methods
Study Design and Data Source
We conducted a retrospective cohort analysis using patient data extracted from the eICU Collaborative Research Database (CRD). This is a large multi-center public database of 200,859 unique ICU patient encounters at 208 hospitals across the US from 2014 to 2015 which is maintained by the Philips eICU Research Institute [19]. The study was exempt from institutional review board approval due to the retrospective design, lack of direct patient intervention, and the security schema, for which the re-identification risk was certified as meeting safe harbor standards by an independent privacy expert (Privacert, Cambridge, MA) (Health Insurance Portability and Accountability Act Certification no. 1031219–2).
We included patients with severe hyponatremia, defined as subjects who had serum [Na+] level ≤ 120 mEq/L during their ICU admission. We excluded patients if they were < 18 years old, on dialysis, discharged from ICU within 24 hours of ICU admission, had associated glucose level ≥ 360 mg/dL, or had no recorded serum [Na+] level in the 36 hours after their first recorded serum [Na+] ≤ 120 mEq/L. We only used serum sodium values and excluded any sodium values from whole blood measurements. We considered only the first ICU admission if a patient had multiple admissions.
Definition of Exposure and Outcomes
The baseline serum [Na+] level was defined as the first hyponatremic (≤ 120 mEq/L) measure at any time point during a patient’s ICU stay or within 4 hours prior to their ICU admission. The serum [Na+] level that was recorded closest to 24 hours after the baseline measurement was used to calculate the 24-hour correction rate. The correction rate was calculated using the following formula:
We set the exposure as “rapid correction,” defined as a serum [Na+] correction rate of > 8 mEq/L/day, and the control as “slow correction,” defined as a serum [Na+] correction rate of ≤ 8 mEq/L/day.
The primary outcome was in-hospital mortality. Secondary outcomes included hospital-free days, ICU-free days, and neurological complications at discharge. Hospital-free days and ICU-free days were defined as the number of days patients spent outside of the hospital and the ICU, respectively, up to 28 days. If patients died during hospitalization, both measures were assigned the value of 0. We defined neurological complications as having any of 8 diagnoses from the International Classification of Diseases, Ninth Revision (ICD-9) at time of discharge from the ICU: other demyelinating diseases of central nervous system (341.8), hemiplegia and hemiparesis (342), other paralytic syndromes (344), epilepsy and recurrent seizure (345), alteration of consciousness (780.0), coma (780.01), persistent vegetative state (780.03), and other coma (780.09).
Data Collection
Additional data including demographics, baseline laboratory values, primary diagnosis, past medical history, prior use of diuretics, ICU type, Glasgow Coma Scale (GCS), ventilation status, and Acute Physiology and Chronic Health Evaluation (APACHE) IV score were extracted from the eICU-CRD (Table 1).
Table 1.
Baseline Characteristics of Study Population
| Rapid correction (>8mEq/L per day) (n = 451) | Slow correction (≤8mEq/L per day) (n = 573) | |
|---|---|---|
| Age, yr, mean (SD) | 45.94 (13.31) | 49.97 (12.97) |
| Sex (male), n (%) | 213 (47.2%) | 287 (50.1%) |
| Ethnicity, n (%) | ||
| Caucasian | 347 (76.9%) | 477 (83.2%) |
| African American | 31 (6.9%) | 27 (4.7%) |
| Asian | 15 (3.3%) | 17 (3.0%) |
| Hispanic | 14 (3.1%) | 18 (3.1%) |
| Other/Unknown | 44 (9.8%) | 34 (5.9%) |
| Unit type, n (%) | ||
| Surgical ICU | 300 (66.5%) | 343 (59.9%) |
| Cardiac ICU | 80 (17.7%) | 113 (19.7%) |
| Medical ICU | 53 (11.8%) | 98 (17.1%) |
| Neurologic ICU | 18 (4.0%) | 19 (3.3%) |
| APACHE VI Score, mean (SD) | 57.91 (25.56) | 59.49 (24.96) |
| GCS, mean (SD) | 12.14 (4.11) | 13.33 (3.13) |
| Ventilation status, n (%) | 115 (26.1%) | 98 (18.3%) |
| Prior diuretics use, n (%) | 21 (4.7%) | 40 (7.0%) |
| Laboratory values, mean (SD) | ||
| Sodium, mEq/L | 116.37 (9.49) | 117.02 (6.37) |
| Potassium, mEq/L | 4.01 (1.11) | 4.21 (1.02) |
| BUN, mg/dL | 22.98 (27.03) | 28.39 (31.03) |
| Creatinine, mg/dL | 1.43 (2.10) | 1.58 (1.91) |
| Glucose, mg/dL | 134.40 (53.31) | 134.71 (56.55) |
| Albumin, g/dL | 3.38 (0.74) | 3.17 (0.73) |
| ALT, unit/L | 72.01 (210.36) | 83.06 (345.99) |
| AST, unit/L | 114.53 (358.86) | 136.51 (633.41) |
| Bicarbonate, mEq/L | 23.12 (6.58) | 23.52 (5.42) |
| Magnesium, mg/dL | 1.75 (0.46) | 1.81 (0.46) |
| Phosphate, mg/dL | 3.23 (1.82) | 3.55 (1.84) |
| Total bilirubin, mg/dL | 1.55 (3.29) | 2.29 (4.65) |
| Primary diagnosis, n (%) | ||
| Metabolic/Electrolyte disorder | 152 (33.7%) | 208 (36.3%) |
| Cardiovascular disease | 86 (19.1%) | 86 (15.0%) |
| Sepsis | 47 (10.4%) | 82 (14.3%) |
| Neurological disease | 77 (17.1%) | 49 (8.6%) |
| Surgical disease | 12 (2.7%) | 12 (2.1%) |
| Others | 77 (17.1%) | 136 (23.7%) |
| Coexisting conditions, n (%) | ||
| CHF | 63 (14.0%) | 126 (22.0%) |
| CKD | 24 (5.3%) | 75 (13.1%) |
| COPD | 67 (14.9%) | 112 (19.5%) |
| Diabetes | 52 (11.5%) | 65 (11.3%) |
| Liver failure | 13 (2.9%) | 26 (4.5%) |
| Neurological diseases | 127 (28.2%) | 117 (20.4%) |
| Stroke | 30 (6.7%) | 44 (7.7%) |
Categorical variables are expressed as the number (%) and continuous variables are presented as the mean ± standard deviation. ALT, alanine aminotransferase; APACHE, Acute Physiology and Chronic Health Evaluation; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CHF, congestive heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; GCS, Glasgow Coma Scale; ICU, intensive care unit; SD, standard deviation
Statistical Analyses
Continuous variables were presented as means ± standard deviations and categorical variables were described as numbers (%). Crude outcomes were compared using Student’s t-tests for continuous variables and chi-square tests for categorical variables. Missing data were addressed by 100 times multiple imputation using the MICE package [20]. For the main analysis, we adjusted for confounders using inverse probability (IP) weighting. As sensitivity analyses, we also used g-formulas and outcome regression models adjusting for deciles of the propensity scores (PS).
IP weighting
We calculated the probability of receiving exposure (i.e. PS) by multivariable logistic regression using all covariates from Table 1 and product terms of baseline sodium and past medical histories. IP weights were constructed by 1/probability of receiving exposure in the exposure group and 1/probability of not receiving exposure in the control group. Then we estimated the marginal absolute difference by IP weighted regression (IP weighting of marginal structural model) for each outcome.
g-formula
For the binary outcomes of in-hospital mortality and neurological complications, we conducted multivariable logistic regressions adjusting for age, GCS, baseline sodium, potassium, blood urea nitrogen (BUN), albumin, congestive heart failure (CHF), prior neurological disease, ventilation status, and APACHE IV score. Then, we estimated the marginal absolute difference by standardization using the empirical distribution of the confounders for each outcome. More specifically, we first set the exposure level of all individuals to “exposure” and estimated probabilities of having outcomes in the population using the logistic regression models. Next, we set the exposure level of all individuals to “control” and estimated probabilities of having outcomes using the same models. Finally, we compared these probabilities and estimated marginal absolute differences. For the continuous outcomes of hospital-free days and ICU-free days, we conducted multivariable linear regressions adjusting for age, sex, ethnicity, GCS, baseline sodium, potassium, magnesium, phosphate, bicarbonate, BUN, albumin, total bilirubin, AST, glucose, primary diagnosis, past medical histories, prior use of diuretics, unit types of ICU, ventilation status, APACHE IV score, and product terms of baseline sodium and past medical histories. Then, we estimated the marginal absolute difference using the linear regression model for each outcome by the same methods described above.
PS adjustment
For the outcome regression models, we used the same estimated PS as the IP weighting. We then created the deciles of the estimated PS and conducted the regression model adjusting for the deciles of the estimated PS to obtain the conditional absolute difference for each outcome.
We iterated these analyses in 1,000 bootstrap samples and calculated means and variances of absolute differences in each imputed dataset [21]. Then we pooled parameter estimates across the 100 imputed datasets using Rubin’s rule and estimated overall absolute differences and 95% confidence intervals (CIs) [22].
Exploratory Analyses
We investigated the associations between the serum [Na+] correction rate and outcomes using generalized additive models adjusting for age and sex. Smoothing parameters were estimated using generalized cross validation for each outcome. The predicted cubic spline curves were described with their 95% CIs for 48-year-old females, which were the mean age and the reference category of sex in the population.
We conducted another exploratory analysis in which correction rates were trichotomized to slow (≤ 8 mEq/L/day), intermediate (8–12 mEq/L/day), and rapid (> 12 mEq/L/day) correction. This was not a pre-specified analysis and was performed after the results of the primary analysis were generated. We adjusted for clinically plausible confounders by multivariable outcome regression models. The slow correction group was set as a reference and point estimate and confidence interval of absolute difference were estimated by 1000 times bootstrap after multiple imputation.
Finally, we conducted subgroup analyses to investigate whether the effects of rapid correction on outcomes were different between high risk of ODS and low to moderate risk of ODS. Previously published expert panel recommendations identified patients with serum sodium concentration <105 mmol/L, hypokalemia, alcoholism, malnutrition, or advanced liver disease as high risk of ODS [8]. We defined patients with baseline serum sodium ≤ 105 mEq/L, baseline serum potassium ≤ 3.5 mEq/L, or past medical history of liver failure as high risk since past history of alcoholism and malnutrition were not available in the dataset. As the sample size of the subgroups was limited, we removed baseline sodium, baseline potassium, past medical history, primary diagnosis, and unit type from the covariates of the model to estimate PS to reduce model instability and used IP weighting of marginal structural models to estimate the effects. These exploratory analyses were also not included in the initial analysis plan but were conducted to investigate the difference in effects across subgroups.
All reported p values were 2-sided, and p values <0.05 were considered to be statistically significant. The analysis was carried out using R software packages (version 3.5.1; R Foundation for Statistical Computing, Vienna, Austria).
Results
Study Cohort Characteristics
Of the 1,832 patients who had serum [Na+] level ≤120 mEq/L during their ICU admission, a total of 1,024 patients were included in the analysis (Figure 1). This represents 0.7% of patients in the eICU-CRD. Among them, 451 patients underwent rapid correction (exposure group) and 573 patients underwent slow correction (control group). The distribution of correction rates is represented in Figure 2(a). The rapid correction group was younger than the slow correction group and tended to have higher albumin and lower GCS, BUN, potassium, baseline sodium, and total bilirubin values. A higher proportion of patients in the rapid correction group required mechanical ventilation. Patients in the rapid correction group had higher proportions of cardiovascular disorders and neurological diseases and lower proportions of sepsis and past medical history of CHF and chronic kidney disease compared to those in the slow correction group. There was also a significant difference in the unit type of ICU between the two groups. Otherwise, baseline characteristics were similar (Table 1). None of the patients had the specific ICD-9 code for ODS (341.8).
Figure 1.
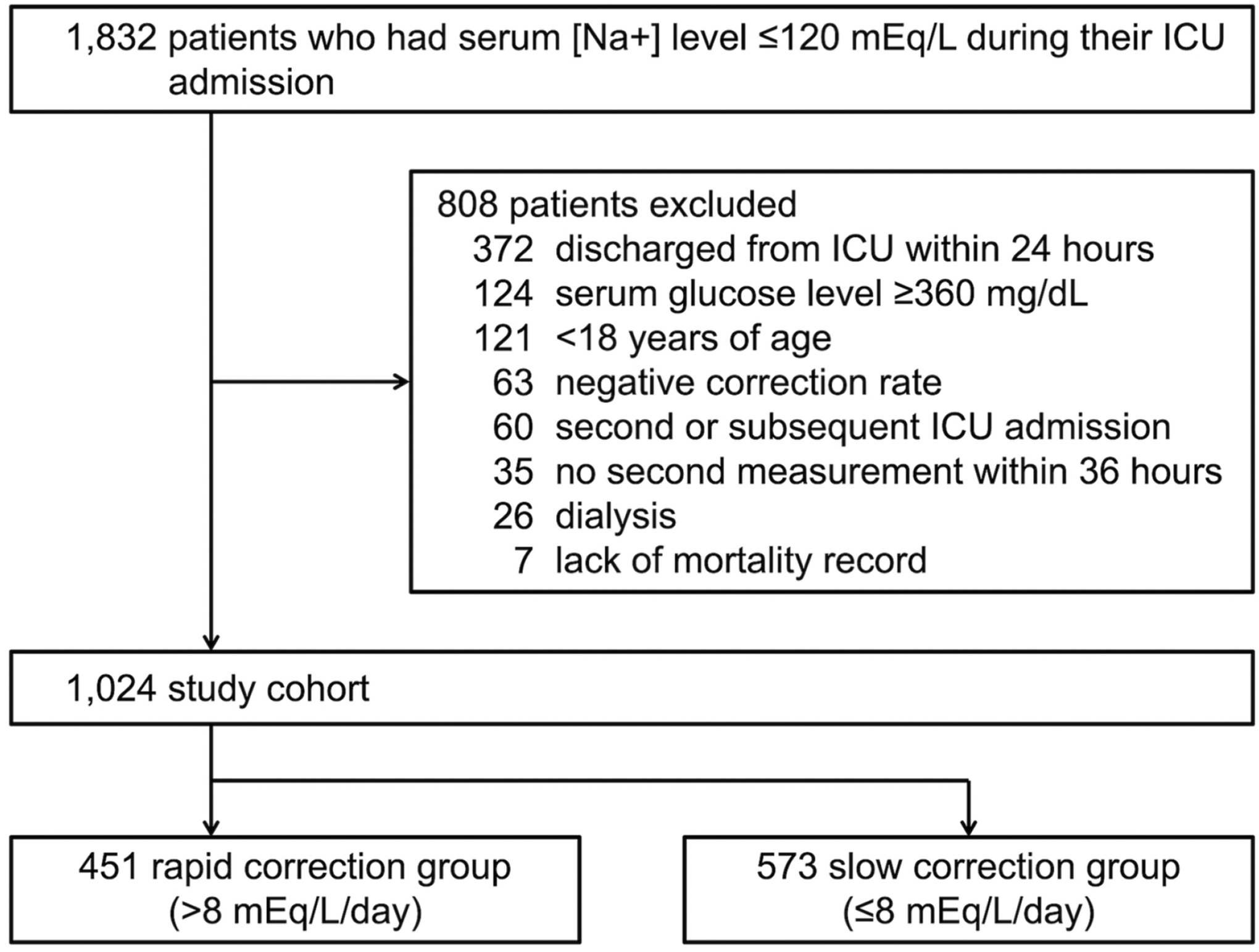
Patient flow diagram. ICU, intensive care unit
Figure 2.
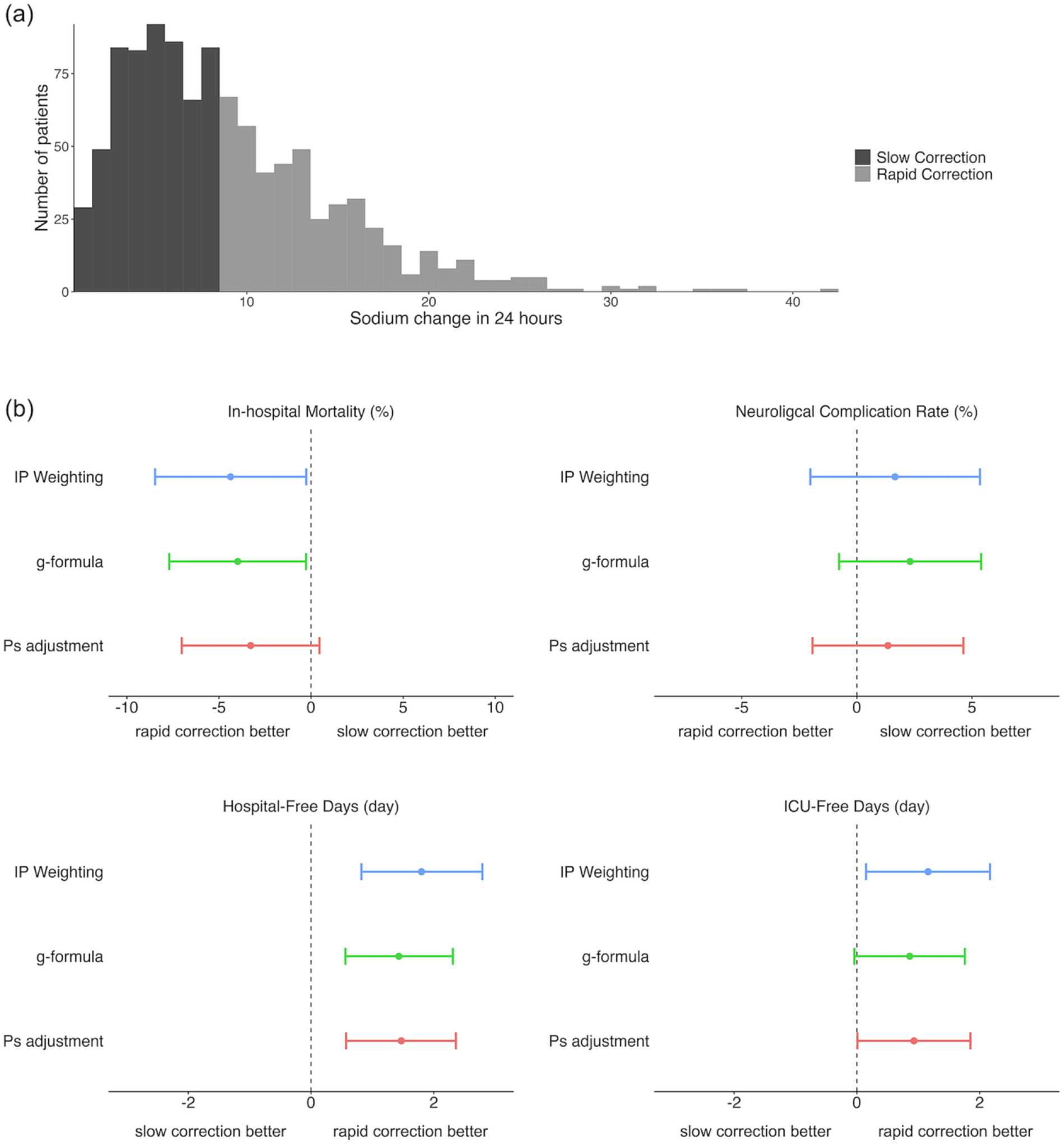
Distribution of serum [Na+] correction rates and association with outcomes of interest applying three models. a) Histogram showing the distribution of sodium corrections rates over 24 hours among the two treatment groups. b) Absolute differences and 95% confidence intervals for in-hospital mortality, neurological complications, hospital-free days, and ICU-free days by treatment group using three models. IP weighting shows the estimated absolute differences and 95% CIs using the IP weighting of marginal structural model. g-formula shows the estimated absolute differences and 95% CIs using the g-formula. PS adjustment shows the estimated absolute differences and 95% CIs using the outcome regression model adjusting for deciles of the PS. CI, confidence interval; ICU, intensive care unit; IP, inverse probability; PS, propensity score
Crude and Adjusted Analyses
Table 2 shows the results of the crude and adjusted analyses. For the crude analysis, rapid correction was associated with lower in-hospital mortality, higher neurological complications, and a greater number of hospital-free days compared to slow correction. ICU-free days were similar between the groups.
Table 2.
Crude and Adjusted Analysis of Correction Rate on Outcomes of Interest
| Outcome | Rapid correction (>8mEq/L/day) (n = 451) | Slow correction (≤8mEq/L/day) (n = 573) | P-value | Adjusted absolute difference | 95% CI |
|---|---|---|---|---|---|
| In-hospital mortality, n (%) | 38 (8.4%) | 77 (13.4%) | 0.015 | −4.37 | −8.47 to 0.26 |
| Neurological complications at discharge, n (%) | 111 (24.6%) | 87 (15.2%) | <0.001 | 1.66 | −2.02 to 5.35 |
| Hospital-free days, mean (SD) | 18.88 (6.14) | 17.90 (6.37) | 0.013 | 1.80 | 0.82 to 2.79 |
| ICU-free days, mean (SD) | 22.82 (4.40) | 22.73 (4.45) | 0.765 | 1.16 | 0.15 to 2.17 |
Crude analysis with categorical variables expressed as the number (%) and continuous variables presented as the mean ± standard deviation. Student’s t-tests were used for continuous variables and chi–square tests were conducted for categorical variables. Adjusted analysis using IP weighting of marginal structural model with absolute difference presented as the number (%) for categorical variables and as number of days for the continuous variables. CI, confidence interval; ICU, intensive care unit; SD, standard deviation
After adjusting for confounders by the IP weighting of marginal structural model, in-hospital mortality (absolute difference, −4.37%; 95% CI, −8.47 to −0.26%) remained significantly lower in the rapid correction group (Table 2, Figure 2(b)). Hospital-free days (1.80 days; 95% CI, 0.82 to 2.79 days) and ICU-free days (1.16 days; 95% CI, 0.15 to 2.17 days) were significantly greater in the rapid correction group. However, sensitivity analyses using g-formula for in-hospital mortality (−3.28%; 95% CI, −7.02 to 0.46%) and PS adjustment for ICU-free days (0.86 days; 95% CI, −0.04 to 1.76 days) showed no significant associations (Figure 2(b)). There were no significant differences in neurological complications (1.66%; 95% CI, −2.02 to 5.35%) between the two groups. The results for hospital-free days and neurological complications were robust across the sensitivity analyses using the g-formula and the outcome regression model adjusting for deciles of the PS.
Modeling Optimal Sodium Correction Rate
Cubic spline curves describing the changes in predicted outcomes over the correction rates adjusting for age and sex using the generalized additive models are shown in Figure 3. We found an inverse U-shaped relationship between the serum [Na+] correction rate and hospital-free days, with the greatest number of hospital-free days observed between 10mEq/L/day and 20mEq/L/day. The cubic spline curve for neurological complications rises linearly with increasing correction rates. Cubic spline curve associations between the serum [Na+] correction rate and mortality and ICU-free days did not provide significant insight regarding an optimal correction rate for these outcomes.
Figure 3.
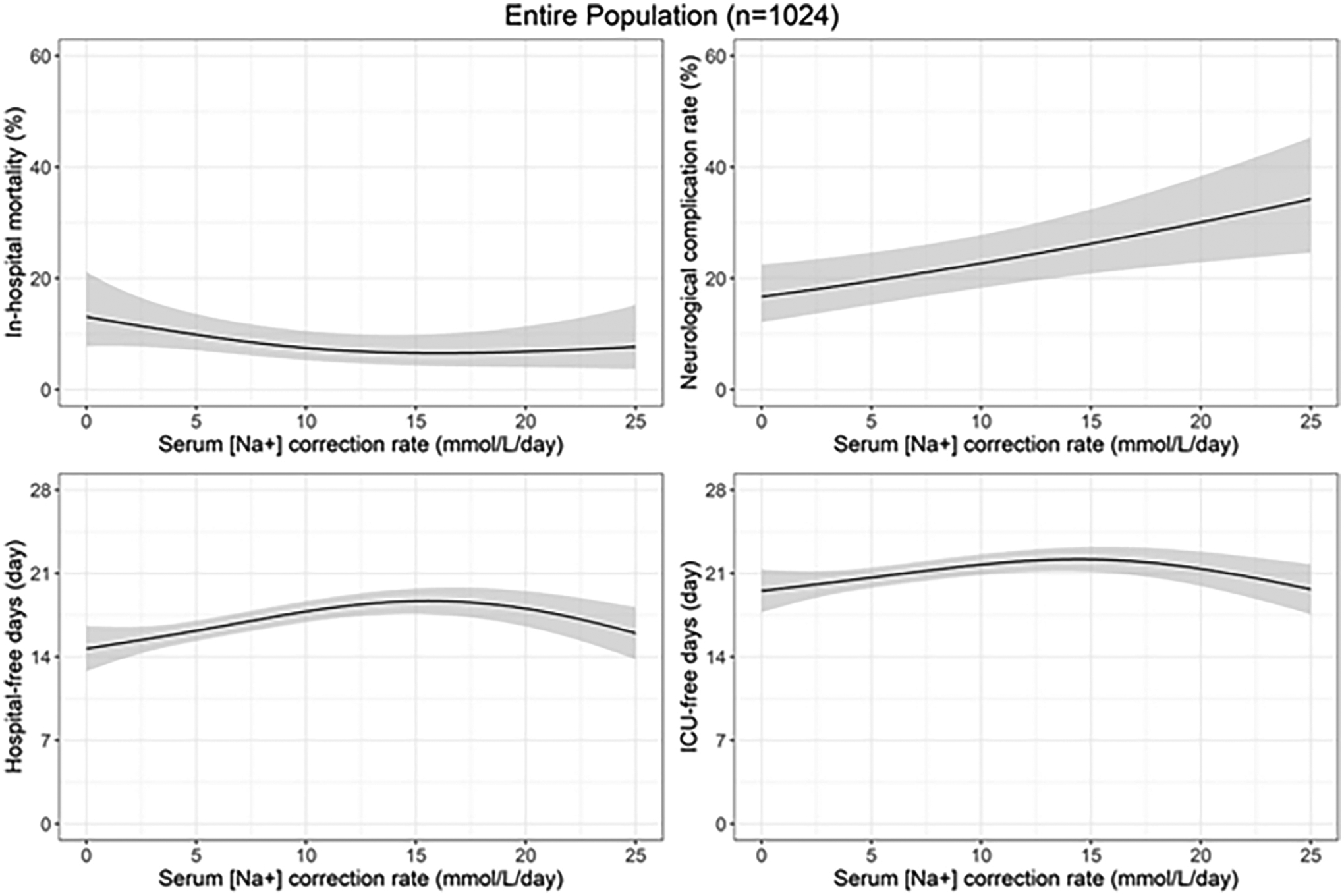
Predicted cubic spline curves using generalized additive models with their 95% CIs describing the changes in predicted outcomes over the correction rates adjusting for age and sex are shown. Age and sex were fixed to 48-year-old females as these were mean age and the reference category of sex in the population. CI, confidence interval; ICU, intensive care unit
Analysis of Trichotomous Rates of Correction
For this secondary analysis, 242 patients underwent correction at > 12 mEq/L/day, 209 underwent correction at 9 to 12 mEq/L/day, and 573 underwent correction at ≤ 8 mEq/L/day. Hospital-free days were significantly greater in the intermediate correction group (1.26 days; 95% CI, 0.19 to 2.34 days) and the rapid correction group (1.25 days; 95% CI, 0.16 to 2.33 days) when compared to the slow correction group (Table 3). There was no significant difference between the 3 groups for other outcomes in the multivariable regression model.
Table 3.
Associations Between Three Categories of Correction Rate and Outcomes
| Outcome | Exposure | Crude analysis | Adjusted absolute difference (95% CI) |
|---|---|---|---|
| In-hospital mortality | Slow correction (≤ 8 mEq/L/day), n=573 | 77 (13.44%) | Reference |
| Intermediate correction (9 to 12 mEq/L/day), n=209 | 18 (8.61%) | −3.05% (−7.63 to 1.53%) | |
| Rapid correction (> 12 mEq/L/day), n=242 | 20 (8.26%) | −2.20% (−6.67 to 2.26%) | |
| Neurological complications at discharge | Slow correction (≤ 8 mEq/L/day), n=573 | 87 (15.18%) | Reference |
| Intermediate correction (9 to 12 mEq/L/day), n=209 | 51 (24.40%) | 2.66% (−1.62 to 6.93%) | |
| Rapid correction (> 12 mEq/L/day), n=242 | 60 (24.79%) | 1.47% (−2.09 to 5.02%) | |
| Hospital-free days | Slow correction (≤ 8 mEq/L/day), n=573 | 20.01 ± 8.65 | Reference |
| Intermediate correction (9 to 12 mEq/L/day), n=209 | 21.08 ± 7.55 | 1.26 days (0.19 to 2.34 days) | |
| Rapid correction (> 12 mEq/L/day), n=242 | 20.99 ± 7.52 | 1.25 days (0.16 to 2.33 days) | |
| ICU-free days | Slow correction (≤ 8 mEq/L/day), n=573 | 15.59 ± 8.35 | Reference |
| Intermediate correction (9 to 12 mEq/L/day), n=209 | 17.33 ± 7.53 | 0.80 days (−0.30 to 1.91 days) | |
| Rapid correction (> 12 mEq/L/day), n=242 | 17.49 ± 7.93 | 0.61 days (−0.49 to 1.72 days) |
Associations between trichotomized exposures and outcomes are shown. Categorical variables are expressed as the number (%) and continuous variables are presented as the mean ± standard deviation in crude analysis. We adjusted for clinically plausible confounders by multivariable outcome regression models setting slow correction group as a reference. Point estimate and confidence interval of adjusted absolute difference were estimated by 1000 times bootstrap after multiple imputation. CI, confidence interval; ICU, intensive care unit
Analysis of Different ODS Risk Subgroups
Among 1,024 patients, 376 patients had one or more risk factors for ODS (baseline serum sodium ≤ 105 mEq/L, baseline serum potassium ≤ 3.5 mEq/L, or past medical history of liver failure) and 648 patients did not have any of these risk factors. Although the risk of neurological complications was generally higher in the high-risk group compared to the low to moderate-risk group, there was no significant difference in the effects of correction rates on neurological outcomes across the two subgroups (Table 4).
Table 4.
Difference in Effects of Rapid Correction on Outcomes in ODS High Risk and Low to Moderate Risk Population
| Outcome | ODS Risk | Crude Analysis | Adjusted Absolute Difference (95% CI) | |
|---|---|---|---|---|
| Rapid Correction | Slow Correction | |||
| In-hospital mortality | High (n=376) | 10/198 (5.05%) | 23/178 (12.92%) | −5.35% (−15.87 to 5.17%) |
| Low to moderate (n=648) | 28/253 (11.07%) | 54/395 (13.67%) | −2.37% (−7.97 to 3.22%) | |
| Neurological complications at discharge | High (n=376) | 57/198 (28.79%) | 32/178 (17.98%) | −0.57% (−10.05 to 8.91%) |
| Low to moderate (n=648) | 54/253 (21.34%) | 55/395 (13.92%) | 2.82% (−1.12 to 6.83%) | |
| Hospital-free days | High (n=376) | 18.45 ± 7.11 | 16.53 ± 8.23 | 1.00 days (−1.24 to 3.24 days) |
| Low to moderate (n=648) | 16.60 ± 8.12 | 15.17 ± 8.37 | 1.21 days (−0.14 to 2.55 days) | |
| ICU-free days | High (n=376) | 22.07 ± 6.36 | 20.38 ± 8.49 | 0.79 days (−1.76 to 3.33 days) |
| Low to moderate (n=648) | 20.22 ± 8.25 | 19.84 ± 8.73 | 0.43 days (−1.00 to 1.85 days) | |
Associations between exposures and outcomes in each subgroup are shown. Categorical variables are expressed as the number (%) and continuous variables are presented as the mean ± standard deviation in crude analysis. We adjusted for clinically plausible confounders using inverse probability weighting of marginal structural models. Point estimate and confidence interval of adjusted absolute difference were estimated by 1000 times bootstrap after multiple imputation. CI, confidence interval; ICU, intensive care unit; ODS, Osmotic Demyelination Syndrome
Discussion
In this study, 0.7% of patients admitted to ICUs in the eICU-CRD had hyponatremia with serum [Na+] ≤ 120 mEq/L during their ICU stay, a rate similar to that seen in other large studies of hyponatremia [3, 23]. More rapid correction was associated with increased hospital-free days after adjusting for confounders in both the primary and secondary analyses. There was also a statistically significant reduced mortality risk and greater ICU-free days, although this significance was lost in the g-formula and PS analyses for each outcome, respectively. Rapid correction was not associated with a significant difference in neurological complications. We also found in exploratory analyses that rapid correction was not significantly associated with neurological complications regardless of the baseline risk of ODS.
Overall, these findings may suggest that correcting serum [Na+] more rapidly than 8 mEq/L/day does not lead to worse outcomes among ICU patients with serum [Na+] ≤ 120 mEq/L, and there is a potential benefit in reducing time in the hospital and ICU, as well as lowering mortality risk. This is similar to findings in smaller studies that showed more rapid serum [Na+] correction was associated with shorter hospital length of stay and lower mortality among emergency department patients [16], and lower mortality among ICU patients [17].
The greater number of hospital-free days in our study and reduced length of stay noted in the prior studies may be a result of patients achieving normal sodium values more quickly and thus are able to leave the hospital sooner. This has the theoretical benefit of decreasing iatrogenic complications for patients, leading to reduced morbidity and mortality, as well as hospital costs [24–26]. The mortality benefit seen in this study should be interpreted cautiously as one of the secondary analyses did not show statistical significance. This association needs further evaluation with future studies given the potential impact on patient outcomes.
These findings also suggest that the risks of overcorrection may be overstated. While ODS is a complication that can arise with too rapid correction of hyponatremia, it was not identified in the diagnosis codes for any of the 1,024 patients in our study with correction rates that were frequently more than double the recommended maximum rate. Our finding was consistent with previous research suggesting that ODS is an extremely rare condition [6, 7, 9]. However, as our search for ODS cases was performed only by ICD-9 review without any additional chart review, there was a risk of missing cases as ICD-9 codes may not always be documented for this diagnosis.
We performed an exploratory analysis trichotomizing the exposure groups to better understand which patients were driving the improved hospital-free days in the rapid correction group, and to see if either subgroup (8–12 mEq/L/day vs >12 mEq/L/day) would potentially have worse outcomes (Table 3). Our particular focus was on the outcomes of the group with correction rates of >12 mEq/L/day, given that this is the maximum recommended 24-hour correction rate in many reviews and guidelines [7, 8, 18, 27, 28], despite the divergent opinion of a recent review suggesting that a rate of 0.75 mEq/L/hour (18 mEq/L/day) is not associated with poor outcomes [29]. The group which corrected at >12 mEq/L/day showed greater hospital free days compared to < 8 mEq/L/day but no difference in other outcomes. Overall, the analysis of trichotomized data does not definitively suggest either benefit or harm associated with more rapid correction rates at over 12 mEq/L/day. We also explored outcomes related to baseline risk of ODS (defined as any of the following: serum [Na+] ≤ 105 mEq/L, baseline serum potassium ≤ 3.5 mEq/L, or past medical history of liver failure), and found no significant difference in any of the outcomes based on this analysis. While this analysis was limited by the small number of patients and lack of records for past history of alcoholism and malnutrition, it does not provide evidence for using different correction rates for this subgroup of patients.
Additional exploratory analyses using generalized additive models represented in Figure 3 provided several additional insights. First, we observed an inverse U-shaped pattern for rate of correction and hospital-free days, with most benefit seen with correction rates between 10 and 20 mEq/L/day. Second, the spline curve for neurological complications has a linear pattern which suggests a possible trend towards increased neurological complications with increasing correction rates. However, when adjusted for multiple confounders in our primary analysis, that association was not observed. These results should be interpreted carefully as we could only adjust for age and sex due to methodological limitations for handling missing values in generalized additive models. As shown in Figure 2(a), the number of patients who had extremely high correction rates (e.g., > 18mEq/L/day) was limited. This led to broader confidence intervals of the effects of correction rates on outcomes in this population (Figure 3). Although we believe that the extremely high correction rates for potentially chronic hyponatremia could be harmful, we could not draw a robust conclusion from our data.
This study has several limitations. We could not differentiate acute versus chronic hyponatremia and determine the precise etiology of the hyponatremia due to lack of reliable urinary [Na+], potassium, and osmolality measurements. The retrospective and observational nature of the study design leaves it subject to bias and residual confounding by indication. Although we conducted IP weighting, g-formula, and outcome regression model adjusting for deciles of the PS, we could not adjust for unmeasured confounders such as acuity of the onset of the hyponatremia and its underlying cause. Additionally, we did not explore whether different techniques of serum [Na+] correction such as hypertonic fluid therapy, desmopressin clamps, or vasopressin receptor antagonists resulted in different outcomes, as these treatment modalities were not documented consistently in the database [30]. The identification of ODS was solely relied on the ICD-9 code, without individual chart to ascertain the outcome. We were unable to obtain information on the severity and causes of neurological complications. The absence of data on correction rates beyond the first 24 hours in the ICU, which is likely to affect outcomes, was another limitation. For our high ODS risk population, we did not have data available for alcoholism or malnutrition (which have been correlated with risk of ODS [8]), so we could not include these risk factors when defining this population.
We cannot make any definitive recommendations on the best correction rate, as our primary model only looked at a dichotomous correction rate, and our trichotomized secondary analysis found no difference between groups and was subject to higher risk of type II error. The cubic spline curves, while suggesting a possible “best” correction rate, had suboptimal adjustment for confounding and large confidence intervals, and therefore no definitive conclusions can be made from these analyses. We had missing data for a number of individuals, and although we made efforts to estimate the unbiased parameters using multiple imputation under the assumption that missingness was conditional on other variables, these imputations may be imperfect. Finally, the prevalence of neurological complications was based on ICD-9 coding without manual chart review. Given the relatively high rates of neurological complications seen in all groups, there was likely over-reporting of neurological complications unrelated to hyponatremia correction.
Conclusions
Among patients admitted to the ICU with severe hyponatremia, a more rapid correction rate of >8 mEq/L over the first 24 hours was not associated with any worse outcomes than lower correction rates. There were more hospital-free days per month, lower mortality risk, and more ICU-free days without a significant difference in neurological complications when compared to a slower correction rate. A maximum correction rate of 8 mEq/L/day may be too conservative, and further investigation with prospective studies should be considered to better understand the benefits and risks of more rapid correction rates in severe hyponatremia.
Highlights.
Retrospective analysis of correction rates for severe hyponatremia in the ICU.
Compared correction rates of >8 mEq/L/day to ≤ 8 mEq/L/day.
Higher rates associated with lower mortality and more hospital and ICU-free days.
No difference in neurological complications based on correction rate.
Acknowledgments
We would like to thank the Massachusetts Institute of Technology Critical Data and its course, “Health Sciences and Technology Course 953: Collaborative Data Science in Medicine,” for allowing this collaboration of authors and for teaching the skills needed to carry out this study. We would like to acknowledge the team behind PhysioNet and eICU-CRD for the availability of the data, and multiple data extraction guidelines.
Funding
This work was supported by the National Institute of Health through NIBIB R01 EB017205. The funding source was not involved in study design, the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the article for publication.
Declarations of Interest
LAC is funded by the National Institute of Health through NIBIB R01 EB017205. The work of NHR is supported by T32-DK007199 and the Doris Duke Charitable Foundation Physician Scientist Fellowship Award Grant # 202182. The work of TK was supported by a Fulbright Japan Graduate Study Program. EM, QX, RS have no declarations of interest.
Abbreviations
- APACHE
Acute Physiology and Chronic Health Evaluation
- BUN
blood urea nitrogen
- CHF
congestive heart failure
- CI
confidence interval
- CRD
Collaborative Research Database
- GCS
Glasgow Coma Scale
- ICD-9
the International Classification of Diseases Ninth Revision
- ICU
intensive care unit
- IP
inverse probability
- Na+
sodium
- ODS
osmotic demyelination syndrome
- PS
propensity score
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
TK: conceptualization, data curation, formal analysis, methodology, supervision, writing - original draft, writing - review & editing manuscript
EM: conceptualization, methodology, supervision, writing - original draft, writing - review & editing manuscript
QX: data curation, formal analysis, methodology
RS: conceptualization, methodology, writing- review & editing manuscript
NHR: conceptualization, methodology, supervision, writing- review & editing manuscript
LAC: conceptualization, methodology, supervision, funding acquisition, writing- review & editing manuscript
Data Availability Statement
All of the code used for data management and analyses is openly shared online for review and re-use. All iterations of the study design are archived with version control. https://github.com/raphaelsherak/Hyponatremia_eicu/blob/master/Hypo_analysis_0629.Rmd
References
- 1.Adrogué HJ, Madias NE. Hyponatremia. N Engl J Med. 2000. May 25;342(21):1581–9. DOI: 10.1056/NEJM200005253422107 [DOI] [PubMed] [Google Scholar]
- 2.Bennani SL, Abouqal R, Zeggwagh AA, Madani N, Abidi K, Zekraoui A, et al. Incidence, causes and prognostic factors of hyponatremia in intensive care. Rev Med Interne. 2003. Apr;24(4):224–9. DOI: 10.1016/s0248-8663(02)00811-1 [DOI] [PubMed] [Google Scholar]
- 3.Funk GC, Lindner G, Druml W, Metnitz B, Schwarz C, Bauer P, et al. Incidence and prognosis of dysnatremias present on ICU admission. Intensive Care Med. 2010. Feb 1;36(2):304–11. DOI: 10.1007/s00134-009-1692-0 [DOI] [PubMed] [Google Scholar]
- 4.Sturdik I, Adamcova M, Kollerova J, Koller T, Zelinkova Z, Payer J. Hyponatraemia is an independent predictor of in-hospital mortality. European J Intern Med. 2014. Apr 1;25(4):379–82. DOI: 10.1016/j.ejim.2014.02.002 [DOI] [PubMed] [Google Scholar]
- 5.Corona G, Giuliani C, Verbalis JG, Forti G, Maggi M, Peri A. Hyponatremia improvement is associated with a reduced risk of mortality: evidence from a meta-analysis. PloS One. 2015;10(4). DOI: 10.1371/journal.pone.0124105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.George JC, Zafar W, Bucaloiu ID, Chang AR. Risk factors and outcomes of rapid correction of severe hyponatremia. Clin J Am Soc Nephrol. 2018. Jul 6;13(7):984–92. DOI: 10.2215/CJN.13061117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sterns RH, Riggs JE, Schochet SS Jr. Osmotic demyelination syndrome following correction of hyponatremia. N Engl J Med. 1986. Jun 12;314(24):1535–42. DOI: 10.1056/NEJM198606123142402 [DOI] [PubMed] [Google Scholar]
- 8.Verbalis JG, Goldsmith SR, Greenberg A, Korzelius C, Schrier RW, Sterns RH, et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med. 2013. Oct 1;126(10):S1–42. DOI: 10.1016/j.amjmed.2013.07.006 [DOI] [PubMed] [Google Scholar]
- 9.Sterns RH. Severe symptomatic hyponatremia: treatment and outcome: a study of 64 cases. Ann Intern Med. 1987. Nov 1;107(5):656–64. DOI: 10.7326/0003-4819-107-5-656 [DOI] [PubMed] [Google Scholar]
- 10.Sterns RH, Cappuccio JD, Silver SM, Cohen EP. Neurologic sequelae after treatment of severe hyponatremia: a multicenter perspective. J Am Soc Nephrol. 1994. Feb 1;4(8):1522–30. DOI: 10.1681/ASN.V481522 [DOI] [PubMed] [Google Scholar]
- 11.Brunner JE, Redmond JM, Haggar AM, Kruger DF, Elias SB. Central pontine myelinolysis and pontine lesions after rapid correction of hyponatremia: a prospective magnetic resonance imaging study. Ann Neurol. 1990. Jan;27(1):61–6. DOI: 10.1002/ana.410270110 [DOI] [PubMed] [Google Scholar]
- 12.Vu T, Wong R, Hamblin PS, Zajac J, Grossmann M. Patients presenting with severe hypotonic hyponatremia: etiological factors, assessment, and outcomes. Hosp Pract. 2009. Jan 1;37(1):128–36. DOI: 10.3810/hp.2009.12.266 [DOI] [PubMed] [Google Scholar]
- 13.Pham PM, Pham PA, Pham SV, Pham PT, Pham PT, Pham PC. Correction of hyponatremia and osmotic demyelinating syndrome: have we neglected to think intracellularly? Clin Exp Nephrol. 2015;19(3):489–495. DOI: 10.1007/s10157-014-1021-y [DOI] [PubMed] [Google Scholar]
- 14.Ayus JC, Arief AI. Chronic hyponatremic encephalopathy in postmenopausal women: association of therapies with morbidity and mortality. JAMA. 1999;281(24):2299–2304. DOI: 10.1001/jama.281.24.2299 [DOI] [PubMed] [Google Scholar]
- 15.George JC, Zafar W, Bucaloiu ID, Chang AR. Risk factors and outcomes of rapid correction of severe hyponatremia. Clin J Am Soc Nephrol. 2018;13(7):984–992. DOI: 10.2215/CJN.13061117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giordano M, Ciarambino T, Priore EL, Castellino P, Malatino L, Cataliotti A, et al. Serum sodium correction rate and the outcome in severe hyponatremia. Am J Emerg Med. 2017. Nov 1;35(11):1691–4. DOI: 10.1016/j.ajem.2017.05.050 [DOI] [PubMed] [Google Scholar]
- 17.Darmon M, Pichon M, Schwebel C, Ruckly S, Adrie C, Haouache H, et al. Influence of early dysnatremia correction on survival of critically ill patients. Shock. 2014. May 1;41(5):394–9. DOI: 10.1097/SHK.0000000000000135 [DOI] [PubMed] [Google Scholar]
- 18.Sterns RH, Nigwekar SU, Hix JK. The treatment of hyponatremia. Semin Nephrol. 2009;29:282–99. DOI: 10.1016/j.semnephrol.2009.03.002 [DOI] [PubMed] [Google Scholar]
- 19.Pollard TJ, Johnson AE, Raffa JD, Celi LA, Mark RG, Badawi O. The eICU Collaborative Research Database, a freely available multi-center database for critical care research. Sci Data. 2018. Sep 11;5:180178. DOI: 10.1038/sdata.2018.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buuren SV, Groothuis-Oudshoorn K. mice: Multivariate imputation by chained equations in R. J Stat Softw. 2010:1–68. DOI: 10.18637/jss.v045.i03 [DOI] [Google Scholar]
- 21.Schomaker M, Heumann C. Bootstrap Inference When Using Multiple Imputation. Stat Med. 2018;37(14):2252–2266. DOI: 10.1002/sim.7654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubin DB. Multiple imputation for nonresponse in surveys. John Wiley & Sons; 2004. Jun 9. DOI: 10.1002/9780470316696 [DOI] [Google Scholar]
- 23.Lansink-Hartgring AO, Hessels L, Weigel J, de Smet AMGA, Gommers D, Panday PVN, et al. Long-term changes in dysnatremia incidence in the ICU: a shift from hyponatremia to hypernatremia. Ann Intensive Care. 2016. Dec 1;6(1):22. DOI: 10.1186/s13613-016-0124-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bai AD, Dai C, Srivastava S, Smith CA, Gill SS. Risk factors, costs and complications of delayed hospital discharge from internal medicine wards at a Canadian academic medical centre: retrospective cohort study. BMC Health Serv Res. 2019. Dec 1;19(1):935. DOI: 10.1186/s12913-019-4760-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curtis JR, Cook DJ, Wall RJ, Angus DC, Bion J, Kacmarek R, et al. Intensive care unit quality improvement: A “how-to” guide for the interdisciplinary team. Crit Care Med. 2006. Jan 1;34(1):211–8. DOI: 10.1186/s12913-019-4760-3 [DOI] [PubMed] [Google Scholar]
- 26.To KB, Napolitano LM. Common complications in the critically ill patient. Surg Clin North Am. 2012. Dec 1;92(6):1519–57. DOI: 10.1016/j.suc.2012.08.018 [DOI] [PubMed] [Google Scholar]
- 27.Spasovski G, Vanholder R, Allolio B, Annane D, Ball S, Bichet D, et al. Clinical practice guideline on diagnosis and treatment of hyponatraemia. Intensive Care Med. 2014;40(3):320–31. doi: 10.1007/s00134-014-3210-2 [DOI] [PubMed] [Google Scholar]
- 28.Nagler EV, Vanmassenhove J, van der Veer SN, Nistor I, Van Biesen W, Webster AC, et al. Diagnosis and treatment of hyponatremia: a systematic review of clinical practice guidelines and consensus statements. BMC Med. 2014;12:1. doi: 10.1186/s12916-014-0231-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hofmaenner DA, Singer M. Challenging management dogma where evidence is nonexistent, weak or outdated. Intensive Care Med. 2022;48(5):548–558. doi: 10.1007/s00134-022-06659-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacMillan TE, Cavalcanti RB. Outcomes in severe hyponatremia treated with and without desmopressin. Am J Med. 2018. Mar 1;131(3):317–e1. DOI: 10.1016/j.amjmed.2017.09.048 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All of the code used for data management and analyses is openly shared online for review and re-use. All iterations of the study design are archived with version control. https://github.com/raphaelsherak/Hyponatremia_eicu/blob/master/Hypo_analysis_0629.Rmd


