Abstract
BACKGROUND.
Obesity and loss of muscle mass are emerging as risk factors for dementia, but the role of adiposity infiltrating skeletal muscles is less clear. Skeletal muscle adiposity increases with older age and especially among black women, a segment of the US population who is also at higher risk for dementia.
METHODS.
In 1634 adults (69–79 years, 48% women, 35% black), we obtained thigh intermuscular adipose tissue (IMAT) via computerized tomography at Years 1 and 6, and Mini-Mental State Exam (3MS) at Years 1, 3, 5, 8 and 10. Linear mixed effects models tested the hypothesis that increased IMAT (Year 1 to 6) would be associated with 3MS decline (Year 5 to 10). Models were adjusted for traditional dementia risk factors at Year 1 (3MS, education, APOe4 allele, diabetes, hypertension, and physical activity), with interactions between IMAT change by race or sex. To assess the influence of other muscle and adiposity characteristics, models accounted for change in muscle strength, muscle area, body weight, abdominal subcutaneous and visceral adiposity, and total body fat mass (all measured in Years 1 and 6). Models were also adjusted for cytokines related to adiposity: leptin, adiponectin, and Interleukine-6.
RESULTS.
Thigh IMAT increased by 4.85 cm2 (Year 1 to 6) and 3MS declined by 3.20 points (Year 6 to 10). The association of IMAT increase with 3MS decline was statistically significant: an IMAT increase of 4.85 cm2 corresponded to a 3MS decline of an additional 3.60 points (p<0.0001), indicating a clinically important change. Interactions by race and sex were not significant.
CONCLUSIONS.
Clinicians should be aware that regional adiposity accumulating in the skeletal muscle may be an important, novel risk factor for cognitive decline in black and white participants independent of changes to muscle strength, body composition and traditional dementia risk factors.
Keywords: intermuscular adiposity, cognitive decline, black, dementia
INTRODUCTION
Adiposity, lower muscle mass and strength are each associated with cognitive decline and dementia1–3. However, less is known about the role of adiposity in skeletal muscle tissue. Studies examining the association between muscle measures with cognitive outcomes have primarily focused on muscle mass and strength4–9. Studies exploring the link between adiposity and cognitive outcomes have primarily focused on total adiposity or abdominal visceral or subcutaneous adiposity, and less on skeletal muscle adiposity10–12.
There is an emerging body of evidence supporting a biologically plausible link, via several different pathways, between muscle adiposity and dementia risk. For example, muscle adiposity increases risk of type 2 diabetes13–15 and hypertension14,16, both of which are known risk factors for dementia. Additionally, ectopic adipose tissue leads to higher circulating levels of cytokines, which have detrimental effects on cognitive function17,18. Recently, two studies have shown an association between higher skeletal muscle adiposity and worse cognitive outcomes; one was a cross-sectional study of cognition in Australian older men19, and the other used neuroimaging measures to assess both cross-sectional20 and longitudinal21 associations among middle-aged participants via the UK Biobank. We propose to further this line of research by assessing longitudinal changes in skeletal muscle adiposity and cognitive function in a biracial cohort of older adults in the United States.
Recent meta-analyses indicate that muscle adiposity adds prognostic value to traditional risk factors for many clinical outcomes in older age, including disability, and COVID-19 severity22,23. Whether the predictive role of muscle adiposity extends to cognitive outcomes is not known, but answering this question is of clinical relevance given the rising prevalence of both adiposity and dementia in the US population and worldwide. We and others have shown that skeletal muscle adiposity increases with older age, even among older adults who lose weight24, and is highly prevalent among older adults of African ancestry25. In the United States, black older adults, and especially black women, are more likely to have both higher muscle adiposity and dementia relative to non-Hispanic white adults of similar age and BMI26,27. Studying the role of skeletal muscle adiposity in cognitive decline among men and women with diverse ethnic backgrounds could uncover novel mechanisms underlying dementia and advance our understanding of racial differences. Unlike brain parenchyma, muscle tissue is more readily accessible, and rapid technological advancements allow for measurements of muscle adiposity via tools that are portable, minimally invasive, and feasible for use in clinical settings28.
Herein, we hypothesize that increases in skeletal muscle adiposity predicts faster cognitive decline in older adults independently of traditional risk factors for dementia, including other muscle or adiposity characteristics. To test this hypothesis, we examined a large biracial sample of older adults participating in the Health, Aging and Body Composition Study. We tested potential pathways linking muscle adiposity and cognitive decline by examining the influence of cardiometabolic conditions (hypertension, diabetes), and adipokines. Although race- and sex-related differences have been reported both for muscle adiposity and dementia, it is not known whether the strength of the association between muscle adiposity and cognitive decline would also differ. To address this limitation, we tested the interaction of skeletal muscle adiposity by race and sex, and examined the associations separately for men and women and black and white participants.
METHODS
The Health, Aging and Body Composition (Health ABC) Study enrolled 3,075 men and women aged 70–79 living in Memphis, Tennessee and Pittsburgh, Pennsylvania to study changes in body composition, functional limitation, disability, and mortality.11 Participants were eligible if they were free of difficulty when walking ¼ of a mile or free of difficulty climbing 10 stairs.11 All participants (60% women, 40% African American) had active follow up starting in 1997 – 1998 (Year 1, or baseline of the study) and ending in 2011.11 Health ABC obtained repeated measures of cognition in Years 1, 3, 5, 8 and 10, and an extensive characterization of body composition at Years 1 and 6, including intermuscular adipose tissue (IMAT), visceral and subcutaneous adiposity, total fat mass, and muscle area. The main independent variable was 5-year change in thigh IMAT (Year 6 minus Year 1), and the main dependent variable was 3MS decline (from Year 5 to Year 10) via linear mixed models (detailed methodology for both are below). Thus, for these analyses, we selected those who received a Computed Tomography (CT) scan in both Years 1 and 6, and had completed the Modified Mini-mental exam (3MS) in Year 1 and at least two follow-up time points.
From the initial sample of 3075 participants, 1634 participants were included in the analytical sample after excluding those who did not have a 3MS at both baseline and at least 2 follow-up times (n=716) and who did not have data on change in thigh muscle adiposity from Year 1 to 6 (n=725). Reasons for lack of follow-up data were primarily due to death or disability. Compared with those excluded (n=1441), the participants included in this analysis were younger (73.4 and 73.9 years), with higher baseline 3MS (91.6 and 88.3 points) and were more likely to be white and to have postsecondary education (p<0.001 for all).
Variables of interest.
Cognitive Function
The 3MS is a pencil and paper test of cognitive function with a score range of 0–100, and higher scores reflecting higher performance in orientation, attention, memory, construction, basic language, verbal fluency, and conceptualization domains. The 3MS is a validated cognitive screening tool, and is widely used as a marker of cognitive change and incident dementia in older adult populations29. The 3MS was assessed in Years 1, 3, 5, 8, and 10, with an average total follow-up time of 9.03 years. Change was computed using linear mixed models. The primary outcome was 3MS change from Year 5 to 10 to address potential confounding due to the temporal overlap of measurements in IMAT and 3MS; 3MS change was examined from Year 1 to 10 in secondary analyses. A decline as small as 1.1 points over 5 years of follow-up is considered clinically meaningful30, with a medium effect size and comparable to other scales to predict AD risk31.
Adiposity Measures
Abdomen and thigh CT scans were obtained at Years 1 and 6 at the Pittsburgh site using a 9800 Advantage Scanner (General Electric, Milwaukee, WI) and either a Somatom Plus 4 (Siemens, Erlangen, Germany) or a Picker PQ 2000S (Marconi Medical Systems, Cleveland, OH) scanner at the Memphis site, with high reliability32,33. Briefly, the scans were conducted at 120 kVp, 200 to 250 mA seconds, at a slice thickness of 10 mm. Areas were calculated by multiplying the number of pixels of a given tissue type by the pixel area using ILD development software (RSI Systems, Boulder, CO). Tissue types were identified on the basis of radiographic density (HU), which was calibrated to distilled water (0 HU) and air (−1000 HU). Thus, higher HU indicated more dense tissue, and less adiposity infiltrating the muscle. Adipose tissue areas were defined as voxels between −150 and −30 HU. After distinguishing fat from lean and bone tissues, the cross-sectional areas of the muscle and IMAT of the right thigh were measured at the level of the mid-femur (Figure 1). A single 10-mm axial image of the right thigh was acquired at the midpoint of the distance between the medial edge of the greater trochanter and the intercondyloid fossa. Thigh IMAT was partitioned from subcutaneous adiposity by drawing a line along the deep fascial plane surrounding the thigh muscle. The total area of nonfat and nonbone tissues within the fascial border was used as a quantification of muscle thigh area (cm2).
Figure 1. CT scans of the mid thigh of two study participants.
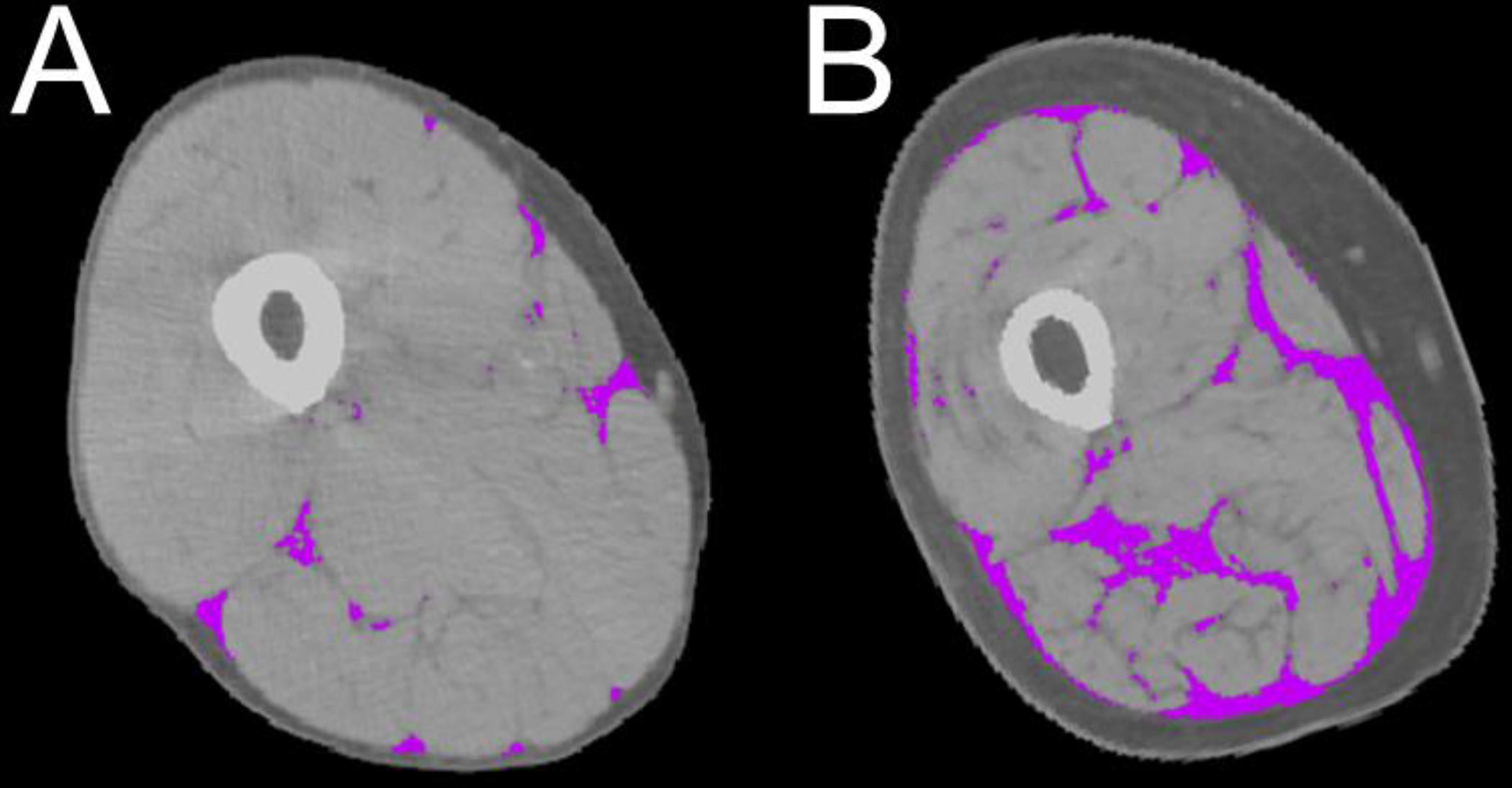
Computer Tomography (CT) scans of the mid thigh of 2 study participants showing intermuscular fat (purple), and subcutaneous fat (darker grey) and muscle parenchyma (lighter gray). Note that, albeit similar in cross-sectional thigh muscle area, adiposity is lower in panel A and higher in panel B.
Abdominal visceral and subcutaneous fat area were measured by CT scans at the L4-L5 level, in Years 1 and 6. Fat areas were calculated by multiplying the number of pixels of a given tissue type by the pixel area using ILD development software (RSI Systems, Boulder, CO). Visceral fat was manually distinguished from subcutaneous fat by tracing along the fascial plane defining the internal abdominal wall. Total body fat mass was measured using fan-beam dual-energy X-ray absorptiometry (model QDR 4.500, software version 8.21; Hologic, Bedford, MA).
Other measures of interest.
Right knee extension muscle strength was measured with an isokinetic dynamometer (Kin-Com dynamometer, 125 AP; Chattanooga, TN) at 60°/s, with start and stop joint angles set at 90° and 30°; the left leg of those with joint replacement or knee pain was tested on the right side. Contraindications to strength testing included: systolic blood pressure greater than or equal to 200 mmHg, diastolic blood pressure greater than or equal to 110 mmHg, history of cerebral aneurysm, cerebral bleeding, bilateral total knee replacement, or severe bilateral knee pain (12.7% of original cohort). The maximum muscle torque (Newton meters) was calculated from the average of three reproducible and acceptable trials from a maximum of six.
Risk factors for cognitive decline.
Information was obtained at Year 1 for self-reported age, race, sex, and for years of school education (grouped into more or less than postsecondary education). In Health ABC, participants were asked to identify their primary racial or ethnic group, choosing from a list of options that included: Asian/Pacific Islander, Black/African-American, White/Caucasian, Latino/Hispanic, “don’t know” or “other” (and specify), and “refused”. Apolipoprotein Allele 4 carrier status (APOe4) was assessed via genotype testing using standard single-nucleotide polymorphism analyses. Presence of diabetes and hypertension were assessed via self-report questionnaires, medications listed, or Health Care Finance Administration diagnosis. Total weekly energy expenditure was calculated as kilocalories per kilogram per week based on self-reported physical activity in the previous 12 months. Body weight and height were measured in patients wearing a hospital gown and without shoes on a calibrated balance beam scale and stadiometer, respectively, and were used to calculate body mass index.
The protocols for cytokine measurement have been previously described34. Participants underwent venipuncture at the baseline visit after an overnight fast, and serum samples were frozen at −70°C. Adiponectin and leptin were measured in duplicate by means of radioimmunoassay (Linco Research Inc, St Charles, Mo), with an intra-assay coefficient of variation of 1.8% to 3.6% for adiponectin and 3.7% to 7.5% for leptin. Interleukin 6 (IL-6) was measured in duplicate using an ultrasensitive enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, Minn). The lower limit of detection was <0.10 pg/mL.
Statistical analyses
Distributions for variables of interest were examined for outliers (>4 standard deviations from the mean) and to determine whether transformation and/or alternate modeling were required.
Linear mixed effect models with random slopes and intercepts were used to model the association of change in thigh IMAT (Year 6- Year 1) with change in 3MS using all available data points from Year 5 through 10 in primary analyses, and from Year 1 through 10 in secondary analyses. The base model independent variables were: baseline 3MS, time, change in IMAT, its interaction with time and change in thigh muscle area. Change in thigh muscle area was added to all models because it can confound the association between adiposity and the outcome. Next, we tested whether the main association was independent of risk factors for cognitive decline: demographics, cardiometabolic conditions (BMI, weight, diabetes, hypertension), sedentary behavior, APOe4, and cytokines associated with adiposity (leptin, adiponectin, IL-6). To limit collinearity between related covariates, models were constructed as follows: we first identified which variables would be associated with 3MS change independently of thigh IMAT change, by examining the coefficients of each variable and its interaction with time in the main model, one at a time. The variables that were significantly associated with 3MS decline in these individual models (e.g., coefficient of the interaction by time p<0.05), entered a final model together. Finally, to assess whether the main association was also independent of change in other muscle or adiposity characteristics, models were adjusted for change (Year 6- Year 1) in muscle strength, subcutaneous adiposity, visceral adiposity, and total fat mass. To avoid potential issues of collinearity, the same approach described above was used.
To compare the statistical association between change in thigh muscle adiposity and change in 3MS between black and white participants, we tested the interaction of race by change in thigh muscle adiposity. The same approach was used to test for sex differences. The main models were repeated separately for black and white participants, and for men and women.
Missing values for follow-up visits were primarily due to death or disability. To address concerns of survivor bias and estimate whether the main association would be dependent on changes in the 3MS slope over time, piecewise regression models were built that accounted for changes in 3MS slope at years 3, 5, and 8.
All analyses were conducted using SAS version 9.4.14
RESULTS
Among the participants included in these analyses, 48% were women and 35% were Black, with an average total follow-up of 9.0±1.8 years (Table 1). Compared with whites, black participants were more likely to: be female, have at least one APOe4 allele, have more comorbidities, received less than postsecondary education, have lower 3MS scores, more thigh IMAT and larger thigh muscle areas at baseline (p<0.05). Compared with whites, black participants also had greater 3MS decline (4.1 (IQR:9.5) vs. 2.5 (IQR:7) points), smaller increases in thigh IMAT, and larger decreases in muscle area and other adiposity measures (p<0.05).
Table 1.
Characteristics of 1634 participants at Year 1
| Mean (standard deviation) or Number (%) | |
|---|---|
| Years follow-up | 9.03 (1.77) |
| Age, years | 73.38 (2.78) |
| Sex, female | 842 (51.53) |
| Race, White | 1070 (65.48) |
| Education, postsecondary | 777 (47.64) |
| APOe4 allele ≥1^ | 421 (27.25) |
| Diabetes, present | 558 (34.15) |
| Hypertension, present | 609 (37.27) |
| Physical Activity, kcal/kg/week | 89.77 (70.34) |
| BMI, kg/m2 | 27.25 (4.56) |
| 3MS, mean points (interquartile range) | 91.60 (9.00) |
APOe4 measured in N = 1545 participants.
In all participants, thigh IMAT increased by 39.0% from Year 1 to Year 6, corresponding to an increase of 4.85 cm2 (0.97 cm2/ year). Over the same period, muscle strength decreased by 14.0% (p<0.05), whereas thigh muscle area remained somewhat stable over time, decreasing by less than 0.5% (Table 2). Decreases in abdominal subcutaneous and visceral adiposity were 3.92% and 6.43%, respectively (p< 0.05, Table 2). From Year 5 to Year 10, 3MS declined by 3.3%, corresponding to a decrease of 3.20 points.
Table 2.
Mean values of muscle and adiposity measures at Year 1 and change over time
| Mean (standard deviation) or Number (%) | Mean difference (standard deviation) and percent difference from year 1 to year 6 | |
|---|---|---|
| Thigh IMAT, cm2 | 19.54 (11.50) | 4.83 (5.81), 39.02% |
| Thigh muscle area, cm2 | 223.83 (55.63) | −9.07 (17.08), −0.04% |
| Thigh muscle strength, nm | 109.81(39.04) | −19.03 (24.21), −15.09% |
| Abdominal adiposity, visceral, cm2 | 143.12(66.85) | −5.66 (40.35), −3.92% |
| Abdominal adiposity, subcutaneous, cm2 | 282.16(115.48) | −19.47 (46.62), −6.43% |
| Weight, kg | 75.51 (14.67) | −1.36 (4.88), −1.69% |
| Total body fat mass, kg | 26.72 (8.37) | −0.08 (3.57) −0.46% |
IMAT: intermuscular adipose tissue.
Older age, less education, and having at least one copy of the APOe4 allele were associated with 3MS decline independently of change in thigh IMAT (Supplemental Table 1). Thus, these variables were included in the model of IMAT change predicting 3MS change. Changes in adiposity measures (abdominal visceral and subcutaneous adiposity, weight, and total fat mass), were also significantly associated with 3MS decline, whereas change in muscle strength, cardiometabolic conditions (diabetes, hypertension) sedentary behavior, or cytokine levels were not (Supplemental Table 1).
There was a significant association between increasing thigh IMAT and declining 3MS, independent of changes in thigh muscle area, muscle strength, and other adiposity measures (Table 3). The association between increasing thigh IMAT with declining 3MS remained statistically significant after adjusting for race, age, education, and APOe4 (coefficient of thigh IMAT* time: 0.0187, p<0.0001). In these models, an increase of 4.83 cm2 in thigh IMAT from Year 1 to 6 corresponded to an additional 3MS decrease of 3.6 points from Year 5 to 10, indicative of clinically meaningful change. Further adjustment for hypertension, diabetes, and cytokines did not substantially modify the results (not shown). Results were similar for 3MS decline from Year 1 to 10 (not shown).
Table 3.
Decline in Modified Minimental Score (from year 5 to year 10) corresponding to an increase in thigh intermuscular adiposity (from year 1 to year 6), before and after adjustment for changes in other adiposity and muscle measures. All p values are <0.001.
| Model 1^ | Model 1+ Change in thigh muscle strength | Model 1+ Change in visceral adiposity | Model 1+ Change in abdominal subcutaneous adiposity | Model 1+ Change in weight | Model 1+ Change in total fat mass | |
|---|---|---|---|---|---|---|
| Mean decline in 3MS points (Standard Deviation) from year 5 to 10 corresponding to the average IMAT increase of 4.83 cm2 from year 1 to 6. | ||||||
| Mean 3MS (SD) | −3.637 (0.207) | −3.582 (0.267) | −3.659 (0.218) | −3.647 (0.224) | −3.635 (0.208) | −3.646 (0.209) |
| Coefficients from linear mixed effect models (SE) of change in IMAT * time predicting change in 3MS | ||||||
| Change IMAT * time | −0.028 (0.007) | −0.027 (0.008) | −0.028 (0.008) | −0.030 (0.008) | −0.027 (0.007) | −0.027 (0.007) |
IMAT: thigh intermuscular adiposity
Includes the following independent variables: change in thigh intermuscular adiposity (year 1 to 6), time, interaction of IMAT change with time, change in thigh intermuscular area (year 1 to 6), and 3MS at year 1.
To visualize the impact of IMAT increase on 3MS decline, the mean 3MS score at each study visit was plotted separately for those with and those without increase in thigh IMAT (change from Year 1 to Year 6 >0 and ≤ 0 cm2, respectively) (Figure 2). Sensitivity analyses with piecewise regression models accounting for changes in 3MS slope yielded similar results.
Figure 2: Minimental score (3MS) by increase in thigh IMAT.
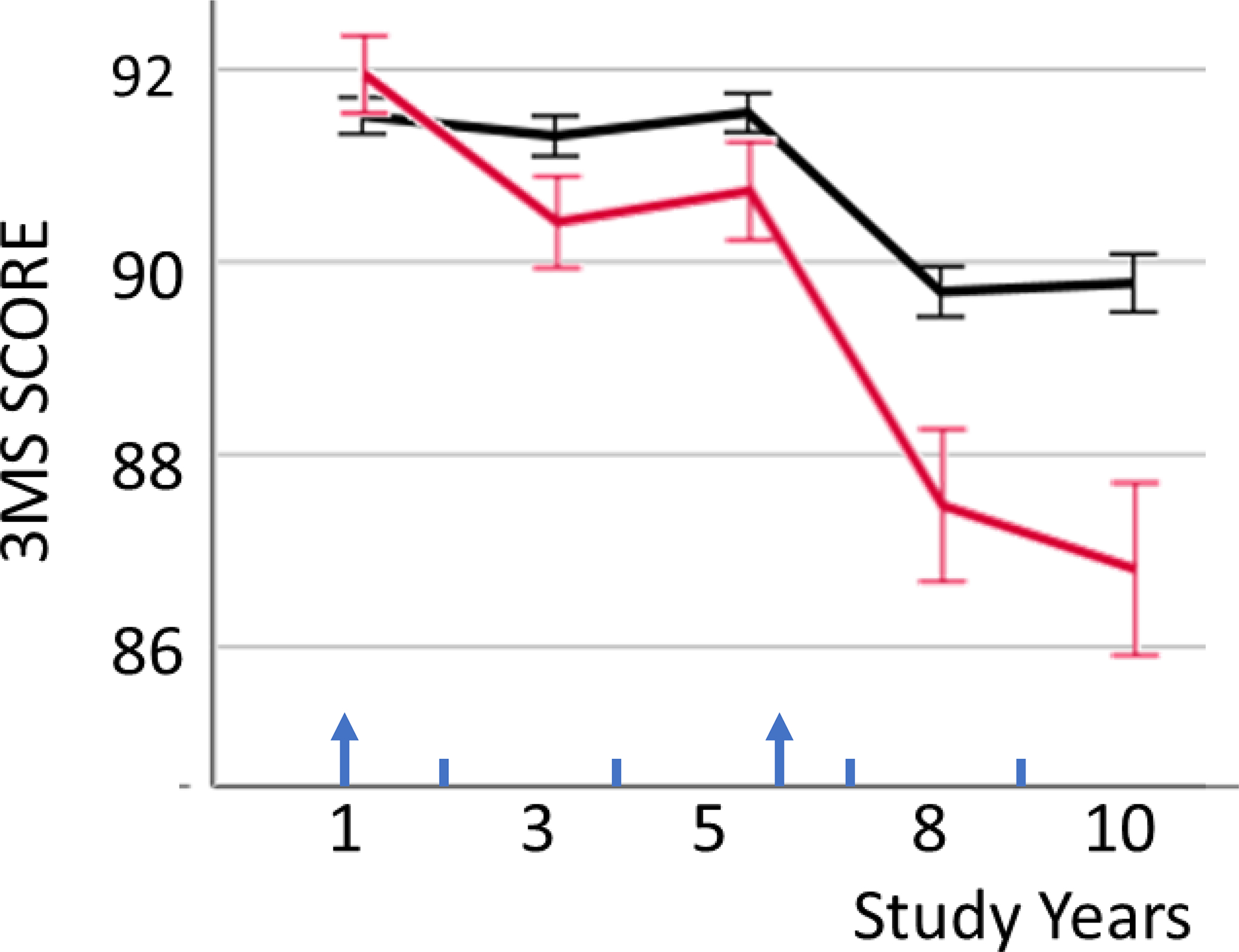
Means (standard errors) of Minimental score (3MS) in study year 1 to 10, plotted separately for those with (red) and those without (black) increase in thigh intermuscular adipose tissue (IMAT) from study year 1 to 6 (red: IMAT change > 0; black: IMAT change ≤0). Arrows indicate the two timepoints of IMAT measurements.
The interaction terms of change in thigh IMAT *time * race and change in thigh IMAT*time * sex predicting 3MS change were not statistically significant (p>0.8). The associations of IMAT increase with 3MS decline were similar in analyses stratified by race or sex (Supplemental Table 2).
DISCUSSION
In this large sample of biracial older adults, we examined longitudinal increase in a marker of skeletal muscle adiposity (as measured by thigh intermuscular adipose tissue) in relation to cognitive decline (as measured by mini-mental state decline) and examined potential explanatory pathways as well as differences by race and gender. We discovered a significant association between increasing skeletal muscle adiposity and cognitive decline, which remained robust after adjusting for known risk factors for cognitive decline, including the presence of the allele APOe4, hypertension, and diabetes. Importantly, associations were also independent of other regional and total adiposity depots, as well as muscle area and strength. Although the size of 3MS decline relative to IMAT increase was relatively small, these differences are clinically meaningful and provide predictive value for incident dementia29–31. Taken together, these findings suggest that increases in skeletal muscle adiposity should be considered among the risk factors for future cognitive decline, with important clinical ramifications.
While muscle adiposity is not yet routinely measured in clinical settings, it is being captured opportunistically on CT scans obtained as part of routine patient care. The utility of these CT measurements have already been validated in many studies of older adults, and could provide clinicians with novel information without additional cost, time, or radiation exposure to patients.23 As rapidly evolving methodologies make measuring skeletal muscle adiposity more feasible and broadly applicable in clinical settings, there will be rich opportunities to test the value of muscle adiposity in prediction models for dementia. Obtaining comprehensive measures of muscle composition in older age is very timely. Both diagnosis and treatment of sarcopenia in older adults are finally transitioning from research settings to becoming part of routine patient care35. Similarly, muscle adiposity could be incorporated into routine assessments and lead to more precise estimates of dementia risk.
These findings contribute to knowledge of the relation between muscle, adiposity, and cognitive outcomes in several ways. Studies on biomarkers for dementia risk underscore the importance of using algorithms that integrate measures from multiple systems and organs to improve dementia prediction. There is a growing recognition that adiposity and loss of muscle mass are risk factors for future dementia risk, each strengthening the algorithms predicting cognitive decline. Our results suggest that muscle adiposity may have a valuable role as a predictor of cognitive decline in addition to (not instead of) other traditional dementia risk factors, with applicability in clinical settings.
To date, only a few studies of cognition have examined adiposity infiltrating skeletal muscles20,21,36, and none examined these associations longitudinally or in a diverse cohort of older adults. Prior studies examining IMAT and cognitive or neuroimaging outcomes have been either cross-sectional20,36 or have only assessed changes over short periods of times21 in ethnically homogenous cohorts. Our findings are novel as they indicate that increasing adiposity accumulation in the skeletal muscle may have unique properties in relation to cognitive decline, for both black and white older adults. Our results also indicate that these effects are unique to muscle adiposity, and distinct from changes in other muscle characteristics (e.g. strength or mass) or other adiposity depots (e.g. visceral or subcutaneous adiposity). This is consistent with prior work showing that the effect of adiposity on health outcomes varies according to the anatomical site and the extent of adipose tissue accumulation37,38,39. The interrelationship of skeletal muscle adiposity with BMI and other ectopic adiposity depots have been previously described as low40, suggesting that there may be something unique to the localization of adiposity within skeletal muscles, and that the clinical consequences of ectopic adiposity may vary by the location of the adipose tissue.
Why would increasing IMAT be associated with declining cognitive function? While our study was not designed to address biological mechanisms or causality, several pathways linking adipose tissue, muscle and brain health exist that are biologically plausible and merit consideration. One possible mechanism may be through adiposity-related detrimental effects on glucose metabolism/ insulin sensitivity41, and cardiometabolic conditions40. Indeed, both diabetes and hypertension are higher in individuals with higher IMAT, both are known dementia risk factors. Additionally, ectopic adipose tissue is a metabolically active tissue, releasing proinflammatory cytokines that have paracrine and endocrine action, and neurodegenerative effects42,43. However, adjusting for hypertension, diabetes, or cytokines did not attenuate the main association in our analyses. Another pathway may be via impaired muscle endocrine properties. With its unique position and close proximity to the muscle fibers, adiposity infiltrating the muscle may impair muscle endocrine properties44,45, altering the homeostasis of circulating muscle-derived cytokines; for example, it may decrease circulating levels of neuroprotective cytokines, such as irisin46,47. Although we adjusted for cytokines that play a critical role in the inflammatory cascade and have well documented links with adiposity, we did not have access to irisin or other muscle-derived cytokines. A comprehensive study of other adipokines and myokines would shed light on potential mechanistic links between increasing muscle adiposity and cognitive decline.
As expected, we found that older age, having at least one APOe4 allele, and having lower levels of school education were significantly associated with cognitive decline, independent of change in thigh IMAT. Contrary to our hypotheses, and in apparent contrast with previously published work, we did not find a significant association between hypertension, diabetes, physical activity, race, or cytokines and cognitive decline. Notably, these models included change in IMAT, whereas prior studies did not. Thus, it is possible that the effect of increasing thigh IMAT on declining 3MS attenuated the impact of these variables on the outcome. It is also possible the shared variance between IMAT, diabetes, hypertension, and sedentary behavior40,48 obscured the independent effects of these conditions on cognitive decline.
We examined race and sex differences in the association between IMAT and cognitive decline for several reasons. First, the prevalence of dementia is higher among blacks relative to non-Hispanic whites, with estimates ranging from 14 to 100%, and even higher for women27. Secondly, skeletal muscle adiposity is higher and increases more with age among black men compared with non-Hispanic Whites, independent of general and central adiposity49,50. Lastly, prior studies did not examine whether racial differences influenced the strength of the association between muscle characteristics and cognition. Consistent with prior studies, black participants in this cohort had more skeletal muscle adiposity and lower 3MS scores as compared with whites. Conversely, we found no evidence for a race-related influence on the association between IMAT increase and cognitive decline, as shown by the results of stratified analyses and interaction terms. It is possible these analyses were not adequately powered to detect differences by race or sex.
This study has limitations. The assessment of cognitive decline relied only on one cognitive test, the 3MS, which captures some, but not all, cognitive domains that change with age. For example, 3MS does not capture processing speed and executive cognitive function. While it would be interesting to examine other cognitive domains in relation to skeletal muscle adiposity, it is unclear whether or why such relationships would vary across different cognitive domains. We have previously shown that incidence of dementia in this cohort is similar to that observed in other cohort studies of similarly aged individuals living in the community51, indicating the 3MS captures a cognitive status that is generalizable to other epidemiological studies of aging. Nonetheless, we cannot exclude that our findings may not apply to other race/ethnic groups and those living outside the United States. Our study also has notable strengths: a longitudinal, biracial design, community-based sampling design, relatively long follow-up period, and extensive measures of regional and global adiposity and putative mediators, including diabetes, hypertension and adipokines.
In conclusion, in a biracial cohort of community dwelling older adults, increasing skeletal muscle adiposity predicted declining cognitive function, independent of traditional risk factors for dementia and other body composition depots. Our results extend prior findings that muscle adiposity adds prognostic value to traditional risk factors for many clinical outcomes22,23. Future studies should assess whether adding muscle adiposity to the assessment of traditional dementia risk factors could improve prediction models of risk of dementia.
Supplementary Material
Supplemental Table 1. Results of linear mixed effect models (regression coefficients) testing the association of each population characteristic at Year 1 (unless otherwise noted) with decline in 3MS from year 5 to year 10.
Supplemental Table 2. Coefficients (p values) from linear mixed effect models testing the associations between change in thigh IMAT (from years 1 to 6) and 3MS decline (from years 5 to 10), stratified by race and gender.
Key Points.
Increasing adiposity in skeletal muscles predicts cognitive decline, independent of overall adiposity or muscle health, and irrespective of demographic factors.
Associations were not modified by diabetes, hypertension, or cytokines.
Why does this matter?
Clinicians should be aware that adiposity distribution is an important risk factor for cognitive decline, distinct from overall adiposity and sarcopenia. While muscle adiposity is not yet routinely measured in clinical settings, it is already being measured opportunistically on clinical CT scans obtained as part of routine patient care. These CT measurements have already been validated in many studies of older adults, thus clinicians could have access to this novel information without additional cost, time, or radiation exposure. As rapidly evolving methodologies are making this measure more feasible and broadly applicable in clinical settings, there are rich opportunities for collecting muscle adiposity and improve prediction models for dementia. Obtaining comprehensive measures of muscle in older age is very timely. Both diagnosis and treatment of sarcopenia in older adults are finally transitioning from research settings to a routine part of patient care. Thus, muscle adiposity could become a component of these routine assessments and provide useful and new information for dementia risk.
Sponsor’s Role
This research was supported by National Institute on Aging (NIA) contracts #N01-AG-6-2101; N01-AG-6-2103; N01-AG-6-2106; NIA grant R01-AG028050; NINR grant R01-NR012459; NIA grant K23AG028966, R01AG037451, R01AG029232). The funders had no role in the design of the study, in the collection, analyses, or interpretation of the data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Conflict of Interest
The authors of this manuscript have no conflicts of interest to report.
REFERENCES
- 1.Sui SX, Williams LJ, Holloway-Kew KL, Hyde NK, Pasco JA. Skeletal Muscle Health and Cognitive Function: A Narrative Review. Int J Mol Sci. 2020;22(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danat IM, Clifford A, Partridge M, et al. Impacts of Overweight and Obesity in Older Age on the Risk of Dementia: A Systematic Literature Review and a Meta-Analysis. Journal of Alzheimer’s disease: JAD. 2019;70(s1):S87–s99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gustafson DR, Luchsinger JA. High adiposity: risk factor for dementia and Alzheimer’s disease? Alzheimer’s research & therapy. 2013;5(6):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nourhashémi F, Andrieu S, Gillette-Guyonnet S, et al. Is there a relationship between fat-free soft tissue mass and low cognitive function? Results from a study of 7,105 women. Journal of the American Geriatrics Society. 2002;50(11):1796–1801. [DOI] [PubMed] [Google Scholar]
- 5.Levine ME, Crimmins EM. Sarcopenic obesity and cognitive functioning: the mediating roles of insulin resistance and inflammation? Current gerontology and geriatrics research. 2012;2012:826398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tolea MI, Chrisphonte S, Galvin JE. Sarcopenic obesity and cognitive performance. Clinical interventions in aging. 2018;13:1111–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee H, Seo HS, Kim REY, Lee SK, Lee YH, Shin C. Obesity and muscle may have synergic effect more than independent effects on brain volume in community-based elderly. European radiology. 2021;31(5):2956–2966. [DOI] [PubMed] [Google Scholar]
- 8.Spauwen PJ, Murphy RA, Jónsson PV, et al. Associations of fat and muscle tissue with cognitive status in older adults: the AGES-Reykjavik Study. Age and ageing. 2017;46(2):250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klinedinst BS, Pappas C, Le S, et al. Aging-related changes in fluid intelligence, muscle and adipose mass, and sex-specific immunologic mediation: A longitudinal UK Biobank study. Brain, behavior, and immunity. 2019;82:396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanaya AM, Lindquist K, Harris TB, et al. Total and regional adiposity and cognitive change in older adults: The Health, Aging and Body Composition (ABC) study. Archives of neurology. 2009;66(3):329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moh MC, Low S, Ng TP, et al. Association of traditional and novel measures of central obesity with cognitive performance in older multi-ethnic Asians with type 2 diabetes. Clinical obesity. 2020;10(2):e12352. [DOI] [PubMed] [Google Scholar]
- 12.Cereda E, Sansone V, Meola G, Malavazos AE. Increased visceral adipose tissue rather than BMI as a risk factor for dementia. Age and ageing. 2007;36(5):488–491. [DOI] [PubMed] [Google Scholar]
- 13.Miljkovic-Gacic I, Gordon CL, Goodpaster BH, et al. Adipose tissue infiltration in skeletal muscle: age patterns and association with diabetes among men of African ancestry. Am J Clin Nutr. 2008;87(6):1590–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miljkovic I, Kuipers AL, Cvejkus R, et al. Myosteatosis increases with aging and is associated with incident diabetes in African ancestry men. Obesity (Silver Spring). 2016;24(2):476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miljkovic I, Kuipers AL, Cvejkus RK, et al. Hepatic and Skeletal Muscle Adiposity Are Associated with Diabetes Independent of Visceral Adiposity in Nonobese African-Caribbean Men. Metab Syndr Relat Disord. 2020;18(6):275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao Q, Zmuda JM, Kuipers AL, et al. Muscle Attenuation Is Associated With Newly Developed Hypertension in Men of African Ancestry. Hypertension. 2017;69(5):957–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raschke S, Eckel J. Adipo-myokines: two sides of the same coin--mediators of inflammation and mediators of exercise. Mediators Inflamm. 2013;2013:320724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishii M, Iadecola C. Adipocyte-derived factors in age-related dementia and their contribution to vascular and Alzheimer pathology. Biochimica et biophysica acta. 2016;1862(5):966–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sui SX, Williams LJ, Holloway-Kew KL, et al. Skeletal Muscle Density and Cognitive Function: A Cross-Sectional Study in Men. Calcified tissue international. 2021;108(2):165–175. [DOI] [PubMed] [Google Scholar]
- 20.Gurholt TP, Kaufmann T, Frei O, et al. Population-based body–brain mapping links brain morphology with anthropometrics and body composition. Translational Psychiatry. 2021;11(1):295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beck D, de Lange A-MG, Alnæs D, et al. Adipose tissue distribution from body MRI is associated with cross-sectional and longitudinal brain age in adults. NeuroImage: Clinical. 2022;33:102949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pinto FCS, Andrade MF, Gatti da Silva GH, et al. Function Over Mass: A Meta-Analysis on the Importance of Skeletal Muscle Quality in COVID-19 Patients. Front Nutr. 2022;9:837719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boutin RD, Lenchik L. Value-Added Opportunistic CT: Insights Into Osteoporosis and Sarcopenia. AJR Am J Roentgenol. 2020;215(3):582–594. [DOI] [PubMed] [Google Scholar]
- 24.Delmonico MJ, Harris TB, Visser M, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90(6):1579–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miljkovic I, Cauley JA, Petit MA, et al. Greater adipose tissue infiltration in skeletal muscle among older men of African ancestry. The Journal of clinical endocrinology and metabolism. 2009;94(8):2735–2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson DE, D’Agostino JM, Bruno AG, Demissie S, Kiel DP, Bouxsein ML. Variations of CT-based trunk muscle attenuation by age, sex, and specific muscle. The journals of gerontology Series A, Biological sciences and medical sciences. 2013;68(3):317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matthews KA, Xu W, Gaglioti AH, et al. Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015–2060) in adults aged ≥65 years. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2019;15(1):17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Correa-de-Araujo R, Harris-Love MO, Miljkovic I, Fragala MS, Anthony BW, Manini TM. The Need for Standardized Assessment of Muscle Quality in Skeletal Muscle Function Deficit and Other Aging-Related Muscle Dysfunctions: A Symposium Report. Frontiers in physiology. 2017;8:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuller LH, Lopez OL, Newman A, et al. Risk factors for dementia in the cardiovascular health cognition study. Neuroepidemiology. 2003;22(1):13–22. [DOI] [PubMed] [Google Scholar]
- 30.Andrew MK, Rockwood K. A five-point change in Modified Mini-Mental State Examination was clinically meaningful in community-dwelling elderly people. J Clin Epidemiol. 2008;61(8):827–831. [DOI] [PubMed] [Google Scholar]
- 31.Andrews JS, Desai U, Kirson NY, Zichlin ML, Ball DE, Matthews BR . Disease severity and minimal clinically important differences in clinical outcome assessments for Alzheimer’s disease clinical trials. Alzheimer’s & dementia (New York, N Y). 2019;5:354–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snijder MB, Visser M, Dekker JM, et al. Low subcutaneous thigh fat is a risk factor for unfavourable glucose and lipid levels, independently of high abdominal fat. The Health ABC Study. Diabetologia. 2005;48(2):301–308. [DOI] [PubMed] [Google Scholar]
- 33.Visser M, Kritchevsky SB, Goodpaster BH, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. Journal of the American Geriatrics Society. 2002;50(5):897–904. [DOI] [PubMed] [Google Scholar]
- 34.Holden KF, Lindquist K, Tylavsky FA, Rosano C, Harris TB, Yaffe K. Serum leptin level and cognition in the elderly: Findings from the Health ABC Study. Neurobiology of aging. 2009;30(9):1483–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guralnik JM, Cawthon PM, Bhasin S, et al. Limited physician knowledge of sarcopenia: A survey. Journal of the American Geriatrics Society. 2023. [DOI] [PubMed] [Google Scholar]
- 36.Kim J, Choi KH, Cho SG, et al. Association of muscle and visceral adipose tissues with the probability of Alzheimer’s disease in healthy subjects. Scientific reports. 2019;9(1):949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Britton KA, Massaro JM, Murabito JM, Kreger BE, Hoffmann U, Fox CS. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. Journal of the American College of Cardiology. 2013;62(10):921–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meigs JB, Wilson PW, Fox CS, et al. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. The Journal of clinical endocrinology and metabolism. 2006;91(8):2906–2912. [DOI] [PubMed] [Google Scholar]
- 39.Gruzdeva O, Borodkina D, Uchasova E, Dyleva Y, Barbarash O. Localization of fat depots and cardiovascular risk. Lipids Health Dis. 2018;17(1):218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miljkovic I, Vella CA, Allison M. Computed Tomography-Derived Myosteatosis and Metabolic Disorders. Diabetes Metab J. 2021;45(4):482–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sachs S, Zarini S, Kahn DE, et al. Intermuscular adipose tissue directly modulates skeletal muscle insulin sensitivity in humans. American journal of physiology Endocrinology and metabolism. 2019;316(5):E866–e879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grant RW, Stephens JM. Fat in flames: influence of cytokines and pattern recognition receptors on adipocyte lipolysis. American journal of physiology Endocrinology and metabolism. 2015;309(3):E205–213. [DOI] [PubMed] [Google Scholar]
- 43.Vettor R, Milan G, Franzin C, et al. The origin of intermuscular adipose tissue and its pathophysiological implications. American journal of physiology Endocrinology and metabolism. 2009;297(5):E987–998. [DOI] [PubMed] [Google Scholar]
- 44.Hamrick MW, McGee-Lawrence ME, Frechette DM. Fatty Infiltration of Skeletal Muscle: Mechanisms and Comparisons with Bone Marrow Adiposity. Front Endocrinol (Lausanne). 2016;7:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim HK, Kim CH. Quality Matters as Much as Quantity of Skeletal Muscle: Clinical Implications of Myosteatosis in Cardiometabolic Health. Endocrinol Metab (Seoul). 2021;36(6):1161–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Konopka AR, Wolff CA, Suer MK, Harber MP. Relationship between Intermuscular Adipose Tissue Infiltration and Myostatin before and after Aerobic Exercise Training. American journal of physiology Regulatory, integrative and comparative physiology. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee SJ. Regulation of muscle mass by myostatin. Annual review of cell and developmental biology. 2004;20:61–86. [DOI] [PubMed] [Google Scholar]
- 48.Vella CA, Michos ED, Sears DD, et al. Associations of Sedentary Behavior and Abdominal Muscle Density: The Multi-Ethnic Study of Atherosclerosis. Journal of physical activity & health. 2018;15(11):827–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Torriani M, Grinspoon S. Racial differences in fat distribution: the importance of intermuscular fat. Am J Clin Nutr. 2005;81(4):731–732. [DOI] [PubMed] [Google Scholar]
- 50.Gallagher D, Kuznia P, Heshka S, et al. Adipose tissue in muscle: a novel depot similar in size to visceral adipose tissue. Am J Clin Nutr. 2005;81(4):903–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hong CH, Falvey C, Harris TB, et al. Anemia and risk of dementia in older adults: findings from the Health ABC study. Neurology. 2013;81(6):528–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Results of linear mixed effect models (regression coefficients) testing the association of each population characteristic at Year 1 (unless otherwise noted) with decline in 3MS from year 5 to year 10.
Supplemental Table 2. Coefficients (p values) from linear mixed effect models testing the associations between change in thigh IMAT (from years 1 to 6) and 3MS decline (from years 5 to 10), stratified by race and gender.


