Abstract
Intimate partner violence (IPV) has been associated with delays throughout the HIV care continuum. This study explored prospective associations between experiences of past-year IPV and two HIV care outcomes in the context of current universal test and treat guidelines using two consecutive rounds of an ongoing HIV surveillance study conducted in the Rakai region of Uganda. Longitudinal logistic regression models examined associations between IPV, use of antiretroviral therapy (ART) and viral load suppression (VS), adjusting for outcome variables at baseline. To address differences in ART retention by IPV, propensity scores were used to create inverse-probability-of-treatment-and-censoring-weighted (IPTCW) models. At baseline, of 1,923 women with HIV (WWH), 34.6%, 26.5%, 13.5% reported past-year verbal, physical and sexual IPV; a lower proportion of persons who experienced physical IPV (79.4%) were VS than those who did not (84.3%; p=0.01). The proportion VS at baseline also significantly differed by exposure to verbal IPV (p=0.03). However, in adjusted longitudinal models, IPV was not associated with lower odds of ART use or VS at follow-up. Among WWH in the Rakai region, IPV does not appear to be a barrier to subsequent ART use or VS. However, given prevalence of IPV in this population, interventions are needed.
Keywords: HIV, treatment adherence, Uganda, Viral Suppression, Intimate Partner Violence
Introduction
Intimate partner violence (IPV), defined as physical, sexual, or verbal violence perpetrated by an intimate partner, is a pervasive public health issue that disproportionately burdens women. Globally, 30% of women have experienced lifetime IPV; this estimate is even higher in sub-Saharan Africa (36.6%) (WHO, 2013).
IPV is linked to HIV, and the relationship is bidirectional. IPV can increase risk of HIV infection directly (e.g., forced intercourse) and indirectly (e.g. reduced ability to safely negotiate condom use) (WHO, 2004). Women living with HIV (WWH) are more likely to experience IPV (Campbell et al., 2008; Li et al., 2014; Maman et al., 2002) and experiencing IPV is associated with delayed and decreased engagement throughout the HIV care continuum. A 2015 meta-analysis found a significant association between experiencing IPV and lower odds of current use of ART (OR 0.79, 95% CI 0.64 – 0.97, p=0.012), ART adherence (OR 0.48, 95% CI 0.30 – 0.75, p=0.044) and viral suppression (VS) (OR 0.64, 95% CI 0.46–0.90, p=0.114) (Hatcher, Smout, Turan, Christofides, & Stockl, 2015). Several causal pathways have been identified to explain poor engagement and retention in the HIV care continuum among victims of IPV.
Fear of violence may lead to delayed partner disclosure of positive serostatus, which can delay care-seeking and lead to poor adherence, as personal safety is prioritized over treatment adherence (Hatcher et al., 2014). Studies have reported that lifetime experience of physical or sexual violence is associated with lower odds of ART use among women (M. H. Cohen et al., 2004) and likelihood of treatment non-adherence increases with each additional lifetime experience of trauma (Mugavero et al., 2006). In addition, a controlling partner may limit access to care and WWH may avoid care-seeking due to shame and denial around the experienced abuse (Lichtenstein, 2006; McCauley, Yurk, Jenckes, & Ford, 1998).
In the Rakai region of South-central Uganda, both IPV and HIV are public health issues. HIV prevalence exceeds the national average (6.2%) at 7.9% with some deeply affected fishing communities experiencing a median prevalence of 49% among women (Chang et al., 2016; Ministry of Health, 2019). Nearly half (49.8%) of women in the region report having experienced some form of lifetime IPV (Kouyoumdjian et al., 2013) and 21% of women report experiencing IPV in the past year (Sabri et al., 2019). Mixed-methods research conducted prior to the implementation of Universal Test and Treat (UTT) in the region found that experiencing past-year IPV reduced engagement in HIV care by 49–56% and ART use by 50–73% among women 30+ years of age (Wirtz et al., 2018). WWH participating in the qualitative component of this study reported that ART use resulted in IPV and indicated that threats of violence contributed to non-adherence (Wirtz et al., 2018).
Despite a robust body of literature supporting the relationship between IPV victimization and poor HIV care outcomes, gaps in evidence exist. The review described above only identified cross-sectional studies and most were conducted in the United States (Hatcher et al., 2015). Understanding the relationship between IPV and HIV care outcomes in sub-Saharan Africa, a setting where young women and girls experience high-risk of both IPV and HIV, is important for public health planning efforts, in terms of improving care outcomes, promoting secondary prevention via VS, and improving delivery of trauma-informed care. The present analysis addresses this urgent gap by exploring associations between experiences of IPV and two HIV care outcomes using data from two rounds of an ongoing HIV surveillance cohort in Rakai, Uganda. Data were collected after the rollout of UTT allowing us to explore associations between experiences of violence and care outcomes in the context of current treatment guidelines. We focus exclusively on experiences of IPV among women; although men experience IPV, the overwhelming majority of IPV is experienced by women and perpetrated by men (generally, and in the study setting) (Koenig et al., 2003; Reed, Gupta, & Silverman, 2014). We hypothesize that women experiencing IPV at baseline will be less likely to subsequently report current ART use and less likely to be VS relative to women who report no past-year IPV.
Materials and methods
Data Collection
The RCCS is a longitudinal open-cohort HIV surveillance study which has previously been described in detail (Wawer et al., 1998). The survey, which is conducted across 40 communities in the Rakai region, includes approximately 20,000 residents in each round which occurs at roughly 24-month intervals. Consenting residents aged 15–49 years are invited to participate. Survey data include sociodemographic information, use of HIV treatment and prevention services, and experience of IPV. HIV rapid testing is performed and post-test HIV counseling, ART treatment initiation and referral to ongoing care are provided at no cost.
The RCCS is reviewed and approved by Institutional Review Boards (IRB) in Uganda: Western IRB, the Uganda Virus Research Institute’s Research and Ethics Committee and the Uganda National Council for Science and Technology.
Analytic Sample
The present study involves secondary analysis of data collected in two consecutive rounds of the RCCS. Round 18 (hereafter, “baseline”) was conducted from August 2016 to May 2018. Round 19 (hereafter, follow-up) was conducted between June 2018 and Nov 2020, with a four-month hiatus due to COVID-19 lockdown in 2020. The analytic sample focused on WWH (known positives and those testing positive in RCCS) at baseline who reported an intimate partnership in the past 12 months, resulting in a sample of 1,923 women.
Measures
The primary exposures of interest pertained to past-year experiences of three categories of IPV at baseline: physical, sexual, and verbal. Ten adapted questions were used from the Conflict Tactics Scales (CTS) (Straus, 1979). IPV were measured by asking, “In the past 12 months has your partner...”:
Verbal IPV (1 item) “verbally abused or shouted at you?”
Physical IPV (6 items) “pushed, pulled, slapped, held you down?”; “punched you with fist or something that could hurt you?”; “kicked or dragged you?”; “tried to strangle or burn you?”; “threatened you with a knife, gun, other weapon?”; and “attacked you with knife, gun, other weapon?”
Sexual IPV (3 items) “used verbal threats to force you to have sex;” “physically forced you to have sex;” or “coerced you to perform other sexual acts when you did not want to.”
The formatting of this module differed between rounds. At baseline, participants were asked the IPV questions in relation to specific past-year intimate partners (up to four). At follow-up participants were asked each IPV item only once, regarding all past-year partners. For baseline, responses were collapsed across intimate partners. The responses for the physical and sexual IPV items were further collapsed so that an individual who responded yes to any of the physical IPV items was a “yes” for past-year physical IPV; similarly, an affirmative response to any of the sexual violence items was a “yes” for past-year sexual IPV.
Dependent variables pertained to HIV care outcomes. “Current ART use” was a dichotomous variable measured by the question, “Are you currently taking antiretrovirals?” Our major variable of interest, VS, defined per Ugandan ministry of health guidelines as a viral load ≤1000 viral copies per mL of blood (PEPFAR, 2016). This variable was collected as a continuous variable, viral load, but dichotomized to increase clinical relevance.
Baseline covariates were identified a priori from the literature and included community type, occupation, marital status, age and household socioeconomic status (an index based on dwelling attributes such as the presence of electricity and divided into tertiles for low, middle, high).
Data Analysis
Analyses were conducted in SAS studio (SAS Institute Inc.). Baseline sociodemographic and independent variables of interest were analyzed, using descriptive statistics, to characterize the analytic sample. Multicollinearity was assessed by examining the intercorrelations between the exposure variables in the model as well as the tolerance and variance inflation factor (VIF).
To test our hypotheses, correlates of current ART use and VS were examined longitudinally, using logistic regression, and adjusting for outcome variables (i.e., current ART use and VS) at baseline. Directed acyclic graphs were used to identify the minimally sufficient set of baseline covariates to adjust for to reduce bias from measured confounders. A variable was considered a confounder if it was predictive of both the exposure and outcome and was not on the causal pathway between them. Next, propensity scores were created based on these measured confounders: age, household socioeconomic status, marital status, and residence. The follow-up round of RCCS was interrupted by the 2020 COVID-19 lockdown which may have impacted follow-up. Fifty-seven percent (n=1083) of those who participated at baseline also participated in follow-up. Chi-squared analysis was performed to identify differences in retention by our main outcome variable, VS as well as our IPV exposures. Statistically significant differences were observed for physical IPV (49% of those reporting physical IPV were lost to follow-up while 41.5% of those not reporting physical IPV were lost to follow-up, p=0.003). Significant differences in retention were also observed for several confounders: age, marital status, community type and household SES. To address attrition as well as confounding, estimated propensity scores were used to create inverse probability weights for both treatment (IPTW) and censoring (IPCW). To create the propensity scores for the IPTWs, an exposure logistic regression model was used where identified confounders were regressed on each IPV exposure. Stabilized and unstabilized IPTWs were applied and compared. Outcomes from stabilized models are reported. Next, propensity scores for the IPCWs, were created by regressing the identified confounders on censoring status. These two weights were then combined to create a single weight that addressed bias from both censoring and the potential confounders (known as inverse-probability-of-treatment-and-censoring weights, IPTCWs). Finally, IPTCW weighted logistic regression models were built to obtain odds ratios.
Multicollinearity
All VIF were less than 2, no tolerance values were <0.1 and no correlations were >0.7, suggesting that there was little multicollinearity between variables.
Results
Descriptive characteristics of the sample at baseline
Just over one-third of participants reported past-year verbal IPV (34.6%). Physical and sexual IPV were reported by 26.5% and 13.5% of participants, respectively. Mean age of participants was 32.8 years (SD 7.2 years). Most (61.8%) were currently married and most (72.0%) had attended at least some of secondary school. The majority (56.3%) were classified as having high socioeconomic status and the most common occupation among participants was housework (45.1%). See Table 1.
Table 1.
Baseline Characteristics of Women Living with HIV (WWH) Participating in Round 18 of the Rakai Community Cohort Study
| Characteristic | Overall (n=1,923) | |
|---|---|---|
|
| ||
| Any Past Year Verbal IPV | ||
| Yes | 665 (34.6%) | |
| No | 1427 (65.4%) | |
| Any Past Year Physical IPV | ||
| Yes | 510 (26.5%) | |
| No | 1413 (73.5%) | |
| Any Past Year Sexual IPV | ||
| Yes | 260 (13.5%) | |
| No | 1663 (86.5%) | |
| Mean Age (SD) | 32.8 (SD 7.2) | |
| Residence | ||
| Agrarian | 721 (37.5%) | |
| Trade | 320 (16.6%) | |
| Fishing | 882 (45.9%) | |
| Education | ||
| No School/Primary | 539 (28.0%) | |
| Secondary or above | 1384 (72.0%) | |
| HH SES | ||
| High | 1082 (56.3%) | |
| Middle | 323 (16.8%) | |
| Low | 517 (26.9%) | |
| Occupation | ||
| Housework | 867 (45.1%) | |
| Trade/shopkeeper | 444 (23.1%) | |
| Bar/Restaurant Worker | 272 (14.1%) | |
| Other | 340 (17.7%) | |
| Marital Status | ||
| Currently Married | 1188 (61.8%) | |
| Previously Married | 643 (33.4%) | |
| Never Married | 92 (4.78%) | |
Figures 1 and 2 show the proportion of women at baseline reporting current ART use and VS, respectively, by exposure to each form of IPV. A greater proportion of persons who did not experience physical IPV in the past year (81.3%) were currently on ART treatment than those who did (77.1%) (p=0.0387). The same pattern was observed for VS; a smaller proportion of those who experienced physical IPV were VS (79.4%) than those who did not (84.3%) (p=0.0117). Across sexual and verbal IPV, we saw the same trend: persons experiencing both forms of IPV were less likely to be on ART and less likely to be VS than their counterparts who did not. However, these differences were not significant for either treatment outcome by exposure to sexual IPV and only VS significantly differed by exposure to verbal IPV (p=0.0302). Overall, from baseline to follow-up, prevalence of current ART use (80.2% vs 95%) and VS (83.0% vs 92.9%) increased.
Figure 1.
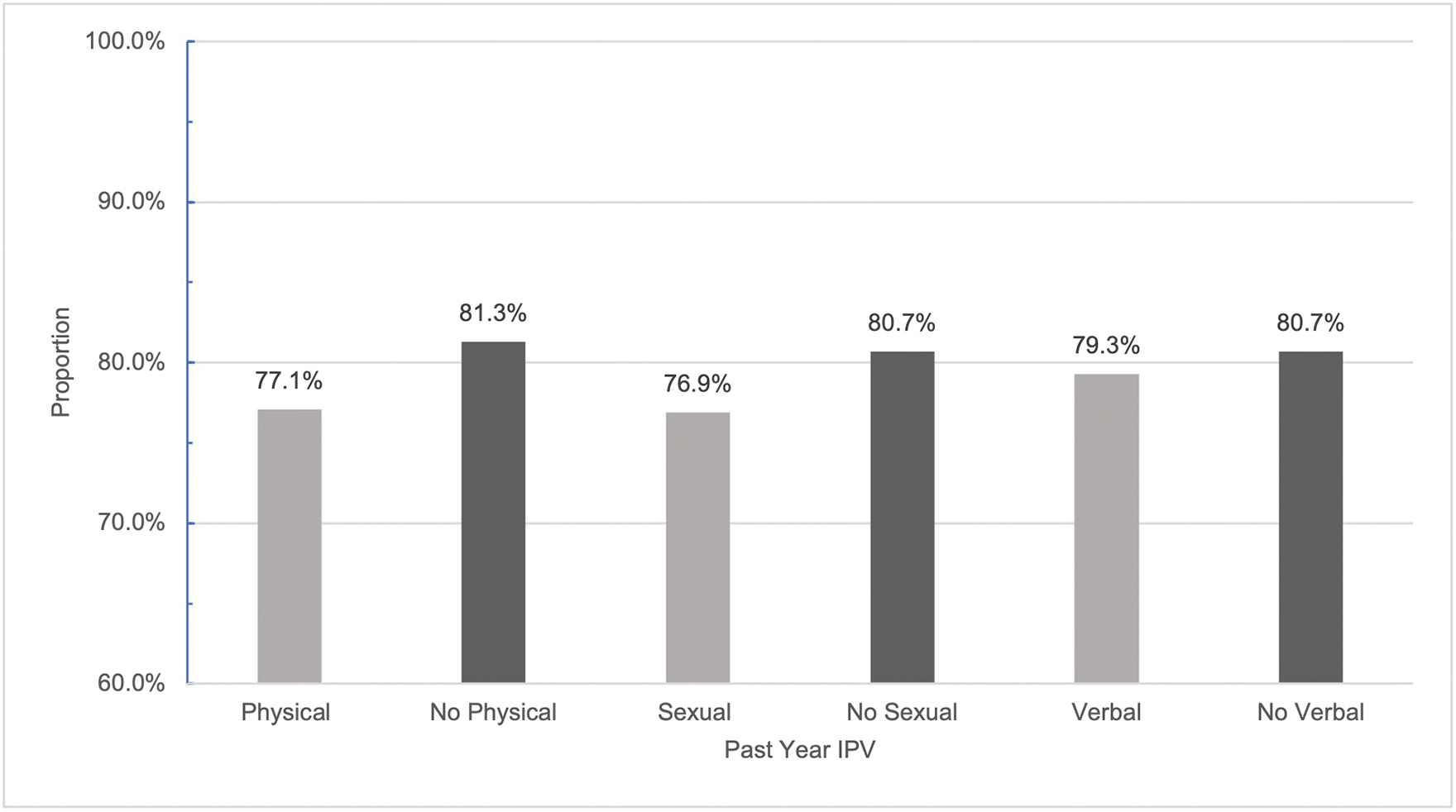
Proportion of WWH currently on ART at baseline by exposure to past year IPV.
Figure 2.
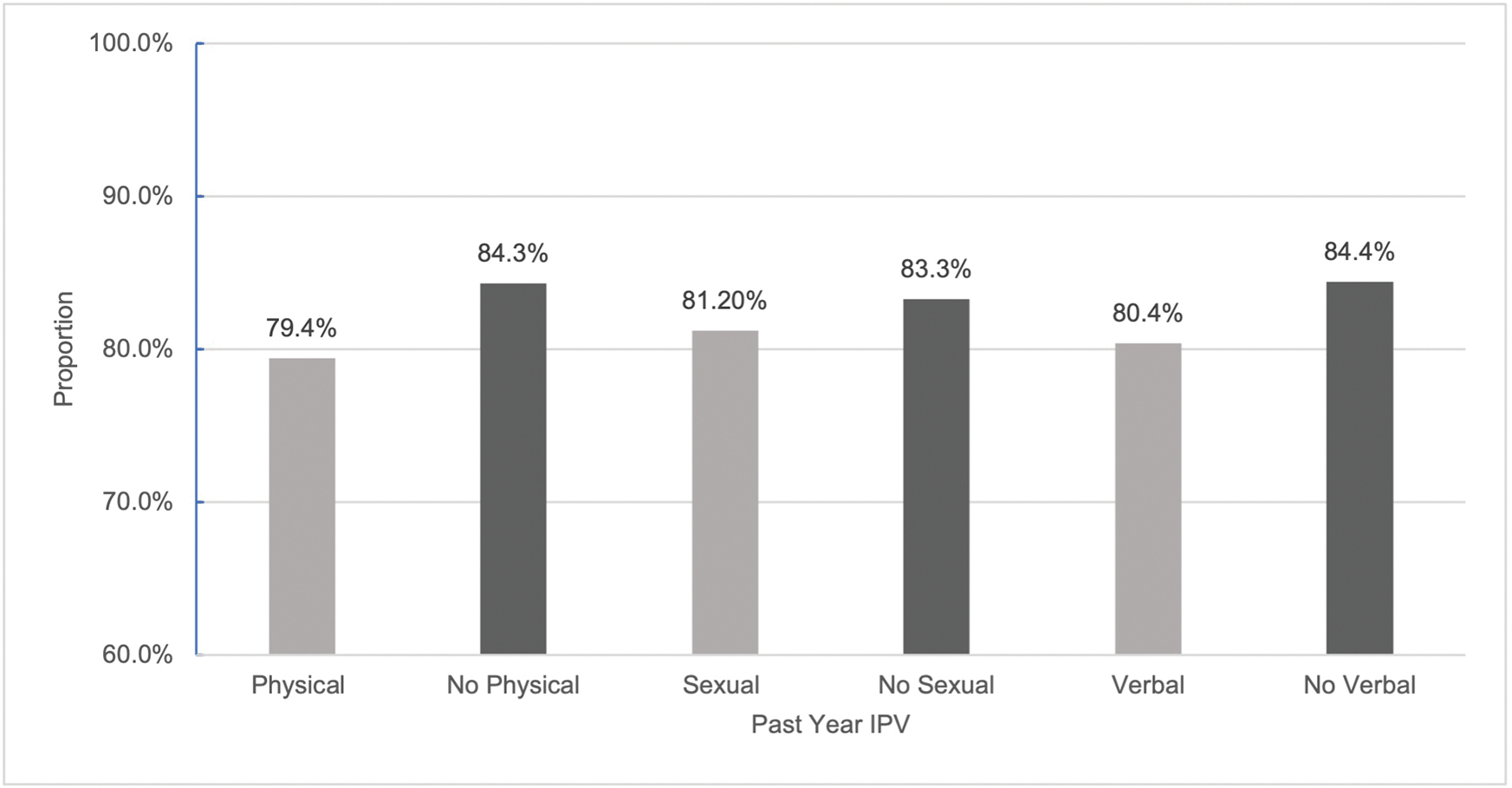
Proportion of WWH who are VS at baseline by exposure to past year IPV.
Multivariable analysis of IPV on current ART use and VS
Tables 2 summarizes the IPTCW weighted regression analysis for the association between past-year IPV at baseline and current ART use and VS at follow-up. None of the IPV exposures were prospectively significantly associated with current ART use or VS at follow-up. Observed associations between IPV and the outcomes were highly insignificant in the adjusted analysis with odds ratios approximating one.
Table 2.
Weighted Prospective Logistic Regression Examining the Relationship between IPV at Baseline and HIV Care Outcomes at Follow-Up (n=1,923)
| Current ART Use | Achievement of VS | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Form of IPV | B | SE | Odds Ratio (95% CI) | P value | B | SE | Odds Ratio (95% CI) | P value | |
|
| |||||||||
| Verbal | |||||||||
| Yes | 0.01 | 0.00 | 1.01 (1.00–1.02) | 0.1237 | 0.00 | 0.01 | 1.00 (0.98–1.02) | 0.8554 | |
| No | -- | -- | 1.00 | -- | |||||
| Physical | |||||||||
| Yes | 0.00 | 0.01 | 1.00 (0.99–1.01) | 0.6145 | 0.00 | 001 | 0.99 (0.98–1.01) | 0.9743 | |
| No | -- | -- | 1.00 | -- | -- | -- | 1.00 | -- | |
| Sexual | |||||||||
| Yes | −0.01 | 0.01 | 0.99 (0.97–1.01) | 0.4935 | −0.01 | 0.01 | 0.99 (0.97–1.01) | 0.4570 | |
| No | -- | -- | 1.00 | -- | -- | -- | 1.00 | -- | |
PS weighted for age, marital status, household SES, community type and censoring
Discussion
The present study, which explored associations between IPV and HIV care outcomes in the context of Uganda’s current HIV treatment guidelines found that IPV was not significantly associated with lower odds of current ART use or VS. The findings do not confirm our hypotheses and are inconsistent with conclusions from a recent meta-analysis (Hatcher et al., 2015). Although Hatcher et al.’s meta-analysis found significant associations between IPV and care outcomes, the individual studies included in the review had less conclusive results. Of five identified studies that measured self-reported “current ART use” in a similar format to our question, none found a statistically significant association with IPV (Blackstock, Blank, Fletcher, Verdecias, & Cunningham, 2015; Illangasekare et al., 2012; Kalokhe et al., 2012; Ramachandran, Yonas, Silvestre, & Burke, 2010; Siemieniuk et al., 2013). Of the seven studies that measured VS, only three found a statistically significant association between VS and IPV (Blackstock et al., 2015; Blank et al., 2015; Espino et al., 2015; Illangasekare et al., 2012; Rose, House, & Stepleman, 2010; Siemieniuk et al., 2013; Sullivan, Messer, & Quinlivan, 2015). It is also important to note that most of the studies in this review were from high-income settings and relied on cross-sectional data, limiting their generalizability. Our findings are also inconsistent with previous longitudinal work in Rakai by Wirtz et al. which found significant associations between IPV and ART use among women >30 years of age (Wirtz et al., 2018).
Although not the focus of this manuscript, a post-hoc analysis explored models in older and younger age strata (results not shown) to see if similar age-related patterns were observed; associations remained insignificant across strata. The ever-evolving HIV care landscape is one possible explanation for observed differences between our study and research by Wirtz et al. in the same setting. In 2016, Uganda aligned their HIV treatment guidelines with the UTT approach (Ministry of Health, 2020). Prior to UTT, persons were referred to HIV care and treatment initiation was delayed until an individual’s CD4 t-cell count reached a certain threshold. These additional steps between diagnosis and ART initiation were identified in earlier work as barriers to optimal treatment engagement for women experiencing violence (Hatcher et al., 2015). For example, a woman fearful of disclosing her HIV positive status to her partner may delay care-seeking (Tam, Amzel, & Phelps, 2015) and once disclosure occurred depending on her relationship agency, her partner may prohibit her from accessing care. Provision of ART at diagnosis may reduce barriers incurred to engage in care and initiate treatment.
Findings have been mixed regarding the relationship between IPV and care outcomes in other studies in sub-Saharan Africa under UTT. A longitudinal study of mothers in South Africa found that IPV did not adversely impact treatment adherence over time (Rotheram-Borus et al., 2019). Conversely, a recent cross-sectional study from Malawi looking at IPV in dyads found significant associations between IPV victimization and treatment non-adherence (Conroy et al., 2020). The authors found the strongest association to non-adherence among participants experiencing bidirectional violence (i.e., when a person is both a perpetrator and victim). Recent work elsewhere in Uganda suggests that bidirectional IPV is common (Bulamba et al., 2019). This phenomenon has not been recently explored in Rakai (Koenig et al., 2003) and shifting gender norms may have impacted patterns and directionality of IPV; if women experiencing bidirectional IPV are at greatest risk of poor care outcomes, the inclusion of data collection around bidirectional IPV may help to identify a subset of WWH who are most likely to experience IPV-related barriers to care.
Although we observed statistically significant differences in our HIV care outcomes by exposure to each form of IPV at baseline, our prospective adjusted analysis, indicated that women who experienced IPV used ART and achieved VS at follow-up at rates comparable to those not experiencing IPV. It is possible that increased support for status disclosure among serodiscordant couples in Rakai in recent years increased ART adherence, as disclosure of status to a partner has been associated with increased partner support for HIV services in Uganda (Naigino et al., 2017). In addition, increased awareness that achieving VS can reduce risk of HIV transmission to close to zero may have motivated serodiscordant partners to be supportive of treatment (M. S. Cohen et al., 2016). Qualitative work with WWH who experience IPV in this setting could shed light on how IPV currently impacts HIV care-seeking behavior and if this has changed in response to changing treatment guidelines.
This analysis had several limitations. The measures for IPV and current ART use are subject to reporting bias. Additionally, the IPV variable was asked differently across rounds and this change may have altered recall; prevalence was higher when persons were asked about individual partners and there is evidence that people have better recall when prompted to remember specific details (Bachman & Saltzman, 1995). This is also an open-cohort study and loss-to-follow-up between rounds is typically high; people migrate in and out of the community and our requirement that people participate in baseline precluded inclusion of those migrating in while allowing for missing data from those migrating out. Rates of loss-to-follow-up were comparable to past rounds and we therefore do not attribute this to the pandemic. Further, our application of censoring weights allowed us to overcome this limitation. Finally, the COVID-19 lockdown disrupted follow-up data collection activities. However, given that we would anticipate an increase in IPV and additional barriers to treatment adherence under the lockdown and our findings were null, the lockdown does not appear to have impacted results. The analysis also had several strengths. The data were collected and analyzed in the context of UTT allowing us to consider the policy implications of our results and VS is an objective measure not prone to measurement bias.
To understand absolute associations between IPV and our outcomes, this study looked at IPV without considering alcohol use, a frequently occurring co-morbidity that is independently associated with poor care outcomes. Although we did not see a significant association between IPV and the care outcomes, additional work is needed to identify whether IPV among specific subsets of women (e.g., those experiencing bidirectional violence) is a driver of worse care outcomes. Qualitative work with IPV-experiencing WWH would be informative in further disentangling the relationship between IPV and HIV care outcomes.
Despite the lack of significant relationship between IPV and HIV care outcomes in our study, IPV remains a critical public health issue disproportionately burdening WWH. Severe IPV among WWH has been associated with a twofold increase in odds of all-cause mortality (Closson et al., 2020). Reducing IPV in this setting is a public health priority and interventions are needed to address this health issue among WWH.
Funding:
This work was supported by National Institutes of Health under grants F31AA028198–01, K01AA024068, R01DA042666, 5R01HD072695, R01AI114438, 5U2GGH000817 and in part by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases.
Footnotes
Disclosure statement
The authors declare no competing interests.
References
- Bachman R, & Saltzman LE (1995). Violence Against Women: Estimates From the Redesigned Survey, Special Report,. Retrieved from Washington, D.C.: [Google Scholar]
- Blackstock OJ, Blank AE, Fletcher JJ, Verdecias N, & Cunningham CO (2015). Considering care-seeking behaviors reveals important differences among HIV-positive women not engaged in care: implications for intervention. AIDS Patient Care STDS, 29 Suppl 1, S20–26. doi: 10.1089/apc.2014.0271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank AE, Fletcher J, Verdecias N, Garcia I, Blackstock O, & Cunningham C (2015). Factors associated with retention and viral suppression among a cohort of HIV+ women of color. AIDS Patient Care STDS, 29 Suppl 1, S27–35. doi: 10.1089/apc.2014.0272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulamba R, Miller A, Mugamba S, Nakigozi G, Kigozi G, Kigozi G, . . . Nalugoda F. (2019). Drivers and risk factors of Intimate Partner Violence (IPV) perpetration and victimization in a Uganda based urban community cohort study. Paper presented at the 24th International Summit on Violence, Abuse and Trauma, La Jolla, California. [Google Scholar]
- Campbell JC, Baty ML, Ghandour RM, Stockman JK, Francisco L, & Wagman J (2008). The intersection of intimate partner violence against women and HIV/AIDS: a review. Int J Inj Contr Saf Promot, 15(4), 221–231. doi: 10.1080/17457300802423224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LW, Grabowski MK, Ssekubugu R, Nalugoda F, Kigozi G, Nantume B, . . . Wawer MJ. (2016). Heterogeneity of the HIV epidemic in agrarian, trading, and fishing communities in Rakai, Uganda: an observational epidemiological study. The Lancet HIV, 3(8), e388–e396. doi: 10.1016/s2352-3018(16)30034-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Closson K, McLinden T, Parry R, Lee M, Gibbs A, Kibel M, . . . Hogg RS. (2020). Severe intimate partner violence is associated with all-cause mortality among women living with HIV. AIDS, 34(10), 1549–1558. doi: 10.1097/QAD.0000000000002581 [DOI] [PubMed] [Google Scholar]
- Cohen MH, Cook JA, Grey D, Young M, Lawrence HH, Tien P, . . . Wilson TE. (2004). Medically eligible women who do not use HAART: the importance of abuse, drug use, and race. American Journal of Public Health, 94(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, . . . Team HS. (2016). Antiretroviral Therapy for the Prevention of HIV-1 Transmission. N Engl J Med, 375(9), 830–839. doi: 10.1056/NEJMoa1600693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy AA, Leddy AM, Darbes LA, Neilands TB, Mkandawire J, & Stephenson R (2020). Bidirectional Violence Is Associated with Poor Engagement in HIV Care and Treatment in Malawian Couples. J Interpers Violence, 886260520959632. doi: 10.1177/0886260520959632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espino SR, Fletcher J, Gonzalez M, Precht A, Xavier J, & Matoff-Stepp S (2015). Violence screening and viral load suppression among HIV-positive women of color. AIDS Patient Care STDS, 29 Suppl 1, S36–41. doi: 10.1089/apc.2014.0275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher AM, Smout EM, Turan JM, Christofides N, & Stockl H (2015). Intimate partner violence and engagement in HIV care and treatment among women: a systematic review and meta-analysis. AIDS, 29(16), 2183–2194. doi: 10.1097/QAD.0000000000000842 [DOI] [PubMed] [Google Scholar]
- Hatcher AM, Woollett N, Pallitto CC, Mokoatle K, Stockl H, MacPhail C, . . . Garcia-Moreno C. (2014). Bidirectional links between HIV and intimate partner violence in pregnancy: implications for prevention of mother-to-child transmission. J Int AIDS Soc, 17, 19233. doi: 10.7448/IAS.17.1.19233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illangasekare S, Tello M, Hutton H, Moore R, Anderson J, Baron J, & Chander G (2012). Clinical and mental health correlates and risk factors for intimate partner violence among HIV-positive women in an inner-city HIV clinic. Womens Health Issues, 22(6), e563–569. doi: 10.1016/j.whi.2012.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalokhe AS, Paranjape A, Bell CE, Cardenas GA, Kuper T, Metsch LR, & del Rio C (2012). Intimate partner violence among HIV-infected crack cocaine users. AIDS Patient Care STDS, 26(4), 234–240. doi: 10.1089/apc.2011.0275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig MA, Lutalo T, Zhao F, Nalugoda F, Wabwire-Mangen F, Kiwanuka N, . . . Gray R. (2003). Domestic violence in rural Uganda: evidence from a community-based study. Bull World Health Organ, 81(1), 53–60. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12640477 [PMC free article] [PubMed] [Google Scholar]
- Kouyoumdjian FG, Calzavara LM, Bondy SJ, O’Campo P, Serwadda D, Nalugoda F, . . . Gray R. (2013). Risk factors for intimate partner violence in women in the Rakai Community Cohort Study, Uganda, from 2000 to 2009. BMC Public Health, 13, 566. doi: 10.1186/1471-2458-13-566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Marshall CM, Rees HC, Nunez A, Ezeanolue EE, & Ehiri JE (2014). Intimate partner violence and HIV infection among women: a systematic review and meta-analysis. J Int AIDS Soc, 17, 18845. doi: 10.7448/IAS.17.1.18845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein B (2006). Domestic violence in barriers to health care for HIV-positive women. AIDS Patient Care STDS, 20(2), 122–132. doi: 10.1089/apc.2006.20.122 [DOI] [PubMed] [Google Scholar]
- Maman S, Mbwambo JK, Hogan NM, Kilonzo GP, Campbell JC, Weiss E, & Sweat MD (2002). HIV-positive women report more lifetime partner violence: findings from a voluntary counseling and testing clinic in Dar es Salaam, Tanzania. Am J Public Health, 92(8), 1331–1337. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12144993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley J, Yurk RA, Jenckes MW, & Ford DE (1998). Inside ‘Pandora’s box’: abused women’s experiences with clinicians and health services. J Gen Intern Med 13, 549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health U.,;. (2019). UGANDA POPULATION-BASED HIV IMPACT ASSESSMENT (UPHIA) 2016–2017: Final Report. Retrieved from Kampala: [Google Scholar]
- Ministry of Health, U.,;. (2020). Consolidated Guidelines for the Prevention and Treatment of HIV and AIDS in Uganda. Retrieved from Kampala: https://uac.go.ug/sites/default/files/Consolidated%20HIV%20Guidelines%202020.pdf [Google Scholar]
- Mugavero M, Ostermann J, Whetten K, Leserman J, Swartz M, Stangl D, & Thielman N (2006). Barriers to antiretroviral adherence: the importance of depression, abuse, and other traumatic events. AIDS Patient Care STDS, 20(6), 418–428. doi: 10.1089/apc.2006.20.418 [DOI] [PubMed] [Google Scholar]
- Naigino R, Makumbi F, Mukose A, Buregyeya E, Arinaitwe J, Musinguzi J, & Wanyenze RK (2017). HIV status disclosure and associated outcomes among pregnant women enrolled in antiretroviral therapy in Uganda: a mixed methods study. Reprod Health, 14(1), 107. doi: 10.1186/s12978-017-0367-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEPFAR. (2016). Standard Operating Procedures on Viral Load Monitoring for ICAP Clinical Staff and Health Care Workers: A Template for Coutnry Adaptation. [Google Scholar]
- Ramachandran S, Yonas MA, Silvestre AJ, & Burke JG (2010). Intimate partner violence among HIV-positive persons in an urban clinic. AIDS Care, 22(12), 1536–1543. doi: 10.1080/09540121.2010.482199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed E, Gupta J, & Silverman JG (2014). Understanding sexual violence perpetration. JAMA Pediatr, 168(6), 581. doi: 10.1001/jamapediatrics.2013.5408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose RC, House AS, & Stepleman LM (2010). Intimate partner violence and its effects on the health of African American HIV-positive women. Psychological Trauma: Theory, Research, Practice, and Policy, 2(4), 311–317. doi: 10.1037/a0018977 [DOI] [Google Scholar]
- Rotheram-Borus MJ, Weichle TW, Wynn A, Almirol E, Davis E, Stewart J, . . . Tomlinson M. (2019). Alcohol, But Not Depression or IPV, Reduces HIV Adherence Among South African Mothers Living with HIV Over 5 Years. AIDS Behav, 23(12), 3247–3256. doi: 10.1007/s10461-019-02617-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabri B, Wirtz AL, Ssekasanvu J, Nonyane BAS, Nalugoda F, Kagaayi J, . . . Wagman JA. (2019). Intimate partner violence, HIV and sexually transmitted infections in fishing, trading and agrarian communities in Rakai, Uganda. BMC Public Health, 19(1), 594. doi: 10.1186/s12889-019-6909-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute Inc. SAS University Edition. Cary, NC.: SAS Institute Inc. Retrieved from https://odamid.oda.sas.com/SASLogon/login?service=https%3A%2F%2Fodamid.oda.sas.com%2FSASODAControlCenter%2Fj_spring_cas_security_check [Google Scholar]
- Siemieniuk RA, Krentz HB, Miller P, Woodman K, Ko K, & Gill MJ (2013). The clinical implications of high rates of intimate partner violence against HIV-positive women. J Acquir Immune Defic Syndr, 64(1), 32–38. doi: 10.1097/QAI.0b013e31829bb007 [DOI] [PubMed] [Google Scholar]
- Straus MA (1979). Measuring intrafamily conflict and violence. The conflict tactics (CT) scales. Journal of Marriage and the Family (41), 75–88. [Google Scholar]
- Sullivan KA, Messer LC, & Quinlivan EB (2015). Substance abuse, violence, and HIV/AIDS (SAVA) syndemic effects on viral suppression among HIV positive women of color. AIDS Patient Care STDS, 29 Suppl 1, S42–48. doi: 10.1089/apc.2014.0278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam M, Amzel A, & Phelps BR (2015). Disclosure of HIV serostatus among pregnant and postpartum women in sub-Saharan Africa: a systematic review. AIDS Care, 27(4), 436–450. doi: 10.1080/09540121.2014.997662 [DOI] [PubMed] [Google Scholar]
- Wawer MJ, Gray RH, Sewankambo NK, Serwadda D, Paxton L, Berkley S, . . . Quinn TC. (1998). A randomized, community trial of intensive sexually transmitted disease control for AIDS prevention, Rakai, Uganda. AIDS, 12(10), 1211–1225. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9677171 [DOI] [PubMed] [Google Scholar]
- WHO. (2004). Intimate Partner IVolence and HIV/AIDS. Retrieved from Geneva, Switzerland: http://www.who.int/hac/techguidance/pht/InfoBulletinIntimatePartnerViolenceFinal.pdf [Google Scholar]
- WHO. (2013). Global and reginal estimate of violence against women: prevlaence and health effects of intimate partner violence and non-partner sexual violence. Retrieved from Geneva: https://apps.who.int/iris/bitstream/handle/10665/85239/9789241564625_eng.pdf;jsessionid=5370F847991660F291CE61D59ECAC2EE?sequence=1 [Google Scholar]
- Wirtz A, Nakyanjo N, Wagman J, Gray R, Ssekubugu R, Serwadda D, . . . Wawer MJ. (2018). The effects of intimate partner violence on HIV care and ART use in Rakai, Uganda. Paper presented at the International AIDS Conference, Amsterdam, Netherlands. [Google Scholar]


