Abstract
Background:
Evidence gaps remain regarding the influence of prenatal psychosocial factors on adverse pregnancy outcomes.
Objective:
To evaluate relationships between psychosocial factors and adverse perinatal outcomes among Kenyan women.
Methods:
We analysed data from a prospective cohort study enrolling HIV-negative women in pregnancy (NCT03070600) in 20 antenatal clinics in Western Kenya. Study nurses assessed depressive symptoms using the Center for Epidemiologic Studies Depression Scale (CESD-10), social support using the Medical Outcomes Survey scale (MOS-SSS), intimate partner violence (IPV) with the Hurt, Insult, Threaten, Scream scale (HITS), and pregnancy outcomes at 6 weeks postpartum. Cox proportional hazards models were used to evaluate relationships between depressive symptoms (moderate-to-severe [MSD, CESD-10 ≥10] and mild-to-severe [Mild-SD, CESD-10 ≥5]), low social support (MOS-SSS <72), and IPV (HITS ≥10) with adverse perinatal outcomes of pregnancy loss, stillbirth, preterm birth (PTB), small for gestational age, and neonatal mortality. We also estimated the population attributable risk.
Results:
Among 4153 women, 23.9% (n = 994) had MSD, 54.7% (n = 2273) mild-SD, 37.3% (n = 1550) low social support, and 7.8% (n = 323) experienced IPV. Pregnancy loss was 5-fold higher among women with MSD (adjusted hazard ratio [HR] 5.04, 95% confidence interval [CI] 2.44, 10.42); 37.4% of losses were attributable to MSD. Mild-SD was associated with PTB (HR 1.39, 95% CI 1.03, 1.87). Stillbirth risk more than doubled among women reporting low social support (HR 2.37, 95% CI 1.14, 4.94).
Conclusions:
Adverse perinatal outcomes were common and associated with prenatal depressive symptoms and low social support in this large cohort of Kenyan mother-infant pairs.
Keywords: Depression, preterm birth, pregnancy loss, perinatal, Kenya, social support
Background
Over 10% of pregnant women experience depression; the burden is higher in low- and middle-income countries (LMIC), particularly in sub-Saharan Africa (SSA) where a quarter of women are depressed during or after pregnancy.1 Changes in reproductive hormones during gestation and innate genetic factors are compounded by stressors, such as inadequate social support and violence by an intimate partner, to impact mental distress during this period.2–4 Maternal mental distress during pregnancy influences a range of adverse maternal and child health outcomes.2 A meta-analysis utilising data from high-income countries (HICs) and LMICs (India and Pakistan) found an association between depression during pregnancy with preterm birth (PTB) and intrauterine growth restriction (which was more than doubled among women with antenatal depression in India and Pakistan).5 Subsequent meta-analyses confirmed these relationships,6,7 additionally identifying maternal depressive symptoms as a predictor of small for gestational age (SGA)7,8 and infant death.9
Adverse perinatal outcomes including pregnancy loss, stillbirth, PTB, and SGA occur more frequently in LMICs and contribute to suboptimal neonatal survival.10,11 However, relationships between psychosocial factors and adverse birth outcomes have been insufficiently evaluated in LMICs, particularly in sub-Saharan Africa where 43% of global neonatal deaths occur.12 The high prevalence of maternal depression in sub-Saharan Africa (25%),1,13 combined with slower gains in neonatal health, make understanding the potential linkages between maternal mental health and perinatal outcomes vital in this region. Routine maternal child health (MCH) services are well-attended in sub-Saharan Africa (>95%),14 offering a high-impact setting for preventing and treating depression in pregnancy for dyadic benefit.15
A recent meta-analysis identified only 3 studies in sub-Saharan Africa (Ethiopia,16 Ghana and Cote d’Ivoire,17 and Kenya18) evaluating antenatal depression and birth outcomes.19 Pooled results (n=1511 participants) indicated an increased risk of PTB with maternal depression in pregnancy.19 These studies had relatively small sample sizes and evaluated few birth outcomes, limiting their scope.
We evaluated relationships between psychosocial factors during pregnancy (depression, low social support, intimate partner violence) and adverse perinatal outcomes of pregnancy loss, stillbirth, PTB, SGA, and neonatal mortality among perinatal women in Kenya.
Methods
Cohort selection
This analysis was nested in the PrEP implementation for Mothers in Antenatal Care study (PrIMA) which was a cluster randomized trial comparing two models for pre-exposure prophylaxis implementation among pregnant women in Western Kenya (NCT03070600).20 Women attending antenatal care (ANC) between Jan 15, 2018, and July 31, 2019 in 20 MCH clinics were screened and enrolled. Eligible women were pregnant, HIV-uninfected, ≥15 years old and were able to provide consent.
Study nurses collected information about demographics, pregnancy history, partner characteristics, and psychosocial factors through questionnaires administered in Kiswahili, Dholuo, or English languages using REDCap surveys.
Exposures
Experience of depressive symptoms was collected during pregnancy (enrolment visit) using the 10-item Center for Epidemiologic Studies Depression Scale (CESD-10), which has been adapted and validated among perinatal and general populations in sub-Saharan Africa.21,22 Participants rated each of 10 items from 0–3 based on past-week frequency (absolute score range: 0–30). Moderate-to-severe depressive symptoms (MSD) were defined as a validated cut-point of 10 or greater (CESD-10 score ≥ 10).23 Mild-to-severe depressive symptoms (Mild-SD) were defined as a cut-point of 5 or greater (CESD-10 score ≥ 5) to identify differences between women with “any” versus “no” depressive symptoms. The 18-item Medical Outcomes Study Social Support Survey (MOS-SSS; range: 18–90), which has been previously used among African women, was used to assess social support.24–26 This variable was dichotomized with a cut-point of <72 denoting low social support (LSS). Scores <72 indicate that women reported not having access “most of the time” to all forms of social support. The 4-item Hurt, Insult, Threaten, Scream Scale, which has been adapted for SSA populations, assessed intimate partner violence (IPV) based on a cut-point of 10 or greater (range: 4–20).27,28
Outcomes
Study nurses determined gestational age at enrolment by ascertainment of last menstrual period (LMP) or fundal height (if assessed). Data on birth outcomes were collected at the 6-week postpartum visit and abstracted from medical records. Information was collected about pregnancy loss (<20 weeks’ gestation), stillbirth (fetal death ≥20 weeks’ gestation), gestational age at delivery (weeks), and birthweight (kg). Late stillbirth was defined as fetal death ≥28 weeks’ gestation. PTB was defined as delivery <37 weeks’ gestation. Infant SGA was defined using the World Health Organization (WHO) Fetal Growth Standards to identify infants in the lowest 10th percentile for birthweight for respective gestational age week and sex.29 Verbal autopsies were performed by a study nurse with the mother or caregiver for pregnancy loss, stillbirth, or neonatal death (up to 28 days post-delivery).
Covariates
We hypothesized that multiple characteristics confound the relationships of interest. Household crowding,30 defined as a ratio of people per room greater than the median (>2 people/room), was used as a marker of socioeconomic status. A validated risk score, developed to predict HIV acquisition among perinatal women in SSA, was used to define high HIV risk.31 Self-perceived risk for HIV acquisition was measured by asking participants “What is your gut feeling about how likely you are to get infected with HIV?”, with five Likert response options. We defined high self-perceived HIV risk as “somewhat likely”, “very likely”, or “extremely likely” compared to “very unlikely” or “extremely unlikely”.32
Data were collected at study visits monthly during pregnancy and at 6 weeks, 14 weeks, 6 months, and 9 months postpartum.
Statistical analysis
Participants were included in the analysis if they had data on gestational age at pregnancy outcome (values >44 weeks were considered missing), did not acquire HIV, had a singleton birth, and had depressive symptom information during pregnancy. Incidence of pregnancy loss and stillbirth were evaluated among those enrolled <20 weeks’ gestation to alleviate selection bias. Similarly, late stillbirth was assessed among those enrolled at <28 weeks, and PTB incidence among participants enrolled at <37 weeks gestation. The SGA analysis was limited to live births with infant birthweight data. Neonatal mortality was evaluated among all live births.
Two different definitions were used for “any adverse perinatal outcome” among different sub-groups. First, we estimated the risks of pregnancy loss, stillbirth, PTB, or neonatal death among all pregnancies (n = 4153) to evaluate the most inclusive number of pregnancies. Second, we separately assessed the occurrence of pregnancy loss, stillbirth, PTB, SGA, or neonatal death in a more restricted group of pregnancies enrolled <20 weeks gestation with birthweight data for live births (n = 625) (Figure 1). By evaluating the most- and least-inclusive groups, we offer a reasonable range of risk for any adverse perinatal outcome.
Figure 1. Flow diagram for PrIMA study population.
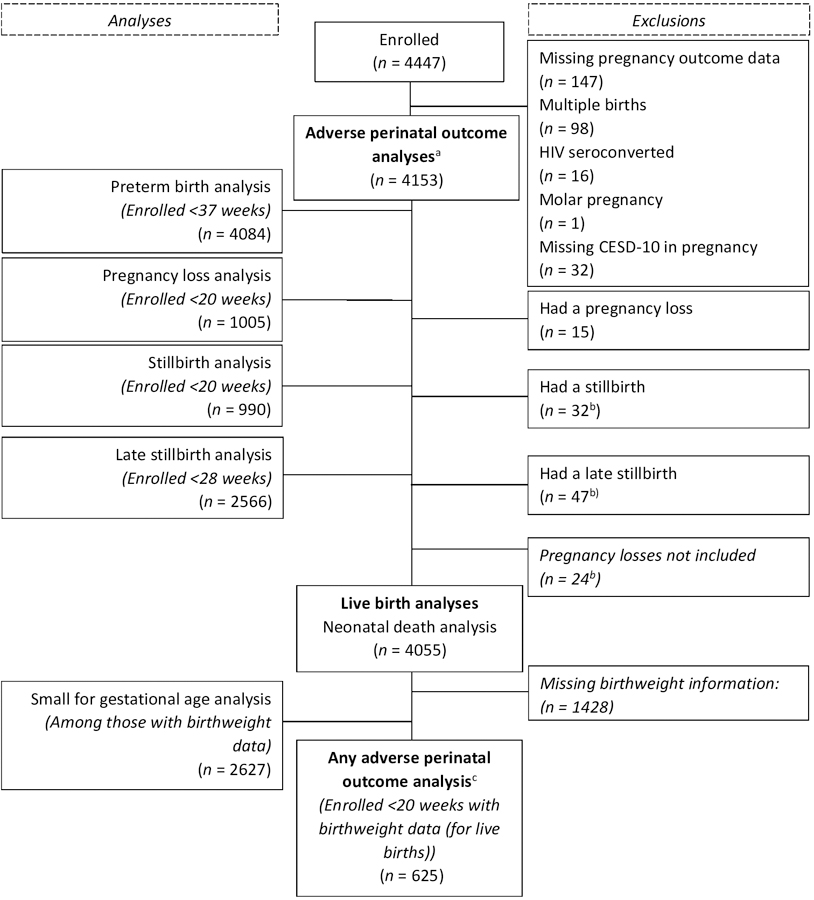
aAny adverse perinatal outcome: pregnancy loss, stillbirth, preterm birth, small for gestational age, or neonatal death; among those enrolled <20 weeks and with birthweight data (for live births).
bThe stillbirth cases included in the “stillbirth” analysis and “late stillbirth” analysis overlap by N = 20. There were N = 24 stillbirths not included in either stillbirth analysis based on ineligible enrollment age.
cAny adverse perinatal outcome: pregnancy loss, stillbirth, preterm birth, or neonatal death; among all eligible pregnancies.
Cox proportional hazards models were used to assess relationships between psychosocial factors of depressive symptom score, mild-SD, MSD, LSS, and IPV with time to pregnancy loss, stillbirth, PTB, SGA, and neonatal death, clustered by the facility. When case counts were <5, regression analyses were not performed. Time from enrolment gestational age to gestational age at the adverse perinatal outcome was used for time-at-risk for cases, except for the neonatal death analyses which used the time from birth. Time-at-risk for non-cases was gestational age at the end of the at-risk period or gestational age at pregnancy end, whichever came first. Gestational age at enrolment served as the start time to account for left truncation.33 Start time was set at 20 weeks gestation for the stillbirth analysis and 28 weeks gestation for the late stillbirth analysis based on the at-risk period. In the “any adverse perinatal outcome” analyses, survival time to neonatal death was gestational age plus time from birth to death (cases) and gestational age plus 28 days postpartum (non-cases).
Variables hypothesized as confounders were included in multivariable models, per our conceptual model (Table 3, Figure 2).2,13,34,35 In each model, the two psychosocial factors that were not being evaluated as the main exposure (MSD, LSS, and/or IPV) were included. Multivariable analyses were performed for relationships between psychosocial factors and adverse perinatal outcomes identified in univariable analyses. Population attributable risk percentages (PAR%) were estimated for each psychosocial risk factor; we did not estimate PAR for protective or continuous variables.
Table 3.
Psychosocial correlates of adverse perinatal outcomes and population attributable risk percentages among PrIMA study participants
| Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|
| HRd (95% CI) | aHRe (95% CI) | PAR (95% CI) | |
| Exposure: CESD-10 score c | |||
| Pregnancy loss (n=1005) | 1.73 (1.13, 2.65) | 2.59 (1.71, 3.92) | |
| Preterm birth (n=4084) | 1.24 (0.99, 1.54) | 1.25 (1.01, 1.55) | |
| Any adverse perinatal outcome (n=4153)a | 1.25 (1.00, 1.56) | 1.28 (1.02, 1.60) | |
| Exposure: Mild-SD vs No Mild-SD | |||
| Preterm birth (n=4084) | 1.46 (1.07, 1.99) | 1.39 (1.03, 1.87) | 17.3% (2.6, 29.8) |
| Any adverse perinatal outcome (n=4153)a | 1.42 (1.07, 1.89) | 1.40 (1.02, 1.90) | 17.6% (2.6, 30.3) |
| Exposure: MSD vs. No MSD | |||
| Pregnancy loss (n=1005) | 2.81 (1.32, 5.99) | 5.04 (2.44, 10.42) | 37.4% (30.3, 43.8) |
| Exposure: LSS vs. higher social support | |||
| Stillbirth (n=990) | 2.06 (1.03, 4.12) | 2.37 (1.14, 4.94) | 29.9% (12.0, 44.1) |
| Late stillbirth (n=2563) | 1.40 (0.99, 1.97) | 1.27 (0.88, 1.85) | |
| Any adverse perinatal outcome (n=625)b | 1.59 (1.32, 1.92) | 1.56 (1.24, 1.97) | 14.0% (8.0, 19.6) |
| Any adverse perinatal outcome (n=4152)a | 1.35 (0.97, 1.88) | 1.34 (0.98, 1.85) | |
| Exposure: IPV vs. No IPV | |||
| Preterm birth (n=4084) | 0.77 (0.59, 0.99) | 0.74 (0.54, 1.01) | |
| Any adverse perinatal outcome (n=4152)a | 0.79 (0.61, 1.02) | 0.77 (0.57, 1.05) |
Any adverse perinatal outcome: pregnancy loss, stillbirth, preterm birth, small for gestational age, or neonatal death; among those enrolled <20 weeks and with birthweight data (for live births).
Any adverse perinatal outcome: pregnancy loss, stillbirth, preterm birth, or neonatal death; among all eligible pregnancies
CESD-10 score was re-scaled to reflect a 10-unit change in score
Hazards Ratios (HR) estimated from Cox Regression, clustered by site
Adjusted HRs were estimated from models including: the exposure of interest (CESD-10 score, Mild-SD, MSD, LSS, IPV) and maternal age (years, continuous), educational attainment (years, continuous), regular employment (yes/no), married/living with a partner (yes/no), household crowding (yes/no), multiparous (yes/no), prior adverse perinatal outcome (pregnancy loss, stillbirth, or preterm birth, yes/no), high HIV risk (yes/no), self-perceived high HIV risk (Extremely/very likely vs Somewhat/very/extremely unlikely), PrEP uptake in pregnancy (yes/no), ANC attendance (Total visits attended by enrollment [pregnancy loss analysis], ≥4 ANC visits attended vs. <4 ANC visits [all other analysis]), and (the other psychosocial factors (MSD, low social support, or intimate partner violence). CESD-10: Center for epidemiologic studies depression scale-10
Mild-SD: Mild-to-severe depressive symptoms
MSD: Moderate-to-severe depressive symptoms
LSS: Low social support
IPV: Intimate partner violence
Figure 2. Conceptual model for depression and adverse perinatal outcomes.
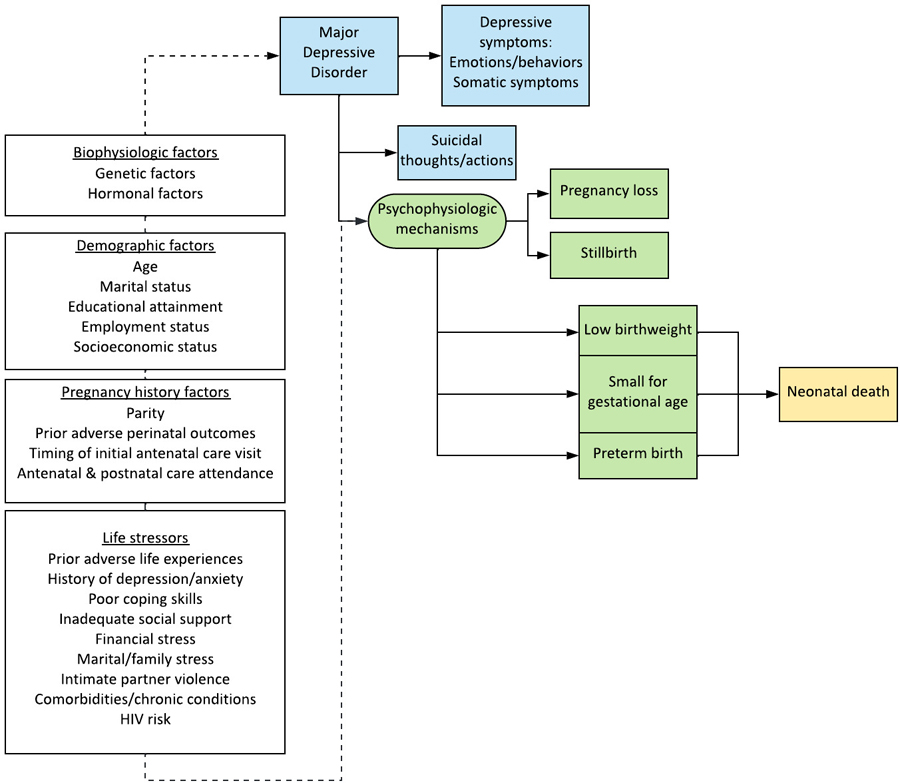
References for figure components:
Major depressive disorder, depressive symptoms:
Fisher J, Cabral de Mello M, Patel V, et al. Prevalence and determinants of common perinatal mental disorders in women in low- and lower-middle-income countries: a systematic review. Bull World Health Organ 2012; 90: 139–149H.
Predictors of peripartum depression:
Fisher J, Cabral de Mello M, Patel V, et al. Prevalence and determinants of common perinatal mental disorders in women in low- and lower-middle-income countries: a systematic review. Bull World Health Organ 2012; 90: 139–149H.
Stein A, Pearson RM, Goodman SH, et al. Effects of perinatal mental disorders on the fetus and child. Lancet 2014; 384: 1800–19.
Lancaster CA, Gold KJ, Flynn HA, Yoo H, Marcus SM, Davis MM. Risk factors for depressive symptoms during pregnancy: a systematic review. Am J Obstet Gynecol 2010; 202: 5–14.
Psychophysiologic mechanisms:
Payne JL, Maguire J. Pathophysiological mechanisms implicated in postpartum depression. Front Neuroendocrinol 2019; 52: 165–80.
Low birthweight:
Jarde A, Morais M, Kingston D, et al. Neonatal Outcomes in Women With Untreated Antenatal Depression Compared With Women Without Depression. JAMA Psychiatry 2016; 73: 826.
Grigoriadis S, VonderPorten EH, Mamisashvili L, et al. The Impact of Maternal Depression During Pregnancy on Perinatal Outcomes. J Clin Psychiatry 2013; 74: e321–41.
Small for gestational age:
Jarde A, Morais M, Kingston D, et al. Neonatal Outcomes in Women With Untreated Antenatal Depression Compared With Women Without Depression. JAMA Psychiatry 2016; 73: 826.
Preterm birth:
Jarde A, Morais M, Kingston D, et al. Neonatal Outcomes in Women With Untreated Antenatal Depression Compared With Women Without Depression. JAMA Psychiatry 2016; 73: 826.
Grigoriadis S, VonderPorten EH, Mamisashvili L, et al. The Impact of Maternal Depression During Pregnancy on Perinatal Outcomes. J Clin Psychiatry 2013; 74: e321–41.
Grote NK, Bridge JA, Gavin AR, Melville JL, Iyengar S, Katon WJ. A Meta-analysis of Depression During Pregnancy and the Risk of Preterm Birth, Low Birth Weight, and Intrauterine Growth Restriction. Arch Gen Psychiatry 2010; 67: 1012.
Infant death:
Jacques N, de Mola CL, Joseph G, Mesenburg MA, da Silveira MF. Prenatal and postnatal maternal depression and infant hospitalization and mortality in the first year of life: A systematic review and meta-analysis. J Affect Disord 2019; 243: 201–8.
Missing data
For participants missing data in <5 out of 10 depressive symptom scale items (11.8%, 492/4185, Appendix 1), item-level scores were imputed as the median score across the participant’s existing items (person-median imputation).36 Person-median imputation has advantages over other scale imputation methods; it does not artificially reduce variability and has been shown to produce similar estimates to multiple imputations.36 Among those missing <8 out of 16 social support scale items (2.6%, 108/4185, Appendix 2), we imputed item-level scores using person-median imputation. Scores were not analysed for participants missing over half of the scale items. Analyses were conducted using Stata 15 by StataCorp, LLC in College Station, Texas.
Ethics approval
The study protocol was approved by the Kenyatta National Hospital-University of Nairobi Ethics Research Committee and the University of Washington Human Subjects Review Committee. All participants provided written informed consent.
Results
Overall, 93.4% (n=4153) of the 4447 pregnant women enrolled in the parent study met the inclusion criteria for this analysis (Figure 1). The median maternal age was 24 years (Interquartile range [IQR]: 21–28) and the median educational attainment 10 years (Table 1). The median gestational age at enrolment was 24 weeks (IQR: 20–30), predominantly determined by LMP (fundal height was used for 2.2% [90/4153] of participants). Most participants were married or living with a partner (84.8%) and were multiparous (74.3%). Half of the women (50.5%) attended their first ANC visit during the second trimester, and most (88.0%) attended at least 4 ANC visits during pregnancy.
Table 1.
Baseline characteristics of PrIMA study participants included in MSD-birth outcomes analysis
| Overall (n=4153) | MSD (n=994) | No MSD (n=3159) | |||||
|---|---|---|---|---|---|---|---|
| Demographic characteristics | N | n (%) or Median (IQR) | Missing n (%) | n | n (%) or Median (IQR) | n | n (%) or Median (IQR) |
| Age (years) | 4151 | 24 (21, 28) | 2 (0.05%) | 993 | 24 (21, 29) | 3158 | 24 (21, 28) |
| Adolescents and young women (<24 years) | 4151 | 2369 (57.1%) | 2 (0.05%) | 993 | 566 (57.0%) | 3158 | 1803 (57.1%) |
| Gestational age (enrollment, weeks) | 4153 | 24 (20, 30) | 0 (0.0%) | 994 | 24 (20, 30) | 3159 | 24 (20, 30) |
| Married or living with a partner | 4119 | 3491 (84.8%) | 34 (0.8%) | 987 | 812 (82.3%) | 3132 | 2679 (85.5%) |
| Completed education (years) | 4074 | 10 (8, 12) | 81 (2.0%) | 971 | 10 (8, 12) | 3101 | 10 (8, 12) |
| Regularly employed | 4101 | 612 (14.9%) | 52 (1.3%) | 982 | 121 (12.3%) | 3119 | 491 (15.7%) |
| Household crowding (≥2 people/room) | 4126 | 1995 (48.4%) | 27 (0.7%) | 989 | 532 (53.8%) | 3137 | 1463 (46.6%) |
| Pregnancy history & factors | |||||||
| Primiparous | 4148 | 1065 (25.7%) | 5 (0.1%) | 993 | 235 (23.7%) | 3155 | 830 (26.3%) |
| Multiparous | 4148 | 3083 (74.3%) | 5 (0.1%) | 993 | 758 (76.3%) | 3159 | 2325 (73.7%) |
| No prior preterm birth | 3083 | 3041 (98.6%) | 0 (0.0%) | 758 | 750 (98.9%) | 2325 | 2291 (98.5%) |
| Prior preterm birth | 3083 | 42 (1.4%) | 0 (0.0%) | 758 | 8 (1.1%) | 2325 | 34 (1.5%) |
| No prior pregnancy loss | 3083 | 2645 (85.9%) | 0 (0.0%) | 758 | 639 (84.3%) | 2325 | 2008 (86.4%) |
| Prior pregnancy loss | 3083 | 436 (14.1%) | 0 (0.0%) | 758 | 119 (5.1%) | 2325 | 317 (13.6%) |
| Trimester of initial antenatal care (ANC) visit | 4153 | 0 (0.0%) | |||||
| First | 4153 | 615 (14.8%) | 0 (0.0%) | 994 | 128 (12.9%) | 3159 | 487 (15.4%) |
| Second | 4153 | 2098 (50.5%) | 0 (0.0%) | 994 | 500 (50.3%) | 3159 | 1598 (50.6%) |
| Third | 4153 | 1440 (34.7%) | 0 (0.0%) | 994 | 366 (36.8%) | 3159 | 1074 (34.0%) |
| Attended at least 4 ANC visits (before pregnancy end) | 4153 | 3655 (88.0%) | 0 (0.0%) | 994 | 876 (88.1%) | 3159 | 2779 (88.0%) |
| HIV risk factors | |||||||
| High HIV riska | 4153 | 1542 (37.1%) | 0 (0.0%) | 994 | 454 (45.7%) | 3159 | 1088 (34.4%) |
| Self-perceived HIV risk | 4146 | 369 (8.9%) | 7 (0.2%) | 993 | 124 (12.5%) | 3153 | 245 (7.8%) |
| Lifetime sexual partners | 4148 | 2 (2, 3) | 5 (0.1%) | 994 | 3 (2, 4) | 3154 | 2 (2, 3) |
| Lifetime sexual partners (>2) | 4148 | 3448 (83.1%) | 5 (0.1%) | 994 | 871 (87.6%) | 3154 | 2577 (81.7%) |
| Partner age difference >10 yearsb | 3182 | 497 (15.6%) | 917 (23.4%) | 736 | 118 (16.0%) | 2446 | 379 (15.5%) |
| Partner HIV-positiveb | 4101 | 176 (4.3%) | 0 (0.0%) | 979 | 67 (6.8%) | 3122 | 109 (3.5%) |
| Partner HIV status unknownb | 4101 | 1296 (31.6%) | 0 (0.0%) | 979 | 370 (37.8%) | 3122 | 926 (29.7%) |
| Sexually transmitted infection (enrollment) | 4146 | 104 (2.5%) | 7 (0.2%) | 992 | 45 (4.5%) | 3154 | 59 (1.9%) |
| PrEP Use in pregnancy | 4153 | 551 (13.3%) | 0 (0.0%) | 994 | 192 (19.3%) | 3159 | 359 (11.4%) |
| Psychosocial characteristics | |||||||
| Ever drink alcohol | 4135 | 168 (4.1%) | 18 (0.4%) | 985 | 50 (5.1%) | 3150 | 118 (3.7%) |
| Social support scorec | 4153 | 75 (63, 88) | 0 (0.0%) | 994 | 70 (55, 81) | 3159 | 78 (66, 89) |
| Low social support (MOS-SSS score <72) | 4153 | 1550 (37.3%) | 0 (0.0%) | 994 | 521 (52.4%) | 3159 | 1029 (32.6%) |
| Intimate partner violenced (HITS score ≥10) | 4148 | 323 (7.8%) | 5 (0.1%) | 993 | 170 (17.1%) | 3155 | 153 (4.8%) |
| CESD-10 score | 4153 | 5 (3, 9) | 0 (0.0%) | 994 | 13 (11, 16) | 3159 | 4 (2, 6) |
| Mild-to-severe depressive symptoms (CESD-10≥5) | 4153 | 2273 (54.7%) | 0 (0.0%) | 994 | 994 (100.0%) | 3159 | 1279 (40.5%) |
| Moderate-to-severe depressive symptoms (CESD-10≥10) | 4153 | 994 (23.9%) | 0 (0.0%) | -- | -- | ||
We evaluated HIV risk using the Pintye et al.31 risk score (high HIV risk: score >6 = “Yes”, score ≤6 = “No”).
Among those with current partners
We evaluated social support using the 18-item Medical Outcomes Study social support score (MOS-SSS), defining low social support as scores below 72 (Low social support: MOS-SSS score score <72 = “Yes”, MOS-SSS score ≥ 72 = “No”).
We evaluated intimate partner violence using the 4-item Hurt, Insult, Threaten, and Scream scale (HITS), defining intimate partner violence as scores of 10 and above (IPV: HITS score ≥10 = “Yes”, HITS score <10 = “No”).
CESD-10: Center for epidemiologic studies depression scale-10
MSD: Moderate-to-severe depressive symptom
About a quarter (23.9%) of women reported MSD during pregnancy (median CESD-10 score: 5, IQR: 3–9). Over 50% of women reported mild SD. Over a third (37.3%) had low social support, and 7.8% reported IPV within two weeks prior to enrolment.
Pregnancy loss
Pregnancy loss was experienced by 1.5% (15/1005) of women enrolled <20 weeks gestation (Table 2), over 111.3 fetus-years of follow-up until 20 weeks gestation (incidence rate [IR] 13.5 pregnancy losses per 100 fetus-years) (Appendix 3). The median gestational age at pregnancy loss was 15.0 weeks (IQR 12.1, 17.7). Women reporting MSD were over twice as likely to experience pregnancy loss compared to those without MSD (Appendix 4). This relationship became stronger after confounding adjustment (Table 3). A ten-unit increase in CESD-10 score was associated with at least 70% higher risk of pregnancy loss (Table 3, Figure 3).
Table 2.
Cumulative incidence of adverse perinatal outcomes among pregnant and postpartum women in Western Kenya in the PrIMA study
| Adverse perinatal outcomes | Exposed | Cases | N | Cumulative incidence (CI) per 1000 pregnancies (95% Confidence interval [CI]) | Unexposed | Cases | N | CI per 1000 pregnancies (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Pregnancy loss (<20 weeks) (n=1005) | Overall | 15 | 990 | 14.9 (8.1, 22.4) | ||||
| MSD | 7 | 223 | 31.4 (15.0, 64.6) | No MSD | 8 | 782 | 10.2 (5.1, 20.3) | |
| Mild-SD | 10 | 525 | 19.0 (10.3, 35.1) | No Mild-SD | 5a | 480 | 10.4 (4.3, 24.8) | |
| LSS | 2a | 333 | 6.0 (1.5, 23.8) | No LSS | 13 | 672 | 19.3 (11.3, −33.1) | |
| IPV | 1a | 80 | 12.5 (1.7, 85.8) | No IPV | 14 | 925 | 15.1 (9.0, 25.4) | |
|
| ||||||||
| Stillbirth (≥ 20 weeks) (n=990) | Overall | 32 | 990 | 32.3 (22.9, 45.4) | ||||
| MSD | 4a | 216 | 18.5 (6.9, 48.5) | No MSD | 28 | 774 | 36.2 (25.1, 51.9) | |
| Mild-SD | 15 | 515 | 29.1 (17.6, 47.8) | No Mild-SD | 17 | 475 | 35.8 (22.3, 56.9) | |
| LSS | 16 | 331 | 48.3 (29.8, 77.6) | No LSS | 16 | 659 | 24.3 (14.9, 39.3) | |
| IPV | 3a | 79 | 37.9 (12.1, 113.0) | No IPV | 29 | 911 | 31.8 (22.2, 45.5) | |
|
| ||||||||
| Late stillbirth (≥28 weeks) (n=2566) | Overall | 47 | 2566 | 18.3 (13.8, 24.3) | ||||
| MSD | 11 | 599 | 18.3 (10.2, 32.9) | No MSD | 36 | 1964 | 18.3 (13.2, 25.3) | |
| Mild-SD | 28 | 1418 | 19.7 (13.6, 28.5) | No Mild-SD | 19 | 1145 | 16.6 (10.6, 25.9) | |
| LSS | 21 | 900 | 23.3 (15.2, 35.5) | No LSS | 26 | 1663 | 15.6 (10.7, 22.9) | |
| IPV | 4a | 200 | 20.0 (7.5, 52.4) | No IPV | 43 | 2359 | 18.2 (13.5, 24.5) | |
|
| ||||||||
| Preterm birth (<37 weeks) (n=4084) | Overall | 780 | 4084 | 191.0 (179.2, 203.3) | ||||
| MSD | 202 | 975 | 207.2 (182.9, 233.8) | No MSD | 578 | 3109 | 185.9 (172.6, 200.0) | |
| Mild-SD | 492 | 2239 | 219.7 (203.1, 237.4) | No Mild-SD | 288 | 1845 | 156.1 (140.2, 173.4) | |
| LSS | 332 | 1522 | 218.1 (198.1, 239.6) | No LSS | 448 | 2562 | 174.9 (160.6, 190.1) | |
| IPV | 48 | 318 | 150.9 (115.5, 194.8) | No IPV | 730 | 3761 | 194.1 (181.8, 207.1) | |
|
| ||||||||
| Small for gestational age (n=2627) | Overall | 263 | 2627 | 100.1 (89.2, 112.2) | ||||
| MSD | 77 | 699 | 110.2 (89.0, 135.6) | No MSD | 186 | 1928 | 96.5 (84.1, 110.5) | |
| Mild-SD | 155 | 1486 | 104.3 (89.7, 120.9) | No Mild-SD | 108 | 1141 | 94.7 (79.0, 113.1) | |
| LSS | 88 | 881 | 99.9 (81.7, 121.5) | No LSS | 175 | 1746 | 100.2 (87.0, 115.2) | |
| IPV | 20 | 225 | 88.9 (57.9, 134.0) | No IPV | 243 | 2400 | 101.3 (89.8, 114.0) | |
|
| ||||||||
| Neonatal death (within 28 days of life) (n=4055) | Overall | 63 | 4055 | 15.5 (12.8, 20.7) | ||||
| MSD | 14 | 967 | 14.5 (8.6, 24.3) | No MSD | 52 | 3088 | 16.8 (12.9, 22.0) | |
| Mild-SD | 40 | 2215 | 18.1 (13.3, 24.5) | No Mild-SD | 26 | 1840 | 14.1 (9.6, 20.7) | |
| LSS | 25 | 1516 | 16.5 (11.2, 24.3) | No LSS | 41 | 2539 | 16.1 (11.9, 21.9) | |
| IPV | 3^ | 315 | 9.5 (3.1, 29.2) | No IPV | 63 | 3735 | 16.9 (13.2, 21.5) | |
|
| ||||||||
| Any adverse perinatal outcome (n=625)b | Overall | 201 | 625 | 321.6 (286.1, 359.3) | ||||
| MSD | 58 | 163 | 335.8 (285.7, 432.8) | No MSD | 143 | 462 | 309.5 (268.9, 353.3) | |
| Mild-SD | 117 | 346 | 338.2 (290.1, 389.8) | No Mild-SD | 84 | 279 | 301.1 (249.9, 357.7) | |
| LSS | 79 | 201 | 393.0 (327.5, 462.6) | No LSS | 122 | 424 | 287.7 (246.5, 332.8) | |
| IPV | 13 | 49 | 265.3 (158.5, 409.0) | No IPV | 188 | 576 | 326.4 (289.2, 365.9) | |
|
| ||||||||
| Any adverse perinatal outcome (n=4153)c | Overall | 1132 | 4153 | 272.6 (259.2, 286.3) | ||||
| MSD | 297 | 994 | 298.8 (271.1, 328.0) | No MSD | 835 | 3159 | 264.3 (249.2, 280.0) | |
| Mild-SD | 694 | 2273 | 305.3 (286.7, 324.6) | No Mild-SD | 438 | 1880 | 233.0 (214.4, 252.6) | |
| LSS | 447 | 1550 | 288.4 (266.4, 311.5) | No LSS | 685 | 2603 | 263.2 (246.6, 280.4) | |
| IPV | 75 | 323 | 232.2 (189.2, 281.6) | No IPV | 1055 | 3825 | 275.8 (261.9, 290.2) | |
For instances with case counts ≤5 in an exposure group, we did not perform regression analyses
Any adverse perinatal outcome: pregnancy loss, stillbirth, preterm birth, small for gestational age, or neonatal death; among those enrolled <20 weeks and with birthweight data (for live births)
Any adverse perinatal outcome: pregnancy loss, stillbirth, preterm birth, or neonatal death; among all eligible pregnancies
CESD-10: Center for epidemiologic studies depression scale-10
CI: Cumulative Incidence per 1000 pregnancies
Exposed/Unexposed: “Exposures” are psychosocial factors including mild-to-severe depressive symptoms (Mild-SD), moderate-to-severe depressive symptoms (MSD), low social support (LSS), and intimate partner violence (IPV)
Mild-SD: Mild-to-severe depressive symptoms
MSD: Moderate-to-severe depressive symptoms
LSS: Low social support
IPV: Intimate partner violence
Figure 3. Associations between depressive symptoms and adverse perinatal outcomes among PrIMA study participants.
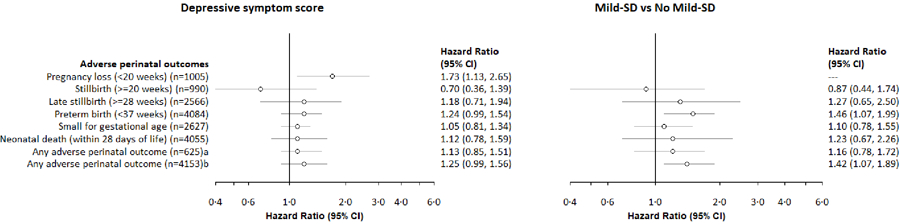
aHR: Hazard ratios are from Cox Regression Models, clustered by facility used for outcomes of: pregnancy loss, stillbirth, late stillbirth, preterm birth, neonatal death, any adverse perinatal outcome
bFor instances with case counts ≤5 in an exposure group, we did not perform regression analyses
cCESD-10 score was re-scaled to reflect a 10-unit change in score
dAny adverse perinatal outcome: pregnancy loss, stillbirth, preterm birth, small for gestational age, or neonatal death; among those enrolled <20 weeks and with birthweight data (for live births)
eAny adverse perinatal outcome: pregnancy loss, stillbirth, preterm birth, or neonatal death; among all eligible pregnancies
CESD-10: Center for epidemiologic studies depression scale-10
Mild-SD: Mild-to-severe depressive symptoms
MSD: Moderate-to-severe depressive symptoms
LSS: Low social support
IPV: Intimate partner violence
Stillbirth
Overall, 3.2% (32/990) of women enrolled <20 weeks gestation experienced stillbirth (Table 2) over 337.9 fetus-years of follow-up starting at 20 weeks gestation (IR: 9.5 stillbirths per 100 fetus-years) (Appendix 3). Stillbirths occurred at a median gestational age of 35.4 weeks (IQR: 25.6, 38.3). Women reporting low social support (LSS) had over double the risk of stillbirth (Table 3, Appendix 5). Late stillbirth occurred in 1.8% (47/2563) of women enrolled <28 weeks gestation over 542.1 fetus-years (IR: 8.7 per 100 fetus-years).
Preterm birth
PTB occurred among 19.1% (780/4084) of mother-infant pairs enrolled <37 weeks gestation (Table 2) over 1099.3 fetus-years of follow-up (IR 71.0 PTB per 100 fetus-years) and at median gestational age of 36.0 weeks (IQR 35.3, 36.0). Mild-SD was associated with increased risk for PTB (Table 3, Figure 3). There was a pattern for higher CESD-10 scores associated with higher risk of PTB, yet this estimate became only marginally more precise after adjustment (Table 3, Figure 3). IPV in pregnancy was inversely related to risk of PTB (Table 3, Appendix 6), yet this relationship did not persist after confounding adjustment.
Small for gestational age
Overall, 64.7% (2627/4055) of live births had birthweight data and were included in the SGA analyses. SGA occurred among 10% (263/2627) mother-infant pairs (Table 2) over 682.9 fetus-years of follow-up (IR 38.5 SGA births per 100 fetus-years). There was no evidence of relationships between psychosocial factors and SGA.
Neonatal death
Among 4,055 live births, 63 deaths occurred in the first 28 days postpartum (1.6%), for a cumulative mortality of 16 deaths per 1000 live births (Table 2). Neonatal deaths took place over 306.8 fetus-years (IR 20.5 cases per 100 fetus-years). The median age at neonatal death was 1 day (IQR 0, 7.5). There was no evidence of relationships between psychosocial factors and neonatal mortality.
Any adverse perinatal outcome
Over a quarter (27.3%, 1132/4153) of pregnancies resulted in at least one adverse perinatal outcome (Table 2), for an incidence rate of 63.8 per 100 fetus-years). Any adverse perinatal outcome was more likely with maternal mild-SD, and a 10-unit higher CESD-10 score (Table 3, Figure 3). In the subset of mother-infant pairs enrolled <20 weeks gestation with birthweight data, 32.2% (201/625) had any adverse perinatal outcome for an incidence rate of 64.7 per 100 fetus-years. Risk of any adverse outcomes was higher among women reporting low social support than those with higher social support (Table 3, Appendix 5).
Population-level impact
Based on population attributable risk proportions, over a third of pregnancy losses were attributable to MSD (Table 3), and a third of stillbirth cases were attributable to low social support. About 17.3% of PTB and 17.6% of any adverse perinatal outcome cases were attributable to having mild SD. Among those enrolled <20 weeks gestation and with birthweight data, 14% of any adverse perinatal outcome cases were attributable to low social support.
Prior to imputing CESD-10 and MOS-SSS items for partial scores, 23.9% of women had MSD and 37.3% had low social support. Those with complete vs. partial CESD-10 data were not meaningfully different (Appendix 7).
Comment
Principal findings
In this large prospective analysis among mother-infant pairs followed from pregnancy through 28 days postpartum, mild- or moderate-to-severe depressive symptoms during pregnancy were common and associated with increased pregnancy loss and PTB. The risk of stillbirth was elevated among women with low levels of social support. Our results extend the global evidence base for the relationships between maternal mental distress and adverse perinatal outcomes, adding novel results on psychosocial factors and birth outcomes to the dearth of data on this topic from the African region. To our knowledge, this is the largest study to evaluate relationships between maternal mental health and >3 birth outcomes among African mother-infant pairs.19 Our findings that maternal depressive symptoms are associated with increased risk of pregnancy loss and PTB, and that lack of social support was associated with increased risk of stillbirth, highlight the potential need for integrated mental health services within MCH settings.
Strengths of the study
Prospective data from a large cohort (>4000) of pregnant women enabled rigorous evaluation of relationships between multiple psychosocial factors and adverse birth outcomes.
Limitations of the data
Selection bias, which disproportionately excluded earlier pregnancy losses and stillbirths, is evident in the cohort since only 24% (1005/4153) of pregnancies were enrolled prior to 20 weeks’ gestation and pregnancy losses were rare (1.5%) compared to the estimated 10–20% of pregnancies that result in spontaneous abortion.37,38 This selection bias may be responsible for the lack of stronger associations with more specific exposure measurement – we had expected to see more of a “dose-response” relationship with higher depressive symptoms (MSD) and adverse pregnancy outcomes compared to Mild-SD.
Further, we found a counterintuitive trend of lower PTB among women with IPV in pregnancy in contrast to findings from other East African settings.39,40 This finding was not retained in multivariable analyses and therefore may have been due to confounding. We also suspect that selection bias played a role in the counterintuitive relationship between IPV and adverse perinatal outcomes, wherein those experiencing both IPV and adverse pregnancy outcomes were disproportionately underrepresented in our sample.
For particularly rare perinatal outcomes and infrequent exposures, our study had modest power to detect associations. We predominantly estimated gestational age as the time between last menstrual period (LMP) and pregnancy end. LMP tends to overestimate gestational age which may have biased our risk estimates.41
We calculated population attributable risk percentages to estimate the proportion of adverse perinatal outcome cases in the population that were potentially attributable to the exposure of interest. PAR% are not additive across risk exposures and, therefore, should be interpreted with that limitation in mind.42
Interpretation
We found about a quarter (24%) of women were depressed during pregnancy, which closely aligned with results from a recent meta-analysis of antenatal depression in Africa (26%).1 Low social support (37%) and IPV (8%) during pregnancy were also similar to estimates from other studies among pregnant women in SSA (23%,43 13.5%,44 respectively). Half of women enrolled in this study initiated ANC during the second trimester, similar to national findings for Kenya (median gestational month of initial ANC: 5.4).14
Pregnancy loss incidence is not routinely evaluated in sub-Saharan Africa. Our finding of 15 pregnancy losses per 1000 pregnancies was about double the estimate from a Kenyan study using the Nairobi Urban Health and Demographic Surveillance System,45 likely due to underreporting and ascertainment challenges within the health system. Our estimate of pregnancy loss aligns with estimates from settings with more advanced monitoring systems for this outcome,46 yet is substantially below the global estimate that 10–20% of pregnancies result in spontaneous abortion.37,38 Our estimate of stillbirth risk was also higher than regional estimates.11 In our study, PTB occurred in 19% of births, somewhat higher than the 12% estimated for SSA overall.47,48 Our finding that 16 neonatal deaths occur per 1000 live births was slightly lower than a 2015 estimate of 22 deaths per 1000 live births.49
We used gestational age estimated by LMP, which has been previously shown to overestimate duration of gestation, yet a recent study in South Africa found LMP provided a reliable and valid estimate of gestational age compared to ultrasound (within 0.2 days).50 We used person-median imputation for CESD-10 and MOS-SSS items among participants missing fewer than half of scale items (12% and 2% of participants, respectively). In simulation studies, this method performs indistinguishably from multiple imputation of item-level psychosocial scale missingness and is recommended to optimize analytic power.36
In this large cohort of mother-infant pairs, maternal depressive symptoms in pregnancy were associated with multiple adverse birth outcomes. To date, few studies have evaluated associations between depression during pregnancy and pregnancy loss,51,52 likely due to challenges with statistical power and timing of measurement. The PrIMA study enrolled over 1000 pregnant women before 20 weeks gestation who were evaluated for pregnancy loss, among whom pregnancy loss risk was substantially increased with MSD. We found nearly 40% of pregnancy losses could be potentially prevented if MSD in pregnancy was eliminated in this population. Pregnancy loss risk increased with higher depressive symptom scores, strengthening inference and suggesting a potential “dose-response” relationship.
As in similar studies, depressive symptoms were not associated with stillbirth in our study. However, stillbirth incidence was double among women reporting low social support. We are not aware of other studies identifying this relationship, though a study in Ethiopia found an association between low social support and low birthweight.16 Social support is modifiable with low-intensity, evidence-based psychological interventions integrated in routine MCH care.53
There is strong global evidence for the influence of depression during pregnancy on PTB,5–7 including one study from Kenya.18 A study in Ghana also found a potential relationship, however the effect size was not substantial and the estimate had low precision.17 We found an association between mild-SD and PTB. There is need for further study of depression and PTB in SSA where >25% of the world’s PTB occur.47
Maternal depressive symptoms may influence pregnancy loss and PTB through physiologic mechanisms like heightened stress hormone levels, and through behavioural responses to depressive symptoms such as inadequate nutrition or insufficient sleep, which can affect foetal growth and length of gestation.54–56 Depressive symptoms may also adversely influence one’s health-seeking behaviour, potentially limiting opportunities for monitoring and early intervention.54
Psychosocial interventions in individual- or group-based formats, led by trained or lay counsellors, are effective in preventing and treating perinatal depression.57–59 Approaches like cognitive behaviour therapy and interpersonal psychotherapy utilize multiple sessions with a counsellor to reduce negative thought processes, improve interpersonal relationships, promote problem-solving, and reduce stress – ultimately disrupting the bio-physiological underpinnings of perinatal depression.57–59 Psychosocial interventions are increasingly evaluated in SSA settings, particularly those utilizing task-shifted models delivered by peer and lay counsellors integrated into well-attended public sector health settings (e.g., MCH or HIV care).60–62 These interventions show promise in SSA,60–62 yet further efforts are needed to rigorously identify successful approaches and expand access through integrated care models to support African perinatal women.
Conclusions
This study contributes new evidence for the relationships between maternal mental distress and birth outcomes contributing to high pregnancy loss and neonatal mortality in LMICs. Among this large cohort of Kenyan mother-infant pairs, we found that having mild- or moderate-to-severe depressive symptoms during pregnancy was common and associated with an elevated risk of pregnancy loss and preterm birth. Those with low social support also had higher risk of stillbirth. Closing the “last mile” in neonatal health should include integrating mental health services into MCH care to reduce maternal depression and depression-related neonatal outcomes. Interventions that increase social support and alleviate depressive symptoms may substantially reduce pregnancy loss, stillbirth, and preterm birth ultimately improving linked mother-infant health.
Synopsis.
Study question
Are psychosocial factors in pregnancy, including depression, low social support, and intimate partner violence associated with adverse perinatal outcomes among Kenyan mother-infant pairs?
What’s already known
Depression in pregnancy is common and is associated with preterm birth and infant death. Yet, these relationships are understudied in sub-Saharan Africa—the region with the highest burden of adverse perinatal outcomes. It is unclear whether maternal psychosocial factors are associated with adverse perinatal outcomes in Kenya.
What this study adds
Our study is the largest to date to assess maternal mental health and >3 birth outcomes among African mother-infant pairs. Novel findings include the relationship between moderate-to-severe depressive symptoms in pregnancy and elevated risk of pregnancy loss, and the relationship between low social support in pregnancy and stillbirth in Kenya.
Funding
This work was supported by the National Institute of Allergy and Infectious Disease (R01 AI125498 to GJS and P30AI027757), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (F31HD101149 to AL, 1F32HD108857 to AL, R01HD100201 to JP and R01 HD094630 to GJS). The funding agencies had no role in the writing of the manuscript or the decision to submit it for publication.
APPENDICES
Appendix 1.
Number of CESD-10 items missing among singleton births with pregnancy outcome data among women who did not acquire HIV by 9 months postpartum in the PrIMA study (n=4185)
| Number of CESD-10 items missing | N (%) |
|---|---|
| 0 | 3661 (87.5%) |
| 1 | 259 (6.2%) |
| 2 | 152 (3.6%) |
| 3 | 67 (1.6%) |
| 4 | 14 (0.3%) |
| 5 | 7 (0.2%) |
| 6 | 2 (0.1%) |
| 7 | 1 (0.02%) |
| 8 | 1 (0.02%) |
| 9 | 0 (0.0%) |
| 10 | 21 (0.5%) |
Appendix 2.
Number of MOS-SSS items missing among singleton births with pregnancy outcome data among women who did not acquire HIV by 9 months postpartum in the PrIMA study (n=4185)
| Number of MOS-SSS items missing | N (%) |
|---|---|
| 0 | 4077 (97.4%) |
| 1 | 80 (1.9%) |
| 2 | 7 (0.2%) |
| 3 | 3 (0.1%) |
| 4 | 18 (0.4%) |
Appendix 3.
Incidence of adverse perinatal outcomes by psychosocial factors among PrIMA study participants
| CESD-10 scorea | ||||||||
|---|---|---|---|---|---|---|---|---|
| Overall | Adverse perinatal outcome | Without adverse perinatal outcome | ||||||
| Adverse perinatal outcomes | Cases | Fetus-years | IR | 95% Confidence interval (CI) | Mean | St. Dev. (SD) | Mean | SD |
| Pregnancy loss (<20 weeks) (n=1005) | 15 | 111.3 | 13.5 | 8.1, 22.4 | 8.6 | 6.5 | 6.2 | 5.4 |
| Stillbirth (≥ 20 weeks) (n=990) | 32 | 337.9 | 9.5 | 6.7, 13.4 | 5.2 | 4.1 | 6.2 | 5.5 |
| Late stillbirth (≥28 weeks) (n=2566) | 47 | 542.1 | 8.7 | 6.5, 11.5 | 6.8 | 5.4 | 6.3 | 5.4 |
| Preterm birth (<37 weeks) (n=4084) | 780 | 1099.3 | 71.0 | 66.1, 76.1 | 7.0 | 5.3 | 6.2 | 5.4 |
| Small for gestational age (n=2627) | 263 | 682.9 | 38.5 | 34.1, 43.5 | 6.8 | 5.5 | 6.6 | 5.6 |
| Neonatal death (within 28 days of life) (n=4055) | 63 | 306.8 | 20.5 | 16.0, 26.3 | 6.6 | 5.4 | 6.3 | 5.4 |
| Any adverse perinatal outcome (n=625)b | 201 | 310.7 | 64.7 | 56.3, 74.3 | 7.0 | 5.6 | 6.5 | 5.7 |
| Any adverse perinatal outcome (n=4153)c | 864 | 1355.1 | 63.8 | 59.6, 68.2 | 7.0 | 5.3 | 6.2 | 5.4 |
| Mild-SD in pregnancy | No Mild-SD in pregnancy | |||||||
| Cases | Fetus-years | IR | 95% CI | Cases | Fetus-years | IR | 95% CI | |
| Pregnancy loss | 10 | 57.1 | 17.5 | 9.4, 32.5 | 5* | 54.1 | 9.2 | 3.8, 22.2 |
| Stillbirth | 15 | 175.1 | 8.6 | 5.2, 14.2 | 17 | 162.8 | 10.4 | 6.5, 16.8 |
| Late stillbirth | 28 | 297.7 | 9.4 | 6.5, 13.6 | 19 | 244.5 | 7.8 | 5.0, 12.2 |
| Preterm birth | 492 | 596.1 | 82.5 | 75.6, 90.2 | 288 | 503.2 | 57.2 | 51.0, 64.2 |
| Small for gestational age | 155 | 389.2 | 39.8 | 34.0, 46.6 | 108 | 293.8 | 36.8 | 30.4, 44.4 |
| Neonatal death | 38 | 167.4 | 22.7 | 16.5, 31.2 | 25 | 139.4 | 17.9 | 12.1, 26.5 |
| Any adverse perinatal outcome (n=625)b | 117 | 170.9 | 68.4 | 57.1, 82.0 | 84 | 139.7 | 60.1 | 48.5, 74.4 |
| Any adverse perinatal outcome (n=4153)c | 540 | 731.4 | 73.8 | 67.9, 80.3 | 324 | 623.7 | 51.9 | 46.6, 57.9 |
| MSD in pregnancy | No MSD in pregnancy | |||||||
| Cases | Fetus-years | IR | 95% CI | Cases | Fetus-years | IR | 95% CI | |
| Pregnancy loss | 7 | 25.9 | 27.1 | 12.9, 56.8 | 8 | 85.4 | 9.4 | 4.7, 18.7 |
| Stillbirth | 4 | 73.6 | 5.4 | 2.0, 14.5 | 28 | 264.3 | 10.6 | 7.3, 15.3 |
| Late stillbirth | 11 | 126.7 | 8.7 | 4.8, 15.7 | 36 | 415.5 | 8.7 | 6.3, 12.0 |
| Preterm birth | 202 | 256.8 | 78.7 | 68.5, 90.3 | 578 | 842.5 | 68.6 | 63.2, 74.4 |
| Small for gestational age | 77 | 180.3 | 42.7 | 34.2, 53.4 | 186 | 502.7 | 37.0 | 32.1, 42.7 |
| Neonatal death | 14 | 73.3 | 19.1 | 11.3, 32.2 | 49 | 233.5 | 21.0 | 15.9, 27.8 |
| Any adverse perinatal outcome (n=625)b | 58 | 79.9 | 72.6 | 56.1, 93.9 | 143 | 230.8 | 62.0 | 52.6, 73.0 |
| Any adverse perinatal outcome (n=4153)c | 224 | 317.1 | 70.6 | 62.0, 80.5 | 640 | 1038.1 | 61.7 | 57.1, 66.6 |
| Low social support in pregnancy | Not low social support in pregnancy | |||||||
| Cases | Fetus-years | IR | 95% CI | Cases | Fetus-years | IR | 95% CI | |
| Pregnancy loss | 2 | 33.5 | 6.0 | 1.5, 23.9 | 13 | 77.7 | 16.7 | 9.7, 28.8 |
| Stillbirth | 16 | 110.9 | 14.4 | 8.8, 23.6 | 16 | 227.1 | 7.0 | 4.3, 11.5 |
| Late stillbirth | 21 | 189.0 | 11.1 | 7.2, 17.0 | 26 | 353.1 | 7.4 | 5.0, 10.8 |
| Preterm birth | 332 | 386.1 | 86.0 | 77.2, 95.8 | 448 | 713.2 | 62.8 | 57.3, 68.9 |
| Small for gestational age | 88 | 215.0 | 40.9 | 33.2, 50.4 | 175 | 467.9 | 37.4 | 32.2, 43.4 |
| Neonatal death | 24 | 114.9 | 20.9 | 14.0, 31.2 | 39 | 191.9 | 20.3 | 14.8, 27.8 |
| Any adverse perinatal outcome (n=625)b | 79 | 96.3 | 82.0 | 65.8, 102.3 | 122 | 214.4 | 56.9 | 47.7, 68.0 |
| Any adverse perinatal outcome (n=4153)c | 363 | 478.4 | 75.9 | 68.5, 84.1 | 501 | 876.7 | 57.1 | 52.4, 62.4 |
| IPV in pregnancy | No IPV in pregnancy | |||||||
| Cases | Fetus-years | IR | 95% CI | Cases | Fetus-years | IR | 95% CI | |
| Pregnancy loss | 1 | 9.0 | 11.1 | 1.6, 78.9 | 14 | 102.3 | 13.7 | 8.1, 32.1 |
| Stillbirth | 3 | 26.9 | 11.1 | 3.6, 34.5 | 29 | 311.0 | 9.3 | 6.5, 13.4 |
| Late stillbirth | 4 | 42.9 | 9.3 | 3.5, 24.8 | 43 | 498.4 | 8.6 | 6.4, 11.6 |
| Preterm birth | 48 | 86.7 | 55.4 | 41.7, 73.5 | 730 | 1011.3 | 72.2 | 67.1, 77.6 |
| Small for gestational age | 20 | 57.0 | 35.1 | 22.6, 54.3 | 243 | 625.3 | 38.9 | 34.3, 44.1 |
| Neonatal death | 3* | 24.0 | 12.5 | 4.0, 38.8 | 60 | 282.4 | 21.2 | 16.5, 27.4 |
| Any adverse perinatal outcome (n=625)b | 13 | 23.8 | 54.7 | 31.7, 94.1 | 188 | 286.9 | 65.5 | 56.8, 75.6 |
| Any adverse perinatal outcome (n=4153)c | 55 | 107.5 | 51.2 | 39.3, 66.6 | 807 | 1246.1 | 64.8 | 60.4, 69.4 |
CESD-10 score was re-scaled to reflect a 10-unit change in score
Any adverse perinatal outcome: miscarriage, stillbirth, preterm birth, small for gestational age, or neonatal death; among those enrolled <20 weeks and with birthweight data
Any adverse perinatal outcome: miscarriage, stillbirth, preterm birth, or neonatal death; among all pregnancies
IR: Incidence rate per 100 fetus-years
Mild-SD: Mild-to-severe depressive symptoms
MSD: Moderate-to-severe depressive symptoms
LSS: Low social support
IPV: Intimate partner violence
Appendix 4. Associations between moderate-to-severe depressive symptoms and adverse perinatal outcomes among PrIMA study participants.
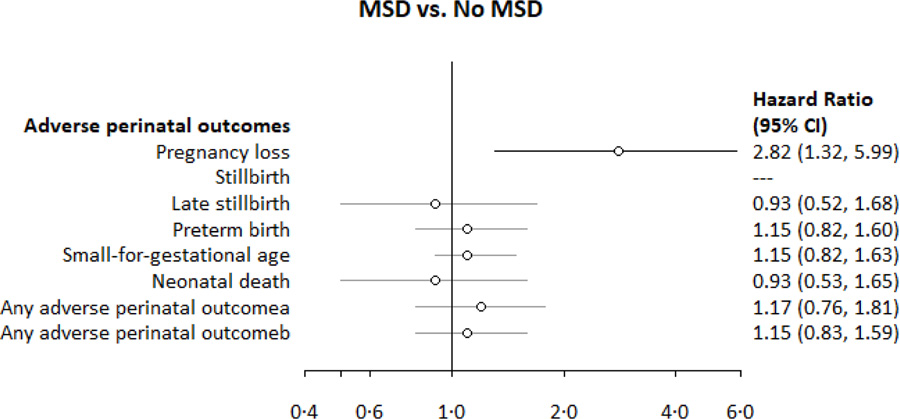
aAny adverse perinatal outcome: pregnancy loss, stillbirth, preterm birth, small for gestational age, or neonatal death; among those enrolled <20 weeks and with birthweight data (for live births)
bAny adverse perinatal outcome: pregnancy loss, stillbirth, preterm birth, or neonatal death; among all eligible pregnancies
HR: Hazard ratios are from Cox Regression Models, clustered by facility used for outcomes of: pregnancy loss, stillbirth, late stillbirth, preterm birth, neonatal death, any adverse perinatal outcome
For instances with case counts ≤5 in an exposure group, we did not perform regression analyses
CESD-10 score was re-scaled to reflect a 10-unit change in score
CESD-10: Center for epidemiologic studies depression scale-10
MSD: Moderate-to-severe depressive symptoms
Appendix 5. Associations between low social support and adverse perinatal outcomes among PrIMA study participants.
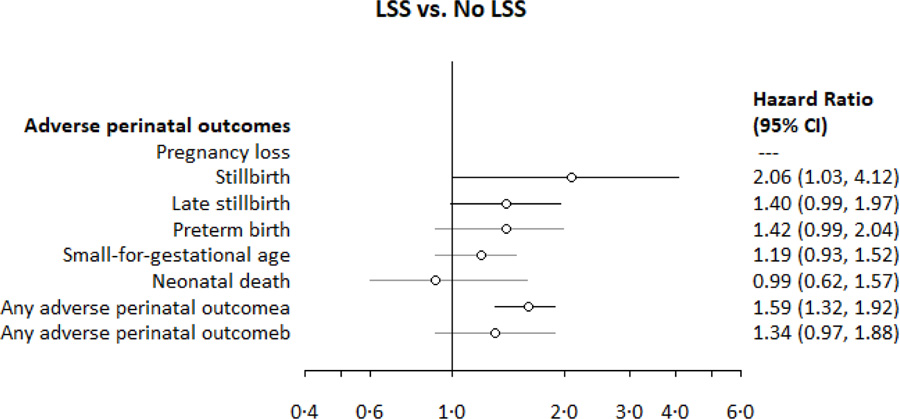
aAny adverse perinatal outcome: pregnancy loss, stillbirth, preterm birth, small for gestational age, or neonatal death; among those enrolled <20 weeks and with birthweight data (for live births)
bAny adverse perinatal outcome: pregnancy loss, stillbirth, preterm birth, or neonatal death; among all eligible pregnancies
HR: Hazard ratios are from Cox Regression Models, clustered by facility used for outcomes of: pregnancy loss, stillbirth, late stillbirth, preterm birth, neonatal death, any adverse perinatal outcome
For instances with case counts ≤5 in an exposure group, we did not perform regression analyses
CESD-10 score was re-scaled to reflect a 10-unit change in score
CESD-10: Center for epidemiologic studies depression scale-10
LSS: Low social support
Appendix 6. Associations between intimate partner violence and adverse perinatal outcomes among PrIMA study participants.
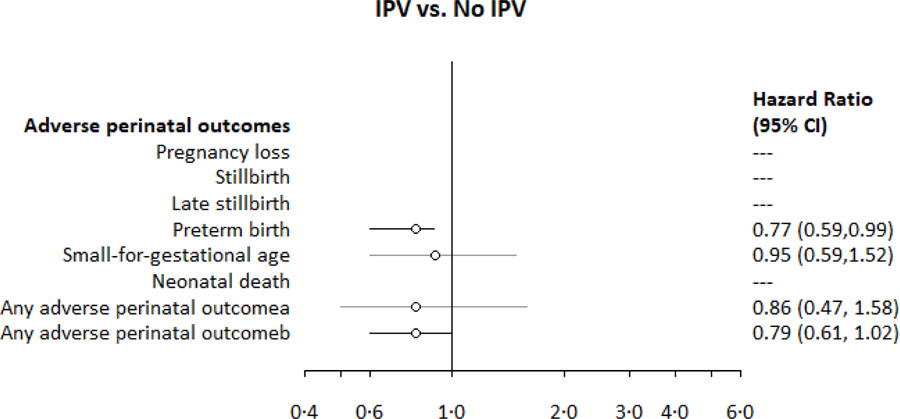
aAny adverse perinatal outcome: pregnancy loss, stillbirth, preterm birth, small for gestational age, or neonatal death; among those enrolled <20 weeks and with birthweight data (for live births)
bAny adverse perinatal outcome: pregnancy loss, stillbirth, preterm birth, or neonatal death; among all eligible pregnancies
HR: Hazard ratios are from Cox Regression Models, clustered by facility used for outcomes of: pregnancy loss, stillbirth, late stillbirth, preterm birth, neonatal death, any adverse perinatal outcome
For instances with case counts ≤5 in an exposure group, we did not perform regression analyses
CESD-10 score was re-scaled to reflect a 10-unit change in score
CESD-10: Center for epidemiologic studies depression scale-10
IPV: Intimate partner violence
Appendix 7.
Baseline characteristics of PrIMA study participants among those with complete CESD-10 information versus partial (n=4153)
| Overall (N=4153) | Complete CESD-10 (N=3661) | Incomplete CESD-10 (N=492) | |
|---|---|---|---|
| Demographic characteristics | n (%) or Median (IQR) | n (%) or Median (IQR) | n (%) or Median (IQR) |
| Age (years) | 24 (21, 28) (n=4151) | 24 (21, 29) (n=3659) | 24 (21, 28) (n=492) |
| Adolescents and young women (<25 years) | 2369 (57.0%) | 2080 (56.8%) | 289 (58.7%) |
| Missing | 2 (<1%) | 2 (0.1%) | 0 (0.0%) |
| Gestational age (enrollment, weeks) | 24 (20, 30) (n=4153) | 24 (20, 30) (n=3661) | 26 (18, 30) (n=492) |
| Married or living with a partner | 3491 (84.1%) | 3083 (84.2%) | 408 (82.9%) |
| Missing | 34 (0.8%) | 33 (0.9%) | 1 (0.2%) |
| Completed education (years) | 10 (8, 12) (n=4072) | 10 (8, 12) (n=3589) | 10 (8, 12) (n=483) |
| Regularly employed | 612 (14.7%) | 517 (14.1%) | 95 (19.3%) |
| Missing | 52 (1.3%) | 44 (1.2%) | 8 (1.6%) |
| Household crowding (≥2 people/room) | 1995 (48.0%) | 1771 (48.4%) | 224 (45.5%) |
| Missing | 27 (0.7%) | 26 (0.7%) | 1 (0.2%) |
| Pregnancy history & factors | |||
| Multiparous | 3083 (74.2%) | 2735 (74.7%) | 348 (70.7%) |
| Missing | 5 (0.1%) | 4 (0.1%) | 1 (0.2%) |
| Prior pregnancy loss | 539 (13.0%) | 468 (12.8%) | 71 (14.4%) |
| Missing | 14 (0.3%) | 12 (0.3%) | 2 (0.4%) |
| Prior preterm birth | 42 (1.0%) | 35 (1.0%) | 7 (1.4%) |
| Trimester of initial antenatal care (ANC) visit | |||
| First | 615 (14.8%) | 523 (14.3%) | 92 (18.7%) |
| Second | 2098 (50.5%) | 1894 (51.7%) | 204 (41.5%) |
| Third | 1440 (34.7%) | 1244 (34.0%) | 196 (39.8%) |
| Infant sex (female) | 1842 (44.3%) | 1633 (44.6%) | 209 (42.5%) |
| Missing | 534 (12.9%) | 462 (12.6%) | 72 (14.6%) |
| HIV risk factors | |||
| Self-perceived high HIV risk | 369 (8.9%) | 320 (8.7%) | 49 (10.0%) |
| Missing | 7 (0.2%) | 4 (0.1%) | 3 (0.6%) |
| Lifetime sexual partners | 2 (2, 3) (n=4148) | 2 (2, 3) (n=3656) | 2 (2, 3) (n=492) |
| Partner HIV-positive* | 176 (4.2%) | 149 (4.1%) | 27 (5.5%) |
| Missing | 52 (1.3%) | 49 (1.3%) | 3 (0.6%) |
| Sexually transmitted infection (enrollment) | 104 (2.5%) | 96 (2.6%) | 8 (1.6%) |
| Missing | 7 (0.2%) | 5 (0.1%) | 2 (0.4%) |
| Psychosocial characteristics | |||
| Low social support (MOS-SSS score <72) | 1504 (36.2%) | 1372 (37.5%) | 132 (26.8%) |
| Missing | 90 (2.2%) | 69 (1.9%) | 21 (4.3%) |
| Intimate partner violence c (HITS score ≥10) | 323 (7.8%) | 289 (7.9%) | 34 (6.9%) |
| Missing | 5 (0.1%) | 5 (0.1%) | 0 (0.0%) |
REFERENCES
- 1.Dadi AF, Wolde HF, Baraki AG, Akalu TY. Epidemiology of antenatal depression in Africa: A systematic review and meta-analysis. BMC Pregnancy Childbirth 2020; 20: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stein A, Pearson RM, Goodman SH, et al. Effects of perinatal mental disorders on the fetus and child. Lancet 2014; 384: 1800–19. [DOI] [PubMed] [Google Scholar]
- 3.Howard LM, Molyneaux E, Dennis C-L, Rochat T, Stein A, Milgrom J. Non-psychotic mental disorders in the perinatal period. Lancet (London, England) 2014; 384: 1775–88. [DOI] [PubMed] [Google Scholar]
- 4.Biaggi A, Conroy S, Pawlby S, Pariante CM. Identifying the women at risk of antenatal anxiety and depression: A systematic review. J Affect Disord 2016; 191: 62–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grote NK, Bridge JA, Gavin AR, Melville JL, Iyengar S, Katon WJ. A Meta-analysis of Depression During Pregnancy and the Risk of Preterm Birth, Low Birth Weight, and Intrauterine Growth Restriction. Arch Gen Psychiatry 2010; 67: 1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grigoriadis S, VonderPorten EH, Mamisashvili L, et al. The Impact of Maternal Depression During Pregnancy on Perinatal Outcomes. J Clin Psychiatry 2013; 74: e321–41. [DOI] [PubMed] [Google Scholar]
- 7.Jarde A, Morais M, Kingston D, et al. Neonatal Outcomes in Women With Untreated Antenatal Depression Compared With Women Without Depression. JAMA Psychiatry 2016; 73: 826. [DOI] [PubMed] [Google Scholar]
- 8.Szegda K, Markenson G, Bertone-Johnson ER, Chasan-Taber L. Depression during pregnancy: a risk factor for adverse neonatal outcomes? A critical review of the literature. J Matern Neonatal Med 2014; 27: 960–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacques N, de Mola CL, Joseph G, Mesenburg MA, da Silveira MF. Prenatal and postnatal maternal depression and infant hospitalization and mortality in the first year of life: A systematic review and meta-analysis. J Affect Disord 2019; 243: 201–8. [DOI] [PubMed] [Google Scholar]
- 10.Tamirat KS, Sisay MM, Tesema GA, Tessema ZT. Determinants of adverse birth outcome in Sub-Saharan Africa: analysis of recent demographic and health surveys. BMC Public Health 2021; 21: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hug L, You D, Blencowe H, et al. Global, regional, and national estimates and trends in stillbirths from 2000 to 2019: a systematic assessment. Lancet 2021; 398: 772–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.You D, Hug L, Ejdemyr S, Beise J, Idele P, UN IGME. Levels & Trends in Child Mortality New York, NY, 2015. http://www.childmortality.org/files_v20/download/IGME%20Report%202015_9_3%20LR%20Web.pdf. [Google Scholar]
- 13.Fisher J, Cabral de Mello M, Patel V, et al. Prevalence and determinants of common perinatal mental disorders in women in low- and lower-middle-income countries: a systematic review. Bull World Health Organ 2012; 90: 139–149H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kenya Demographic and Health Survey. 2015. https://dhsprogram.com/pubs/pdf/fr308/fr308.pdf (accessed Aug 24, 2019).
- 15.Rahman A, Surkan PJ, Cayetano CE, Rwagatare P, Dickson KE. Grand Challenges: Integrating Maternal Mental Health into Maternal and Child Health Programmes. PLoS Med 2013; 10: e1001442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wado YD, Afework MF, Hindin MJ. Effects of Maternal Pregnancy Intention, Depressive Symptoms and Social Support on Risk of Low Birth Weight: A Prospective Study from Southwestern Ethiopia. PLoS One 2014; 9: e96304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bindt C, Guo N, Te Bonle M, et al. No Association between Antenatal Common Mental Disorders in Low-Obstetric Risk Women and Adverse Birth Outcomes in Their Offspring: Results from the CDS Study in Ghana and Côte D’Ivoire. PLoS One 2013; 8: e80711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mochache K, Mathai M, Gachuno O, Stoep A, Kumar M. Depression during pregnancy and preterm delivery: a prospective cohort study among women attending antenatal clinic at Pumwani Maternity Hospital. Ann Gen Psychiatry 2018; 17. DOI: 10.1186/S12991-018-0202-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dadi AF, Akalu TY, Wolde HF, Baraki AG. Effect of perinatal depression on birth and infant health outcomes: a systematic review and meta-analysis of observational studies from Africa. Arch Public Heal 2022; 80: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.JC D, J K, J P, et al. PrEP Implementation for Mothers in Antenatal Care (PrIMA): Study Protocol of a Cluster Randomised Trial. BMJ Open 2019; 9. DOI: 10.1136/BMJOPEN-2018-025122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.BK N, J A, A A, et al. Reliability and validity of the center for epidemiologic studies-depression scale in screening for depression among HIV-infected and -uninfected pregnant women attending antenatal services in northern Uganda: a cross-sectional study. BMC Psychiatry 2014; 14. DOI: 10.1186/S12888-014-0303-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baron EC, Davies T, Lund C. Validation of the 10-item Centre for Epidemiological Studies Depression Scale (CES-D-10) in Zulu, Xhosa and Afrikaans populations in South Africa. BMC Psychiatry 2017; 17: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am J Prev Med; 10: 77–84. [PubMed] [Google Scholar]
- 24.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med 1991; 32: 705–14. [DOI] [PubMed] [Google Scholar]
- 25.Ono M, Matsuyama A, Karama M, Honda S. Association between social support and place of delivery: A cross-sectional study in Kericho, Western Kenya. BMC Pregnancy Childbirth 2013; 13: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaede B, Majeke S, Modeste RRM, Naidoo JR, Titus MJ, Uys LR. Social support and health behaviour in women living with HIV in KwaZulu-Natal. SAHARA J J Soc Asp HIV/AIDS Res Alliance 2006; 3: 362–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rabin RF, Jennings JM, Campbell JC, Bair-Merritt MH. Intimate partner violence screening tools: a systematic review. Am J Prev Med 2009; 36: 439–445.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wandera SO, Tumwesigye NM, Walakira EJ, Kisaakye P, Wagman J. Alcohol use, intimate partner violence, and HIV sexual risk behavior among young people in fishing communities of Lake Victoria, Uganda. BMC Public Health 2021; 21: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiserud T, Piaggio G, Carroli G, et al. The World Health Organization Fetal Growth Charts: A Multinational Longitudinal Study of Ultrasound Biometric Measurements and Estimated Fetal Weight. PLOS Med 2017; 14: e1002220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melki IS, Beydoun HA, Khogali M, Tamim H, Yunis KA, National Collaborative Perinatal Neonatal Network (NCPNN). Household crowding index: a correlate of socioeconomic status and inter-pregnancy spacing in an urban setting. J Epidemiol Community Heal 2004; 58: 476–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pintye J, Drake AL, Kinuthia J, et al. A Risk Assessment Tool for Identifying Pregnant and Postpartum Women Who May Benefit From Preexposure Prophylaxis. Clin Infect Dis 2017; 64: 751–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Napper LE, Fisher DG, Reynolds GL. Development of the perceived risk of HIV scale. AIDS Behav 2012; 16: 1075–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schisterman EF, Cole SR, Ye A, Platt RW. Accuracy Loss Due to Selection Bias in Cohort Studies with Left Truncation. Paediatr Perinat Epidemiol 2013; 27: 491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lancaster CA, Gold KJ, Flynn HA, Yoo H, Marcus SM, Davis MM. Risk factors for depressive symptoms during pregnancy: a systematic review. Am J Obstet Gynecol 2010; 202: 5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Payne JL, Maguire J. Pathophysiological mechanisms implicated in postpartum depression. Front Neuroendocrinol 2019; 52: 165–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bono C, Ried LD, Kimberlin C, Vogel B. Missing data on the Center for Epidemiologic Studies Depression Scale: A comparison of 4 imputation techniques. Res Soc Adm Pharm 2007; 3: 1–27. [DOI] [PubMed] [Google Scholar]
- 37.Everett C. Incidence and outcome of bleeding before the 20th week of pregnancy: prospective study from general practice. BMJ 1997; 315: 32–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X, Chen C, Wang L, et al. Conception, early pregnancy loss, and time to clinical pregnancy: a population-based prospective study. Fertil Steril 2003; 79: 577–84. [DOI] [PubMed] [Google Scholar]
- 39.Sigalla GN, Mushi D, Meyrowitsch DW, et al. Intimate partner violence during pregnancy and its association with preterm birth and low birth weight in Tanzania: A prospective cohort study. PLoS One 2017; 12: e0172540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Desta M, Getaneh T, Memiah P, et al. Is preterm birth associated with intimate partner violence and maternal malnutrition during pregnancy in Ethiopia? A systematic review and meta analysis. Heliyon 2021; 7: e08103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Savitz DA, Terry JW, Dole N, Thorp JM, Maria Siega-Riz A, Herring AH. Comparison of pregnancy dating by last menstrual period, ultrasound scanning, and their combination. Am J Obstet Gynecol 2002; 187: 1660–6. [DOI] [PubMed] [Google Scholar]
- 42.Spiegelman D, Hertzmark E, Wand HC. Point and interval estimates of partial population attributable risks in cohort studies: Examples and software. Cancer Causes Control 2007; 18: 571–9. [DOI] [PubMed] [Google Scholar]
- 43.Osok J, Kigamwa P, Vander Stoep A, Huang KY, Kumar M. Depression and its psychosocial risk factors in pregnant Kenyan adolescents: A cross-sectional study in a community health Centre of Nairobi. BMC Psychiatry 2018; 18. DOI: 10.1186/s12888-018-1706-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Devries KM, Kishor S, Johnson H, et al. Intimate partner violence during pregnancy: Analysis of prevalence data from 19 countries. Reprod Health Matters 2010; 18: 158–70. [DOI] [PubMed] [Google Scholar]
- 45.Iddi S, Kadengye DT, Kiwuwa-Muyingo S, Mutua MK, Asiki G. Associated factors of pregnancy loss in two urban slums of Nairobi: A generalized estimation equations approach. Glob Epidemiol 2020; 2: 100030. [Google Scholar]
- 46.Schummers L, Oveisi N, Ohtsuka MS, et al. Early pregnancy loss incidence in high-income settings: a protocol for a systematic review and meta-analysis. Syst Rev 2021; 10: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chawanpaiboon S, Vogel JP, Moller AB, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Heal 2019; 7: e37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vogel JP, Chawanpaiboon S, Moller A-B, Watananirun K, Bonet M, Lumbiganon P. The global epidemiology of preterm birth 2018. DOI: 10.1016/j.bpobgyn.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 49.Imbo AE, Mbuthia EK, Ngotho DN. Determinants of Neonatal Mortality in Kenya: Evidence from the Kenya Demographic and Health Survey 2014. Int J Matern Child Heal AIDS 2021; 10: 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Macaulay S, Buchmann EJ, Dunger DB, Norris SA. Reliability and validity of last menstrual period for gestational age estimation in a low-to-middle-income setting. J Obstet Gynaecol Res 2019; 45: 217–25. [DOI] [PubMed] [Google Scholar]
- 51.Ross LE, Grigoriadis S, Mamisashvili L, et al. Selected Pregnancy and Delivery Outcomes After Exposure to Antidepressant Medication. JAMA Psychiatry 2013; 70: 436. [DOI] [PubMed] [Google Scholar]
- 52.Kjaersgaard MIS, Parner ET, Vestergaard M, et al. Prenatal Antidepressant Exposure and Risk of Spontaneous Abortion – A Population-Based Study. PLoS One 2013; 8: e72095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chowdhary N, Sikander S, Atif N, et al. The content and delivery of psychological interventions for perinatal depression by non-specialist health workers in low and middle income countries: A systematic review. Best Pract Res Clin Obstet Gynaecol 2014; 28: 113–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dunkel Schetter C, Tanner L. Anxiety, depression and stress in pregnancy: implications for mothers, children, research, and practice. Curr Opin Psychiatry 2012; 25: 141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Diego MA, Field T, Hernandez-Reif M, Schanberg S, Kuhn C, Gonzalez-Quintero VH. Prenatal Depression Restricts Fetal Growth. Early Hum Dev 2009; 85: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gur TL, Palkar AV, Rajasekera T, et al. Prenatal stress disrupts social behavior, cortical neurobiology and commensal microbes in adult male offspring. Behav Brain Res 2019; 359: 886–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Ravesteyn LM, Lambregtse - van den Berg MP, Hoogendijk WJG, Kamperman AM. Interventions to treat mental disorders during pregnancy: A systematic review and multiple treatment meta-analysis. PLoS One 2017; 12: e0173397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sockol LE. A systematic review and meta-analysis of interpersonal psychotherapy for perinatal women. J Affect Disord 2018; 232: 316–28. [DOI] [PubMed] [Google Scholar]
- 59.Sockol LE. A systematic review of the efficacy of cognitive behavioral therapy for treating and preventing perinatal depression. J Affect Disord 2015; 177: 7–21. [DOI] [PubMed] [Google Scholar]
- 60.Cooper PJ, Tomlinson M, Swartz L, et al. Improving quality of mother-infant relationship and infant attachment in socioeconomically deprived community in South Africa: randomised controlled trial. BMJ 2009; 338: b974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rotheram-Borus MJ, Tomlinson M, Le Roux I, Stein JA. Alcohol Use, Partner Violence, and Depression: A Cluster Randomized Controlled Trial Among Urban South African Mothers Over 3 Years. Am J Prev Med 2015; 49: 715–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tomlinson M, Rotheram-Borus MJ, Harwood J, le Roux IM, O’Connor M, Worthman C. Community health workers can improve child growth of antenatally-depressed, South African mothers: a cluster randomized controlled trial. BMC Psychiatry 2015; 15: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]


