Abstract
Blockade of the CD40/CD154 T cell costimulation pathway is a promising approach to supplement or replace current clinical immunosuppression in solid organ transplantation. We evaluated the tolerability and activity of a novel humanized anti-CD154 monoclonal antibody, TNX-1500 (TNX) in a nonhuman primate (NHP) heterotopic cardiac allogeneic (allo) transplant model. TNX contains the rupluzimab Fab and an IgG4 Fc region engineered to reduce binding to FcγRIIa and associated risks of thrombosis. Recipients were treated for six months with standard-dose TNX (sTNX) monotherapy, low-dose monotherapy (loTNX), or loTNX with mycophenolate mofetil (loTNX+MMF). Results were compared to historical data using chimeric hu5c8 monotherapy dosed as for loTNX but discontinued at 3 months. Median survival time was similar for hu5c8 and both loTNX groups, but significantly longer with sTNX (>265 days) than with loTNX (99 days) or loTNX+MMF (88 days) (p<0.05 for both comparisons against sTNX). sTNX prevented anti-donor alloAb elaboration, inhibited chronic rejection, and was associated with a significantly reduced TEFF/TREG ratio relative to loTNX with MMF. No thrombotic complications were observed. This study demonstrates that TNX-1500 was well tolerated, prolongs allograft survival and prevents alloAb production and cardiac allograft vasculopathy in a stringent preclinical NHP heart allo transplant model.
1. Introduction
New approaches are needed to more effectively and safely protect allografts from immune injury. Immunomodulatory strategies that selectively target the CD40/CD154 T cell costimulatory pathways, have shown potential to inhibit T cell-mediated rejection while avoiding side effects associated with conventional immunosuppressive drugs.1–6 Significant prolongation of graft survival in multiple nonhuman primate (NHP) allograft models was achieved by monoclonal antibodies (mAbs) that inhibit the CD40/CD154 co-stimulation pathway by blocking CD154, including hu5c8 (chimeric mouse 5c8/human IgG1),6,7 Rupliuzumab (a humanized 5c8 IgG1),8–12 and IDEC-131 (a humanized IgG1), 7,13. Ruplizumab showed activity in prophylaxis of kidney transplant rejection14,15 and treating autoimmune conditions.16 However clinical development of antibodies targeting CD154 were halted due to an increased risk of thrombotic complications seen in clinical trials and in a preclinical kidney transplant model.14–17 Subsequent studies revealed that immune complexes containing soluble CD154, and anti-CD154 mAbs with IgG1 crystallizable fragment (Fc) interact with the Fc gamma receptor IIa (FcγRIIa/FCGR2A) on platelets to induce thrombosis in human FCGR2A transgenic mice.18
To decrease or eliminate the risk of thrombosis, several groups have engineered the Fc region of anti-CD154 antibodies to down-modulate FcγRIIa-binding. An Fc-disabled aglycosyl version of ruplizumab19 and an Fc-silent anti-CD154 domain antibody20 were not associated with increased thrombotic risk and maintained the ability to block antibody responses, but had reduced activity in prolonging graft survival in NHP kidney transplant models. These findings suggested that some Fc-functionality in anti-CD154 mAb therapeutics contributes to the therapeutic effects in prophylaxis against organ rejection. TNX-1500 (TNX), that contains the humanized 5c8 Fab domain from ruplizumab21 and an IgG4 Fc region engineered to reduce FcγRIIa-binding. Here we report results in a NHP cardiac allotransplant model testing TNX-1500 for its ability to protect grafts against rejection and to preserve function.
2. Materials and Methods
2.1. Animals
The care and treatment of all experimental animals in this study were conducted with the approval of the Institutional Animal Care and Use Committee at Massachusetts General Hospital and were conducted in compliance with National Institutes of Health guidelines for the care and use of laboratory animals. Captive-bred and wild caught cynomolgus monkeys of Chinese and Indonesian origin were used. Males and females weighing 3.3 to 10.0 kg were selected as recipients of ABO blood type-compatible donors. All animals underwent class I and class II major histocompatibility complex (MHC) typing to assure MHC mismatching.
2.2. Heterotopic Heart Allograft Transplantation
All recipient animals underwent heterotopic intra-abdominal cardiac transplantation, as described previously.12,13,22 Postoperatively, graft function (left ventricular systolic and diastolic blood pressure, heart rate) and core temperature were monitored daily by implanted telemetry device (L11-F1, Data Sciences International, St. Paul, MN) until time of graft explant. Laboratory studies, including serum chemistry and complete blood count assessments, were performed weekly and for cause. Open cardiac biopsies were performed by protocol on postoperative days (d) 45 and 90 after transplant when clinical condition allowed. Biopsies were omitted or delayed due to fever, anemia, or weight loss, or in case of earlier graft failure.
2.3. TNX-1500
TNX-1500 (TNX), that contains the humanized 5c8 Fab domain from ruplizumab21 and an IgG4 Fc region engineered to encode amino acid transitions S228P and L235A to prevent chain switching and to reduce FcγRIIa-binding, respectively (Patent Number: WO2021001458A1; Table S-1; Fig S-1). Ref Lassiter et al
2.4. Immunosuppression Treatment Groups and Dosing
“Standard-dose” TNX-1500 (sTNX) was administered intravenously to five cynomolgus monkey heterotopic cardiac recipients at 30 mg/kg twice weekly, given on d0, d3, d7, and d14; and then 20 mg/kg weekly from d21 to d175. A “low-dose maintenance” regimen (loTNX) was explored, giving 10 mg/kg weekly from d21 until, d42 followed by 20 mg/kg monthly from d56 to d168, either alone (n=4) or in combination with mycophenolate mofetil (MMF; 200mg/d po from d1 until time of graft explant; loTNX+MMF, n=4). The results for these three treatment groups were compared to historical data using a hu5c8 reference regimen (n=5) analogous to the loTNX regimen [mouse-human IgG1κ chimeric anti-CD154, the NIH Nonhuman Primate Reagent Resource (Boston, MA)] but with treatment stopped after d84 (Fig 1).
Figure 1: Treatment schedules for anti-CD154 mAb, TNX-1500 and hu5c8.
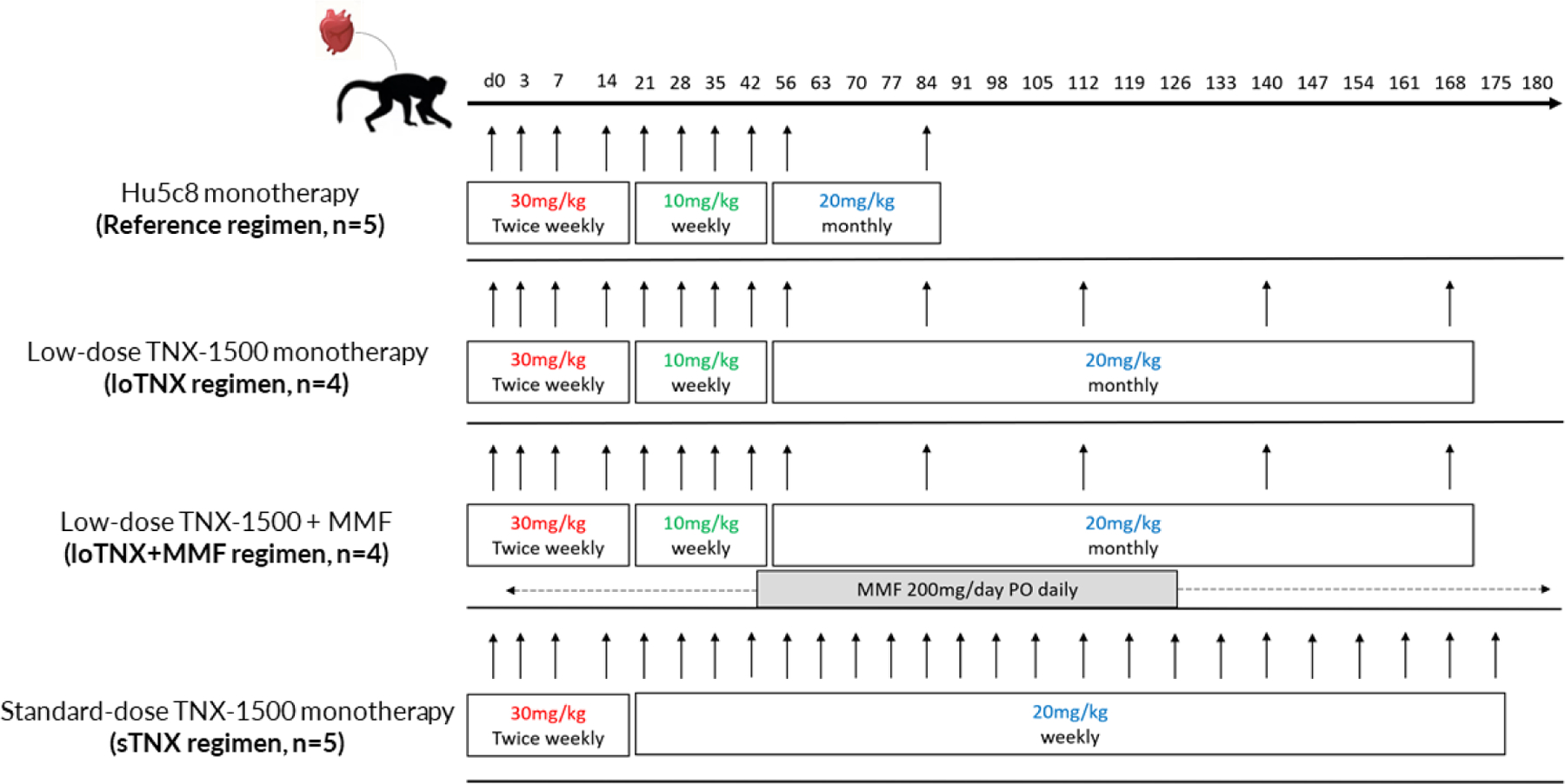
Treatment schedules and dosing regimens for low- (loTNX) and standard-dose (sTNX) maintenance TNX groups are illustrated. An historical hu5c8 regimen is shown for reference.
TNX, TNX-1500; MMF, mycophenolate mofetile; hu5c8, mouse-human IgG1κ chimeric anti-CD154; d, post-transplant days
2.5. Allograft Rejection and Graft Failure Criteria
Graft failure was defined by 1 or more of the following criteria for 2 consecutive days: decline in heart rate greater than 20% below baseline or less than 120 beats per minute, pulse pressure (systolic-diastolic in mmHg) less than 30 mmHg, or nonpalpable graft contraction lasting longer than 12 hours. Grafts were explanted if the animal met graft failure criteria, developed a life-threatening complication, or in two cases at end of study (EOS) on d180. Graft function was monitored after treatment cessation in three recipients in the sTNX group following graft biopsy at EOS on ~d180. When grafts failed before d90, recipients underwent graft removal, and immune responses to the graft donor antigens were monitored in the peripheral blood. Allografts in the historical hu5c8 treatment group were explanted electively at d90 after stopping treatment at d84 (n=1), or at graft failure due to rejection as previously reported.7
2.6. Drug Trough Concentration Measurement
TNX-1500 serum trough concentrations were measured in all study animals at multiple time points throughout the study period using a human IgG4 Fc-specific enzyme-linked immunosorbent assay (Cayman Chemical, Ann Arbor, MI) according to the kit manufacturer’s protocol. These measurements were supplemented by a single dose pharmacokinetic study in treatment-naïve cynomolgus monkeys at three dose levels, 30, 100 and 300 mg/kg (n=2 at each dose level), performed at a contract research organization (Charles River Laboratories).
2.6. Anti-donor Alloantibody Detection
IgM and IgG donor-reactive alloantibody (alloAb) was measured retrospectively by flow cytometry using archived frozen donor splenocytes and recipient serum, as described previously.22,23 Antidonor alloAb was defined by detection of recipient serum IgM or IgG binding to donor CD3+CD20− T cells (expressing MHC Class 1) or CD3−CD20+ B cells (expressing MHC Class 1 and Class 2) with a value consistently greater than twice the pre-transplant donor serum on 2 or more consecutive measurements.24
2.7. Hematology and Lymphocyte Phenotyping
Cell blood counts and T lymphocytes subsets were measured by protocol at regular intervals in freshly collected ethylenediaminetetraacetic acid (EDTA)-blood using automated cell counter (Antech Diagnostics and Hemavet 950FS Hematology Analyzer, Drew Scientific in duplicate) and flow cytometry, respectively, as previous described.24 Peripheral blood cells were stained for CD3, CD4, CD8, CD20, CD25 CD95, CD127, and CD62L (Biolegend, San Diego, CA). Intracellular staining with anti-Foxp3 was performed using a Foxp3 intranuclear staining kit (eBiosciences, San Diego, CA), according to manufacturer’s instructions. Samples were analyzed using a FACS machine (BD Biosciences, San Jose, CA). Data were analyzed using FlowJo software (Treestar, San Carlos, CA).
2.8. Graft Histology
Biopsy and explant cardiac tissue specimens were fixed with 10% formalin, processed for paraffin embedding, and stained with hematoxylin and eosin. Histopathological features of acute cardiac allograft rejection were quantified on a scale of 0 to 3 based on the 2005 International Society for Heart and Lung Transplantation (ISHLT) revised criteria for cardiac allograft rejection.25 Cardiac allograft vasculopathy (CAV) severity was scored from 0 to 3 in each myocardial biopsies and explanted graft specimen, respectively, by 4 independent evaluators, who were blinded with respect to treatment group.7,24 Biopsies with poor tissue preservation or absence of identifiable myocardial tissue were classified as nondiagnostic. Pericardial granulation tissue was excluded from rejection and CAV assessments.
2.9. βTG and soluble P-selectin assays
βTG (Asserachrom β-TG; Diagnostica Stago, Asnieres, France) and soluble P-selectin (CD62; Monkey P-selectin ELISA kit; Invitrogen, Thermo Fisher Scientific, Inc, cat number BMS650) and were measured in plasma collected before transplant and at protocol-driven intervals after transplant by commercial ELISA kit according to the manufacturers’ instructions. The normal reference ranges for human βTG is <50 IU/mL. In healthy humans, the sP-selectin circulates at a concentration of 15 to 100 ng/mL.
2.10. Statistical analysis
Graft survival was evaluated with use of Kaplan-Meier curves by log-rank test for significance analysis. Continuous variables were expressed as mean ± SEM or median and were checked for normality. Variables that were normally distributed were assessed by the 2-tailed unpaired t test to compare 2 groups, or assessed by analysis of variance (ANOVA) to compare 3 or more groups, followed by Tukey’s test for multiple time point comparisons between the groups. Those that were not normally distributed were analyzed using Mann-Whitney non-parametric test. Differences were considered significant when the P value was less than 0.05. All statistical analyses were performed with GraphPad Prism (Version 9.1.2; GraphPad Software, San Diego, CA).
3. Results
3.1. Allograft Survival
The sTNX regimen was associated with significantly prolonged cardiac allograft survival (MST>265d; range 116–305) relative to hu5c8 (133d, range, 90–173, p=0.020), loTNX (99d; range 35–183, p=0.014), or loTNX+MMF (88d; range 58–146, p=0.011) (Fig 2). Outcomes and clinical highlights for individual animals are reported in Table 1.
Figure 2: Allograft survival by treatment regimen.
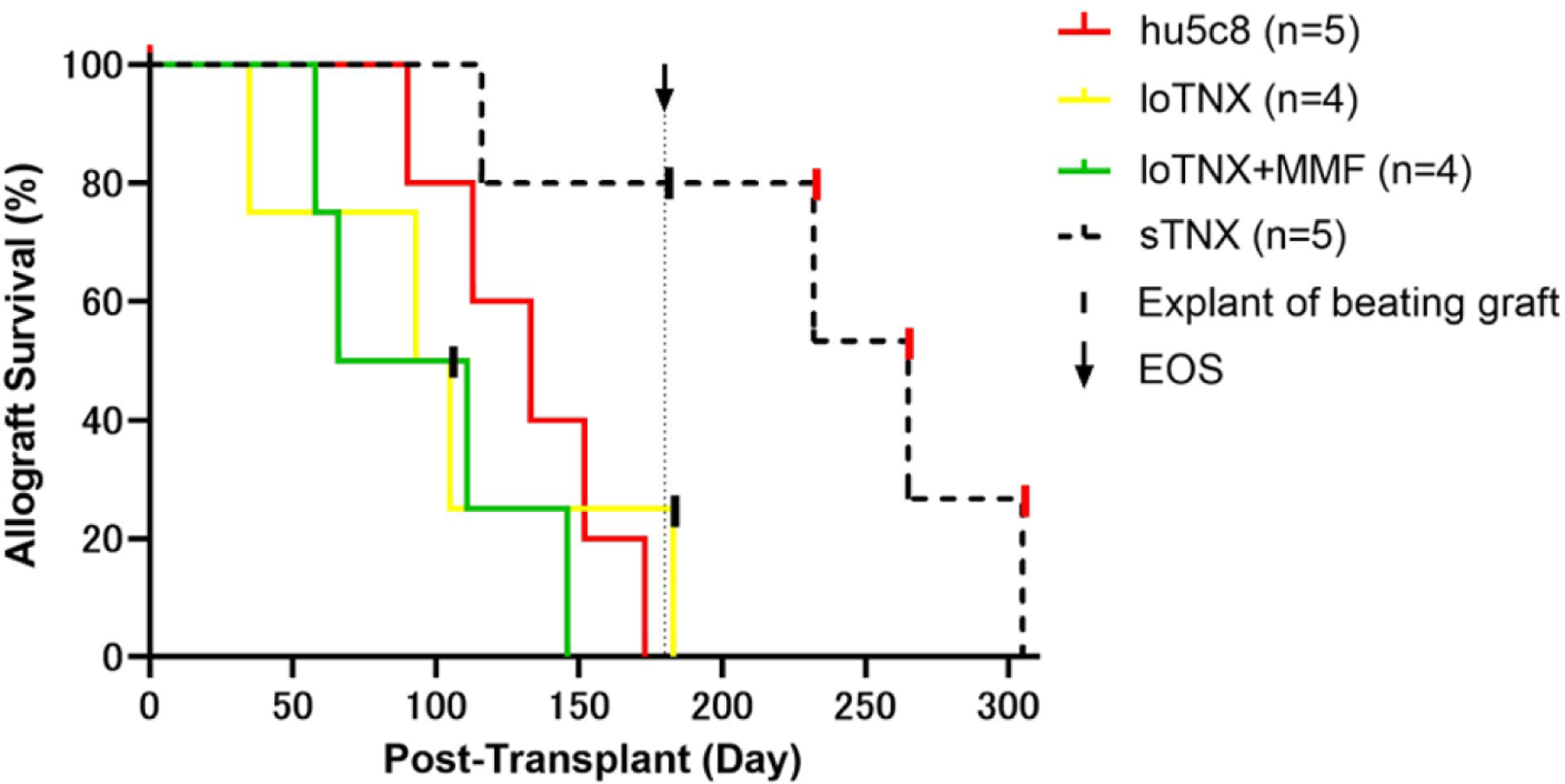
Allograft survival was the time to graft failure or explant, as defined in Methods. Three black vertical hash marks in the loTNX and sTNX treatment groups represent beating grafts electively explanted due to animal health concerns or at EOS as described in Results. Black arrow marks EOS. Three other sTNX grafts rejected on d232, d265 and d305 (red vertical hash) after EOS and cessation of TNX treatment after d175. Allograft MST was significantly longer in the sTNX treatment group (>265d; range 116->287) than with hu5c8 (133d; range 90–173; treatment stopped at d84, p=0.020), loTNX (99d; range 35–183, p=0.014), or loTNX+MMF (88d; range 58–146, p=0.011). Early in our surgical experience, occasional tail lesions associated with cautery grounding pad placement and superficial lower extremity foot pad skin ischemic changes distal to intra-operative limb tethers were observed. These superficial and clinically insignificant lesions were not observed after discontinuing lower extremity external warming during peri-implant aortic occlusion.
TNX, TNX-1500; d, post-transplant days; MST, median survival time; MMF, mycophenolate mofetil; EOS, end of study
Table 1. Individual animal survival and clinical notes.
Allograft survival and clinical notes for individual animals throughout the experiment. TNX, TNX-1500; MMF, mycophenolate mofetil; ACR, acute cellular rejection; AMR, antibody mediated rejection; CVL, central venous line; Bx, biopsy; BW, body weight; d, post-transplant days
| Treatment Group | Animal ID | Graft Survival (days) | Clinical Outcome | Histological diagnosis | Notes |
|---|---|---|---|---|---|
| hu5c8 | MDL5X | 90 | Explanted by protocol at end of study | ACR, CAV | |
| MC710057 | 113 | Explanted due to rejection after end of study | ACR, AMR, CAV | ||
| MDM6A | 133 | Explanted due to rejection after end of study | ACR, CAV | ||
| MDP28 | 152 | Explanted due to rejection after end of study | ACR, AMR, CAV | ||
| MFA6L | 173 | Explanted due to rejection after end of study | ACR, AMR, CAV | ||
| Median | 133 | ||||
| loTNX | M7419 | 93 | Explanted due to rejection | ACR, AMR | |
| M5420 | >105 | Euthanized with beating graft | ACR | BW loss; bowel obstruction | |
| M4520 | >183 | Euthanized at end of study | ACR | ||
| M5220 | 35 | Explanted due to rejection | ACR, AMR | CVL infection, + BC | |
| Median | 99 | ||||
| loTNX+MMF | M8820 | 146 | Explanted due to rejection | ACR, AMR, CAV | |
| M8620 | 111 | Explanted due to rejection | ACR, AMR, CAV | ||
| M8720 | 58 | Explanted due to rejection | ACR, AMR, CAV | CVL infection | |
| M5020 | 66 | Explanted due to rejection | ACR, AMR, CAV | Telemetry device infection | |
| Median | 88 | ||||
| sTNX | M921 | >181 | Elective explant at end of study | No rejection at end of study | |
| M1321 | 305 | Rejection after end of study | No rejection at end of study | d180 Bx 0R | |
| M821 | 265 | Rejection after end of study | No rejection at end of study | d180 Bx 0R | |
| M1721 | 232 | Rejection after end of study | ACR 1R, capillaritis at end of study | d180 Bx 1R | |
| M421 | 116 | Explanted due to rejection | ACR, AMR, early CAV | Endocarditis; +BC | |
| Median | >265 |
With loTNX, 3 of 4 animals maintained stable graft function for more than three months during treatment. M5220 was euthanized on d35 with graft rejection occurring in association with a bacterial central venous line (CVL) infection. M5420 developed a bowel obstruction associated with infection adjacent to the telemetry device about two weeks after protocol biopsy at d90 and was euthanized with a beating graft in which evidence of rejection was detected on terminal pathology. M7419 exhibited graft failure with severe acute cellular and antibody-mediated rejection (ACR3R AMR3) two days after protocol biopsy on d91 revealed ACR 1R AMR 0. M4520 was euthanized at EOS with moderate rejection (ACR2R) in the beating explanted graft.
All grafts in loTNX+MMF animals exhibited ACR and/or AMR during treatment. Early allograft rejection in the two of four animals (M8720, M5020) was associated with long-term antibiotic treatment for drug-resistant bacterial infections associated with their CVL or telemetry device, which were removed at diagnosis and with resolution of the infection. All loTNX+MMF-treated grafts failed in association with severe cellular and/or humoral rejection and in association with severe CAV.
With sTNX, one allograft (M421) exhibited graft failure at d116 in association with recurrent bacteremia from graft mitral valve endocarditis two weeks after discontinuation of prolonged suppressive antibiotic treatment. Endocarditis, diagnosed ultrasonography at d32, was likely triggered initially by a CVL infection seeding the telemetry device. M421’s explant showed regional infarction and diffuse C4d staining consistent with AMR (Fig S2). The remaining four sTNX animals maintained good graft function until EOS. One beating graft (M921) was electively explanted at d181 without evidence of rejection on terminal pathology. Three other grafts showed mild (ACR 1R; M1721) or no rejection (M1321, M821) on d180 biopsies, and rejected at d57 (M1721), d90 (M821), and d130 (M1321) after discontinuation of TNX after d175.
3.2. TNX-1500 Pharmacokinetics
Trough concentrations in all TNX-treated allograft recipients exceeded 600 μg/ml in the second week after transplant in association with dosing at 30 mg/kg twice weekly (Fig 3). TNX trough concentrations for sTNX (mean ± SEM; 711 ± 331 μg/mL) were significantly higher than with either loTNX (498 ± 254; p=0.002) or loTNX+MMF (413 ± 265; p=0.0002). The loTNX regimen was associated with decreasing trough concentrations after d21 in association with tapered dosing. Similarly, hu5c8 serum trough concentrations were greater than 400 μg/mL for the first two weeks, but then remained only greater than 100 μg/mL for the 90 days after transplantation.7 In contrast, the sTNX regimen (20 mg/kg weekly starting on d21) consistently maintained trough concentrations after d28 above 500 µg/mL throughout the remainder of the study (Fig S3).
Figure 3: Mean serum trough concentrations for TNX-1500.
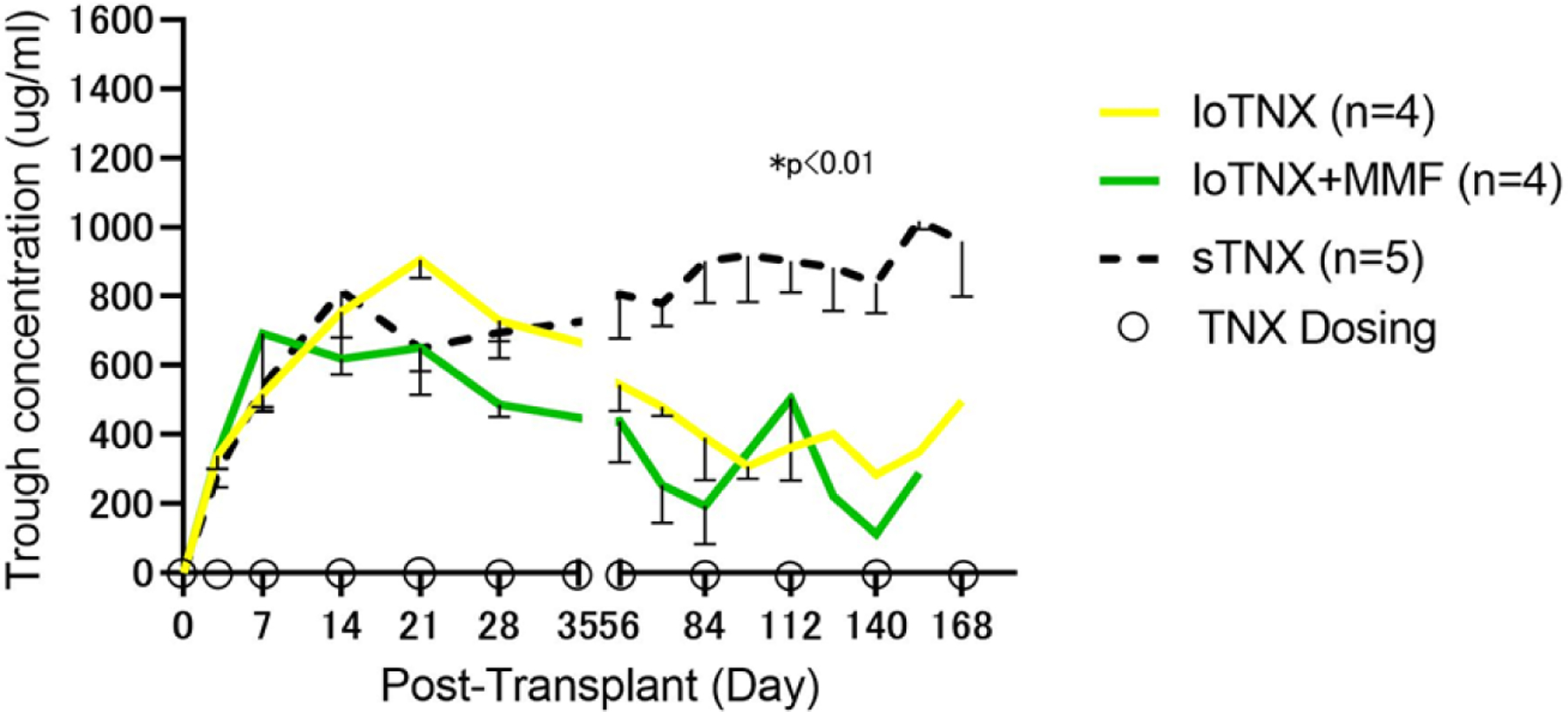
TNX-1500 trough concentrations were measured in serum by a human IgG4 Fc-specific enzyme-linked immunosorbent assay as described in Methods. Mean serum trough levels for loTNX (yellow), loTNX+MMF (green) and sTNX (dashed black) treatment groups, with SEM reflected by a negative error bar for each group. Open circles on the X-axis represent drug dosing in the loTNX regimens as described in Methods. Trough levels in all groups exceeded 600 µg/ml in the second week after transplant in association with dosing at 30 mg/kg twice weekly. TNX trough levels during treatment for sTNX-treated allografts (mean ± SEM; 711 ± 331 μg/mL) were significantly higher than either loTNX-treated (498 ± 254; P = 0.002) or loTNX+MMF-treated allografts (413 ± 265; P = 0.0002).
TNX, TNX-1500; MMF, mycophenolate mofetil; d, post-transplant days.
3.3. Antidonor Alloantibody Elaboration
In serum samples collected weekly and assayed retrospectively, the incidence of production of alloAb binding to the donor MHC Class I (CD3+CD20− T cells) was significantly lower in sTNX (IgM: 0/5, IgG: 0/5, left bottom panels) in association with loTNX (IgG: 2/4, p=0.046, left top panel) or loTNX+MMF (IgM: 2/4, p=0.046, left middle panel) (Fig 4). Similarly, alloAb binding to donor B cells (which express MHC Class II in addition to Class I) was significantly lower in association with sTNX (IgM: 0/5, IgG: 0/5, right bottom panels) relative to loTNX (IgG: 2/4, p=0.046, right top panel).
Figure 4: Antidonor alloAb elaboration.
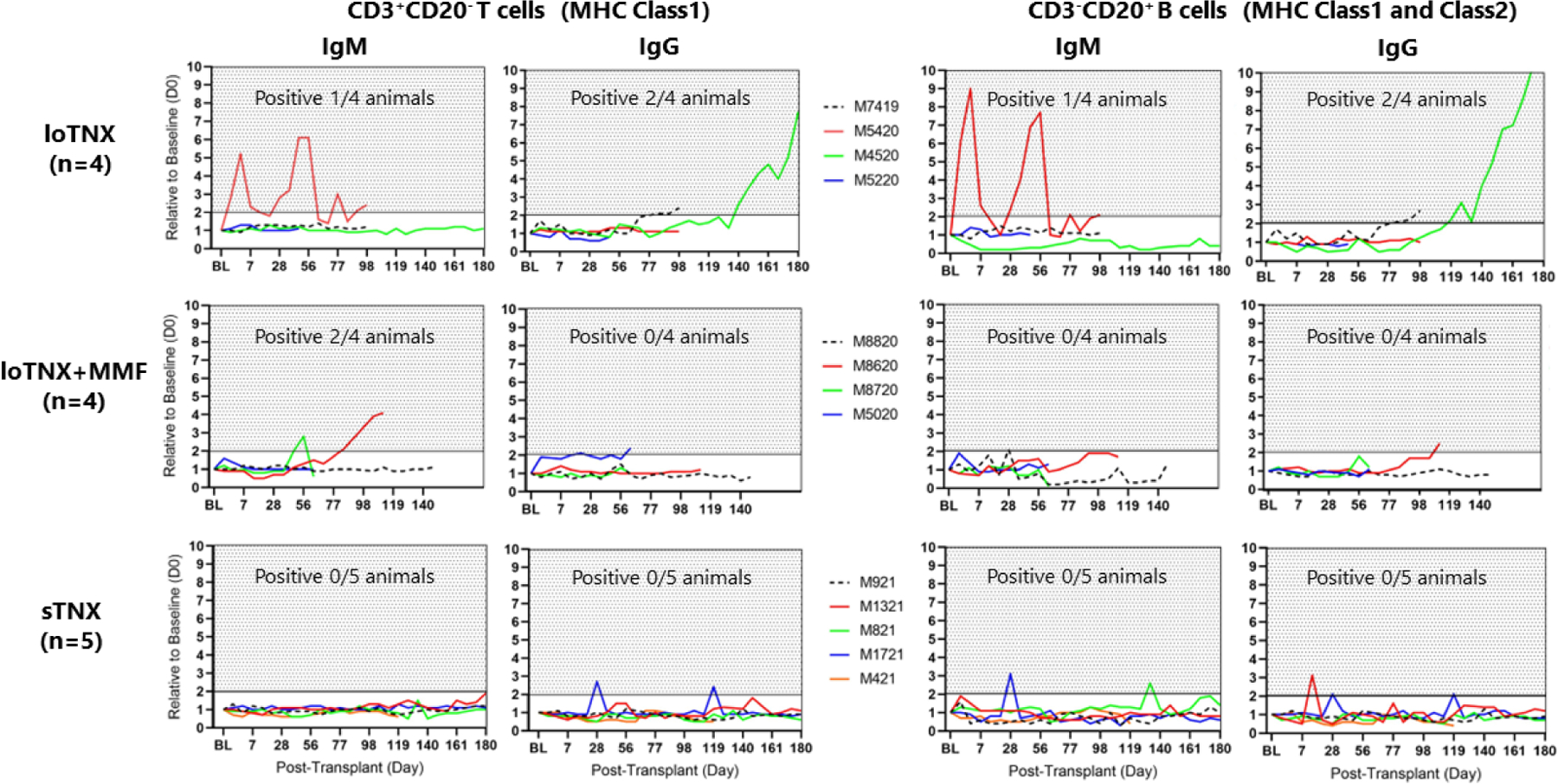
Presence of donor specific alloAb was defined by detection of recipient serum IgM or IgG binding to donor CD3+CD20− T-cells (expressing MHC Class I) or CD3−CD20+ B-cells (expressing MHC Class I and Class II) with a value (fold increase MFI) consistently (on 2 or more consecutive measurements) greater than twice the pre-transplant donor serum baseline (d0). Compared to loTNX (3 of 4 positive for either Class I or Class II) or loTNX+MMF-treated allografts (2 of four positive for Class I, one transiently), sTNX treatment prevented anti-donor antibody elaboration (0/5 animals). M4520 (loTNX regimen) continued beating until d183 despite a rising titer of presumed anti-Class II IgG. Transient detection of alloAb with sTNX may reflect effective inhibition of class switching and affinity maturation and thus efficient CD154 blockade associated with this TNX dosing regimen.
TNX, TNX-1500; MMF, mycophenolate mofetil; d, post-transplant days
With loTNX, alloAb elaboration preceded the explant in 3 of 4 animals (M5420, M7419, M4520). In one (M7419), alloantibody was detected at d77, before rejection and explant at d93. M5420 exhibited preserved graft function despite high levels of antidonor IgM antibody in two peaks, but without evidence of class switching (absence of anti-donor IgG), before being euthanized for a post-biopsy intestinal complication. M4520’s graft continued beating until EOS euthanasia at d183 despite a rising titer of presumed anti-Class II IgG (detected before anti-Class I) at d119, and, surprisingly, in the absence of detectable anti-donor IgM.
With loTNX+MMF, anti-Class I IgM was detected before rejection in 2 animals (M8720, M8620), on d42 and d84, respectively; M8720 exhibited only a transient low IgM titer, whereas M8620 demonstrated class switching (a single ‘positive’ titer) around the time of graft failure. Intermittent low-titer antidonor IgG was observed in M5020 but did not reach prespecified criteria to be classified as ‘positive’.
With sTNX, sustained anti-donor alloAb elaboration was not detected. Possible transient detection of anti-donor IgG (M1721, left bottom panel, M1321; right bottom panel), anti-donor IgM (M821, M1721; right bottom panel) did not meet prespecified criteria for antidonor antibody detection.
3.4. Peripheral Blood T-Cell Phenotype
The percentage of phenotypically naive CD4+ or CD8+ T cells (defined as CD95− CD62L+) and the ratio of CD4+/CD8+ naive T cells did not significantly differ over time between groups (Fig 5A). Relative to sTNX-treated animals, loTNX+MMF-treated animals had a significantly lower proportion of naïve CD4+ (55.3±11.2% vs 37.8±11.2%; p= 0.045) and CD8+ (35.8±7.8% vs 22.4± 5.7%; p=0.018) T-cells at the final time point (Fig S4-A).
Figure 5: T-cell phenotype and regulatory T-cells.
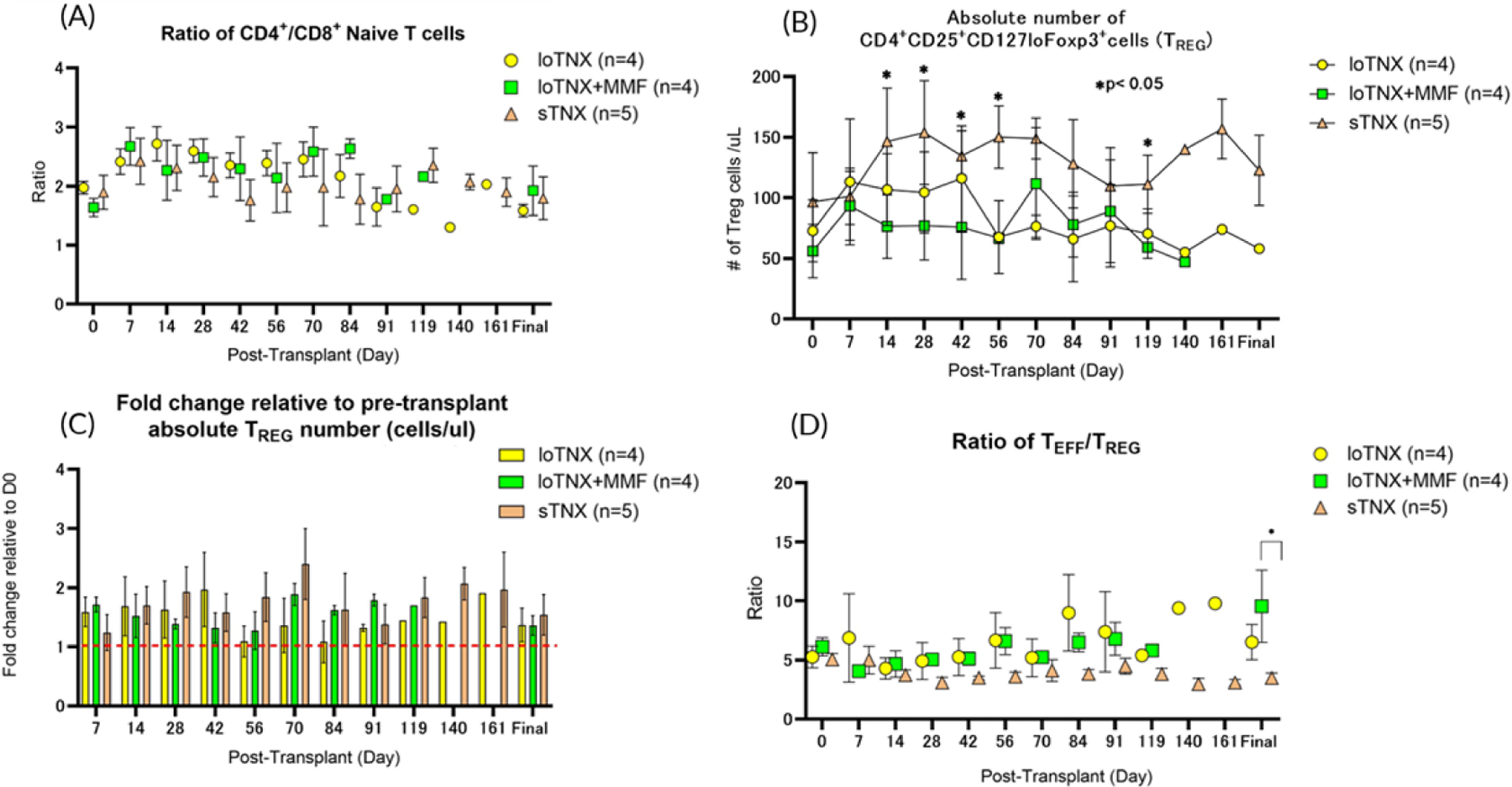
Peripheral blood lymphocyte phenotypes were quantified over time after transplant in loTNX (yellow symbols), loTNX+MMF (green symbols) and sTNX (brown symbols)-treated cardiac allograft recipients. All data are shown as mean ± SEM over time after transplant. (A)The ratio of CD4+/CD8+ naive T cells in all treatment groups remained close to the pretransplant range; no significant differences were observed between the groups throughout the experiment. (B) sTNX was generally associated with an increase in absolute numbers of TREG (defined as CD4+CD25+CD127loFoxp3+) compared to either loTNX or loTNX+MMF, differences that were significant at multiple time points (p<0.05). (C) Numbers of TREG relative to pre-transplantation (d0) increased all treatment groups, but there was no significant difference between the groups at any time point. (D) TREG expanded relative to TEFF in the sTNX-treated group relative to other groups as reflected by a stable and declining TEFF/TREG ratio throughout the experiment, a difference which achieved statistical significance when comparing that ratio at the final time point between sTNX and loTNX+MMF treatment groups (3.5 ± 0.9 vs 9.5 ± 6.1, p=0.038).
TNX, TNX-1500; MMF, mycophenolate mofetil; d, post-transplant days
Animals receiving loTNX+MMF tended to exhibit a higher proportion of terminally differentiated TEMRA (defined as CD95±CD62L−CD4+T cells; 38.8±8.3% vs 28.5± 8.4%; p=0.133) and TCM (defined as CD95+CD62L+CD4+; 22.7±6.7% vs 15.7± 3.3%; p=0.063) than sTNX-treated animals, but these trends were not statistically significant (Fig S4-B, C). During TNX treatment, the absolute numbers of TREG (defined as CD4+CD25+CD127loFoxp3+) in the sTNX treatment group was increased compared to either loTNX or loTNX+MMF treatment groups, differences that were significant at multiple time points (p<0.05) (Fig 5B). The fold change in the absolute TREG number relative to pre-transplant increased in association with loTNX (1.5- to 1.9- fold increase from d7 through d42), loTNX+MMF (1.7-to 1.9-fold increase from d7 through d70) and sTNX (1.2- to 2.1-fold increase from d7 through d140), but there was no significant difference between the groups in this metric (Fig 5C). In the loTNX treatment groups, alone or with MMF, the TEFF/TREG ratio increased slowly and peaked at later time points. In contrast, the TEFF/TREG ratio was significant lower associated with sTNX at the final time point relative to loTNX+MMF (3.5 ± 0.9 vs 9.5 ± 6.1, p=0.034) (Fig 5D).
3.5. Platelet Counts and Activation Markers
Platelet activation after exposure to CD154 antigen/antibody complexes was significantly reduced (P<0.05) in association with TNX1500 relative to murine 5c8 or ruplizumab (h5c8) (Figure S-1), demonstrating that the Fc region modifications in TNX1500 are associated with reduced platelet activation in vitro.
Animals were evaluated daily for any objective evidence of thromboembolic events including limb swelling, respiratory changes, changes in appetite or activity, and skin changes. Platelet counts were measured for each animal throughout the experiment (Fig 6A). None of the animals that received TNX treatment demonstrated sustained thrombocytopenia. Mean platelet counts in all animals were generally within normal range while on therapy; no consistent or statistically significant difference was observed between treatment groups. Platelet activation in vivo, as measured by βTG and soluble P-selectin, was not significantly increased in association with TNX-1500 monotherapy throughout the duration of drug treatment (Fig 6B).
Figure 6: Absolute platelet counts and platelet activation markers measured throughout the experiment.
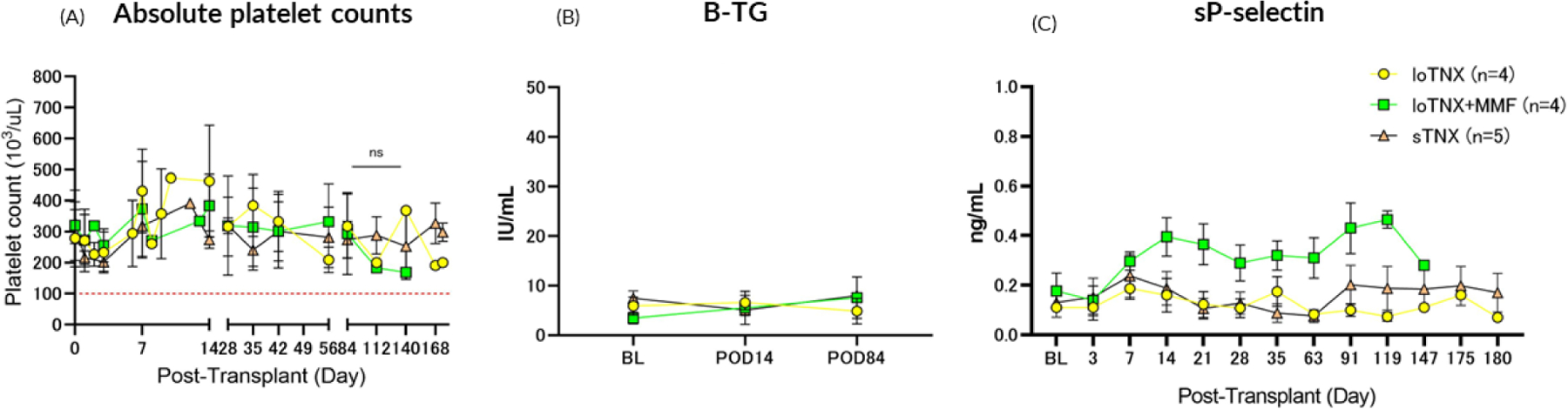
Animals in all treatment groups generally maintained normal platelet counts (above 100 ×103/uL; Fig 6A). Neither sustained thrombocytopenia nor micro- or macrovascular thrombosis were observed during TNX-1500 treatment. Platelet activation, as soluble CD62P and βTG in plasma, remained similar to baseline levels before transplant (BL) in association with either loTNX or stTNX monotherapy (Fig 6B). There was no consistent or statistically significant difference between the TNX monotherapy groups in platelet activation kinetics or in platelet counts throughout treatment relative to baseline BL. The significant sustained elevation in soluble P-selectin in the loTNX+MMF group by the second week and thereafter was unexpected and without obvious explanation.
TNX, TNX-1500; MMF, mycophenolate mofetil
3.6. Allograft Histology
ACR grade increased with increasing time after transplantation in most hearts in the loTNX and loTNX+MMF treatment groups (Fig 7). Two grafts treated with loTNX (M5220, M7419) failed with severe ACR (3R) at explant on d35 and d93, respectively. M5420 was euthanized on d105 with a beating graft due to body weight loss associated with mechanical bowel obstruction; final pathology showed ACR 3R. M4520’s beating graft, explanted on d183 at EOS, exhibited moderate rejection (ACR 2R).
Figure 7. ISHLT scores.
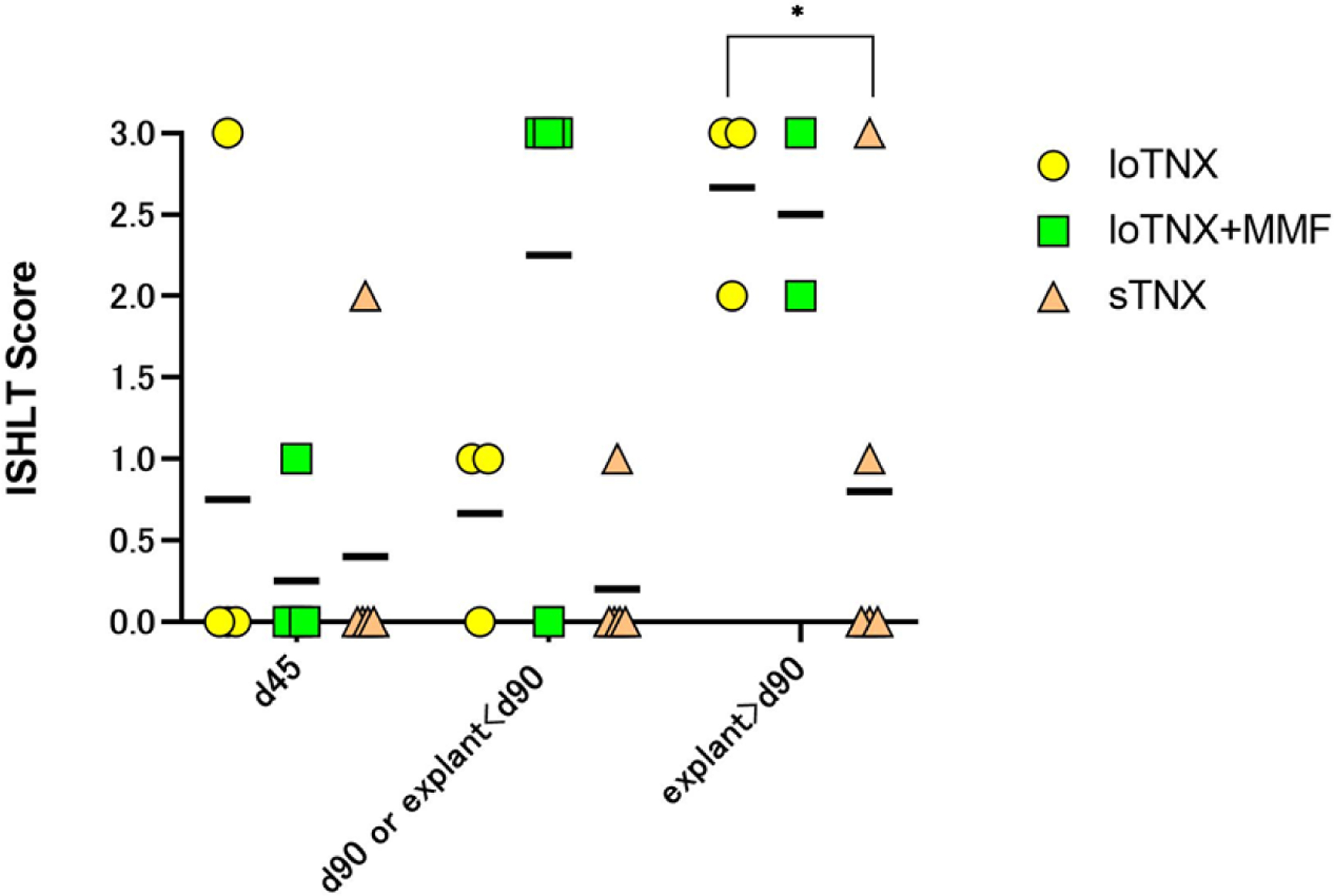
Individual animal and mean (black bars) ISHLT scores at the three protocol-defined time-point biopsies (d45, d90, d180 or earlier explant after day 90), or in for-cause biopsies or explanted failed grafts around those time points. Two grafts in loTNX (yellow symbols) failed with ACR3 at days 35 and 93; one was euthanized on d105 with a beating graft in which evidence of ACR3 was detected on terminal pathology, and the fourth beating graft (2R) was explanted by protocol at 180 days. In loTNX+MMF (green symbols), ACR was moderate (2R) or severe (3R) in the majority of biopsies of beating grafts or in explanted failed grafts after d45. sTNX (brown symbols) was associated with transient moderate rejection (2R resolving to 0R) at d45 in one recipient, and progressive ACR (1R progressing to 3R between day 90 and d116) in association with graft endocarditis and recurrent septicemia after antibiotic cessation at d90. With sTNX, one explanted allograft and 3 end-of-study biopsies around d180 showed absent (0R) or mild rejection (1R). LoTNX had significantly higher mean ISHLT score than sTNX-treated allografts at the time point of subsequent explant after d90 or end-of-study biopsy around d180 (2.7 vs 0.8; *p=0.033). When scores were occasionally discrepant, biopsies were reviewed in conference to discern the source of score variation and adjudicate an agreed score. TNX, TNX-1500, MMF, mycophenolate mofetil; d, post-transplant days; ACR, acute cellular rejection
After d45, all grafts treated with loTNX+MMF exhibited ACR 2R or 3R in the majority of biopsies or in explanted failed grafts. Two grafts (M8720, M5020) exhibited early rejection with ACR on d58 and d66 in association with prior diagnosis of a bacterial CVL or telemetry device infection and ongoing antibiotic treatment after removal of the infected device. The other two grafts (M8820, M8620) exhibited moderate or severe ACR (2R or 3R) on d146 and d111, respectively, in association with declining graft function.
In contrast only two recipients in the sTNX treatment group exhibited ACR >1R. M1721 exhibited self-limited moderate rejection, with ACR 2R at d45 resolving to 0R with ongoing treatment. M421 showed a progressive ACR (1R progressing to 3R) between d90 and d116 in association with graft endocarditis and recurrent septicemia after the discontinuation of antibiotic treatment at d90. With sTNX, one explanted allograft (M921) and 3 EOS-biopsies (M1321, M821, M1721) at d180 showed absent (0R) or mild rejection (1R) (Fig 8); the three grafts surviving for 57–130 days after discontinuation of TNX exhibited severe ACR and AMR at graft failure (CAV not illustrated).
Figure 8. Representative cardiac pathology at explant.
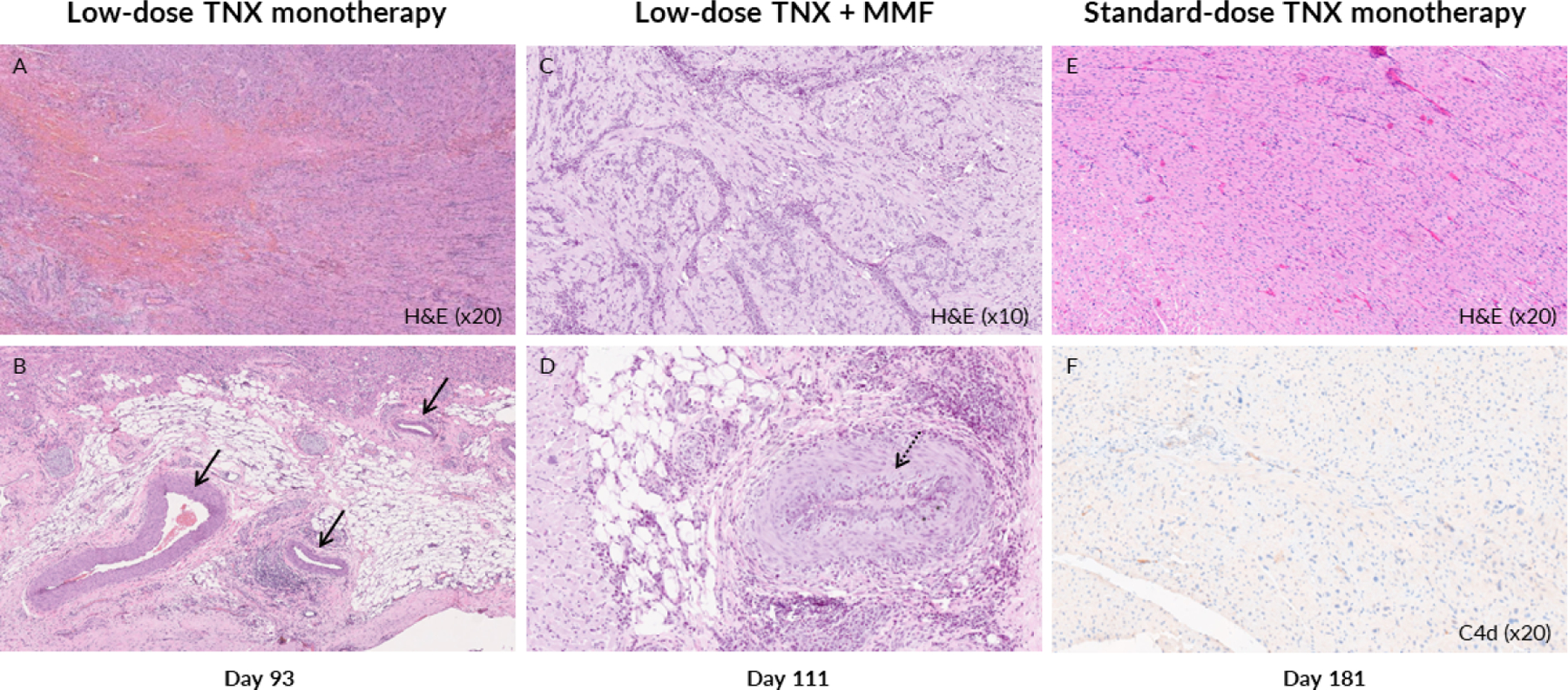
Representative histologic sections are shown of explanted cardiac allografts at the time of graft rejection (left and center panels) or at end of study graft explant (right panels) in association with the 3 Tonix 1500-based treatment regimens evaluated. M7419’s explanted allograft (loTNX; H&E ×20) at day 93 exhibited Grade 3 ACR and AMR with focal interstitial hemorrhage (A); most coronary arteries were histologically normal, without CAV, and intravascular thrombi were not detected (B, black arrows). In contrast, in M8620’s explanted allograft at day 111 (loTNX+MMF; H&E ×10) showed Grade 3 ACR and AMR with diffuse perivascular and spreading interstitial inflammatory cell infiltration (C) and prevalent moderate or severe CAV lesions with intimal hyperplasia (D, dotted black arrow). In association with sTNX, M921’s explanted allograft at day 181 did not show cellular or humoral rejection (E, H&E ×20), CAV (not illustrated), or C4d staining (F, C4d x20).
TNX, TNX-1500; MMF, mycophenolate mofetil; d, post-transplant days; Hematoxylin and eosin, H&E, ACR, acute cellular rejection, AMR, antibody mediated rejection, CAV, cardiac allograft vasculopathy
3.7. Cardiac Allograft Vasculopathy
Overall, mean CAV severity scores increased between d45 and d90 or at explant before d90 in the loTNX+MMF (Fig 9), with higher CAV scores by d90 (mean, 1.5) compared to either loTNX (0.2) or sTNX (0.4). In addition, at graft failure after d90 in two loTNX+MMF animals, CAV score (mean, 2.4) was significantly higher than with either loTNX (0.5, n=3; p=0.007) or in EOS sTNX biopsies (0.7, n=5; p=0.0009). The severity of CAV seen in association with loTNX+MMF is illustrated by M8620’s explanted graft at d111 (Fig 8D), in contrast to a loTNX graft (M7419) failing due to ACR and AMR on d93 (Fig 8B) or an sTNX graft (M921) electively explanted on d181 (CAV not illustrated).
Figure 9. CAV severity scores.
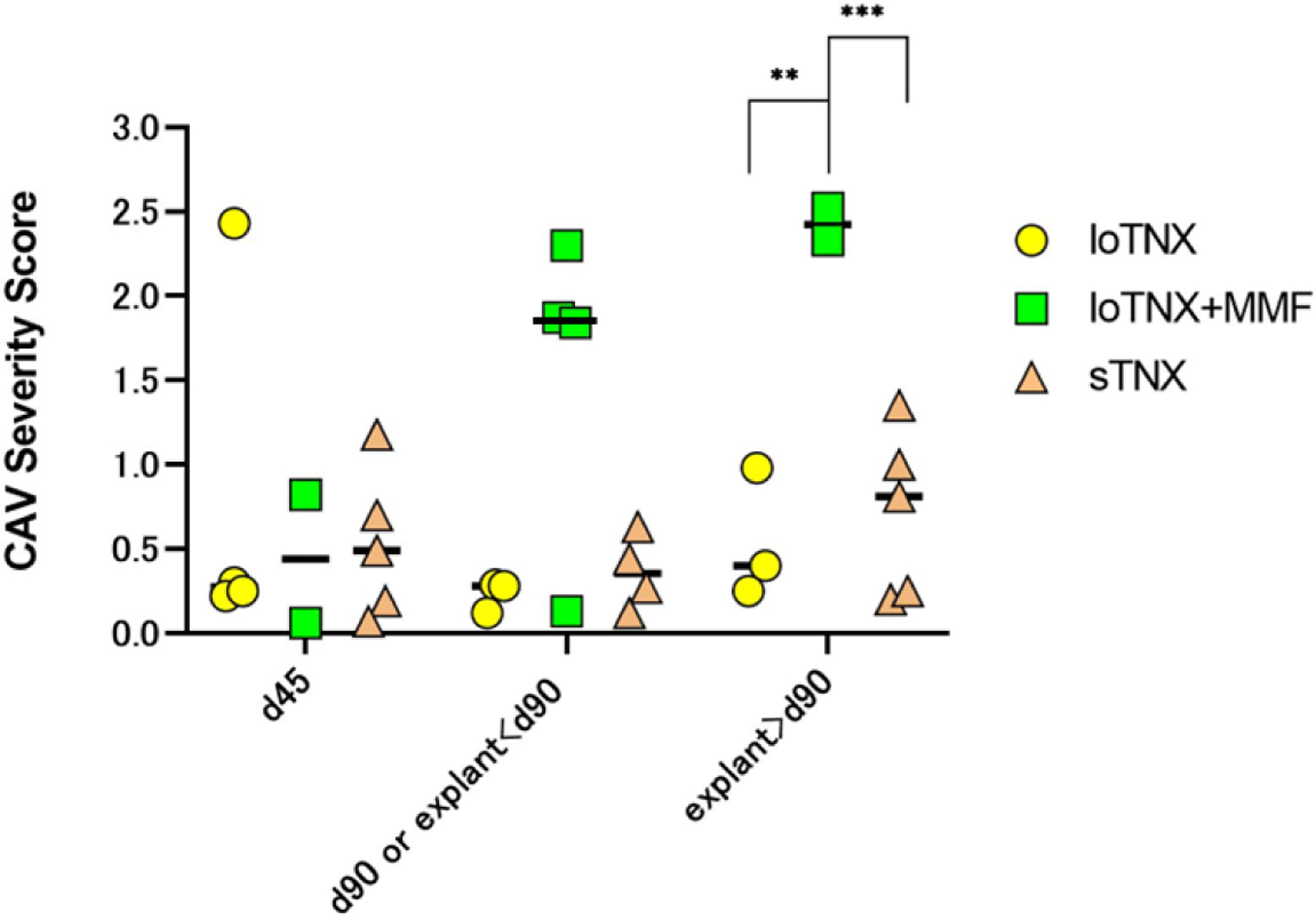
Individual animal CAV severity score and mean (black bars) CAV severity scores at the three protocol-defined time-point biopsies (d45, d90, d180 or earlier explant after day 90), or in for-cause biopsies or explanted failed grafts around those time points. LoTNX+MMF (green symbols) had significantly higher CAV scores (Mean, 2.4) compared to either loTNX (yellow symbols; mean, 0.5; **p=0.007) or sTNX-treated grafts (brown symbols; mean, 0.7; ***p=0.0009) at the time of subsequent explant after d90 or end-of-study biopsy around d180.
TNX, TNX-1500; MMF, mycophenolate mofetil; d, post-transplant days; CAV, cardiac allograft vasculopathy
3.8. Systematic Assessment of Clinical Thrombotic Events
No clinically evident macrovascular thrombotic events attributable to TNX were observed. In M4520 a comprehensive autopsy included gross and histologic examination of the brain and all major organ systems; no macro- or microthrombotic lesions were identified. Macroscopically evident thrombotic lesions were not observed during systematic extracranial autopsy assessment of each other recipient.
4. Discussion
Current success in controlling alloimmune injury to the graft relies on various combinations of potent immunosuppressive drug treatments, each of which is associated with undesirable side effects. Selectively targeting T cell costimulatory pathways has advanced to the clinic with belatacept, which targets the CD28/B7 pathway26,27. In addition to the CD28/B7 pathway, antibodies directed against CD40 and CD154 are being developed to prevent organ transplant rejection, and promote graft survival and function. Several of these molecules appear promising based on results in preclinical NHP animal models.6–13
Despite striking efficacy to prevent pathogenic alloimmunity in preclinical trials, the clinical development of humanized 5c8 (ruplizumab) and other antibodies targeting this pathway were halted due to an increased risk of thrombotic events.14–17 Studies linked the risk of thrombosis with anti-CD154 antibodies to an IgG1 and FcγRIIa-dependent mechanism. Anti-CD154 antibodies with an IgG1 Fc region form immune complexes with soluble CD154, and the immune complexes interact with FcγRIIa and result in thrombosis in FCGR2A transgenic mice.18 Subsequently, a few anti-CD154 mAbs were engineered to modulate FcγRIIa-binding and successfully avoided thrombosis, including a PEGylated Fab,28 an Fc-disabled aglycosyl version of ruplizumab19 and an Fc-silent anti-CD154 domain antibody.20 However, absent or reduced Fc functionality was also associated with reduced efficacy as monotherapy in NHP kidney transplant models.19,20,29–31 Based on these considerations, it was postulated that some Fc functionality was important for facilitating graft survival and function. Therefore, TNX-1500, containing the humanized 5c8 Fab region from ruplizumab,19,21 was combined with a genetically modified IgG4 Fc region which reduces functional interaction with FcγRIIa.Ref Lassiter et al Platelet activation in vitro was reduced in association with TNX1500-antigen complexes relative to either the parent murine 5c8 antibody or the humanized version, ruplizumab (h5c8).Ref Lassiter et al Since laboratory, clinical, and pathologic assessments showed no evidence of platelet activation or related thrombotic complications, our observations support the prediction that TNX1500 is likely to be associated with an improved safety profile in clinical application relative to the parent ruplizumab molecule. Here, in contrast to prior work with Fc-defunctionalized anti-CD154 antibodies, we also describe preserved functional ability of TNX-1500 to prolong graft survival as monotherapy.
TREG expansion potentially contributes to immune protection and may promote durable peripheral regulatory tolerance. Several reports have described increased TREG frequency associated with CD154 targeting.31–34 In this study, sTNX was consistently associated with increased frequency of TREG and reduced frequency of TEMRA in the peripheral blood compared to animals treated with loTNX, alone or with MMF, resulting in a lower TEFF/TREG ratio in the sTNX group by six months after transplant. We hypothesize that promoting peripheral TREG expansion may be one mechanism by which anti-CD154 therapy protect allografts from immune injury. This mechanism may distinguish CD154 blockade from effects associated with CD40 receptor-directed approaches and also potentially treatment with CD28/B7 blockers. Whether the increase in TREG promotes durable peripheral regulatory T-cell tolerance remains to be determined.
Transplantation regimens have traditionally attempted to combine drugs with different mechanisms to reduce the dose of each and increase activity while decreasing side-effects. For example, belatacept is associated with improved long-term renal function in kidney allograft recipients when used in conjunction with standard-dose26 or reduced-dose conventional IS,27 even when a relatively high incidence of early acute rejection was observed. Therefore, we studied a low-dose of TNX with MMF. Importantly, the reduced-intensity loTNX maintenance regimen was less effective than sTNX to prevent histologic evidence of alloimmune injury on protocol biopsies or graft rejection during ongoing treatment. Combining loTNX with MMF did not prevent histologic evidence of alloimmune injury. Indeed, grafts in recipients treated with loTNX with MMF unexpectedly trended to be more rapid progression of CAV relative to loTNX alone despite similar TNX trough levels. Studies currently under way in our lab will test the hypothesis that combining sTNX with MMF has a detrimental effect relative to sTNX alone, perhaps because MMF preferentially interferes with maturation of protective regulatory immunity in the context of CD154 inhibition.
The higher incidence of bacterial infections observed in the loTNX+MMF treatment group relative to either of the other treatment groups introduces a potentially confounding variable. It is possible that CVL-associated infections may have elicited systemic inflammation sufficient to precipitate early graft failure despite the activity of loTNX. Viral infections have been associated with increased incidence of graft rejection in murine, primate and human transplant studies;35 a correlation with bacterial infections is plausible if innate immune activation and the associated systemic inflammatory response are efficient to tip the immune balance of a transplant recipient toward effector/killer function and to suppress emergence of anti-inflammatory and antigen-specific regulatory responses.
CD154 blockade inhibits B cell proliferation, modulates germinal center formation, and prevents antibody isotype switching and affinity maturation.36 Consistent with these known mechanistic effects, in TNX-treated animals where antidonor IgM was transiently detected, class switching between anti-class II IgM and anti-class II IgG was usually not observed. The sTNX regimen was associated with improved control of pathogenic immunity relative to loTNX with or without MMF, as demonstrated by consistent prevention of antidonor alloAb elaboration, reduced ISHLT score and CAV severity scores post-transplant, and the reduced incidence of graft failure during treatment. Of note, relative to our previously reported criterion for detection of anti-donor antibody (staining detected on >10% of donor T-cells), we find that a 2-fold-increase in MFI is more sensitive to detect low levels of anti-donor antibody (Fig. S-5); while this criterion is inherently arbitrary, we conclude that this measured antibody is consequential because its detection was consistently associated with pathology in the graft (ACR, AMR, C4d staining) and a high incidence of graft failure during ongoing loTNX treatment.
In conclusion, we demonstrate that a novel anti-CD154 mAb, TNX-1500 was well tolerated and prevents pathogenic alloimmunity and elaboration of antidonor alloAb in a NHP cardiac allograft model, resulting in prolonged survival with preserved graft function. In addition, TNX appeared to promote peripheral regulatory tolerance with increased TREG. Together these data support clinical evaluation of TNX as a supplement or alternative to currently used small molecule immunosuppressants.
Supplementary Material
A: Freshly collected cynomolgus monkey peripheral blood samples were incubated with anti-CD154 antibody (mouse 5c8, humanized 5c8 or TNX-1500) alone or preformed immune complex (IC) consisting of anti-CD154 antibodies and soluble CD154 (Fig. 1A). B: Using flow cytometry, platelets were gated based on FSC/SSC scatter and CD61 expression. C: Platelet activation was then evaluated by expression of CD62P and PAC-1. Representative dot plots from cynomolgus monkey platelets (upper panels) and human platelets (lower panels) are shown. D: The mean with standard errors of the mean fluorescent intensity (MFI) of CD62+PAC-1+ platelets from cynomolgus monkeys (n=4) and human platelets (n=3) were shown. **p<0.01, NS, not significant. Platelet activation was observed after incubation with hu5c8-sCD154 ICs in both cynomolgus monkeys humans. In contrast, no activation was observed with TNX-1500-sCD154 ICs in either cynomolgus or human platelets.
M421, treated with sTNX, experienced endocarditis diagnosed by ultrasonography on d34 with a mitral valve vegetation in the transplanted heart (A, white arrow). At the time of the diagnosis, the central line and the telemetry device implanted into the abdomen were removed. The animal received sensitivity-guided antibiotic treatment for a total of 8 weeks, with marked shrinkage of the vegetation. On d116, two weeks after discontinuation of prolonged suppressive antibiotic treatment, the animal was euthanized due to recurrent fever with septicemia and graft dysfunction. The explanted allograft showed regional infarction (B, H&E ×20), possible attributable to a septic embolus versus immunologic vascular occlusion, and mild early CAV lesions (C), while C4d staining was present diffusely, including in areas with otherwise normal histology (D, C4d x10). D, post-transplant days, Hematoxylin and eosin, H&E, ACR, acute cellular rejection, AMR, antibody mediated rejection
Serum concentrations of TNX-1500 measured over two weeks after single dose drug administration are shown for cynomolgus monkeys given either a 30, 100, or 300 mg/kg bolus infusion (n=2 per dose level). TNX-1500 clearance ranged from 2.85–5.0 ml/d/kg, and serum half-life from 10.6–15.8 days.
Peripheral blood lymphocyte phenotypes were characterized over time after transplant using flowcytometry in loTNX-treated (yellow), loTNX with MMF-treated (green) and sTNX-treated (black) cardiac allograft recipients. All data are shown with mean ± SEM as a change over time. A) Overall, the percentage of phenotypically naive CD4+ or CD8+ T cells in all treatment groups were within the normal range, but significant differences were observed between sTNX-treated and loTNX alone or with MMF-treated allografts at the final time point [naive CD4+; sTNX 55.3±11.2 vs loTNX+MMF 37.8±11.2%, p=0.045, naive CD8+; sTNX 35.8±7.8 vs loTNX 21.5±7.4, p=0.014]. B, C) Animals receiving loTNX with MMF demonstrated a higher frequency of terminally differentiated CD95±CD62L-CD4+T cells (defined as TEMRA cells) and CD95+CD62L+CD4+ T cells (defined as TCM cells). Significant difference in the percentage of TCM cells at the final time point was observed between loTNX+MMF and loTNX treatment groups (22.7±6.7 vs 14.1±4.9, p=0.035).
TNX, TNX-1500; MMF, mycophenolate mofetil; d, post-transplant days; TEMRA, effector memory T cells;TCM, central memory T cells.
Presence of donor specific alloAb was defined by detection of recipient serum IgM or IgG binding to donor CD3+CD20− T-cells (expressing MHC Class I) or CD3-CD20+ B-cells (expressing MHC Class I and Class II), but classifying staining as positive when >10% of donor cells exhibit consistent (on 2 or more consecutive measurements) staining relative to the pre-transplant recipient serum (d0). By this criterion, alloantibody was only detected in 1 recipient treated with loTNX, whose graft continued beating until elective explant at d183. Detection of low-level binding to donor cells as shown in Figure 4 appears to be consequential in that it was associated with graft pathology and failure. We explain transient low-level detection of alloAb with loTNX in Figure 4 as reflecting adsorption of alloAb to the surviving allograft as well as partial inhibition of class switching and affinity maturation by relatively incomplete CD154 blockade in secondary lymphoid organs in association with the loTNX dosing regimen.
TNX, TNX-1500; MMF, mycophenolate mofetil; d, post-transplant days
TNX-1500 exhibits a 14-fold reduction in FcrIIa binding affinity relative to the parent 5c8 IgG1 molecule. For methods, please see the primary reference. Ref Lassiter et al
Acknowledgments / Funding:
Funding for this study was supported by a sponsored research agreement with Tonix Pharmaceuticals, by NIH P01 HL158504 and by seed funding from the Center for Transplantation Sciences at Massachusetts General Hospital. RC is supported by the Benjamin Research Fellowship from the German Research Foundation (DFG).
Abbreviations:
- NHP
nonhuman primate
- mAb
monoclonal antibody
- Fc
constant fragment
- FcγRIIa
Fc gamma receptor IIa
- MHC
major histocompatibility complex
- d
post-transplant days
- MMF
mycophenolate mofetil
- EOS
end of study
- alloAb
alloantibody
- EDTA
ethylenediaminetetraacetic acid
- CAV
cardiac allograft vasculopathy
- CVL
central venous line
- ACR
acute cellular rejection
- AMR
antibody mediated rejection
- MST
median survival time
- TEFF
effector T cells
- TEMRA
effector memory T cells
- TCM
central memory T cells.
- TREG
regulatory T cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: SML, SF, BD, and PM are paid employees and BM is a paid consultant of Tonix Pharmaceutical LLC. The authors have no other significant financial interests to disclose as defined by NIH and MGH regulations.
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Richard N. Pierson 3rd reports financial support was provided by Tonix Pharmaceuticals. Richard N. Pierson 3rd reports a relationship with Tonix Pharmaceuticals Holding Corp that includes: funding grants. Siobhan Fogarty reports a relationship with Tonix Pharmaceuticals Holding Corp that includes: employment. Bruce Daugherty reports a relationship with Tonix Pharmaceuticals Holding Corp that includes: employment. Seth Lederman reports a relationship with Tonix Pharmaceuticals Holding Corp that includes: employment. Bernd Meibohm reports a relationship with Tonix Pharmaceuticals Holding Corp that includes: consulting or advisory. Ryan Chaban reports a relationship with German Research Foundation that includes: funding grants. Seth Lederman has patent #WO2021001458A1 licensed to TONIX PHARMA HOLDINGS LIMITED.
References
- 1.Li XC, Rothstein DM, Sayegh MH. Costimulatory pathways in transplantation: challenges and new developments. Immunol. Rev 229(1), 271–293 (2009). [DOI] [PubMed] [Google Scholar]
- 2.Lederman S, Yellin MJ, Krichevsky A, Belko J, Lee JJ, Chess L. Identification of a novel surface protein on activated CD4+ T cells that induces contact-dependent B cell differentiation (help). J Exp Med 1992. Apr 1;175(4):1091–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poirier N, Blancho G, Vanhove B. A more selective costimulatory blockade of the CD28-B7 pathway. Transpl. Int 24(1), 2–11 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Kinnear G, Jones ND, Wood KJ. Costimulation blockade: current perspectives and implications for therapy. Transplantation 95(4), 527–535 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ford ML, Adams AB, Pearson TC. Targeting co-stimulatory pathways: transplantation and autoimmunity. Nat. Rev. Nephrol 10(1), 14–24 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang T, Pierson RN 3rd, Azimzadeh AM. Update on CD40 and CD154 blockade in transplant models. Immunotherapy 2015;7(8):899–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.OʼNeill NA, Zhang T, Braileanu G, Sun W, Cheng X, Hershfeld A, Laird CT, Kronfli A, Hock LA, Dahi S, Kubicki N, Sievert E, Hassanein W, Cimeno A, Pierson RN 3rd, Azimzadeh AM. Comparative Evaluation of αCD40 (2C10R4) and αCD154 (5C8H1 and IDEC-131) in a Nonhuman Primate Cardiac Allotransplant Model. Transplantation 2017. Sep;101(9):2038–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirk AD, Harlan DM, Armstrong NN, Davis TA, Dong Y, Gray GS, Hong X, Thomas D, Fechner JH Jr, Knechtle SJ. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc Natl Acad Sci U S A 1997. Aug 5;94(16):8789–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirk AD, Burkly LC, Batty DS, Baumgartner RE, Berning JD, Buchanan K, Fechner JH Jr, Germond RL, Kampen RL, Patterson NB, Swanson SJ, Tadaki DK, TenHoor CN, White L, Knechtle SJ, Harlan DM. Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates. Nat Med 1999. Jun;5(6):686–93. [DOI] [PubMed] [Google Scholar]
- 10.Kenyon NS, Chatzipetrou M, Masetti M, Ranuncoli A, Oliveira M, Wagner JL, Kirk AD, Harlan DM, Burkly LC, Ricordi C. Long-term survival and function of intrahepatic islet allografts in rhesus monkeys treated with humanized anti-CD154. Proc Natl Acad Sci U S A 1999. Jul 6;96(14):8132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elster EA, Xu H, Tadaki DK, Montgomery S, Burkly LC, Berning JD, Baumgartner RE, Cruzata F, Marx R, Harlan DM, Kirk AD. Treatment with the humanized CD154-specific monoclonal antibody, hu5C8, prevents acute rejection of primary skin allografts in nonhuman primates. Transplantation 2001. Nov 15;72(9):1473–8. [DOI] [PubMed] [Google Scholar]
- 12.Pierson RN 3rd, Chang AC, Blum MG, Blair KS, Scott MA, Atkinson JB, Collins BJ, Zhang JP, Thomas DW, Burkly LC, Miller GG. Prolongation of primate cardiac allograft survival by treatment with ANTI-CD40 ligand (CD154) antibody. Transplantation 1999. Dec 15;68(11):1800–5. [DOI] [PubMed] [Google Scholar]
- 13.Azimzadeh AM, Pfeiffer S, Wu G, Schröder C, Zorn GL 3rd, Kelishadi SS, Ozkaynak E, Kehry M, Atkinson JB, Miller GG, Pierson RN 3rd. Alloimmunity in primate heart recipients with CD154 blockade: evidence for alternative costimulation mechanisms. Transplantation 2006. Jan 27;81(2):255–64. [DOI] [PubMed] [Google Scholar]
- 14.Vincenti F New monoclonal antibodies in renal transplantation. Minerva Urol Nefrol 2003. Mar;55(1):57–66. [PubMed] [Google Scholar]
- 15.Knechtle SJ. Development of tolerogenic strategies in the clinic. Philos Trans R Soc Lond B Biol Sci 2005. Sep 29;360(1461):1739–46. doi: 10.1098/rstb.2005.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boumpas DT, Furie R, Manzi S, Illei GG, Wallace DJ, Balow JE, Vaishnaw A; BG9588 Lupus Nephritis Trial Group. A short course of BG9588 (anti-CD40 ligand antibody) improves serologic activity and decreases hematuria in patients with proliferative lupus glomerulonephritis. Arthritis Rheum 2003. Mar;48(3):719–27. [DOI] [PubMed] [Google Scholar]
- 17.Kawai T, Andrews D, Colvin RB, Sachs DH, Cosimi AB. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat Med 2000. Feb;6(2):114. doi: 10.1038/72162. [DOI] [PubMed] [Google Scholar]
- 18.Robles-Carrillo L, Meyer T, Hatfield M, Desai H, Dávila M, Langer F, Amaya M, Garber E, Francis JL, Hsu YM, Amirkhosravi A. Anti-CD40L immune complexes potently activate platelets in vitro and cause thrombosis in FCGR2A transgenic mice. J Immunol 2010. Aug 1;185(3):1577–83. [DOI] [PubMed] [Google Scholar]
- 19.Ferrant JL, Benjamin CD, Cutler AH, Kalled SL, Hsu YM, Garber EA, Hess DM, Shapiro RI, Kenyon NS, Harlan DM, Kirk AD, Burkly LC, Taylor FR. The contribution of Fc effector mechanisms in the efficacy of anti-CD154 immunotherapy depends on the nature of the immune challenge. Int Immunol 2004. Nov;16(11):1583–94. [DOI] [PubMed] [Google Scholar]
- 20.Kim SC, Wakwe W, Higginbotham LB, Mathews DV, Breeden CP, Stephenson AC, Jenkins J, Strobert E, Price K, Price L, Kuhn R, Wang H, Yamniuk A, Suchard S, Farris AB 3rd, Pearson TC, Larsen CP, Ford ML, Suri A, Nadler S, Adams AB. Fc-Silent Anti-CD154 Domain Antibody Effectively Prevents Nonhuman Primate Renal Allograft Rejection. Am J Transplant 2017. May;17(5):1182–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lederman S, Cleary AM, Yellin MJ, Frank DM, Karpusas M, Thomas DW, Chess L. The central role of the CD40-ligand and CD40 pathway in T-lymphocyte-mediated differentiation of B lymphocytes. Curr Opin Hematol 1996. Jan;3(1):77–86. [DOI] [PubMed] [Google Scholar]
- 22.Kelishadi SS, Azimzadeh AM, Zhang T, Stoddard T, Welty E, Avon C, Higuchi M, Laaris A, Cheng XF, McMahon C, Pierson RN 3rd. Preemptive CD20+ B cell depletion attenuates cardiac allograft vasculopathy in cyclosporine-treated monkeys. J Clin Invest 2010. Apr;120(4):1275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azimzadeh AM, Pfeiffer S, Wu GS, Schröder C, Zhou H, Zorn GL 3rd, Kehry M, Miller GG, Rose ML, Pierson RN 3rd. Humoral immunity to vimentin is associated with cardiac allograft injury in nonhuman primates. Am J Transplant 2005. Oct;5(10):2349–59. [DOI] [PubMed] [Google Scholar]
- 24.Azimzadeh AM, Zhang T, Wu G, Kelishadi SS, Stoddard T, OʼNeill N, Nguyen BN, Welty E, Avon C, Higuchi M, Mitchell SL, Hershfeld A, Cheng XF, Kronfli A, Rybak E, Burdorf L, Pierson RN 3rd. Preemptive CD20+ B cell Depletion Attenuates Cardiac Allograft Vasculopathy in CD154-Treated Monkeys. Transplantation 2017. Jan;101(1):63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stewart S, Winters GL, Fishbein MC, Tazelaar HD, Kobashigawa J, Abrams J, Andersen CB, Angelini A, Berry GJ, Burke MM, Demetris AJ, Hammond E, Itescu S, Marboe CC, McManus B, Reed EF, Reinsmoen NL, Rodriguez ER, Rose AG, Rose M, Suciu-Focia N, Zeevi A, Billingham ME. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant 2005. Nov;24(11):1710–20. [DOI] [PubMed] [Google Scholar]
- 26.Vincenti F, Rostaing L, Grinyo J, Rice K, Steinberg S, Gaite L, Moal MC, Mondragon-Ramirez GA, Kothari J, Polinsky MS, Meier-Kriesche HU, Munier S, Larsen CP. Belatacept and Long-Term Outcomes in Kidney Transplantation. N Engl J Med 2016. Jan 28;374(4):333–43. [DOI] [PubMed] [Google Scholar]
- 27.Adams AB, Goldstein J, Garrett C, Zhang R, Patzer RE, Newell KA, Turgeon NA, Chami AS, Guasch A, Kirk AD, Pastan SO, Pearson TC, Larsen CP. Belatacept Combined With Transient Calcineurin Inhibitor Therapy Prevents Rejection and Promotes Improved Long-Term Renal Allograft Function. Am J Transplant 2017. Nov;17(11):2922–2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tocoian A, Buchan P, Kirby H, Soranson J, Zamacona M, Walley R, Mitchell N, Esfandiari E, Wagner F, Oliver R. First-in-human trial of the safety, pharmacokinetics and immunogenicity of a PEGylated anti-CD40L antibody fragment (CDP7657) in healthy individuals and patients with systemic lupus erythematosus. Lupus 2015. Sep;24(10):1045–56. doi: 10.1177/0961203315574558. Epub 2015 Mar 16. [DOI] [PubMed] [Google Scholar]
- 29.Daley SR, Cobbold SP, Waldmann H. Fc-disabled anti-mouse CD40L antibodies retain efficacy in promoting transplantation tolerance. Am J Transplant 2008. Nov;8(11):2265–71. [DOI] [PubMed] [Google Scholar]
- 30.Monk NJ, Hargreaves RE, Marsh JE, Farrar CA, Sacks SH, Millrain M, Simpson E, Dyson J, Jurcevic S. Fc-dependent depletion of activated T cells occurs through CD40L-specific antibody rather than costimulation blockade. Nat Med 2003. Oct;9(10):1275–80. [DOI] [PubMed] [Google Scholar]
- 31.Pinelli DF, Wagener ME, Liu D, Yamniuk A, Tamura J, Grant S, Larsen CP, Suri A, Nadler SG, Ford ML. An anti-CD154 domain antibody prolongs graft survival and induces Foxp3(+) iTreg in the absence and presence of CTLA-4 Ig. Am J Transplant 2013. Nov;13(11):3021–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferrer IR, Wagener ME, Song M, Kirk AD, Larsen CP, Ford ML. Antigen-specific induced Foxp3 + regulatory T cells are generated following CD40/CD154 blockade. Proc Natl Acad Sci USA 2011; 108: 20701–20706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu D, Ferrer IR, Konomos M, Ford ML. Inhibition of CD8+ T cell-derived CD40 signals is necessary but not sufficient for Foxp3+ induced regulatory T cell generation in vivo. J Immunol 2013. Aug 15;191(4):1957–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor PA, Noelle RJ, Blazar BR. CD4(+) CD25(+) immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. J Exp Med 2001;193:1311–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ford ML, Larsen CP. Translating costimulation blockade to the clinic: lessons learned from three pathways. Immunol Rev 2009. May;229(1):294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yazdany J, Davis J. The role of CD40 ligand in systemic lupus erythematosus. Lupus 2004;13(5):377–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A: Freshly collected cynomolgus monkey peripheral blood samples were incubated with anti-CD154 antibody (mouse 5c8, humanized 5c8 or TNX-1500) alone or preformed immune complex (IC) consisting of anti-CD154 antibodies and soluble CD154 (Fig. 1A). B: Using flow cytometry, platelets were gated based on FSC/SSC scatter and CD61 expression. C: Platelet activation was then evaluated by expression of CD62P and PAC-1. Representative dot plots from cynomolgus monkey platelets (upper panels) and human platelets (lower panels) are shown. D: The mean with standard errors of the mean fluorescent intensity (MFI) of CD62+PAC-1+ platelets from cynomolgus monkeys (n=4) and human platelets (n=3) were shown. **p<0.01, NS, not significant. Platelet activation was observed after incubation with hu5c8-sCD154 ICs in both cynomolgus monkeys humans. In contrast, no activation was observed with TNX-1500-sCD154 ICs in either cynomolgus or human platelets.
M421, treated with sTNX, experienced endocarditis diagnosed by ultrasonography on d34 with a mitral valve vegetation in the transplanted heart (A, white arrow). At the time of the diagnosis, the central line and the telemetry device implanted into the abdomen were removed. The animal received sensitivity-guided antibiotic treatment for a total of 8 weeks, with marked shrinkage of the vegetation. On d116, two weeks after discontinuation of prolonged suppressive antibiotic treatment, the animal was euthanized due to recurrent fever with septicemia and graft dysfunction. The explanted allograft showed regional infarction (B, H&E ×20), possible attributable to a septic embolus versus immunologic vascular occlusion, and mild early CAV lesions (C), while C4d staining was present diffusely, including in areas with otherwise normal histology (D, C4d x10). D, post-transplant days, Hematoxylin and eosin, H&E, ACR, acute cellular rejection, AMR, antibody mediated rejection
Serum concentrations of TNX-1500 measured over two weeks after single dose drug administration are shown for cynomolgus monkeys given either a 30, 100, or 300 mg/kg bolus infusion (n=2 per dose level). TNX-1500 clearance ranged from 2.85–5.0 ml/d/kg, and serum half-life from 10.6–15.8 days.
Peripheral blood lymphocyte phenotypes were characterized over time after transplant using flowcytometry in loTNX-treated (yellow), loTNX with MMF-treated (green) and sTNX-treated (black) cardiac allograft recipients. All data are shown with mean ± SEM as a change over time. A) Overall, the percentage of phenotypically naive CD4+ or CD8+ T cells in all treatment groups were within the normal range, but significant differences were observed between sTNX-treated and loTNX alone or with MMF-treated allografts at the final time point [naive CD4+; sTNX 55.3±11.2 vs loTNX+MMF 37.8±11.2%, p=0.045, naive CD8+; sTNX 35.8±7.8 vs loTNX 21.5±7.4, p=0.014]. B, C) Animals receiving loTNX with MMF demonstrated a higher frequency of terminally differentiated CD95±CD62L-CD4+T cells (defined as TEMRA cells) and CD95+CD62L+CD4+ T cells (defined as TCM cells). Significant difference in the percentage of TCM cells at the final time point was observed between loTNX+MMF and loTNX treatment groups (22.7±6.7 vs 14.1±4.9, p=0.035).
TNX, TNX-1500; MMF, mycophenolate mofetil; d, post-transplant days; TEMRA, effector memory T cells;TCM, central memory T cells.
Presence of donor specific alloAb was defined by detection of recipient serum IgM or IgG binding to donor CD3+CD20− T-cells (expressing MHC Class I) or CD3-CD20+ B-cells (expressing MHC Class I and Class II), but classifying staining as positive when >10% of donor cells exhibit consistent (on 2 or more consecutive measurements) staining relative to the pre-transplant recipient serum (d0). By this criterion, alloantibody was only detected in 1 recipient treated with loTNX, whose graft continued beating until elective explant at d183. Detection of low-level binding to donor cells as shown in Figure 4 appears to be consequential in that it was associated with graft pathology and failure. We explain transient low-level detection of alloAb with loTNX in Figure 4 as reflecting adsorption of alloAb to the surviving allograft as well as partial inhibition of class switching and affinity maturation by relatively incomplete CD154 blockade in secondary lymphoid organs in association with the loTNX dosing regimen.
TNX, TNX-1500; MMF, mycophenolate mofetil; d, post-transplant days
TNX-1500 exhibits a 14-fold reduction in FcrIIa binding affinity relative to the parent 5c8 IgG1 molecule. For methods, please see the primary reference. Ref Lassiter et al


