Abstract
The activities of levofloxacin and clarithromycin against 199 penicillin- and macrolide-susceptible and -resistant pneumococci were tested by agar and microdilution methods in air and by disk diffusion and E-test methods in air and CO2. For levofloxacin, ≥99.0% of strains were susceptible at ≤2.0 μg/ml with zone diameters of ≥17 mm, regardless of incubation in air or CO2. Although zone sizes were smaller and E-test MICs were higher for clarithromycin in CO2 than those in air, category differences were minor, and susceptibility rates for clarithromycin were similar to those obtained by agar and microdilution in air (range, 76.9 to 80.9% by all methods). For clarithromycin, adjustment of breakpoints based upon distribution of results resulted in susceptibility rates which were similar by all methods (75.8 to 76.9% susceptible, 0 to 1.5% intermediate, 22.6 to 23.1% resistant). Minor discrepancies were obtained with levofloxacin for one strain (0.5%) by microdilution and two strains (1.0%) by disk diffusion in CO2. For clarithromycin, minor discrepancies were found in three strains (1.5%) by microdilution, seven strains (3.5%) by agar dilution, four strains (2.0%) by E-test in air, six strains (3.0%) by disk diffusion in air, and five strains (2.5%) by disk diffusion in CO2. Major discrepancies occurred with levofloxacin in one strain (0.5%) by microdilution but were not found with clarithromycin. Very major discrepancies were not seen with levofloxacin, but occurred with clarithromycin in five strains (2.5%) by microdilution, three strains (1.5%) by agar dilution, two strains (1.0%) by E-test in air, eight strains (4.0%) by disk diffusion in air, and one strain (0.5%) by disk diffusion in CO2.
Streptococcus pneumoniae continues to be a significant cause of morbidity and mortality in humans; is the leading cause of bacterial pneumonia, sinusitis, and otitis media; and is an important cause of meningitis. The past 5 years have witnessed a dramatic worldwide increase in the incidence of pneumococcal strains which are resistant to penicillin G and other β-lactam and non-β-lactam antimicrobials, such as macrolides, clindamycin, tetracycline, chloramphenicol, and trimethoprim-sulfamethoxazole (1, 2, 7, 8). The problem has been exacerbated by the tendency of these strains to spread from country to country and from continent to continent (11, 12).
In the United States, a recent study has shown that the penicillin MICs for 23.6% of 1,527 clinically significant pneumococci from 30 U.S. centers were ≥0.125 μg/ml, with 14.1% of isolates intermediate and 9.5% of isolates resistant (5). Penicillin-resistant pneumococci are more likely to be resistant to macrolides and other unrelated agents, such as chloramphenicol, tetracycline, and trimethoprim-sulfamethoxazole (5, 9). Erythromycin resistance among pneumococci has increased in the United States from approximately 0.2% in the late 1980s to 5 and 15% in some areas of the country (4, 16). In the survey described above, 20% of penicillin-intermediate pneumococcal strains and 49% of penicillin-resistant strains were erythromycin resistant (5). Pneumococcal strains that are resistant to erythromycin exhibit cross-resistance to all other available macrolides and azalides, such as azithromycin, clarithromycin, and roxithromycin (5, 6, 18).
Currently available quinolones such as ciprofloxacin and ofloxacin are either inactive or are marginally active against pneumococci, because MICs are above or cluster around achievable serum drug levels and susceptibility breakpoints (9, 15, 17, 19). Levofloxacin, the l-isomer of ofloxacin, has been shown to have MICs for all pneumococci, irrespective of their penicillin or macrolide MICs, that are 1 or 2 dilutions lower than those of ciprofloxacin and ofloxacin (15, 17, 19). The compound also possesses favorable kill kinetics against these organisms (17, 19).
Currently, the National Committee for Clinical Laboratory Standards (NCCLS) recommends incubation in air for microdilution testing and CO2 for disk diffusion (13, 14). The manufacturers of the E-test (AB Biodisk, Solna, Sweden) also recommend incubation in CO2 for testing their product against pneumococci, because 5 to 10% of strains do not grow without CO2 on primary isolation. Because CO2 has previously been shown to affect pneumococcal macrolide MICs (6, 18) and may also have an effect on quinolone results (3, 10), we tested the activities of levofloxacin (Fig. 1) and clarithromycin (Fig. 2) against 199 pneumococci by agar dilution and microdilution in air and disk diffusion and E-test in air as well as CO2.
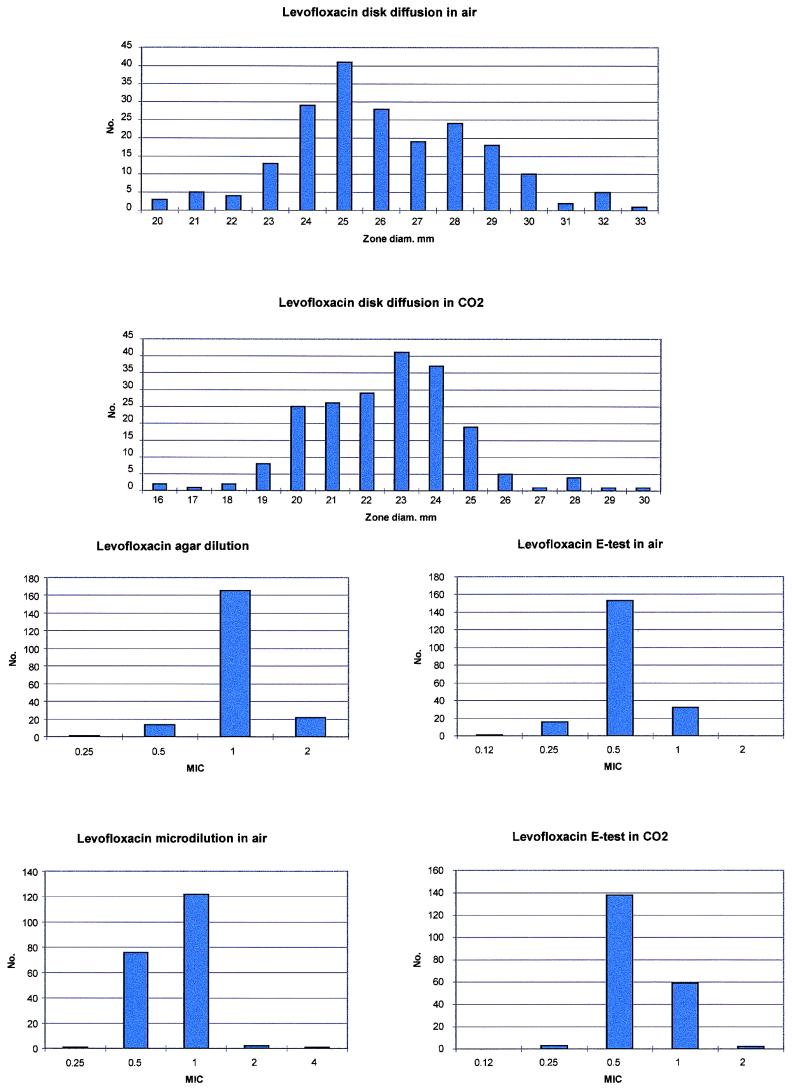
FIG. 1.
Histogram analysis of levofloxacin susceptibilities.
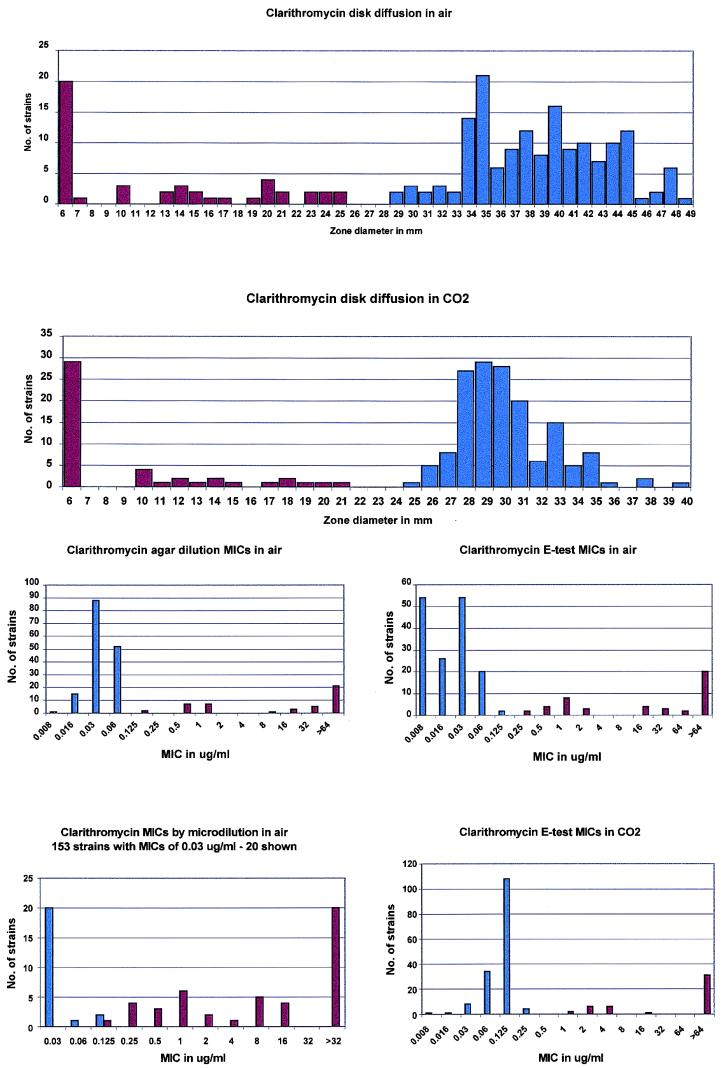
FIG. 2.
Histogram analysis of clarithromycin susceptibilities. Blue bars indicate clarithromycin-susceptible strains, and red bars indicate clarithromycin-resistant strains.
MATERIALS AND METHODS
Bacteria and antibiotics.
Of 199 pneumococcal isolates tested in this study, 70 were recent penicillin-susceptible isolates (MIC, <1.0 μg/ml) from the United States, 70 were penicillin intermediate resistant (MIC, 0.1 to 1.0 μg/ml), and 59 were penicillin resistant (MIC, ≥2.0 μg/ml). Because all strains had been subcultured several times prior to use, all grew well in air. Cultures were frozen at −70°C in double-strength litmus milk (Difco Laboratories, Detroit, Mich.). Levofloxacin susceptibility powder was obtained from Ortho McNeil Pharmaceutical, Raritan, N.J., and clarithromycin was obtained from Abbott Laboratories, North Chicago, Ill.
Interpretation of results.
Breakpoints for levofloxacin and clarithromycin susceptibility were ≤2.0 μg/ml (4.0 μg/ml is intermediate) and ≤0.25 μg/ml (0.5 μg/ml is intermediate), respectively. Corresponding zone diameters for the two compounds were ≥17 mm (14 to 16 mm is intermediate) and ≥21 mm (17 to 20 mm is intermediate), respectively. Because of the different incubation atmospheres for agar and microdilution (air) and CO2 (disk diffusion) currently recommended by the NCCLS (13, 14), the presence or absence of macrolide resistance was defined by disk diffusion with erythromycin disks, because this has been shown to be the most discriminating method (6); for levofloxacin, agar dilution was considered the “gold standard”: the distribution of MICs of all quinolones (including levofloxacin) is unimodal (15, 17, 19).
Agar dilution MICs.
Agar dilution MICs were determined according to standard methods with Mueller-Hinton agar supplemented with 5% sheep blood, incorporating compounds at concentrations from 0.002 to 64 μg/ml in doubling dilutions (9). Inocula were prepared by suspending growth from overnight cultures in sterile saline to the turbidity of a 0.5 McFarland standard. Final inocula contained 104 organisms/spot. Plates were inoculated with a Steers replicator with 3-mm-diameter inoculating pins and incubated overnight at 35°C in air. The lowest concentration of antibiotic at which organisms showed no growth was read as the MIC. Quality control strains (Staphylococcus aureus ATCC 29213, Escherichia coli ATCC 25922, and Streptococcus pneumoniae ATCC 49619) were included in each run.
Microdilution MICs.
Microdilution MICs were determined by the method recommended by the NCCLS (13), using cation-adjusted Mueller-Hinton broth (Difco Laboratories) supplemented with 5% lysed defibrinated horse blood. Suspensions with a turbidity equivalent to that of a 0.5 McFarland standard were prepared by suspending growth from blood agar plates in 2 ml of sterile saline. Suspensions were further diluted 1:10 to obtain a final inoculum of 5 × 105 CFU/well. Trays were incubated overnight in ambient air at 35°C. Standard quality control strains (as described above) were included in each run.
E-test MICs.
Standard methodology was used to determine E-test MICs (3, 10). Mueller-Hinton plates supplemented with added 5% sheep blood (Cleveland Scientific, Cleveland, Ohio) were inoculated with a 0.5 McFarland suspension scraped from plates, and E-test strips (AB Biodisk) were placed on each plate. After overnight incubation at 35°C, the MIC was read as the intersect where the ellipse of growth inhibition intersects the strip. E-test MICs were determined both in air and in CO2.
Disk diffusion.
Disk diffusion testing was performed by standard NCCLS methodology (14), using Mueller-Hinton plates supplemented with 5% added sheep blood (Cleveland Scientific) inoculated with a 0.5 McFarland suspension. After overnight incubation in both air and 5% CO2 at 35°C, zone diameters were measured with calipers.
RESULTS
With erythromycin disk diffusion as the reference method (6), 153 strains were susceptible, 2 were intermediate, and 44 were resistant to erythromycin. The results from the four susceptibility testing methods are presented in Table 1. Table 2 presents the number of strains susceptible, intermediate, and resistant to clarithromycin with the NCCLS breakpoints as well as adjusted breakpoints obtained by inspection of histograms of the data (Fig. 2). As can be seen, use of NCCLS breakpoints with all methods resulted in 153 to 161 strains susceptible, 0 to 7 intermediate, and 32 to 46 resistant to clarithromycin. Analysis of histograms suggested that breakpoints for all methods except the E-test in CO2 should be adjusted. Use of these adjusted breakpoints resulted in improved correlation for all methods, with 151 to 153 susceptible, 0 to 3 intermediate, and 45 to 46 resistant.
TABLE 1.
MICs and susceptibilities by the four methods used in this study
| Antimicrobial | Agar dilution (air)
|
Microdilution (air)
|
% Susceptible by disk diffusiona
|
E-test
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC50/MIC90 (μg/ml)b | % Susceptiblec | MIC50/MIC90 (μg/ml) | % Susceptible | Air | CO2 | Air
|
CO2
|
|||
| MIC50/MIC90 (μg/ml) | % Susceptible | MIC50/MIC90 (μg/ml) | % Susceptible | |||||||
| Levofloxacind | 1/2 | 100 | 1/1 | 99.5 | 100 | 99.0 | 0.5/1 | 100 | 0.5/1 | 100 |
| Clarithromycine | 0.03/>64 | 78.4 | ≤0.03/16 | 79.4 | 80.9 | 77.4 | 0.03/>64 | 77.9 | 0.125/>64 | 76.9 |
Levofloxacin, zone diameter of ≥17 mm; clarithromycin, zone diameter of ≥21 mm.
MIC at which 50% of the isolates are inhibited/MIC at which 90% of the isolates are inhibited.
Percent susceptible to levofloxacin at ≤2.0 μg/ml and clarithromycin at ≤0.25 μg/ml.
Identical irrespective of penicillin or clarithromycin susceptibility status.
As classified by erythromycin disk susceptibility (6), 153 strains were susceptible, 2 were intermediate, and 44 were resistant to erythromycin (76.9% susceptible).
TABLE 2.
Correlation of clarithromycin results with different methods of susceptibility testing
| Methodology | No. of isolates susceptible/ intermediate/ resistant by NCCLS breakpoints or diska | Adjusted breakpointsb | No. of isolates susceptible/ intermediate/ resistant by adjusted breakpoints |
|---|---|---|---|
| Agar dilution | 156/7/36 | ≤0.06/−/≥0.125 | 152/1/45 |
| Microdilution | 158/3/38 | ≤0.06/0.125/≥0.25 | 151/3/45 |
| E-test | |||
| Air | 155/4/40 | ≤0.06/0.125/≥0.25 | 151/2/46 |
| CO2 | 153/0/46 | ≤0.25/0.5/≥1c | 153/0/46 |
| Disk | |||
| Air | 161/6/32 | ≥29/26–28/≤25 | 153/0/46 |
| CO2 | 154/5/40 | ≥25/22–24/≤21 | 153/0/46 |
Levofloxacin MICs were unaffected by the penicillin and clarithromycin susceptibility of strains or by test methodology; ≥99% of strains were susceptible to levofloxacin at ≤2.0 μg/ml or ≥17 mm. Although incubation of disks and E-tests in CO2 led to a slight decrease in zone sizes and increase in MICs, these did not result in differences in category or susceptibility rates (Table 1). The levofloxacin results are also presented graphically in Fig. 1. As can be seen, a unimodal distribution was found by all four methods.
With the clarithromycin disk diffusion method tested by incubation in CO2, 94.4% of penicillin-susceptible strains, 81.4% of penicillin-intermediate strains, and 52.5% of penicillin-resistant strains were susceptible to clarithromycin. Corresponding rates when clarithromycin disks were incubated in air were 96.1, 84.5, and 54.5%, respectively. Although clarithromycin zone sizes were smaller and E-test MICs were higher in CO2 than those in air, susceptibility rates were similar to those obtained by agar and microdilution in air, ranging from 76.9 to 80.9% for all methods (Table 1).
Categorical discrepancies among the four methods (all calculated with levofloxacin agar dilution and erythromycin disk diffusion for clarithromycin as the standard) are presented in Table 3. With levofloxacin, minor discrepancies were obtained in one strain by microdilution and two strains by disk diffusion in CO2, and with clarithromycin, minor discrepancies were obtained in three strains by microdilution, seven strains by agar dilution, four strains by E-test in air, six strains by disk diffusion in air, and five strains by disk diffusion in CO2. Some of the latter strains showed discrepancies in more than one method. Major discrepancies occurred with levofloxacin in one strain by microdilution; no major discrepancies were found with clarithromycin. In contrast, very major discrepancies were not seen with levofloxacin, but did occur with clarithromycin in five strains by microdilution, three strains by agar dilution, two strains by E-test in air, eight strains by disk diffusion in air, and one strain by disk diffusion in CO2.
TABLE 3.
Categorical discrepancies among MIC methods used in this study
| Method | No. of results with categorical discrepanciesa
|
||
|---|---|---|---|
| Minor | Major | Very major | |
| Microdilution | |||
| Levofloxacin | 1 | 1 | 0 |
| Clarithromycin | 3 | 0 | 5 |
| Agar dilution | |||
| Levofloxacin | NAb | NA | NA |
| Clarithromycin | 7 | 0 | 3 |
| E-test | |||
| Air | |||
| Levofloxacin | 0 | 0 | 0 |
| Clarithromycin | 4 | 0 | 2 |
| CO2 | |||
| Levofloxacin | 0 | 0 | 0 |
| Clarithromycin | 0 | 0 | 0 |
| Disk diffusion | |||
| Air | |||
| Levofloxacin | 0 | 0 | 0 |
| Clarithromycin | 6 | 0 | 8 |
| CO2 | |||
| Levofloxacin | 2 | 0 | 0 |
| Clarithromycin | 5 | 0 | 1 |
Major errors, reference method (levofloxacin, agar dilution; clarithromycin, erythromycin disk susceptibility) susceptible and comparison method resistant; very major errors, reference method resistant and comparison method susceptible.
NA, not applicable.
DISCUSSION
As reported previously, levofloxacin MICs were similar in all penicillin categories for pneumococci (15, 17, 19); in contrast, clarithromycin MICs were higher for penicillin-intermediate and, especially, fully resistant strains than those for penicillin-susceptible organisms (5, 18).
The results of the current study show an excellent correlation between the results obtained by disk diffusion, agar dilution, microdilution, and E-test for levofloxacin, with slightly higher MICs on agar than those of other methods. According to current NCCLS guidelines, ≥99% susceptibility rates with the four methods were obtained, irrespective of whether E-test and disk diffusion tests were incubated in air or CO2. Because MICs of levofloxacin for pneumococci cluster 1 dilution below the breakpoint of 2.0 μg/ml (13), the accuracy of a method recommended for use in the clinical laboratory is important. In our hands, both E-test and disk diffusion can be recommended for routine levofloxacin pneumococcal testing. Incubation in CO2 did not result in differences in levofloxacin susceptibility category or susceptibility rates. Bolmström and Karlsson (3) have recently reported that pneumococcal MICs of 12 quinolones (including levofloxacin) agreed within 1 dilution in 95% of cases with or without CO2, with MICs minimally changed by CO2.
Although disk testing by erythromycin is our recommended method for screening of pneumococci for macrolide susceptibility, for macrolide-susceptible strains, clarithromycin has been shown, both by us and by others, to be more active than erythromycin (and also azithromycin, dirithromycin, and roxithromycin) (6, 18, 19).
Incubation in CO2 led to higher E-test MICs and smaller zone diameters with clarithromycin than those with incubation in air. In a previous study, Fasola and coworkers (6) have documented that erythromycin and clindamycin MICs were 1 to 2 dilutions higher in CO2 than in air with both the microdilution and agar dilution MIC methodology. However, as observed by Fasola et al. (6), raised MICs and zone sizes in CO2 did not lead to a significant difference in susceptibility rates of pneumococci when compared to those in air. The observed difference in MICs in and out of CO2 may be due to improved organism growth in CO2 or macrolide inactivation caused by CO2-induced lowering of the pH of the medium. Visalli et al. (18) have shown that agar dilution MICs in air for pneumococci were lower than those in CO2 of erythromycin, azithromycin, clarithromycin, dirithromycin, and roxithromycin. The MICs for many strains showed they were susceptible in air, but MICs were at or near the susceptible breakpoint in CO2. The five very major discrepancies found with clarithromycin by microdilution in air compared to agar dilution may be due to poorer growth in broth than on agar. This phenomenon requires confirmation with more strains and by other workers.
It should be noted that NCCLS macrolide breakpoints for pneumococci have been established for microdilution MICs with incubation in air. There is thus a need for establishment by the NCCLS of macrolide breakpoints for incubation of agar and microdilution tests under CO2, especially for the 5 to 10% of strains which do not grow adequately in air at primary isolation.
ACKNOWLEDGMENT
This study was supported by a grant from the R. W. Johnson Research Institute, Raritan, N.J.
REFERENCES
- 1.Appelbaum P C. Antimicrobial resistance in Streptococcus pneumoniae—an overview. Clin Infect Dis. 1992;15:77–83. doi: 10.1093/clinids/15.1.77. [DOI] [PubMed] [Google Scholar]
- 2.Block S, Hedrick J, Wright P, Finger R, Leggiadro R, Appleton M, Kahn S, Hutcheson R. Drug-resistant Streptococcus pneumoniae—Kentucky and Tennessee, 1993. Morbid Mortal Weekly Rep. 1994;43:23–25. , 31. [PubMed] [Google Scholar]
- 3.Bolmstrom A, Karlsson C. Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. The influence of CO2 incubation on MICs of quinolones tested with S. pneumoniae and H. influenzae, abstr. D-35; p. 89. [Google Scholar]
- 4.Breiman R F, Butler J C, Tenover F C, Elliott J A, Facklam R R. Emergence of drug-resistant pneumococcal infections in the United States. JAMA. 1994;271:1831–1835. [PubMed] [Google Scholar]
- 5.Doern G V, Brueggemann A, Holley H P, Jr, Rauch A M. Antimicrobial resistance of Streptococcus pneumoniae recovered from outpatients in the United States during the winter months of 1994 to 1995: results of a 30-center national surveillance study. Antimicrob Agents Chemother. 1996;40:1208–1213. doi: 10.1128/aac.40.5.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fasola E L, Bajaksouzian S, Appelbaum P C, Jacobs M R. Variation in erythromycin and clindamycin susceptibilities of Streptococcus pneumoniae by four test methods. Antimicrob Agents Chemother. 1997;41:129–134. doi: 10.1128/aac.41.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedland I R, Istre G S. Management of penicillin-resistant pneumococcal infections. Pediatr Infect Dis J. 1992;11:433–435. doi: 10.1097/00006454-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Friedland I R, McCracken G H., Jr Management of infections caused by antibiotic-resistant Streptococcus pneumoniae. N Engl J Med. 1994;331:377–382. doi: 10.1056/NEJM199408113310607. [DOI] [PubMed] [Google Scholar]
- 9.Jacobs M R. Treatment and diagnosis of infections caused by drug-resistant Streptococcus pneumoniae. Clin Infect Dis. 1992;15:119–127. doi: 10.1093/clinids/15.1.119. [DOI] [PubMed] [Google Scholar]
- 10.Jones R N, Erwin M E, Croco J L. Critical appraisal of E test for the detection of fluoroquinolone resistance. J Antimicrob Chemother. 1996;38:21–25. doi: 10.1093/jac/38.1.21. [DOI] [PubMed] [Google Scholar]
- 11.McDougal L K, Facklam R, Reeves M, Hunter S, Swenson J M, Hill B C, Tenover F C. Analysis of multiply antimicrobial-resistant isolates of Streptococcus pneumoniae from the United States. Antimicrob Agents Chemother. 1992;36:2176–2184. doi: 10.1128/aac.36.10.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munoz R, Musser J M, Crain M, Briles D E, Marton A, Parkinson A J, Sorensen U, Tomasz A. Geographic distribution of penicillin-resistant clones of Streptococcus pneumoniae: characterization by penicillin-binding protein profile, surface protein A typing, and multilocus enzyme analysis. Clin Infect Dis. 1992;15:112–118. doi: 10.1093/clinids/15.1.112. [DOI] [PubMed] [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. NCCLS publication no. M7-A4. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk susceptibility tests. NCCLS publication no. M2-A6. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 15.Pankuch G A, Jacobs M R, Appelbaum P C. Activity of CP 99,219 compared to DU-6859a, ciprofloxacin, ofloxacin, levofloxacin, lomefloxacin, tosufloxacin, sparfloxacin and grepafloxacin against penicillin-susceptible and -resistant pneumococci. J Antimicrob Chemother. 1995;35:230–232. doi: 10.1093/jac/35.1.230. [DOI] [PubMed] [Google Scholar]
- 16.Spika J S, Facklam R R, Plikaytis B D, Oxtoby M J the Pneumococcal Surveillance Working Group. Antimicrobial resistance of Streptococcus pneumoniae in the United States, 1979–1987. J Infect Dis. 1991;163:1273–1278. doi: 10.1093/infdis/163.6.1273. [DOI] [PubMed] [Google Scholar]
- 17.Visalli M A, Jacobs M R, Appelbaum P C. MIC and time-kill study of activities of DU-6859a, ciprofloxacin, levofloxacin, sparfloxacin, cefotaxime, imipenem, and vancomycin against nine penicillin-susceptible and -resistant pneumococci. Antimicrob Agents Chemother. 1996;40:362–366. doi: 10.1128/aac.40.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Visalli M A, Jacobs M R, Appelbaum P C. Susceptibility of penicillin-susceptible and -resistant pneumococci to dirithromycin compared with susceptibilities to erythromycin, azithromycin, clarithromycin, roxithromycin, and clindamycin. Antimicrob Agents Chemother. 1997;41:1867–1870. doi: 10.1128/aac.41.9.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Visalli M A, Jacobs M R, Appelbaum P C. Susceptibility of twenty penicillin-susceptible and -resistant pneumococci to levofloxacin, ciprofloxacin, ofloxacin, erythromycin, azithromycin, and clarithromycin by MIC and time-kill. Diagn Microbiol Infect Dis. 1997;28:131–137. doi: 10.1016/s0732-8893(97)00017-5. [DOI] [PubMed] [Google Scholar]