Abstract
Objective:
There is a need for research exploring the temporal trends of nonpulmonary organ dysfunction (NPOD) and biomarkers in order to identify unique predictive or prognostic phenotypes. We examined the associations between the number and trajectories of NPODs and plasma biomarkers of early and late inflammatory cascade activation, specifically plasma interleukin-1 receptor antagonist (IL-1ra) and interleukin-8 (IL-8), respectively, in the setting of acute respiratory failure (ARF).
Design:
Secondary analysis of the RESTORE clinical trial and BALI ancillary study.
Setting:
Multi-center
Patients:
Intubated pediatric patients with ARF
Interventions:
NPODs were evaluated against plasma IL-1ra and IL-8 levels on individual days (1 to 4 days after intubation) and longitudinally across days.
Measurements and Main Results:
Within the BALI cohort, 432 patients had at least one value for IL-1ra or IL-8 within days 0 through 5. 36.6% had a primary diagnosis of pneumonia, 18.5% had a primary diagnosis of sepsis and 8.1% died. Multivariable logistic regression models showed that increasing levels of both plasma IL-1ra and IL-8 were statistically significantly associated with increasing numbers of NPODs (IL-1ra: days 1 – 3; IL-8: days 1 – 4), independent of sepsis diagnosis, severity of oxygenation defect, age and race/ethnicity. Longitudinal trajectory analysis identified 4 distinct NPOD trajectories and 7 distinct plasma IL-1ra and IL-8 trajectories. Multivariable ordinal logistic regression revealed that specific IL-1ra and IL-8 trajectory groups were associated with greater NPOD trajectory group (p=0.004 and p< 0.0001, respectively), independent of severity of oxygenation defect, age, sepsis diagnosis and race/ethnicity.
Conclusions:
Both the inflammatory biomarkers and number of NPODs exhibit distinct trajectories over time with strong associations with one another. These biomarkers and their trajectory patterns may be useful in evaluating the severity of multiple organ dysfunction syndrome in critically ill children and identifying those phenotypes with time-sensitive, treatable traits.
Keywords: multiple organ dysfunction syndrome, Acute Respiratory Distress Syndrome, Pediatric, Interleukin-8, Interleukin 1 Receptor Antagonist Protein, Biomarkers
Summary
Both the plasma inflammatory biomarkers, IL-8 and IL-1ra, and non-pulmonary organ dysfunctions exhibit distinct trajectories over time with strong associations with one another. These trajectory patterns may be useful in identifying subphenotypes of critically ill children with time-sensitive, treatable traits.
Introduction
Multiorgan dysfunction syndrome (MODS) carries a high morbidity and mortality burden in the pediatric intensive care unit (PICU) [1]. Both MODS, and more specifically, nonpulmonary organ dysfunctions (NPODs) are major risk factors for mortality independent of other respiratory findings [2–5]. The inclusion of measures of pulmonary organ dysfunction confounds studies related to MODS in patients with acute respiratory failure (ARF), in particular acute respiratory distress syndrome (ARDS). The 2015 Pediatric Acute Lung Injury Consensus Conference (PALICC) recommended that research explore the use of NPODs in ARDS risk factor analyses [6]. The recently published Pediatric Organ Dysfunction Information Update Mandate (PODIUM) consensus also encouraged research evaluating temporal trends of organ dysfunction, and biomarkers of organ dysfunction, as an approach to identify unique phenotypes with predictive or prognostic value [7].
Inflammatory biomarkers are associated with ARDS development and ARDS outcomes in adults [8–13]. We previously reported that interleukin-1 receptor antagonist (IL-1ra) and interleukin-8 (IL-8) are associated with mortality and duration of mechanical ventilation in children with ARF in the Genetic Variation and Biomarkers in Acute Lung Injury (BALI) study [14, 15], an ancillary study to the Randomized Evaluation for Sedation Titration for Respiratory Failure (RESTORE) clinical trial [16]. IL-1ra blocks the pro-inflammatory cytokine interleukin-1β and is released early in the inflammatory cascade. IL-8 is a key neutrophil chemokine, elaborated somewhat later in the inflammatory cascade and strongly associated with sepsis and MODS in both adults and children. The association of these biomarkers with NPODs in pediatric patients has not been extensively studied.
In this analysis, we first used a more traditional approach to examine the relationship between the number of NPODs and IL-1ra or IL-8 levels on individual days in the setting of ARF in participants of the BALI study. Second, we analyzed the relationship between IL-1ra or IL-8 with the trichotomized outcome (respiratory dysfunction only, concurrent MODS and new MODS) described previously in the RESTORE study patient population [2]. Finally, we used a more novel approach to examine the relationship between NPODs and biomarkers in individuals across multiple days by identifying both biomarker trajectories over time and trajectories of the daily number of NPODs and determining whether there was an association between the two. We hypothesized that elevated IL-1ra and IL-8 would be associated with increasing number of NPODs and that biomarker trajectories with persistently higher biomarker levels would be associated with persistent elevation in the number of NPODs.
Materials and Methods
Study Design
This is a secondary analysis of the BALI study (R01HL0954410), an ancillary study to the multi-center RESTORE randomized controlled trial (U01HL086622). The Institutional Review Boards at all participating sites (see eTable 1) approved this study and informed consent was obtained. The University of Michigan served as the primary IRB for this work (IRBMED HUM00021034, approval date 8/29/2013 to present) in accordance with the institutional ethical standards of the responsible conduct of human research and with the Helsinki Declaration of 1975.
Children eligible for RESTORE included those 2 weeks to 17 years old treated with invasive mechanical ventilation for acute airways and/ or parenchymal lung disease [16]. Children for whom the length of mechanical ventilation was unlikely to be altered by the sedation management protocol being examined in RESTORE (i.e., children who were ventilator dependent on PICU admission or those expected to be extubated within 24hr) were excluded. Thirty-three sites participated in RESTORE, 22 of which were included in BALI.
For the BALI study (n=549), blood samples were obtained from patients initially within 24 hours of consent and then 24 and 48 hours after. Study day 0 was the day of intubation. We included patients with any samples assayed for IL-1ra or IL-8 within the days 0–5 (n=432). Ultimately, biomarker samples from day 0 and day 5 were not included in the analysis due to markedly smaller sample sizes on each of those days. Blood samples were assayed for IL-1ra and IL-8 using a two-antibody sandwich enzyme linked immunosorbent assay as described previously [14].
Outcome Measures and Statistical Analyses
All patients had respiratory failure as part of the RESTORE trial inclusion criteria. Types of NPODs evaluated within RESTORE were cardiovascular, renal, hematologic, neurologic, and hepatic (see eTable 2) [16]. To assess MODS, the number of NPODs on each study day was categorized into 0, 1, 2, and 3 or more. A trichotomized MODS outcome (respiratory dysfunction only, concurrent MODS, and new MODS) previously used in an analysis with the RESTORE database [2] was also examined. Concurrent MODS was defined as extrapulmonary organ dysfunction present on RESTORE study day 0 or 1 (i.e., concurrent with onset of respiratory failure) and new MODS as extrapulmonary organ dysfunction that developed on RESTORE study day 2 to 28.
Bivariate analyses of IL-1ra levels, IL-8 levels, clinical, and demographic variables were performed comparing patients with different numbers of NPODs or trichotomized MODS outcomes using Kruskal-Wallis test with post hoc Dunn’s test [17], Wilcoxon Rank Sum test, or Chi-Square test as indicated. The association of biomarker levels on study days 1 through 4 with the number of NPODs on that study day or with the trichotomized MODS outcome were analyzed using separate multivariable logistic regression models on each day for each biomarker. All multivariable models used log-transformed biomarker levels and were adjusted for oxygenation index (OI) on that study day, continuous age, race/ethnicity, and ARF due to sepsis. If OI was not available, oxygen saturation index (OSI) on that study day was used to estimate OI [18].
To assess the impact of biomarker levels on the number of NPODs across multiple days, separate longitudinal trajectories were identified for the number of NPODs and both biomarker levels. Group-based trajectory modeling identifies groups of individuals with similar profiles across time using maximum likelihood estimation. Poisson trajectories were fit for the daily number of NPODs. As NPOD data were available on all study days, trajectories were fit for study day 0 through day 5 (n=431; one patient excluded because they died on day 0). Due to limited biomarker data on day 0 and day 5, normal trajectories were fit across day 1 through day 4 for log-transformed values of IL-8 and IL-1ra separately, including all subjects with at least 2 days of biomarker data (n=372 for IL-1ra and n=378 for IL-8). Using the PROC TRAJ procedure in SAS [19] longitudinal trajectories were fit in steps, starting with two groups, and increasing until the optimal number of groups was determined. Optimal trajectories were chosen based on goodness-of-fit indices, including the Bayesian Information Criterion (BIC; where less negative scores indicate better fit), posterior probabilities [20], group size, and interpretability. The association of the biomarker trajectory groups with the NPOD trajectory groups was then examined using multivariable ordinal logistic regression models, adjusting for highest OI across day 0 through day 5, continuous age, race/ethnicity, and ARF due to sepsis. All analyses were done in SAS 9.4.
Results
Within the BALI cohort of 549 patients, 432 patients had at least one value for IL-1ra or IL-8 within days 0 through 5 and were used for the primary analyses on individual days. Of these, 378 patients had at least 2 sequential samples between days 1 through 4 and were included in the biomarker trajectory analyses. Demographic and clinical characteristics for the cohort of 432 with biological samples were almost identical to the original BALI cohort (eTable 3). Median age was 4.1 y (IQR 0.7–11y), 46.1% (199/432) were female, 52.5% (225/432) were non-Hispanic white and 16.6% (71/432) were non-Hispanic black. Primary diagnoses associated with ARF were pneumonia (36.6%, 158/432), bronchiolitis (19.4%, 84/432) and sepsis (18.5%, 80/432). Most (68.8%, 297/432) met pediatric acute respiratory distress syndrome (PARDS) criteria and 8.1% (35/432) died. Characteristics of the group used for the biomarker trajectory analyses (n=378) were very similar to the cohort with biological samples used for the primary analyses with slight differences in age, distribution of primary diagnoses, frequency of past medical history of cancer and PRISM score (eTable 3).
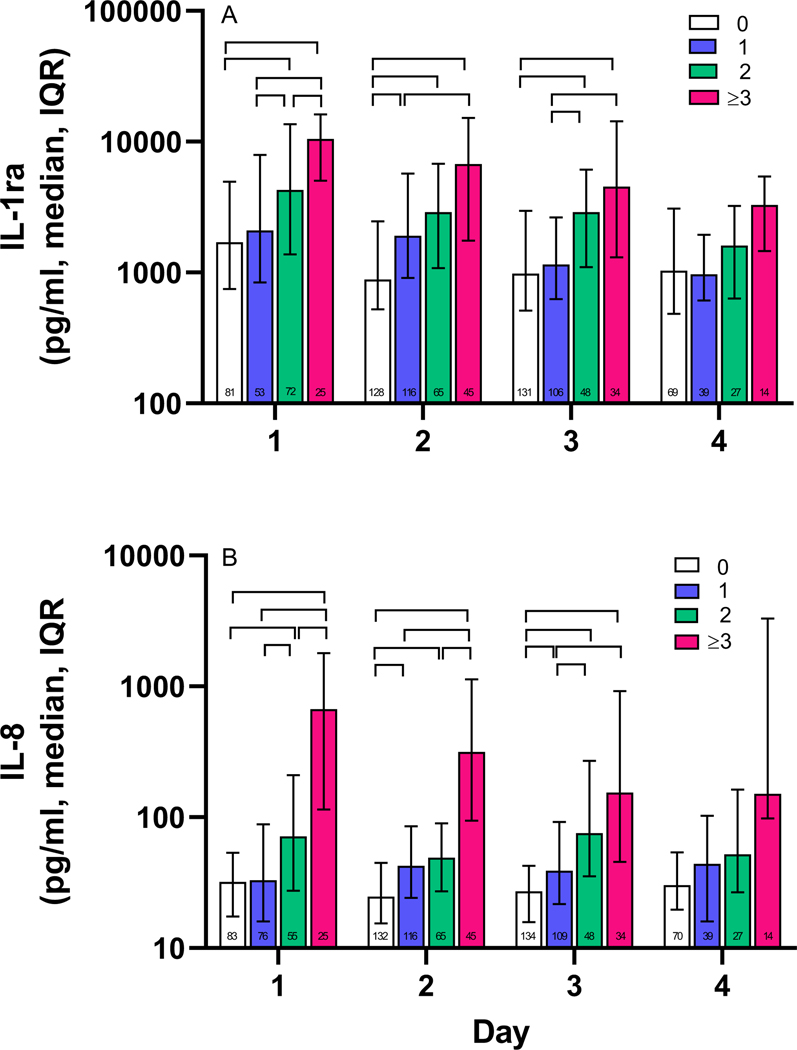
Bivariate analyses on individual study days indicated that elevated plasma levels of IL-1ra were associated with increasing number of NPODs (0, 1, 2, and 3 or more) on study days 1 through 4 (Figure 1a, Kruskal-Wallis test, p<0.001 on days 1 through 3, p<0.05 on day 4). Similarly, bivariate analyses examining IL-8 levels showed an association of elevated levels of IL-8 with increasing number of NPODs (0, 1, 2, and 3 or more) on days 1 through 4 (Figure 1b, Kruskal-Wallis test, p<0.001 on all days).
Figure 1. Plasma biomarker levels are associated with increasing numbers of nonpulmonary organ dysfunctions on days 1 through 3.
A, Comparison of plasma IL-1ra levels in children with 0, 1, 2, and ≥3 NPODs on each day. Day 1 IL-1ra levels were significantly different between all NPOD groups, except for 0 vs 1 NPODs. Day 2 IL-1ra levels were significantly different between all NPOD groups except for 1 vs 2 NPODs and 2 vs 3 or more NPODs. Day 3 IL-1ra levels were all significantly different except for those between 0 vs 1 NPODs and 2 vs 3 or more NPODs. B, Comparison of plasma IL-8 levels in children with 0, 1, 2, and ≥3 NPODs on each day. Day 1 IL-8 levels were significantly different between all NPOD groups. Day 2 IL-8 levels between all groups were significantly different except for 1 vs. 2 NPODS. Day 3 IL-8 levels were significantly different except for 2 vs. ≥3 NPODs. Brackets indicate p <0.05 determined by post-hoc analysis using the Dunn’s test. Numbers in bars indicate the number of subjects per group on each day. Analysis included 432 individuals with at least 1 and up to 3 samples assayed for IL-1ra and/or IL-8 on days 0–5. IL-1ra, interleukin-1 receptor antagonist, NPOD, nonpulmonary organ dysfunction, IL-8, interleukin-8.
Multivariable logistic regression models for the number of NPODs showed that increasing levels of IL-1ra were independently associated with increasing numbers of NPODs on days 1, 2, and 3 (overall type 3 analysis of effects: p=0.007, p<0.001, and p=0.009, respectively). On day 1, elevated levels of IL-1ra were statistically significantly associated with greater odds of having 1 and 3 or more NPODs compared to zero NPODs (Table 1). On day 2, elevated IL-1ra levels were again associated with greater odds of having multiple NPODs. On day 3, elevated IL-1ra levels were associated with greater odds of 3 or more NPODs. Multivariable analyses incorporating plasma IL-8 showed greater and more consistent association of elevated IL-8 with NPODs compared to plasma IL-1ra. Elevated plasma IL-8 levels exhibited a strong and consistently statistically significant association with increasing numbers of NPODs on all days 1 through 4 (overall type 3 analysis of effects: p<0.001 on days 1–3, p=0.001 on day 4; see Table 2 for group comparisons).
Table 1.
Multivariable analysis of association of interleukin-1 receptor antagonist with nonpulmonary organ dysfunctions
| Day | Number of Organ Dysfunctions | Odds Ratio | 95% Confidence Interval | p value |
|---|---|---|---|---|
|
| ||||
| 1 (n=188) |
0 | Reference | ||
| 1 | 1.42 | 1.01–1.99 | 0.04 | |
| 2 | 1.45 | 0.98–2.13 | 0.06 | |
| ≥3 | 2.67 | 1.52–4.68 | <0.001 | |
|
| ||||
| 2 (n=299) |
0 | Reference | ||
| 1 | 1.36 | 1.06–1.75 | 0.02 | |
| 2 | 1.53 | 1.14–2.06 | 0.005 | |
| ≥3 | 2.31 | 1.57–3.39 | <0.001 | |
|
| ||||
| 3 (n=258) |
0 | Reference | ||
| 1 | 0.87 | 0.66–1.16 | 0.34 | |
| 2 | 1.21 | 0.86–1.72 | 0.28 | |
| ≥3 | 1.77 | 1.13–2.77 | 0.01 | |
|
| ||||
| 4 (n=115) |
0 | Reference | ||
| 1 | 1.13 | 0.69–1.86 | 0.64 | |
| 2 | 1.15 | 0.67–1.96 | 0.62 | |
| ≥3 | 2.00 | 0.96–4.17 | 0.06 | |
All models used log-transformed interleukin-1 receptor antagonist levels and were adjusted for ethnicity/race, continuous age, acute respiratory failure due to sepsis and oxygenation index (OI) on that day. If OI was not available, oxygen saturation index (OSI) on that study day was used to estimate OI.
Table 2.
Multivariable analysis of association of interleukin-8 and nonpulmonary organ dysfunctions
| Day | Number of Organ Dysfunctions | Odds Ratio | 95% Confidence Interval | p value |
|---|---|---|---|---|
|
| ||||
| 1 (n=196) |
0 | Reference | ||
| 1 | 1.46 | 1.02–2.07 | 0.04 | |
| 2 | 1.78 | 1.21–2.62 | 0.004 | |
| ≥3 | 3.14 | 1.95–5.04 | <0.001 | |
|
| ||||
| 2 (n=305) |
0 | Reference | ||
| 1 | 1.34 | 1.01–1.76 | 0.04 | |
| 2 | 1.60 | 1.15–2.22 | 0.005 | |
| ≥3 | 3.37 | 2.28–4.97 | <0.001 | |
|
| ||||
| 3 (n=264) |
0 | Reference | ||
| 1 | 1.44 | 1.03–2.00 | 0.03 | |
| 2 | 2.31 | 1.56–3.44 | <0.001 | |
| ≥3 | 3.54 | 2.25–5.56 | <0.001 | |
|
| ||||
| 4 (n=115) |
0 | Reference | ||
| 1 | 2.07 | 1.21–3.53 | 0.008 | |
| 2 | 2.15 | 1.24–3.73 | 0.007 | |
| ≥3 | 3.78 | 1.97–7.27 | <0.001 | |
All models used log-transformed interleukin-8 levels and were adjusted for ethnicity/race, continuous age, acute respiratory failure due to sepsis and oxygenation index (OI) on that day. If OI was not available, oxygen saturation index (OSI) on that study day was used to estimate OI.
Multivariable logistic regression analysis examining the association of biomarker levels with the trichotomized MODS outcome previously used in a secondary analysis focused on MODS risk and RESTORE clinical and demographic data showed that elevated IL-1ra levels were not independently associated with concurrent MODS compared to respiratory dysfunction alone (eTable 4). Elevated IL-8 was independently associated with concurrent MODS compared to respiratory dysfunction alone on days 2 and 4 (eTable 5). Neither IL-1ra nor IL-8 were independently associated with new MODS on any study day, though this may have been due to the small number of individuals with new MODS (n ranging from 14–30 on days 1–4).
Trajectory analyses
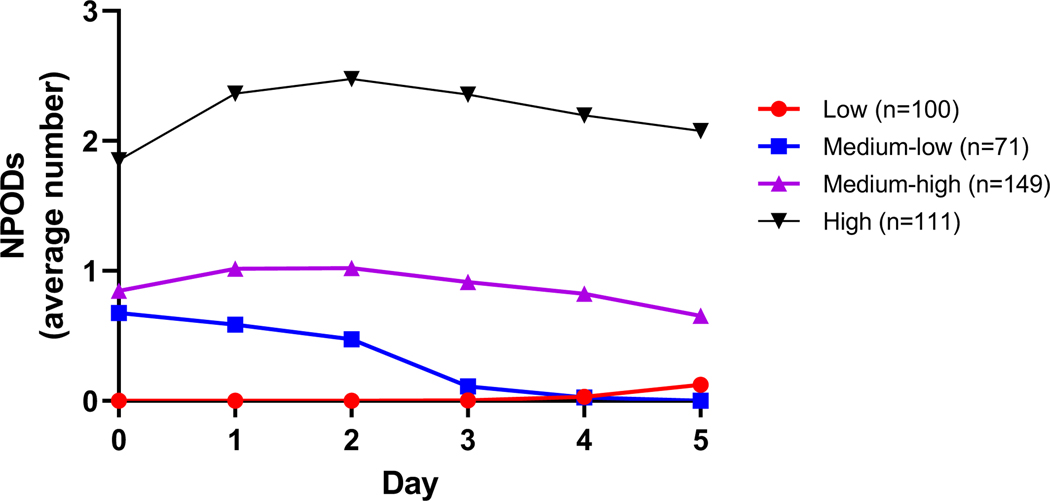
Longitudinal trajectory analysis of the number of NPODs across days 0 through 5 identified four different trajectories (low, medium-low, medium-high, high) with a clear pattern of increasing number of NPODs from the low to high trajectories (eTable 6, Figure 2). NPOD trajectories were primarily distinguished by the number of organ dysfunctions on day 0, with the greatest initial number and persistence of multiple NPODs occurring in the high trajectory, and the medium-low trajectory exhibiting progressively decreasing NPODs over time. Individual NPODs associated with each of the four NPOD trajectories is shown in eTable 7.
Figure 2: Nonpulmonary organ dysfunction trajectory groups.
Trajectory analysis was completed across days 0 through 5 and included 431 patients.
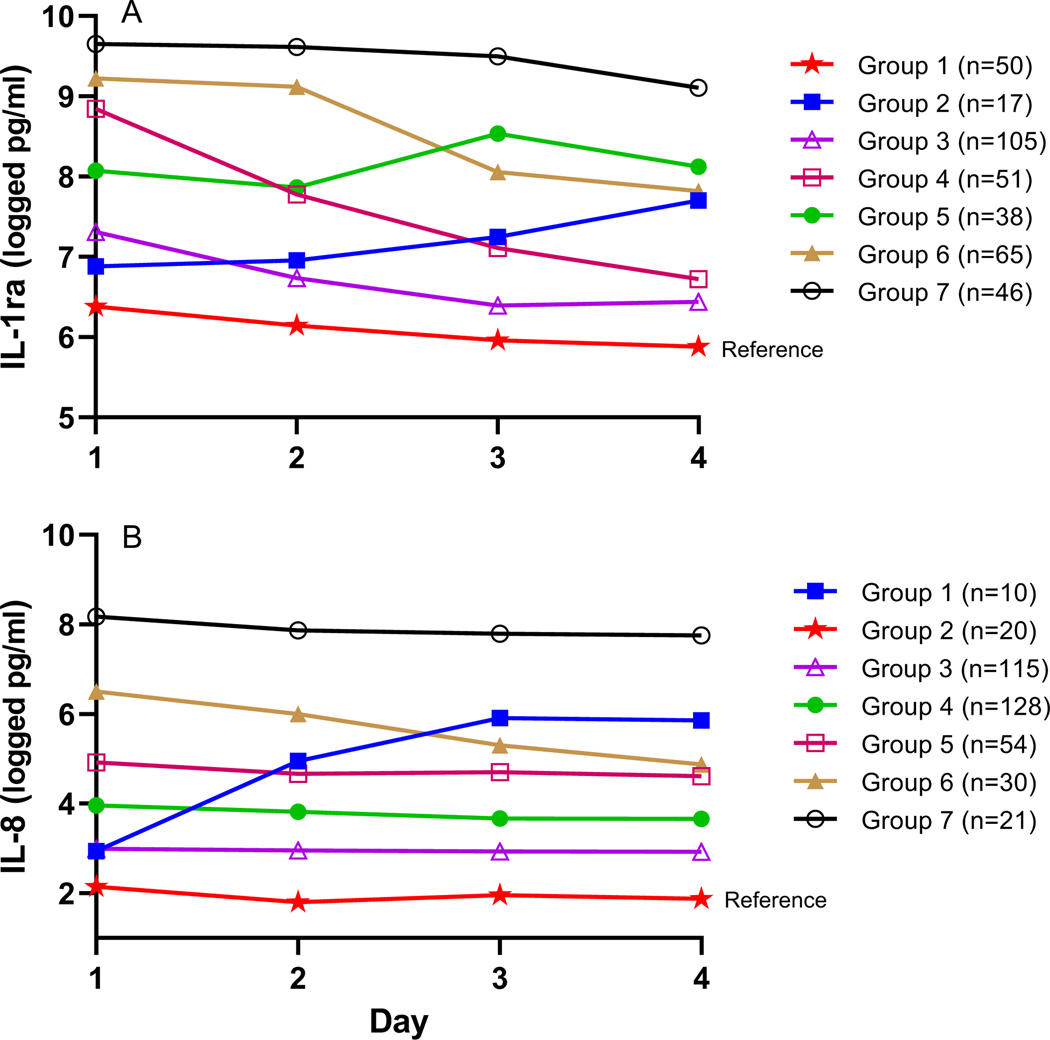
Longitudinal trajectory analysis was also used to assess whether there were specific patterns to the changes in the natural log of the biomarker levels across days 1 through 4. Seven different trajectories were independently identified for IL-1ra and IL-8 (eTable 8, Figures 3a and 3b, eFigure 1 and 2). For IL-1ra, the trajectories were characterized by differing levels on day 1 and various patterns across days: either decreasing (trajectories 1, 3, 4, 6), staying relatively constant (trajectory 7), or increasing (trajectories 2 and 5) over time. Most of the IL-8 trajectories exhibited differing levels on day 1 and marked stability over time. Only two trajectories exhibited large changes over time with both a large increase (trajectory 1) and a large decrease (trajectory 6) in IL-8 level observed.
Figure 3. Cytokine trajectory groups.
A, IL-1ra biomarker trajectory groups across days 1–4 (n=372). Group 1, indicated by stars, was assigned as the reference group for multivariable, ordinal logistic regression. B, IL-8 biomarker trajectory groups across days 1–4 (n=378). Group 2, indicated by stars, was assigned as the reference group. Biomarker levels were measured in natural logged values of pg/mL.
Due to the clear trend of NPODs from the low to high NPOD trajectories, we used multivariable ordinal logistic regression models to determine if biomarker trajectory groups were related to NPOD trajectories. IL-1ra trajectory group was associated with higher NPOD trajectory group (p=0.004), independent of highest OI, age, sepsis diagnosis and race/ethnicity (eTable 9). Using IL-1ra trajectory 1 as the reference group, trajectory 7 had increased odds of being in a higher NPOD trajectory group (OR 3.98, 95% CI 1.77, 8.96). Similarly, IL-8 trajectory group was associated with higher NPOD trajectory group in adjusted models (p< 0.001) (eTable 10) Using IL-8 trajectory 2 as the reference group, IL-8 trajectories 1 (OR 8.04, CI 1.74, 37.12), 5 (OR 6.63, CI 2.30, 12.12), 6 (OR 11.56, CI 3.43, 38.91), and 7 (OR 25.52, CI 5.40, 120.51) had increased odds of being in a higher NPOD trajectory group.
Discussion
In this secondary analysis of children intubated for ARF in the BALI study, we completed an in-depth analysis of inflammatory cytokines representative of inflammatory cascade activation and their relationships to NPODs. Unlike previous studies, our study examined both the association of individual biomarker levels with the number of NPODs for multiple days after intubation and the association of temporal biomarker trajectories with NPOD trajectories over the same time period. When examined on individual days, elevated levels of both IL-1ra and IL-8 were found to be independently associated with increasing numbers of NPODs. When changes over time in NPODs or biomarkers in individual subjects were examined using trajectory analysis, individuals fell into four NPOD trajectories and seven biomarker trajectories for both IL-1ra and IL-8. Specific IL-1ra and IL-8 trajectories were independently associated with NPOD trajectories reflective of progressively increasing numbers of NPODs. These findings offer potential opportunities for future precision medicine approaches to be employed in subsets of patients with specific NPODs trajectories, as both IL-1ra and IL-8 have inhibitors available for use in clinical practice and/or clinical trials for other disease processes.
IL-1ra functions as a competitive inhibitor of the interleukin 1 receptor and elevated levels are generally reflective of early inflammatory cascade activation. Our current work identifies a statistically significant independent association of IL-1ra with increasing numbers of NPODs 1, 2 and 3 days after intubation, but not on day 4, with trajectory analysis revealing the only significant association with higher NPOD trajectory group occurring with the IL-1ra trajectory group with the highest initial and robustly sustained IL-1ra levels. Interestingly, Meyer and colleagues identified a subphenotype of adult sepsis patients with high initial plasma IL-1ra levels who showed a potential 12% mortality benefit when treated with recombinant human interleukin 1 antagonist [21]. Thus, early, extreme elevations of plasma IL-1ra are likely to correlate with an exaggerated pro-inflammatory response. The finding that the strength of the association between number of NPODs and IL-1ra may weaken as days progress may not be surprising as the levels of IL-1ra are highest on day 1 and generally decrease substantially with each additional day [22].
Adult ARDS research supports strong associations between elevated IL-8 and development of MODS, severity of MODS, and MODS outcomes (organ failure free days) [23–25]. In a cohort of severely injured adults 2 to 5 days following blunt trauma, Liu and colleagues recently report four distinct MODS clusters, also differing in inflammatory profiles, including IL-8 [26]. In the pediatric population, Zinter and colleagues found that IL-8 measured within the first 24 hours after PARDS onset was associated with early evidence of multiple organ injury and ICU morbidity, measured by survivor PELOD score [27]. In a large cohort of critically ill pediatric patients with sepsis and MODS, Qin and colleagues used machine learning techniques to identify four distinct sepsis/MODS phenotypes, again differing in inflammatory profiles, including IL-8 but not differing in IL-1ra. All analyses in that investigation relied on plasma specimens measured on the first day of study [28]. Our study extends previous research as we have examined IL-8 levels over multiple days. The association of plasma IL-8 with the number of NPODs on individual days was more consistent than the associations seen with IL-1ra, and remained statistically significant across all four days analyzed. On trajectory analysis, the four IL-8 trajectory groups with the highest plasma IL-8 level on days 3 and 4 were significantly and independently associated with increasing NPOD group trajectories suggesting that IL-8 trajectory groups that either rise to or remain most elevated on days 3 and 4 after intubation (IL-8 trajectory group 1, 5, 6 and 7) are composed of patients with multiorgan system failure of greatest severity. The consistency and significance of the association between IL-8 and NPODs over time suggest that this may be a good biomarker to track, and potentially modulate, in patients with persistent MODS, although the elevation and trajectory of plasma IL-1ra may be more specific for ARF and/or PARDS than other pediatric critical illnesses, such as sepsis.
Taken together, these data indicate that both the cytokines measured and the magnitude of organ system failure, as measured by NPOD trajectory, may identify different phenotypes of disease severity or progression. Our analyses indicated that the associations of both IL-1ra and IL-8 levels with either the number of NPODs or with NPOD trajectory groups are more robust than the association of biomarkers with the trichotomized outcomes of respiratory dysfunction alone, concurrent, or new MODS, the latter group accounting for only 10% of all RESTORE patients [2]. Thus, we speculate that these biomarkers may be more sensitive to the degree of organ dysfunction and not necessarily the onset or “presence” of MODS. Finally, our ordinal logistic regression analyses indicated that, independent of important clinical covariates (severity of oxygenation defect across the first 5 days after intubation, age and sepsis diagnosis), specific cytokine trajectories were also associated with NPOD trajectory identifying not only patients with multiorgan system failure of greatest severity but also those with the greatest potential benefit for timely escalation in therapy if these cytokines and their trajectories could be measured in real time at the bedside.
While these data do not increase the ability for earlier identification of risk for the development of MODS, measurement of these markers can serve as important diagnostic tools in severity of illness assessment. Identification of cytokine and MODS patterns or phenotypes could also prove useful when planning research studies or clinical trials where the target patient population includes the patients with the worst degree of prolonged organ failure and therefore the highest risk of death or severe morbidity. Finally, in combination with further mechanistic study, specifically temporal endotyping, as reported by Wong and colleagues for pediatric septic shock [29], clinicians and researchers can gather needed understanding of the subgroups of patients that may benefit from precision-medicine based, anti-inflammatory therapies. Importantly, functional immunophenotyping must be moved from the research lab to the bedside to use these findings in real time.
This study has some limitations. There were relatively few samples available for day 0 (day of intubation) due to the requirement that consent needed to be obtained for RESTORE before parents/guardians were approached for the BALI study. Consequently, we were unable to examine the association of biomarkers with the number of NPODs at this early time point. With only 11 patients with immunodeficiency and 36 patients with current and/or prior cancer history included in this entire cohort, the impact of these patients could not be fully examined. Also, there was no way to factor in the degree of a specific organ failure as the RESTORE dataset only indicated the presence or absence of a particular organ failure on a particular study day. Due to study sample size, there was limited ability to include race and ethnicity, hence the categorization of race/ethnicity into non-Hispanic white versus other. As well, the data set did not include other measurable markers of social determinants of health. Lastly, the definitions of the various organ dysfunctions were those used by the RESTORE clinical trial [16] and future research would require more precise definitions of NPODs, particularly in relation to the recent PODIUM consensus statements [7].
Conclusions
The 2022 PODIUM Executive Summary [7] champions future research “to identify patterns of organ dysfunction combinations and temporal trends that constitute unique phenotypes associated with worse outcomes.” In this study, we attempt an initial response to this goal. We have demonstrated that increased plasma IL-1ra and IL-8 levels are associated with increasing number of NPODs in pediatric patients with ARF on days 1 through 3 and 1 through 4, respectively. We also demonstrate that in children with ARF, both the levels of inflammatory markers and the number of NPODs exhibit distinct trajectories, also known as phenotypes, over time with biomarker trajectories associated with NPOD trajectories independent of sepsis, age and oxygenation defect. These biomarkers and their trajectory patterns may be useful in evaluating the severity of MODS in critically ill children and identifying those subgroups of patients with potentially time sensitive, treatable traits. Our data underscore the need for functional immunophenotyping partnered with phenotype identification with capability of measurement in real time and not simply in a research laboratory, to identify which patients should/could receive targeted anti-inflammatory, or other endotype specific, treatment in these patients. These data strongly support future investigations with measurement of inflammatory biomarkers early and serially in patients with PARDS.
Supplementary Material
RESEARCH IN CONTEXT.
Multiorgan dysfunction is associated with an increased risk for morbidity and mortality in children with critical illness; nonpulmonary organ dysfunctions are major risk factors for mortality independent of other respiratory findings.
Little is known about trends over time for pediatric organ dysfunctions, biomarker levels and the relationship between the two in children with critical illness.
Trajectory analysis provides a novel way to examine the temporal trends in organ dysfunction and biomarker levels.
AT THE BEDSIDE.
When examined on individual days, elevated levels of both IL-1ra and IL-8 were independently associated with increasing numbers of NPODS.
Four NPOD trajectories were identified primarily distinguished by their initial NPOD level though the medium-low trajectory exhibited decreasing NPODs over time. IL-1ra and IL-8 each had seven trajectories which showed differences in initial levels and different patterns across days.
Trajectory analyses indicated that there is a significant association between specific cytokine trajectories and NPOD trajectories independent of important clinical covariates which may aid in the identification of unique phenotypes in the future.
Acknowledgements:
We would like to thank all the patients and guardians of those patients for their participation in the study. We would also like to acknowledge the contribution of the Biomarkers in Children with Acute Lung Injury study investigators at the sites that participated in the Randomized Evaluation of Sedation Titration for Respiratory Failure (RESTORE) study including: Scot T. Bateman (University of Massachusetts Memorial Children’s Medical Center, Worcester, MA), M. D. Berg (University of Arizona Medical Center, Tucson, AZ), Santiago Borasino (Children’s Hospital of Alabama, Birmingham, AL), G. Kris Bysani (Medical City Children’s Hospital, Dallas, TX), Allison S. Cowl (Connecticut Children’s Medical Center, Hartford, CT), Cindy Darnell Bowens (Children’s Medical Center of Dallas, Dallas, TX), E. Vincent S. Faustino (Yale-New Haven Children’s Hospital, New Haven, CT), Lori D. Fineman (University of California San Francisco Benioff Children’s Hospital at San Francisco, San Francisco, CA), A. J. Godshall (Florida Hospital for Children, Orlando, FL), Ellie Hirshberg (Primary Children’s Medical Center, Salt Lake City, UT), Aileen L. Kirby (Oregon Health & Science University Doernbecher Children’s Hospital, Portland, OR), Gwenn E. McLaughlin (Holtz Children’s Hospital, Jackson Health System, Miami, FL), Shivanand Medar (Cohen Children’s Medical Center of New York, Hyde Park, NY), Phineas P. Oren (St. Louis Children’s Hospital, St. Louis, MO), James B. Schneider (Cohen Children’s Medical Center of New York, Hyde Park, NY), Adam J. Schwarz (Children’s Hospital of Orange County, Orange, CA), Thomas P. Shanley (Ann & Robert H. Lurie, Children’s Hospital of Chicago, Chicago, IL), Lauren R. Sorce (Ann & Robert H. Lurie, Children’s Hospital of Chicago, Chicago, IL), Edward J. Truemper (Children’s Hospital and Medical Center, Omaha, NE), Michele A. Vander Heyden (Children’s Hospital at Dartmouth, Dartmouth, NH), Kim Wittmayer (Advocate Hope Children’s Hospital, IL), Athena Zuppa (Children’s Hospital of Philadelphia, Philadelphia, PA) and the RESTORE data coordination center led by David Wypij, PhD (Department of Biostatistics, Harvard School of Public Health, Boston, MA; Department of Pediatrics, Harvard Medical School, Boston, MA; Department of Cardiology, Boston Children’s Hospital, Boston, MA).
Institution where this work was performed: University of Michigan, Ann Arbor, Michigan
Reprints: will not be ordered
Financial support for this study:
This study was funded by R01HL0954410.
Copyright Form Disclosure:
Drs. Dahmer, Quasney, Curley, and Flori’s institutions received funding from the National Heart, Lung, and Blood Institute. Drs. Dahmer and Sapru’s institutions received funding from the National Institutes of Health (NIH). Dr. Dahmer’s institution received funding from the National Institute of Child Health and Human Development. Drs. Dahmer, Quasney, Sapru, Curley, and Flori received support for article research from the NIH. Dr. Flori disclosed that she is a Board member of Michigan Thoracic Society, Board member of Pediatric Acute Lung Injury and Sepsis Investigators, Taskforce member for Lancet, and a Taskforce member for Society of Critical Care Medicine.
Footnotes
The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Watson R, Crow S, Hartman M, et al. : Epidemiology and Outcomes of Pediatric Multiple Organ Dysfunction Syndrome. Pediatr Crit Care Med 2017; 18:S4–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss S, Asaro L, Flori H, et al. : Multiple Organ Dysfunction in Children Mechanically Ventilated for Acute Respiratory Failure. Pediatr Crit Care Med 2017; 18(4):319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erickson S, Schibler A, Numa A, et al. : Acute lung injury in pediatric intensive care in Australia and New Zealand: A prospective, multicenter, observational study. Pediatr Crit Care Med 2007; 8:317–323. [DOI] [PubMed] [Google Scholar]
- 4.Flori HR, Glidden DV, Rutherford GW, Matthay MA: Pediatric acute lung injury: prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med 2005; 171(9):995–1001. [DOI] [PubMed] [Google Scholar]
- 5.López-Fernández Y, Azagra A, de la Oliva P, et al. : Pediatric Acute Lung Injury Epidemiology and Natural History study: Incidence and outcome of the acute respiratory distress syndrome in children. Crit Care Med 2012; 40:3238–3245. [DOI] [PubMed] [Google Scholar]
- 6.Flori H, Dahmer M, Sapru A, et al. : Comorbidities and assessment of severity of pediatric acute respiratory distress syndrome: proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med 2015; 16:S41–S50. [DOI] [PubMed] [Google Scholar]
- 7.Bembea M, Agus M, Akcan-Arikan A, et al. : Pediatric Organ Dysfunction Information Update Mandate (PODIUM) Contemporary Organ Dysfunction Criteria: Executive Summary. Pediatrics 2022; 149:S1–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park W, Goodman R, Steinberg K, et al. : Cytokine balance in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2001; 164:1896–1903. [DOI] [PubMed] [Google Scholar]
- 9.Parsons P, Moss M, Vannice J, et al. : Circulating IL-1ra and IL-10 levels are increased but do not predict the development of acute respiratory distress syndrome in at-risk patients. Am J Respir Crit Care Med 1997; 155:1469–1473. [DOI] [PubMed] [Google Scholar]
- 10.Donnelly S, Strieter R, Reid P, et al. : The association between mortality rates and decreased concentrations of interleukin-10 and interleukin-1 receptor antagonist in the lung fluids of patients with the adult respiratory distress syndrome. Ann Intern Med 1996; 125:191–196. [DOI] [PubMed] [Google Scholar]
- 11.Ware L, Koyama T, Zhao Z, et al. : Biomarkers of lung epithelial injury and inflammation distinguish severe sepsis patients with acute respiratory distress syndrome. Critical care (London, England) 2013; 17(5):R253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calfee CS, Janz DR, Bernard GR, et al. : Distinct molecular phenotypes of direct vs indirect ARDS in single-center and multicenter studies. Chest 2015; 147(6):1539–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calfee CS, Thompson BT, Parsons PE, et al. : Plasma interleukin-8 is not an effective risk stratification tool for adults with vasopressor-dependent septic shock. Crit Care Med 2010; 38(6):1436–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahmer M, Quasney M, Sapru A, et al. : Interleukin-1 Receptor Antagonist Is Associated With Pediatric Acute Respiratory Distress Syndrome and Worse Outcomes in Children With Acute Respiratory Failure. Pediatr Crit Care Med 2018; 19:930–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flori H, Sapru A, Quasney MW, et al. : A prospective investigation of interleukin-8 levels in pediatric acute respiratory failure and acute respiratory distress syndrome. Critical care (London, England) 2019; 23(1):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Curley MA, Wypij D, Watson RS, et al. : Protocolized sedation vs usual care in pediatric patients mechanically ventilated for acute respiratory failure: a randomized clinical trial. JAMA 2015; 313(4):379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elliott A, Hynan L: A SAS Macro Implementation of a Multiple Comparison post hoc test for Kruskal-Wallis analysis. Comp Meth Prog Bio 2011; 102:75–80. [DOI] [PubMed] [Google Scholar]
- 18.Khemani R, Thomas N, Venkatachalam V, et al. : Comparison of SpO2 to PaO2 based markers of lung disease severity for children with acute lung injury. Crit Care Med 2012; 40:1309–1316. [DOI] [PubMed] [Google Scholar]
- 19.Jones B, Nagin D, Roeder K: A SAS procedure based on mixture models for estimating developmental trajectories. Sociological Methods & Research 2001; 29(3):374–393. [Google Scholar]
- 20.Nagin D: Group-Based Modeling of Development: Harvard University Press; 2005.
- 21.Meyer N, Reilley J, Anderson B, et al. : Mortality Benefit of Recombinant Human Interleukin-1 Receptor Antagonist (rhIL1RA) for Sepsis Varies by Initial IL1RA Plasma Concentration. Crit Care Med 2018; 46(1):21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dinarello C: Overview of the IL‐1 family in innate inflammation and acquired immunity. Immunol Rev 2017; 281:8–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parsons P, Eisner M, Thompson B, et al. : Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Critical Care Medicine 2005; 33:1–6. [DOI] [PubMed] [Google Scholar]
- 24.Marty C, Misset B, Tamion F, et al. : Circulating interleukin-8 concentrations in patients with multiple organ failure of septic and nonseptic origin. Crit Care Med 1994; 22:673–679. [DOI] [PubMed] [Google Scholar]
- 25.Cartin-Ceba R, Hubmayr R, Qin R, et al. : Predictive value of plasma biomarkers for mortality and organ failure development in patients with acute respiratory distress syndrome. J Crit Care 2015; 30(1):219.e211–217. [DOI] [PubMed] [Google Scholar]
- 26.Liu D, Namas R, Vodovotz Y, et al. : Unsupervised Clustering Analysis Based on MODS Severity Identifies Four Distinct Organ Dysfunction Patterns in Severely Injured Blunt Trauma Patients. Front Med 2020; 7(46). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zinter MS, Orwoll BE, Spicer AC, et al. : Incorporating Inflammation into Mortality Risk in Pediatric Acute Respiratory Distress Syndrome. Crit Care Med 2017; 45(5):858–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin Y, Kernan KF, Fan Z, et al. : Machine learning derivation of four computable 24-h pediatric sepsis phenotypes to facilitate enrollment in early personalized anti-inflammatory clinical trials. Critical care (London, England) 2022; 26(1):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong H, Cvijanovich N, Anas N, et al. : Endotype Transitions During the Acute Phase of Pediatric Septic Shock Reflect Changing Risk and Treatment Response. Crit Care Med 2018; 46(3):e242–e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.