Summary
Rapid reaction to microbes invading mucosal tissues is key to protect the host against disease. Respiratory tissue-resident memory T (TRM) cells provide superior immunity against pathogen infection and/or re-infection, due to their presence at the site of pathogen entry. However, there has been emerging evidence that exuberant TRM cell responses contribute to the development of various chronic respiratory conditions including pulmonary sequelae post-acute viral infections. In this review, we have described the characteristics of respiratory TRM cells and processes underlying their development and maintenance. We have reviewed TRM cell protective functions against various respiratory pathogens as well as their pathological activities in chronic lung conditions including post-viral pulmonary sequelae. Furthermore, we have discussed potential mechanisms regulating the pathological activity of TRM cells and proposed therapeutic strategies to alleviate TRM cell-mediated lung immunopathology. We hope that this review provides insights towards the development of future vaccines or interventions that can harness the superior protective abilities of TRM cells, while minimizing the potential for immunopathology, a particularly important topic in the era of coronavirus disease 2019 (COVID-19) pandemic.
Keywords: TRM cells, protection, immunopathology, viral sequelae, lung
1. Introduction
Naïve T cells, including CD8+ and CD4+ T cells, become activated upon recognizing specific peptide-MHC complexes presented by antigen-presenting cells (APCs). Subsequently, the cells proliferate and differentiate into effector T cells, eliminating the pathogen and infected cells via different mechanisms1,2. During acute infection by respiratory viruses such as the respiratory syncytial virus (RSV) and influenza A virus (IAV), antigen-specific CD8+ effector T cell responses in the lungs generally peak at day 8–10 post infection3–6. Then, effector T cells undergo contraction during which most cells undergo apoptosis, whereas a group of effector T cells remain and further differentiate into memory cells - providing long-term protection against reinfection by the same or related pathogens1,2. Memory T cells have remarkable heterogeneity vis-à-vis their circulating patterns, functions, and expression of specific markers. Memory T cells can generally be classified as central memory T cells (TCMs), effector memory T cells (TEMs) and tissue-resident memory T cells (TRMs). In both humans and mice, TCM cells express L-selectin (CD62L) and CC-chemokine receptor 7 (CCR7) and migrate within lymphoid organs7–9. TEM cells exhibited higher basal expression of effector molecules compared to TCM cells10,11 and can circulate throughout the blood, secondary lymphoid organs, and non-lymphoid tissues. TRM cells mainly reside in non-lymphoid tissues and remain primarily parked inside the tissue without entering the circulation12–14. TRM cells have been observed in almost all non-lymphoid tissues including skin12,15–17, lung18–22, gut23–25, brain26,27, and the reproductive tract28,29 of humans and animal models. TRM cells typically express tissue residency-related molecules including CD69 and CD103. CD69 expression on TRM cells prevents tissue exit by suppressing the activity of sphingosine 1-phosphate receptor-1 (S1P1)30,31, while CD103 facilitates TRM tissue retention by binding to E-cadherin - which is usually expressed on epithelial cells16,32. Pulmonary TRM cells can reside in two different sites, the airway and the lung interstitium33. Airway TRM cells and interstitial TRM cells differ in phenotypic markers, turn-over rates, and cytotoxic potentials, but both confer protection against lethal viral rechallenge via the rapid production of antiviral cytokines33–35.
Owing to their presence at the site of viral entry and high levels of expression of effector molecules, respiratory TRM cells provide superior protection against secondary infections compared to TCM and TEM cells. In particular, it has been shown that TRM cells can confer nearly sterilizing immunity if present in sufficient numbers20,36. TRM cells can be rapidly reactivated to kill pathogen-infected cells directly, produce effector cytokines to establish a local antiviral state and/or activate a series of downstream signaling cascades to impede viral replication and dissemination. Therefore, the induction of robust TRM responses may hold promise for development of the next generation of vaccines capable of providing superior and broad protection against different pathogen variants in the mucosal tissue37. However, on the opposite side of the same coin, exuberant or dysregulated TRM responses have also been shown to contribute significantly to lung immunopathology. Emerging evidence has found that TRM cells may drive and/or contribute to the development of several chronic lung diseases including asthma, COPD (Chronic obstructive pulmonary disease) and pulmonary fibrosis. Furthermore, exuberant TRM responses may also result in the development of chronic lung sequelae post-acute viral infections including influenza and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. In this review, we first describe the general characteristics and protective functions of pulmonary TRM cells in the context of respiratory infections. Then, we detail the pathological roles of TRM cells in the development of chronic respiratory disease and persistent sequelae after acute infection, as well as likely underlying mechanisms. Finally, we discuss the potential of targeting pathological TRM cells to resolve or attenuate lung immunopathology in respiratory diseases.
2. Respiratory TRM cell development and characteristics
Naive T cells generally undergo priming, proliferation and differentiation into effector T cells at the lung-draining mediastinal lymph node (mLN) after respiratory viral infection. Activated effector T cells then migrate to the site of infection in the lungs. After pathogen clearance, a group of effector T cells or TRM precursors survive the contraction phase and further differentiate into mature TRM cells. TRM cells are phenotypically and functionally distinct from circulating TCM and TEM cells. For instance, in order to maintain their residence in tissues, respiratory TRM cells downregulate surface molecules such as CD62L, CCR7 and S1P1, which are required for the entry of lymphocytes into circulation38–40. Furthermore, TRM cells gain the expression of tissue residency molecules including CD69, CD103 and CD49a. CD69 antagonizes the function of S1P1 and CD103 is required for binding to epithelial integrin. CD49a, the α1 integrin, binds to collagen IV and facilitates the survival of lung TRM cells41. TRM development and maintenance are also dependent on a number of transcription factors (TFs). Particularly, the PR domain zinc finger protein 1 Blimp-1, and the Blimp-1 homolog, Hobit, have been shown to instruct a transcriptional program required for T cell tissue residency in the respiratory tract15. Runx3, Notch1, the orphan nuclear receptor Nur77, the aryl hydrocarbon receptor (Ahr), and the basic helix-loop-helix family member Bhlhe40 have also been shown to be required for optimal respiratory TRM responses22. Conversely, transcription factors such as the Kruppel-like factor KLF2, the T-cell factor TCF-1 and Eomesodermin (Eomes) can inhibit the expression of the tissue-residency gene program, instead promoting genes associated with T cell circulation39,42,43. Specific pathways and molecules that are important for respiratory CD8+ or CD4+ TRM responses are listed below.
2.1. CD8+ TRM cells
Naïve CD8+ T cells are activated by APCs, particularly the migratory CD103+ conventional dendritic cells (cDC1) in the mLN. The effector T cells then migrate to infected tissues, including the lung parenchyma or airways44,45. The development of TRM cells from infiltrating effector T cells relies on instructional signals from the pulmonary microenvironment. These essential local factors include cognate antigen re-stimulation, proper cytokine milieu and engagement with neighboring cells.
The necessity of local antigen restimulation for establishing lung TRM cells has been demonstrated over multiple studies36,46,47. For instance, it has been shown that intraperitoneal primary influenza virus immunization induces circulating effector T cells, which can be pulled to the respiratory tract with subsequent CpG single intranasal administration. However, intranasal CpG treatment failed to generate robust CD8+ TRM responses unless cognate antigen was combined with CpG in the administration46. These data suggest that antigen re-encounter is essential for the optimal generation of respiratory TRM cells. Besides the requirement of antigen re-encounter in the effector phase, low levels of persistent TCR/MHC-I signaling also facilitates the maintenance of a group of “exhausted-like” lung TRM cells that are specific to the influenza nucleoprotein (NP)36 (further discussion below). Unlike lung parenchymal CD8+ TRM cells, nasal and upper respiratory tract TRM cells seem to develop independent of local antigen re-engagement, and are regulated by TGF-β signaling48.
TGF-β is well-established to be an essential cytokine for the development of CD103+ TRM cells in mucosal tissues such as the skin, gut, and lung via the induction of CD103 expression16,23,49,50. The deficiency of TGFβRII appeared to also diminish CD69+ CD103− TRM cell numbers in lungs36, indicating TGF-β signaling is essential for both CD103+ and CD103− TRM cell development in the respiratory tract. Interestingly, CD103-expressing cDC1s may preferentially promote lung CD8+ TRM cells after viral infection and selectively targeting antigens to CD103+ DCs markedly augmented CD8+ TRM cell development post vaccination – a process dependent on TGF-β signaling49. Moreover, lungs of aged mice express elevated levels of TGF-β compared to young mice, resulting in increased CD8+ TRM cell levels after influenza infection51. Thus, modulating TGF-β signaling in vivo may serve as an effective strategy to boost CD8+ TRM responses. Notably, human lung CD1c+ DCs, but not CD141+ DCs, produce the membrane-bound form of TGF-β1 which can induce CD103 expression in respiratory CD8+ T cells52.
The release of another anti-inflammatory cytokine, IL-10, also enhanced TGF-β expression, thus promoting early commitment of effector T cells to the TRM cell lineage53. IL-10 was shown to suppress early effector T cell expansion and acute inflammation, while promoting CD8+ TRM formation during SARS-CoV-2 infection in rhesus macaques54. Interestingly, a major source of IL-10 during respiratory viral infections is effector CD8+ T cell themselves6,55, indicating autocrine CD8+ T cell IL-10 signaling may also contribute to CD8+ TRM responses. IL-15 is considered a central regulator of memory CD8+ T cells, but TRM cells were believed to be less dependent on IL-15 for maintenance in vivo56. However, activation of IL-15 signaling by IL-15 complexes (IL-15c) treatment stimulated rapid proliferation and expansion of both CD8+ circulating memory and TRM cells57. Interestingly, IL-15 influenced the migratory ability of activated CD8+ T cells into the airway following influenza virus infection58. IL-21 is an important cytokine that has been implicated in facilitating CD8+ effector and memory T cell responses. IL-21 blockade at the memory phase selectively reduced a population of CD8+ TRM cells that are specific to the influenza NP protein. IL-21 functioned to promote BATF expression and NP-specific CD8+ TRM survival, suggesting that IL-21 regulates protective CD8+ TRM responses in an epitope-specific manner within the respiratory tract21.
2.2. CD4+ TRM cells
In contrast to the extensive characterization of CD8+ TRM cell development and maintenance, relatively fewer studies have elucidated CD4+ TRM responses. CD4+ TRM cells also uniformly express CD69, but less CD103 compared to CD8+ TRM cells. The general mechanism underlying respiratory CD4+ TRM cell development resembles that of CD8+ TRM cells. Naïve CD4+ T cells are stimulated by antigen-presenting DCs, mainly the IRF4-expressing CD11b+ CD103− cDC2s59, in the draining mLN. Activated and differentiated CD4+ effector T cells then migrate to the lung, where they further upregulate tissue-resident markers such as CD69, CXCR6, and/or CD103. One distinct characteristic of CD4+ TRM cells compared to CD8+ TRM cells is their ability to differentiate into several TRM-cell subtypes expressing distinct cytokine profiles, including T helper (Th)1 TRM (TRM1), Th2 TRM (TRM2), and Th17 TRM (TRM17) cells, depending on the nature of the pathogen60. Pulmonary TRM1 cells rapidly respond to influenza virus re-infection to produce interferon gamma (IFN-γ)61,62. T-bet+ TRM1 cells are mainly located on the border of the inducible Bronchus-Associated Lymphoid Tissue (iBALT) structure63, and express high levels of CD11a and VLA-1 for their retention and survival64. TRM2 cells are generated in response to allergens or parasitic infections, and usually produce Th2 cytokines such as IL-4, IL-5, and IL-1365,66. Following extracellular bacterial or fungal infection of the respiratory tract, IL-17A-expressing CD4+ TRM cells can be detected in the lungs67. High levels of IL-17- and IL-2-expressing TRM17 cells have also been observed in human lungs after Mycobacterium tuberculosis (M. tuberculosis) infection68.
We recently observed a hybrid CD4+ T cell population expressing features of both follicular helper T cells (TFH) and tissue-resident cells in the lung, which mainly localize within iBALT structures following influenza virus infection60,63. These cells express high levels of IL-21, and their development is dependent both on the TFH transcription factor BCL-6 and the TRM transcriptional factor Bhlhe40. Functionally, this cell population assists lung-resident B cell responses and lung CD8+ TRM formation and maintenance. Based on their gene expression and function, we termed this population “tissue-resident helper T (TRH) cells”21,60,63. Similar to lung TRH cells, IL-21-expressing CD4+ T cells have been observed in the brain and found to influence brain-resident CD8+ T cell development following mouse polyomavirus infection69.
3. Protective role of TRM cells in respiratory tract
Sterilizing protective immunity against pathogen reinfection is usually mediated by pre-existing antibody (Ab) responses, particularly the mucosal Ab response. Insufficient mucosal antibody levels and/or frequent mutations of pathogen surface proteins often facilitate re-infection by the same or related pathogens. In these cases, memory T cell responses are vital for the protection against pathogen dissemination and severe host disease. Indeed, pre-existing memory T cell levels can predict disease severity following influenza exposure in humans70,71. Furthermore, memory T cells induced by vaccination and/or previous infection are believed to be essential for the protection against severe coronavirus disease 2019 (COVID-19)72,73. To this end, both local (TRM) and systemic (TCM and TEM) T cell memory are likely required to provide optimal protection. In the following section, we summarize the protective roles of TRM cells against respiratory pathogen infection (Figure 1).
FIGURE 1. Mucosal immune protection mediated by respiratory TRM cells.
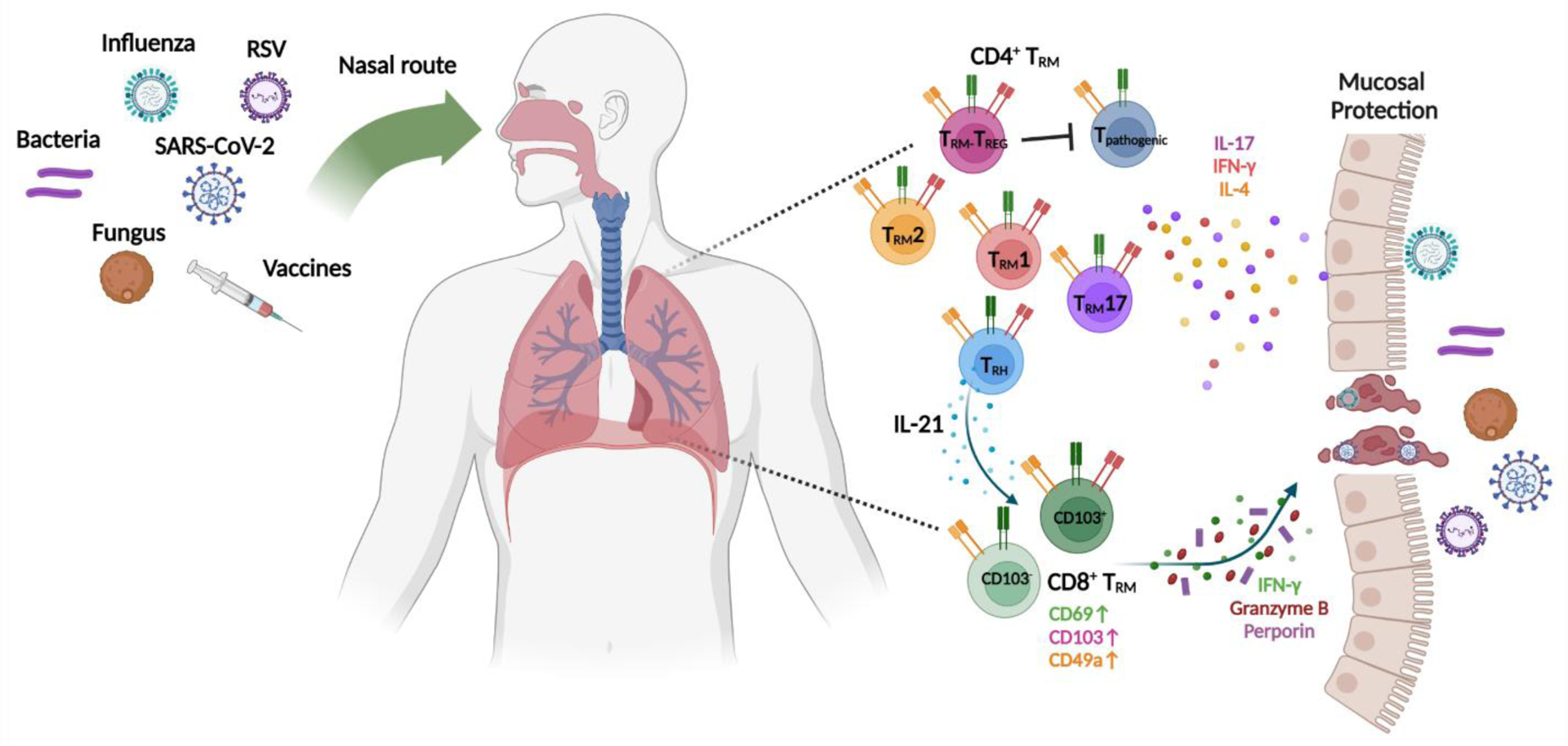
Respiratory infections or intranasal vaccination elicits CD4+ or CD8+ TRM cell responses in the respiratory tract. Based on their cytokine expression, CD4+ TRM cells can be categorized into specific subtypes including TRM1, TRM2, TRM17, TRM-TREG or TRH cells, which confer protection against different categories of pathogens. CD8+ TRM cells can be divided into CD103+ and CD103− sub-populations, and produce a variety of effector and cytotoxic molecules to directly kill pathogen-infected cells and/or induce a tissue anti-pathogen state.
3.1. The protective effect of TRM cells against respiratory viral infection
3.1.1. Respiratory syncytial virus (RSV)
RSV primarily targets children and the elderly, typically causing mild common cold-like symptoms in most individuals. However, it may also result in severe bronchitis, bronchiolitis and even pneumonia in a fraction of individuals74. Several studies have demonstrated a strong protective function of TRM cells against RSV infection75–77. Over 20 years ago, primary RSV infection was shown to induce the development of lung TRM responses78. Subsequent studies have found that RSV-specific CD4+ and CD8+ TRM cells can persist for more than 100 days after infection79. Furthermore, RSV-specific CD8+ TRM cells provided protection against secondary infection in the absence of circulating memory cells79. Similarly, in a study with RSV challenge in healthy volunteers, it was found that the abundance of pre-existing RSV-specific CD8+ TRM cells prior to infection negatively correlated with disease severity and viral load80. Interestingly, RSV-specific TRM recall responses during the secondary viral exposure also relied on the instructional signals from the innate immune responses, specifically the Mitochondrial antiviral-signaling protein (MAVS) signaling-mediated type I interferon responses77. Together these data suggest that TRM cells contribute significantly to protection against secondary RSV infection. Future RSV vaccine candidates focusing on the generation of robust TRM populations within the lung are likely to be effective in protection against symptomatic RSV re-infection. Indeed, in an immunization model with murine cytomegalovirus vector (MCMV-M) encoding the RSV matrix (M) gene, it was found that intranasal vaccination generated robust and durable CD8+ TRM responses which protected against secondary RSV challenge in an IFN-γ dependent manner75.
3.1.2. Influenza virus
Influenza infection causes up to 35.6 million illnesses and 140,000 to 710,000 hospitalizations in the United States alone annually81,82. Influenza virus infection can result in a range of clinical manifestations, ranging from asymptomatic infection to severe lower airway infection, pneumonia and death. In addition to seasonal outbreaks, the emergence of pandemic influenza strains can cause catastrophic illness and death. More than twenty years ago, it was found that influenza infection can elicit robust antigen-specific memory CD8+ T cell responses, which persist for several months after primary infection83. Similarly, influenza specific TRM cells capable of proliferating and producing functional molecules were also observed in human lungs84,85. Numerous studies have demonstrated remarkable protective capabilities of lung CD4+ and CD8+ TRM cells against influenza virus infection, particularly in the context of heterotypic influenza viruses that escape pre-existing antibodies20,22,33,86. Using parabiosis and/or FTY720 treatment to block circulating memory T cell infiltration, studies have found that both CD4+ and CD8+ TRM cells are sufficient to protect against lethal influenza virus re-challenge21,62,87. Moreover, when present in sufficient numbers, TRM cells can provide nearly sterilizing protective immunity against influenza infection88.
Upon viral entry, CD8+ TRM cells that can recognize infected cells are activated to become secondary effector cells, producing functional effector molecules including IFN-γ, tumor necrosis factor alpha (TNF-α), perforin, and granzyme B89. Both CD4+ and CD8+ TRM cells have been shown to rely on IFN-γ for their protective activities against secondary influenza infection, partially due to its function in activating an anti-viral state in the lungs34,90,91.
The reactivation of influenza TRM cells is mainly mediated by the recognition of cognate antigens. Interestingly, lung CD8+ TRM cells are reactivated more quickly, yet less efficiently, than their counterparts in the draining LNs (mLN) during secondary infection92. Reactivated lung TRM cells upregulate antiviral and cytotoxic molecules, while reactivated mLN memory T cells more robustly up-regulated genes involved in proliferation. Thus, lung TRM cells are more specialized in executing rapid anti-viral functions, whereas lymphoid memory T cells may provide sustained responses to counter viral dissemination if lung TRM cells are unable to constrain the virus. Notably, respiratory TRM cells can also be activated by bystander inflammation, particularly type I IFNs, inducing the expression of anti-viral interferon-stimulated-genes (ISGs) and granzymes which may contribute to secondary anti-viral responses92,93. Furthermore, lung CD8+ TRM cells expressed high interferon-induced transmembrane protein 3 (IFITM3) levels compared to spleen memory CD8+ cells following influenza infection. IFITM3 functions to protect CD8+ TRM cells against direct viral infection and IFITM3-deficient lung CD8+ TRM cells are lost during secondary viral infection94.
Respiratory tract CD4+ TRM cells have been reported to exert direct protective functions during secondary viral encounter. When influenza virus antigen-specific memory CD4+ T cells were transferred into a lymphocyte-deficient mouse model, donor CD69+CD11a+ lung CD4+ TRM cells provided greater lung protection compared to splenic memory CD4+ T cells after secondary influenza infection in an IFN-γ dependent manner90. Recently, we found that TRH cells can protect mice from secondary lethal viral infection, likely due to their ability to assist optimal resident memory B cell and CD8+ TRM responses21. Taken together, these results suggest that CD4+ TRM cells are involved in direct and indirect protection against influenza viral infection.
Frequent mutations in the surface hemagglutinin and neuraminidase of influenza virus have endowed the virus with great capacity to evade protective antibody responses elicited by prior infection and/or the current influenza vaccines. Since T cells can recognize more conserved influenza epitopes, the induction of robust memory T cell including TRM responses holds promise for the development of a “universal vaccine”, which can provide strain-independent protection against a broad spectrum of influenza viruses37. Various immunization strategies have been explored for the induction of strong TRM responses in the respiratory tract95. In particular, an adenoviral vector-based influenza vaccination strategy has been shown to generate robust CD8+ TRM responses in the lungs that can be maintained for at least 1 year post vaccination95.
3.1.3. SARS-CoV-2
The current COVID-19 pandemic, caused by the SARS-CoV-2 infection, is estimated to have claimed more than 18 million lives worldwide by 202296. The global scientific community rapidly responded to the crisis and has gained tremendous insight into the mechanisms underlying viral pathogenesis and host responses, including effector and memory T cell responses post SARS-CoV-2 infection and/or vaccination. However, most studies have focused on the immune responses in circulation, and we have comparatively limited information regarding responses in the respiratory tract, the primary site of infection. Using single cell RNA sequencing (scRNAseq), respiratory immune responses in the bronchoalveolar lavage (BAL) fluid of acute COVID-19 patients was examined97. A number of effector CD8+ T cells and potential CD8+ TRM precursors were observed in the BAL. During the acute phase, CD8+ TRM precursors were more prominent in patients with mild COVID-19, whereas cells from severe patients tended to exhibit naïve T cell-like action98. Later, Poon et al. found that SARS-CoV-2-specifc memory T cells were present in multiple tissues of COVID-19 convalescents, including the bone marrow, spleen, lungs, lymph nodes, and blood. Notably, lung tissue harbored the highest number of SARS-CoV-2-specific CD69+ CD103+ CD4+ or CD8+ TRM cells in COVID-19 convalescents. Moreover, lung memory cells exhibited greater functional profiles with distinct cytokine production99, indicating that SARS-CoV-2-specific lung TRM cells may be protective against potential SARS-CoV-2 re-infection. Consistent with this data, we also found the presence of CD69+ CD103+/–CD4+ or CD8+ TRM cells in BAL samples from COVID-19 convalescent patients. BAL CD4+ and CD8+ TRM cells demonstrated significantly greater levels of cytokine production following in vitro SARS-CoV-2-peptide re-stimulation compared to blood memory T cells100, suggesting that SARS-CoV-2-specific T cells are more enriched at the site of infection.
The generation of TRM cells after SARS-CoV-2 infection has also been captured in animal studies101,102. Currently, the function of TRM cells in protection against SARS-CoV-2 reinfection remains controversial in animal studies, depending on the model chosen for the study37. Intranasal vaccination strongly induced lung CD8+ TRM cells with superior polyfunctional phenotypes and conferred partial protection against SARS-CoV-2 challenge with a lethal dose101. On the other hand, another study demonstrated that lung CD8+ TRM induced after severe SARS-CoV-2 infection provided insufficient protection against SARS-CoV-2 reinfection in the K18-hACE2 transgenic mouse infection model103.
Unfortunately, the current mRNA vaccination strategy induces negligible CD4+ or CD8+ TRM cells in the respiratory tract37,104. To this end, intranasal booster immunization with adenovirus expressing spike protein (Ad5-S) or spike protein alone could promote robust lung CD4+ and CD8+ TRM cells in mRNA-immunized animals37,104,105. These studies indicate that vaccination strategies with systemic prime plus a respiratory booster may be effective in generating respiratory TRM responses required for optimal protection against future SARS-CoV-2 variants.
3.2. Protective roles of TRM cells against bacterial and fungal infection
Similar to respiratory viral infection, bacteria that target the respiratory tract have been known to induce TRM cell responses. Unlike viral infections, respiratory CD4+ TRM cells appear to serve a key role in providing local protection against bacterial infections of the respiratory tract compared to CD8+ TRM cells67,106–108. Severe Streptococcus pneumonia (Spn) infection can often cause pneumonia. In mouse models, repeated Spn challenge elicited a robust CD4+ TRM17 response. Interestingly, these CD4+ TRM cells can provide lung region-specific protection against Spn re-infection106. When lung was infected by Spn in a lobe-specific manner, it was found that IL-17-producing CD4+ TRM17 cells were mainly confined to the previously infected lobe, but not throughout the entire lower respiratory tract. Importantly, pneumonia protection was also restricted to the immunologically experienced lobe, indicating that CD4+ TRM cells provide localized but superior tissue protection compared to circulating memory cells106. Mechanistically, CD4+ TRM cells prevented the colonization of pneumococcal bacteria on the mucosa of the respiratory tract via IL-17 mediated neutrophil recruitment107. Furthermore, IL-17 produced by lung CD4+ TRM cells contributed to the control of M. tuberculosis infection in humans68. In the animal model, M. tuberculosis specific CD4+ TRM cells showed enhanced protective effects compared to intravascular counterparts109. Vaccines that can induce lung robust TRM cells provide superior protection against bacteria re-infection. Combination with outer membrane protein from Klebsiella pneumoniae and an adjuvant that strongly induces lung TRM1 and TRM17 cells was shown to confer critical protection against lethal Klebsiella infection110.
Similar to bacterial infections, TRM cells have been identified in tissues after exposure to fungi. Aspergillus fumigatus infection generated two distinct TRM subsets based on their surface marker expression. CD69hi CD103low CD4+ TRM exhibited pathological features, whereas CD69hi CD103hi Foxp3+ resident CD4+ regulatory T cells suppressed the detrimental activities of the CD103low CD4+ TRM cells111. Of note, a DC-based vaccine strategy was found to promote lung TRM17 cell generation, which provided significant protection against highly virulent fungus Cryptococcus gattii112. These results suggest that lung TRM cells can be generated after vaccination for the protection against fungal infections.
4. TRM and chronic respiratory disease
Chronic respiratory diseases such as asthma and chronic obstructive pulmonary disease (COPD) affect hundreds of millions of individuals globally and are a leading cause of mortality and morbidity worldwide. In the past decade, increasing evidence has suggested that respiratory CD4+ and/or CD8+ TRM cells are a major contributor, if not a driver, of the development and/or progression of many chronic respiratory diseases. Below we have summarized our current understanding of the roles of TRM cells in multiple respiratory disease conditions (Figure 2).
FIGURE 2. Contribution of TRM cells to respiratory diseases.
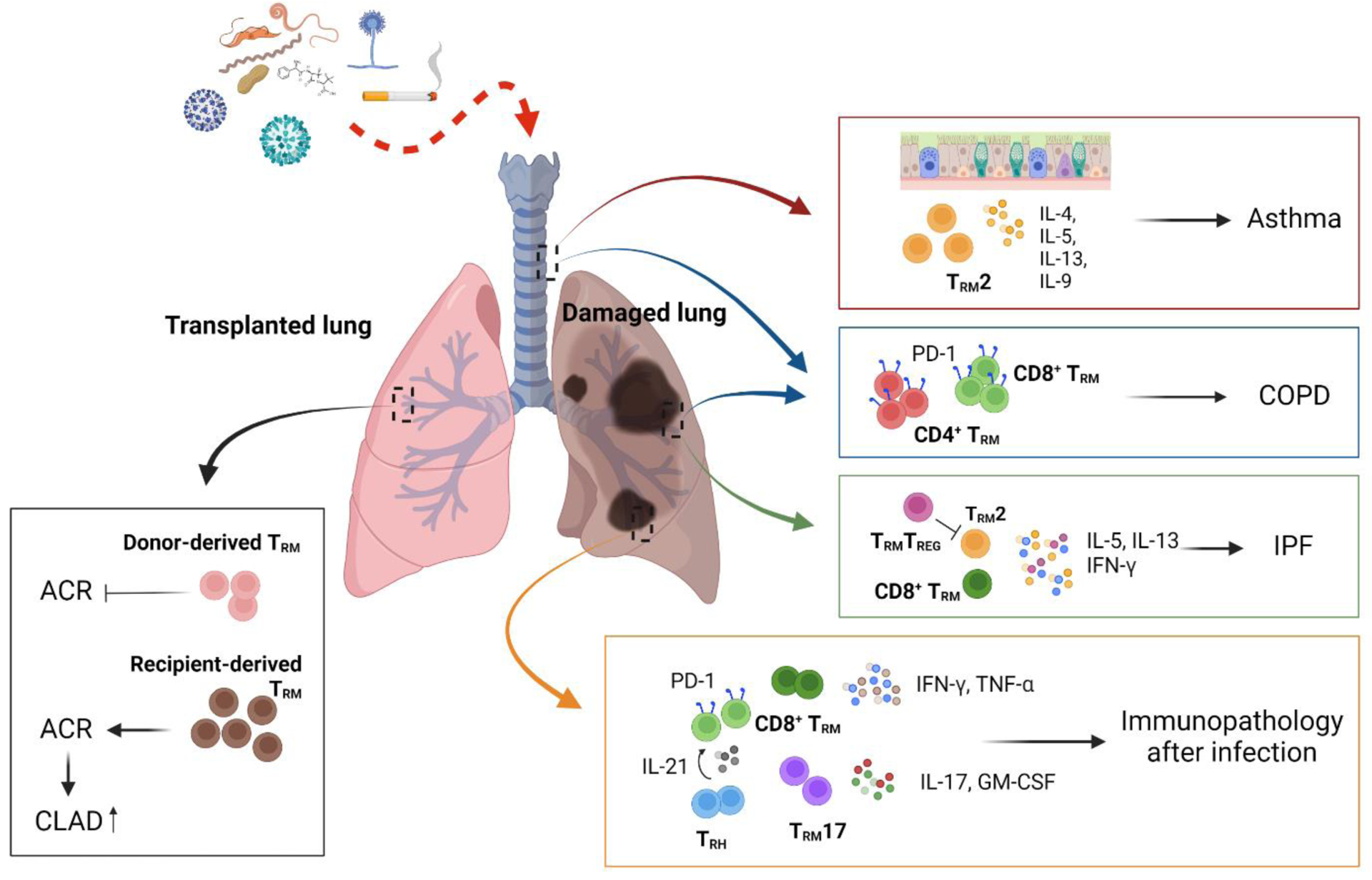
Dysregulated CD4+ or CD8+ TRM cells in the respiratory tract are associated with the development of various respiratory diseases. Reactivation of TRM2 cells that express type 2 cytokines appear to be a driver of asthma symptoms. Elevated CD4+ or CD8+ TRM cell levels likely promote disease progression in COPD. Further, TRM2, TRM17 and/or CD8+ TRM cells are involved in the development of pulmonary fibrosis. In the aftermath of respiratory infection, exuberant CD4+ and/or CD8+ TRM cell responses result in chronic lung immunopathology. In the transplanted lung, TRM cell origin may have distinct functions. Donor-derived TRM cells are likely protective, but recipient-derived TRM cells may promote ACR and CLAD. COPD: chronic obstructive pulmonary disease; ACR: acute cellular rejection; CLAD: chronic lung allograft dysfunction.
4.1. TRM in allergies and asthma
Allergy and asthma are chronic inflammatory disorders in the airway that affect hundreds of millions of people globally113. CD4+ T cells, particularly Th2 cells, are well-known to orchestrate the development of asthma114,115. In response to seasonal exposures to allergens, memory T cells, particularly memory Th2 cells, are believed to mediate intermittent flares of asthma. The role of CD4+ TRM cells in regulating allergic asthma has been the focus of several recent studies. In a mouse model of house dust mite (HDM) induced allergic inflammation, HDM-specific TRM cells were formed after allergen sensitization and persisted in the lung for more than 100 days after initial sensitization116. The development and/or maintenance of these CD4+ TRM cells in the lung was found to be dependent on IL-2 and IL-7 signaling116,117. Importantly, these lung TRM cells were sufficient to promote asthma symptoms, independent of memory cells in secondary lymphoid organs, highlighting the importance of lung CD4+ TRM cells in driving pathology after allergen re-exposure116,118. Comparison of the gene profiles of TCM cells in the lymphoid organs and TRM cells in the lung, Rahimi et al. found that TCM and TRM cells shared a core Th2 gene signature, while TRM cells uniquely expressed a tissue-adaptation signature including genes involved in regulating and interacting with the extracellular matrix65. Both TCM and TRM cells contributed to the recall response after allergen re-exposure, but they appeared to have different functions. Recall of circulating TCM cells promoted perivascular inflammation and eosinophil recruitment, TRM cells augmented peri-bronchial inflammation including mucus metaplasia, airway hyperresponsiveness, and airway eosinophil activation65,116,117.
In a chronic intranasal HDM exposure model, both CD4+ and CD8+ T cells infiltrated into the lungs during the acute phase of challenge, but only CD4+TRM cells persisted in the lungs following cessation of allergen exposure118. Lung CD4+ TRM cells were localized around airways and responded rapidly upon allergen re-exposure, leading to airway hyperresponsiveness, recruitment and activation of other immune cells, and production of IL-4, IL-5, and IL-17118. Additionally, a subset of multi cytokine-producing CD4+ TRM cells also expressed high levels of IL-9, which was critical to mediate rapid allergen recall responses and promoted the infiltration of multiple immune cells into the allergic lung119.
Consistent with mouse data, patients with moderate to severe asthma had increased levels of airway CD4+ T cells expressing TRM markers, compared to subjects with mild asthma and healthy controls - indicating a role of CD4+ TRM cells in asthma pathophysiology120. Furthermore, allergic patients also harbored pathogenic IL-9-epxressing TRM cells that co-expressed the IL-33 receptor, ST2, and multiple other cytokines121,122. Together, pathological CD4+ TRM cells may represent a major driver for the development of airway inflammation and asthmatic symptoms following allergen exposure and re-exposure. Thus, interventions targeting CD4+ TRM development, maintenance and/or their effector activities will be crucial for the development of effective therapies against asthma.
4.2. TRM in COPD and IPF
Idiopathic pulmonary fibrosis (IPF) is a chronic lung disease with progressive fibrotic tissue remodeling and lung scarring123,124. IPF is an irreversible disease and the median survival rate after diagnosis is only 2–4 years125. There are two FDA-approved medications for IPF, but unfortunately neither of them have been shown to extend the median survival time following diagnosis126. Thus, it is an urgent need to better understand the cellular and molecular mechanisms modulating IPF development and progression in order to develop more effective therapeutic interventions. T cells, particularly Th2 and Th17 cells, have been found to promote lung fibrosis in animal models123,127. In a mouse model of fibrosis induced by chronic exposure to Aspergillus fumigatus, lung resident CD4+ TRM cells, but not circulating CD4+ T cells, caused lung inflammation and fibrosis. In particularly, IL-5 and IL-13 producing CD69hi CD103lo CD4+ TRM2 cells mediated fibrotic processes, whereas CD69hi CD103hi lung-resident CD4+ regulatory T cells suppressed the pathological TRM2 responses. These data suggest that CD4+ TRM cells are heterogenous in the lung and their pathological or immunosuppressive effects could be delineated by CD103 expression level111.
In human, the population of CD103+ CD4+ T cells were significantly increased in the airway of patients with fibrotic lung disease128. Both airway CD103+ and CD103− CD4+ T cells highly expressed CD69, but CD103+ CD4+ T cells expressed higher other TRM-associated markers such as CD101, CD49a and VLA-2. Interestingly, the CD103+ CD4+ T cells in the human lungs expressed IFN-γ and exhibited a T-helper 1-like effector phenotype128,129. Consistent with these earlier findings, patients with progressive fibrosing interstitial lung disease (PF-ILD) harbored higher levels of IFN-γ and IL-13-double producing CD4+ T cells in BAL compared to controls. Notably, these BAL IL-13+/IFN-γ+ CD4+ T cells from PF-ILD patients exhibited characteristics of conventional TRM cells130. In addition to CD4+ TRM cells, lungs from IPF patients also had increased levels of CD103+CD8+ TRM cells. Using scRNAseq, it was recently found that both CD4+ and CD8+ TRM as well as CD8+ TEM cells were increased in the lungs of IPF patients. Furthermore, the response to the IFN-γ pathway was enriched in CD4+ TRM and CD8+ TRM cells in IPF, along with other T cell activation and signaling pathways131. These data highlight the potential involvement of both CD4+ and CD8+ TRM cells in pulmonary fibrosis, but their exact protective or pathological functions in the development and/or progression of fibrosis remains to be determined in further studies.
COPD is a chronic lung inflammatory disease affecting the lung parenchyma and small airways, leading to irreversible and progressive airflow limitation. COPD is usually caused by chronic cigarette smoking or long-term inhalation exposure to harmful substances. Both CD8+ and CD4+ T cells have been implicated in the inflammatory response of COPD132. Particularly, the percentage of BAL CD8+ T cells positively correlated with the number of cigarettes smoked per day in male smokers with COPD - indicating a potential causative correlation between smoking and TRM levels. More recently, using mass spectrometry, it was found that both CD103+ CD4+ and CD103+ CD8+ TRM cells were increased in the lungs of COPD patients compared to healthy controls. Also, TRM cells expressing high levels of PD-1 were found within the walls of small airways133,134. Consistent with the human data, cigarette smoking in mice caused elevated accumulation of CD8+ T cells in lungs. Notably, CD8-deficient, but not CD4-deficient, mice had reduced inflammation and airspace enlargement135, suggesting lung tissue CD8+ T cells promote tissue destruction during chronic smoking. Moreover, CD69-deficient mice exhibited reduced inflammation after smoking136. Additionally, in a viral model of COPD exacerbation, it was shown that IFN-γ derived from tissue-resident lymphocytes including TRM cells suppressed alveolar stem cell growth, promoting emphysema exacerbation137. Together, these data suggest a potential role of TRM cells, particularly CD8+ TRM cells, in the development or exacerbation of COPD. Since there are currently no effective therapeutics for COPD, targeting the pathological functions of CD8+ TRM cells may pave the way for the development of potent treatment strategies against COPD in the future.
4.3. TRM in the rejection of lung transplantation
Lung transplantation is performed in individuals with various end-stage lung diseases. However, the long-term survival rate after lung transplant is still relatively low compared to other solid organs due to increased frequency of acute and chronic rejection of the transplants138,139. The lower threshold for activation, along with faster response compared to naïve T cells implicates memory T cells in acute cellular rejection (ACR) of lung transplants140–143. ACR also increases the risk of chronic lung allograft dysfunction (CLAD), which is the major limiting factor to long-term survival after lung transplantation142. Since TRM cells have the potential to mediate rapid immune responses in situ, TRM cells are likely important in orchestrating the allogeneic rejection process after transplantation142,144. Indeed, there are several reports indicating that TRM cells are associated with allograft rejection. For instance, TRM cells mediate allograft rejection after kidney transplantation in mouse models145 and CD103 expression from patients of renal allograft predicted the acute rejection response146.
During lung transplantation, donor T cells expressing TRM markers such as CD69 and CD103 persisted in lung allografts for over 1 year after transplantation147. Furthermore, recipient T cells infiltrating the lungs gradually acquired TRM phenotypes months after transplantation. Interestingly, the long-term persistence of mature donor TRM cells (CD69+ CD103+) was associated with lower incidence of primary graft dysfunction (PGD) and ACR147. In contrast, ACR was characterized by the perivascular infiltration of recipient T cells in the lung. Recipient T cells underwent clonal expansion and expressed high levels of genes related to cytotoxicity, inflammation and tissue residency in the lung allografts - consistent with the notion that recipient T cells mediate lung ACR148,149. Of note, after the administration of systemic glucocorticoids, TRM cells continued to persist for months but gene expression profiles were reprogrammed toward diminished cytotoxic functions148,150. These data suggest that maintaining donor derived TRM and/or preventing the replacement of donor TRM cells with recipient derived TRM cells may be the key to improve clinical outcomes following transplantation144. Also, drugs capable of inducing TRM cell reprogramming towards less inflammatory phenotypes and/or capable of depleting alloreactive TRM cells could be promising to prevent lung allograft rejection and increase long-term survival rates after lung transplantation150.
5. TRM and virus-induced lung immunopathology
Respiratory viral infections are a leading cause of mortality, accounting for more than 2 million deaths globally per year151. Occasionally, viral pandemics, such as influenza pandemics and the current COVID-19 pandemic, could result in even greater burden of disease. Besides the acute diseases caused by viral infections, there is growing evidence indicating the prevalence of chronic pulmonary sequelae after the resolution of primary infection. Exuberant TRM cell, particularly CD8+ TRM cell responses have been recently shown to play a prominent role in driving persistent lung immunopathology after acute viral infection in the respiratory tract.
5.1. Influenza
Influenza infection may cause persistent pulmonary and extra-pulmonary sequelae after the resolution of acute diseases in both humans and animal models152–157. As mentioned above, CD8+ TRM cells express high levels of effector and cytolytic molecular, which potentiate their rapid responses to re-infection. However, enhanced effector molecule expression not only augments their anti-microbial activity, but can also potentially cause bystander inflammation and tissue injury if dysregulated158. In a model of influenza infection in aged mice, we found that aged hosts exhibited persistent inflammatory and fibrotic responses after the resolution of infection. Similar delays in recovery of the lung during aging has been recognized after human pneumonia as well51. RNA-seq analysis found that aged lungs exhibited increased signatures of T cell-associated genes and pro-inflammatory mediators, indicating the induction of excessive TRM cell responses. Indeed, using flow cytometry and parabiosis, we confirmed that aged lungs had both increased antigen-specific and bystander CD8+ TRM cells (both CD69+ CD103+ and CD69+ CD103−) compared to young mice. Interestingly, aged lungs mounted increased TRM responses despite diminished circulating memory counterparts, suggesting that the local tissue environment preferentially supports the development of exuberant TRM responses. The transfer of T cells from young mice into aged lungs resulted in increased TRM responses compared to those of T cells administered into the young lungs, confirming that the aged environment was responsible for this phenomenon51. To this end, elevated TGF-β expression was observed in aged lungs and the transfer of TGFβRII-deficient T cells abrogated the elevated TRM response, indicating that the excessive age-associated CD8+ TRM response was dependent on TGF-β signaling.
Of note, the increased accumulation of CD8+ TRM cells in aged lungs did not provide better protection against heterologous influenza reinfection compared to young counterparts51,159. Using scRNAseq, we found that CD8+ TRM cells in aged lungs exhibited altered phenotypes and lacked a TRM subpopulation that expressed high levels of protective molecules. Furthermore, TRM cells from aged lungs expressed lower levels of downstream TCR signaling genes and appeared to be more senescent in producing effector cytokines in response to peptide mediated TCR, but not phorbol myristate acetate (PMA)/ionomycin, stimulation. Collectively, these data suggest that altered functional capacity, particularly after antigenic restimulation, underlies the impaired protection provided by CD8+ TRM cells during aging. Strikingly, the depletion of CD8+ TRM cells with a high dose of anti-CD8 Ab administration, but not the depletion of circulating CD8+ T cells with a low dose of CD8 Ab administration, alleviated lung inflammation and fibrosis51. In addition, the lungs from aged mice with CD8+ TRM cell depletion showed diminished expression of multiple inflammatory cytokines and chemokines compared to the lungs of mice that received control antibody. Furthermore, CD8+ TRM cell depletion resulted in reduced recruitment of inflammatory monocytes and neutrophils to the tissue. These results together indicate that CD8+ TRM cells in aged lungs are a driver for the development of chronic lung sequelae following primary influenza pneumonia.
After influenza infection, young mice also developed persistent lesions in the lung characterized by patches of inflammatory, mucus hypersecretion and fibrotic regions, resulting in dysplastic epithelial repair160,161. However, the persistence of pathological sequelae in young mice was less frequent and milder compared to aged mice. Interestingly, the depletion of CD8+ T cells at the memory stage did not alter the pathological responses in young mice, suggesting that CD8+ TRM cells may not contribute to chronic lung sequelae in young hosts. Rather, dysregulated myeloid responses appeared to play an important role in the development of chronic lung sequelae after viral pneumonia152,162. However, excessive TRM responses and activity could also cause chronic lung pathology and fibrosis when the brake on TRM cells was released36,51.
To this end, CD8+ TRM cells expressed multiple inhibitory receptors including PD-1163,164. Particularly, CD8+ TRM cells specific to the H2Db-restricted NP366–374 peptide highly expressed PD-1 and other inhibitory receptors including T- cell immunoglobulin and mucin-domain containing-3 (TIM-3) and lymphocyte-activation gene 3 (LAG-3) compared to TRM cells specific to other influenza epitopes and circulating memory cells. Despite viral clearance for over 4 weeks, TRM cells upregulated gene programs similar to “exhausted” or effector-like CD8+ T cells, thus termed as “exhausted-like” TRM cells36,165. NP366–374 -specific “exhausted-like” TRM cells showed persistent activation of TCR signaling at the memory phase. Indeed, these “exhausted-like” TRM cells exhibited persistent low levels of TCR signaling, and the ablation of MHC-I after viral clearance led to diminished PD-1 expression and decreased levels of “exhausted-like” TRM cells. These data suggest that chronic TCR signaling after the clearance of infectious viruses due to persistent NP antigen (given their abundance during primary viral replication), induced the generation of PD-1hi CD8+ “exhausted-like” TRM cells following primary viral infection.
Similar to chronic viral infections where blocking the interaction between PD-1 and PD-L1 increased the expansion of exhausted CD8+ T cells166, inhibition of PD-1 and PD-L1 interaction at the memory stage elevated the abundance of NP366–374-specific “exhausted-like” CD8+ TRM cells, but not other CD8+ TRM cells following influenza infection. PD-L1 blockade also increased the production of effector cytokines, particularly TNF, by NP366–374-specific CD8+ TRM cells and further enhanced their protective function against influenza re-infection. Thus, persistent PD-L1 signaling in CD8+ TRM cells restricted their effector activities and protective functions against secondary infection.
However, the enhanced protection against secondary influenza infection comes at a cost. Anti-PD-L1 treated lungs exhibited enhanced inflammatory and fibrotic sequelae after primary influenza virus infection. Notably, the depletion of CD8+ T cells alleviated tissue pathology, suggesting that enhanced CD8+ TRM cell responses directly caused the chronic sequelae. The selective expansion and increased effector activity of “exhausted-like” NP-specific TRM cells after PD-L1 blockade suggested that the exaggerated responses of TRM cells specific to the NP366–374 epitope are likely the cause of the observed lung sequelae. Thus, high levels of PD-1 expression on certain TRM cells functions to balance the protective versus pathological functions of TRM cells. Notably, PD-1hi CD8+ TRM cells were observed in the pancreas, and reduced PD-1 expression in pancreatic TRM cells was observed in chronic pancreatitis, indicating important roles for PD-1 in constraining TRM cell activity and maintaining tissue homeostasis in humans164. Increased PD-1 expressing-TRM cells were also observed in IPF patients36. Whether dysregulated or diminished PD-1 or PD-L1 signaling also has a role in pulmonary fibrosis development requires future investigation. Additionally, a small percentage of cancer patients receiving immune checkpoint blockade (ICB) develop pneumonitis and fibrosis167,168. It is possible that the activation of preexisting influenza or viral-specific TRM cells, which are abundant in human lungs, may contribute to disease development after receiving ICB. Altogether, these data suggest that the primary function of the expression of inhibitory receptors on TRM cells is to restrain their cytotoxic and pathological activities to promote recovery and maintenance of immune homeostasis. Additionally, lung TRM cells express the cytokine IL-10169, which may endow TRM cell anti-inflammatory or regulatory properties to resolve tissue inflammation after primary viral infection or during the TRM recall responses after secondary viral exposure.
5.2. SARS-CoV-2
The symptoms of acute COVID-19 vary from mild to severe, potentially resulting in death due to respiratory failure. Of note, COVID-19 symptoms may persist, or new symptoms may arise months after recovery from acute diseases, which are generally referred as post-acute sequelae of COVID-19 (PASC) or long COVID. Both acute and long COVID are particularly a concern among older people or people with pre-existing comorbidities157,170,171. Rapid clearance of the SARS-CoV-2 virus and complete recovery of the host requires timely and robust T cell responses, whereas improper T cell responses have been reported to contribute to disease after infection. Dysregulated TRM cell responses have also been reported to promote pulmonary pathology in both acute and chronic stages after SARS-CoV-2 infection.
For instance, immune profiling in the BAL of COVID-19 patients identified that clonally expanded CD4+ TRM17 cells were associated with severe lung damage in COVID-19 patients172. These TRM17 populations exhibited high levels of expression of proinflammatory cytokines such as IL-17A and CSF2 (GM-CSF). These cells also expressed the transcription factor, RBPJ, which is known to regulate Th17 cell pathogenicity173,174. Furthermore, bioinformatics analysis found that TRM17 cells had potential interactions with other tissue-specific immune cells, including macrophages and CD8+ T cells, collectively promoting severe COVID-19. In addition to CD4+ TRM cells, CD8+ TRM-like cells have also been identified in the BAL of acute COVID-19 patients. Hobbit (ZNF683)-expressing CD8+ TRM-like cells likely represented SARS-CoV-2-specific CD8+ T cells and were more enriched in patients with moderate infection compared to patients with severe disease, suggesting that the presence of these cells may be beneficial to the host97.
Bona fide SARS-CoV-2 antigen-specific TRM cells have been detected in the lungs of COVID-19 convalescents after primary infection175. Poon et al showed that SARS-CoV-2–specific memory T cells are maintained across diverse tissue sites99. In a cohort of aged control and COVID-19 convalescents, we found that CD8+ T cells were highly increased in the BAL of COVID-19 convalescents. Most of these BAL CD8+ T cells express CD69 and a portion of those cells co-express CD103, indicating their tissue-residency phenotype. Significant BAL CD8+ TRM cell populations in COVID-19 convalescents produced IFN-γ and TNF-α upon antigenic stimulation in vitro, indicating their polyfunctional features. Compared to the CD69+ CD103+ double positive “conventional” TRM cells, the higher frequency of CD69+ CD103− TRM cell population was observed in the respiratory tract, although quantities of both populations were increased after SARS-CoV-2 infection – consistent with the overall increase in CD8+ T cells within the BAL of COVID-19 convalescents. Of note, majority of this aged COVID-19 convalescents cohort exhibited moderate to severe chronic lung pathology and impaired lung gas exchange function as measured by quantitative computed tomography (CT) and pulmonary function tests. Interestingly, total BAL CD8+ T cells positively correlated with radiographic abnormalities such as ground glass opacification (GGO) or reticular densities and consolidation. BAL CD8+ TRM cells and the CD69+ CD103− sub-population in particular, showed significantly negative correlation with lung function parameters including forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC) and diffusion capacity for carbon monoxide (DLCO) - indicating a detrimental role of TRM cells in recovery after primary SARS-CoV-2 infection.
Using scRNAseq technique, we further analyzed the characteristics of BAL CD8+ T cells. We identified three BAL CD8+ T cell populations expressing tissue-residency gene programs compared with circulating CD8+ T cells in blood, including Hobbit and CD103-expressing conventional TRM cells, CD69+ CD103−/low TRM cells and a population of CXCR6hi effector-like tissue-resident cells. Compared to the CD103+ conventional TRM cells, CD103−/low TRM cells expressed higher levels of cytotoxic and/or inflammatory molecules that can promote inflammation and fibroblast activation after infection. CD103−/low TRM cells also were enriched with TCR signaling downstream genes, indicating that they may receive chronic antigen stimulation from persistent viral antigens and/or auto-antigens. These data suggest that the CD103−/low TRM cells may have higher pathogenic potential than those of conventional TRM cells. The BAL CXCR6hi CD8+ T cells exhibited fewer T cell memory features, but higher effector T cell features compared to other subsets of BAL CD8+ T cells100. Consistent with another study in the context of the liver where CXCR6hi CD8+ T cell population exhibited pathological activity and promoted liver tissue damage176, this subset of BAL CD8+ T cells was enriched in gene signatures associated with inflammation and tissue destruction. Alternatively, these CXCR6hi CD8+ T cells may represent effector CD8+ T cells that provide protective function after a severe SARS-CoV-2 infection since transcriptome-wide association studies (TWAS) studies suggested that lower expression of CXCR6 in CD8+ TRM cells was associated with severe disease development following acute COVID-19177. Nevertheless, our study has demonstrated that exuberant responses of respiratory CD8+ TRM cells likely contributes to impaired lung function and the development of chronic pulmonary sequelae after the resolution of acute COVID-19 in aged individuals100. In a more recent study using proteome profiling of convalescent airway and blood, various inflammatory chemokines and proteins associated with tissue damage were observed to be dysregulated in the airways of COVID-convalescents compared to controls. Notably, albumin and lactate dehydrogenase (LDH), which serve as indicators of ongoing cell death and damage, were increased in the BAL of COVID-19 convalescents with persistent symptoms. Interestingly, different BAL immune cells including CD8+ T cells positively correlated with lung pathophysiology in COVID-19 convalescents with chronic pulmonary symptoms. Similar to our findings, pulmonary TRM cells were found to be elevated in the COVID-19 convalescents with persistent conditions and negatively correlated with certain lung function parameters178. These findings indicate that persistent elevated CD8+ TRM cells in the airway may cause constant damage to the respiratory epithelium, leading to pathology long after recovery from acute disease.
6. Mechanisms regulating TRM pathogenicity in virus-induced lung sequelae
As discussed above, dysregulated TRM cell responses are linked to the development of chronic lung sequelae following the recovery from acute viral infection, particularly during aging. At present, the underlying mechanisms by which lung TRM cells cause the development of chronic disease are largely unknown and require further mechanistical studies in animal models (Figure 3).
FIGURE 3. Potential mechanisms regulating TRM cell-mediated pathogenesis in lung sequelae after infection.
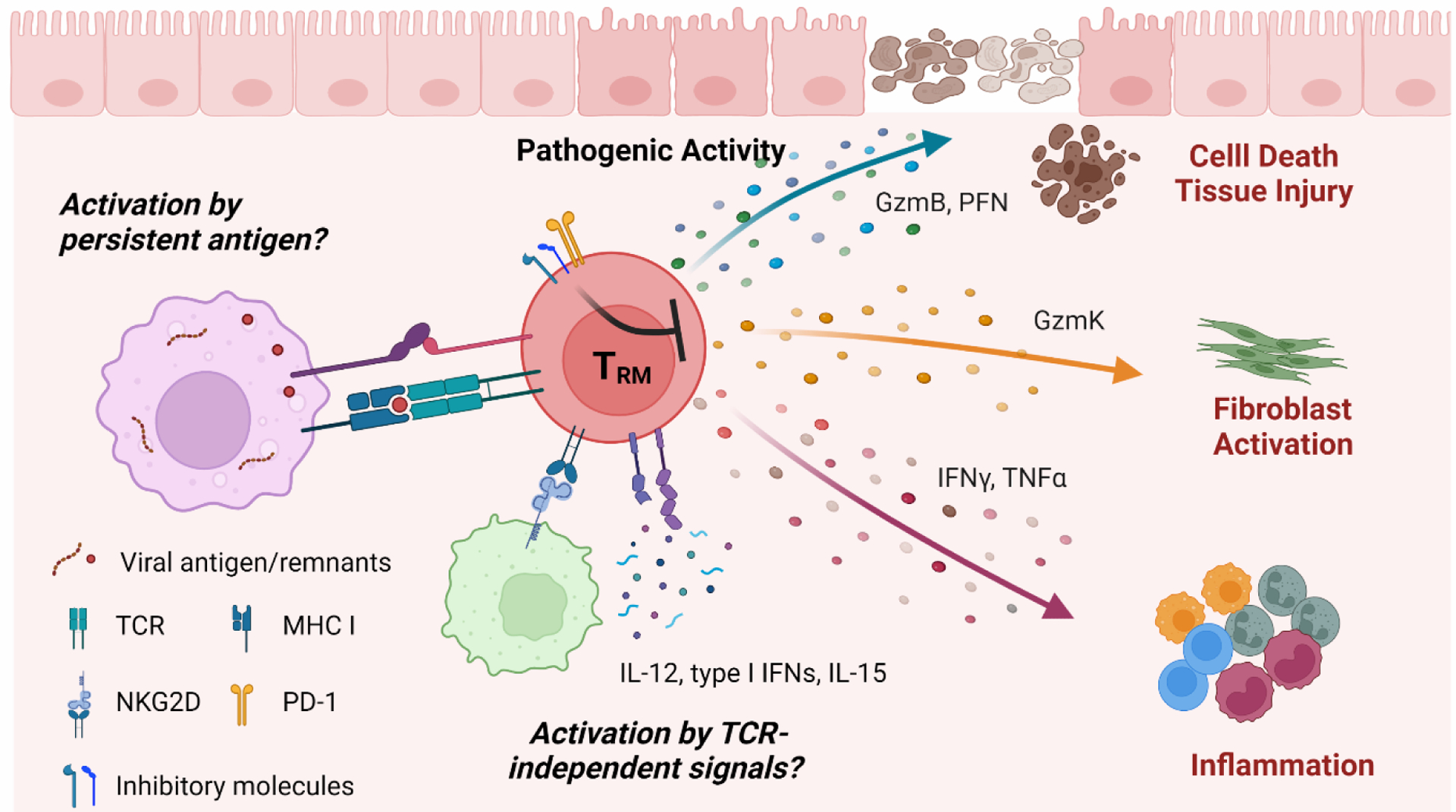
Persistent antigen presence or environmental cues such as inflammatory cytokines stimulate TRM cells to produce a variety of pathogenic molecules, leading to development chronic inflammatory and/or fibrotic lung pathology.
Although transcriptional analyses have suggested that circulating CD8+ terminally differentiated effector cells (TEMRA) may exhibit greater cytolytic activity than TRM cells in human179,180, mouse TRM cells exhibited higher expression of cytotoxic molecules including Granzyme B and perforin compared to circulating memory cells40. It is possible that the activity of exuberant TRM cells in the lung can result in tissue microinjury, inducing increased cell death due to constant release of cytotoxic molecules. To this end, it is worth noting that persistent non-healing epithelial microinjuries may further induce a cascade of inflammatory and fibrotic responses123,181, which is believed to be a major driver of pulmonary fibrosis. Furthermore, TRM cells produce high levels of pro-inflammatory cytokines such as TNF, CCL3 and IFN-γ, which may be needed to optimally mediate their protective functions, but also potentially lead to chronic tissue inflammation and lung damage under certain conditions34,51. Of note, T cells, particularly memory T cells, usually do not constitutively release cytotoxic molecules and/or produce cytokines without antigenic or inflammatory stimuli despite gene expression182,183. Thus, antigenic or environmental cues in the tissue are needed to perpetuate the inflammatory cascade.
To this end, it is increasingly recognized that acute respiratory viral infection may result in chronic deposition of viral antigens, remnants and/or establish a persistent virus reservoir. For instance, the persistence of viral RNA and/or antigen in the lung was observed months after influenza virus infection184. Indeed, persistent inflammatory and fibrotic foci were shown to be enriched with influenza viral RNAs months after viral clearance, suggesting an association between viral genes/antigens and tissue damage152. Influenza viral antigens, particularly the NP protein, was shown to be deposited in an irradiation-resistant lung structural cell type after infectious viral clearance184. Using Nur77-GFP transgenic mice, we further showed that lung TRM cells specific to the NP366–374 peptide had persistent low levels of TCR signaling as evidenced by the GFP expression for more than one month after influenza infection. Furthermore, the ablation of MHC I signaling resulted in diminished NP366–374 -specific TRM cells, indicating chronic TCR signaling at the memory phase is critical for their maintenance in the lung36. Notably, the potential of persistent TCR stimulation in driving chronic disease is balanced by high expression of inhibitory molecules such as PD-1 on TRM cells in young hosts36,185. However, the activity of these checkpoints is likely insufficient to completely curb the pathological activities of exuberant TRM cell responses upon chronic stimulation by persistent antigens in aged hosts. Additionally, aging may further delay the clearance of viral antigens and/or viral remnants, resulting in elevated or extended antigenic stimulation to TRM cells. All these mechanisms may contribute to the age-associated pathological roles of TRM cells after viral pneumonia.
Emerging evidence has also suggested that SARS-CoV-2 infection may lead to persistent deposition of viral antigens, remnants and even reservoirs. A recent study using a large number of autopsy samples demonstrated that SARS-CoV-2 virus could potentially replicate in multiple respiratory and non-respiratory tissues as late as 230 days following symptom onset186. Furthermore, SARS-CoV-2 viral RNAs and/or antigens have been detected in the gastrointestinal (GI) tract, liver, and olfactory epithelium in COVID-19 convalescents, although the persistence of viral remnants or antigens in the respiratory tract remain unknown. The CD69+ CD103− TRM subset found in the BAL of aged COVID-19 convalescents was highly enriched with TCR signaling pathway genes, indicating antigen-mediated stimulation of TRM cells after resolution of primary SARS-CoV-2 infection100. Therefore, it is possible that persistent antigen deposited in the lung may drive chronic activation of this TRM subset, thereby promoting the development of post COVID-19 lung sequelae.
Alternatively, viral antigen-independent signals may play a role in regulating chronic lung sequelae after primary respiratory viral infection. It is possible that the antigenic signal to TRM cells may be derived from an autoantigen. Such a possibility is particularly relevant given the fact that antibodies against autoantigen have been frequently reported after SARS-CoV-2 infection187,188. Furthermore, memory T cells can be activated or stimulated by inflammatory cytokines such as IL-12, type I IFNs and/or IL-15, independent of TCR signaling189. Certain co-stimulatory molecules such as NKG2D can also activate memory T cells independent of TCR. Thus, it is possible that persistent tissue inflammation following viral infection stimulates TRM cell activation even after viral clearance, resulting in tissue destruction and chronic lung sequelae. Notably, IL-15 has been shown to be a survival and activation signal for a CXCR6hi TRM -like subset that was enriched with gene programs involved in tissue inflammation in the liver190,191. Persistent IL-15 levels may promote the development and/or function of these T cells in the respiratory tract, contributing to the development of chronic lung sequelae after primary viral pneumonia including SARS-CoV-2 infection.
While TRM cells in other tissue such as skin, liver or intestinal mucosal are stable and long-lasting, pulmonary antigen-specific TRM cells are relatively short-lived and steadily decline over time after the primary infection22,192–195. However, it is important to note that this phenomenon was documented using animal models, and whether this is true in humans after respiratory viral infections remains to be fully established. Nevertheless, given the potential danger of persistent and over-reactive TRM cells in chronic lung conditions, lung TRM cell decrease may be beneficial to facilitate the complete recovery and return to tissue homeostasis after acute infection. Particularly, higher order mammals such as primates and humans are likely to encounter many episodes of pulmonary infection throughout their lifetime. Then, a steady decline of potentially dangerous TRM cells in the lung is likely an evolutionarily benefit to avoid chronic accumulation of tissue pathology in a critical organ following multiple pathogen exposures. Therefore, even though TRM cells are very powerful in protecting against viral infection and should be harnessed by future vaccine strategies, it is crucial to carefully calibrate their activities to avoid potential collateral damage.
As discussed, TRM cells in the lung have both protective and pathological functions. Currently, little is known on whether these activities are coupled or not, i.e. mediated by the same molecular pathways and/or the same cells. To this end, TRM cell heterogeneity is being increasingly appreciated196–198 with several studies revealing various subsets of respiratory TRM cells in mice and humans18,22,163. For instance, we found at least three TRM or TRM-like subsets in the BAL of COVID-19 convalescents100. Although CD69+ CD103− and CXCR6hi TRM-like cells exhibited higher levels of autoinflammatory features, the conventional CD69+ CD103+ TRM cells appeared to be less inflammatory based on gene expression. Therefore, we speculate that the pathological and protective functions of TRM cells could potentially be, at least in part, mediated by different TRM cell subsets. Furthermore, different molecules may also separate the protective and detrimental activities of TRM cells in the lungs. TNF can cause epithelial apoptosis and is considered to be a major factor contributing to fibroblast activation199,200. Furthermore, Granzyme K is highly expressed by age-associated T cells and can also activate fibroblasts201,202. However, it is less cytotoxic against virus-infected cells compared to other granzymes201,202. Thus, it is possible that constitutive TNF and Granzyme K expression by TRM cells preferentially leads to chronic tissue pathology rather than protection against secondary infection. In contrast, IFN-γ production by TRM cells mediates their protective function against secondary viral infection, although its effects in tissue injury or fibrosis have not been tested. Thus far, there is no solid experimental evidence supporting the uncoupling of protection and pathogenicity of TRM cells, but this would be an extremely important to study in the future. If this is true, it would be imperative to promote protective TRM subset function and/or effector molecule expression, while selective dampening the activity of pathological TRM subsets after primary viral infection or following vaccination. Alternatively, the determinant of TRM protective function versus pathogenic activity may be simply the time. TRM may exert their antiviral functions to provide beneficial effects at the early times after infection, whereas prolonged engagement of the same TRM cells or the “protective” molecules in TRM cells causes deleterious outcome to the host after the clearance of infectious virus. Further studies are required to examine all these potential possibilities.
7. Targeting TRM to resolve or attenuate immunopathology in respiratory tract
As discussed, exuberant TRM responses contribute significantly to various lung conditions. Thus, means targeting dysregulated TRM responses may be a promising strategy to mitigate the burden of lung diseases. In this section, we discuss potential approaches that may be employed to diminish exuberant respiratory TRM cell responses and/or their pathogenic activities, which can likely lead to the development of new immunomodulatory treatments for various lung conditions in the future.
7.1. Targeting chronic antigenic signaling in TRM cells
As discussed above, viral antigen persistence is likely a reason for the persistence and stimulation of exuberant TRM cell responses after viral pneumonia. Evidence for this notion comes from both animal models as well as recent studies in the COVID-19 pandemic. Thus, interventions that can target and eliminate viral reservoirs and/or antigen persistence may be a useful strategy in ameliorating pathology due to uncontrolled TRM responses. To this end, treatment with anti-viral drugs that can block viral replication may be efficacious in preventing chronic sequelae. Paxlovid is an FDA-approved anti-viral drug capable of reducing SARS-CoV-2 burden during primary infection. Treatment with nirmatrelvir, the anti-viral component of Paxlovid, within 5 days post symptoms onset has been shown to reduce the overall risk of the development of PASC including respiratory symptoms such as shortness of breath203,204. Currently, Paxlovid is still under active investigation with regards to its function in dampening PASC in a large cohort of COVID-19 patients203,204 (NCT05595369). Similarly, oseltamivir (Tamiflu), an anti-viral drug for influenza infection, may be employed to decrease the incidence of post influenza lung sequelae205,206. Of note, most of antiviral drugs function to inhibit active viral replication. Thus, they are likely most effective in eliminating antigen reservoirs and mitigating chronic sequelae upon administration during early disease, when the host harbors substantial levels of replicating virus. It is still unknown whether they may be used to reduce antigen deposition, TRM cell activation, and lung pathology after the clearance of infectious virus.
To this end, vaccination may be employed to target viral reservoirs, remnants and/or antigen persistence. Indeed, emerging evidence has suggested that COVID-19 convalescents with ongoing PASC may benefit from vaccination207,208. In this case, it is possible that vaccination-induced humoral and/or cellular immunity may accelerate the clearance of viral remnants and/or antigen in the tissue, thereby diminishing TRM cell activation and lung sequelae.
Other modalities that can dampen TCR signaling can also be potentially employed to curb TRM cell pathogenicity and lung sequelae. For instance, Teplizumab, an FDA-approved anti-CD3 antibody that can induce T cell anergy209,or abatacept (CTLA-4 Ig) may be potentially employed to inhibit TCR signaling in TRM cells to mitigate lung pathology210,211. Small molecule inhibitors targeting downstream TCR signaling may also be useful in diminishing exuberant TRM cell activity.
7.2. Potential strategies to target pathological TRM cell persistence and maintenance
Notch signaling has been shown to promote the maintenance of lung TRM cells after viral infection32,212. To this end, exuberant Notch signaling has been associated with chronic inflammatory diseases including lung cancer, asthma, and pulmonary fibrosis213,214. Thus, interventions targeting Notch signaling may attenuate immunopathology caused by lung TRM cells. AL101 is a potent and selective inhibitor of gamma secretase required for Notch signaling and has been granted by FDA as an orphan drug designation for the treatment of patients with adenoid cystic carcinoma (NCT03691207). Antibodies against Notch ligands may also be effective in diminishing exuberant TRM cell responses and associated lung disease.
As mentioned before, TGF-β signaling is required for the development and maintenance of lung TRM cell responses. We found that increased TGF-β expression in aged lung is responsible for the increased levels of TRM cells during aging. Additionally, TGF-β signaling is considered as the most important driver for pulmonary fibrosis215,216. Therefore, it is reasonable to assume that the blockade of TGF-β signaling may help to dampen pathogenic TRM responses and diminish lung pathology and fibrosis after viral infection. However, neutralization of TGF-β and/or inhibition of TGF-β downstream signaling has been shown to be extremely toxic to hosts due to the diverse roles of TGF-β in tissue homeostasis. To this end, therapies targeting specific TGF-β activation pathways, including the blockade of β6 or β8 integrin function may be less toxic but functional to diminish pathological TRM responses in lung viral sequelae or pulmonary fibrosis.
IL-21 is an important cytokine that can potently augment CD8+ T cell responses. IL-21 is usually derived from activated CD4+ T cells, especially TFH cells, and can mediate CD4+ T cell help for CD8+ T cell activation and/or maintenance, particularly in with the context of chronic antigen deposition217. Influenza infection induced the development of IL-21 producing TRH cells in the lungs, and IL-21 blockade selectively decreased the number of CD8+ TRM cells that received persistent antigenic signals in the lung after influenza infection21. Therefore, the blocking IL-21 activity in the respiratory tract may serve to selectively dampen pathological TRM cell responses, thereby diminishing chronic lung sequelae. Conversely, IL-21 has been shown to promote pathogenic CD8+ T cells after bleomycin administration. Furthermore, the blockade of IL-21 function ameliorated CD8+ T cell-mediated lung fibrosis after bleomycin administration in mice218. A human monoclonal antibody, avizakimab, that can inhibit IL-21 bioactivity, is currently in phase 2 clinical trials for systemic lupus erythematosus (SLE)219 (NCT03371251). It would be worth exploring the utility of IL-21 mAb to prevent chronic lung pathology including lung fibrosis following viral infection.
7.3. Targeting CD8+ TRM effector activities
CD8+ TRM cells highly express multiple effector molecules, which upon dysregulation could cause chronic inflammatory and/or fibrotic responses. Thus, interventions neutralizing the effector molecules released by CD8+ TRM cells and/or their downstream signaling may potentially mitigate CD8+ TRM-mediated lung pathology. TNF has been recognized as an important driver of tissue immunopathology during acute influenza and SARS-CoV-2 infection220–222. TNF is also considered to be an important contributor to pulmonary fibrosis and anti-TNF treatment has been investigated in several pulmonary disease models220. However, its role in TRM-mediated immunopathology has not been firmly established. Nevertheless, we found that a main function of PD-1 on TRM cells was to counter-balance TNF production, suggesting that excess production of TNF by CD8+ TRM cells may be detrimental after viral pneumonia. Furthermore, the CXCR6hi TRM-like cells identified in the BAL of COVID-19 convalescents, not only correlated with impaired lung function, but also produced high levels of TNF100, indicating TNF may contribute to adverse outcomes after acute COVID-19. To this end, TNF neutralizing monoclonal Ab such as adalimumab or infliximab may be used for the treatment of TRM-mediated lung pathology. Notably, TNF blockade may also exacerbate certain lung diseases and so further studies are required to definitively address the beneficial versus adverse effects of TNF in regulating TRM-mediated immunopathology223–225.
Even though IFN-γ per se exhibits little fibrogenic activities as discussed above, excessive production of IFN-γ has been associated with the development of lung injury in the late phase of SARS-CoV-1 infection226. T cell-derived IFN-γ and macrophage interactions have been implicated in driving the immunopathology after influenza and SARS-CoV-2 infection227,228. Moreover, IFN-γ was found to be persistently elevated in COVID-19 convalescents exhibiting PASC symptoms229,230, suggesting that IFN-γ neutralizing Abs, i.e. emapalumab-lzsg (Gamifant), which is a FDA approved monoclonal antibody, or its receptor blocking Abs maybe employed to block TRM-induced immunopathology. Alternatively, inhibitors blocking the IFN-γ downstream signaling such as JAK inhibitors including tofacitinib and baricitinib, may also be utilized. Granzyme K (GzmK) is a pro-inflammatory granzyme capable of stimulating inflammatory activities of other cell types231. In particular, GzmK has been shown to induce inflammatory cytokine secretion and proliferation of human lung fibroblasts232. Further, GzmK-derived from age-associated CD8+ T cells can promote the senescent phenotype of aged stromal cells233. We observed that GzmK is highly produced in the BAL CD69+ CD103− TRM cell subset of COVID-19 convalescents100,202. Thus, inhibitors or Abs that can neutralize GzmK activity may be promising to dampen TRM-mediated lung immunopathology. Additionally, inhibitors targeting other cytotoxic granzymes such as Granzyme B may also be utilized if studies implicate the cytotoxic activities of TRM cells in lung immunopathology.
7.4. Targeting CD4+ TRM pathogenic activities
As discussed above, CD4+ TRM cells could also potentially contribute to lung pathology in various chronic lung diseases as well as the post-viral sequelae. Currently, the cues required for the development and/or maintenance of respiratory CD4+ TRM cells are relatively less understood compared to CD8+ TRM cells. We expect that some of the interventions inhibiting CD8+ TRM activation and maintenance in the lungs, including the suppression of persistent antigenic signaling, may also be effective in mitigating persistent CD4+ TRM responses. Below we mainly focus on the potential countermeasures that can inhibit the effector activities of CD4+ TRM cells.
As stated, CD4+ TRM cells can be categorized based on their cytokine production. TRM17 cells, which produce IL-17, can potentially activate lung inflammatory and fibrogenic responses, largely dependent on the recruitment of neutrophils, as evidenced by several studies172,234–236. Furthermore, IL-17, TRM17 and neutrophils have been implicated in acute COVID-19 as well as PASC237–239. Additionally, GM-CSF production by TRM17 cells may contribute to the development of lung pathology in COVID-19172,240. Therefore, Abs such as tildrakizumab that can block IL-23, which is required for Th17 maintenance and activity, may be used to diminish TRM17-mediated lung pathology. Alternatively, IL-17 neutralizing Ab (such as secukinumab and ixekizumab), IL-17 receptor blocking Ab (such as brodalumab) and/or GM-CSF blocking Ab (such as Lenzilumab and mavrilimumab) could be potentially employed to mitigate TRM17-mediated immunopathology.
Besides TRM17 cells, TRM2 cells have been implicated in driving asthma related pathologies and lung fibrosis. Additionally, type 2 cytokines have been implicated in the development of pulmonary sequelae of viral pneumonia including COVID-19152,241–243. Thus, the blockade of TRM2 effector activities, i.e. inhibiting the function of TRM2-released cytokines, would likely be effective in dampening TRM2-mediated tissue pathology. To this end, IL-5 Ab, IL-13 Ab or IL-13 receptor Abs, have been approved for treating moderate to severe asthma, and may be further repurposed to treat other lung conditions including fibrosis and post viral sequelae. IL-9 is increasingly being appreciated as an important mediator of TRM2-mediated lung inflammation during chronic asthma119. Abs targeting IL-9 or IL-9 receptor may also be utilized for treating TRM2-associated pathology, particularly during acute exacerbation of chronic asthma. IL-9 is also upregulated in PASC patients and whether the inhibition of IL-9 activity can be employed to treat pulmonary sequelae warrants further investigation. Other means that can potentially mitigate TRM2 activity such as the blockade of IL-33 or ST-2 may also be employed to selectively dampen immunopathology caused by TRM2 cells.
8. Conclusion
Without a doubt, TRM cells are extremely powerful in terms of their ability to protect against respiratory viral infections and re-infections. However, there is increasing evidence indicating that detrimental role of uncontrolled TRM cell responses, either quantitatively or qualitatively, in the development of immunopathology and/or chronic lung diseases. Exuberant TRM cell activity has been reported in several human inflammatory and fibrotic diseases of the respiratory tract. The phenotype and functions of these cells are highly influenced by several parameters including age, antigen persistence, tissue milieu, and disease conditions. Emerging data also suggests that respiratory TRM cells exhibit remarkable phenotypic and functional heterogeneity, which may dictate their beneficial versus pathological functions. The burgeoning burden of chronic lung sequelae in COVID-19 convalescents over the course of the pandemic necessitates the rapid understanding of the mechanisms underlying TRM cell-mediated protection and immunopathology. With these insights, we will be able to develop new therapeutic avenues or vaccines, that harnessing the potent protective functions of TRM cells, while minimizing their pathological activity in the lungs.
Acknowledgements
This work is in part supported by the US National Institutes of Health grants AI147394, AG047156, AG069264, AI112844, and AI154598 to J.S. and by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HV22C0183 and HI22C1510) to Y.M.S. All figures were created with BioRender.com. We thank Harish Narasimhan and Kamyar Sharifi for their critical reading of the manuscript.
Conflict of interest statement
J.S. is a consultant for the Teneofour company and receives fundings from Icosavax, which do not directly involve with this work.
References
- 1.Braciale TJ, Sun J, Kim TS. Regulating the adaptive immune response to respiratory virus infection. Nat Rev Immunol. 2012;12(4):295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim TS, Sun J, Braciale TJ. T cell responses during influenza infection: getting and keeping control. Trends Immunol. 2011;32(5):225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang J, Srikiatkhachorn A, Braciale TJ. Visualization and characterization of respiratory syncytial virus F-specific CD8(+) T cells during experimental virus infection. J Immunol. 2001;167(8):4254–4260. [DOI] [PubMed] [Google Scholar]
- 4.Knudson CJ, Weiss KA, Hartwig SM, Varga SM. The pulmonary localization of virus-specific T lymphocytes is governed by the tissue tropism of infection. J Virol. 2014;88(16):9010–9016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flynn KJ, Belz GT, Altman JD, Ahmed R, Woodland DL, Doherty PC. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity. 1998;8(6):683–691. [DOI] [PubMed] [Google Scholar]
- 6.Sun J, Madan R, Karp CL, Braciale TJ. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat Med. 2009;15(3):277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benichou G, Gonzalez B, Marino J, Ayasoufi K, Valujskikh A. Role of Memory T Cells in Allograft Rejection and Tolerance. Front Immunol. 2017;8:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jameson SC, Masopust D. Understanding Subset Diversity in T Cell Memory. Immunity. 2018;48(2):214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts AD, Ely KH, Woodland DL. Differential contributions of central and effector memory T cells to recall responses. J Exp Med. 2005;202(1):123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–712. [DOI] [PubMed] [Google Scholar]
- 11.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291(5512):2413–2417. [DOI] [PubMed] [Google Scholar]
- 12.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10(5):524–530. [DOI] [PubMed] [Google Scholar]
- 13.Masopust D, Choo D, Vezys V, et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med. 2010;207(3):553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheuk S, Schlums H, Gallais Serezal I, et al. CD49a Expression Defines Tissue-Resident CD8(+) T Cells Poised for Cytotoxic Function in Human Skin. Immunity. 2017;46(2):287–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mackay LK, Minnich M, Kragten NA, et al. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science. 2016;352(6284):459–463. [DOI] [PubMed] [Google Scholar]
- 16.Mackay LK, Rahimpour A, Ma JZ, et al. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat Immunol. 2013;14(12):1294–1301. [DOI] [PubMed] [Google Scholar]
- 17.Strobl J, Gail LM, Kleissl L, et al. Human resident memory T cells exit the skin and mediate systemic Th2-driven inflammation. J Exp Med. 2021;218(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snyder ME, Farber DL. Human lung tissue resident memory T cells in health and disease. Curr Opin Immunol. 2019;59:101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson KG, Sung H, Skon CN, et al. Cutting edge: intravascular staining redefines lung CD8 T cell responses. J Immunol. 2012;189(6):2702–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu T, Hu Y, Lee YT, et al. Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J Leukoc Biol. 2014;95(2):215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Son YM, Cheon IS, Wu Y, et al. Tissue-resident CD4(+) T helper cells assist the development of protective respiratory B and CD8(+) T cell memory responses. Sci Immunol. 2021;6(55). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng MZM, Wakim LM. Tissue resident memory T cells in the respiratory tract. Mucosal Immunol. 2022;15(3):379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang N, Bevan MJ. Transforming growth factor-beta signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity. 2013;39(4):687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.FitzPatrick MEB, Provine NM, Garner LC, et al. Human intestinal tissue-resident memory T cells comprise transcriptionally and functionally distinct subsets. Cell Rep. 2021;34(3):108661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyu Y, Zhou Y, Shen J. An Overview of Tissue-Resident Memory T Cells in the Intestine: From Physiological Functions to Pathological Mechanisms. Front Immunol. 2022;13:912393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wakim LM, Woodward-Davis A, Bevan MJ. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc Natl Acad Sci U S A. 2010;107(42):17872–17879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Urban SL, Jensen IJ, Shan Q, et al. Peripherally induced brain tissue-resident memory CD8(+) T cells mediate protection against CNS infection. Nat Immunol. 2020;21(8):938–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuzen D, Arck PC, Thiele K. Tissue-resident immunity in the female and male reproductive tract. Semin Immunopathol. 2022;44(6):785–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shin H, Iwasaki A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature. 2012;491(7424):463–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matloubian M, Lo CG, Cinamon G, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427(6972):355–360. [DOI] [PubMed] [Google Scholar]
- 31.Shiow LR, Rosen DB, Brdickova N, et al. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440(7083):540–544. [DOI] [PubMed] [Google Scholar]
- 32.Hombrink P, Helbig C, Backer RA, et al. Programs for the persistence, vigilance and control of human CD8(+) lung-resident memory T cells. Nat Immunol. 2016;17(12):1467–1478. [DOI] [PubMed] [Google Scholar]
- 33.Gilchuk P, Hill TM, Guy C, et al. A Distinct Lung-Interstitium-Resident Memory CD8(+) T Cell Subset Confers Enhanced Protection to Lower Respiratory Tract Infection. Cell Rep. 2016;16(7):1800–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McMaster SR, Wilson JJ, Wang H, Kohlmeier JE. Airway-Resident Memory CD8 T Cells Provide Antigen-Specific Protection against Respiratory Virus Challenge through Rapid IFN-gamma Production. J Immunol. 2015;195(1):203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wein AN, McMaster SR, Takamura S, et al. CXCR6 regulates localization of tissue-resident memory CD8 T cells to the airways. J Exp Med. 2019;216(12):2748–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Z, Wang S, Goplen NP, et al. PD-1(hi) CD8(+) resident memory T cells balance immunity and fibrotic sequelae. Sci Immunol. 2019;4(36). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang J, Sun J. Lung tissue-resident memory T cells: the gatekeeper to respiratory viral (re)-infection. Curr Opin Immunol. 2022;80:102278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Behr FM, Kragten NAM, Wesselink TH, et al. Blimp-1 Rather Than Hobit Drives the Formation of Tissue-Resident Memory CD8(+) T Cells in the Lungs. Front Immunol. 2019;10:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skon CN, Lee JY, Anderson KG, Masopust D, Hogquist KA, Jameson SC. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat Immunol. 2013;14(12):1285–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yenyuwadee S, Sanchez-Trincado Lopez JL, Shah R, Rosato PC, Boussiotis VA. The evolving role of tissue-resident memory T cells in infections and cancer. Sci Adv. 2022;8(33):eabo5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ray SJ, Franki SN, Pierce RH, et al. The collagen binding alpha1beta1 integrin VLA-1 regulates CD8 T cell-mediated immune protection against heterologous influenza infection. Immunity. 2004;20(2):167–179. [DOI] [PubMed] [Google Scholar]
- 42.Zhang J, Lyu T, Cao Y, Feng H. Role of TCF-1 in differentiation, exhaustion, and memory of CD8(+) T cells: A review. FASEB J. 2021;35(5):e21549. [DOI] [PubMed] [Google Scholar]
- 43.Parga-Vidal L, Behr FM, Kragten NAM, et al. Hobit identifies tissue-resident memory T cell precursors that are regulated by Eomes. Sci Immunol. 2021;6(62). [DOI] [PubMed] [Google Scholar]
- 44.Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol. 2013;31:137–161. [DOI] [PubMed] [Google Scholar]
- 45.Iborra S, Martinez-Lopez M, Khouili SC, et al. Optimal Generation of Tissue-Resident but Not Circulating Memory T Cells during Viral Infection Requires Crosspriming by DNGR-1(+) Dendritic Cells. Immunity. 2016;45(4):847–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takamura S, Yagi H, Hakata Y, et al. Specific niches for lung-resident memory CD8+ T cells at the site of tissue regeneration enable CD69-independent maintenance. J Exp Med. 2016;213(13):3057–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McMaster SR, Wein AN, Dunbar PR, et al. Pulmonary antigen encounter regulates the establishment of tissue-resident CD8 memory T cells in the lung airways and parenchyma. Mucosal Immunol. 2018;11(4):1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pizzolla A, Nguyen THO, Smith JM, et al. Resident memory CD8(+) T cells in the upper respiratory tract prevent pulmonary influenza virus infection. Sci Immunol. 2017;2(12). [DOI] [PubMed] [Google Scholar]
- 49.Wakim LM, Smith J, Caminschi I, Lahoud MH, Villadangos JA. Antibody-targeted vaccination to lung dendritic cells generates tissue-resident memory CD8 T cells that are highly protective against influenza virus infection. Mucosal Immunol. 2015;8(5):1060–1071. [DOI] [PubMed] [Google Scholar]
- 50.Mackay LK, Wynne-Jones E, Freestone D, et al. T-box Transcription Factors Combine with the Cytokines TGF-beta and IL-15 to Control Tissue-Resident Memory T Cell Fate. Immunity. 2015;43(6):1101–1111. [DOI] [PubMed] [Google Scholar]
- 51.Goplen NP, Wu Y, Son YM, et al. Tissue-resident CD8(+) T cells drive age-associated chronic lung sequelae after viral pneumonia. Sci Immunol. 2020;5(53). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu CI, Becker C, Wang Y, et al. Human CD1c+ dendritic cells drive the differentiation of CD103+ CD8+ mucosal effector T cells via the cytokine TGF-beta. Immunity. 2013;38(4):818–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thompson EA, Darrah PA, Foulds KE, et al. Monocytes Acquire the Ability to Prime Tissue-Resident T Cells via IL-10-Mediated TGF-beta Release. Cell Rep. 2019;28(5):1127–1135 e1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nelson CE, Foreman TW, Kauffman KD, et al. IL-10 suppresses T cell expansion while promoting tissue-resident memory cell formation during SARS-CoV-2 infection in rhesus macaques. bioRxiv. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun J, Cardani A, Sharma AK, et al. Autocrine regulation of pulmonary inflammation by effector T-cell derived IL-10 during infection with respiratory syncytial virus. PLoS Pathog. 2011;7(8):e1002173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schenkel JM, Fraser KA, Casey KA, et al. IL-15-Independent Maintenance of Tissue-Resident and Boosted Effector Memory CD8 T Cells. J Immunol. 2016;196(9):3920–3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jarjour NN, Wanhainen KM, Peng C, et al. Responsiveness to interleukin-15 therapy is shared between tissue-resident and circulating memory CD8(+) T cell subsets. Proc Natl Acad Sci U S A. 2022;119(43):e2209021119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Verbist KC, Cole CJ, Field MB, Klonowski KD. A role for IL-15 in the migration of effector CD8 T cells to the lung airways following influenza infection. J Immunol. 2011;186(1):174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim TS, Braciale TJ. Respiratory dendritic cell subsets differ in their capacity to support the induction of virus-specific cytotoxic CD8+ T cell responses. PLoS One. 2009;4(1):e4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Son YM, Sun J. Co-Ordination of Mucosal B Cell and CD8 T Cell Memory by Tissue-Resident CD4 Helper T Cells. Cells. 2021;10(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Turner DL, Bickham KL, Thome JJ, et al. Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol. 2014;7(3):501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Teijaro JR, Turner D, Pham Q, Wherry EJ, Lefrancois L, Farber DL. Cutting edge: Tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J Immunol. 2011;187(11):5510–5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Swarnalekha N, Schreiner D, Litzler LC, et al. T resident helper cells promote humoral responses in the lung. Sci Immunol. 2021;6(55). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chapman TJ, Topham DJ. Identification of a unique population of tissue-memory CD4+ T cells in the airways after influenza infection that is dependent on the integrin VLA-1. J Immunol. 2010;184(7):3841–3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rahimi RA, Nepal K, Cetinbas M, Sadreyev RI, Luster AD. Distinct functions of tissue-resident and circulating memory Th2 cells in allergic airway disease. J Exp Med. 2020;217(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Steinfelder S, Rausch S, Michael D, Kuhl AA, Hartmann S. Intestinal helminth infection induces highly functional resident memory CD4(+) T cells in mice. Eur J Immunol. 2017;47(2):353–363. [DOI] [PubMed] [Google Scholar]
- 67.Amezcua Vesely MC, Pallis P, Bielecki P, et al. Effector T(H)17 Cells Give Rise to Long-Lived T(RM) Cells that Are Essential for an Immediate Response against Bacterial Infection. Cell. 2019;178(5):1176–1188 e1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ogongo P, Tezera LB, Ardain A, et al. Tissue-resident-like CD4+ T cells secreting IL-17 control Mycobacterium tuberculosis in the human lung. J Clin Invest. 2021;131(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ren HM, Kolawole EM, Ren M, et al. IL-21 from high-affinity CD4 T cells drives differentiation of brain-resident CD8 T cells during persistent viral infection. Sci Immunol. 2020;5(51). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wild K, Smits M, Killmer S, et al. Pre-existing immunity and vaccine history determine hemagglutinin-specific CD4 T cell and IgG response following seasonal influenza vaccination. Nat Commun. 2021;12(1):6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Patel MM, York IA, Monto AS, Thompson MG, Fry AM. Immune-mediated attenuation of influenza illness after infection: opportunities and challenges. Lancet Microbe. 2021;2(12):e715–e725. [DOI] [PubMed] [Google Scholar]
- 72.Kingstad-Bakke B, Lee W, Chandrasekar SS, et al. Vaccine-induced systemic and mucosal T cell immunity to SARS-CoV-2 viral variants. Proc Natl Acad Sci U S A. 2022;119(20):e2118312119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McMahan K, Yu J, Mercado NB, et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature. 2021;590(7847):630–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mazur NI, Terstappen J, Baral R, et al. Respiratory syncytial virus prevention within reach: the vaccine and monoclonal antibody landscape. Lancet Infect Dis. 2023;23(1):e2–e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kinnear E, Lambert L, McDonald JU, Cheeseman HM, Caproni LJ, Tregoning JS. Airway T cells protect against RSV infection in the absence of antibody. Mucosal Immunol. 2018;11(1):249–256. [DOI] [PubMed] [Google Scholar]
- 76.Morabito KM, Ruckwardt TR, Redwood AJ, Moin SM, Price DA, Graham BS. Intranasal administration of RSV antigen-expressing MCMV elicits robust tissue-resident effector and effector memory CD8+ T cells in the lung. Mucosal Immunol. 2017;10(2):545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Varese A, Nakawesi J, Farias A, et al. Type I interferons and MAVS signaling are necessary for tissue resident memory CD8+ T cell responses to RSV infection. PLoS Pathog. 2022;18(2):e1010272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ostler T, Hussell T, Surh CD, Openshaw P, Ehl S. Long-term persistence and reactivation of T cell memory in the lung of mice infected with respiratory syncytial virus. Eur J Immunol. 2001;31(9):2574–2582. [DOI] [PubMed] [Google Scholar]
- 79.Luangrath MA, Schmidt ME, Hartwig SM, Varga SM. Tissue-Resident Memory T Cells in the Lungs Protect against Acute Respiratory Syncytial Virus Infection. Immunohorizons. 2021;5(2):59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jozwik A, Habibi MS, Paras A, et al. RSV-specific airway resident memory CD8+ T cells and differential disease severity after experimental human infection. Nat Commun. 2015;6:10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rolfes MA, Foppa IM, Garg S, et al. Annual estimates of the burden of seasonal influenza in the United States: A tool for strengthening influenza surveillance and preparedness. Influenza Other Respir Viruses. 2018;12(1):132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Myers MA, Smith AP, Lane LC, et al. Dynamically linking influenza virus infection kinetics, lung injury, inflammation, and disease severity. Elife. 2021;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cerwenka A, Morgan TM, Dutton RW. Naive, effector, and memory CD8 T cells in protection against pulmonary influenza virus infection: homing properties rather than initial frequencies are crucial. J Immunol. 1999;163(10):5535–5543. [PubMed] [Google Scholar]
- 84.Pizzolla A, Nguyen TH, Sant S, et al. Influenza-specific lung-resident memory T cells are proliferative and polyfunctional and maintain diverse TCR profiles. J Clin Invest. 2018;128(2):721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Purwar R, Campbell J, Murphy G, Richards WG, Clark RA, Kupper TS. Resident memory T cells (T(RM)) are abundant in human lung: diversity, function, and antigen specificity. PLoS One. 2011;6(1):e16245.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang M, Li N, He Y, Shi T, Jie Z. Pulmonary resident memory T cells in respiratory virus infection and their inspiration on therapeutic strategies. Front Immunol. 2022;13:943331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li C, Zhu B, Son YM, et al. The Transcription Factor Bhlhe40 Programs Mitochondrial Regulation of Resident CD8(+) T Cell Fitness and Functionality. Immunity. 2019;51(3):491–507 e497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zens KD, Chen JK, Farber DL. Vaccine-generated lung tissue-resident memory T cells provide heterosubtypic protection to influenza infection. JCI Insight. 2016;1(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu X, Wu P, Shen Y, Jiang X, Xu F. CD8(+) Resident Memory T Cells and Viral Infection. Front Immunol. 2018;9:2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Teijaro JR, Verhoeven D, Page CA, Turner D, Farber DL. Memory CD4 T cells direct protective responses to influenza virus in the lungs through helper-independent mechanisms. J Virol. 2010;84(18):9217–9226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jiang L, Liu L, Zhang M, et al. Prompt Antiviral Action of Pulmonary CD8+ T(RM) Cells Is Mediated by Rapid IFN-gamma Induction and Its Downstream ISGs in the Lung. Front Immunol. 2022;13:839455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Low JS, Farsakoglu Y, Amezcua Vesely MC, et al. Tissue-resident memory T cell reactivation by diverse antigen-presenting cells imparts distinct functional responses. J Exp Med. 2020;217(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kohlmeier JE, Cookenham T, Roberts AD, Miller SC, Woodland DL. Type I interferons regulate cytolytic activity of memory CD8(+) T cells in the lung airways during respiratory virus challenge. Immunity. 2010;33(1):96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wakim LM, Gupta N, Mintern JD, Villadangos JA. Enhanced survival of lung tissue-resident memory CD8(+) T cells during infection with influenza virus due to selective expression of IFITM3. Nat Immunol. 2013;14(3):238–245. [DOI] [PubMed] [Google Scholar]
- 95.Pizzolla A, Wakim LM. Memory T Cell Dynamics in the Lung during Influenza Virus Infection. J Immunol. 2019;202(2):374–381. [DOI] [PubMed] [Google Scholar]
- 96.Collaborators C-EM. Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020–21. Lancet. 2022;399(10334):1513–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liao M, Liu Y, Yuan J, et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med. 2020;26(6):842–844. [DOI] [PubMed] [Google Scholar]
- 98.Wauters E, Van Mol P, Garg AD, et al. Discriminating mild from critical COVID-19 by innate and adaptive immune single-cell profiling of bronchoalveolar lavages. Cell Res. 2021;31(3):272–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Poon MML, Rybkina K, Kato Y, et al. SARS-CoV-2 infection generates tissue-localized immunological memory in humans. Sci Immunol. 2021;6(65):eabl9105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cheon IS, Li C, Son YM, et al. Immune signatures underlying post-acute COVID-19 lung sequelae. Sci Immunol. 2021;6(65):eabk1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhuang Z, Lai X, Sun J, et al. Mapping and role of T cell response in SARS-CoV-2-infected mice. J Exp Med. 2021;218(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Joag V, Wijeyesinghe S, Stolley JM, et al. Cutting Edge: Mouse SARS-CoV-2 Epitope Reveals Infection and Vaccine-Elicited CD8 T Cell Responses. J Immunol. 2021;206(5):931–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Roberts LM, Jessop F, Wehrly TD, Bosio CM. Cutting Edge: Lung-Resident T Cells Elicited by SARS-CoV-2 Do Not Mediate Protection against Secondary Infection. J Immunol. 2021;207(10):2399–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tang J, Zeng C, Cox TM, et al. Respiratory mucosal immunity against SARS-CoV-2 after mRNA vaccination. Sci Immunol. 2022;7(76):eadd4853.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mao T, Israelow B, Pena-Hernandez MA, et al. Unadjuvanted intranasal spike vaccine elicits protective mucosal immunity against sarbecoviruses. Science. 2022;378(6622):eabo2523.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Smith NM, Wasserman GA, Coleman FT, et al. Regionally compartmentalized resident memory T cells mediate naturally acquired protection against pneumococcal pneumonia. Mucosal Immunol. 2018;11(1):220–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.O’Hara JM, Redhu NS, Cheung E, et al. Generation of protective pneumococcal-specific nasal resident memory CD4(+) T cells via parenteral immunization. Mucosal Immunol. 2020;13(1):172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sakai S, Kauffman KD, Schenkel JM, et al. Cutting edge: control of Mycobacterium tuberculosis infection by a subset of lung parenchyma-homing CD4 T cells. J Immunol. 2014;192(7):2965–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dubois MF, Mezger V, Morange M, Ferrieux C, Lebon P, Bensaude O. Regulation of the heat-shock response by interferon in mouse L cells. J Cell Physiol. 1988;137(1):102–109. [DOI] [PubMed] [Google Scholar]
- 110.Iwanaga N, Chen K, Yang H, et al. Vaccine-driven lung TRM cells provide immunity against Klebsiella via fibroblast IL-17R signaling. Sci Immunol. 2021;6(63):eabf1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ichikawa T, Hirahara K, Kokubo K, et al. CD103(hi) T(reg) cells constrain lung fibrosis induced by CD103(lo) tissue-resident pathogenic CD4 T cells. Nat Immunol. 2019;20(11):1469–1480. [DOI] [PubMed] [Google Scholar]
- 112.Ueno K, Urai M, Sadamoto S, et al. A dendritic cell-based systemic vaccine induces long-lived lung-resident memory Th17 cells and ameliorates pulmonary mycosis. Mucosal Immunol. 2019;12(1):265–276. [DOI] [PubMed] [Google Scholar]
- 113.Holgate ST, Polosa R. Treatment strategies for allergy and asthma. Nat Rev Immunol. 2008;8(3):218–230. [DOI] [PubMed] [Google Scholar]
- 114.Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol. 2004;22:789–815. [DOI] [PubMed] [Google Scholar]
- 115.Lloyd CM, Hessel EM Functions of T cells in asthma: more than just T(H)2 cells. Nat Rev Immunol. 2010;10(12):838–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hondowicz BD, An D, Schenkel JM, et al. Interleukin-2-Dependent Allergen-Specific Tissue-Resident Memory Cells Drive Asthma. Immunity. 2016;44(1):155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yeon SM, Halim L, Chandele A, et al. IL-7 plays a critical role for the homeostasis of allergen-specific memory CD4 T cells in the lung and airways. Sci Rep. 2017;7(1):11155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Turner DL, Goldklang M, Cvetkovski F, et al. Biased Generation and In Situ Activation of Lung Tissue-Resident Memory CD4 T Cells in the Pathogenesis of Allergic Asthma. J Immunol. 2018;200(5):1561–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ulrich BJ, Kharwadkar R, Chu M, et al. Allergic airway recall responses require IL-9 from resident memory CD4(+) T cells. Sci Immunol. 2022;7(69):eabg9296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Smyth LJ, Eustace A, Kolsum U, Blaikely J, Singh D. Increased airway T regulatory cells in asthmatic subjects. Chest. 2010;138(4):905–912. [DOI] [PubMed] [Google Scholar]
- 121.Seumois G, Ramirez-Suastegui C, Schmiedel BJ, et al. Single-cell transcriptomic analysis of allergen-specific T cells in allergy and asthma. Sci Immunol. 2020;5(48). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wambre E, Bajzik V, DeLong JH, et al. A phenotypically and functionally distinct human T(H)2 cell subpopulation is associated with allergic disorders. Sci Transl Med. 2017;9(401). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Desai O, Winkler J, Minasyan M, Herzog EL. The Role of Immune and Inflammatory Cells in Idiopathic Pulmonary Fibrosis. Front Med (Lausanne). 2018;5:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wynn TA. Integrating mechanisms of pulmonary fibrosis. J Exp Med. 2011;208(7):1339–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Richeldi L, Collard HR, Jones MG. Idiopathic pulmonary fibrosis. Lancet. 2017;389(10082):1941–1952. [DOI] [PubMed] [Google Scholar]
- 126.Shenderov K, Collins SL, Powell JD, Horton MR. Immune dysregulation as a driver of idiopathic pulmonary fibrosis. J Clin Invest. 2021;131(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Paun A, Bergeron ME, Haston CK. The Th1/Th17 balance dictates the fibrosis response in murine radiation-induced lung disease. Sci Rep. 2017;7(1):11586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Braun RK, Foerster M, Grahmann PR, Haefner D, Workalemahu G, Kroegel C. Phenotypic and molecular characterization of CD103+ CD4+ T cells in bronchoalveolar lavage from patients with interstitial lung diseases. Cytometry B Clin Cytom. 2003;54(1):19–27. [DOI] [PubMed] [Google Scholar]
- 129.Szabo PA, Miron M, Farber DL. Location, location, location: Tissue resident memory T cells in mice and humans. Sci Immunol. 2019;4(34). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sikkeland LIB, Qiao SW, Ueland T, et al. Lung CD4+ T-cells in patients with lung fibrosis produce pro-fibrotic interleukin-13 together with interferon-gamma. Eur Respir J. 2021;57(3). [DOI] [PubMed] [Google Scholar]
- 131.Serezani APM, Pascoalino BD, Bazzano JMR, et al. Multiplatform Single-Cell Analysis Identifies Immune Cell Types Enhanced in Pulmonary Fibrosis. Am J Respir Cell Mol Biol. 2022;67(1):50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Forsslund H, Mikko M, Karimi R, et al. Distribution of T-cell subsets in BAL fluid of patients with mild to moderate COPD depends on current smoking status and not airway obstruction. Chest. 2014;145(4):711–722. [DOI] [PubMed] [Google Scholar]
- 133.Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2016;138(1):16–27. [DOI] [PubMed] [Google Scholar]
- 134.Richmond B, Serezani A, Schaff J, Blackwell T. Tissue resident memory T cells are increased in the lungs of COPD patients. ERJ Open Research. 2022;8(suppl 8):247. [Google Scholar]
- 135.Maeno T, Houghton AM, Quintero PA, Grumelli S, Owen CA, Shapiro SD. CD8+ T Cells are required for inflammation and destruction in cigarette smoke-induced emphysema in mice. J Immunol. 2007;178(12):8090–8096. [DOI] [PubMed] [Google Scholar]
- 136.Tsuyusaki J, Kuroda F, Kasuya Y, et al. Cigarette smoke-induced pulmonary inflammation is attenuated in CD69-deficient mice. J Recept Signal Transduct Res. 2011;31(6):434–439. [DOI] [PubMed] [Google Scholar]
- 137.Wang C, Hyams B, Allen NC, et al. Dysregulated lung stroma drives emphysema exacerbation by potentiating resident lymphocytes to suppress an epithelial stem cell reservoir. Immunity. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bos S, Vos R, Van Raemdonck DE, Verleden GM. Survival in adult lung transplantation: where are we in 2020? Curr Opin Organ Transplant. 2020;25(3):268–273. [DOI] [PubMed] [Google Scholar]
- 139.Studer SM, Levy RD, McNeil K, Orens JB. Lung transplant outcomes: a review of survival, graft function, physiology, health-related quality of life and cost-effectiveness. Eur Respir J. 2004;24(4):674–685. [DOI] [PubMed] [Google Scholar]
- 140.Martinu T, Chen DF, Palmer SM. Acute rejection and humoral sensitization in lung transplant recipients. Proc Am Thorac Soc. 2009;6(1):54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cheng L, Guo H, Qiao X, et al. T cell immunohistochemistry refines lung transplant acute rejection diagnosis and grading. Diagn Pathol. 2013;8:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Greer M, Werlein C, Jonigk D. Surveillance for acute cellular rejection after lung transplantation. Ann Transl Med. 2020;8(6):410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Beura LK, Rosato PC, Masopust D. Implications of Resident Memory T Cells for Transplantation. Am J Transplant. 2017;17(5):1167–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Miller CL O JM, Allan JS, Madsen JC. Novel approaches for long-term lung transplant survival. Front Immunol. 2022;13:931251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Abou-Daya KI, Tieu R, Zhao D, et al. Resident memory T cells form during persistent antigen exposure leading to allograft rejection. Sci Immunol. 2021;6(57). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ding R, Li B, Muthukumar T, et al. CD103 mRNA levels in urinary cells predict acute rejection of renal allografts. Transplantation. 2003;75(8):1307–1312. [DOI] [PubMed] [Google Scholar]
- 147.Snyder ME, Finlayson MO, Connors TJ, et al. Generation and persistence of human tissue-resident memory T cells in lung transplantation. Sci Immunol. 2019;4(33). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Snyder ME, Moghbeli K, Bondonese A, et al. Modulation of tissue resident memory T cells by glucocorticoids after acute cellular rejection in lung transplantation. J Exp Med. 2022;219(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pipeling MR, West EE, Osborne CM, et al. Differential CMV-specific CD8+ effector T cell responses in the lung allograft predominate over the blood during human primary infection. J Immunol. 2008;181(1):546–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Stuve P, Hehlgans T, Feuerer M. Alloreactive Tissue-resident Memory T Cells in Solid Organ Transplantation: Do They Light the Fire? Transplantation. 2022;106(10):1890–1891. [DOI] [PubMed] [Google Scholar]
- 151.Giraud-Gatineau A, Colson P, Jimeno MT, et al. Comparison of mortality associated with respiratory viral infections between December 2019 and March 2020 with that of the previous year in Southeastern France. Int J Infect Dis. 2020;96:154–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Keeler SP, Agapov EV, Hinojosa ME, Letvin AN, Wu K, Holtzman MJ. Influenza A Virus Infection Causes Chronic Lung Disease Linked to Sites of Active Viral RNA Remnants. J Immunol. 2018;201(8):2354–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kanegai CM, Xi Y, Donne ML, et al. Persistent Pathology in Influenza-Infected Mouse Lungs. Am J Respir Cell Mol Biol. 2016;55(4):613–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Luyt CE, Combes A, Becquemin MH, et al. Long-term outcomes of pandemic 2009 influenza A(H1N1)-associated severe ARDS. Chest. 2012;142(3):583–592. [DOI] [PubMed] [Google Scholar]
- 155.Liu W, Peng L, Liu H, Hua S. Pulmonary Function and Clinical Manifestations of Patients Infected with Mild Influenza A Virus Subtype H1N1: A One-Year Follow-Up. PLoS One. 2015;10(7):e0133698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Narasimhan H, Wu Y, Goplen NP, Sun J. Immune determinants of chronic sequelae after respiratory viral infection. Sci Immunol. 2022;7(73):eabm7996. [DOI] [PubMed] [Google Scholar]
- 157.Wei X, Narasimhan H, Zhu B, Sun J. Host Recovery from Respiratory Viral Infection. Annu Rev Immunol. 2023. [DOI] [PubMed] [Google Scholar]
- 158.Behr FM, Chuwonpad A, Stark R, van Gisbergen K. Armed and Ready: Transcriptional Regulation of Tissue-Resident Memory CD8 T Cells. Front Immunol. 2018;9:1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ballesteros-Tato A, Leon B, Lee BO, Lund FE, Randall TD. Epitope-specific regulation of memory programming by differential duration of antigen presentation to influenza-specific CD8(+) T cells. Immunity. 2014;41(1):127–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Qiao J, Zhang M, Bi J, et al. Pulmonary fibrosis induced by H5N1 viral infection in mice. Respir Res. 2009;10(1):107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Barr J, Gentile ME, Lee S, et al. Injury-induced pulmonary tuft cells are heterogenous, arise independent of key Type 2 cytokines, and are dispensable for dysplastic repair. Elife. 2022;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Huang S, Goplen NP, Zhu B, et al. Macrophage PPAR-gamma suppresses long-term lung fibrotic sequelae following acute influenza infection. PLoS One. 2019;14(10):e0223430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yuan R, Yu J, Jiao Z, et al. The Roles of Tissue-Resident Memory T Cells in Lung Diseases. Front Immunol. 2021;12:710375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Weisberg SP, Carpenter DJ, Chait M, et al. Tissue-Resident Memory T Cells Mediate Immune Homeostasis in the Human Pancreas through the PD-1/PD-L1 Pathway. Cell Rep. 2019;29(12):3916–3932 e3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15(8):486–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682–687. [DOI] [PubMed] [Google Scholar]
- 167.Zhang Q, Tang L, Zhou Y, He W, Li W. Immune Checkpoint Inhibitor-Associated Pneumonitis in Non-Small Cell Lung Cancer: Current Understanding in Characteristics, Diagnosis, and Management. Front Immunol. 2021;12:663986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wass RE, Lang D, Horner A, Lamprecht B. Checkpoint inhibitor pneumonitis: Short review of literature and case report. memo - Magazine of European Medical Oncology. 2022;15(1):62–66. [Google Scholar]
- 169.Kumar BV, Ma W, Miron M, et al. Human Tissue-Resident Memory T Cells Are Defined by Core Transcriptional and Functional Signatures in Lymphoid and Mucosal Sites. Cell Rep. 2017;20(12):2921–2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mansell V, Hall Dykgraaf S, Kidd M, Goodyear-Smith F. Long COVID and older people. Lancet Healthy Longev. 2022;3(12):e849–e854. [DOI] [PubMed] [Google Scholar]
- 171.Bull-Otterson L, Baca S, Saydah S, et al. Post–COVID Conditions Among Adult COVID-19 Survivors Aged 18–64 and ≥65 Years — United States, March 2020–November 2021. In: MMWR Morb Mortal Wkly Rep. Vol 71.2022:713–717. [Google Scholar]
- 172.Zhao Y, Kilian C, Turner JE, et al. Clonal expansion and activation of tissue-resident memory-like Th17 cells expressing GM-CSF in the lungs of severe COVID-19 patients. Sci Immunol. 2021;6(56). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Stockinger B, Omenetti S. The dichotomous nature of T helper 17 cells. Nat Rev Immunol. 2017;17(9):535–544. [DOI] [PubMed] [Google Scholar]
- 174.Meyer Zu Horste G, Wu C, Wang C, et al. RBPJ Controls Development of Pathogenic Th17 Cells by Regulating IL-23 Receptor Expression. Cell Rep. 2016;16(2):392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Grau-Exposito J, Sanchez-Gaona N, Massana N, et al. Peripheral and lung resident memory T cell responses against SARS-CoV-2. Nat Commun. 2021;12(1):3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Dudek M, Pfister D, Donakonda S, et al. Auto-aggressive CXCR6(+) CD8 T cells cause liver immune pathology in NASH. Nature. 2021;592(7854):444–449. [DOI] [PubMed] [Google Scholar]
- 177.Dai Y, Wang J, Jeong HH, Chen W, Jia P, Zhao Z. Association of CXCR6 with COVID-19 severity: delineating the host genetic factors in transcriptomic regulation. Hum Genet. 2021;140(9):1313–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Vijayakumar B, Boustani K, Ogger PP, et al. Immuno-proteomic profiling reveals aberrant immune cell regulation in the airways of individuals with ongoing post-COVID-19 respiratory disease. Immunity. 2022;55(3):542–556 e545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Doan Ngoc TM, Tilly G, Danger R, et al. Effector Memory-Expressing CD45RA (TEMRA) CD8. J Am Soc Nephrol. 2022;33(12):2211–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Poon MML, Caron DP, Wang Z, et al. Tissue adaptation and clonal segregation of human memory T cells in barrier sites. Nat Immunol. 2023;24(2):309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sharif R Overview of idiopathic pulmonary fibrosis (IPF) and evidence-based guidelines. Am J Manag Care. 2017;23(11 Suppl):S176–S182. [PubMed] [Google Scholar]
- 182.Berard M, Tough DF. Qualitative differences between naive and memory T cells. Immunology. 2002;106(2):127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2(4):251–262. [DOI] [PubMed] [Google Scholar]
- 184.Kim TS, Hufford MM, Sun J, Fu YX, Braciale TJ. Antigen persistence and the control of local T cell memory by migrant respiratory dendritic cells after acute virus infection. J Exp Med. 2010;207(6):1161–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yao S, Jiang L, Moser EK, et al. Control of pathogenic effector T-cell activities in situ by PD-L1 expression on respiratory inflammatory dendritic cells during respiratory syncytial virus infection. Mucosal Immunol. 2015;8(4):746–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Stein SR, Ramelli SC, Grazioli A, et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature. 2022;612(7941):758–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Damoiseaux J, Dotan A, Fritzler MJ, et al. Autoantibodies and SARS-CoV2 infection: The spectrum from association to clinical implication: Report of the 15th Dresden Symposium on Autoantibodies. Autoimmun Rev. 2022;21(3):103012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wang EY, Mao T, Klein J, et al. Diverse functional autoantibodies in patients with COVID-19. Nature. 2021;595(7866):283–288. [DOI] [PubMed] [Google Scholar]
- 189.Lee H, Jeong S, Shin EC. Significance of bystander T cell activation in microbial infection. Nat Immunol. 2022;23(1):13–22. [DOI] [PubMed] [Google Scholar]
- 190.Holz LE, Prier JE, Freestone D, et al. CD8(+) T Cell Activation Leads to Constitutive Formation of Liver Tissue-Resident Memory T Cells that Seed a Large and Flexible Niche in the Liver. Cell Rep. 2018;25(1):68–79 e64. [DOI] [PubMed] [Google Scholar]
- 191.Koda Y, Teratani T, Chu PS, et al. CD8(+) tissue-resident memory T cells promote liver fibrosis resolution by inducing apoptosis of hepatic stellate cells. Nat Commun. 2021;12(1):4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Mackay LK, Stock AT, Ma JZ, et al. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc Natl Acad Sci U S A. 2012;109(18):7037–7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Bartsch LM, Damasio MPS, Subudhi S, Drescher HK. Tissue-Resident Memory T Cells in the Liver-Unique Characteristics of Local Specialists. Cells. 2020;9(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Bergsbaken T, Bevan MJ. Proinflammatory microenvironments within the intestine regulate the differentiation of tissue-resident CD8(+) T cells responding to infection. Nat Immunol. 2015;16(4):406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Slutter B, Van Braeckel-Budimir N, Abboud G, Varga SM, Salek-Ardakani S, Harty JT. Dynamics of influenza-induced lung-resident memory T cells underlie waning heterosubtypic immunity. Sci Immunol. 2017;2(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Konjar S, Ficht X, Iannacone M, Veldhoen M. Heterogeneity of tissue resident memory T cells. Immunol Lett. 2022;245:1–7. [DOI] [PubMed] [Google Scholar]
- 197.Milner JJ, Toma C, He Z, et al. Heterogenous Populations of Tissue-Resident CD8(+) T Cells Are Generated in Response to Infection and Malignancy. Immunity. 2020;52(5):808–824 e807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Szabo PA, Levitin HM, Miron M, et al. Single-cell transcriptomics of human T cells reveals tissue and activation signatures in health and disease. Nat Commun. 2019;10(1):4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Pilling D, Vakil V, Cox N, Gomer RH. TNF-alpha-stimulated fibroblasts secrete lumican to promote fibrocyte differentiation. Proc Natl Acad Sci U S A. 2015;112(38):11929–11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Drakopanagiotakis F, Xifteri A, Polychronopoulos V, Bouros D. Apoptosis in lung injury and fibrosis. Eur Respir J. 2008;32(6):1631–1638. [DOI] [PubMed] [Google Scholar]
- 201.Mogilenko DA, Shpynov O, Andhey PS, et al. Comprehensive Profiling of an Aging Immune System Reveals Clonal GZMK(+) CD8(+) T Cells as Conserved Hallmark of Inflammaging. Immunity. 2021;54(1):99–115 e112. [DOI] [PubMed] [Google Scholar]
- 202.Jonsson AH, Zhang F, Dunlap G, et al. Granzyme K(+) CD8 T cells form a core population in inflamed human tissue. Sci Transl Med. 2022;14(649):eabo0686.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Xie Y, Choi T, Al-Aly Z. Nirmatrelvir and the Risk of Post-Acute Sequelae of COVID-19. medRxiv. 2022:2022.2011.2003.22281783. [Google Scholar]
- 204.Peluso MJ, Anglin K, Durstenfeld MS, et al. Effect of Oral Nirmatrelvir on Long COVID Symptoms: 4 Cases and Rationale for Systematic Studies. Pathog Immun. 2022;7(1):95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Marois I, Cloutier A, Garneau E, Lesur O, Richter MV. The administration of oseltamivir results in reduced effector and memory CD8+ T cell responses to influenza and affects protective immunity. FASEB J. 2015;29(3):973–987. [DOI] [PubMed] [Google Scholar]
- 206.Kaiser L, Wat C, Mills T, Mahoney P, Ward P, Hayden F. Impact of oseltamivir treatment on influenza-related lower respiratory tract complications and hospitalizations. Arch Intern Med. 2003;163(14):1667–1672. [DOI] [PubMed] [Google Scholar]
- 207.Zisis SN, Durieux JC, Mouchati C, Perez JA, McComsey GA. The Protective Effect of Coronavirus Disease 2019 (COVID-19) Vaccination on Postacute Sequelae of COVID-19: A Multicenter Study From a Large National Health Research Network. Open Forum Infect Dis. 2022;9(7):ofac228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Al-Aly Z, Bowe B, Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med. 2022;28(7):1461–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Herold KC, Bundy BN, Long SA, et al. An Anti-CD3 Antibody, Teplizumab, in Relatives at Risk for Type 1 Diabetes. N Engl J Med. 2019;381(7):603–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Goenka R, Xu Z, Samayoa J, et al. CTLA4-Ig-Based Bifunctional Costimulation Inhibitor Blocks CD28 and ICOS Signaling to Prevent T Cell Priming and Effector Function. J Immunol. 2021;206(5):1102–1113. [DOI] [PubMed] [Google Scholar]
- 211.Stephen-Victor E, Das M, Karnam A, Pitard B, Gautier JF, Bayry J. Potential of regulatory T-cell-based therapies in the management of severe COVID-19. Eur Respir J. 2020;56(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Hombrink P, Helbig C, Backer RA, et al. Erratum: Programs for the persistence, vigilance and control of human CD8(+) lung-resident memory T cells. Nat Immunol. 2017;18(2):246. [DOI] [PubMed] [Google Scholar]
- 213.Christopoulos PF, Gjolberg TT, Kruger S, Haraldsen G, Andersen JT, Sundlisaeter E. Targeting the Notch Signaling Pathway in Chronic Inflammatory Diseases. Front Immunol. 2021;12:668207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Xu K, Moghal N, Egan SE. Notch signaling in lung development and disease. Adv Exp Med Biol. 2012;727:89–98. [DOI] [PubMed] [Google Scholar]
- 215.Budi EH, Schaub JR, Decaris M, Turner S, Derynck R. TGF-beta as a driver of fibrosis: physiological roles and therapeutic opportunities. J Pathol. 2021;254(4):358–373. [DOI] [PubMed] [Google Scholar]
- 216.Sheppard D Transforming growth factor beta: a central modulator of pulmonary and airway inflammation and fibrosis. Proc Am Thorac Soc. 2006;3(5):413–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zander R, Kasmani MY, Chen Y, et al. Tfh-cell-derived interleukin 21 sustains effector CD8(+) T cell responses during chronic viral infection. Immunity. 2022;55(3):475–493 e475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Brodeur TY, Robidoux TE, Weinstein JS, Craft J, Swain SL, Marshak-Rothstein A. IL-21 Promotes Pulmonary Fibrosis through the Induction of Profibrotic CD8+ T Cells. J Immunol. 2015;195(11):5251–5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Hussaini A, Mukherjee R, Berdieva DM, Glogowski C, Mountfield R, Ho PTC. A Double-Blind, Phase I, Single Ascending Dose Study to Assess the Safety, Pharmacokinetics, and Pharmacodynamics of BOS161721 in Healthy Subjects. Clin Transl Sci. 2020;13(2):337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Hussell T, Pennycook A, Openshaw PJ. Inhibition of tumor necrosis factor reduces the severity of virus-specific lung immunopathology. Eur J Immunol. 2001;31(9):2566–2573. [DOI] [PubMed] [Google Scholar]
- 221.Karki R, Sharma BR, Tuladhar S, et al. Synergism of TNF-alpha and IFN-gamma Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell. 2021;184(1):149–168 e117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Gawish R, Starkl P, Pimenov L, et al. ACE2 is the critical in vivo receptor for SARS-CoV-2 in a novel COVID-19 mouse model with TNF- and IFNgamma-driven immunopathology. Elife. 2022;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Huggett MT, Armstrong R. Adalimumab-associated pulmonary fibrosis. Rheumatology (Oxford). 2006;45(10):1312–1313. [DOI] [PubMed] [Google Scholar]
- 224.Dias OM, Pereira DA, Baldi BG, et al. Adalimumab-induced acute interstitial lung disease in a patient with rheumatoid arthritis. J Bras Pneumol. 2014;40(1):77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Perez-Alvarez R, Perez-de-Lis M, Diaz-Lagares C, et al. Interstitial lung disease induced or exacerbated by TNF-targeted therapies: analysis of 122 cases. Semin Arthritis Rheum. 2011;41(2):256–264. [DOI] [PubMed] [Google Scholar]
- 226.Theron M, Huang KJ, Chen YW, Liu CC, Lei HY. A probable role for IFN-gamma in the development of a lung immunopathology in SARS. Cytokine. 2005;32(1):30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Schmit T, Guo K, Tripathi JK, et al. Interferon-gamma promotes monocyte-mediated lung injury during influenza infection. Cell Rep. 2022;38(9):110456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Grant RA, Morales-Nebreda L, Markov NS, et al. Circuits between infected macrophages and T cells in SARS-CoV-2 pneumonia. Nature. 2021;590(7847):635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Peluso MJ, Lu S, Tang AF, et al. Markers of Immune Activation and Inflammation in Individuals With Postacute Sequelae of Severe Acute Respiratory Syndrome Coronavirus 2 Infection. J Infect Dis. 2021;224(11):1839–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Phetsouphanh C, Darley DR, Wilson DB, et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat Immunol. 2022;23(2):210–216. [DOI] [PubMed] [Google Scholar]
- 231.Bouwman AC, van Daalen KR, Crnko S, Ten Broeke T, Bovenschen N. Intracellular and Extracellular Roles of Granzyme K. Front Immunol. 2021;12:677707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Cooper DM, Pechkovsky DV, Hackett TL, Knight DA, Granville DJ. Granzyme K activates protease-activated receptor-1. PLoS One. 2011;6(6):e21484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Camell CD. Taa Cells and Granzyme K: Old Players with New Tricks. Immunity. 2021;54(1):6–8. [DOI] [PubMed] [Google Scholar]
- 234.Linden A, Dahlen B. Interleukin-17 cytokine signalling in patients with asthma. Eur Respir J. 2014;44(5):1319–1331. [DOI] [PubMed] [Google Scholar]
- 235.Mi S, Li Z, Yang HZ, et al. Blocking IL-17A promotes the resolution of pulmonary inflammation and fibrosis via TGF-beta1-dependent and -independent mechanisms. J Immunol. 2011;187(6):3003–3014. [DOI] [PubMed] [Google Scholar]
- 236.Zhang J, Wang D, Wang L, et al. Profibrotic effect of IL-17A and elevated IL-17RA in idiopathic pulmonary fibrosis and rheumatoid arthritis-associated lung disease support a direct role for IL-17A/IL-17RA in human fibrotic interstitial lung disease. Am J Physiol Lung Cell Mol Physiol. 2019;316(3):L487–L497. [DOI] [PubMed] [Google Scholar]
- 237.Maione F, Casillo GM, Raucci F, et al. Interleukin-17A (IL-17A): A silent amplifier of COVID-19. Biomed Pharmacother. 2021;142:111980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Queiroz MAF, Neves P, Lima SS, et al. Cytokine Profiles Associated With Acute COVID-19 and Long COVID-19 Syndrome. Front Cell Infect Microbiol. 2022;12:922422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.George PM, Reed A, Desai SR, et al. A persistent neutrophil-associated immune signature characterizes post-COVID-19 pulmonary sequelae. Sci Transl Med. 2022;14(671):eabo5795. [DOI] [PubMed] [Google Scholar]
- 240.Temesgen Z, Assi M, Shweta FNU, et al. GM-CSF Neutralization With Lenzilumab in Severe COVID-19 Pneumonia: A Case-Cohort Study. Mayo Clin Proc. 2020;95(11):2382–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, Ruiz C, Melguizo-Rodriguez L. SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Pavel AB, Glickman JW, Michels JR, Kim-Schulze S, Miller RL, Guttman-Yassky E. Th2/Th1 Cytokine Imbalance Is Associated With Higher COVID-19 Risk Mortality. Front Genet. 2021;12:706902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Kim EY, Battaile JT, Patel AC, et al. Persistent activation of an innate immune response translates respiratory viral infection into chronic lung disease. Nat Med. 2008;14(6):633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]


