Abstract
Background:
Atopic dermatitis (AD) is a chronic inflammatory skin condition with a highly variable clinical phenotype.
Objective:
This study aimed to identify historical and clinical features and biomarkers associated with AD severity.
Methods:
A US registry of extensively phenotyped AD participants (0.73-80 yrs) were enrolled at nine academic centers. Information on family and personal medical history, examination, skin swabs (culture), and serum biomarkers were collected to evaluate their association with AD severity.
Results:
AD participants (N=2,862), whose disease was categorized as mild (11.6%), moderate (58.0%), or severe (30.4%) based on Rajka-Langeland (RJL) scoring were enrolled. The trend test, when adjusting for gender, race, and age, demonstrated severity was strongly (p≤0.04) associated with a personal/family history of allergic disorders, history of alopecia, exposure to passive smoke, ocular herpes infection, skin bacterial and viral infections, and history of arrythmia. Features observed more frequently (p≤0.002), as a function of severity, included skin infections (impetigo, HPV and MCV), S. aureus colonization, excoriations, hyperlinear palms, ichthyosis, blepharitis, conjunctivitis, ectropion, and wheezing. Serum IgE, allergen and food (≤6 yrs) Phadiatop and eosinophilia were strongly linked to severity (p<0.001).
Conclusion:
In a diverse US AD population, severity was associated with a history of atopic disorders, skin and extracutaneous bacterial and viral infections (by history and physical examination), higher IgE, eosinophilia and allergen sensitization, atopic skin manifestations (i.e., excoriation, hyperlinear palms and ichthyosis), and atopic ocular features (i.e., blepharitis, conjunctivitis, and ectropion) as well as asthma findings (i.e., wheezing). Data from our prospective registry significantly advances our understanding of AD phenotypes and endotypes, which is critical to achieve optimal management.
Keywords: Atopic dermatitis, disease severity, endotype, Rajka-Langeland score, registry, infection, comorbidity, biomarkers, allergen sensitization, Staphylococcus aureus
Introduction
Atopic dermatitis (AD) is one of the most common chronic inflammatory skin disorders with highly variable clinical features. Population-based surveys have suggested that AD prevalence in the United States is about 7.3% in adults and 10.7% (8.7% to 18.1%) in children.(1) Clinically, AD is characterized by eczematous skin lesions, pruritus, and sleep disturbance, with a relapsing/remitting or persistent clinical course. Infectious complications of AD include both bacterial and viral infections and can be limited to skin or uncommonly involve other sites (e.g., osteomyelitis, infectious arthritis, and endocarditis).(2, 3)
Many studies have confirmed the negative impact of AD on quality of life, which typically correlates with objective measures of disease severity. The economic burden of AD is high, due to out-of-pocket costs, health care utilization, and indirect costs from lost days at work or school.(4) The complexity and heterogeneity of AD have led to an interest in identifying clinically-meaningful subpopulations to guide prognosis and treatment decisions. Skin and serum biomarker studies demonstrate that AD is heterogeneous based on unique molecular endotypes (5) with differences based on age of onset and racial background (6-9). A better understanding of how clinical features might reflect endotypes will not only help us better understand AD pathophysiology but also holds the promise of a more precision medicine approach to treatment.
Several studies suggest that AD severity correlates with risks for mental health disorders, some non-allergic comorbidities including skin infection (9, 10), and more severe allergic comorbidities (i.e., asthma, hay fever/allergic rhinitis, and food allergy). Often these retrospective studies are limited by a relatively imprecise AD definition, lack of physical examination and validated assessments of AD severity, or by a limited focus on one or two specific comorbidities.
An objective of the National Institute of Allergy and Infectious Diseases (NIAID)-funded AD Research Network (ADRN) Registry was to investigate endotypes as well as clinical and historical characteristics of different AD sub-phenotypes. Previously, we reported results of a cross-sectional study investigating the clinical and biomarker characteristics of AD participants with and without cutaneous S. aureus colonization (based on routine clinical laboratory culture techniques) compared to a noncolonized, non-AD population. S. aureus-colonized AD participants had greater skin barrier impairment, systemic immune activation, type 2 immune deviation, and allergen sensitization than those who were not colonized (11). Additionally, this study validated previous findings that AD severity strongly correlated with S. aureus colonization. An earlier ADRN study had also demonstrated that AD severity was associated with an increased risk of eczema herpeticum (12).
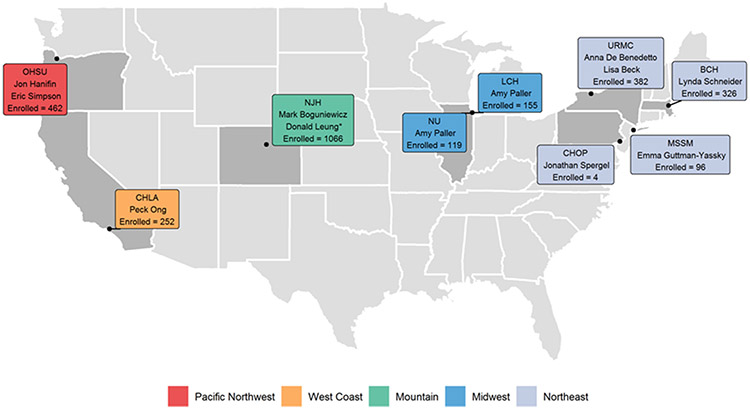
We report our observations from a large (N=2,862), deeply phenotyped, pediatric and adult AD registry recruited at nine geographically-diverse academic AD centers (Figure 1). We systematically and prospectively documented AD features, including physical examination, past medical history, skin culture results, and peripheral blood biomarkers in an AD cohort stratified by disease severity using the Rajka-Langeland score (RJL).
Figure 1. Nine US academic centers involved in AD subject recruitment for the Atopic Dermatitis Research Network (ADRN)-02 Registry study.
Each box represents a single center with principal investigator(s) and enrollment totals. A total of 2,862 AD participants were enrolled. OHSU=Oregon Health Sciences University, CHLA=Children’s Hospital of Los Angeles, NJH=National Jewish Health, NU=Northwestern University/ LCH=Lurie Children’s Hospital, URMC=University of Rochester Medical Center, CHOP=Children’s Hospital of Pennsylvania, MSSM=Mt. Sinai School of Medicine, BCH=Boston Children’s Hospital.
Methods
Study Design
This was a multi-center, clinical registry study designed to examine factors associated with susceptibility of AD participants to cutaneous viral dissemination and bacterial colonization/infection as well as biomarkers related to AD sub-phenotypes (ClinicalTrials.gov Identifier: NCT01494142 [ADRN02]). Participants were recruited from nine US academic centers (Figure 1) after protocol approval at the institutional review board at each site. Written informed consent was provided by the participant or parent/legal guardian, and written assent was provided by the participant, as applicable, before participation.
Population
We enrolled AD participants with a history of eczema herpeticum (EH; 8 months to 80 years of age) or without a history of EH (3 years to 80 years of age) as defined in the ADRN Standard Diagnostic Criteria(12). Key exclusion criteria included: history of any systemic immunological illness, active systemic malignancy, skin disease other than AD that might compromise the stratum corneum barrier or had a first degree relative already enrolled in the study. The full inclusion and exclusion criteria for this study are available on clinicaltrials.gov (NCT01494142).
Additional methodological details including study assessments, diagnostic criteria, case report forms, skin swab collection, biomarker collection and statistical analysis can be found in the Online Repository.
Results
General Characteristics of the Population
A total of 2,862 AD participants (0.73 to 80 years, inclusive) were enrolled (Figure 1). Table I details the demographics for both the total population and stratified by disease severity (using RJL scoring (Mild 3-4, Moderate 4.5-7.5, Severe 8-9)). We found that RJL scoring, which uses historical data, strongly correlated with EASI scores (R=0.72; P<0.001) performed at the same visit (Figure E1). Moderate severity group was the largest (n=1660), followed by Severe (n=869) and Mild (n=333). No geographic region recruited <250 participants; however, differences were observed across regions, with the largest numbers recruited from the Mountain region (NJH) which recruited the most Moderate (n=566) and Severe (n=497) participants (Table I).
Table I.
Demographics and Baseline Characteristics
| Rajka-Langeland |
|||||
|---|---|---|---|---|---|
| Overall N = 2,862 |
Mild (3-4) N = 333 |
Moderate (4.5-7.5) N = 1,660 |
Severe (8-9) N = 869 |
P-value1 | |
| Regional Site2 | <0.001 | ||||
| Mountain | 1,066 (37%) | 3 (0.9%) | 566 (34%) | 497 (57%) | |
| Northeast | 808 (28%) | 143 (43%) | 493 (30%) | 172 (20%) | |
| Pacific Northwest | 462 (16%) | 45 (14%) | 286 (17%) | 131 (15%) | |
| North Mid-West | 274 (9.6%) | 46 (14%) | 184 (11%) | 44 (5.1%) | |
| West Coast | 252 (8.8%) | 96 (29%) | 131 (7.9%) | 25 (2.9%) | |
| Age3 | 21.0 (10.0, 35.0) | 23.0 (10.0, 35.0) | 23.0 (11.0, 36.0) | 17.0 (8.0, 34.0) | <0.001 |
| Age at Enrollment2 | <0.001 | ||||
| 0-5 years | 347 (12%) | 34 (10%) | 172 (10%) | 141 (16%) | |
| 6-11 years | 521 (18%) | 61 (18%) | 277 (17%) | 183 (21%) | |
| 12-17 years | 374 (13%) | 36 (11%) | 221 (13%) | 117 (13%) | |
| 18-64 years | 1,560 (55%) | 189 (57%) | 958 (58%) | 413 (48%) | |
| 65 years and greater | 60 (2.1%) | 13 (3.9%) | 32 (1.9%) | 15 (1.7%) | |
| Sex2 | <0.001 | ||||
| Female | 1,652 (58%) | 206 (62%) | 1,002 (60%) | 444 (51%) | |
| Male | 1,210 (42%) | 127 (38%) | 658 (40%) | 425 (49%) | |
| Race2 | <0.001 | ||||
| White | 1,718 (60%) | 182 (55%) | 939 (57%) | 597 (69%) | |
| Black | 822 (29%) | 120 (36%) | 522 (31%) | 180 (21%) | |
| Multi-racial | 156 (5.5%) | 17 (5.1%) | 94 (5.7%) | 45 (5.2%) | |
| Asian | 98 (3.4%) | 7 (2.1%) | 66 (4.0%) | 25 (2.9%) | |
| Other | 68 (2.4%) | 7 (2.1%) | 39 (2.3%) | 22 (2.5%) | |
| Ethnicity2 | 0.002 | ||||
| Hispanic or Latino | 607 (21%) | 95 (29%) | 345 (21%) | 167 (19%) | |
| Not Hispanic or Latino | 2,255 (79%) | 238 (71%) | 1,315 (79%) | 702 (81%) | |
| BMI (kg/m2)3 | 23.6 (18.7, 29.3) | 24.1 (19.5, 29.0) | 24.0 (19.2, 29.7) | 22.5 (17.5, 28.4) | <0.001 |
| EASI3 | 7.6 (3.0, 16.6) | 1.6 (0.9, 2.7) | 5.6 (2.9, 9.7) | 21.9 (14.6, 29.6) | <0.001 |
| EASI Group2 | <0.001 | ||||
| 0-1 (Clear, Almost Clear) | 190 (6.6%) | 107 (32%) | 83 (5.0%) | 0 (0%) | |
| 1.1-7 (Mild) | 1,165 (41%) | 214 (64%) | 917 (55%) | 34 (3.9%) | |
| 7.1-21 (Moderate) | 986 (34%) | 11 (3.3%) | 605 (36%) | 370 (43%) | |
| >21 (Severe) | 521 (18%) | 1 (0.3%) | 55 (3.3%) | 465 (54%) | |
Exact Cochran-Armitage Trend Test for categorical variables; Linear Regression for continuous variables
n (%)
Median (25th percentile,75th percentile)
Although all age categories were represented, adults accounted for the majority of the population. Besides the ≥65 years age group, which comprised only 2.1% of the population, all other age categories had accounted for ≥12% of the population. The median age at enrollment was 21 years, but those participants with Severe disease were slightly younger (median age 17 years; p<0.001). More females were enrolled than males (58% vs. 42%) (Table I). The population was diverse ethnically and racially, with Black participants comprising 29% and those of Hispanic ethnicity 21%. White participants were overrepresented in the Severe group, while Black participants were underrepresented. The non-Hispanic population was slightly overrepresented in the Severe group.
Comorbidities and Environment
Table II reports the prevalence of comorbidities and environmental factors in participants stratified by RJL severity. A significant stepwise increase, adjusted for gender, race, and age, in the prevalence of allergic rhinitis, asthma and eosinophilic esophagitis was observed as disease severity increased (p<0.01). The association between severity and reported food allergy was also strong while recognizing the many limitations of self-reported food allergy. The prevalence of mental health conditions (anxiety or depression) was also higher with increasing disease severity (p=0.003).
Table II.
Personal History of Comorbidities & Home Environment
| Rajka-Langeland |
|||||||
|---|---|---|---|---|---|---|---|
| Overall N = 2,862 n (%) |
Mild (3-4) N = 333 n (%) |
Moderate (4.5-7.5) N = 1,660 n (%) |
Severe (8-9) N = 869 n (%) |
P-value1 | OR2 | 95% CI2 | |
| History of Allergic Rhinitis or Hay Fever | 1,639 (57%) | 133 (40%) | 922 (56%) | 584 (67%) | <0.001 | 1.70 | (1.50-1.93) |
| History of Food Allergy | 1,343 (47%) | 108 (32%) | 704 (42%) | 531 (61%) | <0.001 | 1.87 | (1.64-2.12) |
| History of Asthma | 1,320 (46%) | 127 (38%) | 718 (43%) | 475 (55%) | <0.001 | 1.41 | (1.25-1.59) |
| History of Eosinophilic Esophagitis | 19 (1%) | 0 (0%) | 6 (0%) | 13 (1%) | 0.003 | 4.04 | (1.69-11.30) |
| History of Anxiety or Depression | 616 (22%) | 60 (18%) | 348 (21%) | 208 (24%) | 0.003 | 1.26 | (1.08-1.48) |
| History of Hair Loss/Alopecia | 165 (6%) | 19 (6%) | 90 (5%) | 56 (6%) | 0.012 | 1.42 | (1.08-1.86) |
| History of Alopecia Areata3 | 62/164 (38%) | 9/19 (47%) | 35/90 (39%) | 18/55 (33%) | 0.3 | 0.74 | (0.42-1.29) |
| History of Thyroid Disease | 104 (4%) | 13 (4%) | 68 (4%) | 23 (3%) | 0.5 | 0.88 | (0.63-1.23) |
| History of Hypertension | 278 (10%) | 35 (11%) | 171 (10%) | 72 (8%) | 0.4 | 1.11 | (0.87-1.41) |
| History of Coronary Artery Disease/Heart Attack/Stenting/Angioplasty/Bypass | 22 (1%) | 5 (2%) | 8 (0%) | 9 (1%) | 0.2 | 1.69 | (0.77-3.79) |
| History of Arrhythmia | 48 (2%) | 2 (1%) | 28 (2%) | 18 (2%) | 0.032 | 1.72 | (1.05-2.85) |
| History of Smoking3 | 673/2,069 (33%) | 73/254 (29%) | 406/1,249 (33%) | 194/566 (34%) | 0.055 | 1.17 | (1.00-1.38) |
| Current Smoking Status3 | -- | -- | -- | -- | 0.004 | 1.50 | (1.14-1.98) |
| Current | 316/673 (47%) | 24/73 (33%) | 194/406 (48%) | 98/194 (51%) | |||
| Former | 357/673 (53%) | 49/73 (67%) | 212/406 (52%) | 96/194 (49%) | |||
| Someone Smokes in Home | 552 (19%) | 38 (11%) | 328 (20%) | 186 (21%) | <0.001 | 1.43 | (1.22-1.68) |
| Dog Owner | 1,075 (38%) | 108 (32%) | 601 (36%) | 366 (42%) | 0.14 | 1.10 | (0.97-1.25) |
| Cat Owner | 519 (18%) | 57 (17%) | 311 (19%) | 151 (17%) | 0.3 | 0.93 | (0.79-1.09) |
Trend Test
OR = Odds Ratio, CI = Confidence Interval
n/N (%)
Participant smoking status and smoking in the household significantly increased with AD severity (p=0.004 and p<0.001 respectively). A history of alopecia was reported by 6% of AD participants and associated with disease severity (p=0.012); but only 38% of those participants specifically reported having alopecia areata. Notably a history of the two autoimmune conditions, thyroid disease (p=0.5), and alopecia areata (p=0.3), did not correlate with disease severity. Only one participant reported insulin-dependent diabetes. Neither dog or cat ownership (p=0.14 and p=0.3 respectively) correlated with disease severity. Participant-reported history of arrythmia also showed a significant trend with disease severity (p=0.032).
Ocular Disease History
Previous studies revealed that participants with AD demonstrate higher rates of ocular disorders than the non-AD population. However, the prevalence of ocular disorders (Table III) in our study did not increase with AD severity (i.e., cataracts, keratoconus, and retinal detachment), other than a history of HSV ocular infections (p<0.001), adjusted for gender, race, and age, which was most often reported in the Severe group.
Table III.
History of Ocular Problems
| Rajka-Langeland | |||||||
|---|---|---|---|---|---|---|---|
| Overall N = 2,862 n/N (%) |
Mild (3-4) N = 333 n/N (%) |
Moderate (4.5-7.5) N = 1,660 n/N (%) |
Severe (8-9) N = 869 n/N (%) |
P-value1 | OR2 | 95% CI2 | |
| History of Serious Eye Problems | 190/2,849 (7%) | 20/331 (6%) | 109/1,652 (7%) | 61/866 (7%) | 0.2 | 1.18 | (0.91-1.52) |
| History of Cataracts | 61/190 (32%) | 6/20 (30%) | 31/109 (28%) | 24/61 (39%) | 0.3 | 1.35 | (0.74-2.49) |
| History of Keratoconus | 13/190 (7%) | 1/20 (5%) | 5/109 (5%) | 7/61 (11%) | 0.14 | 2.16 | (0.80-6.43) |
| History of Retinal Detachment | 15/190 (8%) | 1/20 (5%) | 8/109 (7%) | 6/61 (10%) | 0.5 | 1.42 | (0.58-3.64) |
| History of Herpes Simplex Infection of the Eye | 40/2,862 (1%) | 0/333 (0%) | 10/1,660 (1%) | 30/869 (3%) | <0.001 | 7.53 | (3.87-16.16) |
Trend Test
OR = Odds Ratio, CI = Confidence Interval
Family History
The majority (≥ 66%) of our AD participants reported a family history of atopic conditions (i.e., asthma [AS], allergic rhinitis [AR] or hay fever, and AD) (Table IV). The history of each atopic condition was more common in participants with more severe disease: AR (p<0.001), AS (p=0.001) and AD (p=0.001). Two percent of AD participants reported a family history of eczema herpeticum (EH), but this history had no relationship to AD severity (p=0.4).
Table IV.
Family History
| Rajka-Langeland |
|||||||
|---|---|---|---|---|---|---|---|
| Overall N = 2,862 n/N (%) |
Mild (3-4) N = 333 n/N (%) |
Moderate (4.5-7.5) N = 1,660 n/N (%) |
Severe (8-9) N = 869 n/N (%) |
P-value1 | OR2 | 95% CI2 | |
| Family History of Atopic Dermatitis or Eczema | 2,025/2,855 (71%) | 218/333 (65%) | 1,168/1,656 (71%) | 639/866 (74%) | 0.001 | 1.24 | (1.09-1.42) |
| Family History of Allergic Rhinitis or Hay Fever | 1,923/2,855 (67%) | 166/333 (50%) | 1,106/1,656 (67%) | 651/866 (75%) | <0.001 | 1.62 | (1.41-1.85) |
| Family History of Asthma | 1,872/2,856 (66%) | 200/333 (60%) | 1,076/1,657 (65%) | 596/866 (69%) | 0.001 | 1.24 | (1.09-1.41) |
| Family History of Eczema Herpeticum | 53/2,856 (2%) | 9/333 (3%) | 28/1,657 (2%) | 16/866 (2%) | 0.4 | 0.84 | (0.54-1.30) |
Trend Test
OR = Odds Ratio, CI = Confidence Interval
S. aureus Colonization and Infectious Diseases
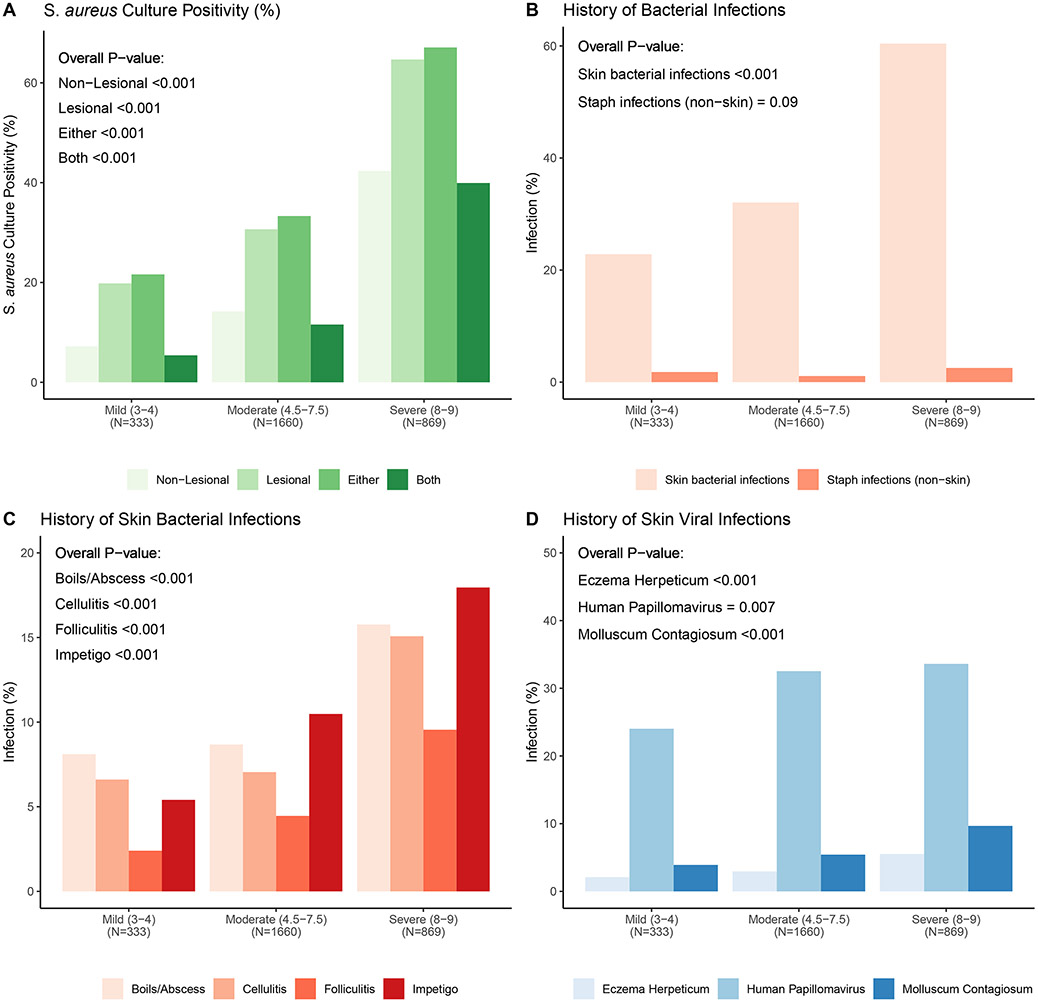
AD disease severity strongly correlated with S. aureus skin colonization, any skin infection (bacterial or viral) and non-skin bacterial infection (Figure 2). Among the 2,862 AD participants, 21.9%, 39.7%, 19.5% and 42.2% had S. aureus colonization on non-lesional, lesional, both sites, and either site, respectively (Figure E2). S. aureus colonization rates for both non-lesional and lesional skin increased in a stepwise fashion with increasing disease severity. Participants with Severe AD (N=869) had the highest colonization rates of 42.3% and 64.7% from non-lesional and lesional sites, respectively (Figure 2A and Figure E2). Methicillin-resistant S. aureus (MRSA) was isolated in 3.8% of all AD samples with similar percentages in Mild (2.1%) and Moderate (2.7%), but greater percentages in Severe (6.4%) AD group (Table E2; p<0.001).
Figure 2. Relationship between AD severity as assessed by RJL score on bacterial or viral infections.
Based on their RJL score AD participants were characterized as either Mild, Moderate or Severe. A. The percentage of S. aureus culture positive skin swabs from non-lesional, lesional, either site or both sites increased in a stepwise fashion as a function of AD severity. B. History of skin bacterial infections and extra-cutaneous S. aureus infections increased with AD severity. C. The percentage of AD participants reporting a history of any of four types of bacterial skin infections increased in participants with greater AD severity. D. A greater percentage of AD participants with more severe disease reported a history of the three most common cutaneous viral infections (i.e. human papilloma virus, molluscum contagiosum and herpes simplex virus).
AD disease severity was strongly associated with a history of clinically relevant infections. Overall, 39.6% reported a bacterial skin infection requiring oral or intravenous antibiotics, with Severe AD participants having the highest reported lifetime incidence at 60.4% (Figure 2B; p<0.001). Disease severity significantly correlated with the risk of non-cutaneous S. aureus infections and cutaneous bacterial infections, although the frequencies of the former were low. Of the bacterial infections reported on the skin, all clinical presentations (i.e., folliculitis, boils/abscesses, cellulitis, and impetigo) were more frequently observed on direct examination as disease severity increased (Figure 2C; p<0.001). Infections were most commonly observed on the extremities, followed by the head and neck, and infrequently on the chest and back (Table E4). The most common non-cutaneous S. aureus infection was bloodstream (1.4%) and bones/osteomyelitis and infected orthopedic hardware (both 0.2%); all were more common in the Severe group (p<0.001).
AD severity strongly correlated with a history of common skin viral infections (Figure 2D). A history of EH was reported in 3.6% of the total population, and the risk of EH significantly increased with severity (p<0.001). Severe AD participants reported the highest lifetime incidence (5.5%; Figure 2D). Similarly, infections with molluscum contagiosum virus (MCV) and human papillomavirus (HPV) were reported more often with higher AD severity (Figure 2D; p<0.001 and p=0.007; respectively).
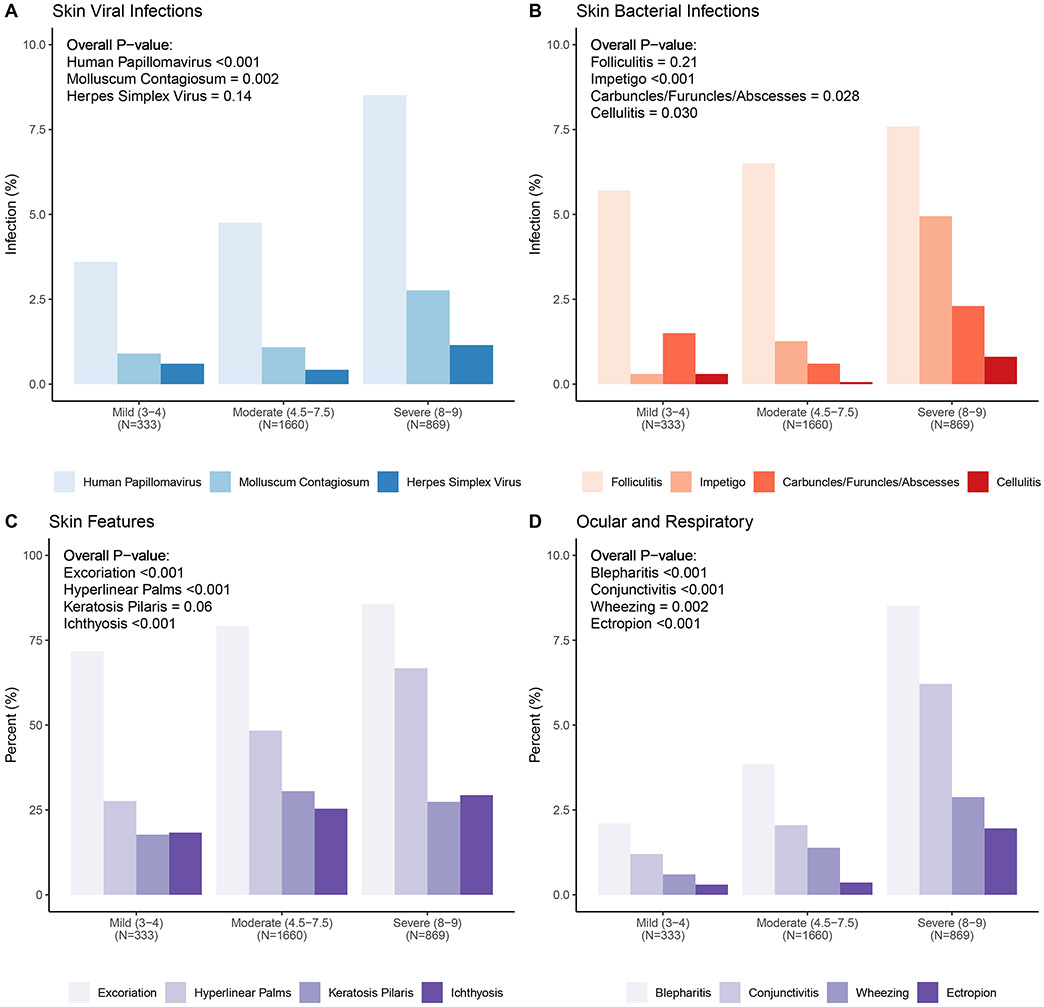
Physical Examination Features
All enrolled participants underwent a comprehensive physical examination by a licensed physician recognized as an AD expert (Figure 1). The most common viral infection observed was verruca vulgaris, seen in 5.8% of registry participants, most often on the upper extremities (Figure 3A; Table E3). MCV lesions were seen in 1.6% of participants and evenly distributed on the extremities and trunk. Probable HSV infections were observed in 0.7%, with most lesions on the head (Table E3). Only cutaneous wart and MCV skin infections increased as a function of AD severity (p<0.001, p=0.002, respectively). Folliculitis was the most common feature of infection (6.7%), followed by impetigo (2.3%). Carbuncles/furuncles/abscesses or cellulitis were only seen in a few AD participants (≤0.7%) (Figure 3B; Table E3). AD-associated skin features were most commonly excoriation (80.3%), hyperlinear palms (51.5%), keratosis pilaris (28.1%), and ichthyosis (25.8%) (Figure 3C; Table E3). All of these features were observed more commonly in AD participants with more severe disease (p<0.001), except keratosis pilaris (p=0.06). Blepharitis, conjunctivitis, and ectropion were also observed more often in more severe AD (Figure 3D; Table E3; p<0.001). Wheezing, audible in 1.7% of all enrolled participants, was 4-fold higher in Severe vs Mild AD participants (Figure 3D; Table E3; p=0.002).
Figure 3. Relationship between AD severity as assessed by RJL score on physical exam features.
A. Three viral infections (HPV, MCV and HSV) were observed more commonly in AD participants with more severe disease. B. AD participants with greater disease severity more frequently had clinical features suggestive of a bacterial infection (folliculitis, impetigo, carbuncles/furuncles/abscesses, or cellulitis). C. Skin features (excoriation, hyperlinear palms, keratosis pilaris and ichthyosis) frequently observed in AD participants were observed more frequently in AD participants with more severe disease. D. A number of ocular (blepharitis, conjunctivitis and ectropion) and respiratory (wheezing) features were identified more frequently as a function of disease severity.
Blood biomarkers
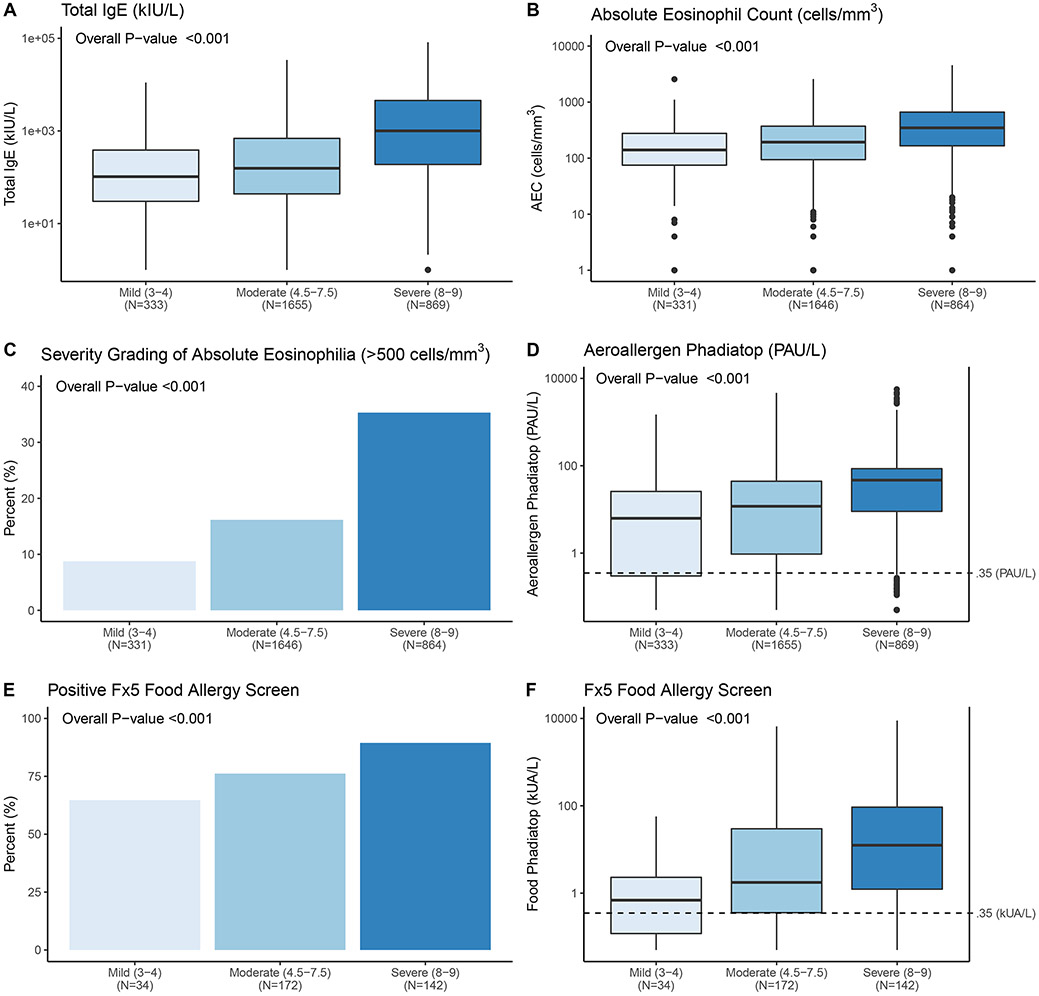
Total IgE levels increased dramatically in a stepwise fashion with RJL severity (Figure 4A; p<0.001). Circulating eosinophil counts, both absolute values and dichotomized (as high versus low), also increased with AD severity (Figure 4B & C; p<0.001). The majority of participants (>83%) had detectable aeroallergen sensitization (≥0.35 PAU/L cutoff for the Immunocap Phadiatop) and the absolute value increased as a function of AD severity (Figure 4D). The Food Phadiatop (Fx5), (only performed on AD participants ≤6 years; N=348), demonstrated that more than 80% of our childhood AD population was sensitized to common food allergens. Both percent positive (≥0.35 kUA/L; Figure 4E) and absolute values (Figure 4F) showed a correlation with disease severity (p<0.001).
Figure 4. Relationship between AD severity and blood biomarkers.
A. Serum total IgE increased with AD severity. B & C. Peripheral blood absolute eosinophil count (AEC) and percentage of participants with AEC > 500 cells/mm3 significantly increased with AD severity. D. Allergen sensitization, as measured by the multi-aeroallergen Phadiatop test, increased as AD severity increased. E. The percentage of positive Fx5 food allergen tests increased with AD severity. This test was only performed on AD participants ≤ 6 years of age (N=348). F. The absolute Fx5 value increased as a function of AD severity in this pediatric cohort. Top and bottom of boxes represent first and third quartiles, respectively, the center line represents the median, whiskers represent minimum to maximum excluding any outliers (greater than 1.5*IQR from the first or third quartiles).
Discussion
We report the clinical and biomarker features that strongly associate with AD severity. These findings came from an NIH-funded, large, prospective, highly phenotyped AD registry that recruited infant to elderly participants (8 months to 80 years) at nine academic AD centers of excellence, led by allergists (n=4) and dermatologists (n=5) (Figure 1). A number of AD registries or cohorts have been developed worldwide (overview, Table E1). Many of these have collected (or are currently collecting) clinical information, disease severity metrics, and even biospecimens. The strength of this registry is that it employed strict definitions, a common and detailed case report form, and careful attention to methodologic standards for disease severity assessments (performed by MDs with prior experience in these measures), standardized skin swabbing techniques, and centralized laboratories for all laboratory measures.
The Rajka-Langeland AD severity score was used because it considers a longer time frame, while still providing good sensitivity to detect changes (13). RJL includes information on the course of disease during the previous year, thus providing insights into the chronicity of the condition. This is unique compared to other measures that provide one point in time (EASI and SCORAD) or one-week (Patient Oriented Eczema Measure [POEM]) assessments of severity. Furthermore, RJL was shown to be a valid and reliable scoring system, with good internal consistency and responsiveness in both children and adults with AD, consistent with our observation that it strongly correlated with EASI (Figure E1) (13).
This study demonstrated that disease severity stratification is likely the result of key biological and familial/genetic differences, with severe AD having strong associations with systemic type 2 biomarkers, allergen sensitization, risk of concomitant allergic and non-allergic comorbidities, bacterial and viral infections, and physical examination findings. We also identified previously reported comorbidities with AD that did not associate with severity such as a history of autoimmune diseases, hypertension, and cardiovascular disease (1, 14-16). These observations should encourage mechanistic studies to explore why some AD comorbidities are strongly linked to severity and others are not. These data also suggest that therapeutics addressing the underlying disease severity may positively impact a multitude of associated conditions that represent a significant burden for patients with AD.
The majority (65%) of the recruited participants came from the Mountain and Northeast regions of the US, representing five of the nine academic centers. NJH, a tertiary referral center for severe AD, enrolled the highest number and greatest severity of AD. Centers with a pediatric dermatology focus, such as West Coast (CHLA) and Northeast (BCH and CHOP), enrolled a higher proportion of Mild participants, likely reflecting the greater frequency of mild disease in the pediatric population. Southern regions were not well represented in our registry. Despite this, racial and ethnic diversity closely reflected the US Census (2020) with 21% of participants reporting Hispanic ethnicity (Census 18.7%), 60% White (Census 61.6%), 29% Black (Census 12.4%) and 3.4% Asian (Census 7.2%) (17). Lastly, recruited participants had a median BMI at the upper limit of normal, but lower than the US average of 26.5-26.6 (adult men and women, respectively). Notably, the Severe AD group had the lowest BMI [21, 22], which contrasts with publications suggesting AD prevalence and severity associates with obesity (18, 19).
While skin infections are a well-known comorbidity in AD, few studies report the lifetime or point prevalence from physical examination and even fewer address the relationship to disease severity. We found strong associations between disease severity and both a history and presence of skin infections on examination. Our data reveal that lifetime prevalence of bacterial skin infections is about 20% for participants with Mild disease and increases to >60% for participants with Severe disease. This high historical burden of skin infections was accompanied by a surprisingly high burden of active bacterial skin infections. The prevalence of bacterial skin infections by examination strongly correlated with disease severity with all four clinical features of infection showing a stepwise increase in rates based on disease severity. Almost 20% of participants with Severe disease had signs of active bacterial skin infection, including folliculitis (7.6%), impetigo (4.9%), furuncles or abscesses (1.2%) and cellulitis (0.8%; Table E3). The high prevalence of active bacterial skin infection is likely explained by S. aureus colonization, revealed by culture of lesional and non-lesional sites. A systematic review estimated that 70% of patients have lesional S. aureus colonization, although historical figures vary, with severity being a modifying factor (20). Our study suggests that the rate of colonization largely depends on underlying disease severity, with only participants with Severe disease having a high degree of colonization based on routine culture techniques.
Disease severity also impacted the history of skin viral infections. Warts (HPV) were the most common skin infection across all severity levels (> 24% lifetime prevalence). The role of AD in predisposition to cutaneous warts is unclear. Although the association of warts with AD is controversial (21-23), a large population-based study found an increased risk of warts only in AD participants with concomitant atopic comorbidities (23), suggesting an influence of AD severity, as we found in our cohort. Molluscum contagiosum was second in frequency, followed by eczema herpeticum (HSV). Published data regarding the role of AD and the risk of molluscum are conflicting; a systematic review suggested that AD predisposes patients to molluscum infection, but studies from Japan and Brazil failed to find an association (24-26). Our study found molluscum infections, based on history or physical examination, strongly associated with increasing AD severity, an observation not previously described.
Non-cutaneous bacterial infections also correlated with disease severity lending support to the idea that AD participants with high S. aureus skin bioburden may become bacteremic and seed distant organs. A study of >1 million children found AD to be a significant risk factor for S. aureus bacteremia (27). Another study of >30,000 participants undergoing anterior cruciate ligament surgery found that having AD imparted a >7-fold increased odds of surgical site infection, greater than that seen for diabetes or obesity (28). A cross-sectional US study also found AD to be an independent risk factor for non-cutaneous infections, with the highest risk in AD participants with comorbid atopic diseases (23). AD severity was not captured in that study, but one might assume that the presence of more comorbid allergic disease indicates more severe AD, aligning with our study data.
Type 2 inflammation, a key driver of AD severity, could contribute to the increased skin colonization and infections in AD participants (29) inhibiting expression of epidermal barrier proteins and antimicrobial peptides, especially with greater AD severity (30, 31). We assessed type 2 immune skewing by measuring serum total IgE, aeroallergen and food IgE sensitization, and circulating eosinophil counts, all of which increased significantly with disease severity. Why AD severity impacts non-cutaneous infection risk is poorly understood. Theoretically, greater S. aureus bioburden (Figure 2) (32-34), combined with a defective skin barrier and more excoriation in the Severe group (Figure 3), could lead to a transient, but clinically significant S. aureus bacteremia. Indeed, S. aureus can even be seen in the subcutaneous tissues in AD participants and an AD mouse model (ovalbumin-sensitized filaggrin deficient flaky tail mouse) (35).
The greater type 2 skewing, observed in more severe disease, is likely a key driver or consequence of the higher rates of allergic comorbidities. The prevalence of all allergic comorbidities being strongly associated with AD severity, is in line with work by Silverberg and Simpson that found AD severity strongly associated with both the prevalence and severity of atopic comorbidities (36, 37). Unfortunately, patient-reported food allergy is fraught with overestimation and lack of specificity (38-41). Future analyses will refine the definition of food allergy beyond self-report.
Additional novel associations observed in our study include the significant association between household and to a lesser degree, participant smoking (p=0.004), with disease severity. The association between AD and active or passive smoke exposure has been found in several studies and confirmed by meta-analysis (42, 43). Tobacco smoke negatively impacts both skin barrier and immune function, and therefore may plausibly contribute to disease severity (44, 45), although several other confounders may explain the association in addition to reverse causation. Moreover, our findings did not demonstrate an association between dog (p=0.14) or cat (p=0.3) ownership and severity of AD. However, one study demonstrated that having indoor dogs was associated with worsening of AD symptoms (46). Despite concerns that dog and cat allergens could be drivers of AD severity, it is interesting to note that 38% of all ADRN registry participants reported having a dog which is consistent with 2017-2018 pet ownership data that found that 38.4% of US households have a dog, whereas only 18% of our registry participants reported a cat in their home which is lower than the US household rate of 25.4%.
Other notable findings in this analysis include an association between mental health comorbidities and AD severity. While the rates of depression or anxiety were consistent with that reported in the general population (22% vs 27%, respectively), we observed the greatest rates were observed in those with more severe disease (47). This is consistent with previous publications (1). A novel association between AD severity and a history of arrythmias (p=0.032) was observed in our study. Two previous studies observed an association between AD and atrial fibrillation/flutter with both studies revealing a stronger association with more severe disease(48, 49). Like previous studies, the frequency of the comorbidity is rare (2%), but our study observed a higher odds of the condition (OR>3) in our participants with severe disease. The mechanism for this association is not known, but systemic inflammation has been considered to explain similar associations observed in psoriasis and rheumatoid arthritis (50). Another novel association observed was a history of alopecia (p=0.012) in general, not alopecia areata (p=0.3). Possible explanations for this association include inflammatory alopecia similar to psoriatic alopecia or telogen effluvium from various causes including systemic steroid use that may occur more commonly in severe disease (51).
Lastly, several health issues identified by history did not associate with AD severity. These included specific eye conditions (cataracts, keratoconus, and retinal detachment); despite 4-to 180-fold higher rates in AD than historical data in healthy individuals, the rates did not change with disease severity (52-54). The overall burden of eye disease was high, especially a history of HSV infections reported in 1% (40/2,862) of our AD population and much higher than for the general population (0.078%) (52). Retinal detachment was reported in 8% (15/190) compared with historical US rates of 0.0125% (53). Similarly, keratoconus has an estimated US prevalence of 0.054%, but in our AD population was 7% (13/190) (54). Although these ocular conditions are clearly associated with AD, their frequency is not significantly altered by AD severity. Prevalence of autoimmune disease (thyroid disease and alopecia areata) also did not correlate with disease severity, although associations of thyroid disease (15) and alopecia areata (55-57) with AD overall have been noted in the past. Taken together, these data suggest that some comorbidities result from the high degree of underlying type 2 inflammation (i.e., AD severity), while other comorbidities share common pathogenic features outside of type 2 inflammation.
Our study is limited by its cross-sectional nature making it impossible to address whether any observed associations with AD severity are in fact causal. Reducing disease severity with inflammation-specific therapies should help elucidate the downstream effects of specific immunological pathways on some of these severity-related features. We recognize the potential selection bias based on recruitment exclusively from academic AD referral centers. Although we were pleased with a broad representation of ages, races, ethnicities, gender, and geography (US only) in our registry, we would have preferred more in the cohort with mild severity who were elderly, Asians, or living in the Southern US. Lastly, although we recruited a large number of AD participants, we were not powered to determine the role AD severity plays on history or presence of less common conditions (e.g., EH, keratoconus, or IDDM).
In summary, we demonstrate that AD severity, as assessed by RJL score, is associated strongly with: (1) personal history of comorbidities (i.e., arrhythmia, alopecia without areata, and depression or anxiety), (2) personal and family history of atopic disorders, (3) skin and extracutaneous bacterial and viral infections (both by history and physical examination), (4) type 2 blood biomarkers and allergen sensitization, (5) atopic skin manifestations (i.e., excoriation, hyperlinear palms, keratosis pilaris, and ichthyosis), (6) atopic ocular features (i.e., blepharitis, conjunctivitis, and ectropion), and (7) asthma features (i.e. wheezing). It is equally notable that we did not see an association of AD severity with: (1) a history of ocular problems (i.e., cataracts, keratoconus, or retinal detachment), or (2) a history of autoimmune conditions (i.e., thyroid abnormalities, alopecia with areata or diabetes), or (3) pet ownership (i.e., dog or cat). These data identify several clinical and historical features strongly associated with AD participants who have more severe disease. This will enable better prognostication and clinical guidance and may lead to new opportunities to identify AD cohorts to target for disease prevention and/or modification strategies.
Supplementary Material
Highlight Box.
1. What is already known about this topic?
The severity of atopic dermatitis correlates with many quality of life measures in AD, but how severity impacts other clinical and historical features of the disease are not fully characterized.
2. What does this article add to our knowledge?
This large, prospective registry demonstrates that AD severity directly correlates associates with allergen sensitization, type 2 immunity, other atopic conditions, S. aureus colonization, and a history and/or presence of bacterial or viral skin infections.
3. How does this study impact current management guidelines?
Disease severity strongly associates with Staphylococcus aureus colonization a history of both viral and bacterial infections as well as IgE sensitization.
Acknowledgments
The authors would like to acknowledge the helpful discussions with Marshall Plaut, MD, PhD, Wendy Davidson, Ph.D., Joy Laurienzo-Panza, RN, BSN (NIAID), Max Seibold, PhD (NJH) and Raif Geha, MD, PhD (Harvard) as well as assistance of Rebecca Field (OHSU), Keli Johnson BS CCRP (Rho, Inc), Jennifer Martin BS (Rho, Inc) throughout the writing process. A big thanks to Takeshi Yoshida, PhD who oversaw the serum biorepository for this study and our coordinators who worked tirelessly to identify and enroll our participants (Jean Sauvain, Alicia Papalia, Lisa Heughan, Donia Attia, Patricia Taylor, Susan Leung, Caroline Bronchick, Anna Ward, Amanda Mundy, Laura Kuzma, Dalila Ortega, Sandra Figueroa, Sydney Brown, Michelle Lam, Courtney Rooney, Bonnie Johnson, Anjali Shroff, Matthew Gagliotti, and Victoria Godinez-Puig). Our biggest thanks to the participants who continue to inspire us with new ideas and whose motivation to see a better tomorrow for atopic patients is an inspiration to us all.
Funding:
This work was supported by the National Institutes of Health (NIAID Funding Mechanism: HHSN272201000020C [DYL], HHSN272201000017C, UL1TR002369, U01AI152011 and U19AI117673 [LAB]).
Abbreviations:
- AD
atopic dermatitis
- ADRN
Atopic Dermatitis Research Network
- CBC
complete blood count
- AEC
absolute eosinophil count
- CRF
case report form
- CSC
clinical study consortium
- DACI
Dermatology, Allergy and Clinical Immunology Laboratory
- DAIT
Division of Allergy, Immunology and Transplantation
- EASI
Eczema Area and Severity Index
- EDC
electronic data capture
- EH
eczema herpeticum
- HPV
human papilloma virus
- HSV
herpes simplex virus
- IgE
immunoglobulin E
- IRB
institutional review board
- L
lesional skin
- MCV
molluscum contagiosum virus
- MRSA
methicillin-resistant Staphylococcus aureus
- MSSA
methicillin-sensitive Staphylococcus aureus
- NIAID
National Institute of Allergy and Infectious Diseases
- NIH
National Institutes of Health
- NL
non-lesional skin
- PI
principal investigator
- RAST
radioallergosorbent test
- RJL
Rajka-Langeland Score
- SACCC
statistical and clinical coordinating center
Footnotes
Conflicts of Interest: ADB – Consult for dMed Biopharmaceutical Co, Ltd; Grant or clinical trial support from Dermira, Kiniksa, Novartis, and Pfizer. ELS – Consultant for AbbVie, Amgen, Arena Pharmaceuticals, Aslan Pharma, Boston Consulting Group, Collective Acumen, LLC (CA), Dermira, Eli Lilly, Evidera, Excerpta Medica, Forte Bio RX, Galderma, GlaxoSmithKline, Incyte, Janssen, Kyowa Kirin Pharmaceutical Development, Leo Pharm, Medscape LLC, Merck, Pfizer, Physicians World LLC, Regeneron, Roivant, Sanofi-Genzyme, Trevi therapeutics, Valeant, WebMD. Investigator for AbbVie, Amgen, Arcutis, Aslan, Castle Biosciences, Corevita, Dermavant, Dermira, Eli Lilly, Incyte, Kyowa Hakko Kirin, Leo Pharmaceuticals, Merck, Novartis, Pfizer, Regeneron, Sanofi, TARGET-DERM, Tioga, and Vanda. MB - Investigator for Regeneron, Incyte. Consultant for Regeneron, Sanofi-Genzyme, Abbvie, Leo, Lilly, Pfizer, Janssen. PYO -Consultant for Incyte, Abbvie, Janssen; Investigator for Regeneron, Sanofi Genzyme, Leo, Incyte. LCS - Investigator – Regeneron (dupilumab), DBV Technologies (Viaskin peanut patch), Grants – Genentech, Pfizer. Advisory Boards-FARE, Ukko, Amagma, DBV Technologies, Alladapt, Immunotherapeutics, Leo Pharmaceuticals, Biothea Therapeutics. ASP - Investigator for AbbVie, AnaptysBio, Dermavant, Eli Lilly, Incyte, and Janssen; Consultant with honorarium for Abbvie, Acrotech, Almirall, Arcutis, Azitra, BiomX, Boeringer Ingelheim, Botanix, Bristol Myers Squibb, Catawba, Eli Lilly, Gilead, Incyte, Janssen, Leo, Novartis, Pfizer, RAPT, Regeneron, Sanofi/Genzyme, Seanergy, Union; Data Safety Monitoring Board for AbbVie, Bausch, and Galderma. EGY - Employee of Mount Sinai and has received research funds (grants paid to the institution) from Abbvie, Almirall, Amgen, AnaptysBio, Asana Biosciences, AstraZeneca, Boehringer-Ingelheim, Celgene, Dermavant, DS Biopharma, Eli Lilly, Galderma, Glenmark/Ichnos Sciences, Innovaderm, Janssen, Kiniksa, Kyowa Kirin, Leo Pharma, Novan, Novartis, Pfizer, Ralexar, Regeneron Pharmaceuticals, Inc., Sienna Biopharma, UCB and Union Therapeutics/Antibiotx; and is a consultant for Abbvie, Aditum Bio, Almirall, Alpine, Amgen, Arena, Asana Biosciences, AstraZeneca, Bluefin Biomedicine, Boehringer-Ingelheim, Boston Pharmaceuticals, Botanix, Bristol-Meyers Squibb, Cara Therapeutics, Celgene, Clinical Outcome Solutions, DBV, Dermavant, Dermira, Douglas Pharmaceutical, DS Biopharma, Eli Lilly, EMD Serono, Evelo Bioscience, Evidera, FIDE, Galderma, GSK, Haus Bioceuticals, Ichnos Sciences, Incyte, Kyowa Kirin, Larrk Bio, Leo Pharma, Medicxi, Medscape, Neuralstem, Noble Insights, Novan, Novartis, Okava Pharmaceuticals, Pandion Therapeutics, Pfizer, Principia Biopharma, RAPT Therapeutics, Realm, Regeneron Pharmaceuticals, Inc., Sanofi, SATO Pharmaceutical, Sienna Biopharma, Seanegy Dermatology, Seelos Therapeutics, Serpin Pharma, Siolta Therapeutics, Sonoma Biotherapeutics, Sun Pharma, Target PharmaSolutions and Union Therapeutics, Vanda Pharmaceuticals, Ventyx Biosciences, Vimalan. Consultant for Abbvie, Aditum Bio, Almirall, Amgen, Asana Biosciences, AstraZeneca, Boehringer-Ingelheim, Cara Therapeutics, Celgene, Concert, DBV, Dermira, DS Biopharma, Eli Lilly, EMD Serono, Galderma, Ichnos Sciences, Incyte Kyowa Kirin, Leo Pharma, Pandion Therapeutics, Pfizer, RAPT Therapeutics, Regeneron Pharmaceuticals, Inc., Sanofi, Sienna Biopharma, Target PharmaSolutions and Union Therapeutics. JMS - Grant support from Regeneron, Sanofi, Novartis, FARE and NIH. Consultant agreement with Regeneron, Sanofi, Novartis, Leo Pharma. KCB - Receives royalties from UpToDate. DYML - Consultant for AbbVie Pharma, Amagma Therapeutics, Incyte, Boehringer Ingelheim, Evomune, Genentech, Leo, Sanofi-Genzyme, and Zoetis. LAB - Consultant for Allakos, Arena Pharmaceuticals, DermTech, Evelo Biosciences, Galderma, Incyte, Janssen, LEO Pharma, Merck, Numab Therapeutics, Pfizer, Rapt Therapeutics, Regeneron, Ribon Therapeutics, Sanofi/Genzyme, Sanofi-Aventis, Stealth BioTherapeutics, Trevi Therapeutics, Union Therapeutics and Xencor. DMC member – Novartis. Investigator for Abbvie, Astra-Zeneca, DermTech, Kiniksa, Pfizer, Regeneron, Ribon Therapeutics and Sanofi.
References
- 1.Chiesa Fuxench ZC, Block JK, Boguniewicz M, Boyle J, Fonacier L, Gelfand JM, et al. Atopic Dermatitis in America Study: A Cross-Sectional Study Examining the Prevalence and Disease Burden of Atopic Dermatitis in the US Adult Population. J Invest Dermatol. 2019;139(3):583–90. [DOI] [PubMed] [Google Scholar]
- 2.Wang V, Boguniewicz J, Boguniewicz M, Ong PY. The infectious complications of atopic dermatitis. Ann Allergy Asthma Immunol. 2021;126(1):3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ong PY, Leung DY. Bacterial and Viral Infections in Atopic Dermatitis: a Comprehensive Review. Clin Rev Allergy Immunol. 2016;51(3):329–37. [DOI] [PubMed] [Google Scholar]
- 4.Silverberg JI. Health Care Utilization, Patient Costs, and Access to Care in US Adults With Eczema: A Population-Based Study. JAMA Dermatol. 2015;151(7):743–52. [DOI] [PubMed] [Google Scholar]
- 5.Bieber T, D'Erme AM, Akdis CA, Traidl-Hoffmann C, Lauener R, Schappi G, et al. Clinical phenotypes and endophenotypes of atopic dermatitis: Where are we, and where should we go? J Allergy Clin Immunol. 2017;139(4S):S58–S64. [DOI] [PubMed] [Google Scholar]
- 6.He H, Del Duca E, Diaz A, Kim HJ, Gay-Mimbrera J, Zhang N, et al. Mild atopic dermatitis lacks systemic inflammation and shows reduced nonlesional skin abnormalities. J Allergy Clin Immunol. 2021;147(4):1369–80. [DOI] [PubMed] [Google Scholar]
- 7.Zhou L, Leonard A, Pavel AB, Malik K, Raja A, Glickman J, et al. Age-specific changes in the molecular phenotype of patients with moderate-to-severe atopic dermatitis. J Allergy Clin Immunol. 2019;144(1):144–56. [DOI] [PubMed] [Google Scholar]
- 8.Sanyal RD, Pavel AB, Glickman J, Chan TC, Zheng X, Zhang N, et al. Atopic dermatitis in African American patients is TH2/TH22-skewed with TH1/TH17 attenuation. Ann Allergy Asthma Immunol. 2019;122(1):99–110 e6. [DOI] [PubMed] [Google Scholar]
- 9.Noda S, Suarez-Farinas M, Ungar B, Kim SJ, de Guzman Strong C, Xu H, et al. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. J Allergy Clin Immunol. 2015;136(5):1254–64. [DOI] [PubMed] [Google Scholar]
- 10.Yu SH, Silverberg JI. Association between Atopic Dermatitis and Depression in US Adults. J Invest Dermatol. 2015;135(12):3183–6. [DOI] [PubMed] [Google Scholar]
- 11.Simpson EL, Villarreal M, Jepson B, Rafaels N, David G, Hanifin J, et al. Patients with Atopic Dermatitis Colonized with Staphylococcus aureus Have a Distinct Phenotype and Endotype. J Invest Dermatol. 2018;138(10):2224–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beck LA, Boguniewicz M, Hata T, Schneider LC, Hanifin J, Gallo R, et al. Phenotype of atopic dermatitis subjects with a history of eczema herpeticum. J Allergy Clin Immunol. 2009;124(2):260–9, 9 e1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silverberg JI, Lei D, Yousaf M, Janmohamed SR, Vakharia PP, Chopra R, et al. Measurement properties of the Rajka-Langeland severity score in children and adults with atopic dermatitis. Br J Dermatol. 2021;184(1):87–95. [DOI] [PubMed] [Google Scholar]
- 14.Thyssen JP, Toft PB, Halling-Overgaard AS, Gislason GH, Skov L, Egeberg A. Incidence, prevalence, and risk of selected ocular disease in adults with atopic dermatitis. J Am Acad Dermatol. 2017;77(2):280–6 e1. [DOI] [PubMed] [Google Scholar]
- 15.Narla S, Silverberg JI. Association between atopic dermatitis and autoimmune disorders in US adults and children: A cross-sectional study. J Am Acad Dermatol. 2019;80(2):382–9. [DOI] [PubMed] [Google Scholar]
- 16.Simon de Lusignan HA, Broderick Conor, Dennis John, McGovern Andrew, Feeney Claire, Flohr Carsten,. Atopic dermatitis and risk of autoimmune conditions: Population-based cohort study. Journal of Allergy and Clinical Immunology. 2022(ISSN 0091-6749). [DOI] [PubMed] [Google Scholar]
- 17.NICHOLAS JONES RM, RAMIREZ ROBERTO, RÍOS-VARGAS MERARYS. Bureau USC. 2020 Census Illuminates Racial and Ethnic Composition of the Country. Censusgov 2021. . 2021. [Google Scholar]
- 18.Zhang A, Silverberg JI. Association of atopic dermatitis with being overweight and obese: a systematic review and metaanalysis. J Am Acad Dermatol. 2015;72(4):606–16 e4. [DOI] [PubMed] [Google Scholar]
- 19.Ali Z, Suppli Ulrik C, Agner T, Thomsen SF. Is atopic dermatitis associated with obesity? A systematic review of observational studies. J Eur Acad Dermatol Venereol. 2018;32(8):1246–55. [DOI] [PubMed] [Google Scholar]
- 20.Totte JE, van der Feltz WT, Hennekam M, van Belkum A, van Zuuren EJ, Pasmans SG. Prevalence and odds of Staphylococcus aureus carriage in atopic dermatitis: a systematic review and meta-analysis. Br J Dermatol. 2016;175(4):687–95. [DOI] [PubMed] [Google Scholar]
- 21.Williams H, Pottier A, Strachan D. Are viral warts seen more commonly in children with eczema? Arch Dermatol. 1993;129(6):717–20. [PubMed] [Google Scholar]
- 22.Larsson PA, Liden S. Prevalence of skin diseases among adolescents 12--16 years of age. Acta Derm Venereol. 1980;60(5):415–23. [PubMed] [Google Scholar]
- 23.Silverberg JI, Silverberg NB. Childhood atopic dermatitis and warts are associated with increased risk of infection: a US population-based study. J Allergy Clin Immunol. 2014;133(4):1041–7. [DOI] [PubMed] [Google Scholar]
- 24.Olsen JR, Gallacher J, Piguet V, Francis NA. Epidemiology of molluscum contagiosum in children: a systematic review. Fam Pract. 2014;31(2):130–6. [DOI] [PubMed] [Google Scholar]
- 25.Hayashida S, Furusho N, Uchi H, Miyazaki S, Eiraku K, Gondo C, et al. Are lifetime prevalence of impetigo, molluscum and herpes infection really increased in children having atopic dermatitis? J Dermatol Sci. 2010;60(3):173–8. [DOI] [PubMed] [Google Scholar]
- 26.Seize MB, Ianhez M, Cestari Sda C. A study of the correlation between molluscum contagiosum and atopic dermatitis in children. An Bras Dermatol. 2011;86(4):663–8. [DOI] [PubMed] [Google Scholar]
- 27.Oestergaard LB, Schmiegelow MDS, Bruun NE, Skov R, Andersen PS, Larsen AR, et al. Staphylococcus aureus Bacteremia in Children Aged 5-18 Years-Risk Factors in the New Millennium. J Pediatr. 2018;203:108–15 e3. [DOI] [PubMed] [Google Scholar]
- 28.Kawata M, Sasabuchi Y, Taketomi S, Inui H, Matsui H, Fushimi K, et al. Atopic dermatitis is a novel demographic risk factor for surgical site infection after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2018;26(12):3699–705. [DOI] [PubMed] [Google Scholar]
- 29.Weidinger S, Beck LA, Bieber T, Kabashima K, Irvine AD. Atopic dermatitis. Nat Rev Dis Primers. 2018;4(1):1. [DOI] [PubMed] [Google Scholar]
- 30.Lisa A Beck MJC, Amagai Masayuki, De Benedetto Anna, Kabashima Kenji, Hamilton Jennifer D., Rossi Ana B.,. Type 2 Inflammation Contributes to Skin Barrier Dysfunction in Atopic Dermatitis. JID Innovations. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshida T, Beck LA, De Benedetto A. Skin barrier defects in atopic dermatitis: From old idea to new opportunity. Allergol Int. 2022;71(1):3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alexander H, Paller AS, Traidl-Hoffmann C, Beck LA, De Benedetto A, Dhar S, et al. The role of bacterial skin infections in atopic dermatitis: expert statement and review from the International Eczema Council Skin Infection Group. Br J Dermatol. 2020;182(6):1331–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gernez Y, Freeman AF, Holland SM, Garabedian E, Patel NC, Puck JM, et al. Autosomal Dominant Hyper-IgE Syndrome in the USIDNET Registry. J Allergy Clin Immunol Pract. 2018;6(3):996–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Callewaert C, Nakatsuji T, Knight R, Kosciolek T, Vrbanac A, Kotol P, et al. IL-4Ralpha Blockade by Dupilumab Decreases Staphylococcus aureus Colonization and Increases Microbial Diversity in Atopic Dermatitis. J Invest Dermatol. 2020;140(1):191–202 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakatsuji T, Chen TH, Two AM, Chun KA, Narala S, Geha RS, et al. Staphylococcus aureus Exploits Epidermal Barrier Defects in Atopic Dermatitis to Trigger Cytokine Expression. J Invest Dermatol. 2016;136(11):2192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silverberg JI, Simpson EL. Association between severe eczema in children and multiple comorbid conditions and increased healthcare utilization. Pediatr Allergy Immunol. 2013;24(5):476–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paller AS, Mina-Osorio P, Vekeman F, Boklage S, Mallya UG, Ganguli S, et al. Prevalence of type 2 inflammatory diseases in pediatric patients with atopic dermatitis: Real-world evidence. J Am Acad Dermatol. 2021. [DOI] [PubMed] [Google Scholar]
- 38.Bock SA. Prospective appraisal of complaints of adverse reactions to foods in children during the first 3 years of life. Pediatrics. 1987;79(5):683–8. [PubMed] [Google Scholar]
- 39.Osborne NJ, Koplin JJ, Martin PE, Gurrin LC, Lowe AJ, Matheson MC, et al. Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. J Allergy Clin Immunol. 2011;127(3):668–76 e1-2. [DOI] [PubMed] [Google Scholar]
- 40.Eggesbo M, Botten G, Halvorsen R, Magnus P. The prevalence of CMA/CMPI in young children: the validity of parentally perceived reactions in a population-based study. Allergy. 2001;56(5):393–402. [DOI] [PubMed] [Google Scholar]
- 41.McGowan EC KC. Prevalence of self-reported food allergy in the National Health and Nutrition Examination Survey (NHANES) 2007-2010. J Allergy Clin Immunol. 2013;Nov;132(5):1216–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee A, Lee SY, Lee KS. Association of secondhand smoke exposure with allergic multimorbidity in Korean adolescents. Sci Rep. 2020;10(1):16409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kantor R, Kim A, Thyssen JP, Silverberg JI. Association of atopic dermatitis with smoking: A systematic review and meta-analysis. J Am Acad Dermatol. 2016;75(6):1119–25 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rajagopalan P, Nanjappa V, Raja R, Jain AP, Mangalaparthi KK, Sathe GJ, et al. How Does Chronic Cigarette Smoke Exposure Affect Human Skin? A Global Proteomics Study in Primary Human Keratinocytes. OMICS. 2016;20(11):615–26. [DOI] [PubMed] [Google Scholar]
- 45.Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol. 2002;2(5):372–7. [DOI] [PubMed] [Google Scholar]
- 46.Cid BJ, Perez-Mateluna G, Iturriaga C, Zambrano MJ, Vives MI, Valenzuela PM, et al. Is there an association between indoor allergens and the severity of atopic dermatitis? Int J Dermatol. 2019;58(4):433–9. [DOI] [PubMed] [Google Scholar]
- 47.Statistics. National Institute of Mental Health; [Available from: https://www.nimh.nih.gov/health/statistics [Google Scholar]
- 48.Silverwood RJ, Forbes HJ, Abuabara K, Ascott A, Schmidt M, Schmidt SAJ, et al. Severe and predominantly active atopic eczema in adulthood and long term risk of cardiovascular disease: population based cohort study. BMJ. 2018;361:k1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmidt SAJ, Olsen M, Schmidt M, Vestergaard C, Langan SM, Deleuran MS, et al. Atopic dermatitis and risk of atrial fibrillation or flutter: A 35-year follow-up study. J Am Acad Dermatol. 2020;83(6):1616–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lazzerini PE, Capecchi PL, Laghi-Pasini F. Systemic inflammation and arrhythmic risk: lessons from rheumatoid arthritis. Eur Heart J. 2017;38(22):1717–27. [DOI] [PubMed] [Google Scholar]
- 51.George SM, Taylor MR, Farrant PB. Psoriatic alopecia. Clin Exp Dermatol. 2015;40(7):717–21. [DOI] [PubMed] [Google Scholar]
- 52.Liesegang TJ, Melton LJ 3rd, Daly PJ, Ilstrup DM. Epidemiology of ocular herpes simplex. Incidence in Rochester, Minn, 1950 through 1982. Arch Ophthalmol. 1989;107(8):1155–9. [DOI] [PubMed] [Google Scholar]
- 53.Haimann MH, Burton TC, Brown CK. Epidemiology of retinal detachment. Arch Ophthalmol. 1982;100(2):289–92. [DOI] [PubMed] [Google Scholar]
- 54.Kennedy RH, Bourne WM, Dyer JA. A 48-year clinical and epidemiologic study of keratoconus. Am J Ophthalmol. 1986;101(3):267–73. [DOI] [PubMed] [Google Scholar]
- 55.Lu Z, Zeng N, Cheng Y, Chen Y, Li Y, Lu Q, et al. Atopic dermatitis and risk of autoimmune diseases: a systematic review and meta-analysis. Allergy Asthma Clin Immunol. 2021;17(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Drucker AM, Thompson JM, Li WQ, Cho E, Li T, Guttman-Yassky E, et al. Incident alopecia areata and vitiligo in adult women with atopic dermatitis: Nurses' Health Study 2. Allergy. 2017;72(5):831–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wei YH, Tai YH, Dai YX, Chang YT, Chen TJ, Chen MH. Bidirectional association between alopecia areata and atopic dermatitis: A population-based cohort study in Taiwan. Clin Exp Allergy. 2020;50(12):1406–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.