Abstract
Purpose:
Cancer-related worry (concerns related to cancer and its late effects) (CRW) is prevalent among childhood cancer survivors. Elevated cancer-related worry has been associated with self-reported suboptimal physical activity. The aim of this investigation was to describe associations between cancer-related worry and objectively assessed physical activity in childhood cancer survivors.
Methods:
CRW was assessed at a baseline evaluation using six survey items. Weekly minutes of moderate and vigorous physical activity were captured by actigraphy 5.25-years (3.8–8.0) later. Factor analysis was used to identify types of worry; multiple regression determined independent associations between cancer-related worry and moderate and vigorous physical activity adjusting for sex, race, diagnosis, age at baseline, anxiety level at baseline, self-reported physical activity at baseline, and pain interference at baseline.
Results:
Participants (n=1,223) were an average of 30.9-years (SD=6.9) at baseline and 36.1-years (SD=7.1) at follow-up. Thirty-seven percent (37%) were survivors of leukemia, 26% of non-central nervous system solid tumors, 19% of lymphoma, 11% of central nervous system tumors, and 6% of other malignancies. Two types of cancer-related worry were identified: “Body-Focused” and “General Fear”. “Body-Focused” CRW (β=−19.6, p=0.012), endorsing pain interference (β=−27.7, p=0.002) at baseline and having a diagnosis of CNS tumor (β=−41.3, p=0.0003) or non-CNS solid tumor (β=−19.4, p=0.02) were negatively associated with physical activity at follow-up.
Conclusions:
Cancer-related worry related to bodily function and appearance is associated with decreased physical activity. Clinicians should consider the potential negative impact of cancer-related worry on physical activity levels and provide behavioral counseling.
Keywords: CANCER-RELATED WORRY, PHYSICAL ACTIVITY, CHILDHOOD CANCER SURVIVORS, LONGITUDINAL, ACTIGRAPHY
INTRODUCTION
Advances in therapeutic strategies and supportive care have led to increased survival among children with cancer.(1) Childhood cancer survivors (referred to hereafter as survivors) are at risk for both physical and psychological late effects of treatment. Among these, impaired cardiopulmonary fitness,(2) obesity,(3) low lean muscle mass,(4–11) muscle weakness,(6, 11–14) and fatigue(15, 16) are common. Adverse psychological outcomes include anxiety, depression and pain.(17) Many of these conditions are associated with cancer or prior treatment exposures,(18–21) but lifestyle and health behaviors can influence the magnitude of risk.(22–24)
Cancer-related worry (CRW) is a prevalent psychological outcome following treatment for childhood cancer, with reports ranging from 33–62% for various domains of CRW in samples of survivors.(25–28) CRW is a complex psychological outcome and has been linked to both pain and anxiety in survivors.(29) Cancer-related worry (CRW), along with pain and anxiety, may have potential positive or negative effects by either stimulating engagement in or avoidance of healthy behaviors. Due to differences in individual response to CRW and the potential impact on health behavior, investigations into worry and correlates to concomitant health behaviors have been conducted among survivors of childhood cancer.(30, 31) In the St. Jude Lifetime Cohort Study (SJLIFE),(28) an analysis of 3,211 survivors of varying diagnoses, mean age 31.2±8.4 years, mean years since diagnosis 22.8±8.3, the prevalence of CRW was 33%. In a model adjusted for chronic conditions, elevated worry about physical problems due to cancer was associated with lower likelihood to report meeting physical activity (PA) guidelines (RR, 0.92; 95% CI, 0.86–0.98).(28) However, this study utilized cross-sectional data and longitudinal research is needed to identify appropriate interventions to optimize participation in PA.
Evaluating the predictive value of CRW for subsequent PA among survivors is important to understanding progression of causal associations. Ideally, as self-reported PA is prone to measurement error,(32) objectively ascertained PA data will improve the precision of the estimated association and help determine if an intervention to address CRW might have meaningfully impact on improving PA outcomes. Therefore, the purpose of this investigation was to describe the longitudinal association between self-reported CRW at a baseline timepoint and objectively ascertained PA obtained at five-year follow-up.
METHODS
Inclusion and Exclusion Criteria
Participants for this study were recruited from a subset of SJLIFE, participating in an ancillary study evaluating progression of frailty in survivors (IRB: Pro00004536).(33) The St. Jude Lifetime Cohort Study (SJLIFE) protocols involve a comprehensive, clinical assessment performed on the campus of St. Jude Children’s Research Hospital (SJCRH) as well as completion of surveys containing self-reported demographic information as well as patient reported outcomes.
Eligible participants had a baseline SJLIFE visit between January 1, 2008 and June 30, 2013 when 18–45-years of age, were at least 10-years post-original diagnosis, had completed worry related questions at the baseline visit, and wore an accelerometer to capture PA data at the follow-up visit. Pregnant or lactating women were ineligible. Study measures and documents were approved by the SJCRH Institutional Review Board (IRB). Participants provided written informed consent prior to study activities.
Measures
Cancer-related worry (CRW).
Cancer-related worry (CRW) was assessed at baseline using 6 questionnaire items: 1) “I have general fears about cancer”, 2) “I am worried about my cancer coming back”, 3) “I mostly worry about my cancer and it’s treatment right before I go for a check-up”, 4) “I am concerned about physical problems related to my cancer”, 5) “I am worried about my appearance”, and 6) “Do you currently have anxieties/fears as a result of your cancer or similar illness, or it’s treatment?” The first five items were on a Likert scale from “strongly disagree” to “strongly agree”, and the final item was on a Likert scale from 1 (“no anxiety/fears”) to 5 (“very many, extreme anxiety/fears”). The previous study in SJLIFE utilized 3 items to assess CRW; for the purposes of exploring multidimensional CRW, the present study utilized 6. Factor analysis with varimax rotation was used to determine factor loadings among the six CRW variables of interest. This process resulted in two factors: “General Fear” (comprised of items: “I have general fears about cancer”; “I am worried about my cancer coming back”; “I mostly worry about my cancer and it’s treatment right before I go for a check-up”) and “Body-Focused” (comprised of items: “I am concerned about physical problems related to my cancer”; “I am worried about my appearance”; “Do you currently have anxieties/fears as a result of your cancer or similar illness, or it’s treatment?”) (see Supplemental Table 1, Supplemental Digital Content, Factor loadings). Mean factor scores were created for each participant by taking the average of each factors’ respective items. Average factor scores greater than three (corresponding to greater than “neutral” response) were classified as endorsing CRW in factor domains of “General Fear” and/or “Body-Focused.” CRW data was also available at the follow-up point.
Follow-up Physical Activity
Average daily levels of moderate and vigorous physical activity (MVPA) were evaluated with actigraphy as part of the parent study. Participants wore a triaxial accelerometer (ActiGraph Activity Monitor Devices (RRID:SCR_008399) wGT3X-BT; ActiGraph, LLC, Pensacola, FL) over the right hip during waking hours for 7 days,(34, 35) removing it for bathing. The device collects linear accelerations in three planes of movement as counts per a preset epoch. Epochs were set at 60-seconds. ActiLife software (ActiGraph Activity Monitor Devices (RRID:SCR_008399) ver. 6.11.5; Actigraph, LLC; Pensacola, FL) was used to process the accelerometer data with thresholds for MVPA set at 1,952 and 5,725 counts per epoch. A valid wear day was defined as 10 or more hours of activity monitor wear.(36) Some participants from the parent study (n=171 out of 1432) were ineligible for this study due to missing or corrupt accelerometers at follow-up. Average daily minutes of MVPA were then used to quantify average MVPA (minutes) per week for each participant by multiplying daily value by 7 days. Seven days was selected as a representative measurement period in alignment with previous findings in healthy adults.(35)
Covariates
Psychological and Somatic Symptoms.
Baseline anxiety was assessed using the anxiety subscale of the Brief Symptom Inventory (BSI-18).(37) T-scores were created for each participant,(37) and scores ≥63 (top 10th percentile) were classified as elevated anxiety.
Baseline pain interference was assessed using the following item from the Short-Form Health Survey (SF-36)(38): “During the past 4-weeks, how much did pain interfere with your normal work (including both work outside the home and housework)?” Responses were designated on a 5-point Likert scale ranging from “not at all” to “extremely.” Responses greater than 3 (“moderately”) were classified as indicating pain interference.
Baseline Physical Activity Level.
Participants’ PA level at baseline was collected via self-report focusing on the frequency and amount of moderate to vigorous weekly PA using standard questions from National Health and Nutrition Examination Survey Physical Activity Questionnaire.(39) This survey assesses physical activity during the past 7 days. Self-reported estimated minutes per week of moderate to vigorous physical activity were calculated from participants’ responses. These values were utilized to create a binary variable to identify if participants met Centers for Disease Control and Prevention (CDC) PA guidelines (150-minutes of moderate and/or 75-minutes of vigorous exercise per week(40)) at baseline.
Demographics and Clinical Data.
Primary diagnosis and age at diagnosis were obtained from medical records. Presence of chronic conditions was assessed at SJLIFE clinical visit. Chronic conditions were graded according to SJLIFE modified National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) criteria.(41) Age at baseline assessment, age at follow-up assessment, sex, and race were obtained via survey.
Analysis
Demographics and diagnosis-related characteristics of participants were summarized using descriptive statistics. T-test and Chi square statistics were calculated to compare demographic factors between participants and non-participants. Dichotomous variables for average CRW factor scores (endorsing or not endorsing CRW) were used for analysis. PA was quantified in daily minutes of MVPA.
Multivariable linear regression was utilized to evaluate associations between baseline variables of interest and PA levels five-years later. A priori, the following variables assessed at baseline were selected for use in the regression model: sex, race, diagnosis group, age, anxiety, pain interference, self-reported PA, and presence of cardiac, pulmonary, musculoskeletal, neurological, and endocrine conditions. Of these, the presence of chronic health conditions, endorsing anxiety, and the “General Fear” factor of CRW were determined in preliminary analyses to be insignificant and dropped from the model (see Supplemental Table 2, Supplemental Digital Content, Multivariate regression analysis of physical activity levels - preliminary model). Average MVPA minutes per week was the outcome in both models. Participants with missing data for any covariates were excluded from analysis. Analysis was conducted with SAS (Statistical Analysis System (RRID:SCR_008567) ver. 9.4, Cary, NC).
RESULTS
Participant Demographic Data
A total of 1,223 participants (49% female) were eligible for analysis (Figure 1). Participants were an average of 30.9 years (SD=6.9) at baseline and 36.1 years (SD=7.1) at follow-up; average follow-up time was 5.3 years (SD=0.7). Thirty-seven percent (37%) were survivors of leukemia, 26% of non-central nervous system (CNS) solid tumors, 19% of lymphoma, 11% of CNS tumors, and 6% of other malignancies. Treatments included chemotherapy (71%), surgery (3%), and radiation therapy (28%). At the follow-up assessment, three-fourths (75.3%) of the current sample were not adhering to recommended levels of PA. On average, participants wore the accelerometers 6.5 (SD= 1.2) calendar days. Participants and non-participants differed on age at baseline and age at follow-up assessment, racial distribution, and frequency of pulmonary conditions at baseline (Table 1).
Figure 1 –
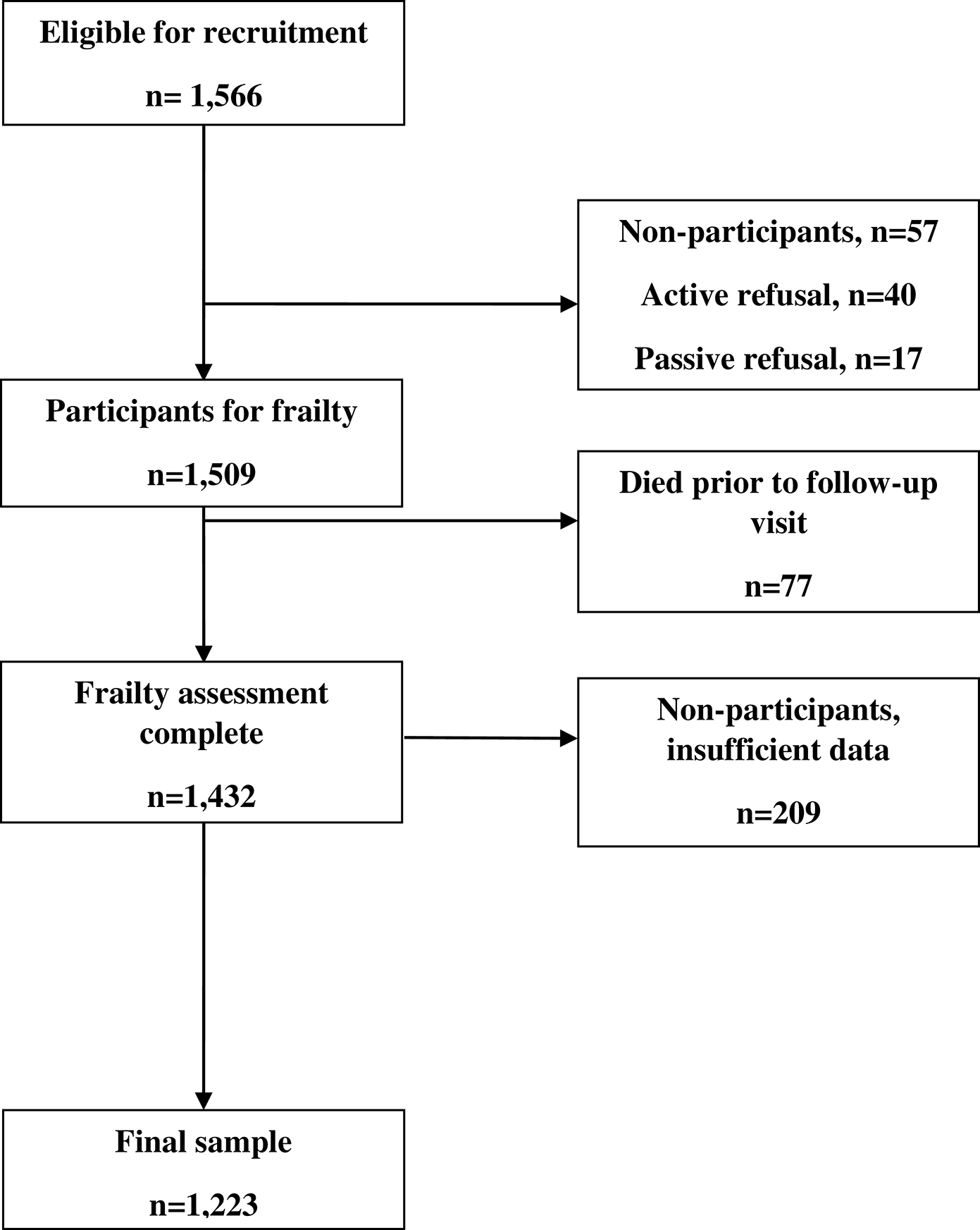
Study flow diagram. n: number
Table 1.
Population Characteristics of Participants and Non-Participants
| Participants (n=1223) | Non-Participants (n=209) | P value | |||
|---|---|---|---|---|---|
| Characteristic | Mean (SD) | No. (%) | Mean (SD) | No. (%) | |
| Age at diagnosis (y) | 8.12 (5.46) | 7.84 (5.35) | 0.49 | ||
| Age at baseline evaluation (y) | 30.91 (6.86) | 28.57 (6.21) | <0.001* | ||
| Age at follow-up evaluation (y) | 36.14 (7.13) | 33.71 (6.39) | <0.001* | ||
| Sex Male Female |
622 (50.94) 601 (49.06) |
110 (52.63) 99 (47.37 |
0.64 | ||
| Race White Black Other |
1028 (84.06) 185 (15.13) 10 (0.01) |
171 (81.82) 32 (15.31) 6 (2.87) |
0.03* | ||
| Diagnosis Leukemia Non-CNS solid tumor Lymphoma CNS tumor Other |
455 (37.20) 319 (26.08) 235 (19.22) 140 (11.45) 74 (6.05) |
93 (44.50) 56 (26.79) 36 (17.22) 16 (7.66) 8 (3.83) |
0.31 | ||
| Radiation Yes None |
342 (27.96) 881 (72.04) |
48 (22.97) 161 (77.03) |
0.13 | ||
| Chemotherapy Yes None |
840 (68.68) 383 (31.32) |
140 (66.99) 69 (33.01) |
0.63 | ||
| Surgery Yes None |
34 (2.78) 1189 (97.22) |
7 (3.35) 2202 (96.65) |
0.65 | ||
| Cardiac Condition Grade 3+ at baseline Yes No |
142 (11.61) 1081 (88.39) |
20 (9.57) 189 (90.43) |
0.39 | ||
| Endocrine Condition Grade 3+ at baseline Yes No |
745 (60.92) 478 (39.08) |
135 (64.59) 74 (35.41) |
0.31 | ||
| Pulmonary Condition Grade 3+ at baseline Yes No |
114 (9.32) 1109 (90.68) |
9 (4.31) 200 (95.69) |
0.02* | ||
| Musculoskeletal Condition Grade 3+ at baseline Yes No |
185 (15.13) 1038 (84.87) |
33 (15.79) 176 (84.21) |
0.81 | ||
| Neurological Condition Grade 3+ at baseline Yes No |
167 (13.65) 1056 (86.35) |
22 (10.53) 187 (89.47) |
0.22 | ||
| Worry Scores “General Fear” “Body-Focused” |
2.79 (1.07) 2.56 (0.88) |
||||
| Physical Activity Level MVPA per day MVPA per week |
15.66 (17.10) 109.59 (119.66) |
||||
denotes statistical significance (p<0.05)
CNS: central nervous system; <: less than; MVPA: moderate and vigorous physical activity; N: number; %: percent; +: plus; p: probability; SD: standard deviation; y: year
Cancer-Related Worry
Average scores for the “General Fear” factor were 2.8 (SD=1.1) and 2.6 (SD= 0.9) for the “Body-Focused” factor. Twenty-five percent (25%) of survivors endorsed “Body-Focused” CRW while 40% of endorsed “General Fear” CRW. At follow-up, average minutes of MVPA per day was 16±17, and average minutes of MVPA per week was 109±120 (Table 1). Survivors who did not complete the questionnaire containing worry items were excluded from regression analysis (n=23). All survivors completed the anxiety questionnaire items and only 13 survivors did not complete the pain interference item.
Analyses of Physical Activity Level
In multivariable models adjusting for sex, race, diagnosis group, and baseline assessments of age, pain interference, and meeting CDC PA guidelines, endorsing the “Body-Focused” factor was negatively associated with PA minutes at follow-up (β=−19.6, SE=7.8, p=0.01). Endorsing pain interference (β=−27.7, p=0.002) at baseline and having a diagnosis of CNS tumor (β=−41.3, p=0.0003) or non-CNS solid tumor (β=−19.4, p=0.02) were negatively associated with PA minutes at follow-up. Meeting PA guidelines at baseline was positively associated with PA minutes at follow-up (β=24.8, p=0.0002). Black race (β=−24.1, p=0.009), female sex (β=−56.5, p<0.0001), and age at baseline (β=−1.8, p=0.0002) were negatively associated with PA minutes at follow-up. Full results are displayed in Table 2.
Table 2.
Multivariate Regression Analysis of Physical Activity Levels
| Independent Variables | Estimate (β) | Standard Error (SE) | t value | Pr> |t| |
|---|---|---|---|---|
| Body-Focused CRW | −19.64 | 7.83 | −2.51 | 0.012* |
| Age at baseline | −1.84 | 0.50 | −3.70 | 0.0002* |
| Gender Male vs. Female |
−56.48 |
6.63 |
−8.52 |
<0.0001* |
| Diagnosis Group CNS Tumor vs. Leukemia Lymphoma vs. Leukemia Non-CNS Tumor vs. Leukemia Other vs. Leukemia |
−41.30 7.63 −19.44 −9.87 |
11.26 9.23 8.25 14.15 |
−3.67 0.83 −2.36 −0.70 |
0.0003* 0.41 0.02* 0.49 |
| Race Black vs. White Other vs. White |
−24.06 −27.80 |
9.22 35.75 |
−2.61 −0.78 |
0.01* 0.44 |
| Pain interference Endorsing vs. Not endorsing |
−27.65 |
9.10 |
−3.04 |
0.0024* |
| Baseline physical activity minutes Not meeting CDC guidelines vs. Meeting CDC guidelines |
24.84 |
6.71 |
3.70 |
0.0002* |
denotes statistical significance (p<0.05)
β: Beta; CDC: Centers for Disease Control and Prevention; CNS: central nervous system; CRW: Cancer-related worry; <: less than; Pr: the probability of; Pr> |t|: p-value associated with the value in the t value column; SE: standard error; t: t-statistic; vs.: versus
Additional analyses were conducted to assess associations between CRW at follow-up and PA minutes; in multivariable models adjusting for sex, race, diagnosis group, age at follow-up, anxiety at follow-up, pain interference at follow-up, meeting CDC PA guidelines at baseline, and presence of cardiac, pulmonary, musculoskeletal, neurological, and endocrine conditions, neither “Body-Focused” nor “General Fear” CRW at follow-up were associated with PA minutes. To further contextualize these results, self-reported PA was then calculated (using the same method described above for baseline self-reported PA) and classified into meeting vs. not meeting CDC PA guidelines for logistic regression models of self-reported PA at follow-up. In multivariable models adjusting for sex, race, diagnosis group, age at follow-up, anxiety at follow-up, pain interference at follow-up, meeting CDC PA guidelines at baseline, and presence of cardiac, pulmonary, musculoskeletal, neurological, and endocrine conditions, those who endorsed the “Body-Focused” CRW factor at follow-up were less likely to meet CDC PA guidelines (OR: 0.66, CI 0.47, 0.95). Full results are displayed in Supplemental Tables 3 and 4 (see Supplemental Digital Content, Multivariate regression analysis of physical activity levels – model measures at follow-up, and Multinomial logistic regression for self-reported physical activity).
DISCUSSION
We provide evidence of an association between CRW and objectively measured PA in a longitudinal paradigm. A sizable portion of childhood cancer survivors in this sample endorsed the two types of CRW identified in factor analysis with 40% endorsing “General Fear” and 25% endorsing “Body-Focused” CRW. The frequency observed in this sample is consistent with other reports from childhood cancer survivors in the literature, ranging from 33 to 62% for various domains of CRW.(25–28) Three-fourths (75.3%) of the current sample was not adhering to recommended levels of PA at follow-up, which is larger than frequencies obtained with self-report in the survivor population (over half, 52%),(42) and exceeds evidence reported in the general population (~50%).(43)
Survivors in this sample were more likely to endorse concerns with “General Fear” CRW than “Body-Focused” CRW. Nonetheless, we observed a negative association between “Body-Focused” CRW and PA was observed, compared to a non-significant association between “General Fear” CRW and PA. These results suggest a unique contribution of CRW related to bodily function, consequences of treatment on bodily function, and appearance to engagement in PA. Additionally, we observed a negative association between baseline pain interference and PA; this association should be taken in context of the use of the single item from the SF-36 (“During the past 4-weeks, how much did pain interfere with your normal work (including both work outside the home and housework?”) and therefore could be limited in precision and reliability. In previous study of CRW in childhood cancer survivors, strong associations were found between CRW and pain interference also assessed with the same single item from the SF-36.(28) While the observed association follows that which is observed in the general population for overall pain endorsement and PA(44), our study focuses only on perceived pain interference, not overall endorsement. Pain is a complex construct, and future studies should consider using tools that assess multiple dimensions of pain to understand the effects on PA behavior in childhood cancer survivors. Finally, we observed lower physical activity levels in individuals with CNS and non-CNS solid tumor diagnoses. This finding is consistent with evidence reported in other studies of physical activity levels in childhood cancer survivors, in which lower levels of physical activity were observed in survivors of CNS and bone tumors.(42, 45, 46)
Our study is unique to the larger literature because we evaluated the temporal nature of the association between CRW and PA, ascertained PA with accelerometry, and accounted for baseline PA. These findings, as well as others (31), shed light on the potential for CRW to predict negative or health-adverse behaviors. Data gathered in 272 adolescent childhood cancer survivors indicated that worry pertaining to physical problems, appearance, cancer recurrence, health care access, and finding physician care was associated with increased substance abuse one year later.(31) Future studies should consider assessing the effect of CRW on health behaviors longitudinally.
Our additional analyses utilizing cross-sectional CRW and objectively measured PA revealed no association between CRW and PA. However, when self-report PA data from this sample was utilized as an outcome cross-sectionally, CRW was negatively associated with meeting CDC PA guidelines. We previously showed that CRW was negatively associated with self-report of meeting the CDC guidelines for PA in a cohort of 3,211 survivors.(28) Another group of authors, in a sample of 215 adolescent and young adult survivors of childhood cancer, reported an association between self-reported leisure time PA and lessened cancer-related worry.(30) We did not query survivors about perceived benefits of PA participation. However, Finnegan and colleagues, in a study among 117 young adult survivors, suggest that the association between CRW and PA behavior is moderated by perceived benefits of physical activity. In their study, using a sample of convenience where 80% of participants reported meeting PA guidelines, survivors who endorsed higher levels of worry and who perceived fewer benefits of PA were less likely to be active when compared to survivors who worried less and perceived more benefits of PA.(47) Together, these data suggest that there are differences in the associations between CRW and objectively and subjectively assessed PA at cross-sectional and longitudinal timepoints. Future studies should consider these differences when observing impact of CRW on PA.
Interventions to address worry have been developed for use in the general population, and utilize methods such as Ecological Momentary Assessment (EMA) and Cognitive Behavioral Therapy (CBT).(48, 49) These interventions have observed effects of interventions on worry in relation to health behaviors, such as smoking, alcohol consumption, and diet. As reported in a recent meta-analysis, these interventions to address worry or rumination demonstrate a moderate heterogenous effect for health behaviors such as smoking, alcohol intake, and dietary intake.(48) While these effects are seen in health behaviors other than PA, health behaviors do tend to follow a clustered pattern. Some of these interventions on worry have been reworked to function in different populations of cancer survivors, such as breast cancer survivors, but with a focus on health outcomes (i.e., blood pressure) rather than health behaviors.(50) However, repurposing these interventions to focus on unique aspects of worry in childhood cancer survivorship and coupling these interventions with PA or exercise intervention has yet to be explored. Incorporating purposeful PA into interventions that focus on worry could provide childhood cancer survivors with an outlet to address worry. For example, resistance training activities could be incorporated to offer participants with a way to address worry related to muscle weakness or physical function. Framing PA as a mechanism by which to address worry related to body function could be helpful to maximizing the potential impact of PA on worry in this population, as worry related to physical function and/or appearance was an issue in this sample. However, the longitudinal data collected from this sample would suggest that focus should be on long-term engagement in physical activity to address worry and building physical activity into long-term habits.
Limitations
This investigation is not without limitations. First, although representative of patients treated at SJCRH from 1962 to 2012, this sample was not ethnically diverse (84% Non-Hispanic White). A more diverse sample would help make broader interpretations of the impact of CRW on PA. Secondly, selection bias is possible because not all eligible participants wore the accelerometer at the follow-up timepoint. Participants in this study were slightly older, more likely to be White, and more likely to have chronic pulmonary conditions than non-participants. While having chronic pulmonary conditions was not associated with objectively measured physical activity, age at baseline and Black race were associated with reduced physical activity. The estimates observed in this study may be inflated (if younger non-participants experienced CRW) or biased toward the null hypothesis (if Black non-participants experienced CRW). Previously reported data from the SJLIFE cohort indicates that by age 50, childhood cancer survivors have experienced (on average) 17 chronic health conditions.(51) Given the average age (36 years) of this sample, these conclusions might not be generalizable to survivors of older age with potentially more chronic disease burden. As this investigation utilized data from a previous study that focused on frailty, these data should be interpreted considering the distribution of frailty in that cohort (13.6% frail at 5-year follow-up).(33) The assessment of CRW utilized in this study is not a validated measure, but rather are a set of questions developed specifically for the SJLIFE cohort study; although, it is of note that few measures of CRW are validated in adult survivors of childhood cancer. While a 5-year follow-up period does give a glimpse at long-term behavior, longer follow-up durations may yield different results. Further studies should attempt longer term follow-up to obtain data regarding the role of CRW in PA over a longer time, and potential changes over the lifespan. Further, the use of self-reported PA as a baseline measure of PA is a limitation of this study; however, self-report PA at baseline and objectively measured PA at follow-up were associated (r=0.18, p=<0.0001). The present results do not lend themselves to the experiences of participants prior to baseline assessment, and the data are not available regarding how long a participant experienced CRW prior to the baseline assessment. The duration of CRW may affect PA and was not captured in our study. Finally, while CRW items themselves give a glimpse into what body-focused worry impacts, this investigation does not provide specifics of how that concept manifests itself for survivors (i.e., specific thoughts, specific persuasions towards PA, etc.). Qualitatively collecting this information may enlighten these results and potentially provide more specific avenues for intervention.
CONCLUSIONS
In the present sample of 1,223 childhood cancer survivors, cancer-related worry (CRW) is negatively associated with objectively measured PA longitudinally. These data indicate that worry related to bodily function and appearance could provide valuable insights into long-term PA behavior in childhood cancer survivors. Physical activity (PA) practitioners could consider the potential negative impact of this type of CRW when working with this population. Further research seeking to characterize determinants of body focused CRW may provide insight into specific avenues of intervention for CRW in the context of PA and/or exercise.
Supplementary Material
Acknowledgements:
This study was funded by National Institutes of Health, NCI T32 CA225590 (Krull KR), NCI U01 CA195547 (Hudson MM/Ness KK), NCI R01 CA174851 (Ness KK), and the American Lebanese Syrian Associated Charities (ALSAC). The authors would like to thank Tracie Gatewood for organization of this submission. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The results of the present study do not constitute an endorsement by the American College of Sports Medicine.
Footnotes
Conflict of Interest: The authors make no disclosures.
SUPPLEMENTAL DIGITAL CONTENT
SDC 1: Supplemental Digital Content.docx
Table S1 – Factor loadings
Table S2 - Multivariate regression analysis of physical activity levels - preliminary model
Table S3 - Multivariate regression analysis of physical activity levels – model measures at follow-up
Table S4 - Multinomial logistic regression for self-reported physical activity
REFERENCES
- 1.Surveillance, Epidemiology, and End Results Program. Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2018 Sub (2000–2016) <Katrina/Rita Population Adjustment> [Internet]. 2019. Available from: https://seer.cancer.gov/data-software/documentation/seerstat/nov2018/.
- 2.Hoffman MC, Mulrooney DA, Steinberger J, Lee J, Baker KS, Ness KK. Deficits in physical function among young childhood cancer survivors. J Clin Oncol. 2013;31(22):2799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mostoufi-Moab S, Seidel K, Leisenring WM, et al. Endocrine abnormalities in aging survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2016;34(27):3240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandez-Pineda I, Hudson MM, Pappo AS, et al. Long-term functional outcomes and quality of life in adult survivors of childhood extremity sarcomas: a report from the St. Jude Lifetime Cohort Study. J Cancer Surviv. 2017;11(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meacham LR, Gurney JG, Mertens AC, et al. Body mass index in long-term adult survivors of childhood cancer: a report of the Childhood Cancer Survivor Study. Cancer. 2005;103(8):1730–9. [DOI] [PubMed] [Google Scholar]
- 6.Ness KK, Baker KS, Dengel DR, et al. Body composition, muscle strength deficits and mobility limitations in adult survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2007;49(7):975–81. [DOI] [PubMed] [Google Scholar]
- 7.Tonorezos ES, Robien K, Eshelman-Kent D, et al. Contribution of diet and physical activity to metabolic parameters among survivors of childhood leukemia. Cancer Causes Control. 2013;24(2):313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boland AM, Gibson TM, Lu L, et al. Dietary protein intake and lean muscle mass in survivors of childhood acute lymphoblastic leukemia: report from the St. Jude Lifetime Cohort Study. Phys Ther. 2016;96(7):1029–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chemaitilly W, Li Z, Huang S, et al. Anterior hypopituitarism in adult survivors of childhood cancers treated with cranial radiotherapy: a report from the St. Jude Lifetime Cohort Study. J Clin Oncol. 2015;33(5):492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ness KK, Krull KR, Jones KE, et al. Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer: a report from the St. Jude Lifetime Cohort Study. J Clin Oncol. 2013;31(36):4496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scheede-Bergdahl C, Jagoe RT. After the chemotherapy: potential mechanisms for chemotherapy-induced delayed skeletal muscle dysfunction in survivors of acute lymphoblastic leukaemia in childhood. Front Pharmacol. 2013;4:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hovi L, Era P, Rautonen J, Siimes MA. Impaired muscle strength in female adolescents and young adults surviving leukemia in childhood. Cancer. 1993;72(1):276–81. [DOI] [PubMed] [Google Scholar]
- 13.Schoenmakers M, Takken T, Gulmans V, et al. Muscle strength and functional ability in children during and after treatment for acute lymphoblastic leukemia or T-cell non-Hodgkin lymphoma: a pilot study. Cancer Ther. 2006;4:241–8. [Google Scholar]
- 14.Talvensaari KK, Jämsen A, Vanharanta H, Lanning M. Decreased isokinetic trunk muscle strength and performance in long-term survivors of childhood malignancies: correlation with hormonal defects. Arch Phys Med Rehabil. 1995;76(11):983–8. [DOI] [PubMed] [Google Scholar]
- 15.Daniel LC, Brumley LD, Schwartz LA. Fatigue in adolescents with cancer compared to healthy adolescents. Pediatr Blood Cancer. 2013;60(11):1902–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomlinson D, Zupanec S, Jones H, O’Sullivan C, Hesser T, Sung L. The lived experience of fatigue in children and adolescents with cancer: a systematic review. Support Care Cancer. 2016;24(8):3623–31. [DOI] [PubMed] [Google Scholar]
- 17.Brinkman TM, Li C, Vannatta K, et al. Behavioral, social, and emotional symptom comorbidities and profiles in adolescent survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2016;34(28):3417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phillips SM, Padgett LS, Leisenring WM, et al. Survivors of childhood cancer in the United States: prevalence and burden of morbidity. Cancer Epidemiol Biomarkers Prev. 2015;24(4):653–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tai E, Buchanan N, Townsend J, Fairley T, Moore A, Richardson LC. Health status of adolescent and young adult cancer survivors. Cancer. 2012;118(19):4884–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309(22):2371–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ness KK, Gurney JG. Adverse late effects of childhood cancer and its treatment on health and performance. Annu Rev Public Health. 2007;28:279–302. [DOI] [PubMed] [Google Scholar]
- 22.Fisher RS, Rausch JR, Ferrante AC, et al. Trajectories of health behaviors across early childhood cancer survivorship. Psychooncology. 2019;28(1):68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ford JS, Barnett M, Werk R. Health behaviors of childhood cancer survivors. Children (Basel). 2014;1(3):355–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tonorezos ES, Ford JS, Wang L, et al. Impact of exercise on psychological burden in adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer. 2019;125(17):3059–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibson TM, Li C, Armstrong GT, et al. Perceptions of future health and cancer risk in adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer. 2018;124(16):3436–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cunningham SJ, Patton M, Schulte F, Richardson PA, Heathcote LC. Worry about somatic symptoms as a sign of cancer recurrence: prevalence and associations with fear of recurrence and quality of life in survivors of childhood cancer. Psychooncology. 2021;30(7):1077–85. [DOI] [PubMed] [Google Scholar]
- 27.Wang R, Syed IA, Nathan PC, Barr RD, Rosenberg-Yunger ZR, Klassen AF. Exploring cancer worry in adolescent and young adult survivors of childhood cancers. J Adolesc Young Adult Oncol. 2015;4(4):192–9. [DOI] [PubMed] [Google Scholar]
- 28.McDonnell GA, Brinkman TM, Wang M, et al. Prevalence and predictors of cancer-related worry and associations with health behaviors in adult survivors of childhood cancer. Cancer. 2021;127(15):2743–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brinkman TM, Zhu L, Zeltzer LK, et al. Longitudinal patterns of psychological distress in adult survivors of childhood cancer. Br J Cancer. 2013;109(5):1373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paxton RJ, Jones LW, Rosoff PM, Bonner M, Ater JL, Demark-Wahnefried W. Associations between leisure-time physical activity and health-related quality of life among adolescent and adult survivors of childhood cancers. Psychooncology. 2010;19(9):997–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cox CL, McLaughlin RA, Steen BD, Hudson MM. Predicting and modifying substance use in childhood cancer survivors: application of a conceptual model. Oncol Nurs Forum. 2006;33(1):51–60. [DOI] [PubMed] [Google Scholar]
- 32.Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delaney A, Howell CR, Krull KR, et al. Progression of frailty in survivors of childhood cancer: a St. Jude Lifetime Cohort report. J Natl Cancer Inst. 2021;113(10):1415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumahara H, Schutz Y, Ayabe M, et al. The use of uniaxial accelerometry for the assessment of physical-activity-related energy expenditure: a validation study against whole-body indirect calorimetry. Br J Nutr. 2004;91(2):235–43. [DOI] [PubMed] [Google Scholar]
- 35.Matthews CE, Ainsworth BE, Thompson RW, Bassett DR Jr. Sources of variance in daily physical activity levels as measured by an accelerometer. Med Sci Sports Exerc. 2002;34(8):1376–81. [DOI] [PubMed] [Google Scholar]
- 36.Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, Mcdowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–8. [DOI] [PubMed] [Google Scholar]
- 37.Derogatis LR. BSI 18, Brief Symptom Inventory 18 : administration, scoring and procedures manual. Minneapolis, MN: NCS Pearson, Inc.; 2001. [Google Scholar]
- 38.Ware JE Jr. SF-36 health survey update. Spine (Phila Pa 1976). 2000;25(24):3130–9. [DOI] [PubMed] [Google Scholar]
- 39.U.S. Department of Health and Human Services CfDCaP, National Center for Health Statistics. National Health and Nutrition Examination Survey Data, NHANES 2017–2018 Procedure Manuals. . [Google Scholar]
- 40.Piercy KL, Troiano RP, Ballard RM, et al. The Physical Activity Guidelines for Americans. JAMA. 2018;320(19):2020–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hudson MM, Ehrhardt MJ, Bhakta N, et al. Approach for classification and severity grading of long-term and late-onset health events among childhood cancer survivors in the St. Jude Lifetime Cohort. Cancer Epidemiol Biomarkers Prev. 2017;26(5):666–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ness KK, Leisenring WM, Huang S, et al. Predictors of inactive lifestyle among adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer. 2009;115(9):1984–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.U.S. Department of Health and Human Services. 2018 Physical Activity Guidelines Advisory Committee Scientific Report Washington, DC: U.S. Department of Health and Human Services, 2018. [Google Scholar]
- 44.Landmark T, Romundstad P, Borchgrevink PC, Kaasa S, Dale O. Associations between recreational exercise and chronic pain in the general population: evidence from the HUNT 3 study. Pain. 2011;152(10):2241–7. [DOI] [PubMed] [Google Scholar]
- 45.Odame I, Duckworth J, Talsma D, et al. Osteopenia, physical activity and health-related quality of life in survivors of brain tumors treated in childhood. Pediatr Blood Cancer. 2006;46(3):357–62. [DOI] [PubMed] [Google Scholar]
- 46.Gerber LH, Hoffman K, Chaudhry U, et al. Functional outcomes and life satisfaction in long-term survivors of pediatric sarcomas. Arch Phys Med Rehabil. 2006;87(12):1611–7. [DOI] [PubMed] [Google Scholar]
- 47.Finnegan L, Wilkie DJ, Wilbur J, Campbell RT, Zong S, Katula S. Correlates of physical activity in young adult survivors of childhood cancers. Oncol Nurs Forum. 2007;34(5):E60–9. [DOI] [PubMed] [Google Scholar]
- 48.McCarrick D, Prestwich A, Prudenzi A, O’Connor DB. Health effects of psychological interventions for worry and rumination: a meta-analysis. Health Psychol. 2021;40(9):617–30. [DOI] [PubMed] [Google Scholar]
- 49.Querstret D, Cropley M. Assessing treatments used to reduce rumination and/or worry: a systematic review. Clin Psychol Rev. 2013;33(8):996–1009. [DOI] [PubMed] [Google Scholar]
- 50.Campbell TS, Labelle LE, Bacon SL, Faris P, Carlson LE. Impact of mindfulness-based stress reduction (MBSR) on attention, rumination and resting blood pressure in women with cancer: a waitlist-controlled study. J Behav Med. 2012;35(3):262–71. [DOI] [PubMed] [Google Scholar]
- 51.Bhakta N, Liu Q, Ness KK, et al. The cumulative burden of surviving childhood cancer: an initial report from the St Jude Lifetime Cohort Study (SJLIFE). Lancet. 2017;390(10112):2569–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


