Abstract
Objective
This study evaluated frontal behavioral symptoms, via the FrSBe self-report, in military personnel with and without history of blast-related mild traumatic brain injury (mild TBI).
Methods
Prospective observational cohort study of combat-deployed service members leveraging 1-year and 5-year demographic and follow up clinical outcome data.
Results
The blast mild TBI group (n=164) showed greater frontal behavioral symptoms, including clinically elevated apathy, disinhibition, and executive dysfunction, at five-year follow-up, compared to a group of combat-deployed controls (n=107) without mild TBI history or history of blast exposure. We also explored change in behavioral symptoms over a 4-year span, which showed clinically significant increases in disinhibition in the blast mild TBI group, whereas the control group did not show significant increases in symptoms over time.
Conclusion
Our findings add to the growing evidence that a proportion of individuals who sustain mild TBI experience persistent behavioral symptoms. We also offer a demonstration of a novel use of the FrSBe as a tool for longitudinal symptom monitoring in a military mild TBI population.
Keywords: mild traumatic brain injury, executive function, long term outcomes, military service members, frontal behavioral symptoms
Introduction
Traumatic brain injury (TBI) is the most common injury in modern military conflicts(1). Specifically, mild TBI comprises 82.3% of the nearly 380,000 head injuries that were reported between 2000–2017 in the US military(2). Estimates range from approximately 17–20% of military personnel from Operations Enduring Freedom, Iraqi Freedom, and New Dawn (OEF/OIF/OND) who reported at least one mild TBI during deployment(3, 4). The prevalence of mild TBI incidents has led to the Department of Defense describing mild TBI as a “signature injury” of OEF/OIF/OND(5). In one study, more than 75% of reported mild TBI incidents were associated with blast exposures(6). During recent conflicts in Iraq and Afghanistan, there has been a clear increase in blast-related injuries due to use of improvised explosive devices (IEDs) that may result in chronic post-concussive conditions and neuropathology(7, 8). Eighty percent of a convenience sample of US soldiers who served in these conflicts reported receiving at least one blast exposure within 100 meters of their location during their deployment (9). These findings have emphasized the need to understand the impact of blast and non-blast related mild TBI in combat-deployed military personnel, including behavioral symptoms commonly associated with frontal lobe dysfunction.
It is well established that the frontal cortex is vulnerable in all TBI (3), and in blast-related injuries in particular (10). Studies examining frontal behavioral symptoms in mild TBI and blast-related injury are of high importance, as veterans diagnosed with mild TBI often report frontal neurobehavioral symptoms such as emotional lability, disinhibition, and difficulties with executive functioning. Longitudinal studies, including our own work, have suggested behavioral and emotional symptoms may persist even after the period of expected recovery from mild TBI(11). The mild TBI literature suggests that people who sustain a mild TBI experience relatively predictable progressive recovery with return to baseline within 3 months post-injury(12). However, more recent studies have found evidence of a higher proportion of individuals who experience symptoms beyond three months(13). In the TRACK-TBI cohort study, Nelson and colleagues (12) found that 53% of participants with mild TBI reported functional impairments 12 months post-injury, and Mikolic and colleagues found that 51% of their mild TBI group of the CENTER-TBI study demonstrated GOSE scores less than 8 after 6 months post-injury, suggesting persistent functional difficulties after the expected recovery window(13). Recently, in a comprehensive review of the TBI literature, Maas and colleagues and the Lancet Neurology Commission reported that approximately 50% of adults who sustain a mild TBI do not recover to pre-injury levels of health after six months, challenging the long-standing belief that mild TBI does not cause chronic symptoms or functional limitations(14). In our own work with military personnel, we found that over a 5-year period, 30% of participants with history of blast mild TBI declined into a worse disability bracket, and statistically significant increases in psychiatric and neurobehavioral symptoms were reported at 5-year follow-up visits(11).
The Frontal Systems Behavioral Scale (FrSBe) is a validated measure of symptoms and behaviors associated with frontal lobe damage (15). It includes self- and observer-report (“Family” rating) versions and can be further analyzed with three domain-based scores that assess clusters of symptoms related to apathy, disinhibition, and executive dysfunction. The FrSBe has been widely used to assess neurobehavioral symptoms at baseline and post-injury and evaluate and monitor change in behaviors and symptoms following injury or damage to frontal systems. For example, Reid-Arndt and colleagues showed that symptoms on the FrSBe predicted functional outcomes in patients with a self-reported history of TBI (16). Karr and colleagues focused on Iraq and Afghanistan veterans with blast-related mild TBI and found that 82% of the group had clinically elevated post-injury total score on the FrSBe, a significant increase from 11% of the group pre-injury(17). In a study by Lengenfelder and colleagues, thirty-three people with moderate-to-severe TBI reported increased post-injury scores for both self and family ratings in all three domains, apathy, disinhibition, and executive dysfunction(3).
While the FrSBe was designed to assess premorbid behavior and behavior post-injury, we have employed it as a measure of current symptoms. This represents a relatively novel use of the FrSBe to measure frontal-network behavioral symptoms in a longitudinal setting. Our intention is to use the FrSBe to assess persistence versus resolution of behavioral symptoms in our participant sample over time. A search for similar approaches yielded limited studies. One study of community-based severe TBI sample found that most participants demonstrated stability of apathy symptoms over a 10-month period(18). The literature also indicates two studies of patients with Parkinson’s disease demonstrating worsening of behavioral symptoms in the progression of the disease process(19, 20). To our knowledge, this study is the first reported use of the FrSBe as a longitudinal measure assessed in a military service member sample with and without mild TBI over multiple time points.
The aim of the present study was to examine the extent of resultant frontal behavioral symptoms (as measured by the FrSBe) at 5-years post-injury in military personnel who sustained blast-related mild TBI during combat deployment. We hypothesized that participants with history of blast mild TBI would demonstrate higher symptom endorsement on the FrSBe at 5-year follow-up, compared to non-blast, combat-deployed controls, which would support impact of mild TBI above and beyond environmental factors that were also experienced by the combat-deployed force as a whole. Because the FrSBe was added later into the study protocol (i.e., during Year 1 data collection), we focused on more robust Year 5 FrSBe data in our two primary participant groups, blast-related mild TBI and the non-blast controls. We also conducted exploratory analyses of Year 1 FrSBe data, as well as change over time from Year 1 to Year 5 FrSBe scores within and between groups. In these analyses, we hypothesized that the non-blast control group would demonstrate stability, or possibly, a decrease in behavioral symptoms from Year 1 to Year 5. However, based on our prior work that demonstrated persistence or worsening, rather than resolution, of cognitive and psychological symptoms at 5-year follow-up, we hypothesized that the blast mild TBI group would exhibit an increase in behavioral symptoms via the FrSBe from Year 1 to Year 5. Our study provides a unique perspective in that the FrSBe rating scale is utilized not simply to assess change between pre- and post-injury, but to monitor the trajectory of post-injury symptoms in combat-deployed military personnel with history mild TBI compared to controls with comparable environmental exposures but without history of TBI or blast exposure.
Materials and Methods
A secondary analysis was performed of data obtained from the “EValuation Of Longitudinal outcomes in mild TBI active-duty military and VEterans” (EVOLVE) study examining the long-term effects of combat-related mild TBI which has been described in detail elsewhere(21–23). All methods were carried out in accordance with approved Institutional Review Board human subjects protocols and informed consent was obtained from all participants at enrollment (University of Washington, STUDY00001323, FWA #00006878).
Participants:
Briefly, all participants were military service members deployed between November 2008 and July 2013 and enrolled in the study while in Afghanistan or after they were medically evacuated to Landstuhl Regional Medical Center in Landstuhl, Germany. In the full longitudinal study, participants were assigned to 1 of 4 study groups based on clinical assessment. Two primary groups were blast-related mild TBI (Blast mild TBI group), and combat-deployed service members without any TBI or blast exposure (Non-blast control group). The two exploratory participant groups were not included in the current analysis (mild TBI from non-blast mechanisms, exposure to blast but without mild TBI). Diagnosis of mild TBI was made by trained medical personnel (24) immediately after the event, according to the United States Department of Defense definition of mild, uncomplicated TBI (i.e., loss of consciousness lasting 30 minutes or less, alteration of consciousness less than 24 hours, post-traumatic amnesia less than 24 hours, Glasgow Coma Scale of 13–15, unremarkable CT or MRI findings)(25). Mild TBI participants were enrolled on average 4–9 days post-injury depending on evacuation status. Participants in the non-blast control group were either enrolled during combat deployment or underwent medical evacuation for noncombat diagnoses such as dermatitis or gastrointestinal illness. Upon clinical evaluation they were found to be without signs and symptoms suggestive of acute TBI. Exclusion criteria for all groups included prior TBI or psychiatric diagnosis.
Study Methods:
In-person clinical evaluations were conducted 1 year and 5 years after enrollment during deployment. The Self-Rating form of the Frontal Systems Behavior Scale (FrSBe)(26, 27) was administered as part of this evaluation. Participants completed the self-report form for their current symptoms at Year 5 follow-up, and a smaller subgroup completed this measure at Year 1 (n=81). The FrSBe is a 46-item behavior rating scale that captures frontal behavioral symptoms commonly attributed to damage to frontal lobe networks. The FrSBe is designed to assess premorbid behavior and behavior post-injury and demonstrates utility in numerous populations including patients with neurological disorders, psychiatric disorders with frontal systems impairment, and head injury(15). Each item represents a description of a possible symptom (e.g., “Doing things impulsively”, “Lost interest in things”), and is reported on a scale of 1, “Almost Never” to 5 “Almost Always”; thus, higher scores reflect greater symptom endorsement. The assessment results in a total composite score, as well as subscale scores in the three behavioral domains of “Apathy,” “Disinhibition,” and “Executive Dysfunction” with raw to T-score conversion corrected for age, gender, and education. T-scores below 60 are considered within normal limits, T-scores of 60 to 64 indicate borderline clinical significance, and T-scores of 65 or greater are considered clinically elevated and reflect significant frontal systems abnormalities. Clinically-elevated scores on the Apathy subscale suggest problems with initiation, persistence, loss of energy, anhedonia, lack of concern about self-care, and/or blunted affective expression. Elevated scores on the Disinhibition subscale suggest impulsivity, hyperactivity, socially inappropriate behavior, emotional lability, or irritability. Lastly, higher scores on the Executive Dysfunction subscale suggest difficulties with organization, planning, sequencing, problem solving, insight, mental flexibility, or self-monitoring of ongoing behavior(15).
Statistical Analyses:
Differences in patient characteristics between the blast mild TBI and non-blast controls were assessed for statistical significance using Fisher’s exact tests for categorical variables and Mann-Whitney tests for continuous variables. Linear regression adjusting for age and education was used to analyze differences between the two groups on the 5-year and 1-year FrSBe scores, using all data available at that assessment. Spaghetti plots and unadjusted analyses of change within group between 1 and 5 years were performed including only subjects with data at both years using paired t-tests. Mixed-effects linear regression adjusting for age and education was used to model the FrSBe scores at 1 and 5 years, including all cases with FrSBe assessed at either time and assuming random subject slopes and an unstructured correlation matrix; the interaction between group and year assessed whether the scores changed differentially between the groups. Normality of the residuals was observed for all FrSBe models. For all analyses a two-sided significance level of 0.05 was used and no adjustments were made for multiple comparisons.
Data Availability
Data used in the analysis of this manuscript can be made available to interested parties through data requests submitted to the corresponding author.
Results
From 2008–2013, a total of 591 participants were enrolled in the EVOLVE study. Of these, 271 participants were in the primary groups and completed the FrSBe as a part of their 5-year follow-up evaluation (164 blast mild TBI, 107 non-blast control). Participant demographic, information including mean age, sex, race, education, and branch of military service, are presented in Table 1.
Table 1.
Demographic Characteristics of Participants at 5 Year Follow-up
| Non-Blast Control n=107 | Blast Mild TBI N=164 | Full Sample n=271 | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| M | sd | M | sd | M | sd | |
|
| ||||||
| Age (years)* | 36.2 | 7.8 | 31.7 | 6.8 | 33.5 | 7.6 |
| Education (years)* | 15.9 | 2.7 | 13.6 | 1.7 | 14.5 | 2.4 |
|
|
||||||
| % | % | % | ||||
|
|
||||||
| Gender* | ||||||
| Female | 16% | 4% | 8% | |||
| Male | 84% | 96% | 92% | |||
| Race/Ethnicity | ||||||
| White | 76% | 74% | 75% | |||
| African American | 15% | 8% | 11% | |||
| Hispanic/Latino | 9% | 15% | 13% | |||
| Asian/Pacific Islander | 0% | 2% | 1% | |||
| Other | 0% | 1% | 0% | |||
| Military Branch* | ||||||
| Army | 56% | 85% | 73% | |||
| Navy | 20% | 1% | 8% | |||
| Air Force | 16% | 1% | 7% | |||
| Marines | 8% | 13% | 11% | |||
Note.
statistically significant group difference at p < .05
M=Mean
sd=Standard Deviation
Primary Analyses
Group Differences at Year 5 Follow-up:
Average T-scores for the blast mild TBI group were clinically elevated (i.e., T>65) for FrSBe total score (T-score Mean= 69.5, sd=19.3) and the Executive Dysfunction subscore (T-score Mean=68.2, sd=18.5). The FrSBe Apathy subscore was just below clinical elevation for the blast mild TBI group (T-score Mean=64.9, sd=19.0). In contrast, the non-blast control group did not show clinical elevations on FrSBe total (T-score Mean=54.0, sd=15.2) or any of the three FrSBe subscores (Apathy: T-score Mean=53.2, sd=15.8; Disinhibition: T-score Mean=52.7, sd=13.1); Executive Dysfunction: T-score Mean=53.7, sd=13.0). All means and standard deviations for group differences at Year 1 and Year 5 are presented in Supplemental Material.
Overall, mean group differences were found for all FrSBe variables and are visualized for total and domain subscores in Figure 1. The blast mild TBI group demonstrated significantly higher FrSBe Total scores (MeanΔ =+15.6, 95% CI=11.2–19.9, p<.001), compared to the non-blast control group. Similar results were observed in examining the subscale scores of the three FrSBe domains. On the FrSBe Apathy subscale at Year 5, the blast mild TBI group reported significantly higher apathy-related symptoms (MeanΔ = +11.8, 95% CI=7.4–16.1, p<.001) compared to the non-blast control group. On the FrSBe Disinhibition subscale at year 5, the blast mild TBI group endorsed significantly higher scores than the non-blast control group (MeanΔ = +10.7, 95% CI=7.4–14.1, p<.001). On the FrSBe Executive Dysfunction subscale at Year 5, the blast mild TBI group again reported significantly higher scores than the non-blast control group (MeanΔ = +14.5, 95% CI=10.5–18.6, p<.001).
Figure 1: FrSBe T-scores at 5-Year Follow-up for Blast Mild TBI and Non-blast Controls.
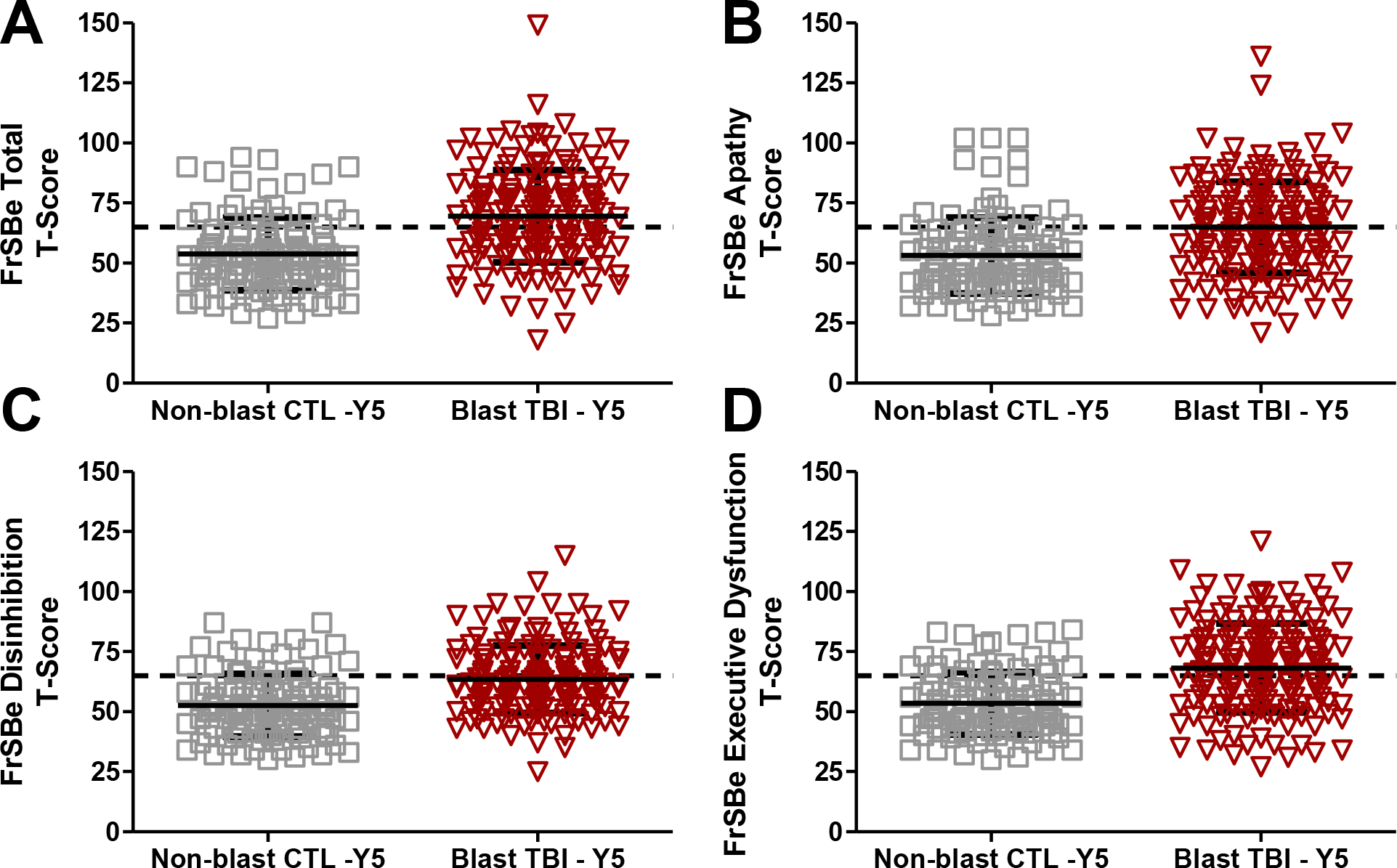
Note. A) FrSBe Total T-scores; p<.001. B) FrSBe Apathy T-scores; p<.001. C) FrSBe Disinhibition T-scores; p<.001. D) FrSBe Executive Dysfunction T-scores; p<.001.
Dashed horizontal line = Clinical elevation threshold of T>65
Exploratory Analyses
During the 1-year follow-up, 124 participants completed the FrSBe as the assessment was introduced later in the study. Of those, 81 were in the primary groups (44 blast mild TBI, 37 non-blast control) with 59 completing both 1-year and 5-year FrSBe evaluations. We explored trends in Year 1 data, as well as change in FrSBe scores from Year 1 to Year 5 follow-up.
Group Differences at Year 1:
At Year 1, average T-scores for the blast mild TBI group were clinically elevated (i.e., T>65) for FrSBe total score (T-score Mean=65.5, sd=23.7) and the Executive Dysfunction subscore (T-score Mean=67.4, sd=22.1). The FrSBe Apathy subscore (T-score Mean=59.4, sd=20.0) and Disinhibition subscore (T-score Mean=58.9, sd=16.4) were not clinically elevated for the blast mild TBI group. In addition, the non-blast control group did not show clinical elevations on FrSBe total (T-score Mean=49.8, sd=15.3) or any of the three FrSBe subscores (Apathy: T-score Mean=48.7, sd=13.8; Disinhibition: T-score Mean=47.6, sd=13.8); Executive Dysfunction: T-score Mean=52.2, sd=14.9).
As shown in Figure 2, the blast mild TBI group demonstrated significantly higher FrSBe Total scores, compared to the non-blast control group (MeanΔ = +15.7, 95% CI=6.8–24.6, p=.001). On the FrSBe Apathy subscale at Year 1, the blast mild TBI group reported significantly higher apathy-related symptoms compared to the non-blast control group (MeanΔ = +10.7, 95% CI=3.1–18.3, p=.007). On the FrSBe Disinhibition subscale, the blast mild TBI group endorsed significantly higher scores than the non-blast control group (MeanΔ = +11.3, 95% CI=4.6–18.0, p=.001). On the FrSBe Executive Dysfunction subscale, the blast mild TBI group again reported significantly higher scores than the non-blast control group (MeanΔ = +15.2, 95% CI=6.8–23.6, p=.001).
Figure 2: FrSBe T-scores at 1-Year Follow-up for Blast Mild TBI and Non-blast Controls.
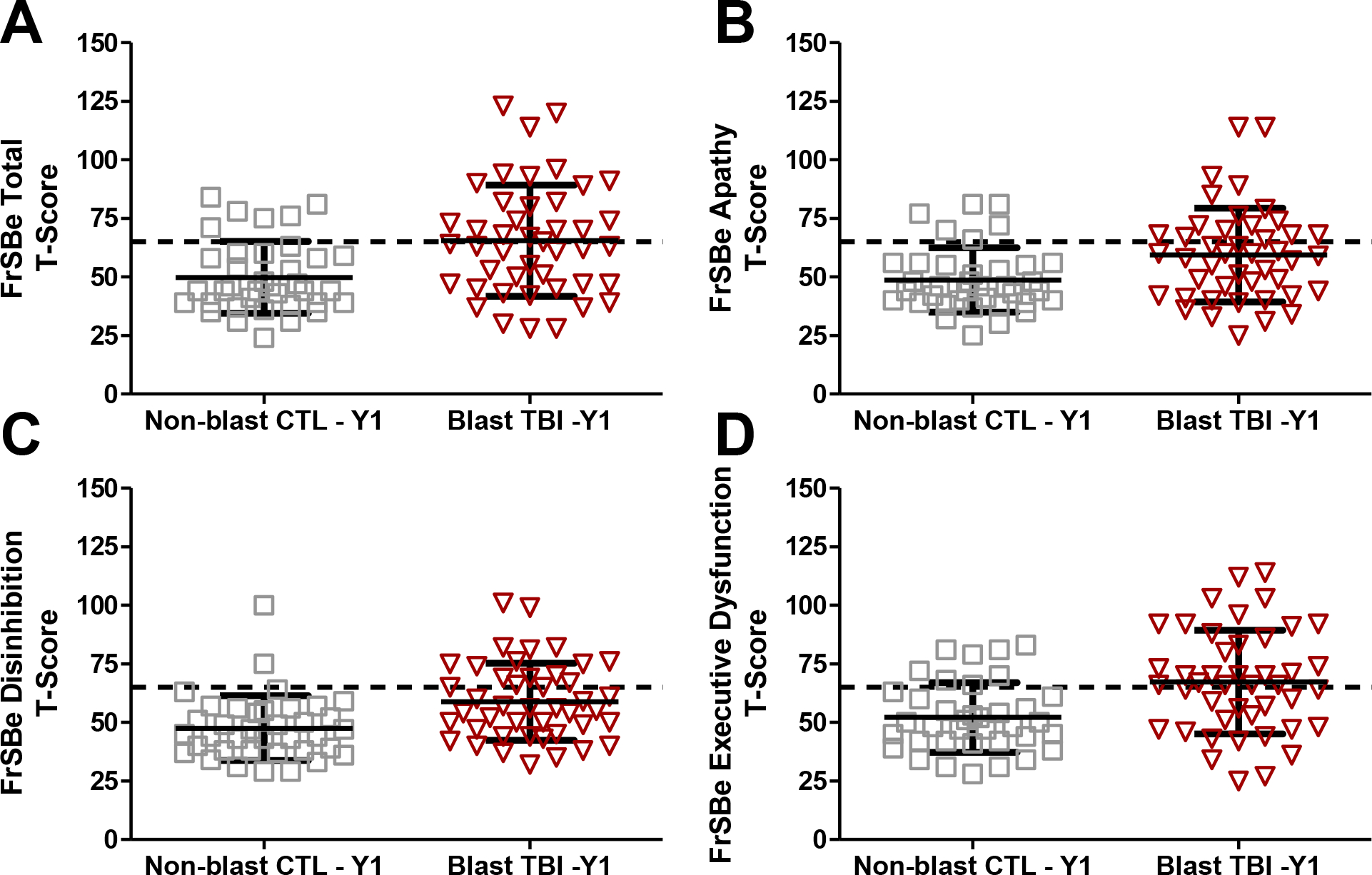
Note. A) FrSBe Total T-scores; p=.001. B) FrSBe Apathy T-scores; p=.007. C) FrSBe Disinhibition T-scores; p=.001. D) FrSBe Executive Dysfunction T-scores; p=.001.
Dashed horizontal line = Clinical elevation threshold of T>65
Change in FrSBE Scores from Year 1 to Year 5:
We also evaluated whether significant changes in scores were present from Year 1 to Year 5 within groups (Figure 3). For the blast mild TBI group, significantly higher FrSBe Disinhibition scores were reported at Year 5 compared to Year 1 (MeanΔ = +6.29; 95% CI 1.29–11.29; p=.015), and there was a trend in the same direction for Total FrSBe score (MeanΔ = +5.32; 95% CI −0.68–11.33, p=.080). Although there were higher mean T-scores for Apathy and Executive Function subscores, these changes were not statistically significant for the blast mild TBI group (p’s>.05). Although in the same direction, none of the mean T-score changes were significant for the non-blast control group (p’s>.05). We also investigated between group comparisons of Year 1 to Year 5 change. There was no suggestion of differential change between the groups (Modeled interactions between −.47 and .01, adjusted p-values all >.80; see Supplemental Material).
Figure 3: Change in FrSBe T-scores from 1-Year to 5-Year Follow-up for Blast Mild TBI and Non-blast Controls.
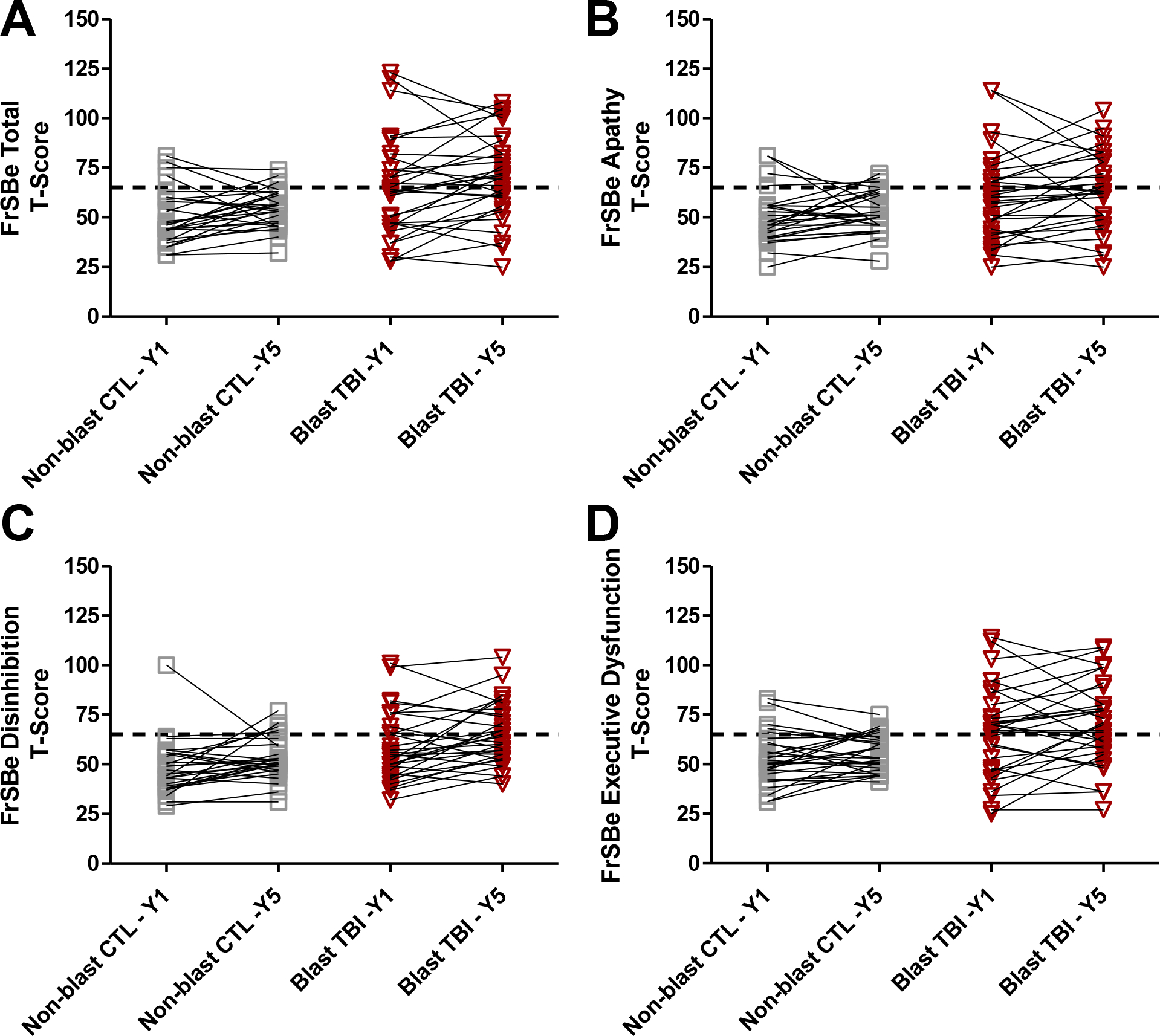
Note. A) FrSBe Total T-scores; Non-blast controls, p=.094, Blast mild TBI, p=.080. B) FrSBe Apathy T-scores; Non-blast controls, p=.182, Blast mild TBI, p=.236. C) FrSBe Disinhibition T-scores; Non-blast controls, p=.089 Blast mild TBI, p=.015. D) FrSBe Executive Dysfunction T-scores; Non-blast controls, p=.186, Blast mild TBI, p=.172.
Dashed horizontal line = Clinical elevation threshold of T>65
Discussion
This study evaluated differences of frontal behavioral symptoms between two groups of combat-deployed military personnel with and without history of blast-induced mild TBI. We found that the blast mild TBI group showed clinically-elevated frontal behavioral symptoms at 5 years post-injury, including those indicative of apathy, disinhibition, and difficulties with executive functioning. These symptoms were significantly higher when compared to combat-deployed controls without history of TBI or blast exposure. This is consistent with prior studies, including several large civilian and military cohort TBI studies, including on showing veterans with blast-related injuries often report several frontal network behavioral symptoms including irritability and disinhibition, amongst others(8, 13). Our primary findings also support the growing evidence that a proportion of individuals who sustain mild TBI experience persistent symptoms and do not follow the expected recovery of full symptom resolution in 3–6 months post-injury(14). This raises not only a call for better understanding of mild TBI mechanisms and symptom trajectories, but also the appropriate post-injury care to promote recovery.
We also found evidence of trends for the blast mild TBI group demonstrating increases in frontal behavioral symptoms between 1- and 5-years post injury. This phenomenon appears to be driven mostly by disinhibition, with lesser presence of apathy or executive functioning symptoms in their self-report. This is concerning, given that individuals with prior TBI are at risk for future TBIs, and disinhibited behavior may lead to further risk-taking behavior increasing the possibility of additional injuries. Interestingly, the non-blast control group also showed increases in behavioral symptom endorsement, but these did not reach statistical significance or averages above the clinical thresholds. This raises questions about co-morbid presence of mental or physical health conditions that may be contributing to frontal behavioral symptom endorsement.
Mild TBI is associated with high rates of co-occurring mental health disorders, including, and most commonly, PTSD(28). Many military veterans with a history of blast related TBI experience a higher PTSD symptom severity (17). Studies have shown that military veterans with comorbid mild TBI and PTSD, as opposed to PTSD alone or mild TBI alone, are at greater risk for experiencing long lasting subjective distress (29). In the present study we found clinically elevated scores at Year 1 follow-up for the control group, as well as non-significant increases in symptom endorsement from Year 1 to Year 5. One explanation could be the comorbid presence of psychiatric symptoms. The current study did not address potential drivers of persistent symptoms. As such, given the high frequency of clinical comorbidity, further research into the neural networks of both PTSD and mild TBI, as well as the potential interaction effects of PTSD and mild TBI on the duration and severity of symptoms would be of high utility.
Lastly, our study was one of few to use the FrSBe to assess long-term frontal neurobehavioral symptoms, as opposed to its initial design to assess pre- and post-injury symptoms. To our knowledge, this is also the first study to apply this longitudinal approach with the FrSBe to evaluate frontal behavioral symptoms in a longitudinal combat-deployed military cohort study. We have demonstrated the potential for using the FrSBe to assess frontal behavioral symptoms beyond the immediate post-injury period and incorporating more detailed assessment of these symptoms in observational and interventional studies of TBI, including mild TBI, to better understand progression or resolution of symptoms over time. While the present study does not represent a formal validation of the FrSBe measure for longitudinal monitoring of behavioral symptoms, it creates a foundation for future studies to consider analyses of this type of use.
There are several limitations to our conclusions. This study did not explore specific causal mechanisms for persistence, and in some cases increases, in behavioral symptoms. Our intention was to lay the groundwork for further models to explore causal or mechanistic research questions. For example, symptoms of depression or PTSD may be contributing differently in our groups, including the co-morbid presence of mild TBI. The study participants represent a rather homogenous group, predominantly Non-Hispanic White males, and may not be generalizable to other racial/ethnic groups. The Year 1 data is also limited in group size and represents exploratory analyses and repeating the analyses of changes with larger group sizes is recommended. In addition, we acknowledge the limitation of using a single behavioral instrument such as the FrSBe in such a complex study. The FrSBe includes a total score and three defined behavioral domains; however, additional symptoms that are common in mild TBI, such as irritability, are not separately captured, but instead included under the loadings of the defined domains. Future studies should consider inclusion of additional or alternative measures that assess other common symptoms more specifically. This study also comes with several strengths, including the relatively large group of combat-deployed control subjects with similar environmental exposures in military settings. This allows for stronger conclusions to be drawn about differences in groups who sustained blast-related mild TBI compared to those with similar combat, environment, and military experience. In addition, rather than retrospective reporting of injury history and symptoms, data for these participants was obtained shortly after injury during enrollment, and behavioral symptom reports represent the present rather than retrospective recall.
Future directions include evaluating models of mild TBI outcomes, such as moderating or mediating factors of psychiatric symptoms (e.g., PTSD, depression) or neuroimaging findings, as predictors driving post-injury symptom prognosis. Future studies should focus on the neural networks and pathophysiology connecting mild TBI to frontal network symptoms and could also focus on the effect and pathophysiology of comorbid PTSD and mild TBI have on the severity and duration of frontal symptoms. In addition, future studies should consider pursuit of models that address cognitive functioning (by neuropsychological tests) presenting co-morbidly with neuropsychiatric and behavioral symptoms in the post-injury periods of military personnel with and without history of mild TBI, including differentiation of blast versus non-blast injury mechanism. The EVOLVE study continues to conduct follow-up visits, now in Year 10, and we anticipate opportunities to re-evaluate our current findings with a longer time interval to better understand trajectories of physical, emotional, and behavioral symptoms in combat-deployed military personnel.
Supplementary Material
Acknowledgements:
The authors thank the service members, their families, commanding officers, and clinical providers; and the EVOLVE Study clinical support team, including Dr. Beverly Scott, Katelyn Kern, Brie Sullivan, Max Tuvloff, Manny Kaur, Morgan Hall, and Sarah Conger for whom compensation was provided for their contributions to the study.
Funding details:
This work was supported by the National Institute of Neurological Disorders and Stroke under Grant R01NS091618 awarded to C. Mac Donald.
Biographies
Carolyn M. Parsey, PhD
Dr. Parsey is a board-certified neuropsychologist with expertise in assessment of cognitive functioning. She is an Assistant Professor in the Department of Neurology at the University of Washington School of Medicine, where she conducts both clinical research and direct patient care with adults presenting with a variety of neurologic conditions. She is a member of the Clinical Core for the Alzheimer’s Disease Research Center at the University of Washington, and she collaborates on multiple large scale studies assessing cognition in aging, neurodegenerative disease, and traumatic brain injury.
Hyun Jin Kang, BA
Mr. Kang is a psychometrist at Regional Epilepsy Center of Harborview Medical Center. Under the supervision of Dr. Parsey and three other clinical neuropsychologists, he administers and scores a variety of clinical batteries of neuropsychological tests in mainly evaluating patients suffering from memory and movement disorders and epilepsy.
Jessica C. Eaton, MD
Dr. Eaton is a graduate of the University of Louisville, where she studied Physics and English. She then remained at the University of Louisville for medical school before becoming a resident in Neurological Surgery at the University of Washington.
Margaret E. McGrath, MD
Dr. McGrath completed her undergraduate degree in biochemistry at the University of Montana. She subsequently graduated from the University of Washington School of Medicine. She is a resident in Neurological Surgery at the University of Washington.
Jason Barber, MS
Mr. Barber is a biostatistican in the Department of Neurological Surgery at Harborview Medical Center in Seattle, WA. He has over 25 years of experience in the management, curation, and statistical analysis of clinical and outcome data collected from studies of traumatic brain injury (TBI) and spinal cord injury (SCI).
Nancy R. Temkin, PhD
Dr. Temkin is a biostatistician and traumatic brain injury (TBI) researcher with over 45 years of experience designing, implementing, and analyzing clinical trials of treatments for TBI and longitudinal observational studies of TBI outcomes. She has been involved with studies in military and civilian populations in the United States and Latin America. She is a professor in the Department of Neurological Surgery at the University of Washington School of Medicine and in the Department of Biostatistics at the University of Washington School of Public Health in Seattle, WA.
Christine L. Mac Donald, PhD
Dr. Mac Donald is a Neurotraumatologist with expertise in advanced neuroimaging methods and the application to neurodegenerative diseases, in particular traumatic brain injury (TBI). She split her time throughout her early faculty career with a position in Trauma Surgery at Landstuhl Regional Army Medical Center in Germany where for 5 years she worked screening and evaluating medically evacuated combat casualties of war for traumatic brain injury and conducted early clinical trials in new imaging diagnostics. She is now a Professor and Endowed Chair, as well as the Vice Chair for Research in the department of Neurological Surgery at the University of Washington School of Medicine and Harborview Medical Center in Seattle, WA. She has over ten years of experience conducting clinical trials and observational studies, and for the past decade has been managing and directing large scale, multi-center, international clinical research studies in the United States, Italy, Germany, and Afghanistan.
Footnotes
Disclosure Statement: The authors report there are no competing interests to declare.
References
- 1.Hayward P Traumatic brain injury: the signature of modern conflicts. Lancet neurology. 2008;7(3):200–1. doi: 10.1016/S1474-4422(08)70032-2. [DOI] [PubMed] [Google Scholar]
- 2.System MH. DOD TBI Worldwide Numbers 2022 [cited 2022 October 22nd]. Available from: https://health.mil/Military-Health-Topics/Centers-of-Excellence/Traumatic-Brain-Injury-Center-of-Excellence/DOD-TBI-Worldwide-Numbers.
- 3.Lengenfelder J, Arjunan A, Chiaravalloti N, Smith A, DeLuca J. Assessing frontal behavioral syndromes and cognitive functions in traumatic brain injury. Appl Neuropsychol Adult. 2015;22(1):7–15. doi: 10.1080/23279095.2013.816703. [DOI] [PubMed] [Google Scholar]
- 4.Lindquist LK, Love HC, Elbogen EB. Traumatic Brain Injury in Iraq and Afghanistan Veterans: New Results From a National Random Sample Study. The Journal of neuropsychiatry and clinical neurosciences. 2017;29(3):254–9. doi: 10.1176/appi.neuropsych.16050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Government US. DOD TBI Special Report [cited 2022 October 27]. Available from: https://dod.defense.gov/News/Special-Reports/0315_tbi/.
- 6.Wilk JE, Thomas JL, McGurk DM, Riviere LA, Castro CA, Hoge CW. Mild traumatic brain injury (concussion) during combat: lack of association of blast mechanism with persistent postconcussive symptoms. The Journal of head trauma rehabilitation. 2010;25(1):9–14. Epub 2010/01/07. doi: 10.1097/HTR.0b013e3181bd090f. [DOI] [PubMed] [Google Scholar]
- 7.Clark AL, Merritt VC, Bigler ED, Bangen KJ, Werhane M, Sorg SF, et al. Blast-Exposed Veterans With Mild Traumatic Brain Injury Show Greater Frontal Cortical Thinning and Poorer Executive Functioning. Frontiers in neurology. 2018;9:873. doi: 10.3389/fneur.2018.00873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elder GA, Cristian A. Blast-related mild traumatic brain injury: mechanisms of injury and impact on clinical care. Mt Sinai J Med. 2009;76(2):111–8. doi: 10.1002/msj.20098. [DOI] [PubMed] [Google Scholar]
- 9.Fortier CB, Amick MM, Kenna A, Milberg WP, McGlinchey RE. Correspondence of the Boston Assessment of Traumatic Brain Injury-Lifetime (BAT-L) clinical interview and the VA TBI screen. J Head Trauma Rehabil. 2015;30(1):E1–7. Epub 2013/12/18. doi: 10.1097/htr.0000000000000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DePalma RG, Hoffman SW. Combat blast related traumatic brain injury (TBI): Decade of recognition; promise of progress. Behav Brain Res. 2018;340:102–5. Epub 2016/08/25. doi: 10.1016/j.bbr.2016.08.036. [DOI] [PubMed] [Google Scholar]
- 11.Mac Donald CL, Barber J, Patterson J, Johnson AM, Parsey C, Scott B, et al. Comparison of Clinical Outcomes 1 and 5 Years Post-Injury Following Combat Concussion. Neurology. 2021;96(3):e387–e98. doi: 10.1212/WNL.0000000000011089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carroll LJ, Cassidy JD, Peloso PM, Borg J, von Holst H, Holm L, et al. Prognosis for mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. Journal of rehabilitation medicine : official journal of the UEMS European Board of Physical and Rehabilitation Medicine. 2004(43 Suppl):84–105. doi: 10.1080/16501960410023859. [DOI] [PubMed] [Google Scholar]
- 13.Nelson LD, Temkin NR, Dikmen S, Barber J, Giacino JT, Yuh E, et al. Recovery After Mild Traumatic Brain Injury in Patients Presenting to US Level I Trauma Centers: A Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) Study. JAMA Neurol. 2019;76(9):1049–59. doi: 10.1001/jamaneurol.2019.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maas AIR, Menon DK, Manley GT, Abrams M, Akerlund C, Andelic N, et al. Traumatic brain injury: progress and challenges in prevention, clinical care, and research. Lancet neurology. 2022;21(11):1004–60. doi: 10.1016/S1474-4422(22)00309-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grace J Frontal Systems Behavior Scale. In: Kreutzer JS, DeLuca J, Caplan B, editors. Encyclopedia of Clinical Neuropsychology. New York, NY: Springer; 2011. [Google Scholar]
- 16.Reid-Arndt SA, Nehl C, Hinkebein J. The Frontal Systems Behaviour Scale (FrSBe) as a predictor of community integration following a traumatic brain injury. Brain Inj. 2007;21(13–14):1361–9. Epub 2007/12/11. doi: 10.1080/02699050701785062. [DOI] [PubMed] [Google Scholar]
- 17.Karr JE, Rau HK, Shofer JB, Hendrickson RC, Peskind ER, Pagulayan KF. Variables associated with subjective cognitive change among Iraq and Afghanistan war Veterans with blast-related mild traumatic brain injury. J Clin Exp Neuropsychol. 2019;41(7):680–93. doi: 10.1080/13803395.2019.1611740. [DOI] [PubMed] [Google Scholar]
- 18.Arnould A, Rochat L, Azouvi P, Van der Linden M. Longitudinal Course and Predictors of Apathetic Symptoms after Severe Traumatic Brain Injury. Archives of clinical neuropsychology : the official journal of the National Academy of Neuropsychologists. 2018;33(7):808–20. doi: 10.1093/arclin/acx122. [DOI] [PubMed] [Google Scholar]
- 19.Wee N, Kandiah N, Acharyya S, Chander RJ, Ng A, Au WL, et al. Baseline predictors of worsening apathy in Parkinson’s disease: A prospective longitudinal study. Parkinsonism & related disorders. 2016;23:95–8. doi: 10.1016/j.parkreldis.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Pluim CF, Nakhla MZ, Split M, Filoteo JV, Litvan I, Moore RC, et al. Changes in Self- and Informant-Reported Frontal Behaviors in Parkinson’s Disease: A Longitudinal Study. J Geriatr Psychiatry Neurol. 2022;35(1):89–101. doi: 10.1177/0891988720964257. [DOI] [PubMed] [Google Scholar]
- 21.Mac Donald CL, Adam OR, Johnson AM, Nelson EC, Werner NJ, Rivet DJ, et al. Acute post-traumatic stress symptoms and age predict outcome in military blast concussion. Brain : a journal of neurology. 2015;138(Pt 5):1314–26. doi: 10.1093/brain/awv038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mac Donald CL, Johnson AM, Wierzechowski L, Kassner E, Stewart T, Nelson EC, et al. Outcome Trends after US Military Concussive Traumatic Brain Injury. Journal of neurotrauma. 2017;34(14):2206–19. doi: 10.1089/neu.2016.4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macdonald CL, Johnson AM, Nelson EC, Werner NJ, Fang R, Flaherty SF, et al. Functional status after blast-plus-impact complex concussive traumatic brain injury in evacuated United States military personnel. Journal of neurotrauma. 2014;31(10):889–98. doi: 10.1089/neu.2013.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dempsey K, Dorlac W, Martin K, Fang R, Fox C, CBennett B, et al. Landstuhl Regional Medical Center: Traumatic Brain Injury Screening Program. J Trauma Nursing. 2009;16(1):6–12. [DOI] [PubMed] [Google Scholar]
- 25.DoD. Clinical Practice Guideline: Management of Concussion/mild Traumatic Brain Injury. In: DOD DoVA, editor. Washington, DC: 2009. [Google Scholar]
- 26.Carvalho JO, Ready RE, Malloy P, Grace J. Confirmatory factor analysis of the Frontal Systems Behavior Scale (FrSBe). Assessment. 2013;20(5):632–41. Epub 2013/06/27. doi: 10.1177/1073191113492845. [DOI] [PubMed] [Google Scholar]
- 27.Grace J, Stout J, Malloy P. Assessing Frontal Lobe Behavioral Syndromes with the Frontal Lobe Personality Scale. Assessment. 1999;6(3):269–84. [DOI] [PubMed] [Google Scholar]
- 28. Stein MB, Jain S, Giacino JT, Levin H, Dikmen S, Nelson LD, et al. Risk of Posttraumatic Stress Disorder and Major Depression in Civilian Patients After Mild Traumatic Brain Injury: A TRACK-TBI Study. JAMA psychiatry. 2019;76(3):249–58. Epub 2019/01/31. doi: 10.1001/jamapsychiatry.2018.4288. Aptinyx, Bionomics, Dart Neuroscience, Healthcare Management Technologies, Janssen, Neurocrine Biosciences, Oxeia Biopharmaceuticals, Pfizer, and Resilience Therapeutics in the past 3 years; owns founders shares and stock options in Resilience Therapeutics; and has stock options in Oxeia Biopharmaceuticals. Dr Manley discloses grants from the US Department of Defense (TBI Endpoints Development Initiative, grant #W81XWH-14–2-0176; TRACK-TBI Precision Medicine, grant #TBD; and TRACK-TBI Network, grant # W81XWH-15–9-0001); a grant from National Institutes of Health–National Institute of Neurological Disorders and Stroke (TRACK-TBI, grant #U01NS086090); reports being on the National Football League (NFL) scientific advisory board; reports support from the US Department of Energy for a traumatic brain injury precision medicine collaboration; has received an unrestricted gift from the NFL to the University of California, San Francisco Foundation to support research efforts of the TRACK-TBI Network; and has also received funding from NeuroTruama Sciences LLC to support TRACK-TBI data curation efforts. Dr Foreman reports speaking fees from UCB Pharma. Dr McAllister reports grants from University of California, San Francisco, during the conduct of the study. Dr Seabury is a consultant for Precision Health Economics, which provides consulting services to pharmaceutical manufacturers and other companies in the life sciences industry; has received research funding through unrestricted gifts to the Schaeffer Center for Health Policy and Economics at the University of Southern California from Alkermes and Verily; reports that partial support for his work is provided through an unrestricted grant to the Department of Ophthalmology from Research to Prevent Blindness; and reports grants from National Institute of Neurological Disorders and Stroke. Dr Vespa reports grants from National Institutes of Health during the conduct of the study. Dr Zafonte received royalties from Oakstone Publishing for an educational CD, “Physical Medicine and Rehabilitation: A Comprehensive Review”; serves on the scientific advisory board of Myomo, Oxeia Biopharmaceuticals, ElMindA, and BioDirection; and evaluates patients in the Massachusetts General Hospital Brain and Body TRUST Program funded by the NFL Players Association and receives funding from the Football Players Health Study at Harvard University, which is funded by the NFL Players Association. No other disclosures were reported.
- 29.Merritt VC, Jurick SM, Crocker LD, Hoffman SN, Keller AV, DeFord N, et al. Evaluation of objective and subjective clinical outcomes in combat veterans with and without mild TBI and PTSD: A four-group design. J Clin Exp Neuropsychol. 2019;41(7):665–79. Epub 2019/05/16. doi: 10.1080/13803395.2019.1610161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used in the analysis of this manuscript can be made available to interested parties through data requests submitted to the corresponding author.


