Abstract
Objectives:
The treatment for chronic limb-threatening ischemia (CLTI) has changed dramatically in the last few decades with a shift towards an endovascular-first approach and aggressive revascularization to achieve limb salvage. As the size of the CLTI population and intervention rates increase, patients will continue to experience technical failure (TF). Here, we describe the natural history of patients after TF of endovascular intervention for CLTI.
Methods:
We conducted a retrospective cohort study of patients with CLTI that attempted endovascular intervention or bypass at our multidisciplinary limb salvage center from 2013 to 2019. Patient characteristics were collected according to the Society for Vascular Surgery’s reporting standards. Primary outcomes included survival, limb salvage, wound healing, and revascularization patency. Product-limit Kaplan-Meier estimated survival functions for these outcomes, and between-group comparisons were made using Mantel-Cox log-rank nonparametric tests.
Results:
We identified 242 limbs from 220 unique patients that underwent primary bypass (n=30) or attempted endovascular intervention (n=212) at our limb salvage center. Endovascular intervention was a TF in 31 (14.6%) limbs. Following TF, 13 limbs underwent secondary bypass and 18 limbs were managed medically. Patients that experienced TF tended to be older (p<0.001), male (p=0.003), current tobacco users (p=0.014), have longer lesions (p=0.001), and have chronic total occlusions of target arteries (p<0.001) as compared to those who experienced technical success (TS). Furthermore, the TF group had worse limb salvage (p=0.047) and slower wound healing (p=0.028), but their survival was not different. Survival, limb salvage, and wound healing were not different in patients who received secondary bypass or medical management after TF. The secondary bypass group was older (p=0.012) and had a lower prevalence of tibial disease (p=0.049) than the primary bypass group and trended towards decreased survival, limb salvage, and wound healing (p=0.059, p=0.083, and p=0.051, respectively).
Conclusions:
Increased age, male sex, current tobacco use, longer arterial lesions, and occluded target arteries are associated with TF of endovascular intervention. Limb salvage and wound healing are relatively poor after TF of endovascular intervention, but survival appears comparable to patients who experience TS. Secondary bypass may not always rescue patients after TF, though our sample size limits statistical power. Interestingly, patients who received a secondary bypass after TF trended towards decreased survival, limb salvage, and wound healing compared to primary bypass.
Keywords: CLTI, Natural History, Technical Failure, Endovascular
Table of Contents Summary
We report an immediate technical failure rate of 14.6% after attempted endovascular intervention in a retrospective study of 212 limbs with chronic limb-threatening ischemia. Outcomes were less favorable as compared to patients that experienced technical success, and the benefit of subsequent bypass appeared minimal.
Introduction
Chronic limb-threatening ischemia (CLTI) is the most severe variant of peripheral artery disease (PAD), and its prevalence has rapidly grown in line with an aging population and increased diabetes rates on a global scale.1,2 Outcomes for CLTI are unfavorable, with high rates of mortality, adverse cardiovascular events, and amputations.3–8 Thus, CLTI presents a significant economic burden on healthcare systems, requiring frequent clinical observation, chronic wound care, and staged surgical interventions.9–11
Treatment for CLTI has changed dramatically in the last few decades with a shift towards an endovascular approach as the primary intervention strategy, which has been associated with improving outcomes.7,12–16 However, an endovascular-first strategy remains controversial, especially given the recent conclusions from the BEST-CLI trial identifying the superiority of open bypass if a single segment of adequate great saphenous vein (GSV) can be harvested.6,7,12,17 Furthermore, although the technical failure (TF) rates in most device trials are negligible, the real-world experience of BEST-CLI demonstrates that TF can be as high as 15%, even with optimal patient selection.6 As we continue aggressive attempts at revascularization to improve the lives of patients with CLTI, the number of patients whose endovascular intervention is a TF will rise.
Despite recent advancements, information on the natural course of patients with CLTI is limited.3,18 While the natural history of untreated patients with CLTI was reported a few years ago, a paucity of data exists on the course of patients who attempt and fail endovascular intervention. We aimed to explore the natural history of patients with CLTI after the TF of endovascular intervention.
Methods
Institution Information
After approval from an Institutional Review Board (The Ohio State University Biomedical Sciences Institutional Review Board protocol number 2019H0219), a retrospective cohort study was conducted of patients with CLTI who were managed at our multidisciplinary limb salvage center from 2013 to 2019. Our Limb Preservation Program team consists of vascular surgery, podiatry, and a fully staffed wound center providing both inpatient and outpatient care at a single location. The building contains an inpatient hospital, a vascular lab, operating rooms, catheterization labs, and a dedicated limb salvage inpatient floor. Initial patient evaluation includes consultation with vascular and podiatric physicians, arterial and venous noninvasive testing, debridement, prosthetic and orthotic evaluation, and scheduling of coordinated treatment. The Program is purposefully located in a community with high rates of renal failure, diabetes mellitus and CLTI, and the ability to provide coordinated expedited care to these patients who often have difficulty with follow up and compliance is central to our treatment approach. For example, clinic occurs on Tuesdays, and our angio suite block is on Fridays, allowing scheduling of treatment within three days while still accommodating individual patient needs, including withholding of anticoagulation, hydration for renal failure, treatment for dye allergies, and arranging transport. Furthermore, when bypass is considered after angiography, we perform vein mapping and partner with cardiologists to initiate risk stratification in the recovery area immediately after angiogram. This minimizes time between diagnosis and intervention, limiting clinical progression in the interim. At all points in the treatment process, a patient-centered shared decision-making model is used. Patients prioritize benefits they hope to achieve (e.g. prevent amputation, eliminate pain) and harms they hope to avoid (e.g. prolonged hospitalization, multiple procedures, procedural complications) based upon their unique past experiences and personal priorities.19 When these patient-centered priorities conflict with recommended treatments or when there is clinical equipoise between more than one treatment, a compromise treatment course accommodating the patient’s priorities is selected and agreed upon by both the surgeon and patient.
Evidence-based Wound Healing Society protocols are used in the treatment of all patients.20 All patients are seen every two to four weeks for wound care until healed. Vascular clinical and noninvasive arterial examination occurs every three months for the first year after revascularization. After the first year, vascular follow-up occurs every six months with noninvasive arterial assessment.
Limb loss was defined as any above-ankle amputation. Loss of patency was defined as a loss of a previously palpable pedal pulse, a velocity ratio >2.5 on arterial duplex, a drop in ABI of >0.15, or when repeat intervention was performed in the target arterial path.
Patient Characteristics
Patient information was collected according to SVS Reporting standards. Additionally, functional status is excluded from our report because approximately 85% of patient charts did not contain this information. For endovascular interventions, lesion characteristics including lengths and presence of chronic total occlusion (CTO) were reviewed.
Surgical and Endovascular Intervention
Patients undergoing attempts at limb salvage underwent either attempted endovascular intervention or primary bypass per the judgment of the operating surgeon and multidisciplinary limb salvage team using our patient-centered shared decision-making model. Specifically, if initial angiogram demonstrated multilevel long-segment CTO that included the trifurcation and the patient was suspected to have acceptable operative risk, angiography would be terminated after the diagnostic portion. Vein mapping and initiation of cardiac risk stratification were performed immediately after angiography in the recovery area. If there was adequate single-segment GSV and acceptable operative risk, primary bypass would be recommended.
Target arterial paths for endovascular intervention were overwhelmingly chosen based on the healthiest outflow vessel, including the peroneal artery. Rarely, when multiple tibial recanalizations were equally possible, attempts at endovascular revascularization were angiosome-directed. In these cases, pedal choke vessel status was assessed intraoperatively and used to guide target selection. Patients that received attempted endovascular intervention were classified into two groups, a technical success (TS) group and a TF group. TF was defined as the inability to restore in-line flow to the affected foot for anterior tibial and posterior tibial targets, the inability to restore in-line flow to the affected ankle for peroneal targets, or residual (>50%) stenosis after intervention along the target arterial path.6,21
Following TF of endovascular intervention, patients immediately underwent vein mapping and initiation of cardiac risk evaluation while in the recovery area. The decision to undergo secondary bypass was based on our patient-centered shared decision-making model and heavily informed by vein status and cardiac risk evaluation. All patients who underwent secondary bypass had bypass within two weeks of TF, and most patients received secondary bypass within one week. Patients that did not undergo secondary bypass pursued medical management.
Primary outcomes included mortality, limb salvage, wound healing, and revascularization patency if applicable. Outcomes were compared between the TS and TF groups, between the secondary bypass and medical management groups, and between the primary and secondary bypass groups.
Statistical Analysis
Two-sample independent t-tests were used to compare continuous variables (shown as mean ± standard deviation), and Chi-squared or Fisher’s exact tests were used for categorical variables (shown as category counts and percentages). Product-limit Kaplan-Meier estimated survival functions, and between-group comparisons were made using Mantel-Cox log-rank nonparametric tests. Unadjusted data was used for all analyses. A p-value less than 0.05 was considered statistically significant. All statistical analyses were performed using SPSS.
Results
Interventions Performed
Our multidisciplinary limb salvage clinic attempted limb salvage in 242 limbs from 220 unique patients between 2013 and 2019. The overall cohort had an average age of 65.2 ± 11.6 years, 65.3% were male, 57.7% were White, 41.5% were Black, 40.8% were current smokers or quit less than 10 years ago, 78.3% had diabetes mellitus, 90.0% had hypertension, 20.4% required dialysis due to chronic kidney disease, and 86.2% had hyperlipidemia. SVS cardiac status stages 1, 2, 3, and 4 comprised 36.5%, 8.7%, 39.8%, and 14.9% of the cohort, respectively. SVS pulmonary status stages 1, 2, 3, and 4 comprised 67.8%, 11.3%, 13.4%, and 7.5% of the cohort, respectively. Lastly, SVS WIfI stages 1, 2, 3, and 4 comprised 17.1%, 20.1%, 29.1%, and 33.7% of the cohort, respectively.
Primary bypass was done on 30 limbs (12.4%) from 29 patients, and endovascular intervention was attempted on 212 limbs (87.6%) from 191 patients (Figure 1). Endovascular intervention was a TS in 181 limbs (85.4%) from 160 patients and a TF in 31 limbs (14.6%) from 31 patients. Causes of TF included an inability to cross the lesion (n=28), and persistent >50% stenosis after intervention (n=3). Of the 31 limbs for which endovascular intervention failed, 13 limbs (42.0%) received a secondary bypass, and 18 limbs (58.0%) were medically managed.
Figure 1.
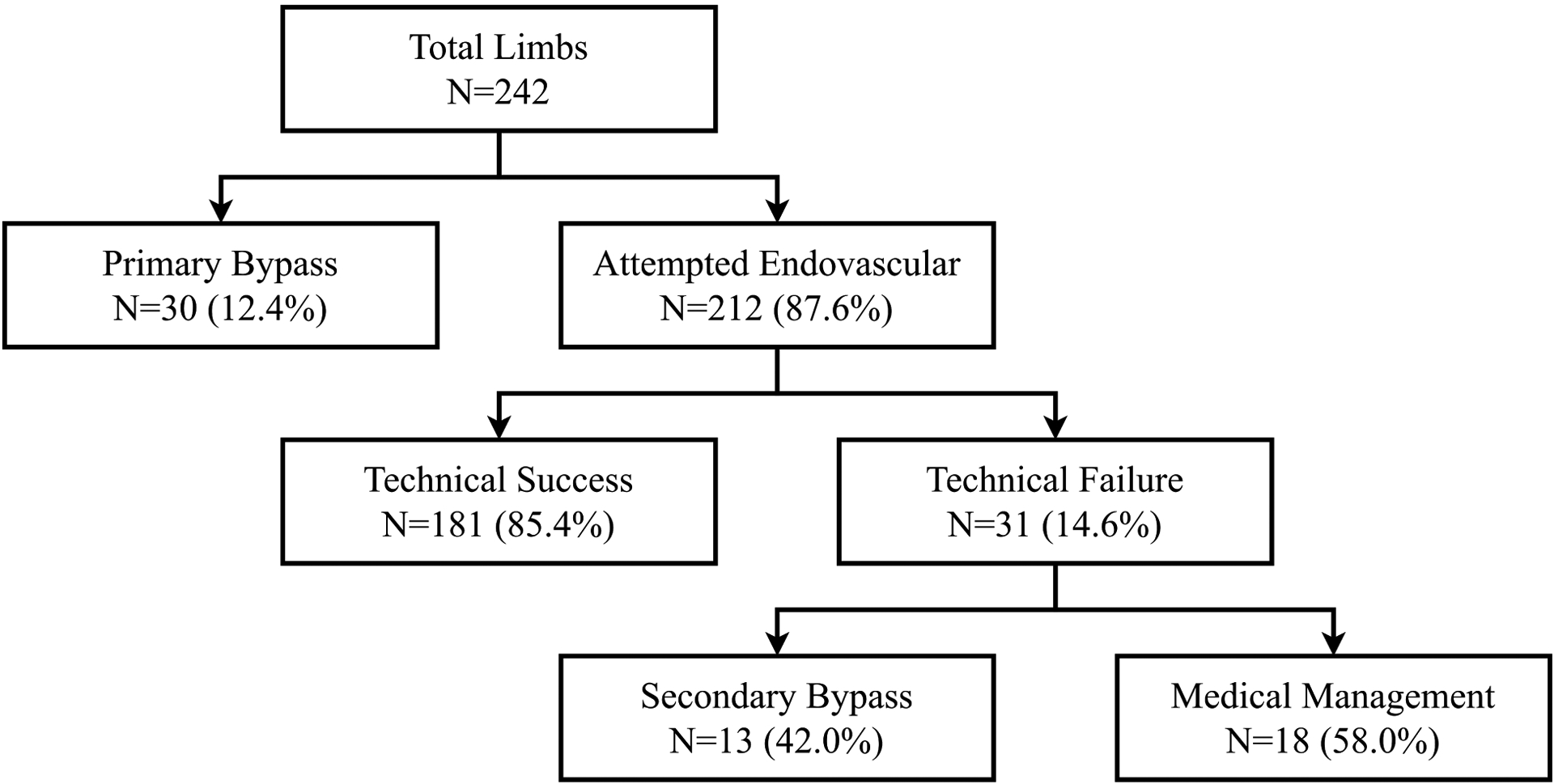
Numbers of limbs that underwent primary bypass and attempted endovascular intervention are displayed. Following technical failure of endovascular intervention, patients underwent either secondary bypass or medical management.
Outcome of Endovascular Intervention
Characteristics of patients that underwent attempted endovascular intervention are shown in Table I. Patients that experienced a TF of endovascular intervention tended to be older (72.52 ± 12.7 vs. 64.72 ± 11.1, p<0.001). Males comprised a greater proportion of the TF group than the TS group (87.1% vs. 59.7%, p=0.003). Recent or current tobacco use was also higher in the TF group (58.1% vs. 34.8%, p=0.014). Lesions were longer in the TF group as compared to the TS group (25.51 ± 14.95 cm vs. 15.35 ± 11.65 cm, p=0.001). Additionally, the TF group had a greater proportion of CTOs (100% vs. 42.5%, p<0.001). Ethnicity, diabetes mellitus, hypertension, dialysis dependence, hyperlipidemia, cardiac status, pulmonary status, SVS WIfI stage, and level of atherosclerotic disease did not differ between the TS and TF groups.
Table I:
Characteristics of patients that underwent attempted endovascular intervention are shown.
| Technical Failure | Technical Success | ||
|---|---|---|---|
| Patient Characteristics | N=31 | N=181 | p-value |
| Age | 72.5 ± 12.7 | 64.7 ± 11.1 | <0.001* |
| Male Sex | 27 (87.1%) | 108 (59.7%) | 0.003* |
| Ethnicity | 0.939 | ||
| White | 19 (61.3%) | 105 (58.3%) | |
| Black | 12 (38.7%) | 73 (40.6%) | |
| OtherΔ | 0 (0%) | 2 (1.2%) | |
| Current Tobacco Use or Quit <10 years ago | 18 (58.1%) | 62 (34.8%) | 0.014* |
| Diabetes Mellitus (DM) | 20 (66.7%) | 114 (80.0%) | 0.102 |
| Hypertension (HTN) | 25 (89.3%) | 159 (89.8%) | 0.930 |
| Dialysis Dependent | 8 (26.7%) | 37 (21.1%) | 0.499 |
| Hyperlipidemia (HLD) | 27 (90.0%) | 155 (86.6%) | 0.607 |
| Cardiac Statusβ | 0.548 | ||
| SVS Grade 1 | 13 (41.9%) | 61 (33.9%) | |
| SVS Grade 2 | 1 (3.2%) | 14 (7.8%) | |
| SVS Grade 3 | 11 (35.5%) | 79 (43.9%) | |
| SVS Grade 4 | 6 (19.4%) | 26 (14.4%) | |
| Pulmonary Statusβ | 0.446 | ||
| SVS Grade 1 | 21 (70.0%) | 116 (64.1%) | |
| SVS Grade 2 | 1 (3.3%) | 25 (13.8%) | |
| SVS Grade 3 | 5 (16.7%) | 25 (13.8%) | |
| SVS Grade 4 | 3 (10.0%) | 15 (8.3.%) | |
| SVS WIfI | 0.969 | ||
| Stage 1 | 4 (19%) | 28 (18.3%) | |
| Stage 2 | 5 (23.8%) | 32 (20.9%) | |
| Stage 3 | 5 (23.8%) | 44 (28.8%) | |
| Stage 4 | 7 (33.3%) | 49 (32%) | |
| Level of Disease† | |||
| Aortoiliac | 3 (9.7%) | 22 (12.2%) | 0.693 |
| Femoropopliteal | 21 (67.7%) | 139 (76.8%) | 0.279 |
| Tibial | 16 (51.6%) | 77 (42.5%) | 0.347 |
| Lesion Length (cm) | 25.51 ± 14.95 | 15.35 ± 11.65 | 0.001* |
| CTO Present | 31 (100%) | 77 (42.5%) | <0.001* |
Patients with unknown characteristics were excluded from applicable denominators.
Statistically significant difference between groups (p<0.05).
Excluded from analysis.
Reported according to SVS standards.21
Some patients had disease at multiple levels.
Outcomes for patients who attempted endovascular intervention included mortality, limb salvage, and wound healing (Figure 2). The mean survival in the TS and TF groups was 52 and 42 months, respectively (p=0.087). The mean time to major amputation was longer in the TS group compared to the TF group (58 vs. 45 months, respectively; p=0.047). The mean time to wound healing was shorter in the TS group compared to the TF group (25 vs. 38 months, respectively; p=0.028).
Figure 2.
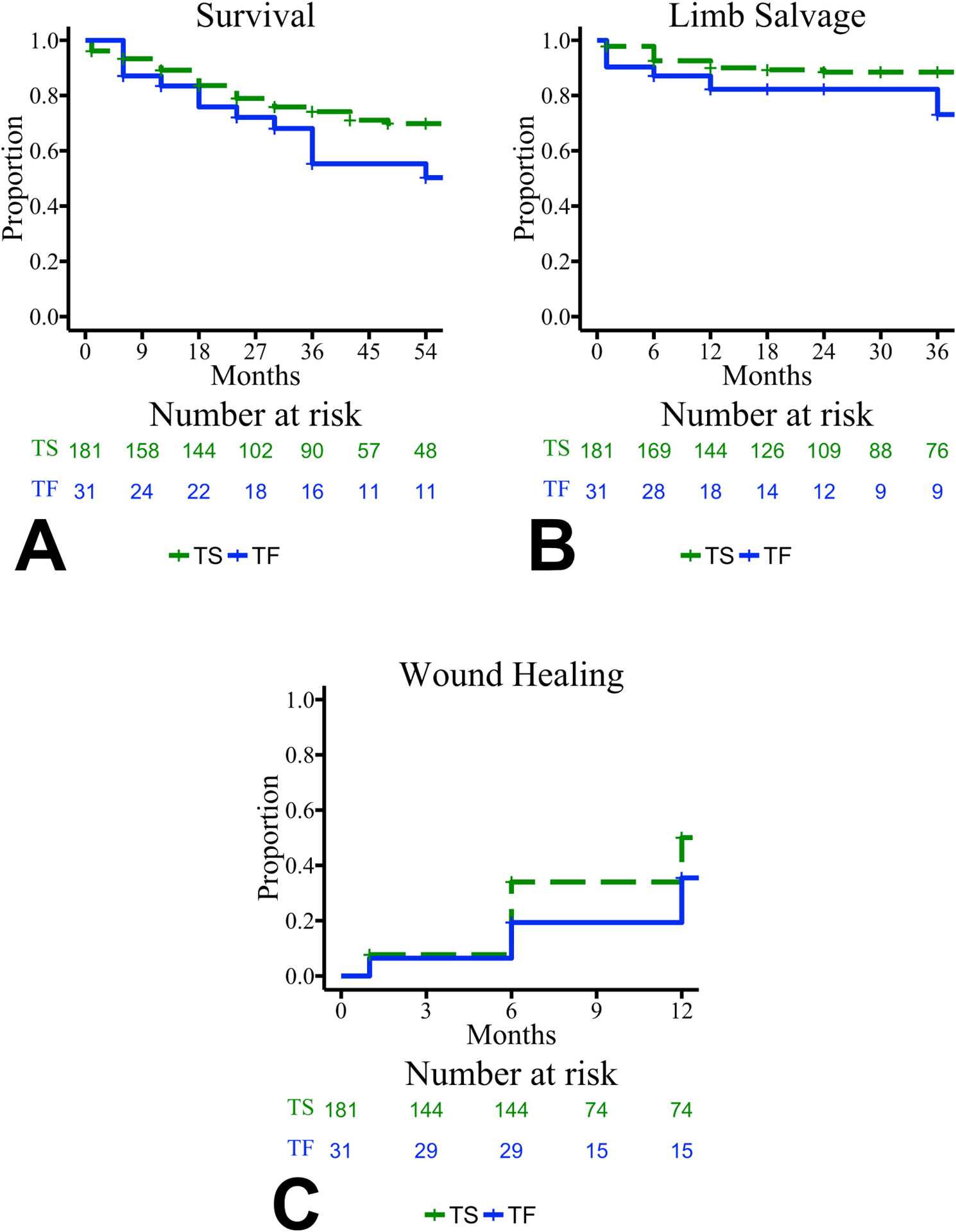
Kaplan-Meier plots are shown for the primary outcomes of patients who attempted endovascular intervention: survival (A), limb salvage (B), and wound healing (C). Functions are grouped by technical result of endovascular intervention: technical success (TS) shown in dashed green and technical failure (TF) shown in solid blue. Censored values are shown with “+”. Curves are truncated where standard error exceeded 10%. Survival functions did not differ between groups (p=0.087), but limb salvage and wound healing functions differed between groups (p=0.047 and p=0.028, respectively).
Course After Technical Failure of Endovascular Intervention
After TF, no patients had an alteration of their anatomy or progression of their SVS WIfI stage. Patients that went on to receive secondary bypass after TF had a higher prevalence of diabetes mellitus (91.7% vs. 50.0%, p=0.018) and a lower prevalence of tibial disease (30.8% vs. 66.7%, p=0.048) as compared to the medical management after TF group. Age, sex, ethnicity, tobacco use, hypertension, dialysis dependence, hyperlipidemia, cardiac status, pulmonary status, SVS WIfI stage, aortoiliac disease, and femoropopliteal disease did not differ between the secondary bypass and medical management groups.
Outcomes were not statistically significantly different between the medical management and secondary bypass groups. The mean survival in the medical management and secondary bypass groups was 41 and 45 months, respectively (p=0.448). The mean time to major amputation was 46 months in both groups (p=0.824). The mean time to wound healing in the medical management and secondary bypass groups was 33 and 41 months, respectively (p=0.691).
Primary and Secondary Bypass Groups
Patients that received bypass after TF tended to be older (70.62 ± 13.8 vs. 60.77 ± 10.0, p=0.012) compared to those that received primary bypass. Primary and secondary bypass groups did not differ by sex, ethnicity, tobacco use, diabetes mellitus, hypertension, dialysis dependence, hyperlipidemia, cardiac status, or SVS WIfI stage. Patients that underwent primary bypass had a higher prevalence of tibial disease than the secondary bypass group (63.3% vs. 30.8%, p=0.049), but the prevalence of aortoiliac and femoropopliteal disease did not differ between the primary and secondary bypass groups. Additionally, there were no technical failures in the primary or secondary bypass groups.
Outcomes were not statistically significantly different between the primary and secondary bypass groups. The mean survival in the primary and secondary bypass groups was 61 and 45 months, respectively (p=0.059). The mean time to major amputation in the primary and secondary bypass groups was 62 and 47 months, respectively (p=0.083). The mean time to wound healing in the primary and secondary bypass groups was 24 and 41 months, respectively (p=0.051). The mean time to loss of bypass patency in the primary and secondary bypass groups was 54 and 37 months, respectively (p=0.404).
Discussion
We report a TF rate of 14.6% for patients with CLTI undergoing endovascular intervention. As compared to the TS group, the TF group was older, had a greater proportion of males and current tobacco users, had longer arterial lesions, and had more CTOs. While overall survival was not linked to TF, this group experienced higher amputation rates and slower wound healing. Following TF, outcomes were not different between those who received secondary bypass versus medical management. Finally, outcomes in the secondary bypass group trended towards being less favorable compared to the primary bypass group.
While intermediate and long-term failure rates caused by restenosis or recurrent disease after endovascular intervention are well described, immediate TF rates following attempted endovascular intervention for CLTI are often under discussed outside of randomized controlled trials. It is likely the near 0% TF rates reported in many industry-sponsored device trials are a function of their highly selected populations and strict inclusion criteria that probably underestimate real-world TF rates. TF most commonly results from the inability to cross the arterial lesion or immediate failure of recanalization and has been linked to calcification and anatomic severity of disease.21–23 Real-world failure rates appear to have decreased in the last 20 years, with the 2005 BASIL RCT reporting the highest rate at 27%, and our TF rate of 14.6% is in good agreement with the recent BEST-CLI trial’s report of 15% TF.6,7,14
Our findings agree with other reports that increased age is associated with TF.4 Additionally, the TF group had a greater proportion of males and recent tobacco users (i.e., quit <10 years ago) which is expected given the independent association of these characteristics with arterial calcification: the best predictor of TF.22,23 Age, male sex, and tobacco use are important patient characteristics as they are also strongly associated with worse overall outcomes in CLTI along with other characteristics and comorbidities including chronic kidney disease, congestive heart failure, poor nutrition, non-White race, and high WIfI stage.4,21,24,25 While our work did not identify differences in WIfI stage between the TS and TF groups, WIfI stage is useful in predicting the benefit of revascularization.26 This finding suggests the WIfI classification system is less suited to predicting TF despite its documented utility in predicting benefit of revascularization. Unsurprisingly, limb salvage and wound healing were less favorable in our TF group as compared to the TS group, and instead resembled outcomes of patients with untreated CLTI.18
We did not identify differences in disease level with TF, but the TF group suffered from longer arterial lesions and CTOs more commonly compared to the TS group. Clinical experience supports these observations, as lesion lengths and CTOs are associated with higher TF rates. However, it is likely that other anatomic characteristics (e.g., calcification, runoff vessel quality, and patency of pedal vasculature) and risk factors not captured in this study also influenced the outcome of our TF group.
Outcomes were not statistically significantly different between patients who went on to receive a secondary bypass or manage their CLTI medically, though group sizes limited statistical power. Interestingly, this contrasts with generally improved outcomes in patients who receive a bypass after mid or long-term restenosis or reocclusion of endovascular intervention, which has been the focus of past work.27,28 Loss of target zone availability after failed endovascular intervention may worsen outcomes of subsequent bypass. Additionally, the temporal delay between TF and secondary bypass could influence outcomes for certain patients. If wounds worsen due to delayed revascularization, the feasibility of limb salvage after bypass may be affected. We did not find changes in anatomy or WIfI stage for any patients that received secondary bypass after TF, and all patients received their bypass less than two weeks after TF. Given this, and the fact there were no differences in WIfI stage between the TS and TF groups in the first place, it is hard to link differences in wound status to outcomes after medical management or secondary bypass based on our data.
It is possible the intensity of our wound care regimen may have masked the benefit of secondary bypass. The benefits of a standardized, systematic approach to wound care are well-documented, so the every two to four week multidisciplinary wound care follow up provided at our institution to the medical management group may have limited the detection of a benefit to secondary bypass.29–31 However, all patients, including secondary bypass patients, received the same level of care and follow up prescribed by Wound Healing Society guidelines, so we are unable to determine the extent to which wound care modified our results.20 Furthermore, the benefits of revascularization for CLTI are clear, especially with regards to amputation rates.1,32 Therefore, the comparable outcomes between patients who received medical management or secondary bypass after TF in our work may be due to small sample sizes and excellent wound care instead of true non-inferiority of medical management. While we suspect secondary bypass may be the superior approach in many cases based on clinical experience, large multi-institution studies are essential to determine the optimal course for patients after TF. In the meantime, decisions for secondary bypass should be made using a patient-centered shared decision-making model that incorporates patient preferences (e.g., life expectancy, prioritized outcomes, etc.), anatomic evaluation (e.g., WIfI stage, vein availability, runoff vessel quality), and clinical context (e.g., operative risk).
The secondary bypass group was older and had more tibial disease than the primary bypass group. While outcomes between the groups were not statistically different, the secondary bypass group trended towards increased mortality, shorter time to major amputation, and slower wound healing. Though small cohort sizes limited the detection of meaningful differences between these two groups, the clinically meaningful differences in outcomes should prompt continued investigation. Possible explanations for these trends include damage to target zones from the prior attempted endovascular intervention or a bias to select clinically sicker patients for initial endovascular intervention over bypass, resulting in a more comorbid secondary bypass group. Furthermore, the trends in differences between these groups may be explained given the age difference, a documented risk factor for poor outcomes after bypass.1,3,4 Our results are in slight disagreement with the BASIL data on secondary bypass outcomes after TF of endovascular intervention, which suggested a clinically failed angioplasty did not affect the results of any subsequent surgical intervention.7 On subgroup analysis of BASIL participants with infrapopliteal disease, no differences were noted between patients who received a primary bypass as compared to patients who received a secondary bypass, though only eight patients received a secondary bypass.14 Moreover, the high one-year amputation and death rates in the group that underwent secondary bypass suggest palliative limb care or primary amputation may have been the preferable course for some patients.
Selection bias is the most significant limitation of our retrospective study. The judgments to offer endovascular intervention, primary bypass, secondary bypass, and medical management were made by a vascular surgeon in the context of a patient-centered shared decision-making model. However, its impact on the TF and TS groups should be minimal as both were offered endovascular intervention, and our main conclusions based on these groups should be sound. Regarding TF rates, our definition of in-line flow is not as strict as a definition of in-line flow to the affected angiosome, which likely results in decreased TF rates as compared to a dataset utilizing an angiosome-based definition. Additional anatomic characteristics other than lesion length and presence of CTO undoubtedly also impact TF rates, and clinical events following TF may further affect the outcomes we observed.
Selection bias most significantly affects our conclusions in the secondary bypass versus medical management and primary versus secondary bypass groups. Our analyses in these groups is also greatly limited by our small sample sizes, so conclusions pertaining to these groups should be taken carefully in the context of individualized patient care. Lastly, none of the predictors are independently associated with our outcomes due to small sample sizes and the lack of a robust multivariable model. Future work in collaboration with other institutions is necessary to generate a larger patient base with sufficient statistical power to inform clinical decisions.
Conclusion
CLTI is a highly morbid condition experienced by patients late in life. While work in the past few decades has identified characteristics of patients best suited for endovascular treatment, a meaningful minority of patients experience TF. We report detailed long-term outcomes of this patient population, and the natural history of these patients resembles untreated patients. The complex clinical course after TF presents challenges for management, and future work is necessary to identify patients who are likely to experience TF or benefit from bypass thereafter.
ARTICLE HIGHLIGHTS.
Type of Research:
Single-center retrospective cohort study
Key Findings:
Of the 212 limbs that underwent attempted endovascular intervention to manage their chronic limb-threatening ischemia, 31 (14.6%) experienced immediate technical failure. As compared to technically successful interventions, patients after technical failure experienced worse limb salvage (p=0.047) and wound healing (p=0.028). Outcomes were not affected by subsequent bypass.
Take home Message:
Primary limb salvage outcomes are worse after immediate technical failure of endovascular intervention for chronic limb-threatening ischemia, and patients may not be rescued by subsequent bypass.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by National Institutes of Health award R01 HL135103 [MR Stacy].
Footnotes
Declaration of Conflicting Interests
The Authors declare that there is no conflict of interest.
Presentation Information: This study was presented as a poster at the 2021 Vascular Annual Meeting of the Society for Vascular Surgery, San Diego, California, Aug. 18–21, 2021.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Conte MS, Bradbury AW, Kolh P, White JV, Dick F, Fitridge R, et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. J Vasc Surg. 2019. Jun;69(6):3S–125S.e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fowkes FGR, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. The Lancet. 2013. Oct;382(9901):1329–40. [DOI] [PubMed] [Google Scholar]
- 3.Stella J, Engelbertz C, Gebauer K, Hassu J, Meyborg M, Freisinger E, et al. Outcome of patients with chronic limb-threatening ischemia with and without revascularization. Vasa. 2020. Mar;49(2):121–7. [DOI] [PubMed] [Google Scholar]
- 4.Smet N, Fourneau I, Roeleveld H, Boonman-de Winter L, Schraepen C, Favoreel M, et al. Age-Dependent Outcome of First-Line Endovascular and Surgical Revascularization Strategies in Chronic Limb-Threatening Ischemia. Ann Vasc Surg. 2022. Sep;85:133–45. [DOI] [PubMed] [Google Scholar]
- 5.Mills JL, Conte MS, Armstrong DG, Pomposelli FB, Schanzer A, Sidawy AN, et al. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: Risk stratification based on Wound, Ischemia, and foot Infection (WIfI). J Vasc Surg. 2014. Jan;59(1):220–234.e2. [DOI] [PubMed] [Google Scholar]
- 6.Farber A, Menard MT, Conte MS, Kaufman JA, Powell RJ, Choudhry NK, et al. Surgery or Endovascular Therapy for Chronic Limb-Threatening Ischemia. N Engl J Med. 2022 Dec 22;387(25):2305–16. [DOI] [PubMed] [Google Scholar]
- 7.Bradbury AW. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. 2005 Dec 3;366(9501):1925–34. [DOI] [PubMed] [Google Scholar]
- 8.Weissler EH, Wang Y, Gales JM, Feldman DN, Arya S, Secemsky EA, et al. Cardiovascular and Limb Events Following Endovascular Revascularization Among Patients ≥65 Years Old: An American College of Cardiology PVI Registry Analysis. J Am Heart Assoc. 2022 Jun 20;11(12):e024279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vadia R, Malyar N, Stargardt T. Cost-utility analysis of early versus delayed endovascular intervention in critical limb-threatening ischemia patients with rest pain. J Vasc Surg. 2023 Jan 1;77(1):299–308.e2. [DOI] [PubMed] [Google Scholar]
- 10.Popplewell MA, Andronis L, Davies HOB, Meecham L, Kelly L, Bate G, et al. Procedural and 12-month in-hospital costs of primary infra-popliteal bypass surgery, infrapopliteal best endovascular treatment, and major lower limb amputation for chronic limb threatening ischemia. J Vasc Surg. 2022 Jan 1;75(1):195–204. [DOI] [PubMed] [Google Scholar]
- 11.Russell DA. Modernizing vascular services to meet the demands of a changing disease burden. Br J Surg. 2021 Jun 22;108(6):593–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nienaber JJ, Smith CY, Cha S, Correa M, Rowse PG, Bailey KR, et al. Population-Based Trends in Amputations and Revascularizations for Peripheral Artery Disease From 1990 to 2009. Mayo Clin Proc. 2022. May;97(5):919–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moxey PW, Hofman D, Hinchliffe RJ, Jones K, Thompson MM, Holt PJE. Epidemiological study of lower limb amputation in England between 2003 and 2008. Br J Surg. 2010 Jul 28;97(9):1348–53. [DOI] [PubMed] [Google Scholar]
- 14.Popplewell MA, Davies HOB, Narayanswami J, Renton M, Sharp A, Bate G, et al. A Comparison of Outcomes in Patients with Infrapopliteal Disease Randomised to Vein Bypass or Plain Balloon Angioplasty in the Bypass vs. Angioplasty in Severe Ischaemia of the Leg (BASIL) Trial. Eur J Vasc Endovasc Surg. 2017. Aug;54(2):195–201. [DOI] [PubMed] [Google Scholar]
- 15.Schanzer A, Conte MS. Critical Limb Ischemia. Curr Treat Options Cardiovasc Med. 2010. Jun;12(3):214–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arain SA, White CJ. Endovascular therapy for critical limb ischemia. Vasc Med. 2008. Aug;13(3):267–79. [DOI] [PubMed] [Google Scholar]
- 17.Kim TI, Kiwan G, Mohamedali A, Zhang Y, Dardik A, Guzman RJ, et al. Multiple Reinterventions for Claudication are Associated with Progression to Chronic Limb-Threatening Ischemia. Ann Vasc Surg. 2021. Apr;72:166–74. [DOI] [PubMed] [Google Scholar]
- 18.Abu Dabrh AM, Steffen MW, Undavalli C, Asi N, Wang Z, Elamin MB, et al. The natural history of untreated severe or critical limb ischemia. J Vasc Surg. 2015. Dec;62(6):1642–1651.e3. [DOI] [PubMed] [Google Scholar]
- 19.Corriere MA, Kim GY, Byrnes ME, Sales A, Keith D, Ip EH, et al. Focus group study of factors relevant to treatment decisions and experiences among patients with symptomatic peripheral artery disease. J Vasc Surg. 2022. Nov;76(5):1316–24. [DOI] [PubMed] [Google Scholar]
- 20.Federman DG, Ladiiznski B, Dardik A, Kelly M, Shapshak D, Ueno CM, et al. Wound Healing Society 2014 update on guidelines for arterial ulcers. Wound Repair Regen Off Publ Wound Heal Soc Eur Tissue Repair Soc. 2016;24(1):127–35. [DOI] [PubMed] [Google Scholar]
- 21.Stoner MC, Calligaro KD, Chaer RA, Dietzek AM, Farber A, Guzman RJ, et al. Reporting standards of the Society for Vascular Surgery for endovascular treatment of chronic lower extremity peripheral artery disease. J Vasc Surg. 2016. Jul;64(1):e1–21. [DOI] [PubMed] [Google Scholar]
- 22.Itoga NK, Kim T, Sailer AM, Fleischmann D, Mell MW. Lower extremity computed tomography angiography can help predict technical success of endovascular revascularization in the superficial femoral and popliteal artery. J Vasc Surg. 2017. Sep;66(3):835–843.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato Y, Morishita T, Tan M, Hayashi T, Miwa T, Hieda S, et al. Prediction of Technical Failure of Inframalleolar Angioplasty in Patients with Chronic Limb Threatening Ischaemia. Eur J Vasc Endovasc Surg. 2022. Jun;63(6):852–63. [DOI] [PubMed] [Google Scholar]
- 24.Miyata T, Kumamaru H, Mii S, Kinukawa N, Miyata H, Shigematsu K, et al. Prediction Models for Two Year Overall Survival and Amputation Free Survival After Revascularisation for Chronic Limb Threatening Ischaemia. Eur J Vasc Endovasc Surg. 2022. Oct;64(4):367–76. [DOI] [PubMed] [Google Scholar]
- 25.Joshi GS, Zhang SM, Wang K, El Khoury R, Cataneo J, Jacobs CE, et al. Predictors of Amputation-free Survival after Endovascular Intervention for Chronic Limb-Threatening Ischemia in the Modern era. Ann Vasc Surg. 2022. Oct;86:268–76. [DOI] [PubMed] [Google Scholar]
- 26.Mayor J, Chung J, Zhang Q, Montero-Baker M, Schanzer A, Conte MS, et al. Using the Society for Vascular Surgery Wound, Ischemia, and foot Infection classification to identify patients most likely to benefit from revascularization. J Vasc Surg. 2019. Sep;70(3):776–785. e1. [DOI] [PubMed] [Google Scholar]
- 27.Bodewes TCF, Ultee KHJ, Soden PA, Zettervall SL, Shean KE, Jones DW, et al. Perioperative outcomes of infrainguinal bypass surgery in patients with and without prior revascularization. J Vasc Surg. 2017. May;65(5):1354–1365.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee KB, Chaudhry S, Lala S, Ricotta JJ, Sidawy AN, Amdur RL, et al. Failed Prior Endovascular Interventions Do Not Affect 30-day Cardiovascular or Limb-related Outcomes of Infrainguinal Bypasses for Chronic Limb Threatening Ischemia. Ann Vasc Surg. 2021. Feb;71:315–20. [DOI] [PubMed] [Google Scholar]
- 29.Trautner C, Haastert B, Mauckner P, Gätcke LM, Giani G. Reduced Incidence of Lower-Limb Amputations in the Diabetic Population of a German City, 1990–2005. Diabetes Care. 2007 Oct 1;30(10):2633–7. [DOI] [PubMed] [Google Scholar]
- 30.Anichini R, Zecchini F, Cerretini I, Meucci G, Fusilli D, Alviggi L, et al. Improvement of diabetic foot care after the Implementation of the International Consensus on the Diabetic Foot (ICDF): Results of a 5-year prospective study. Diabetes Res Clin Pract. 2007. Feb;75(2):153–8. [DOI] [PubMed] [Google Scholar]
- 31.Canavan RJ, Unwin NC, Kelly WF, Connolly VM. Diabetes- and Nondiabetes-Related Lower Extremity Amputation Incidence Before and After the Introduction of Better Organized Diabetes Foot Care. DIABETES CARE. 2008;31(3). [DOI] [PubMed] [Google Scholar]
- 32.Almasri J, Adusumalli J, Asi N, Lakis S, Alsawas M, Prokop LJ, et al. A systematic review and meta-analysis of revascularization outcomes of infrainguinal chronic limb-threatening ischemia. J Vasc Surg. 2018. Aug;68(2):624–33. [DOI] [PubMed] [Google Scholar]


