Abstract
Objective:
The worldwide practice and impact of non-invasive ventilation (NIV) in pediatric acute respiratory distress syndrome (PARDS) is unknown. We sought to describe NIV use and associated clinical outcomes in PARDS.
Design:
Planned ancillary study to the 2016/2017 prospective Pediatric Acute Respiratory Distress Syndrome Incidence and Epidemiology (PARDIE) study.
Setting:
145 international pediatric intensive care units (PICU).
Patients:
Patients with newly diagnosed PARDS admitted during 10 study weeks.
Interventions:
None
Measurements and Main Results:
Children were categorized by their respiratory support at PARDS diagnosis into NIV or invasive mechanical ventilation (IMV) groups. Of 708 subjects with PARDS, 160 (23%) received NIV at PARDS diagnosis (NIV group). NIV failure rate (defined as tracheal intubation or death) was 84/160 (53%). Higher non-respiratory pediatric logistic organ dysfunction (PELOD-2) score, arterial partial pressure of oxygen to fractional inspired oxygen ratio (PaO2/FiO2 ) <100 at PARDS diagnosis, immunosuppression, and male sex were independently associated with NIV failure. NIV failure was 100% among patients with non-respiratory PELOD-2 score >2, PaO2/FiO2 <100, and immunosuppression all present. Among patients with PaO2/FiO2 >100, children in the NIV group had shorter total duration of NIV and IMV, than the IMV at initial diagnosis group. We failed to identify associations between NIV use and PICU survival in a multivariable Cox regression analysis (Hazard Ratio 1.04 [95% confidence interval 0.61-1.80]) or mortality in a propensity score matched analysis (p=0.369).
Conclusions:
Use of NIV at PARDS diagnosis was associated with shorter exposure to IMV in children with mild to moderate hypoxemia. Even though risk of NIV failure was high in some children, we failed to identify greater hazard of mortality in these patients.
Keywords: respiratory distress, pediatric critical care, mechanical ventilation, immunosuppression, tracheal intubation
Summary.
NIV use is common at PARDS diagnosis (20%) although 50% of patients fail NIV support. In the PARDIE cohort, NIV failure was associated with hypoxemia severity, non-pulmonary organ dysfunction, and immunosuppression. NIV exposure was not associated with increased mortality.
For over two decades, use of non-invasive ventilation (NIV) for patients with severe bronchiolitis in pediatric intensive care units (PICUs) has been associated with an important reduction in the use of invasive mechanical ventilation (IMV)(1). For many other pediatric respiratory conditions, NIV has now become the primary modality of respiratory support in the PICU (2, 3) with the goal to prevent tracheal intubation and complications related to IMV and deeper sedation (4). However, NIV is also associated with potential risks related to delay in securing the airway, intolerance of the interface, and reduced unloading of high patient effort possibly contributing to lung injury (5). Failure of NIV is associated with increased mortality and longer duration of IMV (6, 7). Over twenty years ago, patients with immunosuppression and acute respiratory distress syndrome (ARDS) were considered good candidates for NIV trials as mortality rates with IMV support was high (8). Although NIV is often used in children with immunosuppression and pediatric ARDS (PARDS), NIV failure is common and there is little new evidence supporting this practice (9).
The severity of hypoxemia is a major risk factor for NIV failure (10, 11). In the last 10 years, NIV failure in children with severe PARDS has been reported to exceed 50% (11–13). Therefore, it is crucial to identify additional characteristics of hypoxemic children where the likelihood of NIV failure is high, and the risk of NIV may outweigh potential benefits. Recently, the second Pediatric Acute Lung Injury Consensus Conference (PALICC-2) highlighted the limited evidence on NIV use in PARDS, emphasizing the need for further research (14, 15). Therefore, as a planned ancillary study of the 2016/2017 Pediatric Acute Respiratory Distress Syndrome Incidence and Epidemiology (PARDIE) study (16), we aimed to describe contemporary use of NIV in PARDS. Our three objectives were: 1) to describe the characteristics associated with use of NIV, rather than use of IMV, at the initial diagnosis of PARDS; 2) to identify factors associated with NIV failure; and 3), to explore the association between NIV use and mortality and duration of ventilatory support.
MATERIAL AND METHODS
Study design
PARDIE was a prospective, observational, cohort study carried out in 145 international PICUs (17). The study enrolled children with a new diagnosis of PARDS – according to the 2015 PALICC criteria – during ten distinct weeks between May 2016 and June 2017. Detailed methods are published elsewhere (16). The Children’s Hospital Los Angeles (CHLA) Institutional Review Board (CHLA 16-0043) approved the study, titled “Pediatric ARDS Incidence and Epidemiology (PARDIE)” on February 8, 2016, and procedures were followed in accordance with the ethical standards of the responsible committee and with the Helsinki Declaration of 1975. All PARDIE patients were included in the current study and categorized based on the respiratory support used at the time of PARDS diagnosis, NIV or IMV. In accordance with the 2015 PALICC guidelines, in patients on NIV, only those with a full-face mask interface and either continuous positive airway pressure (CPAP) ≥5 cm H2O, or bi-level ventilation were eligible to be diagnosed with PARDS (17).
Data collection
Patient characteristics and respiratory parameters were collected for the first 3 days. Oxygenation severity was classified at the time of PARDS diagnosis according to the Berlin arterial partial pressure of oxygen (PaO2) to fractional inspired oxygen (FiO2) strata: <100 mmHg (severe), 101-200 mmHg (moderate), and 201-300 mmHg (mild), or their equivalent using the ratio of pulse oximetry oxygen saturation (SpO2) to FiO2 (SpO2/ FiO2) strata (16, 17). NIV failure was defined as the occurrence of tracheal intubation or death in the PICU, whichever occurred first.
Statistical analysis
The characteristics and outcomes of patients were reported using the median (interquartile range, IQR) or mean ± standard deviation (SD), then compared using Wilcoxon rank-sum or Student t-tests. Categorical variables were reported using number (percentage) and compared using Chi-square or Fisher’s exact tests. Details of the analysis plan are provided in the online data supplement.
To assess risk factors for NIV failure in the NIV group, we conducted univariate generalized estimating equation (GEE) analyses with failure as the dependent variable. We then conducted a multivariable GEE analysis considering all independent variables that were statistically significant in univariate analyses. In patients who failed NIV, we explored the association between NIV duration and mortality using a GEE analysis.
We calculated duration of NIV, IMV, and total ventilatory support (sum of NIV and IMV), duration of PICU stay, and PICU mortality for both the NIV and IMV groups. We performed a Cox regression analysis to assess the effect of NIV exposure on time between PARDS diagnosis and PICU mortality adjusting for risk factors with imbalanced distributions across the two groups.
We used a propensity score matching approach to assess the association between NIV use and mortality, matching 1:1 on the logit propensity score that was developed for NIV use. We used McNemar’s test to assess differences in mortality between matched NIV and IMV groups. We conducted a GEE analysis using mortality as a dependent variable in the entire group of patients, adjusting for the same covariates used in the propensity score model. Finally, we conducted a subgroup analysis to assess the association between NIV use and mortality in immunosuppressed patients, using a GEE analysis adjusted for the mortality risk factors identified in the entire population.
All analyses were clustered by site. In all multivariable analyses, we included the non-respiratory pediatric logistic organ dysfunction (PELOD-2) score as a covariate rather than the PELOD-2 score given inclusion of oxygenation metric covariates (18). Statistical analyses were conducted using SAS software version 9.4 (SAS Institute, Cary, NC, USA). Two-sided p-values <0.05 were considered significant.
RESULTS
Patient characteristics and NIV use
Of 708 patients included in the original PARDIE study, 160 (23%) were supported with NIV at the time of PARDS diagnosis (eFigure 1). Compared to IMV patients (Table 1), NIV patients were older and more frequently had chronic pulmonary disease, neuromuscular disease, oncologic disease, and immunosuppression, while they were less frequently born prematurely (all p <0.01). The causes of PARDS also differed, with pneumonia more common in the NIV patients. The use of NIV was associated with higher initial blood pH and lower PRISM-IV, PELOD-2, and non-respiratory PELOD-2 scores (all p<0.01). There were no differences in arterial partial pressure of carbon dioxide (PaCO2) or PaO2/FiO2 ratio between the two groups.
Table 1.
Characteristics of patients with PARDS treated with non-invasive ventilation (NIV) and invasive mechanical ventilation (IMV) at the time of PARDS diagnosis.
| Variable | NIV N=160 |
IMV N=548 |
P value |
|---|---|---|---|
| Age (years) | 7.1 (2.5-13.7) | 2.2 (0.6-8.5) | <0.0001 |
| Sex / Male | 94 (59%) | 340 (62%) | 0.471 |
| Primary cause of PARDS | 0.004 | ||
| Pneumonia | 112 (70%) | 298 (54%) | |
| Sepsis | 22 (14%) | 114 (21%) | |
| Aspiration | 13 (8%) | 39 (7%) | |
| Trauma | 1 (1%) | 26 (5%) | |
| Pancreatitis | 1 (1%) | 2 (0%) | |
| Near drowning | 0 (0%) | 5 (1%) | |
| Transfusion Related Lung Injury | 0 (0%) | 1 (0%) | |
| Other | 11 (7%) | 63 (11%) | |
| Comorbidities | |||
| Prematurity | 16 (10%) | 115 (21%) | 0.002 |
| Chronic pulmonary disease | 64 (40%) | 133 (24%) | <0.0001 |
| Chronic respiratory support before PICU admission | 34 (21%) | 86 (16%) | .099 |
| Neuro-muscular disease | 47 (29%) | 75 (14%) | <0.0001 |
| Congenital cardiac disease | 18 (11%) | 60 (11%) | 0.915 |
| Left ventricular dysfunction | 9 (6%) | 31 (6%) | 0.988 |
| Acquired heart disease | 7 (4%) | 49 (9%) | 0.060 |
| Oncologic disease | 25 (16%) | 34 (6%) | <0.001 |
| Immunosuppression | 34 (21%) | 61 (11%) | <0.001 |
| - With oncologic disease | 19 (12%) | 30 (5%) | |
| - Stem cell transplants | 12 (7%) | 14 (3%) | |
| Time of PARDS diagnosis since PICU admission (d) | 0.2 (0.0-0.9) | 0.25 (0.0-1.7) | 0.032 |
| Initial PaO2/FiO2 ratio | 132 ± 70 | 142 ± 84 | 0.150 |
| PARDS Berlin PaO2/FiO2 groups | 0.854 | ||
| PaO2/FiO2 201-300 mmHg | 37 (23%) | 129 (24%) | |
| PaO2/FiO2 101-200 mmHg | 57 (36%) | 206 (38%) | |
| PaO2/FiO2 <100 mmHg | 66 (41%) | 213 (39%) | |
| Bilateral infiltrates on chest imaging | 110 (68.7) | 413 (75.4) | 0.094 |
| Initial pH, mean ± SD; number of values * | 7.34 ± 0.10 ; 100 | 7.30 ± 0.12 ; 453 | 0.001 |
| Initial PCO2 (mmHg), mean ± SD; number of values | 50.4 ± 18.0 ; 98 | 52.5 ± 19.5 ; 452 | 0.316 |
| PRISM-IV Score, median (IQR); number of values | 5 (1-9) ; 142 | 8 (3-14) ; 492 | <0.0001 |
| PELOD-2 Score, median (IQR); number of values | 2 (0-4) ; 138 | 6 (5-8) ; 483 | <0.0001 |
| Non-Respiratory PELOD-2 score | 2 (0 - 3) | 2 (2 - 4) | <0.0001 |
Number of values are reported when missing values were higher than 5%.
Data are presented as median (interquartile range), mean ± standard deviation, or as number (percentage), as appropriate. PARDS pediatric acute respiratory distress syndrome, NIV noninvasive ventilation, IMV invasive mechanical ventilation, IQR interquartile range, PICU pediatric intensive care unit.
The proportion of PARDS patients on NIV varied widely between sites (median 18%, IQR 0-36%, in sites with at least 4 patients enrolled) and across countries (eFigure 2), but did not differ between world bank income classification of the country (eTable 1). The use of NIV in PARDS patients was more frequent in sites that used NIV more frequently in the overall PICU population. NIV characteristics and settings used during the first 3 days are reported in eTable 2.
NIV failure
NIV failure occurred in 84/160 (53%) patients on NIV at PARDS diagnosis, including 80 patients who required intubation and 4 patients who died without intubation. These 4 deaths had severe oncologic or neurological disorders, and documented limitations in support were clearly captured in two of the patients. Table 2 shows that in univariate analyses NIV failure was associated with male sex, immunosuppression, lower initial PaO2/FiO2 ratio, PaO2/FiO2 <100 mmHg, bilateral radiological infiltrates, higher pediatric risk of mortality (PRISM-IV) score, and higher non-respiratory PELOD-2 score at PARDS diagnosis. On multivariable analysis (Table 2), immunosuppression, PaO2/FiO2 <100, non-respiratory PELOD-2 score, and male sex were independently associated with NIV failure. Regarding the site characteristics, the frequency of NIV use in the site (in the entire PICU population, not just PARDS patients) was not associated with NIV failure rate in the PARDS cohort (odds ratio 0.80 [0.61-1.05]). Patients from sites in low- or middle-income countries however had a higher risk of NIV failure: 13/16 (81%), versus 71/144 (49%), odds ratio 4.46 (1.44-13.9).
Table 2.
Characteristics associated with noninvasive (NIV) failure in univariate analyses and multivariable GEE.
| Variable | NIV Failure N=84 |
NIV success N=76 |
Odds Ratio (95% CI) |
P value |
Adjusted Odds Ratio (95% CI) |
P value |
|---|---|---|---|---|---|---|
| Age (years) | 7.3 (2.0-13.9) | 6.9 (3.4-13.7) | 0.99 (0.95-1.05) | 0.931 | ||
| Sex / Male | 55 (65%) | 39 (51%) | 1.80 (1.01-3.20) | 0.045 | 2.39 (1.15-4.95) | 0.019 |
| PARDS Risk Factor | 0.218 | |||||
| Pneumonia | 55 (65%) | 57 (75%) | ref | |||
| Sepsis | 16 (19%) | 6 (8%) | 2.76 (0.96-7.94) | 0.059 | ||
| Aspiration | 7 (8%) | 6 (8%) | 1.21 (0.43-3.41) | 0.720 | ||
| Other | 6 (7%) | 7 (9%) | 0.89 (0.29-2.70) | 0.835 | ||
| Comorbidities | ||||||
| Prematurity | 7 (8%) | 9 (12%) | 0.68 (0.21-2.16) | 0.510 | ||
| Chronic pulmonary disease | 28 (33%) | 36 (47%) | 0.56 (0.27-1.13) | 0.106 | ||
| Chronic respiratory support before PICU admission | 17 (20%) | 17 (22%) | 0.88 (0.33-2.34) | 0.799 | ||
| Neuro-muscular disease | 27 (32%) | 20 (26%) | 1.33 (0.72-2.44) | 0.365 | ||
| Congenital cardiac disease | 7 (8%) | 11 (14%) | 0.54 (0.18-1.59) | 0.261 | ||
| LV dysfunction | 7 (8%) | 2 (3%) | 3.36 (0.39-29.2) | 0.271 | ||
| Acquired heart disease | 4 (5%) | 3 (4%) | 1.22 (0.32-4.65) | 0.774 | ||
| Oncologic disease | 17 (20%) | 8 (10%) | 2.16 (0.79-5.89) | 0.134 | ||
| Immunosuppression | 27 (32%) | 7 (9%) | 4.67 (1.61-13.5) | 0.005 | 3.75 (1.04-13.44) | 0.043 |
| Initial PaO2/FiO2 ratio | 118 ± 66 | 147 ± 70 | 0.94 (0.90-0.99) | 0.010 | ||
| PARDS Berlin PF groups | 0.008 | |||||
| PaO2/FiO2 201-300 mmHg | 14 (17%) | 23 (30%) | Ref. | Ref. | ||
| PaO2/FiO2 101-200 mmHg | 26 (31%) | 31 (41%) | 1.38 (0.60-3.14) | 0.445 | 1.35 (0.53-3.41) | 0.531 |
| PaO2/FiO2 <100 mmHg | 44 (52%) | 22 (29%) | 3.29 (1.46-7.37) | 0.004 | 3.01 (1.08-8.37) | 0.034 |
| Bilateral infiltrates | 65 (77%) | 45 (59%) | 2.36 (1.29-4.29) | 0.005 | 1.59 (0.80-3.16) | 0.187 |
| Initial pH | 7.33 (7.28-7.38) | 7.37 (7.33-7.40) | 0.58 (0.33-1.02) | 0.058 | ||
| Initial PCO2 (mmHg) | 51.9 ± 19.2 | 47.3 ± 15.0 | 1.02 (0.99-1.04) | 0.243 | ||
| PRISM-IV Score | 6 (3-13) | 2.5 (0-7) | 1.12 (1.04-1.21) | 0.003 | ||
| Non-Respiratory PELOD-2 | 2 (0-4) | 2 (0-2) | 1.22 (1.05- 1.41) | 0.008 | 1.19 (1.005-1.42) | 0.044 |
| PELOD-2 score | 3 (0-5) | 2 (0-2) | 1.34 (1.18-1.51) | <0.001 | ||
| Mode BIPAP vs CPAP | 34 vs 5 | 40 vs 9 | 1.53 (0.43-5.50) | 0.515 |
Data are presented as median (1st quartile-3rd quartile), mean ± standard deviation, or as number (percentage), as appropriate. The effect estimates for continuous variables are per 1 unit increase.
NIV noninvasive ventilation, GEE generalized estimated equation, CI confidence interval, PARDS pediatric acute respiratory distress syndrome, PICU pediatric intensive care unit, LV left ventricular, BIPAP bilevel positive airway pressure, CPAP continuous positive airway pressure.
Figure 1 illustrates the interaction between immunosuppression, severe hypoxemia, and non-respiratory PELOD-2 score >2. Inspection of the Venn diagram shows that the NIV success rate was 42/66 (64%) when none of these 3 factors were present. When only one of these three risk factors was present the cumulative success rate was 28/57 (49%). When a combination of any two risk factors was present the cumulative success rate was 6/31 (19%). When all three risk factors were present there were no successes (0/6, 0%). The proportion of NIV failure and the mortality rate depending on SpO2/FiO2 and PaO2/FiO2 ratio are illustrated in Figure 2.
Figure 1.
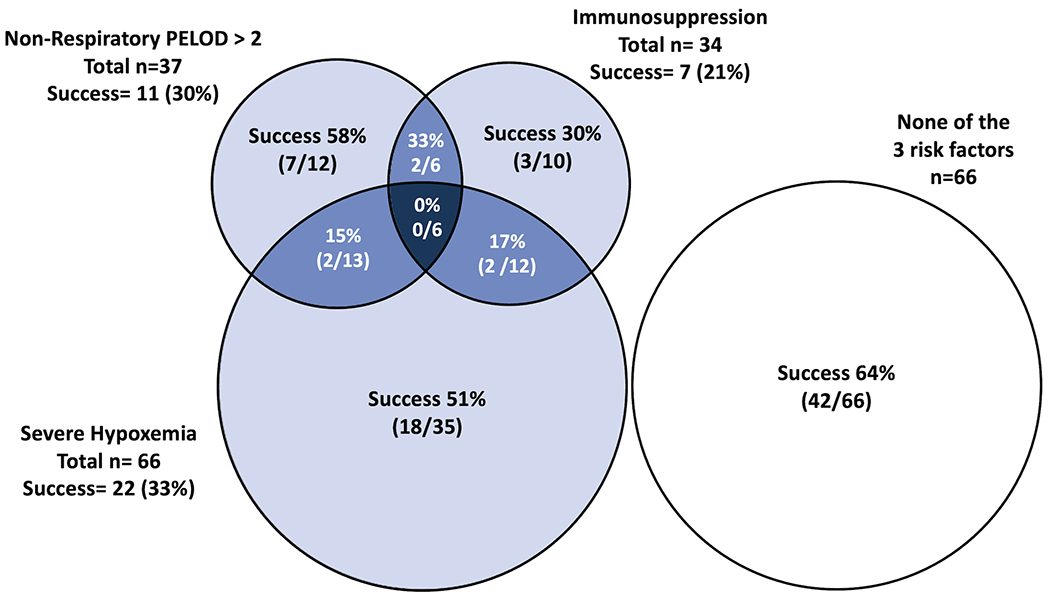
Number of patients treated with non-invasive ventilation (NIV) at PARDS diagnosis and success rate, depending on the presence of a high non-respiratory PELOD-2 score, immunosuppression, severe hypoxemia (PaO2/FiO2 <100), a combination of these factors.
Figure 2.
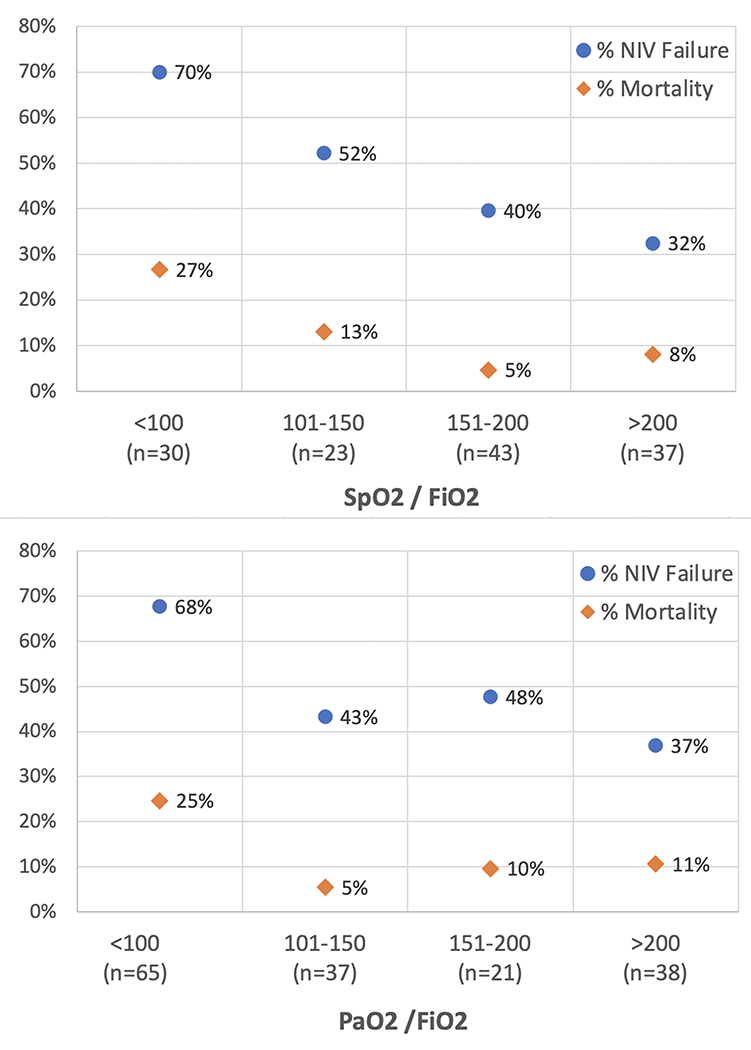
Proportion of NIV failure and mortality rate depending on initial SpO2/FiO2 ratio (upper panel) and PaO2/FiO2 ratio (lower panel).
In cases of NIV failure, tracheal intubation occurred after a median of 10 (IQR 2-26) hours of NIV. Higher non-respiratory PELOD-2 score was the only factor independently associated with earlier intubation in multivariable modeling (eTable 3). NIV failure occurred before 6 hours in 39/84 (47%) cases, between 6 and 24 hours in 23/84 (27%) cases, and after 24 hours of NIV in 22/84 (26%) cases, and the mortality rates were 23%, 26%, and 42% in these subgroups, respectively (eFigure 3). In a multivariable GEE analysis restricted to this NIV failure group, immunosuppression was independently associated with mortality (p=0.001) while the duration of NIV prior to intubation (for each hour) had an odds ratio of 1.32 (0.98-1.78, p=0.064) for mortality (eTable 4).
PICU Outcome
PICU mortality rates were not different between NIV and IMV groups overall or within each oxygenation severity strata (Table 3). eFigure 4 illustrates the duration of total ventilatory support in surviving patients by PaO2/FiO2 strata. As detailed in Table 3, the total duration of ventilation support was shorter in the NIV group overall, with a significant difference for the mild and moderate severity strata only. Length of PICU stay was also shorter in the NIV group overall, with a significant difference in the mild severity strata. Compared to the NIV success group, the NIV failure group exhibited qualitatively worse PICU outcomes overall (Table 3). Duration of ventilation and length of PICU stay for the entire group (including non-survivors) are reported in eTable 5.
Table 3.
Main PICU outcomes in patients with PARDS treated with noninvasive ventilation (NIV) and invasive mechanical ventilation (IMV).
| IMV group (n=548) |
Entire NIV group (n=160) |
P value | NIV Success group (n=76) |
NIV failure group (n=84) |
|
|---|---|---|---|---|---|
| PICU Mortality rate | |||||
| All severity strata | 97/548 (18%) | 24/160 (15%) | 0.425 | 0/76 (0) | 24/84 (29%) |
| PaO2/FiO2 201-300 mmHg group | 14/129 (11%) | 4/37 (11%) | 0.994 | 0/23 (0) | 4/14 (29%) |
| PaO2/FiO2 101-200 mmHg group | 31/206 (15%) | 4/57 (7%) | 0.114 | 0/31 (0) | 4/26 (15%) |
| PaO2/FiO2 <100 mmHg group | 52/213 (24%) | 16/66 (24%) | 0.977 | 0/22 (0) | 16/44 (36%) |
| Total duration of ventilatory support in PICU survivors (days) | |||||
| All severity strata | 7.0 (4.0-11.9) | 5.6 (2.6-9.6) | <0.001 | 3.2 (1.9-6.9) | 8.0 (4.9-13.1) |
| PaO2/FiO2 201-300 mmHg group | 6.1 (4.1-11.6) | 5.2 (2.0-7.5) | 0.015 | 2.5 (1.5-6.3) | 7.0 (5.5-10.1) |
| PaO2/FiO2 101-200 mmHg group | 6.6 (3.6-10.9) | 5.2 (2.2-9.6) | 0.019 | 2.8 (1.6-6.2) | 9.7 (4.9-14.2) |
| PaO2/FiO2 <100 mmHg group | 7.5 (4.2-13.0) | 6.9 (3.3-10.2) | 0.158 | 4.4 (2.6-8.0) | 8.2 (4.7-13.8) |
| Duration of NIV in PICU survivors (days) | |||||
| All severity strata | 0 | 1.6 (0.2-4.4) | - | 3.2 (1.9-6.9) | 0.2 (0.0-0.9) |
| PaO2/FiO2 201-300 mmHg group | 0 | 1.8 (0.8-5.4) | - | 2.5 (1.5-6.3) | 0.8 (0.4-1.6) |
| PaO2/FiO2 101-200 mmHg group | 0 | 1.8 (0.4-4.1) | - | 2.8 (1.6-6.2) | 0.4 (0.1-1.0) |
| PaO2/FiO2 <100 mmHg group | 0 | 0.7 (0.1-4.4) | - | 4.4 (2.6-8.0) | 0.1 (0.0-0.4) |
| Duration of invasive MV in PICU survivors (days) | |||||
| All severity strata | 7.0 (4.0-11.9) | 0.0 (0.0-6.6) | - | 0 | 7.5 (4.3-12.5) |
| PaO2/FiO2 201-300 mmHg group | 6.1 (4.1-11.6) | 0.0 (0.0-3.3) | - | 0 | 5.5 (3.9-9.9) |
| PaO2/FiO2 101-200 mmHg group | 6.6 (3.6-10.9) | 0.0 (0.0-6.5) | - | 0 | 7.5 (4.3-13.9) |
| PaO2/FiO2 <100 mmHg group | 7.5 (4.2-13.0) | 2.5 (0.0-9.0) | - | 0 | 8.1 (4.7-13.8) |
| Length of PICU stay after PARDS diagnosis in PICU survivors (days) | |||||
| All severity strata | 10.2 (6.4-19.2) | 8.3 (5.4-15.1) | 0.028 | 6.3 (3.8 - 9.6) | 15.1 (8.0-23.9) |
| PaO2/FiO2 201-300 mmHg group | 9.6 (6.3-19.5) | 7.9 (4.2-11.0) | 0.025 | 5.5 (2.8-8.5) | 16.1 (9.1-23.8) |
| PaO2/FiO2 101-200 mmHg group | 9.7 (5.9-17.6) | 9.8 (5.3-18.1) | 0.628 | 5.5 (3.8-10.1) | 18.3 (10.7-39.1) |
| PaO2/FiO2 <100 mmHg group | 11.1 (7.2-19.8) | 8.5 (6.4-14.7) | 0.129 | 7.0 (5.5-10.2) | 12.2 (3.0-43.4) |
The mortality results are reported as Number of deaths/Number of patients (proportion). Durations of ventilation support are presented as median (interquartile range).
NIV noninvasive ventilation, IMV invasive mechanical ventilation, PICU pediatric intensive care unit.
In a multivariable Cox analysis (eTable 6) adjusted for age, PARDS risk factor, comorbidities, PARDS severity class, initial pH, and non-respiratory PELOD score, exposure to NIV was not associated with shorter survival time (p=0.877). Chronic pulmonary disease, immunosuppression, severe PaO2/FiO2 class, initial pH, and non-respiratory PELOD score were independently associated with earlier death. In a sensitivity analysis, censoring the Cox regression at 90 days instead of PICU discharge did not change the results (data not shown).
A propensity score analysis for NIV exposure was conducted, with a greedy matching 1:1 on the logit propensity score obtained by logistic regression, based on all variables presented in eTable 7. Propensity-score matching was possible for 89 patients treated with NIV (56% of cohort) matched to 89 patients with PARDS not exposed to NIV (eFigure 5). The rate of NIV failure in the children who could be matched was 67%, illustrating the selection of a relatively severe subgroup (eTable 7). In these propensity-score matched groups, the mortality rate was 19% in the NIV group vs 25% in the IMV group (p=0.369). In the survivors of these groups, the length of PICU stay and ventilation duration did not differ. A GEE analysis conducted in the entire group of 708 patients, adjusting for the variables used in propensity score matching and clustering for site, also revealed no difference in mortality rates depending on NIV exposure (odds ratio 0.81, 95% CI 0.41-1.58).
Subgroup analysis
In immunosuppressed patients, the mortality rate was 38% (13/34) when initially treated with NIV (and 48% in the subgroup of children with NIV failure), and 51% (31/61) with primary IMV (p=0.238). When adjusting for baseline non-respiratory PELOD-2, PaO2/FiO2 severity group, and pH, NIV exposure was not associated with mortality (adjusted odds ratio: 1.26 [95% CI: 0.47-3.36], p=0.650). A sensitivity analysis including all oncologic patients (in addition to the immunosuppressed patients) provided similar results (data not shown).
DISCUSSION
This study about using NIV as initial respiratory support for cases of PARDS is important and the largest reported to date, albeit reflecting contemporary practice in the 2016/2017 PARDIE dataset. Overall, 23% of children with PARDS were supported with NIV at the time of PARDS diagnosis. Previous data for comparison are limited, but this prevalence is significantly higher than the 14/165 (8.5%) observed in a 2007 international cross-sectional study of acute lung injury in children (19). It is also significantly higher than the 436/2813 (15.5%) identified in the 2014 point prevalence study of NIV use in adults with ARDS (20). In pediatric practice, older age was associated with NIV use, which may be explained by the difficulty using NIV in younger patients (21), more frequent use of nasal modes in younger patients (thereby excluding them from the PARDS diagnosis), as well as the different diagnoses observed in older children. In our PARDIE study, NIV was particularly used in patients with chronic pulmonary disease, neuromuscular disease, immune-oncologic disease, and lower illness severity scores.
The similar distribution of PaCO2 and PaO2/FiO2 strata in the NIV and IMV groups was more surprising. In 2015, the PALICC experts did not recommend the use of NIV in children with severe PARDS (22). This result may be partly explained by patients being placed on NIV before the occurrence of severe PARDS. It is also possible that a trial of NIV was attempted in these patients to assess their response to recruitment. Of note, while the rate of intubation in the patients with severe hypoxemia was high (66%), when no other risk factors were present about half the patients avoided intubation. The recent PALICC-2 conference updated the recommendations on NIV use and now suggests that NIV should be used for a time-limited trial. Transition to IMV should occur when patients do not have improvement within the first 6 hours of NIV, particularly in those with severe PARDS when earlier transition to IMV should be considered (14, 15).
Independent risk factors associated with NIV failure included severe hypoxemia, immunosuppression, non-respiratory PELOD-2 score>2, and male sex. Severe hypoxemia has been consistently reported as a risk factor for NIV failure (10, 11, 13, 23). The risk of NIV failure associated with non-pulmonary organ dysfunction is also consistent with previous studies that reported higher NIV failure rates in the presence of sepsis (10, 12, 23, 24), vasoactive agents (25), or higher PRISM scores (10, 12, 23). Immunosuppression was associated with both NIV use and NIV failure in this study. NIV has long been advocated in this population (8, 12, 26) because of poor outcomes of these patients after intubation. Previous studies demonstrating high mortality rates (>70%) of immunosuppressed patients who fail an NIV trial have raised concerns about the adverse impact of delaying intubation (9, 26), including significant risk of cardiac arrest (25). Moreover, a randomized controlled trial assessing the potential benefit of early PICU admission for CPAP in immunosuppressed children with respiratory failure provided no evidence in favor of this intervention (27). In the present study, the NIV failure rate in immunosuppressed patients was particularly high (79%). However, this high failure rate was not associated with an increased risk of mortality when adjusting for other risk factors. Additionally, the mortality rate in primarily intubated patients was also high (51%) and was similar to that observed in immunosuppressed patients who failed NIV and were subsequently intubated (48%). Our study does not allow us to make a conclusion about the benefit of NIV in this subpopulation. However, an important finding in our study is the cumulative burden of risk factors of immunosuppression, severe hypoxemia, and multiple organ failure, and clinicians should be particularly cautious when managing patients with two or more of these risk factors on NIV.
The duration of ventilatory support and PICU length of stay were shorter in the NIV group overall, and especially in patients with baseline PaO2/FiO2 >100 mmHg. This signal may support the use of NIV in this population, although it should be tempered by the fact that the NIV group appeared to be less severely ill at PARDS diagnosis. In patients with risk factors, the rate of NIV failure was relatively high, and the patients with NIV failure exhibited relatively poor outcomes, therefore the question of potential harm caused by the NIV exposure in this population is particularly important. In addition to the risk associated with intubation delay and instability, use of NIV could theoretically expose patients to the risk of NIV-induced lung injury, when the spontaneous ventilatory drive is high enough to induce excessive transpulmonary pressure or tidal volume (5, 28).
In adult patients with severe ARDS, NIV exposure is associated with an increased risk of death (20, 29). In a secondary analysis of a large pediatric prospective trial, pre-intubation exposure to NIV was also associated with increased mortality (30). However, only intubated patients were included and the outcome of patients with NIV success was therefore not considered. Somewhat reassuringly, we did not observe any increased risk in mortality associated with NIV use, while using several strategies to adjust for other risk factors. This does not confirm that NIV is without risk in patients with PARDS. The NIV duration was quite short in the NIV failure group, and we can speculate that the clinical teams avoided inappropriately prolonged NIV in most instances. While the duration of NIV exposure prior to intubation was not an independent risk factor for mortality in the NIV failure group, the upper limit of the 95% confidence interval for the Odds Ratio (1.32 [0.98-1.78], p=0.064) shows what we cannot exclude as a potentially clinically meaningful effect which did not reach statistical significance because of limited power and sample size. These findings are consistent with PALICC-2 recommendations (14), in which tracheal intubation should be considered rapidly in patients with PARDS treated with NIV who do not show early clinical improvement.
We observed a particularly high failure rate in sites in low- and middle-income countries. This subgroup was small, and further studies are needed to confirm the generalizability of this finding and to explore potential reasons for this failure rate, particularly with respect to specific diseases, available equipment, monitoring, and personnel (15, 31).
This study has several limitations. First, its observational design precludes conclusions on causality of observed associations. The use of NIV is intrinsically linked with baseline condition and clinician preferences, and residual confounding in the multivariable analyses cannot be excluded. Second, although this is the largest cohort of PARDS patients treated with NIV, some analyses were limited by the sample size. Third, participation in the ancillary studies was voluntary and the burden of data collection was high. This may have led to a selection bias, and this limited some sub-analyses; in particular, we could not assess the prognostic impact of the patient response (improvement or not) after NIV exposure, nor explore the potential impact of high patient effort on the lung injury. Fourth, consistent with PALICC definitions (17), only patients with a full-face or an oro-nasal mask were included. It is possible that some patients with similar clinical condition were only diagnosed as “at risk for PARDS” because they were supported with other nasal interface (32). It is uncertain how our findings translate to this population. Last, the data captured in PARDIE relates to practice and management in 2016/2017.
CONCLUSIONS
This ancillary study of a 2016/2017 international dataset shows that a 1-in-5 children with PARDS are first managed with NIV, with close to 50% success rate. While use of NIV is associated with some positive outcomes, other findings call for caution. These include the high failure rate when multiple risk factors are present, and the potentially poor outcomes of NIV failure patients. Interventional controlled trials appear particularly warranted to confirm the role of NIV in PARDS patients at high risk, including patients with severe PARDS, immunosuppression, and/or multi-organ dysfunction.
Supplementary Material
“Research in Context”.
The worldwide use of non-invasive ventilation (NIV) in pediatric acute respiratory distress syndrome (PARDS) is unknown.
NIV may prevent tracheal intubation in some patients, but in others its use may contribute to lung injury (i.e., in the case of reduced unloading of high patient effort) or instability due to NIV failure.
The characteristics of PARDS patients who may benefit from NIV, rather than be harmed, is also uncertain.
“What this Study Means”.
In this large international observational study from 2016/2017, 23% of children with PARDS were initially supported by NIV.
Half of these NIV-supported patients were eventually placed on IMV, with hypoxemia severity, non-respiratory PELOD-2 score, immunosuppression, and male sex being independently associated with NIV failure.
Although children intubated after NIV failure have a high risk of adverse events, NIV exposure in children with PARDS was not independently associated with higher mortality.
Acknowledgments:
The authors thank Margaret Klein for the supervision of the statistical analysis of the main study and the ancillary studies.
Financial support.
University of Southern California Clinical Translational Science Institute, Sainte Justine Children’s Hospital, University of Montreal, Canada, Réseau en Santé Respiratoire du Fonds de Recherche Québec-Santé, and Children’s Hospital Los Angeles, Department of Anesthesiology and Critical Care Medicine.
Copyright Form Disclosure:
Dr. Emeriaud’s institution received funding from Fonds de recherche du Quebec Santé (research public agency award) and Maquet. Dr. Pons-Òdena’s institution received funding from Medtronic; he received funding from Philips Respironics. Drs. Bhalla and Killien received support for article research from the National Institutes of Health. Dr. Shein received funding from Hill Ward Henderson. Dr. Killien’s institution received funding from the National Institutes of Child Health and Human Development. Dr. Rowan’s (institution received funding from the National Heart, Lung, and Blood Institute (K23). Dr. Lin received funding from ROMTech. Dr. Napolitano’s institution received funding from Drager, Actuated Medical, Philips/Respinronics, and VeroBiotech. Dr. Khemani received funding from Orange Med and Bayer. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Footnotes
PARDIE investigators are listed in Appendix (in the supplemental data content).
References
- 1.Essouri S, Laurent M, Chevret L, et al. Improved clinical and economic outcomes in severe bronchiolitis with pre-emptive nCPAP ventilatory strategy. Intensive Care Med 2014;40(1):84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolfler A, Calderini E, Iannella E, et al. Evolution of Noninvasive Mechanical Ventilation Use: A Cohort Study Among Italian PICUs. Pediatr Crit Care Med 2015;16(5):418–427. [DOI] [PubMed] [Google Scholar]
- 3.Mayordomo-Colunga J, Pons-Odena M, Medina A, et al. Non-invasive ventilation practices in children across Europe. Pediatr Pulmonol 2018;53(8):1107–1114. [DOI] [PubMed] [Google Scholar]
- 4.Boghi D, Kim KW, Kim JH, et al. Noninvasive Ventilation for Acute Respiratory Failure in Pediatric Patients: A Systematic Review and Meta-Analysis. Pediatr Crit Care Med 2023;24(2):123–132. [DOI] [PubMed] [Google Scholar]
- 5.Brochard L, Slutsky A, Pesenti A. Mechanical Ventilation to Minimize Progression of Lung Injury in Acute Respiratory Failure. Am J Respir Crit Care Med 2017;195(4):438–442. [DOI] [PubMed] [Google Scholar]
- 6.Payen V, Jouvet P, Lacroix J, et al. Risk factors associated with increased length of mechanical ventilation in children. Pediatr Crit Care Med 2012;13(2):152–157. [DOI] [PubMed] [Google Scholar]
- 7.Demoule A, Girou E, Richard JC, et al. Benefits and risks of success or failure of noninvasive ventilation. Intensive Care Med 2006;32(11):1756–1765. [DOI] [PubMed] [Google Scholar]
- 8.Hilbert G, Gruson D, Vargas F, et al. Noninvasive continuous positive airway pressure in neutropenic patients with acute respiratory failure requiring intensive care unit admission. Crit Care Med 2000;28(9):3185–3190. [DOI] [PubMed] [Google Scholar]
- 9.Rowan CM, Gertz SJ, McArthur J, et al. Invasive Mechanical Ventilation and Mortality in Pediatric Hematopoietic Stem Cell Transplantation: A Multicenter Study. Pediatr Crit Care Med 2016;17(4):294–302. [DOI] [PubMed] [Google Scholar]
- 10.Mayordomo-Colunga J, Pons M, Lopez Y, et al. Predicting non-invasive ventilation failure in children from the SpO(2)/FiO(2) (SF) ratio. Intensive Care Med 2013;39(6):1095–1103. [DOI] [PubMed] [Google Scholar]
- 11.Munoz-Bonet JI, Flor-Macian EM, Brines J, et al. Predictive factors for the outcome of noninvasive ventilation in pediatric acute respiratory failure. Pediatr Crit Care Med 2010;11(6):675–680. [DOI] [PubMed] [Google Scholar]
- 12.Piastra M, De Luca D, Pietrini D, et al. Noninvasive pressure-support ventilation in immunocompromised children with ARDS: a feasibility study. Intensive Care Med 2009;35(8):1420–1427. [DOI] [PubMed] [Google Scholar]
- 13.Essouri S, Chevret L, Durand P, et al. Noninvasive positive pressure ventilation: five years of experience in a pediatric intensive care unit. Pediatr Crit Care Med 2006;7(4):329–334. [DOI] [PubMed] [Google Scholar]
- 14.Emeriaud G, Lopez-Fernandez YM, Iyer NP, et al. Executive Summary of the Second International Guidelines for the Diagnosis and Management of Pediatric Acute Respiratory Distress Syndrome (PALICC-2). Pediatr Crit Care Med 2023;24(2):143–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carroll CL, Napolitano N, Pons-Odena M, et al. Noninvasive Respiratory Support for Pediatric Acute Respiratory Distress Syndrome: From the Second Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med 2023;24(Supplement 1 2S):S135–S147. [DOI] [PubMed] [Google Scholar]
- 16.Khemani RG, Smith L, Lopez-Fernandez YM, et al. Paediatric acute respiratory distress syndrome incidence and epidemiology (PARDIE): an international, observational study. Lancet Respir Med 2019;7(2):115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khemani RG, Smith LS, Zimmerman JJ, et al. Pediatric acute respiratory distress syndrome: definition, incidence, and epidemiology: proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med 2015;16(5 Suppl 1):S23–40. [DOI] [PubMed] [Google Scholar]
- 18.Leclerc F, Duhamel A, Deken V, et al. Nonrespiratory pediatric logistic organ dysfunction-2 score is a good predictor of mortality in children with acute respiratory failure. Pediatr Crit Care Med 2014;15(7):590–593. [DOI] [PubMed] [Google Scholar]
- 19.Santschi M, Jouvet P, Leclerc F, et al. Acute lung injury in children: therapeutic practice and feasibility of international clinical trials. Pediatr Crit Care Med 2010;11(6):681–689. [DOI] [PubMed] [Google Scholar]
- 20.Bellani G, Laffey JG, Pham T, et al. Noninvasive Ventilation of Patients with Acute Respiratory Distress Syndrome. Insights from the LUNG SAFE Study. Am J Respir Crit Care Med 2017;195(1):67–77. [DOI] [PubMed] [Google Scholar]
- 21.Pons-Odena M, Medina A, Modesto V, et al. [What are the most reliable predictive factors of non-invasive ventilation failure in paediatric intensive care units?]. An Pediatr (Barc) 2019;91(5):307–316. [DOI] [PubMed] [Google Scholar]
- 22.Essouri S, Carroll C, Group FtPALICCP. Non-invasive support and ventilation for Pediatric Acute Respiratory Distress Syndrome : Proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med 2015;16((5 suppl 1)):S102–110. [DOI] [PubMed] [Google Scholar]
- 23.Lum LC, Abdel-Latif ME, de Bruyne JA, et al. Noninvasive ventilation in a tertiary pediatric intensive care unit in a middle-income country. Pediatr Crit Care Med 2011;12(1):e7–13. [DOI] [PubMed] [Google Scholar]
- 24.Zeng JS, Qian SY, Wong JJ, et al. Non-Invasive Ventilation in Children with Paediatric Acute Respiratory Distress Syndrome. Ann Acad Med Singap 2019;48(7):224–232. [PubMed] [Google Scholar]
- 25.Rowan CM, Fitzgerald JC, Agulnik A, et al. Risk Factors for Noninvasive Ventilation Failure in Children Post-Hematopoietic Cell Transplant. Front Oncol 2021;11:653607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pancera CF, Hayashi M, Fregnani JH, et al. Noninvasive ventilation in immunocompromised pediatric patients: eight years of experience in a pediatric oncology intensive care unit. J Pediatr Hematol Oncol 2008;30(7):533–538. [DOI] [PubMed] [Google Scholar]
- 27.Peters MJ, Agbeko R, Davis P, et al. Randomized Study of Early Continuous Positive Airways Pressure in Acute Respiratory Failure in Children With Impaired Immunity (SCARF) ISRCTN82853500. Pediatr Crit Care Med 2018;19(10):939–948. [DOI] [PubMed] [Google Scholar]
- 28.Gattinoni L. Ventilation-induced lung injury exists in spontaneously breathing patients with acute respiratory failure: We are not sure. Intensive Care Med 2017;43(2):256–258. [DOI] [PubMed] [Google Scholar]
- 29.Dumas G, Lemiale V, Rathi N, et al. Survival in Immunocompromised Patients Ultimately Requiring Invasive Mechanical Ventilation: A Pooled Individual Patient Data Analysis. Am J Respir Crit Care Med 2021;204(2):187–196. [DOI] [PubMed] [Google Scholar]
- 30.Kopp W, Gedeit RG, Asaro LA, et al. The Impact of Preintubation Noninvasive Ventilation on Outcomes in Pediatric Acute Respiratory Distress Syndrome. Crit Care Med 2021;49(5):816–827. [DOI] [PubMed] [Google Scholar]
- 31.Morrow BM, Agulnik A, Brunow de Carvalho W, et al. Diagnostic, Management, and Research Considerations for Pediatric Acute Respiratory Distress Syndrome in Resource-Limited Settings: From the Second Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med 2023;24(12 Suppl 2):S148–S159. [DOI] [PubMed] [Google Scholar]
- 32.Shein SL, Maddux AB, Klein MJ, et al. Epidemiology and Outcomes of Critically Ill Children at Risk for Pediatric Acute Respiratory Distress Syndrome: A Pediatric Acute Respiratory Distress Syndrome Incidence and Epidemiology Study. Crit Care Med 2022;50(3):363–374. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


