Abstract
Melanoma, a lethal malignancy that arises from melanocytes, exhibits a multiplicity of clinico-pathologically distinct subtypes in sun-exposed and non-sun-exposed areas. Melanocytes are derived from multipotent neural crest cells and are present in diverse anatomical locations, including skin, eyes, and various mucosal membranes. Tissue-resident melanocyte stem cells and melanocyte precursors contribute to melanocyte renewal. Elegant studies using mouse genetic models have shown that melanoma can arise from either melanocyte stem cells or differentiated pigment-producing melanocytes depending on a combination of tissue and anatomical site of origin and activation of oncogenic mutations (or overexpression) and/or the repression in expression or inactivating mutations in tumor-suppressors. This variation raises the possibility that different subtypes of human melanomas (even subsets within each subtype) may also be a manifestation of malignancies of distinct cells of origin. Melanoma is known to exhibit phenotypic plasticity and trans-differentiation (defined as a tendency to differentiate into cell lineages other than the original lineage from which the tumor arose) along vascular and neural lineages. Additionally, stem cell-like properties such as pseudo-epithelial-to-mesenchymal (EMT-like) transition and expression of stem cell-related genes have also been associated with the development of melanoma drug resistance. Recent studies that employed reprogramming melanoma cells to induced pluripotent stem cells have uncovered potential relationships between melanoma plasticity, trans-differentiation, and drug resistance and implications for cell or origin of human cutaneous melanoma. This review provides a comprehensive summary of the current state of knowledge on melanoma cell of origin and the relationship between tumor cell plasticity and drug resistance.
Keywords: melanoma-derived iPSC, BRAF/MEK inhibitors, drug resistance, melanoma cell of origin, melanoma plasticity and trans-differentiation, melanoma stem cells
Introduction
Melanoma is the leading cause of death from diseases of the skin, the largest organ in humans, with the highest incidence in the U.S. and other Western countries. Melanoma incidence increased 270% from 1973 to 2002 and continues to increase (Rastrelli et al., 2014). Melanoma is caused by the malignant transformation of melanocytes, the skin-resident pigment-producing cells that accumulate mutations leading to abnormal proliferation (Vultur and Herlyn, 2013).
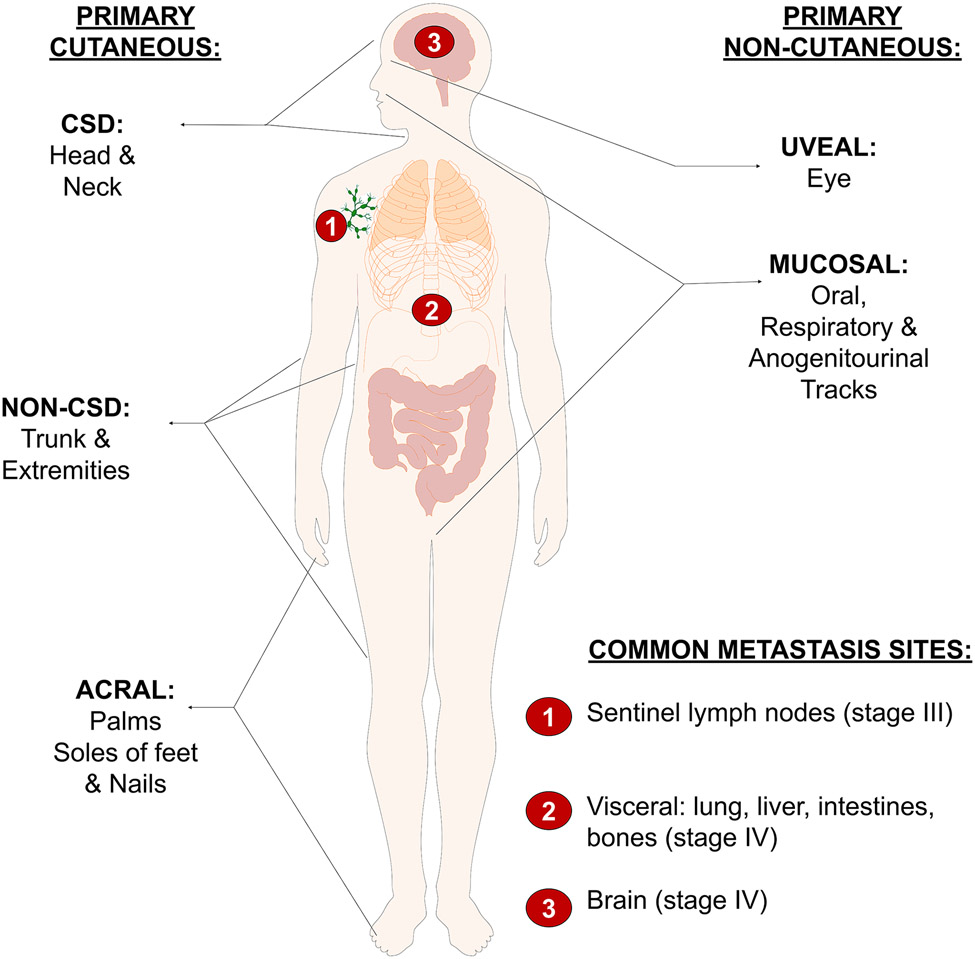
Cutaneous melanoma is most commonly diagnosed in areas of sun-exposed skin, and it is broadly classified into two main categories based on the anatomical location of its occurrence: chronically sun-damaged (CSD) and non-CSD (Shain and Bastian, 2016). Accordingly, CSD melanomas usually arise on areas of the skin that are naturally prone to long-term exposure to UV radiation throughout life, in particular head and neck regions, mainly in individuals 55 years and older. CSD melanomas typically exhibit a lentiginous (slow) growth pattern, and these lesions are called lentigo maligna melanoma. Acral (palms, soles of feet, or under finger or toenails) and mucosal (respiratory, gastrointestinal, or anogenitourinary tracts) melanomas also have a lentiginous manifestation; however, they do not exhibit sun damage and are classified by different criteria (see below). In contrast to CSD melanocytic lesions, non-CSD melanomas occur in younger individuals in body areas that typically have less frequent or low sun exposure, such as proximal extremities and the trunk. Non-CSD melanomas are classified as superficial spreading melanoma (Shain and Bastian, 2016).
Genomic analyses demonstrated a highly specific distribution of distinct driver mutations among different melanoma subtypes. For example, >60% of all cutaneous melanomas harbor oncogenic mutation T1796A in exon 15 of v-raf murine sarcoma viral oncogene homolog B1 (BRAF), leading to a substitution of valine by glutamic acid at position 600. This change produces a mutant protein designated as BRAFV600E that is constitutively active and results in hyperactivation of mitogen-activated protein kinase (MAPK) signaling, a critical component for cell survival and proliferation. In contrast, CSD melanomas often harbor mutations in Neurofibromin 1 (NF1), Neuroblastoma RAS viral oncogene homolog (NRAS), nonV600E BRAF, or mast/stem cell growth factor receptor (KIT) genes (Bastian, 2014). (Flaherty et al., 2012; Robert et al., 2015). The reasons for the particular pattern of these mutations are unknown. It is also unknown whether these clinical and pathological melanoma subtypes represent different mechanisms of tumor initiation or malignancies that reflect different cells of origin. Patients with BRAF mutant melanoma do not respond to traditional chemotherapy such as dacarbazine (DTIC) and show a poor prognosis (Serrone et al., 2000; Houben et al., 2004; Vultur and Herlyn, 2013). However, therapies specifically directed to inhibit oncogenic BRAFV600E (with inhibitors Vemurafenib, Dabrafenib, and Encorafenib; termed collectively hereafter as BRAFi) are highly effective in the short term. Unfortunately, melanoma tumors in some patients show intrinsic resistance to these inhibitors, and most patients sensitive to the initial treatment with these drugs eventually acquire resistance to BRAFi and even to combined therapies of BRAFi with inhibitors of downstream MAPK pathway targeting MEK 1/2 (Trametinib, Cobimetinib, and Binimetinib; termed collectively hereafter as MEKi). Resistance of melanoma to BRAFi/MEKi and aggressiveness of recurrent metastatic tumors are known to be associated with activation of stem cell pathways and the presence of cancer stem cells (CSC), also known as melanoma stem cells (MSC) (Roesch et al., 2010). Therefore, targeting MSC was proposed as an effective strategy for treating melanoma (Schatton and Frank, 2008). Furthermore, self-renewal, cellular plasticity, pluripotency, and trans-differentiation, which are features associated with stem cells, have been implicated in melanoma tumor progression and drug resistance (Kemper et al., 2014; Roesch et al., 2016; Tsoi et al., 2018). However, it is not known whether differences in melanoma plasticity and variable expression of CSC/MSC markers are also related to melanoma cells of origin. Recently, reprogramming of melanoma cells in vitro to induced pluripotent stem-like cells and re-differentiation to a melanocytic lineage has been shown to have the potential to uncover the relationship between melanoma cell plasticity and drug resistance and melanoma cell of origin (Castro-Pérez et al., 2019). In this review, we discuss cellular and molecular pathology-based subtypes of melanoma, and the relationship between the specific patterns of oncogene mutations, developmental precursors of melanocytes, and melanoma cell of origin. We suggest that reprogramming melanoma cells to iPSC might shed some light on the relationship between melanoma cell of origin and the diversity of oncogenic driver mutations in melanoma subtypes.
Developmental origin and anatomical distribution of melanocytes
Melanoma arises from melanocytes, specialized cells that produce melanin pigment. Melanocytes in the skin are found predominantly in two distinct locations: the epidermis and hair follicles, where they produce melanin to be exported to epidermal keratinocytes and hair shafts, respectively (Haass et al., 2005; Slominski et al., 2004). Melanocytes are also found in other anatomical locations, including the eyes, inner ear, oral and sinonasal mucosa, anogenital tracts, and the central nervous system (Aoki et al., 2009; Mort et al., 2015). During the neurulation process, melanoblasts, the embryonic melanocyte precursors, develop from the neural crest (NC), a transitory embryonic structure that arises from the neuroectoderm under the influence of the notochord (Dupin et al., 2003; O'Rahilly and Muller, 2007; Young et al., 2009). NC cells are multipotent stem cells with highly migratory and proliferative features, and their developmental fate is determined by their antero-posterior anatomic location (Figure 1). Accordingly, NC cells that migrate along the dorsal pathway in the trunk give rise to peripheral neurons, glial cells, and cutaneous melanocytes in the epidermis and the hair follicles, a source of melanocyte stem cells and melanocytic precursors (Theveneau and Mayor, 2012; Sauka-Spengler and Bronner, 2010). Interestingly, although epidermal melanocytes are recognized as molecularly distinct from dermal melanocytes, it is not yet clear if they emerge from distinct precursors (Aoki et al., 2009; Cichorek et al., 2013). A remarkable variety of cells emerge from NC cells that migrate along the ventral pathway, including Schwann cell precursors (SCP), neurons, endocrine glands, smooth muscles, and some cutaneous melanocytes (Adameyko et al., 2009; Theveneau and Mayor, 2012; Harris and Erickson, 2007). In the head region, the diversity of cells that emerge from cranial NC cells and migrate along the dorsal path is even more remarkable, contributing to the formation of bone, cartilage, smooth muscle, connective tissue, glandular tissues, neurons, glia, and melanocytes in the inner ear, the oral cavity and the scalp skin (Cordero et al., 2011; Snider and Mishina, 2014; Young et al., 2009). Interestingly, cranial melanocytes also emerge from cells that are distinct SCP (Adameyko et al., 2012). Additionally, retinal pigment epithelial cells are a distinct melanocytic lineage that emerges directly from cranial NC-derived SCP. and from the neural tube (NT). NC-derived melanocytes also populate some internal anatomical sites, such as the meninges (Cramer and Fesyuk, 2012; Dupin et al., 2003). In addition, based on studies on humans with pigmentary mosaic disorders, it was proposed that a population of melanocyte precursors might emerge in earlier developmental lineages, presumably from mesodermal lineage primitive streak at the time of gastrulation (Kinsler and Larue, 2018). However, more studies in human and animal genetic models are needed to confirm this mesodermal origin of melanocytes.
Figure 1. Embryonic origin of melanocytes.
Melanocyte development starts from multipotent neural crest cells (NCC) and gives rise to several cell types and tissues. Migrating NCC precursors of melanocytes include melanoblasts and a common precursor of Schwann cells that populate the epidermis, dermis, brain, eye, ear mucosa, and other tissues/organs proposed by dorsal and ventral pathway. (SMT = somites; NT = neural tube; NC = notochord).
Given the multiplicity of cell precursors that can give rise to cells of the melanocyte lineages, their plasticity, and migratory patterns, it is tempting to argue that the diversity of oncogenic drivers in melanoma and the subtypes they cause is a manifestation of the diverse origins of melanocytes. Additionally, the various epigenetic modifications acquired during embryonic development and/or microenvironment interactions could also determine the activation of specific driver oncogenes. Consistent with this notion of a diverse cell of origin of melanoma subtypes, expression of markers of stem cells, NC and neural lineages, and different epigenetic patterns have been reported to be associated with the plasticity of melanocytes, melanoma, and drug resistance (Yaar and Park, 2012; Johannessen et al., 2013; Caramel et al., 2013; Larribère et al., 2018; Larribere and Utikal, 2019).
Diversity of Melanoma Subtypes
The original classification of cutaneous melanoma proposed by Clark and colleagues (Clark et al., 1986) was based on morphologic aspects of the early growth phase and the body site of primary melanocytic lesions and distinguished four main types: superficial spreading melanoma (SSM), lentigo malignant melanoma (LMM), nodular melanoma (NM) and acral-lentiginous melanoma (ALM). The current histopathological classification of melanocytic neoplasms is based on the evidence of chronic sun damage exposure and the anatomical location of the lesions. The 2018 World Health Organization classification of cutaneous, mucosal, and uveal melanoma identifies nine distinct subtypes distinguishing them by their epidemiology, clinical and histologic morphology, and genomic characteristics (Elder et al., 2020). However, recent evidence suggests that certain tumors might not unequivocally fit into these broad categories based on the origin and distribution of melanocytes and their characteristics (Figure 2). It is not well understood whether these histologically distinct lesions all arise from melanocytes at the same stage of differentiation (even melanocytes residing in different anatomic substructures within the skin like follicular and interfollicular or scale and interscale regions as in mouse tail and may exhibit different susceptibilities to transformation) or represent malignancies of a different cell of origin. For example, cells from skin-resident melanocyte precursors, melanocyte stem cells, or terminally differentiated melanocytes (Grichnik et al., 2014; Kulesa et al., 2006; Yu et al., 2010). As described above, cutaneous melanomas are most frequently diagnosed on hair-bearing skin. Melanoma can also arise in non-hair bearing glabrous skin such as palms, soles of feet, or under fingers, finger/toe-nails, and mucosal surfaces such as respiratory gastrointestinal or genitourinary tracts, ocular sites such as choroid, the ciliary body, and the iris (Shain and Bastian, 2016; Bastian, 2014). Therefore, a better understanding of the relationship between the cellular origin of different melanoma subtypes could improve melanoma classification, diagnosis, and prognosis.
Figure 2. Multiple anatomic locations and types of melanomas.
The most common type of melanoma (cutaneous, non-acral) arises from the skin and it is classified as CSD and non-CSD. Other cutaneous melanomas include acral melanoma. Melanoma is also diagnosed in the eye (uveal) mucosa (mucous membrane). Each melanoma subtype exhibits specific patterns of oncogene driver mutations.
Different clinical and histopathological subsets of melanocytic lesions harbor highly unique patterns of oncogenic driver mutations (Bastian, 2014). For example, common acquired nevi have the highest frequency of BRAF mutation, while lesions associated with sun-induced damage and acral lesions exhibit mostly KIT mutations (Beadling et al., 2008; Curtin et al., 2006; Torres-Cabala et al., 2009), suggesting that biologically distinct types of melanocytic neoplasms arise due to different oncogenic mutations susceptibilities to transformation. However, it is unclear whether these distinct melanocytic lesions all arise from mature, differentiated melanocytes or originate from different precursors along the melanocytic lineage (Grichnik et al., 2014). A more detailed understanding of the biology of the developmentally diverse melanocytes and the clinico-pathological and molecular diversity of melanomas that arise at different anatomical locations may answer these questions.
Diversity and Prevalence of Driver Mutations in Melanoma Sub-Types
An impressive array of concerted international collaborations aimed at creating a comprehensive catalog of genes responsible for melanomagenesis and progression of various human cancers has resulted in whole exome and whole genome sequences of matched sets of tumor-normal samples. Analyzing more than 3,080 tumor-normal genomic data from 27 cancer types, it was discovered that extraordinary variation exists in the frequency and spectrum of mutations across the genome among cancer types (Lawrence et al., 2013). For example, as expected, pediatric cancers, as this group is least exposed yet to mutagens compared to older patients, showed the lowest frequencies of mutation rates (approximately 0.1/Mb). In contrast, at the opposite end of the spectrum, melanoma showed the highest rate of mutations exceeding 100/Mb, followed by lung cancers with the second highest mutational rate. It is important to note that the mutation frequencies vary more than 1,000-fold between the lowest (pediatric) and the highest (melanoma). The higher mutation rate frequency in lung cancers might be attributable to chronic exposure to the well-known carcinogens in tobacco smoke, whereas the highest rate of mutations in melanoma is attributed to exposure to ultraviolet radiation. However, the remarkable variation in melanoma mutations might be attributed to other biological factors, such as melanomas subtypes, including those that arise in areas not associated with ultraviolet skin exposure. A total of 121 different melanomas subtypes were included in the analyses. Based on these observations, it is appealing to hypothesize that these high rates and diversity of mutations could also reflect the multiplicity of cell lineages derived from N.C. and perhaps other developmental origins.
More recent studies included those led by The Cancer Genome Atlas (TCGA), including whole exomes of 33 cancer types and 1,100 tumor samples (Hutter and Zenklusen, 2018) and the Pan-Cancer Analysis of Whole genomes (PCAWG) (a combined effort from TCGA and the International Cancer Genome Consortium ICGC) database contains 2,658 whole genomes from 38 types of primary cancers and their matched normal tissues (Campbell et al., 2020). In melanoma, these studies confirmed previously known driver mutations representing over 40 mutated genes (Shaughnessy et al., 2018).
These studies have classified driver mutations in cutaneous melanoma into four major subclasses (percent melanomas): 1) BRAF (>60%) associated with non-CSD, 2) NRAS (28%), and 3) NF1 (14%), both associated with C.S.D., and 4) triple-wild type (15%) (lack BRAF, NRAS, or NF1 mutations), found more frequently in C.S.D. melanomas (Akbani et al., 2015; Hayward et al., 2017; Shaughnessy et al., 2018; Lawrence et al., 2013; Cirenajwis et al., 2017). However, triple wild-type (of the above three genes) melanomas harbor mutations more commonly in GNA11, GNAQ, SF3B1, or KIT genes, which are, interestingly, also frequent drivers of uveal melanoma (Akbani et al., 2015; Cirenajwis et al., 2017; Hayward et al., 2017) (Table 1).
Table 1.
Frequency of major melanoma mutations in different melanoma subtypes
| Subtype | Inc. | Mutation | Freq. | Common Variants | Manifest. | Exclusive mutations |
|---|---|---|---|---|---|---|
| Cutaneous | 91.2 | BRAF | 60 | V600E (80-90%) | Non-CSD | NRAS/NF1 |
| (non-acral) | NRAS | 28 | Q61K, Q61R | CSD | BRAF/PTEN | |
| NF1 | 14 | Del/inactivation | CSD | BRAF | ||
| Triple WT | 15 | GNAQ/GNA11/KIT | CSD | BRAF/NRAS/NF1 | ||
| KIT | 28 | L576P (70%) | CSD | BRAF/NRAS | ||
| PTEN | 14 | Del/inactivation | Unk/ND | Unk/ND | ||
| MITF | 10 | Amplif/Activation | Unk/ND | Unk/ND | ||
| CDKN2A | 2 | Del/inactivation | Familial | Unk/ND | ||
| TERT | 29 (all) | Amplif/Activation | Unk/ND | Unk/ND | ||
| TP53 | 20 (all) | Del/inactivation | Unk/ND | Unk/ND | ||
| Mucosal | 1.3 | KIT | 39 | L576P, K642E | Non-CSD | BRAF/NRAS/PDGFRA |
| PDGFRA | 4 | Amplif/Activation | Non-CSD | KIT | ||
| GNAQ | 4.6 | Q209L (92%) | Non-CSD | GNA11 | ||
| GNA11 | 4.9 | Q209L (92%) | Non-CSD | GNAQ | ||
| SF3B1 | 20-35 | R625H/S/C | Non-CSD | Unk/ND | ||
| Acral | 2.3 | CCND1 | 45 | Amplification | Non-CSD | Unk/ND |
| KIT | 36 | L576P, activation | Non-CSD | BRAF/NRAS | ||
| PDGFRA | 7 | Amplif/activation | Non-CSD | KIT | ||
| Uveal | 5.2 | GNAQ | 33 | Q209L (90%) | CSD | BRAF/NRAS/GNA11 |
| GNA11 | 39 | Q209L (90%) | CSD | BRAF/NRAS/GNAQ | ||
| BAP1 | 45 | Del/inactivation | CSD | EIF1AX/SF3B1 | ||
| EIF1AX | 14-20 | A11T, N4S | CSD | SF3B1/BAP1 | ||
| SF3B1 | 22 | R625C/H/L | CSD | EIF1AX/BAP1 |
Inc. = incidence (%); Freq. = Frequency (%), except for TERT and TP53, representing all melanomas; Manifest = manifestation; Exclusive mutations = Mutually exclusive mutations with mutations/genes in column 3; Del = deletion; Amplif = amplification; Unk = unknown; ND = not determined; CSD = chronic sun damage.
The signaling cascades affected by these oncogenic mutations in different melanoma subtypes have been extensively investigated. The BRAF protein is a serine/threonine protein kinase that downstream activates the MAPK/ERK pathway. The V600E mutation represents 80-90% of all BRAF mutations in melanoma (Cheng et al., 2018; Greaves et al., 2013). NRAS is a member of the family of G-regulatory proteins. NRAS mutations Q61K and Q61R cause constitutive activation leading to BRAF stimulation and consequently causing hyperactivation of the MAPK pathway (Ellerhorst et al., 2011; Polakis and McCormick, 1993). BRAF and NRAS mutations rarely co-occur in the same tumor (Raaijmakers et al., 2016). NF1 is a tumor suppressor gene that encodes the protein neurofibromin 1, and it negatively regulates RAS by converting activated RAS-GTP to inactive RAS-GDP. Inactivating mutations of NF1 result in constitutive activation of both MAPK and phosphoinositide 3-kinase (PI3K) pathways, thus causing a similar effect as BRAF and NRAS driving mutations (Ars et al., 2003; Reddy et al., 2017; Shaughnessy et al., 2018 Brastianos et al., 2015; Davies, 2012; Wee et al., 2009). Other genes, such as telomerase reverse transcriptase (TERT), essential for chromosomal stability, exhibit amplifications or activating mutations in 29% of melanomas (Vinagre et al., 2013). Mutations in cyclin-dependent kinases (CDKs) like CDK inhibitor 2A (CDKN2A) and cyclin-dependent kinase 4 (CDK4), both implicated in susceptibility to familial melanoma, represent about 2% of mutations (Harland et al., 2014; Hayward et al., 2017). Other common genetic alterations in cutaneous melanoma include the loss of the phosphatase and tensin homolog (PTEN), a tumor suppressor and a key regulator of the PI3K pathway, found in about 14% of melanomas (Birck et al., 2000; Shain et al., 2015). PTEN mutations frequently co-occur with BRAF mutations but not with NRAS (Reddy et al., 2017). Additionally, amplifications of the microphthalmia-associated transcription factor (MITF), the melanocyte master regulator required for melanocyte development, are found in 10% of melanomas (Garraway et al., 2005; Wellbrock and Arozarena, 2015). Tumor protein 53 (TP53) inactivating mutations are present in approximately 19% of melanomas (Hodis et al., 2012).
Mucosal and acral melanomas representing 1.3% and 2% of all melanomas, respectively, are genetically more similar to each other than they are to other melanoma subtypes. Activating mutations in KIT (39% of mucosal and 36% of acral melanomas) are the most frequent drivers in these melanomas, with the L576P present in 70% of KIT mutated tumors. KIT activating mutations cause dimerization of the receptor activating MAPK/ERK and PI3K/AKT/mTOR. Other genes that exhibit alterations in these melanomas include the platelet-derived growth factor receptor a polypeptide (PDGFRA) and cyclin D1 (CCND1) (Dominiak et al., 2016; Reddy et al., 2017; Shaughnessy et al., 2018; Vazquez Vde et al., 2016). Some cases also exhibit mutations in GNAQ/GNA11 genes (Kim et al., 2014; Sheng et al., 2016).
KIT mutations are mutually exclusive with BRAF and NRAS mutations but cause similar effects on MAPK and PI3K pathways (Carvajal et al., 2011; Natali et al., 1992; Shaughnessy et al., 2018). Genetic alterations in the PDGFRA gene are predominantly amplifications and are present in 7% of acral and 4% of mucosal melanomas (Dai et al., 2013). Mutations in the PDGFRA gene are mutually exclusive with KIT mutations and are associated with non-CSD lesions (Dai et al., 2013; Merkel and Gerami, 2017). Mutations in the CCND1 gene (Cyclin D1) are mostly amplifications and are present in approximately 45% of acral melanomas (Smalley et al., 2008; Sauter et al., 2002).
Melanomas may also arise from melanocytes in two anatomical locations of the eye: the uvea/uveal tract and the conjunctiva. Human eye consists of three distinct concentric layers: (1) the outer layer known as the sclera, which comprises the transparent cornea and the opaque white connective tissue; (2) the middle layer composed of the uvea/uveal tract, comprising the iris anteriorly (composed of an anterior layer called the stroma containing melanocytes and a posterior pigmented epithelial layer), an intermediate structure called the ciliary body (surrounding the iris and located behind the sclera), and the choroid located posteriorly surrounding the middle layer of the eyeball, which supports and nourishes the retina. And (3) the inner layer composed of the retina, which consists of the retinal pigment epithelium (RPE) and the neural retina (Hu et al., 2002: Hu, 2005; Istrate et al., 2020). The RPE is a monolayer of pigment cells that lies between the uveal tract and the neural retina, and although they are in close proximity (uveal melanocytes in the middle and RPE in the inner layer), they have different developmental origins. Melanocytes are derived from the neural crest, while retinal pigment cells share their embryonic origin with the neural retina, arising from the anterior neural plate (Zuber et al., 2003; Martinez-Morales et al., 2004; Esteve and Bovalenta, 2006; Cechmanek and McFarlane, 2017).
In uveal melanomas the most commonly mutated genes are GNAQ, GNA11, BAP1, EIF1AX, and SF3B1 (Shaughnessy et al., 2018). Mutations in GNAQ and GNA11 genes are present in 33% and 39% of uveal melanomas, respectively, and most commonly in position Q209L (Moore et al., 2018; Reddy et al., 2017; Shoushtari and Carvajal, 2014). Mutations in GNAQ and GNA11 converge on the MAPK/ERK pathway, thus upregulating the M.A.P. kinase pathway, similarly to BRAF and NRAS mutations (Van Raamsdonk et al., 2009; Van Raamsdonk et al., 2010). Mutations in GNAQ/GNA11 are also associated with chronic solar damage (CSD) (de Lange et al., 2015). Mutations in GNAQ and GNA11 genes are mutually exclusive and rarely found in the same tumor (Shaughnessy et al., 2018). Inactivating mutations or loss of the tumor suppressor BRCA1-associated protein 1 (BAP1) occur in 45% of uveal melanomas due to the monosomy in chromosome 3 (Ransohoff et al., 2016; Scheuermann et al., 2010; Shaughnessy et al., 2018). Uveal melanomas also exhibit mutations in eukaryotic translation initiation factor 1A X-linked (EIF1AX) and six splicing factor 3b subunit 1 (SF3B1) at 14% and 22% frequency, respectively (Field et al., 2018; Hintzsche et al., 2017; Kong et al., 2014; Quek et al., 2019; Rose et al., 2018; Yavuzyigitoglu et al., 2016; Doherty et al., 2018; Field et al., 2018; Shaughnessy et al., 2018).
There are also melanocytes located in the conjunctiva, the thin mucous membrane that lines the inside of the eyelids and covers the surface of the sclera (the white of the eye), and they may give rise to conjunctival melanoma. Conjunctival melanocytes are derived from the neural crest as uveal and cutaneous melanocytes, although they migrate in different waves (Iwamoto et al., 2002; Hu et al., 2007). Although more population studies are needed, current evidence suggests that the most common driver mutations in conjunctival melanoma are in the BRAF (~30-70%), NF1 (~20-30%), NRAS (~17%), and c-KIT (~8%) genes that lead to constitutive activation of the MAPK pathway as well as mutations that result in activation of the PI3K/AKT/mTOR signaling pathway (Gkiala and Palioura, 2020).
Melanoma is the most highly mutated malignancy, and given the remarkable diversity and highly specific patterns of oncogene mutations among subtypes arising from diverse anatomical locations, we argue that this phenomenon may have a relationship with specific competence requirements like transcriptional, epigenetic programs, and tissue microenvironment of different cells of origin for malignant transformation.
Significance of Diverse Patterns of Melanoma Mutations
Despite the detailed knowledge gained on the landscape of genetic alterations in melanoma, a relatively small number of clinically relevant and effective drugs are available to treat melanoma. The etiology and interactions of mutations that control melanoma onset and their significance to cell of origin, progression, and therapeutic vulnerabilities are not yet fully understood (Akbani et al., 2015; Shaughnessy et al., 2018). Understanding the complexity of mutations in melanoma continues to be a challenge with implications for deciphering the cell or origin and improving patient care (Davis et al., 2018). Interestingly, melanoma subtypes not only exhibit highly unique driver mutations, but these mutations are often exclusive among them, i. e., do not co-exist in the same tumor (Table 1). Moreover, some oncogenic mutations that produce a melanoma subtype in a specific tissue do not cause melanoma in other tissue (Huang et al., 2015; Weiss et al., 2022). Melanoma clinical and molecular subtypes -cutaneous-non-CSD, cutaneous-CSD, mucosal/acral, and uveal melanoma- exhibit distinct biological properties, including metastatic properties and disease progression, and have different prognoses.
The skin is a complex organ harboring a rich array of different cell types and distinct populations of stem cells. For example, populations of skin stem cells that behave spatio-temporally in different manners under healthy and pathological conditions suggest stem cell population heterogeneity with different properties. These skin stem cells produce cell lineages that depend on signals from their local environment but can give rise to all epidermal lineages in response to appropriate stimuli (Li et al., 2004; Mannik et al., 2010). It is known, for instance, that certain human and rodent skin stem cells have the ability to differentiate towards fat, bone, muscle, cartilage, hematopoietic cells, and neural lineages, including NC cells (Waters et al., 2007). Additionally, developmental lineage plasticity and tissue microenvironment appear to influence the susceptibility to specific oncogenic mutations and malignant transformation. Consistent with this notion, it is interesting to note that in a mouse genetic model, activation of oncogenic Gnaq (frequently found in uveal melanomas) was reported to induce proliferation of melanocytes in the eye and meninges but caused an opposite effect in skin melanocytes, which exhibited decreased proliferation and survival with 39% fewer cells than normal (Huang et al., 2015).
Recent studies also showed that the ability to induce malignancy by oncogenic depends on cellular context (Weiss et al., 2022; Fowler et al., 2020). In these studies, DNA sequencing demonstrated that BRAF mutations are enriched in cutaneous melanomas while CRKL amplifications are enriched in acral melanomas. Transgenic zebrafish models supported these findings. It was shown that CRKL-driven tumors are predominantly formed in the fins, the evolutionary anatomic equivalent of limbs and acral location. These data indicate that melanocytes in these acral locations are uniquely susceptible to CRKL mutations. Moreover, RNA-seq data revealed that a transcriptional program controlled by HOXB13 positional identity genes synergized with CRKL to amplify cancer-related signaling pathways (IGF1R–PI3K), making these melanocytes susceptible to malignant transformation to only certain oncogenic mutations (Weiss et al., 2022). The authors also discussed that these results could alternatively be explained by the lineage-specific chromatin state of acral melanocytes that impede their oncogenic competence from responding to activation of MAPK signaling (Baggiolini et al., 2021). Differences in the skin microenvironment were also considered to contribute to the oncogenic mutation specificity in melanomagenesis. Consistent with this view, recent studies also showed a role for keratinocytes in promoting the escape of oncogene-induced senescence in melanocytes (Sadangi et al., 2022). These observations not only suggest differential predisposition of melanocytes to malignant transformation by specific oncogenic mutations but also that differences in the tissue microenvironment, cell of origin, and plasticity could influence melanoma tumor initiation.
Furthermore, published reports suggest that the predisposition of different melanomas to metastasize could be related to the oncogenic driver and/or cell of origin. In particular, it is known that cutaneous BRAF-mutant tumors have a higher tendency to metastasize to the brain than BRAF WT tumors (Ribas and Flaherty, 2011). Conversely, uveal melanoma exhibiting GNAQ or GNA11 mutations predominantly metastasizes through the hematogenous spread, and the most common sites of involvement are the liver, lung, and bone (Harbour, 2012; Reddy et al., 2017). These differences between cutaneous and uveal melanomas in their mode and sites of metastasis and the oncogenic driver mutations they harbor are intriguing because, based on their anatomical proximity to the site of the primary tumor, the brain would be expected to be a more frequent or natural site for metastasis of uveal melanomas than for the spread of BRAF mutant cutaneous melanomas. Similarly, inactivating mutations or deletion of the tumor suppressor PTEN co-existing with BRAF mutation have been reported in humans and mouse models to correlate with a shorter time to brain metastasis (Krepler et al., 2017; Bucheit et al., 2014). Furthermore, activating mutations in PI3K, which is regulated by PTEN, have also been implicated in brain metastasis, causing a similar effect as PTEN inactivating mutations (Brastianos et al., 2015). These observations suggested not only that the patterns of metastasis may be related to melanoma driver mutation but also to the melanoma subtype, which in turn is related to the cell of origin. Consistent with this notion, mouse subcutaneous xenograft models of metastasis showed that after inoculation of human melanoma cells, BRAF mutant melanoma cells exhibit, compared to triple-negative melanoma, a significantly higher predisposition to invade the mouse tissue. Several studies have suggested that the differences in the predisposition to metastasize to specific tissues and organs might not just be an indicator of the specificity of driver oncogene but also be an intrinsic biological property of different lineages of melanocytes from which melanoma arises. For example, histological observations showed that melanoma precursor lesions, such as melanocytic nevi, are already capable of dissemination and exhibit some limited colonization capacity. In particular, small deposits of melanocytes are routinely found as a “benign metastasis” in lymph nodes and are known as nodal nevi, which are removed from patients without any history of melanoma (Bautista et al., 1994; Carson et al., 1996). These nodal nevi typically only exhibit BRAFV600E mutation and are especially common in the sentinel lymph node of patients with melanoma that originate from or are associated with nevi (Holt et al., 2004), suggesting that nevus cells could disseminate even before the primary tumor has formed (Taube et al., 2009). These observations also suggest that the differential capacity to disseminate might be related to the cell of origin of melanoma subtypes and not entirely an acquired feature during tumor progression.
Melanoma Cell of Origin: Human studies
One limitation in understanding melanocyte development and melanoma cell of origin in humans is that all studies are conducted in animal models (Le Douarin and Kalcheim, 1999; Le Douarin et al., 2004). Although there is very little information on cell of origin derived directly from humans, the concept that human melanomas may have distinct cells of origin was proposed by Houghton et al. (1982) using a panel of surface antigen markers in melanoma cells and melanocytes in different stages of development. In this study, the authors characterized in a comparative analysis the expression of cell surface antigens (termed M1-M10) recognized by a panel of antibodies on metastatic melanoma cells and fetal, neonatal and adult melanocytes. They grouped the antigens into 4 categories: (1) Antigens not expressed on newborn and adult melanocytes and expressed by subsets of melanoma: HLA-DR and melanoma antigens M-l, M-2, and M-3. (2) Antigens expressed on newborn melanocytes but not adult melanocytes: early melanocyte antigens M-4 to M-8. (3) Antigens expressed on adult melanocytes and only weakly (or not at all) on newborn melanocytes: mature melanocyte antigens M-9 and M-10. (4) And antigens detected equally on both newborn and adult melanocytes: melanocytic lineage markers M-11 to M-34. Based on this diversity of expression patterns, it was proposed that antigens present only on melanomas but not on early, intermediate, or adult melanocytes could represent markers of melanocytic precursors, such as presumptive melanoblasts, and a subset of melanomas that express these antigens may arise from such melanocyte precursors or immature melanocytes. However, the expression of antigens, such as HLA-DR, that do not correspond to the neural crest and melanocyte differentiation markers may result from aberrant gene expression during malignant transformation and/or tumor progression. For nearly three decades, not much progress was made in this area until the recent availability of genetic mouse models and elegant methods for in vivo lineage tracing of melanocytes.
Lineage Tracing of Melanoma Cell of Origin
The first indication that the diversity in mechanisms of melanoma tumor initiation could be related to differences in the cell of origin came from genetic models of mouse melanoma. These models were first developed using a Tyr-SV40E oncogene expression in ocular and cutaneous mouse melanomas (Bradl et al., 1991; Mintz and Silvers, 1993). More recently, employing BrafV600E/Pten−/− mouse melanoma model, it was demonstrated that melanocytic tumors develop from melanoma-competent melanocyte stem cells (MCSC) upon stimulation by UVB, which induces MCSC activation and translocation from the hair follicle to the epidermis by an inflammation-dependent process (Moon et al., 2017). These observations suggested that hair follicle MCSC, but not differentiated adult epidermal melanocyte, are the cell of origin of cutaneous melanoma that develops in mouse skin upon UV exposure.
Conversely, by employing mouse genetic models with elegant fluorescent reporters to study cell of origin of tumors in BrafV600E/Pten−/− mice, Köhler et al. 2017 showed that MCSCs in the bulge were not susceptible to oncogenic transformation by BrafV600E (Kohler et al., 2017). A possible explanation for this observation is that the Cre recombinase, which is driven by a Tyr promoter, is not activated in MCSC that do not express Tyr. Consistent with this notion, a population of differentiated pigmented melanocytic cells in the hair follicle matrix were found to be the targets of malignant transformation when activated by depilation producing multiclonal hyperproliferative lesions. Moreover, these authors studied tail melanocyte cells, which are distributed in two distinct locations- the scale areas and the interscale regions. Importantly, the scale (parakeratotic) and the interscale (orthokeratotic) represent interfollicular regions of the mouse tail epidermis compartments with two cell lineages maintained by distinct stem cell populations (Gomez et al., 2013). The scale melanocytes are pigmented and show dendritic morphology, whereas interscale melanocytes are composed of non-pigmented and pigmented cells. Interestingly, although Braf oncogene activation and Pten loss could be induced in both populations of melanocytes, hyperproliferative lesions developed only from scale melanocytes and exhibited radial growth. These data showed that melanocytes in very close proximity and existing in similar tissue microenvironments but originating from different stem cell lineages exhibit differences in susceptibility for melanoma initiation even under the same oncogene driver. These studies highlight the need for detailed analyses of gene expression profiles of melanocytes at diverse anatomical locations and changes in gene expression in response to intrinsic and extrinsic stimuli in the context of their lineage and microenvironment (Soengas and Patton, 2017).
Reprogramming Cancer Cells into iPSC: a Model for Assessing Melanoma Cell of Origin, Plasticity and Drug Resistance.
The concept of cell of origin in cancer refers to normal cells that first acquire the mutations required for malignant transformation and tumor initiation (Visvader, 2011). In order to identify the cells of origin of cancers, it is critical to understand the normal lineage progression that occurs during the development and adulthood and the specific stage at which cells are prone/susceptible to malignant transformation. For instance, previous reports on the cell of origin of chronic myeloid leukemia (CML) indicated that BCR/ABL translocation occurred in hematopoietic stem cells; however, if this translocation occurred in committed progenitor cells, it gave rise to acute lymphoid leukemia (ALL) instead (Tough et al., 1963; Li et al., 1999). Similarly, different types of brain tumors are formed when a specific mutation occurs in neural stem cells, neural progenitor cells, oligodendrocyte progenitors, or neuroepithelial stem cells (Alcantara-Llaguno et al., 2009, Alcantara-Llaguno et al., 2015; Huang et al., 2019). In several other cases (mentioned below), such as colon cancer, mutations in the tumor suppressor gene of Apc result in tumor formation only in the intestine but not in other cell types (Hashimoto et al., 2017).
In the past decade, the ability to generate patient-derived induced pluripotent cells (iPSC), which exhibit self-renewal, unlimited proliferation, and the capacity to differentiate into nearly all cell types and to generate functional organoids in vitro and in vivo, has revolutionized disease modeling to understand mechanisms of disease pathogenesis and rapid drug screening (Liu et al., 2018; Siller et al., 2013). iPSCs have been used to model cancer cell heterogeneity, plasticity, tumor progression, drug resistance, and for drug screening (Campbell et al., 2020; Czerwińska et al., 2018; Kim, 2015; Sharkis et al., 2012). Also, iPSC reprogramming as a strategy to study cancer cell of origin has been successfully employed in several cancers with different strategies (Friedmann-Morvinski and Verma, 2014; Pan et al., 2017; Czerwińska et al., 2018; Kim and Schaniel, 2018; Marin-Navarro et al., 2018). One strategy to study cell of origin in cancer with iPSC is by direct reprogramming of tumor cells. Reports indicate that the cell of origin affects the efficiency of reprogramming and pluripotency of iPSC as a result of retention of some epigenetic marks generating iPSCs that are more amenable to express markers related to the cell of origin and have more tendency to differentiate toward the original lineage (Kim et al., 2010; Polo et al., 2010; Bar-Nur et al., 2011; Hargus et al., 2014; Nukaya et al., 2015; Hu et al., 2016; Wang et al., 2018; Chlebanowska et al., 2020; Ruiz et al., 2012). In the context of melanoma, this strategy could elucidate potential differences in cell of origin, tumor plasticity, and heterogeneity, as suggested by some studies (Castro-Pérez et al., 2019; Bernhardt et al., 2017).
Another strategy successfully applied to investigate cell of origin in cancer with iPSC is to study the effect of oncogenic mutations in different cell types/lineages as candidates of cell of origin. It is known that the spectrum of oncogenic driver mutations differs among cancers in different organs, suggesting the requirement for specific competent cells of origin for their malignant transformation (Friedmann-Morvinski and Verma, 2014; Hashimoto et al., 2017), and we have revised here this possibility among different melanoma subtypes (see above). This effect of an oncogenic mutation in different cell types has been investigated using iPSCs in several cancers, including iPSC-derived from non-small cell lung cancer (NSCLC) and sarcoma cells, that although retaining the oncogenic mutations; they also required differentiation to the cellular context of the cell of origin (e.g., transcriptional, epigenetic programs, tissue microenvironment) for acquisition and progression of malignant behavior (Mahalingam et al., 2012; Zhang et al. 2013). Another work generated iPSCs from patients with Acute myeloid leukemia (AML) carrying MLL-gene rearrangements; however, only AML-iPS cells that were differentiated into hematopoietic cells gave rise to leukemia in mice, whereas differentiation into other cellular lineages did not (Chao et al., 2017). Furthermore, other reports demonstrated that AML-derived iPSC could be used to map in detail the recapitulation of cell stage-specific progression by introducing or correcting mutations (Kotini et al., 2017). Similarly, glioblastoma- and pancreatic ductal adenocarcinoma (PDAC)-derived iPSCs retain malignancy after differentiation into the context of their cell of origin (Stricker et al., 2013; Kim et al., 2013). In another study, iPSC generated to model cell of origin in colon cancer showed that disruption of tumor suppressor Apc in reprogrammed cells resulted in neoplastic growth that was exclusive to the intestine but not to other cell types. Similarly, iPSC-derived neural precursor cells (NPCs) led to malignant transformation when introduced relevant mutations limited to NPCs and did not have such an effect on any other neural or non-neural cell type (Funato et al., 2014).
Cancer cell-derived c-iPSC and re-differentiation of c-iPSC to normal counterparts have also been employed to study tumorigenicity, plasticity, and mechanisms of drug resistance (Gong et al., 2019). For instance, iPSC derived from sarcoma cells showed terminal differentiation and loss of tumorigenicity (Zhang et al., 2013); pancreatic cancer cell-derived iPSCs showed stable differentiation and loss of tumorigenicity (Miyoshi et al., 2010). B cell lymphoma and leukemia-derived iPSC exhibited loss of tumorigenicity (Rapino et al., 2013), hepatocellular carcinoma-derived iPSC showed recovery of normal functions and the capacity for liver regeneration (Cheng et al., 2019), and breast cancer-derived iPSC showed adipocyte functions and loss of metastatic potential (Ishay-Ronen et al., 2019). Furthermore, iPSC-derived from colorectal carcinoma cells showed differentiation to benign phenotype and reduction of tumorigenic potential (Zhou et al., 2017); melanoma iPSC exhibited tumorigenicity loss and increased drug resistance (Bernhardt et al., 2017; Utikal et al., 2009). However, reprogramming cancer cells to iPSC has some limitations, such as intrinsic heterogeneity of the parental tumor cell population, low reprogramming efficiency, and restricted differentiation of iPSC. Barriers to reprogramming present in normal somatic cells can often be more pronounced in cancer cells (Liu et al., 2018). For example, oncogene activation, oncogene-induced cellular senescence, cancer cell-selective signaling pathways such as TGF-β signaling, repression of transcription factors such as c-Jun, and certain microRNAs can act as roadblocks to reprogramming. Moreover, epigenetic factors such as DNA methylation and histone modifications can also impede the generation of iPSCs (Banito et al., 2009; Haridhasapavalan et al., 2020; Liu et al., 2015; Mosteiro et al., 2016).
Leukemia-derived iPSCs strategy was employed to study cancer plasticity in relation to the cell of origin and differential response to imatinib in chronic myeloid leukemia (CML) (Suknuntha et al., 2015; Chao et al., 2017). Reprogramming of CML cells to iPSC followed by differentiation into hematopoietic lineages generated primitive hematopoietic cells with leukemia stem cells features similar to cancer stem cells and exhibited resistance to tyrosine kinase inhibitor imatinib, which is used to treat CML. These studies led to the discovery of the survival factor olfactomedin 4 (OLFM4) as a new and potential therapeutic target to overcome resistance to imatinib in CML (Suknuntha et al., 2015). Overall, these studies showed that it is possible to successfully reprogram cancer cells into iPSC, differentiate them into lineages of the original cell and generate non-tumorigenic normal cells.
Reprogramming melanoma cells into iPSC: a model for understanding melanoma cell of origin, plasticity, and trans-differentiation
As mentioned above, several strategies can be used to study cell of origin and plasticity in cancer and melanoma. As some epigenetic marks related to the cell of origin might be retained by melanoma-iPSC, making them more prompt to express and differentiate into lineages of the cell of origin. Therefore, direct reprogramming of melanoma cells could elucidate potential differences in cell of origin, tumor plasticity, and heterogeneity, as suggested by some studies (Castro-Pérez et al., 2019; Bernhardt et al., 2017).
Another reprogramming strategy used in cancer to study cell of origin is by evaluating the role of driver mutations in reprogramming, differentiation/plasticity, and tumorigenesis. For instance, studies by Castro-Pérez et al., 2019 demonstrated that BRAFV600E expression blocks iPSC reprogramming in melanocytes, while the addition of BRAF inhibitor vemurafenib facilitates the reprogramming of BRAFV600E mutant melanoma cell lines. These findings open the possibility of investigating the role of specific mutations in reprogramming, differentiation, and cell of origin in melanoma, as reported in other cancer-derived iPSC studies Hashimoto et al., 2017; Chao et al., 2017). For example, mutations of specific melanoma subtypes could be induced or corrected in cells with CRISPR/Cas9 before or after reprogramming and evaluate their role as cell of origin after differentiation into different iPSC-differentiated cells such as melanoblasts, melanocytes, keratinocytes, fibroblasts or other tissue/lineages. Additionally, the role of these melanoma mutant-iPSCs derived cells could be modeled for cell of origin in melanomagenesis in vivo in specific tissues with xenograft mice as well as in vitro with co-culture models using, for instance, microfluidics platforms and 3D organoids (Nissan et al., 2011; Hill et al., 2015; Bourland et al., 2018; Sadangi et al., 2022). These strategies have the advantage of testing the oncogenic competence of normal cells that might not be easily obtained from live patients (like eye melanocytes, NCCs or melanoblasts during developmental stages) and harboring specific mutations rather than tumor cells containing multiple unknown mutations or even hESCs which have more limited accessibility and different mutations.
As mentioned above, plasticity of cancers has also been evaluated using the strategy of reprogramming. The relevance of phenotypic plasticity and trans-differentiation have recently been highlighted as new functionally distinct hallmarks of cancer pathogenesis (Hanahan, 2022). These are capabilities normally restricted by lineage-specific developmental programs. However, they may be unlocked in cancer by, for example, dedifferentiation back to progenitor/stem-like cell states or changing their morphology to become recognizable as elements of another tissue (Yuan et al., 2019). In order to address plasticity, trans-differentiation properties, and drug resistance in melanoma, (Castro-Pérez et al., 2019) employed the iPSC reprogramming strategy. In these studies, skin-derived fibroblast and melanocytes and primary and metastatic human patient-derived melanoma cell lines were reprogrammed into iPSC, and both fibroblast-derived iPSC and melanocyte-derived iPSC were found to readily re-differentiate to pigment-producing melanocytes. However, only a subset of melanoma cell lines was able to generate iPSC, albeit less efficiently than skin fibroblasts and melanocytes. Primary melanoma cells were more efficiently reprogrammed into iPSC than metastatic melanoma cells. In a set of genetically matched primary and metastatic melanoma cells isolated from the same patient, primary melanoma cells efficiently generated iPSC, whereas metastatic cells did not. Melanoma-derived iPSC exhibited pluripotency to differentiate into the primordial germ cells of ectoderm, mesoderm, and endoderm in vitro. Therefore, we asked whether melanoma-iPSC could differentiate back into melanocytes since several studies protocols demonstrating that both ESC and iPSC can be differentiated into melanocytes have been reported (Fang et al., 2006; Ohta et al., 2011; Yang et al., 2011). We found that upon directed differentiation to melanocytes (in vitro), melanoma-iPSC showed neural-like plastic differentiation (Figure 3). These melanoma-iPSC-differentiated cells co-expressed neuronal and melanocyte markers, including MITF, MAP2, neuronal tubulin (TUBB3), and even glial marker GFAP. The expression of these markers varied among different melanoma lines. For example, some melanoma-iPSC-differentiated cells exhibited deficient expression of melanocyte lineage markers, whereas, in others, melanocyte markers expression was barely detectable, but the expression of markers of terminal neuronal differentiation MAP2 and TUBB3 was prominent. It is known that melanocytes derived from human iPSCs transplanted into athymic mouse skin can produce pigmented melanocytic aggregates and express melanocyte markers (Kawakami et al., 2018), suggesting skin microenvironment influences the elaboration of the melanocyte differentiation program. When xenotransplanted into mouse skin, melanoma iPSC-differentiated cells, which show limited melanocyte differentiation in vitro, formed amelanotic tumors that exhibit weak expression of MITF but not key melanocyte differentiation markers TYR, TYRP1, TYRP2, and MART1). In contrast, these tumors exhibit strong expression of neural markers PAX3, TUBB3, MAP2, and GFAP and melanoma stem cell markers SOX9, CD271, and ALDH1, suggesting plasticity to differentiate along multiple lineages. Culture of melanoma-iPSC in media that promote neuronal differentiation facilitated elaboration of neural markers of terminal neural differentiation as evident by the expression of MAP2, TUBB3, SYN1 (Synapsin 1), and GFAP. Melanoma-iPSC generated from different melanoma cell lines exhibited varying patterns of differentiation in melanocyte or neuronal differentiation medium in vitro, in the mouse xenograft skin microenvironment (in vivo). These varying patterns might be a possible indication of different cells of origin among the cell lines analyzed. We argue that cellular plasticity and capacity to trans-differentiate may provide a window into the potential differences in the cell of origin of melanoma subtypes.
Figure 3. Melanoma-derived iPSC to model cell of origin and drug resistance.
(A) Melanocytes populate multiple locations as their diverse developmental origins from NCC Melanomas emerge from multiple locations of melanocytes. Reprogramming skin-derived fibroblasts and melanocytes into iPSC and differentiation into melanocytes results in efficient iPSC reprogramming and melanocyte differentiation. However, melanoma cells reprogrammed to iPSC and directed to differentiate back to melanocytes do not exhibit melanocyte differentiation but neural/NCC-like mixed phenotype. Melanoma iPSC-differentiated cells show increased expression of neural/NCC and melanoma stem cell-related markers and decreased expression of melanocytic lineage markers. (B) During malignant transformation, melanocytes acquire BRAFV600E oncogenic mutation and other mutations such as PTEN loss. These cells are initially sensitive to vemurafenib, but after treatment with the inhibitor, the drug induces changes in expression related to cellular plastic changes generating a stem cell-like transitional state. After this transition, many cells survive, have made plastic changes with stem-like characteristics, and exhibit acquired resistance to the drug (top). Reprogramming of BRAFV600E melanoma cells induces the formation of iPSC colonies. iPSC generated do not differentiate into melanocytes but exhibit neural/NCC-like plasticity and show acquired resistance (bottom).
Tumor cell plasticity and drug resistance
The cellular plasticity of cancer cells is often associated with phenotypic heterogeneity and drug resistance. However, the specific mechanisms associated with phenotypic plasticity that promote the survival and proliferation of drug-resistant melanoma cells have not yet been elucidated (Granados et al., 2020). In a certain respect, this plasticity to switch from the proliferative potential of metastatic and drug-resistant melanoma cells is similar to the switch, in reverse, i. e., differentiation of embryonic stem cells (ESC)/tissue stem cells (TSC)/induced pluripotent stem cells (iPSC) with self-renewal and pluripotency. Factors and mechanisms that control pluripotency and unlimited self-renewal of ESC/iPSC have been extensively investigated. For example, it was reported that signaling networks involving crosstalk between several pathways culminate in a finely balanced molecular switch that determines the fate of pluripotent cells (Dalton, 2013). In melanoma cells, genes that promote undifferentiated and cancer stem cell-like phenotypes have been reported to be associated with targeted therapy resistance in melanoma-derived induced pluripotent cancer cells (Hüser et al., 2018). Using a strategy of reprogramming human melanoma cells to iPSC showed that culturing melanoma-derived iPSCs in melanocyte differentiation medium produces neural-like lineages that become intrinsically resistant to MAPK inhibitors (Castro-Pérez et al., 2019), suggesting crosstalk between signal pathways involved in trans-differentiation and mechanisms that render melanoma cells resistant to these drugs. These observations suggest that targeting such pathways can block or reverse the emergence of new cell lineage states and make melanoma cells retain sensitivity to these cancer treatments. Trans-differentiation to neuroendocrine phenotype has been reported to act as a treatment resistance mechanism in prostate and lung cancers (Davies et al., 2021; Quintanal-Villalonga et al., 2020; Sequist et al., 2011; Zou et al., 2017). It is not known whether melanoma trans-differentiation also acts as a treatment resistance mechanism.
Conclusions/Perspectives:
Induced pluripotent stem cell technology has revolutionized regenerative medicine and disease modeling by reprogramming normal and patient-derived cells to iPSC. In particular, the cancer cell-derived iPSC strategy has recently been employed to understand the mechanism of plasticity, drug resistance, and drug screening. Melanocytic neoplasms present a diverse clinical, pathological, and molecular subtype of malignant melanomas that exhibit different patterns of oncogene mutations in a highly specific manner, raising the question of whether these subtypes have a different cell of origin. Reprogramming melanoma-derived iPSC and characterizing their plasticity by directed differentiation is an attractive strategy for understanding the cell of origin in melanoma. A better understanding of the cell of origin could help refine the diagnosis, prognosis, and mechanisms of drug resistance in melanoma. Melanoma-derived iPSC could lead to the identification of genes, proteins, and cellular pathways that control senescence, pluripotency, and oncogenic potential and allow the development and design of novel therapeutics that can overcome drug resistance in malignant melanoma, the primary clinical challenge in this disease.
Acknowledgments
This work was supported partially by Veteran Affairs Merit Award I01BX004921, U.S. National Institutes of Health grant 1P30AR066524, and Department of Defense grant W81XWH-18-PRCRP-IASF (CA181014) (to V.S.). E.C.-P. is supported by INDICASAT-AIP GRANT 015-2020; National Secretary for Science, Technology, and Innovation (SENACYT, Panama) grant PFID-FID-2021-177; and by Sistema Nacional de Investigación (SNI-SENACYT, Panama). C.M.-S. is supported by a Graduate Scholarship from SENACYT-Panama and by the Master Program in Molecular Biology, University of Panama.
Footnotes
Conflict of Interest Statement: No conflict of interest to declare.
REFERENCES
- Adameyko I, Lallemend F, Aquino JB, Pereira JA, Topilko P, Muller T, Fritz N, Beljajeva A, Mochii M, Liste I, et al. (2009). Schwann cell precursors from nerve innervation are a cellular origin of melanocytes in skin. Cell 139, 366–379. [DOI] [PubMed] [Google Scholar]
- Adameyko I, Lallemend F, Furlan A, Zinin N, Aranda S, Kitambi SS, Blanchart A, Favaro R, Nicolis S, Lubke M, et al. (2012). Sox2 and Mitf cross-regulatory interactions consolidate progenitor and melanocyte lineages in the cranial neural crest. Development 139, 397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbani R, Akdemir Kadir C., Aksoy BA, Albert M, Ally A, Amin Samirkumar B., Arachchi H, Arora A, Auman JT, Ayala B, et al. (2015). Genomic Classification of Cutaneous Melanoma. Cell 161, 1681–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcantara-Llaguno S, Chen J, Kwon CH, Jackson EL, Li Y, et al. 2009. Malignant astrocytomas originate from neural stem/progenitor cells in a somatic tumor suppressor mouse model. Cancer Cell. Jan 6;15(1):45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcantara-Llaguno SR, Wang Z, Sun D, Chen J, Xu J, et al. 2015. Adult Lineage-Restricted CNS Progenitors Specify Distinct Glioblastoma Subtypes. Cancer Cell. Oct 12;28(4):429–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki H, Yamada Y, Hara A, and Kunisada T (2009). Two distinct types of mouse melanocyte: differential signaling requirement for the maintenance of non-cutaneous and dermal versus epidermal melanocytes. Development 136, 2511–2521. [DOI] [PubMed] [Google Scholar]
- Ars E, Kruyer H, Morell M, Pros E, Serra E, Ravella A, Estivill X, and Lazaro C (2003). Recurrent mutations in the NF1 gene are common among neurofibromatosis type 1 patients. J Med Genet 40, e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggiolini A, Callahan SJ, Montal E, Weiss JM, Trieu T, et al. , 2021. Developmental chromatin programs determine oncogenic competence in melanoma. Science. Sep 3;373(6559):eabc1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banito A, Rashid ST, Acosta JC, Li S, Pereira CF, Geti I, Pinho S, Silva JC, Azuara V, Walsh M, et al. (2009). Senescence impairs successful reprogramming to pluripotent stem cells. Genes Dev 23, 2134–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Nur O, Russ HA, Efrat S and Benvenisty N. 2011. Epigenetic memory and preferential lineage-specific differentiation in induced pluripotent stem cells derived from human pancreatic islet beta cells. Cell Stem Cell 9: 17–23. [DOI] [PubMed] [Google Scholar]
- Bastian BC (2014). The molecular pathology of melanoma: an integrated taxonomy of melanocytic neoplasia. Annu Rev Pathol 9, 239–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista NC, Cohen S, and Anders KH (1994). Benign melanocytic nevus cells in axillary lymph nodes. A prospective incidence and immunohistochemical study with literature review. Am J Clin Pathol 102, 102–108. [DOI] [PubMed] [Google Scholar]
- Beadling C, Jacobson-Dunlop E, Hodi FS, Le C, Warrick A, Patterson J, Town A, Harlow A, Cruz F 3rd, Azar S, et al. (2008). K.I.T. gene mutations and copy number in melanoma subtypes. Clin Cancer Res 14, 6821–6828. [DOI] [PubMed] [Google Scholar]
- Bernhardt M, Novak D, Assenov Y, Orouji E, Knappe N, Weina K, Reith M, Larribere L, Gebhardt C, Plass C, et al. (2017). Melanoma-Derived iPCCs Show Differential Tumorigenicity and Therapy Response. Stem Cell Reports 8, 1379–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birck A, Ahrenkiel V, Zeuthen J, Hou-Jensen K, and Guldberg P (2000). Mutation and allelic loss of the PTEN/MMAC1 gene in primary and metastatic melanoma biopsies. J Invest Dermatol 114, 277–280. [DOI] [PubMed] [Google Scholar]
- Bourland J, Fradette J, Auger FA. 2018. Tissue-engineered 3D melanoma model with blood and lymphatic capillaries for drug development. Sci Rep. Sep 4;8(1):13191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradl M, Klein-Szanto A, Porter S, Mintz B. 1991. Malignant melanoma in transgenic mice. Proc Natl Acad Sci USA. Jan 1;88(1):164–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brastianos PK, Carter SL, Santagata S, Cahill DP, Taylor-Weiner A, Jones RT, Van Allen EM, Lawrence MS, Horowitz PM, Cibulskis K, et al. (2015). Genomic Characterization of Brain Metastases Reveals Branched Evolution and Potential Therapeutic Targets. Cancer Discov 5, 1164–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucheit AD, Chen G, Siroy A, Tetzlaff M, Broaddus R, Milton D, Fox P, Bassett R, Hwu P, Gershenwald JE, et al. (2014). Complete loss of PTEN protein expression correlates with shorter time to brain metastasis and survival in stage IIIB/C melanoma patients with BRAFV600 mutations. Clin Cancer Res 20, 5527–5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell PJ, Getz G, Korbel JO, Stuart JM, Jennings JL, Stein LD, Perry MD, Nahal-Bose HK, Ouellette BFF, Li CH, et al. (2020). Pan-cancer analysis of whole genomes. Nature 578, 82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramel J, Papadogeorgakis E, Hill L, Browne GJ, Richard G, Wierinckx A, Saldanha G, Osborne J, Hutchinson P, Tse G, et al. (2013). A switch in the expression of embryonic EMT-inducers drives the development of malignant melanoma. Cancer Cell 24, 466–480. [DOI] [PubMed] [Google Scholar]
- Carson KF, Wen DR, Li PX, Lana AM, Bailly C, Morton DL, and Cochran AJ (1996). Nodal nevi and cutaneous melanomas. Am J Surg Pathol 20, 834–840. [DOI] [PubMed] [Google Scholar]
- Carvajal RD, Antonescu CR, Wolchok JD, Chapman PB, Roman RA, Teitcher J, Panageas KS, Busam KJ, Chmielowski B, Lutzky J, et al. (2011). K.I.T. as a therapeutic target in metastatic melanoma. Jama 305, 2327–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Pérez E, Rodríguez CI, Mikheil D, Siddique S, McCarthy A, Newton MA, and Setaluri V (2019). Melanoma Progression Inhibits Pluripotency and Differentiation of Melanoma-Derived iPSCs Produces Cells with Neural-like Mixed Dysplastic Phenotype. Stem Cell Reports 13, 177–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cechmanek PB, McFarlane S. 2017. Retinal Pigment Epithelium Expansion Around the NeuralRetina Occurs in Two Separate Phases With DistinctMechanisms. Dev Dyn. Aug;246(8):598–609. [DOI] [PubMed] [Google Scholar]
- Chao MP, Gentles AJ, Chatterjee S, Lan F, Reinisch A, Corces MR, Xavy S, Shen J, Haag D, Chanda S, et al. (2017). Human AML-iPSCs Reacquire Leukemic Properties after Differentiation and Model Clonal Variation of Disease. Cell Stem Cell 20, 329–344.e327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Lopez-Beltran A, Massari F, MacLennan GT, and Montironi R (2018). Molecular testing for BRAF mutations to inform melanoma treatment decisions: a move toward precision medicine. Mod Pathol 31, 24–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z, He Z, Cai Y, Zhang C, Fu G, Li H, Sun W, Liu C, Cui X, Ning B, et al. (2019). Conversion of hepatoma cells to hepatocyte-like cells by defined hepatocyte nuclear factors. Cell Res 29, 124–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlebanowska P, Sułkowski M, Skrzypek K, Tejchman A, Muszyńska A, et al. 2020. Origin of the Induced Pluripotent Stem Cells Affects Their Differentiation into Dopaminergic Neurons. Int. J. Mol. Sci 21, 5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichorek M, Wachulska M, and Skoniecka A (2013). Heterogeneity of neural crest-derived melanocytes. Central European Journal of Biology 8, 315–330. [Google Scholar]
- Cirenajwis H, Lauss M, Ekedahl H, Törngren T, Kvist A, Saal LH, Olsson H, Staaf J, Carneiro A, Ingvar C, et al. (2017). NF1-mutated melanoma tumors harbor distinct clinical and biological characteristics. Molecular oncology 11, 438–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark WH Jr., Elder DE, and Van Horn M (1986). The biologic forms of malignant melanoma. Hum Pathol 17, 443–450. [DOI] [PubMed] [Google Scholar]
- Cordero DR, Brugmann S, Chu Y, Bajpai R, Jame M, and Helms JA (2011). Cranial neural crest cells on the move: their roles in craniofacial development. Am J Med Genet A 155a, 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer SF, and Fesyuk A (2012). On the development of neurocutaneous units--implications for the histogenesis of congenital, acquired, and dysplastic nevi. Am J Dermatopathol 34, 60–81. [DOI] [PubMed] [Google Scholar]
- Curtin JA, Busam K, Pinkel D, and Bastian BC (2006). Somatic activation of K.I.T. in distinct subtypes of melanoma. J Clin Oncol 24, 4340–4346. [DOI] [PubMed] [Google Scholar]
- Czerwińska P, Mazurek S, and Wiznerowicz M (2018). Application of induced pluripotency in cancer studies. Rep Pract Oncol Radiother 23, 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Kong Y, Si L, Chi Z, Cui C, Sheng X, Mao L, Li S, Lian B, Yang R, et al. (2013). Large-scale analysis of PDGFRA mutations in melanomas and evaluation of their sensitivity to tyrosine kinase inhibitors imatinib and crenolanib. Clin Cancer Res 19, 6935–6942. [DOI] [PubMed] [Google Scholar]
- Dalton S (2013) Signaling networks in human pluripotent stem cells. Curr Opin Cell Biol. 25: 241–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Dan-Ning, Savage Howard E., and Roberts Joan E.. Uveal Melanocytes, Ocular Pigment Epithelium, and Müller Cells in Culture: In Vitro Toxicology. International Journal of Toxicology, 21:465–472, 2002. [DOI] [PubMed] [Google Scholar]
- Davies A, Nouruzi S, Ganguli D, Namekawa T, Thaper D, Linder S, Karaoglanoglu F, Omur ME, Kim S, Kobelev M, Kumar S, Sivak O, Bostock C, Bishop J, Hoogstraat M, Talal A, Stelloo S, van der Poel H, Bergman AM, Ahmed M, Fazli L, Huang H, Tilley W, Goodrich D, Feng FY, Gleave M, He HH, Hach F, Zwart W, Beltran H, Selth L, and Zoubeidi A (2021) An androgen receptor switch underlies lineage infidelity in treatment-resistant prostate cancer. Nat Cell Biol. 23: 1023–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies MA (2012). The role of the PI3K-AKT pathway in melanoma. Cancer J 18, 142–147. [DOI] [PubMed] [Google Scholar]
- de Lange MJ, Razzaq L, Versluis M, Verlinde S, Dogrusoz M, Bohringer S, Marinkovic M, Luyten GP, de Keizer RJ, de Gruijl FR, et al. (2015). Distribution of GNAQ and GNA11 Mutation Signatures in Uveal Melanoma Points to a Light Dependent Mutation Mechanism. PLoS One 10, e0138002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EJ, Johnson DB, Sosman JA, Chandra S. Melanoma: What do all the mutations mean?. Cancer. 2018;124(17):3490–3499. doi: 10.1002/cncr.31345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty RE, Alfawaz M, Francis J, Lijka-Jones B, and Sisley K (2018). Genetics of Uveal Melanoma. In Noncutaneous Melanoma, Scott JF, and Gerstenblith MR, eds. (Brisbane (A.U.): Codon Publications The Authors.). [PubMed] [Google Scholar]
- Dominiak NR, Wick MR, and Smith MT (2016). Mucosal melanomas: Site-specific information, comparisons with cutaneous tumors, and differential diagnosis. Semin Diagn Pathol 33, 191–197. [DOI] [PubMed] [Google Scholar]
- Dupin E, Real C, Glavieux-Pardanaud C, Vaigot P, and Le Douarin NM (2003). Reversal of developmental restrictions in neural crest lineages: transition from Schwann cells to glial-melanocytic precursors in vitro. Proc Natl Acad Sci U S A 100, 5229–5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder DE, Bastian BC, Cree IA, Massi D, Scolyer RA. 2020. The 2018 World Health Organization Classification of Cutaneous, Mucosal, and Uveal Melanoma: Detailed Analysis of 9 Distinct Subtypes Defined by Their Evolutionary Pathway. Arch Pathol Lab Med. Apr;144(4):500–522. [DOI] [PubMed] [Google Scholar]
- Ellerhorst JA, Greene VR, Ekmekcioglu S, Warneke CL, Johnson MM, Cooke CP, Wang LE, Prieto VG, Gershenwald JE, Wei Q, et al. (2011). Clinical correlates of NRAS and BRAF mutations in primary human melanoma. Clin Cancer Res 17, 229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteve P, Bovalenta P. 2006. Secreted inducers in vertebrate eye development: more functions for old morphogens. Curr Opin Neurobiol 1:13–19. [DOI] [PubMed] [Google Scholar]
- Fang D, Leishear K, Nguyen TK, Finko R, Cai K, et al. 2006. Defining the conditions for the generation of melanocytes from human embryonic stem cells. Stem Cells. Jul;24(7):1668–77. [DOI] [PubMed] [Google Scholar]
- Field MG, Durante MA, Anbunathan H, Cai LZ, Decatur CL, Bowcock AM, Kurtenbach S, and Harbour JW (2018). Punctuated evolution of canonical genomic aberrations in uveal melanoma. Nat Commun 9, 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty KT, Robert C, Hersey P, Nathan P, Garbe C, Milhem M, Demidov LV, Hassel JC, Rutkowski P, Mohr P, et al. (2012). Improved survival with M.E.K. inhibition in BRAF-mutated melanoma. N Engl J Med 367, 107–114. [DOI] [PubMed] [Google Scholar]
- Fowler JC, King C, Bryant C, Hall MWJ, Sood R, et al. , 2021. Selection of Oncogenic Mutant Clones in Normal Human Skin Varies with Body Site. Cancer Discov. Feb;11(2):340–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann-Morvinski D, Verma IM. 2014. Dedifferentiation and reprogramming: origins of cancer stem cells. EMBO Rep 15: 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funato K, Major T, Lewis PW, Allis CD, Tabar V. 2014. Use of human embryonic stem cells to model pediatric gliomas with H3.3K27M histone mutation. Science. Dec 19;346(6216):1529–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garraway LA, Widlund HR, Rubin MA, Getz G, Berger AJ, Ramaswamy S, Beroukhim R, Milner DA, Granter SR, Du J, et al. (2005). Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma. Nature 436, 117–122. [DOI] [PubMed] [Google Scholar]
- Gkiala A, Palioura S. 2020. Conjunctival Melanoma: Update on Genetics, Epigenetics and Targeted Molecular and Immune-Based Therapies. Clin Ophthalmol. Oct 9;14:3137–3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez C, Chua W, Miremadi A, Quist S, Headon DJ, and Watt FM (2013). The interfollicular epidermis of adult mouse tail comprises two distinct cell lineages that are differentially regulated by Wnt, Edaradd, and Lrig1. Stem Cell Reports 1, 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong L, Yan Q, Zhang Y, Fang X, Liu B, and Guan X (2019). Cancer cell reprogramming: a promising therapy converting malignancy to benignity. Cancer Commun (Lond) 39, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves WO, Verma S, Patel KP, Davies MA, Barkoh BA, Galbincea JM, Yao H, Lazar AJ, Aldape KD, Medeiros LJ, et al. (2013). Frequency and spectrum of BRAF mutations in a retrospective, single-institution study of 1112 cases of melanoma. The Journal of molecular diagnostics : J.M.D 15, 220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grichnik JM, Ross AL, Schneider SL, Sanchez MI, Eller MS, and Hatzistergos KE (2014). How, and from which cell sources, do nevi really develop? Exp Dermatol 23, 310–313. [DOI] [PubMed] [Google Scholar]
- Haass NK, Smalley KS, Li L, and Herlyn M (2005). Adhesion, migration and communication in melanocytes and melanoma. Pigment Cell Res 18, 150–159. [DOI] [PubMed] [Google Scholar]
- Hanahan D 2022. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022 Jan;12(1):31–46. [DOI] [PubMed] [Google Scholar]
- Harbour JW (2012). The genetics of uveal melanoma: an emerging framework for targeted therapy. Pigment Cell Melanoma Res 25, 171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargus G, Ehrlich M, Araúzo-Bravo MJ, Hemmer K, Hallmann AL, et al. , 2014. Origin-dependent neural cell identities in differentiated human iPSCs in vitro and after transplantation into the mouse brain. Cell Rep. 8, 1697–1703. [DOI] [PubMed] [Google Scholar]
- Haridhasapavalan KK, Raina K, Dey C, Adhikari P, and Thummer RP (2020). An Insight into Reprogramming Barriers to iPSC Generation. Stem Cell Rev Rep 16, 56–81. [DOI] [PubMed] [Google Scholar]
- Harland M, Cust AE, Badenas C, Chang YM, Holland EA, Aguilera P, Aitken JF, Armstrong BK, Barrett JH, Carrera C, et al. (2014). Prevalence and predictors of germline CDKN2A mutations for melanoma cases from Australia, Spain and the United Kingdom. Hered Cancer Clin Pract 12, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris ML, and Erickson CA (2007). Lineage specification in neural crest cell pathfinding. Dev Dyn 236, 1–19. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Yamada Y, Semi K, Yagi M, Tanaka A, et al. , 2017. Cellular context-dependent consequences of Apc mutations on gene regulation and cellular behavior. Proc Natl Acad Sci USA. Jan 24;114(4):758–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward NK, Wilmott JS, Waddell N, Johansson PA, Field MA, Nones K, Patch AM, Kakavand H, Alexandrov LB, Burke H, et al. (2017). Whole-genome landscapes of major melanoma subtypes. Nature 545, 175–180. [DOI] [PubMed] [Google Scholar]
- Hill DS, Robinson ND, Caley MP, Chen M, O'Toole EA, et al. 2015. A Novel Fully Humanized 3D Skin Equivalent to Model Early Melanoma Invasion. Mol Cancer Ther. Nov;14(11):2665–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintzsche JD, Gorden NT, Amato CM, Kim J, Wuensch KE, Robinson SE, Applegate AJ, Couts KL, Medina TM, Wells KR, et al. (2017). Whole-exome sequencing identifies recurrent SF3B1 R625 mutation and comutation of NF1 and K.I.T. in mucosal melanoma. Melanoma Res 27, 189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodis E, Watson IR, Kryukov GV, Arold ST, Imielinski M, Theurillat JP, Nickerson E, Auclair D, Li L, Place C, et al. (2012). A landscape of driver mutations in melanoma. Cell 150, 251–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt JB, Sangueza OP, Levine EA, Shen P, Bergman S, Geisinger KR, and Creager AJ (2004). Nodal melanocytic nevi in sentinel lymph nodes. Correlation with melanoma-associated cutaneous nevi. Am J Clin Pathol 121, 58–63. [DOI] [PubMed] [Google Scholar]
- Houben R, Becker JC, Kappel A, Terheyden P, Brocker EB, Goetz R, and Rapp UR (2004). Constitutive activation of the Ras-Raf signaling pathway in metastatic melanoma is associated with poor prognosis. J Carcinog 3, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu DN. 2005, Photobiology of ocular melanocytes and melanoma. Photochem. Photobiol.81:506–509. [DOI] [PubMed] [Google Scholar]
- Hu DN, McCormick SA, Seedor JA, Ritterband DC, Shah MK. 2007. Isolation, purification and cultivation of conjunctival melanocytes. Exp. Eye Res, 84, pp. 655–662. [DOI] [PubMed] [Google Scholar]
- Hu S, Zhao MT, Jahanbani F, Shao NY, Lee WH, et al. 2016. Effects of cellular origin on differentiation of human induced pluripotent stem cell-derived endothelial cells. JCI Insight. Jun 2;1(8):e85558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JL, Urtatiz O, and Van Raamsdonk CD (2015). Oncogenic G Protein GNAQ Induces Uveal Melanoma and Intravasation in Mice. Cancer Res 75, 3384–3397. [DOI] [PubMed] [Google Scholar]
- Huang M, Tailor J, Zhen O, Gillmor AH, Milleret ML al. 2019. Engineering Genetic Predisposition in Human Neuroepithelial Stem Cells Recapitulates Medulloblastoma Tumorigenesis. Cell Stem Cell. Sep 5;25(3):433–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüser L, Sachindra S, Granados K, Federico A, Larribère L, Novak D, Umansky V, Altevogt P, and Utikal J (2018). SOX2-mediated upregulation of CD24 promotes adaptive resistance toward targeted therapy in melanoma. Int J Cancer 143, 3131–3142. [DOI] [PubMed] [Google Scholar]
- Hutter C, and Zenklusen JC (2018). The Cancer Genome Atlas: Creating Lasting Value beyond Its Data. Cell 173, 283–285. [DOI] [PubMed] [Google Scholar]
- Ishay-Ronen D, Diepenbruck M, Kalathur RKR, Sugiyama N, Tiede S, Ivanek R, Bantug G, Morini MF, Wang J, Hess C, et al. (2019). Gain Fat-Lose Metastasis: Converting Invasive Breast Cancer Cells into Adipocytes Inhibits Cancer Metastasis. Cancer Cell 35, 17–32.e16. [DOI] [PubMed] [Google Scholar]
- Istrate M, Vlaicu B, Poenaru M, Hasbei-Popa M, Salavat MC, et al. , 2020.. Photoprotection role of melanin in the human retinal pigment epithelium. Imaging techniques for retinal melanin. Rom J Ophthalmol. Apr-Jun;64(2):100–104. [PMC free article] [PubMed] [Google Scholar]
- Iwamoto S, Burrows RC, Grossniklaus HE, Orcutt J, Kalina RE, Boehm M, Bothwell MA, Schmidt R,. 2002. Immunophenotype of conjunctival melanomas: comparisons with uveal and cutaneous melanomas. Arch. Ophthalmol, 120, pp. 1625–1629. [DOI] [PubMed] [Google Scholar]
- Johannessen CM, Johnson LA, Piccioni F, Townes A, Frederick DT, Donahue MK, Narayan R, Flaherty KT, Wargo JA, Root DE, et al. (2013). A melanocyte lineage program confers resistance to M.A.P. kinase pathway inhibition. Nature 504, 138–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper K, de Goeje PL, Peeper DS, and van Amerongen R (2014). Phenotype switching: tumor cell plasticity as a resistance mechanism and target for therapy. Cancer Res 74, 5937–5941. [DOI] [PubMed] [Google Scholar]
- Kim CY, Kim DW, Kim K, Curry J, Torres-Cabala C, and Patel S (2014). GNAQ mutation in a patient with metastatic mucosal melanoma. B.M.C. Cancer 14, 516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Schaniel C. 2018. Modeling Hematological Diseases and Cancer With Patient-Specific Induced Pluripotent Stem Cells. Front. Immunol 9:2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Hoffman JP, Alpaugh RK, Rhim AD, Reichert M, et al. 2013. An iPSC line from human pancreatic ductal adenocarcinoma undergoes early to invasive stages of pancreatic cancer progression. Cell Rep. 3:2088–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ (2015). Applications of iPSCs in Cancer Research. Biomark Insights 10, 125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Doi A, Wen B, Ng K, Zhao R, et al. 2010. Epigenetic memory in induced pluripotent stem cells. Nature, 467, 285–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsler VA, and Larue L (2018). The patterns of birthmarks suggest a novel population of melanocyte precursors arising around the time of gastrulation. Pigment Cell Melanoma Res 31, 95–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler C, Nittner D, Rambow F, Radaelli E, Stanchi F, Vandamme N, Baggiolini A, Sommer L, Berx G, van den Oord JJ, et al. (2017). Mouse Cutaneous Melanoma Induced by Mutant BRaf Arises from Expansion and Dedifferentiation of Mature Pigmented Melanocytes. Cell Stem Cell 21, 679–693.e676. [DOI] [PubMed] [Google Scholar]
- Kong Y, Krauthammer M, and Halaban R (2014). Rare SF3B1 R625 mutations in cutaneous melanoma. Melanoma Res 24, 332–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotini AG, Chang CJ, Chow A, Yuan H, Ho TC, et al. 2017. Stage-Specific Human Induced Pluripotent Stem Cells Map the Progression of Myeloid Transformation to Transplantable Leukemia. Cell Stem Cell. Mar 2;20(3):315–328.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krepler C, Sproesser K, Brafford P, Beqiri M, Garman B, Xiao M, Shannan B, Watters A, Perego M, Zhang G, et al. (2017). A Comprehensive Patient-Derived Xenograft Collection Representing the Heterogeneity of Melanoma. Cell Rep 21, 1953–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulesa PM, Kasemeier-Kulesa JC, Teddy JM, Margaryan NV, Seftor EA, Seftor RE, and Hendrix MJ (2006). Reprogramming metastatic melanoma cells to assume a neural crest cell-like phenotype in an embryonic microenvironment. Proc Natl Acad Sci U S A 103, 3752–3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larribère L, Kuphal S, Sachpekidis C, Hüser L, Bosserhoff A, and Utikal J (2018). Targeted Therapy-Resistant Melanoma Cells Acquire Transcriptomic Similarities with Human Melanoblasts. Cancers (Basel) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larribere L, and Utikal J (2019). Stem Cell-Derived Models of Neural Crest Are Essential to Understand Melanoma Progression and Therapy Resistance. Front Mol Neurosci 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, Carter SL, Stewart C, Mermel CH, Roberts SA, et al. (2013). Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 499, 214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Pouliot N, Redvers R, and Kaur P (2004). Extensive tissue-regenerative capacity of neonatal human keratinocyte stem cells and their progeny. The Journal of clinical investigation 113, 390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Ilaria RL Jr, Million RP, Daley GQ, Van Etten RA. 1999. The P190, P210, and P230 forms of the BCR/ABL oncogene induce a similar chronic myeloid leukemia-like syndrome in mice but have different lymphoid leukemogenic activity. J Exp Med. May 3;189(9):1399–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Han Q, Peng T, Peng M, Wei B, Li D, Wang X, Yu S, Yang J, Cao S, et al. (2015). The oncogene c-Jun impedes somatic cell reprogramming. Nat Cell Biol 17, 856–867. [DOI] [PubMed] [Google Scholar]
- Liu M, Tu J, Gingold JA, Kong CSL, and Lee DF (2018). Cancer in a dish: progress using stem cells as a platform for cancer research. Am J Cancer Res 8, 944–954. [PMC free article] [PubMed] [Google Scholar]
- Mahalingam D, Kong CM, Lai J, Tay LL, Yang H, et al. 2012. Reversal of aberrant cancer methylome and transcriptome upon direct reprogramming of lung cancer cells. Sci Rep. 2:592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannik J, Alzayady K, and Ghazizadeh S (2010). Regeneration of multilineage skin epithelia by differentiated keratinocytes. J Invest Dermatol 130, 388–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Navarro A, Susanto E, Falk A, Wilhelm M. 2018. Modeling cancer using patient-derived induced pluripotent stem cells to understand development of childhood malignancies. Cell Death Discov. Feb 1;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Morales JR, Rodrigo I, Bovalenta P. 2004. Eye develop-ment: a view from the retina pigmented epithelium. BioEssays 26:766–777. [DOI] [PubMed] [Google Scholar]
- Merkel EA, and Gerami P (2017). Malignant melanoma of sun-protected sites: a review of clinical, histological, and molecular features. Lab Invest 97, 630–635. [DOI] [PubMed] [Google Scholar]
- Mintz B, and Silvers WK (1993). Transgenic mouse model of malignant skin melanoma. Proc Natl Acad Sci U S A 90, 8817–8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi N, Ishii H, Nagai K, Hoshino H, Mimori K, Tanaka F, Nagano H, Sekimoto M, Doki Y, and Mori M (2010). Defined factors induce reprogramming of gastrointestinal cancer cells. Proc Natl Acad Sci U S A 107, 40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon H, Donahue LR, Choi E, Scumpia PO, Lowry WE, Grenier JK, Zhu J, and White AC (2017). Melanocyte Stem Cell Activation and Translocation Initiate Cutaneous Melanoma in Response to U.V. Exposure. Cell Stem Cell 21, 665-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AR, Ran L, Guan Y, Sher JJ, Hitchman TD, Zhang JQ, Hwang C, Walzak EG, Shoushtari AN, Monette S, et al. (2018). GNA11 Q209L Mouse Model Reveals RasGRP3 as an Essential Signaling Node in Uveal Melanoma. Cell Rep 22, 2455–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mort RL, Jackson IJ, and Patton EE (2015). The melanocyte lineage in development and disease. Development 142, 1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosteiro L, Pantoja C, Alcazar N, Marión RM, Chondronasiou D, Rovira M, Fernandez-Marcos PJ, Muñoz-Martin M, Blanco-Aparicio C, Pastor J, et al. (2016). Tissue damage and senescence provide critical signals for cellular reprogramming in vivo. Science 354. [DOI] [PubMed] [Google Scholar]
- Natali PG, Nicotra MR, Winkler AB, Cavaliere R, Bigotti A, and Ullrich A (1992). Progression of human cutaneous melanoma is associated with loss of expression of c-kit proto-oncogene receptor. Int J Cancer 52, 197–201. [DOI] [PubMed] [Google Scholar]
- Nissan X, Larribere L, Saidani M, Hurbain I, Delevoye C, et al. 2011. Functional melanocytes derived from human pluripotent stem cells engraft into pluristratified epidermis. Proc Natl Acad Sci U A. Sep 6;108(36):14861–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nukaya D, Minami K, Hoshikawa R, Yokoi N, Seino S. Preferential gene expression and epigenetic memory of induced pluripotent stem cells derived from mouse pancreas. Genes Cells. 2015. May;20(5):367–81. [DOI] [PubMed] [Google Scholar]
- Ohta S, Imaizumi Y, Okada Y, Akamatsu W, Kuwahara R, et al. 2011. Generation of human melanocytes from induced pluripotent stem cells. PLoS One. Jan 13;6(1):e16182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rahilly R, and Muller F (2007). The development of the neural crest in the human. J Anat 211, 335–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis P, and McCormick F (1993). Structural requirements for the interaction of p21ras with G.A.P., exchange factors, and its biological effector target. J Biol Chem 268, 9157–9160. [PubMed] [Google Scholar]
- Polo JM, Liu S, Figueroa ME, Kulalert W, Eminli S, et al. 2010. Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nat. Biotechnol 28, 848–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quek C, Rawson RV, Ferguson PM, Shang P, Silva I, Saw RPM, Shannon K, Thompson JF, Hayward NK, Long GV, et al. (2019). Recurrent hotspot SF3B1 mutations at codon 625 in vulvovaginal mucosal melanoma identified in a study of 27 Australian mucosal melanomas. Oncotarget 10, 930–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintanal-Villalonga A, Chan JM, Yu HA, Pe'er D, Sawyers CL, Sen T, and Rudin CM (2020) Lineage plasticity in cancer: a shared pathway of therapeutic resistance. Nat Rev Clin Oncol. 17: 360–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raaijmakers MI, Widmer DS, Narechania A, Eichhoff O, Freiberger SN, Wenzina J, Cheng PF, Mihic-Probst D, Desalle R, Dummer R, et al. (2016). Co-existence of BRAF and NRAS driver mutations in the same melanoma cells results in heterogeneity of targeted therapy resistance. Oncotarget 7, 77163–77174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff KJ, Jaju PD, Tang JY, Carbone M, Leachman S, and Sarin KY (2016). Familial skin cancer syndromes: Increased melanoma risk. J Am Acad Dermatol 74, 423–434; quiz 435-426. [DOI] [PubMed] [Google Scholar]
- Rapino F, Robles EF, Richter-Larrea JA, Kallin EM, Martinez-Climent JA, and Graf T (2013). C/EBPalpha induces highly efficient macrophage trans-differentiation of B lymphoma and leukemia cell lines and impairs their tumorigenicity. Cell Rep 3, 1153–1163. [DOI] [PubMed] [Google Scholar]
- Rastrelli M, Tropea S, Rossi CR, and Alaibac M (2014). Melanoma: epidemiology, risk factors, pathogenesis, diagnosis and classification. In Vivo 28, 1005–1011. [PubMed] [Google Scholar]
- Reddy BY, Miller DM, and Tsao H (2017). Somatic driver mutations in melanoma. Cancer 123, 2104–2117. [DOI] [PubMed] [Google Scholar]
- Ribas A, and Flaherty KT (2011). BRAF targeted therapy changes the treatment paradigm in melanoma. Nat Rev Clin Oncol 8, 426–433. [DOI] [PubMed] [Google Scholar]
- Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, Lichinitser M, Dummer R, Grange F, Mortier L, et al. (2015). Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 372, 30–39. [DOI] [PubMed] [Google Scholar]
- Roesch A, Fukunaga-Kalabis M, Schmidt EC, Zabierowski SE, Brafford PA, Vultur A, Basu D, Gimotty P, Vogt T, and Herlyn M (2010). A temporarily distinct subpopulation of slow-cycling melanoma cells is required for continuous tumor growth. Cell 141, 583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch A, Paschen A, Landsberg J, Helfrich I, Becker JC, and Schadendorf D (2016). Phenotypic tumour cell plasticity as a resistance mechanism and therapeutic target in melanoma. Eur J Cancer 59, 109–112. [DOI] [PubMed] [Google Scholar]
- Rose AM, Luo R, Radia UK, Kalirai H, Thornton S, Luthert PJ, Jayasena CN, Verity DH, Coupland SE, and Rose GE (2018). Detection of mutations in SF3B1, EIF1AX and GNAQ in primary orbital melanoma by candidate gene analysis. B.M.C. Cancer 18, 1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz S, Diep D, Gore A, Panopoulos AD, Montserrat N, et al. 2012. Identification of a specific reprogramming-associated epigenetic signature in human induced pluripotent stem cells. Proc Natl Acad Sci USA 109:16196–16201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadangi S, Milosavljevic K, Castro-Perez E, Lares M, Singh M, et al. , 2022. Role of the Skin Microenvironment in Melanomagenesis: Epidermal Keratinocytes and Dermal Fibroblasts Promote BRAF Oncogene-Induced Senescence Escape in Melanocytes. Cancers; 14(5):1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauka-Spengler T, and Bronner M (2010). Snapshot: neural crest. Cell 143, 486–486.e481. [DOI] [PubMed] [Google Scholar]
- Sauter ER, Yeo UC, von Stemm A, Zhu W, Litwin S, Tichansky DS, Pistritto G, Nesbit M, Pinkel D, Herlyn M, et al. (2002). Cyclin D1 is a candidate oncogene in cutaneous melanoma. Cancer Res 62, 3200–3206. [PubMed] [Google Scholar]
- Schatton T, and Frank MH (2008). Cancer stem cells and human malignant melanoma. Pigment Cell Melanoma Res 21, 39–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuermann JC, de Ayala Alonso AG, Oktaba K, Ly-Hartig N, McGinty RK, Fraterman S, Wilm M, Muir TW, and Muller J (2010). Histone H2A deubiquitinase activity of the Polycomb repressive complex PR-DUB. Nature 465, 243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, Bergethon K, Shaw AT, Gettinger S, Cosper AK, Akhavanfard S, Heist RS, Temel J, Christensen JG, Wain JC, Lynch TJ, Vernovsky K, Mark EJ, Lanuti M, Iafrate AJ, Mino-Kenudson M, and Engelman JA (2011) Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 3: 75ra26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrone L, Zeuli M, Sega FM, Cognetti F. 2000. Dacarbazine-based chemotherapy for metastatic melanoma: thirty-year experience overview. J Exp Clin Cancer Res. Mar;19(1):21–34. [PubMed] [Google Scholar]
- Shain AH, and Bastian BC (2016). From melanocytes to melanomas. Nat Rev Cancer 16, 345–358. [DOI] [PubMed] [Google Scholar]
- Shain AH, Yeh I, Kovalyshyn I, Sriharan A, Talevich E, Gagnon A, Dummer R, North J, Pincus L, Ruben B, et al. (2015). The Genetic Evolution of Melanoma from Precursor Lesions. N Engl J Med 373, 1926–1936. [DOI] [PubMed] [Google Scholar]
- Sharkis SJ, Jones RJ, Civin C, and Jang YY (2012). Pluripotent stem cell-based cancer therapy: promise and challenges. Sci Transl Med 4, 127ps129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaughnessy M, Klebanov N, and Tsao H (2018). Clinical and therapeutic implications of melanoma genomics. Journal of Translational Genetics and Genomics. [Google Scholar]
- Sheng X, Kong Y, Li Y, Zhang Q, Si L, Cui C, Chi Z, Tang B, Mao L, Lian B, et al. (2016). GNAQ and GNA11 mutations occur in 9.5% of mucosal melanoma and are associated with poor prognosis. Eur J Cancer 65, 156–163. [DOI] [PubMed] [Google Scholar]
- Shoushtari AN, and Carvajal RD (2014). GNAQ and GNA11 mutations in uveal melanoma. Melanoma Res 24, 525–534. [DOI] [PubMed] [Google Scholar]
- Siller R, Greenhough S, Park IH, and Sullivan GJ (2013). Modelling human disease with pluripotent stem cells. Curr Gene Ther 13, 99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Tobin DJ, Shibahara S, and Wortsman J (2004). Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev 84, 1155–1228. [DOI] [PubMed] [Google Scholar]
- Smalley KS, Lioni M, Dalla Palma M, Xiao M, Desai B, Egyhazi S, Hansson J, Wu H, King AJ, Van Belle P, et al. (2008). Increased cyclin D1 expression can mediate BRAF inhibitor resistance in BRAF V600E-mutated melanomas. Mol Cancer Ther 7, 2876–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider TN, and Mishina Y (2014). Cranial neural crest cell contribution to craniofacial formation, pathology, and future directions in tissue engineering. Birth Defects Res C Embryo Today 102, 324–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soengas MS, and Patton EE (2017). Location, Location, Location: Spatio-Temporal Cues That Define the Cell of Origin in Melanoma. Cell Stem Cell 21, 559–561. [DOI] [PubMed] [Google Scholar]
- Stricker SH, Feber A, Engstrom PG, Caren H, Kurian KM, et al. 2013. Widespread resetting of DNA methylation in glioblastoma-initiating cells suppresses malignant cellular behavior in a lineage-dependent manner. Genes Dev. 27:654–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suknuntha K, Ishii Y, Tao LH, Hu KJ, McIntosh BE, Yang D, Swanson S, Stewart R, Wang JYJ, Thomson J, et al. (2015). Discovery of survival factor for primitive chronic myeloid leukemia cells using induced pluripotent stem cells. Stem Cell Research 15, 678–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JM, Begum S, Shi C, Eshleman JR, and Westra WH (2009). Benign nodal nevi frequently harbor the activating V600E BRAF mutation. Am J Surg Pathol 33, 568–571. [DOI] [PubMed] [Google Scholar]
- Theveneau E, and Mayor R (2012). Neural crest delamination and migration: from epithelium-to-mesenchyme transition to collective cell migration. Dev Biol 366, 34–54. [DOI] [PubMed] [Google Scholar]
- Torres-Cabala CA, Wang WL, Trent J, Yang D, Chen S, Galbincea J, Kim KB, Woodman S, Davies M, Plaza JA, et al. (2009). Correlation between K.I.T. expression and K.I.T. mutation in melanoma: a study of 173 cases with emphasis on the acral-lentiginous/mucosal type. Mod Pathol 22, 1446–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoi J, Robert L, Paraiso K, Galvan C, Sheu KM, Lay J, Wong DJL, Atefi M, Shirazi R, Wang X, et al. (2018). Multi-stage Differentiation Defines Melanoma Subtypes with Differential Vulnerability to Drug-Induced Iron-Dependent Oxidative Stress. Cancer Cell 33, 890–904.e895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tough IM, Jacobs PA, Court-Brown WM, Baikie AG, Williamson ER. 1963. Cytogenetic studies on bone-marrow in chronic myeloid leukaemia. Lancet. Apr 20;1(7286):844–6. [DOI] [PubMed] [Google Scholar]
- Utikal J, Maherali N, Kulalert W, and Hochedlinger K (2009). Sox2 is dispensable for the reprogramming of melanocytes and melanoma cells into induced pluripotent stem cells. Journal of Cell Science 122, 3502–3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Raamsdonk CD, Bezrookove V, Green G, Bauer J, Gaugler L, O'Brien JM, Simpson EM, Barsh GS, and Bastian BC (2009). Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature 457, 599–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Raamsdonk CD, Griewank KG, Crosby MB, Garrido MC, Vemula S, Wiesner T, Obenauf AC, Wackernagel W, Green G, Bouvier N, et al. (2010). Mutations in GNA11 in uveal melanoma. N Engl J Med 363, 2191–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez Vde L, Vicente AL, Carloni A, Berardinelli G, Soares P, Scapulatempo C, Martinho O, and Reis RM (2016). Molecular profiling, including TERT promoter mutations, of acral lentiginous melanomas. Melanoma Res 26, 93–99. [DOI] [PubMed] [Google Scholar]
- Vinagre J, Almeida A, Populo H, Batista R, Lyra J, Pinto V, Coelho R, Celestino R, Prazeres H, Lima L, et al. (2013). Frequency of TERT promoter mutations in human cancers. Nat Commun 4, 2185. [DOI] [PubMed] [Google Scholar]
- Visvader JE. 2011. Cells of origin in cancer. Nature. Jan 20;469(7330):314–22. [DOI] [PubMed] [Google Scholar]
- Vultur A, and Herlyn M (2013). SnapShot: melanoma. Cancer Cell 23, 706–706.e701. [DOI] [PubMed] [Google Scholar]
- Wang L, Hiler D, Xu B, AlDiri I, Chen X, et al. 2018. Retinal Cell Type DNA Methylation and Histone Modifications Predict Reprogramming Efficiency and Retinogenesis in 3D Organoid Cultures. Cell Rep. 22, 2601–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters JM, Richardson GD, and Jahoda CA (2007). Hair follicle stem cells. Semin Cell Dev Biol 18, 245–254. [DOI] [PubMed] [Google Scholar]
- Wee S, Jagani Z, Xiang KX, Loo A, Dorsch M, Yao YM, Sellers WR, Lengauer C, and Stegmeier F (2009). PI3K pathway activation mediates resistance to M.E.K. inhibitors in KRAS mutant cancers. Cancer Res 69, 4286–4293. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Hunter MV, Cruz NM, Baggiolini A, Tagore M, et al. , 2022. Anatomic position determines oncogenic specificity in melanoma. Nature Apr;604(7905):354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellbrock C, and Arozarena I (2015). Microphthalmia-associated transcription factor in melanoma development and MAP-kinase pathway targeted therapy. Pigment Cell Melanoma Res 28, 390–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaar M, and Park H-Y (2012). Melanocytes: A Window into the Nervous System. Journal of Investigative Dermatology 132, 835–845. [DOI] [PubMed] [Google Scholar]
- Yang R, Jiang M, Kumar SM, Xu T, Wang F, et al. 2011. Generation of melanocytes from induced pluripotent stem cells. J Invest Dermatol. Dec;131(12):2458–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavuzyigitoglu S, Koopmans AE, Verdijk RM, Vaarwater J, Eussen B, van Bodegom A, Paridaens D, Kilic E, and de Klein A (2016). Uveal Melanomas with SF3B1 Mutations: A Distinct Subclass Associated with Late-Onset Metastases. Ophthalmology 123, 1118–1128. [DOI] [PubMed] [Google Scholar]
- Young HM, Anderson RB, and Newgreen DF (2009). Neural Crest. In Encyclopedia of Neuroscience, Squire LR, ed. (Oxford: Academic Press; ), pp. 123–133. [Google Scholar]
- Yu H, Kumar SM, Kossenkov AV, Showe L, and Xu X (2010). Stem cells with neural crest characteristics derived from the bulge region of cultured human hair follicles. J Invest Dermatol 130, 1227–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S, Norgard RJ, Stanger BZ. Cellular plasticity in cancer. Cancer Discov 2019;9:837–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Vandana JJ, Lacko L, Chen S. Modeling cancer progression using human pluripotent stem cell-derived cells and organoids. Stem Cell Res. Dec;49:102063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Cruz FD, Terry M, Remotti F, and Matushansky I (2013). Terminal differentiation and loss of tumorigenicity of human cancers via pluripotency-based reprogramming. Oncogene 32, 2249–2260, 2260.e2241-2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Abdouh M, Arena V, Arena M, and Arena GO (2017). Reprogramming Malignant Cancer Cells toward a Benign Phenotype following Exposure to Human Embryonic Stem Cell Microenvironment. PLoS One 12, e0169899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou M, Toivanen R, Mitrofanova A, Floch N, Hayati S, Sun Y, Le Magnen C, Chester D, Mostaghel EA, Califano A, Rubin MA, Shen MM, and Abate-Shen C (2017) Trans-differentiation as a Mechanism of Treatment Resistance in a Mouse Model of Castration-Resistant Prostate Cancer. Cancer Discov. 7: 736–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber M, Gestri G, Viczian A, Barsacchi G, Harris W. 2003. Specification of the vertebrate eye by a network of eye field transcription factors. Development 13:5155–5167. [DOI] [PubMed] [Google Scholar]