Abstract
Objective:
To examine sex differences in risk factors for anorexia nervosa (AN).
Method:
This population-based study involved 44,743 individuals (6,239 AN cases including 5,818 females and 421 males, and 38,504 controls including 18,818 females and 19,686 males) born in Denmark between May 1981 and December 2009. Follow-up began on the individual’s sixth birthday and ended at AN diagnosis, emigration, death, or 31st December 2016, whichever occurred first. Exposures included socioeconomic status (SES), pregnancy, birth, and early childhood factors based on data from Danish registers, and psychiatric and metabolic polygenic risk scores (PRS) based on genetic data. Hazard ratios were estimated using weighted Cox proportional hazards models stratified by sex (assigned at birth), with AN diagnosis as the outcome.
Results:
The effects of early life exposures and PRS on AN risk were comparable between females and males. Although we observed some differences in the strength and direction of effects, there were no significant interactions between sex and SES, pregnancy, birth, or early childhood exposures. The effects of most PRS on AN risk were highly similar between the sexes. We observed significant sex-specific effects of parental psychiatric history and body mass index PRS, though effects did not survive corrections for multiple comparisons.
Conclusions:
Risk factors for AN are comparable between females and males. Collaboration across countries with large registers is needed to further investigate sex-specific effects of genetic, biological, and environmental exposures on AN risk, including exposures in later childhood and adolescence as well as the additive effects of exposures.
Keywords: Anorexia nervosa, epidemiology, risk factors, polygenic risk, sex differences
Introduction
Anorexia nervosa (AN) is a severe mental disorder characterized by extremely low body weight, distorted body image, and intense fear of weight gain (Koch et al., 2022; Zipfel et al., 2015). AN is associated with medical complications (Momen et al., 2022; Schaumberg et al., 2017), psychiatric comorbidity (Schaumberg et al., 2017), and the highest relative mortality risk of any mental disorder besides substance use disorders (Chesney et al., 2014). Less than half of individuals with AN fully recover, with many remaining chronically ill or only partially recovered (Berkman et al., 2007; Watson & Bulik, 2013). Given the treatment challenges, morbidity, and mortality associated with AN, it is essential that risk factors for AN are well understood.
Previous studies have elucidated the complex interplay between genetics and environment in the etiology of AN (Bulik et al., 2015; Zipfel et al., 2015). Twin-based heritability estimates range from 48–74%, indicating that genetic factors explain a large proportion of phenotypic variance (Bulik et al., 2015). Several prenatal, perinatal, and early childhood factors are associated with increased AN risk, including shorter gestational age (Foley et al., 2001), older parental age (Javaras et al., 2017; Larsen et al., 2021), prematurity (Larsen et al., 2021; Raevuori et al., 2014), Caesarean section (C-section) (Larsen et al., 2021), and higher parental socioeconomic status (SES) (Koch et al., 2022). However, it is unclear whether associations between these exposures and AN risk are comparable between females and males. Since AN is diagnosed more frequently in females than in males (Zerwas et al., 2015), research on AN risk is often limited by small male samples, which precludes reliable conclusions about AN risk in males and perpetuates a female-centric understanding of AN. Although AN is diagnosed more frequently in females, AN in males can be more disabling (Wu et al., 2020) and have an earlier age of onset (Kinasz et al., 2016; Yilmaz et al., 2022). Sex differences in the prevalence and clinical presentation of AN warrant examination of sex-specific risk factors.
A review has concluded that genetic contributions to eating disorders (EDs) vary significantly between the sexes, with twin studies indicating that differential exposure to sex steroid hormones likely contributes to between-sex variation (Culbert et al., 2021). Sex-differentiated effects in genetic risk for AN have been investigated using polygenic risk scores (PRS), which provide an estimate of genetic liability to complex traits (Johnson et al., 2022). A genome-wide association study (GWAS) has shown no sex differences in the polygenic architecture of AN based on AN PRS (Watson et al., 2019), whereas a population-based study has demonstrated sex-specific effects of AN PRS on AN-related behaviours, though the sample size was small (Yilmaz et al., 2022). More research with larger samples is needed to examine sex-differentiated effects in the association between AN PRS and AN risk as well as other potentially relevant PRS for phenotypes that are genetically correlated with AN, like major depressive disorder (MDD), body mass index (BMI), and type 2 diabetes mellitus (T2DM) (Watson et al., 2019; Yilmaz et al., 2022).
Past research has also highlighted sex differences in prenatal and perinatal risk factors for AN. A Danish register-based study reported that amniotic fluid disorder during pregnancy was a risk factor for AN in males only, whereas older parental age, maternal genitourinary tract infections during pregnancy, maternal smoking during pregnancy, C-section, shorter gestational age, and congenital malformation were risk factors in females only (Larsen et al., 2021). A Swedish cohort study reported a significant linear association between paternal age at delivery and AN risk among women only (Javaras et al., 2017). An examination of genetic risk alongside these early life factors could provide valuable insights into the relative contribution of exposures.
This study examined sex differences in risk factors for AN by linking Danish register data on SES, pregnancy, birth, and early childhood exposures, with genetic data on psychiatric and metabolic PRS. Based on previous studies (Javaras et al., 2017; Larsen et al., 2021), we hypothesized that early life exposures would be more strongly associated with AN risk in females than males. We also expected sex-specific effects of PRS on AN risk.
Methods
Data Sources
This population-based study was conducted by linking data from Danish national registers. Every Danish citizen is assigned a registration number at birth or upon migration to Denmark, which facilitates data linkage across registers. Data on sex assigned at birth (henceforth referred to as sex), place of birth, dates of birth, death, and migration, and parents’ identity were obtained from the Danish Civil Registration System (CRS) (Pedersen, 2011), established in 1968. Data on diagnoses of mental disorders and medical conditions were obtained from the Danish National Patient Register (NPR) (Lynge et al., 2011) and the Danish Psychiatric Central Research Register (PCRR) (Mors et al., 2011). The NPR and PCRR have registered inpatient contacts since 1977 and 1969, respectively, and outpatient and emergency contacts since 1995. In these registers, diagnoses have been classified according to the International Classification of Diseases–Eighth Revision (ICD-8) until December 1993 and the Tenth Revision (ICD-10) since January 1994 (WHO, 2016).
Data on parental education and income were derived from the Danish Population Education Register (Jensen & Rasmussen, 2011) and the Danish Income Statistics Register (Baadsgaard & Quitzau, 2011), respectively. Data on pregnancy and birth outcomes were obtained from the Danish Medical Birth Register (MBR) (Bliddal et al., 2018). Data on childhood adversities were derived from the CRS, NPR, PCRR, Integrated Database for Labour Market Research (Petersson et al., 2011), and Register for Support for Children and Adolescents.
Genomic samples used to calculate PRS were obtained from the Danish Neonatal Screening Biobank, which stores dried blood spots collected during routine post-birth screening from nearly all infants born in Denmark since 1981 (Nørgaard-Pedersen & Hougaard, 2007). Principal components used in PRS calculation were obtained using the methods proposed by Privé et al. (2020) (Privé, Luu, et al., 2020). This study was approved by the Danish Data Protection Agency and Danish Health Data Authority.
Study Population
The study population included AN cases from the Danish branches of the Anorexia Nervosa Genetics Initiative (ANGI) (Thornton et al., 2018) and Eating Disorders Genetics Initiative (EDGI) (Bulik et al., 2021), as well as a randomly selected sub-cohort of controls from the iPSYCH2015 case-cohort sample (Bybjerg-Grauholm et al., 2020). The ANGI and EDGI cases included all individuals born in Denmark between 1981 and 2009, who were alive and residing in Denmark on their first birthday and diagnosed with AN (ICD-10: F50.0, F50.1) after their sixth birthday and before 31st December 2016. We only included ANGI and EDGI cases who had been diagnosed with AN after their sixth birthday. The iPSYCH2015 sub-cohort includes a random sample of individuals from a population of all singletons born in Denmark between 1981 and 2009, who were alive and residing in Denmark on their first birthday. Individuals in the sub-cohort who were diagnosed with AN after their sixth birthday and before 31st December 2016 were included as AN cases in this study. Individuals who had not been genotyped due to missing blood spots in the Biobank or whose genotype did not pass quality control were excluded. We only included individuals in the sub-cohort who were alive and residing in Denmark on their sixth birthday. Figure 1 presents the flow of case and control selection. Follow-up began on the individual’s sixth birthday and ended on the date the individual was diagnosed with AN, emigrated, died, or 31st December 2016, whichever date occurred first.
Figure 1.
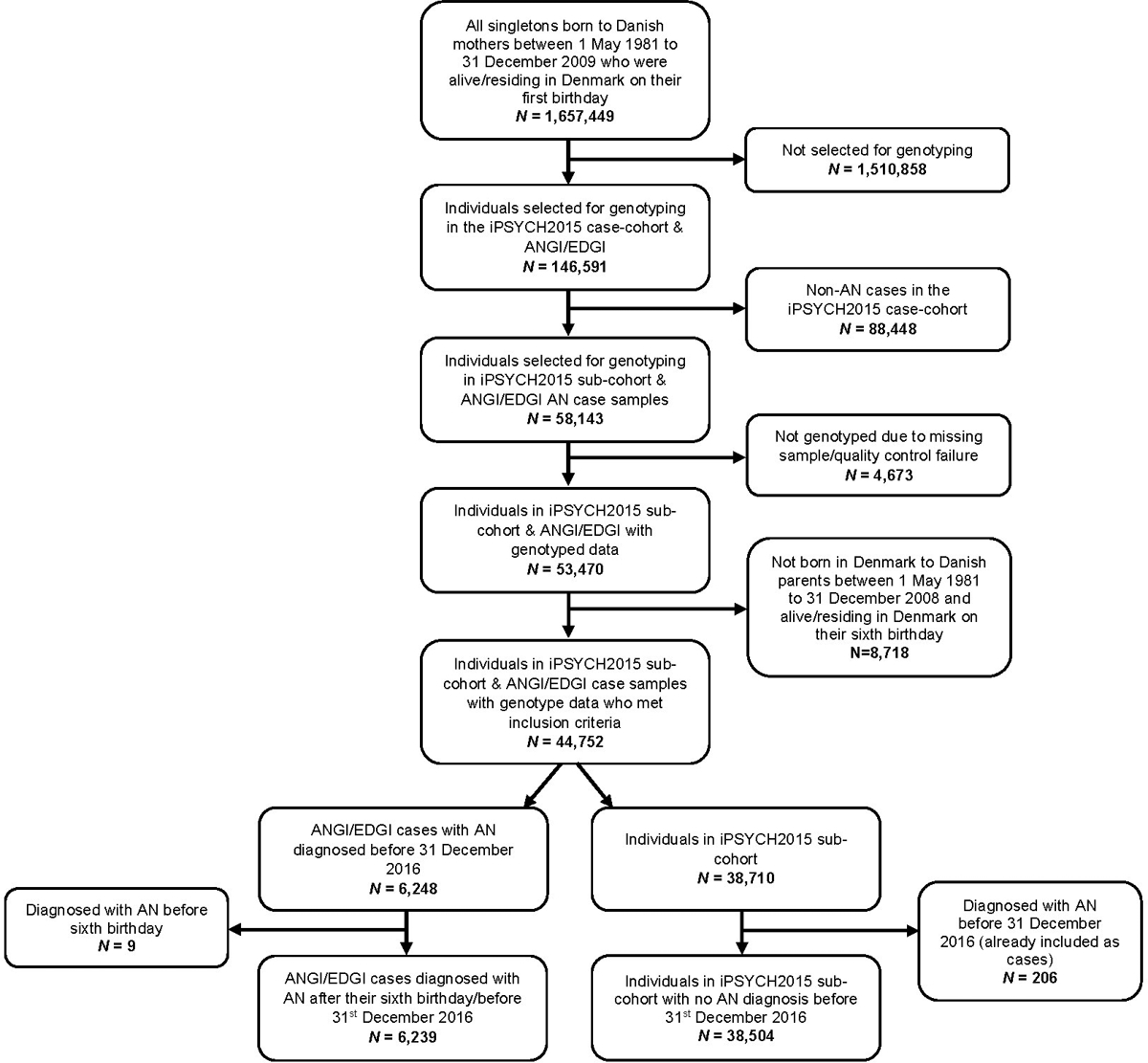
Flow of case and control selection.
Exposures
We examined several SES, pregnancy, birth, and early childhood variables, and relevant psychiatric and metabolic PRS (see Table 1) based on past studies showing sex-differentiated effects in these exposures on AN risk (Javaras et al., 2017; Larsen et al., 2021; Watson et al., 2019; Yilmaz et al., 2022). All exposures were defined at the start of follow-up (i.e., the individual’s sixth birthday) to ensure that exposures occurred prior to the outcome.
Table 1.
Definitions of Exposure Variables.
| Exposure | Description | Data Source (Register) | Levels |
|---|---|---|---|
| SES | |||
| Urbanicity | Municipality where the index person was born categorized according to degree of urbanization (Vassos et al., 2016) | CRS | Capital city (i.e., Copenhagen); Capital suburb; Provincial city (>100,000 residents); Provincial town (>10,000 residents); Rural area |
| Parental education level | Parents’ highest level of education in the year the index person turned 6 years old | Population Education Register | Elementary school; High school or vocational school; Academic degree |
| Parental income | Parents’ income in the year the index person turned 6 years old categorized according to the Danish population by age, calendar year, and sex | Income Statistics Register | First quintile; Second quintile; Third quintile; Fourth quintile; Fifth quintile |
| Parental Psychiatric History | |||
| Parental psychiatric history | Parent diagnosed with a mental disorder before the index person turned 6 years old | NPR/PCRR | Eating, depressive, or anxiety disorder; Mental disorder other than an eating, depressive, or anxiety disorder; No mental disorder |
| Pregnancy and Birth Factors | |||
| Maternal infection during pregnancy | Mother admitted with an infection in the 280 days prior to the index person’s birth | NPR | Yes; No |
| Gestational age | Index person’s gestational age | MBR | <35 weeks; 35–36 weeks; 37–41 weeks; ≥42 weeks |
| Birthweight | Index person’s birthweight | MBR | <2000 grams; 2000–2499 grams; 2500–3999 grams; ≥4000 grams |
| Maternal age at birth | Mother’s age at birth of the index person | CRS | <21 years; 21–24 years; 25–29 years; 30–34 years; ≥35 years |
| Paternal age at birth | Father’s age at birth of index person | CRS | <21 years; 21–24 years; 25–29 years; 30–34 years; 35–39 years; ≥40 years |
| Caesarean section | Index person delivered by Caesarean section | MBR | Yes; No |
| Congenital malformations | Index person diagnosed with any congenital malformation before they turned 6 years old* | NPR | Yes; No |
| Early Childhood Factors | |||
| Childhood infection | Index person admitted with an infection between birth and 6 years of age | NPR | Yes; No |
| Childhood Adversity Index | Number of adversities^ the index person was exposed to before 6 years of age | CRS, NPR, PCRR, Integrated Database for Labour Market Research, Register for Support for Children and Adolescents | No adversities; 1 adversity; ≥2 adversities |
| Polygenic risk scores (PRS) | |||
| AN, OCD, MDD, educational attainment, BMI, HDL cholesterol, and T2DM PRS | Index person’s calculated PRS | Danish Newborn Screening Biobank | (Continuous) |
Abbreviations: AN = Anorexia nervosa; BMI = Body mass index; CRS = Civil Registration System; HDL = High-density lipoprotein; MBR = Medical Birth Register; MDD = Major depressive disorder; NPR = National Patient Register; OCD = Obsessive-compulsive disorder; PCRR = Psychiatric Central Research Register; T2DM = Type 2 diabetes mellitus.
Data only available for individuals born after 1994.
Types of adversities include death of a parent, childhood abuse (defined by diagnostic codes related to neglect, abandonment, physical abuse, sexual abuse, psychological abuse, and other maltreatment), parent permanently leaving the workforce (defined by parent receiving a permanent pension), parental chronic somatic disease (defined by diagnostic codes related to the 18 somatic conditions in the Charlson Comorbidity Index), placement in out-of-home care, in-home care, and family disruption (defined as child not sharing an address with both legal parents).
SES variables.
Urbanicity, parental education level, and parental income were included as SES-related exposures.
Parental psychiatric history.
We had originally classified parental psychiatric history into three categories: 1) parent(s) with an ED; 2) parent(s) with a mental disorder other than an ED; and 3) parents with no mental disorders. However, there were too few parents with an ED to examine this category while complying with Danish legislation regarding personally identifiable information. Accordingly, we broadened these categories to include: 1) parent(s) with an ED (ICD-10: F50.x; ICD-8: 306.50, 306.58, 306.59) or a mental disorder known to co-occur with EDs (Keski-Rahkonen & Mustelin, 2016), including a depressive (ICD-10: F32-F39; ICD-8: 296.09, 296.29, 298.09, 300.49) or anxiety disorder (ICD-10: F40-F48; ICD-8: 300.09, 300.29, 300.39); 2) parent(s) with a mental disorder other than an eating, depressive, or anxiety disorder (ICD-10: F00-F31, F49, F51-F99; ICD-8: 290–315 excluding ICD-8 codes listed previously); or 3) parents with no mental disorders.
Pregnancy and birth variables.
Maternal infection during pregnancy (see ICD codes in Supplemental Table 1), gestational age, birthweight, parental age at birth, C-section, and congenital malformation (ICD-10: Q00-Q89; ICD-8: 740.0–759.9) were included as pregnancy- and birth-related exposures.
Early childhood variables.
Childhood infection (see Supplemental Table 1) and exposure to adversities were examined. We investigated seven childhood adversities (death of a parent, childhood abuse, parent permanently leaving the workforce, parental chronic somatic disease, placement in out-of-home care, in-home care, and family disruption), based on these events being highlighted as risk factors for other mental disorders (Dahl et al., 2017; Debost et al., 2019). Details on how these adversities were assessed are summarized in Table 1 and detailed in Larsen et al. (2021). Adversities were coded dichotomously depending on the individual’s exposure, then the total number of adversities were summed to calculate the Childhood Adversity Index (Debost et al., 2019).
Polygenic risk scores.
Several PRS were examined as genetic exposures, including AN PRS (Watson et al., 2019) and PRS for phenotypes that are genetically correlated with AN, including obsessive-compulsive disorder (OCD) (IOCDF-GC/OCGAS, 2018), MDD (Wray et al., 2018), educational attainment (Lee et al., 2018), BMI (Yengo et al., 2018), high-density lipoprotein (HDL) cholesterol (Spracklen et al., 2017), and T2DM (Xue et al., 2018).
Outcome
The outcome was a diagnosis of AN (ICD-10: F50.0, F50.1) in the NPR or PCRR. Date of onset was defined as the admission date for the individual’s first inpatient or outpatient contact after their sixth birthday leading to a discharge diagnosis of AN.
Statistical Analysis
Individuals with missing data on paternal education (3.3%), maternal education (2.2%), paternal income (0.8%), maternal income (0.3%), and birthweight (0.9%) were excluded from analyses. Hazard ratios (HRs) with 95% confidence intervals (CIs) were estimated using sex-stratified weighted Cox proportional hazards models with age as the underlying time scale. Wald tests were performed assuming the same effect from one level of the exposure to the level above, and p-values were reported. Given the small male sample, we conducted post-hoc power calculations (Chow et al., 2017) that revealed this sample was powered to detect HRs between 1.27 (with 50% exposed to a risk factor) and 1.50 (with 10% exposed), based on a total male sample of N=20,107 and an overall AN diagnosis probability of 0.0209. Additional Wald tests were conducted to examine interactions between sex and exposures. All analyses were adjusted for birth year (≤1994, 1995–1999, 2000–2004, ≥2005).
PRS were calculated based on summary statistics from GWASs of the selected discovery traits (IOCDF-GC/OCGAS, 2018; Lee et al., 2018; Watson et al., 2019; Wray et al., 2018; Yengo et al., 2018). The genotyping and imputation of variants is detailed elsewhere (Bybjerg-Grauholm et al., 2020; Pedersen et al., 2018). The number of single nucleotide polymorphisms (SNPs) was restricted to the HapMap3 set of variants provided by the European-ancestry linkage disequilibrium (LD) reference in LDpred2 (Privé, Arbel, et al., 2020), with 1,054,330 SNPs. PRS were derived using LDpred2-auto (Privé, Arbel, et al., 2020). PRS were standardized by calculating the mean and standard deviation in the random sub-cohort: (observed value – mean) / standard deviation. Associations between PRS and AN risk were estimated using weighted Cox models adjusted for the first ten genomic principal components to account for population stratification and the origin of the sample. Inverse probability weights (Kalbfleisch & Lawless, 1988) were used to adjust for the iPSYCH2015 case-cohort design and sampling scheme, where AN cases were more likely to be selected for genotyping than non-cases (approximately 1:34) (Bybjerg-Grauholm et al., 2020). Analyses were conducted in R Version 4.1.1.
Results
The study population comprised 44,743 individuals, including 6,239 AN cases and 38,504 controls. Of the AN cases, 93.3% were female (n=5,818) and 6.8% were male (n=421). In the control group, 48.9% were female (n=18,818) and 51.1% were males (n=19,686). Age at AN diagnosis ranged from 6 to 35 years (M=18, SD=4) among female cases, and 7 to 33 years (M=16, SD=4) among male cases. Estimated HRs with 95% CIs for associations between exposures and AN diagnosis by sex are presented in Table 2. Estimates in the non-stratified sample are provided in Supplemental Table 2. Estimates when all exposures were entered into the model simultaneously are provided in Supplemental Table 3. When exposures were entered simultaneously, gestational age <35 weeks (HR=1.44 [95% CI=0.98–2.11]), AN PRS (1.38 [1.33–1.42]), living in a provincial city (1.32 [1.19–1.46]), and parental history of an eating, depressive, or anxiety disorder (1.32 [1.12–1.55]) were most strongly associated with increased AN risk in females. Among males, we observed the largest associations with gestational age <35 weeks (2.44 [0.96–6.22]), paternal age <21 years (1.89 [0.83–4.30]), and parental history of a mental disorder other than an eating, depressive, or anxiety disorder (1.79 [1.25–2.57).
Table 2.
Estimated hazard ratios (HRs) and 95% confidence intervals (CIs) for associations between exposures and anorexia nervosa among individuals born in Denmark between 1981 and 2009.
| Males | Females | Sex Difference | Sex Difference | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposure | n controls | n cases | HR | 95% CI | p-value* | n controls | n cases | HR | 95% CI | p-value* | p-value* | Adjusted p-value^ |
| Socioeconomic Status | ||||||||||||
| Maternal Education Level | ||||||||||||
| Elementary school | 4,934 (25.1%) | 92 (21.9%) | 1 | (ref) | 4,597 (24.4%) | 1327 (22.8%) | 1 | (ref) | ||||
| High school or vocational school | 8,257 (41.9%) | 175 (41.6%) | 1.29 | (0.99, 1.68) | 7,903 (42.0%) | 2349 (40.4%) | 1.20 | (1.11, 1.29) | ||||
| Academic degree | 6,252 (31.8%) | 154 (36.6%) | 1.61 | (1.23, 2.11) | .002 | 6,124 (32.5%) | 2071 (35.6%) | 1.49 | (1.37, 1.62) | <.001 | .413 | .640 |
| Paternal Education Level | ||||||||||||
| Elementary school | 4,278 (21.7%) | 83 (19.7%) | 1 | (ref) | 4,111 (21.8%) | 1124 (19.3%) | 1 | (ref) | ||||
| High school or vocational school | 9,627 (48.9%) | 198 (47.0%) | 1.10 | (0.85, 1.43) | 9,285 (49.3%) | 2738 (47.1%) | 1.17 | (1.08, 1.27) | ||||
| Academic degree | 5,318 (27.0%) | 135 (32.1%) | 1.44 | (1.09, 1.90) | .018 | 5,016 (26.7%) | 1835 (31.5%) | 1.57 | (1.44, 1.71) | <.001 | .985 | .985 |
| Paternal Income Quintile | ||||||||||||
| 1st quintile | 2,169 (11.0%) | 47 (11.2%) | 1 | (ref) | 2,063 (11.0%) | 565 (9.7%) | 1 | (ref) | ||||
| 2nd quintile | 3,513 (17.8%) | 67 (15.9%) | 0.91 | (0.62, 1.33) | 3,281 (17.4%) | 905 (15.6%) | 1.04 | (0.92, 1.18) | ||||
| 3rd quintile | 4,245 (21.6%) | 83 (19.7%) | 0.94 | (0.65, 1.36) | 4,113 (21.9%) | 1233 (21.2%) | 1.16 | (1.04, 1.31) | ||||
| 4th quintile | 4,696 (23.9%) | 97 (23.0%) | 1.01 | (0.71, 1.44) | 4,420 (23.5%) | 1352 (23.2%) | 1.21 | (1.08, 1.36) | ||||
| 5th quintile | 4,938 (25.1%) | 127 (30.2%) | 1.25 | (0.89, 1.76) | .167 | 4,831 (25.7%) | 1730 (29.7%) | 1.41 | (1.26, 1.57) | <.001 | .850 | .969 |
| Maternal Income Quintile | ||||||||||||
| 1st quintile | 2,306 (11.7%) | 45 (10.7%) | 1 | (ref) | 2,176 (11.6%) | 687 (11.8%) | 1 | (ref) | ||||
| 2nd quintile | 3,952 (20.1%) | 95 (22.6%) | 1.25 | (0.87, 1.79) | 3,776 (20.1%) | 1086 (18.7%) | 0.95 | (0.85, 1.06) | ||||
| 3rd quintile | 4,636 (23.5%) | 101 (24.0%) | 1.17 | (0.82, 1.67) | 4,326 (23.0%) | 1259 (21.6%) | 0.98 | (0.88, 1.09) | ||||
| 4th quintile | 4,410 (22.4%) | 81 (19.2%) | 0.99 | (0.68, 1.43) | 4,361 (23.2%) | 1330 (22.9%) | 1.05 | (0.94, 1.17) | ||||
| 5th quintile | 4,341 (22.1%) | 99 (23.5%) | 1.23 | (0.86, 1.76) | .424 | 4,142 (22.0%) | 1441 (24.8%) | 1.21 | (1.09, 1.34) | <.001 | .246 | .640 |
| Urbanicity | ||||||||||||
| Rural area | 7,086 (36.0%) | 144 (34.2%) | 1 | (ref) | 6,799 (36.1%) | 1838 (31.6%) | 1 | (ref) | ||||
| Capital | 2,254 (11.4%) | 57 (13.5%) | 1.28 | (0.94, 1.75) | 2,023 (10.8%) | 752 (12.9%) | 1.48 | (1.34, 1.64) | ||||
| Capital suburb | 2,484 (12.6%) | 48 (11.4%) | 0.94 | (0.68, 1.31) | 2,412 (12.8%) | 848 (14.6%) | 1.32 | (1.20, 1.45) | ||||
| Provincial city | 2,300 (11.7%) | 58 (13.8%) | 1.25 | (0.92, 1.70) | 2,219 (11.8%) | 833 (14.3%) | 1.44 | (1.31, 1.59) | ||||
| Provincial town | 5,523 (23.1%) | 114 (27.1%) | 1.01 | (0.79, 1.29) | .302 | 5,343 (28.4%) | 1547 (26.6%) | 1.10 | (1.02, 1.19) | <.001 | .427 | .640 |
| Parental Psychiatric Diagnosis | ||||||||||||
| No disorder | 13,795 (70.1%) | 360 (85.5%) | 1 | (ref) | 16,753 (89.0%) | 5215 (89.6%) | 1 | (ref) | ||||
| Eating, depressive, or anxiety disorder | 5,080 (25.8%) | 24 (5.7%) | 1.45 | (0.95, 2.22) | 1,127 (6.0%) | 276 (4.7%) | 1.35 | (1.17, 1.57) | ||||
| Other mental disorder | 1,003 (5.1%) | 37 (8.8%) | 1.60 | (1.13, 2.26) | .009 | 938 (5.0%) | 327 (5.6%) | 1.01 | (0.89, 1.16) | <.001 | .024 | .361 |
| Pregnancy and Birth Factors | ||||||||||||
| Gestational Age | ||||||||||||
| <35 weeks | 304 (1.5%) | 12 (2.9%) | 1.95 | (1.09, 3.52) | 285 (1.5%) | 103 (1.8%) | 1.22 | (0.97, 1.54) | ||||
| 35–36 weeks | 605 (3.1%) | 13 (3.1%) | 1.01 | (0.58, 1.77) | 505 (2.7%) | 148 (2.5%) | 1.00 | (0.83, 1.21) | ||||
| 37–41 weeks | 17,053 (86.6%) | 367 (87.2%) | 1 | (ref) | 16,330 (86.8%) | 5073 (87.2%) | 1 | (ref) | ||||
| ≥42 weeks | 1,724 (8.8%) | 29 (6.9%) | 0.75 | (0.51, 1.10) | .059 | 1,698 (9.0%) | 494 (8.5%) | 0.88 | (0.79, 0.98) | .031 | .342 | .640 |
| Maternal Age at Birth | ||||||||||||
| <21 years | 733 (3.7%) | 19 (4.5%) | 1.17 | (0.72, 1.89) | 702 (3.7%) | 170 (2.9%) | 0.65 | (0.55, 0.78) | ||||
| 21–24 years | 3,202 (16.3%) | 60 (14.3%) | 0.86 | (0.64, 1.17) | 3,095 (16.4%) | 891 (15.3%) | 0.79 | (0.72, 0.86) | ||||
| 25–29 years | 7,715 (39.2%) | 157 (37.3%) | 1 | (ref) | 7,234 (38.4%) | 2372 (40.8%) | 1 | (ref) | ||||
| 30–34 years | 5,790 (29.4%) | 136 (32.3%) | 1.21 | (0.96, 1.53) | 5,566 (29.6%) | 1763 (30.3%) | 1.07 | (0.99, 1.15) | ||||
| ≥35 years | 2,246 (11.4%) | 49 (11.6%) | 1.18 | (0.85, 1.63) | .203 | 2,221 (11.8%) | 622 (10.7%) | 0.99 | (0.90, 1.1) | <.001 | .192 | .640 |
| Paternal Age at Birth | ||||||||||||
| <21 years | 239 (1.2%) | 8 (1.9%) | 1.68 | (0.81, 3.48) | 262 (1.4%) | 60 (1.0%) | 0.68 | (0.51, 0.91) | ||||
| 21–24 years | 1,662 (8.4%) | 38 (9.0%) | 1.12 | (0.77, 1.62) | 1,620 (8.6%) | 448 (7.7%) | 0.82 | (0.72, 0.92) | ||||
| 25–29 years | 6,156 (31.3%) | 120 (28.5%) | 1 | (ref) | 5,914 (31.4%) | 1870 (32.1%) | 1 | (ref) | ||||
| 30–34 years | 6,808 (34.6%) | 150 (35.6%) | 1.18 | (0.92, 1.5) | 6,407 (34.0%) | 1989 (34.2%) | 1.08 | (1.01, 1.17) | ||||
| 35–39 years | 3,427 (17.4%) | 67 (15.9%) | 1.08 | (0.80, 1.47) | 3,249 (17.3%) | 1008 (17.3%) | 1.12 | (1.03, 1.23) | ||||
| ≥40 years | 1,394 (7.1%) | 38 (9.0%) | 1.47 | (1.02, 2.14) | .315 | 1,366 (7.3%) | 443 (7.6%) | 1.16 | (1.03, 1.31) | <.001 | .177 | .640 |
| Caesarean Section | ||||||||||||
| No | 17,588 (89.3%) | 384 (91.2%) | 1 | (ref) | 16,911 (89.9%) | 5312 (91.3%) | 1 | (ref) | ||||
| Yes | 2,098 (10.7%) | 37 (8.8%) | 0.94 | (0.67, 1.32) | .720 | 1,907 (10.1%) | 506 (8.7%) | 1.16 | (1.04, 1.29) | .008 | .733 | .917 |
| Birthweight | ||||||||||||
| <2000 g | 210 (1.1%) | 7 (1.7%) | 1.65 | (0.77, 3.54) | 219 (1.2%) | 67 (1.2%) | 1.02 | (0.77, 1.35) | ||||
| 2000–2499 g | 405 (2.1%) | 9 (2.1%) | 1.04 | (0.53, 2.04) | 476 (2.5%) | 159 (2.7%) | 1.06 | (0.88, 1.28) | ||||
| 2500–3999 g | 14,678 (74.6%) | 312 (74.1%) | 1 | (ref) | 15,287 (81.2%) | 4760 (81.8%) | 1 | (ref) | ||||
| ≥4000 g | 1,724 (8.8%) | 93 (22.1%) | 1.03 | (0.82, 1.30) | .638 | 2,795 (14.9%) | 810 (13.9%) | 0.99 | (0.91, 1.08) | .934 | .605 | .825 |
| Maternal Infection During Pregnancy | ||||||||||||
| No | 18,944 (96.2%) | 409 (97.1%) | 1 | (ref) | 18,126 (96.3%) | 5610 (96.4%) | 1 | (ref) | ||||
| Yes | 742 (3.8%) | 12 (2.9%) | 0.81 | (0.45, 1.44) | .468 | 692 (3.7%) | 208 (3.6%) | 1.11 | (0.94, 1.31) | .220 | .366 | .640 |
| Congenital Malformations | ||||||||||||
| No | 18,133 (92.1%) | 388 (92.2%) | 1 | (ref) | 17,778 (94.5%) | 5559 (95.5%) | 1 | (ref) | ||||
| Yes | 1,553 (7.9%) | 33 (7.8%) | 1.07 | (0.75, 1.53) | .709 | 1,040 (5.5%) | 259 (4.5%) | 0.94 | (0.81, 1.08) | .376 | .324 | .640 |
| Childhood Factors | ||||||||||||
| Childhood Infection | ||||||||||||
| No | 14,366 (73%) | 305 (72.4%) | 1 | (ref) | 14,748 (78.4%) | 4548 (78.2%) | 1 | (ref) | ||||
| Yes | 5,320 (27%) | 116 (27.6%) | 1.07 | (0.86, 1.33) | .524 | 4,070 (21.6%) | 1270 (21.8%) | 1.13 | (1.05, 1.22) | .001 | .904 | .969 |
| Childhood Adversity Index | ||||||||||||
| No adversities | 13,795 (70.1%) | 285 (67.7%) | 1 | (ref) | 13,129 (59.8%) | 4061 (69.8%) | 1 | (ref) | ||||
| 1 adversity | 5,080 (25.8%) | 122 (29.0%) | 1.16 | (0.94, 1.44) | 4,957 (26.3%) | 1536 (26.4%) | 0.99 | (0.92, 1.06) | ||||
| ≥2 adversities | 811 (4.1%) | 14 (3.3%) | 0.83 | (0.48, 1.43) | .275 | 732 (3.9%) | 221 (3.8%) | 1.04 | (0.89, 1.22) | .797 | .278 | .640 |
|
Polygenic Risk Scores
|
||||||||||||
| Anorexia nervosa | 19,686 (100%) | 421 (100%) | 1.48 | (1.33, 1.64) | <.001 | 18,818 (100%) | 5,818 (100%) | 1.45 | (1.40, 1.49) | <.001 | .111 | .111 |
| Obsessive-compulsive disorder | 19,686 (100%) | 421 (100%) | 1.11 | (1.01, 1.23) | .034 | 18,818 (100%) | 5,818 (100%) | 1.16 | (1.12, 1.19) | <.001 | .086 | .111 |
| Major depressive disorder | 19,686 (100%) | 421 (100%) | 1.14 | (1.03, 1.25) | .011 | 18,818 (100%) | 5,818 (100%) | 1.11 | (1.08, 1.15) | <.001 | .109 | .111 |
| Educational attainment | 19,686 (100%) | 421 (100%) | 1.24 | (1.11, 1.37) | <.001 | 18,818 (100%) | 5,818 (100%) | 1.19 | (1.15, 1.22) | <.001 | .060 | .111 |
| Body mass index | 19,686 (100%) | 421 (100%) | 0.83 | (0.76, 0.92) | <.001 | 18,818 (100%) | 5,818 (100%) | 0.77 | (0.75, 0.80) | <.001 | .036 | .111 |
| High-density lipoprotein cholesterol | 19,686 (100%) | 421 (100%) | 1.02 | (0.92, 1.13) | .722 | 18,818 (100%) | 5,818 (100%) | 1.04 | (1.01, 1.07) | .019 | .068 | .111 |
| Type 2 diabetes | 19,686 (100%) | 421 (100%) | 0.92 | (0.84, 1.02) | .106 | 18,818 (100%) | 5,818 (100%) | 0.91 | (0.89, 0.94) | <.001 | .062 | .111 |
Bolded HRs indicate statistically significant effects.
P-values derived from Wald tests.
P-values with corrections for multiple testing using the Benjamini-Hochberg procedure.
SES
Overall, sex differences in the effect of SES were not significant (p=.246-.985). In both sexes, AN risk increased with each level of maternal education (males: HRs=1.29–1.61, p=.002; females: HRs=1.20–1.49, p <.001) and paternal education (males: HRs=1.10–1.44, p=.018; females: HRs=1.17–1.57, p <.001). We observed a significant linear association between paternal income and AN risk in females (HRs=1.04–1.41, p <.001). Among males, we observed decreased risk with paternal income in the second and third quintiles (HRs=0.91–0.94, ps >.05) and increased risk in the fourth and fifth quintiles (HRs=1.01–1.25, ps >.05). There was a similar pattern between maternal income and AN risk in females: decreased risk in the second and third quintiles (HRs=0.95–0.98; ps >.05), and increased risk in the fourth and fifth quintiles (HRs=1.05–1.21; p=.406 and p <.001, respectively). Among males, AN risk increased with each maternal income quintile (HRs=1.17–1.25, ps <.05) besides the fourth quintile (0.99 [0.68–1.43]). Females who lived in the capital, a capital suburb, a provincial city, or a provincial town had significantly higher risk of AN (HRs=1.10–1.48, p >.001) than those living in a rural area. A similar trend was seen in males (HRs=1.01–1.28, p=.302), besides those living in a capital suburb (0.94 [0.68–1.31]).
Parental Psychiatric History
Having a parent with a mental disorder was a significant risk factor for AN in both females (p <.001) and males (p=.009). We observed a stronger effect of parental history of an eating, depressive, or anxiety disorder in males (1.45 [0.95–2.22]) than females (1.35 [1.17–1.57]), and a large effect of parental history of a mental disorder other than an eating, depressive, or anxiety disorder in males (1.60 [1.13–2.26]) that was not present in females (1.01 [0.89–1.16]). These differences could explain the significant interaction between sex and parental psychiatric history (p=.024), though this interaction did not survive multiple testing corrections (p=.361).
Pregnancy and Birth Factors
Overall, sex differences in the effects of pregnancy and birth factors were not significant (p=.177-.733). Gestational age was inversely associated with AN risk in both sexes; AN risk increased with gestational age <35 weeks (females: 1.22 [0.97–1.54]; males: 1.95 [1.09–3.52]) and decreased with gestational age ≥42 weeks (females: 0.88 [0.79–0.98]; males: 0.75 [0.51–1.10]). Among females, we observed significantly decreased risk of AN with maternal age <25 years (HRs=0.65–0.79, ps <.001), slightly increased risk with maternal age 30–34 years (1.07 [0.99–1.15]), and no effect of maternal age ≥35 years (0.99 [0.90–1.10]). Among males, we observed increased risk of AN in all maternal age groups (HRs=1.07–1.21, ps <.05) compared with maternal age 25–29 years, besides maternal age 21–24 years (0.86 [0.64–1.17]).
We observed a significant linear association between paternal age at birth and AN risk in females, with decreased risk with paternal age <25 years (HRs=0.68–0.87; ps <.05) and increased risk with paternal age >30 years (HRs=1.08–1.16, ps <.05). In males, AN risk was increased with all paternal age groups (HRs=1.08–1.68, p=.315) compared with paternal age 25–29 years. C-section was associated with significantly increased risk of AN in females (1.16 [1.04–1.29]) and slightly reduced risk in males (0.94 [0.67–1.32]), although CIs were wide and included unity. In both sexes, we observed increased risk of AN with birthweight <2,500g (males: HRs=1.04–1.65, ps >.05; females: HRs=1.02–1.06, ps >.05). Maternal infection during pregnancy was associated with increased AN risk in females (1.11 [0.94–1.31]) and decreased risk in males (0.81 [0.45–1.44]). Although the direction of effect was opposite, both CIs were wide and contained unity. Finally, congenital malformation was associated with decreased AN risk in females (0.94 [0.81–1.08]) and increased risk in males (1.07 [0.75–1.53]), though CIs were wide and included unity.
Early Childhood Factors
Sex differences in early childhood exposures were not significant (p=.278-.904). Childhood infection was associated with increased risk of AN in both females (1.13 [1.05–1.22]) and males (1.07 [0.86–1.33]). HRs indicate a positive association with one childhood adversity in males (1.16 [0.94–1.44]) but not females (0.99 [0.92–1.06]), whereas ≥2 adversities were associated with decreased risk in males (0.83 [0.48–1.43]) and slightly increased risk in females (1.04 [0.89–1.22]). Although the direction of effect differed between the sexes, CIs overlapped.
PRS
Apart from BMI PRS, sex differences in the effects of PRS were not significant (p=0.060–0.111). Effect sizes for PRS were highly similar between the sexes. In both females and males, AN risk was significantly higher with each 1 SD increase in AN PRS (females: 1.45 [1.40–1.49]; males: 1.48 [1.33–1.63]), OCD PRS (females: 1.16 [1.12–1.19]; males: 1.11 [1.01–1.23]), MDD PRS (females: 1.11 [1.08–1.15]; males: 1.14 [1.03–1.25]), and educational attainment PRS (females: 1.19 [1.15–1.22]; males: 1.24 [1.11–1.37]), and significantly decreased with BMI PRS (females: 0.77 [0.75–0.80]; males: 0.83 [0.76–0.92]. We observed a significant interaction between sex and BMI PRS, which could be explained by the stronger negative association in females than males, though this interaction did not survive multiple testing corrections (p=.111). In both sexes, we observed comparable associations between HDL cholesterol PRS and increased AN risk (females: 1.04 [1.01–1.07]; males: 1.02 [0.92–1.13]) as well as T2DM PRS and decreased AN risk (females: 0.91 [0.89–0.94]; males: 0.92 [0.84–1.02]), though these effects were only significant in females.
Discussion
To the best of our knowledge, this represents the first study of interactions between sex and risk factors for AN in a nationwide sample. Overall, the results indicate that risk factors for AN are comparable between females and males. There were no significant interactions between sex and SES, pregnancy, birth, or early childhood exposures. For both sexes, AN risk generally increased with higher parental education, parental income, and urbanicity. Gestational age was inversely associated with AN risk in both sexes. Among females, parental age <25 years at birth was associated with decreased AN risk and parental age >30 years with increased risk, whereas we generally observed increased AN risk in both parental age <25 and >30 years among males. The direction of effects for C-section, maternal infection, and congenital malformation differed between females and males, though sex differences remained non-significant. Childhood infection was associated with increased risk of AN in both females and males, whereas childhood adversity was differentially (though not significantly) associated with AN between the sexes. Supplemental Material 4 presents further discussion of significant effects in sex-stratified analyses.
Consistent with studies demonstrating familial coaggregation between AN and other mental disorders (Duncan et al., 2017; Koch et al., 2015; Watson et al., 2019; Zhang et al., 2021), we found that parental history of a mental disorder was a significant risk factor for AN in both sexes, with larger effects in males. We observed a significant interaction between sex and parental psychiatric history, which was likely explained by the large effect of parental history of a mental disorder other than an eating, depressive, or anxiety disorder in males that was not present in females. This effect is relatively novel but consistent with a cohort study showing a higher incidence of EDs in males than females when parents had substance abuse or somatoform disorders (Bould et al., 2015). However, this interaction did not survive multiple testing corrections. It is possible that the categories used to classify parental psychiatric history could have impacted on these results. Further research with larger male samples is needed to examine which parental disorders are differentially related to AN risk, which could be achieved via collaboration between countries with nationwide registers. The effects of PRS on AN risk were highly similar between the sexes. In females and males, AN risk was higher with each 1 SD increase in PRS for AN, OCD, MDD, educational attainment, and HDL cholesterol, and lower with each 1 SD increase in T2DM and BMI PRS. Overall, the results imply that associations between these PRS and AN risk are comparable between females and males. In relation to AN PRS, this result is consistent with genetic studies (Watson et al., 2019; Yilmaz et al., 2022) and implies that, based on current GWAS data, there is no evidence to suggest that the genetic architecture of AN differs between the sexes. AN PRS were calculated using summary statistics derived in a predominantly female cohort, which could be a limitation. The results show a positive association between HDL cholesterol PRS and AN risk in both sexes (albeit marginally, HRs=1.02–1.04), in contrast to a GWAS reporting a genetic correlation in the opposite direction (rg = −0.24) (Watson et al., 2019). We observed a significant interaction between sex and BMI PRS, which was likely driven by the stronger negative association between BMI PRS and AN risk in females. This result provides support for the hypothesis that genomic variation influencing body composition and AN liability may be differentially active in females (Hübel et al., 2019). However, this interaction between sex and BMI PRS did not survive multiple testing corrections.
A key strength of this study is the focus on sex-specific risk factors for AN, an understudied topic that has important clinical implications for early identification and prevention. Some studies on AN risk have been conducted with females and males collapsed across analyses (Koch et al., 2022) or with analyses on female data only (Brown et al., 2020), which has precluded investigation of sex-specific risk factors. Another strength of this study is the use of Danish national registers, which provide data on all Danish citizens from birth to death, thus eliminating loss to follow-up and selection and reporting biases. Diagnoses in the Danish registers are known to be reliable with high positive predictive values (Bock et al., 2009); thus, we can be confident in the validity of mental disorders and medical conditions determined in this study. The validity of Danish education and income registers are very high (Jensen & Rasmussen, 2011), as are standard measures (e.g., gestational age) and well-defined procedures (e.g., C-section) in the MBR (Bliddal et al., 2018). Conversely, childhood adversity is likely underreported so register data may only reflect more severe cases (Bengtsson et al., 2020); these results should be interpreted with caution. Register-based hospital contacts used to define AN reflect the date of diagnosis rather than the true date of onset. The registers only record hospital-based diagnoses, which likely reflect more severe AN cases and may not capture individuals with subthreshold disorders, those who do not seek treatment, and individuals undergoing treatment in primary care and private services. This could also influence associations with SES-related exposures as some effects (e.g., parental education) could be explained by high-resourced groups being more likely to access treatment (Sonneville & Lipson, 2018), though this may be less likely in Denmark where healthcare is free.
This study was limited with respect to the small male sample, though this was expected given that AN is diagnosed more frequently in females (Hudson et al., 2007; Watson et al., 2021). Nonetheless, diagnostic criteria may not adequately represent how EDs manifest in males, since EDs have historically been viewed as disorders primarily affecting females (Sangha et al., 2019). Baker et al. (2009) suggest that females and males express EDs at different thresholds, which may not be captured by assessment tools such as the Eating Disorder Inventory (EDI) (Baker et al., 2009), which emphasizes body areas that females are more likely to express dissatisfaction with. These sex differences could impact on the detection and treatment of AN, as supported by a Finnish cohort study demonstrating that males with EDs were much less likely to receive treatment (Silén et al., 2021). It may be that stronger sex differences emerge with environmental and biological exposures in late childhood and adolescence. For example, AN risk may be more heavily influenced by sociocultural factors such as weight-related societal pressure in adolescent females compared with adolescent males (Kinasz et al., 2016; McCabe & Ricciardelli, 2005). Pubertal status and timing are also known to play a much larger role in AN risk among adolescent girls than boys (Klump, 2013; Klump et al., 2012), though we were unable to examine this in the current study. Future studies should tease apart the additive and synergistic effects of biological/genetic and environmental exposures.
Overall, this study revealed few sex differences in risk factors for AN, including SES, pregnancy, birth, and early childhood factors, and psychiatric and metabolic PRS. This implies that risk factors for AN are comparable between females and males. Although the results highlight significant sex differences in the effects of parental psychiatric history and BMI PRS, these effects did not survive corrections for multiple comparisons. There were some differences in the strength and direction of effects between females and males, though we generally observed comparable trends in both sexes, particularly in the effects of PRS. This study is limited by the small male sample and thus lower statistical power needed to detect effects in males. Broader diagnostic issues may also contribute to less reliable detection of AN in males and consequently less frequent diagnosis. Population-based research with larger samples is needed to further investigate sex-specific effects of biological and environmental exposures on AN risk, including exposures in later childhood and adolescence. Future studies on AN should also focus more broadly on improving recruitment of males with AN to better understand its etiology. Taken together, these investigations could contribute to improved early identification and reduced duration of illness via timely prevention and management of AN.
Supplementary Material
Public Significance Statement.
Sex differences in the prevalence and clinical presentation of anorexia nervosa (AN) warrant examination of sex-specific risk factors. This population-based study indicates that the effects of polygenic risk and early life exposures on AN risk are comparable between females and males. Collaboration between countries with large registers could help to further investigate sex-specific AN risk factors and contribute to improved early identification.
Acknowledgements:
The authors gratefully acknowledge the Psychiatric Genomics Consortium (PGC) as well as the research participants and employees of 23andMe, Inc., for providing the summary statistics used to generate the major depressive disorder polygenic risk scores. ZY acknowledges grant support from the National Institute of Mental Health (NIMH; R01MH120170) and Independent Research Fund Denmark (DFF) Sapere Aude. CMB acknowledges grant support from the NIMH (Grant No. R56MH129437; R01MH120170; R01MH124871; R01MH119084; R01MH118278), Swedish Research Council (Vetenskapsrådet; Grant No. 538-2013-8864), and Lundbeck Foundation (Grant No. R276-2018-4581). BJV acknowledges grant support from the Lundbeck Foundation (Grant No. R335-2019-2339).
Financial Support:
iPSYCH data were supported by grants from the Lundbeck Foundation (Grant No. R102-A9118, R155-2014-1724, and R248-2017-2003), Aarhus University and University Hospital, and Copenhagen University and University Hospital. The Anorexia Nervosa Genetics Initiative (ANGI) was an initiative of the Klarman Family Foundation. Further anorexia nervosa genotype data were supported by a grant from the Lundbeck Foundation (Grant No. R276-2018-4581).
Footnotes
Competing Interests: CMB reports: Shire (grant recipient, Scientific Advisory Board member); Lundbeck Foundation (grant recipient); Pearson (author, royalty recipient); Equip Health Inc. (Stakeholder Advisory Board).
Contributor Information
Hannah Chatwin, National Centre for Register-Based Research (NCRR), Aarhus University, Aarhus, Denmark.
Katrine Holde, National Centre for Register-Based Research (NCRR), Aarhus University, Aarhus, Denmark.
Zeynep Yilmaz, National Centre for Register-Based Research (NCRR), Aarhus University, Aarhus, Denmark; Department of Biomedicine, Aarhus University, Aarhus, Denmark; Department of Psychiatry, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA.
Janne Tidselbak Larsen, National Centre for Register-Based Research (NCRR), Aarhus University, Aarhus, Denmark; Lundbeck Foundation Initiative for Integrative Psychiatric Research (iPSYCH), Aarhus University, Aarhus, Denmark.
Clara Albiñana, National Centre for Register-Based Research (NCRR), Aarhus University, Aarhus, Denmark; Lundbeck Foundation Initiative for Integrative Psychiatric Research (iPSYCH), Aarhus University, Aarhus, Denmark.
Bjarni Jóhann Vilhjálmsson, National Centre for Register-Based Research (NCRR), Aarhus University, Aarhus, Denmark; Bioinformatics Research Centre, Aarhus University, Aarhus, Denmark.
Preben Bo Mortensen, National Centre for Register-Based Research (NCRR), Aarhus University, Aarhus, Denmark; Lundbeck Foundation Initiative for Integrative Psychiatric Research (iPSYCH), Aarhus University, Aarhus, Denmark; Centre for Integrated Register-based Research (CIRRAU), Aarhus University, Aarhus, Denmark.
Laura M. Thornton, Department of Psychiatry, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA
Cynthia M. Bulik, Department of Psychiatry, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden; Department of Nutrition, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA.
Liselotte Vogdrup Petersen, National Centre for Register-Based Research (NCRR), Aarhus University, Aarhus, Denmark; Lundbeck Foundation Initiative for Integrative Psychiatric Research (iPSYCH), Aarhus University, Aarhus, Denmark.
Data Availability Statement:
Access to data requires application to the Danish Health Data Authority and the Danish Data Protection Agency.
References
- Baker JH, Maes HH, Lissner L, Aggen SH, Lichtenstein P, & Kendler KS (2009). Genetic risk factors for disordered eating in adolescent males and females. Journal of Abnormal Psychology, 118(3), 576–586. 10.1037/a0016314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson J, Byberg S, Carstensen B, De Stavola BL, Svensson J, Jørgensen ME, & Rod NH (2020). Accumulation of childhood adversities and type 1 diabetes risk: a register-based cohort study of all children born in Denmark between 1980 and 2015. International Journal of Epidemiology, 49(5), 1604–1613. 10.1093/ije/dyaa138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman ND, Lohr KN, & Bulik CM (2007). Outcomes of eating disorders: A systematic review of the literature. International Journal of Eating Disorders, 40(4), 293–309. 10.1002/eat.20369 [DOI] [PubMed] [Google Scholar]
- Bliddal M, Broe A, Pottegård A, Olsen J, & Langhoff-Roos J (2018). The Danish Medical Birth Register. European Journal of Epidemiology, 33(1), 27–36. 10.1007/s10654-018-0356-1 [DOI] [PubMed] [Google Scholar]
- Bock C, Bukh J, Vinberg M, Gether U, & Kessing L (2009). Validity of the diagnosis of a single depressive episode in a case register. Clinical Practice and Epidemiology in Mental Health, 5(1), 4. 10.1186/1745-0179-5-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bould H, Koupil I, Dalman C, Destavola B, Lewis G, & Magnusson C (2015). Parental mental illness and eating disorders in offspring. International Journal of Eating Disorders, 48(4), 383–391. 10.1002/eat.22325 [DOI] [PubMed] [Google Scholar]
- Brown M, Hochman A, & Micali N (2020). Emotional instability as a trait risk factor for eating disorder behaviors in adolescents: Sex differences in a large-scale prospective study. Psychological Medicine, 50(11), 1783–1794. 10.1017/s0033291719001818 [DOI] [PubMed] [Google Scholar]
- Bulik C, Yilmaz Z, & Hardaway A (2015). Genetics and epigenetics of eating disorders. Advances in Genomics and Genetics, 131. 10.2147/agg.s55776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulik CM, Thornton LM, Parker R, Kennedy H, Baker JH, Macdermod C, Guintivano J, Cleland L, Miller AL, Harper L, Larsen JT, Yilmaz Z, Grove J, Sullivan PF, Petersen LV, Jordan J, Kennedy MA, & Martin NG (2021). The Eating Disorders Genetics Initiative (EDGI): study protocol. BMC Psychiatry, 21(1). 10.1186/s12888-021-03212-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bybjerg-Grauholm J, Bøcker Pedersen C, Bækvad-Hansen M, Giørtz Pedersen M, Adamsen D, Søholm Hansen C, Agerbo E, Grove J, Als TD, Schork AJ, Buil A, Mors O, Nordentoft M, Werge T, Børglum AD, Hougaard DM, & Mortensen PB (2020). The iPSYCH2015 Case-Cohort sample: updated directions for unravelling genetic and environmental architectures of severe mental disorders. medRxiv, 2020.2011.2030.20237768. 10.1101/2020.11.30.20237768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baadsgaard M, & Quitzau J (2011). Danish registers on personal income and transfer payments. Scandinavian Journal of Public Health, 39(7_suppl), 103–105. 10.1177/1403494811405098 [DOI] [PubMed] [Google Scholar]
- Chesney E, Goodwin GM, & Fazel S (2014). Risks of all-cause and suicide mortality in mental disorders: a meta-review. World Psychiatry, 13(2), 153–160. 10.1002/wps.20128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow S-C, Shao J, Wang H, & Lokhnygina Y (2017). Sample size calculations in clinical research. Chapman and Hall/CRC. [Google Scholar]
- Culbert KM, Sisk CL, & Klump KL (2021). A Narrative Review of Sex Differences in Eating Disorders: Is There a Biological Basis? Clinical Therapeutics, 43(1), 95–111. 10.1016/j.clinthera.2020.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl SK, Larsen JT, Petersen L, Ubbesen MB, Mortensen PB, Munk-Olsen T, & Musliner KL (2017). Early adversity and risk for moderate to severe unipolar depressive disorder in adolescence and adulthood: A register-based study of 978,647 individuals. Journal of Affective Disorders, 214, 122–129. 10.1016/j.jad.2017.03.014 [DOI] [PubMed] [Google Scholar]
- Debost J-C, Larsen JT, Munk-Olsen T, Mortensen PB, Agerbo E, & Petersen LV (2019). Childhood infections and schizophrenia: The impact of parental SES and mental illness, and childhood adversities. Brain, Behavior, and Immunity, 81, 341–347. 10.1016/j.bbi.2019.06.031 [DOI] [PubMed] [Google Scholar]
- Duncan L, Yilmaz Z, Gaspar H, Walters R, Goldstein J, Anttila V, Bulik-Sullivan B, Ripke S, Thornton L, Hinney A, Daly M, Sullivan PF, Zeggini E, Breen G, Bulik CM, Duncan L, Yilmaz Z, Gaspar H, Walters R, … Bulik CM (2017). Significant Locus and Metabolic Genetic Correlations Revealed in Genome-Wide Association Study of Anorexia Nervosa. American Journal of Psychiatry, 174(9), 850–858. 10.1176/appi.ajp.2017.16121402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley DL, Thacker LR, Aggen SH, Neale MC, & Kendler KS (2001). Pregnancy and perinatal complications associated with risks for common psychiatric disorders in a population-based sample of female twins. American Journal of Medical Genetics, 105(5), 426–431. 10.1002/ajmg.1402 [DOI] [PubMed] [Google Scholar]
- Hudson JI, Hiripi E, Pope HG, & Kessler RC (2007). The Prevalence and Correlates of Eating Disorders in the National Comorbidity Survey Replication. Biological Psychiatry, 61(3), 348–358. 10.1016/j.biopsych.2006.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübel C, Gaspar HA, Coleman JRI, Finucane H, Purves KL, Hanscombe KB, Prokopenko I, Graff M, Ngwa JS, Workalemahu T, O’Reilly PF, Bulik CM, & Breen G (2019). Genomics of body fat percentage may contribute to sex bias in anorexia nervosa. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 180(6), 428–438. 10.1002/ajmg.b.32709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IOCDF-GC/OCGAS. (2018). Revealing the complex genetic architecture of obsessive-compulsive disorder using meta-analysis. Molecular Psychiatry, 23(5), 1181–1188. 10.1038/mp.2017.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javaras KN, Rickert ME, Thornton LM, Peat CM, Baker JH, Birgegård A, Norring C, Landén M, Almqvist C, Larsson H, Lichtenstein P, Bulik CM, & D’Onofrio BM (2017). Paternal age at childbirth and eating disorders in offspring. Psychological Medicine, 47(3), 576–584. 10.1017/s0033291716002610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen VM, & Rasmussen AW (2011). Danish education registers. Scandinavian Journal of Public Health, 39(7_suppl), 91–94. 10.1177/1403494810394715 [DOI] [PubMed] [Google Scholar]
- Johnson D, Wilke MAP, Lyle SM, Kowalec K, Jorgensen A, Wright GEB, & Drögemöller BI (2022). A Systematic Review and Analysis of the Use of Polygenic Scores in Pharmacogenomics. Clinical Pharmacology & Therapeutics, 111(4), 919–930. 10.1002/cpt.2520 [DOI] [PubMed] [Google Scholar]
- Kalbfleisch JD, & Lawless JF (1988). Likelihood analysis of multi-state models for disease incidence and mortality. Statistics in Medicine, 7(1–2), 149–160. 10.1002/sim.4780070116 [DOI] [PubMed] [Google Scholar]
- Keski-Rahkonen A, & Mustelin L (2016). Epidemiology of eating disorders in Europe: prevalence, incidence, comorbidity, course, consequences, and risk factors. Current Opinion in Psychiatry, 29(6), 340–345. 10.1097/yco.0000000000000278 [DOI] [PubMed] [Google Scholar]
- Kinasz K, Accurso EC, Kass AE, & Le Grange D (2016). Does Sex Matter in the Clinical Presentation of Eating Disorders in Youth? Journal of Adolescent Health, 58(4), 410–416. 10.1016/j.jadohealth.2015.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL (2013). Puberty as a critical risk period for eating disorders: A review of human and animal studies. Hormones and Behavior, 64(2), 399–410. 10.1016/j.yhbeh.2013.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Culbert KM, Slane JD, Burt SA, Sisk CL, & Nigg JT (2012). The effects of puberty on genetic risk for disordered eating: evidence for a sex difference. Psychological Medicine, 42(3), 627–637. 10.1017/s0033291711001541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch SV, Larsen JT, Mouridsen SE, Bentz M, Petersen L, Bulik C, Mortensen PB, & Plessen KJ (2015). Autism spectrum disorder in individuals with anorexia nervosa and in their first- and second-degree relatives: Danish nationwide register-based cohort-study. British Journal of Psychiatry, 206(5), 401–407. 10.1192/bjp.bp.114.153221 [DOI] [PubMed] [Google Scholar]
- Koch SV, Larsen JT, Plessen KJ, Thornton LM, Bulik CM, & Petersen LV (2022). Associations between parental socioeconomic‐, family‐, and sibling status and risk of eating disorders in offspring in a Danish national female cohort. International Journal of Eating Disorders, 55(8), 1130–1142. 10.1002/eat.23771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen JT, Bulik CM, Thornton LM, Koch SV, & Petersen L (2021). Prenatal and perinatal factors and risk of eating disorders. Psychological Medicine, 51(5), 870–880. 10.1017/s0033291719003945 [DOI] [PubMed] [Google Scholar]
- Lee JJ, Wedow R, Okbay A, Kong E, Maghzian O, Zacher M, Nguyen-Viet TA, Bowers P, Sidorenko J, Karlsson Linnér R, Fontana MA, Kundu T, Lee C, Li H, Li R, Royer R, Timshel PN, Walters RK, Willoughby EA, … Cesarini D (2018). Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nature Genetics, 50(8), 1112–1121. 10.1038/s41588-018-0147-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynge E, Sandegaard JL, & Rebolj M (2011). The Danish National Patient Register. Scandinavian Journal of Public Health, 39(7_suppl), 30–33. 10.1177/1403494811401482 [DOI] [PubMed] [Google Scholar]
- McCabe MP, & Ricciardelli LA (2005). A prospective study of pressures from parents, peers, and the media on extreme weight change behaviors among adolescent boys and girls. Behaviour research and therapy, 43(5), 653–668. 10.1016/j.brat.2004.05.004 [DOI] [PubMed] [Google Scholar]
- Momen NC, Plana-Ripoll O, Bulik CM, McGrath JJ, Thornton LM, Yilmaz Z, & Petersen LV (2022). Comorbidity between types of eating disorder and general medical conditions. The British Journal of Psychiatry, 220(5), 279–286. 10.1192/bjp.2021.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mors O, Perto GP, & Mortensen PB (2011). The Danish Psychiatric Central Research Register. Scandinavian Journal of Public Health, 39(7_suppl), 54–57. 10.1177/1403494810395825 [DOI] [PubMed] [Google Scholar]
- Nørgaard-Pedersen B, & Hougaard DM (2007). Storage policies and use of the Danish Newborn Screening Biobank. Journal of Inherited Metabolic Disease, 30(4), 530–536. 10.1007/s10545-007-0631-x [DOI] [PubMed] [Google Scholar]
- Pedersen CB (2011). The Danish Civil Registration System. Scandinavian Journal of Public Health, 39(7_suppl), 22–25. 10.1177/1403494810387965 [DOI] [PubMed] [Google Scholar]
- Pedersen CB, Bybjerg-Grauholm J, Pedersen MG, Grove J, Agerbo E, Bækvad-Hansen M, Poulsen JB, Hansen CS, McGrath JJ, Als TD, Goldstein JI, Neale BM, Daly MJ, Hougaard DM, Mors O, Nordentoft M, Børglum AD, Werge T, & Mortensen PB (2018). The iPSYCH2012 case–cohort sample: new directions for unravelling genetic and environmental architectures of severe mental disorders. Molecular Psychiatry, 23(1), 6–14. 10.1038/mp.2017.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersson F, Baadsgaard M, & Thygesen LC (2011). Danish registers on personal labour market affiliation. Scandinavian Journal of Public Health, 39(7_suppl), 95–98. 10.1177/1403494811408483 [DOI] [PubMed] [Google Scholar]
- Privé F, Arbel J, & Vilhjálmsson BJ (2020). LDpred2: better, faster, stronger. Bioinformatics, 36(22–23), 5424–5431. 10.1093/bioinformatics/btaa1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privé F, Luu K, Blum MG, McGrath JJ, & Vilhjálmsson BJ (2020). Efficient toolkit implementing best practices for principal component analysis of population genetic data. Bioinformatics, 36(16), 4449–4457. 10.1093/bioinformatics/btaa520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raevuori A, Linna MS, & Keski-Rahkonen A (2014). Prenatal and perinatal factors in eating disorders: A descriptive review. International Journal of Eating Disorders, 47(7), 676–685. 10.1002/eat.22323 [DOI] [PubMed] [Google Scholar]
- Robinson N, & Bergen SE (2021). Environmental Risk Factors for Schizophrenia and Bipolar Disorder and Their Relationship to Genetic Risk: Current Knowledge and Future Directions. Front Genet, 12, 686666. 10.3389/fgene.2021.686666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangha S, Oliffe JL, Kelly MT, & McCuaig F (2019). Eating Disorders in Males: How Primary Care Providers Can Improve Recognition, Diagnosis, and Treatment. American Journal of Men’s Health, 13(3), 155798831985742. 10.1177/1557988319857424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaumberg K, Welch E, Breithaupt L, Hübel C, Baker JH, Munn-Chernoff MA, Yilmaz Z, Ehrlich S, Mustelin L, Ghaderi A, Hardaway AJ, Bulik-Sullivan EC, Hedman AM, Jangmo A, Nilsson IAK, Wiklund C, Yao S, Seidel M, & Bulik CM (2017). The Science Behind the Academy for Eating Disorders’ Nine Truths About Eating Disorders. European Eating Disorders Review, 25(6), 432–450. 10.1002/erv.2553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silén Y, Sipilä PN, Raevuori A, Mustelin L, Marttunen M, Kaprio J, & Keski‐Rahkonen A (2021). Detection, treatment, and course of eating disorders in Finland: A population‐based study of adolescent and young adult females and males. European Eating Disorders Review, 29(5), 720–732. 10.1002/erv.2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonneville KR, & Lipson SK (2018). Disparities in eating disorder diagnosis and treatment according to weight status, race/ethnicity, socioeconomic background, and sex among college students. International Journal of Eating Disorders, 51(6), 518–526. 10.1002/eat.22846 [DOI] [PubMed] [Google Scholar]
- Spracklen CN, Chen P, Kim YJ, Wang X, Cai H, Li S, Long J, Wu Y, Wang YX, Takeuchi F, Wu JY, Jung KJ, Hu C, Akiyama K, Zhang Y, Moon S, Johnson TA, Li H, Dorajoo R, … Sim X. (2017). Association analyses of East Asian individuals and trans-ancestry analyses with European individuals reveal new loci associated with cholesterol and triglyceride levels. Human Molecular Genetics, 26(9), 1770–1784. 10.1093/hmg/ddx062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton LM, Munn-Chernoff MA, Baker JH, Juréus A, Parker R, Henders AK, Larsen JT, Petersen L, Watson HJ, Yilmaz Z, Kirk KM, Gordon S, Leppä VM, Martin FC, Whiteman DC, Olsen CM, Werge TM, Pedersen NL, Kaye W, … Bulik CM (2018). The Anorexia Nervosa Genetics Initiative (ANGI): Overview and methods. Contemporary Clinical Trials, 74, 61–69. 10.1016/j.cct.2018.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassos E, Agerbo E, Mors O, & Pedersen CB (2016). Urban–rural differences in incidence rates of psychiatric disorders in Denmark. British Journal of Psychiatry, 208(5), 435–440. 10.1192/bjp.bp.114.161091 [DOI] [PubMed] [Google Scholar]
- Watson HJ, & Bulik CM (2013). Update on the treatment of anorexia nervosa: review of clinical trials, practice guidelines and emerging interventions. Psychological Medicine, 43(12), 2477–2500. 10.1017/s0033291712002620 [DOI] [PubMed] [Google Scholar]
- Watson HJ, Palmos AB, Hunjan A, Baker JH, Yilmaz Z, & Davies HL (2021). Genetics of eating disorders in the genome-wide era. Psychological Medicine, 51(13), 2287–2297. 10.1017/s0033291720005474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson HJ, Yilmaz Z, Thornton LM, Hübel C, Coleman JRI, Gaspar HA, Bryois J, Hinney A, Leppä VM, Mattheisen M, Medland SE, Ripke S, Yao S, Giusti-Rodríguez P, Hanscombe KB, Purves KL, Adan RAH, Alfredsson L, Ando T, … Bulik CM (2019). Genome-wide association study identifies eight risk loci and implicates metabo-psychiatric origins for anorexia nervosa. Nature Genetics, 51(8), 1207–1214. 10.1038/s41588-019-0439-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. (2016). International Statistical Classification of Diseases and Related Health Problems (10th Ed.) https://doi.org/https://icd.who.int/browse10/2016/en
- Wray NR, Ripke S, Mattheisen M, Trzaskowski M, Byrne EM, Abdellaoui A, Adams MJ, Agerbo E, Air TM, Andlauer TMF, Bacanu SA, Bækvad-Hansen M, Beekman AFT, Bigdeli TB, Binder EB, Blackwood DRH, Bryois J, Buttenschøn HN, Bybjerg-Grauholm J, … Sullivan PF (2018). Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nature Genetics, 50(5), 668–681. 10.1038/s41588-018-0090-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Liu J, Li S, Ma H, & Wang Y (2020). Trends in the prevalence and disability-adjusted life years of eating disorders from 1990 to 2017: results from the Global Burden of Disease Study 2017. Epidemiology and Psychiatric Sciences, 29. 10.1017/s2045796020001055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue A, Wu Y, Zhu Z, Zhang F, Kemper KE, Zheng Z, Yengo L, Lloyd-Jones LR, Sidorenko J, Wu Y, McRae AF, Visscher PM, Zeng J, & Yang J (2018). Genome-wide association analyses identify 143 risk variants and putative regulatory mechanisms for type 2 diabetes. Nature Communications, 9(1), 2941. 10.1038/s41467-018-04951-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yengo L, Sidorenko J, Kemper KE, Zheng Z, Wood AR, Weedon MN, Frayling TM, Hirschhorn J, Yang J, & Visscher PM (2018). Meta-analysis of genome-wide association studies for height and body mass index in ~700000 individuals of European ancestry. Human Molecular Genetics, 27(20), 3641–3649. 10.1093/hmg/ddy271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz Z, Schaumberg K, Halvorsen M, Goodman EL, Brosof LC, Crowley JJ, Mathews CA, Mattheisen M, Breen G, Bulik CM, Micali N, & Zerwas SC (2022). Predicting eating disorder and anxiety symptoms using disorder-specific and transdiagnostic polygenic scores for anorexia nervosa and obsessive-compulsive disorder. Psychological Medicine, 1–15. 10.1017/s0033291721005079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerwas S, Larsen JT, Petersen L, Thornton LM, Mortensen PB, & Bulik CM (2015). The incidence of eating disorders in a Danish register study: Associations with suicide risk and mortality. Journal of Psychiatric Research, 65, 16–22. 10.1016/j.jpsychires.2015.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Larsen JT, Kuja-Halkola R, Thornton L, Yao S, Larsson H, Lichtenstein P, Petersen LV, Bulik CM, & Bergen SE (2021). Familial co-aggregation of schizophrenia and eating disorders in Sweden and Denmark. Molecular Psychiatry, 26(9), 5389–5397. 10.1038/s41380-020-0749-x [DOI] [PubMed] [Google Scholar]
- Zipfel S, Giel KE, Bulik CM, Hay P, & Schmidt U (2015). Anorexia nervosa: aetiology, assessment, and treatment. The Lancet Psychiatry, 2(12), 1099–1111. 10.1016/S2215-0366(15)00356-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Access to data requires application to the Danish Health Data Authority and the Danish Data Protection Agency.


