Abstract
We describe an electrical running down phenomenon and also a consistent spectral change (in the aperiodic component of the power spectrum) derived from chronic interictal electrocorticography (ECoG), after surgery in a patient with drug-resistant epilepsy. These data were recorded using a closed-loop neurostimulation system that was implanted after resection. The patient has been seizure-free for 2.5 years since resection without requiring the neurostimulator to be turned on. Concurrently, there was an exponential decrease in the number of epileptiform electrographic detections recorded by the device, particularly over the first 26 weeks, indicative of an electrical ‘running-down’ phenomenon as the brain adapted to an extended period of seizure freedom. We also find that the aperiodic exponent of the power spectrum gradually decreases over time. The aperiodic component of intracranial ECoG may represent a novel marker of epileptogenicity, independent of seizures.
Keywords: responsive neurostimulation, focal epilepsy, broadband power, aperiodic component of spectrum, FOOOF, running-down
Introduction
Biomarkers prognosticating the course of epilepsy after resection are being investigated. ‘Running down’ has been described as a clinical feature in which there is a gradual decrease of the apparent seizure frequency after surgical intervention, and this has been described as a positive predictor of eventual seizure freedom [1–4]. Time domain features including interictal activity [5–7] and frequency domain features including power changes [8] and phase coupling changes [9] may also be important for prognostication. Characterization of spectral features requires parametrization of both spectral periodic and aperiodic features [10].
The Responsive Neurostimulation (RNS) system (Neuropace, Mountain View, CA) offers a means to chronically record interictal and ictal activity and search for such biomarkers. Here we illustrate how the chronic intracranial recordings offered by the RNS system can be used to track ongoing abnormal activity and the raw data used to derive a set of spectral features that may be used to study seizure outcomes. Specifically, we documented epileptiform electrographic events (referred to as ‘detections’) captured by the RNS device in a patient after resection and found that those events decreased with time (which we refer to as an electrical “running-down” feature [11], not the running down of clinical seizures). We also computed a novel spectral feature of the electroencephalographic (ECoG) data, the aperiodic exponential component [10,12].
Methods and case illustration
The patient was a 41-year-old woman with drug-resistant epilepsy whose seizures began at 4 years of age. Seizures initially presented with staring spells which then progressed to a feeling of “falling out of a chair”. Of note, the patient was born 7 weeks premature, but she achieved normal developmental milestones and is otherwise healthy. She was controlled on medications until college when she began to have generalized seizures without aura. She first underwent resection of a right frontal lobe seizure focus at age 30 after intracranial monitoring with stereotactic depth electrodes. After 6 months, her focal awareness seizures returned and were mostly nocturnal with some daytime episodes. At age 33, she underwent remapping with depth electrodes and surgery that extended the margins of her prior resection cavity. She continued to have daily seizures and nightly auras consisting of feelings of uneasiness, confusion, and out-of-body experience, particularly during the early morning hours. She also heard a buzzing or ringing and felt her jaw clenching with these episodes, but maintained consciousness.
Magnetic resonance imaging of the brain at this time showed progressive encephalomalacia surrounding the resection cavity in the lateral frontal lobe and volume loss of the right hippocampus, fornix, and mammillary body. She underwent evaluation using stereotactically placed depth electrodes (SEEG) at age 38, which showed 19 focal ictal discharges from the anterior, deep edge of the resection cavity, from orbitofrontal cortex. There were sharp waves from that anterior, medial, deep area of the resection cavity as well as from the bilateral hippocampi and left frontal lobe. She underwent a final extension of the resection volume up to motor cortex posteriorly and placement of an RNS system with strip electrodes at the resection margins including on motor cortex (Figure 1A). Middle frontal gyrus and orbitofrontal cortex were resected and strip electrodes were placed on the superior frontal gyrus (SFG) and inferior frontal gyrus (IFG; Figure 1, A and B). A postoperative CT was obtained to confirm electrode positions and that there was no surgical complication including hematoma formation. Pathological analysis of the tissue showed focal cortical dysplasia type IIb. In the 2.5 years since resection, the patient has been electrographically seizure-free. She had three typical auras that were not captured on the RNS device (and the magnet was not used). Whether these represented brief and localized (but uncaptured) epileptiform events versus nonepileptic events is unknown. If true epileptiform events, whether they were not captured because they were too brief or because they occurred in unsampled areas is also unknown. Her RNS stimulation has remained off, and her ECoG data are reviewed periodically with the neurology team.
Figure 1. Total detections over weeks derived from electrodes surrounding the resection margins on the superior and inferior frontal gyri.
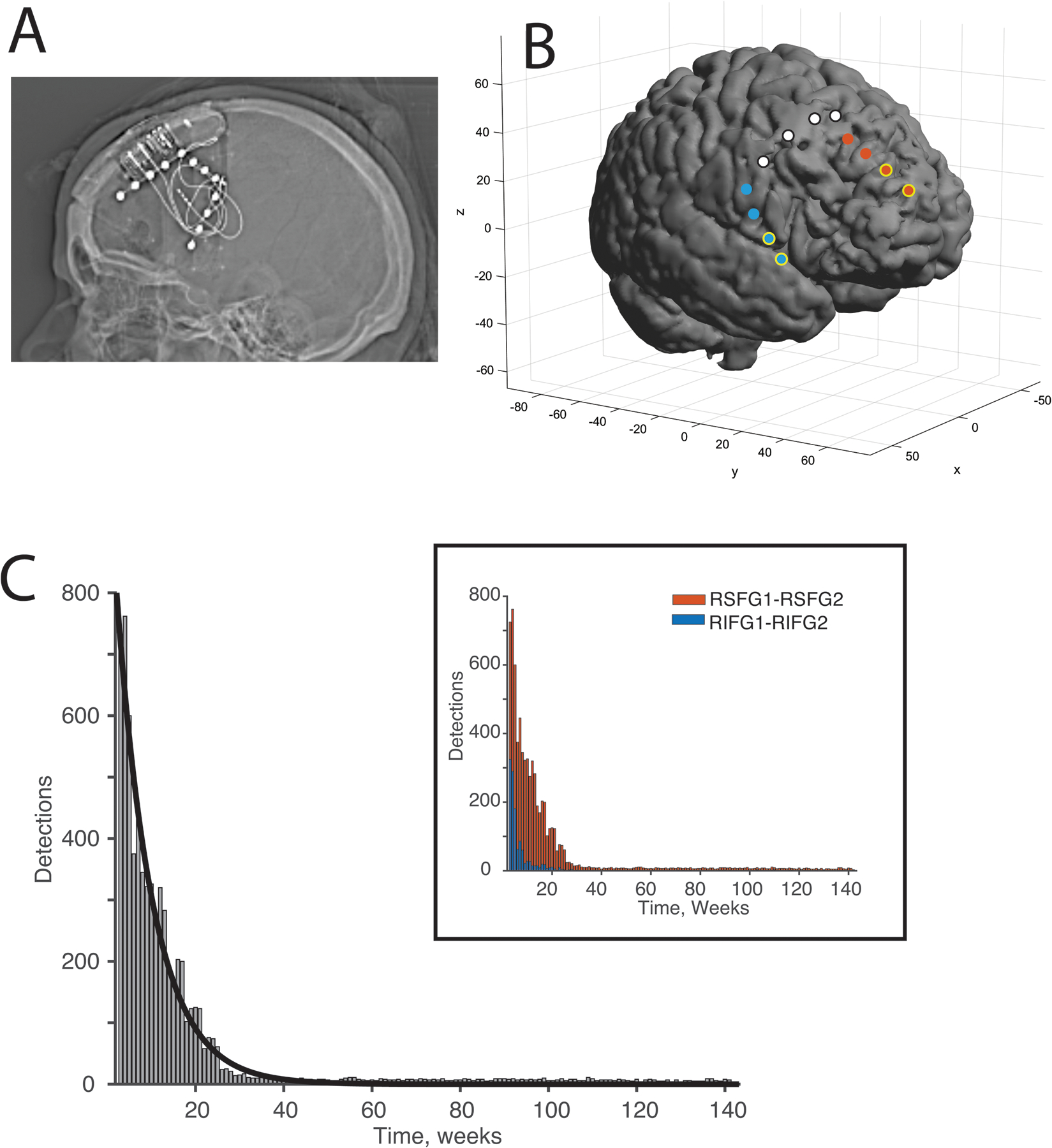
A) Skull x-ray showing the device orientation and electrodes. The strip electrode on the posterior margin remained disconnected. B) Three-dimensional reconstruction of the strip electrodes around the resection cavity shown. Note that the resection area is larger than that shown because a postoperative MRI was not obtained. This reconstruction model of the resection cavity is based on the preoperative MRI and prior resection cavity. Electrodes in red (superior frontal gyrus) or blue (inferior frontal gyrus) with yellow surround are those used for detections. Electrodes in white are disconnected.” (C) Histogram showing total detections recording from both electrodes combined recorded over weeks after implantation of RNS system. Plot and curve fit starts from four weeks after surgery. An exponential curve was fit to the data. Note the substantial decrease in detections by 26 weeks. (Inset) Histogram shows detections from the two channel pairs, one from the superior frontal gyrus (SFG) and one from inferior frontal gyrus (IFG).
The patient’s antiepileptic medications were decreased between years 1 and 2 after implant. Before the final resection, the patient was taking lacosamide (150 mg three times a day), carbamazepine (300 mg twice a day), and clobazam (10 mg AM/15 mg PM). At most recent follow-up, her antiseizure medications (ASMs) have been decreased to lacosamide (75 mg AM/150 mg PM), carbamazepine (300 mg twice a day), and clobazam (5 mg AM/10 mg PM).
Ambulatory ECoG data from the RNS consist of four data channels derived using a bipolar configuration (i.e., data channel 1 was contact 1– contact 2 and data channel 2 was contact 3 – contact 4) on each of the two leads, sampled at 250 Hz. Only scheduled ECoGs that do not contain detections, recorded at specific times of the day, were used for spectral analysis. ‘Long episode’ data (an event lasting longer than 30 seconds) were not used for analysis, though one sample is provided for reference (Figure S1). Data were collected at 1 AM, 7 AM, 1 PM, and 7 PM, and the patient downloaded her data roughly daily.
The PDMS data were reviewed, and detections were marked using the line-length detector with a sensitivity of 75%, with a detection window of 4.096 s. The line-length detector can detect changes in power and frequency relative to a prior 2-minute time window. Note that detection parameters were not changed during this time and no spike detector or frequency detector were used. Detections were recorded from both the right SFG and right IFG, the two regions covered by the strip electrodes (Figure 1B). Detections counts were derived from the PDMS data and were visually inspected. The rate of total detections over time was modeled (Figure 1C). The impedances were noted as well (Figure S2A).
The power spectra for all scheduled ECoGs that did not have a detection were calculated with the median Welch’s procedure from the NeuroDSP python package (compute_spectrum, https://neurodsp-tools.github.io/neurodsp/index.html). The ECoG power spectra were then parameterized using the fitting oscillations and one over f (FOOOF) python package (https://fooof-tools.github.io/fooof/index.html) [10]. The FOOOF algorithm incorporates parameterization of the aperiodic or nonoscillatory power characterized by 1/f using the exponent. The aperiodic exponent represents the negative slope of 1/fx, where X is the negative slope of the log-log power spectrum. This parameter may have physiological correlates to neuronal population spiking and the integration of synaptic currents [13].
Data for this report were derived from the electronic medical record. All components of the care were part of routine medical management. Patient consent was obtained per University of Utah Institutional Review Board regulations.
Results
The number of weekly detections from the channels bordering the resection cavity were recorded over time and decreased rapidly over the first 26 weeks (exponential decay = weekly detections (w) = 1038(e−0.117t)) with an adjusted R2 value of 0.97, where t is weeks after implant (half-life is 5.92 weeks; Figure 1C). The detections captured were based on a line length detection filter. Visual inspection of the detection ECog traces showed interictal epileptiform activity on average in 9 of 10 traces (see Figure 2 for examples).
Figure 2.
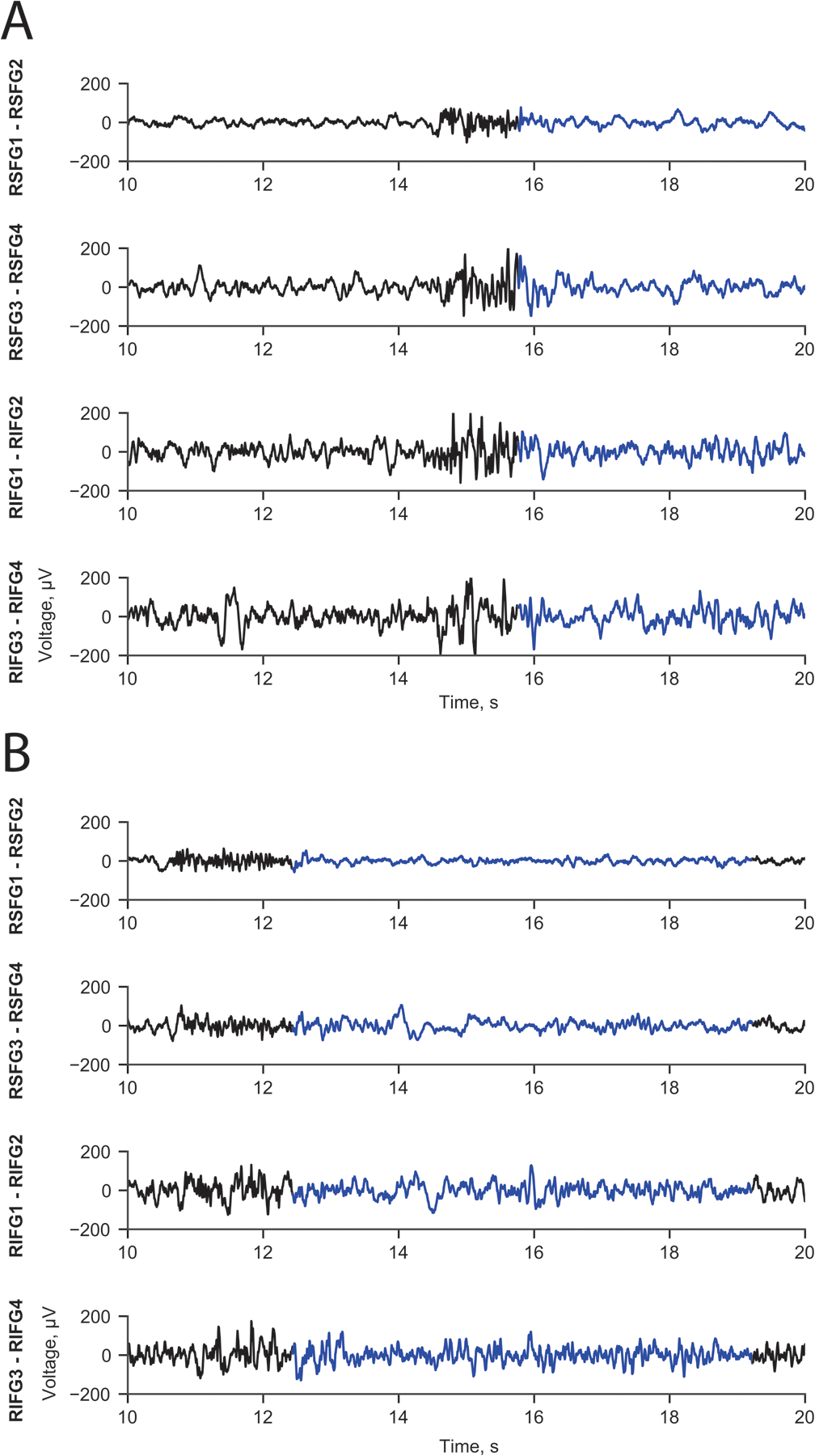
Line length change detector captured interictal epileptiform activity. Panel A shows a burst of low amplitude, high frequency activity on SFG1-SFG2 and polyspike-wave electrical feature across the other three channels. Panel B also shows a burst of low amplitude, high frequency activity on SFG1-SFG2 and spike-wave activity on the other channels. Detector on SFG1 – SFG2.
The clips of data from scheduled recordings that did not contain a detection (Figure 3A, left panel) were used to compute the aperiodic exponent of the spectrum using the FOOOF algorithm (Figure 3A, right panel) described above. The aperiodic exponent calculated for each data clip decreased over time samples for all channels (Figure 3B). This change was not correlated systematically with changes in impedance (Figure S2B).
Figure 3. The aperiodic component of the power spectrum during scheduled ECoGs.
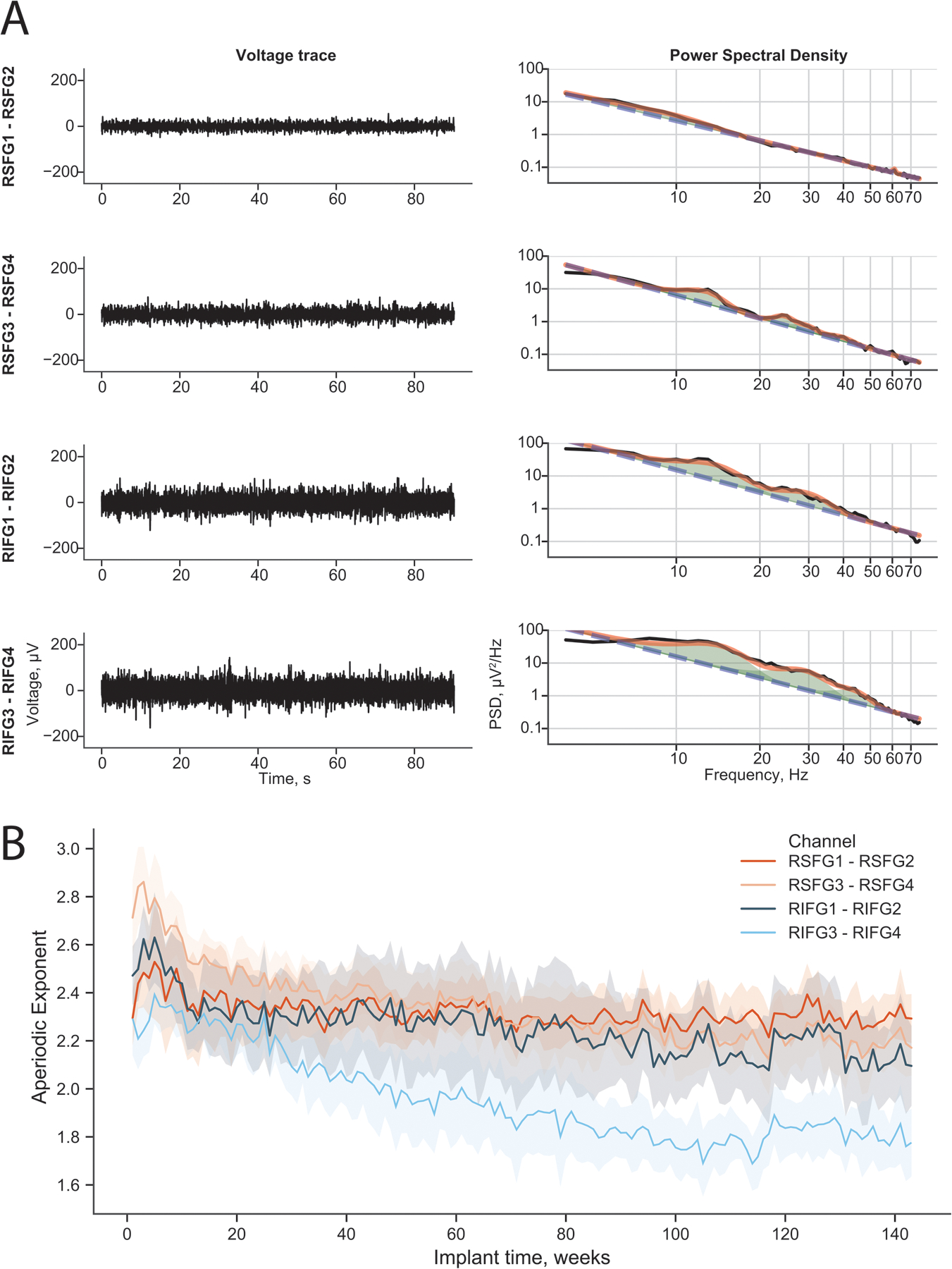
(A) (Left panels) Illustrative intracranial ECoG recorded by the RNS during a scheduled recording without detection for all four channels. (Right panels) The corresponding power spectra for those ECoGs are shown in black. The full FOOOF model is shown in orange and the aperiodic fit in blue dashed lines. The axes are log-log to show the linear aperiodic relationship. (B) The aperiodic exponent of the FOOOF model for all scheduled ECoGs (except those with detections) since implantation. The mean and standard deviation during each week plotted over time. The aperiodic exponent decreased over weeks since implantation in all channels. The data are binned by week, and the data clouds represent the standard deviation during that week.
Discussion
In this report, we have calculated a spectral measure called the ‘aperiodic component’ using chronic intracranially recorded data from the RNS device implanted in a patient with focal epilepsy. The patient underwent several resections during her clinical course. After the most recent resection described above, RNS electrodes were placed on the margins of the resection cavity for chronic monitoring and stimulation if needed. The patient has remained free of disabling seizures and stimulation has not been turned on over the 2.5 years follow-up. Additionally, the number of detections showed an exponential decrease over time, reaching a nadir at 26 weeks (Figure 1C).
The aperiodic exponential component, which is the slope of the broadband power of recording data, has been related to the balance in excitatory and inhibitory network components [10,12]. Theoretically, if there is an increase in the ratio of excitatory to inhibitory components of the network, the exponent is lower and there is a ‘flattening’ of the broadband spectra (i.e., the 1/f ‘noise’ feature is flatter) [10]. In this patient, there was a flattening of the aperiodic exponential component over time, reaching a nadir around 26 weeks. Lower exponent values indicate increased relative excitatory activity within the network. This finding suggests there was a shift in network excitatory-to-inhibitory activity balance, and this may be related to decreased detections and perhaps seizure freedom. To our knowledge, this is the first report of the long-term changes in the aperiodic spectral component modulation recorded for epilepsy using chronic human intracranial recordings.
These data were recorded during a seizure-free period, and the majority of excitatory-to-inhibitory balance modeling is done during ictal time periods. Thus, it is unclear whether our findings should be reflective of ictal time parameters. Additionally, whether seizures are related to increased inhibitory activity or excitatory activity is not clear [14–18]. The balance may be different depending on whether the recording is from the seizure initiation zone or the propagation zone and when the recording is done relative to the timing of the seizure occurrence [19]. In one other case report of a child with focal epilepsy due to focal cortical dysplasia type II undergoing magnetoencephalography, the authors derived the aperiodic component of the spectrum preictally and during a seizure [20]. Importantly, the preictal exponent is flatter than the ictal component, supporting our results. Areas of normal brain also showed lower exponent values [20], which is consistent with our finding. Responders to VNS treatment showed decreased periodic component derived from scalp EEG compared to non-responders [21]. Additionally, if the running-down phenomenon indicates decoupling and deactivation of other epileptogenic sites after the primary focus was resected, decreased detections may also be a biomarker of seizure freedom. Data from additional patients are needed to explore these questions.
With regards to the decreasing detections, Geller et al. [11] also reported a patient who underwent a limited resection near eloquent language areas and RNS implantation. That patient was seizure-free after implantation, and stimulation was not turned on. The patient was monitored for two years, and detections based also on line-length reached a nadir around 200 days, which is very similar to our case of roughly 182 days. A running-down phenomenon has been described after epilepsy surgery such that, after the primary seizure focus has been resected, there is continued clinical seizure activity that eventually remits over time [1]. This is presumably supported by tissue that was epileptogenic but not quite ‘autonomous’ enough to become a sustained seizure focus. This tissue might have been part of the original seizure focus and not completely resected or from another focus [22]. The physiological running-down phenomenon has been described in humans, and its related activation has been recorded with chronic recordings [11,23]. One concern is that the decrease in detections could be related to changes in impedance between the electrode and tissue. Impedance measurements decreased for some of the contacts and not others in these data (Figure S2A).
Finally, could detections or the aperiodic component be used to guide withdrawal of ASMs after surgery? We decreased the dosing of our patient’s ASMs starting one year after surgery by cutting lacosamide down by 25% and clobazam down by 60%. Geller et al. [11] described reducing levetiracetam by 50% at 13 months and discontinuing lacosamide at 18 months in their patient. Both patients in these reports had focal epilepsy with resection, RNS implantation but not activation, and seizure freedom two years out from surgery. More data are needed, but chronic recordings may be a means to guide ASM withdrawal.
Conclusion
Here we describe a patient with focal epilepsy who underwent multiple resections and finally placement of a RNS device with electrodes surrounding the resection cavity. The patient remained free of disabling seizures after device implantation and never required stimulation to be turned on. The chronic recordings were used to derive a novel parameter called the aperiodic component of the power spectra. We also determined the number of detections the patient had per week. In this patient, the aperiodic component exponent decreased over time. The number of detections, which electrically appear to relate to interictal events, also decreased. It remains to be seen if the aperiodic component is a useful biomarker for determining the likelihood of seizure freedom or timing of medication withdrawal.
Supplementary Material
Acknowledgments
We thank Kristin Kraus for her editorial assistance.
Funding sources for JDR: NIH/NINDS K23 NS114178 and R21 NS113031. DNA: NINDS F32 NS 114322. CMC: NSF Graduate Research Fellowship 1747505.
Footnotes
Conflicts of Interest
Bornali Kundu, Chantel Charlebois, Daria Anderson and Angela Peters have no disclosures. John Rolston has consulted for Medtronic, NeuroPace, and Corlieve Therapeutics.
Ethics Approval Statement
We confirm that we have read the Journal’s position on issues in ethical publication and affirm that this report is consistent with those guidelines.
Patient Consent Statement
Patient consent was obtained per University of Utah Institutional Review Board regulations.
Data Availability Statement
All data pertinent to this report are available in the paper.
References
- [1].Salanova V, Rasmussen T, Andermann F. The running down phenomenon in temporal lobe epilepsy. Adv Neurol 1999;81:165–9. [PubMed] [Google Scholar]
- [2].Özkara Ç, Uzan M, Benbir G, Yeni N, Oz B, Hanoǧlu L, et al. Surgical outcome of patients with mesial temporal lobe epilepsy related to hippocampal sclerosis. Epilepsia 2008;49:696–9. doi: 10.1111/j.1528-1167.2007.01503.x. [DOI] [PubMed] [Google Scholar]
- [3].Fauser S, Essang C, Altenmüller DM, Staack AM, Steinhoff BJ, Strobl K, et al. Long-term seizure outcome in 211 patients with focal cortical dysplasia. Epilepsia 2015;56:66–76. doi: 10.1111/epi.12876. [DOI] [PubMed] [Google Scholar]
- [4].Mihara T, Usui N, Matsuda K, Tottori T, Kondo A, Terada K, et al. A classification system for verifying the long-term efficacy of resective surgery for drug-resistant seizures. Epilepsy Res 2018;141:23–30. doi: 10.1016/j.eplepsyres.2018.01.019. [DOI] [PubMed] [Google Scholar]
- [5].Bautista RED, Cobbs MA, Spencer DD, Spencer SS. Prediction of surgical outcome by interictal epileptiform abnormalities during intracranial EEG monitoring in patients with extrahippocampal seizures. Epilepsia 1999;40:880–90. doi: 10.1111/j.1528-1157.1999.tb00794.x. [DOI] [PubMed] [Google Scholar]
- [6].Holmes MD, Kutsy RL, Ojemann GA, Wilensky AJ, Ojemann LM. Interictal, unifocal spikes in refractory extratemporal epilepsy predict ictal origin and postsurgical outcome. Clin Neurophysiol 2000;111:1802–8. doi: 10.1016/S1388-2457(00)00389-8. [DOI] [PubMed] [Google Scholar]
- [7].Smith EH, Liou JY, Merricks EM, Davis TS, Thomson K, Greger B, et al. Human interictal epileptiform discharges are bidirectional traveling waves echoing ictal discharges. Elife 2022;11:e73541. doi: 10.7554/eLife.73541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kokkinos V, Sisterson ND, Wozny TA, Richardson RM. Association of closed-loop brain stimulation neurophysiological features with seizure control among patients with focal epilepsy. JAMA Neurol 2019;76:800–8. doi: 10.1001/jamaneurol.2019.0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Khambhati AN, Shafi A, Rao VR, Chang EF. Long-term brain network reorganization predicts responsive neurostimulation outcomes for focal epilepsy. Sci Transl Med 2021;13:abf6588. doi: 10.1126/scitranslmed.abf6588. [DOI] [PubMed] [Google Scholar]
- [10].Donoghue T, Haller M, Peterson EJ, Varma P, Sebastian P, Gao R, et al. Parameterizing neural power spectra into periodic and aperiodic components. Nat Neurosci 2020;23:1655–65. doi: 10.1038/s41593-020-00744-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Geller AS, Friedman D, Fang M, Doyle WK, Devinsky O, Dugan P. Running-down phenomenon captured with chronic electrocorticography. Epilepsia Open 2018;3:528–34. doi: 10.1002/epi4.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gao R, Peterson EJ, Voytek B. Inferring synaptic excitation/inhibition balance from field potentials. Neuroimage 2017;158:70–8. doi: 10.1016/j.neuroimage.2017.06.078. [DOI] [PubMed] [Google Scholar]
- [13].Donoghue T, Dominguez J, Voytek B. Electrophysiological frequency band ratio measures conflate periodic and aperiodic neural activity. Eneuro 2020;7:ENEURO.0192–20.2020. doi: 10.1523/eneuro.0192-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Niemeyer JE, Gadamsetty P, Chun C, Sylvester S, Lucas JP, Ma H, et al. Seizures initiate in zones of relative hyperexcitation in a zebrafish epilepsy model. Brain 2022;145: 2347–60. doi: 10.1093/brain/awac073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lillis KP, Kramer MA, Mertz J, Staley KJ, White JA. Pyramidal cells accumulate chloride at seizure onset. Neurobiol Dis 2012;47:358–66. doi: 10.1016/J.NBD.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Miri ML, Vinck M, Pant R, Cardin JA. Altered hippocampal interneuron activity precedes ictal onset. Elife 2018;7:e40750. doi: 10.7554/eLife.40750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Elahian B, Lado NE, Mankin EA, Vangala S, Misra A, Moxon KA, et al. Low-voltage fast seizures in humans begin with increased interneuron firing. Ann Neurol 2018;84:588–600. doi: 10.1002/ana.25325.Low-Voltage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Toyoda I, Fujita S, Thamattoor AK, Buckmaster PS. Unit activity of hippocampal interneurons before spontaneous seizures in an animal model of temporal lobe epilepsy. J Neurosci 2015;35:6600–18. doi: 10.1523/JNEUROSCI.4786-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Shao LR, Habela CW, Stafstrom CE. Pediatric epilepsy mechanisms: Expanding the paradigm of excitation/inhibition imbalance. Children 2019;6:23. doi: 10.3390/children6020023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].van Heumen S, Moreau JT, Simard-Tremblay E, Albrecht S, Dudley RWR, Baillet S. Case report: aperiodic fluctuations of neural activity in the ictal MEG of a child with drug-resistant fronto-temporal epilepsy. Front Hum Neurosci 2021;15:646426. doi: 10.3389/fnhum.2021.646426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Coa R, La Cava SM, Baldazzi G, Polizzi L, Pinna G, Conti C, et al. Estimated EEG functional connectivity and aperiodic component induced by vagal nerve stimulation in patients with drug-resistant epilepsy. Front Neurol 2022;13:1030118. doi: 10.3389/fneur.2022.1030118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jehi L The epileptogenic zone: concept and definition. Epilepsy Curr 2018;18:12–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ung H, Baldassano SN, Bink H, Krieger AM, Vitale F, Wu C, et al. Intracranial EEG fluctuates over months after implanting electrodes. J Neural Eng 2017;14. doi: 10.1088/1741-2552/aa7f40.Intracranial. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data pertinent to this report are available in the paper.


