Abstract
On September 21, 2022, the U.S. Food and Drug Administration (FDA) granted accelerated approval to selpercatinib (Retevmo®, Eli Lilly and Company) for the treatment of adult patients with locally advanced or metastatic solid tumors with a rearranged during transfection (RET) gene fusion that have progressed on or following prior systemic treatment or who have no satisfactory alternative treatment options. The approval was based on data from Study LOXO-RET-17001 (LIBRETTO-001; NCT03157128), an international, non-randomized, multi-cohort clinical trial which included patients with advanced solid tumors harboring RET alterations. The overall response rate (ORR) in 41 patients with locally advanced or metastatic RET fusion-positive solid tumors other than non-small cell lung cancer (NSCLC) or thyroid cancer was 44% (95% CI: 28%, 60%) with median duration of response 24.5 months (95% CI: 9.2, not evaluable). Patients with 10 of 14 tumor types with a variety of fusion partners had objective responses, including patients with the following tumors: pancreatic adenocarcinoma, colorectal, salivary, unknown primary, breast, soft tissue sarcoma, bronchial carcinoid, ovarian, small intestine, and cholangiocarcinoma. The recommendation for approval was supported by results from LIBRETTO-001 in patients with RET fusion-positive NSCLC and thyroid cancer, which formed the basis of prior approvals in these tumor types. The most common adverse reactions (>25%) were edema, diarrhea, fatigue, dry mouth, hypertension, abdominal pain, constipation, rash, nausea, and headache. This is the first tissue agnostic approval of a RET-directed targeted therapy.
Introduction
Rearranged during transfection (RET) gene fusions are rare oncogenic alterations observed most commonly in patients with papillary thyroid cancer (5–10%) and non-small cell lung cancer (NSCLC; 1–2%).1,2 In other solid tumors, RET gene fusions are observed in <1% of patients, but are a distinct population given they are typically mutually exclusive of other oncogenic drivers.3 Due to the rarity of RET fusions in solid tumors other than NSCLC and thyroid cancer, there is limited knowledge regarding differences in prognosis attributable to the presence of RET fusions. However, for most patients with advanced solid tumors that have progressed on or following prior systemic treatment, prognosis is poor. Five-year overall survival rates for the most common solid tumors which are diagnosed at a distant stage range from 3% to 40%.4
While selpercatinib and pralsetinib received accelerated approval in 2020 for patients with RET fusion-positive NSCLC and thyroid cancer, there were no approved therapies for other tumor types which harbor RET fusions.5,6 Available treatment options for patients with advanced RET fusion-positive solid tumors who have progressed on prior therapy are similar to those used for patients without a specific driver mutation. However, the effectiveness of these treatments has not been specifically studied in this sub-population. Second-line or greater therapies per National Comprehensive Cancer Network (NCCN) guidelines vary by tumor type and are generally chemotherapy-based with overall responses rates less than 30%.7
Selpercatinib is an oral inhibitor of the RET receptor tyrosine kinase. Herein, we provide a summary of FDA’s review of the marketing application that led to the approval of selpercatinib for the treatment of adult patients with locally advanced or metastatic solid tumors with a RET gene fusion, the first tissue agnostic indication for a RET-directed targeted therapy.
Regulatory History
On May 8, 2020, FDA granted accelerated approval to selpercatinib for the treatment of adult patients with metastatic RET fusion-positive NSCLC, the treatment of adult and pediatric patients 12 years of age and older with advanced or metastatic RET-mutant medullary thyroid cancer (MTC) who require systemic therapy, and the treatment of adult and pediatric patients 12 years of age and older with advanced or metastatic RET fusion-positive thyroid cancer who require systemic therapy and who are radioactive iodine-refractory (if radioactive iodine is appropriate).5 Selpercatinib received Orphan Drug Designation for tissue agnostic RET fusion-positive solid tumors on May 19, 2022. The supplemental new drug application (sNDA) was submitted on May 31, 2022. The sNDA review included use of the Assessment Aid to facilitate FDA review,8 and received Priority Review designation.9
Mechanism of Action
Selpercatinib is an oral kinase inhibitor of the RET receptor tyrosine kinase. In cellular assays, selpercatinib inhibited RET at approximately 60-fold lower concentrations than FGFR1 and 2 and approximately 8-fold lower concentration than VEGFR3.5 Certain chromosomal rearrangements involving in-frame fusions of RET with various partners can result in constitutively activated chimeric RET fusion proteins that can act as oncogenic drivers by promoting cell proliferation of tumor cell lines. In in vitro and in vivo tumor models, selpercatinib demonstrated anti-tumor activity in cells harboring constitutive activation of RET proteins resulting from gene fusions and mutations, including CCDC6-RET, KIF5B-RET, RET V804M, and RET M918T.5 In addition, selpercatinib showed anti-tumor activity in mice intracranially implanted with a patient-derived RET fusion-positive tumor.5
Clinical Pharmacology
The approved recommended dosage of selpercatinib is 120 mg orally twice daily for patients with body weight less than 50 kg and 160 mg orally twice daily for patients with body weight of 50 kg or greater. Selpercatinib may be taken with or without food unless coadministered with a proton pump inhibitor (PPI). Dosage modifications for use with concomitant medications are included in product labeling.5
No clinically significant differences in the pharmacokinetics of selpercatinib were observed based on age (15 to 92 years), sex, or mild, moderate, or severe renal impairment (eGFR ≥15 to 89 mL/min estimated by Modification of Diet in Renal Disease equation). Dosage reduction to 80 mg orally twice daily is recommended for patients with severe hepatic impairment.
The recommended dosage was supported by population pharmacokinetics (PopPK), tumor size, and exposure-response (E-R) modeling conducted using patient data from Study LIBRETTO-001, as detailed in the original FDA multi-disciplinary review for selpercatinib.10 The tissue agnostic sNDA included updated selpercatinib PopPK and E-R analyses, which were consistent with previous submissions for safety results. E-R analyses for efficacy were inconclusive in the subset of patients with RET fusion-positive solid tumors other than NSCLC and thyroid cancer due to the small number of evaluable patients.
Clinical Trials
The supplemental NDA approval of selpercatinib was based on results of Study LOXO-RET-17001 (LIBRETTO-001; NCT03157128), an international, non-randomized, multi-cohort clinical trial which included patients with advanced solid tumors harboring RET alterations. The major efficacy outcome measure was ORR according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, as evaluated by blinded independent central review (BICR). Additional efficacy outcome measures included duration of response (DOR) by BICR. The investigators’ analyses of Study LIBRETTO-001 have been published.11 The analyses below represent FDA’s independent analyses of data submitted by the Applicant in the sNDA.
Demographics, Disease Characteristics and Prior Treatment
The primary efficacy analysis population included 41 patients with locally advanced or metastatic RET fusion-positive solid tumors other than NSCLC and thyroid cancer that had progressed on or following prior systemic treatment or who had no satisfactory alternative treatment options (Table 1). In the efficacy population, 14 different tumor types were represented (range 1 to 11 patients per type), including: pancreatic adenocarcinoma, colorectal, salivary gland, unknown primary, breast, sarcoma, xanthogranuloma, bronchial carcinoid, ovarian, small intestine, cholangiocarcinoma, pulmonary carcinosarcoma, rectal neuroendocrine and carcinoma of the skin (Figure 1). Patients received a median of 2 prior therapies (range 0 to 9). The majority of patients (95%) had metastatic disease; one patient had Stage III colorectal cancer and one patient had xanthogranuloma of unknown stage. More than half of patients were enrolled in the U.S., and demographic and baseline disease characteristics of the primary efficacy population generally reflect the known profile of these patients in the U.S. However, given the small population, rarity of these cancers, and limited knowledge regarding the specific incidence of RET fusions by race and ethnicity, some population variability may be expected.
Table 1: Baseline Demographics and Disease Characteristics in the Efficacy and Safety Populations of Study LIBRETTO-001.
| Efficacy Population N = 41 | Safety Population N = 796 | |
|---|---|---|
| Sex, n (%) | ||
| Female | 22 (54) | 390 (49) |
| Male | 19 (46) | 406 (51) |
| Age at diagnosis (years), median [range] | 50 [21, 85] | 59 [15, 92] |
| Race, n (%) | ||
| Asian | 10 (24) | 185 (23) |
| Black or African American | 2 (5) | 25 (3) |
| White | 28 (68) | 545 (69) |
| Ethnicity, n (%) | ||
| Hispanic or Latino | 3 (7) | 40 (5) |
| Not Hispanic or Latino | 37 (90) | 737 (93) |
| Region, n (%) | ||
| Asia | 12 (29) | 185 (23) |
| Europe | 3 (7) | 123 (15) |
| North America | 26 (63) | 473 (59) |
| ECOG performance status at study entry, n (%) | ||
| 0 | 14 (34) | 294 (37) |
| 1 | 25 (61) | 464 (58) |
| History of Metastatic Disease | ||
| Yes | 95% | 98% |
| Number of Prior Lines of Therapy, median [range] | 2 [0, 9] | 1 [0, 15] |
ECOG = Eastern Cooperative Oncology Group
Source: U.S. Food and Drug Administration. sNDA Multi-disciplinary Review and Evaluation and Approval Package: RETEVMO (selpercatinib).19
Figure 1:
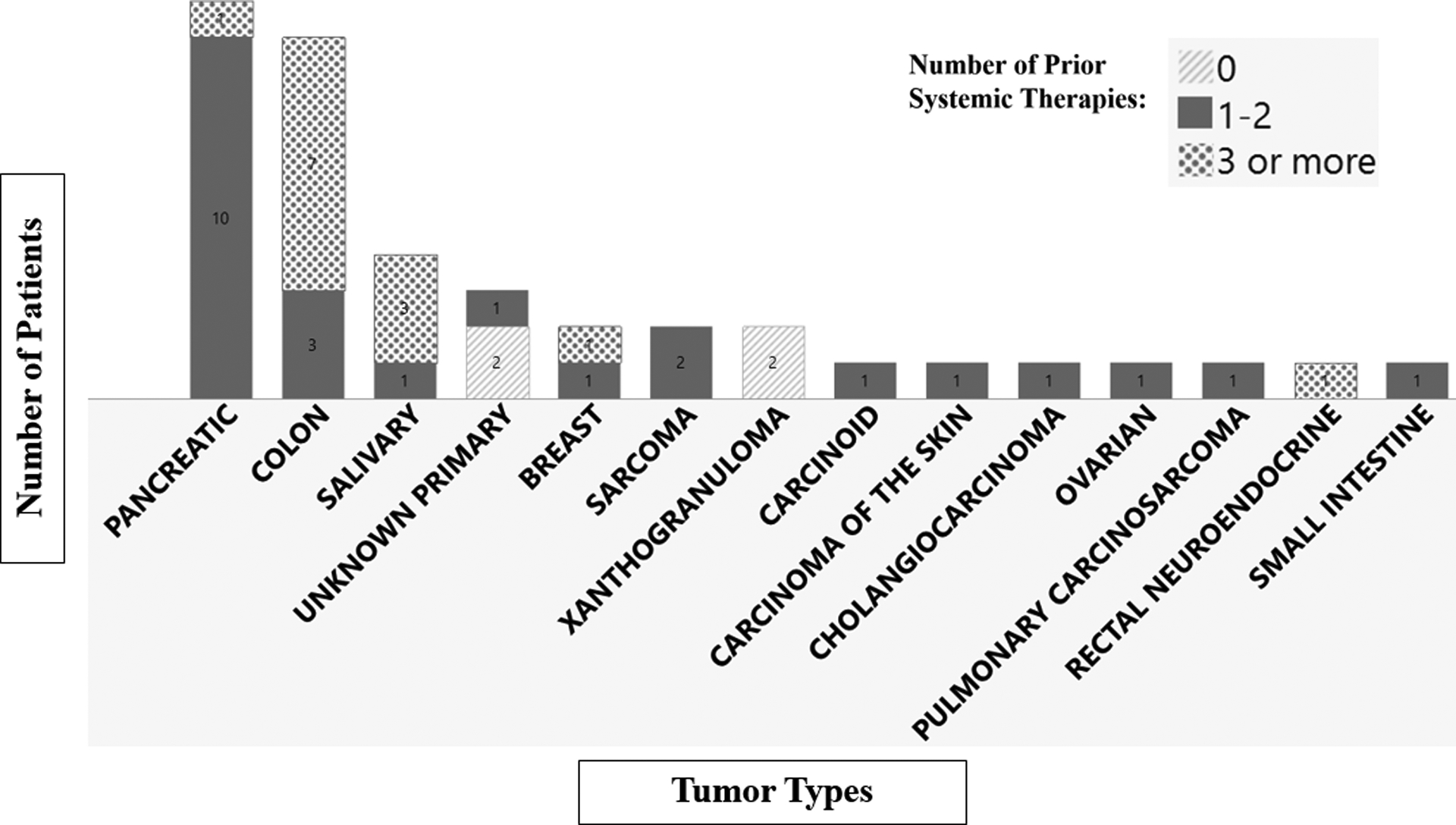
Tumor Types and Number of Prior Therapies in the Tissue Agnostic Efficacy Population (N=41). Source: U.S. Food and Drug Administration. sNDA Multi-disciplinary Review and Evaluation and Approval Package: RETEVMO (selpercatinib).19
Efficacy Results
The confirmed ORR per RECIST 1.1 as determined by BICR was 44% (95% CI 28, 60), and the median DOR was 24.5 months (95% CI 9.2, NE) (Table 2). The observed proportion of patients remaining in response per BICR was 67% (95% CI 41, 87) at 6 months and 56% (95% 31, 78) at 12 months, respectively. Patients with 10 of 14 tumor types with a variety of fusion partners had objective responses by BICR (Table 3). The most common RET fusion partners were NCOA4 (N=16), CCDC6 (N=6), and KIF5B (N=4). Due to the small number of patients in each subgroup, no conclusions could be drawn regarding differences in efficacy by fusion partner.
Table 2: Efficacy Results in Patients with RET Fusion-Positive Solid Tumors in Study LIBRETTO-001.
| RETEVMO (n = 41) | |
|---|---|
| Overall Response Rate1 (95% CI) | 44% (28, 60) |
| Complete response | 4.9% |
| Partial response | 39% |
| Duration of Response | |
| Median in months (95% CI) | 24.5 (9.2, NE) |
| % with ≥6 months2 | 67% |
Confirmed overall response rate assessed by BIRC.
Based on observed duration of response.
NE = not estimable
Source: U.S. product labeling, RETEVMO (selpercatinib)5
Table 3: Efficacy Results by Tumor Type in Patients with RET Fusion-Positive Solid Tumors in Study LIBRETTO-001.
| Tumor Type | Patients (n = 41) | ORR1,2 | DOR Range (months) | |
|---|---|---|---|---|
| n (%) | 95% CI | |||
| Pancreatic adenocarcinoma | 11 | 6 (55%) | (23, 83) | 2.5, 38.3+ |
| Colorectal | 10 | 2 (20%) | (2.5, 56) | 5.6, 13.3 |
| Salivary | 4 | 2 (50%) | (7, 93) | 5.7, 28.8+ |
| Unknown primary | 3 | 1 (33%) | (0.8, 91) | 9.2 |
| Breast | 2 | PR, CR | NA | 2.3+, 17.3 |
| Sarcoma (soft tissue) | 2 | PR, SD | NA | 14.9+ |
| Xanthogranuloma | 2 | NE, NE | NA | NA |
| Carcinoid (bronchial) | 1 | PR | NA | 24.1+ |
| Carcinoma of the skin | 1 | NE | NA | NA |
| Cholangiocarcinoma | 1 | PR | NA | 5.6+ |
| Ovarian | 1 | PR | NA | 14.5+ |
| Pulmonary carcinosarcoma | 1 | NE | NA | NA |
| Rectal neuroendocrine | 1 | NE | NA | NA |
| Small intestine | 1 | CR | NA | 24.5 |
denotes ongoing response.
Confirmed overall response rate assessed by BIRC.
Best overall response for each patient is presented for tumor types with ≤2 patients.
CI = confidence interval, CR = complete response, DOR = duration of response, NA = not applicable, NE = not evaluable, ORR = overall response rate, PR = partial response, SD = stable disease.
Source: U.S. product labeling, RETEVMO (selpercatinib)5
Safety Results
The overall safety population included 796 patients treated with selpercatinib in Study LIBRETTO-001, including the 41 patients in the primary efficacy population (Table 1). The most common (≥ 25%) treatment-emergent adverse events were edema, diarrhea, fatigue, dry mouth, hypertension, abdominal pain, constipation, rash, nausea, and headache. The most common (≥ 5%) Grade 3 or 4 laboratory abnormalities were decreased lymphocytes, increased alanine aminotransferase (ALT), increased aspartate aminotransferase (AST), decreased sodium, and decreased calcium. U.S. product labeling includes warnings for hepatotoxicity, interstitial lung disease/pneumonitis, hypertension, QTc interval prolongation, hemorrhagic events, hypersensitivity, tumor lysis syndrome, risk of impaired wound healing, and hypothyroidism. There were no significant safety concerns identified during sNDA review requiring risk management beyond labeling or warranting consideration for a Risk Evaluation and Mitigation Strategy (REMS).
Regulatory Insights
This is the first approval of an oral targeted therapy for patients with RET fusion-positive solid tumors other than NSCLC and thyroid cancer, and the seventh tissue agnostic indication in the U.S. Other drugs with tissue agnostic indications include pembrolizumab17, larotrectinib13, entrectenib14, dostarlimab15, and combination therapy with dabrafenib and trametinib16 (in chronological order of approval).
Consideration of a tissue agnostic indication in oncology requires a robust understanding of the following across cancer types: biology, pathophysiology of the molecular alteration, incidence and distribution of the molecular alteration, meaningful PK or pharmacodynamic (PD) differences, efficacy and safety.17 The draft guidance for industry published in October 2022, entitled “Tissue Agnostic Drug Development in Oncology”, describes FDA’s current thinking on the development of drugs that target a specific molecular alteration across multiple cancer types as defined, for example, by organ, tissue, or tumor type.18
In determining the appropriateness of a tissue agnostic indication for selpercatinib, FDA considered ORR, in conjunction with durability of response, to be an endpoint reasonably likely to predict clinical benefit in this setting (Table 4).19 FDA acknowledged some uncertainty regarding the magnitude of responses expected in extremely rare tumor types represented in the efficacy population and in tumor types not represented in the efficacy population. At this time, there remains uncertainty as to the rationale for the lower response rate observed in patients with colorectal cancer compared to other solid tumors (e.g., chance finding due to the small number of patients with this tumor type enrolled, or biological factors such as resistance mechanisms or differences in number of prior therapies).
Table 4: FDA Benefit-Risk Analysis.
| Dimension | Evidence and Uncertainties | Conclusions and Reasons |
|---|---|---|
| Analysis of Condition |
|
|
| Current Treatment Options |
|
|
| Benefit |
|
|
| Risk and Risk Management |
|
|
Source: Adapted from U.S. Food and Drug Administration, sNDA Multi-disciplinary Review and Evaluation and Approval Package: RETEVMO (selpercatinib).19
Exploratory analyses investigating the impact of genomic profile on ORR suggest that RET fusion partner (specifically the presence of NCOA4), TP53 mutation status, and tumor mutational burden (TMB), may each have an impact on response to selpercatinib. However, the exploratory nature of the analyses, relatively low number of patients with each tumor type, and complexities of combining data across several different molecular platforms preclude definitive conclusions from genomic profiling data collected in this trial.
Given the rarity of RET fusion-positive solid tumors, the magnitude of response observed in patients with NSCLC and thyroid cancer, and the diversity of tumor types with accompanying standard of care therapies, a randomized trial was not considered feasible. Considering the strength of the scientific evidence across a wide variety of tumor types including NSCLC and thyroid cancer, lack of available treatment options for patients with rare non-NSCLC and non-thyroid RET fusion-positive solid tumors, and acceptable safety profile of selpercatinib in this setting, the totality of the evidence favored approval of a tissue agnostic indication. Under 21 Code of Federal Regulations (CFR) 314 Subpart H, accelerated approval is subject to the requirement that the Applicant study the biological product further to verify and describe its clinical benefit.20 Data from additional patients with non-NSCLC, non-thyroid RET fusion-positive solid tumors, including a sufficient number of patients with tumor types for which responses require additional characterization (e.g., colorectal cancer, esophagogastric cancer, and glioma), will be submitted as a post-marketing requirement (PMR) to verify clinical benefit across tumor types. The PMR will include available data regarding RET fusion partners and co-occurring genetic alterations for all patients.
Selpercatinib for advanced RET fusion-positive NSCLC
Contemporaneously with the tissue agnostic approval of selpercatinib, the indication for the treatment of patients with locally advanced or metastatic NSCLC with a RET gene fusion which received accelerated approval in 2020, was granted regular approval. The recommendation for regular approval was based on additional data with longer duration of follow-up demonstrating substantial durability of responses, supported by a consistent, substantial ORR, in both treatment-naïve and previously treated patients enrolled in Study LIBRETTO-001.
The primary efficacy population included 69 patients with RET fusion-positive NSCLC who were naïve to systemic therapy (increased from the accelerated approval population of 39 patients) and 247 patients with RET fusion-positive NSCLC previously treated with platinum chemotherapy (increased from the accelerated approval population of 105 patients). The updated assessment of DOR provided an additional follow-up time of approximately 18 months from the data cut-off date for the data supporting accelerated approval. The confirmed ORR per RECIST 1.1 as determined by BICR in the treatment-naïve population was 84% (95% CI: 73%, 92%) and the median DOR was 20.2 months (95% CI: 13.0, NE). The confirmed ORR per RECIST 1.1 as determined by BICR in the previously treated population was 61% (95% CI: 55%, 67%) and the median DOR was 28.6 months (95% CI: 20.4, NE).
The observed DOR demonstrated a beneficial effect on a clinically meaningful endpoint, supported by consistency of ORR results in the larger efficacy population relative to the results supporting accelerated approval. FDA determined these results were sufficient to verify the clinical benefit of selpercatinib in the rare, genetically defined subgroup of patients with locally advanced or metastatic RET fusion-positive NSCLC.
The FDA Center for Devices and Radiological Health (CDRH) contemporaneously approved a Companion Diagnostic (Oncomine™ Dx Target Test; Thermo Fisher Scientific) to detect RET fusions in NSCLC and thyroid cancer and to detect RET mutations in medullary thyroid cancer for the safe and effective use of selpercatinib.21 The clinical review team determined it was in the best interest of U.S. patients to approve selpercatinib for the treatment of other RET fusion-positive solid tumors before one or more companion diagnostic assays were ready for submission. A post‐marketing commitment (PMC) was issued to provide adequate analytical and clinical validation results from clinical trial data to support labeling of a companion diagnostic test to identify patients with other RET fusion-positive solid tumors who may benefit from selpercatinib.
Conclusion
Selpercatinib is the first approved targeted therapy for patients with RET fusion-positive solid tumors other than NSCLC and thyroid cancer. The observed response rate and durability of responses, in the setting of a genetically based biologic rationale and the existing approvals in advanced RET fusion-positive NSCLC and thyroid cancer, are clinically meaningful in the context of the poor prognosis of the disease and limited available therapies. A clinical trial intended to verify the clinical benefit of selpercatinib in this population through more precise estimation of the overall response rate and mature response duration is currently ongoing with the final report submission due in December 2025.
Footnotes
Disclosure of Potential Conflicts of Interest: The authors report no financial interests or relationships with the commercial sponsors of any products discussed in this report.
References
- 1.Li AY, McCusker MG, Russo A, Scilla KA, Gittens A, Arensmeyer K, Mehra R, Adamo V, Rolfo C. RET fusions in solid tumors. Cancer Treat Rev. 2019. Dec;81:101911. doi: 10.1016/j.ctrv.2019.101911. [DOI] [PubMed] [Google Scholar]
- 2.Subbiah V, Yang D, Velcheti V, Drilon A, Meric-Bernstam F. State-of-the-Art Strategies for Targeting RET-Dependent Cancers. J Clin Oncol. 2020. Apr 10;38(11):1209–1221. doi: 10.1200/JCO.19.02551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belli C, Anand S, Gainor JF, Penault-Llorca F, Subbiah V, Drilon A, Andrè F, Curigliano G. Progresses Toward Precision Medicine in RET-altered Solid Tumors. Clin Cancer Res. 2020. Dec 1;26(23):6102–6111. doi: 10.1158/1078-0432.CCR-20-1587. [DOI] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021. Jan;71(1):7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 5.U.S. Food and Drug Administration. Drugs@FDA [database on the internet]. Selpercatinib USPI Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/213246s008lbl.pdf.
- 6.U.S. Food and Drug Administration. Drugs@FDA [database on the internet]. Pralsetinib USPI Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/214701s000lbl.pdf.
- 7.National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology. Available at: https://www.nccn.org/guidelines/category_1.
- 8.U.S. Food and Drug Administration. Assessment Aid. Available at: https://www.fda.gov/about-fda/oncology-center-excellence/assessment-aid.
- 9.U.S. Food and Drug Administration. Priority Review. https://www.fda.gov/patients/fast-track-breakthrough-therapy-accelerated-approval-priority-review/priority-review.
- 10.U.S. Food and Drug Administration. Drugs@FDA [database on the internet]. Selpercatinib Multi-Disciplinary Review and Evaluation, May 2020. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/213246Orig1s000TOC.cfm.
- 11.Subbiah V, Wolf J, Konda B, Kang H, Spira A, Weiss J, Takeda M, Ohe Y, Khan S, Ohashi K, Soldatenkova V, Szymczak S, Sullivan L, Wright J, Drilon A. Tumour-agnostic efficacy and safety of selpercatinib in patients with RET fusion-positive solid tumours other than lung or thyroid tumours (LIBRETTO-001): a phase 1/2, open-label, basket trial. Lancet Oncol. 2022. Oct;23(10):1261–1273. doi: 10.1016/S1470-2045(22)00541-1. [DOI] [PubMed] [Google Scholar]
- 12.U.S. Food and Drug Administration. Drugs@FDA [database on the internet]. Pembrolizumab USPI Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/125514s132lbl.pdf.
- 13.U.S. Food and Drug Administration. Drugs@FDA [database on the internet]. Larotrectinib USPI Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/210861s008lbl.pdf.
- 14.U.S. Food and Drug Administration. Drugs@FDA [database on the internet]. Entrectenib USPI Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/212725s006lbl.pdf.
- 15.U.S. Food and Drug Administration. Drugs@FDA [database on the internet]. Dostarlimab USPI Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761174s003s004lbl.pdf.
- 16.U.S. Food and Drug Administration. Drugs@FDA [database on the internet]. Dabrafenib USPI Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/202806s025lbl.pdf.
- 17.Lemery S, Fashoyin-Aje L, Marcus L, Casak S, Schneider J, Theoret M, Kluetz P, Pazdur R, Beaver JA. Development of Tissue-Agnostic Treatments for Patients with Cancer. Annu. Rev. Cancer Biol 2022. Apr;6(1):147–165. doi: 10.1146/annurev-cancerbio-060921-021828. [DOI] [Google Scholar]
- 18.U.S. Food and Drug Administration. Tissue Agnostic Drug Development in Oncology: Draft Guidance for Industry. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/tissue-agnostic-drug-development-oncology.
- 19.U.S. Food and Drug Administration. sNDA Multi-disciplinary Review and Evaluation and Approval Package: RETEVMO (selpercatinib), September 2022. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/213246Orig1s008ltr.pdf.
- 20.Code of Federal Regulations, Title 21, Volume 7, Part 601, Subpart E, Sec 601.41. Available at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=601.41.
- 21.U.S. Food and Drug Administration. Summary of Safety and Effectiveness Data, PMA P160045. Available at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=p160045s031. [Google Scholar]
- 22.Kato S, Subbiah V, Marchlik E, Elkin SK, Carter JL, Kurzrock R. RET Aberrations in Diverse Cancers: Next-Generation Sequencing of 4,871 Patients. Clin Cancer Res. 2017. Apr 15;23(8):1988–1997. doi: 10.1158/1078-0432.CCR-16-1679. [DOI] [PubMed] [Google Scholar]


