Abstract
Hybridomas secreting specific monoclonal antibodies (MAbs) to Vibrio cholerae serogroup O139 were produced. Six monoclones (hybridomas) secreting MAbs specific only to lipopolysaccharide of V. cholerae O139 strains and which did not cross-react to 137 strains of other enteric microorganisms were obtained. These clones were designated 12F5-G11, 12F5-G2, 15F5-H5, 5B9-F8, 14C9-D2, and 6D2-D8. The immunoglobulin (Ig) heavy chain isotypes secreted by these clones were IgG2b, IgG2b, IgG2b, IgM, IgG2b, and IgG3, respectively. Clone 12F5-G11 was selected for mass production of MAb, which was used as a detection reagent in the antigen detection assay for diagnosis of cholera caused by V. cholerae O139, and this assay was compared to the conventional bacterial isolation method. Five batches of rectal swab cultures in alkaline-peptone water were collected from 6,497 patients with watery diarrhea. These were 6,310 patients admitted to Bamrasnaradura Infectious Diseases Hospital, 16 patients from Krung Thon Hospital, 78 patients from Bangkok Children’s Hospital, 19 patients from Karen refugee camps, and 74 Indian patients from the National Institute of Cholera and Enteric Diseases, Calcutta, India. The V. cholerae O139 isolations from the rectal swab cultures and the antigen detection assays (i.e., the MAb-based dot-blot ELISA) were performed by different persons of different laboratories, and the results were revealed after all specimens had been tested. Of the 6,497 samples tested, the dot-blot ELISA correctly identified 42 of 42 V. cholerae O139-positive samples and gave a result of positive for three samples which were culture negative for V. cholerae O139. The diagnostic sensitivity, specificity, and efficacy of the dot-blot ELISA were 100, 99.95, and 99.26%, respectively. The ELISA is easy to perform and relatively inexpensive. It can test multiple samples at a single time, does not require special equipment, and does not produce great quantities of contaminated waste. Most of all, it reduces the diagnostic time from at least 2 days for the bacterial isolation to less than 90 min. The assay is recommended as a rapid screening test of cholera cases caused by V. cholerae O139.
Vibrio cholerae of serogroup O139 has been shown to share several properties with the serogroup O1 biotype El Tor organisms, including the genotypes which encode for cholera toxin and toxin-coregulated pili (13), the clinical disease caused by them (2) and the epidemic potential (1). However, organisms of this serogroup fail to agglutinate with the specific serogroup O1 antiserum (2, 7). Intensive genetic analysis has provided evidence that O139 arose from an El Tor V. cholerae strain by acquisition of a novel DNA which was inserted into and replaced part of the O-antigen gene cluster at the rfb region (encoding the O-antigen synthesis of the V. cholerae O1) of the recipient strain (3). The part of this novel DNA was sequenced, and two open reading frames, ontA and ontB, were found. These genes encode for O-antigen and capsule synthesis, respectively. Thus, the O139 strains produce O antigen different from that of the O1 antigen, which explains the nonreactivity of the O139 bacteria with the specific antiserum to V. cholerae O1. Furthermore, like many non-O1 but unlike the O1 strains, the O139 organisms produce a polysaccharide capsular material (17). Epidemiological data also showed a significant age group difference between the patients whose cholera was caused by the O1 and by the O139 organisms. The majority of O139-infected patients were adults while those with cholera caused by V. cholerae O1 were children (1). This information, together with the evidence that the adult population residing in the areas in which V. cholerae O1 is endemic is susceptible to O139 infection, implies that the natural immunity to O1 bacteria does not cross-protect against the disease caused by O139 bacteria (1, 14). Thus, there is a need for a separate vaccine against V. cholerae O139 infections.
Because V. cholerae O139 bacteria are similar to the O1 organisms in their epidemic potential and clinical patterns, rapid detection of cholera caused by them is also necessary for controlling an explosive outbreak, which may occur within a day or two after a single unrecognized cholera case. The standard method used extensively to detect V. cholerae is the bacterial culture method. In common practice, when a cholera case is suspected in an area remote from an available microbiology laboratory, a few drops of stool or rectal swabs are put in a transport medium, e.g., Cary-Blair, and sent to the laboratory. The bacteria are then transferred to an enrichment medium such as alkaline peptone solution, allowed to grow for 6 to 8 h, and then cultured on specific media such as (taurocholake-citrate-bile salt-sucrose (TCBS) agar. After 16 to 24 h of incubation at 37°C, typical colonies may be stained and tested for V. cholerae biochemical reactions. For final identification, the bacteria are subjected to agglutination tests against serogroup-specific antisera. The whole process of V. cholerae isolation and identification takes at least 2 days, and by that time the disease may have spread explosively. Therefore, as is true for cholera caused by V. cholerae O1, rapid detection of cholera caused by the O139 strain or its carriers is needed for controlling the disease.
In this report, a rapid, simple, specific, sensitive, and inexpensive dot ELISA similar in principle and concept to that for V. cholerae O1 previously reported by our group (4) has been developed to detect the V. cholerae O139 antigen in stools of patients with watery diarrhea by using the specific monoclonal antibody (MAb) directed against O139 lipopolysaccharide (LPS).
MATERIALS AND METHODS
LPS was prepared from V. cholerae serogroup O139 strain TH 166 isolated from a cholera patient in 1993. The phenol-water extraction method of Westphal and Jann (18) was followed throughout. The single extracted LPS was reextracted two more times until no protein could be detected by the method of Lowry et al. (11).
Whole-cell lysates (Ly) were prepared from the organisms listed in Table 1. Bacterial cells obtained from growth at 37°C for 4 h in Trypticase soy broth were lysed in distilled water to give an optical density (OD) at 540 nm of 2.0. The preparations were subjected to ultrasonication at 20 kHz for 10 min. Dry weight and protein contents of all preparations were determined. Extract of Entamoeba histolytica trophozoites was kindly provided by Wanchai Maleewong, Department of Parasitology, Faculty of Medicine, Kohn Kaen University, Thailand. All of the Ly and the E. histolytica extracts were used for cross-reactivity testing of the MAbs and for checking specificity of the MAb-based dot ELISA. Log-phase cultures of V. cholerae O139 were obtained by inoculating a loopful of the organisms from an overnight plate into 10 ml of alkaline peptone broth and incubating it at 37°C for 4 h in a shaking incubator. Viable counts were made by dilution plating. These whole cells were used for determining the analytical sensitivity of the dot ELISA.
TABLE 1.
Bacterial strains and Entamoeba histolytica used for preparing the whole-cell lysates
| Strain | Strain | Strain | ||
|---|---|---|---|---|
| V. cholerae | ||||
| O139 TH 166 | ||||
| O139 MO 45 | ||||
| O139 NG 1619/93 | ||||
| O139 NG 1671/93 | ||||
| O139 NG ACD/93 | ||||
| O139 NGTH 1380 | ||||
| O139 W 1163 | ||||
| O139 W 1164/93 | ||||
| O139 W 1165/93 | ||||
| Classical, Inaba 569B | ||||
| El Tor, Inaba 519/35 | ||||
| El Tor, Inaba 20/37 | ||||
| El Tor, Inaba 4/32 | ||||
| El Tor, Inaba 1514/32 | ||||
| El Tor, Inaba 3030/33 | ||||
| El Tor, Inaba 4459/36 | ||||
| El Tor, Inaba 609/34 | ||||
| El Tor, Inaba 1271/34 | ||||
| El Tor, Ogawa 17/33 | ||||
| El Tor, Ogawa 58/38 | ||||
| El Tor, Ogawa 66/38 | ||||
| El Tor, Ogawa 67/38 | ||||
| El Tor, Ogawa 68/38 | ||||
| El Tor, Ogawa 69/38 | ||||
| El Tor, Ogawa 1580/35 | ||||
| El Tor, Ogawa 287/35 | ||||
| NG 1051a | ||||
| NG 518/35 | ||||
| NG 523/35 | ||||
| NG 524/35 | ||||
| NG 526/35 | ||||
| NG 527/35 | ||||
| NG 528/35 | ||||
| NG 532/35 | ||||
| NG 534/35 | ||||
| NG 537/35 | ||||
| NG 538/35 | ||||
| NG 542/35 | ||||
| NG 545/35 | ||||
| NG 556/35 | ||||
| NG 559/35 | ||||
| NG 564/35 | ||||
| NG 566/35 | ||||
| NG 567/35 | ||||
| NG 577/35 | ||||
| NG 592/35 | ||||
| NG Ca 385 | ||||
| NG 1166 |
NG, nonagglutinatable vibrio.
W, water isolate.
Young adult BALB/c mice (6 to 7 weeks old) were kindly supplied by the Armed Forces Research Institute of Medical Sciences (AFRIMS), U.S. Component, Bangkok. For the hybridoma production five mice were immunized. Before immunization, the mice were bled individually via the retro-orbital plexus, and the sera were collected, pooled, and used as a pool of negative control serum. After bleeding, each mouse was injected intraperitoneally with 0.2 ml of a mixture of equal volume of the Ly (1 mg/ml in normal saline solution) of V. cholerae O139 TH 166 and Freund’s complete adjuvant. The mice were reimmunized two more times at 2-week intervals using the same dose and route of the immunogen but with Freund’s incomplete adjuvant. Three weeks after the third immunization, they were bled and their sera were assayed for antibody titers against the homologous antigen by an indirect enzyme-linked immunosorbent assay (ELISA) (5). The immune mouse showing the highest titer was used as a spleen cell donor in hybridoma production while the others were bled and their sera were pooled and used as a pool of positive control serum (PS). Three days before the cell fusion, the immune mouse was given an intravenous injection of a half dose of the immunogen in normal saline solution.
Three days after the intravenous booster, the immune mouse was bled and the serum was subsequently used as an immune serum (IS). The animal was then sacrificed by cervical dislocation. Spleen cells were fused with P3x-63-Ag8 myeloma cells by using polyethylene glycol 4000 as a fusogen at a spleen cell/myeloma cell ratio of 10:1 for production of hybridomas as previously described (4, 5). Culture fluids from culture wells containing growing hybrids were collected and screened for antibodies against the homologous antigen. Cells from the antibody-positive wells were subjected to cloning by the limiting dilution method using spleen cells of a nonimmune BALB/c mouse as feeder cells. Supernatants from these clones (hybridomas) were retested against the homologous antigens as well as a panel of the heterologous antigens for cross-reactivity by indirect ELISA. Antigenic specificity of the MAb secreted by an individual clone was determined by Western blotting against the sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)-separated homologous antigen.
An indirect ELISA was used for determining antibody titers of sera from immunized mice and for detecting antibodies in the cell culture supernatants in positive hybrid screening. The technique was also used for determining specificity versus cross-reactivity of the MAb. The microtiter plates were coated with appropriate antigen (in 10 μg of carbonate-bicarbonate buffer [pH 9.6]/ml) listed in Table 1. LPS of V. cholerae O139 TH 166 was also included in the test, which served as a positive control. The antigen-sensitized plates were incubated at 37°C in a humid chamber for 2 h and at 4°C overnight. The unbound antigens were washed off with phosphate-buffered saline (PBS) containing 0.5% Tween-20 (PBST). The unoccupied sites on plates were blocked with 1% bovine serum albumin (BSA) in PBS and incubated at 37°C for 1 h. The excess BSA was washed off, and 100 μl of antibody preparation (serially diluted IS or PS, undiluted cell culture supernatant) and fresh culture medium, which served as a negative control or blank, were added to the appropriate wells. The antigen-antibody reaction was allowed to take place for 1 h at 37°C. After washing thoroughly with PBST, 100 μl of a 1:1,000 dilution of rabbit anti-mouse immunoglobulin (Ig)-horseradish peroxidase conjugate (Dakopatts, Glostrup, Denmark) in PBS containing 0.2% BSA and 0.2% gelatin was added to each well and incubated as described above for 1 h. The unbound conjugate was removed by washing with PBST. The enzyme substrate was added to all wells (100 μl per well). The reaction was allowed to take place in the dark for 30 min and was then stopped by adding 50 μl of 1 N NaOH per well. The OD of each well was measured at 492 nm with an ELISA reader (Titertek Multiscan MCC/340, Helsinki, Finland) against the blank. The ELISA titer of the antibody preparation was the highest dilution of the antibody which gave an OD that was >0.05. One indirect ELISA unit was defined as the smallest amount of the antibody which gave a positive indirect ELISA reaction.
Dot ELISA was used for detecting V. cholerae O139 antigen in the rectal swab specimens and for testing the cross-reactivity of the antibodies produced by the growing hybridomas. It was performed as described previously (4). A 3-μl aliquot of the sample (boiled V. cholerae whole cells, Ly, or specimen) was dotted onto the nitrocellulose (NC) strip (nitrocellulose membrane; Bio-Rad, Richmond, Calif.), and the strip was air dried. The positive control was 3 μl of V. cholerae O139 TH 166 Ly (10 μg of PBS/ml); the negative control was 3 μl of appropriate medium or diluent (fresh alkaline peptone solution, RPMI 1640 medium, or PBS). The NC strip was blocked with 0.5% BSA in PBS (pH 7.4), incubated at room temperature for 10 min, and washed with three changes of 0.1 M Tris-HCl (pH 7.5). The strip was then incubated with the MAb (at the concentration of 80 indirect ELISA units/ml in the experiments for detecting the V. cholerae O139 antigen in patients’ specimens; the culture supernatant of the hybridoma was used undiluted in the cross-reactivity checking) for 20 min with occasional rocking, washed with PBS (pH 7.4) as described above, and then incubated with the rabbit anti-mouse Ig-enzyme conjugate. After 10 min, the strip was washed with PBS (pH 7.4) three times and once with 0.15 M Tris (pH 9.6) and was then immersed in a substrate solution for color development for 5 min, washed with distilled water, and air dried. Positive reactions appeared as blue spots while negative reactions appeared as spots of brown or other (nonspecific) colors or as clear areas.
The SDS-PAGE was carried out in a vertical slab gel apparatus (Bio-Rad) according to the method of Laemmli (10). A 4% stacking gel and 10% acrylamide separating gel were used in the procedure. Western blotting was performed by transblotting the SDS-PAGE-separated Ly or LPS of V. cholerae O139 TH 166 from the gel to an NC membrane (15). The unoccupied sites on the NC were blocked by soaking it in a solution of 0.5% gelatin and 0.2% BSA in PBS for 1 h. After washing with PBST, the NC membrane was treated with antibody preparation (at an appropriate dilution of immune serum or culture supernatant of the hybridoma) at room temperature for 1 h. After washing thoroughly, the NC was put in a solution of rabbit anti-mouse Ig-horseradish peroxidase conjugate (Dakopatts) at a dilution of 1:1,000 in PBS (pH 7.4) containing 1% BSA and 1% gelatin for 30 min at room temperature. The NC was washed with phosphate buffer (pH 7.6) before being placed in a substrate solution for 5 min, washed with distilled water, and air dried.
Five batches of rectal swab specimens were collected from patients with acute watery diarrhea. The first batch consisted of specimens collected from 6,310 patients admitted to Bamrasnaradura Infectious Diseases Hospital, Nonthaburi province, Thailand; the second batch consisted of 16 rectal swabs of 16 patients admitted to Krung Thon Hospital, Bangkok; the third batch consisted of rectal swabs of 78 patients from the Children’s Hospital, Bangkok; the fourth batch consisted of swabs of 19 patients from two Karen Refugee Camps in Rachaburi province, Central Thailand; and the fifth batch was collected from 74 Indian patients from the National Institute of Cholera and Enteric Diseases, Calcutta, India. Each rectal swab was put in 4 ml of an alkaline peptone solution and enriched for approximately 6 to 8 h. One milliliter of the enriched culture was boiled for 30 min and sent to the Department of Microbiology and Immunology, Faculty of Tropical Medicine, Mahidol University, Bangkok, where it was kept frozen at −20°C for subsequent V. cholerae O139 antigen detection by dot ELISA performed by a scientist who was not among the ones who performed the bacterial cultures. The remaining portion of the alkaline peptone was used to complete V. cholerae O139 isolation according to the World Health Organization guidelines (19) by different bacteriologists of the hospital or health centers mentioned above, except the bacterial cultures of specimens from the refugee camps were performed at AFRIMS in Bangkok. The results of the dot ELISA and the V. cholerae O139 isolation were revealed and compared when all specimens had been completely tested.
The sensitivity, specificity, and efficacy of the dot ELISA were evaluated in comparison with the bacterial isolation method by using the method of Galen (6).
RESULTS
Antibody titers against the homologous antigen of the immunized mice were assessed by the indirect ELISA 3 weeks after the last intraperitoneal booster. Mice no. 1, 2, and 3 had titers of 1:12,800 and the fifth mouse had a titer of 1:25,600. These four immune mice were bled via the retro-orbital plexi, and their sera were pooled and used as the PS. Mouse no. 4 had the highest titer (1:51,200); thus, it was used as a splenocyte donor in the hybridoma production. This mouse was given an intravenous booster with the immunogen at half the amount of the intraperitoneal dose 3 days before cell fusion. On the day of cell fusion, the mouse was bled, and the serum was used as the IS. A total of 1.68 × 108 spleen cells were obtained from the mouse, which were mixed with 1.68 × 107 myeloma cells in the cell fusion procedure. The cell mixture was aliquoted into a total of 1,800 tissue culture wells in 96-well tissue culture plates. Of the 1,800 wells, 1,188 had growing hybrids (66%). The culture supernatants from these 1,188 wells were tested for antibodies against the Ly of the homologous V. cholerae O139, and the fluids from 500 wells (27.8%) were positive. Of the 500 wells, only 42 wells had cell culture supernatants with indirect ELISA OD of >0.100. These were subjected to the cloning, and a total of six specific hybridomas were obtained. These were designated clones 12F5-G11, 12F5-G2, 12F5-H5, 5B9-F8, 14C9-D2, and 6C2-D8. The MAbs secreted by these hybridomas were isotyped by using the mouse typer subisotyping kit (Bio-Rad). The isotypes were IgG2b for the clones 12F5-G11, 12F5-G2, and 12F5-H5; IgM for 5B9-F8; IgG2a for 14C9-D2, and IgG3 for 6C2-D8. All of the Ig molecules carried kappa-type light chains (Table 2). The antibody titers presented in the culture supernatants when the six monoclones had grown to the stationary phase are shown also in Table 2. The titers were consistent; several subcultures of these clones always yielded the same titers. The clone 12F5-G11 was propagated further for bulk production of the V. cholerae O139-specific MAb while the other clones were kept frozen in a liquid nitrogen tank.
TABLE 2.
Specific monoclones, their secreted Ig isotypes, and the indirect ELISA titers of their culture supernatants at stationary phase of growth
| Monoclones | Ig isotype
|
Reciprocal indirect ELISA titer | |
|---|---|---|---|
| H chain | L chain | ||
| 12F5-G11a | IgG2b | Kappa | 1,024 |
| 12F5-G2 | IgG2b | Kappa | 256 |
| 15F5-H5 | IgG2b | Kappa | 256 |
| 5B9-F8 | IgM | Kappa | 512 |
| 14C9-D2 | IgG2a | Kappa | 256 |
| 6C2-D8 | IgG3 | Kappa | 256 |
The monoclone the culture supernatant of which was used in the dot ELISA for detecting V. cholerae O139 antigen in the specimens.
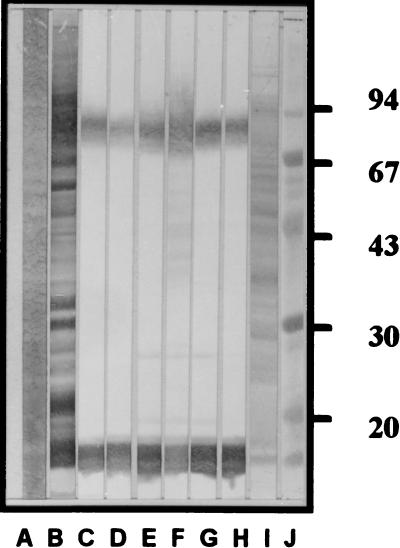
The MAbs of all six clones reacted to the Ly and LPS of the V. cholerae O139 TH 166 and also to the Ly of all V. cholerae O139 tested but did not react to the Ly of any other heterologous antigens listed in Table 1; thus, they were specific to V. cholerae O139. Figure 1 illustrates the antigenic specificities of the MAbs from these clones as tested against the SDS-PAGE-separated whole-cell lysate of V. cholerae TH 166. The same patterns of antigenic specificities were seen when the MAbs were tested against the TH 166 LPS (data not shown).
FIG. 1.
Western blot patterns of MAbs from the six specific monoclones against SDS-PAGE-separated V. cholerae O139 strain TH 166 whole-cell Ly. Lanes: A, separated Ly stained by Con A-enzyme conjugate; B through H, Western blot patterns of immune serum of mouse no. 4 and MAbs from clones 12F5-G11, 12F5-G2, 15F5-H5, 5B9-F8, 14C9-D2, and 6C2-D8, respectively; I, separated Ly stained with amido black; J, standard molecular weight markers (numbers at right are relative molecular masses × 10−3).
The MAbs secreted from the hybridoma 12F5-G11 (MAb12F5-G11) were used as a detection reagent in the dot ELISA performed on the Ly of all organisms listed in Table 1. The dot ELISAs were positive only with the Ly of the homologous organism and of the other V. cholerae O139 and were negative for the antigens of the V. cholerae O1, non-O1-non-O139 V. cholerae, other vibrios, and other enteric organisms. The smallest amounts of the homologous V. cholerae O139 and its LPS which could be detected by the dot ELISA were 30 cells (1 × 104 CFU/ml) and 0.48 ng, respectively. The dot ELISA was still positive when tested on a 20-μl dot containing 200 V. cholerae cells and 10,000 or more cells of normal flora Escherichia coli.
The MAb-based dot ELISA was performed on the five batches of the stool samples for comparison with the bacterial culture method. The dot ELISA correctly detected 5 V. cholerae O139 culture-positive samples from the 6,310 specimens collected from Bamrasnaradura Infectious Diseases Hospital (the first batch of specimens). Both tests were negative for 6,304 samples. However, one sample was culture negative but dot-ELISA positive. The sensitivity and specificity of the dot ELISA were 100 and 99.98%, respectively. The two tests were in perfect agreement when tested on the second batch of the specimens; testing found that 16 samples collected from patients admitted to Krung Thon Hospital were positive for V. cholerae O139. Among the 78 stool samples from the Bangkok Children’s Hospital, 1 was positive for V. cholerae O139 and the others were negative by both tests, thus showing perfect agreement of the two assays (the sensitivity and specificity were both 100%). There was no positive V. cholerae O139 sample among the 19 samples of the fourth batch of the specimens (collected from the Karen refugee camps). Although the sensitivity could not be calculated from the result because there was no positive case, the two methods were in perfect agreement (100% specificity). The dot ELISA correctly identified 20 culture-positive and 52 culture-negative specimens of the fifth batch. However, the dot ELISA was positive for two culture-negative samples. The sensitivity and specificity of the dot ELISA compared with the bacterial isolation method were 100 and 96.29%, respectively. The overall results of the sample testing by the dot ELISA in comparison to the culture method are shown in Table 3. Of the 6,497 samples tested, the dot ELISA correctly identified 42 of 42 V. cholerae culture-positive samples and gave a result of positive for 3 samples which were culture negative for V. cholerae O139. Thus, the diagnostic sensitivity, specificity, and efficacy of the dot ELISA were 100, 99.95, and 99.26%, respectively.
TABLE 3.
Comparison of the results of the dot-blot ELISA performed on the 6,497 specimens and the results of the V. cholerae O139 isolation method
| Dot-blot ELISA resulta | Culture for V. cholerae O139
|
Total | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 42 | 3 | 45 |
| Negative | 0 | 6,452 | 6,452 |
| Total | 42 | 6,455 | 6,497 |
The sensitivity, specificity, and efficacy of the dot ELISA were 100, 99.5, and 99.26%, respectively.
DISCUSSION
V. cholerae O139, like V. cholerae O1, can spread rapidly and cause explosive outbreaks. The conventional culture method to detect cholera takes at least 2 days, by which time the disease might have spread widely. The rapid detection of cholera cases or asymptomatic carriers is needed for epidemic control and disease surveillance.
It is known that V. cholerae O139 arose from the El Tor V. cholerae O1 by a novel DNA insertion and replacement of a portion of the DNA for an O1 antigen synthesis (3). Specific antibodies to the serogroup O1 do not react with the V. cholerae O139 or their LPS (8). It is necessary, therefore, to have anti-V. cholerae O139-specific antibodies, either polyclonal or monoclonal, for use in the final identification of the isolated V. cholerae in the conventional bacterial culture. The serogroup-specific polyclonal antibodies to the V. cholerae O139 are usually prepared by repeated immunization of the animals, e.g., rabbits, over an extended period of time by using the V. cholerae whole cells as the immunogen. The cross-reacting antibodies in the immune serum are then extensively absorbed by a panel of heterologous antigens, especially the V. cholerae O1, and O2 to O138 and the other enteric pathogens. Such absorption usually results in the decrease of the antibody titer of the immune serum and sometimes was incomplete (antibodies to V. cholerae of other groups were still present, especially the antibody to O55 and/or the antirough mutant). Therefore, MAbs directed to the specific O antigen of V. cholerae O139 should be a more reliable source of highly specific reagent for the immunological purposes (9).
Applications of MAbs specific to V. cholerae have been partly outlined by Holme and Gustafsson (9). MAbs can be used instead of polyclonal antibodies in the final step of the conventional culture method for identifying the serogroups of the isolated organisms and for inhibiting the darting movement of the living organisms in the motility-inhibition test for rapid detection of V. cholerae in diarrheic stool. MAbs may be used in other immunoassays for rapid detection of the bacteria in stools or other specimens, e.g., food, water, or other environmental samples. Rapid recognition of cholera cases and V. cholerae sources is important, because epidemiological work such as active prevention and control measures can be promptly arranged before the occurrence of any outbreak. Moreover, serogroup-specific MAbs may be useful in monitoring the quantities of their respective O antigens at various stages of vaccine preparations, especially a refined antigen vaccine, or they may be used for screening of genetically modified mutants produced for various purposes.
In hybridoma technology for the production of the MAbs, it is known that the quality of the MAbs depends on various factors including the source, form, route, and nature of the immunogen as well as the immunization schedule. Pure immunogens are not always essential or even preferred to the crude immunogen. From our past experience in producing V. cholerae serogroup O1-specific MAbs (MAbs to antigen A of the LPS molecule), it was found that the most suitable immunogen was the bacterial whole-cell lysate and not the pure LPS. Immunization of the mice with pure LPS (T-independent immunogen) resulted in low antibody titers predominantly of the IgM isotype, the type with comparatively low binding affinity to the antigen. In this study, therefore, the V. cholerae serogroup O139 whole-cell lysate was used as the immunogen, and the same dose, route, and schedule of immunization as those described previously (4) were applied. It was found that the percentages of the growing polyhybrids (66%) and the antibody-positive polyhybrids (27.8%) were higher than when we produced V. cholerae serogroup O1-specific hybridomas. In this study, we cloned only 8.4% of the positive polyhybrids (cells from 42 wells with OD of >1.00 out of the 500 wells with antibody-positive polyhybrids), and up to six specific hybridoma clones (monoclones) were obtained. Western blot analysis revealed that although these monoclones produce antibodies of different isotypes, i.e., IgG2a, IgG2b, and IgM (Table 2), they were specific to the same antigenic epitope, i.e., the LPS molecule at the location of O-side chain (Fig. 1, lanes C to H, bottom) connected to the core polysaccharide (Fig. 1, lanes C to H, top) (16). Antigenic cross-reactivity testing by both indirect (microplate) and dot-blot (membrane) ELISA revealed that the MAbs of these six clones were reactive only to the whole-cell lysates and LPS prepared from V. cholerae serogroup O139 and did not react to V. cholerae of other serogroups, other bacteria, E. histolytica extract, and even normal stool extract, indicating 100% analytical specificity of the MAbs to the V. cholerae O139 antigen.
The MAbs secreted from the clone 12F5-G11 (MAb12F5-G11) which were IgG2b (the isotype known to have high binding affinity to the homologous antigen) were used in a dot ELISA for detecting varying concentrations and numbers of V. cholerae O139, respectively. The lowest amounts of the LPS and cells which could be detected by the assay were 0.48 ng and 30 cells (104 CFU/ml), respectively. The analytical sensitivity of the dot ELISA was higher than the MAb-based coagglutination test (107 CFU/ml) and slightly better than the Bengal SMART test (5 × 105 CFU/ml) (12, 13).
In this study, there were three culture-negative samples that tested positive by the dot ELISA. Thus, the diagnostic specificity of the dot ELISA compared to the culture method was 99.95% and the sensitivity was 100% (the dot ELISA correctly identified 42 culture-positive samples). The discrepant results of the two tests on these specimens might be due to the fact that the culture method could detect only living cells while the dot ELISA detects any form of the V. cholerae LPS. For successful culture, a sufficient number of living V. cholerae organisms must first be picked up by the rectal swab (which can absorb about 0.1 to 0.2 ml of fluid). Second, an adequate number of living vibrios must be contained in the alkaline-peptone solution after the enrichment period to assure that one or more living cells are presented in the aliquot subsequently streaked onto the selective medium, such as TCBS agar. In the situation of prior treatment with antibiotics, the causative vibrios in the patients’ intestinal tracts might have been killed, but adequate numbers of the organisms, their antigens, and the cholera toxin might still be present. These antigens could be picked up by the swabs, which were then placed into 4 ml of the alkaline peptone solution. It is known that during the purgation period of cholera, the patient may harbor as many as 108 to 109 vibrios/ml; thus, the 0.1 to 0.2 ml of rectal fluid absorbed by the swab should contain a sufficient number of the vibrios to yield a positive result by the dot ELISA even though all or most of them were dead and could not grow in the enrichment medium. Experiments with multiplex PCR using primers designed from the rfb and ctx genes of V. cholerae O139 (unpublished data) have revealed that the three culture-negative, dot-ELISA-positive specimens contained the respective DNA fragments to the primers, and appropriate amplicons were obtained after completion of the reactions. This finding indicates the higher sensitivity of the MAb-based dot ELISA than the conventional culture method for diagnosis of cholera or detection of the vibrios in rectal swabs from patients.
From this study, the MAbs directed against the V. cholerae O139 epitope located at the junction of the O-side chain and the core polysaccharide of the LPS molecule were produced. Their best representative, i.e., MAb12F5-G11, was used in the dot ELISA to detect V. cholerae O139 in the rectal swab samples of the diarrheic patients and gave 100, 99.95, and 99.26% sensitivity, specificity, and efficacy, respectively, compared with the conventional bacterial isolation method. The ELISA is easy to perform, relatively inexpensive, sensitive, and specific. It allows the testing of multiple samples at a single time. It requires no special equipment and does not produce as much contaminated waste for disposal as does the culture method. Most of all, it reduces the test time from at least 2 days for the culture to less than 90 min. The assay is recommended as a rapid screening test of cholera cases caused by V. cholerae O139.
ACKNOWLEDGMENTS
The work was financially supported by the National Center for Genetic Engineering and Biotechnology, National Science and Technology Development Agency, Ministry of Science, Technology and Environment, Thailand.
We thank L. Pang, AFRIMS, Bangkok, for reviewing the manuscript.
REFERENCES
- 1.Albert M J, Ansaruzzaman M, Bardhan P K, Faruque A S G, Faruque S M, Islam M S, Mahalanabis D, Sack R B, Salam M A, Siddique A K, Yunus M D, Zaman K. Large epidemic of cholera-like disease in Bangladesh caused by Vibrio cholerae O:139 synonym Bengal. Lancet. 1993;342:387–390. [PubMed] [Google Scholar]
- 2.Albert M J, Siddique A K, Islam M S, Faruque A S G, Ansaruzzaman M, Faruque S M, Sack R B. Large outbreak of clinical cholera due to V. cholerae non-O:1 in Bangladesh. Lancet. 1993;341:704. doi: 10.1016/0140-6736(93)90481-u. [DOI] [PubMed] [Google Scholar]
- 3.Bik E M, Bunschoten A E, Gouw R D, Mooi F R. Genesis of the novel epidemic Vibrio cholerae O:139 strain: evidence for horizontal transfer of genes involved in polysaccharide synthesis. EMBO J. 1995;14:209–216. doi: 10.1002/j.1460-2075.1995.tb06993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaicumpa W, Thattiyapong A, Supawat K, Chongsa-nguan M, Kalambaheti T, Eampokalap B, Ruangkunaporn Y, Sricharmorn S, Tapchaisri P. Rapid detection of V. cholerae O:1. Serodiag Immunother Infect Disease. 1994;7:161–172. [Google Scholar]
- 5.Chaicumpa W, Thin-inta W, Khusmith S, Tapchaisri P, Echeverria P, Kalambaheti T, Chongsa-nguan M. Detection with monoclonal antibody of Salmonella typhi antigen 9 in specimens from patients. J Clin Microbiol. 1988;26:1824–1830. doi: 10.1128/jcm.26.9.1824-1830.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galen R S. The predictive values and efficiency of laboratory testing. Pediatr Clin N Am. 1980;27:861–869. doi: 10.1016/s0031-3955(16)33930-x. [DOI] [PubMed] [Google Scholar]
- 7.Garg S, Saha P K, Ramamurthy T, Deb B C, Nair G B, Shimada T, Takeda Y. Nationwide prevalence of the new epidemic strain of Vibrio cholerae O:139 Bengal in India. J Infection. 1993;27:108–109. doi: 10.1016/0163-4453(93)94398-u. [DOI] [PubMed] [Google Scholar]
- 8.Hisatsune K, Kondo S, Isshiki Y, Iguchi T, Kawamata Y, Shimada T. O-antigenic lipopolysaccharide of Vibrio cholerae O:139 Bengal, a new epidemic strain for recent cholera in the Indian subcontinent. Biochem Biophys Res Commun. 1993;3:1309–1315. doi: 10.1006/bbrc.1993.2395. [DOI] [PubMed] [Google Scholar]
- 9.Holme T, Gustafsson B. Monoclonal antibodies against group- and type-specific antigens of Vibrio cholerae O:1. In: Macario A J L, de Macario E C, editors. Monoclonal antibodies against bacteria. Vol. 1. New York, N.Y: Academic Press Inc.; 1985. pp. 167–189. [Google Scholar]
- 10.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 11.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 12.Qadri F, Chowdhury A, Hossain J, Chowdhury K, Azim T, Shimada T, Islam K M N, Sack R B, Albert M J. Development and evaluation of rapid monoclonal antibody-based coagglutination test for direct detection of Vibrio cholerae O139 synonym Bengal in stool samples. J Clin Microbiol. 1994;32:1589–1590. doi: 10.1128/jcm.32.6.1589-1590.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qadri F, Hasan J A K, Hossain J, Chowdhury A, Begum Y M, Azim T, Loomis L, Sack R B, Albert M J. Evaluation of the monoclonal antibody-based kit Bengal SMART for rapid detection of Vibrio cholerae O139 synonym Bengal in stool samples. J Clin Microbiol. 1995;33:732–734. doi: 10.1128/jcm.33.3.732-734.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siddique A K, Zaman K, Baqui A H, Akram K, Mutsuddy P, Eusof A, Haider K, Islam S, Sack R B. Cholera epidemics in Bangladesh. J Diarrhoeal Dis Res. 1992;10:79–86. [PubMed] [Google Scholar]
- 15.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheet: procedure and some applications. Proc Natl Acad Sci USA. 1973;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsai H, Frasch C E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 17.Weintraub A, Widmalm G, Jansson P E, Jansson M, Hultenby K, Albert M J. Vibrio cholerae O139 possesses a capsular polysaccharide which may confer increased virulence. Microb Pathog. 1994;16:235–241. doi: 10.1006/mpat.1994.1024. [DOI] [PubMed] [Google Scholar]
- 18.Westphal O, Jann K. Bacterial lipopolysaccharide extraction with phenol-water and further applications of the procedure. Methods Carbohydr Chem. 1965;5:83–91. [Google Scholar]
- 19.World Health Organization. Guidelines for the laboratory diagnosis of cholera. Geneva, Switzerland: World Health Organization; 1974. [Google Scholar]