Summary
Patients with chronic myeloid leukaemia in chronic phase (CML-CP) who have a sustained deep molecular response (DMR) are eligible to discontinue treatment and attempt treatment-free remission (TFR). In the DASFREE study (ClinicalTrials.gov; NCT01850004), the 2-year TFR rate after dasatinib discontinuation was 46%; here we present the 5-year update. Patients with a stable DMR after ≥ 2 years of dasatinib therapy discontinued treatment and were followed for 5 years. At a minimum follow-up of 60 months, in 84 patients discontinuing dasatinib, the 5-year TFR rate was 44% (n = 37). No relapses occurred after month 39 and all evaluable patients who relapsed and restarted dasatinib (n = 46) regained a major molecular response in a median of 1.9 months. The most common adverse event during the off-treatment period was arthralgia (18%, 15/84); a total of 15 withdrawal events were reported in 9 patients (11%). At the 5-year final follow-up, almost half of the patients who discontinued dasatinib after a sustained DMR maintained TFR. All evaluable patients who experienced a relapse quickly regained a DMR after restarting dasatinib, demonstrating that dasatinib discontinuation is a viable and potentially long-term option in patients with CML-CP. The safety profile is consistent with the previous report.
Keywords: Dasatinib, deep molecular response, treatment-free remission, major molecular response, chronic phase chronic myeloid leukaemia
Introduction
The introduction of tyrosine kinase inhibitors (TKIs) allowed optimal disease control in patients with chronic myeloid leukaemia (CML) in the chronic phase (CML-CP), thereby allowing them to have a life expectancy similar to the general population.1, 2 Achieving a deep molecular response (DMR), defined as a 4- or 4.5-log reduction in BCR::ABL1 levels (≤ 0.01% or ≤ 0.0032% International Scale [IS], respectively), is a treatment goal for patients with CML-CP who wish to discontinue treatment safely. Treatment with second-generation TKIs is associated with increased rates of deep and sustained molecular responses.3–5 However, long-term treatment with TKIs is associated with an increased risk of adverse events (AEs)3–5 (such as pleural effusion with dasatinib) and can also result in moderate to severe symptom burden (per the MD Anderson Symptom Inventory questionnaire for patients with CML).6 In addition, reduced adherence due to the cost of treatment can occur with long-term TKI treatment.7 As a result, discontinuation of TKI treatment, or attempting treatment-free remission (TFR), is increasingly becoming a preferred option among patients with CML-CP with a sustained DMR.
EURO-SKI is the largest international trial to date investigating TFR, with 758 patients discontinuing TKI treatment after first-line (1L) or second-line (2L) imatinib, dasatinib, or nilotinib (including 14 and 62 patients discontinuing 1L and 2L dasatinib, respectively). After 3 years, the overall molecular recurrence-free rate was 46%.8 In the AFTER-SKI study following up 111 patients from EURO-SKI who maintained TFR, 99 patients (89%) were still in TFR after a further 3 years.9 Other TFR studies with long-term follow-ups, such as the STIM and A-STIM studies with imatinib, have reported TFR rates of 38% (5-year TFR) to 46% (estimated 7-year TFR).10, 11 In addition, the ENESTfreedom trial showed that 43% of patients who entered the TFR phase after discontinuing 1L nilotinib remained in TFR at 5 years.12 Across all long-term TFR studies, most relapses occurred within the first 6–24 months, with late relapses being uncommon.10–14
Based on the successful TFR observed in long-term follow-ups, the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®), the ESMO guidelines and the ELN recommendations now consider TFR a potential treatment goal.15–17 Eligible patients (aged ≥ 18 years) with CML-CP and prior evidence of quantifiable BCR::ABL1 transcripts are required to have had an optimal response to 1L TKI therapy (with no treatment failure or 2L TKI therapy if intolerance was the only reason for switching [ELN recommendations only]), prior TKI treatment for 3 years (NCCN Guidelines®) to > 5 years (ESMO guidelines/ELN recommendations; > 4 years for second-generation TKI [ELN recommendations]) and a sustained DMR (MR4 or better) for 2 years or more prior to TFR attempt. Patients are also required to undergo regular molecular monitoring using a reliable quantitative reverse transcriptase-polymerase chain reaction (qPCR) test with a sensitivity of detection of at least MR4.5.
DASFREE (ClinicalTrials.gov; NCT01850004) is an open-label, single-arm, phase 2 study of dasatinib discontinuation in patients with CML-CP in DMR,18 and is the largest clinical trial to date to assess TFR after dasatinib discontinuation. In total, 84 patients with a DMR after 1L or 2L dasatinib (including patients with prior resistance to 1L therapy) discontinued treatment. In the 2-year update, TFR rates at 1 year (primary endpoint) and 2 years post-dasatinib discontinuation were 48% and 46%, respectively. Of the 46 patients who lost a major molecular response (MMR; BCR::ABL1 < 0.1% IS), 45 restarted treatment. At the time of primary analysis, 44 of these patients had regained MMR and 43 had regained MR4.5 after a median (range) of 2 (1–4) and 3 (2–18) months, respectively (data were not available for one patient). The 2-year progression-free survival (PFS) rate was 99% and no patients progressed to advanced phase or blast crisis (CML-AP/BC); however, one patient died from ovarian cancer 1 month after discontinuing the study. The 2-year update also identified age, duration of prior dasatinib and prior line of dasatinib therapy as potential predictors for maintaining TFR over the 2-year study period. Here we report the final 5-year results from this study.
Methods
Study design and eligibility
The full study design and eligibility criteria have been published previously.18 Key eligibility criteria included patients ≥ 18 years old, who had received dasatinib for at least 2 years prior to study entry, with confirmed MR4.5 at enrolment and for ≥ 1 year prior to study entry. Additionally, patients were required to have achieved a 1-log reduction in BCR::ABL1 levels from start of dasatinib treatment (determined by local standards) or BCR::ABL1 < 10% (IS) by 3 months with current dasatinib therapy (1L or 2L and beyond [2L+]). Once all eligibility criteria were met, patients discontinued dasatinib and were monitored for at least 5 years. If MMR was lost, dasatinib treatment was restarted at the same dose as received directly before discontinuation.18 A univariate analysis was conducted to identify potential baseline predictors of maintaining TFR.
Study endpoints and assessments
The primary endpoint was the proportion of patients who maintained MMR at 1 year (TFR rate) and has been previously published in Shah et al., 2019.18 Secondary endpoints included event-free survival, BCR::ABL1 kinetics, rate of transformation, PFS and overall survival. Key exploratory endpoints were frequency of AEs and serious AEs, both after discontinuation and after restarting dasatinib treatment; withdrawal events; rate of MMR recapture after dasatinib discontinuation; and identification of predictive factors for TFR maintenance. Withdrawal events were captured via a specific field in the case report form.
Statistical analyses
Statistical analyses (including details on univariate analysis) were conducted as described previously.18 Kaplan–Meier and Brookmeyer–Crowley methods were used to estimate time-to-event endpoints and a Cox proportional hazards model was used to estimate hazard ratios, 95% confidence intervals and p values for exploratory analyses. Rates of MMR and MR4.5 regained after treatment reinitiation were reported by cumulative incidences.
Ethics
All patients provided written informed consent in accordance with the Declaration of Helsinki and local guidelines before study entry. The study protocol was approved by all Institutional Review Boards of each participating centre, as well as each centre’s Ethics Committee and competent national authority.
Results
Patient characteristics and disposition
In total, 84 patients were enrolled between February 2014 and June 2016 and discontinued treatment. A detailed description of the patient population can be found in Shah et al., 2019.18 Baseline characteristics are shown in Table 1. Forty-four per cent (n = 37) of patients received 1L dasatinib and 56% (n = 47) received subsequent (2L+) dasatinib. Of the 47 patients who received 2L+ dasatinib, 53% (n = 25) switched to dasatinib due to resistance and 38% (n = 18) due to intolerance; most patients switched from imatinib (94%, n = 44).
Table 1.
Baseline characteristics
| Patients who maintained TFR (n = 37) |
Patients who relapsed (n = 47) |
Enrolled patients (N = 84) |
|
|---|---|---|---|
| Age | |||
| Median, years (range) | 60.0 (24–80) | 52.0 (28–77) | 52.0 (24–80) |
| < 65 years, n (%) | 24 (64.9) | 40 (85.1) | 64 (76.2) |
| ≥ 65 years, n (%) | 13 (35.1) | 7 (14.9) | 20 (23.8) |
| Sex, male, n (%) | 18 (48.6) | 29 (61.7) | 47 (56.0) |
| ECOG PS, n (%) | |||
| 0 | 32 (86.5) | 34 (72.3) | 66 (78.6) |
| 1 | 3 (8.1) | 9 (19.1) | 12 (14.3) |
| Not reported | 2 (5.4) | 4 (8.5) | 6 (7.1) |
| Line of therapy, n (%) | |||
| First | 17 (45.9) | 20 (42.6) | 37 (44.0) |
| Subsequent | 20 (54.1) | 27 (57.4) | 47 (56.0) |
| Resistanta | 11 (55.0) | 14 (51.9) | 25 (53.2) |
| Intoleranta | 8 (40.0) | 10 (37.0) | 18 (38.3) |
| Othera | 1 (5.0) | 3 (11.1) | 4 (8.5) |
| Prior treatment, n (%) | |||
| Imatinib | — | — | 44 (52.4) |
| Ponatinib | — | — | 1 (1.2) |
| Imatinib + nilotinib | — | — | 2 (2.4) |
| IFN-α | — | — | 0 |
| Sokal score,b n (%) | |||
| Low | 22 (59.5) | 32 (68.1) | 54 (64.3) |
| Intermediate | 12 (32.4) | 12 (25.5) | 24 (28.6) |
| High | 3 (8.1) | 2 (4.3) | 5 (6.0) |
| Unknown | 0 | 1 (2.1) | 1 (1.2) |
| Median time in prior MR4.5, months (range) | 29.6 (13.0–105.9) | 28.9 (13.8–116.3) | 28.9 (13.0–116.3) |
| Median time from diagnosis to discontinuation, months (range) | 74.6 (28.6–156.0) | 65.2 (28.5–243.7) | 69.2 (28.5–243.7) |
| Median dose at discontinuation, mg (range) | 100.0 (20–100) | 100.0 (20–150) | 100.0 (20–150) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; IFN-α, interferon-alpha; MR4.5, molecular response with 4.5-log reduction of BCR::ABL1 transcripts; PS, performance status; TFR, treatment-free remission.
Percentage calculated based on the number of patients receiving subsequent therapy.
After 35 patients were enrolled, the protocol was amended to allow for enrolment of patients with high Sokal scores.
Among enrolled patients, 24 discontinued the study early and 60 discontinued at end of study per planned protocol (Table S1). Of the patients who discontinued early, four discontinued due to AEs associated with dasatinib after restarting treatment and four discontinued the study due to achieving the maximum clinical benefit per investigator discretion after regaining MR4.5 with restarting dasatinib. There were no discontinuations due to death. All patients except one who discontinued were evaluated for response prior to discontinuation; that patient withdrew from the study 1 month after restarting dasatinib and did not undergo molecular assessment.
Efficacy
At a minimum follow-up of 60 months (database lock: December 2021), the Kaplan–Meier estimate of the 5-year TFR rate was 44% (95% CI: 33%–54%) (Figure 1), with 44% (n = 37) of patients maintaining MMR. There were 24 patients (38%) aged < 65 years and 13 patients (65%) aged ≥ 65 years who maintained TFR. When analysed by prior lines of dasatinib therapy, patients with 1L or 2L+ treatment had similar TFR rates at the 5-year timepoint (46% vs 42%, respectively). The TFR rate at 5 years was also similar in patients with 2L+ dasatinib who switched either due to intolerance or resistance (44% for both).
Figure 1.
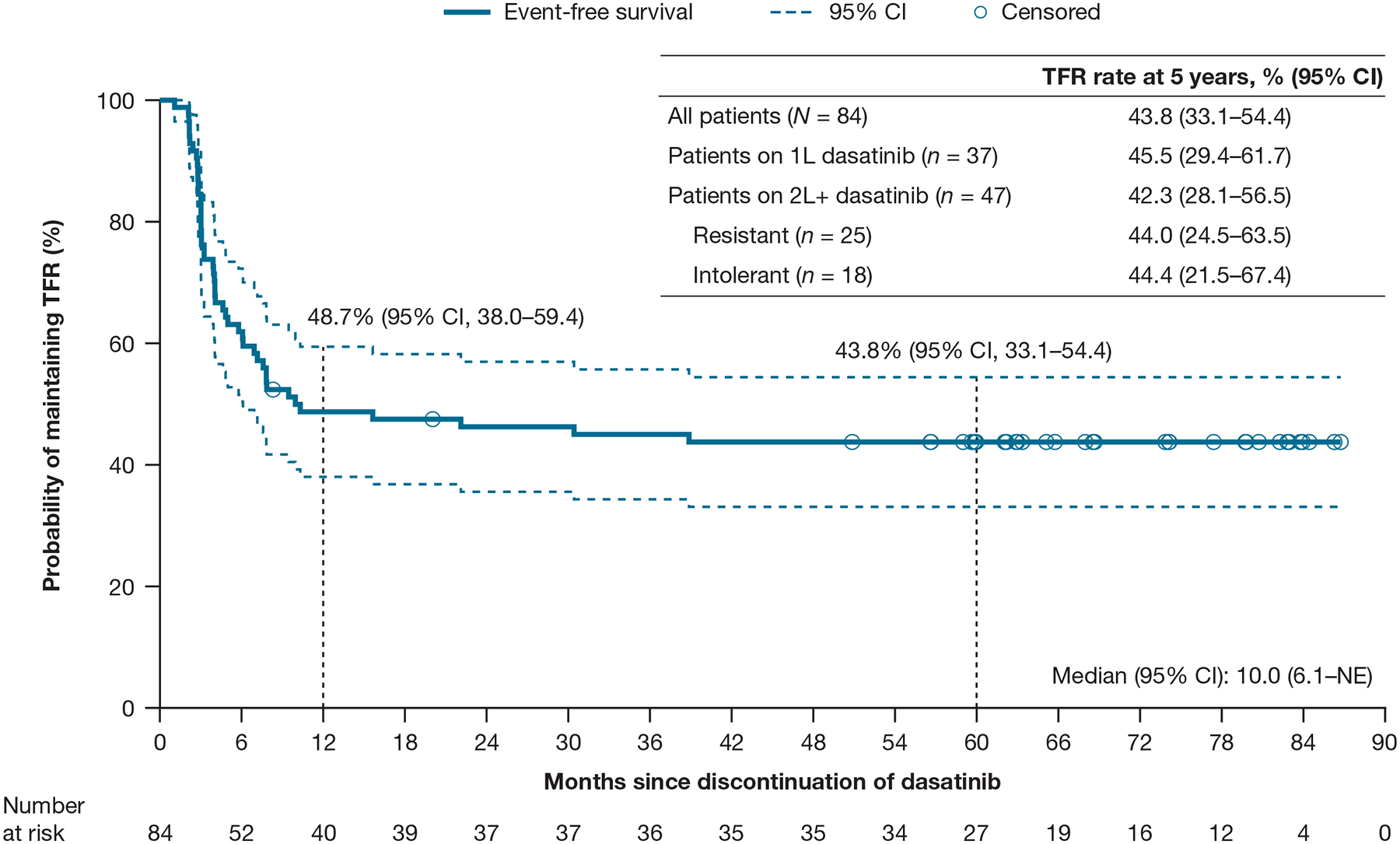
Treatment-free remission (EFS) rate
Adapted with permission from Shah, et al., HemaSphere 2022.27 © 2022 Wolters Kluwer Health Inc.
Abbreviations: 1L, first-line; 2L, second-line; CI, confidence interval; EFS, event-free survival; NE, not evaluable; TFR, treatment-free remission.
Overall, 56% (n = 47) of patients lost MMR and all restarted treatment with dasatinib. Median (range) time to loss of MMR was 3.9 (1.1–38.9) months (Figure 2). No relapses occurred later than 39 months after discontinuation of dasatinib and no patients lost a complete haematologic response. All evaluable patients (n = 46) regained MMR and MR4.5 in a median (range) of 1.9 months (0.9–3.7) and 3.3 months (1.5–29.6), respectively (Figure 2). In the 31 evaluable patients who maintained TFR 5 years after dasatinib discontinuation, 12 also maintained MR4.5 while 19 lost MR4.5 but maintained MMR.
Figure 2.
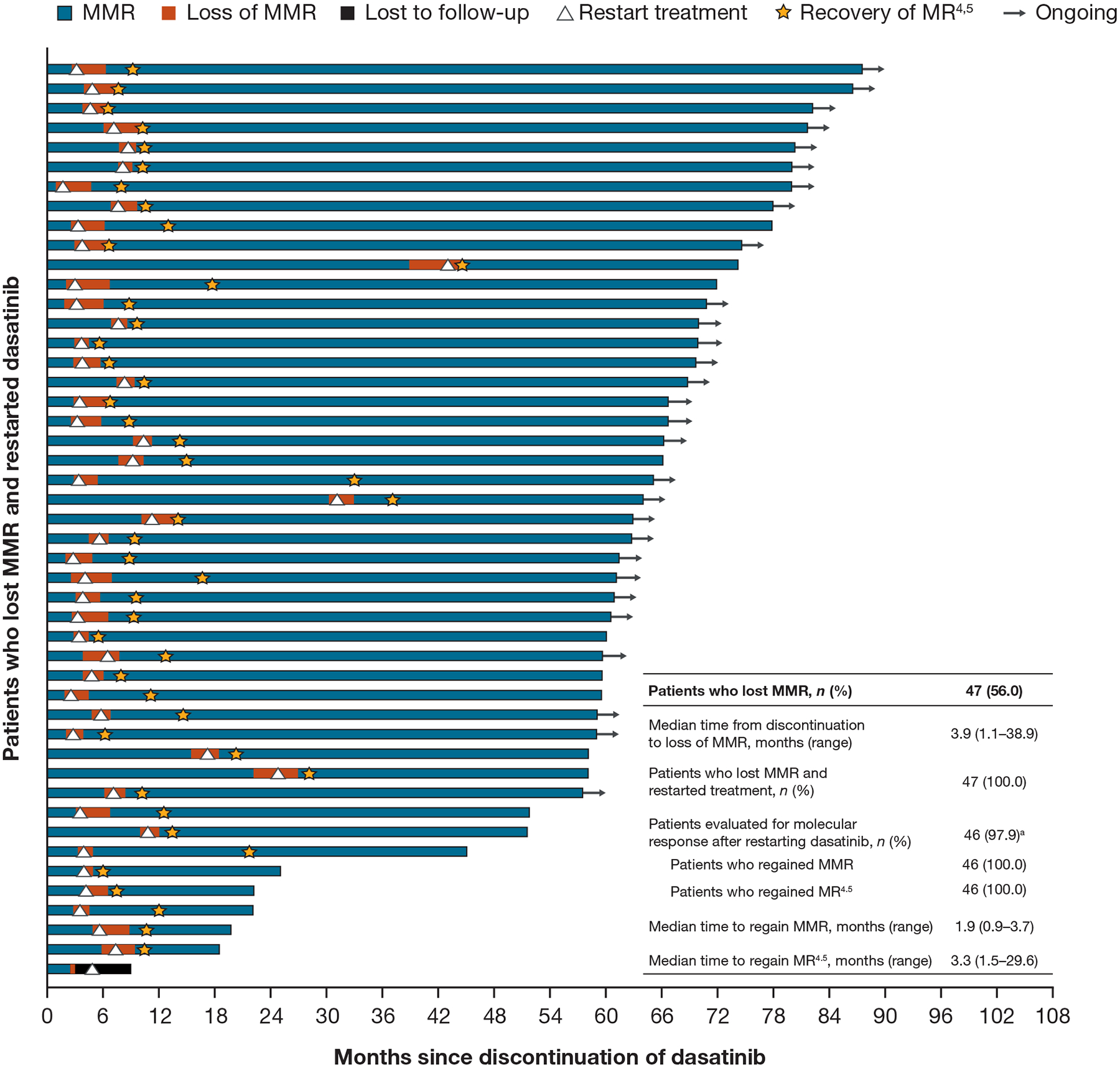
Kinetics of loss and recovery of MMR/MR4.5
Abbreviations: MMR, major molecular response; MR4.5, molecular response with 4.5-log reduction in BCR::ABL1 transcript levels.
aOne patient lost MMR and restarted treatment, but only had one follow-up polymerase chain reaction assessment 1 month after restart and thus could not be evaluated for response.
The PFS rate was 99% at 5 years (Figure 3). No new events were reported after the 2-year follow-up and no patients progressed to CML-AP/BC. One patient died due to causes unrelated to CML (ovarian cancer) after restarting dasatinib and regaining MMR.
Figure 3.
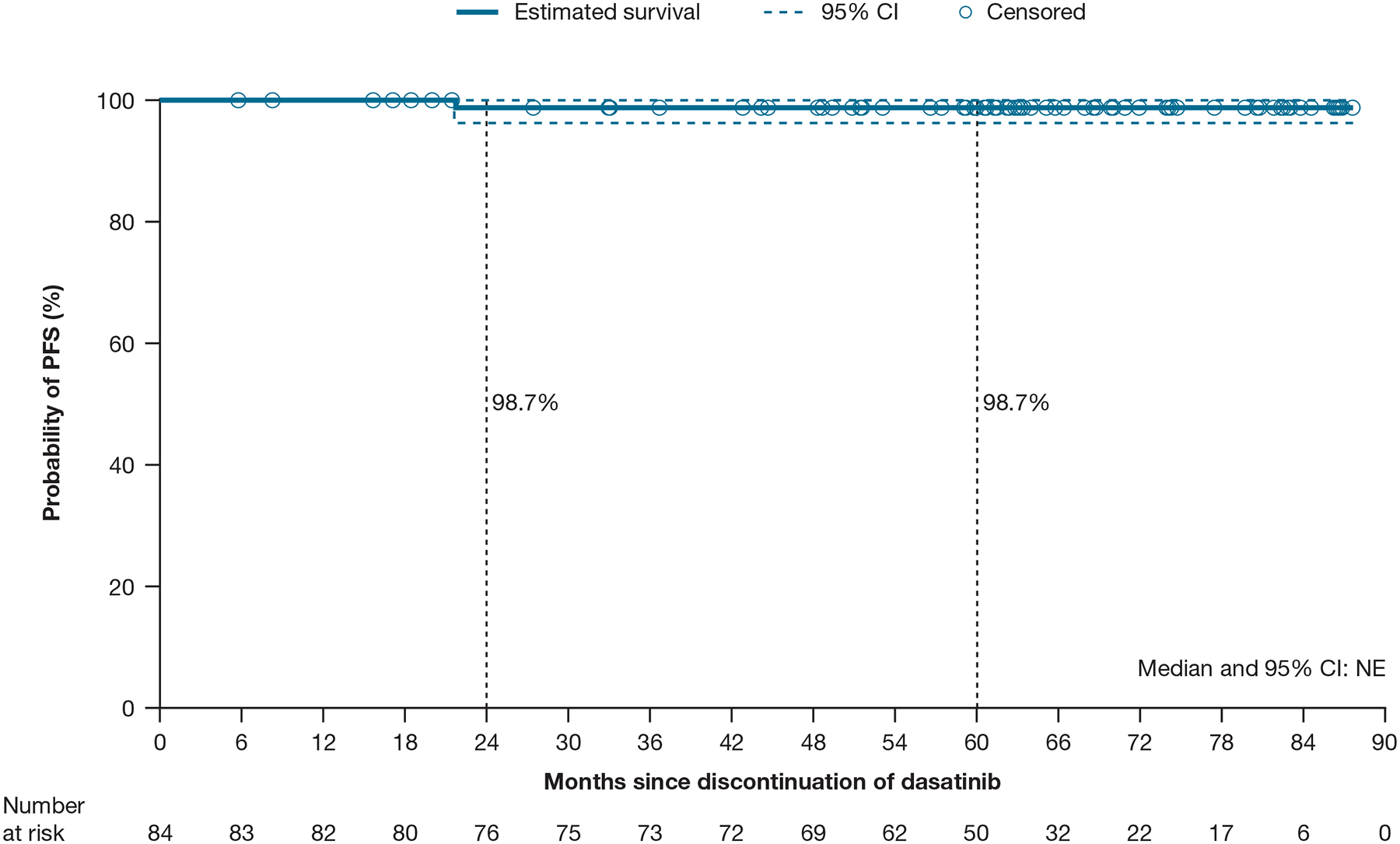
Kaplan–Meier estimate of PFS
Abbreviations: CI, confidence interval; NE, not estimable; PFS, progression-free survival.
As part of the study, a univariate analysis of baseline characteristics (sex, age, duration of prior dasatinib, duration of prior TKI, prior line of dasatinib therapy and Sokal score) was performed to identify potential predictors of achieving TFR during the 5-year study period (Figure 4). Patients aged ≥ 65 years, those who received 1L dasatinib, or those with a duration of prior dasatinib equal to or above the median had more favourable TFR rates and these were identified as potential predictive factors of maintaining TFR at 5 years. The duration of prior dasatinib was numerically higher in patients who maintained MMR versus those who did not (Table S2); however, the study was not designed to perform a statistical comparison between the two groups and interpretation is limited by small sample sizes (Figure 4).
Figure 4.
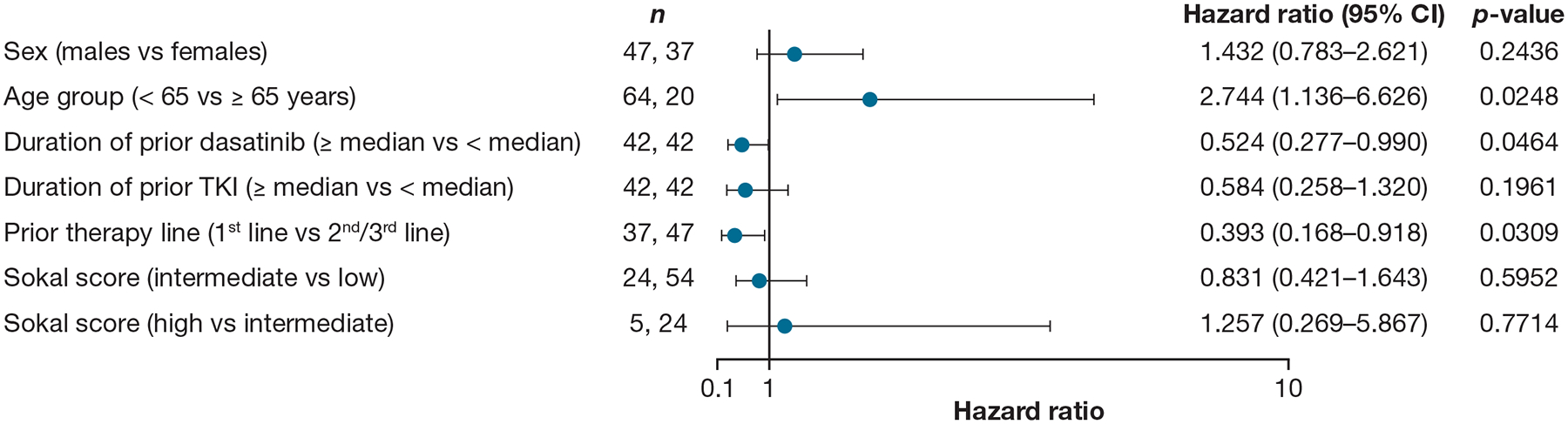
Univariate analysis of TFR during the 5-year study period
Based on Cox proportional hazards model with EFS as dependent variable and sex, age group, duration of prior dasatinib, duration of prior TKI, prior therapy line and Sokal score as predictor variables. Hazard ratios are estimated for male over female, age < 65 over age ≥ 65 years, duration of prior dasatinib ≥ median over < median, duration of prior TKI ≥ median over < median, first line of therapy over 2nd-/3rd-line of therapy, Sokal score of intermediate over low and Sokal score of high over intermediate.
Numbers of patients (n) for each covariate are based on non-missing information.
Abbreviations: CI, confidence interval; EFS, event-free survival; TFR, treatment-free remission; TKI, tyrosine kinase inhibitor.
Safety
All-cause AEs that occurred in ≥ 5% of patients are shown in Table 2. In total, 58 patients (69%) who were off dasatinib treatment and 42 patients (89%) who restarted dasatinib experienced any grade AEs. Fifteen patients (18%) who were off treatment and 13 patients (28%) who restarted dasatinib experienced grade 3/4 AEs. Any grade musculoskeletal and connective tissue AEs occurred in 39% (n = 33) of patients discontinuing dasatinib and in 32% (n = 15) of the patients who restarted treatment; 4% (n = 3) and 2% (n = 1), respectively, reported grade 3/4 events. Arthralgia and arterial hypertension at any grade were the most common off-treatment AEs, occurring in 18% (n = 15) and 13% (n = 11) of patients, respectively; no grade 3/4 arthralgia events were reported and 2 grade 3/4 arterial hypertension events were reported. Pleural effusion (any grade) was experienced by 1 patient (1%) who was off treatment and 4 patients (9%) who restarted dasatinib.
Table 2.
All-causality AEs occurring in ≥ 5% of patients and withdrawal events
| Patients off treatment after discontinuing dasatinib (n = 84) | Patients on treatment after restarting dasatinib (n = 47) | |||
|---|---|---|---|---|
| Any grade | Grade 3/4 | Any grade | Grade 3/4 | |
| Patients with AEs, n (%) | 58 (69.0) | 15 (17.9) | 42 (89.4) | 13 (27.7) |
| Musculoskeletal and connective tissue disorders | 33 (39.3) | 3 (3.6)a | 15 (31.9) | 1 (2.1) |
| Arthralgia | 15 (17.9) | 0 | 6 (12.8) | 0 |
| Back pain | 7 (8.3) | 0 | 4 (8.5) | 0 |
| Myalgia | 6 (7.1) | 0 | 6 (12.8) | 0 |
| Vascular disorders | 11 (13.1) | 2 (2.4) | 6 (12.8) | 0 |
| Arterial hypertension | 11 (13.1) | 2 (2.4) | 4 (8.5) | 0 |
| Nervous system disorders | 18 (21.4) | 2 (2.4) | 16 (34.0) | 3 (6.4) |
| Headache | 8 (9.5) | 0 | 6 (12.8) | 0 |
| Dizziness | 4 (4.8) | 0 | 3 (6.4) | 0 |
| Infections and infestations | 23 (27.4) | 2 (2.4) | 21 (44.7) | 3 (6.4) |
| Nasopharyngitis | 7 (8.3) | 0 | 7 (14.9) | 0 |
| Sinusitis | 4 (4.8) | 0 | 4 (8.5) | 0 |
| Upper respiratory tract infection | 2 (2.4) | 0 | 4 (8.5) | 0 |
| Viral infection | 1 (1.2) | 0 | 3 (6.4) | 0 |
| Skin and subcutaneous tissue disorders | 19 (22.6) | 0 | 7 (14.9) | 0 |
| Rash | 6 (7.1) | 0 | 5 (10.6) | 0 |
| Investigations | 17 (20.2) | 3 (3.6) | 12 (25.5) | 5 (10.6) |
| Weight increased | 6 (7.1) | 0 | 2 (4.3) | 1 (2.1) |
| General disorders and administration site conditions | 13 (15.5) | 0 | 20 (42.6) | 0 |
| Pyrexia | 4 (4.8) | 0 | 4 (8.5) | 0 |
| Oedema peripheral | 3 (3.6) | 0 | 3 (6.4) | 0 |
| Asthenia | 2 (2.4) | 0 | 5 (10.6) | 0 |
| Fatigue | 2 (2.4) | 0 | 10 (21.3) | 0 |
| Psychiatric disorders | 9 (10.7) | 1 (1.2) | 13 (27.7) | 0 |
| Depression | 3 (3.6) | 1 (1.2) | 3 (6.4) | 0 |
| Insomnia | 2 (2.4) | 0 | 3 (6.4) | 0 |
| Gastrointestinal disorders | 13 (15.5) | 0 | 19 (40.4) | 0 |
| Constipation | 2 (2.4) | 0 | 6 (12.8) | 0 |
| Diarrhoea | 1 (1.2) | 0 | 4 (8.5) | 0 |
| Haemorrhoids | 1 (1.2) | 0 | 4 (8.5) | 0 |
| Vomiting | 1 (1.2) | 0 | 3 (6.4) | 0 |
| Metabolism and nutrition disorders | 9 (10.7) | 0 | 10 (21.3) | 0 |
| Hypercholesterolaemia | 1 (1.2) | 0 | 3 (6.4) | 0 |
| Respiratory, thoracic and mediastinal disorders | 7 (8.3) | 0 | 12 (25.5) | 1 (2.1) |
| Pleural effusion | 1 (1.2) | 0 | 4 (8.5) | 1 (2.1) |
| Patients with withdrawal events (n = 9) | ||||
| Withdrawal events,b n | 15 | |||
| Median time from discontinuation to withdrawal event onset/worsening, months (range) | 3.7 (0.3–17.9) | |||
| Withdrawal events resolved, n | 10 | |||
| Resolution before restarting dasatinib | 5 | |||
| Resolution on or after restarting dasatinib | 5 | |||
| Spontaneous resolution without medication (other than a TKI) | 8 | |||
| Resolution after non-TKI/non-analgesic medicationc | 2 | |||
| Median time from withdrawal event onset to resolution, months (range) | 6.0 (0.5–69.5) | |||
Abbreviations: AE, adverse event; TKI, tyrosine kinase inhibitor.
Two intervertebral disc protrusion, one bone pain and one rotator cuff syndrome.
AEs occurring and/or worsening after dasatinib was discontinued were considered withdrawal events, as determined by the investigator.
One withdrawal event was an arterial hypertension event that resolved with antihypertensive therapy.
Treatment-related AEs of any grade were experienced by 25 patients (53%) who restarted dasatinib, with 4 patients (9%) experiencing a grade 3/4 event and only 2 patients (4%) reporting treatment-related serious AEs. AEs leading to dasatinib discontinuation after restarting treatment occurred in 5 patients (11%); 4 (9%) were due to treatment-related pleural effusion and 1 (2%) was due to metastasis from primary ovarian cancer unrelated to treatment. There were no deaths due to CML and no new deaths of any cause reported after the 2-year follow-up.
A total of 15 withdrawal events were reported in 9 patients (11%), all within 18 months of discontinuing treatment; the median (range) time from discontinuation to withdrawal event was 3.7 (0.3–17.9) months (Table 2). Ten events resolved in a median (range) time of 6.0 (0.5–69.5) months; 5 before restarting dasatinib and 5 while on or after restarting dasatinib. Only 2 events required concomitant medication.
Discussion
TFR is an increasingly desirable goal among patients with CML-CP in DMR due to the symptom burden, costs and potential toxicities associated with long-term TKI treatment. DASFREE is the largest trial to date to examine TFR in patients with CML-CP discontinuing dasatinib therapy in any line. With a 5-year TFR rate of 44%, the results of the long-term follow-up of DASFREE demonstrate the feasibility of TFR in patients treated with dasatinib with a stable MR4.5 for at least 12 months and the viability of discontinuing dasatinib in the 2L after prior resistance to 1L therapy. Since the 2-year follow-up, only one patient experienced a relapse (at month 39) and all patients who lost MMR (56%) were able to regain DMR quickly after restarting dasatinib. The safety profile was consistent with previous reports and no new withdrawal events were reported in the long-term follow-up. Overall, these results highlight the success and safety of TFR in patients with CML-CP in DMR after treatment with dasatinib across any line of therapy.
Long-term results of DASFREE are similar to those seen in the 5-year follow-up of the ENESTfreedom and ENESTop studies, which explored TFR after the discontinuation of 1L and 2L nilotinib, respectively (5-year TFR rate: 43% in both studies).12, 19 Similar to DASFREE, ENESTfreedom and ENESTop recruited patients with a sustained MR4.5, but unlike DASFREE, patients were not required to have a 1-log reduction in BCR::ABL1 levels from start of treatment or BCR::ABL1 < 10% (IS) at 3 months after starting therapy. The patient populations for ENESTfreedom and ENESTop also included patients with Eastern Cooperative Oncology Group performance status 0–2 (compared with 0–1 in DASFREE) and had more stringent criteria regarding patient health such as requiring adequate end organ function, having electrolyte values ≥ lower limit of normal and having normal marrow function.20, 21
The results from DASFREE are also similar to those from the > 6 year follow-up of the STIM1 (5-year TFR rate of 38%)10 and the A-STIM (5-year TFR rate of 51%) observational studies,11 both of which evaluated the feasibility of discontinuing imatinib. It should be noted that direct comparisons between clinical trials must be done with caution due to differences in study designs and protocols.
In the ENESTfreedom, ENESTop, STIM1 and DASFREE trials, most relapses occurred within the first 6 months and no progression to CML-AP/BC was observed.10–12, 19 Only one relapse occurred after the 2-year DASFREE follow-up, consistent with results from previous TFR studies where most relapses occurred within the first 2 years.11, 13, 14, 22, 23 Although uncommon, late relapses do occur during TFR; from the study by Rousselot et al.,11 the kinetics of relapse in patients treated with dasatinib were similar to those of early relapse in the DASFREE study. Results from the AFTER-SKI study suggested that molecular status (MR4) after 3 years was highly predictive of late relapse (loss of MMR during years 3–6); in that study, 11 of the 13 patients not maintaining MR4 at 3 years lost MMR versus 1 of 98 patients who had maintained MR4.9 In DASFREE, only 4 of 23 patients with loss of MR4.5 3 years after dasatinib discontinuation did not maintain MMR at 5 years.
The prospective DESTINY trial had a cohort of patients with MMR as well as MR4 attempting discontinuation, unlike the majority of other TFR trials.23 However, the TFR rate at 3 years was just 36% in the MMR group versus 72% in the MR4 group. Patients in DESTINY also had a period of TKI dose de-escalation before discounting treatment completely, but it was inconclusive as to whether this increased the success of maintaining TFR. Although EURO-SKI and DESTINY have shorter follow-up periods than DASFREE and the patients’ treatment history comprises a mix of dasatinib, imatinib and nilotinib, the results from DASFREE are largely consistent with these trials.
In DASFREE, the 5-year TFR rate for patients receiving 2L+ dasatinib was 42%, which is consistent with TFR rates seen previously in the Japanese prospective trial monitoring TFR after 2L dasatinib (DADI, N = 88, estimated 3-year TFR rate of 44%).14 In the DADI trial, patients who switched to dasatinib from imatinib due to resistance had a lower TFR rate than those who switched due to intolerance (8% vs 56%). The STOP2G-TKI trial evaluating TFR after DMR with dasatinib or nilotinib also showed that patients with 2L+ dasatinib or nilotinib who had intolerance or resistance to 1L therapy had a significantly lower 2-year TFR rate (36%) versus patients who were not intolerant/resistant (77%, p = 0.002).13 However, in DASFREE, the 5-year TFR rate was the same for patients who switched to dasatinib due to intolerance or resistance to prior therapy (44%) and was similar to that for patients who received 1L dasatinib. Successful TFR has also been observed in a previous study of patients with CML with prior resistance.24 Traditionally, 2L treatment, particularly after resistance to 1L therapy, has precluded patients with CML from attempting TFR, as it is associated with a poorer prognosis. However, results from DASFREE and previous studies suggest that some patients with prior resistance to 1L TKI therapy may successfully attempt TFR providing they achieved a DMR with 2L treatment.
AEs were seen in 58 patients (69%) discontinuing dasatinib, with musculoskeletal and connective tissue disorders being the most common events (39%). Musculoskeletal events are commonly seen after discontinuation of TKI treatment. Although the rates vary between trials, musculoskeletal events were reported at a similar rate in DASFREE as other trials for TFR (30%–53%).19, 21–23 Of note, however, all events in DASFREE were assessed by the investigator per the case report form, with most events determined to have an alternate aetiology; consequently, just 15 events in 9 patients (11%) were classified as withdrawal events. Most withdrawal events resolved and no new withdrawal events occurred after the 2-year follow-up.18
A univariate analysis of TFR rates during the 5-year study period of DASFREE identified older age (≥ 65 years), 1L dasatinib therapy and a prior duration of dasatinib equal to or above the median as potentially predictive of maintaining TFR. This is consistent with the 2-year results and previous studies.10, 14, 22, 23, 25 Previously, older patients have been shown to have a better prognosis than younger patients with CML.26 The Cox model methodology used in this study analysed the trend in TFR rates over the entire study period. Although the TFR rate at 5 years was similar between patients receiving 1L and 2L dasatinib, the initial difference reported at the 2-year analysis18 was carried forward using this model, indicating that, overall, 1L dasatinib therapy was potentially predictive of maintaining TFR.
The potential predictors of TFR success differ across TFR studies. This may be due to the small sample sizes in some studies, but also due to the differing patient populations and eligibility criteria. DASFREE included a heterogenous population of patients treated with 1L and 2L+ dasatinib, patients with prior resistance and intolerance to different 1L treatments and patients with different BCR::ABL1 levels at the start of dasatinib treatment.
This study has several limitations. Some of the data in this study were collected retrospectively and this may account for some of the differences between DASFREE and other TFR studies. In addition, although several baseline factors were identified as potentially predictive of maintaining TFR in this study, the small sample size does not allow a statistical comparison and the data should be interpreted with caution.
Findings from the 5-year follow-up of DASFREE showed that discontinuation of dasatinib is a viable option for patients with CML-CP in sustained DMR after first- or subsequent-line dasatinib. About half of the patients who discontinued dasatinib maintained TFR, and in those who lost MMR, retreatment was successful, with all evaluable patients regaining MMR and MR4.5 after dasatinib was restarted. Finally, the overall safety profile was consistent with the known safety profile of dasatinib and no new withdrawal events were reported since the 2-year follow-up.
Supplementary Material
Acknowledgments
The authors thank the patients who participated in this study and the clinical study teams. This study was sponsored and funded by Bristol Myers Squibb. Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number P30CA082103. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Medical writing and editorial support were provided by Flint Stevenson-Jones, PhD of Caudex, funded by Bristol Myers Squibb.
Disclosures
NS: research funding from Bristol Myers Squibb. VGG: payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from Bristol Myers Squibb, Incyte, Novartis and Pfizer; payment for expert testimony from Bristol Myers Squibb, Incyte, Novartis and Pfizer; support for attending meetings and/or travel from Bristol Myers Squibb, Incyte, Novartis and Pfizer; participation on a data safety monitoring board or advisory board from Bristol Myers Squibb, Incyte, Novartis and Pfizer. AJV: nothing to declare. SL: research funding from Bristol Myers Squibb. SS: research funding from Bristol Myers Squibb, Incyte and Novartis; honoraria from Novartis, Bristol Myers Squibb, Incyte, Pfizer and Roche. DR: payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from Incyte, Novartis and Pfizer; participation in a data safety monitoring board or advisory board for Incyte, Novartis and Pfizer. FXM: nothing to declare. MYL: consultant and/or promotional speaker for AstraZeneca, Beigene, Bristol Myers Squibb, Dova, Epizyme, Janssen, Jazz, Morphosys, Novartis, Seagen and Takeda. MTGC: payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from Bristol Myers Squibb and participation on a data safety monitoring board or advisory board from Bristol Myers Squibb. MJM: Grants from Bristol Myers Squibb, Novartis and Sun Pharma/SPARC. Consulting fees: Bristol Myers Squibb, Novartis, Pfizer and Takeda; payment/honoraria from Bristol Myers Squibb, Novartis, Pfizer and Takeda; travel support from Bristol Myers Squibb, Novartis, Pfizer and Takeda. OS and PMR: employees and stockholders of Bristol Myers Squibb. JHL: payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from Incyte, Pfizer and Takeda.
Data availability statement
The Bristol Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
References
- 1.Bower H, Björkholm M, Dickman PW, Hoglund M, Lambert PC, Andersson TM-L. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J Clin Oncol. 2016;34:2851–7. [DOI] [PubMed] [Google Scholar]
- 2.Sasaki K, Strom SS, O’Brien S, Jabbour E, Ravandi F, Konopleva M, et al. Relative survival in patients with chronic-phase chronic myeloid leukaemia in the tyrosine-kinase inhibitor era: analysis of patient data from six prospective clinical trials. Lancet Haematol. 2015;2:e186–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cortes JE, Saglio G, Kantarjian HM, Baccarani M, Mayer J, Boque C, et al. Final 5-year study results of DASISION: the dasatinib versus imatinib study in treatment-naive chronic myeloid leukemia patients trial. J Clin Oncol. 2016;34:2333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hochhaus A, Saglio G, Hughes TP, Larson RA, Kim DW, Issaragrisil S, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30:1044–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brümmendorf TH, Cortes JE, Milojkovic D, Gambacorti-Passerini C, Clark RE, le Coutre PD, et al. Bosutinib (BOS) versus imatinib for newly diagnosed chronic phase (CP) chronic myeloid leukemia (CML): final 5-year results from the Bfore trial. Blood. 2020;136:41–2. [Google Scholar]
- 6.Williams LA, Garcia Gonzalez AG, Ault P, Mendoza TR, Sailors ML, Williams JL, et al. Measuring the symptom burden associated with the treatment of chronic myeloid leukemia. Blood. 2013;122:641–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dusetzina SB, Winn AN, Abel GA, Huskamp HA, Keating NL. Cost sharing and adherence to tyrosine kinase inhibitors for patients with chronic myeloid leukemia. J Clin Oncol. 2014;32:306–11. [DOI] [PubMed] [Google Scholar]
- 8.Mahon F-X, Richter J, Hochhaus A, Panayiotidis P, Medina de Almeida A, Mayer J, et al. FINAL analysis of a PAN European STOP tyrosine kinase inhibitor trial in chronic myeloid leukemia: The EURO-SKI Study. Blood. 2021;138:633. [Google Scholar]
- 9.Richter J, Lübking A, Söderlund S, Lotfi K, Markevärn B, Själander A, et al. Molecular status 36 months after TKI discontinuation in CML is highly predictive for subsequent loss of MMR—final report from AFTER-SKI. Leukemia. 2021;35:2416–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Etienne G, Guilhot J, Rea D, Rigal-Huguet F, Nicolini F, Charbonnier A, et al. Long-term follow-up of the French Stop Imatinib (STIM1) study in patients with chronic myeloid leukemia. J Clin Oncol. 2017;35:298–305. [DOI] [PubMed] [Google Scholar]
- 11.Rousselot P, Loiseau C, Delord M, Cayuela JM, Spentchian M. Late molecular recurrences in patients with chronic myeloid leukemia experiencing treatment-free remission. Blood Adv. 2020;4:3034–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Radich JP, Hochhaus A, Masszi T, Hellmann A, Stentoft J, Casares MTG, et al. Treatment-free remission following frontline nilotinib in patients with chronic phase chronic myeloid leukemia: 5-year update of the ENESTfreedom trial. Leukemia. 2021;35:1344–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rea D, Nicolini FE, Tulliez M, Guilhot F, Guilhot J, Guerci-Bresler A, et al. Discontinuation of dasatinib or nilotinib in chronic myeloid leukemia: interim analysis of the STOP 2G-TKI study. Blood. 2017;129:846–54. [DOI] [PubMed] [Google Scholar]
- 14.Okada M, Imagawa J, Tanaka H, Nakamae H, Hino M, Murai K, et al. Final 3-year results of the dasatinib discontinuation trial in patients with chronic myeloid leukemia who received dasatinib as a second-line treatment. Clin Lymphoma Myeloma Leuk. 2018;18:353–60 e1. [DOI] [PubMed] [Google Scholar]
- 15.Hochhaus A, Baccarani M, Silver RT, Schiffer C, Apperley JF, Cervantes F, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34:966–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hochhaus A, Saussele S, Rosti G, Mahon FX, Janssen J, Hjorth-Hansen H, et al. Chronic myeloid leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv41–iv51. [DOI] [PubMed] [Google Scholar]
- 17.Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Chronic Myeloid Leukemia Version 1.2023. © National Comprehensive Cancer Network, Inc. 2022. All rights reserved. Accessed August 18, 2022. [Google Scholar]
- 18.Shah NP, Garcia-Gutierrez V, Jimenez-Velasco A, Larson S, Saussele S, Rea D, et al. Dasatinib discontinuation in patients with chronic-phase chronic myeloid leukemia and stable deep molecular response: the DASFREE study. Leuk Lymphoma. 2020;61:650–9. [DOI] [PubMed] [Google Scholar]
- 19.Hughes TP, Clementino NCD, Fominykh M, Lipton JH, Turkina AG, Moiraghi EB, et al. Long-term treatment-free remission in patients with chronic myeloid leukemia after second-line nilotinib: ENESTop 5-year update. Leukemia. 2021;35:1631–42. [DOI] [PubMed] [Google Scholar]
- 20.Ross DM, Masszi T, Gómez Casares MT, Hellmann A, Stentoft J, Conneally E, et al. Durable treatment-free remission in patients with chronic myeloid leukemia in chronic phase following frontline nilotinib: 96-week update of the ENESTfreedom study. J Cancer Res Clin Oncol. 2018;144:945–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahon FX, Boquimpani C, Kim DW, Benyamini N, Clementino NCD, Shuvaev V, et al. Treatment-free remission after second-line nilotinib treatment in patients with chronic myeloid leukemia in chronic phase: results from a single-group, phase 2, open-label study. Ann Intern Med. 2018;168:461–70. [DOI] [PubMed] [Google Scholar]
- 22.Saussele S, Richter J, Guilhot J, Gruber FX, Hjorth-Hansen H, Almeida A, et al. Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukaemia (EURO-SKI): a prespecified interim analysis of a prospective, multicentre, non-randomised, trial. Lancet Oncol. 2018;19:747–57. [DOI] [PubMed] [Google Scholar]
- 23.Clark RE, Polydoros F, Apperley JF, Milojkovic D, Rothwell K, Pocock C, et al. De-escalation of tyrosine kinase inhibitor therapy before complete treatment discontinuation in patients with chronic myeloid leukaemia (DESTINY): a non-randomised, phase 2 trial. Lancet Haematol. 2019;6:e375–e83. [DOI] [PubMed] [Google Scholar]
- 24.Claudiani SF Apperley J, Khan A, Khorashad J, Milojkovic D. Prolonged treatment-free remission in chronic myeloid leukemia patients with previous BCR-ABL1 kinase domain mutations. Haematologica. 2020;105:e225–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mori S, Vagge E, le Coutre P, Abruzzese E, Martino B, Pungolino E, et al. Age and dPCR can predict relapse in CML patients who discontinued imatinib: the ISAV study. Am J Hematol. 2015;90:910–4. [DOI] [PubMed] [Google Scholar]
- 26.Pemmaraju N, Kantarjian H, Shan J, Jabbour E, Quintas-Cardama A, Verstovsek S, et al. Analysis of outcomes in adolescents and young adults with chronic myelogenous leukemia treated with upfront tyrosine kinase inhibitor therapy. Haematologica. 2012;97:1029–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah NP, García-Gutiérrez V, Jiménez-Velasco A, Saussele S, Rea D, Mahon F-X, et al. Treatment-free remission (TFR) after dasatinib in patients with chronic myeloid leukemia in chronic phase (CML-CP) and deep molecular response (DMR): final 5-year results of DASFREE. HemaSphere. 2022;6:595–6 P700. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Bristol Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.


