Abstract
Objective
Both clopidogrel and atorvastatin metabolism are rooted in hepatic cytochrome p450 activation.There are published reports of atorvastatin interfering with clopidogrel metabolism by inhibiting the activation of clopidogrel. This in turn would decrease the therapeutic effect of clopidogrel potentially resulting in an increase in thrombotic events in patients who are taking both medications. The emergence of viscoelastic assays, such as Thromboelastography with platelet mapping (TEG-PM), have been utilized to identify prothrombotic states and may provide insight into a patient’s microvascular coagulation profile. The aim of this prospective, observational study was to delineate the differences in platelet function between patients on clopidogrel alone versus those on clopidogrel and atorvastatin in patients that are undergoing peripheral revascularization.
Methods
All patients undergoing revascularization between December 2020 and August 2022 were prospectively evaluated. Patients on clopidogrel and atorvastatin were compared to those on clopidogrel alone. Serial perioperative TEG-PM analysis was performed up to six months postoperatively and the platelet function in terms of percent inhibition was evaluated in both groups. Statistical analysis was performed using unpaired t-test to identify differences in platelet function.
Results
Over the study period, a total of 182 patients were enrolled. Of this cohort 72 patients met study criteria. 87 samples from the 72 patients were analyzed. 31(43.05%) patients were on clopidogrel alone and 41(56.94%) were on clopidogrel and atorvastatin. Patients on clopidogrel alone showed significantly greater platelet inhibition compared to those on clopidogrel and atorvastatin [49.01% vs. 34.54%, p=.03]. There was no statistical difference in platelet inhibition between groups in terms of aspirin use alone versus aspirin and atorvastatin.
Conclusions
Patients on clopidogrel and atorvastatin showed significantly less platelet inhibition compared to those on clopidogrel alone, supporting the concept that atorvastatin may interfere with the therapeutic effect of clopidogrel. Patients taking atorvastatin may require an alternative antiplatelet therapy regimen that does not include clopidogrel to achieve adequate thromboprophylaxis.
Keywords: Peripheral artery disease, thromboelastography, thrombosis, clopidogrel, atorvastatin
1. Introduction
Peripheral artery disease (PAD) is associated with numerous cardiovascular risk factors including: advanced age, diabetes, smoking, arterial hypertension, hyperlipidemia, and chronic kidney disease resulting in frequent poly-pharmacy in this population.1 Drug interactions are common and it is important to understanding the compounded or contradictory impact of various medications in these patients. In the PAD patient cohort, two of the most commonly prescribed medications are the antiplatelet medication clopidogrel and the lipid lowered drug atorvastatin given that antiplatelet and anti-cholesterol therapy are the mainstay of medical therapy for PAD.2
Clopidogrel works by binding irreversibly to the platelet P2Y12 receptor, inhibiting adenosine diphosphate (ADP) mediated platelet activation and aggregation.3 It is a pro-drug that requires hepatic cytochrome p450 (CYP) metabolic activation, specifically CYP2C19 and CYP3A4/5, to produce the active metabolite and exerts its function. This process in the CYP isoenzymes can be dampened by a drug to drug interactions which may affect the clinical effectiveness of clopidogrel. Specifically, atorvastatin, a lipophilic statin, is metabolized by CYP3A4 has been shown to interfere with the CYP metabolic activation of clopidogrel decreasing the clinical effectiveness and/or impact of clopidogrel to inhibit platelet.2,4,5 This decrease in the therapeutic effect of clopidogrel has the potential to impact the frequency of thrombotic events in the PAD patient population unwittingly as the patients being prescribed both atorvastatin and clopidogrel may not be gleaning the complete clinical effect of clopidogrel due to the drug interaction with atorvastatin however the impact of this drug interaction on platelet inhibition has never been quantified. We included patients on atorvastatin given that the literature supports atorvastatin having an impact on clopidogrel activation but the literature is not as clear about another statins.
The emergence of viscoelastic assays, such as Thromboelastography with platelet mapping (TEG-PM), allows for point of care assessment of platelet aggregation and inhibition secondary to antiplatelet therapy.6 TEG-PM has two components: arachidonic acid inhibition, which is sensitive to aspirin, and ADP inhibition, which is sensitive to clopidogrel. This assay has been utilized to identify prothrombotic states and may provide insight into a patient’s microvascular coagulation profile. Previous research has shown that different percentages of platelet inhibition can lead to different outcomes and an ADP platelet inhibition less than 29.2% seems to be associated with higher thrombosis rates in patients with PAD undergoing revascularization. At the same time the ability of obtain a quantification of platelet reactivity helps to select a better combination of medications.7 The aim of this prospective, observational study was to delineate the differences in platelet function in terms of percentage of platelet inhibition between patients on clopidogrel alone versus those on clopidogrel and atorvastatin in patients that are undergoing peripheral revascularization to detail the impact of the drug interaction in the PAD patient population.
2. Methods
2.1. Study population:
Patients at a large tertiary institution, older than 18 years, undergoing elective, emergent or urgent lower extremity revascularization procedures within the vascular surgery department were prospectively enrolled and followed clinically between December 2020 and August 2022. Data analysis comparing patients on clopidogrel and atorvastatin to those on clopidogrel alone was performed retrospectively. Exclusion criteria included the inability to provide informed consent, inability to undergo serial blood draws, other antiplatelet medication different than clopidogrel or aspirin, other statins different than atorvastatin and pregnancy (Table 1.). Patients were considered screen failures and excluded from analysis if they did not have a successful revascularization due to the inability to endovascularly access. The study protocol, including the consent form, was approved by the institutional review board (Mass General Brigham IRB # 2020P000263). Prior to participation in the study, a written informed consent was obtained from each patient or the patient’s legally authorized representative if the patient was unable to provide consent.
Table 1.
Study Inclusion and Exclusion Criteria
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
2.2. Procedures:
Following enrollment, a 5cc blood samples was collected preoperatively on the day of surgery and then serial TEG-PM analysis was then performed in the follow-up period at one month, three months and six months. Whole blood samples were tested using the TEG®6S Haemostasis Analyzer (Haemonetics Corp., Boston MA). Citrated multichannel cartridges without lysis, measuring time to clot formation (R time), clot strengthening (K time and α-angle) and maximal clot amplitude (MA), and percent lysis at 30 minutes (%lysis) were chosen to assess for prothrombotic states. PlateletMapping® cartridges were assayed with heparinized blood to quantify platelet function using both arachidonic acid (AA), adenosine diphosphate (ADP) assays. The use of antithrombotic medication was recorded and associated with specific TEG-PM samples and not individual patients as some patients underwent changes in their antithrombotic regimen throughout the study period; we excluded patients on other antiplatelets different than clopidogrel or aspirin. Samples were considered to be associated with atorvastatin and/or clopidogrel if they were obtained from patients that were taking the medications consistently. The metrics of TEG-PM analysis were taken from the different time points. TEG-PM parameters were compared in the group of patients on atorvastatin and clopidogrel, with the group of patients on clopidogrel alone.
2.3. Statistical analysis:
Student’s t test was used for continuous variables. Bar chart analysis was the performed to visually assess the distribution of Platelet Mapping values by cohort.
3. Results
3.1. Study population:
Over the study period, a total of one hundred and eighty-two patients were enrolled, one hundred and ten were excluded due to screen failure or the absence of the medication in study (Table 1). Seventy-two patients were included, 31 (43.05%) patients were on clopidogrel alone and 41 (56.94%) patients were on clopidogrel and atorvastatin from which 87 TEG-PM samples were analyzed. In the clopidogrel alone cohort 27 patients had 1 sample collected and 4 patients had 2 samples collected; in the clopidogrel and atorvastatin cohort 30 patients had 1 sample collected and 11 patients had 2 samples collected. This samples were collected a different timepoints (preoperative, 1 month, 3 month or 6 months visit) (Figure 1.). Some patients were lost at follow up and/or had medication changes that resulted in the ending of the study.
Figure 1.
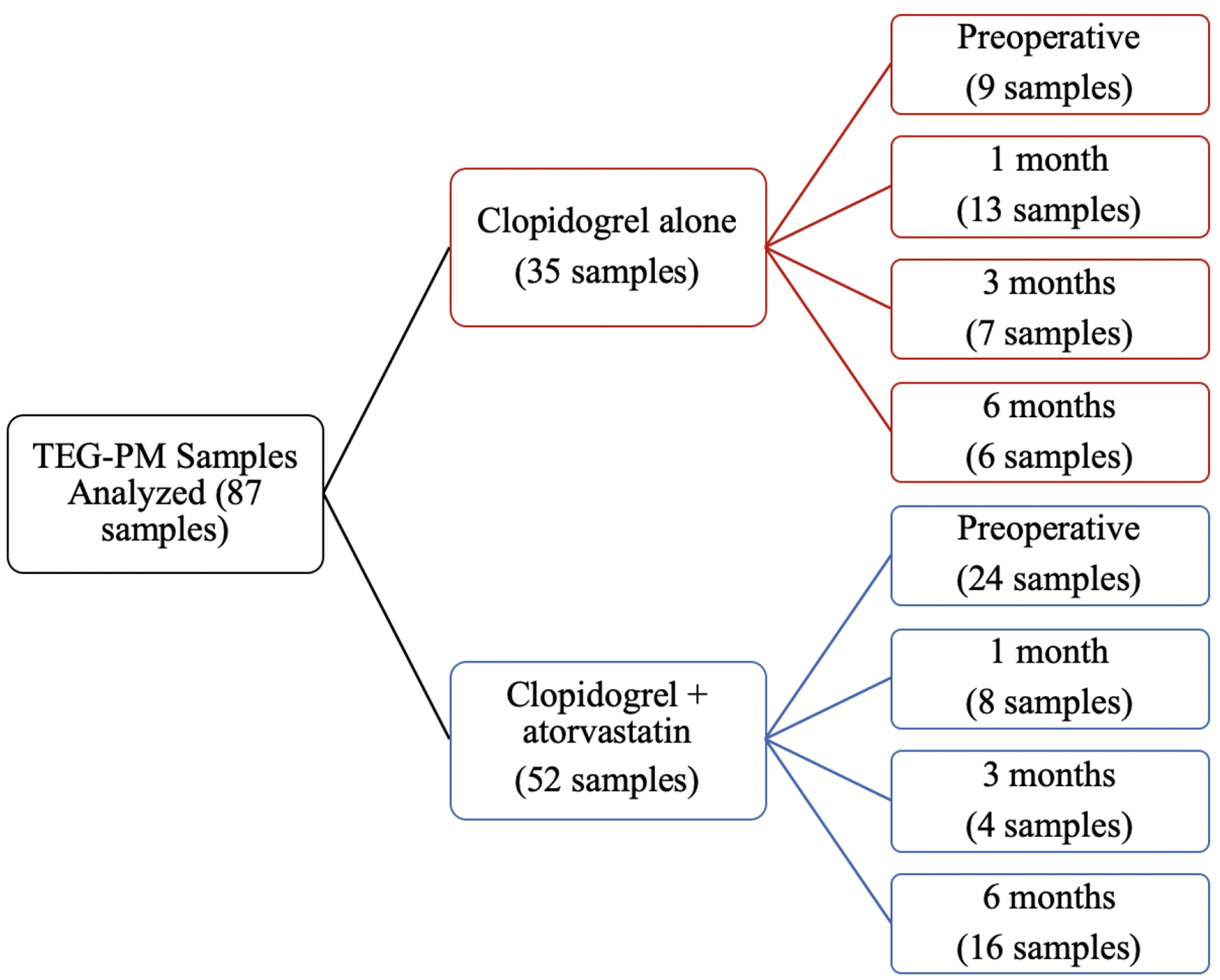
Timeline of TEG-PM sample collections.
The study population was represented by 21(29.16%) women and 51 (70.83%) men. The age group ranged from 39 to 87 with an average of 67.76 years old; women on average were older than men (71 vs 66 years respectively). For the ethnicity 87.5% of patients identify themselves as White, 4.16% as African Americans, 4.16% as Hispanic or Latino, 2.8% as Asian and 1.4% as other (Table 2.).
Table 2.
Baseline Demographics
| N | % | Clopidogrel Only | Clopidogrel + Atorvastatin | p value | ||
|---|---|---|---|---|---|---|
| Sex | Female | 21 | 29.17 | 9 | 12 | 1.00 |
| Male | 51 | 70.83 | 22 | 29 | ||
| Ethnicity | White | 63 | 87.50 | 27 | 36 | 1.00 |
| African American | 3 | 4.17 | 2 | 1 | ||
| Asian | 2 | 2.78 | 1 | 1 | ||
| Hispanic or Latino | 3 | 4.17 | 0 | 3 | ||
| Other | 1 | 1.39 | 1 | 0 | ||
| Tobaco Use | Current within the last year (> or = 1 pack a day) | 8 | 11.11 | 3 | 5 | 0.51 |
| Current within the last year (< 1 pack a day) | 6 | 8.33 | 3 | 3 | ||
| None | 11 | 15.28 | 6 | 5 | ||
| Past, quit >10 year ago | 28 | 38.89 | 12 | 16 | ||
| Past, quit 1 to 10 years ago | 19 | 26.39 | 7 | 12 | ||
| Diabetes | None | 27 | 37.50 | 11 | 16 | 0.80 |
| Type 1 | 3 | 4.17 | 2 | 1 | ||
| Type 2 controlled by insulin | 27 | 37.50 | 10 | 17 | ||
| Type 2 not requiring insulin | 12 | 16.67 | 6 | 6 | ||
| Type 2 Uncontrolled | 3 | 4.17 | 2 | 1 | ||
| Hypertension | None (normotensive) | 5 | 6.94 | 2 | 3 | 1.00 |
| Controlled with 1 drug | 26 | 36.11 | 13 | 13 | ||
| Controlled with 2 drugs | 23 | 31.94 | 10 | 13 | ||
| Requires 3 or more drugs | 18 | 25 | 6 | 12 | ||
| Renal Function | GFR > 90 | 31 | 43.06 | 13 | 18 | 1.00 |
| GFR 60 to 89 | 13 | 18.06 | 3 | 10 | ||
| GFR 30 to 59 | 18 | 25.00 | 9 | 9 | ||
| GFR 15 to 29 | 4 | 5.56 | 2 | 2 | ||
| GFR<15 or patient is on dialysis | 6 | 8.33 | 3 | 3 | ||
| Hyperlipidemia | None | 9 | 12.50 | 3 | 6 | 0.72 |
| Yes, without drug treatment | 3 | 4.17 | 3 | 0 | ||
| Yes, requiring dietary treatment | 2 | 2.78 | 0 | 2 | ||
| Yes, requiring drug treatment | 58 | 80.56 | 25 | 33 | ||
| History of Miocardial Infarction | No | 55 | 76.39 | 21 | 34 | 1.00 |
| Yes | 17 | 23.61 | 6 | 11 |
The majority of patients were former smokers (65.27%) or never smoked (15.27%); the rest were current smokers of < or > than a 1 pack a day (8.33% and 11.11% respectively) (Table 2.).
The more prevalent comorbidities in the study population were: Hypertension (93.05% of patients), Hyperlipidemia (87.5% of patients), Diabetes (62.5% of patients), Impaired renal function (56.94% of patients) and History of Myocardial Infarction (23.61% of patients) (Table 2.).
The type of procedures were elective (93.1%) and urgent (6.9%). The type of intervention was represented in its majority by Endovascular (61.11%), followed by Combined (20.83%) and Open (18.05%). Further classification of the intervention was represented by Balloon Angioplasty (33.65%), Self-expandable Stent (20.19%), Endarterectomy (18.26%), Bypass (10.57%), Drug-eluting Balloon (7.69%), Balloon Expandable Stent (4.80%) and Deep Venous Arterialization (4.80%) (Table 3.).
Table 3.
Type of Vascular Intervention
| N | % | ||
|---|---|---|---|
| Intervention | Endovascular | 44 | 61.11 |
| Combined | 15 | 20.83 | |
| Open | 13 | 18.06 | |
| Procedure | Balloon Angioplasty | 35 | 33.65 |
| Drug-eluting Balloon | 8 | 7.69 | |
| Balloon Expandable Stent | 5 | 4.81 | |
| Self-expandable Stent | 21 | 20.19 | |
| Endarterectomy | 19 | 18.27 | |
| Bypass | 11 | 10.58 | |
| Deep Venous Arterialization (DVA) | 5 | 4.81 |
3.2. TEG-PM parameters:
Samples included for analysis came from the perioperative period up to six months postoperatively. Platelet function in terms of percent inhibition was evaluated in both groups. Overall patients on clopidogrel alone showed significantly greater platelet inhibition compared to those on clopidogrel and atorvastatin [49.01% (±31.74) vs. 34.54% (±30.98) p=.03] (Graph 1.). There was no statistical difference in platelet inhibition between groups in terms of aspirin use alone versus aspirin and atorvastatin.
Graph 1.

Percentage of platelet inhibition by clopidogrel only and clopidogrel plus atorvastatin
Clopidogrel Only
Clopidogrel + atorvastatin
4. Discussion
Patients with PAD are a vulnerable population that often presents with multiple risk factors and comorbidities, requiring an integral treatment from lifestyle changes, medical therapy and ultimately surgical intervention. As shown in our study population the most common comorbidities were hypertension, hyperlipidemia and diabetes, which are very important risk factors for the development of PAD. Antiplatelets and lipid lowering drugs represent an essential combination for medical therapy in patients with PAD and it is crucial to obtain their full pharmacologic effect in order to achieve their therapeutic goals and avoid progression and/or complications of the disease.
The CYP isoenzymes in the liver are responsible for the metabolism of numerous drugs before they are excreted in the kidneys. This process can be affected by the use of multiple medications that are routed through the same subset of enzymes from this complex. The results obtained from the 87 samples analyzed in our study showed that the simultaneous use of atorvastatin and clopidogrel, interferes with clopidogrel’s activation, resulting in a decreased ability of platelet inhibition. These results are important because patients may not be receiving the full effect of clopidogrel which may result in lower platelet inhibition and higher rates of thrombosis. Building on previous research from our coagulation laboratory, we have recently shown that among patients with PAD undergoing revascularization, the increased platelet reactivity and decreased platelet inhibition (<29.2%), was predictive of subacute postoperative graft/stent thrombosis, principle that reinforce the concept that the antiplatelet therapy should have an optimal effect in order to decrease the risk of these events and at the same time keeping in mind that most of the patients are treated simultaneously with atorvastatin what may interfere with clopidogrel effect.7 Lau et al, measured platelet aggregation in patients undergoing coronary stent implantation and showed that atorvastatin competitively inhibits clopidogrel activation by being metabolized in the same CYP3A4 and suggest that the use of a statin not metabolized by the same isoenzyme (pravastatin) should be consider for patients treated with clopidogrel.5
Different studies performed in patients with coronary artery disease undergoing percutaneous coronary intervention, showed that there is was no difference in platelet reactivity in the groups treated with a CYP3A4-metabolized statin vs groups treated with a CYP3A4 non-metabolized statin.8–11 Wenaweser et al, concluded that drug-drug interaction between dual antiplatelet therapy (aspirin and clopidogrel) and statins seems not to be associated with stent thrombosis in patients with previous coronary stent thrombosis.9 Zhang et al, showed that patients with a non-ST-segment elevated acute coronary syndrome and have a drug-eluting stent implantation, did not have a decrease in antiplatelet efficacy of clopidogrel when treated simultaneously with clopidogrel, atorvastatin and lansoprazole over a period of 6 months.11
TEG is an FDA approved viscoelastic assay, available in multiple centers and points of care, easy to use and help to asses platelet reactivity in patients taking antiplatelet medications. However, an ideal test on blood coagulation does not yet exist and some confounding factors that affect platelet function in patients taking clopidogrel including genetic alleles and SNPs might have an effect in the study results and represent a limitation that should be approach in future studies. Clopidogrel resistance is a common phenomena identified in up to 40% of patients taking this medication, and in this cohort it might benefit to have a clopidogrel resistance test to get a better accuracy of the results. Another limitation that should be taken in consideration for future research is the sample size and the patient population that it is not generalizable, since our cohort was based on patients with PAD undergoing revascularization and also that the reported medications used by the patient and their actual compliance may differ.
5. Conclusion
Patients on clopidogrel and atorvastatin showed significantly less platelet inhibition compared to those on clopidogrel alone with TEG-PM, supporting the concept that atorvastatin may interfere with the therapeutic effect of clopidogrel. Patients with PAD taking atorvastatin may require an alternative antiplatelet therapy regimen that does not include clopidogrel or the use of a statin that is not metabolized in the same CPY isoenzymes than clopidogrel to achieve adequate thromboprophylaxis. Even when there are some studies in patients with coronary artery disease showing no difference in platelet reactivity with the use of these two drugs, there is lack of research about this topic in patients with peripheral artery disease and their clinical outcomes overtime with the simultaneous use of atorvastatin and clopidogrel.
ARTICLE HIGHLIGHTS.
Key Findings:
Endovascular, open and combined interventions in 72 patients with peripheral artery disease (PAD). Antithrombotic prophylaxis includes antiplatelet therapy. Patients on Clopidogrel alone showed significantly greater platelet inhibition compared to those on Clopidogrel and Atorvastatin [49.01% vs. 34.54%, p=.03].
Take home Message:
Patients with PAD taking Atorvastatin may require an alternative antiplatelet therapy regimen that does not include Clopidogrel or the use of a statin that is not metabolized in the same liver isoenzymes than Clopidogrel to achieve adequate thromboprophylaxis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang GJ, Shaw PA, Townsend RR, et al. The Associations between Peripheral Artery Disease and Physical Outcome Measures in Men and Women with Chronic Kidney Disease. Ann Vasc Surg 2016; 35: 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clarke TA, Waskell LA. The metabolism of clopidogrel is catalyzed by human cytochrome P450 3A and is inhibited by atorvastatin. Drug Metab Dispos 2003; 31: 53–59. [DOI] [PubMed] [Google Scholar]
- 3.Sangkuhl K, Klein TE, Altman RB. clopidogrel pathway. Pharmacogenet Genomics 2010; 20: 463–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leoncini M, Toso A, Maioli M, et al. Statin and clopidogrel pharmacological interaction. G Ital Cardiol 2013; 14: 574–584. [DOI] [PubMed] [Google Scholar]
- 5.Lau WC, Waskell LA, Watkins PB, et al. atorvastatin reduces the ability of clopidogrel to inhibit platelet aggregation: a new drug-drug interaction. Circulation 2003; 107: 32–37. [DOI] [PubMed] [Google Scholar]
- 6.Collyer TC, Gray DJ, Sandhu R, et al. Assessment of platelet inhibition secondary to clopidogrel and aspirin therapy in preoperative acute surgical patients measured by Thrombelastography® Platelet Mapping™. Br J Anaesth 2009; 102: 492–498. [DOI] [PubMed] [Google Scholar]
- 7.Majumdar M, Hall RP, Feldman Z, et al. Predicting Arterial Thrombotic Events Following Peripheral Revascularization Using Objective Viscoelastic Data. J Am Heart Assoc 2023; 12: e027790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ovrakh T, Serik S, Kochubiei O. Impact of atorvastatin and rosuvastatin on residual onclopidogrel treatment platelet reactivity in patients with ischemic heart disease and type 2 diabetes mellitus after acute coronary syndrome. Georgian Med News 2017; 7–14. [PubMed] [Google Scholar]
- 9.Wenaweser P, Windecker S, Billinger M, et al. Effect of atorvastatin and pravastatin on platelet inhibition by aspirin and clopidogrel treatment in patients with coronary stent thrombosis. Am J Cardiol 2007; 99: 353–356. [DOI] [PubMed] [Google Scholar]
- 10.Karaźniewicz-Łada M, Rzeźniczak J, Główka F, et al. Influence of statin treatment on pharmacokinetics and pharmacodynamics of clopidogrel and its metabolites in patients after coronary angiography/angioplasty. Biomedicine & Pharmacotherapy 2019; 116: 108991. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J-R, Wang D-Q, Du J, et al. Efficacy of clopidogrel and Clinical Outcome When clopidogrel Is Coadministered With atorvastatin and Lansoprazole: A Prospective, Randomized, Controlled Trial. Medicine 2015; 94: e2262. [DOI] [PMC free article] [PubMed] [Google Scholar]


