Abstract
Background:
MRT5005, a codon-optimized CFTR mRNA, delivered by aerosol in lipid nanoparticles, was designed as a genotype-agnostic treatment for CF lung disease.
Methods:
This was a randomized, double-blind, placebo-controlled Phase 1/2 study performed in the US. Adults with 2 severe class I and/or II CFTR mutations and baseline ppFEV1 values between 50 and 90% were randomized 3:1 (MRT5005: placebo). Six dose levels of MRT5005 (4, 8, 12, 16, 20, and 24 mg) or placebo (0.9% Sodium Chloride) were administered by nebulization. The single ascending dose cohort was treated over a range from 8–24 mg; the multiple ascending dose cohort received five weekly doses (range 8–20 mg); and the daily dosing cohort received five daily doses (4 mg).
Results:
A total of 42 subjects were assigned to MRT5005 (31) or placebo (11). A total of 14 febrile reactions were observed in 10 MRT5005-treated participants, which were mild (3) or moderate (11) in severity; two subjects discontinued related to these events. Additionally, two MRT5005-treated patients experienced hypersensitivity reactions, which were managed conservatively. The most common treatment emergent adverse events were cough and headache. No consistent effects on FEV1 were noted.
Conclusions:
MRT5005 was generally safe and well tolerated through 28 days of follow-up after the last dose, though febrile and hypersensitivity reactions were noted. The majority of these reactions resolved within 1–2 days with supportive care allowing continued treatment with MRT5005 and careful monitoring. In this small first-in-human study, FEV1 remained stable after treatment, but no beneficial effects on FEV1 were observed.
Keywords: Cystic Fibrosis, aerosol, mRNA therapy, gene therapy
1. Introduction
Treatment of cystic fibrosis (CF) has markedly improved, especially with the introduction of cystic fibrosis transmembrane conductance regulator (CFTR) modulators (1, 2). However, even the most broadly active CFTR modulator combinations are incapable of treating a segment of the population, estimated to encompass around 7% of non-Hispanic white individuals with CF (3, 4) and a greater percentage of those who are of non-white racial or ethnic backgrounds (2). For such patients and those that do not tolerate modulators, genetic therapies such as gene editing, delivery of the full CFTR gene, or messenger RNA (mRNA) therapies are of particular interest.
The goal of mRNA therapy is to deliver CFTR mRNA to CFTR expressing epithelial cells. Due to the unstable nature of mRNA, therapeutics are usually formulated within a protective lipid nanoparticle (LNP) (5). Because protein expression is transient (6, 7), CF mRNA must be dosed repeatedly, enabling dose interruptions or adjustments. The power of mRNA therapies has recently been demonstrated with the development of two widely used mRNA-based COVID-19 vaccines (8, 9). Other applications, such as the production of antibodies or metabolic enzymes, are also under investigation (10–16).
MRT5005 is a biosynthetic, codon-optimized mRNA (CO-hCFTR) encoding for the CFTR, which is delivered by nebulization as an LNP-formulated aerosol. In vitro and in vivo experiments in non-CF rodents and non-human primates indicated expression of CFTR, as measured by western blot and immunohistochemical detection of CFTR in the proximal and distal airways after treatment with inhaled MRT5005 (17). Human embryonic kidney cells transfected with MRT5005 showed robust CFTR protein expression. In Fischer rat thyroid gland cells grown under polarizing conditions, Ussing chamber experiments indicated functional restoration of CFTR activity with MRT5005 treatment and to levels that would suggest the potential for bioactivity in humans; however, these responses could not be consistently repeated in primary human bronchial epithelial cells derived from CF donors, a finding thought to be related to the challenge of achieving substantial CFTR activity in terminally differentiated airway cells with non-viral transfection at the time of this study’s conduct. RESTORE-CF (NCT03375047) was the first-in-human, first-in-patient clinical trial of inhaled MRT5005 in adults with CF; it was also the first clinical trial of any inhaled mRNA replacement therapy for people with CF.
This three-part study consisted of Part A (single ascending doses; testing 4 dose levels), Part B (multiple ascending doses; consisting of 5 weekly doses, testing 4 dose levels) and Part D (daily repeat dosing; consisting of 5 daily doses, testing only the 4 mg dose). Part C (intended to evaluate the presence of exogenous CFTR mRNA and CFTR protein expression in airway epithelial cells harvested by bronchoscopy following multiple-dose treatment) was not conducted. Here we report interim data representing the safety, tolerability, pharmacokinetic, immunological and spirometry data of all study participants in the RESTORE-CF trial up to 1 month after the last dose. Some of this data has been reported in abstracts (17–19).
2. Methods
2.1. Study design:
RESTORE-CF (NCT03375047) was a randomized, double-blind, placebo-controlled, combined single and multiple ascending dose (SAD and MAD) trial, conducted in 3 parts. The primary goal was to evaluate the safety and tolerability of MRT5005. MRT5005 was administered to subjects at 6 dose levels: 4, 8, 12, 16, 20, and 24 mg of CO-hCFTR mRNA (nominal dose levels) corresponding to approximate nebulization times of 22, 44, 66, 89, 110, and 133 minutes, respectively. In Part A, single doses of 8, 16, 20, and 24 mg were administered. Part B consisted of 5 weekly doses of 8, 12, 16, and 20 mg; and Part D consisted of 5 daily doses of 4 mg (DD). The originally planned Part C was not executed because a risk-benefit assessment did not consider bronchoscopy justified in this early stage of development.
The trial design called for one-year follow-up; however, this interim report is focused on the follow-up period during the first month after each participant’s last dose. This cut-off was chosen to match the short half-life of mRNA therapeutics and to avoid confounding of safety and tolerability assessments of MRT5005 by stochastic events more likely related to the natural history of CF.
The study was conducted at 16 US centers. All dosing was performed under observation in research clinics. Ethics approval was obtained from Western Institutional Review Board or local IRBs as required. As MRT5005 is an mRNA therapy, study materials were also reviewed by each center’s Institutional Biosafety Committee, per National Institutes of Health guidelines.
2.2. Dose escalation:
A protocol safety review committee (PSRC) reviewed safety data to make dose escalation decisions. In addition, there was a sponsor-independent data monitoring committee (DMC) to safeguard the interests of study participants in conjunction with the PSRC.
2.3. Trial population:
Adult males and females with clinically stable CF, two severe class I and/or II CFTR mutations and baseline percent predicted forced expiratory volume in one second (ppFEV1) between 50 and 90% were enrolled. Concomitant ivacaftor monotherapy was prohibited from all study parts, while concomitant triple combination therapy (elexacaftor-tezacaftor-ivacaftor, ETI) was prohibited from Parts A and B, but not Part D. Concomitant lumacaftor-ivacaftor or tezacaftor-ivacaftor was acceptable for all study parts for those with two F508del CFTR mutations.
2.4. Investigational treatment:
MRT5005 is a LNP suspension consisting of CO-hCFTR mRNA, the active drug, formulated with 3 lipids (imidazole cholesterol ester (ICE), dioleoyl phosphatidylethanolamine (DOPE), and 1,2-Dimyristoyl-sn-glycero-3-methoxypolyethylene glycol (DMG-PEG2000) that comprise the LNP (20). The nominal dose of MRT5005 is based on the mass of CO-hCFTR mRNA. Sterile normal saline solution (Sodium Chloride Inhalation Solution, USP 0.9%) was used as the placebo comparator. The volume of placebo and number of nebulizers used to deliver placebo matched that of the MRT5005 dosing group to maintain blinding.
2.5. Randomization and masking:
In all 3 study parts, participants were randomized to receive either MRT5005 or placebo in a 3:1 ratio. The Investigators and all study staff involved in the evaluation of subject eligibility, administration of study medication, and assessment of study outcomes were blinded to treatment assignment.
2.6. Outcome measures:
Definition of adverse events (AE), severe AEs (SAE), and febrile reactions are described in the Supplementary Methods. Other safety assessments included vital signs, pulse oximetry, clinical laboratory evaluations, serum inflammatory markers (Part B, 20 mg and Part D only), electrocardiogram, 2-view chest radiograph, and spirometry (see Supplementary Methods for details on the timing of spirometry in each study part). Whole blood samples were quantitatively analyzed for CO-hCFTR mRNA and ICE. Serum samples were assessed by enzyme-linked immunosorbent assay (ELISA) for antibodies to CFTR protein and/or PEG. CFTR-specific T cell responses were assessed using peripheral blood mononuclear cells. See Supplementary Methods for additional details.
Serum samples were analyzed for inflammatory markers on an exploratory basis (Part B, 20 mg and Part D only), including interferon (IFN)-γ, interleukin (IL)-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-13, and tumor necrosis factor (TNF)-α, using ELISA (MSD V-PLEX Proinflammatory Panel 1).
2.7. Statistical Analysis:
All analyses were descriptive. This report comprises data up to 1 month after the last dose in each study participant, but subjects were followed, per protocol, for one year after the last dose for ongoing additional safety evaluations.
3. Results:
3.1. Demographics and disposition
Between 15 May 2018 and 22 February 2021, 53 people with CF were consented (see Figure 1, CONSORT diagram) and 42 were randomized to receive treatment (MRT5005: n =31; placebo: n = 11, Table 1). Intervention was discontinued in 4 individuals (MRT5005: 2 patients due to AE and 1 patient due to the COVID-19 pandemic; placebo: 1 patient due to the pandemic). All 42 participants were included in the analysis dataset.
Figure 1.
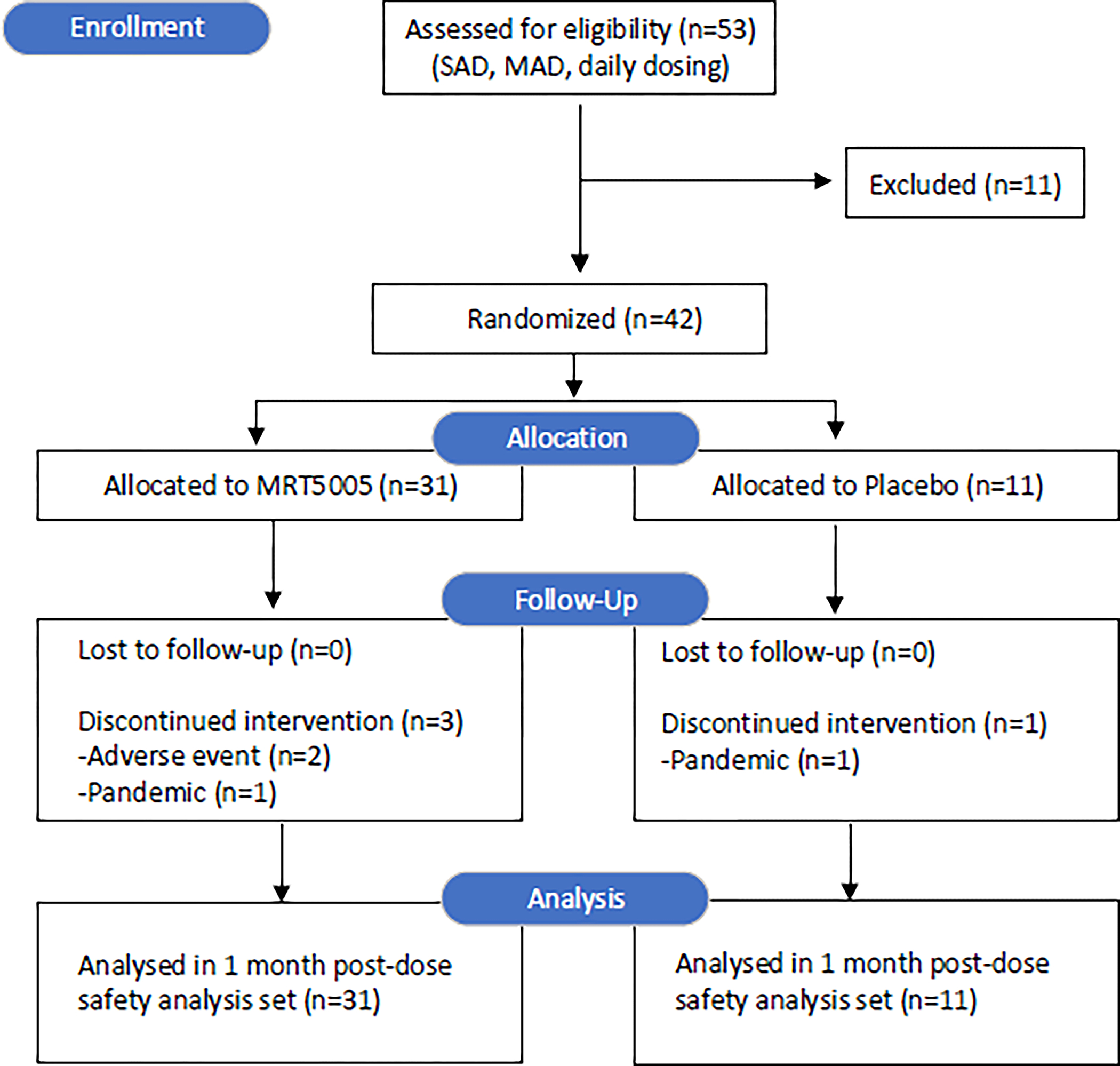
CONSORT diagram of individuals assessed and enrolled in RESTORE-CF. Thank you very much for all of the work you are doing in bringing this to press.
Table 1.
Demographics
| Characteristic | MRT5005 | Placebo |
|---|---|---|
| (n=31) | (n=11) | |
| Age, mean (SD) | 27.8 (7.41) | 29.3 (13.27) |
| Sex, n (%) | ||
| Female | 17 (54.8) | 6 (54.5) |
| Race, n (%) | ||
| White | 31 (100) | 10 (90.9) |
| Black or African-American | 0 | 1 (9.1) |
| Genotype | ||
| Class I/Class I | 2 (6.5) | 3 (27.3) |
| Class I/Class II | 8 (25.8) | 5 (45.5) |
| Class II/Class II | 20 (64.5) | 3 (27.3) |
| Class II/Undetermined a | 1 (3.2) | 0 |
| CFTR modulator use at baseline, n (%) | 19 (61.3) | 4 (36.4) |
| Percent predicted FEV1 at baseline, n (%) b | ||
| < 70 | 20 (64.5) | 7 (63.6) |
| ≥ 70 | 11 (35.5) | 4 (36.4) |
F508del/Q452P mutation.
Baseline value was defined as the average of the results from testing on Day -1 and at pre-dose on Day 1; Daily Dosing cohort baseline ppFEV1 based on pre-dose Day 1 only.
Two study participants were replaced. Both replacements were in Part B: 1 who withdrew after a febrile reaction (16 mg cohort) and 1 who was discontinued due to pandemic-related site closure (12 mg cohort).
The trial population was equally balanced between MRT5005 and placebo in terms of age (average age: 27.8 ±7.4 for MRT5005; 29.3 ± 13.3 for placebo) and sex (54.8 % females for MRT5005; 54.5 % for placebo). As per the inclusion criteria, participants had severe Class I and/or II mutations (Supplementary Table 1), with the most prevalent genotype being F508del/F508del (23/42 participants). One participant had an uncharacterized mutation (Q452P), which was considered, based on evidence of a severe CF phenotype, to be compatible with the inclusion criteria. The majority of participants were using CFTR modulator therapy.
Triple combination modulator therapy with ETI was approved during the course of the trial; for that reason, only the participants in Part D (Daily Dose cohort) were allowed to take this therapy.
3.2. Safety and tolerability:
3.2.1. Overall safety:
The majority of AE reported (up until 1 month after the last dose) were mild to moderate (Table 2). There were 249 treatment-emergent adverse events (TEAE) reported in 29 (93.5%) MRT5005-treated participants versus 48 TEAE in 10 (90.9%) placebo participants. The MRT5005 group reported more moderate events (66 events in 16 participants [51.6%]) than the placebo group (4 events in 3 participants [27.3%]), which was related to the incidence of febrile/hypersensitivity reactions.
Table 2.
Safety overview of RESTORE-CF
| TEAE through 1 Month Post-Dosea | Pooled MRT5005 | Pooled Placebo | ||
|---|---|---|---|---|
| (n=31) | (n=11) | |||
| Patients with any TEAE | 29 (93.5) | 10 (90.9) | ||
| Mild, n (%) | 11 (35.5) | 7 (63.6) | ||
| Moderate, n (%) | 16 (51.6) | 3 (27.3) | ||
| Severe b , n (%) | 2 (6.5) | 0 (0.0) | ||
| Patients with serious TEAE c , n (%) | 1 (3.2) | 0 (0.0) | ||
| Patients with TEAE Leading to Discontinuation, n (%) | 2 (6.5) | 0 (0.0) | ||
| TEAE by Preferred Term (with ≥ 10 events in Pooled MRT5005 group) | Number of Events | Patients n (%) | Number of Events | Patients n (%) |
| Cough | 29 | 16 (51.6) | 3 | 2 (18.2) |
| Headache | 26 | 16 (51.6) | 2 | 2 (18.2) |
| Pyrexia | 15 | 9 (29.0) | 0 | 0 (0.0) |
| Chills | 14 | 10 (32.2) | 0 | 0 (0.0) |
| Chest Discomfort | 11 | 4 (12.9) | 1 | 1 (9.1) |
| Pulmonary Exacerbation | 10 | 9 (29.0) | 1 | 1 (9.1) |
| Wheezing | 10 | 6 (19.4) | 2 | 2 (18.2) |
The 1-month post-dose time interval is defined as through Day 29 for SAD, Day 57 for MAD, and Day 32 for Daily Dosing Cohort.
Pulmonary exacerbation (20mg SAD), myalgia upper back (20mg MAD).
Pulmonary exacerbation (20mg SAD).
Only one potentially drug-related SAE was reported, in which a 20 mg SAD study participant developed a pulmonary exacerbation, rated “severe” and “possibly related to treatment”, on day 23 after dosing. This required hospitalization during the end of the 1-month post-dose follow-up period; this exacerbation was effectively treated with intravenous amikacin and cefepime. There were no AE of grade 4 or higher.
The most frequently observed AE, occurring at least 10 times in the pooled MRT5005 treated group, were cough, headache, pyrexia, chills, chest discomfort, pulmonary exacerbation and wheezing (Table 2). Due to the small number of participants per group, no clear between-group dose-response could be identified (See Supplementary Tables 17, 18, 19), with the exception of febrile reactions, which tended to occur more frequently at higher doses.
Four study participants discontinued treatment early. Two MRT5005-treated participants discontinued treatment because of AE (one with febrile reaction after the first dose in the 16 mg MAD group, one with fever/hypersensitivity after the 3rd of 5 planned doses). The other two discontinuations (one placebo, one MRT5005) were due to COVID-related closures of study sites. All patients received the planned number of doses, with two exceptions in the daily dose group where 2 patients received 4 out of 5 planned doses: one 12 mg MAD patient missed the final dose due to COVID-related closure of the study site and one daily dose patient missed the 2nd daily dose due to weather-related study center closure.
3.2.2. Febrile reactions
For the purposes of this study, “febrile reactions” were defined by the MedDRA terms “body temperature increased” or “pyrexia”, in combination with at least one other systemic symptom such as headache, arthralgia, myalgia, fatigue, chills, nausea or vomiting, starting within 24 h of study medication administration. In Part A, participants were monitored overnight, and thus temperature checks could be reliably obtained. In Part B and Part D, study participants were outpatients, and temperatures were not methodically measured. Thus, the definition of “febrile reactions” was expanded to include the term “body temperature increased”. Figure 2 shows the occurrence of febrile reactions. In total, 14 febrile reactions were reported in 10 participants, all MRT5005-treated. These were either mild (3 events) or moderate (11 events) and were treated with standard medications such as acetaminophen, ibuprofen and, in the case of nausea, with ondansetron. Pyrexia typically started within 4–14 h after the end of the dose and resolved within 1–2 days after the dose.
Figure 2.
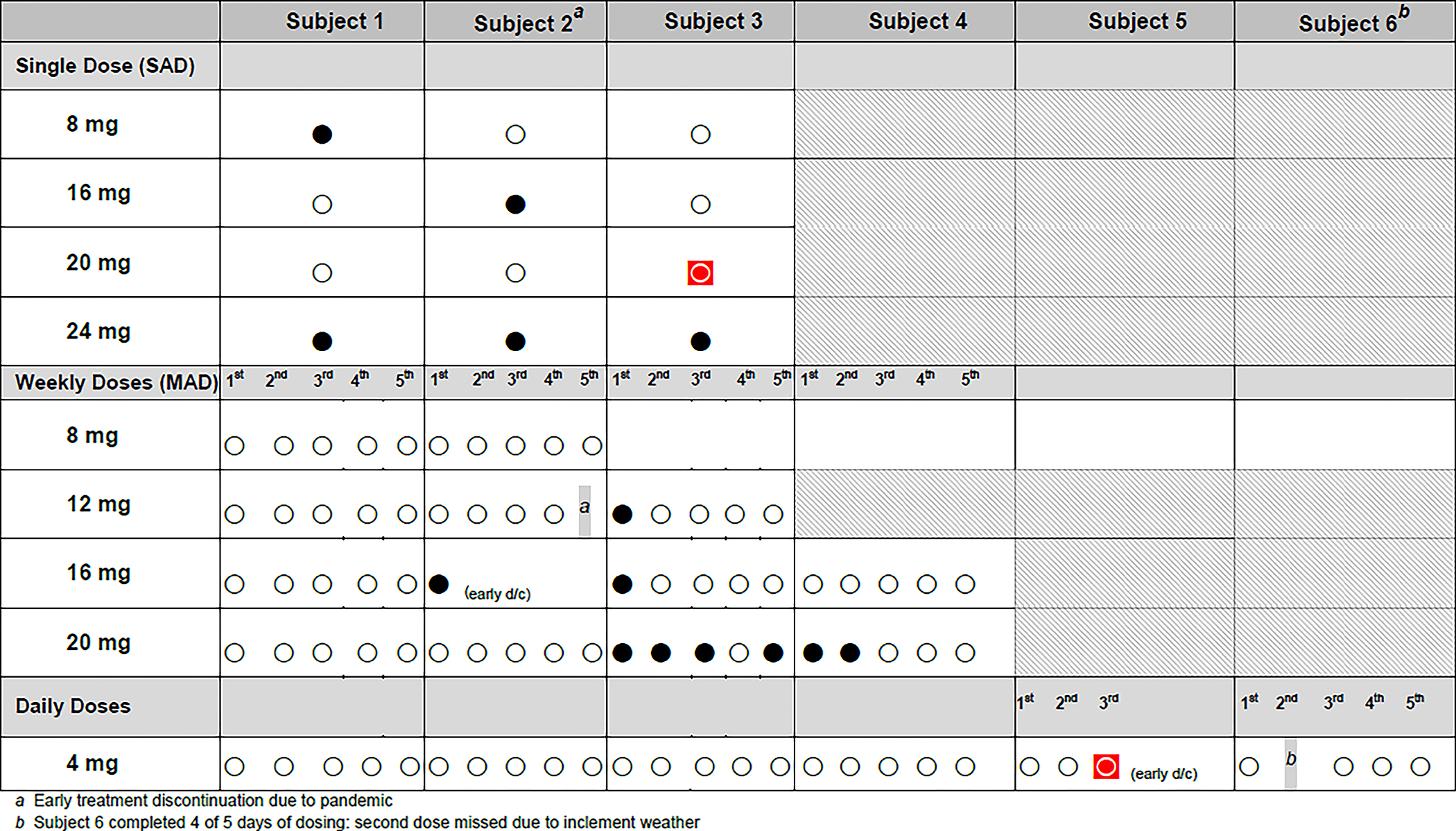
Overview of febrile and hypersensitivity reactions in RESTORE-CF. Every circle represents a completed MRT5005 dose in an individual patient. Empty circles represent uneventful doses, black circles represent doses followed by a febrile reaction, and the two red circles encased in squares represent doses followed by a hypersensitivity reaction. One participant (subject 2) in the 12 mg MAD group received only 4 doses due to COVID-related site closure (footnote a). One participant in the 16 mg MAD group (subject 2) discontinued after 1 dose due to a febrile reaction. One participant in the Daily Dose group (subject 6) received only 4/5 doses due to inability to travel to the site for one of the doses due to inclement weather (footnote b), and one participant (subject 5) discontinued treatment after 3 doses due to a hypersensitivity reaction.
Febrile reactions tended to occur more frequently at higher dose levels. Participants who experienced a first febrile reaction did not consistently have subsequent febrile reactions with repeat dosing (Figure 2). Individual case descriptors are described online.
3.2.3. Hypersensitivity reactions
In addition to the febrile reactions described above, another two study participants (one 20 mg SAD participant, one daily dose participant after the third daily 4 mg dose) experienced hypersensitivity reactions, both considered moderate in severity. In both the hypersensitivity reaction also fulfilled the definition of a febrile reaction; these two participants developed an urticarial rash on the chest, arms, and hands. In both symptoms appeared within several hours after dosing, and most symptoms, including the rash, resolved the next day. Additional details regarding these cases are described online.
Hypersensitivity reactions were not associated with clinically significant laboratory abnormalities. These events were not considered to be anaphylactic reactions due to the lack of airway compromise and the independent DMC concurred with this evaluation.
3.3. PK, immunogenicity and inflammation markers
Pharmacokinetics of both CO-hCFTR mRNA and one of the lipids in the LNP (ICE) were assessed. Traces of CO-hCFTR mRNA and/or ICE were detected occasionally in 14 study participants receiving MRT5005 across the SAD, MAD, and DD cohorts (See Tables 2–7 in the Supplementary Results). No clear dose-dependency was observed, and there were no signs of accumulation in the MAD or DD cohorts.
No pattern of immunogenicity to CFTR or PEG was identified. Anti-CFTR antibodies, anti-PEG antibodies, and CFTR-sensitized T cell activation were detected sporadically across the SAD, MAD, and DD cohorts. However, positive values were also occasionally observed in placebo participants and at baseline, indicating the need to develop improved assays (e.g., specificity) (See Tables 8–16 in the Supplementary Results).
After the observation of febrile reactions, a small panel of inflammatory markers was added by protocol amendment, starting with the last of the SAD cohorts (20 mg) and the DD cohort. No consistent patterns of inflammatory markers were observed, and there was no correlation with febrile reactions, hypersensitivity reactions, or other AE.
There was no relationship between febrile or hypersensitivity reactions and the presence of CO-hCFTR mRNA or ICE in blood samples nor did any other biomarkers of immunogenicity correlate with the presence of CO-hCFTR mRNA or ICE in biospecimens.
3.4. Spirometry
Lung function testing by spirometry was performed throughout the study according to ATS/ERS guidelines (21), including repeatedly after the single doses, and before and after each one of the multiple doses. In the DD group, spirometry was performed before each of the 5 doses, and 2 hours after the first dose). Although there were transient increases in FEV1 after the SAD cohort that appeared dose-dependent (Figure 3A), these changes were not replicated in the MAD cohort (Figure 3B–3C).
Figure 3A.
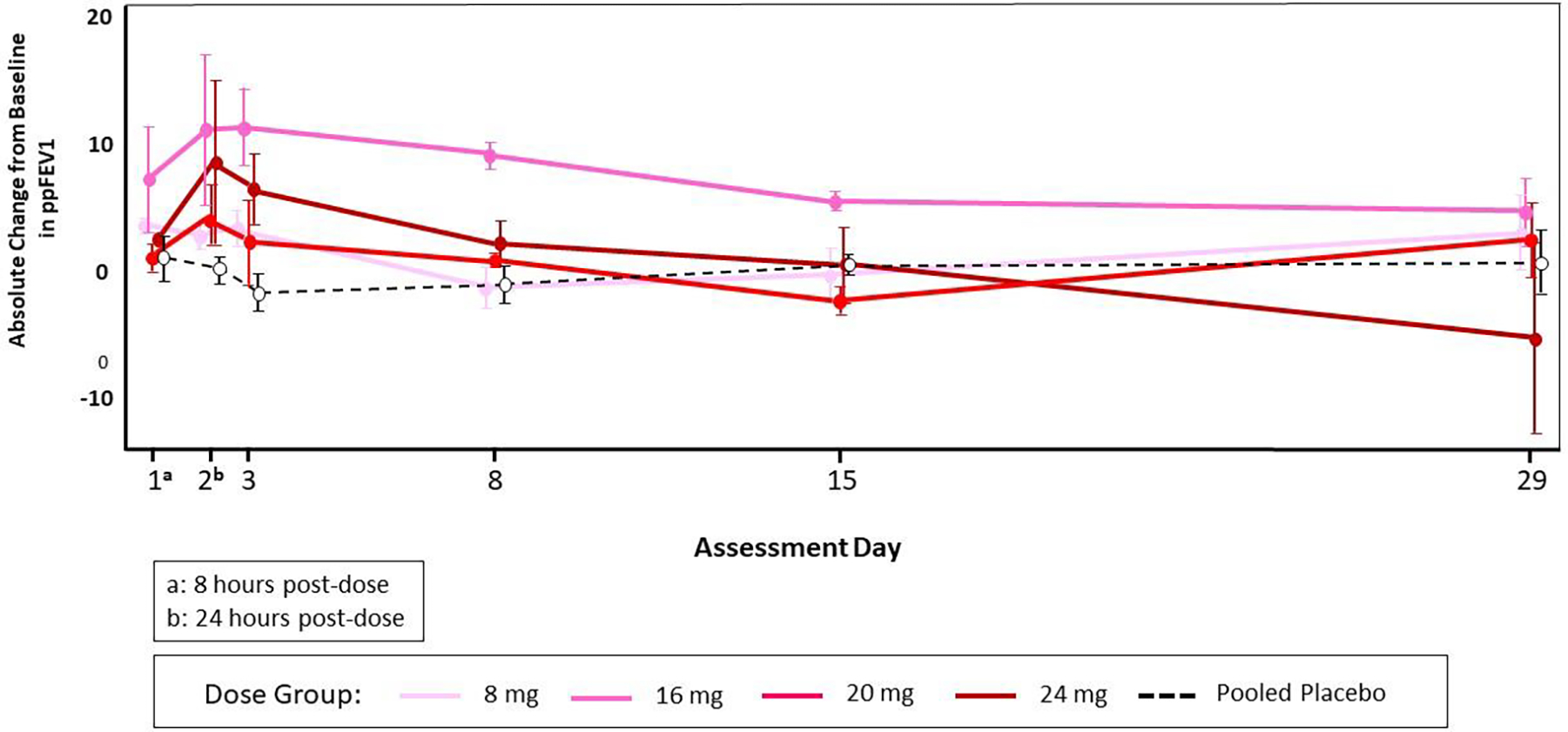
Mean and Standard Error of Absolute Change from Baseline in ppFEV1 by Dose Group and Visit through Day 29 (Single Ascending Dose Cohort)
Note: The baseline value was defined as the average of the results from testing on Day -1 and at pre-dose on Day 1.
Figure 3B.
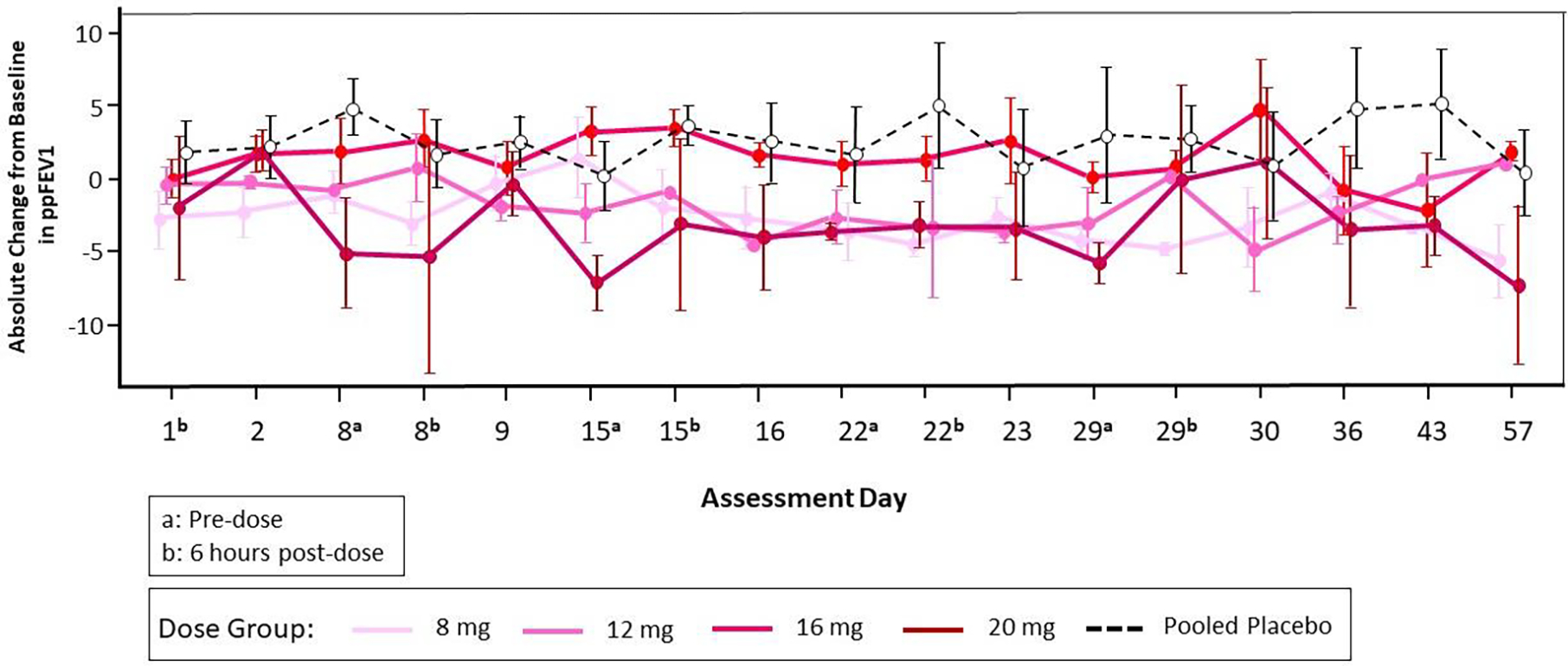
Mean and Standard Error of Absolute Change from Baseline in ppFEV1 by Dose Group and Visit through Day 57 (Part B, Multiple Ascending Doses).
Note: The baseline value was defined as the average of the results from testing on Day -1 and at pre-dose on Day 1.
Figure 3C.
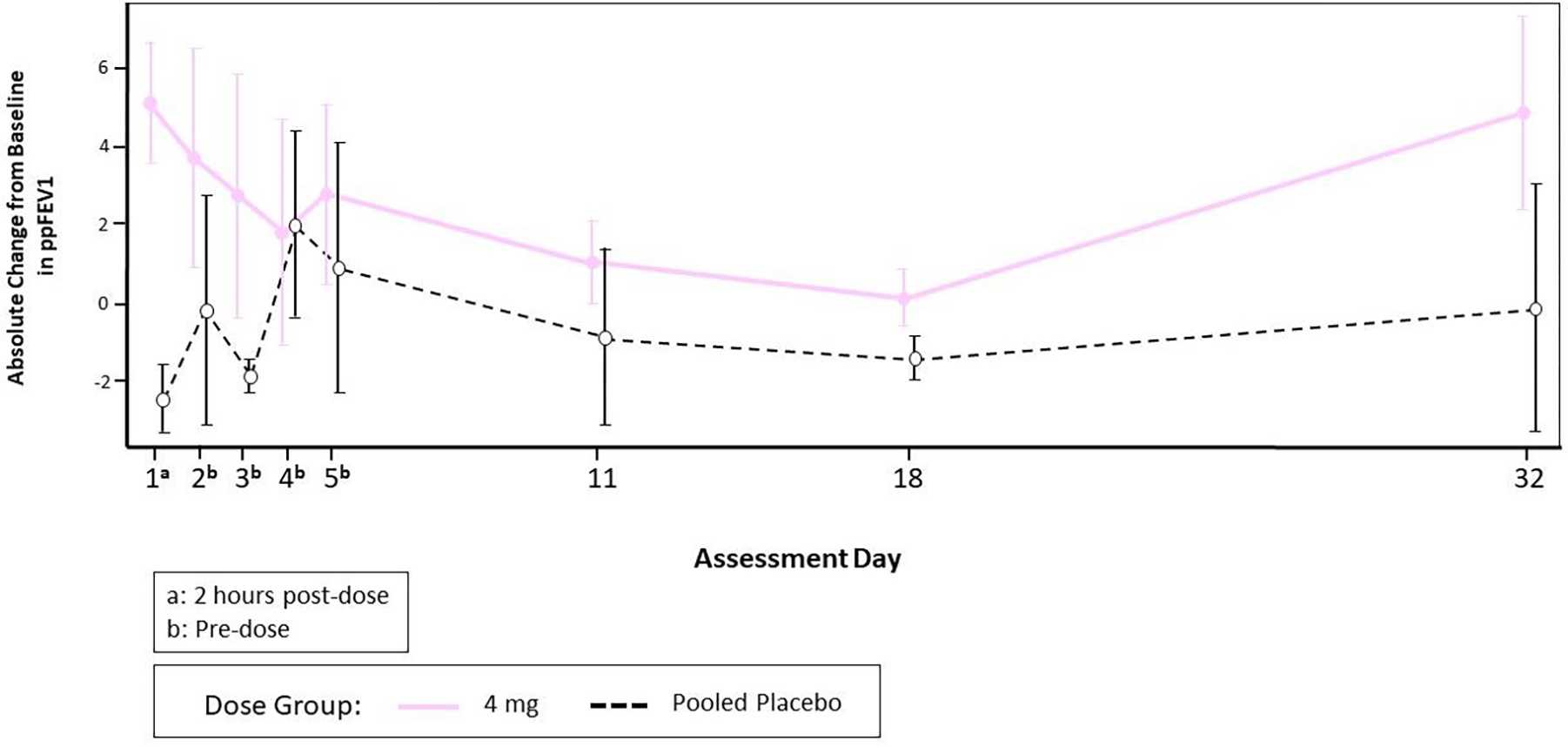
Mean and Standard Error of Absolute Change from Baseline in ppFEV1 by Dose Group and Visit through Day 32 (Daily Dosing Cohort)
Note: The baseline value was defined as the result from testing pre-dose on Day 1.
4. Discussion
This is the first trial of inhaled mRNA in people with CF. This first-in-human, first-in-patient study evaluated MRT5005 between 4 and 24 mg, as SAD (8-16-20-24 mg), MAD (8-12-16-20 mg for 5 weeks), and DD (4 mg for 5 days). MRT5005 was generally well tolerated, with only one SAE (pulmonary exacerbation) being noted within one month of dosing. However, several participants developed febrile reactions hours after dosing, in some cases accompanied by nausea and/or vomiting, signs and symptoms that were not predicted by pre-clinical toxicology studies in rats and non-human primates. Two episodes also met criteria for hypersensitivity reaction, although neither met criteria for anaphylactic reaction. All febrile/hypersensitivity reactions were mild-to-moderate in severity and symptoms resolved within 1–2 days, similar to what was reported by Alton et al in the evaluation of CFTR DNA-lipid complex therapy (22). The biochemistry and inflammatory panels did not show a consistent pattern of abnormalities. Neither leukocytosis nor activation of complement or other inflammatory cascades were detected. Our ability to investigate the febrile reactions was limited by the fact that an inflammatory panel was not instituted until later in the trial and was conducted at a single time point.
Special attention is focused on two questions related to these febrile reactions. The first question is whether pretreatment with nonsteroidal anti-inflammatory medications can prevent or mitigate these reactions. Limited data from one patient suggests that this may be possible. It should be noted that CF patients in prior trials with DNA-lipid complexes also experienced transient febrile reactions, effectively managed through symptomatic treatment (23). The second question is whether sporadic febrile reactions may be predicted in advance. Due to the limited sample size, of which only a minor proportion developed febrile reactions, no predisposing factors could be identified, so this question was not settled. However, it appears that within a given person, febrile reactions may or may not recur with subsequent dosing, which argues against a predictably susceptible phenotype for these events (24). Febrile reactions, including chills, were also observed, albeit less frequently, in large clinical trials and an observational study of intramuscular mRNA vaccines against COVID-19 (9, 25, 26). In those studies, there was a clearly increased incidence of fever after the second injection, potentially indicating sensitization.
It is not clear whether the mRNA or the lipid component is responsible for these febrile reactions. Similar reactions have been described in gene therapy studies in CF with lipid formulations (22, 23). It is known that mRNA can activate the immune system via Toll-Like Receptors (27). Lipids can likewise be pro-inflammatory, particularly in the context of airway deposition (28), and in this study it was not possible to distinguish between these two essential components of MRT5005.
Spirometry testing was performed on a frequent basis, as is appropriate for inhaled therapies for which bronchospasm is an inherent risk (29, 30). MRT5005 treatment did not precipitate bronchospasm nor provide any appreciable improvement in airflow. In the SAD part, where FEV1 was frequently measured in the first days after the single dose, an apparent increase, with occasional values as high as 20% above baseline, was noted in some patients. However, in the MAD and DD cohorts, this was not confirmed, and evidence of sustained improvements in lung function was not observed. Examination of subgroups by genotype or use of CFTR modulators did not reveal a more responsive population. We conclude that the variability of FEV1 when measured frequently over a short time span has the potential to be misleading and reiterates the importance of placebo-controlled trials with use of this effort-dependent maneuver.
Although the trial follow-up is one year after the last dose for safety reasons, we believe that one month follow up after the last dose is an appropriate window for the evaluation of both efficacy and drug-related AE. This is due to the self-limited bioactivity of mRNA, which is a labile molecule (31).
Nebulization time was approximately 20 minutes per 4 mg, necessitating a substantial administration time for the highest doses tested. Considering the need to treat young patients with CF, who would not be able to tolerate prolonged weekly dosing events, a daily dose group of 4 mg was also studied, which offered the potential to improve delivery by daily sequential administration.
Pharmacokinetic evaluation of MRT5005 in the blood was uninformative, as only occasional trace levels of either mRNA or lipid could be detected. This was expected for an inhaled macromolecular complex.
There was no evidence that MRT5005 induced an immune response or generation of auto-antibodies.
It is not yet clear why the effects of laboratory studies demonstrating efficacy in CF models and adequate expression in non-CF animals did not translate to clinical efficacy among human CF subjects, though we posit some potential explanations here. The study was not powered or intended to detect a change in FEV1; rather, with only 3 active subjects per dose group, determining safety and tolerability were the principal objectives. It should be noted that in Part D of the trial (in an effort to facilitate recruitment) participants were permitted to be on a stable dose of highly effective modulator therapy (ETI), which could have masked beneficial effects of the study drug on pulmonary function in these subjects. Nevertheless, no meaningful trends in FEV1 were observed across any dose group, and treatment failed to show sufficient effects on lung function on which to predicate well-powered follow up studies. At the time of the study’s conduct, multiple groups had reported the challenge of transfection with LNP particles in primary human bronchial epithelial cells, a highly predictive preclinical model system for small molecule CFTR modulators, even when delivery and protein expression could be achieved with respiratory delivery in animal models. In retrospect, it seems likely that adequate expression within differentiated bronchial epithelium was not achieved with the formulation administered in this study, resulting in the absence of substantial and consistent changes in FEV1. Future studies evaluating alternative lipids that overcome barriers presented by terminally differentiated epithelium, in addition to achieving adequate protein expression in CF animals, could help improve the potential for translation to humans. It is also possible that other factors contributed, such as impairment of drug entry into airway surface cells by the CF mucus barrier, limited delivery to secretory cell types that participate in vectorial ion transport, or limited duration of action of delivered mRNA, although the present study offers no specific supportive evidence of these possibilities. The inclusion of biomarkers related to CFTR expression or delivery in future studies involving CFTR mRNA therapy could help answer questions, if efficacy by FEV1 is not definitive.
In summary, mRNA in LNP can be safely inhaled, although it has shown a propensity for inducing febrile and hypersensitivity reactions. Preclinical and toxicology work should be designed to optimize the formulation for tissue-specific tropism and to minimize inflammatory responses. Future studies of efficacy of inhaled mRNA in people with CF should be mindful to enroll enough participants to account for the short-term variability in airflow that is characteristic of the disease, and where feasible, include airway samples to confirm drug delivery and establish proof-of-concept. Development of bioanalytical techniques for the direct assessment of expression and function of the desired protein in the target cells will be helpful in providing mechanistic proof-of-concept and selection of the optimal doses to advance for further therapeutic development.
Supplementary Material
Highlights.
CFTR mRNA was delivered by aerosol in lipid nanoparticles to cystic fibrosis adults
In this small first-in-human study treatment was generally safe and well tolerated
Quickly resolving fever and hypersensitivity reactions were noted in some subjects
Lung function remained stable after treatment but no benefit was observed
Acknowledgements
The authors acknowledge the support of the Cystic Fibrosis Foundation Clinical Trials Network and Emily’s Entourage.
Role of funding sources: Translate Bio provided funds for the study but did not have a role in the interpretation or reporting of results.
Funding:
This study was funded by Translate Bio. Infrastructure grants were funded by the Cystic Fibrosis Foundation (CFF) and NIH P30DK072482
Abbreviations:
- AE
adverse event(s)
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- mRNA
messenger RNA
- CO-hCFTR
Codon-optimized human recombinant CFTR mRNA
- CRISPR
clustered regularly interspersed short palindromic repeats
- DD
daily dose
- DMG-PEG-2K
1,2 dimyristoyl-sn-glycerol methoxypropylene glycol
- DOPE
1,2 dioleoyl-sn-glycero-3phosphoethanolamine
- ETI
elexacaftor-tezacaftor-ivacaftor
- ICE
imidazole cholesterol ester
- LPN
protective lipid nanoparticle
- MAD
multiple ascending dose
- ppFEV1
percent predicted forced expiratory volume in 1 second
- SAD
single ascending dose
- TEAE
treatment-emergent adverse event(s)
Footnotes
Declaration of Competing Interest
The authors have completed ICMJE disclosure of interest forms. MJ, JL, KDM, and SZN report no competing interests. JBZ, CB, JCC, DD, NL, SL, SMR, KAM, KSM, and MSS report clinical trial funding from Translate Bio to their institution for this study. MSS, EBM, KAM, and JCC report support for the current manuscript from Emily’s Entourage. DD reports research contracts with Insmed, Inc., Aridis Pharmaceuticals, Armata Pharmaceuticals, and 4D Molecular Therapeutics and fees from the CFF for his role on the Data Safety Monitoring Board (DSMB). DD is a member of the DSMB at the University of Pennsylvania. KSM reports research grants to her institution from the CFF in partnership with 4D Molecular Therapeutics, Abbvie, Aridis Pharmaceuticals, Armata Pharmaceuticals, Boeringer-Ingelheim, Corbus, Insmed, Laurent Pharmaceuticals, Novartis, Eloxx, Vertex Pharmaceuticals, Savara, and Proteostasis for unrelated research. SMR reports research grants to his institution from the CFF in partnership with Novartis, Galapagos/Abbvie, Synedgen/Synspira, Eloxx, Vertex Pharmaceuticals, Ionis, and Astra Zenica for unrelated research. SMR reports consulting fees for clinical trial design and conduct from Novartis, Galapagos/Abbvie, Synedgen/Synspira, Vertex Pharmaceuticals, Renovion, Ionis, Cystetic Medicines, and Arcturus. JBZ reports fees from the CFF for his role on the DSMB and research grants from the CFF in partnership with Laurent Pharmaceuticals, Savara, AzureRx Biopharma, Aridis Pharmaceuticals, and Vertex Pharmaceuticals for unrelated research but no personal payments. AB, EBM and MV were full-time employees of Translate Bio during the conduct of the study. MV was an employee of Rho, Inc. and is currently an employee of Krystal Biotech. MV reports stock options provided to employees of Translate Bio and Krystal Biotech. MV is former board member of the CFF Central Carolinas Chapter. AB is currently on the Board of Directors for Pieris Pharmaceuticals. CB reports research grants from the CFF and CFF Therapeutic Development Network (CFFTDN) in partnership with Vertex pharmaceuticals for unrelated research but no personal payments. MSS reports research grants to his institution from the CFF in partnership with Vertex Pharmaceuticals and consulting fees from Vertex Pharmaceuticals. MVI reports research grants from the CFF and CFFTDN.
To advance drug development and a search for a cure, the CFF has contracts with several companies to help fund the development of potential treatments and/or cures for CF. Pursuant to these contracts, CFF may receive milestone-based payments, equity interests, royalties on the net sales of therapies, and/or other forms of consideration. Resulting revenue received by CFF is used in support of their mission.
Declaration of Competing Interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bell SC, Mall MA, Gutierrez H, Macek M, Madge S, Davies JC, et al. The future of cystic fibrosis care: a global perspective. Lancet Respir Med. 2020;8(1):65–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cystic Fibrosis Foundation Patient Registry - 2021 Annual Data Report 2022 ed. Bethesda, Maryland; 2022. [Google Scholar]
- 3.McGarry ME, McColley SA. Cystic fibrosis patients of minority race and ethnicity less likely eligible for CFTR modulators based on CFTR genotype. Pediatr Pulmonol. 2021;56(6):1496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mall MA, Mayer-Hamblett N, Rowe SM. Cystic Fibrosis: Emergence of Highly Effective Targeted Therapeutics and Potential Clinical Implications. Am J Respir Crit Care Med. 2020;201(10):1193–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hou X, Zaks T, Langer R, Dong Y. Lipid nanoparticles for mRNA delivery. Nat Rev Mater. 2021;6(12):1078–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Houseley J, Tollervey D. The many pathways of RNA degradation. Cell. 2009;136(4):763–76. [DOI] [PubMed] [Google Scholar]
- 7.Hargrove JL, Schmidt FH. The role of mRNA and protein stability in gene expression. Faseb j. 1989;3(12):2360–70. [DOI] [PubMed] [Google Scholar]
- 8.Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, et al. An mRNA Vaccine against SARS-CoV-2 - Preliminary Report. N Engl J Med. 2020;383(20):1920–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Hoecke L, Roose K. How mRNA therapeutics are entering the monoclonal antibody field. J Transl Med. 2019;17(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.An D, Schneller JL, Frassetto A, Liang S, Zhu X, Park JS, et al. Systemic Messenger RNA Therapy as a Treatment for Methylmalonic Acidemia. Cell Rep. 2017;21(12):3548–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farelli JD, Asrani KH, Isaacs C, deBear JS, Stahley MR, Shah A, et al. Leveraging Rational Protein Engineering to Improve mRNA Therapeutics. Nucleic Acid Ther. 2018;28(2):74–85. [DOI] [PubMed] [Google Scholar]
- 13.Jiang L, Park JS, Yin L, Laureano R, Jacquinet E, Yang J, et al. Dual mRNA therapy restores metabolic function in long-term studies in mice with propionic acidemia. Nat Commun. 2020;11(1):5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prieve MG, Harvie P, Monahan SD, Roy D, Li AG, Blevins TL, et al. Targeted mRNA Therapy for Ornithine Transcarbamylase Deficiency. Mol Ther. 2018;26(3):801–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roseman DS, Khan T, Rajas F, Jun LS, Asrani KH, Isaacs C, et al. G6PC mRNA Therapy Positively Regulates Fasting Blood Glucose and Decreases Liver Abnormalities in a Mouse Model of Glycogen Storage Disease 1a. Mol Ther. 2018;26(3):814–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu X, Yin L, Theisen M, Zhuo J, Siddiqui S, Levy B, et al. Systemic mRNA Therapy for the Treatment of Fabry Disease: Preclinical Studies in Wild-Type Mice, Fabry Mouse Model, and Wild-Type Non-human Primates. Am J Hum Genet. 2019;104(4):625–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbier A, DeRosa F, Karve S, Smith L, Askew K, Kaza N, et al. In vitro and in vivo evaluation of an mRNA therapeutic for the treatment of patients with cystic fibrosis. Pediatr Pulmonol. 2018;53(S2):253–4. [Google Scholar]
- 18.Zuckerman JB, McCoy K, Schechter MS, Dorgan D, Jain M, Macdonald KD, et al. Safety and tolerability of a single dose of MRT5005, an inhaled CFTR mRNA therapeutic, in adult CF patients. Pediatr Pulmonol. 2019;54:A515. [Google Scholar]
- 19.Rowe SM, Dorgan D, Lascano J, Zuckerman JB, McCoy K, Jain M, et al. Safety and tolerability of single and repeated doses of MRT5005, an inhaled CFTR mRNA replacement therapy, in adult CF patients. J Cyst Fibros. 2021;20:s257. [Google Scholar]
- 20.Barbier A, Heartlein M, DeRoss F, Abysalh J, Dias A, Karve S, et al. Treatment of cystic fibrosis by delivery of nebulized mRNA encoding CFTR. Patent Application Publication. United States: Translate Bio, Inc.; 2022:1–151. [Google Scholar]
- 21.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–38. [DOI] [PubMed] [Google Scholar]
- 22.Alton E, Armstrong DK, Ashby D, Bayfield KJ, Bilton D, Bloomfield EV, et al. Repeated nebulisation of non-viral CFTR gene therapy in patients with cystic fibrosis: a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir Med. 2015;3(9):684–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alton EW, Stern M, Farley R, Jaffe A, Chadwick SL, Phillips J, et al. Cationic lipid-mediated CFTR gene transfer to the lungs and nose of patients with cystic fibrosis: a double-blind placebo-controlled trial. Lancet. 1999;353(9157):947–54. [DOI] [PubMed] [Google Scholar]
- 24.Jimenez-Rodriguez TW, Garcia-Neuer M, Alenazy LA, Castells M. Anaphylaxis in the 21st century: phenotypes, endotypes, and biomarkers. J Asthma Allergy. 2018;11:121–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenblum HG, Gee J, Liu R, Marquez PL, Zhang B, Strid P, et al. Safety of mRNA vaccines administered during the initial 6 months of the US COVID-19 vaccination programme: an observational study of reports to the Vaccine Adverse Event Reporting System and v-safe. Lancet Infect Dis. 2022;22(6):802–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303(5663):1529–31. [DOI] [PubMed] [Google Scholar]
- 28.Marchiori E, Zanetti G, Mano CM, Hochhegger B. Exogenous lipoid pneumonia. Clinical and radiological manifestations. Respir Med. 2011;105(5):659–66. [DOI] [PubMed] [Google Scholar]
- 29.Meeker DP, Wiedemann HP. Drug-induced bronchospasm. Clin Chest Med. 1990;11(1):163–75. [PubMed] [Google Scholar]
- 30.Zhang YG, Wright WJ, Tam WK, Nguyen-Dang TH, Salome CM, Woolcock AJ. Effect of inhaled preservatives on asthmatic subjects. II. Benzalkonium chloride. Am Rev Respir Dis. 1990;141(6):1405–8. [DOI] [PubMed] [Google Scholar]
- 31.Rohner E, Yang R, Foo KS, Goedel A, Chien KR. Unlocking the promise of mRNA therapeutics. Nat Biotechnol. 2022;40(11):1586–600. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


