Abstract
Quantitative traits are measurable characteristics distributed along a continuous scale thought to relate to underlying biology. There is growing interest in the use of quantitative traits in behavioral and psychiatric research, particularly in research on conditions diagnosed based on reports of behaviors, including autism. This brief commentary describes quantitative traits, including defining what they are, how we can measure them, and key considerations for their use in autism research. Examples of measures include behavioral report scales like the Social Responsiveness Scale and Broader Autism Phenotype Questionnaire, as well as biological measurements, like certain neuroimaging metrics; such measures can capture quantitative traits or constructs like the broader autism phenotype, social communication, and social cognition. Quantitative trait measures align with the Research Domain Criteria (RDoC) approach and can be used in autism research to help gain a better understanding of causal pathways and biological processes. They can also identify genetic and environmental factors involved in such pathways, and lead to an understanding of influences on traits across the entire population. Finally, in some cases, they may be used in gauging treatment response, and assist screening and clinical characterization of phenotype. In addition, practical benefits of quantitative trait measures include improved statistical power relative to categorical classifications and (for some measures) efficiency. Ultimately, research across autism fields may benefit from incorporating quantitative trait measures as a complement to categorical diagnosis to advance understanding of autism and neurodevelopment.
Lay summary:
Many characteristics lie along a spectrum. Quantitative traits are measurable characteristics that lie along a continuous scale and may relate to underlying biology. This brief commentary summarizes the concept of quantitative traits, ways to measure them, and how they can be used in autism research. Examples of commonly used quantitative trait measures in autism research include questionnaires capturing social behaviors, as well as certain biological measurements. These measurements capture features or traits known to differ in autism, like social cognition. Quantitative trait measures can be used in autism research to help gain a better understanding of potential causes and pathways; to identify genetic and environmental factors involved in the development of autism; and to better understand patterns of behaviors and co-occurring factors. Ultimately, research across autism fields may benefit from incorporating quantitative trait measures to learn more about autism and neurodevelopment.
Autism is a neurodevelopmental condition with varied presentations and complex causes. Autism is diagnosed according to a set of behavioral features that can vary widely from individual to individual, but is unified by challenges in social communication and the presence of restricted, repetitive patterns of behavior and interests. According to current diagnostic criteria, autism is recognized as a spectrum of conditions that range in severity and support needs. Traditionally, autism and other neurodevelopmental and psychiatric diagnoses have been conceptualized as binary “yes/no” conditions. However, this would tend to imply an underlying “on/off” biology, and does not align well with the wide variability in phenotype (defined as a set of observable characteristics), or at the least, a categorization that does not capture the nuance (Hyman, 2021). This binary categorization also does not align with evidence that core traits related to the autism phenotype extend into the general population (Constantino and Todd, 2003; Reiersen et al., 2007, Robinson et al., 2011a, Robinson et al., 2011b; English, et al. 2021). The “spectrum” terminology addresses some of this variability, but only within those above the threshold for diagnosis, and still in practice results in categories. This then begs the question (in discussion for decades, eg, (Constantino et al., 2000; Baron-Cohen et al., 2001; Dawson et al., 2002; Hyman, 2021, Wing, 1988) ), can autism or key features of the diagnosis be captured along a continuous scale? And what does it even mean to consider autism or underlying related traits continuously, or quantitatively?
Though biologic measures do not factor into the current diagnostic criteria, there are known biological underpinnings to autism, as demonstrated by autism’s high heritability, genetic contributors (Hallmayer et al., 2011; Tick et al., 2016; Thapar and Rutter, 2021; Vashisth and Chahrour, 2023) and association with alterations of brain morphology and growth (Zwaigenbaum, et al. 2005; Wolff, et al. 2012; Donovan and Basson 2017). Changes to diagnostic criteria, and debates around nosology/ classification of not only autism but also related conditions, have persisted for decades (McKusick, 1969; Volkmar and McPartland, 2014; Marquand et al., 2016). Recently, there have been calls to shift the research focus to incorporate continuous measures, or quantitative traits (Insel et al., 2010; Cuthbert and Insel, 2013), driven by the theory that such traits may be more closely tied to underlying biologic processes than are diagnostic categories. Use of quantitative trait measures may therefore contribute to our understanding of autism etiology (or causes) and could lead to innovative targeted services.
What are autism-related quantitative traits?
A quantitative measure is an assessment tool that yields a continuous score.
A quantitative trait is a measurable characteristic, defined along a continuous scale, that is influenced by genetic and environmental factors. Classic examples of quantitative traits include height, weight, and blood pressure. Endophenotype is a related term that tends to be used (often in genetic studies) to refer to specific heritable biologic markers. Endophenotypes are sometimes also referred to as intermediate phenotypes, e.g., measurable characteristics intermediate between the behavioral phenotype and the underlying genetics (Gottesman and Gould, 2003; Cannon and Keller, 2006). While there is overlap across these terms, here quantitative trait is distinguished as the broader term capturing both behavioral and biologic traits, and endophenotype as more specific to biologic traits.
Returning to the question of whether and how autism can be considered on a continuous scale, before delving into prior work on this topic, we might start by first considering how autism is diagnosed according to standard clinical measures. Detailed developmental histories and clinical observations assess a series of core behaviors as present or absent, the degree to which they are present, and when. Summary scores based on these ratings determine whether an individual falls above or below a defined threshold for diagnosis indicative of substantial differences, impairment, or impact on functioning. Clinician expertise and judgement provide the final step in weighing these observations and determining whether an individual is given the diagnosis. Behavioral quantitative trait measures are in some ways an extension of diagnostic rating systems to capture traits and variability, except that they do so across the entire population and not just in those being assessed for diagnosis.
In autism, quantitative traits include constructs or underlying processes central to autism like social communication and social functioning (Sucksmith et al., 2011). These may be measured by behavioral questionnaires, and in some instances, biologic assessments like certain neuroimaging measures. Characteristics associated with autism have strong and multifactorial genetic contributions, so they should be measurable across the entire source population from which diagnosed individuals arise. In general, these quantitative traits lie along a distribution that may look something like the below figure (though not all traits may follow a normal distribution, and it is also worth noting that distributional properties of a continuous measure are tied to sampling characteristics). Following this model, the relationship between a quantitative trait measure and the diagnosis would be that diagnosed individuals fall in the upper range of the underlying trait distribution, an observation that has been supported for several quantitative trait measures of the autism phenotype (Constantino et al., 2000; Spiker et al., 2002; English et al., 2021).
A brief, non-exhaustive list of some commonly used quantitative measures that can be considered for use as capturing quantitative traits in autism is provided in Table 1. These measures broadly fall into behavioral-based questionnaires and more biologically-based measurements that may also capture quantitative traits. Further characterizations can be made based on whether such measures capture central aspects of the autism phenotype, such as social reciprocity, or social communication, or related components, such as verbal or cognitive abilities or motor functioning. Some of the behavioral measures seek to assess the autism phenotype more comprehensively, e.g., overall “broad autism traits,” and also incorporate subscales, which offer opportunities for measurement of multiple trait dimensions or domains (such as the SRS, or the more recently developed CATI). Some of these measures are primarily used for screening (and therefore are typically implemented as threshold/binary categorization measures), while others are more routinely used as continuous trait scores and thus better fit the quantitative trait measure classification. The SRS and the BAP-Q are used for both purposes, but are most commonly implemented as continuous trait scores, with strong evidence that they reliably capture an autism-related quantitative trait over the range of the population. While some autism severity scores (such as the ADOS severity score) yield a continuous score, they are not optimally designed to capture variability across the entire population (e.g., including trait variability with adequate range to capture and analyze differences in those without autism. In addition, such diagnostically-oriented measures are often not feasible to conduct in large studies or large samples without indication for autism assessment).
Table 1.
Common quantitative measures that may be used to capture quantitative traits in autism research1
| Measure | Brief description | Ages for use | Construct or QT(s) being measured2 | Reference(s)3 |
|---|---|---|---|---|
| Behavioral Measures | ||||
| Autism Spectrum Quotient (ASQ/AQ) | 50-item self-report questionnaire that assesses extent of expression of autism-spectrum traits in an individual. (Freely available) | 16yrs+ | BAP (total score); Social communication and attention (via Social Skill, Attention Switching, Attention to Detail, Communication, and Imagination subscales) |
(Baron-Cohen et al. 2001) |
| Autism Spectrum Screening Questionnaire (ASSQ) | 27-item informant report questionnaire designed for screening purposes for “high functioning” autism and Asperger syndrome with no intellectual disability. Assessments completed by parents or teachers. (Freely available). *Primarily used as a screening measure |
6–17yrs | Autistic traits/BAP; social interaction and communication, RRB | (Ehlers et al. 1999) |
| The Broad Autism Phenotype Questionnaire (BAP-Q) | 36-item self or informant report questionnaire to identify ASD traits that are expressed in the general population and among individuals with ASD. | ≥ 18 years | BAP (total score); Personality traits and social language skills (via aloof personality, pragmatic language, and rigid personality subscales) | (Hurley et al. 2007) |
| Childhood Autism Spectrum Test (CAST) | 37-item self or informant report tool designed for screening ASD in children in non-clinical settings to identify possible ASD cases in the general population for research settings. (Freely available). *Primarily used as a screening measure |
5–11 yrs | BAP | (Scott et al. 2002) |
| Comprehensive Autistic Trait Inventory (CATI) | 42-item self-report scale capturing autistic traits, developed and validated in adults. Each item rated on a 5-point Likert scale. 6 subscales (7 items each). (Freely available). | ≥ 18 years | BAP (total score); Social communication, repetitive behaviors, cognitive rigidity, sensory sensitivity (via subscales as listed, with addition of social camouflage) | (English et al. 2021) |
| Social Responsiveness Scale (SRS) | 65-item self or informant report questionnaire designed for use in affected and general population samples, yielding a single score of autism-related phenotype as well as subdomain scores. | 2.5–4.5yrs (preschool), 4–18 (school age), 18+ (adult) | BAP, Social reciprocity (total scores); Social awareness, social motivation, social cognition, social communication, and RRB (subscales) | (Constantino 2012) |
| Social and Communication Disorders Checklist (SCDC) | 12-item informant reporting questionnaire designed for assessing reciprocal social interaction and communication skills in children. | 3–19yrs | Social communication | (Skuse et al. 2005) |
| Quantitive Checklist for Autism in Toddlers (Q-CHAT) | 25-item parent report scale designed for assessing autistic traits across the population in toddlers and preschoolers. Used both for early screening and as a general population trait measure.(Freely available). | 18–24 months | Autistic traits/BAP | (Allison et al. 2008) |
| Biological measurements | ||||
| Anatomical MRI | Measures of brain growth, size, structure. Magnetic resonance imaging (MRI) of brain anatomy can generate quantitative measures of early brain and CSF enlargement, altered growth of structures involved in social cognition (e.g., amygdala), and white matter connectivity supporting behavioral development (e.g., motor, repetitive behaviors, sensory processing). These measures have been related to autistic traits and severity, as well as diagnosis. | Social cognition and autistic traits | (Shen et al., 2013; Hazlett et al., 2017; Wolff et al., 2012) | |
| functional Near-Infrared Spectroscopy (fNIRS) Signature | fNIRS measures changes in cerebral blood flow and hemoglobin oxygenation levels indicative of neural activity to measure brain activity and connectivity; the quantitative measure is the blood-oxygen-level-dependent (BOLD) signal, which directly measures changes in the brain blood flow; has been associated with functional connectivity and socio-communicative skills in autism. | Social cognition | (Conti et al. 2022) | |
| fMRI | functional Magnetic Resonance Imaging (fMRI) measures changes in blood flow as an indicator of brain activity; quantitative measure is the BOLD signal ; may capture social processing and cognition in autism. | Social processing and social cognition | (Santana et al. 2022; Ammons et al. 2021; Jassim et al. 2021) | |
| Motor assessments | Various metrics of movements and movement dynamics, which may capture social processing in autism. | Social processing | (Zhao et al. 2018;Krishnappa et al. 2023 ) | |
| Select eye-tracking and pupillometry measures3 | Measurements of gaze fixation and duration of fixation; measurement of changes in pupil diameter (mm) in response to a stimulus; a longer latency of the pupil and altered fixation have been associated with autism. May relate to social processing. | Social processing | (Frazier et al. 2018; Hamner and Vivanti 2019; de Vries et al. 2020; Ma et al. 2021; Mastergeorge et al. 2021; Setien-Ramos et al. 2022) | |
| Genetic factors | Genetic data may be used in multiple ways to capture associations with complex traits. Quantitative trait locus (QTL) analyses seek to link complex phenotypes to genetic markers, for example. In autism, multifactorial involvement of many genes has been established, and studies using QTLs have suggested key biological pathways and linked biology to phenotypic characteristics. Studies have also linked genetic signatures in autism or autism risk genes to neurologic characteristics associated with autism. | Multiple, including BAP, social communication and interaction, RRB, verbal and non-verbal communication | (Duvall et al. 2007; Bourgeron 2015; Andrews et al. 2017; Guo et al. 2018; Lee et al. 2022) | |
Table not exhaustive of all potential quantitative measures in autism. Additional quantitative behavioral measures, such as the Childhood Autism Rating Scale (CARS; Schopler et al., 1980), and the Broader Phenotype Autism Symptom Scale (BPASS) (Dawson et al., 2007) not included in table given use in general population samples lacking, with use for screening for the former and development and use in affected families for the latter.
Abbreviations: QT=quantitative trait; BAP= broader autism phenotype (construct); RRB= restricted repetitive behaviors. Note “autistic traits” used in addition to or rather than BAP where described or suggested by the measure.
References provided are not an exhaustive list but example(s) of validation studies or use of the measure. For further summary of quantitative traits in autism, see also (Sucksmith, Roth et al. 2011) Certain eye-tracking measures may be better considered as behavioral assessments or responses; see Hamner and Vivanti, 2019 for a summary of eye-tracking technology and the related constructs assessed.
Key considerations
It is worth noting – and addressing head on- that there is some debate in the field regarding the use and validity of quantitative trait measures in autism research (Constantino, 2011; Waterhouse et al., 2016; Constantino, 2021; Lord and Bishop, 2021; Mottron, 2021; Waterhouse, 2021). This debate is inherently tied to different theories regarding the nature of autism itself -how we define autism, its causes, and how it develops. Briefly, there is the view that autism is qualitatively distinct from typical variation in neurodevelopment, and utilizing broader, less specific outcome measures may detract from understanding of autism; and then there is the view, perhaps best summarized under the liability threshold model, that autism represents one end of a spectrum of trait(s) across the entire population. Less discussed (Kim et al., 2019; Constantino, 2021a) is the consideration that both “sides” to this debate may hold some truth and value (e.g., it is possible that in some cases, such as in syndromic forms of autism with simple monogenic causes, etiology follows the former, and in others, such as non-syndromic cases, it follows the latter), or that studying broader variation need not detract from also studying subgroups or more refined categories. The topic regarding how quantitative traits fit within autism conceptualization deserves further attention in separate work (Constantino, 2021a; Hyman, 2021; Vivanti and Messinger, 2021). However, the complex genetics, the varied phenotype, and the aforementioned evidence supporting traits central to autism extending throughout the population, lend substantial weight to the latter viewpoint, and the general utility of quantitative traits.
The debate can also at least in part be attributed to some clarifications that can be made regarding quantitative traits. Briefly, one key concern against the use of trait measures has been lack of specificity to the autism spectrum disorder (ASD) diagnosis. Individuals with other conditions, such as anxiety disorders or ADHD, may register high levels of autism traits (though often with a developmental course that differs from that seen in autistic individuals). However, individuals confirmed with ASD diagnoses also are known to have high co-occurrence rates of other behavioral conditions, and thus, overlap in the underlying continuous trait measures related to those diagnoses is therefore also expected. While quantitative trait measures may carry some “noise,” they may also be picking up signals representing actual shared etiologic pathways (Bolton et al., 1998; Ronald et al., 2008). Future work should address these more etiologically-framed comparisons and considerations of autism quantitative traits.
It is also important to recognize that different quantitative trait measures carry different strengths and weaknesses, and the validity, reliability, and related properties of various quantitative trait measures will differ. Measurement errors and biases cannot be fully avoided in any behavioral trait measure (nor the existing diagnostic process itself), and so the relative impacts of such errors and biases therefore needs to be placed within context. Furthermore, sample characteristics and study settings will play into comparison of traits or observed findings based on trait scores across populations. (For example, to expect the same screening performance in a high likelihood sample as a general population sample overlooks the increased clinical follow-up in the former; expecting the same estimates of associations in populations with differing exposure or unmeasured confounder profiles overlooks the role of these in detection and magnitude of associations. Context and confounders matter in comparisons). Alternatively, there could be true differences in psychometric properties and measurement across different subgroups of individuals with autism, such as those with co-occurring intellectual disability or non-speaking populations. While work addressing measurement invariance has been conducted for many quantitative trait measures (primarily focused on age and sex), further work is needed addressing potential subgroup differences. Psychometric considerations of individual quantitative trait measures are addressed in other publications, for example (Skuse et al., 2005; Bolte et al., 2008)). Finally, is also worth noting that some biomarkers associated with severity of autism may represent consequences of the condition, rather than intermediate or underlying causes; thus, attention to timing of processes is needed (Constantino, 2021b). The remainder of this article will focus on clarifying the utility and purpose of quantitative traits in autism research.
Why and how should quantitative traits be used in autism research?
A main motivation for use of quantitative traits, in autism research and more broadly, consistent with the National Institute of Mental Health’s Research Domain Criteria (RDoC) approach (Insel et al., 2010; Cuthbert and Insel, 2013), is to advance understanding of underlying, biologically driven processes. Quantitative trait measures, in being theoretically more related to these processes, may increase the likelihood of identifying associations with factors involved in the development of the autism phenotype and help bridge the gap in our understanding of the complex etiology(ies) of autism. Quantitative trait measures also preserve characterization of variability and avoid of loss of information that comes with binary categorization. Additional motivations for use of quantitative trait measures include (see also (Sagiv et al., 2015; Constantino, 2021)): improved statistical power, relative to analyses based on case status or categories, reduced potential for outcome misclassification in a continuous score due to not grouping individuals with a varied phenotype, and in some cases, reduced potential for selection biases relative to studies recruiting based on outcome status, and increased efficiency and feasibility of phenotype assessment.
Keeping these motivating factors in mind, there are several ways in which quantitative traits may be harnessed to advance autism research. First, quantitative trait measures may be used as outcomes in both genetic and environmental studies seeking to better understand the causes of autism and autism-related traits, and to provide insights into biological pathways. This may include identification of biological markers related to autism (Constantino, 2021b). For example, genetic analyses have revealed methylation signatures in brain tissue enriched for immune-related pathways (Andrews et al., 2017), and have identified multiple loci related to quantitative measures such as the SRS (Duvall et al., 2007). Environmental studies of ubiquitous chemicals with evidence for influences on neurodevelopment have also found associations with autism-related traits, including as measured by the SRS and the SCQ (Sagiv et al., 2018; Kim et al., 2021). As noted above, utilization of quantitative trait measures in this manner also improves statistical power, because these measures do not rely on categories or counts of people, but instead provide continuous values for analysis on everyone in a study sample. Thus, studies seeking to detect associations and identify novel factors and genetic variants related to likelihood of autism may benefit from the use of quantitative traits (Sagiv et al., 2015; Taylor et al., 2021).
A second - related - way that quantitative traits may be used is to examine how common exposures may influence subtler aspects of the phenotype, or identify potential subthreshold effects. As observed for the relationship between early life lead exposure and cognitive development, “little shifts” in the underlying trait distribution can have large impacts at the population level, and public health efforts to reduce or prevent these shifts (e.g., by the phaseout of leaded gasoline) had population benefits far beyond reductions in numbers of children classified as cognitively impaired (Bellinger, 2008; Lanphear, 2018). The public health implications of this dimensional approach are well depicted in Figure 2 of Lanphear (2018). Importantly, quantitative trait measures are uniquely suited to capture these broader population-level effects. Furthermore, for quantitative trait measures capturing the autism phenotype, these also allow us to identify individuals who, while falling below the threshold for diagnosis, may still benefit from access to services. Importantly, capturing variability and seeking to better support those below a threshold does not imply there is not also the need to address limitations in existing supports for those above the threshold. We can strive to advance support (in differing ways) for all levels of functioning and abilities- but only if our research includes study of this variability.
Third, quantitative trait measures can be utilized in comparative approaches that draw conclusions based not only on state, but also on trait. For example, we may compare the association between factor X (suspected to influence neurodevelopment) and autism diagnosis, with the association between factor X and a quantitative trait measure. This comparison may help to address whether the factor is associated with an effect above a threshold consistent with clinical diagnosis, or rather, a shift in the trait distribution across the population, as has been suggested for example with obstetric complications (Zwaigenbaum et al., 2002; Lyall et al., 2022). Commonalities or differences across these outcome metrics may also inform our understanding of specificity of associations and/or of underlying genetic and neurobiologic pathways. Furthermore, comparability of associations with contributing etiologic factors across quantitative trait measures and diagnosis, as has been seen for some quantitative traits related to autism (Lundstrom et al., 2012; Taylor et al., 2021; Lyall et al., 2022), would argue in favor of such quantitative traits capturing pathways involved in the diagnosis. More of this type of comparative work within the same study populations is needed. In addition, comparing measures across general population and autistic samples is also useful in further resolving influences on variability.
Finally, quantitative trait measures may be used to gain a better understanding or further characterization of the autism phenotype. This may include use in clinical settings where implementing assessment batteries across a number of domains might aid in differential diagnosis or identification of symptom clusters or unique subgroups that could translate into better targeted treatments or more focused systems of support. Using measures that capture quantitative traits in the general population is also informative to clinical recommendations. In addition, brief quantitative trait behavioral questionnaires may offer opportunities for measurement of autism-related phenotype or related traits in settings where lengthier assessments may be prohibited; examples in large population-based studies include shortened versions of the SRS being applied in the NIH’s Environmental influences on Child Health Outcomes (ECHO) program (Lyall et al., 2021, Kaat et al., 2023), the ABCD study (Sharp et al., 2023), and the Netherland’s Generation R cohort (Alemany et al., 2021). While biological signatures of autism are not known in most cases, and may not be comparable for all cases given the complexity and diversity represented in the diagnosis, incorporating quantitative traits based on biologic measures may help to define unique subgroups consistent with an RDoC approach. On the other hand, quantitative trait measures may also help to identify commonalities across neurodevelopmental disorders, which could challenge current diagnostic constructs and point to the need for continued evolution of nosological systems (Kendler, 2009; Hyman, 2021).
Conclusions
Quantitative trait measures capture continuous traits with the goal of characterizing the autism phenotype, or aspects of it, along a continuum. Key motivations for using quantitative traits in autism (and in any complex condition) research are to gain a better understanding of etiology, and/or to characterize the phenotype in a way that more fully captures variability (including beyond a clinical construct or threshold) and may be more tied to underlying biology. Work to optimize quantitative trait measures in autism, and to better understand the etiology of autism, is still ongoing. However, several quantitative trait measures exist that well characterize the autism phenotype, or components of it, with numerous examples in studies of risk factors, developmental course, and treatment strategies. Ultimately, leveraging multiple types of outcome measures that capture the autism phenotype is likely to help us more rapidly advance the field towards better understanding and support of autism and developmental variability.
Figure 1:
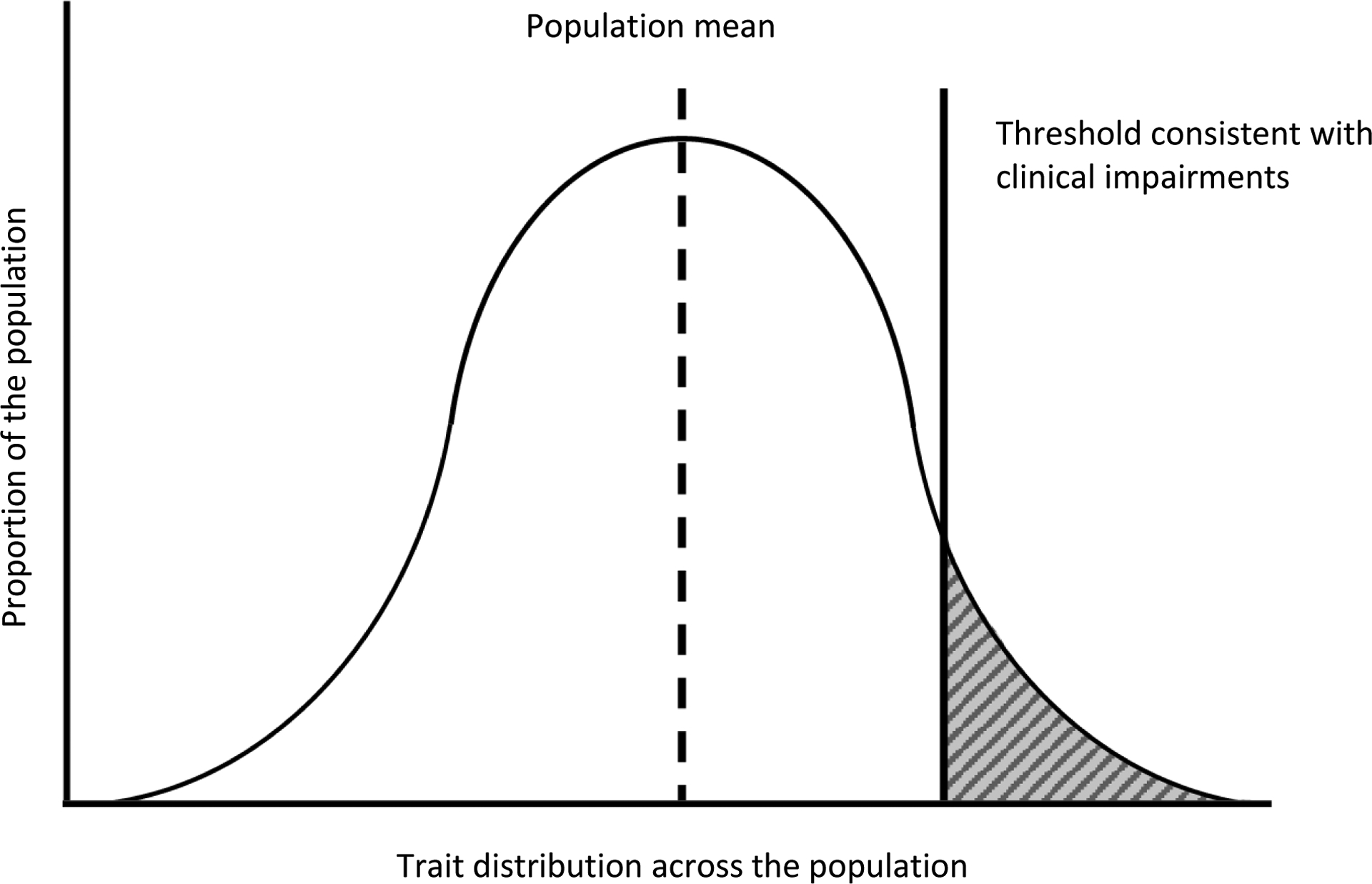
Example of a quantitative trait distribution and classic trait-liability threshold model
Example plot showing the distribution of a hypothetical quantitative trait in the population. Liability for the condition increases at the upper tail of the distribution, and above a defined threshold diagnosis is defined. The Y axis represents the proportion of the population that has a given trait score or trait at a given measured level, while the X axis represents the scale or range of the measured quantitative trait.
Acknowledgements:
The author thanks Drs. Craig Newschaffer, M. Daniele Fallin, Brian Lee, Christine Ladd-Acosta, Giacomo Vivanti, Diana Robins, and Mark Shen for their feedback on this manuscript, and Dr. Marisa Patti and Ms. Juliette Rando for their assistance in Table preparation. No conflicts of interest to report. This work was supported by funding through NIEHS R01ES032469.
Data availability statement:
N/A – no data were included as part of this commentary.
References
- Alemany S, Blok E, Jansen PR, Muetzel RL, White T. Brain morphology, autistic traits, and polygenic risk for autism: A population-based neuroimaging study. Autism Res. 2021. Oct;14(10):2085–2099. doi: 10.1002/aur.2576. Epub 2021 Jul 26. [DOI] [PubMed] [Google Scholar]
- Allison C, Baron-Cohen S, Wheelwright S, Charman T, Richler J, Pasco G and Brayne C (2008). “The Q-CHAT (Quantitative CHecklist for Autism in Toddlers): a normally distributed quantitative measure of autistic traits at 18–24 months of age: preliminary report.” J Autism Dev Disord 38(8): 1414–1425. [DOI] [PubMed] [Google Scholar]
- Ammons CJ, Winslett ME, Kana RK. Neural responses to viewing human faces in autism spectrum disorder: A quantitative meta-analysis of two decades of research. Neuropsychologia. 2021. Jan 8;150:107694. doi: 10.1016/j.neuropsychologia.2020.107694. Epub 2020 Nov 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews SV, Ellis SE, Bakulski KM, Sheppard B, Croen LA, Hertz-Picciotto I, Newschaffer CJ, Feinberg AP, Arking DE, Ladd-Acosta C, Fallin MD. Cross-tissue integration of genetic and epigenetic data offers insight into autism spectrum disorder. Nat Commun. 2017. Oct 24;8(1):1011. doi: 10.1038/s41467-017-00868-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J Autism Dev Disord. 2001. Feb;31(1):5–17. doi: 10.1023/a:1005653411471. Erratum in: J Autism Dev Disord 2001 Dec;31(6):603. [DOI] [PubMed] [Google Scholar]
- Bellinger DC. Very low lead exposures and children’s neurodevelopment. Curr Opin Pediatr. 2008. Apr;20(2):172–7. doi: 10.1097/MOP.0b013e3282f4f97b. [DOI] [PubMed] [Google Scholar]
- Bolte S, Poustka F and Constantino JN (2008). “Assessing autistic traits: cross-cultural validation of the social responsiveness scale (SRS).” Autism Res 1(6): 354–363. [DOI] [PubMed] [Google Scholar]
- Bolton PF, Pickles A, Murphy M and Rutter M (1998). “Autism, affective and other psychiatric disorders: patterns of familial aggregation.” Psychol Med 28(2): 385–395. [DOI] [PubMed] [Google Scholar]
- Bourgeron T (2015). “From the genetic architecture to synaptic plasticity in autism spectrum disorder.” Nat Rev Neurosci 16(9): 551–563. [DOI] [PubMed] [Google Scholar]
- Cannon TD and Keller MC (2006). “Endophenotypes in the genetic analyses of mental disorders.” Annu Rev Clin Psychol 2: 267–290. [DOI] [PubMed] [Google Scholar]
- Constantino JN. The quantitative nature of autistic social impairment. Pediatr Res. 2011. May;69(5 Pt 2):55R–62R. doi: 10.1203/PDR.0b013e318212ec6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN (2021a). “Response to “A Radical Change in Our Autism Research Strategy is Needed: Back to Prototypes” by Mottron et al. (2021).” Autism Res 14(10): 2221–2223. [DOI] [PubMed] [Google Scholar]
- Constantino JN. (2021b) New guidance to seekers of autism biomarkers: an update from studies of identical twins. Mol Autism. 2021 Apr 19;12(1):28. doi: 10.1186/s13229-021-00434-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Gruber C (2012). Social Responsiveness Scale, Second Edition Los Angeles, CA, Western Psychological Services. [Google Scholar]
- Constantino JN, Przybeck T, Friesen D and Todd RD (2000). “Reciprocal social behavior in children with and without pervasive developmental disorders.” J Dev Behav Pediatr 21(1): 2–11. [DOI] [PubMed] [Google Scholar]
- Constantino JN and Todd RD (2003). “Autistic traits in the general population: a twin study.” Arch Gen Psychiatry 60(5): 524–530. [DOI] [PubMed] [Google Scholar]
- Conti E, Scaffei E, Bosetti C, Marchi V, Costanzo V, Dell’Oste V, Mazziotti R, Dell’Osso L, Carmassi C, Muratori F, Baroncelli L, Calderoni S, Battini R. Looking for “fNIRS Signature” in Autism Spectrum: A Systematic Review Starting From Preschoolers. Front Neurosci. 2022. Mar 2;16:785993. doi: 10.3389/fnins.2022.785993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN and Insel TR (2013). “Toward the future of psychiatric diagnosis: the seven pillars of RDoC.” BMC Med 11: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Estes A, Munson J, Schellenberg G, Bernier R, Abbott R. Quantitative assessment of autism symptom-related traits in probands and parents: Broader Phenotype Autism Symptom Scale. J Autism Dev Disord. 2007. Mar;37(3):523–36. doi: 10.1007/s10803-006-0182-2. [DOI] [PubMed] [Google Scholar]
- Dawson G, Webb S, Schellenberg GD, Dager S, Friedman S, Aylward E and Richards T (2002). “Defining the broader phenotype of autism: genetic, brain, and behavioral perspectives.” Dev Psychopathol 14(3): 581–611. [DOI] [PubMed] [Google Scholar]
- de Vries L, Fouquaet I, Boets B, Naulaers G and Steyaert J (2020). “Autism spectrum disorder and pupillometry: A systematic review and meta-analysis.” Neurosci Biobehav Rev 120(1873–7528 (Electronic)): 479–508. [DOI] [PubMed] [Google Scholar]
- Donovan AP and Basson MA (2017). “The neuroanatomy of autism - a developmental perspective.” J Anat 230(1): 4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvall JA, Lu A, Cantor RM, Todd RD, Constantino JN and Geschwind DH (2007). “A quantitative trait locus analysis of social responsiveness in multiplex autism families.” Am J Psychiatry 164(4): 656–662. [DOI] [PubMed] [Google Scholar]
- Ehlers S, L. Gillberg C Fau - Wing and Wing L(1999). “A screening questionnaire for Asperger syndrome and other high-functioning autism spectrum disorders in school age children.” (0162–3257 (Print)). [DOI] [PubMed]
- English MCW, Gignac GE, Visser TAW, Whitehouse AJO, Enns JT and Maybery MT (2021). “The Comprehensive Autistic Trait Inventory (CATI): development and validation of a new measure of autistic traits in the general population.” Mol Autism 12(1): 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier TW, Klingemier EW, Parikh S, Speer L, Strauss MS, Eng C, Hardan AY and Youngstrom EA (2018). “Development and Validation of Objective and Quantitative Eye Tracking-Based Measures of Autism Risk and Symptom Levels.” J Am Acad Child Adolesc Psychiatry 57(11): 858–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman I and Gould TD (2003). “The endophenotype concept in psychiatry: etymology and strategic intentions.” Am J Psychiatry 160(4): 636–645. [DOI] [PubMed] [Google Scholar]
- Guo H, Wang T, Wu H, Long M, Coe BP, Li H, Xun G, Ou J, Chen B, Duan G, Bai T, Zhao N, Shen Y, Li Y, Wang Y, Zhang Y, Baker C, Liu Y, Pang N, Huang L, Han L, Jia X, Liu C, Ni H, Yang X, Xia L, Chen J, Shen L, Li Y, Zhao R, Zhao W, Peng J, Pan Q, Long Z, Su W, Tan J, Du X, Ke X, Yao M, Hu Z, Zou X, Zhao J, Bernier RA, Eichler EA-O and Xia K (2018). “Inherited and multiple de novo mutations in autism/developmental delay risk genes suggest a multifactorial model.” Mol Autism 13(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, Miller J, Fedele A, Collins J, Smith K, Lotspeich L, Croen LA, Ozonoff S, Lajonchere C, Grether JK and Risch N (2011). “Genetic heritability and shared environmental factors among twin pairs with autism.” Arch Gen Psychiatry 68(11): 1095–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamner T and Vivanti G (2019). “Eye-Tracking Research in Autism Spectrum Disorder: What Are We Measuring and for What Purposes?” Communication Disorders Quarterly. [Google Scholar]
- Hazlett HC, Gu H, Munsell BC, Kim SH, Styner M, Wolff JJ, Elison JT, Swanson MR, Zhu H, Botteron KN, Collins DL, Constantino JN, Dager SR, Estes AM, Evans AC, Fonov VS, Gerig G, Kostopoulos P, McKinstry RC, Pandey J, Paterson S, Pruett JR, Schultz RT, Shaw DW, Zwaigenbaum L, Piven J; IBIS Network; Clinical Sites; Data Coordinating Center; Image Processing Core; Statistical Analysis. Early brain development in infants at high risk for autism spectrum disorder. Nature. 2017. Feb 15;542(7641):348–351. doi: 10.1038/nature21369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley RS, Losh M Fau - Parlier M, Parlier JS Fau - Reznick M, Reznick J Fau - Piven Js and Piven J (2007). “The broad autism phenotype questionnaire.” (0162–3257 (Print)). [DOI] [PubMed]
- Hyman SE (2021). “Psychiatric Disorders: Grounded in Human Biology but Not Natural Kinds.” Perspect Biol Med 64(1): 6–28. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C and Wang P (2010). “Research domain criteria (RDoC): toward a new classification framework for research on mental disorders.” Am J Psychiatry 167(7): 748–751. [DOI] [PubMed] [Google Scholar]
- Jassim N, Baron-Cohen S, Suckling J. Meta-analytic evidence of differential prefrontal and early sensory cortex activity during non-social sensory perception in autism. Neurosci Biobehav Rev 2021. Aug;127:146–157. doi: 10.1016/j.neubiorev.2021.04.014. Epub 2021 Apr 19. [DOI] [PubMed] [Google Scholar]
- Kaat AJ, Croen LA, Constantino J, Newshaffer CJ and Lyall K (2023). “Modifying the social responsiveness scale for adaptive administration.” Qual Life Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS (2009). “An historical framework for psychiatric nosology.” Psychol Med 39(12): 1935–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Keifer C, Rodriguez-Seijas C, Eaton N, Lerner M and Gadow K (2019). “Quantifying the Optimal Structure of the Autism Phenotype: A Comprehensive Comparison of Dimensional, Categorical, and Hybrid Models.” J Am Acad Child Adolesc Psychiatry 58(9): 876–886.e872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JI, Lee J, Lee KS, Lee YA, Shin CH, Hong YC, Kim BN, Lim YH. Association of phthalate exposure with autistic traits in children. Environ Int. 2021. Dec;157:106775. doi: 10.1016/j.envint.2021.106775. Epub 2021 Jul 24. [DOI] [PubMed] [Google Scholar]
- Krishnappa Babu PA-O, Di Martino JM, Chang Z, Perochon S, Aiello R, Carpenter KLH, Compton S, Davis N, Franz L, Espinosa S, Flowers J, Dawson G and Sapiro G (2023). “Complexity analysis of head movements in autistic toddlers.” J Child Psychol Psychiatry 64(1): 156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanphear BP. The impact of toxins on the developing brain. Annu Rev Public Health. 2015. Mar 18;36:211–30. doi: 10.1146/annurev-publhealth-031912-114413. Epub 2015 Jan 12. [DOI] [PubMed] [Google Scholar]
- Lee IA-O, Koelliker E and Kong SA-O (2022). “Quantitative trait locus analysis for endophenotypes reveals genetic substrates of core symptom domains and neurocognitive function in autism spectrum disorder.” Transl Psychiatry 12(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C and Bishop SL (2021). “Let’s Be Clear That “Autism Spectrum Disorder Symptoms” Are Not Always Related to Autism Spectrum Disorder.” Am J Psychiatry 178(8): 680–682. [DOI] [PubMed] [Google Scholar]
- Lundstrom S, Chang Z, Rastam M, Gillberg C, Larsson H, Anckarsater H and Lichtenstein P (2012). “Autism spectrum disorders and autistic like traits: similar etiology in the extreme end and the normal variation.” Arch Gen Psychiatry 69(1): 46–52. [DOI] [PubMed] [Google Scholar]
- Lyall K, Hosseini M, Ladd-Acosta C, Ning X, Catellier D, Constantino JN, Croen LA, Kaat AJ, Botteron K, Bush NR, Dager SR, Duarte CS, Fallin MD, Hazlett H, Hertz-Picciotto I, Joseph RM, Karagas MR, Korrick S, Landa R, Messinger D, Oken E, Ozonoff S, Piven J, Pandey J, Sathyanarayana S, Schultz RT, St John T, Schmidt R, Volk H and Newschaffer CJ (2021). “Distributional Properties and Criterion Validity of a Shortened Version of the Social Responsiveness Scale: Results from the ECHO Program and Implications for Social Communication Research.” J Autism Dev Disord 51(7): 2241–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall K, Ning X, Aschner JL, Avalos LA, Bennett DH, Bilder DA, Bush NR, Carroll KN, Chu SH, Croen LA, Dabelea D, Daniels JL, Duarte C, Elliott AJ, Fallin MD, Ferrara A, Hertz-Picciotto I, Hipwell AE, Jensen ET, Johnson SL, Joseph RM, Karagas M, Kelly RS, Lester BM, Margolis A, McEvoy CT, Messinger D, Neiderhiser JM, O’Connor TG, Oken E, Sathyanarayana S, Schmidt RJ, Sheinkopf SJ, Talge NM, Turi KN, Wright RJ, Zhao Q, Newschaffer C, Volk HE, Ladd-Acosta C and Environmental O Influences On Child Health Outcomes (2022). “Cardiometabolic Pregnancy Complications in Association With Autism-Related Traits as Measured by the Social Responsiveness Scale in ECHO.” Am J Epidemiol 191(8): 1407–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Gu H and Zhao JA-O (2021). “Atypical gaze patterns to facial feature areas in autism spectrum disorders reveal age and culture effects: A meta-analysis of eye-tracking studies.” Autism Res 14(12): 2625–2639. [DOI] [PubMed] [Google Scholar]
- Marquand AF, Wolfers T, Mennes M, Buitelaar J and Beckmann CF (2016). “Beyond Lumping and Splitting: A Review of Computational Approaches for Stratifying Psychiatric Disorders.” Biol Psychiatry Cogn Neurosci Neuroimaging 1(5): 433–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KB, Hammal Z, Ren G, Cohn JF, Cassell J, Ogihara M, Britton JC, Gutierrez A and Messinger DS (2018). “Objective measurement of head movement differences in children with and without autism spectrum disorder.” 9(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastergeorge AM, Kahathuduwa C and Blume J (2021). “Eye-Tracking in Infants and Young Children at Risk for Autism Spectrum Disorder: A Systematic Review of Visual Stimuli in Experimental Paradigms.” J Autism Dev Disord 51(8): 2578–2599. [DOI] [PubMed] [Google Scholar]
- McKusick VA (1969). “On lumpers and splitters, or the nosology of genetic disease.” Perspect Biol Med 12(2): 298–312. [DOI] [PubMed] [Google Scholar]
- Mottron L (2021). “A radical change in our autism research strategy is needed: Back to prototypes.” Autism Res 14(10): 2213–2220. [DOI] [PubMed] [Google Scholar]
- Reiersen AM, Constantino JN, Volk HE and Todd RD (2007). “Autistic traits in a population-based ADHD twin sample.” J Child Psychol Psychiatry 48(5): 464–472. [DOI] [PubMed] [Google Scholar]
- Robinson EB, Koenen KC, McCormick MC, Munir K, Hallett V, Happe F, Plomin R and Ronald A (2011a). “Evidence that autistic traits show the same etiology in the general population and at the quantitative extremes (5%, 2.5%, and 1%).” Arch Gen Psychiatry 68(11): 1113–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson EB, Munir K, Munafò MR, Hughes M, McCormick MC and Koenen KC (2011b). “Stability of autistic traits in the general population: further evidence for a continuum of impairment.” Journal of the American Academy of Child and Adolescent Psychiatry 50(4): 376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald A, Simonoff E, Kuntsi J, Asherson P and Plomin R (2008). “Evidence for overlapping genetic influences on autistic and ADHD behaviours in a community twin sample.” J Child Psychol Psychiatry 49(5): 535–542. [DOI] [PubMed] [Google Scholar]
- Sagiv SK, Harris MH, Gunier RB, Kogut KR, Harley KG, Deardorff J, Bradman A, Holland N, Eskenazi B. Prenatal Organophosphate Pesticide Exposure and Traits Related to Autism Spectrum Disorders in a Population Living in Proximity to Agriculture. Environ Health Perspect. 2018. Apr 25;126(4):047012. doi: 10.1289/EHP2580. Erratum in: Environ Health Perspect. 2018 Jul 05;126(7):079001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv SK, Kalkbrenner AE and Bellinger DC (2015). “Of decrements and disorders: assessing impairments in neurodevelopment in prospective studies of environmental toxicant exposures.” Environ Health 14: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana CP, de Carvalho EA, Rodrigues ID, Bastos GS, de Souza AD, de Brito LL. rs-fMRI and machine learning for ASD diagnosis: a systematic review and meta-analysis. Sci Rep. 2022. Apr 11;12(1):6030. doi: 10.1038/s41598-022-09821-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott FJ, Baron-Cohen S, Bolton P, Brayne C. The CAST (Childhood Asperger Syndrome Test): preliminary development of a UK screen for mainstream primary-school-age children. Autism. 2002. Mar;6(1):9–31. doi: 10.1177/1362361302006001003. Erratum in: Autism. 2002 Dec;6(4):following table of contents. [DOI] [PubMed] [Google Scholar]
- Setien-Ramos I, Lugo-Marín JA-O, Gisbert-Gustemps L, Díez-Villoria E, Magán-Maganto M, Canal-Bedia R and Ramos-Quiroga JA (2022). “Eye-Tracking Studies in Adults with Autism Spectrum Disorder: A Systematic Review and Meta-analysis.” J Autism Dev Disord. [DOI] [PubMed] [Google Scholar]
- Sharp TH, Elsabbagh M, Pickles A and Bedford R (2023). “The subcortical correlates of autistic traits in school-age children: a population-based neuroimaging study.” Mol Autism 14(1): 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuse DH, Mandy WP, Scourfield J. Measuring autistic traits: heritability, reliability and validity of the Social and Communication Disorders Checklist. Br J Psychiatry. 2005. Dec;187:568–72. doi: 10.1192/bjp.187.6.568. [DOI] [PubMed] [Google Scholar]
- Shen MD, Nordahl CW, Young GS, Wootton-Gorges SL, Lee A, Liston SE, Harrington KR, Ozonoff S, Amaral DG. Early brain enlargement and elevated extra-axial fluid in infants who develop autism spectrum disorder. Brain. 2013. Sep;136(Pt 9):2825–35. doi: 10.1093/brain/awt166. Epub 2013 Jul 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiker D, Lotspeich LJ, Dimiceli S, Myers RM, Risch N. Behavioral phenotypic variation in autism multiplex families: evidence for a continuous severity gradient. Am J Med Genet. 2002. Mar 8;114(2):129–36. doi: 10.1002/ajmg.10188. [DOI] [PubMed] [Google Scholar]
- Sucksmith E, Roth I and Hoekstra RA (2011). “Autistic traits below the clinical threshold: re-examining the broader autism phenotype in the 21st century.” Neuropsychol Rev 21(4): 360–389. [DOI] [PubMed] [Google Scholar]
- Taylor SC, Steeman S, Gehringer BN, Dow HC, Langer A, Rawot E, Perez L, Goodman M, Smernoff Z, Grewal M, Eshraghi O, Pallathra AA, Oksas C, Mendez M, Gur RC, Rader DJ, Bucan M, Almasy L, Brodkin ES. Heritability of quantitative autism spectrum traits in adults: A family-based study. Autism Res. 2021. Aug;14(8):1543–1553. doi: 10.1002/aur.2571. Epub 2021 Jul 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapar A and Rutter M (2021). “Genetic Advances in Autism.” J Autism Dev Disord 51(12): 4321–4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tick B, Bolton P, Happe F, Rutter M and Rijsdijk F (2016). “Heritability of autism spectrum disorders: a meta-analysis of twin studies.” J Child Psychol Psychiatry 57(5): 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vashisth S and Chahrour MH (2023). “Genomic strategies to untangle the etiology of autism: A primer.” Autism Res 16(1): 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivanti G and Messinger DS (2021). “Theories of Autism and Autism Treatment from the DSM III Through the Present and Beyond: Impact on Research and Practice.” J Autism Dev Disord 51(12): 4309–4320. [DOI] [PubMed] [Google Scholar]
- Volkmar FR and McPartland JC (2014). “From Kanner to DSM-5: autism as an evolving diagnostic concept.” Annu Rev Clin Psychol 10: 193–212. [DOI] [PubMed] [Google Scholar]
- Waterhouse L (2021). “Is autism a unitary biological entity? A revised and extended response to “A radical change in our autism research strategy is needed: Back to prototypes” (Mottron, 2021, Autism Research).” Autism Res 14(10): 2241–2242. [DOI] [PubMed] [Google Scholar]
- Waterhouse L, London E and Gillberg C (2016). “ASD Validity.” Rev J Autism Dev Disord 3: 302–329. [Google Scholar]
- Wing L The continuum of autistic characteristics. In: Schopler E, Mesibov GB, eds. Diagnosis and assessment in autism. Plenum Press; 1988:91–110. [Google Scholar]
- Wolff JJ, Gu H, Gerig G, Elison JT, Styner M, Gouttard S, Botteron KN, Dager SR, Dawson G, Estes AM, Evans AC, Hazlett HC, Kostopoulos P, McKinstry RC, Paterson SJ, Schultz RT, Zwaigenbaum L and Piven J (2012). “Differences in white matter fiber tract development present from 6 to 24 months in infants with autism.” Am J Psychiatry 169(6): 589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Zhu Z, Zhang X, Tang H, Xing J, Hu X, Lu J, Qu X. Identifying Autism with Head Movement Features by Implementing Machine Learning Algorithms. J Autism Dev Disord. 2022. Jul;52(7):3038–3049. doi: 10.1007/s10803-021-05179-2. Epub 2021 Jul 11. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J and Szatmari P (2005). “Behavioral manifestations of autism in the first year of life.” Int J Dev Neurosci 23(2–3): 143–152. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Szatmari P, Jones MB, Bryson SE, MacLean JE, Mahoney WJ, Bartolucci G and Tuff L (2002). “Pregnancy and birth complications in autism and liability to the broader autism phenotype.” J Am Acad Child Adolesc Psychiatry 41(5): 572–579. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
N/A – no data were included as part of this commentary.


