Abstract
Objective:
To quantify the roles and relationships between age at implantation, duration of deafness (DoD), and daily processor use via data logging on speech recognition outcomes for post-lingually deafened adults with cochlear implants.
Study Design:
Retrospective case review
Setting:
Cochlear implant (CI) program at a tertiary medical center
Patients:
Six-hundred and fourteen post-lingually deafened adult ears with CIs (mean age = 63 years, 44% female) were included.
Main Outcome Measure(s):
A stepwise multiple regression analysis was completed to investigate the combined effects of age, DoD, and daily processor use on CI-aided speech recognition (Consonant-Nucleus-Consonant [CNC] monosyllables and AzBio sentences).
Results:
Results indicated that only daily processor use was significantly related to CNC word scores (R2 = .194, p<.001) and AzBio in quiet scores (R2 = .198, p<.001), while neither age nor DoD were significantly related. Additionally, there was no significant relationship between daily processor use, age at implantation, or DoD and AzBio sentences in noise (R2 = .026, p=.005).
Conclusions:
Considering the clinical factors of age at implantation, DoD, and daily processor use, only daily processor use significantly predicted the ~20% of variance in postoperative outcomes (CI aided speech recognition) accounted for by these clinical factors.
Keywords: cochlear implant, audiology, duration of deafness, duration of daily processor use, age at implantation, outcomes
Introduction
Cochlear implants (CIs) aim to restore functional hearing by improving both audibility and speech understanding for patients with significant sensorineural hearing loss. While most patients receive significant benefit from cochlear implantation (e.g., 1–3), there exists a high variability in degree of benefit. This variability is especially prevalent when measuring outcomes using speech recognition scores 4,5, as performance can range from 0–100% for post-lingually deafened adults.
For a number of years there has been a long line of research dedicated to better understanding what factors could be contributing to this variability in order to better predict postoperative outcomes for CI users6. Evidence in this field suggests that both age at implantation7,8 and duration of deafness (DoD) 2,8–13 are negatively correlated with adult CI outcomes. These findings have been corroborated by systematic reviews and meta-analyses, but even a recent conglomerate analysis of 36 separate studies yielded only 1802 patients. Therefore, much of this evidence is limited by smaller sample sizes.
A larger multicenter study by Goudey and colleagues2 examining associations between 21 different preoperative factors and postoperative speech recognition for 2735 adult CI recipients also found DoD to be a significant predictor of outcomes; however, even with all of these predictive factors combined, only 12% of the variance in outcomes was accounted for by their model. One important limitation of these findings is the exclusion of other factors which have also been found to be significantly correlated to post-operative outcomes. These other factors include top down processing 14–16, underlying neural health 17–22, electrode placement 3,5,23–26, and duration of daily processor use 27,28. Despite this myriad of factors affecting adult CI outcomes, most are fixed variables and therefore remain largely outside of clinician or patient influence. In contrast, duration of daily processor use is the only malleable factor that has been shown to improve speech recognition even in experienced CI users 29. To date, the existing evidence highlighting the influence of age of implantation and DoD on CI outcomes has not controlled for these other factors that are known to impact patient outcomes – such as duration of daily processor use. Therefore, to better understand patient-related considerations and counsel patients on how to best achieve optimal outcomes, the roles of and relationships between age at implantation, DoD, and daily processor use must be examined in a large cohort.
In the current study, our first aim was to quantify the relationships between speech recognition outcomes and the clinical predictor variables of age at implantation, DoD, and daily processor use in post-lingually deafened adults. Consistent with previous literature, we hypothesized that age at implantation and DoD would be negatively correlated with CI-only speech recognition scores whereas daily processor use would be positively correlated with CI-only speech recognition scores. Our second aim was to quantify the amount of unique variance each of these clinical predictor variables had on CI-only speech recognition scores. We hypothesized that there would be a collective significant effect of using age at implantation, DoD, and daily processor use in predicting CI outcomes using speech recognition scores.
Materials & Methods
Participants
A retrospective chart review of our clinical CI database (3162 total patients) was preformed, identifying 589 patients (614 ears) with pertinent variables available for inclusion. These variables included: biological sex (44% female), age at implantation (mean = 63 years, range: 17–96 years), hours of processor use per day, DoD, and CI-only speech recognition (see Table 1). Exclusion criteria included age (younger than 18 years at data collection), revision surgery, unilateral hearing loss, and prelingual onset of deafness. Patients were implanted between 2012 and 2021 and included all three CI manufacturers: 225 Advanced Bionics (Valencia, CA), 306 Cochlear (Sydney, Australia), and 83 MED-EL (Innsbruck, Austria).
Table 1.
Participant demographics by manufacturer.
| Advanced Bionics | Cochlear Americas | MED-EL | |
|---|---|---|---|
| Biological Sex (% female) |
40% | 47% | 41% |
| Age at Implantation (mean years) |
64.4 | 62.0 | 65.0 |
| Duration of Deafness (median years) |
3.0 | 3.0 | 3.0 |
| Duration of Daily Processor Use (mean hours/day) |
9.9 | 10.9 | 9.8 |
| CI-Only Speech Recognition (mean % correct) |
CNC: 48% AzBio Quiet: 57% AzBio Noise: 24% |
CNC: 49% AzBio Quiet: 64% AzBio Noise: 28% |
CNC: 44% AzBio Quiet: 59% AzBio Noise: 31% |
Clinical Predictor Variables
Daily Processor Use
The mean number of hours of processor use per day was collected from clinical reports, which was extracted from data logging information in the CI programming software. If the patient routinely used more than one processor, data logging from all processors was summed. For each patient, data logging information nearest to one-year-post-implant-activation was utilized (mean = 9.5 months).
Duration of Deafness
Duration of deafness (DoD) was operationally defined as either the amount of time the patient reported significant difficultly with hearing aids or the amount of time since a significant change in hearing was noted (i.e. sudden sensorineural hearing loss). In some clinical reports, qualifiers were used to quantify duration, these included: recently, couple, few, several, and many. For these instances, the team of cochlear implant clinicians at the tertiary care center were surveyed to develop clinic norms for these qualifiers. The survey revealed on average that “recently” represented one (day, month, and/or year), “couple” represented two, “few” represented four, “several” represented six, and “many” represented ten. Using these qualifiers and subsequent clinic norms, DoD was measured for the remaining participants for whom a numerical value was not reported in the chart (107 of 614). DoD as a percentage of the participant’s life was also calculated by dividing the DoD by the participant’s age at implantation.
CI Outcomes: Speech Recognition
Speech recognition testing was collected from clinical reports. All testing was completed in a sound treated booth through a single loudspeaker at zero degrees azimuth positioned approximately one meter from the participant. Testing followed the revised Minimum Speech Test Battery (MSTB) for adult CI recipients 30 for the CI-aided ear alone. For bilateral CI users, each ear was evaluated independently. This included Consonant-Nucleus-Consonant (CNC) monosyllabic word recognition31 (50-word lists) in quiet and AzBio sentence recognition32 (20-sentence lists) in quiet and in +5 dB signal-to-noise ratio (SNR) multi talker babble. Prior to all testing, a Larson Davis LxT sound level meter was used to calibrate speech materials for a presentation level of 60 dB SPL—as a sound level meter is included in each clinical sound booth. In an effort to try and minimize the influence of floor and ceiling effects, these speech recognition scores were converted from percent correct to rationalized arcsine units or RAU 33 prior to statistical analyses.
Statistical Approach
A series of individual regression analyses were first completed to assess the zero-order correlations between variables. Independent sample t-tests and one-way ANOVAs were used to evaluate the effects of biological sex and manufacturer, respectively. Then, a stepwise multiple regression was completed to investigate the effects of age, DoD, and daily processor use on CI-aided speech recognition. Given our large sample size, a conservative alpha level of 0.001 was selected prior to analyses in effort to strictly limit the possibility of Type I error. All distributions were normally distributed (skewness < 1, kurtosis < 3), except DoD which was found to be negatively skewed (skewness = 3.157, kurtosis = 12.513). Therefore, DoD was transformed (using log10 transformation) prior to statistical analyses. Additionally, all distributions were found to be homoscedastic (Breusch Pagan test, p > .05). All statistical analyses were completed in IBM SPSS Statistics Version 2734.
Results
Clinical Predictor Variables
Daily Processor Use
Daily processor use ranged from 0 to 23 hours, with a mean of 10.4 hours (SD = 4.1 hours). It should be noted that three participants wore their processors more than 20 hours a day, all in effort to reduce tinnitus during sleep. There was no significant effect of biological sex on daily processor use (t(612)=−1.769, p=.077), as females used their CIs for a mean of 10.1 hours and males used their CIs for a mean of 10.6 hours per day. There was no significant effect of manufacturer on daily processor use (F(2,611)=5.218, p=.005, η2=.017), with Advanced Bionics users’ having a mean of 9.9 hours per day while Cochlear users’ mean was 10.9 hours per day and MED-EL users’ mean was 9.8 hours 35.
Duration of Deafness
Duration of deafness (DoD) for these post-lingually deafened adults ranged from 0.6 to 74 years, with a median of 3.0 years (interquartile range = 7.0 years). Despite this positively skewed sample, the were still 55 individuals in this sample with a DoD of 20 years or more. There was no significant effect of biological sex on DoD (t(612)=−.539, p=.590). Males and females both had a median DoD of 3.0 years. There was also no significant effect of manufacturer on DoD (F(2,611)=.258, p=.773), and all three manufacturers had a median DoD of 3.0 years.
DoD was also quantified as a percentage of the participant’s life, which ranged from <1% to 95%, with a median of 4.93%. There was no significant effect of biological sex (t(612)=.316, p=.752) or manufacturer (F(2,611)=.207, p=.813) on DoD as percentage of life. The mean DoD deafness as a percentage of life was 5.1% for females and 4.8% for males. Advanced Bionics users’ median DoD as a percentage of life was 5.2%, Cochlear users’ mean was 4.8%, and MED-EL users’ mean was 4.8%.
CI Outcomes: Speech Recognition
Speech recognition scores were collected at the same time as data logging, at a mean of 9.5 months post implantation. Across all participants, the mean raw scores for CNC words, AzBio sentences in quiet, and AzBio sentences in noise at +5 dB SNR were 47.9%, 60.7%, and 27.0%, respectively.
Individual Relationships Between Clinical Predictor Variables and CI Outcomes
The relationships between speech recognition scores (RAU converted CNC, AzBio in quiet, and AzBio in noise) and age at implantation, daily processor use, and DoD were all independently evaluated using a series of individual regression analyses. Only age at implantation and hours of processor use per day were significantly related to speech recognition outcomes, whereas DoD (CNC scores: r(602)=−.075, p=.067, 95% CI [−.154 .005]) and DoD as a percentage of life were not related (CNC scores: r(602)=−.051, p=.207, 95% CI [−.131 .029]; Figure 1). Daily processor use had the strongest relationship with speech recognition scores and was found to have a statistically significant positive correlation with CNC scores (r(602)=.441, p<.001, 95% CI [.374 .503]) and AzBio in quiet scores (r(553)=.445, p<.001, 95% CI [.375 .509]), but not AzBio in noise scores (r(298)=.162, p=.005, 95% CI [.050 .271]). Age at implantation had a weaker relationship with speech recognition scores and was only found to have a significant negative correlation with CNC scores (r(602)=−.144, p<.001, 95% CI [−.222 −.065]), but not AzBio in quiet (r(553)=−.125, p=.003, 95% CI [−.206 −.042]) or AzBio in noise scores (r(298)=−.125, p=.031, 95% CI [−.236 −.012]).
Figure 1.
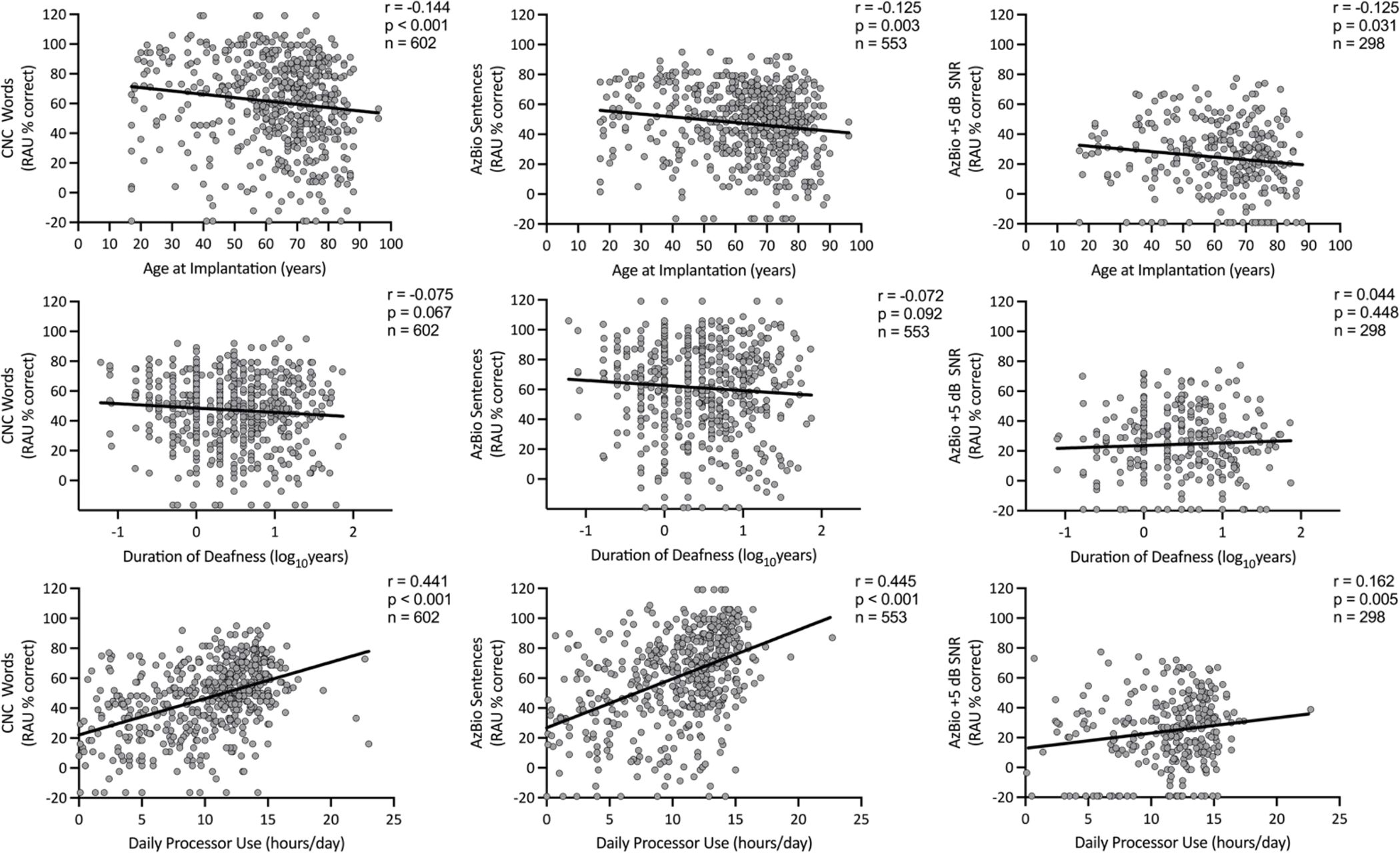
Scatterplots with regression lines examining the independent relationships between age at implantation (years), duration of deafness (log10 years), and daily processor use (hours/day) on CI-aided speech recognition outcomes (CNC words, RAU % correct; AzBio Sentences, RAU % correct; AzBio +5 dB SNR, RAU % correct).
Unique Variance in CI Outcomes Explained by Clinical Predictor Variables
A multiple regression analysis was completed to further investigate the predictive value of the clinical variables of age at implantation, DoD, and daily processor use on CI-aided speech recognition. Since DoD as a percentage of life had a weaker correlation to speech outcomes than DoD, it was excluded from the model. Results indicated that only daily processor use significantly contributed to the model predicting CNC word scores (R2 = .194, p<.001) and AzBio sentences in quiet scores (R2 = .198, p<.001)(Table 2). There was no collective significant relationship between daily processor use, age at implantation or DoD and AzBio sentences in noise (R2 = .026, p=0.005).
Table 2.
Results of stepwise multiple regression demonstrating variance in post-operative speech recognition outcomes (CNC words in quiet, AzBio sentences in quiet, and AzBio sentences in +5 dB SNR of noise) explained jointly and uniquely by daily processor use, age at implantation and duration of deafness.
| Unique Variance Explained |
Total Variance Explained | |||
|---|---|---|---|---|
| Daily Processor Use | Age | Duration of Deafness | ||
|
| ||||
| CNC Words Quiet | 19.4%* | 1.2% | 0.7% | 21.3%* |
| AzBio Sentences Quiet | 19.8%* | 0.8% | 0.6% | 21.2%* |
| AzBio Sentences Noise | 2.6% | 0% | 0% | 2.6% |
Note.
p < .001
Discussion
This study had two primary aims. The first aim was to quantify the relationships between speech recognition outcomes and the clinical predictor variables of age at implantation, DoD, and daily processor use in post-lingually deafened adults. The second aim was to quantify the amount of unique variance each of these clinical predictor variables had on CI-only speech recognition scores. To investigate these aims, we completed a retrospective chart review examining post-implantation speech recognition scores (mean = 9.5 months) for 614 ears (589 patients). Mean daily processor use was 10.4 hours per day, consistent with previous reports of this population 27,36. While DoD, operationally defined as duration of difficulty with hearing aids, varied from 0.6 to 74 years, the data were skewed with a median of 3.0 years. This broad range between deafness and implantation, however, is consistent with other reports which have demonstrated ranges from 0.1 to 77 years 37. The mean, however, is lower than has previously been reported in large cohorts 2, which may reflect the referrals and pipeline between diagnosis and implantation at this tertiary medical center. Furthermore, the speech recognition outcomes for this group also remains consistent with the literature for large clinical populations, as our mean CNC word recognition was 47.9% compared to previous reports of 47.2% 38, 50.0% 27, 50.9% 39 and 55.7% 40. The consistency between the cohort of participants in this study and the extant literature on the population of post lingually deafened adults provides validity for cross-study comparisons.
Only Age at Implantation and Daily Processor Use Were Significantly Correlated With Speech Recognition
Regarding the first aim, we found that age at implantation and hours of daily processor use were the only two clinical factors assessed that significantly helped to predict CI-only aided speech recognition outcomes. There was no significant relationship between DoD and post-operative outcomes for this cohort. Of these clinical variables, only age at implantation and DoD are known pre-operatively. From these two variables, only age at implantation had a small negative relationship with post-operative speech recognition, which was only significant for CNC words (r(602)=−.139, p<.001). When these relationships were re-examined just for individuals with longer DoD (at least one standard deviation above the mean: 11+ years), daily processor use was the only factor to be significantly correlated with post-operative speech recognition in quiet (CNC words: r(109)=.554, p<.001; AzBio sentences in quiet: r(102)=.521, p<.001).
While age at implantation was the only pre-operatively known factor found to have an independent effect on speech recognition outcomes in this sample, it should be noted that age at implantation and DoD are thought to have a synergistic relationship. Goudey and colleagues2 demonstrated that for individuals with longer durations of deafness in the contralateral ear (more than 32 years), CI-aided speech outcomes in the implanted ear were worse for younger recipients than older recipients. Conversely, for individuals with shorter durations of deafness (less than 2.5 years), age did not impact CI-aided speech recognition. Therefore, while often evaluated in isolation of each other, the effects of age of implantation and DoD should likely be evaluated in conjunction.
When this synergistic relationship was re-examined in the current study, results for the ipsilateral ear showed the opposite trend that Goudey and colleagues2 found for the contralateral ear. For individuals with longer DoD, more than one standard deviation above the mean ( >11.42 years), age and word recognition had an inverse relationship (see Figure 2). That is, patients with longer DoDs and increased age demonstrated worse post-operative CI-aided word recognition performance. Within this group, however, a large degree of variability was observed, as illustrated by the 95% confidence interval. As a result, the relationship to other groups (mean DoD and one standard deviation below the mean), with less variability, should be interpreted with caution. For individuals with shorter (one standard deviation below the mean, <0.67 years) or average (2.84 years) DoDs, age had a weaker relationship with post-operative word recognition. This difference may be driven by a difference in methodology. While Goudey and colleagues2 used multiple different metrics to determine DoD across sites and compared the contralateral ear to the performance of the implanted ear, we used a single method for determining DoD and only compared DoD to CI-aided outcomes in the same ear. Despite these differences, it should be noted that both the current study and Goudey and colleagues concluded that there may be an interaction between age and DoD. As a result, regardless of the ear used (ipsilateral or contralateral), in future research and clinical settings age and DoD should not be considered in isolation.
Figure 2.
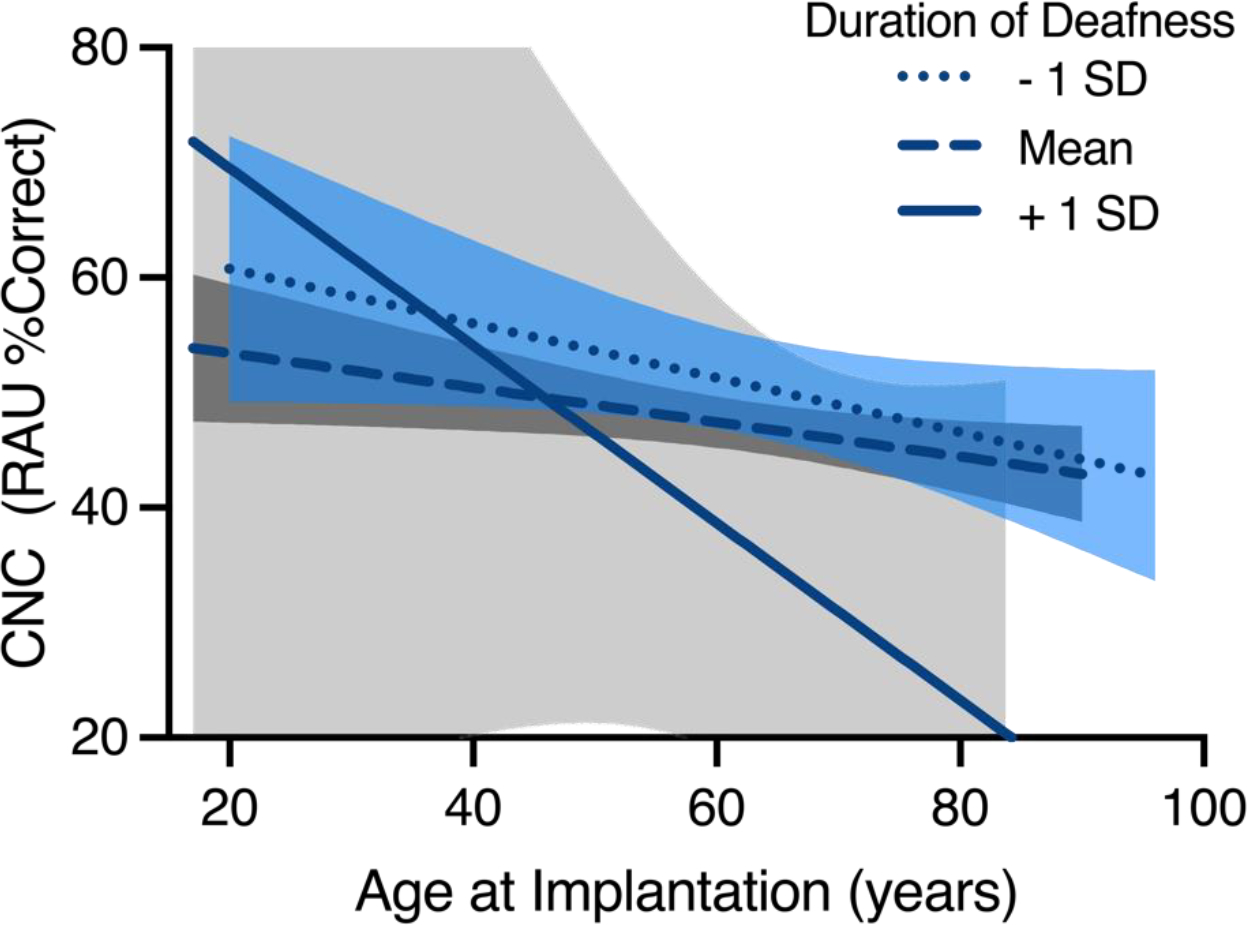
Interaction effect of age at implantation and CI-aided word recognition as a function of duration of deafness (DoD). Each of the three lines shows the linear regression comparing age at implantation and CI-aided word recognition for the following groups: DoD at least one standard deviation below the mean, DoD one standard deviation around the mean, DoD at least one standard deviation above the mean. The shaded regions around the lines of best fit denote the 95% confidence intervals derived from the models.
The strongest correlations in the current study were seen between speech recognition and daily processor use, for both words (r(602)=.441, p<.001) and sentences (r(553)=.445, p<.001) in quiet. The strength of these relationships are similar to what has been previously reported by Schvartz-Leyzac and colleagues28 (r=.432 for CNC words; 2019), but smaller than what was reported by Holder and colleagues27 (rs=.610 for CNC words; 2020). Schvartz-Leyzac and colleagues only had 177 individuals from one implant manufacturer, whereas Holder and colleagues had 300 individuals from three different implant manufacturers. Since the sample size is much larger in this study, it may be more representative of the population than these previous studies. Despite the variance in the strength of the correlation between daily processor use and speech outcomes across studies, the strength still remains notably large across studies (r=.432-.610).
Daily Processor Use Explained More Variance in CI Outcomes than Age and Duration of Deafness Combined
In regard to the second aim, quantifying the amount of unique variance in CI-only speech recognition each of these clinical variables predicted, results indicated that only daily processor use significantly predicted outcomes in the multivariate model. Altogether, daily processor use significantly predicted more variance in CNC word recognition performance (19.4%) than that predicted by age at implantation (1.2%) and DoD (0.7%) summed together. This also remained true for AzBio sentence recognition, but only in quiet (daily processor use [19.8%], age [0.8%], DoD [0.6%]). Variance for speech recognition in noise was poorly predicted from the current statistical model, likely indicating that additional factors are at play (e.g., neurocognitive processes).
These findings suggest that daily processor use is a better predictor of post-operative speech recognition than other clinical factors commonly investigated and routinely collected in clinical settings. While the 21.3% of total variance in outcomes predicted by daily processor use in this sample is less than that observed by Holder and colleagues27, it is more than the total variance predicted by the 16 other clinical factors (excluding daily processor use) examined by Goudey and colleagues (12%)2.
Limitations
Despite the strength of the correlations observed in this study, causality remains unknown. While Holder and Gifford29 demonstrated that a consistent increase in daily processor use yielded significant improvements in speech recognition scores, in the current study daily processor use was only associated with improved outcomes. The design of the present study did not allow for the causality of this association to be determined. Despite this design limitation, it is likely that daily processor use is a driving factor in the performance differences on speech recognition measures given mounting evidence in the referenced literature 27–29. An additional limitation of this study lies in the measurement of DoD. The concept of perceivable change in hearing status, such as deafness, is difficult to define. It is even more troublesome to quantify a duration of this hard to define concept of deafness. This difficulty has also been seen in existing literature. For Goudey and colleagues2, the definition used to quantify DoD even varied across all three sites. While the operational definition in this study attempted to combat this issue by using duration of significant difficulty with hearing aids (in addition to significant change in audiogram if the records were provided), it is not a perfect solution. Furthermore, this calculation relied on both patient and clinician report. Therefore, the finding that DoD was not significantly correlated with speech recognition in quiet or noise may in part be influenced by the difficulty in calculating DoD. In the future, clinics should consider refining how DoD is defined to better aid our understanding and documentation of this variable.
It should be noted that this study only examined the effects of these clinical factors in the post-lingually deafened adult population. Previous literature has already found significant effects of DoD in the prelingually deafened population41. For individuals born with severe to profound hearing loss, shorter durations of deafness and early implantation are correlated with better post-operative performance. Children who are implanted earlier perform better on a number of clinical assessments, including speech recognition, than their peers who are implanted later in childhood, adolescence, or adulthood. Given this existing evidence for the pre-lingual population, the findings of the current study must not be confused with this separate population.
Conclusion & Clinical Implications
When re-examining the traditionally considered factors of age at implantation and DoD alongside daily processor use, only daily processor use significantly contributed to the model - predicting nearly 21% of variance in postoperative outcomes for post-lingually deafened adult CI users. Individual correlations for age at implantation and DoD revealed only a small relationship and no relationship, respectively. In contrast, daily processor was significantly related to speech recognition outcomes for both words and sentences in quiet.
Controlling for daily processor use, neither DoD nor age of implantation play as significant of a role in CI-only aided speech recognition outcomes for this large cohort. This reflects a pivotal change in our understanding of the relative importance of patient factors with regards to adult post-lingual CI recipients. Therefore, as early as pre-operative counseling CI centers should stress the importance of daily processor use. This early patient education can instill in patients how their own compliance with full time use is related to their outcomes. Furthermore, research examining the multitude of factors that influence CI outcomes should be designed with daily processor use in mind. As shown in this study, prior conclusions about what factors influence CI outcomes may have been confounded by the influence of daily processor use.
FINANCIAL MATERIAL & SUPPORT:
NIH R01 DC13117; PI: René H. Gifford, PhD
Footnotes
CONFLICT(S) OF INTEREST TO DECLARE:
None directly related to this case series
JTH: Advanced Bionics and Cochlear (consultant); DSH: Med El, Cochlear, and Advanced Bionics (consultant). RHG: Cochlear & Advanced Bionics (Audiology Advisory Board), Frequency Therapeutics (Clinical Advisory Board)
INSITUTIONAL REVIEW BOARD:
IRB# 221520
References
- 1.Dunn CC, Perreau A, Gantz B, Tyler RS. Benefits of localization and speech perception with multiple noise sources in listeners with a short-electrode cochlear implant. J Am Acad Audiol. 2010;21(1):44–51. doi: 10.3766/jaaa.21.1.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goudey B, Plant K, Kiral I, et al. A MultiCenter Analysis of Factors Associated with Hearing Outcome for 2,735 Adults with Cochlear Implants. Trends Hear. 2021;25:233121652110375–23312165211037524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leigh JR, Dettman SJ, Dowell RC. Evidence-based guidelines for recommending cochlear implantation for young children: Audiological criteria and optimizing age at implantation. Int J Audiol. 2016;55 Suppl 2:S9–S18. doi: 10.3109/14992027.2016.1157268 [DOI] [PubMed] [Google Scholar]
- 4.Gaylor JM, Raman G, Chung M, et al. Cochlear implantation in adults: a systematic review and meta-analysis. JAMA Otolaryngol-- Head Neck Surg. 2013;139(3):265–272. doi: 10.1001/jamaoto.2013.1744 [DOI] [PubMed] [Google Scholar]
- 5.Holden LK, Finley CC, Firszt JB, et al. Factors affecting open-set word recognition in adults with cochlear implants. Ear Hear. 2013;34(3):342–360. doi: 10.1097/AUD.0b013e3182741aa7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boisvert I, Reis M, Au A, Cowan R, Dowell RC. Cochlear implantation outcomes in adults: A scoping review. PloS One. 2020;15(5):e0232421. doi: 10.1371/journal.pone.0232421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blamey P, Arndt P, Bergeron F, et al. Factors affecting auditory performance of postlinguistically deaf adults using cochlear implants. Audiol Neurootol. 1996;1(5):293–306. doi: 10.1159/000259212 [DOI] [PubMed] [Google Scholar]
- 8.Blamey P, Artieres F, Başkent D, et al. Factors affecting auditory performance of postlinguistically deaf adults using cochlear implants: an update with 2251 patients. Audiol Neurootol. 2013;18(1):36–47. doi: 10.1159/000343189 [DOI] [PubMed] [Google Scholar]
- 9.Friedland DR, Venick HS, Niparko JK. Choice of ear for cochlear implantation: the effect of history and residual hearing on predicted postoperative performance. Otol Neurotol Off Publ Am Otol Soc Am Neurotol Soc Eur Acad Otol Neurotol. 2003;24(4):582–589. doi: 10.1097/00129492-200307000-00009 [DOI] [PubMed] [Google Scholar]
- 10.Lazard DS, Vincent C, Venail F, et al. Pre-, per- and postoperative factors affecting performance of postlinguistically deaf adults using cochlear implants: a new conceptual model over time. PloS One. 2012;7(11):Article e48739. doi: 10.1371/journal.pone.0048739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leung J, Wang NY, Yeagle JD, et al. Predictive models for cochlear implantation in elderly candidates. Arch Otolaryngol Head Neck Surg. 2005;131(12):1049–1054. doi: 10.1001/archotol.131.12.1049 [DOI] [PubMed] [Google Scholar]
- 12.Roditi RE, Poissant SF, Bero EM, Lee DJ. A Predictive Model of Cochlear Implant Performance in Postlingually Deafened Adults. Otol Neurotol. 2009;30(4). https://journals.lww.com/otology-neurotology/Fulltext/2009/06000/A_Predictive_Model_of_Cochlear_Implant_Performance.4.aspx [DOI] [PubMed] [Google Scholar]
- 13.Rubinstein JT, Parkinson WS, Tyler RS, Gantz BJ. Residual speech recognition and cochlear implant performance: effects of implantation criteria. Am J Otol. 1999;20(4):445–452. [PubMed] [Google Scholar]
- 14.Moberly AC, Houston DM, Harris MS, Adunka OF, Castellanos I. Verbal working memory and inhibition-concentration in adults with cochlear implants. Laryngoscope Investig Otolaryngol. 2017;2(5):254–261. doi: 10.1002/lio2.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Neill ER, Kreft HA, Oxenham AJ. Cognitive factors contribute to speech perception in cochlear-implant users and age-matched normal-hearing listeners under vocoded conditions. J Acoust Soc Am. 2019;146(1):195. doi: 10.1121/1.5116009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhan KY, Lewis JH, Vasil KJ, et al. Cognitive Functions in Adults Receiving Cochlear Implants: Predictors of Speech Recognition and Changes After Implantation. Otol Neurotol. 2020;41(3). https://journals.lww.com/otology-neurotology/Fulltext/2020/03000/Cognitive_Functions_in_Adults_Receiving_Cochlear.11.aspx [DOI] [PubMed] [Google Scholar]
- 17.Fitzpatrick DC, Campbell AP, Choudhury B, et al. Round window electrocochleography just before cochlear implantation: Relationship to word recognition outcomes in adults. Otol Neurotol. 2014;35(1):64–71. doi: 10.1097/MAO.0000000000000219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldwyn JH, Bierer SM, Bierer JA. Modeling the electrode-neuron interface of cochlear implants: effects of neural survival, electrode placement, and the partial tripolar configuration. Hear Res. 2010;268(1–2):93–104. doi: 10.1016/j.heares.2010.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones GL, Won JH, Drennan WR, Rubinstein JT. Relationship between channel interaction and spectral-ripple discrimination in cochlear implant users. J Acoust Soc Am. 2013;133(1):425–433. doi: 10.1121/1.4768881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfingst BE, Hughes AP, Colesa DJ, Watts MM, Strahl SB, Raphael Y. Insertion trauma and recovery of function after cochlear implantation: Evidence from objective functional measures. IEB Kyoto. 2015;330:98–105. doi: 10.1016/j.heares.2015.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walia A, Shew MA, Kallogjeri D, et al. Electrocochleography and cognition are important predictors of speech perception outcomes in noise for cochlear implant recipients. Sci Rep. 2022;12(1):3083. doi: 10.1038/s41598-022-07175-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Won JH, Humphrey EL, Yeager KR, et al. Relationship among the physiologic channel interactions, spectral-ripple discrimination, and vowel identification in cochlear implant users. J Acoust Soc Am. 2014;136(5):2714–2725. doi: 10.1121/1.4895702 [DOI] [PubMed] [Google Scholar]
- 23.Chakravorti S, Noble JH, Gifford RH, et al. Further evidence of the relationship between cochlear implant electrode positioning and hearing outcomes. Otol Neurotol. 2019;40(5):617–624. doi: 10.1097/MAO.0000000000002204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finley CC, Holden TA, Holden LK, et al. Role of electrode placement as a contributor to variability in cochlear implant outcomes. Otol Neurotol Off Publ Am Otol Soc Am Neurotol Soc Eur Acad Otol Neurotol. 2008;29(7):920–928. doi: 10.1097/MAO.0b013e318184f492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riggs WJ, Hiss MM, Skidmore J, et al. Utilizing Electrocochleography as a Microphone for Fully Implantable Cochlear Implants. Sci Rep. 2020;10(1):3714. doi: 10.1038/s41598-020-60694-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wanna GB, Noble JH, Carlson ML, et al. Impact of electrode design and surgical approach on scalar location and cochlear implant outcomes. The Laryngoscope. 2014;124 Suppl 6(0 6):S1–7. doi: 10.1002/lary.24728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holder JT, Dwyer NC, Gifford RH. Duration of Processor Use Per Day Is Significantly Correlated With Speech Recognition Abilities in Adults With Cochlear Implants. Otol Neurotol Off Publ Am Otol Soc Am Neurotol Soc Eur Acad Otol Neurotol. 2020;41(2):e227–e231. doi: 10.1097/MAO.0000000000002477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schvartz-Leyzac KC, Conrad CA, Zwolan TA. Datalogging Statistics and Speech Recognition During the First Year of Use in Adult Cochlear Implant Recipients. Otol Neurotol Off Publ Am Otol Soc Am Neurotol Soc Eur Acad Otol Neurotol. 2019;40(7):e686–e693. doi: 10.1097/MAO.0000000000002248 [DOI] [PubMed] [Google Scholar]
- 29.Holder JT, Gifford RH. Effect of Increased Daily Cochlear Implant Use on Auditory Perception in Adults. J Speech Lang Hear Res. 2021;64(10):4044–4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minimum Speech Test Battery For Adult Cochlear Implant Users. Published online 2011. Accessed May 4, 2022. http://www.auditorypotential.com/MSTBfiles/MSTBManual2011-06-20%20.pdf
- 31.Peterson GE, Lehiste I. Revised CNC lists for auditory tests. J Speech Hear Disord. 1962;27:62–70. doi: 10.1044/jshd.2701.62 [DOI] [PubMed] [Google Scholar]
- 32.Spahr AJ, Dorman MF, Litvak LM, et al. Development and validation of the AzBio sentence lists. Ear Hear. 2012;33(1):112–117. doi: 10.1097/AUD.0b013e31822c2549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Studebaker GA. A “rationalized” arcsine transform. J Speech Hear Res. 1985;28(3):455–462. doi: 10.1044/jshr.2803.455 [DOI] [PubMed] [Google Scholar]
- 34.SPSS. [Computer Software]. Version 27.0.1.0. Armonk, NY: IBM; 2020. [Google Scholar]
- 35.Cohen J Statistical Power Analysis for the Behavioral Sciences. Routledge Academic; 1988. [Google Scholar]
- 36.Busch T, Vanpoucke F, van Wieringen A. Auditory Environment Across the Life Span of Cochlear Implant Users: Insights From Data Logging. J Speech Lang Hear Res JSLHR. 2017;60(5):1362–1377. doi: 10.1044/2016_JSLHR-H-16-0162 [DOI] [PubMed] [Google Scholar]
- 37.Bernhard N, Gauger U, Romo Ventura E, et al. Duration of deafness impacts auditory performance after cochlear implantation: A meta-analysis. Laryngoscope Investig Otolaryngol. 2021;6(2):291–301. doi: 10.1002/lio2.528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cusumano C, Friedmann DR, Fang Y, Wang B, Roland JTJ, Waltzman SB. Performance Plateau in Prelingually and Postlingually Deafened Adult Cochlear Implant Recipients. Otol Neurotol Off Publ Am Otol Soc Am Neurotol Soc Eur Acad Otol Neurotol. 2017;38(3):334–338. doi: 10.1097/MAO.0000000000001322 [DOI] [PubMed] [Google Scholar]
- 39.Shafieibavani E, Goudey B, Kiral I, et al. Predictive models for cochlear implant outcomes: Performance, generalizability, and the impact of cohort size. Trends Hear. 2021;25:23312165211066176. doi: 10.1177/23312165211066174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gifford RH, Shallop JK, Peterson AM. Speech recognition materials and ceiling effects: considerations for cochlear implant programs. Audiol Neurootol. 2008;13(3):193–205. doi: 10.1159/000113510 [DOI] [PubMed] [Google Scholar]
- 41.Teoh SW, Pisoni DB, Miyamoto RT. Cochlear implantation in adults with prelingual deafness. Part I. Clinical results. The Laryngoscope. 2004;114(9):1536–1540. doi: 10.1097/00005537-200409000-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]


