Abstract
Ovulation is a cyclical biological rupture event fundamental to fertilization and endocrine function. During this process, the somatic support cells that surround the germ cell undergo a remodelling process that culminates in breakdown of the follicle wall and release of a mature egg. Ovulation is driven by known proteolytic and inflammatory pathways as well as structural alterations to the follicle vasculature and the fluid-filled antral cavity. Ovulation is one of several types of systematic remodelling that occur in the human body that can be described as rupture. Although ovulation is a physiological form of rupture, other types of rupture occur in the human body which can be pathological, physiological, or both. In this review, we use intracranial aneurysms and chorioamniotic membrane rupture as examples of rupture events that are pathological or both pathological and physiological, respectively, and compare these to the rupture process central to ovulation. Specifically, we compared existing transcriptomic profiles, immune cell functions, vascular modifications, and biomechanical forces to identify common processes that are conserved between rupture events. In our transcriptomic analysis, we found 12 differentially expressed genes in common among two different ovulation data sets and one intracranial aneurysm data set. We also found three genes that were differentially expressed in common for both ovulation data sets and one chorioamniotic membrane rupture data set. Combining analysis of all three data sets identified two genes (Angptl4 and Pfkfb4) that were upregulated across rupture systems. Some of the identified genes, such as Rgs2, Adam8, and Lox, have been characterized in multiple rupture contexts, including ovulation. Others, such as Glul, Baz1a, and Ddx3x, have not yet been characterized in the context of ovulation and warrant further investigation as potential novel regulators. We also identified overlapping functions of mast cells, macrophages, and T cells in the process of rupture. Each of these rupture systems share local vasoconstriction around the rupture site, smooth muscle contractions away from the site of rupture, and fluid shear forces that initially increase and then decrease to predispose one specific region to rupture. Experimental techniques developed to study these structural and biomechanical changes that underlie rupture, such as patient-derived microfluidic models and spatiotemporal transcriptomic analyses, have not yet been comprehensively translated to the study of ovulation. Review of the existing knowledge, transcriptomic data, and experimental techniques from studies of rupture in other biological systems yields a better understanding of the physiology of ovulation and identifies avenues for novel studies of ovulation with techniques and targets from the study of vascular biology and parturition.
Keywords: ovulation, rupture, aneurysm, chorioamniotic membrane, follicle, parturition
I. INTRODUCTION
The process of ovulation is a highly spatiotemporally coordinated event that culminates in rupture of the wall of the ovarian follicle, which consists of somatic support cells, and expulsion of the gamete and its surrounding specialized cumulus cells (Duffy et al., 2019; Richards, 2005; Richards et al., 1998; Russell & Robker, 2007). The rupture event itself takes place over the course of several hours after the hormonal trigger of ovulation, but the formation of a site within the follicle predisposed to rupture occurs earlier in folliculogenesis (Duffy et al., 2019; Espey, 1967; Martin & Talbot, 1987; Richards et al., 1998). The process of follicle rupture is characterized by extracellular matrix (ECM) reorganization, inflammation, and structural changes that occur in a distinct spatiotemporal pattern, a process that is relatively unique in the context of normal physiological processes in the body (Brown et al., 2010; Duffy et al., 2019; Lind et al., 2006; Park et al., 2020; Richards, Liu & Shimada, 2008). In addition to ovulation, multiple other types of rupture that mirror some of the features of ovulation exist in humans. Many of these ruptures are pathological, such as rupture of intracranial aneurysms (ICAs). Rupture of the chorioamniotic membrane at the time of parturition can be either physiological or pathological depending on the timing of the rupture. Despite the clear differences between the circumstances of ovulation, aneurysms, and chorioamniotic membrane rupture, there are broad similarities relating to structural remodelling and inflammation that link these processes. In this review, we use ovulation, ICAs, and chorioamniotic membrane rupture as examples of rupture systems that are respectively physiological, pathological, or both, to inform common mechanisms.
Although similarities exist between these examples on a pathophysiological level, there are no current studies that compare transcriptomic pathways that lead to rupture across these systems. The process of rupture in ICAs and chorioamniotic membranes has been well characterized because the consequences of rupture in these systems can be life threatening (Lindekleiv et al., 2015; Siegler, Weiner & Solt, 2020). There have been relatively few transcriptomic studies on ovulation and even fewer assessing what makes a follicle rupture or fail to rupture at a spatiotemporal level. In this review, we use data identified in the context of ICAs and chorioamniotic membranes and compare it with the limited data sets available for ovulation to identify pathways that are central to the concept of rupture and remodelling. Through this strategy we identify potential gene targets for future mechanistic studies and drug discovery and develop a new framework for the study of the spatiotemporal transcriptomic changes that underlie rupture.
In addition to the spatiotemporal transcriptomic changes that underlie rupture in each of these systems, there is evidence of structural and biomechanical changes that predispose rupture to occur. These changes include spatial alterations in pressure and fluid shear forces, vasoconstriction of microvasculature, and contraction of smooth muscle cells (Cavender & Murdoch, 1988; Cebral et al., 2011; Chowdhury et al., 2014; Martin & Talbot, 1981b; Matousek et al., 2001; Shojima et al., 2004). In this review, we identify where parallels exist that may provide a greater understanding of the basic biology of each condition, particularly in ovulation where few modern studies on biomechanical and structural changes associated with follicle rupture have been conducted. Additional discoveries about follicle rupture derived from this review could also aid in the understanding of ovarian conditions associated with altered follicle rupture, such as luteinized unruptured follicle syndrome or reproductive aging. These comparisons do not mean that these rupture processes are identical or that all of the identified parallels are exact. However, we argue that new insights can be drawn and new experiments can be designed when commonalities are explored, and knowledge is shared across these distinct fields.
II. OVERVIEW OF RUPTURE SYSTEMS
(1). Overview of ovulation and follicle rupture
The follicle is the functional unit of the ovary and consists of an oocyte surrounded by somatic support cells known as the granulosa and theca cells (Rodgers & Irving-Rodgers, 2010; Russell & Robker, 2007; Smith et al., 2011). During the process of oogenesis and folliculogenesis, the germ cell and somatic cells grow in a coordinated fashion ultimately to form a fully differentiated pre-ovulatory antral follicle (DiZerega & Hodgen, 1981; Richards et al., 1998; Rimon-Dahari et al., 2016; Rodgers & Irving-Rodgers, 2010). The follicle wall is composed of granulosa cells that support oocyte development and hormone production, a basement membrane enriched in collagen, and layers of theca cells that facilitate androgen synthesis (Fig. 1A) (Lind et al., 2006; Richards et al., 1998; Smith et al., 2011). As the follicle grows and matures, the support cells multiply and follicular fluid is pumped into the follicle as a result of angiogenesis and contractile activity of smooth muscle-like cells (Duffy et al., 2019; Espey, 1967; Martin & Talbot, 1981a, 1987; Talbot & Chacon, 1982). This period of follicle and oocyte development marks the follicular phase of the menstrual cycle and lasts for approximately 14 days in humans (Richards et al., 1998). At the end of this phase in the menstrual cycle, there is a surge of luteinizing hormone (LH) which induces ECM degradation, disruption of the basement membrane and ovarian surface epithelium, disruption and thinning of somatic cell layers, and immune cell infiltration, all of which facilitate ovulation (Fig. 1A) (Direito et al., 2013; Duffy et al., 2019; Espey, 1967; Lind et al., 2006; Park et al., 2020; Richards et al., 2008, 1998; Russell & Robker, 2007). Ovulation is complete when a cumulus oocyte complex (COC) is released from the ovulatory follicle at the surface of the ovary (Richards et al., 1998). The COC initially remains attached to the surface of the ovary and is gradually transported through the oviduct by ciliated cells (Bylander et al., 2015; Croxatto, 2002; Kölle et al., 2009; Lyons, Saridogan & Djahanbakhch, 2006; Talbot, Geiske & Knoll, 1999). After ovulation, the unruptured parts of the follicle involute to form the corpus luteum, a temporary endocrine structure that produces oestrogen and progesterone to support early pregnancy (Niswender et al., 2000; Plant, Zeleznik & Albertini, 2015; Richards et al., 1998; Stocco, Telleria & Gibori, 2007). The average woman will undergo ovulation nearly 400 times during her lifetime, averaging around 12 ovulations per year for around 40 fertile years (Clow, Hurst & Fleming, 2002). This number may be lower depending on pregnancies, ovarian pathologies, and use of contraceptive medications that block ovulation.
Fig. 1.
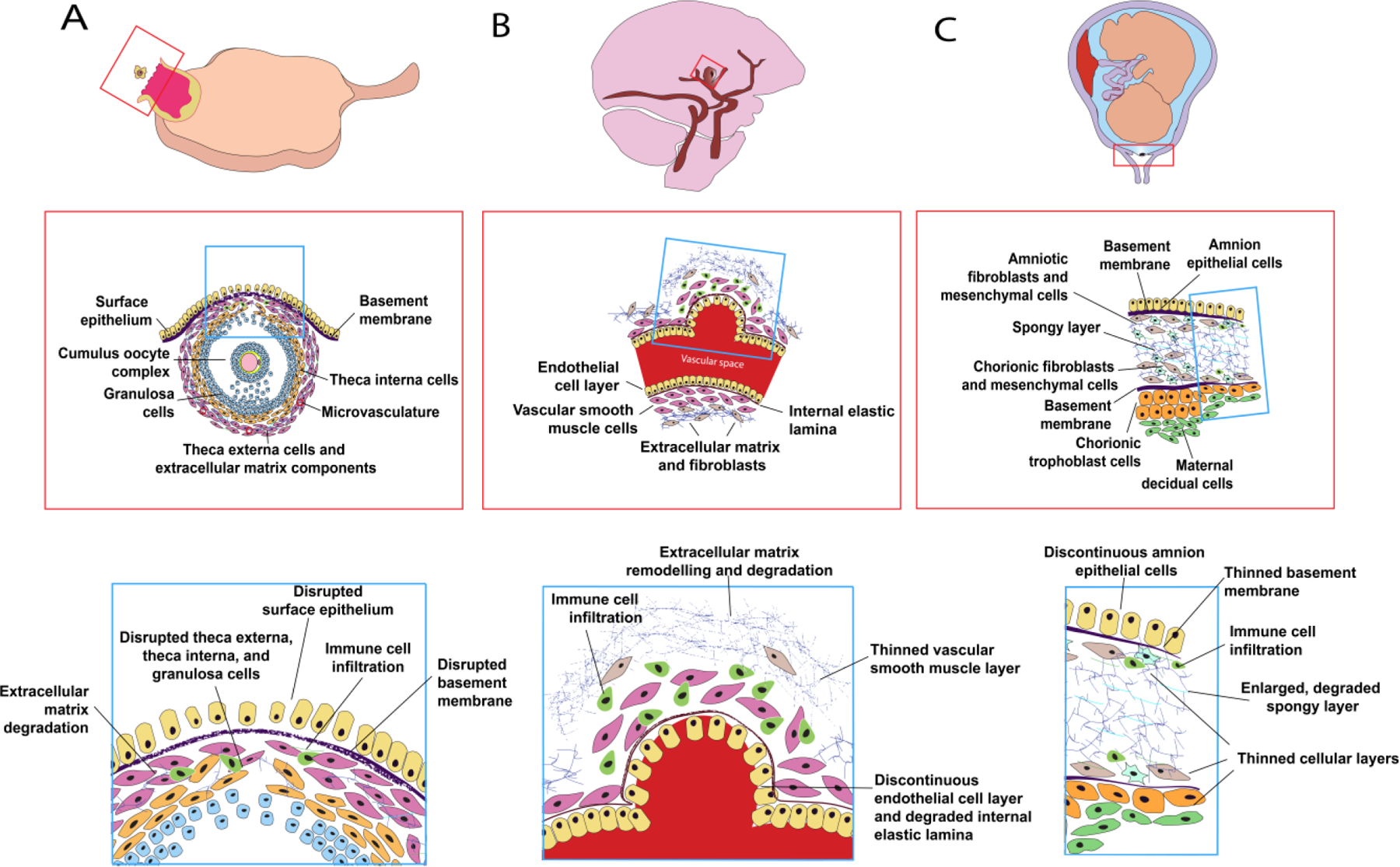
Comparison of rupture systems for (A) ovulation, (B) intracranial aneurysms, and (C) chorioamniotic membranes. The orange box identifies the region of interest. The blue box indicates the specific zone with rupture potential. These rupture events can be described as physiological (A), pathological (B), or both (C). All three types of rupture consist of similar remodelling processes, including disrupted cellular layers, remodelling of the extracellular matrix, and immune cell infiltration.
Although the structural changes associated with follicle rupture and ovulation are most distinct after the LH surge, structural asymmetry develops prior to this hormone surge during the follicular phase. This asymmetry can be seen during the formation of the antral cavity (DiZerega & Hodgen, 1981; Rimon-Dahari et al., 2016). Prior to ovulation, one side of the follicle, the future site of rupture, begins to widen and swell as the layer of cells thins, while the other side, the future site of corpus luteum formation, remains compact with a thicker cellular layer (Duffy et al., 2019). Preliminary work to understand the mechanism of the asymmetry that underlies follicular development and rupture has largely focused on the temporal changes that contribute to follicular growth and remodelling. The asymmetry appears to be driven by inflammatory mediators, ECM remodelling, and angiogenic changes that regulate antral fluid flow (Duffy et al., 2019). The consistent pattern of these asymmetrical changes implies that there is a functionally and molecularly distinct sidedness to the ovulatory follicle prior to rupture.
The combination of inflammatory signalling, fluid pressure, and ECM remodelling that occurs in a spatiotemporal pattern in follicle rupture mirrors the changes that occur in other well-characterized pathological and physiological rupture events in the human body. Understanding follicle rupture on a spatiotemporal level could provide insight into conditions including luteinized unruptured follicle syndrome and ovarian aging which involve impaired follicle rupture and subfertility (Mara et al., 2020; Marik & Hulka, 1978). It may also uncover targets for development of novel non-hormonal contraceptives that inhibit follicle rupture without interfering with hormone production and luteinization.
(2). Overview of intracranial aneurysm formation and rupture
ICAs are structurally diseased regions of neurovascular tissue that form when, due to a variety of complex factors, a section of an intracranial blood vessel stretches, and the vessel wall weakens (Keedy, 2006). The most common type of ICA is a saccular aneurysm, which forms due to breakdown of internal layers of the blood vessel wall (Keedy, 2006). Saccular aneurysms most commonly form in a specific region of the brain arterial system termed the Circle of Willis (Keedy, 2006). Arteries within the Circle of Willis consist of an inner layer of endothelial cells, a layer of elastic tissue called the internal elastic lamina, a layer of vascular smooth muscle cells, and an ECM layer with fibroblasts (Fig. 1B) (Savastano et al., 2018). When an aneurysm forms, the structure of the vasculature changes due to ECM remodelling and degradation, degraded internal elastic lamina, disrupted endothelial layer, thinned smooth muscle layer, and immune cell infiltration (Fig. 1B) (Keedy, 2006; Savastano et al., 2018). Aneurysms may be asymptomatic until the point at which they rupture. When an ICA within the Circle of Willis ruptures, it causes a subarachnoid haemorrhage which can trigger a haemorrhagic stroke (Savastano et al., 2018). It is difficult to estimate the frequency of unruptured ICAs accurately as they are often asymptomatic and only discovered incidentally. Broad estimates suggest that 1–5% of the population may have an ICA and the incidence of subarachnoid haemorrhages, frequently caused by ruptured ICA, was reported as 7.9 per 100,000 person-years, a measurement of the incidence rate relative to the duration of a study, in one large study (Etminan et al., 2019; Wiebers, 2003). Subarachnoid haemorrhages due to ruptured aneurysm cause around 5% of all reported strokes (Nieuwkamp et al., 2009). The rate at which ICA rupture occurs varies widely but is estimated to be around 1.6% annually (Korja, Lehto & Juvela, 2014). Although ruptured aneurysms pose a higher morbidity and mortality rate than those that do not rupture, the one-year mortality rate for patients with unruptured aneurysms was found in one study to be 2.7% compared to 15.6% for patients with ruptured aneurysms (Lindekleiv et al., 2015). Mechanistic studies of ICA suggest that a combination of inflammatory signalling, fluid-derived shear stress on arterial walls, and ECM remodelling cause structural changes in the blood vessel walls that lead to ICA formation (Kanematsu et al., 2011; Kurki et al., 2011; Medero et al., 2020; Savastano et al., 2018; Shojima et al., 2004). This remodelling often occurs asymmetrically and creates a particular region of weakness in the vessel wall that makes it susceptible to rupture (Nehls et al., 1985; Zhang et al., 2016). Understanding the mechanisms of ICA rupture could lead to the development of treatment approaches that target the likely rupture zone of the aneurysm and reduce the risk of rupture in patients with identified ICAs.
(3). Overview of physiological and pathological chorioamniotic membrane rupture
The chorioamniotic membrane forms early in fetal development when two membranes, the chorion and the amnion, fuse at 12 weeks gestation (Bourne, 1962; Bryant-Greenwood, 1998). The chorion (trophoblast layer, basement membrane, fibroblasts, and mesenchymal cells) forms the outermost layer of the amniotic sac and the amnion (spongy layer, fibroblasts, mesenchymal cells, basement membrane, and epithelial cells) forms the inner lining of the sac that fills with amniotic fluid and cushions the growing fetus (Fig. 1C) (Bourne, 1962; Bryant-Greenwood, 1998; Kumar et al., 2016). Rupture of the chorioamniotic membrane occurs as a result of epithelial cell disruption, immune infiltration, thinning of basement membrane and cellular layers, and degradation of the spongy layer components (Fig. 1C) (Kumar et al., 2016; Menon & Richardson, 2017). In a full-term pregnancy, rupture of the chorioamniotic membrane occurs at the onset of labour (Plant et al., 2015). This rupture can also happen pathologically if it occurs prior to labour. Premature rupture of membranes (PROM) is a condition where the chorioamniotic membrane ruptures after 37 weeks gestation but prior to the onset of labour (Menon & Richardson, 2017). Preterm premature rupture of membranes (PPROM) is a condition where the chorioamniotic membrane ruptures before 37 weeks gestation (Menon & Richardson, 2017). PROM occurs in an estimated 8% of all pregnancies in the USA and PPROM occurs in 2–3% of all pregnancies in the USA (Siegler et al., 2020). Both PROM and PPROM carry risk of intrauterine infection that can cause serious complications for the mother (Siegler et al., 2020). PPROM can also be accompanied by abruptio placentae, where the placenta detaches from the uterine wall (Major et al., 1995). PPROM also carries serious risks for the fetus, including respiratory distress, haemorrhage, impaired neurodevelopment, and fetal demise (Siegler et al., 2020). In around 1% of cases, PPROM can occur prior to 22 weeks gestation when the fetus is pre-viable (Bendix et al., 2015; Siegler et al., 2020; Waters & Mercer, 2009). This carries a much higher risk of fetal demise, estimated at 57.7% of cases prior to 22 weeks compared to 14.4% of cases between 22 and 37 weeks (Siegler et al., 2020). Membrane rupture, both pathological and physiological, is associated with inflammatory pathways, ECM remodelling, and mechanical forces from smooth muscle contractions (Bryant-Greenwood, 1998; Joyce, Moore & Sacks, 2009; Kumar et al., 2016). There is evidence that pathological rupture of the chorioamniotic membrane is associated with a pro-inflammatory state and accelerated remodelling (Gomez-Lopez et al., 2011; Kumar et al., 2016; Moore et al., 2009). The rupture zone is also spatially organized with rupture occurring most often at the region of the membrane over the cervix (Kumar et al., 2016; Lappas et al., 2008; McLaren, Taylor & Bell, 1999). Understanding the spatiotemporal dynamics of chorioamniotic membrane rupture could lead to the development of rupture prediction tools and treatments to prevent premature rupture of membranes.
III. TRANSCRIPTOMIC DATA SET COMPARISON FOR DIFFERENT RUPTURE SYSTEMS
To assess common pathways between non-ovulation rupture systems and ovulation, we first surveyed the literature to find appropriate data sets for comparison. Several molecular pathways have been implicated in assessment of rupture risk of ICAs. While many of these pathways have been identified by examining expression patterns of individual genes within aneurysmal tissues, some studies have attempted to assess expression in a more comprehensive manner. Oligonucleotide microarrays have been used to compare expression of transcripts of ruptured and unruptured ICAs to control tissues and found no significant differences (Krischek et al., 2008; Shi et al., 2009). Studies with slightly higher sample sizes found less than 20 differentially expressed genes (DEGs) between samples (Marchese et al., 2010; Pera et al., 2010). By contrast, another study identified over 1,400 DEGs between ruptured and unruptured ICA walls (Kurki et al., 2011) (Table 1). This difference is likely due to the larger sample size and updated microarray probes used by Kurki et al. (2011). This data set provides a wealth of information that can be used to identify pathways that contribute to the process of rupture.
Table 1.
Overview of transcriptomic data sets selected for our comparison of three rupture systems.
| Data set | Rupture System | Results | Method | Organism | Sample details | GEO accession number |
|---|---|---|---|---|---|---|
| Kurki et al. (2011) | Intracranial aneurysm (ICA) | 1,426 DEGs between ruptured and unruptured ICA walls | Microarray | Human | 11 ruptured and 8 unruptured saccular ICA vascular walls | GSE13353 |
| Nhan-Chang et al. (2010) | Chorioamniotic membrane rupture | 677 DEGs between the ruptured zone above the cervix and a distal site after spontaneous rupture of membranes at term | Microarray | Human | 20 matched sets of tissue samples of the chorion and amnion from the site of chorioamniotic membrane rupture and a region distant from the rupture site | – |
| Liu et al. (2017) | Ovulation | 2,888 DEGs between preovulatory follicles and anovulatory follicles from a Pgr-KO model | RNAseq | Zebrafish | Isolated follicular cells (theca and granulosa) from three wildtype and three Pgr-KO female zebrafish prior to ovulation | – |
| Park et al. (2020) | Ovulation | 720 DEGs between mural granulosa cells from Esr2-Pgr-KO mice and wildtype mice 6 h after ovulation | Single-cell RNAseq | Mouse | Single cell dissociation of eight ovaries from wildtype C57BL/6 mice and Esr2-Pgr-KO mice 6 h after induction of ovulation with hCG, differentially expressed gene list based on ovulatory mural granulosa cells | GSE145107 |
GEO, gene expression omnibus; DEG, differentially expressed gene; Esr, estrogen receptor; hCG, human chorionic gonadotropin; KO, knockout; Pgr, progestin receptor; RNAseq, RNA sequencing.
The data sets investigating chorioamniotic membrane rupture vary because chorioamniotic membrane rupture can be both physiological and pathological. Many comprehensive transcriptomic data sets relating to chorioamniotic membrane rupture focus on pathological rupture because of a compelling clinical interest in developing screening methods and treatments for PROM and PPROM. A comparison of chorioamnion samples from four term and four preterm deliveries identified 252 upregulated and 18 downregulated genes in the preterm group (Pereyra et al., 2019). Another study characterized differential microRNA (miRNA) expression in patients at term without labour, at term with labour, and preterm with labour using microarray analysis (Montenegro et al., 2009). They identified dozens of miRNAs that may be involved in post-transcriptional regulation of genes leading to chorioamniotic membrane rupture. One study focused on spontaneous rupture of membranes at term and assessed regional differences in the chorioamniotic membranes at the site over the cervix that ruptures and at sites distal to the rupture zone (Nhan-Chang et al., 2010) (Table 1). They identified 677 DEGs between ruptured and non-ruptured sites in their microarray analysis and selected several genes for validation using quantitative reverse transcription polymerase chain reaction (qRT-PCR) (Nhan-Chang et al., 2010). This data set is well suited for comparison to other rupture systems as it investigated the mechanism of spatial differences in rupture within a physiological rupture system.
Although there have yet to be any comprehensive analyses of the transcriptomic changes that differentiate the specific ruptured zone on the apical side of the follicle from the unruptured zone in the context of ovulation, there are several studies that have interrogated transcriptomic changes associated with the general rupture process. One study compared ovulating follicles to an anovulatory model induced by knockout of the nuclear progestin receptor (Pgr) in a zebrafish (Danio rerio) model (Liu et al., 2017) (Table 1). This study found 2,888 DEGs between the ruptured and unruptured follicles. A Pgr-knockout model was also used in the mouse to interrogate the anovulatory phenotype. One study performed bulk RNA-sequencing on granulosa cells isolated from hormonally stimulated mouse ovaries at 10 h post-injection of human chorionic gonadotropin (hCG) (Smith et al., 2022), and another study performed single-cell RNA-sequencing on dissociated whole ovaries at 6 h post-hCG injection (Park et al., 2020). The latter study used markers specific to ovulatory mural granulosa cells to isolate this population for analysis of DEGs between wildtype mice and anovulatory ESR2-PgrKO mice, making this study particularly suitable for comparison to other ruptured tissues due to the demonstrated purity of the mural granulosa cell population (Table 1). Other knockouts of genes essential for ovulation, including endothelin-2 and the heterodimeric transcription factors known as core binding factors, are either not fully anovulatory or cause alterations to other key ovulatory functions such as luteinization (Cacioppo et al., 2017; Lee-Thacker et al., 2020). For the purposes of this review, we compared the data sets of Liu et al. (2017) and Park et al. (2020) for ovulation, with those of Kurki et al. (2011) for ICAs and Nhan-Chang et al. (2010) for chorioamniotic membrane rupture as these studies were designed explicitly to compare ruptured versus unruptured tissue (Table 1). Note that the ovulation data sets represent data from zebrafish and mice. Since suitable data from human ovulatory follicles are not yet available, the use of two different species for comparisons with human tissues allows more robust conclusions. Park et al. (2020) presented their data as positive values showing upregulated genes in unruptured tissue, whereas data for the other three studies were presented with positive values indicating upregulation in ruptured tissue. We therefore transformed the values from Park et al. (2020) to reflect the same convention used in the other studies, for ease of comparison.
(1). Common transcriptomic changes across rupture systems
Using the selected data sets, comparisons were made between the reported DEGs to identify pathways that may be common between rupture processes (Fig. 2A, B). A comparison of the transcriptional changes reported in Kurki et al. (2011) and Liu et al. (2017) revealed 120 genes (68 upregulated, 52 downregulated) that were differentially expressed in the same direction in both studies (Fig. 2A). A comparison between Kurki et al. (2011) and Park et al. (2020) revealed 42 genes (32 upregulated, 10 downregulated) that were differentially expressed in the same direction in both studies (Fig. 2A). From these three studies, there was a total of 12 overlapping genes (Fig. 2A; Table 2; see online supporting information, Table S1). A comparison between Nhan-Chang et al. (2010) and Liu et al. (2017) found 48 genes (30 upregulated, 18 downregulated) that were differentially expressed in the same direction in both studies (Fig. 2B). A comparison between Nhan-Chang et al. (2010) and Park et al. (2020) found 18 genes (10 upregulated, 8 downregulated) that were differentially expressed in the same direction in both studies (Fig. 2B). When the lists of genes generated from the chorioamniotic membrane rupture–ovulation analyses were compared, there was a total of three overlapping genes (Fig. 2B; Table 3; Table S2). There were two genes that overlapped across all comparisons (Fig. 2C). Although not a focus of this review, there were 60 genes in common between Kurki et al. (2011) and Nhan-Chang et al. (2010) that did not overlap with either ovulation data set; these genes may elucidate processes related to rupture that are distinct from those involved in ovulation (Table S3).
Fig. 2.
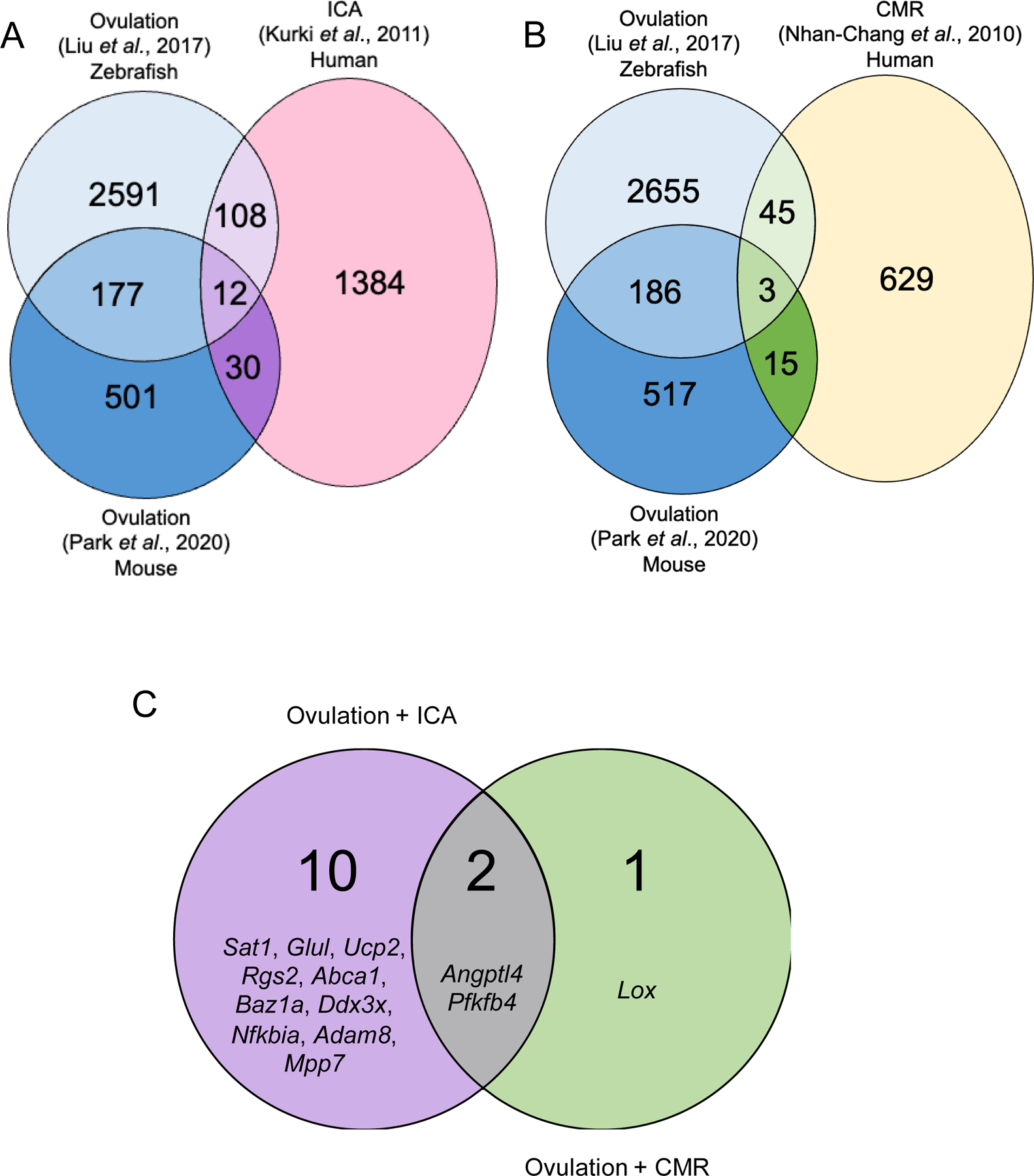
Comparison of the selected data sets for different rupture systems: Kurki et al. (2011) for intracranial aneurysm (ICA); Nhan-Chang et al. (2010) for chorioamniotic membrane rupture (CMR); and both Liu et al. (2017) and Park et al. (2020) for ovulation. Data sets were compared to identify overlapping genes, and these lists were filtered for genes with changes in expression in the same direction (i.e. both upregulated or both downregulated). The Venn diagrams show which genes were in common for the comparison of ovulation and ICA (A) and the comparison between ovulation and CMR (B). Two genes (Angptl4, and Pfkfb4) were upregulated in all data sets (C).
Table 2.
List of upregulated (+) and downregulated (−) genes in common among the two ovulation data sets and the intracranial aneurysms (ICA) data set.
| Gene | Gene function | Ovulation (Liu et al., 2017) Log2FC | Ovulation (Liu et al., 2017) P-value | Ovulation (Park et al., 2020) Log2FC | Ovulation (Park et al., 2020) P-value | ICA (Kurki et al., 2011) Log2FC | ICA (Kurki et al., 2011) P-value |
|---|---|---|---|---|---|---|---|
| Sat1 | N1-acetyltransferase | 1.093 | 0.01 | 0.330 | <0.0001 | 2.539 | 0.0002 |
| Glul | Ligase | 4.073 | <0.0001 | 0.989 | <0.0001 | 2.296 | 0.0003 |
| Ucp2 | Mitochondrial protein | 3.170 | <0.0001 | 0.940 | <0.0001 | 2.195 | 0.01 |
| Rgs2 | GPCR regulator | 7.205 | <0.0001 | 0.821 | <0.0001 | 1.922 | 0.01 |
| Angptl4 | Secreted protein | 1.792 | <0.0001 | 0.317 | <0.0001 | 1.811 | 0.002 |
| Abca1 | ATP binding | 3.389 | <0.0001 | 0.312 | <0.0001 | 1.736 | 0.007 |
| Baz1a | Chromatin assembly | 1.390 | 0.002 | 0.656 | <0.0001 | 1.269 | 0.008 |
| Ddx3x | Helicase | 1.603 | <0.0001 | 0.334 | <0.0001 | 0.824 | 0.0007 |
| Nfkbia | NFKB inhibitor | 2.861 | <0.0001 | 0.450 | <0.0001 | 0.799 | 0.04 |
| Pfkfb4 | Kinase | 2.596 | <0.0001 | 0.447 | <0.0001 | 0.705 | 0.04 |
| Adam8 | Metalloproteinase | 3.550 | <0.0001 | 0.811 | 0.03 | 0.623 | 0.05 |
| Mpp7 | Scaffold protein | −1.210 | 0.006 | −0.325 | <0.0001 | −1.184 | 0.001 |
FC, fold change; GPCR, G-protein coupled receptor; NFKB, nuclear factor kappa beta.
Liu et al. (2017) and Kurki et al. (2011) denoted genes as upregulated in ruptured tissue relative to unruptured tissue. Park et al. (2020) used the opposite convention, with genes denoted as upregulated in unruptured relative to ruptured tissue. We therefore have transformed the results of Park et al. (2020) to reflect the convention used in the other studies for ease of comparison.
Table 3.
List of upregulated and downregulated genes in common among two ovulation data sets and the chorioamniotic membrane rupture (CMR) data set.
| Gene | Gene function | Ovulation (Liu et al., 2017) Log2FC | Ovulation (Liu et al., 2017) P-value | Ovulation (Park et al., 2020) Log2FC | Ovulation (Park et al., 2020) P-value | CMR (Nhan-Chang et al., 2010) Log2FC | CMR (Nhan-Chang et al., 2010) P-Value |
|---|---|---|---|---|---|---|---|
| Angptl4 | Secreted protein | 1.792 | <0.0001 | 0.317 | <0.0001 | 2.40 | 0.0009 |
| Lox | Oxidase | 1.924 | 0.0002 | 0.275 | 0.005 | 2.30 | 0.001 |
| Pfkfb4 | Kinase | 2.596 | <0.0001 | 0.447 | <0.0001 | 1.50 | 0.009 |
FC, fold change.
Liu et al. (2017) and Nhan-Chang et al. (2010) denoted genes as upregulated in ruptured tissue relative to unruptured tissue. Park et al. (2020) used the opposite convention, with genes denoted as upregulated in unruptured relative to ruptured tissue. We therefore have transformed the results of Park et al. (2020) to reflect the convention used in the other studies for ease of comparison.
Although the majority of these genes in common between ovulation and other rupture systems are unique to each comparison, two transcripts were present in all comparisons (Fig. 2C). Angiopoietin like 4 (Angptl4) is an angiogenic factor and lipid metabolism regulator that has been characterized in cumulus cells and in cases of polycystic ovarian syndrome (PCOS) (Al-Edani et al., 2014; Chaemsaithong et al., 2013; Haddad et al., 2006; Jiang et al., 2022; Kremer et al., 2016; Nhan-Chang et al., 2010). It is also associated with arteriovenous malformations in the brain that can lead to ICAs and is present in multiple transcriptomic studies of aneurysm rupture (Estrelinha, Hinterseher & Kuivaniemi, 2014; Gäbel et al., 2017; Mikhak et al., 2011; Xu et al., 2017). It is also upregulated in the cervix and myometrium during labour and may initiate labour within the myometrium (Bollopragada et al., 2009; Mittal et al., 2010; Weiner et al., 2010). 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4 (Pfkfb4) is involved in stress and hypoxia responses within cells (Minchenko et al., 2004). Pfkfb4 has been characterized in multiple transcriptomic studies from peri-ovulatory granulosa cells and is upregulated in cumulus cells following hCG stimulation (Cruz et al., 2017; Poulsen et al., 2020). It is also enriched during subarachnoid haemorrhage following ICAs (Yan et al., 2021). Pfkfb4 is also known to participate in initiating myometrial contractions during term labour (Weiner et al., 2010).
Although the list of genes in common between multiple rupture data sets and ovulation is small, the observed overlap provides insight into pathways and specific transcripts that are enriched in all these rupture systems and suggests avenues for deeper investigation. Assessing both the unique features of each rupture system and the similarities to ovulation, both from a transcriptomic level and a functional or structural level, will provide additional insights into the biology of all these rupture systems.
There are some limitations to our analysis. For example, the data sets compared here are not perfectly analogous in how they assessed rupture or rupture potential. Kurki et al. (2011) compared ruptured and unruptured ICA tissue from different individuals while Nhan-Chang et al. (2010) compared ruptured and unruptured zones from the chorioamniotic membranes of the same individual. Liu et al. (2017) and Park et al. (2020) assessed ruptured and unruptured follicles using a genetic knockout model that exhibits an anovulatory phenotype. Additionally, Liu et al. (2017) used a zebrafish model, Park et al. (2020) a mouse model, while Kurki et al. (2011) and Nhan-Chang et al. (2010) presented data from humans. However, Liu et al. (2017) did note substantial overlap between zebrafish, human, and mouse pre-ovulatory data sets. Zebrafish are widely used in studies of reproductive function and fertility due to the relative ease of genetic manipulation and high genetic conservation with humans, estimated to be as high as 70% (Hajam et al., 2021; Santoriello & Zon, 2012). Although zebrafish do not exhibit the processes of COC expansion, luteinization, or implantation which occur in humans following ovulation, zebrafish ovaries contain follicles that rupture via core molecular signatures that are conserved in mammalian ovulation (Hajam et al., 2021). Park et al. (2020) used a mouse model and single-cell RNA sequencing that allowed specific analysis of the transcriptome of the mural granulosa cells that make up the rupturing follicle wall. However, this data set was limited to samples taken 6 h following induction of ovulation with hCG. In mice, complete follicle rupture occurs at around 12 h post-hCG stimulation, so this data set may be missing key transcriptomic signatures of late follicle rupture (Smith et al., 2022).
IV. FEATURES OF INTRACRANIAL ANEURYSM FORMATION AND RUPTURE THAT PARALLEL OVULATION
We identified 120 genes that were upregulated or downregulated in both the Kurki et al. (2011) ICA and Liu et al. (2017) ovulation data sets (Fig. 2A), and 43 genes that were upregulated and downregulated in common for Kurki et al. (2011) and the Park et al. (2020) ovulation data set. These shared transcripts could provide potential targets for future studies in either system. ICAs and ovulation also share unique features other than their transcriptomic signatures. These rupture systems have similarities in immune cell infiltration and biomechanical fluid forces involved in formation of the rupture site and that contribute to eventual rupture.
(1). Differentially expressed genes shared between ICAs and ovulation
Twelve genes were upregulated or downregulated in common among the two ovulation data sets and the ICA data set (Table 2; see Table S1 for the complete list of shared genes for each comparison). Of these 12 overlapping transcripts, 11 were upregulated in the ruptured group compared to the unruptured group. Two of these, Angptl4 and Pfkfb4, were also found in the chorioamniotic membrane rupture data set (see Section III.1).
Of the remaining nine, spermidine/spermine N1-acetyltransferase 1 (Sat1) is upregulated in response to hCG in both bovine and human granulosa tissue (Lussier et al., 2017; Poulsen et al., 2020). It has also been characterized in thoracic aortic aneurysm rupture as part of the ferroptosis pathway and plays a role in the response to intracerebral haemorrhage following ICA rupture (Ren et al., 2022; Wang et al., 2022). Uncoupling protein 2 (Ucp2) is reduced in cases of impaired ovulation associated with fetal exposure to hypothyroidism and mediates apoptosis, gap junction integrity, and progesterone synthesis in cumulus cells (Ge et al., 2017; Meng et al., 2016; Rousset et al., 2003). In the context of ICAs, it is upregulated within the ruptured region of aneurysm walls and in cerebral ischemia following haemorrhage (Deierborg Olsson et al., 2008; Kurki et al., 2011). Regulator of G protein signalling 2 (Rgs2), NFKB inhibitor alpha (Nfkbia), and ADAM metallopeptidase domain 8 (Adam8) all increase in response to the hormonal trigger of ovulation in multiple mammalian models (Hernandez-Gonzalez et al., 2006; Hughes & Murphy, 2021; Kim, Bagchi & Bagchi, 2009; Paciolla et al., 2011; Park et al., 2020; Sayasith, Sirois & Lussier, 2014; Sriraman et al., 2008; Ujioka et al., 2000). Nfkbia and Adam8 are poorly understood in the context of aneurysm rupture, but Rgs2 is known to be upregulated in response to high wall shear stress and is associated with development of hypertension, which is a major risk factor for ICA development and rupture (Cañes et al., 2021; Dolan et al., 2012; Heximer et al., 2003; Leng et al., 2021; Tang et al., 2003). ATP binding cassette subfamily A member 1 (Abca1) is part of the peroxisome proliferator-activated receptor gamma (PPAR-gamma) pathway that mediates cholesterol influx in the ovary, is increased in early luteal stages, and is associated with altered fertility when mutated (Horihata et al., 2017; Miettinen, Rayburn & Krieger, 2001; Puttabyatappa, VandeVoort & Chaffin, 2010). It plays a similar cholesterol-transporting role in arterial walls (Bekelis et al., 2016; Ollikainen et al., 2016; Synowiec et al., 2016). Cholesterol processing plays a role in driving risk of rupture, and some variants of Abca1 have been shown to be associated with decreased aneurysm risk (Bekelis et al., 2016; Ollikainen et al., 2016; Synowiec et al., 2016). Glutamate-ammonia ligase (Glul), bromodomain adjacent to zinc finger domain A1 (Baz1a), and DEAD-box helicase 3 X-linked (Ddx3x) have been characterized in some reproductive contexts, but there are no published studies establishing a role during ovulation (Bonnet et al., 2008; Pan et al., 2006; Yu et al., 2019). Conversely, there is evidence for involvement of three transcripts in the context of aneurysm formation, rupture, or response to rupture (Arnold et al., 2017; Krischek et al., 2008; Li et al., 2017; Yan et al., 2021; Zhong et al., 2022; Zhou et al., 2022). MAGUK P55 scaffold protein 7 (Mpp7), the only transcript downregulated in the ruptured group, is responsive to oestrogen receptor signalling in granulosa cells and responsive to versican in cumulus cells (Binder et al., 2013; Dunning et al., 2015). It was additionally found to be enriched in aneurysms generally compared to control vascular tissue (Pera et al., 2010; Zhong et al., 2021).
Although the list of overlapping genes for the ovulation–ICA comparisons is relatively small, the transcripts that are present in only one of these comparisons may also yield useful insights. One of the top enriched transcripts in ruptured tissue that is present in both Kurki et al. (2011) and Liu et al. (2017) is pentraxin 3 (Ptx3) (Table S1). This gene is reported to play an essential role in cumulus cell expansion, i.e. expansion of the layer of specialized granulosa cells around the oocyte that is required for ovulation and fertilization (Baranova et al., 2014; Richards et al., 2008; Salustri et al., 2004). Ptx3-knockout mice are subfertile but produce oocytes that are capable of in vitro fertilization, suggesting that its expression is essential for ovulation (Garlanda et al., 2002; Russell & Robker, 2007; Salustri et al., 2004; Varani et al., 2002). In ICAs, Ptx3 has been most widely characterized in the context of subarachnoid haemorrhages, a potentially fatal consequence of ICA rupture. Elevated levels of Ptx3 after subarachnoid haemorrhages are associated with vasospasm and increased mortality (Argın et al., 2017; Kati et al., 2020; Zanier et al., 2011). Although questions remain due to its reported presence in only one ovulation study in our comparisons, its presence in at least one comparison suggests that the overlapping gene lists may capture known regulators of the process of rupture in addition to novel transcripts of interest.
Because many of these overlapping transcripts participate in pathways driving ovulation and in aneurysm formation, rupture, and response to rupture, these transcripts warrant further experimental investigation. Future studies could investigate the roles of the transcripts listed in Table 2 in driving the process of follicle rupture. The additional 108 and 31 genes generated from the two ICA–ovulation comparisons (Fig. 2A) may yield additional targets for study with roles in the process of rupture during ovulation (Table S1).
(2). Patterns of immune cell infiltration in ICAs and ovulation
Immune-related processes are known to be involved in both the ruptured vascular wall and ruptured follicles. Immune-related pathways may include the infiltration of external immune cells into the rupture system. Ruptured ICAs have larger populations of neutrophils, monocytes, M0 macrophages, and M2 macrophages (Shan et al., 2021). Most leukocytes that infiltrate aneurysmal walls are macrophages, and mice with depleted macrophages had a lower incidence of aneurysms (Kanematsu et al., 2011). Mast cells play a key role in aneurysm rupture and stabilization of mast cell degranulation may be a potential therapeutic option for preventing rupture (Furukawa et al., 2020). Similar classes of immune cells have been characterized in the ovary at the time of ovulation. Mast cells are of particular significance in the ovary as they produce three substantial mediators of ovulation: histamine, serotonin, and interleukin-8 (IL-8) (Morikawa et al., 1981; Szukiewicz et al., 2007). Within the ovary, serotonin stimulates progesterone production and plays a role in ovulation induction (Schmidt et al., 1988; Terranova et al., 1990). Histamine also plays a role in ovulation, possibly through stimulation of contractile cells within the follicle and through vasodilatory effects on follicle vasculature (Schmidt, Kannisto & Owman, 1990; Schmidt et al., 1987; Wallach, Wright & Hamada, 1978). The cytokine IL-8 stimulates an immune response, largely through recruitment of neutrophils (Szukiewicz et al., 2007). Mast cells also produce other enzymes such as tryptase and chymase that may contribute to ECM degradation leading up to ovulation (Brännström & Enskog, 2002).
(3). Involvement of biomechanical forces in ICA and ovulation
The biomechanical forces and structural changes that contribute to ICA formation and rupture have been well characterized by studies investigating the pathophysiology of ICAs and stratification of patient risk. ICAs form in conditions of high neurovascular wall shear stress (WSS) paired with a weakened vascular endothelium due to cigarette smoke exposure, genetic disorders, or chronic hypertension (Keedy, 2006). WSS is a quantification of the force per unit area of fluid on a vascular wall and represents the mechanical forces to which the vascular endothelial cells are exposed (Papaioannou & Stefanadis, 2005). When high WSS is present in intracranial vessels, it causes endothelial cell damage and turnover, ECM degradation, medial thinning, and mural cell apoptosis (Dolan et al., 2012; Jamous et al., 2005; Kolega et al., 2011; Meng et al., 2011; Wang et al., 2009). These structural changes resulting from high WSS lead to changes in vessel geometry that facilitate aneurysm formation (Kulcsár et al., 2011; Shojima et al., 2004; Zhang et al., 2016). Once formed, the type and magnitude of WSS changes substantially in different regions of the aneurysm (Kawaguchi et al., 2012; Shimogonya et al., 2009). After initiation, aneurysms exhibit a decrease in the magnitude of WSS within the aneurysm compared to the adjacent non-aneurysmal vessel and a concomitant increase in oscillatory shear index (OSI), a non-dimensional parameter used to quantify disturbances in flow (Kawaguchi et al., 2012; Meng et al., 2014). By contrast, the magnitude of WSS at the point at which the aneurysm connects to the rest of the blood vessel is high (Kawaguchi et al., 2012; Meng et al., 2014). This indicates that there are distinct regions within the aneurysm: a region of lower WSS and high OSI that may rupture and produce a haemorrhagic stroke and a region of high WSS at the mouth of the aneurysm that is not prone to rupture.
Low WSS leads to structural changes and remodelling that predispose aneurysms to rupture. Low WSS paired with high OSI leads to a proinflammatory state with increased reactive oxygen species, immune cell infiltration, and matrix metalloproteinase (MMP) production by inflammatory cells (Galis et al., 1994; Gui et al., 2012; Malek, Alper & Izumo, 1999; Ross, 1999). Low WSS also leads to endothelial cell disruption and smooth muscle proliferation and migration (Chiu & Chien, 2011; Frosen et al., 2012). A certain magnitude of WSS is also necessary to maintain the level of remodelling needed for homeostatic maintenance of vessel walls (Chiu & Chien, 2011). When the WSS falls below this cut-off, the remodelling becomes deleterious and cellular elements of the vascular wall undergo apoptosis (Chiu & Chien, 2011).
Additionally, the magnitude of WSS and OSI within the aneurysm has an impact on rupture risk. Use of computational modelling with patient imaging data from 210 ICAs determined that maximum WSS was higher in ruptured aneurysms relative to unruptured aneurysms (Cebral et al., 2011). Another study similarly found that WSS averaged 2.92 N/m2 in ruptured aneurysms relative to 1.48 N/m2 in unruptured aneurysms, although both values were lower than the measured WSS in non-aneurysmal vessels (Shojima et al., 2004). This suggests that low WSS may lead to remodelling that weakens the wall of the aneurysm but that the WSS and OSI must still rise to a certain level to provide the necessary force for rupture.
Although the WSS associated with ovulation has not been well characterized, similar factors may contribute to follicle rupture. The follicle rupture site in hamster follicles undergoes three stages of structural and biomechanical changes during ovulation induction (Martin & Talbot, 1987). The first stage, occurring in the first 8 h following ovulation induction, involves substantial swelling of the antral cavity and uniform thinning around the entire follicle (Martin & Talbot, 1987). The second stage, over the next 4 h, is characterized by halted antral cavity growth and degeneration of the apical region of the follicle (Martin & Talbot, 1987). Finally, in the last 2 h, the basal region of the follicle thickens, and the apical region breaks apart and ruptures (Martin & Talbot, 1987). Early studies of intrafollicular pressure did not identify a substantial change within ovulatory follicles, but they noted that the follicle wall changes in thickness and extensibility which may alter the wall stress and contribute to additional remodelling that leads to rupture (Espey & Lipner, 1963; Rondell, 1964). A more recent study found a substantial increase in intrafollicular pressure after ovulation induction (Matousek et al., 2001). Additionally, the driving force of intrafollicular pressure is directly related to blood flow within the follicle (Rodgers & Irving-Rodgers, 2010). Use of transvaginal ultrasonography with colour Doppler imaging to visualize regional blood flow in human follicles identified increased blood flow to the follicle base and decreased blood flow to the follicle apex during ovulation (Brannstrom et al., 1998). Although the intrafollicular pressure is generally increased in the follicle after ovulation induction, this pressure may be asymmetrically distributed and thus may have regions of differing WSS that could play a role in formation of the rupture zone.
Biomechanical changes are also similar between follicle rupture and rupture of ICAs. Both systems have an early phase of high pressure and WSS followed by a period of lower biomechanical forces that promote remodelling and predispose them to rupture. Novel methods for studying ovulation and follicle rupture in a controlled manner, such as in vitro follicle growth and rupture assays, will be important to understand the biomechanics of follicle rupture at a higher resolution (Skory et al., 2015). Understanding patterns of altered blood pressure may have implications for luteinized unruptured follicle syndrome, a condition that is often incidentally identified in cases of unexplained infertility associated with follicles that fail to rupture despite being able to form a corpus luteum (Marik & Hulka, 1978). A case report of medication-induced luteinized unruptured follicle syndrome in patients taking paracetamol, an over-the-counter pain medication, found that there was reduced systolic blood velocity in these unruptured follicles as a result of vasodilation (Bourne et al., 1991). The mechanism of luteinized unruptured follicle syndrome has yet to be comprehensively characterized (Marik & Hulka, 1978). WSS modelling and fluid flow analysis techniques derived from studies of ICAs could provide novel insights into the basic biology of ovulation and ovulation-associated pathologies.
The study of ICAs has also generated novel methods that could be used to understand ovarian follicle rupture better. For example, four-dimensional (4D) flow-imaging modalities have been used to assess fluid dynamics during aneurysm formation, growth, and rupture (Brindise et al., 2019; Medero et al., 2020; Vivas et al., 2022). The data from this imaging are used to create in silico models of how changes in flow affect progression of the aneurysm. Microfluidic models have also been adapted to assess how fluid shear stress alters the morphology and transcriptomic signatures of vascular endothelial cells in vitro (Doherty et al., 2021). Ovulation studies could benefit from high-resolution modelling of the impact of fluid pressures and flows on formation of a rupture site. Recently, optical coherence tomography (OCT), which is used to assess aneurysm structure and model flow dynamics, has been adapted to reproductive biology contexts (Hartmann et al., 2019; Liu et al., 2019; Real et al., 2013; Umezu & Larina, 2022). Intravital OCT was used to study COC transit through the oviduct following ovulation and more recently to visualize follicle rupture and transport of COCs through the ovarian bursa to the oviduct (Burton et al., 2015; Umezu, Wang & Larina, 2022). Additional modalities, including doppler-equipped intravital imaging, could be used to assess specific fluid dynamics within the ovulatory follicle. These data could then be used to assess the role that fluid-derived biomechanical forces may play in follicular wall rupture. Additionally, microfluidic studies using flow metrics generated from the imaging data could assess the impact of fluid shear forces on the structure and function of granulosa cells derived from follicle walls. These studies could use strategies from ICA modelling, such as generating three-dimensional (3D)-printed scaffolds that recapitulate ovarian follicle geometry (Doherty et al., 2021; Jamous et al., 2005; Vivas et al., 2022). Microfluidic technology could also be used in ovulation to assess how fluid dynamics change the structure and function of granulosa cells.
V. FEATURES OF CHORIOAMNIOTIC MEMBRANE RUPTURE THAT PARALLEL OVULATION
The comparison between the data sets of Nhan-Chang et al. (2010) and Liu et al. (2017) revealed 48 genes that were upregulated or downregulated in common (Fig. 2B), while the comparison between Nhan-Chang et al. (2010) and Park et al. (2020) revealed 18 genes that were upregulated or downregulated in common. Some of these transcripts have already been characterized in chorioamniotic membranes and ovulation. In addition to the transcriptomic data from chorioamniotic membrane rupture, other features both physiologically at term and in cases of pathological preterm labour parallel ovulation, including altered smooth muscle cell contractility and immune cell infiltration.
(1). Differentially expressed genes shared between chorioamniotic membrane rupture and ovulation
The three upregulated genes found in both ovulation data sets and in the chorioamniotic membrane rupture data set are listed in Table 3 (see Table S2 for the complete list of shared genes for each comparison). Two of the three upregulated genes, Angptl4 and Pfkfb4, were also identified in the ovulation–ICA comparisons (see Section IV.1). Since ovulation and chorioamniotic rupture both represent physiological forms of rupture, it is of interest to consider the other identified overlapping gene, Lox, which was not present in the ovulation–ICA comparisons and thus may be involved in processes that are unique to physiological rupture. Lysyl oxidase (Lox) functions in the crosslinking of collagens and elastin (Lucero & Kagan, 2006). Lox is responsive to several regulators of ovulation, including transforming growth factor beta 1 (TGF-ß1), follicle stimulating hormone (FSH), growth differentiation factor 9 (GDF-9), and Activin A in both human and rat granulosa cells (Fang et al., 2016; Harlow et al., 2003; Slee et al., 2001). Lox has also been shown to play a role in remodelling associated with rupture of chorioamniotic membranes during parturition (Polettini et al., 2016). Specifically, it is responsive to cortisol produced by the amnion (Liu et al., 2016a). It also participates in cyclooxygenase-2 pathways and prostaglandin E2 signalling in fetal membranes to regulate the breakdown of membranes during rupture (Liu et al., 2016b). Although relatively few transcripts overlapped completely between the two ovulation and chorioamniotic rupture comparisons, there were 60 transcripts that overlapped in only one of these two comparisons (Fig. 2B, Table S2). One of these transcripts is prostaglandin-endoperoxide synthase 2 (Ptgs2), which was upregulated in the ruptured tissue in both Nhan-Chang et al. (2010) and Liu et al. (2017). Ptgs2 is a major driver of ovulation that is temporally expressed during the LH surge and downregulated shortly afterwards (Park et al., 2020; Richards, 1997; Wong & Richards, 1991). Functional blocking of Ptgs2 impairs ovulation and has been proposed as a potential contraceptive (Armstrong & Grinwich, 1972; Downs & Longo, 1982; Duffy, 2015; Mikuni et al., 1998; Sogn et al., 1987). In chorioamniotic membranes, Ptgs2 regulates the activity of MMPs that facilitate membrane rupture (Li et al., 2007). Ptgs2 messenger RNA (mRNA) is upregulated in term-labour relative to non-labour membranes (Takahashi et al., 2021). Although this transcript was not identified in the comparison between Nhan-Chang et al. (2010) and the other ovulation data set (Park et al., 2020) and is thus not a robust finding, its presence in at least one of our comparisons implies that it may be involved in the process of rupture. Further research into the genes identified in Table 3 and Table S2 may elucidate novel regulators of follicle rupture during ovulation, and more generally in processes that may be unique to physiological rupture.
(2). Patterns of immune cell infiltration in chorioamniotic membrane rupture and ovulation
Immune cell infiltration plays a major role in chorioamniotic membrane rupture. Intrauterine infection increases the likelihood of preterm chorioamniotic membrane rupture and is associated with immune cell infiltration. Neutrophils are associated with preterm labour in the setting of intrauterine infection (Hamilton et al., 2012). Macrophages are also present in different regions of the uterus and chorioamniotic membranes leading up to parturition (Bokström et al., 1997). Macrophages facilitate remodelling of the supracervical region of the chorioamniotic membranes that leads to weakening and predisposes this region to rupture during parturition (Hamilton et al., 2012; Mackler et al., 1999). This weakening is controlled largely by activation of macrophage-derived MMPs that are major drivers of ECM remodelling and degradation (Gonzalez et al., 2011; Payne et al., 2012). Macrophages also play a role in remodelling after membrane rupture (Shynlova et al., 2013; Timmons, Akins & Mahendroo, 2010). T lymphocytes are another key immune mediator in chorioamniotic membrane rupture. In term parturition, these cells infiltrate the chorioamniotic membranes and are concentrated at the rupture zone (Gomez-Lopez et al., 2011, 2013b). However, in preterm rupture of membranes, there is less infiltration by T lymphocytes and more by granulocytes (Gomez-Lopez et al., 2013a,b). This suggests that alterations in immune cell infiltration may play a role in pathological forms of chorioamniotic membrane rupture.
These patterns of immune cell infiltration parallel features of ovulation where macrophage infiltration occurs in the vasculature and theca cell layer (Brännström & Enskog, 2002; Brannstrom, Mayrhofer & Robertson, 1993). Macrophage density increases in the corpus luteum after ovulation, suggesting they may play a similar role in repairing tissue after rupture in the ovulatory follicle (Brannstrom et al., 1994; Cavender & Murdoch, 1988). T lymphocyte infiltration also occurs in ovulatory follicles, particularly in the theca vasculature (Best et al., 1996). Interestingly, the number of T lymphocytes is significantly decreased in infertile women with polycystic ovarian syndrome (Li et al., 2019). In both chorioamniotic membrane rupture and ovulation, pathological forms of rupture are associated with alterations in T lymphocyte counts.
(3). Involvement of smooth muscle contraction in chorioamniotic membrane rupture and ovulation
Biomechanical forces play a role in the rupture of chorioamniotic membranes, both at term and in pathological preterm rupture, although the exact mechanism may be variable. Early research hypothesized that uterine contractions during labour lead to chorioamniotic membrane rupture through mechanical stretching and tearing of the membranes (Joyce et al., 2009). However, this cannot be the only mechanism as rupture occurs prior to labour-associated contractions in 10% of term deliveries and 40% of preterm deliveries (Joyce et al., 2009). Additionally, an in vitro study of chorioamniotic membrane strength found that acute mechanical stretching in fact strengthens chorioamniotic membranes (Pandey et al., 2007). It is now thought that biomechanical stretch plays a role in chorioamniotic membrane rupture through an alternative mechanism: induction of inflammation and remodelling.
Chorioamniotic membranes have a well-characterized weak zone above the cervical region that forms prior to rupture (Kumar et al., 2016). The weakened zone forms due to disruption of collagen, increased collagenase activity, and inflammation (Kumar et al., 2016). This remodelling occurs prior to labour, the point in pregnancy at which it is subjected to the highest mechanical stretching force (Kumar et al., 2016). The weakened zone is found even in chorioamniotic membranes from caesarian sections that have not undergone labour (McLaren et al., 1999). There is evidence that it is not the acute, high-magnitude forces produced by contractions that lead to this weakened zone but rather it is the smaller, cyclical forces from smooth muscle cell contractions that occur throughout gestation (Kumar et al., 2016; Pandey et al., 2007). Chronic stretching of chorioamniotic membranes, particularly in the region overlying the cervix, induces IL-8 signalling that dictates remodelling of the region (Maehara et al., 1996). Increased Cox2 expression, prostaglandin E2 (PGE2) release, and collagenase activity occurs following mechanical stretching of chorioamniotic membranes (Chowdhury et al., 2014; El Maradny et al., 1996). Chronic stretching of the chorioamniotic membranes as part of gestation is also associated with inflammation, ECM remodelling, and some degree of protection from apoptosis until shortly before delivery (Kendal-Wright, Hubbard & Bryant-Greenwood, 2008). Mechanical deformation of collagen in the chorioamniotic membrane makes the collagen more susceptible to degradation by stretching the fibres and exposing more crosslinking sites to collagen-degrading enzymes (Joyce et al., 2009). Once the weakened zone is formed, rupture may be triggered either by mechanical forces of labour or by an increased inflammatory state, often implicated in pathological ruptures of chorioamniotic membranes.
Smooth muscle contractions also play a role in ovulation, with a series of morphological changes occurring in the ovulatory follicle (Martin & Talbot, 1981b). Smooth muscle cells are only present in the basal region of the follicle, particularly in the theca externa layer, and this region of the follicle is constricted at the time of rupture (Martin & Talbot, 1981b). In hamster follicles, a substantial decrease in intrafollicular pressure coincides with contraction of the smooth muscle cells at the follicle base (Schroeder & Talbot, 1982). Within human periovulatory follicles, non-vascular smooth muscle cells are found in the theca externa and expression of key components of the endothelin system which regulates contractions is high (Choi et al., 2011).
There is evidence for smooth muscle involvement in ovulation. In one study of in vivo ovulation in a hamster model, drugs that inhibit smooth muscle contraction blocked ovulation (Martin & Talbot, 1981a). Drugs that alter calcium availability within the follicle also inhibited ovulation due to the involvement of calcium transport in smooth muscle contraction (Martin & Talbot, 1981a). Endothelin-2 induces smooth muscle contraction, and an endothelin2-knockout mouse model exhibited reduced rates of ovulation and luteinization (Cacioppo et al., 2017). However, there are no studies that link biomechanical force production by smooth muscle contraction in the theca externa to changes in structure and pathway expression of the apical region of the follicle. In ex vivo models of rupture of chorioamniotic membranes, manipulation of mechanical stretch forces exerted on chorioamniotic membranes had a direct impact on pathways related to inflammation and ECM remodelling (Chowdhury et al., 2014; El Maradny et al., 1996; Joyce et al., 2009; Kendal-Wright et al., 2008; Maehara et al., 1996). One study of human cervical fibroblasts cultured on plates with flexible silicon bottoms found that cyclical mechanical stretch forces led to increased production of hyaluronan, which is necessary for cervical ripening during parturition (Takemura et al., 2005). Smooth muscle-like contraction as well as hyaluronan production, largely by cumulus cells within the ovulatory follicle, are both essential for ovulation (Choi et al., 2011; Hess, Chen & Larsen, 1999; Martin & Talbot, 1981a,b; Richards, 2005; Russell & Robker, 2007; Talbot & Chacon, 1982). Adapting ex vivo stretching studies from the study of parturition may provide evidence for an additional mechanism that drives alterations in ECM composition and molecular signatures of ovulation within the ovulatory follicle. Further analysis of the role of smooth muscle contraction and mechanical stretch within periovulatory follicles using ex vivo application of mechanical force and stretch forces to cells isolated from the follicle wall may provide additional insights into fundamental mechanisms of ovulation as well as elucidate potential targets for contraceptive development.
Due to the significant role of mechanical stretch in chorioamniotic membrane rupture, several methods have been developed to introduce stretch forces to membranes in vitro and assess how morphology, ECM composition, and transcriptomic signatures change with stretching (Chowdhury et al., 2014; Kumar et al., 2016). In addition, a puncture-testing method has been adapted from the textile industry to assess how small forces and stretch distend the chorioamniotic membrane to study the biomechanical properties of this tissue (Burzle, Mazza & Moore, 2014). Testing of mechanical stretch in the context of ovulation and the ovary could also elucidate mechanisms of impaired ovulation associated with advanced reproductive age. There is evidence for defects in follicle rupture associated with ovarian aging, demonstrated by an increased incidence of antral follicles with expanded cumulus oocyte complexes that have failed to rupture along with oocytes trapped in luteinized unruptured follicles (Mara et al., 2020). There is also evidence for impaired epithelial layer remodelling and wound healing as well as altered proliferation and apoptosis (Mara et al., 2020). These studies were performed in mice treated with pregnant mare serum gonadotropin (PMSG), a FSH analogue, prior to induction of ovulation with hCG, a LH analogue. This hyperstimulation and superovulation led to recruitment and ovulation of a larger number of follicles in one cycle. The finding that ovulatory defects are observed with advanced age even in the context of superovulation demonstrates that these defects are driven by factors other than age-related changes in the hormonal stimulation of ovulation. The mechanisms for these phenotypes are still under investigation but could be due to increased inflammation and stromal fibrosis, and subsequent ovarian tissue stiffness, which is known to increase with age (Amargant et al., 2020; Mara et al., 2020). Acute treatment with anti-fibrotic agents improves ovulation outcomes with advanced reproductive age (Umehara et al., 2022). Previous studies in the ovary have used instrumental indentation, atomic force microscopy, and shear wave elastography techniques to quantify stiffness (Amargant et al., 2020; Ansardamavandi et al., 2020; Gargus et al., 2020; Sumbul et al., 2022). Additional techniques used to study cervical and chorioamniotic membrane changes during parturition could also be adapted to the study of ovulation. These include Raman spectroscopy, which has been used non-invasively in in vivo studies of cervical remodelling and avoids the necessity for invasive measurements of biomechanical properties of the cervix (O’Brien et al., 2017). Other techniques may include studies of tissue deformability using aspiration distension or strain (static) elastography to assess the distensibility of the ovarian follicle wall, both of which have been used in studies of parturition (Chowdhury et al., 2014; Feltovich & Carlson, 2017). Understanding how aging impacts distensibility of ovarian follicle walls may provide insights into the mechanisms behind age-associated follicle rupture defects.
Studies of chorioamniotic membrane rupture have increased our understanding of transcriptomic signatures around the time of rupture. Many studies have identified differences in signalling pathways between the supracervical rupture site compared to sites distant to rupture (Arikat et al., 2006; Lappas et al., 2008; Moore et al., 2009). This technique could be adapted to the study of follicle rupture by using spatial transcriptomics in ovulatory follicles across the time course of ovulation. It could also be used to isolate transcriptomic signatures of tissue specific to the region of follicle rupture as well as tissue from a region distant to the rupture site in ovulatory follicles. Together, methods for testing the role of mechanical stretch forces on granulosa cells as well as spatial transcriptomic studies of rupture in ovulation will enable us to understand the process of rupture at high resolution.
VI. DISCUSSION
The observed parallels in the transcriptomic signatures, immune profiles, and structural forces that underlie rupture in ICAs, chorioamniotic membranes during parturition, and ovarian follicles during ovulation extend our understanding of ovulation (Fig. 3). The current understanding is that ovulation involves ECM remodelling, inflammation, and structural forces that drive follicle rupture. Comparing biology across pathological and physiological examples of rupture in the human body demonstrates that these general pathways are conserved. In addition, these comparisons highlight key genes that are conserved across multiple comparisons, including Angptl4, Pfkfb4, and Lox. Several of the identified genes have been characterized previously in rupture contexts, whereas others, including Glul, Baz1a, and Ddx3x, are not yet characterized and should be explored further in the context of ovarian follicle rupture. Comparisons of the biomechanical and structural forces that underlie rupture also identify broad similarities in WSS decreases leading up to rupture and mechanical stretch of smooth muscle-like cells away from the site of rupture. The role that these forces play and their potential for clinical and diagnostic use is better understood in the context of ICA and chorioamniotic membrane rupture than in ovulation. As a result of the similarities between rupture systems discussed herein, ex vivo methods that have been developed to study ICAs and chorioamniotic membrane rupture should be paired with existing ex vivo ovulation techniques to advance the field of ovulation biology (Converse et al., 2022; Skory et al., 2015). The gaps identified in this review, specifically in the context of the biomechanical and contractile forces involved, should be explored further to enhance our understanding of the process of ovulation.
Fig. 3.
Understanding common mechanisms of rupture between pathological and physiological rupture systems in addition to novel methods of studying rupture can be used to inform potential innovations in studies of ovarian biology and ovulation.
Novel insights may arise from a deeper investigation of poorly characterized genes from this transcriptomic data set, the role of immune cells and immune modulation, and the impact of biomechanical forces on the process of follicle rupture, particularly with reference to the pathology of conditions associated with abnormal or impaired follicle rupture such as luteinized unruptured follicle syndrome and ovarian aging (Mara et al., 2020; Marik & Hulka, 1978). Learning more about ovulation may elucidate drug targets for ovarian pathologies as well as the development of ovulation-modifying drugs, such as non-hormonal contraceptives. Although the process of ovulation is exceedingly complex, many of its features are reflected in other processes that can be described as rupture events.
Our analysis suggests that ICAs and chorioamniotic membrane rupture share several transcriptomic and non-transcriptomic features with ovulation. Given these parallels between rupture systems and ovulation, there should be a focus on sharing insights to advance each of these fields. Much work has been done to understand rupture in the context of ICAs and chorioamniotic membranes in part because of the risk of morbidity and mortality in these conditions. Ovulation biologists should continue to examine findings from other rupture systems to identify relevant research questions and methods pioneered in other fields that study pathological and physiological rupture.
While such comparisons are compelling, they are not without caveats. First, comparisons of DEGs may not be as effective as direct transcriptomic comparisons of sequencing data. Future studies of the genes identified in this analysis should include validation of findings from the referenced transcriptomic data sets. Second, the designs of the transcriptomic analyses used herein differed in some respects (e.g. comparing tissues from the same individual/organism versus two different organisms; generating ruptured/unruptured phenotypes using knockout models versus using patient samples; or using RNA-Seq versus microarray techniques). Third, there are fundamental differences in the biology of these systems, specifically in relation to timing and pathophysiology. In humans, ovulation occurs over a period of hours, and while rupture of fetal membranes also takes place on this timescale, the formation of a rupture site may begin earlier in gestation. By contrast, rupture of ICAs occurs over a matter of years, or may not happen at all. Despite these differences in timing, the rupture events follow a similar pattern: initiation, growth and development of the rupture site, and finally rupture. With regard to pathophysiology, ovulation differs from the other rupture systems in that it is a purely physiological process. ICAs are always pathological whereas chorioamniotic membrane rupture can be pathological or physiological. Thus, while all rupture processes may not be the same, they do have some parallels that could be explored to further our understanding of each rupture system.
VII. CONCLUSIONS
Intracranial aneurysm (ICA) rupture, chorioamniotic membrane rupture, and ovulation can all be described as rupture events.
These rupture events have overlapping transcriptomic signals (Angptl4, Pfkfb4) relating to vascularization, lipid metabolism, apoptotic regulation, and hypoxia response.
Our transcriptomic comparison has identified several novel transcripts that have not yet been characterized in the context of ovulation, including Glul, Baz1a, and Ddx3x.
ICA and chorioamniotic rupture share overlapping structural and biomechanical features with ovulation, including spatially distributed wall shear stress in both ICAs and ovulation and smooth muscle contractions away from the rupture site in both chorioamniotic membrane rupture and ovulation.
While there are limitations to this analysis, overlapping findings between these rupture systems and ovulation suggest that knowledge from these fields may be used to inform novel studies on ovulation, ovarian disorders, and ovulation-related drug development.
Specific methods that have been developed in various rupture systems including microfluidic studies, computational flow modelling, biomechanical stretch testing, and spatially oriented transcriptomic comparisons may be useful to advance the study of ovulation.
Supplementary Material
Complete list of overlapping upregulated and downregulated genes in comparisons between two ovulation data sets and one data set for intracranial aneurysm (ICA).
Complete list of overlapping upregulated and downregulated genes in comparisons between the intracranial aneurysm (ICA) and chorioamniotic membrane rupture (CMR) data sets.
Complete list of overlapping upregulated and downregulated genes in comparisons between two ovulation data sets and one data set for chorioamniotic membrane rupture (CMR).
ACKNOWLEDGEMENTS
The authors would like to thank Caroline Kratka, Camille Mulcahy, Hoi Chang Lee, Chanakarn Suebthawinkul, Tracy Zhou, Danielle Pi, and Luisa Iruela-Arispe for their insights. This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under Award Number T32HD094699 and the Bill & Melinda Gates Foundation Grant INV-003385. Under the grant conditions of the Foundation, a Creative Commons Attribution 4.0 Generic License has already been assigned to the Author Accepted Manuscript version that might arise from this submission. The authors declare no conflict of interest.
REFERENCES
- Al-Edani T, Assou S, Ferrieres A, Bringer Deutsch S, Gala A, Lecellier CH, Ait-Ahmed O & Hamamah S (2014). Female aging alters expression of human cumulus cells genes that are essential for oocyte quality. Biomedical Research International 2014, 964614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amargant F, Manuel SL, Tu Q, Parkes WS, Rivas F, Zhou LT, Rowley JE, Villanueva CE, Hornick JE & Shekhawat GS (2020). Ovarian stiffness increases with age in the mammalian ovary and depends on collagen and hyaluronan matrices. Aging Cell 19(11), e13259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansardamavandi A, Tafazzoli-Shadpour M, Omidvar R & Nili F (2020). An AFM-based nanomechanical study of ovarian tissues with pathological conditions. International Journal of Nanomedicine 15, 4333–4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argın CE, Aygün A, Katipoğlu B, Tatlı Ö, Yaman H, Menteşe A, Gazioğlu G, Çakmak VA, Örem A & Gündüz A (2017). Diagnostic value of pentraxin-3 in patients with spontaneous subarachnoid and intracerebral hemorrhage. Eurasian Journal of Emergency Medicine 16(3), 133. [Google Scholar]
- Arikat S, Novince RW, Mercer BM, Kumar D, Fox JM, Mansour JM & Moore JJ (2006). Separation of amnion from choriodecidua is an integral event to the rupture of normal term fetal membranes and constitutes a significant component of the work required. American Journal of Obstetrics and Gynecology 194(1), 211–217. [DOI] [PubMed] [Google Scholar]
- Armstrong DT & Grinwich DL (1972). Blockade of spontaneous and LH-induced ovulation in rats by indomethacin, an inhibitor of prostaglandin biosynthesis. Prostaglandins 1(1), 21–28. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Cassis LA, Eghbali M, Reue K & Sandberg K (2017). Sex hormones and sex chromosomes cause sex differences in the development of cardiovascular diseases. Arteriosclerosis, Thrombosis, and Vascular Biology 37(5), 746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranova NS, Inforzato A, Briggs DC, Tilakaratna V, Enghild JJ, Thakar D, Milner CM, Day AJ & Richter RP (2014). Incorporation of pentraxin 3 into hyaluronan matrices is tightly regulated and promotes matrix cross-linking. Journal of Biological Chemistry 289(44), 30481–30498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekelis K, Kerley-Hamilton JS, Teegarden A, Tomlinson CR, Kuintzle R, Simmons N, Singer RJ, Roberts DW, Kellis M & Hendrix DA (2016). MicroRNA and gene expression changes in unruptured human cerebral aneurysms. Journal of Neurosurgery 125(6), 1390–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendix J, Hegaard H, Bergholt T & Langhoff-Roos J (2015). Expectant management of PPROM and major complications before planned delivery: A retrospective cohort study. Journal of Obstetrics and Gynaecology 35(6), 570–577. [DOI] [PubMed] [Google Scholar]
- Best CL, Pudney J, Welch WR, Burger N & Hill JA (1996). Localization and characterization of white blood cell populations within the human ovary throughout the menstrual cycle and menopause. Human Reproduction 11(4), 790–797. [DOI] [PubMed] [Google Scholar]
- Binder AK, Rodriguez KF, Hamilton KJ, Stockton PS, Reed CE & Korach KS (2013). The absence of er-β results in altered gene expression in ovarian granulosa cells isolated from in vivo preovulatory follicles. Endocrinology 154(6), 2174–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokström H, Brännström M, Alexandersson M & Norström A (1997). Leukocyte subpopulations in the human uterine cervical stroma at early and term pregnancy. Human Reproduction 12(3), 586–590. [DOI] [PubMed] [Google Scholar]
- Bollopragada S, Youssef R, Jordan F, Greer I, Norman J & Nelson S (2009). Term labor is associated with a core inflammatory response in human fetal membranes, myometrium, and cervix. American Journal of Obstetrics and Gynecology 200(1), 104.e101–104.e111. [DOI] [PubMed] [Google Scholar]
- Bonnet A, Lê Cao KA, SanCristobal M, Benne F, Robert-Granié C, Law-So G, Fabre S, Besse P, De Billy E, Quesnel H, Hatey F & Tosser-Klopp G (2008). In vivo gene expression in granulosa cells during pig terminal follicular development. Reproduction 136(2), 211–224. [DOI] [PubMed] [Google Scholar]
- Bourne G (1962). The foetal membranes: a review of the anatomy of normal amnion and chorion and some aspects of their function. Postgraduate Medical Journal 38(438), 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne T, Reynolds K, Waterstone J, Okokon E, Jurkovic D, Campbell S & Collins WP (1991). Paracetamol‐associated luteinized unruptured follicle syndrome: effect on intrafollicular blood flow. Ultrasound in Obstetrics and Gynecology: The Official Journal of the International Society of Ultrasound in Obstetrics and Gynecology 1(6), 420–425. [DOI] [PubMed] [Google Scholar]
- Brännström M & Enskog A (2002). Leukocyte networks and ovulation. Journal of Reproductive Immunology 57(1), 47–60. [DOI] [PubMed] [Google Scholar]
- Brannstrom M, Mayrhofer G & Robertson SA (1993). Localization of leukocyte subsets in the rat ovary during the periovulatory period. Biology of Reproduction 48(2), 277–286. [DOI] [PubMed] [Google Scholar]
- Brannstrom M, Pascoe V, Norman RJ & McClure N (1994). Localization of leukocyte subsets in the follicle wall and in the corpus luteum throughout the human menstrual cycle. Fertility and Sterility 61(3), 488–495. [PubMed] [Google Scholar]
- Brannstrom M, Zackrisson U, Hagstrom HG, Josefsson B, Hellberg P, Granberg S, Collins WP & Bourne T (1998). Preovulatory changes of blood flow in different regions of the human follicle. Fertility and Sterility 69(3), 435–442. [DOI] [PubMed] [Google Scholar]
- Brindise MC, Rothenberger S, Dickerhoff B, Schnell S, Markl M, Saloner D, Rayz VL & Vlachos PP (2019). Multi-modality cerebral aneurysm haemodynamic analysis: in vivo 4D flow MRI, in vitro volumetric particle velocimetry and in silico computational fluid dynamics. Journal of the Royal Society Interface 16(158), 20190465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown HM, Dunning KR, Robker RL, Boerboom D, Pritchard M, Lane M & Russell DL (2010). ADAMTS1 cleavage of versican mediates essential structural remodeling of the ovarian follicle and cumulus-oocyte matrix during ovulation in mice. Biology of reproduction 83(4), 549–557. [DOI] [PubMed] [Google Scholar]
- Bryant-Greenwood G (1998). The extracellular matrix of the human fetal membranes: structure and function. Placenta 19(1), 1–11. [DOI] [PubMed] [Google Scholar]
- Burton JC, Wang S, Stewart CA, Behringer RR & Larina IV (2015). High-resolution three-dimensional in vivo imaging of mouse oviduct using optical coherence tomography. Biomedical Optics Express 6(7), 2713–2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzle W, Mazza E & Moore JJ (2014). About puncture testing applied for mechanical characterization of fetal membranes. Journal of Biomechanical Engineering 136(11), 111009. [DOI] [PubMed] [Google Scholar]
- Bylander A, Gunnarsson L, Shao R, Billig H & Larsson DGJ (2015). Progesterone-mediated effects on gene expression and oocyte-cumulus complex transport in the mouse fallopian tube. Reproductive Biology and Endocrinology 13(1), 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JA, Lin P-CP, Hannon PR, McDougle DR, Gal A & Ko C (2017). Granulosa cell endothelin-2 expression is fundamental for ovulatory follicle rupture. Scientific Reports 7(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cañes L, Martí-Pàmies I, Ballester-Servera C, Alonso J, Serrano E, Briones AM, Rodríguez C & Martínez-González J (2021). High NOR-1 (neuron-derived orphan receptor 1) expression strengthens the vascular wall response to angiotensin ii leading to aneurysm formation in mice. Hypertension 77(2), 557–570. [DOI] [PubMed] [Google Scholar]
- Cavender JL & Murdoch WJ (1988). Morphological studies of the microcirculatory system of periovulatory ovine follicles. Biology of Reproduction 39(4), 989–997. [DOI] [PubMed] [Google Scholar]
- Cebral JR, Mut F, Weir J & Putman C (2011). Quantitative characterization of the hemodynamic environment in ruptured and unruptured brain aneurysms. American Journal of Neuroradiology 32(1), 145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaemsaithong P, Madan I, Romero R, Than NG, Tarca AL, Draghici S, Bhatti G, Yeo L, Mazor M, Kim CJ, Hassan SS & Chaiworapongsa T (2013). Characterization of the myometrial transcriptome in women with an arrest of dilatation during labor. Journal of Perinatal Medicine 41(6), 665–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu JJ & Chien S (2011). Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiological Reviews 91(1), 327–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D-H, Kim EK, Kim K-H, Lee K-A, Kang D-W, Kim HY, Bridges P & Ko C (2011). Expression pattern of endothelin system components and localization of smooth muscle cells in the human pre-ovulatory follicle. Human Reproduction 26(5), 1171–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury B, David A, Thrasivoulou C, Becker D, Bader D & Chowdhury T (2014). Tensile strain increased COX-2 expression and PGE2 release leading to weakening of the human amniotic membrane. Placenta 35(12), 1057–1064. [DOI] [PubMed] [Google Scholar]
- Clow O, Hurst P & Fleming J (2002). Changes in the mouse ovarian surface epithelium with age and ovulation number. Molecular and Cellular Endocrinology 191(1), 105–111. [DOI] [PubMed] [Google Scholar]
- Converse A, Zaniker EJ, Amargant F & Duncan FE (2022). Recapitulating folliculogenesis and oogenesis outside the body: encapsulated in vitro follicle growth. Biology of Reproduction 108(1), 5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxatto HB (2002). Physiology of gamete and embryo transport through the Fallopian tube. Reproductive BioMedicine Online 4(2), 160–169. [DOI] [PubMed] [Google Scholar]
- Cruz M, Requena A, Agudo D & García-Velasco JA (2017). Type of gonadotropin used during controlled ovarian stimulation induces differential gene expression in human cumulus cells: a randomized study. European Journal of Obstetrics & Gynecology and Reproductive Biology 215, 124–133. [DOI] [PubMed] [Google Scholar]
- Deierborg Olsson T, Wieloch T, Diano S, Warden CH, Horvath TL & Mattiasson G (2008). Overexpression of UCP2 protects thalamic neurons following global ischemia in the mouse. Journal of Cerebral Blood Flow & Metabolism 28(6), 1186–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Direito A, Bailly S, Mariani A & Ecochard R (2013). Relationships between the luteinizing hormone surge and other characteristics of the menstrual cycle in normally ovulating women. Fertility and Sterility 99(1), 279–285. [DOI] [PubMed] [Google Scholar]
- DiZerega GS & Hodgen GD (1981). Folliculogenesis in the primate ovarian cycle. Endocrine Reviews 2(1), 27–49. [DOI] [PubMed] [Google Scholar]
- Doherty EL, Aw WY, Hickey AJ & Polacheck WJ (2021). Microfluidic and organ-on-a-chip approaches to investigate cellular and microenvironmental contributions to cardiovascular function and pathology. Frontiers in Bioengineering and Biotechnology 9, 624435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan JM, Sim FJ, Meng H & Kolega J (2012). Endothelial cells express a unique transcriptional profile under very high wall shear stress known to induce expansive arterial remodeling. American Journal of Physiology Cell Physiology 302(8), C1109–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs SM & Longo FJ (1982). Effects of indomethacin on preovulatory follicles in immature, superovulated mice. American Journal of Anatomy 164(3), 265–274. [DOI] [PubMed] [Google Scholar]
- Duffy DM (2015). Novel contraceptive targets to inhibit ovulation: the prostaglandin E2 pathway. Human Reproduction Update 21(5), 652–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy DM, Ko C, Jo M, Brannstrom M & Curry TE (2019). Ovulation: parallels with inflammatory processes. Endocrine Reviews 40(2), 369–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunning KR, Watson LN, Zhang VJ, Brown HM, Kaczmarek AK, Robker RL & Russell DL (2015). Activation of mouse cumulus-oocyte complex maturation in vitro through EGF-like activity of versican1. Biology of Reproduction 92(5), 1–10. [DOI] [PubMed] [Google Scholar]
- El Maradny E, Kanayama N, Halim A, Maehara K & Terao T (1996). Stretching of fetal membranes increases the concentration of interleukin-8 and collagenase activity. American Journal of Obstetrics and Gynecology 174(3), 843–849. [DOI] [PubMed] [Google Scholar]
- Espey LL (1967). Ultrastructure of the apex of the rabbit graafian follicle during the ovulatory process. Endocrinology 81(2), 267–276. [DOI] [PubMed] [Google Scholar]
- Espey LL & Lipner H (1963). Measurements of intrafollicular pressures in the rabbit ovary. American Journal of Physiology 205, 1067–1072. [DOI] [PubMed] [Google Scholar]
- Estrelinha M, Hinterseher I & Kuivaniemi H (2014). Gene expression studies in human abdominal aortic aneurysm. Reviews in Vascular Medicine 2(3), 77–82. [Google Scholar]
- Etminan N, Chang H-S, Hackenberg K, de Rooij NK, Vergouwen MDI, Rinkel GJE & Algra A (2019). Worldwide incidence of aneurysmal subarachnoid hemorrhage according to region, time period, blood pressure, and smoking prevalence in the population: a systematic review and meta-analysis. JAMA Neurology 76(5), 588–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Chang H-M, Cheng J-C, Klausen C, Leung PCK & Yang X (2016). Transforming growth factor-β1 increases lysyl oxidase expression by downregulating MIR29A in human granulosa lutein cells. Reproduction 152(3), 205–213. [DOI] [PubMed] [Google Scholar]
- Feltovich H & Carlson L (2017). New techniques in evaluation of the cervix. Seminars in Perinatology 41(8), 477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frosen J, Tulamo R, Paetau A, Laaksamo E, Korja M, Laakso A, Niemela M & Hernesniemi J (2012). Saccular intracranial aneurysm: pathology and mechanisms. Acta Neuropathologica 123(6), 773–786. [DOI] [PubMed] [Google Scholar]
- Furukawa H, Wada K, Tada Y, Kuwabara A, Sato H, Ai J, Lawton MT & Hashimoto T (2020). Mast cell promotes the development of intracranial aneurysm rupture. Stroke 51(11), 3332–3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gäbel G, Northoff BH, Weinzierl I, Ludwig S, Hinterseher I, Wilfert W, Teupser D, Doderer SA, Bergert H, Schönleben F, Lindeman JHN & Holdt LM (2017). Molecular fingerprint for terminal abdominal aortic aneurysm disease. Journal of the American Heart Association 6(12), e006798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galis ZS, Sukhova GK, Lark MW & Libby P (1994). Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. Journal of Clinical Investigation 94(6), 2493–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargus ES, Jakubowski KL, Arenas GA, Miller SJ, Lee SSM & Woodruff TK (2020). Ultrasound shear wave velocity varies across anatomical region in ex vivo bovine ovaries. Tissue Engineering Part A 26(13–14), 720–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlanda C, Hirsch E, Bozza S, Salustri A, De Acetis M, Nota R, Maccagno A, Riva F, Bottazzi B & Peri G (2002). Non-redundant role of the long pentraxin PTX3 in anti-fungal innate immune response. Nature 420(6912), 182–186. [DOI] [PubMed] [Google Scholar]
- Ge H, Zhang F, Duan P, Zhu N, Zhang J, Ye F, Shan D, Chen H, Lu X, Zhu C, Ge R & Lin Z (2017). Mitochondrial uncoupling protein 2 in human cumulus cells is associated with regulating autophagy and apoptosis, maintaining gap junction integrity and progesterone synthesis. Molecular and Cellular Endocrinology 443, 128–137. [DOI] [PubMed] [Google Scholar]
- Gomez-Lopez N, Hernandez-Santiago S, Lobb AP, Olson DM & Vadillo-Ortega F (2013a). Normal and premature rupture of fetal membranes at term delivery differ in regional chemotactic activity and related chemokine/cytokine production. Reproductive Sciences 20(3), 276–284. [DOI] [PubMed] [Google Scholar]
- Gomez-Lopez N, Vadillo-Perez L, Hernandez-Carbajal A, Godines-Enriquez M, Olson DM & Vadillo-Ortega F (2011a). Specific inflammatory microenvironments in the zones of the fetal membranes at term delivery. American Journal of Obstetrics and Gynecology 205(3), 235 e215–224. [DOI] [PubMed] [Google Scholar]
- Gomez-Lopez N, Vega-Sanchez R, Castillo-Castrejon M, Romero R, Cubeiro-Arreola K & Vadillo-Ortega F (2013b). Evidence for a role for the adaptive immune response in human term parturition. American Journal of Reproductive Immunology 69(3), 212–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JM, Dong Z, Romero R & Girardi G (2011b). Cervical remodeling/ripening at term and preterm delivery: the same mechanism initiated by different mediators and different effector cells. PLoS One 6(11), e26877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui T, Shimokado A, Sun Y, Akasaka T & Muragaki Y (2012). Diverse roles of macrophages in atherosclerosis: from inflammatory biology to biomarker discovery. Mediators of Inflammation 2012, 693083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad R, Tromp G, Kuivaniemi H, Chaiworapongsa T, Kim YM, Mazor M & Romero R (2006). Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. American Journal of Obstetrics and Gynecology 195(2), 394 e391–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajam YA, Rani R, Sharma P, Kumar R & Verma SK (2021). Zebrafish (danio rerio): a versatile model for reproductive biology. Recent updates in molecular Endocrinology and Reproductive Physiology of Fish: An Imperative step in Aquaculture, 105–120. [Google Scholar]
- Hamilton S, Oomomian Y, Stephen G, Shynlova O, Tower CL, Garrod A, Lye SJ & Jones RL (2012). Macrophages infiltrate the human and rat decidua during term and preterm labor: evidence that decidual inflammation precedes labor. Biology of Reproduction 86(2), 39. [DOI] [PubMed] [Google Scholar]
- Harlow CR, Rae M, Davidson L, Trackman PC & Hillier SG (2003). Lysyl oxidase gene expression and enzyme activity in the rat ovary: regulation by follicle-stimulating hormone, androgen, and transforming growth factor-β superfamily members in vitro. Endocrinology 144(1), 154–162. [DOI] [PubMed] [Google Scholar]
- Hartmann K, Stein K-P, Neyazi B & Sandalcioglu IE (2019). Aneurysm architecture: first in vivo imaging of human cerebral aneurysms with extravascular optical coherence tomography. Cerebrovascular Diseases 48(1–2), 26–31. [DOI] [PubMed] [Google Scholar]
- Hernandez-Gonzalez I, Gonzalez-Robayna I, Shimada M, Wayne CM, Ochsner SA, White L & Richards JS (2006). Gene expression profiles of cumulus cell oocyte complexes during ovulation reveal cumulus cells express neuronal and immune-related genes: does this expand their role in the ovulation process? Molecular Endocrinology 20(6), 1300–1321. [DOI] [PubMed] [Google Scholar]
- Hess KA, Chen L & Larsen WJ (1999). Inter-α-inhibitor binding to hyaluronan in the cumulus extracellular matrix is required for optimal ovulation and development of mouse oocytes. Biology of Reproduction 61(2), 436–443. [DOI] [PubMed] [Google Scholar]
- Heximer SP, Knutsen RH, Sun X, Kaltenbronn KM, Rhee M-H, Peng N, Oliveira-dos-Santos A, Penninger JM, Muslin AJ & Steinberg TH (2003). Hypertension and prolonged vasoconstrictor signaling in RGS2-deficient mice. The Journal of Clinical Investigation 111(4), 445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horihata K, Yoshioka S, Sano M, Yamamoto Y, Kimura K, Skarzynski DJ & Okuda K (2017). Expressions of lipoprotein receptors and cholesterol efflux regulatory proteins during luteolysis in bovine corpus luteum. Reproduction, Fertility and Development 29(7), 1280–1286. [DOI] [PubMed] [Google Scholar]
- Hughes CHK & Murphy BD (2021). Nuclear receptors: key regulators of somatic cell functions in the ovulatory process. Molecular Aspects of Medicine 78, 100937. [DOI] [PubMed] [Google Scholar]
- Jamous MA, Nagahiro S, Kitazato KT, Satoh K & Satomi J (2005). Vascular corrosion casts mirroring early morphological changes that lead to the formation of saccular cerebral aneurysm: an experimental study in rats. Journal of Neurosurgery 102(3), 532–535. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Pan Y, Li P, Zheng Y, Bian Y, Wang W, Wu G, Song T & Shi Y (2022). ANGPTL4 expression in ovarian granulosa cells is associated with polycystic ovary syndrome. Frontiers in Endocrinology 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce EM, Moore JJ & Sacks MS (2009). Biomechanics of the fetal membrane prior to mechanical failure: review and implications. European Journal of Obstetrics and Gynecology and Reproductive Biology 144(Suppl 1), S121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanematsu Y, Kanematsu M, Kurihara C, Tada Y, Tsou TL, van Rooijen N, Lawton MT, Young WL, Liang EI, Nuki Y & Hashimoto T (2011). Critical roles of macrophages in the formation of intracranial aneurysm. Stroke 42(1), 173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kati C, Yardan T, Akdemir HU, Duran L, Aydin K & Yilman M (2020). Pentraxin-3 level in subarachnoid haemorrhage: Is it a prognostic factor? Journal of Pakistan Medical Association 70(984). [DOI] [PubMed] [Google Scholar]
- Kawaguchi T, Nishimura S, Kanamori M, Takazawa H, Omodaka S, Sato K, Maeda N, Yokoyama Y, Midorikawa H & Sasaki T (2012). Distinctive flow pattern of wall shear stress and oscillatory shear index: similarity and dissimilarity in ruptured and unruptured cerebral aneurysm blebs. Journal of neurosurgery 117(4), 774–780. [DOI] [PubMed] [Google Scholar]
- Keedy A (2006). An overview of intracranial aneurysms. Mcgill Journal of Medicine 9(2), 141–146. [PMC free article] [PubMed] [Google Scholar]
- Kendal-Wright C, Hubbard D & Bryant-Greenwood G (2008). Chronic stretching of amniotic epithelial cells increases pre-B cell colony-enhancing factor (PBEF/visfatin) expression and protects them from apoptosis. Placenta 29(3), 255–265. [DOI] [PubMed] [Google Scholar]
- Kim J, Bagchi IC & Bagchi MK (2009). Signaling by hypoxia-inducible factors is critical for ovulation in mice. Endocrinology 150(7), 3392–3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolega J, Gao L, Mandelbaum M, Mocco J, Siddiqui AH, Natarajan SK & Meng H (2011). Cellular and molecular responses of the basilar terminus to hemodynamics during intracranial aneurysm initiation in a rabbit model. Journal of Vascular Research 48(5), 429–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kölle S, Dubielzig S, Reese S, Wehrend A, König P & Kummer W (2009). Ciliary transport, gamete interaction, and effects of the early embryo in the oviduct: ex vivo analyses using a new digital videomicroscopic system in the cow. Biology of Reproduction 81(2), 267–274. [DOI] [PubMed] [Google Scholar]
- Korja M, Lehto H & Juvela S (2014). Lifelong rupture risk of intracranial aneurysms depends on risk factors: a prospective Finnish cohort study. Stroke 45(7), 1958–1963. [DOI] [PubMed] [Google Scholar]
- Kremer PH, Koeleman BP, Rinkel GJ, Diekstra FP, van den Berg LH, Veldink JH & Klijn CJ (2016). Susceptibility loci for sporadic brain arteriovenous malformation: a replication study and meta-analysis. Journal of Neurology, Neurosurgery, and Psychiatry 87(7), 693–696. [DOI] [PubMed] [Google Scholar]
- Krischek B, Kasuya H, Tajima A, Akagawa H, Sasaki T, Yoneyama T, Ujiie H, Kubo O, Bonin M & Takakura K (2008). Network-based gene expression analysis of intracranial aneurysm tissue reveals role of antigen presenting cells. Neuroscience 154(4), 1398–1407. [DOI] [PubMed] [Google Scholar]
- Kulcsár Z, Ugron A, Marosfői M, Berentei Z, Paal G & Szikora I (2011). Hemodynamics of cerebral aneurysm initiation: the role of wall shear stress and spatial wall shear stress gradient. American Journal of Neuroradiology 32(3), 587–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D, Moore RM, Mercer BM, Mansour JM, Redline RW & Moore JJ (2016). The physiology of fetal membrane weakening and rupture: insights gained from the determination of physical properties revisited. Placenta 42, 59–73. [DOI] [PubMed] [Google Scholar]
- Kurki MI, Häkkinen S-K, Frösen J, Tulamo R, von Und Zu Fraunberg M, Wong G, Tromp G, Niemelä M, Hernesniemi J & Jääskeläinen JE (2011). Upregulated signaling pathways in ruptured human saccular intracranial aneurysm wall: an emerging regulative role of Toll-like receptor signaling and nuclear factor-κB, hypoxia-inducible factor-1A, and ETS transcription factors. Neurosurgery 68(6), 1667–1676. [DOI] [PubMed] [Google Scholar]
- Lappas M, Odumetse TL, Riley C, Reti NG, Holdsworth-Carson SJ, Rice GE & Permezel M (2008). Pre-labour fetal membranes overlying the cervix display alterations in inflammation and NF-kappaB signalling pathways. Placenta 29(12), 995–1002. [DOI] [PubMed] [Google Scholar]
- Lee-Thacker S, Jeon H, Choi Y, Taniuchi I, Takarada T, Yoneda Y, Ko C & Jo M (2020). Core Binding Factors are essential for ovulation, luteinization, and female fertility in mice. Scientific Reports 10(1), 9921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng W, Fan D, Ren Z & Li Q (2021). Identification of upregulated NF-κB inhibitor alpha and IRAK3 targeting lncRNA following intracranial aneurysm rupture-induced subarachnoid hemorrhage. BMC Neurology 21(1), 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Unlugedik E, Bocking AD, Challis JRG (2007). The role of prostaglandins in the mechanism of lipopolysaccharide-induced pro-MMP9 secretion from human placenta and fetal membrane cells. Biology of Reproduction 76(4), 654–659. [DOI] [PubMed] [Google Scholar]
- Li Z, Huang G, Chen L, Tan H, Wang Z, Liu L, Shi Y, Yin C & Wang Q (2017). Construction of transcriptional regulatory network in the intracranial aneurysms. International Journal of Clinical and Experimental Medicine 10(1), 1583–1591. [Google Scholar]
- Li Z, Peng A, Feng Y, Zhang X, Liu F, Chen C, Ye X, Qu J, Jin C, Wang M, Qiu H, Qi Y, Huang J & Yang Q (2019). Detection of T lymphocyte subsets and related functional molecules in follicular fluid of patients with polycystic ovary syndrome. Scientific Reports 9(1), 6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind AK, Weijdegård B, Dahm-Kähler P, Mölne J, Sundfeldt K & Brännström M (2006). Collagens in the human ovary and their changes in the perifollicular stroma during ovulation. Acta Obstetricia et Gynecologica Scandinavica 85(12), 1476–1484. [DOI] [PubMed] [Google Scholar]
- Lindekleiv H, Mathiesen EB, Forde OH, Wilsgaard T & Ingebrigtsen T (2015). Hospital volume and 1-year mortality after treatment of intracranial aneurysms: a study based on patient registries in Scandinavia. Journal of Neurosurgery 123(3), 631–637. [DOI] [PubMed] [Google Scholar]
- Liu C, Guo C, Wang W, Zhu P, Li W, Mi Y, Myatt L & Sun K (2016a). Inhibition of lysyl oxidase by cortisol regeneration in human amnion: implications for rupture of fetal membranes. Endocrinology 157(10), 4055–4065. [DOI] [PubMed] [Google Scholar]
- Liu C, Zhu P, Wang W, Li W, Shu Q, Chen Z-J, Myatt L & Sun K (2016b). Inhibition of lysyl oxidase by prostaglandin E2 via EP2/EP4 receptors in human amnion fibroblasts: implications for parturition. Molecular and Cellular Endocrinology 424, 118–127. [DOI] [PubMed] [Google Scholar]
- Liu DT, Brewer MS, Chen S, Hong W, Zhu Y (2017). Transcriptomic signatures for ovulation in vertebrates. General and Comparative Endocrinology 247, 74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zheng Y, An Q, Song Y & Leng B (2019). Optical coherence tomography for intracranial aneurysms: a new method for assessing the aneurysm structure. World Neurosurgery 123, e194–e201. [DOI] [PubMed] [Google Scholar]
- Lucero HA & Kagan HM (2006). Lysyl oxidase: an oxidative enzyme and effector of cell function. Cellular and Molecular Life Sciences 63(19), 2304–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussier JG, Diouf MN, Lévesque V, Sirois J & Ndiaye K (2017). Gene expression profiling of upregulated mRNAs in granulosa cells of bovine ovulatory follicles following stimulation with hCG. Reproductive Biology Endocrinology 15(1), 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons RA, Saridogan E & Djahanbakhch O (2006). The reproductive significance of human Fallopian tube cilia. Human Reproduction Update 12(4), 363–372. [DOI] [PubMed] [Google Scholar]
- Mackler AM, Iezza G, Akin MR, McMillan P & Yellon SM (1999). Macrophage trafficking in the uterus and cervix precedes parturition in the mouse. Biology of Reproduction 61(4), 879–883. [DOI] [PubMed] [Google Scholar]
- Maehara K, Kanayama N, Maradny EE, Uezato T, Fujita M & Terao T (1996). Mechanical stretching induces interleukin-8 gene expression in fetal membranes: a possible role for the initiation of human parturition. European Journal of Obstetrics & Gynecology and Reproductive Biology 70(2), 191–196. [DOI] [PubMed] [Google Scholar]
- Major CA, de Veciana M, Lewis DF & Morgan MA (1995). Preterm premature rupture of membranes and abruptio placentae: is there an association between these pregnancy complications? American Journal of Obstetrics and Gynecology 172(2), 672–676. [DOI] [PubMed] [Google Scholar]
- Malek AM, Alper SL & Izumo S (1999). Hemodynamic shear stress and its role in atherosclerosis. Journal of the American Medical Association 282(21), 2035–2042. [DOI] [PubMed] [Google Scholar]
- Mara JN, Zhou LT, Larmore M, Johnson B, Ayiku R, Amargant F, Pritchard MT & Duncan FE (2020). Ovulation and ovarian wound healing are impaired with advanced reproductive age. Aging 12(10), 9686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchese E, Vignati A, Albanese A, Nucci CG, Sabatino G, Tirpakova B, Lofrese G, Zelano G & Maira G (2010). Comparative evaluation of genome-wide gene expression profiles in ruptured and unruptured human intracranial aneurysms. Journal of Biological Regulators and Homeostatic Agents 24(2), 185–195. [PubMed] [Google Scholar]
- Marik J & Hulka J (1978). Luteinized unruptured follicle syndrome: a subtle cause of infertility. Fertility and Sterility 29(3), 270–274. [DOI] [PubMed] [Google Scholar]
- Martin GG & Talbot P (1981a). Drugs that block smooth muscle contraction inhibit in vivo ovulation in hamsters. Journal of Experimental Zoology 216(3), 483–491. [DOI] [PubMed] [Google Scholar]
- Martin GG & Talbot P (1981b). The role of follicular smooth muscle cells in hamster ovulation. Journal of Experimental Zoology 216(3), 469–482. [DOI] [PubMed] [Google Scholar]
- Martin GG & Talbot P (1987). Formation of the rupture site in preovulatory hamster follicles: morphological and morphometric analysis of thinning of the granulosa and thecal layers. Gamete Research 17(4), 303–320. [DOI] [PubMed] [Google Scholar]
- Matousek M, Carati C, Gannon B & Brannstrom M (2001). Increased intrafollicular pressure after hCG stimulation by use of a novel method for intra-follicular pressure measurements in the rat ovary. Biology of Reproduction 64, 158–158. [DOI] [PubMed] [Google Scholar]
- McLaren J, Taylor DJ & Bell SC (1999). Increased incidence of apoptosis in non-labour-affected cytotrophoblast cells in term fetal membranes overlying the cervix. Human Reproduction 14(11), 2895–2900. [DOI] [PubMed] [Google Scholar]
- Medero R, Ruedinger K, Rutkowski D, Johnson K & Roldan-Alzate A (2020). In vitro assessment of flow variability in an intracranial aneurysm model using 4D flow MRI and tomographic PIV. Annals of Biomedical Engineering 48(10), 2484–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng H, Metaxa E, Gao L, Liaw N, Natarajan SK, Swartz DD, Siddiqui AH, Kolega J & Mocco J (2011). Progressive aneurysm development following hemodynamic insult. Journal of Neurosurgery 114(4), 1095–1103. [DOI] [PubMed] [Google Scholar]
- Meng H, Tutino V, Xiang J & Siddiqui A (2014). High WSS or low WSS? Complex interactions of hemodynamics with intracranial aneurysm initiation, growth, and rupture: toward a unifying hypothesis. American Journal of Neuroradiology 35(7), 1254–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng L, Rijntjes E, Swarts H, Bunschoten A, van der Stelt I, Keijer J & Teerds K (2016). Dietary-induced chronic hypothyroidism negatively affects rat follicular development and ovulation rate and is associated with oxidative stress. Biology of Reproduction 94(4). [DOI] [PubMed] [Google Scholar]
- Menon R & Richardson LS (2017). Preterm prelabor rupture of the membranes: a disease of the fetal membranes. Seminars in Perinatology 41(7), 409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen HE, Rayburn H & Krieger M (2001). Abnormal lipoprotein metabolism and reversible female infertility in HDL receptor (SR-BI)–deficient mice. The Journal of Clinical Investigation 108(11), 1717–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhak B, Weinsheimer S, Pawlikowska L, Poon A, Kwok P-Y, Lawton MT, Chen Y, Zaroff JG, Sidney S & McCulloch CE (2011). Angiopoietin-like 4 (ANGPTL4) gene polymorphisms and risk of brain arteriovenous malformations. Cerebrovascular Diseases 31(4), 338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikuni M, Pall M, Peterson CM, Peterson CA, Hellberg P, Brännström M, Richards JS & Hedin L (1998). The selective prostaglandin endoperoxide synthase-2 inhibitor, NS-398, reduces prostaglandin production and ovulation in vivo and in vitro in the rat1. Biology of Reproduction 59(5), 1077–1083. [DOI] [PubMed] [Google Scholar]
- Minchenko O, Opentanova I, Minchenko D, Ogura T, Esumi H (2004). Hypoxia induces transcription of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-4 gene via hypoxia-inducible factor-1α activation. FEBS Letters 576(1–2), 14–20. [DOI] [PubMed] [Google Scholar]
- Mittal P, Romero R, Tarca AL, Gonzalez J, Draghici S, Xu Y, Dong Z, Nhan-Chang C-L, Chaiworapongsa T, Lye S, Kusanovic JP, Lipovich L, Mazaki-Tovi S, Hassan SS, Mesiano S & Kim CJ (2010). Characterization of the myometrial transcriptome and biological pathways of spontaneous human labor at term. Journal of Perinatal Medicine 38(6), 617–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montenegro D, Romero R, Kim SS, Tarca AL, Draghici S, Kusanovic JP, Kim JS, Lee DC, Erez O, Gotsch F, Hassan SS & Kim CJ (2009). Expression patterns of microRNAs in the chorioamniotic membranes: a role for microRNAs in human pregnancy and parturition. Journal of Pathology 217(1), 113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RM, Redline RW, Kumar D, Mercer BM, Mansour JM, Yohannes E, Novak JB, Chance MR & Moore JJ (2009). Differential expression of fibulin family proteins in the para-cervical weak zone and other areas of human fetal membranes. Placenta 30(4), 335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa H, Okamura H, Takenaka A, Morimoto K & Nishimura T (1981). Histamine concentration and its effect on ovarian contractility in humans. International Journal of Fertility 26(4), 283–286. [PubMed] [Google Scholar]
- Nehls DG, Flom RA, Carter LP & Spetzler RF (1985). Multiple intracranial aneurysms: determining the site of rupture. Journal of Neurosurgery 63(3), 342–348. [DOI] [PubMed] [Google Scholar]
- Nhan-Chang C-L, Romero R, Tarca AL, Mittal P, Kusanovic JP, Erez O, Mazaki-Tovi S, Chaiworapongsa T, Hotra J & Than NG (2010). Characterization of the transcriptome of chorioamniotic membranes at the site of rupture in spontaneous labor at term. American Journal of Obstetrics and Gynecology 202(5), e461–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkamp DJ, Setz LE, Algra A, Linn FHH, de Rooij NK & Rinkel GJE (2009). Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta-analysis. The Lancet Neurology 8(7), 635–642. [DOI] [PubMed] [Google Scholar]
- Niswender GD, Juengel JL, Silva PJ, Rollyson MK & McIntush EW (2000). Mechanisms controlling the function and life span of the corpus luteum. Physiological reviews 80(1), 1–29. [DOI] [PubMed] [Google Scholar]
- O’Brien CM, Herington JL, Brown N, Pence IJ, Paria BC, Slaughter JC, Reese J & Mahadevan-Jansen A (2017). In vivo Raman spectral analysis of impaired cervical remodeling in a mouse model of delayed parturition. Scientific Reports 7(1), 6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollikainen E, Tulamo R, Lehti S, Lee-Rueckert M, Hernesniemi J, Niemelä M, Ylä-Herttuala S, Kovanen PT & Frösen J (2016). Smooth muscle cell foam cell formation, apolipoproteins, and ABCA1 in intracranial aneurysms: implications for lipid accumulation as a promoter of aneurysm wall rupture. Journal of Neuropathology & Experimental Neurology 75(7), 689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paciolla M, Boni R, Fusco F, Pescatore A, Poeta L, Ursini MV, Lioi MB & Miano MG (2011). Nuclear factor-kappa-B-inhibitor alpha (NFKBIA) is a developmental marker of NF-κB/p65 activation during in vitro oocyte maturation and early embryogenesis. Human Reproduction 26(5), 1191–1201. [DOI] [PubMed] [Google Scholar]
- Pan H, Zhu L, Deng Y & Pollard JW (2006). Microarray analysis of uterine epithelial gene expression during the implantation window in the mouse. Endocrinology 147(10), 4904–4916. [DOI] [PubMed] [Google Scholar]
- Pandey V, Jaremko K, Moore RM, Mercer BM, Stetzer B, Kumar D, Fox JM, Mansour JM & Moore JJ (2007). The force required to rupture fetal membranes paradoxically increases with acute in vitro repeated stretching. American Journal of Obstetrics and Gynecology 196(2), e161–167. [DOI] [PubMed] [Google Scholar]
- Papaioannou TG & Stefanadis C (2005). Vascular wall shear stress: basic principles and methods. Hellenic Journal of Cardiology 46(1), 9–15. [PubMed] [Google Scholar]
- Park CJ, Lin P-C, Zhou S, Barakat R, Bashir ST, Choi JM, Cacioppo JA, Oakley OR, Duffy DM, Lydon JP & Ko CJ (2020). Progesterone receptor serves the ovary as a trigger of ovulation and a terminator of inflammation. Cell Reports 31(2), 107496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne KJ, Clyde LA, Weldon AJ, Milford TA & Yellon SM (2012). Residency and activation of myeloid cells during remodeling of the prepartum murine cervix. Biology of Reproduction 87(5), 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pera J, Korostynski M, Krzyszkowski T, Czopek J, Slowik A, Dziedzic T, Piechota M, Stachura K, Moskala M & Przewlocki R (2010). Gene expression profiles in human ruptured and unruptured intracranial aneurysms: what is the role of inflammation? Stroke 41(2), 224–231. [DOI] [PubMed] [Google Scholar]
- Pereyra S, Sosa C, Bertoni B & Sapiro R (2019). Transcriptomic analysis of fetal membranes reveals pathways involved in preterm birth. BMC Medical Genomics 12(1), 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant TM, Zeleznik AJ & Albertini DF (2015). Knobil and Neill’s Physiology of Reproduction (Fourth edition. ed.). Elsevier/Academic Press. [Google Scholar]
- Polettini J, Silva MG, Kacerovsky M, Syed TA, Saade GR & Menon R (2016). Screening of lysyl oxidase (LOX) and lysyl oxidase like (LOXL) enzyme expression and activity in preterm prelabor rupture of fetal membranes. Journal of Perinatal Medicine 44(1), 99–109. [DOI] [PubMed] [Google Scholar]
- Poulsen LC, Botkjaer JA, Ostrup O, Petersen KB, Andersen CY, Grondahl ML & Englund ALM (2020). Two waves of transcriptomic changes in periovulatory human granulosa cells. Human Reproduction 35(5), 1230–1245. [DOI] [PubMed] [Google Scholar]
- Puttabyatappa M, VandeVoort CA & Chaffin CL (2010). hCG-Induced down-regulation of PPARγ and liver x receptors promotes periovulatory progesterone synthesis by macaque granulosa cells. Endocrinology 151(12), 5865–5872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Real E, Eguizabal A, Pontón A, Díez MC, Val-Bernal JF, Mayorga M, Revuelta JM, López-Higuera JM & Conde OM (2013). Optical coherence tomography assessment of vessel wall degradation in thoracic aortic aneurysms. Journal of Biomedical Optics 18(12), 126003. [DOI] [PubMed] [Google Scholar]
- Ren S, Chen Y, Wang L & Wu G (2022). Neuronal ferroptosis after intracerebral hemorrhage. Frontiers in Molecular Biosciences 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JS (1997). Editorial: sounding the alarm—does induction of prostaglandin endoperoxide synthase-2 control the mammalian ovulatory clock?. Endocrinology 138(10), 4047–4048. [DOI] [PubMed] [Google Scholar]
- Richards JS (2005). Ovulation: New factors that prepare the oocyte for fertilization. Molecular and Cellular Endocrinology 234(1), 75–79. [DOI] [PubMed] [Google Scholar]
- Richards JS, Liu Z & Shimada M (2008). Immune-like mechanisms in ovulation. Trends in Endocrinology & Metabolism 19(6), 191–196. [DOI] [PubMed] [Google Scholar]
- Richards JS, Russell DL, Robker RL, Dajee M & Alliston TN (1998). Molecular mechanisms of ovulation and luteinization. Molecular and Cellular Endocrinology 145(1–2), 47–54. [DOI] [PubMed] [Google Scholar]
- Rimon-Dahari N, Yerushalmi-Heinemann L, Alyagor L & Dekel N (2016). Ovarian folliculogenesis. Molecular Mechanisms of Cell Differentiation in Gonad Development, 167–190. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ & Irving-Rodgers HF (2010). Formation of the ovarian follicular antrum and follicular fluid. Biology of Reproduction 82(6), 1021–1029. [DOI] [PubMed] [Google Scholar]
- Rondell P (1964). Follicular pressure and distensibility in ovulation. American Journal of Physiology 207, 590–594. [DOI] [PubMed] [Google Scholar]
- Ross R (1999). Atherosclerosis--an inflammatory disease. New England Journal of Medicine 340(2), 115–126. [DOI] [PubMed] [Google Scholar]
- Rousset S, Alves-Guerra M-C, Ouadghiri-Bencherif S, Kozak LP, Miroux B, Richard D, Bouillaud F, Ricquier D & Cassard-Doulcier A-M (2003). Uncoupling protein 2, but not uncoupling protein 1, is expressed in the female mouse reproductive tract. Journal of Biological Chemistry 278(46), 45843–45847. [DOI] [PubMed] [Google Scholar]
- Russell DL & Robker RL (2007). Molecular mechanisms of ovulation: co-ordination through the cumulus complex. Human Reproduction Update 13(3), 289–312. [DOI] [PubMed] [Google Scholar]
- Salustri A, Garlanda C, Hirsch E, De Acetis M, Maccagno A, Bottazzi B, Doni A, Bastone A, Mantovani G, Beck Peccoz P, Salvatori G, Mahoney DJ, Day AJ, Siracusa G, Romani L & Mantovani A (2004). PTX3 plays a key role in the organization of the cumulus oophorus extracellular matrix and in in vivo fertilization. Development 131(7), 1577–1586. [DOI] [PubMed] [Google Scholar]
- Santoriello C & Zon LI (2012). Hooked! Modeling human disease in zebrafish. The Journal of Clinical Investigation 122(7), 2337–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savastano LE, Bhambri A, Wilkinson DA & Pandey AS (2018). Biology of cerebral aneurysm formation, growth, and rupture. Intracranial Aneurysms, 17–32. [Google Scholar]
- Sayasith K, Sirois J & Lussier JG (2014). Expression and regulation of regulator of g-protein signaling protein-2 (RGS2) in equine and bovine follicles prior to ovulation: molecular characterization of RGS2 transactivation in bovine granulosa cells. Biology of Reproduction 91(6). [DOI] [PubMed] [Google Scholar]
- Schmidt G, Kannisto P & Owman C (1990). Histaminergic effects on the isolated rat ovarian artery during the estrous cycle. Biology of Reproduction 42(5–6), 762–768. [DOI] [PubMed] [Google Scholar]
- Schmidt G, Kannisto P, Owman C & Sjöberg n. o. (1988). Is serotonin involved in the ovulatory process of the rat ovary perfused in vitro? Acta Physiologica Scandinavica 132(2), 251–256. [DOI] [PubMed] [Google Scholar]
- Schmidt G, Kannisto P, Owman C & Walles B (1987). Characterization of histamine receptors mediating contraction and relaxation of the ovarian follicle wall. International Journal of Fertility 32(5), 399–406. [PubMed] [Google Scholar]
- Schroeder PC & Talbot P (1982). Intrafollicular pressure decreases in hamster preovulatory follicles during smooth muscle cell contraction in vitro. Journal of Experimental Zoology 224(3), 417–426. [DOI] [PubMed] [Google Scholar]
- Shan D, Guo X, Yang G, He Z, Zhao R, Xue H & Li G (2021). Integrated transcriptional profiling analysis and immune-related risk model construction for intracranial aneurysm rupture. Frontiers in Neuroscience 15, 613329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C, Awad IA, Jafari N, Lin S, Du P, Hage ZA, Shenkar R, Getch CC, Bredel M & Batjer HH (2009). Genomics of human intracranial aneurysm wall. Stroke 40(4), 1252–1261. [DOI] [PubMed] [Google Scholar]
- Shimogonya Y, Ishikawa T, Imai Y, Matsuki N & Yamaguchi T (2009). Can temporal fluctuation in spatial wall shear stress gradient initiate a cerebral aneurysm? A proposed novel hemodynamic index, the gradient oscillatory number (GON). Journal of Biomechanics 42(4), 550–554. [DOI] [PubMed] [Google Scholar]
- Shojima M, Oshima M, Takagi K, Torii R, Hayakawa M, Katada K, Morita A & Kirino T (2004). Magnitude and role of wall shear stress on cerebral aneurysm: computational fluid dynamic study of 20 middle cerebral artery aneurysms. Stroke 35(11), 2500–2505. [DOI] [PubMed] [Google Scholar]
- Shynlova O, Nedd-Roderique T, Li Y, Dorogin A & Lye SJ (2013). Myometrial immune cells contribute to term parturition, preterm labour and post-partum involution in mice. Journal of Cell and Molecular Medicine 17(1), 90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegler Y, Weiner Z & Solt I (2020). ACOG practice bulletin No. 217: prelabor rupture of membranes. Obstetrics & Gynecology 136(5), 1061. [DOI] [PubMed] [Google Scholar]
- Skory RM, Xu Y, Shea LD & Woodruff TK (2015). Engineering the ovarian cycle using in vitro follicle culture. Human Reproduction 30(6), 1386–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slee RB, Hillier SG, Largue P, Harlow CR, Miele G & Clinton M (2001). Differentiation-dependent expression of connective tissue growth factor and lysyl oxidase messenger ribonucleic acids in rat granulosa cells. Endocrinology, 142(3), 1082–1089. [DOI] [PubMed] [Google Scholar]
- Smith KM, Dinh DT, Akison LK, Nicholls M, Dunning KR, Morimoto A, Lydon JP, Russell DL & Robker RL (2022). Intraovarian, Isoform-Specific Transcriptional Roles of Progesterone Receptor in Ovulation. Cells 11(9), 1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RP, Turek PJ, Netter FH & Machado CAG (2011). The Netter Collection of Medical Illustrations. Volume 1, Reproductive System (2nd edition. ed.). Elsevier. [Google Scholar]
- Sogn JH, Curry TE Jr., Brännström M, Lemaire WJ, Koos RD, Papkoff H & Janson PO (1987). Inhibition of follicle-stimulating hormone-induced ovulation by indomethacin in the perfused rat ovary. Biology of Reproduction 36(3), 536–542. [DOI] [PubMed] [Google Scholar]
- Sriraman V, Eichenlaub-Ritter U, Bartsch JW, Rittger A, Mulders SM & Richards JS (2008). Regulated expression of ADAM8 (a Disintegrin and Metalloprotease Domain 8) in the mouse ovary: evidence for a regulatory role of luteinizing hormone, progesterone receptor, and epidermal growth factor-like growth factors. Biology of Reproduction 78(6), 1038–1048. [DOI] [PubMed] [Google Scholar]
- Stocco C, Telleria C & Gibori G (2007). The molecular control of corpus luteum formation, function, and regression. Endocrine Reviews 28(1), 117–149. [DOI] [PubMed] [Google Scholar]
- Sumbul HE, Avci BS, Bankir M, Pekoz BC, Gulumsek E & Koc AS (2022). Ovarian stiffness is significantly increased in polycystic ovary syndrome and related with anti-mullerian hormone: a point shear wave elastography study. Ultrasound Quarterly 38(1), 83–88. [DOI] [PubMed] [Google Scholar]
- Synowiec E, Wojcik KA, Wójcik R, Wiśniewski K, Fila M, Tokarz P, Bieśkowski M, Jaskolski D & Blasiak J (2016). Expression and variability of lipid metabolism genes in intracranial aneurysm. Cellular and Molecular Biology 62(4), 73–82. [PubMed] [Google Scholar]
- Szukiewicz D, Pyzlak M, Klimkiewicz J, Szewczyk G & Maslinska D (2007). Mast cell-derived interleukin-8 may be involved in the ovarian mechanisms of follicle growth and ovulation. Inflammation Research 56, S35–36. [DOI] [PubMed] [Google Scholar]
- Takahashi E, Okuno T, Fukii H, Makino S, Takahashi M, Ohba M, Saeki K, Itakura A, Takeda S, Yokomizo T (2021). Up-regulation of cytosolic prostaglandin E synthase in fetal-membrane and amniotic prostaglandin E2 accumulation in labor. PLOS One 16(4), e0250638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura M, Itoh H, Sagawa N, Yura S, Korita D, Kakui K, Kawamura M, Hirota N, Maeda H & Fujii S (2005). Cyclic mechanical stretch augments hyaluronan production in cultured human uterine cervical fibroblast cells. Molecular Human Reproduction 11(9), 659–665. [DOI] [PubMed] [Google Scholar]
- Talbot P & Chacon RS (1982). In vitro ovulation of hamster oocytes depends on contraction of follicular smooth muscle cells. Journal of Experimental Zoology 224(3), 409–415. [DOI] [PubMed] [Google Scholar]
- Talbot P, Geiske C & Knoll M (1999). Oocyte pickup by the mammalian oviduct. Molecular Biology of the Cell 10(1), 5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M, Wang G, Lu P, Karas RH, Aronovitz M, Heximer SP, Kaltenbronn KM, Blumer KJ, Siderovski DP, Zhu Y & Mendelsohn ME (2003). Regulator of G-protein signaling-2 mediates vascular smooth muscle relaxation and blood pressure. Nature Medicine 9(12), 1506–1512. [DOI] [PubMed] [Google Scholar]
- Terranova P, Uilenbroek JTJ, Saville L, Horst D & Nakamura Y (1990). Serotonin enhances oestradiol production by hamster preovulatory follicles in vitro: effects of experimentally induced atresia. Journal of Endocrinology 125(3), 433–438. [DOI] [PubMed] [Google Scholar]
- Timmons B, Akins M & Mahendroo M (2010). Cervical remodeling during pregnancy and parturition. Trends in Endocrinology and Metabolism 21(6), 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujioka T, Russell DL, Okamura H, Richards JS & Espey LL (2000). Expression of regulator of G-protein signaling protein-2 gene in the rat ovary at the time of ovulation. Biology of Reproduction 63(5), 1513–1517. [DOI] [PubMed] [Google Scholar]
- Umehara T, Winstanley YE, Andreas E, Morimoto A, Williams EJ, Smith KM, Carroll J, Febbraio MA, Shimada M & Russell DL (2022). Female reproductive life span is extended by targeted removal of fibrotic collagen from the mouse ovary. Science Advances 8(24). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezu K & Larina IV (2022). Optical coherence tomography for dynamic investigation of mammalian reproductive processes. Molecular Reproduction and Development 90, 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezu K, Wang S & Larina IV (2022). Dynamic volumetric imaging of ovulated egg transport to the fallopian tube with optical coherence tomography. Dynamics and Fluctuations in Biomedical Photonics XIX PC119590H. [Google Scholar]
- Varani S, Elvin JA, Yan C, DeMayo J, DeMayo FJ, Horton HF, Byrne MC & Matzuk MM (2002). Knockout of pentraxin 3, a downstream target of growth differentiation factor-9, causes female subfertility. Molecular Endocrinology 16(6), 1154–1167. [DOI] [PubMed] [Google Scholar]
- Vivas A, Mikhal J, Ong GM, Eigenbrodt A, van der Meer AD, Aquarius R, Geurts BJ & Boogaarts HD (2022). Aneurysm-on-a-chip: setting flow parameters for microfluidic endothelial cultures based on computational fluid dynamics modeling of intracranial aneurysms. Brain Sciences 12(5), 603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach EE, Wright KH & Hamada Y (1978). Investigation of mammalian ovulation with an in vitro perfused rabbit ovary preparation. American Journal of Obstetrics and Gynecology 132(7), 728–738. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhang H, Yang P, Lu C, Liu Y, Zhang Y, Xie Y, Xu Z & Hu J (2022). Identification of the key ferroptosis-related genes in the pathogenesis of thoracic aortic dissection and thoracic aortic aneurysm using integrated bioinformatic analysis and experimental verification. Preprint from Research Square. [Google Scholar]
- Wang Z, Kolega J, Hoi Y, Gao L, Swartz DD, Levy EI, Mocco J & Meng H (2009). Molecular alterations associated with aneurysmal remodeling are localized in the high hemodynamic stress region of a created carotid bifurcation. Neurosurgery 65(1), 169–177; discussion 177–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters TP & Mercer BM (2009). The management of preterm premature rupture of the membranes near the limit of fetal viability. American Journal of Obstetrics and Gynecology 201(3), 230–240. [DOI] [PubMed] [Google Scholar]
- Weiner CP, Mason CW, Dong Y, Buhimschi IA, Swaan PW & Buhimschi CS (2010). Human effector/initiator gene sets that regulate myometrial contractility during term and preterm labor. American Journal of Obstetrics and Gynecology 202(5), e471–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebers DO (2003). Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. The Lancet 362(9378), 103–110. [DOI] [PubMed] [Google Scholar]
- Wong WYL & Richards JS (1991). Evidence for two antigenically distinct molecular weight variants of prostaglandin H synthase in the rat ovary. Molecular Endocrinology 5(9), 1269–1279. [DOI] [PubMed] [Google Scholar]
- Xu Z, Li H, Song J, Han B, Wang Z, Cao Y, Wang S & Zhao J (2017). Meta-analysis of microarray-based expression profiles to identify differentially expressed genes in intracranial aneurysms. World Neurosurgery 97, 661–668. [DOI] [PubMed] [Google Scholar]
- Yan Z, Wu Q, Cai W, Xiang H, Wen L, Zhang A, Peng Y, Zhang X & Wang H (2021). Identifying critical genes associated with aneurysmal subarachnoid hemorrhage by weighted gene co-expression network analysis. Aging 13(18), 22345–22360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K-L, Zhang X-L, Tan X-M, Ji M-M, Chen Y, Liu M-M, Yu Z-L & Zhu Y-Q (2019). Distinctive genes involved in steroidogenesis associated with follicular abnormal development in polycystic ovary syndrome model. Reproductive and Developmental Medicine 03(03), 141–147, C142–C143. [Google Scholar]
- Zanier ER, Brandi G, Peri G, Longhi L, Zoerle T, Tettamanti M, Garlanda C, Sigurta A, Valaperta S, Mantovani A, De Simoni MG & Stocchetti N (2011). Cerebrospinal fluid pentraxin 3 early after subarachnoid hemorrhage is associated with vasospasm. Intensive Care Medicine 37(2), 302–309. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Jing L, Zhang Y, Liu J & Yang X (2016). Low wall shear stress is associated with the rupture of intracranial aneurysm with known rupture point: case report and literature review. BMC Neurology 16(1), 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong A, Ding N, Zhou Y, Yang G, Peng Z, Zhang H & Chai X (2021). Identification of hub genes associated with the pathogenesis of intracranial aneurysm via integrated bioinformatics analysis. International Journal of General Medicine 14, 4039–4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Peng P, Zhang M, Han D, Yang H, Yan X & Hu S (2022). Effect of S-nitrosylation of RIP3 induced by cerebral ischemia on its downstream signaling pathway. Journal of Stroke and Cerebrovascular Diseases 31(7), 106516. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Chai H, Hu Y, Liu R, Jiang H, Fan R, Chen W, Chen X & Huang F (2022). Overexpressed DDX3x promotes abdominal aortic aneurysm formation and activates AKT in ApoE knockout mice. Biochemical and Biophysical Research Communications 634, 138–144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Complete list of overlapping upregulated and downregulated genes in comparisons between two ovulation data sets and one data set for intracranial aneurysm (ICA).
Complete list of overlapping upregulated and downregulated genes in comparisons between the intracranial aneurysm (ICA) and chorioamniotic membrane rupture (CMR) data sets.
Complete list of overlapping upregulated and downregulated genes in comparisons between two ovulation data sets and one data set for chorioamniotic membrane rupture (CMR).


