Abstract
INTRODUCTION:
Examining motor and cognitive decline in separate models may underestimate their associations.
METHODS:
In a single trivariate model we examined the levels and rates of decline of three phenotypes, sensor-derived total daily physical activity, motor abilities and cognition in 1007 older adults during 6 years of follow-up. In 477 decedents, we repeated the model adding fixed terms for indices of nine brain pathologies.
RESULTS:
Simultaneous rates of decline of all three phenotypes showed the strongest correlations with shared variance of up to 50%. Brain pathologies explained about 3% of the variance of declining daily physical activity, 9% of declining motor abilities and 42% of cognitive decline.
DISCUSSION:
The rates of declining cognitive and motor phenotypes are strongly correlated and measures of brain pathologies account for only a small minority of their decline. Further work is needed to elucidate the biology underlying correlated cognitive and motor decline in aging adults.
Keywords: Cognition, physical activity, motor abilities, aging, neuropathology
1. BACKGROUND
Daily physical activity as well as motor and cognitive abilities decline in most older adults.[1–3] Prior studies have generally focused on the association of baseline level of daily physical activity with longitudinal changes in either cognitive or motor abilities, but some have examined dual decline. [4–7] Accumulating evidence suggests that both lower levels and more rapid cognitive and motor decline increase the risk of dementia.[6] Yet, prior modeling has usually compared the rates of change of motor function and cognition derived from separate models.[6, 7] This approach assumes that the observed cognitive and motor decline occur independently and may therefore underestimate their actual associations.[8]
Motor function is controlled by dissociable neural control systems which begin in the brain and extend to muscles in the periphery, the final effector of all movement.[9] Decisions to initiate volitional behavior are distinct from an individual’s motor abilities. Thus, an individual with poor motor abilities may nonetheless manifest a higher quantity of total daily physical activity compared to an individual with good motor abilities, who is sedentary, electing to sit on a couch for the entire day. This dissociation between motor abilities and daily physical activity highlights the importance of examining both phenotypes as their associations with cognition may vary. Yet, we are unaware of any studies that have examined the extent that level and person-specific rates of change of all three phenotypes in a single model.
The associations between worsening cognitive ability, motor ability, and daily physical activity may not be causal if these phenotypes share common risk factors or underlying pathologies. There is increasing recognition that the negative effects of accumulating Alzheimer’s disease and Alzheimer’s disease related dementia (ADRD) brain pathologies in aging brains can affect not only cognition but diverse aging phenotypes including motor phenotypes.[10] This underscores the importance of determining to what extent ADRD pathologies, accumulating in aging brains, may account for the correlated decline of cognitive and motor phenotypes.
This study used data from 1007 older persons participating in the Rush Memory and Aging Project, a community-based longitudinal cohort study that collects all three phenotypes and obtains autopsy in decedents to collect indices of ADRD pathologies.[11] The current analysis employed a single analytic framework that incorporated repeated clinical observations of all three phenotypes to estimate the correlation of their levels and rates of change.[12] The correlated decline between these phenotypes may not be causal, but may reflect the negative effects of a common underlying pathologic basis. So, in further analysis, we repeated our analyses in the subset of decedents who underwent brain autopsy to examine to what extent markers of ADRD pathologies are associated with the levels and rates of change of each of the three phenotypes. Finally, we examined if the person-specific rates of change (slopes) of these phenotypes derived from a single model were differentially associated with the probability of common adverse health outcomes during this study.
2. METHODS
2.1. Participants
Participants are from the Rush Memory and Aging Project who agreed to annual clinical exams and autopsy at the time of death. The study was approved by the IRB of Rush University Medical Center. Written in-formed consent was obtained as well as uniform anatomical gift act. The study was conducted in accordance with the latest version of the Declaration of Helsinki. While the study began enrolling participants in 1997, actigraphy was not added until 2005. [11] Participation in the annual follow-up evaluations exceeds 90% of survivors and the autopsy rate exceeds 80%.
In these analyses, the analytic baseline is the first cycle in which all three phenotypes were available. At the time of these analyses, of 2,258 participants recruited since actigraphy was added, 20 had not completed baseline testing and 840 did not have actigraphy testing. To enable longitudinal analyses, we excluded 315 of 1,398 who had completed initial actigraphy testing, but did not have a follow-up testing session. An additional 76 individuals were excluded as they did not have 2 or more follow-up exams of cognition and/or motor abilities leaving 1,007 for analyses in the current study. This is illustrated in the consort diagram included as Figure e1).
2.2. Assessment of Total Daily Physical Activity
All movement was measured 24hours/day for up to 10 days with an activity monitor worn on the non-dominant wrist which recorded average activity counts every 15s (Actical®; Mini Mitter, Bend, OR). Total daily physical activity was the average of sums of all daily activity counts.[4]
2.3. Assessment of Motor Abilities
Ten motor performances were assessed. These included grip and pinch strength. Dexterity of the arms was based on the number of pegs placed in the Purdue Pegboard in thirty seconds and finger tapping for ten seconds. We measured the time and number of steps taken to walk eight feet and turn 360°. Participants stood on each leg for ten seconds to assess balance. Then they were requested to stand on their toes for ten seconds and the time standing was recorded. These measures were scaled and averaged to obtain a summary global motor score as previously described. [2, 3]
2.4. Assessment of Cognition and Clinical Diagnoses
An annual uniform structured clinical evaluation includes medical history, neurologic examination, and neuropsychological performance tests. [11] Nineteen cognitive tests were assessed and scores from nineteen tests were used to create a composite measure of global cognitive function to minimize floor and ceiling effects.[13] Cognitive diagnoses were made in a three-step process. Cognitive testing was scored by a computer program and the results were reviewed by a neuropsychologist to diagnose cognitive impairment. Then participants were evaluated by a physician who used all available cognitive and clinical data to classify cognitive status. [13] Individuals with cognitive impairment who did not meet dementia criteria were diagnosed with mild cognitive impairment (MCI). Individuals without dementia or MCI were classified as having no cognitive impairment (NCI). [13]
2.5. Assessment of Comorbidities and Other Covariates
Sex and years of education were recorded at the baseline interview. Age in years was computed from self-reported date of birth and clinical evaluation date. A previously validated categorical measure of parkinsonism was constructed based on the number of the four parkinsonian signs present based on the exam of 26 items from a modified UPDRS assessment.[14] A parkinsonian sign was present if two or more of its items were scored as a mild or more severe abnormality. Parkinsonism was present if at least two of the four parkinsonian signs were present.[14] Mobility disability was assessed using the Rosow-Breslau scale, which assesses three activities: walking up and down a flight of stairs, walking a half mile, and doing heavy housework like washing windows, walls, or floors. [15]
2.6. Assessment of Brain Pathologies
Brain removal, tissue sectioning and preservation, and a uniform gross and microscopic exam with quantification of post-mortem indices followed a standard protocol as detailed in a prior publication. [2] We collected indices of four neurodegenerative and five cerebrovascular pathologies.
Neurodegenerative pathologies
PD pathology:
Nigral neuronal loss -Dissection of diagnostic blocks included a hemisection of midbrain including substantia nigra. Nigral neuronal loss was assessed in the substantia nigra in the mid to rostral midbrain near or at the exit of the 3rd nerve using hematoxylin and eosin (H&E) stain and 6 micron sections using a semi-quantitative scale (none, mild, moderate, severe).[16] Seven regions (substantia nigra, amygdala, anterior cingulate cortex, entorhinal cortex, midfrontal cortex, superior or middle temporal cortex, inferior parietal cortex) were assessed for Lewy bodies using α-synuclein immunostaining as previously described.[16] The presence of PD pathology was based on the presence of Lewy bodies in any of the seven regions examined with moderate-severe nigral neuronal loss as described in prior publications.[17]
Alzheimer’s Disease Pathology:
A modified Bielschowsky silver stain was used to visualize neuritic plaques, diffuse plaques, and neurofibrillary tangles in five cortical areas (hippocampus, entorhinal, midfrontal, middle temporal, and inferior parietal). Neuritic and diffuse plaques, and neurofibrillary tangles were counted in the region that appeared to have the maximum density of each pathology as previously described. A standardized score was created for each neuropathology in each region by dividing the raw count by the standard deviation of the mean for the same neuropathology in the same region. These measures were averaged to create a continuous composite measure of AD pathology as previously described.[13] We dichotomized the presence of pathologic AD based on NIA-AA. [18]
TDP-43:
Immunohistochemistry was used to identify the presence of TDP-43 pathology in four brain regions (amygdala, entorhinal cortex, hippocampus CA1 and subiculum and the dentate nucleus) and four neocortical areas (anterior temporal pole (ATPC), midtemporal cortex, orbitofrontal and midfrontal cortex). TDP-43 distribution was grouped in to three stages and staging was based on (1:localized to amygdala only, 2: extended to other limbic regions, and 3: extended to neocortical regions).[19, 20] The presence of TDP-43 pathology was based on extension beyond the amygdala.
Hippocampal Sclerosis:
The presence of hippocampal sclerosis was identified in a coronal section of the mid-hippocampus at the level of the lateral geniculate body by severe neuronal loss and gliosis on H&E-stained sections in CA1 or subiculum[21].
Cerebrovascular pathologies
Macroinfarcts:
Gross examination documented the number of visualized macroinfarcts (macroscopic or gross infarcts). Each gross infarct was then confirmed on microscopic examination and classified by age. Only chronic infarcts were included in the analyses as dichotomous variables.
Microinfarcts:
Microscopic examination also allowed for the identification of microinfarcts, which were, by definition, not visible to the naked eye and identified only under microscopy. Blocks from a minimum of nine regions including midfrontal, middle temporal, entorhinal, hippocampal and inferior parietal cortices, anterior cingulate, thalamus, basal ganglia and midbrain, were paraffin embedded, cut, mounted on slides and stained with H&E for the purpose of documentation of presence and number of microinfarcts. Location and age of microinfarcts were recorded. For analyses in this study, only chronic infarcts were considered and infarcts were classified as present or absent.[22].
Atherosclerosis was based on visual inspection of the large vessels at the Circle of Willis at the base of the brain including: vertebral, basilar, posterior cerebral, middle cerebral, and anterior cerebral arteries and their proximal branches. Severity was graded with a semi-quantitative scale from 0–3 (none, mild, moderate or severe) For descriptive purposes atherosclerosis was present if severity was moderate or severe.
Arteriolosclerosis was assessed in the basal ganglia. We evaluated the vessels of the anterior basal ganglia with a semiquantitative grading system from (0–3 none, mild, moderate or severe as described previously. [23] For descriptives purposes arteriolosclerosis was present if moderate or severe changes were observed.
Cerebral amyloid angiopathy was assessed in sections from 4 neocortical regions (i.e. mid frontal, mid temporal, angular and calcarine) were immunostained for β-amyloid (4G8; 1:9000, Covance Labs, Madison, WI, USA; 6F/3DDako; 1:50, North America Inc., Carpinteria, CA, USA; and 10D5; 1:600, Elan Pharmaceuticals, San Francisco, CA, USA). Parenchymal and meningeal vessels were assessed separately for β-amyloid deposition. Severity was assessed with a 4-level semiquantitative measure, rated as none, mild, moderate, and severe. CAA was present if moderate or severe amyloid angiopathy were identified[24].
2.7. Statistical Analyses
Initial review of the data suggested that measures of total daily physical activity required logarithm transformation prior to these analyses. The parent study design employed rolling admissions. So, our primary analysis aligned data from all the participants at the analytic baseline, i.e., the first cycle at which all three phenotypes were obtained. Trivariate linear mixed-effects models were used to estimate simultaneously the levels and rates of change of motor abilities, daily physical activity and cognition, and the correlations of level of and change in all three outcomes were characterized by a joint distribution of the random effects.[8, 12] These models examined the simultaneous rate of change in daily physical activity, motor abilities and cognition over the same interval of time. Subsequent analyses controlled for demographic (age, sex, education).
Further analyses focused on the autopsy group. We aligned their data at the last visit before death. We added terms for all the postmortem indices together in this single model to examine which postmortem indices were independently associated with the rates of change of daily physical activity, motor abilities and cognition. The rates of change in both models were based on the repeated measures collected during the course of the study. Models were examined graphically and analytically and assumptions were judged to be adequately met. A priori level of statistical significance was 0.05. Programming was done in SAS version 9.4 (SAS Institute Inc, Cary, NC).[25]
3. RESULTS
3.1. Modeling longitudinal trajectories of three phenotypes in a single model
This study examined the longitudinal trajectories of cognition, motor abilities and total daily physical activity of 1007 older adults; 530 did not undergo autopsy and a subset of 477 underwent autopsy (Figure e1). Table 1 summarizes and compares the baseline demographic and clinical characteristics of these two groups. At baseline, the autopsy group was older, had a larger percentage of males and fewer years of formal education. The autopsy group had lower levels of baseline cognition and motor abilities and more individuals with cognitive impairment. Baseline total daily physical activity was similar in both groups.
Table 1.
Baseline Clinical Characteristics and Postmortem Indices in Autopsy Subset
| No Autopsy Mean (SD)/# (%) | Autopsy a Mean (SD)/# (%) | |
|---|---|---|
| Baseline Clinical Characteristics | ||
| Age (years) | 77.6 (7.06) | 84.1 (5.64) & |
| Female sex | 423 (80%) | 343 (72%) ^ |
| Education (years) | 15.4 (3.13) | 14.9 (2.88) & |
| Global motor score (standardized score) | 1.11 (0.22) | 0.94 (0.20) & |
| Global cognitive score (standardized score) | 0.26 (0.54) | −0.01 (0.56) & |
| Actigraphy (log) | 0.87 (0.62) | 0.84 (0.61) NS |
| Dementia (%) | 13 (2%) | 26 (5%) ^ |
| MCI (%) | 84 (16%) | 100 (21%) * |
| NCI (%) | 431 (82%) | 349 (73%) ^ |
| Postmortem Neurodegenerative Disease Pathology Present | ||
| Alzheimer’s disease pathology | 309 (64.8%) | |
| Parkinson’s disease pathology | 25 (5.2%) | |
| TDP-43 pathology | 183 (38.4%) | |
| Hippocampal sclerosis | 47 (9.8%) | |
| Postmortem Cerebrovascular Disease Pathology Present | ||
| Chronic macroinfarct | 183 (38.4%) | |
| Chronic microinfarct | 181 (38.0%) | |
| Arteriolosclerosis | 135 (28.3%) | |
| Cerebral amyloid angiopathy | 166 (34.8%) | |
| Atherosclerosis | 113 (23.7%) |
t-test was used to compare continuous measures and Chi-square was used for ordinal measures
p<0.05
p<0.01
p<0.001
Brain pathologies were dichotomized as discussed in the methods (Section 2.6).
The entire analytic cohort was followed for an average of 6 years (5.8 years, SD=3.11 years). The median number of follow-up exams for cognition was 6 (IQR 4; range 2–14); for motor abilities was 5 (IQR 4; range 2–13) and for total daily physical activity was 5 (IQR 4; range 2–13).
The single model that we employed provides results for fifteen different correlations between the six measures of level and rate of change of the three phenotypes. To simplify our discussion, we organized the results into three groups of correlations that are highlighted in different colors in Table 2. These groups included: a) correlations of baseline level of function of the three phenotypes (yellow), b) correlations of baseline function of each phenotype with the rate of change of all three phenotypes (orange) and c) correlations of simultaneous rates of change (slopes) of the three phenotypes (blue). Person-specific paths of change (grey lines) and mean paths of change predicted by the model (bolded black line) for all three phenotypes are illustrated in Figure 1 (upper row; A-C).
Table 2.
Associations of baseline and slopes of cognition, motor abilities and total daily physical activity
| Trivariate linear mixed-effects models in living older adults | |||||
|---|---|---|---|---|---|
| Mot bl | TDA bl | Cog slp | Mot slp | TDA slp | |
| Cog bl | 0.46* 0.38* |
0.18* 0.14* |
0.35* 0.27* |
0.17^ 0.11NS |
0.30* 0.19* |
| Mot bl | - | 0.43* 0.39* |
0.31* 0.12^ |
0.06NS −0.10NS |
0.41* 0.26* |
| TDA bl | - | 0.03NS −0.07NS |
0.09NS 0.01NS |
0.11NS 0.04NS |
|
| Cog slp | - | 0.63* 0.60* |
0.53* 0.47* |
||
| Mot slp | - | 0.70* 0.69* |
|||
This table shows the results from two trivariate models using data from all participants in the current analytic cohort (N=1007). This model provides correlations of level of function at the analytic baseline and rate of change (slopes) for three phenotypes, cognitive function (Cog); motor abilities (Mot) and total daily physical activity (TDA). The first model was unadjusted for demographic covariates (age, sex and education) and the second model was adjusted for demographic covariates. Upper correlation in each cell is the correlation in a model without adjustment for the demographic covariates, age, sex and education. Lower correlation in each cell is the correlation derived from a model that adjusted for demographic covariates. Each models yield fifteen pairs of correlations derived from modeling level and slopes of function for the three clinical phenotypes based on repeated clinical total daily physical activity measures during follow-up of about six years. Three groups of correlations are obtained: a) the correlations of the three phenotypes at BL (yellow); b) the six correlations between level of function at BL for each of the three phenotypes with the rates of change (slopes) of each of the three phenotypes (orange) and c) the correlations of the rates of change (slopes) between each of the three phenotypes (blue). Each cell shows the estimated correlation:
<0.001
<0.01
NS >0.05.
Figure 1. Simultaneous decline of cognition, motor abilities and total daily activity.
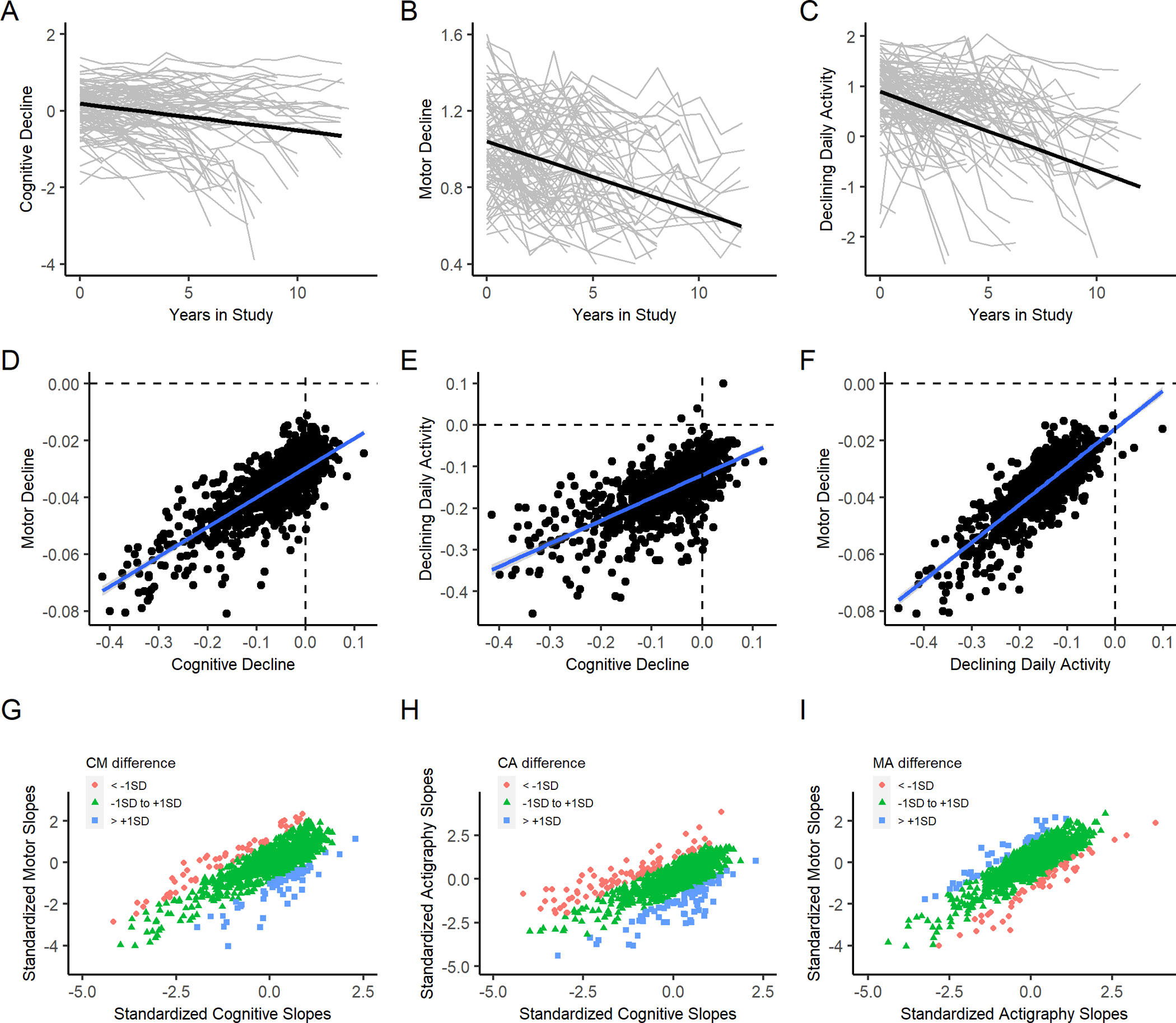
Upper Row (A-C) show the longitudinal trajectories of repeated measures of cognition (A) motor abilities (B) and total daily physical activity (C) during the study. Crude longitudinal trajectories of repeated measures of each of the three phenotypes (gray lines) and mean paths of decline predicted for each phenotype (black lines). Middle Row (D-F) shows the inter-relationship between the slopes extracted from a single trivariate model that examined the simultaneous rates of change for the three phenotypes. Each panel in the row shows a scatter plot of participants based on their person-specific rate of change (slope) in a pair of the examined phenotypes (black circles) and a regression line (blue) is superimposed on each of the three panels. D shows the slopes of cognitive decline (X axis) and motor decline (Y axis); E shows the slopes of cognitive decline (X axis) and total daily activity and F shows the slopes of declining daily activity (X axis) and motor decline (Y axis). Bottom Row (G-I): Using standardized slopes, the bottom row extends the associations illustrated in the middle row to show the extent of discrepancy between the three pairs of slopes. Slopes were considered non-discrepant if the pair of slopes was within 1 SD of one another (green). Slopes were discrepant their slopes were greater than 1 SD (blue) or less than 1 SD (red). As in the middle row, G) shows the slopes of cognitive-motor decline (CM); H shows the slopes of cognitive-daily physical activity decline (CA); I shows the slopes of motor-daily physical activity decline (MA). About 80% (n=775, 77%) showed non-discrepant slopes.
3.2. Associations of baseline level of function of the three phenotypes
The three pairs of baseline associations were modest (Table 2). Estimated percent shared variance can be obtained by squaring the correlation and multiplying by 100. At baseline, individuals with poorer motor impairment tended to have lower daily physical activity but shared only about 20% of the estimated variance. Baseline cognition was related to baseline motor abilities with 20% of shared variance. In contrast, cognition and daily physical activity were weakly correlated with only about 3% shared variance. These associations were unchanged after controlling for age, sex, and education (Table 2).
3.3. Associations of baseline function and rates of change in the other phenotypes.
On average, the log-transformed total daily physical activity decreased by about 0.16 units/yr (Estimate −0.158, S.E 0.004, p<0.001); motor abilities decreased by about 0.04 units/yr (Estimate −0.037, S.E. 0.0008, p<0.001); cognition declined about 0.07 units/yr (Estimate −0.070, S.E. 0.003, p<0.001).
To compare the rates of decline for the three phenotypes we calculated the rate of change as a percentage of the baseline SD of each phenotype. Comparing to the baseline SD, total daily physical activity on average decreased by about 27%/yr, motor abilities on average decreased by about 18% /yr and cognition on average declined about 21%/yr.
Baseline level of function showed less consistent associations with the rate of change in the other phenotypes. Baseline daily physical activity was not associated with change in any of the three phenotypes. In contrast baseline cognition was associated with the rate of change of all three phenotypes but accounted for 12% or less of shared variance. Baseline motor abilities was related to the rate of change in cognition and daily physical activity accounted for about 15% or less of shared variance. (Table 2).
3.4. Associations of the rates of change of the three phenotypes
All individuals in the analytic cohort showed negative slopes for motor abilities, and all but three individuals showed a negative slope for total daily physical activity. In contrast, while most individuals (829 of 1007, 82%) showed a negative slope for cognition (−0.09, SD, 0.078) almost 20% did not show declining cognition as illustrated by the inspection of the many values observed to the right of the vertical dashed showing individuals with cognitive slopes greater than zero (Figures 1D & 1E).
The rates of change of the three phenotypes showed the strongest correlations (Table 2) ranging from 0.53 to 0.70 accounting for 28%-49% of shared variance. In further modeling we added terms to control for age, sex and education. Adding demographic terms did not change the associations described above (Table 2). Increasing age was associated with faster decline of all three phenotypes but not sex or education (Table e1).
3.5. Frequencies of person-specific slope discrepancies between the three phenotypes
Discrepancies in the person-specific slopes of the three phenotypes are difficult to appreciate in the figures showing their correlated decline Figure 1 (middle row). We applied a previously published approach to the slope data to quantify the extent that the three pairs of person-specific slopes are discrepant.[26] First, we standardized the three slopes. If a pair of slopes were within 1 SD then they were characterized as non-discrepant are shown in green; if one of slopes was more than +1 SD higher than the other, the pair was considered discrepant shown in blue and if one of the slopes was less than −1 SD than the other it is also discrepant but is shown in red.
Almost 80% of adults (n=775, 77%) showed non-discrepant green slopes (Cognitive-Motor n=893, 89%; Cognitive-Activity n=844, 84%; Motor-Activity n=922, 92%). There were about 10% of individuals with cognitive slopes greater than +1 SD than one or both motor phenotypes (blue) and 10% with cognitive slopes less than −1 SD from one or both of the motor phenotypes (green). These data can be visualized in the lower row of Figure 1 (lower row) highlighting the heterogeneity of the different pairs of slopes in individuals that cannot be appreciated in the figures shown in the middle row of Figure 1.
The varied combinations and frequencies of the three pairs of slopes observed are shown in a bar chart (Table e2). In an effort to visualize the pairs of discrepant slopes for the three phenotypes, we stratified the rates of decline for each of the three phenotypes based on whether their slope was above or below the its median rate of decline (Table e3). It was difficult to visualize the slope discrepancies between cognition and both motor phenotypes at the same time with a 3D figure (Figure e2). To address this difficulty, since there were two motor phenotypes that could be discrepant from one another as well as from cognition, we defined three patterns of motor decline. Fast motor decline (blue) was present if both rates of decline were below their median; slow motor decline was present if both rates of decline were above their median (green) and intermediate motor decline was present if one motor phenotype was below and the other was above their median rate of decline (scarlet).
We examined the relationship of slow or fast cognitive decline with the three patterns of motor decline. Figure 2 illustrates the slope discrepancies for adults with fast and slow cognitive decline based on the median slope for cognitive decline. About 75% of adults with fast cognitive decline showed fast motor decline of both motor abilities and daily physical activity. In contrast, there was slightly more discrepancies between slopes of individuals with slow cognitive decline and declining motor phenotypes as only about 60% with slower cognitive decline also showed slower motor decline.
Figure 2. Frequency of slow and fast cognitive decline with motor decline e.
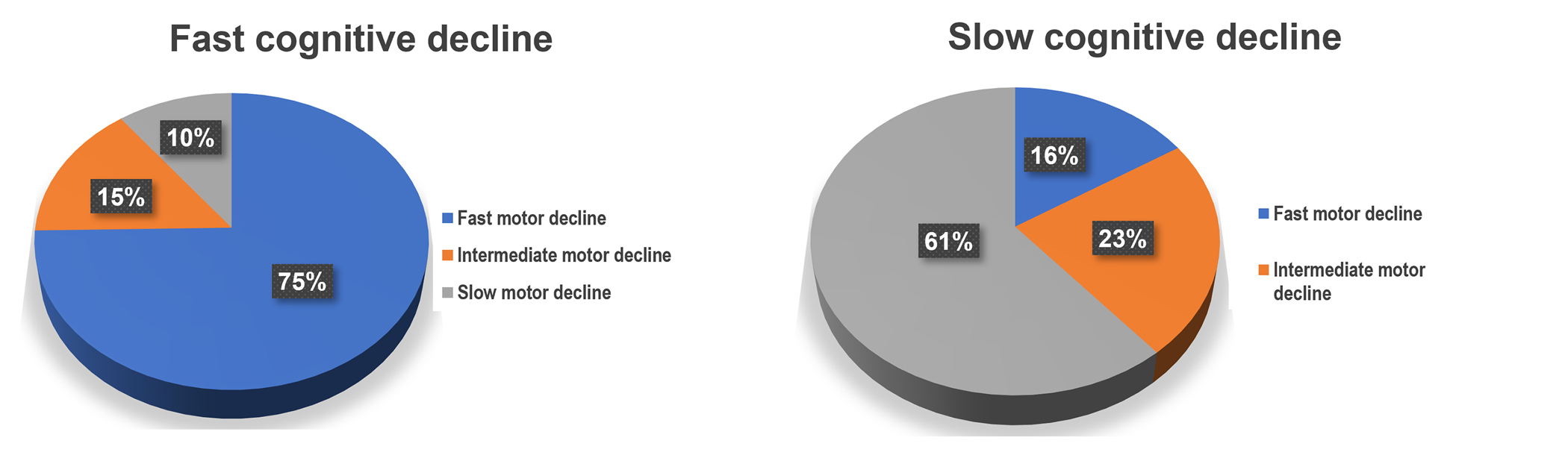
To visualize the inter-relationship of declining cognition and both motor phenotypes, we stratified the rates of decline based on slopes above or below the median rate of decline for each of the three phenotypes. Since the two motor slopes might vary from one another, we defined three patterns of motor decline. Fast motor decline was present if both rates of motor decline were below the median; slow motor decline was present if both rates of decline were above the median; intermediate motor decline was present if one phenotype was below and the other was above their median rate of decline. Most adults with faster cognitive decline, showed faster decline of both motor phenotypes. While, most older adults with slower cognitive decline also showed slower motor decline, a larger percentage showed discrepancies between the rates of slow cognitive decline and motor decline.
3.6. Postmortem ADRD pathologies and trajectories of all three clinical phenotypes
A shared pathologic basis might account for the strong association between declining daily physical activity, motor abilities and cognitive function. There were 477 of 1007 cases included in these analyses who underwent a brain autopsy with a median postmortem interval of 9.7 hours (SD=9.21 hours). Average age at death 91.8 years (SD=5.94 years). Follow-up averaged 7.1 years (SD=3.1 years). On average, last cognitive testing was 1.2 (SD=1.26) years before death; last motor testing was 2.3 (SD=1.92) years before death and last daily physical activity testing was 2.5 (SD=2.25) years before death.
At death, 65% (n=309) of participants showed postmortem changes consistent with a pathologic diagnosis of AD based on a modified National Institute on Aging and Alzheimer’s Association (NIA-AA) criteria.[18] For descriptive purposes we dichotomized the presence or absence of each of the nine pathologies as described above (2.6). The frequencies of each of the nine brain pathologies are summarized in Table 1. The median number of ADRD pathologies in participants was 3 (interquartile range 2,4); about 80% of participants having at least 2 pathologies (Number of pathologies: 0, 5%; 1, 17%, 2, 24%, 3, 22%, 4, 18%, 5, 9%, 6 or 7 6%). As illustrated in Table e4, there were 149 unique combinations of these nine pathologies; more than half of these combinations (80/149, 54%) were unique combinations that occurred in only one decedent.
3.7. Associations of levels and rates of change for all three phenotypes in decedents
We repeated the model described above in the entire analytic cohort (Sections 3.2–3.4) to examine the inter-relationship of the simultaneous rates of change of the three phenotypes in the subset of decedents who underwent autopsy at the time of death.
As observed in our analysis of the entire analytic cohort, the strongest association from the 15 correlations obtained from the trivariate model in decedents were the associations of the three slope measures ranging from 0.50–0.70. The twelve other correlations obtained from the trivariate model focusing on decedents were stronger and more consistent in all three groups of correlations.
3.8. Associations of ADRD pathologies with level and rates of change of the phenotypes
In further analyses, to examine associations of ADRD pathologies with the longitudinal trajectories of all three phenotype, we added nine terms for each of the ADRD pathologies indices measured as well as their interaction with time, the rate of change of the clinical phenotypes. The results for this single complex model are summarized in two tables, one focusing on the level and slopes of the clinical trajectories of all three phenotypes and the second focuses on the associations of ADRD pathologies with the level and slopes of and the clinical trajectories of all three phenotypes.
Table 3 summarizes the results for the 15 correlations of the levels and slope measures for all three phenotypes in decedents in models with demographic covariates alone and with the addition of terms for the nine ADRD pathologies. The inclusion of terms for ADRD pathologies did not change the 15 correlations between the levels and rates of change in all three phenotypes in decedents were unchanged after the addition of terms for ADRD pathologies (Table 3).
Table 3.
Associations of baseline and slopes of cognition, motor abilities and total daily physical activity in autopsied decedents (n=477)
| Mot lv | TDA lv | Cog slp | Mot slp | TDA slp | |
|---|---|---|---|---|---|
| Cog lv | 0.60* 0.59* |
0.42* 0.37* |
−0.79* −0.86* |
−0.51* −0.51* |
−0.44* −0.43* |
| Mot lv | - | 0.68* 0.71* |
−0.44* −0.45* |
−0.47* −0.43* |
−0.62* −0.63* |
| TDA lv | - | −0.35* −0.29* |
−0.48* −0.52* |
−0.85* −0.83* |
|
| Cog slp | - | 0.54* 0.56* |
0.53* 0.50* |
||
| Mot slp | - | 0.63* 0.70* |
This table shows the results from two trivariate models using data from decedents (n=477) that examined the correlation of the level of function at last visit before death (LV) and rate of change (slopes) for three phenotypes, cognitive function (Cog); motor abilities (Mot) and total daily physical activity (TDA). The first model (upper correlation in each cell) included terms for demographic covariates (age, sex and education) and nine terms for ADRD pathologies and their interaction with time. The results of each of the individual pathologies and the rate of change in each of the three phenotypes are shown in Table 3. To compare the results in decedents alone to the entire analytic cohort, the lower correlation in each cell shows the results from a second trivariate model in decedents adjusted for age, sex and education and their interaction with time without terms for ADRD pathologies. The model in decedents for the results in the lower portion of each cell is equivalent to the model results also shown in the lower portion of each cell for the entire cohort in Table 1. The table shows fifteen pairs of correlations derived from modeling level of function for the three clinical phenotypes based on repeated clinical measures during study follow-up of about six years. Three groups of correlations are derived: a) the correlations of the three phenotypes at BL (yellow); b) the six correlations between level of function at last visit before death (LV) for each of the three phenotypes with the rates of change (slopes) of each of the three phenotypes (orange) and c) the correlations of the rates of change (slopes) between each of the three phenotypes (blue).
<0.001
<0.01
NS >0.05
Table 4 summarizes the associations of the ADRD pathologies with levels and rates of decline for the trivariate model that included both terms for demographics and ADRD pathologies. ADRD pathologies showed that ADRD pathologies were associated with faster decline for all three phenotypes. Yet, the specific pathologies associated with each of the phenotypes varied and there was minimal overlap in their associations with the same ADRD pathologies. More rapid decline of daily physical activity was only associated with cerebral amyloid angiopathy (CAA). Declining motor abilities was faster in the presence of PD, hippocampal sclerosis and also CAA pathologies. A faster rate of cognitive decline was associated with five ADRD pathologies including AD, PD, TDP-43, HS, and arteriolosclerosis (Table 4).
Table 4.
Associations of brain pathologies with level and rate of change in daily physical activity, motor abilities and cognition *
| Pathology | Daily Physical Activity Estimate, (SE), p-Value | Motor Abilities Estimate, (SE), p-Value | Cognition Estimate, (SE), p-Value | |||
|---|---|---|---|---|---|---|
| Level before death | Rate of decline | Level before death | Rate of decline | Level before death | Rate of decline | |
| Parkinson’s disease pathology | −0.379 (0.220) 0.085 |
−0.048 (0.029) 0.101 |
−0.113, (0.047) 0.015* |
−0.021 (0.007) 0.002* |
−0.652, (0.185) <0.001* |
−0.081 (0.020) <0.001* |
| Alzheimer’s disease pathology | 0.077 (0.086) 0.371 |
−0.007 (0.010) 0.530 |
−0.037, (0.018) 0.034* |
−0.003 (0.002) 0.256 |
−0.964 (0.073) <0.001* |
−0.100 (0.008) <0.001* |
| TDP-43 | −0.018 (0.109) 0.869 |
0.005 (0.013) 0.674 |
−0.006 (0.022) 0.770 |
−0.005 (0.003) 0.103 |
−0.245 (0.093) 0.009* |
−0.020 (0.010) 0.038* |
| Hippocampal Sclerosis | −0.386 (0.175) 0.027* |
−0.029 (0.020) 0.144 |
−0.108 (0.037) 0.003* |
−0.010 (0.004) 0.020* |
−0.832 (0.148) <0.001* |
−0.057 (0.015) <0.001* |
| Macroinfarcts | −0.200 (0.105) 0.056 |
−0.023 (0.013) 0.074 |
−0.010 (0.021) 0.135 |
−0.0001 (0.003) 0.761 |
−0.093 (0.100) 0.303 |
−0.011 (0.009) 0.256 |
| Microinfarcts | −0.015 (0.102) 0.879 |
0.011 (0.012) 0.345 |
−0.051 (0.021) 0.015* |
−0.002 (0.003) 0.543 |
−0.143 (0.088) 0.103 |
−0.006 (0.009 0.528 |
| Atherosclerosis | −0.093 (0.121) 0.439 |
−0.003 (0.015) 0.851 |
−0.037 (0.025) 0.134 |
−0.002 (0.003) 0.517 |
−0.179 (0.104) 0.085 |
−0.014 (0.012) 0.187 |
| Arteriolosclerosis | −0.083 (0.113) 0.461 |
−0.019 (0.013) 0.146 |
−0.060 (0.023) 0.010* |
−0.005 (0.003) 0.102 |
−0.203 (0.100) 0.036* |
−0.022 (0.010) 0.027* |
| Cerebral amyloid angiopathy | −0.170 (0.107) 0.112 |
−0.027 (0.013 0.013* |
−0.012 (0.022) 0.568 |
−0.006 (0.003) 0.039* |
0.027 (0.091) 0.764 |
−0.004 (0.009) 0.642 |
Estimated from a single trivariate random coefficient model which included terms for baseline and rate of change in total daily physical activity, motor abilities and cognition from 477 decedents with postmortem brain exam. This model included terms for demographics (age, sex, education) and 9 indices of ADRD pathologies and their interactions with the rate of change in in total daily physical activity, motor abilities and cognition. Each column shows the associations for the nine pathologies and the level and rate of decline for one of the three clinical phenotypes. Each cell shows the estimate, standard error (S.E.) and p-Value for either the association of the level of function at the last visit before death or the rate of clinical decline during the study and their association with the pathology listed in the beginning of the row in the left-hand column. P-values <0.05 are bolded. The terms for time before death, age, sex and education and their interaction with time before death that were included in the model are not shown.
One of nine ADRD pathologies was associated with total daily physical activity decline; this accounted for 3% of the variance of the rate of change in total daily physical activity as compared to demographics alone; 3 of nine ADRD pathologies accounted for 9% of the variance of the rate of change in motor abilities and 5 of nine ADRD pathologies accounted for 42% of the variance of the rate of change in cognitive decline (Figure 3).
Figure 3. Variance of declining cognitive, motor abilities and daily physical activity accounted for by ADRD brain pathologies.
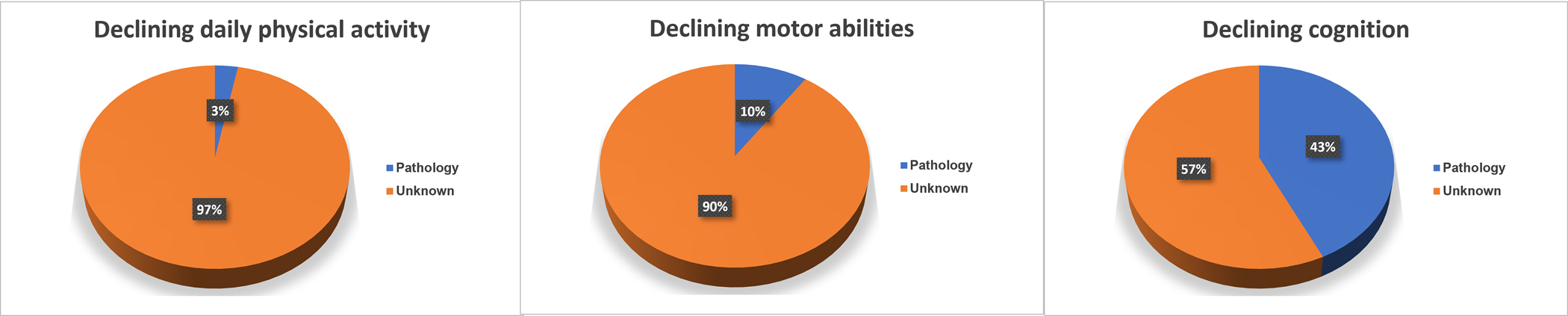
This figure summarizes the additional variance of decline explained by the measures of nine brain pathologies (blue) as compared to a model with demographic measures age, sex, and education and their interaction with the annual rate of change alone for each of the three phenotypes. The majority of decline especially for motor phenotypes remained unexplained.
3.9. Slopes of phenotypic decline and the odds of adverse health outcomes at the last visit.
We examined a series of regression models controlling for age, sex and education that compared the odds of four adverse health outcomes at last visit proximate to death. We standardized the slopes for each of the three phenotypes so that their effect size could be compared. We examined each of the slopes alone and then in a single model with terms for all three slopes.
Examining the three standardized slopes in separate models showed that less negative slopes for all three phenotypes were associated with a reduced odds of each of the four adverse health outcomes (MCI, AD dementia, mobility disability and parkinsonism) at the last visit during this study. Closer inspection of the estimates showed that a slower slope of cognitive decline was the strongest predictor of a lower odds of MCI or ADD at the last visit. In contrast, slower decline of total daily physical activity was the strongest predictor of a lower odds of mobility disability and parkinsonism at the last visit. Similar findings were observed when all three slopes were included in a single model (Table 5).
Table 5.
Associations of slopes and probability of adverse health outcomes at last visit
| Outcome | Slope term from Table 1 | Model 1 OR (95th CI) Estimate (S.E., p-Value) |
Model 2 OR (95th CI) Estimate (S.E., p-Value) |
Model 3 OR (95th CI) Estimate (S.E., p-Value) |
Model 4 OR (95th CI) Estimate (S.E., p-Value) |
|---|---|---|---|---|---|
| Mild cognitive impairment | Cognition | 0.32 (0.26, 0.39) −1.141 (0.107, <0.001) |
0.22 (0.16, 0.29) −1.532 (0.154, <0.001) |
||
| Motor abilities | 0.59 (0.51, 0.69) −0.525 (0.078, <0.001) |
1.67(1.211,2.31) 0.514(0.166,0.002) |
|||
| Daily physical activity | 0.62 (0.53, 0.73) −0.476 (0.080, <0.001) |
0.94 (0.71,1.24) −0.061 (0.142, 0.668) |
|||
| Alzheimer’s disease dementia | Cognition | 0.09 (0.06, 0.12) −2.437 (0.181, <0.001) |
0.07 (0.05, 0.12) −2.59 (0.238, p<0.001) |
||
| Motor abilities | 0.24 (0.19, 0.30) −1.427 (0.116, <0.001) |
1.50 (0.91, 2.47) 0.408 (0.254, 0.108) |
|||
| Daily physical activity | 0.32 (0.26, 0.39) −1.154 (0.106, <0.001) |
0.75 (0.50, 1.14) −0.285 (0.212, 0.179) |
|||
| Mobility Disability | Cognition | 0.60 (0.47, 0.77) −0.507 (0.122, <0.001) |
0.63 (0.44, 0.90) −0.462 (0.800 0.010) |
||
| Motor abilities | 0.73 (0.60, 0.89) −0.311 (0.102, 0.002) |
2.87 (1.901, 4.331) 1.054 (0.210, <0.001) |
|||
| Daily physical activity | 0.44 (0.34, 0.56) −0.828 (0.124, <0.001) |
0.24 (0.17, 0.355) −1.418 (0.195, <0.001) |
|||
| Parkinsonism | Cognition | 0.67 (0.57, 0.78) −0.400 (0.079, <0.001) |
0.52 (0.41, 0.669) −0.653 (0.128, <0.001) |
||
| Motor abilities | 0.83 (0.72, 0.95) −0.191 (0.071, 0.007) |
3.53 (2.52, 4.95) 1.263 (0.172, <0.001) |
|||
| Daily physical activity | 0.56 (0.48, 0.66) −0.560 (0.084, p<0.001) |
0.28 (0.21, 0.379) −1.266 (0.150, <0.001) |
Each row shows the probability of one of the four outcomes listed in the first column on the left and shows a series of logistic models including terms for age at death, sex and education (not shown) as well as the slope terms extracted from the trivariate model described in the text. Each cell shows the odds ratio and the 95th% confidence interval and the estimate, standard error (S.E.) and p-Value for the slope terms included in each of the four models. Inspection of the model estimates (Models 1–3) show that a slower slope of cognitive decline was the strongest predictor of a lower odds of MCI or ADD at the last visit. In contrast, slower decline of total daily physical activity was the strongest predictor of a lower odds of mobility disability and parkinsonism at the last visit. Similar findings were observed when all three slopes were included in a single model. When all three phenotypes were included in the same model, we noticed the estimates of the slopes for motor abilities all flipped from negative to positive. This is likely due to the correlation among the three phenotypes. Note that the magnitude of the coefficients of motor abilities are always smaller than the strongest predictor with negative coefficients. We can interpret the positive coefficients of motor abilities as a difference term between the strongest predictor and motor abilities. Specifically, we found that the risk of MCI and ADD depend not only on the slope of cognitive decline itself but also the relative difference of the cognitive and motor decline, i.e., those whose cognition declined slower than motor abilities had lower risk of MCI and ADD at the last visit. The slope of total daily physical activity did contribute to the probability of MCI or ADD when the slope of cognition and motor abilities were in the same model. In contrast, a slower rate of decline for total daily physical activity was the stronger predictor of a lower probability of mobility disability and parkinsonism than either the slope of cognition or motor abilities.
4. DISCUSSION
Many older adults manifest simultaneous decline in several cognitive and motor phenotypes. Therefore, this study employed a single model to assess the correlated decline of total daily physical activity, motor abilities and cognitive function in about 1000 community-dwelling older adults followed for about six years. Most participants manifested progressive decline of all three phenotypes that was more rapid with increasing age. Of the multiple correlations derived from our modeling, the slopes of the three phenotypes showed the strongest correlations. When slopes were modeled together, cognition was the main driver of the odds of cognitive impairment during the study, while total daily physical activity was the main driver of the odds of mobility disability or parkinsonism. This suggests that incorporating slope measures into risk models could facilitate targeted treatments for distinct adverse outcomes. In decedents undergoing autopsy, all three phenotypes showed faster decline with a higher burden of ADRD pathologies. Yet, there was minimal overlap between the pathologies shared by the three phenotypes. This suggests that the accumulation of ADRD pathologies in aging brains only accounts for a small minority of the correlated cognitive and motor decline observed. Further work is needed to elucidate the biology underlying correlated decline in aging adults.
The current study extends prior studies in several important ways. First, we report the person-specific rates of changes of sensor metrics that capture all exercise and habitual physical activity during continuous recordings of daily living of older adults. This circumvents the limitations of self-report questionnaires used in the very old adults. Second, in addition to quantitative metrics of physical activity, our analyses assessed motor abilities that is related but distinct motor phenotype.[27] The associations of daily volitional physical activity and its potential person-specific benefits are likely to vary with an individual’s underlying cognitive and motor abilities.[28–30] Thirdly, person-specific changes in daily physical activity, motor abilities and cognition are not independent, but rather occur in the same individual and are affected by many of the same environmental, medical, genetic and physiologic risk factors. To match this interdependency, in contrast to prior modeling, we provide novel data about the correlated change of three related phenotypes using a single model. [8] This modeling approach allowed us to characterize the extent to which the slopes of different pairs of phenotypes might be discrepant i.e., that one of the slopes decline faster or slower than the other. Fourth, leveraging the postmortem data available in the subset of decedents, we provide novel data about whether ADRD brain pathologies are differentially associated with the person-specific levels and slopes for all three clinical phenotypes using clinical and pathology measures from the same individuals.[31, 32] Fifth, few studies have examined whether simultaneous derived slopes of three important aging phenotypes from the same individuals are differentially associated with varied adverse health outcomes.
The model used in this study assessed the levels and person-specific slopes of the trajectories of three phenotypes. The model provides 15 different correlations between levels and slopes for each of the three phenotypes. Inspection of these different correlations showed that the strongest associations were between the three slope measures. Similar findings were observed for a prior study that employed bivariate modeling of aging phenotypes.[8] Similarly, the strongest associations from a model limited to decedents were between the three slope measures. Yet, unexpectedly, the other 12 correlations in the matrix from decedents showed stronger and more consistent correlations when compared to our primary analysis from the entire analytic cohort that included twice as many individuals.
The differences between the correlations observed in the entire cohort and decedents may derive from how the data was aligned for both models. Both models in the decedents and the entire cohort used the same longitudinal data. The only difference in the two models is that in decedents the intercept used to align the data was the level of function at the time of death and the intercept used in the entire cohort was level of function at the analytic baseline. It is likely that closer to death, the phenotypic heterogeneity among participants may decrease due to shared biologic mechanisms that contribute to death as compared to their level differences at baseline. This would explain why the primary difference between the two matrices are the 12 correlations that examine the associations among levels and slopes and not the correlations among the three pairs of slopes. This notion is supported by prior work that has shown that heterogeneous phenotypes may show convergence of their trajectories closer to impending death.[33] So, in addition to biologic factors that may contribute to the heterogeneity of motor and cognitive decline, how the data is aligned may impact results that are obtained.
This study provides novel data about the associations of nine ADRD pathologies with the rates of decline of three phenotypes in the same individuals. The current analyses emphasize that ADRD pathologies have a negative effect not only on cognition, but can also adversely affect non-cognitive phenotypes in the same individual.[10] These data also draw attention to the fact that like dementia, motor decline in old adults are not commonly due to a single brain pathology.[17] Similar to our prior studies that examined these phenotypes separately, the variance of decline accounted for by ADRD pathologies in the current single model ranged from more than 40% of the variance of cognitive decline to less than 10% for either of the two motor phenotypes.[1, 34, 35] This highlights the need to determine if degenerative changes in motor systems outside the brain more fully explain the pathologic basis of late-life motor decline.[23, 36]
Prior studies have generally examined the associations of ADRD pathologies with a single phenotype. [31, 32, 35, 37] A novel aspect of the current study was analysis leveraging available postmortem indices of ADRD pathologies seeking to identify shared pathologies that may account for the correlated decline of the three clinical phenotypes. Results from the single model that was employed emphasizes the differential associations of varied ADRD pathologies with the three clinical phenotypes. There was minimal overlap in the pathologies associated with these phenotypes and no single pathology was related to all three phenotypes (Table 4). The minimal overlap of shared pathologies and the small percentage of variance accounted for motor phenotypes, suggests that the strong correlated decline between these phenotypes is unaccounted for by the negative effect of shared ADRD pathologies. Moreover, the varied combinations of ADRD pathologies that accumulate in aging brains highlight the difficulty in identifying common combinations of brain pathologies (Table e4). These results emphasize the need for further work focusing on genes and proteins that may not have a “pathologic footprint” but may contribute to the correlated decline of these phenotypes.[38]
The current results extend recent work which has highlighted the importance of dual decline for identifying adults at risk of adverse health outcomes.[6] Motor abilities are robust but non-specific predictors of adverse health outcome.[39, 40] In prior work, we found that adding baseline motor abilities to models that include terms for cognitive function did not appreciably improve the prediction of Alzheimer’s dementia (ADD) or MCI.[41] In the current study we found that models of all three slopes alone or together showed that slower cognitive decline was the strongest predictor of a lower odds of ADD or MCI during the study. This is consistent with our prior study that focused on baseline data alone.[41] In contrast, total daily physical activity was the strongest predictor of the odds of mobility disability and parkinsonism during the study. Thus, incorporating slope measures into risk models may improve the identification of adults at risk for distinct adverse health outcomes, offering the potential for targeted treatments.
This study has important limitations. First, participants were very old and findings may not translate to younger older adults. The participants in these studies were selected and longitudinal studies of more diverse populations are needed. Brain imaging was not examined and structural changes may be an important contributor of correlated decline. Most of the post-mortem indices were obtained from traditional cognitive brain regions and may underestimate the association of pathologies with motor phenotypes. The study also has strengths that lend confidence in the findings. Large numbers of men and women were tested. The availability of post-mortem indices in a large number of decedents provides novel data about whether motor and cognitive decline share a common pathologic basis. However, this study cannot determine if the associations with pathology are causal or proxies for unmeasured variables.
CONCLUSION
Using a single model, this study highlights the correlated decline of total daily physical activity sensor metrics, motor and cognitive abilities observed in more than 1000 community-dwelling older adults. Incorporating these slope measures into risk models may lead to targeted treatments for distinct health outcomes. In decedents undergoing autopsy, all three phenotypes showed faster decline with a higher burden of ADRD pathologies. Yet, there was minimal overlap between the pathologies shared by the three phenotypes. This suggests that the accumulation of ADRD pathologies in aging brains only accounts for a small minority of the correlated decline documented in aging adults. Further work is needed to elucidate the biology underlying correlated decline of cognitive and motor phenotypes.
Supplementary Material
ACKNOWLEDGMENTS
We thank all the participants in the Rush Memory and Aging Project and Religious Order Study. We also thank the staff of the Rush Alzheimer’s Disease Center.
FUNDING SOURCES
This work was supported by National Institute of Health grants R01AG17917, R01AG79133, R01AG56352, R01AG75728, K01AG054700. The Illinois Department of Public Health. The funding organizations had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
CONFLICTS
The authors have no conflicts of interest to report for this manuscript.
CONSENT STATEMENT
The study was approved by the IRB of Rush University Medical Center. Written in-formed consent was obtained as well as uniform anatomical gift act. The study was conducted in accordance with the latest version of the Declaration of Helsinki.
REFERENCES
- [1].Buchman AS, Wilson RS, Yu L, James BD, Boyle PA, Bennett DA (2014) Total daily activity declines more rapidly with increasing age in older adults. Arch Gerontol Geriatr 58, 74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Buchman AS, Wang T, Yu L, Leurgans SE, Schneider JA, Bennett DA (2020) Brain pathologies are associated with both the rate and variability of declining motor function in older adults. Acta Neuropathol 140, 587–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zammit AR, Lei Y, Petyuk V, Schneider JA, De Jager PL, Klein HU, Bennett DA, Buchman AS (2022) Cortical Proteins and Individual Differences in Cognitive Resilience in Older Adults. Neurology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Buchman AS, Boyle PA, Yu L, Shah RC, Wilson RS, Bennett DA (2012) Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology 78, 1323–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Oveisgharan S, Dawe RJ, Leurgans SE, Yu L, Schneider JA, Bennett DA, Buchman AS (2020) Total daily physical activity, brain pathologies, and parkinsonism in older adults. PLoS One 15, e0232404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tian Q, Montero-Odasso M, Buchman AS, Mielke MM, Espinoza S, DeCarli CS, Newman AB, Kritchevsky SB, Rebok GW, Resnick SM, Thambisetty M, Verghese J, Ferrucci L (2023) Dual cognitive and mobility impairments and future dementia - Setting a research agenda. Alzheimers Dement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Clouston SAP, Brewster P, Kuh D, Richards M, Cooper R, Hardy R, Rubin MS, Hofer SM (2013) The Dynamic Relationship Between Physical Function and Cognition in Longitudinal Aging Cohorts. Epidemiologic Reviews 35, 33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Buchman AS, Yu L, Wilson RS, Boyle PA, Schneider JA, Bennett DA (2014) Brain pathology contributes to simultaneous change in physical frailty and cognition in old age. J Gerontol A Biol Sci Med Sci 69, 1536–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rothwell JC (2012) Overview of neurophysiology of movement control. Clin Neurol Neurosurg 114, 432–435. [DOI] [PubMed] [Google Scholar]
- [10].Albers MW, Gilmore GC, Kaye J, Murphy C, Wingfield A, Bennett DA, Boxer AL, Buchman AS, Cruickshanks KJ, Devanand DP, Duffy CJ, Gall CM, Gates GA, Granholm AC, Hensch T, Holtzer R, Hyman BT, Lin FR, McKee AC, Morris JC, Petersen RC, Silbert LC, Struble RG, Trojanowski JQ, Verghese J, Wilson DA, Xu S, Zhang LI (2015) At the interface of sensory and motor dysfunctions and Alzheimer’s disease. Alzheimers Dement 11, 70–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bennett DA, Buchman AS, Boyle PA, Barnes LL, Wilson RS, Schneider JA (2018) Religious Orders Study and Rush Memory and Aging Project. J Alzheimers Dis 64, S161–S189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sammel M, Lin X, Ryan L (1999) Multivariate linear mixed models for multiple outcomes. Statistics in Medicine 18, 2479–2492. [DOI] [PubMed] [Google Scholar]
- [13].Bennett DA, Wilson RS, Boyle PA, Buchman AS, Schneider JA (2012) Relation of neuropathology to cognition in persons without cognitive impairment. Ann Neurol 72, 599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Buchman AS, Wilson RS, Shulman JM, Leurgans SE, Schneider JA, Bennett DA (2016) Parkinsonism in Older Adults and Its Association With Adverse Health Outcomes and Neuropathology. J Gerontol A Biol Sci Med Sci 71, 549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rosow I, Breslau N (1966) A Guttman health scale for the aged. J Gerontol 21, 556–559. [DOI] [PubMed] [Google Scholar]
- [16].Buchman AS, Nag S, Shulman JM, Lim AS, VanderHorst VG, Leurgans SE, Schneider JA, Bennett DA (2012) Locus coeruleus neuron density and parkinsonism in older adults without Parkinson’s disease. Mov Disord 27, 1625–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Buchman AS, Yu L, Wilson RS, Leurgans SE, Nag S, Shulman JM, Barnes LL, Schneider JA, Bennett DA (2019) Progressive parkinsonism in older adults is related to the burden of mixed brain pathologies. Neurology 92, e1821–e1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Thies B, Trojanowski JQ, Vinters HV, Montine TJ (2012) National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimer’s and Dementia 8, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Nag S, Yu L, Wilson RS, Chen EY, Bennett DA, Schneider JA (2017) TDP-43 pathology and memory impairment in elders without pathologic diagnoses of AD or FTLD. Neurology 88, 653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Nag S, Yu L, Boyle PA, Leurgans SE, Bennett DA, Schneider JA (2018) TDP-43 pathology in anterior temporal pole cortex in aging and Alzheimer’s disease. Acta Neuropathol Commun 6, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nag S, Yu L, Capuano AW, Wilson RS, Leurgans SE, Bennett DA, Schneider JA (2015) Hippocampal sclerosis and TDP-43 pathology in aging and Alzheimer disease. Ann Neurol 77, 942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Arvanitakis Z, Leurgans SE, Barnes LL, Bennett DA, Schneider JA (2011) Microinfarct pathology, dementia, and cognitive Systems. Stroke 42, 722–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Buchman AS, Leurgans SE, Nag S, VanderHorst V, Kapasi A, Schneider JA, Bennett DA (2017) Spinal Arteriolosclerosis Is Common in Older Adults and Associated With Parkinsonism. Stroke 48, 2792–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yu L, Boyle PA, Nag S, Leurgans S, Buchman AS, Wilson RS, Arvanitakis Z, Farfel JM, De Jager PL, Bennett DA, Schneider JA (2015) APOE and cerebral amyloid angiopathy in community-dwelling older persons. Neurobiol Aging 36, 2946–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].SAS Institute Inc (2002–2003) SAS Institute Inc., Cary, NC. [Google Scholar]
- [26].Han SD, Boyle PA, James BD, Yu L, Barnes LL, Bennett DA (2016) Discrepancies between cognition and decision making in older adults. Aging Clin Exp Res 28, 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Buchman AS, Yu L, Wilson RS, Lim A, Dawe RJ, Gaiteri C, Leurgans SE, Schneider JA, Bennett DA (2019) Physical activity, common brain pathologies, and cognition in community-dwelling older adults. Neurology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Buchman AS, Yu L, Wilson RS, Lim A, Dawe RJ, Gaiteri C, Leurgans SE, Schneider JA, Bennett DA (2019) Physical activity, common brain pathologies, and cognition in community-dwelling older adults. Neurology 92, e811–e822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Buchman AS, Wilson RS, Leurgans SE, Bennett DA, Barnes LL (2015) Change in Motor Function and Adverse Health Outcomes in Older African Americans. Exp Gerontol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Fleischman DA, Yang J, Arfanakis K, Arvanitakis Z, Leurgans SE, Turner AD, Barnes LL, Bennett DA, Buchman AS (2015) Physical activity, motor function, and white matter hyperintensity burden in healthy older adults. Neurology 84, 1294–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Buchman AS, Yu L, Oveisgharan S, Farfel JM, Schneider JA, Bennett DA (2021) Person-Specific Contributions of Brain Pathologies to Progressive Parkinsonism in Older Adults. J Gerontol A Biol Sci Med Sci 76, 615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Boyle PA, Yu L, Wilson RS, Leurgans SE, Schneider JA, Bennett DA (2018) Person-specific contribution of neuropathologies to cognitive loss in old age. Ann Neurol 83, 74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wilson RS, Segawa E, Hizel LP, Boyle PA, Bennett DA (2012) Terminal dedifferentiation of cognitive abilities. Neurology 78, 1116–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Buchman AS, Dawe RJ, Yu L, Lim A, Wilson RS, Schneider JA, Bennett DA (2018) Brain Pathology Is Related To Total Daily Physical Activity In Older Adults. Neurology 90, e1911–e1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Boyle PA, Wang T, Yu L, Wilson RS, Dawe R, Arfanakis K, Schneider JA, Bennett DA (2021) To what degree is late life cognitive decline driven by age-related neuropathologies? Brain 144, 2166–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Buchman AS, Nag S, Leurgans SE, Miller J, VanderHorst V, Bennett DA, Schneider JA (2017) Spinal Lewy body pathology in older adults without an antemortem diagnosis of Parkinson’s disease. Brain Pathol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Buchman AS, Dawe RJ, Yu L, Lim A, Wilson RS, Schneider JA, Bennett DA (2018) Brain pathology is related to total daily physical activity in older adults. Neurology 90, e1911–e1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Buchman AS, Bennett DA (2022) Mixed Neuropathologies, Neural Motor Resilience and Target Discovery for Therapies of Late-Life Motor Impairment. Front. Hum. Neurosci. 16, 853330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Middleton A, Fritz SL, Lusardi M (2015) Walking speed: the functional vital sign. J Aging Phys Act 23, 314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Abellan van Kan G, Rolland Y, Andrieu S, Bauer J, Beauchet O, Bonnefoy M, Cesari M, Donini LM, Gillette Guyonnet S, Inzitari M, Nourhashemi F, Onder G, Ritz P, Salva A, Visser M, Vellas B (2009) Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging 13, 881–889. [DOI] [PubMed] [Google Scholar]
- [41].Yang J, Oveisgharan S, Liu X, Wilson RS, Bennett DA, Buchman AS (2022) Risk Models Based on Non-Cognitive Measures May Identify Presymptomatic Alzheimer’s Disease. J Alzheimers Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


